Abstract
Background
Posthepatectomy liver failure (PHLF) contributes significantly to morbidity and mortality after liver surgery. Standardized assessment of preoperative liver function is crucial to identify patients at risk. These European consensus guidelines provide guidance for preoperative patient assessment.
Methods
A modified Delphi approach was used to achieve consensus. The expert panel consisted of hepatobiliary surgeons, radiologists, nuclear medicine specialists, and hepatologists. The guideline process was supervised by a methodologist and reviewed by a patient representative. A systematic literature search was performed in PubMed/MEDLINE, the Cochrane library, and the WHO International Clinical Trials Registry. Evidence assessment and statement development followed Scottish Intercollegiate Guidelines Network methodology.
Results
Based on 271 publications covering 4 key areas, 21 statements (at least 85 per cent agreement) were produced (median level of evidence 2− to 2+). Only a few systematic reviews (2++) and one RCT (1+) were identified. Preoperative liver function assessment should be considered before complex resections, and in patients with suspected or known underlying liver disease, or chemotherapy-associated or drug-induced liver injury. Clinical assessment and blood-based scores reflecting liver function or portal hypertension (for example albumin/bilirubin, platelet count) aid in identifying risk of PHLF. Volumetry of the future liver remnant represents the foundation for assessment, and can be combined with indocyanine green clearance or LiMAx® according to local expertise and availability. Functional MRI and liver scintigraphy are alternatives, combining FLR volume and function in one examination.
Conclusion
These guidelines reflect established methods to assess preoperative liver function and PHLF risk, and have uncovered evidence gaps of interest for future research.
Posthepatectomy liver failure is a significant contributor to morbidity and mortality after liver resections, and a standardized assessment of preoperative liver function is crucial. These inaugural European consensus guidelines provide guidance for clinicians involved in the perioperative care of patients undergoing liver surgery. Based on 271 publications, 21 statements were produced through a structured modified Delphi process implementing systematic evidence assessment and expert opinion.
Introduction
Liver resection represents a potentially curative treatment option for a variety of primary and secondary hepatic malignancies. Hepatic surgery has become safer over recent decades as a result of the evolution in preoperative and intraoperative techniques and strategies, leading to decreased morbidity and mortality rates1,2. Posthepatectomy liver failure (PHLF), however, still remains one of the most frequent causes of major morbidity, with a reported incidence of between 9 and 30 per cent after extended resections. PHLF remains a major determinant of postoperative death3–6. Given the potentially fatal consequences of PHLF and the limited treatment options available, preoperative liver function assessment is paramount to identify patients at increased risk of PHLF, ultimately reducing negative outcomes. A comprehensive review7 of PHLF has pointed out the change in criteria and test modalities applied, the lack of uniform consensus on definition and standard reporting used in practice, as well as limitations in prevention and treatment for the condition. The currently most commonly accepted criteria for PHLF were proposed by the International Study Group of Liver Surgery (ISGLS)8,9. According to the ISGLS, PHLF is defined by an increased bilirubin level and international normalized ratio (INR) on or after postoperative day 5, and is graded by clinical severity (grades A, B, and C).
Multiple patient-, liver- and surgery-related factors are associated with the development of PHLF, as reflected by the variety of reported incidence rates. Parenchymal function and volume of the future liver remnant (FLR) are of major importance. Most liver function risk models, such as the Child–Pugh score, were originally established for assessing the severity of underlying liver disease. They were not developed to stratify the perioperative risk of PHLF and are therefore inaccurate for liver resections10,11. Scores based on posthepatectomy blood parameters, such as the peak 712, 50–5013, and 3–6014 criteria, or perioperative maximum lactate values15,16, have been shown to predict the development of severe PHLF in the early postoperative phase, but are not directly applicable to preoperative risk assessment.
The unmet need for universally applicable assessment tools is demonstrated by the large number of different approaches used in surgical units. Risk stratification with classical volumetric assessment of the FLR, calculated as a proportion of total liver volume17 or in relation to bodyweight18, serves as a widely accepted and standardized foundation for surgical planning. Volumetry might be sufficient in patients without any underlying liver disease. This method, however, does not give direct information on actual liver function. Patients more frequently present with increased age, co-morbidities19, and pre-existing liver parenchymal damage, for example owing to obesity or neoadjuvant systemic treatment20. With efforts to push the boundaries of liver surgery and advancing technical complexity21, preoperative quantification of liver function is necessary. Potential methods include use of clinical risk stratification algorithms22, established and novel biomarkers15,23, as well as various radiological24 and nuclear medicine imaging25 techniques, and functional liver tests26.
To date, no consensus recommendations have been proposed regarding the ideal tools for preoperative PHLF risk assessment in different clinical settings7. This inaugural European guideline, formulated through a structured consensus process that summarizes the published evidence and implements expert opinions, aims to provide guidance for individualized risk stratification in patients scheduled for hepatic resection.
Methods
Selection of committee members
The proposal and structural process of this consensus guideline have been described previously27. Briefly, the steering committee selected members of the expert panel, validation committee, and writing group based on their experience and expertise in the field of clinical, multidisciplinary management of patients undergoing liver surgery, and scientific contribution to liver function assessment and PHLF research. Care was taken to ensure a balanced mix of participants’ characteristics in terms of demographics (age, sex, country), clinical specialty, and experience. The guideline development involved liver surgeons, radiologists, nuclear medicine specialists, and hepatologists from 11 European countries. The formal process was overseen by an experienced statistician/epidemiologist. All individuals made a declaration of interest regarding potential financial and non-financial conflicts related to the guideline content.
Selection of key topics to develop in the consensus
After an exploratory PubMed literature review by the steering committee (March 2021), four key areas were identified covering different topics of preoperative liver function assessment: indications and timing of assessment; static blood markers; functional and morphological imaging; and functional and morphological non-imaging tests. Each area was stratified further into subtopics.
The experts were assigned to individual groups according to their scientific and clinical profile. Under the direction of elected group leaders, each group undertook a systematic literature search of the PubMed/MEDLINE database, Cochrane library, and the WHO International Clinical Trials Registry Platform between June and August 2021 according to prespecified Medical Subject Heading (MeSH) search terms, expanded by individual keywords related to subtopics. The publications were prefiltered according to the inclusion and exclusion criteria (supplementary material and Table S1).
Methodology for guideline development
The methodology followed a previously described process28. Three validated methods were integrated: the Scottish Intercollegiate Guidelines Network (SIGN) methodology for the assessment of evidence and development of guideline statements29; the modified Delphi method for achieving expert consensus30,31; and the AGREE II Global Rating Scale instrument32 for assessment of methodological quality and external validation of final statements.
Inclusion and grading of evidence
Included studies were assessed and graded according to SIGN methodology by evaluating the study quality and evidence level according to the SIGN grading system (Tables S2 and S3). The resulting evidence tables were reviewed by the steering committee and the methodologist to ensure correct application of the inclusion and exclusion criteria and gradings. The working groups then created considered judgement forms to summarize the evidence, quality ratings, limitations and strengths of individual studies, resulting statements, strength of recommendations, and future areas of scientific interest.
Modified Delphi process
The key questions and proposed guideline statements with attached judgement forms were sent out to the whole expert panel for a stepwise Delphi process with anonymous voting. It allowed every member to agree or disagree with the statement, and to make comments and recommendations for changes. An agreement level of 85 per cent in each Delphi stage was considered appropriate to ensure a balance between consensus and voting progress. In a total of three Delphi rounds, statements reaching 85 per cent agreement were excluded from further discussion, whereas statements scoring below this level were reviewed by the steering committee and working group leaders, and revised accordingly for inclusion in the next Delphi round.
At the final virtual preconference meeting, the remaining statements were again discussed and reviewed, and then adopted for the on-site conference. This conference was held as part of the Surger-I-nnsbruck International Meeting on Liver Surgery on 26–27 May 2022 in Innsbruck, Austria. Here, all key questions, statements, and preliminary paragraphs drafted by the four groups were summarized with their underlying evidence, and presented to the committee and audience. All congress participants could discuss and ultimately vote electronically on their individual support for the statements. The presentations, discussions, and voting results were reviewed by the validation committee, which provided final thoughts and comments on each statement before endorsing it. This final voting of the conference participants and the validation committee was considered by the writing and steering committee for refinement of the wording of individual statements and expert comment paragraphs for the published manuscript.
Validation
The entire guideline process was quality controlled by the validation committee (Supplementary Figures). All members of the panel and a patient representative (G. Lobenscheg, from patient support group L(i)eberleben, Tyrol, Austria) reviewed the draft before submitting it for publication. The editorial staff of the publisher was involved during the process in terms of formal structure and layout27. The guideline development was endorsed by the Scientific and Research Committee of the European–African Hepato-Pancreato-Biliary Association (E-AHPBA), the Board of Directors of the European Society of Surgical Oncology (ESSO), and the Secretary General of the European Society for Surgical Research (ESSR).
Overall results of Delphi process
A total of 10 814 publications were reviewed by the four working groups, resulting in final inclusion of 271 included manuscripts (Supplementary Results—Literature Review).
After three online Delphi rounds, all but one statement had achieved the 85 per cent agreement level. This statement was reviewed, discussed, and then finally included after reaching sufficient agreement during the on-site conference. All 21 statements were supported by the validation committee and included in the document after final revision. The overall methodological quality of guideline development was considered high to very high by all members of the validation committee (Supplementary Figures).
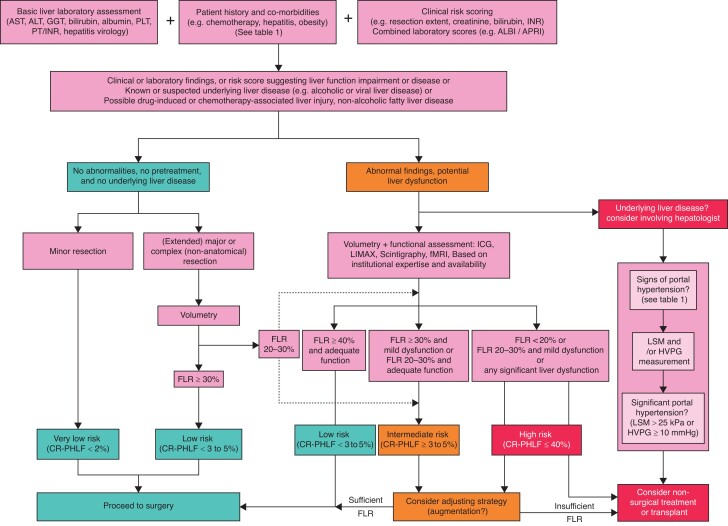
Each clinical question is answered with a short overall statement, including the grade of recommendation and the level of evidence, followed by an expert panel comment including a selection of relevant publications related to the question. A complete list of references for each topic can be found in Supplementary Results—Literature Review. The results are concluded by a proposed clinical algorithm (Fig. 1 and Table 1), and a list of future research questions (Table 2).
Fig. 1.
Clinical algorithm for preoperative liver function assessment in patients in whom hepatectomy is planned 7,17,22,23,33–42
ALBI, albumin–bilirubin grade; ALT, alanine aminotransferase; APRI, aspartate aminotransferase/platelet ratio index; AST, aspartate aminotransferase; CR-PHLF, clinically relevant posthepatectomy liver failure; FLR, future liver remnant; fMRI, functional MRI; GGT, γ-glutamyl transferase; HVPG, hepatic venous pressure gradient; ICG, indocyanine green; LSM, liver stiffness measurement; PLT, platelet count; PT/INR, prothrombin time/international normalized ratio.
Table 1.
Important clinical considerations for functional assessment
|
|
|
|
CALI, chemotherapy-associated liver injury; SOS, sinusoidal obstruction syndrome; NASH, non-alcoholic steatohepatitis.
Table 2.
Future research questions
| Area of research | Comments |
|---|---|
| General topics | |
| Comparison of different functional tests | Design studies with high-level evidence comparing different functional tests (such as established and exploratory blood markers, ICG, LiMAx®, scintigraphy or functional MRI) in different cohorts and surgical settings |
| Timing of liver assessment | Try to define the ideal time point for liver function assessment, especially in patients with preoperative systemic therapy or liver augmentation strategies |
| Cost analysis comparisons | Compare costs and potential cost-saving effects of different assessment tools in order to improve patient selection, outcome, and economic challenges |
| Individual topics | |
| Blood marker dynamics | Assess the kinetics of established and newer blood parameters for liver function prediction over time in patients with preoperative systemic therapy or liver augmentation strategies |
| Impact of new systemic treatment options on perioperative liver function and liver function assessment | How do immuno(chemo)therapy protocols, or targeted therapies influence perioperative liver function, the results of functional tests, and the overall rate of PHLF? |
| Nutritional assessment | What are appropriate definitions and cut-offs for nutritional indices in the setting of liver surgery, and what is their association with PHLF? |
| Sarcopenia | Is there a direct connection between sarcopenia, upfront reduced liver function, and impaired regeneration capacity? Does it influence liver augmentation strategies? |
| Influence of MIS on relevance of preoperative liver function assessment | Compare the relevance of PHLF in different cohorts of MIS versus open resections and the respective value of PHLF risk scores and functional assessment tools in these evolving scenarios |
| Patients with cholestatic livers | Develop and validate assessment tools (for example nuclear medicine tracers) that are not influenced by reduced biliary excretion rates |
| Liver stiffness measurement in patients without HCC | Assess the value of liver stiffness measurement in patients for example with cholangiocarcinoma, or in patients after neoadjuvant chemotherapy for colorectal cancer liver metastases |
ICG, indocyanine green; PHLF, posthepatectomy liver failure; MIS, minimally invasive surgery; HCC, hepatocellular carcinoma.
Indications and timing of liver function assessment
Patient selection
Q1: In which patients should preoperative functional assessment of the liver be performed routinely before liver surgery?
Preoperative liver function assessment should be considered in patients scheduled for a major or extended major or complex hepatectomy or with suspected or known underlying liver disease, or suspected or known drug-induced (DILI) or chemotherapy-associated (CALI) liver injury such as sinusoidal obstructive syndrome or chemotherapy-associated steatohepatitis (CASH).
Recommendation grade: Strong | median level of evidence: 2+ (range 3 to 2++).
Expert panel comment
When defined according to the currently most widely accepted classifications of the ISGLS and the 50–50 criteria, the incidence of PHLF ranges between 8 and 12 per cent8,13. PHLF is inversely related to the quality and quantity of the FLR after liver resection. With regard to volume, in patients with no underlying liver disease, a standardized FLR volume of at least 20 per cent17 or a FLR volume to bodyweight ratio of 0.5 per cent or higher has been described to be sufficient for safe extended hepatectomy18. However, cirrhosis or any (non-cirrhotic) underlying liver disease, including fibrosis, steatosis, and DILI, challenge these defined cut-offs. For patients with steatotic livers, the risk of developing PHLF increases almost three-fold43. Similarly, intensive preoperative combination chemotherapy (for at least 6 months) including, for example, oxaliplatin and/or irinotecan has been associated with a 10-fold increased risk of PHLF after major hepatectomy in patients with a standardized FLR volume below 44 per cent33. The EORTC 40983 trial34 reported a significant increase in postoperative morbidity after 3 months of preoperative FOLFOX (Folinic Acid, Fluorouracil and Oxaliplatin) chemotherapy. Further caution is recommended in patients with cholestatic liver diseases (for example, cholangiocarcinoma or primary sclerosing cholangitis), as these are often associated with reduced liver function and may themselves also affect the validity of several types of functional analysis44,45. In all above scenarios, FLR volumetry should be complemented by functional tests25,46.
Q2: What is the value of clinical risk models in selecting patients who could potentially benefit from liver function testing before hepatectomy?
Preoperative risk stratification models combining clinical information with routine laboratory values may be considered for selecting patients for a detailed treatment pathway with additional dynamic liver function testing. For example, the Birmingham score includes the extent of resection as well as serum creatinine and bilirubin levels, and the INR22 of patients before liver surgery. The aspartate aminotransferase/platelet ratio index (APRI) to albumin–bilirubin grade (ALBI) score23 represents another validated option, which uses a composite ratio of serum levels of aminotransferases, albumin, and bilirubin23.
Recommendation grade: Strong | median level of evidence: 2+ (range 2+).
Expert panel comment
Composite mathematical scores are used in clinical practice to assess the severity of underlying liver disease, and hence determine the outcome and prognosis after liver resection. Application of the Child–Pugh score and Model for End-stage Liver Disease (MELD), which were developed primarily for management of patients with cirrhosis, are of limited use in management of fibrotic, steatotic and non-cirrhotic livers, and do not specifically predict PHLF after elective liver resection47,48. Still, diagnosing underlying liver diseases helps in selecting patients for further functional liver tests. Risk score calculators specific to PHLF, which include purely preoperative parameters based on the type of resection planned, patient demographics, and routine laboratory values, are useful in identifying high-risk patients who will benefit from further functional assessment, or adjustment of treatment strategies (such as consideration of minimally invasive surgical access, or parenchymal-sparing or 2-stage approaches)22,23,49.
Q3: What is the role of combining clinical information (clinical signs and laboratory results) with imaging for statistical models predicting PHLF?
Statistical models integrating FLR volume with imaging, clinical or laboratory parameter surrogates of liver function or portal hypertension allow preoperative prediction of PHLF, and should be considered in patients with a suspected or known underlying liver disease (for example alcohol-associated liver disease, non-alcoholic steatohepatitis, CASH), especially when progressed to cirrhosis.
Recommendation grade: Conditional | median level of evidence: 2+ (range 2+).
Expert panel comment
The cause of PHLF is multifactorial, and closely related to the volume and function of the remnant liver. This is reflected by various—either imaging or blood parameter-based—tools aiming to predict PHLF50–52. The combination of volumetric and laboratory data offers a non-invasive tool with more accurate prediction of PHLF. Numerous risk factors have been identified, including clinical signs of portal hypertension on imaging or specific laboratory parameters. In patients who underwent resection for hepatocellular carcinoma (HCC), an albumin–indocyanine green (ICG) evaluation grade of 2 combined with clinical signs of portal hypertension was shown to result in a six-fold increased rate of PHLF47. In this regard, several nomograms have been developed, including the criterium clinical signs of portal hypertension, providing good performance in predicting PHLF and outperforming Child–Pugh and MELD-based algorithms53,54.
Q4: Should functional liver assessment be performed in patients scheduled to receive a volume augmentation strategy?
Assessment of liver function should be considered in all patients in whom any type of hepatic volume modulation is planned.
Recommendation grade: Strong | median level of evidence: 2+ (range 2− to 2++).
Expert panel comment
A variety of procedures have been proposed to increase the volume of the FLR to mitigate the risks of extended liver resections. Portal vein embolization (PVE) or ligation, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), and double vein embolization have been introduced successfully and adopted into clinical routine by several centres21,55,56. The growth in volume achieved by these augmentation procedures, however, may not correlate with an actual concordant increase in remnant liver function57,58. Functional liver assessment tests provide an estimate of overall parenchymal quality in these circumstances, and may help in selecting patients for such advanced resections. For calculation of the predicted FLR functional capacity in relation to total liver function, either dynamic tests need to be combined with quantitative liver volume assessment (volumetry), or a functional test with specific assessment of regional functional capacity (such as hepatobiliary scintigraphy (HBS)) should be performed59. Repeated functional testing before augmentation, and again before surgery, should be considered to better estimate dynamic changes.
Timing of assessment
Q5: Is there an ideal time point for assessing liver function before surgery?
An ideal time point for preoperative metabolic liver function tests cannot be defined precisely in general, and it should be adapted to individual clinical scenarios.
Recommendation grade: Conditional | median level of evidence: 2+ (range 3 to 1+).
Expert panel comment
To date, there has been no published comparative study providing high-level evidence specifically on the ideal preoperative time point for performing liver function tests for optimal accuracy of prediction. Most studies investigating preoperative liver function undertook such tests a few days to a couple of weeks before the planned surgery to obtain relevant results as close as possible to the time of surgery. Timing of liver function testing, however, should also consider any type of previous treatment or potential recent liver injury (CALI or DALI), or volume modulation strategy. For example, patients who have recently received preoperative chemotherapy for colorectal liver metastases might still be in a liver function recovery phase within the first few weeks after the last dose of chemotherapy. Usually, liver function is considered stable 4–6 weeks after treatment, although further improvement might be noted beyond 6–8 weeks35,60,61. The liver recovery time should be taken into consideration when liver function tests are performed timely (for example 2 weeks) after chemotherapy. Furthermore, in the case of volume modulation strategies, the dynamics over time—also known as kinetic growth rate—especially within the first weeks after PVE have been shown to be of particular importance in regard to overall FLR growth. This might also indicate that the gain in liver function might not be linear within the first weeks after such modulation59,62,63. Therefore, in certain scenarios, testing liver function early enough, or even in a dynamic fashion at more than one time point to potentially allow adaptation of planned strategies, might be advisable. In the event of longer waiting times to improve liver function (for example over 4 weeks), oncological restaging with new imaging is advisable to assess the stability and/or progression of disease.
Static blood markers for liver function assessment
Established blood parameters
Q6: Do routine blood tests before liver resection help identify patients at risk of postoperative liver dysfunction?
Preoperative basic blood parameters should be measured routinely to assist in preoperative patient assessment.
Recommendation grade: Strong | median level of evidence 2− (range 2− to 2+).
Expert panel comment
Single parameters lack a direct, let alone linear, predictive association with postoperative liver dysfunction. It should, however, be noted that single parameters, such as platelet or prealbumin levels, have been demonstrated to show predictive potential for postoperative liver dysfunction and adverse outcomes in patients undergoing resection for HCC64,65, or in patients with Child–Pugh A cirrhosis undergoing resection for primary liver cancers66. In more heterogeneous patient populations, single parameters lack sensitivity (low positive predictive value) and might be combined with dynamic liver function tests or other preoperative laboratory values (see question below) to improve their predictive value67.
Q7: Does a combination of routine, preoperative laboratory parameters improve the predictive potential for postoperative liver dysfunction?
Preoperative risk assessment should be considered using existing scores based on multiple basic blood parameters, as these deliver higher predictive potential than single markers. For example, combining albumin, bilirubin, and platelet measurements in the APRI/ALBI score has higher predictive value for PHLF than single blood parameters.
Recommendation grade: Strong | median level of evidence 2+ (range 2− to 2+).
Expert panel comment
Combinations of routine, preoperative laboratory measurements such as the APRI score68 and the ALBI score69 are easily applicable in a daily routine, and have been shown to better predict postoperative liver dysfunction than single blood-based parameters. Recent literature has shown an even better predictive value for PHLF and associated mortality when these two scores are combined (APRI/ALBI). In a large cohort study23,35 of more than 12 000 patients (8638 in evaluation cohort and in 3517 validation cohort), the area under the receiver operating characteristic curve (AUC-ROC) for APRI/ALBI was 0.689 regarding ISGLS PHLF grade C, and 0.735 regarding liver dysfunction-associated 30-day mortality. More importantly, the value of the APRI/ALBI score in estimating postoperative mortality risk has not only been described for HCC resections70,71 but also for other specific indications, such as perihilar and intrahepatic cholangiocarcinoma, as well as for colorectal cancer metastases after neoadjuvant treatment23,35. Smart phone application tools have been designed to allow specific risk assessment, as risk cut-offs differ between operative indications. The broad applicability and low costs of assessing the APRI/ALBI score could be particularly helpful in low-resource settings.
Q8: Do freely available calculators of blood parameter-based scores facilitate easy clinical translation?
Preoperative risk assessment using complex scores can be performed with existing smart phone calculators, which allow easy translation of these scoring systems into routine clinical practice. Of note, these digital applications have not yet received regulatory approval for clinical use in humans, and so should not be applied in routine clinical practice, unless approved for specific trials.
Recommendation grade: Strong | median level of evidence 2+ (range 2+ (only 1 publication)).
Expert panel comment
The feasibility and reliability, for example, of APRI/ALBI score calculation on a smart phone has been established in a large cohort of patients23. To accurately predict postoperative liver dysfunction, the extent of liver resection is included as a variable. Furthermore, disease type-specific prediction models are available in the application, resulting not only in varying score cut-offs for different indications but also allowing a gradual risk assessment for the individual patient. The expert panel noted that these risk score calculators have not yet been approved for routine clinical practice, as they were mainly designed for research purposes. However, in the future, the available applications could aid in risk stratification for patients in whom liver resection is planned.
Q9: Does the predictive value of blood parameter-based scores allow identification of patients who do not need any further liver function testing before liver resection?
Depending on the extent of planned liver resection, the condition of the FLR (Child A cirrhosis or less severe condition), and the absence of concerns flagged on standard cross-sectional imaging, normal preoperative basic blood-based parameters may be used to rule out the need for further testing before resection.
Recommendation grade: Conditional | median level of evidence 2+ (range 2− to 2+).
Expert panel comment
In this regard, scores including combinations, as opposed to single blood-based parameters, are preferred (for example levels of platelets, albumin, and bilirubin), compared with platelet counts only. Examples are the APRI68 and ALBI69 scores, and the recently proposed APRI/ALBI combined score23,70. Based on routine basic laboratory measurements, they are easily applicable and have been shown to have predictive value for PHLF in patients undergoing liver resection for various indications, including a subset of patients with virtually no relevant risk of PHLF. Preoperative cross-sectional imaging, potentially including volumetry, represents an indispensable prerequisite in this scenario to ensure a sufficient FLR. Given the complexity and limited data available on high-risk patients, this statement is limited to minor and standard major resections. In complex hepatic resections and patients with suspected extensive underlying liver disease, additional liver function assessment should be performed.
Exploratory blood parameters
Q10: Do newer markers show promising potential to improve preoperative risk assessment in an easy blood-based fashion?
Multiple recently identified biomarkers could improve preoperative risk assessment in the future. Currently, there are insufficient data to recommend routine clinical use of these markers.
Recommendation grade: Conditional | median level of evidence: 2+ (range 2− to 2++).
Expert panel comment
Recently identified markers, such as von Willebrand factor (vWF) antigen72, serotonin73, ADAMTS1374, serum GP7375, M2BPGi76, type IV collagen 7S77, and microRNA panels78, have been studied in a very heterogeneous manner based on small or medium-sized cohorts. A number of these markers have demonstrated very good predictive performance (for example, the AUC-ROC of vWF-antigen for PHLF is 0.725)72, and some studies also included independent validation cohorts. Further confirmatory and comparative research is, however, needed.
Q11: Are routine or newer blood-based parameters or scores including these variables able to monitor liver function recovery dynamically?
Newer blood-based parameters (such as microRNA panels) or combined scores of traditional markers (such as APRI/ALBI score) could be used to monitor liver function recovery dynamically after neoadjuvant chemotherapy or in the first step of liver volume modulation strategies. Currently, these results remain exploratory and need further validation in larger cohorts and subgroups of patients.
Recommendation grade: Conditional | median level of evidence: 2+ (range 2+).
Expert panel comment
There is exploratory evidence to suggest that a miRNA signature panel including a ratio of two markers (miRNA 151a-5p to 192-5p or 122-5p), or the APRI/ALBI score can predict the risk of PHLF before operation. They may also be used to monitor liver function recovery dynamically after neoadjuvant chemotherapy, or during the first step after liver volume modulation strategies (such as ALPPS) in conjunction with cross-sectional imaging23,35,78. These miRNA panels have been validated internally in a prospective cohort, and a CE-certified assay kit is now available (hepatoMiR®), but external validation studies are still ongoing. The APRI/ALBI score has been validated in a large cohort from the American College of Surgeons National Surgical Quality Improvement Program database; comparative studies with other tests are awaited. Further exploratory studies including external validation should be encouraged to assess the validity in different cohorts. At this point, volume reassessment and liver function testing after volume modulation therapies still represent the standard before proceeding to the second-stage procedure. Given the easy assessment by means of blood tests and the relevance of potential PHLF after major hepatic resections, new markers are urgently needed.
Functional and morphological imaging
Morphological imaging
Q12: What is the role of liver volumetry before hepatectomy?
In patients at risk (Q1, Q2, and Fig. 1), accurate estimation of FLR volume using CT or MRI should be performed to predict PHLF as it forms an important part of the risk–benefit analysis.
Recommendation grade: Strong | median level of evidence: 2+ (range 2− to 2+).
Expert panel comment
Preoperative estimation of the FLR volume may give insight into risk of PHLF, potential for liver regeneration, and overall outcomes. Patient history (indication for resection, previous surgery), biochemistry, and other indicators of operative suitability greatly influence the interpretation of estimated FLR, and it is important to include this clinical context. FLR volume may be calculated in relation to the bodyweight of the patient79, or can be related to the total preoperative liver volume80 as measured on cross-sectional imaging (excluding tumour volume) or as estimated by a variety of mathematical formulae81.
Further research is required, for example to confirm the additional benefit of combining invasive functional imaging assessments with calculated or estimated FLR, to externally validate multiparametric MRI overlaid on volume, and to evaluate radiomics incorporating multimodal analysis of imaging with clinical and biochemical markers.
Q13: What is the role of liver stiffness measurement before hepatectomy?
Liver stiffness measurement (LSM, commonly assessed by transient elastography) as a predictor of PHLF should be considered in the preoperative risk evaluation of patients with (suspected) underlying liver disease, especially in patients with HCC.
Recommendation grade: Strong (HCC); conditional (other entities) | median level of evidence: 2+ (range 2− to 2+).
Expert panel comment
Direct LSM can be performed based on ultrasonography by transient elastography or two-dimensional shear wave elastography82. A less established method is magnetic resonance elastography83. Most evaluations dealt with the possibility of estimating the risk of PHLF using the transient elastography method, and this is also the most widely used and validated method for assessment of underlying liver disease according to recent guidelines for hepatologists84,85. As transient elastography is usually performed on the right liver lobe, a large right-sided liver lesion may affect test results significantly.
The available perioperative literature mainly comes from Asian countries with a high incidence of HCC. The studies are therefore mostly concerned with hepatectomy for HCC86,87. In general, high LSM values predicted worse outcomes after hepatectomy82. Compared with other tests, such as ICG clearance, LSM was better in the prediction of major complications. It was also superior to invasive measurement of the hepatic venous pressure gradient (HVPG) in predicting PHLF. LSM showed a significant correlation with PHLF. Liver stiffness cut-off values calculated in these studies82,83,86,88–90 to stratify patients at low versus high risk of postoperative complications including PHLF varied significantly between 4.3 and 14 kPA. One study91 evaluated the combined value of LSM and functional liver assessment using the LiMAx® test (Humedics, Berlin, Germany), and showed a good, inverse correlation between the LSM fibrosis grade and LiMAx® values.
Data on the value of LSM in predicting PHLF in patients without HCC are scarce, as these patients represent a minority in the cohorts evaluated, and no adequately powered specific subgroup analysis has been performed92,93. For example, shear wave elastography results did not correlate significantly with hepatic steatosis or postchemotherapy changes such as sinusoidal obstruction in patients with colorectal cancer liver metastases in one single-centre retrospective analysis92. There may, however, be a benefit of elastography in evaluating liver functional reserve in patients with cholangiocarcinoma and no underlying cirrhosis93. To address these specific questions, studies providing high-level evidence with larger numbers of patients and control groups are needed.
Q14: Is sarcopenia, as defined by cross-sectional imaging analysis, predictive of PHLF after liver resection?
Owing to insufficient evidence, heterogeneity in measurement, and an unclear causal relationship with PHLF, the use of sarcopenia as a direct predictor of PHLF is currently not recommended.
Recommendation grade: Strong | median level of evidence: 2+ (range 2− to 2+).
Expert panel comment
Sarcopenia is a body composition parameter describing a relatively low skeletal muscle mass due to atrophy or fatty degeneration. This may be a result of multiple conditions including cancer, cirrhosis, chronic inactivity or general malnutrition. Many studies have linked sarcopenia with poor clinical outcome in several scenarios, especially in oncological diseases and after surgical procedures. Regarding the biological plausibility, sarcopenia per se is not directly linked to PHLF, which has more powerful and causal predictors.
Sarcopenia has been shown to correlate with inferior outcome regarding postoperative morbidity after liver resection including PHLF in some studies, and therefore could turn out to be a valuable parameter in a multifactorial risk score94. Some very recent studies95–99 have noted a smaller total functional liver volume in sarcopenic patients, and a slower kinetic growth rate of the FLR after PVE. At present, there is no standardization of measurement of sarcopenia, and no automatization is available, although it can be derived from standard cross-sectional imaging. This necessitates extra time for parameter extraction, and it is subject to further studies proving its superiority over other indices of patient condition.
Functional imaging
Q15: What is the role of functional MRI with strongly hepatocyte-specific contrast agent (for example gadoxetic acid) before hepatectomy?
Liver MRI with hepatocyte-specific, hepatobiliary contrast media provides an estimation of the function of the FLR and can be used to improve prediction of PHLF.
Recommendation grade: Conditional | median level of evidence: 2+ (range 2+ to 2++).
Expert panel comment
Liver MRI with hepatocyte-specific contrast media has been approved for the detection and characterization of liver lesions, showing excellent sensitivity in both primary and secondary liver tumours while delineating liver anatomy. Currently, two agents are available on the market. Gadoxetic acid (Gd-EOB-DTPA) has a hepatic excretion rate of 50 per cent (and 50 per cent renal), compared with a 5 per cent hepatic rate (and 95 per cent renal) for gadobenic acid. Therefore, intravenously injected Gd-EOB-DTPA has been used in relevant studies on preoperative liver function assessment.
Gd-EOB-DTPA distributes in the early dynamic phases in a similar manner to a regular extracellular contrast agent, followed by hepatocellular uptake of the compound via organic anion-transporting polypeptides (OATP1B1 and OATP1B3). Thereby, MRI can be used to quantify the hepatocellular contrast uptake as a surrogate for actual liver function in combination with high-resolution three-dimensional anatomical (and tumour) imaging in the same sequence. Additionally, a full liver MRI work-up and even magnetic resonance elastography of the liver is possible.
For quantification of hepatocellular uptake, signal intensities are measured in the liver volume of interest (usually the planned FLR) and normalized using signal intensity measurements in psoas muscle or spleen. The uptake ratio compares precontrast and postcontrast values, and can be adjusted to the actual volume of the liver partition of interest, resulting in an absolute or relative regional liver function measurement.
This approach has been shown to be predictive of the risk of developing PHLF after anatomical resection of HCC, and in hepatectomies performed after PVE for volume augmentation24,100. In a prospective trial24 with 36 patients, the MRI-derived functional FLR outperformed pure volumetry, the ALBI score, and the LiMAx® test in predicting PHLF. A recent systematic review101 including 15 studies on the topic confirmed a high predictive value of Gd-EOB-DTPA MRI, but also described obvious heterogeneity between the studies.
The expert panel noted that Gd-EOB-DTPA is approved for liver imaging for focal hepatic lesions but has not been officially approved for liver function assessment, which, therefore, remains an off-label use (resulting in a conditional recommendation). However, liver MRI with hepatocyte-specific contrast media could potentially represent a promising, one-stop examination for preoperative planning of hepatectomy.
Further studies should evaluate the exact protocols for acquisition and analysis of images using Gd-EOB-DTPA-enhanced MRI for liver function measurement (sequences, value, time point), the overall value in patients without HCC, for example those with steatosis or after chemotherapy for colorectal liver metastases, and the limitations of the technique in patients with bilirubinaemia or renal insufficiency.
Q16: What is the role of scintigraphy as a test of hepatic function in predicting PHLF?
Liver scintigraphy, including single-photon emission CT (SPECT) using 99mTc-labelled galactosyl human serum albumin (GSA) or 99mTc-labelled mebrofenin, can be considered as a regional liver function test, especially in patients with limited FLR, the presence of underlying liver disease, and before and after liver volume modulation strategies (for example PVE, ALPPS) as part of planning before intended major hepatectomy. An important limitation of the use of mebrofenin is the presence of a raised bilirubin level, as this hampers interpretation of the biliary excretion rate.
Recommendation grade: Conditional | median level of evidence: 2+ (range 2− to 2+).
Expert panel comment
Nuclear imaging studies of the liver have the advantage of time-resolved quantifiable dynamic measurements and/or three-dimensional volume imaging of the liver (SPECT), nowadays often combined with integrated CT acquisition (SPECT/CT). Thereby, they provide simultaneous morphological (visual) and physiological (quantitative functional) information. As a result, regional (segmental) differentiation allows specific functional assessment of the FLR102,103. In general, there are two tracers in use for this indication. Mebrofenin is used most widely in Europe and the USA, whereas the GSA tracer is mainly used in Japan104. Both can identify patients at risk of PHLF, who might benefit from liver-augmenting techniques105–107.
In 99mTc-labelled mebrofenin HBS, mebrofenin is taken up into hepatocytes through the action of organic anion transporters OATP1B1 and OATP1B3103,108. An important limitation in the use of mebrofenin is a significantly raised bilirubin level, which leads to competitive uptake and can result in falsely low measurements. 99mTc-labelled GSA binds to asialoglycoprotein receptors on viable hepatocytes109. SPECT/CT scintigraphy with either 99mTc-labelled GSA or 99mTc-labelled mebrofenin compensates for misestimation owing to the regional heterogeneity of liver function108,110. The techniques remain limited by lack of an agreed standard for acquisition and analysis, as at least mebrofenin scintigraphy is used in only a limited number of centres. Moreover, recent studies111 have suggested that contrast-enhanced liver MRI may have comparable value to HBS in assessing growth and function.
Q17: Is invasive measurement of hepatic venous pressure gradient useful in the work-up for liver resection?
HVPG measurement can be considered in patients scheduled for liver resection in whom liver cirrhosis with portal hypertension is suspected, which is a major risk factor for PHLF.
Recommendation grade: Conditional | median level of evidence: 2+ (range 2− to 2+).
Expert panel comment
HVPG measures portal venous hypertension in fibrotic or cirrhotic livers, and so the evidence has been acquired mainly from patients with HCC. After first reports in the 1990s by the Barcelona group36,112, it has been included in the European (European Association for the Study of the Liver) and American (American Association for the Study of the Liver Diseases) guidelines for the management of HCC. Since then, a few studies113–116 have been published. Most used a cut-off of at least 10 mmHg to define clinically significant portal hypertension with a markedly increased risk of postoperative death and reduced overall survival, but this has not been generally accepted for all patient subgroups. Although the HVPG is predictive of PHLF in patients with cirrhosis, and some centres have reported regular HVPG measurement in defined patient groups, HVPG has not become a general standard test with a universally agreed cut-off. The test is invasive and can result in complications, although major morbidity is rare in experienced centres (below1 per cent even in combination with simultaneous transjugular liver biopsy)117. If standardized and performed in trained hands, it produces a valid and robust quantification of portal venous pressure. Substitution of this invasive test by other methods of assessing portal pressure-related risk regarding PHLF has been proposed (for example use of single serum markers such as thrombocytes, vWF, or using elastography or CT/MRI findings of portal hypertension) and this may also help to reduce the use of invasive HVPG measurements in selected patients with borderline constellations. There seems to be a subset of patients with a potentially increased risk of PHLF who have normal findings in non-invasive tests, but who still present with portal hypertension when assessed by HVPG, underlining the persisting value of this invasive test.
Functional and morphological non-imaging tests
Indocyanine green clearance
Q18: What is the role of indocyanine green in preoperative assessment of liver function before liver resection?
ICG clearance testing can be used to assess overall liver function. To estimate remnant liver function, ICG needs to be combined with volumetry.
Recommendation grade: Strong (major hepatectomies); conditional (minor hepatectomies) | median level of evidence: 2+ (range 2− to 2+).
Expert panel comment
Several studies118–120 investigated preoperative ICG clearance mainly in major but also minor hepatectomies. The results showed that it reflects global liver function, and that impaired preoperative ICG clearance is associated with a higher rate of postoperative complications including liver dysfunction. However, additional CT/MRI volumetry is necessary in order to estimate FLR function121,122. Benefits of ICG clearance include its availability, reproducibility, relatively low costs, and negligible invasiveness and risk for patients. Nevertheless, most studies had a retrospective design that was prone to selection bias. Varying ICG retention rates after 15 min and plasma disappearance rate cut-offs were proposed and different endpoints were assessed in these studies. Thus, adequate expertise with this technique and its interpretation is required. ICG clearance is affected by hyperbilirubinaemia and cholestasis, resulting in less reliable results in patients presenting with these conditions123. Furthermore, in the event of potentially inhomogeneous parenchymal function (for example after volume modulation strategies or in patients with portal vein thrombosis), calculation of estimated FLR function on the basis of overall liver function through ICG measurement and volumetry may be affected118,124.
LiMAx®
Q19: What is the role of LiMAx® in the assessment of liver function before liver resection?
LiMAx® can be used to estimate overall liver function, and may improve risk stratification and outcomes in both major and minor hepatectomies. To estimate remnant liver function, LiMAx® needs to be combined with volumetry.
Recommendation grade: Strong | median level of evidence: 2− (range 2− to 1+).
Expert panel comment
The LiMAx® test is based on hepatospecific metabolism of the 13C-labelled substrate methacetin (a prodrug of paracetamol and substrate for the hepatic cytochrome P450 1A2 system) and can be used to assess global liver function. Additional CT/MRI volumetry is needed in order to estimate FLR function26,125,126. Prospective validation of LiMAx® demonstrated a reduction in PHLF and PHLF-related mortality26. In a multicentre RCT127, systematic perioperative use of LiMAx® (before resection and within 6 h after surgery in the recovery room) increased the rate of patients being transferred directly to a general ward rather than to the ICU. This was also associated with decreased severe postoperative morbidity rates, and led to a shorter duration of ICU stay and overall hospital stay. An algorithm combining LiMAx® with volumetry allows prediction of PHLF and probability of postoperative death. It enables stratification of patients into groups with a low, intermediate or high risk of PHLF, according to underlying liver disease and type of resection (in particular HCC with underlying cirrhosis, complex hepatectomy including ALPPS, and hepatectomy after neoadjuvant chemotherapy)26,125. Strengths of LiMAx® include its high reproducibility and lack of relevant risks to patients. The expert panel noted that, to date, all available evidence on the LiMAx® test has been published from German-speaking countries, which may influence its external validity. Currently, the availability of this tool may be limited to certain areas around Europe.
Similar to ICG, in the event of potentially inhomogeneous parenchymal function (for example after volume modulation strategies or in patients with portal vein thrombosis), caution is advised when calculating the estimated FLR on the basis of overall liver function through LiMAx® measurement and volumetry as the results might be skewed.
Non-tumoral liver biopsy
Q20: What is the role of non-tumoral liver biopsy in preoperative assessment of liver function before liver resection?
Non-tumoral liver biopsy should not be performed routinely as part of the liver functional assessment before liver surgery as it is not an adequate test for assessing preoperative liver function.
Recommendation grade: Strong | median level of evidence: 2− (range 2− to 2+).
Expert panel comment
Liver biopsy has been used historically to stage fibrosis and to identify cirrhosis. As chronically impaired liver function most commonly occurs in patients with histological cirrhosis (METAVIR F4 or Ishak F5–F6), non-invasive elastography methods128, and serum fibrosis scores (such as the Fibrosis-4 score calculated based on age, platelet count, aspartate and alanine aminotransferase levels)129 have largely replaced liver biopsy because they can identify cirrhosis reliably. Moreover, no studies have demonstrated that liver biopsy (beyond the identification of cirrhosis) has an independent prognostic role in prediction of PHLF. Biopsy is associated with patient discomfort and a clinically relevant, although small, risk of bleeding. Thus, there is no evidence to support a role for non-tumoral liver biopsy as part of the routine assessment of liver function regarding the risk of PHLF130–133. Liver biopsy, however, still has its role in the diagnosis and treatment of underlying liver disease (for example in diagnosis of autoimmune or cholestatic liver disease).
Non-imaging-guided nutritional assessment
Q21: What is the role of non-imaging-guided nutritional assessment alone in preoperative assessment of liver function before liver resection?
Non-imaging-guided nutritional assessment alone should not necessarily be undertaken as part of the routine functional assessment of the liver before hepatic resection as it is not an adequate test for assessment of preoperative liver function. Still, it is generally established that malnutrition has a negative effect on overall postoperative outcomes.
Recommendation grade: Conditional | median level of evidence: 2+ (range 2+).
Expert panel comment
There is overall inconsistency regarded whether albumin and prealbumin are associated with PHLF, arising from discordant results and significant heterogeneity (including different levels of albumin and prealbumin measured, and lack of detailed multivariable analysis)64,66,134,135. Moreover, it is well established that patients with cirrhosis commonly present with hypoalbuminaemia unrelated to malnutrition. Thus, the quality of evidence for use of nutritional assessment in the specific setting of presurgical liver function assessment is suboptimal, and a strong recommendation cannot be made regarding its routine use in predicting PHLF. However, validated nutritional scoring systems are likely to be of prognostic value in any major surgery. This should be investigated in the specific setting of liver surgery to predict PHLF. The available literature, however, suggests that other postoperative outcome parameters, but not specifically PHLF, are influenced by the nutritional status136. Thus, nutritional assessment may have a significant role in the preoperative work-up as malnutrition represents a modifiable preoperative risk factor.
Discussion
These inaugural European guidelines for liver function assessment before hepatectomy aim to fill the gap in clinical guidance regarding tools for preoperative assessment of the risk of PHLF development when planning liver resections7,27. The resulting consensus presented is based on a systematic literature search of published evidence, supplemented by opinions and clinical knowledge from international experts in the field. Divided into 4 sections and several subtopics covering patient selection, serum markers, and morphological and non-morphological analysis, a total of 21 statements were generated. The level of agreement of at least 85 per cent per statement represents a comparatively high threshold for consensus declarations, specifically chosen to increase the clinical relevance and broad applicability of these guidelines. The literature study led to the sobering realization that most retrieved publications have a low-to-medium level of evidence. For all clinical questions, the median evidence level ranged between 2− and 2+ (case–control or cohort studies), with only a few systematic reviews (level 2++), and only one subject (LiMAx®) including 1+ level evidence (1 RCT)127.
Which specific assessment tools should be relied on in clinical work? Is there a one-fits-all solution? These pressing questions could not be answered adequately for all clinical scenarios owing to a lack of high-level evidence. At present, the consensus cannot recommend a universal single assessment tool by which to perfectly identify and stratify all patients at risk of developing PHLF. The present guidelines provide a basket of established and widely accepted functional assessment methods and a framework for clinical decision-making as summarized in the proposed algorithm. The guidelines primarily aim to raise general awareness of PHLF, and to encourage the development of an individualized institutional approach for preoperative liver function assessment.
In all clinical scenarios, cross-sectional imaging (CT and MRI) serves as a foundation for preoperative surgical planning, as it depicts individual-patient anatomy and tumour characteristics. Furthermore, it allows quantitative calculation of estimated FLR volume based on the intended resection lines. Imaging studies may identify features of portal hypertension and cirrhosis, including liver surface nodularity, splenomegaly, portosystemic collaterals, and ascites. The presence of such findings correlates strongly with postoperative complications, and in particular with PHLF. Although essential for the diagnosis and staging of underlying disease, assessing the actual function of the liver by most conventional imaging techniques is very limited. Decisions regarding the risk of surgery and outcome–benefit analysis should therefore incorporate preoperative imaging findings but not rely on them exclusively.
The literature suggests that liver function testing should be considered in at least all complex or major hepatectomies, as well as in specific subsets of patients who have had preoperative systemic treatment, have suspected underlying liver disease, or are candidates for operative strategies involving augmentation techniques. The information gathered from actual functional assessment not only supports clinicians in better characterizing the risk of PHLF but also aids in counselling patients when discussing different treatment pathways. Conversely, in proposed low-risk patients, simple calculation of scores in basic risk models, such as the Birmingham or APRI/ALBI score based on clinical information and standard blood parameters, may be sufficient to estimate a negligible risk of clinically relevant (ISGLS grade B or C) PHLF with no compulsory need for further testing. This may allow clinicians to save healthcare costs and preclude patients from undergoing unnecessary examinations22,23. At the other end of the spectrum, in-depth preoperative functional testing may substantially alter treatment strategies, as it potentially leads to exclusion of patients from surgical procedures if (very) poor liver function is detected. In specific clinical scenarios, it could also support the recent tendency for more liberal use of volume augmentation strategies in high-risk patients, such as those with hilar cholangiocarcinoma137.
In terms of limitations of these guidelines, it is of critical importance to note the involved experts’ demographic and geographical composition, and their clinical specialties. Most participants came from high-income countries and worked in high-volume hospitals with specialized liver surgery units. Their clinical experience and available options regarding functional assessment techniques may therefore not be directly transferable to low- or medium-volume surgical units, or low-income countries. Even within this group of experts, type and frequency of functional assessment methods used in their respective units differed greatly, ranging from applying only very simple assessment methods in specifically selected high-risk patients, to incorporation of extensive algorithms for broad functional assessment of all patients scheduled for hepatectomy. Consequently, the present work aimed to summarize structurally the existing evidence level of available publications. All in all, this guideline represents the lowest common denominator of expert opinions to provide clinical guidance for a broad spectrum of surgeons with an interest in liver surgery working in different countries and individual hospital settings.
The heterogeneity in clinical strategies within the consensus group was also apparent during discussions around the definition of major resections, usually taken as meaning resection of at least three liver segments. In reality, however, this definition requires critical adjustment in light of increasingly complex vascular or minimally invasive surgical techniques in the context of contemporary perioperative care138–140. The panel also noted that varying local health policies heavily influence the availability and choice of perioperative assessment tools. This particularly holds true for liver function testing, which represents a high-cost field where the level of evidence available is low.
Further limitations of these guidelines arise from the methodology applied. By definition, incorporating a modified Delphi approach and expert opinions carries some risk of bias in interpretation of the literature review, especially as a large number of available publications were published by members of the consensus group. Involvement of a neutral validation committee and full transparency through declaration of conflict of interest for each co-author addressed this potential limitation.
Considering the level of available evidence and the current speed of innovation in the field of liver function assessment, the authors estimate a lifespan of approximately 5 years for these guidelines. Throughout the guideline development process, areas of limited evidence and open clinical questions were revealed, providing ideas for potential future research topics (Table 2). Comparative analysis of the effectiveness and costs of different testing modalities should be a priority to produce clinically relevant, high-level evidence. Assessing dynamics and the ideal timing of functional assessment after systemic treatment35 or augmentation strategies could further reduce the rate of PHLF in high-risk patients. The resulting insights may also help in correctly judging the value of future therapeutic dietary and pharmaceutical interventions, or new surgical strategies that aim to improve patient safety by maximizing the potential of a patients’ liver function in the perioperative phase140–143.
Supplementary Material
Acknowledgements
F.P. and M.M. contributed equally to this study. The authors thank G. Lobenscheg from the patient support group L(i)eberleben in Tyrol, Austria, for reviewing the final draft of the manuscript as a patient representative.
Contributor Information
Florian Primavesi, Department of Visceral, Transplant and Thoracic Surgery, Medical University of Innsbruck, Innsbruck, Austria; Department of General, Visceral and Vascular Surgery, Centre for Hepatobiliary Surgery, Vöcklabruck, Austria.
Manuel Maglione, Department of Visceral, Transplant and Thoracic Surgery, Medical University of Innsbruck, Innsbruck, Austria.
Federica Cipriani, Hepatobiliary Surgery Division, San Raffaele Scientific Institute, Milan, Italy.
Timm Denecke, Department of Diagnostic and Interventional Radiology, University Medical Centre Leipzig, Leipzig, Germany.
Christian E Oberkofler, Swiss Hepatopancreatobiliary Transplant Centre, Department of Surgery, University Hospital Zürich, Zürich, Switzerland; Vivévis AG—Visceral, Tumour and Robotic Surgery, Clinic Hirslanden Zürich, Zürich, Switzerland.
Patrick Starlinger, Department of Surgery, Division of Hepatobiliary and Pancreatic Surgery, Mayo Clinic, Rochester, Minnesota, USA; Centre of Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria.
Bobby V M Dasari, Department of Hepatobiliary–pancreatic and Liver Transplantation Surgery, University of Birmingham, Birmingham, UK.
Jan Heil, Department of General, Visceral, Transplant and Thoracic Surgery, Goethe University Frankfurt, University Hospital, Frankfurt, Germany.
Olivia Sgarbura, Department of Surgical Oncology, Cancer Institute of Montpellier, University of Montpellier, Montpellier, France; IRCM, Institut de Recherche en Cancérologie de Montpellier, INSERM U1194, Université de Montpellier, Institut Régional du Cancer de Montpellier, Montpellier, France.
Kjetil Søreide, Department of Gastrointestinal Surgery, Hepatopancreatobiliary Unit, Stavanger University Hospital, Stavanger, Norway; Department of Clinical Medicine, University of Bergen, Bergen, Norway.
Rafael Diaz-Nieto, Liver Surgery Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Constantino Fondevila, General and Digestive Surgery Service, Hospital Universitario La Paz, IdiPAZ, CIBERehd, Madrid, Spain.
Adam E Frampton, Hepatopancreatobiliary Surgical Unit, Royal Surrey NHS Foundation Trust, Guildford, UK; Section of Oncology, Department of Clinical and Experimental Medicine, University of Surrey, Guildford, UK.
Dominik Geisel, Department of Radiology, Charité–Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Benjamin Henninger, Department of Radiology, Medical University of Innsbruck, Innsbruck, Austria.
Amelia J Hessheimer, General and Digestive Surgery Service, Hospital Universitario La Paz, IdiPAZ, CIBERehd, Madrid, Spain.
Mickaël Lesurtel, Department of Hepatopancreatobiliary Surgery and Liver Transplantation, Beaujon Hospital, Assistance Publique-Hôpitaux de Paris, University of Paris Cité, Clichy, France.
Damian Mole, Hepatopancreatobiliary Surgery Unit, Department of Clinical Surgery, University of Edinburgh, Edinburgh, UK.
Robert Öllinger, Department of Surgery, Charité–Universitätsmedizin Berlin, Berlin, Germany.
Pim Olthof, Department of Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands; Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, the Netherlands.
Thomas Reiberger, Division of Gastroenterology and Hepatology, Department of Medicine III and CD-Lab for Portal Hypertension and Liver Fibrosis, Medical University of Vienna, Vienna, Austria.
Andreas A Schnitzbauer, Department of General, Visceral, Transplant and Thoracic Surgery, Goethe University Frankfurt, University Hospital, Frankfurt, Germany.
Christoph Schwarz, Department of General Surgery, Division of Visceral Surgery, Medical University Vienna, Vienna, Austria.
Ernesto Sparrelid, Department of Clinical Science, Intervention and Technology, Division of Surgery and Oncology, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Martin Stockmann, Department of Surgery, Charité–Universitätsmedizin Berlin, Berlin, Germany; Department of General, Visceral and Vascular Surgery, Evangelisches Krankenhaus Paul Gerhardt Stift, Lutherstadt Wittenberg, Germany.
Stéphanie Truant, Department of Digestive Surgery and Transplantation, CHU Lille, Lille University, Lille, France; CANTHER Laboratory ‘Cancer Heterogeneity, Plasticity and Resistance to Therapies’ UMR-S1277, Team ‘Mucins, Cancer and Drug Resistance’, Lille, France.
Luca Aldrighetti, Hepatobiliary Surgery Division, San Raffaele Scientific Institute, Milan, Italy.
Eva Braunwarth, Department of Visceral, Transplant and Thoracic Surgery, Medical University of Innsbruck, Innsbruck, Austria.
Mathieu D’Hondt, Department of Digestive and Hepatobiliary/Pancreatic Surgery, Groeninge Hospital Kortrijk, Kortrijk, Belgium.
Michelle L DeOliveira, Swiss Hepatopancreatobiliary Transplant Centre, Department of Surgery, University Hospital Zürich, Zürich, Switzerland.
Joris Erdmann, Department of Surgery, Amsterdam UMC, Cancer Centre Amsterdam, the Netherlands.
David Fuks, Department of Digestive, Hepatobiliary and Endocrine Surgery, Assistance Publique-Hôpitaux de Paris Centre Hopital Cochin, Paris, France.
Thomas Gruenberger, Department of Surgery, Clinic Favoriten, Hepatopancreatobiliary Centre, Health Network Vienna and Sigmund Freud Private University, Vienna, Austria.
Klaus Kaczirek, Department of General Surgery, Division of Visceral Surgery, Medical University Vienna, Vienna, Austria.
Hassan Malik, Liver Surgery Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Dietmar Öfner, Department of Visceral, Transplant and Thoracic Surgery, Medical University of Innsbruck, Innsbruck, Austria.
Nuh N Rahbari, Department of Surgery, University Hospital Mannheim, University of Heidelberg, Medical Faculty Mannheim, Mannheim, Germany.
Georg Göbel, Department of Medical Statistics, Informatics, and Health Economics, Medical University of Innsbruck, Innsbruck, Austria.
Ajith K Siriwardena, Regional Hepato-Pancreato-Biliary Unit, Manchester Royal Infirmary, Manchester, UK.
Stefan Stättner, Department of Visceral, Transplant and Thoracic Surgery, Medical University of Innsbruck, Innsbruck, Austria; Department of General, Visceral and Vascular Surgery, Centre for Hepatobiliary Surgery, Vöcklabruck, Austria.
Funding
The authors have no funding to declare.
Author contributions
Florian Primavesi (Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing—original draft), Manuel Maglione (Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation), Federica Cipriani, Timm Denecke, Christian Oberkofler, Patrick Starlinger, Bobby Dasari, Jan Heil, Olivia Sgarbura, Kjetil Søreide, Rafael Diaz-Nieto, Constantino Fondevila, Adam Frampton, Dominik Geisel, Benjamin Henninger, Amelia Hessheimer, Mickaël Lesurtel, Damian Mole, Robert Öllinger, Pim B. Olthof, Thomas Reiberger, Andreas Schnitzbauer, Christoph Schwarz, Ernesto Sparrelid, Martin Stockmann, Stéphanie Truant, Luca Aldrighetti, Eva Braunwarth, Mathieu D’Hondt, Michelle DeOliveira, Joris Erdmann, David Fuks, Thomas Gruenberger, Klaus Kaczirek, Hassan Malik, Dietmar Öfner, Nuh Rahbari, Georg Göbel, Ajith Siriwardena, and Stefan Stättner (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing—original draft, Writing—review & editing).
Disclosure
T.D. received honoraria as speaker/advisory board member and travel support to scientific conferences from Bayer, Siemens, and Canon; and research support from b.e. imaging and Guerbet. P.S. holds a patent for a microRNA-based kit to predict liver dysfunction (hepatoMiR®) and advised the company TAmiRNA distributing this kit; for this project, Austrian Research Promotion Agency FFG and Vienna Business agency funding was received. He has been involved in the development of a smart phone application to calculate the APRI/ALBI score together with the company Howto Health, but did not and is not receiving any financial support from these companies at the time of this submission. A.J.H. and C.F. have received research funding from Guanguong Shunde Innovative Design Institute and Instituto de Salud Carlos III. D.G. received honoraria and travel expenses from Bayer (Berlin, Germany). T.R. received grant support from Abbvie, Boehringer-Ingelheim, Gilead, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, Siemens, and W. L. Gore & Associates; speaking honoraria from Abbvie, Gilead, Intercept, Roche, MSD, and W. L. Gore & Associates; consulting/advisory board fees from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, and Siemens; and travel support from Abbvie, Boehringer-Ingelheim, Gilead, and Roche. T.G. received consulting fees from Humedics, Roche, AstraZeneca, Servier, Incyte, and BMS; speaking honoraria from Humedics, AstraZeneca, Roche, Servier, Olympus, Baxter, and BMS; and travel support from Roche, and AstraZeneca. M.S. is the inventor of the LiMAx® test and a consultant/medical advisor to Humedics (Berlin, Germany). The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
There are no new or original data in this manuscript, list of references are available via the Supplementary material at BJS online.
References
- 1. Kingham TP, Correa-Gallego C, D’Angelica MI, Gonen M, DeMatteo RP, Fong Yet al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4152 resections for malignancy. J Am Coll Surg 2015;220:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lassen K, Nymo LS, Olsen F, Brudvik KW, Fretland AA, Soreide K. Contemporary practice and short-term outcomes after liver resections in a complete national cohort. Langenbecks Arch Surg 2019;404:11–19 [DOI] [PubMed] [Google Scholar]
- 3. Gilg S, Sandstrom P, Rizell M, Lindell G, Ardnor B, Stromberg Cet al. The impact of post-hepatectomy liver failure on mortality: a population-based study. Scand J Gastroenterol 2018;53:1335–1339 [DOI] [PubMed] [Google Scholar]
- 4. Dokmak S, Fteriche FS, Borscheid R, Cauchy F, Farges O, Belghiti J. 2012 liver resections in the 21st century: we are far from zero mortality. HPB (Oxford) 2013;15:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJet al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 2011;13:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188–1200 [DOI] [PubMed] [Google Scholar]
- 7. Sparrelid E, Olthof PB, Dasari BVM, Erdmann JI, Santol J, Starlinger Pet al. Current evidence on posthepatectomy liver failure: comprehensive review. BJS Open 2022;6:zrac142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam Ret al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–724 [DOI] [PubMed] [Google Scholar]
- 9. Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Buchler MWet al. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol 2011;18:3640–3649 [DOI] [PubMed] [Google Scholar]
- 10. Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BDet al. Albumin–bilirubin versus Child–Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725–734 [DOI] [PubMed] [Google Scholar]
- 11. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HLet al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol 2015;33:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam Set al. Hepatic insufficiency and mortality in 1059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854–862; discussion 862–854 [DOI] [PubMed] [Google Scholar]
- 13. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse Det al. The ‘50–50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824–828; discussion 828–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gyoeri GP, Pereyra D, Braunwarth E, Ammann M, Jonas P, Offensperger Fet al. The 3–60 criteria challenge established predictors of postoperative mortality and enable timely therapeutic intervention after liver resection. Hepatobiliary Surg Nutr 2019;8:111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niederwieser T, Braunwarth E, Dasari BVM, Pufal K, Szatmary P, Hackl Het al. Early postoperative arterial lactate concentrations to stratify risk of post-hepatectomy liver failure. Br J Surg 2021;108:1360–1370 [DOI] [PubMed] [Google Scholar]
- 16. Connolly C, Stattner S, Niederwieser T, Primavesi F. Systematic review on peri-operative lactate measurements to predict outcomes in patients undergoing liver resection. J Hepatobiliary Pancreat Sci 2020;27:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJet al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–548 [DOI] [PubMed] [Google Scholar]
- 18. Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst Oet al. Remnant liver volume to body weight ratio >or=0.5%: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg 2007;204:22–33 [DOI] [PubMed] [Google Scholar]
- 19. Golse N, Bucur PO, Adam R, Castaing D, Sa Cunha A, Vibert E. New paradigms in post-hepatectomy liver failure. J Gastrointest Surg 2013;17:593–605 [DOI] [PubMed] [Google Scholar]
- 20. Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour Pet al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118–124 [DOI] [PubMed] [Google Scholar]
- 21. Chebaro A, Buc E, Durin T, Chiche L, Brustia R, Didier Aet al. Liver venous deprivation or associating liver partition and portal vein ligation for staged hepatectomy?: A retrospective multicentric study. Ann Surg 2021;274:874–880 [DOI] [PubMed] [Google Scholar]
- 22. Dasari BVM, Hodson J, Roberts KJ, Sutcliffe RP, Marudanayagam R, Mirza DFet al. Developing and validating a pre-operative risk score to predict post-hepatectomy liver failure. HPB (Oxford) 2019;21:539–546 [DOI] [PubMed] [Google Scholar]
- 23. Starlinger P, Ubl DS, Hackl H, Starlinger J, Nagorney DM, Smoot RLet al. Combined APRI/ALBI score to predict mortality after hepatic resection. BJS Open 2021;5:zraa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theilig D, Steffen I, Malinowski M, Stockmann M, Seehofer D, Pratschke Jet al. Predicting liver failure after extended right hepatectomy following right portal vein embolization with gadoxetic acid-enhanced MRI. Eur Radiol 2019;29:5861–5872 [DOI] [PubMed] [Google Scholar]
- 25. Truant S, Baillet C, Gnemmi V, Fulbert M, Turpin A, Dardenne Set al. The impact of modern chemotherapy and chemotherapy-associated liver injuries (CALI) on liver function: value of 99mTc-labelled-mebrofenin SPECT-hepatobiliary scintigraphy. Ann Surg Oncol 2021;28:1959–1969 [DOI] [PubMed] [Google Scholar]
- 26. Jara M, Reese T, Malinowski M, Valle E, Seehofer D, Puhl Get al. Reductions in post-hepatectomy liver failure and related mortality after implementation of the LiMAx algorithm in preoperative work-up: a single-centre analysis of 1170 hepatectomies of one or more segments. HPB (Oxford) 2015;17:651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Primavesi F, Stattner S, Maglione M. European guidelines for assessment of liver function before hepatectomy. Br J Surg 2023;110:166–168 [DOI] [PubMed] [Google Scholar]
- 28. Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov Ret al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 2018;268:11–18 [DOI] [PubMed] [Google Scholar]
- 29. Scottish Intercollegiate Guidelines Network. SIGN 50: A Guideline Developer’s Handbook.http://www.sign.ac.uk/guidelines/fulltext/50/index.html (accessed 1 March 2021)
- 30. Dalkey NC. An experimental application of the Delphi method to the use of experts. Manage Sci 1963;9:458–467 [Google Scholar]
- 31. Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol 2021;11:116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. AGREE II-GRS Instrument . Approval of Guidelines Research and Evaluation (AGREE). http://www.agreetrust.org/resource-centre/agree-ii-grs-instrument/ (accessed 1 March 2021)
- 33. Narita M, Oussoultzoglou E, Fuchshuber P, Pessaux P, Chenard MP, Rosso Eet al. What is a safe future liver remnant size in patients undergoing major hepatectomy for colorectal liver metastases and treated by intensive preoperative chemotherapy? Ann Surg Oncol 2012;19:2526–2538 [DOI] [PubMed] [Google Scholar]
- 34. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier Pet al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pereyra D, Rumpf B, Ammann M, Perrodin SF, Tamandl D, Haselmann Cet al. The combination of APRI and ALBI facilitates preoperative risk stratification for patients undergoing liver surgery after neoadjuvant chemotherapy. Ann Surg Oncol 2019;26:791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JCet al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018–1022 [DOI] [PubMed] [Google Scholar]
- 37. Saadat LV, Brajcich BC, Liu Y, Ko C, D’Angelica MI. Defining the risk of liver failure after minor hepatectomy: a NSQIP analysis of 7029 patients. HPB (Oxford) 2021;23:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vigano L, Torzilli G, Troisi R, Aldrighetti L, Ferrero A, Majno Pet al. Minor hepatectomies: focusing a blurred picture: analysis of the outcome of 4471 open resections in patients without cirrhosis. Ann Surg 2019;270:842–851 [DOI] [PubMed] [Google Scholar]
- 39. Murtha-Lemekhova A, Fuchs J, Feiler S, Schulz E, Teroerde M, Kalkum Eet al. Is metabolic syndrome a risk factor in hepatectomy? A meta-analysis with subgroup analysis for histologically confirmed hepatic manifestations. BMC Med 2022;20:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang J, Zhang Z, Shang D, Liao Y, Yu P, Li Jet al. A novel nomogram for prediction of post-hepatectomy liver failure in patients with resectable hepatocellular carcinoma: a multicenter study. J Hepatocell Carcinoma 2022;9:901–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prodeau M, Drumez E, Duhamel A, Vibert E, Farges O, Lassailly Get al. An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J Hepatol 2019;71:920–929 [DOI] [PubMed] [Google Scholar]
- 42. Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SGet al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol 2021;116:723–732 [DOI] [PubMed] [Google Scholar]
- 43. Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JNet al. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford) 2018;20:462–469 [DOI] [PubMed] [Google Scholar]
- 44. Sumiyoshi T, Shima Y, Okabayashi T, Noda Y, Hata Y, Murata Yet al. Functional discrepancy between two liver lobes after hemilobe biliary drainage in patients with jaundice and bile duct cancer: an appraisal using 99mTc-GSA SPECT/CT fusion imaging. Radiology 2014;273:444–451 [DOI] [PubMed] [Google Scholar]
- 45. Iacono C, Ruzzenente A, Campagnaro T, Bortolasi L, Valdegamberi A, Guglielmi A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg 2013;257:191–204 [DOI] [PubMed] [Google Scholar]
- 46. Wang LJ, Yan XL, Li J, Wang K, Xing BC. Indocyanine green clearance test for the preoperative assessment of chemotherapy-related hepatic injury in patients with colorectal liver metastasis. Cancer Manag Res 2020;12:3237–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shirata C, Kokudo T, Arita J, Akamatsu N, Kaneko J, Sakamoto Yet al. Albumin–indocyanine green evaluation (ALICE) grade combined with portal hypertension to predict post-hepatectomy liver failure. Hepatol Res 2019;49:942–949 [DOI] [PubMed] [Google Scholar]
- 48. Wang YY, Zhao XH, Ma L, Ye JZ, Wu FX, Tang Jet al. Comparison of the ability of Child–Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol 2018;118:440–445 [DOI] [PubMed] [Google Scholar]
- 49. Li B, Qin Y, Qiu Z, Ji J, Jiang X. A cohort study of hepatectomy-related complications and prediction model for postoperative liver failure after major liver resection in 1441 patients without obstructive jaundice. Ann Transl Med 2021;9:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu Y, Hu X, Li J, Dong R, Bai X. An improved scoring system based on platelet–albumin–bilirubin in predicting posthepatectomy liver failure outcomes. Dig Dis 2021;39:258–265 [DOI] [PubMed] [Google Scholar]
- 51. Chapelle T, Op de Beeck B, Driessen A, Roeyen G, Bracke B, Hartman Vet al. Estimation of the future remnant liver function is a better tool to predict post-hepatectomy liver failure than platelet-based liver scores. Eur J Surg Oncol 2017;43:2277–2284 [DOI] [PubMed] [Google Scholar]
- 52. Tanaka S, Iimuro Y, Hirano T, Hai S, Suzumura K, Fujimoto J. Prediction of postoperative hepatic failure after liver resection for hepatocellular carcinoma: significance of the aspartate aminotransferase-to-platelet ratio index. Hepatogastroenterology 2014;61:755–761 [PubMed] [Google Scholar]
- 53. Ye JZ, Mai RY, Guo WX, Wang YY, Ma L, Xiang BDet al. Nomogram for prediction of the International Study Group of Liver Surgery (ISGLS) grade B/C posthepatectomy liver failure in HBV-related hepatocellular carcinoma patients: an external validation and prospective application study. BMC Cancer 2020;20:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang YY, Xiang BD, Ma L, Zhong JH, Ye JZ, Wang Ket al. Development and validation of a nomogram to preoperatively estimate post-hepatectomy liver dysfunction risk and long-term survival in patients with hepatocellular carcinoma. Ann Surg 2021;274:e1209–e1217 [DOI] [PubMed] [Google Scholar]
- 55. Rassam F, Olthof PB, van Lienden KP, Bennink RJ, Erdmann JI, Swijnenburg RJet al. Comparison of functional and volumetric increase of the future remnant liver and postoperative outcomes after portal vein embolization and complete or partial associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Transl Med 2020;8:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Graaf W, van Lienden KP, van den Esschert JW, Bennink RJ, van Gulik TM. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg 2011;98:825–834 [DOI] [PubMed] [Google Scholar]
- 57. Olthof PB, Tomassini F, Huespe PE, Truant S, Pruvot FR, Troisi RIet al. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: liver volume overestimates liver function. Surgery 2017;162:775–783 [DOI] [PubMed] [Google Scholar]
- 58. Sparrelid E, Jonas E, Tzortzakakis A, Dahlen U, Murquist G, Brismar Tet al. Dynamic evaluation of liver volume and function in associating liver partition and portal vein ligation for staged hepatectomy. J Gastrointest Surg 2017;21:967–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tomassini F, Giglio MC, De Simone G, Montalti R, Troisi RI. Hepatic function assessment to predict post-hepatectomy liver failure: what can we trust? A systematic review. Updates Surg 2020;72:925–938 [DOI] [PubMed] [Google Scholar]
- 60. Jara M, Bednarsch J, Malinowski M, Pratschke J, Stockmann M. Effects of oxaliplatin-based chemotherapy on liver function—an analysis of impact and functional recovery using the LiMAx test. Langenbecks Arch Surg 2016;401:33–41 [DOI] [PubMed] [Google Scholar]
- 61. Takamoto T, Hashimoto T, Sano K, Maruyama Y, Inoue K, Ogata Set al. Recovery of liver function after the cessation of preoperative chemotherapy for colorectal liver metastasis. Ann Surg Oncol 2010;17:2747–2755 [DOI] [PubMed] [Google Scholar]
- 62. Hasselgren K, Sandstrom P, Rosok BI, Sparrelid E, Lindell G, Larsen PNet al. Future liver remnant (FLR) increase in patients with colorectal liver metastases is highest the first week after portal vein occlusion: FLR increase in patients with CRLM is highest the first week after PVO. J Gastrointest Surg 2019;23:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kambakamba P, Stocker D, Reiner CS, Nguyen-Kim TD, Linecker M, Eshmuminov Det al. Liver kinetic growth rate predicts postoperative liver failure after ALPPS. HPB (Oxford) 2016;18:800–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li JD, Diao YK, Li J, Wu H, Sun LY, Gu WMet al. Association between preoperative prealbumin level and postoperative mortality and morbidity after hepatic resection for hepatocellular carcinoma: a multicenter study from a HBV-endemic area. Am J Surg 2021;221:1024–1032 [DOI] [PubMed] [Google Scholar]
- 65. Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC IIet al. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg 2011;212:638–648; discussion 648–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang L, Li J, Yan JJ, Liu CF, Wu MC, Yan YQ. Prealbumin is predictive for postoperative liver insufficiency in patients undergoing liver resection. World J Gastroenterol 2012;18:7021–7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nanashima A, Tobinaga S, Abo T, Nonaka T, Takeshita H, Hidaka Set al. Reducing the incidence of post-hepatectomy hepatic complications by preoperatively applying parameters predictive of liver function. J Hepatobiliary Pancreat Sci 2010;17:871–878 [DOI] [PubMed] [Google Scholar]
- 68. Amptoulach S, Gross G, Sturesson C, Rissler P, Kalaitzakis E. Preoperative aspartate aminotransferase-to-platelet ratio index predicts perioperative liver-related complications following liver resection for colorectal cancer metastases. Scand J Surg 2017;106:311–317 [DOI] [PubMed] [Google Scholar]
- 69. Andreatos N, Amini N, Gani F, Margonis GA, Sasaki K, Thompson VMet al. Albumin–bilirubin score: predicting short-term outcomes including bile leak and post-hepatectomy liver failure following hepatic resection. J Gastrointest Surg 2017;21:238–248 [DOI] [PubMed] [Google Scholar]
- 70. Mai RY, Wang YY, Bai T, Chen J, Xiang BD, Wu GBet al. Combination of ALBI and APRI to predict post-hepatectomy liver failure after liver resection for HBV-related HCC patients. Cancer Manag Res 2019;11:8799–8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Luo H, Li C, Chen L. Preoperative albumin–bilirubin grade combined with aspartate aminotransferase-to-platelet count ratio index predict outcomes of patients with hepatocellular carcinoma within Milan criteria after liver resection. Biosci Trends 2019;13:176–181 [DOI] [PubMed] [Google Scholar]
- 72. Starlinger P, Pereyra D, Haegele S, Braeuer P, Oehlberger L, Primavesi Fet al. Perioperative von Willebrand factor dynamics are associated with liver regeneration and predict outcome after liver resection. Hepatology 2018;67:1516–1530 [DOI] [PubMed] [Google Scholar]
- 73. Starlinger P, Assinger A, Haegele S, Wanek D, Zikeli S, Schauer Det al. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology 2014;60:257–266 [DOI] [PubMed] [Google Scholar]
- 74. Haegele S, Fuxsteiner J, Pereyra D, Koeditz C, Rumpf B, Schuetz Cet al. Elevated ADAMTS13 activity is associated with poor postoperative outcome in patients undergoing liver resection. Sci Rep 2018;8:16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ke MY, Wu XN, Zhang Y, Wang S, Lv Y, Dong J. Serum GP73 predicts posthepatectomy outcomes in patients with hepatocellular carcinoma. J Transl Med 2019;17:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Okuda Y, Taura K, Yoshino K, Ikeno Y, Nishio T, Yamamoto Get al. Usefulness of Mac-2 binding protein glycosylation isomer for prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma. Ann Surg 2017;265:1201–1208 [DOI] [PubMed] [Google Scholar]
- 77. Ishii M, Itano O, Shinoda M, Kitago M, Abe Y, Hibi Tet al. Pre-hepatectomy type IV collagen 7S predicts post-hepatectomy liver failure and recovery. World J Gastroenterol 2020;26:725–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Starlinger P, Hackl H, Pereyra D, Skalicky S, Geiger E, Finsterbusch Met al. Predicting postoperative liver dysfunction based on blood-derived microRNA signatures. Hepatology 2019;69:2636–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee JW, Lee JH, Park Y, Lee W, Kwon J, Song KBet al. Risk factors of posthepatectomy liver failure for perihilar cholangiocarcinoma: risk score and significance of future liver remnant volume-to-body weight ratio. J Surg Oncol 2020;122:469–479 [DOI] [PubMed] [Google Scholar]
- 80. Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Cho CKet al. Comparison of remnant to total functional liver volume ratio and remnant to standard liver volume ratio as a predictor of postoperative liver function after liver resection. Korean J Hepatobiliary Pancreat Surg 2013;17:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Olthof PB, van Dam R, Jovine E, Campos RR, de Santibanes E, Oldhafer Ket al. Accuracy of estimated total liver volume formulas before liver resection. Surgery 2019;166:247–253 [DOI] [PubMed] [Google Scholar]
- 82. Wong JS, Wong GL, Chan AW, Wong VW, Cheung YS, Chong CNet al. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg 2013;257:922–928 [DOI] [PubMed] [Google Scholar]
- 83. Sato N, Kenjo A, Kimura T, Okada R, Ishigame T, Kofunato Yet al. Prediction of major complications after hepatectomy using liver stiffness values determined by magnetic resonance elastography. Br J Surg 2018;105:1192–1199 [DOI] [PubMed] [Google Scholar]
- 84. European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado . EASL–ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–264 [DOI] [PubMed] [Google Scholar]
- 85. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair; EASL Governing Board representative; Panel members. EASL Clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J Hepatol 2021;75:659–689 [DOI] [PubMed] [Google Scholar]
- 86. Chong CC, Wong GL, Chan AW, Wong VW, Fong AK, Cheung YSet al. Liver stiffness measurement predicts high-grade post-hepatectomy liver failure: a prospective cohort study. J Gastroenterol Hepatol 2017;32:506–514 [DOI] [PubMed] [Google Scholar]
- 87. Nishio T, Taura K, Koyama Y, Tanabe K, Yamamoto G, Okuda Yet al. Prediction of posthepatectomy liver failure based on liver stiffness measurement in patients with hepatocellular carcinoma. Surgery 2016;159:399–408 [DOI] [PubMed] [Google Scholar]
- 88. Lei JW, Ji XY, Hong JF, Li WB, Chen Y, Pan Yet al. Prediction of posthepatectomy liver failure using transient elastography in patients with hepatitis B related hepatocellular carcinoma. BMC Gastroenterol 2017;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shen Y, Zhou C, Zhu G, Shi G, Zhu X, Huang Cet al. Liver stiffness assessed by shear wave elastography predicts postoperative liver failure in patients with hepatocellular carcinoma. J Gastrointest Surg 2017;21:1471–1479 [DOI] [PubMed] [Google Scholar]
- 90. Han H, Hu H, Xu YD, Wang WP, Ding H, Lu Q. Liver failure after hepatectomy: a risk assessment using the pre-hepatectomy shear wave elastography technique. Eur J Radiol 2017;86:234–240 [DOI] [PubMed] [Google Scholar]
- 91. Heucke N, Wuensch T, Mohr J, Kaffarnik M, Arsenic R, Sinn Bet al. Non-invasive structure–function assessment of the liver by 2D time-harmonic elastography and the dynamic Liver MAximum capacity (LiMAx) test. J Gastroenterol Hepatol 2019;34:1611–1619 [DOI] [PubMed] [Google Scholar]
- 92. Hocquelet A, Frulio N, Gallo G, Laurent C, Papadopoulos P, Salut Cet al. Point-shear wave elastography predicts liver hypertrophy after portal vein embolization and postoperative liver failure. Diagn Interv Imaging 2018;99:371–379 [DOI] [PubMed] [Google Scholar]
- 93. Sugiura R, Kuwatani M, Nishida M, Hirata K, Sano I, Kato Set al. Correlation between liver elasticity by ultrasound elastography and liver functional reserve. Ultrasound Med Biol 2019;45:2704–2712 [DOI] [PubMed] [Google Scholar]
- 94. Berardi G, Antonelli G, Colasanti M, Meniconi R, Guglielmo N, Laurenzi Aet al. Association of sarcopenia and body composition with short-term outcomes after liver resection for malignant tumors. JAMA Surg 2020;155:e203336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Meister FA, Lurje G, Verhoeven S, Wiltberger G, Heij L, Liu WJet al. The role of sarcopenia and myosteatosis in short- and long-term outcomes following curative-intent surgery for hepatocellular carcinoma in a European cohort. Cancers (Basel) 2022;14:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martin D, Maeder Y, Kobayashi K, Schneider M, Koerfer J, Melloul Eet al. Association between CT-based preoperative sarcopenia and outcomes in patients that underwent liver resections. Cancers (Basel) 2022;14:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Heil J, Heid F, Bechstein WO, Bjornsson B, Brismar TB, Carling Uet al. Sarcopenia predicts reduced liver growth and reduced resectability in patients undergoing portal vein embolization before liver resection—a DRAGON collaborative analysis of 306 patients. HPB (Oxford) 2022;24:413–421 [DOI] [PubMed] [Google Scholar]
- 98. Denbo JW, Kim BJ, Vauthey JN, Tzeng CW, Ma J, Huang SYet al. Overall body composition and sarcopenia are associated with poor liver hypertrophy following portal vein embolization. J Gastrointest Surg 2021;25:405–410 [DOI] [PubMed] [Google Scholar]
- 99. Schulze-Hagen M, Truhn D, Duong F, Keil S, Pedersoli F, Kuhl CKet al. Correlation between sarcopenia and growth rate of the future liver remnant after portal vein embolization in patients with colorectal liver metastases. Cardiovasc Intervent Radiol 2020;43:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Araki K, Harimoto N, Kubo N, Watanabe A, Igarashi T, Tsukagoshi Met al. Functional remnant liver volumetry using Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) predicts post-hepatectomy liver failure in resection of more than one segment. HPB (Oxford) 2020;22:318–327 [DOI] [PubMed] [Google Scholar]
- 101. Wang Q, Wang A, Sparrelid E, Zhang J, Zhao Y, Ma Ket al. Predictive value of gadoxetic acid-enhanced MRI for posthepatectomy liver failure: a systematic review. Eur Radiol 2022;32:1792–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rassam F, Olthof PB, Bennink RJ, van Gulik TM. Current modalities for the assessment of future remnant liver function. Visc Med 2017;33:442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sharma P. Value of liver function tests in cirrhosis. J Clin Exp Hepatol 2022;12:948–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gupta M, Choudhury PS, Singh S, Hazarika D. Liver functional volumetry by Tc-99m mebrofenin hepatobiliary scintigraphy before major liver resection: a game changer. Indian J Nucl Med 2018;33:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Graaf W, van Lienden KP, Dinant S, Roelofs JJ, Busch OR, Gouma DJet al. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg 2010;14:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Olthof PB, Coelen RJS, Bennink RJ, Heger M, Lam MF, Besselink MGet al. 99mTc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford) 2017;19:850–858 [DOI] [PubMed] [Google Scholar]
- 107. Tomassini F, D’Asseler Y, Linecker M, Giglio MC, Castro-Benitez C, Truant Set al. Hepatobiliary scintigraphy and kinetic growth rate predict liver failure after ALPPS: a multi-institutional study. HPB (Oxford) 2020;22:1420–1428 [DOI] [PubMed] [Google Scholar]
- 108. de Graaf W, van Lienden KP, van Gulik TM, Bennink RJ. 99mTc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med 2010;51:229–236 [DOI] [PubMed] [Google Scholar]
- 109. Onodera Y, Takahashi K, Togashi T, Sugai Y, Tamaki N, Miyasaka K. Clinical assessment of hepatic functional reserve using 99mTc DTPA galactosyl human serum albumin SPECT to prognosticate chronic hepatic diseases—validation of the use of SPECT and a new indicator. Ann Nucl Med 2003;17:181–188 [DOI] [PubMed] [Google Scholar]
- 110. Yoshida M, Shiraishi S, Sakamoto F, Beppu T, Utsunomiya D, Okabe Het al. Assessment of hepatic functional regeneration after hepatectomy using 99mTc-GSA SPECT/CT fused imaging. Ann Nucl Med 2014;28:780–788 [DOI] [PubMed] [Google Scholar]
- 111. Wang Q, Brismar TB, Gilg S, Jonas E, Nilsson H, Tzortzakakis Aet al. Multimodal perioperative assessment of liver function and volume in patients undergoing hepatectomy for colorectal liver metastasis: a comparison of the indocyanine green retention test, 99mTc mebrofenin hepatobiliary scintigraphy and gadoxetic acid enhanced MRI. Br J Radiol 2022;95:20220370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434–1440 [DOI] [PubMed] [Google Scholar]
- 113. Procopet B, Fischer P, Horhat A, Mois E, Stefanescu H, Comsa Met al. Good performance of liver stiffness measurement in the prediction of postoperative hepatic decompensation in patients with cirrhosis complicated with hepatocellular carcinoma. Med Ultrason 2018;20:272–277 [DOI] [PubMed] [Google Scholar]
- 114. Cucchetti A, Cescon M, Golfieri R, Piscaglia F, Renzulli M, Neri Fet al. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J Hepatol 2016;64:79–86 [DOI] [PubMed] [Google Scholar]
- 115. Boleslawski E, Petrovai G, Truant S, Dharancy S, Duhamel A, Salleron Jet al. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br J Surg 2012;99:855–863 [DOI] [PubMed] [Google Scholar]
- 116. Llop E, Berzigotti A, Reig M, Erice E, Reverter E, Seijo Set al. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol 2012;56:103–108 [DOI] [PubMed] [Google Scholar]
- 117. Stift J, Semmler G, Walzel C, Mandorfer M, Schwarzer R, Schwabl Pet al. Transjugular aspiration liver biopsy performed by hepatologists trained in HVPG measurements is safe and provides important diagnostic information. Dig Liver Dis 2019;51:1144–1151 [DOI] [PubMed] [Google Scholar]
- 118. Yokoyama Y, Nishio H, Ebata T, Igami T, Sugawara G, Nagino M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg 2010;97:1260–1268 [DOI] [PubMed] [Google Scholar]
- 119. Schwarz C, Plass I, Fitschek F, Punzengruber A, Mittlbock M, Kampf Set al. The value of indocyanine green clearance assessment to predict postoperative liver dysfunction in patients undergoing liver resection. Sci Rep 2019;9:8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Haegele S, Reiter S, Wanek D, Offensperger F, Pereyra D, Stremitzer Set al. Perioperative non-invasive Indocyanine green-clearance testing to predict postoperative outcome after liver resection. PLoS One 2016;11:e0165481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, Moon DBet al. Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg 2015;19:1305–1314 [DOI] [PubMed] [Google Scholar]
- 122. Kobayashi Y, Kiya Y, Nishioka Y, Hashimoto M, Shindoh J. Indocyanine green clearance of remnant liver (ICG-Krem) predicts postoperative subclinical hepatic insufficiency after resection of colorectal liver metastasis: theoretical validation for safe expansion of Makuuchi’s criteria. HPB (Oxford) 2020;22:258–264 [DOI] [PubMed] [Google Scholar]
- 123. de Graaf W, Hausler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJet al. Transporters involved in the hepatic uptake of 99mTc-mebrofenin and indocyanine green. J Hepatol 2011;54:738–745 [DOI] [PubMed] [Google Scholar]
- 124. Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lock JF, Malinowski M, Seehofer D, Hoppe S, Rohl RI, Niehues SMet al. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg 2012;397:1297–1304 [DOI] [PubMed] [Google Scholar]
- 126. Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke Met al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg 2009;250:119–125 [DOI] [PubMed] [Google Scholar]
- 127. Stockmann M, Vondran FWR, Fahrner R, Tautenhahn HM, Mittler J, Bektas Het al. Randomized clinical trial comparing liver resection with and without perioperative assessment of liver function. BJS Open 2018;2:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Reiberger T, Ferlitsch A, Payer BA, Pinter M, Schwabl P, Stift Jet al. Noninvasive screening for liver fibrosis and portal hypertension by transient elastography—a large single center experience. Wien Klin Wochenschr 2012;124:395–402 [DOI] [PubMed] [Google Scholar]
- 129. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner Jet al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325 [DOI] [PubMed] [Google Scholar]
- 130. Gu J, Zhang E, Liang B, Zhang Z, Chen X, Huang Z. Effectiveness comparison of indocyanine green retention test with the cirrhotic severity scoring in evaluating the pathological severity of liver cirrhosis in patients with hepatocellular carcinoma and Child–Pugh grade A liver function. World J Surg Oncol 2020;18:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Golse N, El Bouyousfi A, Marques F, Bancel B, Mohkam K, Ducerf Cet al. Large hepatocellular carcinoma: does fibrosis really impact prognosis after resection? J Visc Surg 2018;155:265–273 [DOI] [PubMed] [Google Scholar]
- 132. Clarke CN, Choi H, Hou P, Davis CH, Ma J, Rashid Aet al. Using MRI to non-invasively and accurately quantify preoperative hepatic steatosis. HPB (Oxford) 2017;19:706–712 [DOI] [PubMed] [Google Scholar]
- 133. Gassmann P, Spieker T, Haier J, Schmidt F, Mardin WA, Senninger N. Prognostic impact of underlying liver fibrosis and cirrhosis after curative resection of hepatocellular carcinoma. World J Surg 2010;34:2442–2451 [DOI] [PubMed] [Google Scholar]
- 134. Chin KM, Allen JC, Teo JY, Kam JH, Tan EK, Koh Yet al. Predictors of post-hepatectomy liver failure in patients undergoing extensive liver resections for hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg 2018;22:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Greco E, Nanji S, Bromberg IL, Shah S, Wei AC, Moulton CAet al. Predictors of peri-operative morbidity and liver dysfunction after hepatic resection in patients with chronic liver disease. HPB (Oxford) 2011;13:559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sim JH, Kim SH, Jun IG, Kang SJ, Kim B, Kim Set al. The association between prognostic nutritional index (PNI) and intraoperative transfusion in patients undergoing hepatectomy for hepatocellular carcinoma: a retrospective cohort study. Cancers (Basel) 2021;13:2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Olthof PB, Aldrighetti L, Alikhanov R, Cescon M, Groot Koerkamp B, Jarnagin WRet al. Portal vein embolization is associated with reduced liver failure and mortality in high-risk resections for perihilar cholangiocarcinoma. Ann Surg Oncol 2020;27:2311–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Linn YL, Wu AG, Han HS, Liu R, Chen KH, Fuks Det al. Systematic review and meta-analysis of difficulty scoring systems for laparoscopic and robotic liver resections. J Hepatobiliary Pancreat Sci 2022;30:36–59 [DOI] [PubMed] [Google Scholar]
- 139. Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JWet al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford) 2011;13:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Joliat GR, Kobayashi K, Hasegawa K, Thomson JE, Padbury R, Scott Met al. Guidelines for perioperative care for liver surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations 2022. World J Surg 2023;47:11–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chan BKY, Elmasry M, Forootan SS, Russomanno G, Bunday TM, Zhang Fet al. Pharmacological activation of Nrf2 enhances functional liver regeneration. Hepatology 2021;74:973–986 [DOI] [PubMed] [Google Scholar]
- 142. Jotten L, Steinkraus KC, Traub B, Graf S, Mihaljevic AL, Kornmann Met al. Impact of perioperative steroid administration in patients undergoing elective liver resection: meta-analysis. BJS Open 2022;6:zrac139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Beppu T, Nitta H, Hayashi H, Imai K, Okabe H, Nakagawa Set al. Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol 2015;50:1197–1205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no new or original data in this manuscript, list of references are available via the Supplementary material at BJS online.