Summary
Background
Avoidant restrictive food intake disorder (ARFID) is a new eating disorder with a heterogeneous clinical presentation. It is unclear which patient characteristics contribute to its heterogeneity.
Methods
To identify these patient characteristics, we performed symptom-level correlation and driver-level regression analyses in our cross-sectional study in up to 261 ARFID patients (51% female; median age = 12.7 years) who were assessed at the Maudsley Centre for Child and Adolescent Eating Disorders, London between November 2019 and July 2022.
Findings
Symptoms across the three drivers 1) avoidance based on sensory characteristics of food; 2) apparent lack of interest in eating; and 3) concern about aversive consequences positively correlated with each other. Patients' anxiety traits showed the greatest positive correlations with symptoms of concern about aversive consequences of eating. Patient sex was not significantly associated with any of the three ARFID drivers. Patients with comorbid autism spectrum disorder (ASD; 28%) showed more food-related sensory sensitivities (RR = 1.26) and greater lack of interest in eating (RR = 1.18) than those of patients without ASD (49%).
Interpretation
In our clinical sample, the ARFID drivers occurred together and did not show clinically meaningful differences between the sexes. ASD may accentuate food-related sensory sensitivities and lack of interest, but may not drive a completely different symptom presentation. ARFID is multi-faceted and heterogenous, requiring a comprehensive multidisciplinary assessment to sufficiently understand the drivers of the restrictive eating behaviour. Results need replication in larger samples with more statistical power.
Funding
None.
Keywords: Heterogeneity, Clinical presentation, Panic disorder, Biological sex, Eating disorder, Driver, ARFID
Research in context.
Evidence before this study
Avoidant restrictive food intake disorder (ARFID) is a relatively new eating disorder diagnosis, first formally recognised in 2013. Consequently, ARFID is an emerging topic in current research. We searched PubMed and Google Scholar from 2012 to 2023 for articles using the following search terms: “Avoidant Restrictive Food Intake Disorder”; “ARFID clinical characteristics” “ARFID and sex”; ARFID and psychiatric comorbidity”. To date, several studies have relied on case series or retrospective medical reviews to understand the clinical presentation of ARFID. Conclusions from previous research suggest greater use of standardised and dimensional measures of ARFID symptomatology is needed to understand the heterogeneity in the clinical presentation of ARFID and associations between patient sex and psychiatric comorbidities.
Added value of this study
We assessed our patients with a standardised clinical procedure consecutively. We assessed symptoms of all three ARFID drivers and demonstrated their co-occurrence. Additionally, we showed that certain anxiety traits, such as those of panic disorder and generalised anxiety, are more strongly associated with concern about aversive consequences when eating. In our clinical sample, the drivers were not significantly associated with patients’ sex, while comorbid ASD in patients with ARFID may accentuate sensory sensitivity and lack of interest in eating.
Implications of all the available evidence
The occurrence of all ARFID symptoms in concert supports the multi-dimensional disorder model in research and clinical practice. With this model researchers may capture the heterogeneity of the ARFID clinical presentations. Grouping ARFID patients based on their drivers neglects the multi-faceted nature of the eating disorder. ARFID patients require a standardised assessment with a multidisciplinary team to fully understand their symptom presentation and drivers of their avoidant and restrictive eating. The team should consist of mental health professionals, medical doctors, and dietitians. The even split between males and females in our clinical sample, should raise awareness for health care professionals to watch out for ARFID in males. As we only detected slight differences between ARFID patients with and without ASD, no special assessment appears indicated; however, treatment may require adaptation to their symptom accentuation.
Introduction
Avoidant restrictive food intake disorder (ARFID) is a feeding and eating disorder recognised since 2013 that presents with substantial heterogeneity across the life span.1, 2, 3, 4 Individuals with ARFID consume a restricted amount or variety of food which can adversely impact weight, growth, nutrition, and psychosocial functioning, and may impair individual or family well-being. The main treatment recommendation is a form of out-patient psycho-behavioural therapy.5 However, at present no empirically tested treatments exist and consequently treatment standards are absent.6,7
Individuals with ARFID do not restrict or avoid food due to distress about body weight or shape, setting them apart from individuals with anorexia nervosa and bulimia nervosa.8,9 By contrast, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and the International Classification of Diseases 11th Revision (ICD-11) highlight three main drivers for ARFID symptomatology: 1) avoidance based on sensory characteristics of food (e.g., taste, texture, appearance); 2) apparent lack of interest in eating and food; and 3) concern about aversive consequences of eating, including, for example, choking or vomiting.1, 2, 3
Multiple research studies have categorised patients into one of the three ARFID drivers, resulting in a limited understanding of whether specific associations amongst ARFID symptoms and drivers exist.10,11 The dimensional disorder model of ARFID theorises that patients show heterogeneous presentations with varying symptom combinations across the three drivers.12, 13, 14 For example, the sensory sensitivities driver has been found to commonly co-occur with a lack of interest in eating or food.11 At present, our knowledge of the heterogeneity in ARFID is limited.15
ARFID drivers have been reported to vary with age. For example, the sensory sensitivities and lack of interest drivers are associated with a younger age of onset.11,16 Moreover, both drivers actuate restrictive eating that can lead to nutritional deficiencies and slow growth during childhood.11,16 Additionally, patients with concern about aversive consequences often have an acute onset with weight loss.11 While the lack of interest and concern about aversive consequences drivers are more often associated with low weight, ARFID occurs across the whole weight spectrum.10,11,17
The prevalence of ARFID varies depending on the underlying sample.18,19 In a population-based study in Switzerland, 3.2% of school age children had self-reported ARFID with an equal sex distribution: 41% males and 59% females.12 In a retrospective clinical study across seven adolescent eating disorder clinics in the United States of America and in Canada, 13.8% of patients qualified for an ARFID diagnosis with more female ARFID patients (71%) than males (29%).20 Sex differences have been described not only in the occurrence of ARFID, but also within its symptom drivers. In a surveillance study in Canada, male ARFID patients showed more food avoidance caused by sensory sensitivities than females.16 This contradicts retrospective clinical findings which found no sex differences in the sensory sensitivities or lack of interest drivers.10,11 By contrast, patients with concern about aversive consequences are reported to be more often female than male.11 These reported sex differences in prevalence and mixed findings regarding the ARFID drivers warrant further in-depth investigations.
Individuals with ARFID may present with comorbidities, including gastrointestinal symptoms, anxiety disorders (∼71%), and neurodiversity, such as autism spectrum disorder (ASD; ∼13%).11,21 In children and adolescents with ARFID, anxiety disorders are reported to be associated with the sensory sensitivities and the concern about aversive consequences drivers.21 The concern about aversive consequences driver of ARFID may be underpinned by fear-based anxiety and avoidance or restriction of food due to perception of threat (such as choking or vomiting) from eating.22 In comparison, generalised anxiety and heightened arousal may contribute to reduced appetite and interest in food or an increased hypervigilance to the sensory characteristics (e.g., taste, texture, appearance) of food.22, 23, 24, 25 However, detailed investigations of associations between ARFID symptoms and traits of fear-based anxiety (a core symptom of social anxiety, panic disorder, and separation anxiety) and distress-based anxiety (the core symptom of generalised anxiety) are missing.
A systematic review of ARFID and ASD showed that the three drivers of ARFID can present differently in patients with ASD. For example, food related sensory sensitivities were the most commonly reported.26 Sensory sensitivities, a core feature of ASD, may increase hypersensitivities to the sensory characteristics (e.g., taste, texture, appearance) of food or present as hyposensitivity to interoceptive sensory information, resulting in a lack of interest in eating and food.27,28 Further research is required to identify whether primary drivers of eating restriction differ across patients with and without ASD.26
Healthcare professionals report unfamiliarity with the ARFID diagnosis limiting their confidence in their clinical assessment of patients.29,30 Our research contributes to our existing knowledge by describing the socio-demographic and clinical characteristics of children and adolescents with ARFID. Past research has been constrained by small sample sizes, non-standardised clinical assessment, and retrospective reviews of medical records.10,11,14,20,21 We assessed and diagnosed our patients with a standardised procedure combining parent report and clinical validation at the South London and Maudsley (SLaM) National Health Service (NHS) ARFID outpatient service.
Based on previous research, we formulated the following hypotheses:
On the symptom level, we hypothesised that ARFID drivers do not drive the avoidant and restrictive eating in isolation. By contrast, symptoms across the three ARFID drivers measured on the Pica, ARFID, Rumination Disorder—ARFID—Questionnaire (PARDI-AR-Q) would be positively correlated. We hypothesised that the concern about aversive consequences and lack of interest in eating symptoms of ARFID would negatively correlate with BMI, whereas the sensory sensitivity symptoms of ARFID would positively correlate with nutritional deficiencies.
We hypothesised that anxiety traits in ARFID patients would be positively correlated with ARFID symptoms. We hypothesised that fear-based anxiety would positively correlate with the concern about aversive consequences symptoms, whereas generalised anxiety would positively correlate with symptoms representing sensory sensitivities and a lack of interest in eating.
On the driver level, we hypothesised that the concern about aversive consequences driver would be associated with female sex and that the sensory sensitivities driver and the lack of interest driver would show no association with sex.
We hypothesised that ASD in patients with ARFID is associated with more sensory sensitivities and lack of interest than in patients without ASD. We expected no association of the concern about aversive consequences driver with ASD.
Methods
Sample
Our sample comprised 261 child and adolescent ARFID patients (234 full diagnosis, 27 subthreshold) consecutively attending the Maudsley Centre for Child and Adolescent Eating Disorders (MCCAED) ARFID outpatient service in London, United Kingdom. This service accepts self-referrals, parent/carer referrals, and professional referrals. Patients were clinically diagnosed with a standardised procedure at first assessment, which included baseline questionnaires and a 90-min multidisciplinary face-to-face assessment of eating behaviour, developmental history, and associated psychosocial, medical, and dietetic risk. This was followed by a multidisciplinary case discussion resulting in a diagnosis. Patients were not reimbursed for their participation. This study did not have exclusion criteria. We computed each analysis using pairwise complete cases to retain the largest sample size with the largest statistical power. Therefore, we report the number of participants per analysis.
Ethics
Our study is a clinical audit and was ethically approved by the appropriate local Child and Adolescent Mental Health Service Clinical Governance Approval committee on the 3rd of June 2021. As part of data collection, caregivers consent to inclusion of data in publications of clinical audits and evaluations to improve service quality.
Pre-registration
This research project was preregistered on the Open Science Framework (OSF) on the 1st of July 2021: https://osf.io/rkn58/, including an outline of the study’s rationale, aims, hypotheses, and proposed methodology. OSF makes research hypotheses and methodology more transparent, improving reproducibility of research findings.31
Data collection
We collected the cross-sectional data routinely from parents and clinicians when patients first presented to the outpatient service between November 2019 and July 2022. All questionnaires are publicly available: RCADS (https://www.corc.uk.net/outcome-experience-measures/revised-childrens-anxiety-and-depression-scale-rcads/); Current View (https://www.corc.uk.net/outcome-experience-measures/current-view/); PARDI-AR-Q (https://mccaed.slam.nhs.uk/professionals/resources/featured-resources/; Supplementary Material S1).
Demographics and anthropometrics
Patient biological sex and date of birth were reported by parents and verified during clinical assessment. Patients’ age was calculated from their date of birth. Patient height in metres and weight in kilograms were recorded by clinicians at assessment; due to Covid-19 restrictions, 12 (6%) were measured by General Practitioners and 119 (57%) were parent-reported. BMI-for-age z-scores were derived from the UK 1990 (UK90) growth reference data32, 33, 34 using the R package ‘childsds’.35
ARFID symptomatology
Parents completed the parent version of the PARDI-AR-Q.36,37 This measure of ARFID symptoms includes yes/no questions regarding weight loss, difficulty maintaining weight, slow growth, nutritional deficiencies, reliance on oral supplements or enteral feeding, and psychosocial impairment corresponding to the DSM-5 criteria (A1–A4). The three ARFID drivers (i.e., sensory sensitivities, lack of interest, and concern about aversive consequences) are measured by three questions each (nine questions in total) and two questions inquire about severity of psychosocial difficulty on a 0–6 scale. The questions do not have a standard scale response, it varies between questions with 0 indicating no endorsement and 6 indicating highest endorsement (details, Supplementary Material S1). We calculated three sum scores; one for each driver ranging from 0 to 18.
Anxiety traits
We measured patient anxiety using the Revised Children’s Anxiety and Depression Scale-parent (RCADS-P) questionnaire.38,39 Parents of children ≥8 years answered the RCADS-P which asks how often the young person experiences thoughts and feelings on a 4-point scale (0 [never] to 4 [always]) capturing symptoms, such as worries, sadness, physical symptoms (i.e., heart racing, breathing difficulties, appetite, sleep, tiredness), specific fears, sleep problems, concentration difficulties, panic, and night scares. We included the RCADS-P subscales separation anxiety, social phobia, generalised anxiety, and panic disorder and calculated t-scores based on gender and grade level.38
Autism spectrum disorder
Clinicians recorded comorbid ASD on the Current View Tool Complexity Factor: pervasive developmental disorder (Asperger’s or ASD).40 If patients did not have a clinical ASD diagnosis but the clinicians observed ASD features or the patient was awaiting an ASD assessment, clinicians recorded it as ‘suspected’. This resulted in three categories: (no ASD diagnosis (reference category); suspected ASD; and ASD).
Data analysis
Symptom level analysis
Data were analysed in R, version 4.2.1.41 We report means, standard deviations, medians, interquartile ranges, and ranges for continuous variables and frequencies for categorical variables (Table 1). We calculated correlations using pairwise complete observations among BMI-for-age z-scores, ARFID symptoms and severity of psychosocial difficulty, and anxiety t-scores. We calculated biserial correlations between dichotomous and continuous variables; polychoric correlations between categorical variables; and polyserial correlations between categorical and continuous variables using the ‘hetcor’ function from the ‘polycor’ R package.42 We calculated Spearman’s rank correlations between continuous variables using the ‘rcorr’ function from the ‘Hmisc’ R package.43 The number of pairwise complete observations for each individual correlation ranged from 124 (44.1% missing) to 222 (0% missing; Supplementary Table S1). We present female-only and male-only correlation matrices as a sensitivity analysis to account for the potential confounding effect of sex on the correlations between ARFID symptoms and anxiety traits (Supplementary Figure S1).
Table 1.
Demographic and clinical characteristics of child and adolescent avoidant restrictive food intake disorder (ARFID) outpatients.
| Characteristic | N | N = 261a |
|---|---|---|
| Patient biological sex | 261 | |
| Male | 128 (49%) | |
| Female | 133 (51%) | |
| Age in years | 253 | |
| Mean (SD) | 12.2 (4.1) | |
| Median (IQR) | 12.7 (9.2 to 15.8) | |
| Range | 2.9, 17.8 | |
| Skewness | −0.45 | |
| Kurtosis | −0.92 | |
| Comorbid autism spectrum disorder (ASD) diagnosis | 261 | |
| No ASD diagnosis | 127 (49%) | |
| Suspected ASD | 60 (23%) | |
| ASD | 74 (28%) | |
| BMI-for-age z-score | 242 | |
| Mean (SD) | −1.05 (1.63) | |
| Median (IQR) | −1.07 (−2.25 to −0.01) | |
| Range | −6.75, 4.07 | |
| Skewness | 0.07 | |
| Kurtosis | 0.22 | |
| A1: weight loss or difficulty maintaining weight/growth | 221 | |
| No | 51 (23%) | |
| Yes | 170 (77%) | |
| A2: nutritional deficiency | 184 | |
| No | 122 (66%) | |
| Yes | 62 (34%) | |
| A3: enteral feeding or oral supplement | 221 | |
| No | 134 (61%) | |
| Yes | 87 (39%) | |
| A4: psychosocial impairment | 191 | |
| No | 52 (27%) | |
| Yes | 139 (73%) |
BMI = body mass index; SD = standard deviation; IQR = interquartile range.
n (%).
Driver level analysis
To test whether patient sex and comorbid ASD are associated with ARFID drivers, we regressed the three drivers on 1) sex whilst adjusting for patient age and 2) ASD diagnosis whilst adjusting for patient age and sex. We adjusted our regression models based on previous research showing differences in the outcome variables associated with age and sex.11,16 After inspecting our non-normal outcome distributions, we tested for overdispersion and zero-inflation and computed negative binomial regressions for both the sensory sensitivities and the lack of interest driver and a zero-inflated negative binomial regression for the concern about aversive consequences driver. The sensory sensitivities regression included 222 patients and the lack of interest and concern about aversive consequence regressions included 221 patients each.
Missing data
We computed an attrition analysis between patients included in the regression analyses and those excluded due to missing data which detected no differences (Supplementary Table S2).
Multiple testing correction
To correct for multiple testing, we chose a family-wise approach: symptom vs. driver level. We calculated the number of independent traits in the correlation matrix and used this number to adjust our alpha threshold using the Bonferroni method (alpha = 0.05/11 = 0.005). For the regression analysis, we performed 6 regressions: alpha = 0.05/6 = 0.008.
Role of the funding
We received no funding for this research process. As the study had no funder, no funder had any role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. Rosie Watts, Tanith Archibald, Cate Kelly, Harry Moss, Alfonce Munuve, Mohammed Uddin, Rachel Bryant-Waugh, and Christopher Hübel had full access to the data and verified the data. Rosie Watts, Rachel Bryant-Waugh, and Christopher Hübel hold responsibility for the decision to submit for publication.
Results
Symptom level results
Demographic and clinical characteristics
Of our ARFID patients, 133 (51%) were female and 128 (49%) male with a median age of 12.7 years (IQR = 9.2–15.8). Of the young people, 74 (28%) had comorbid ASD and a further 60 (23%) had suspected ASD. The most reported ARFID diagnostic criterion was A1 ‘weight loss or difficulty maintaining weight or growth’ (77%), and the sample had a median BMI-for-age z-score of −1.07 (IQR = −2.25 to −0.01), which means that the BMI value that splits the sample in half was one standard deviation below the population mean. The least common diagnostic criterion reported was A2 ‘nutritional deficiencies’ (34%). Reliance on enteral feeding or oral supplements (A3), which may correct nutritional deficiencies, was reported in 39% and psychosocial impairment (A4) in 73% of patients (Table 1).
Correlations
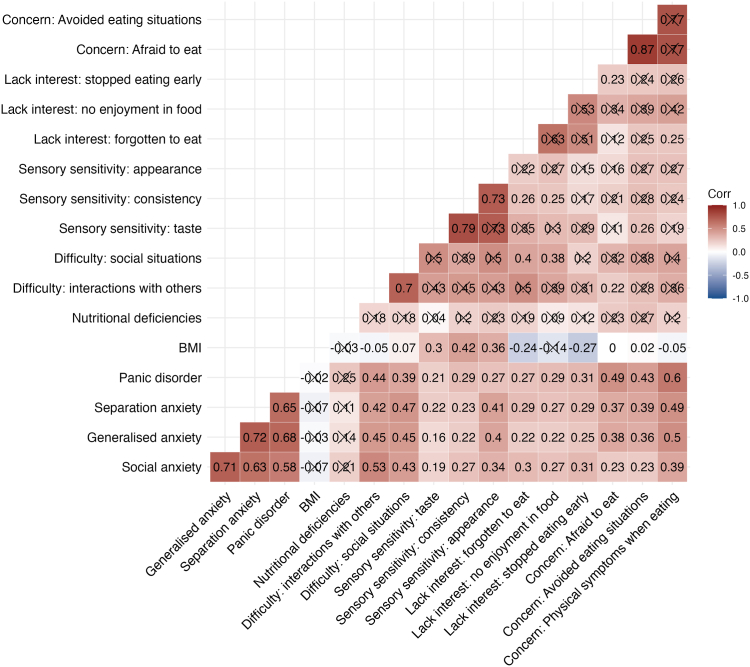
Symptoms on the concern about aversive consequences driver showed the highest positive intra-driver correlations; for example, ‘afraid to eat’ and ‘avoided eating situations’ (r = 0.87; Fig. 1; P-values are reported in Supplementary Table S3). The positive correlations among the three sensory sensitivities symptoms ranged from 0.73 to 0.79. The lack of interest driver symptoms correlated between 0.51 and 0.63, but they did not reach statistical significance at our current sample size and predefined P value threshold. The positive across-driver correlations among symptoms of the three ARFID drivers ranged from 0.11 to 0.42, demonstrating that the drivers are not mutually exclusive.
Fig. 1.
Heterogeneous correlation matrix across avoidant restrictive food intake disorder (ARFID) symptoms. Heterogeneous correlation matrix using pairwise complete observations between symptoms of ARFID measured on the PARDI-AR-Q, BMI-for-age z-scores and comorbid anxiety traits in a clinical sample of children and adolescents ARFID outpatients. BMI = body mass index; Correlations crossed out did not reach statistical significance at our pre-defined P value threshold of 0.005 after decomposing the matrix to detect the number of traits and performing Bonferroni adjustment.
Patient BMI-for-age z-scores positively correlated with experiencing symptoms of sensory sensitivities to the taste (r = 0.30), consistency (r = 0.42), and appearance of food (r = 0.36). In contrast, lower BMI-for-age z-scores were associated with patients more often forgetting to eat (r = −0.24) and stopping eating early (r = −0.27). Patient BMI-for-age z-scores had stronger negative correlations with forgetting to eat and stopping eating early in males than females (Supplementary Figure S1).
Fear-based (panic disorder, separation anxiety, and social anxiety) and distress-based (generalised) anxiety t-scores measured on the RCADS were positively correlated with all symptoms of ARFID (Fig. 1). Panic disorder traits showed the highest positive correlations with the three symptoms of concern about aversive consequences: 1) physical feelings of panic and anxiety when eating, 2) feeling afraid to eat, and 3) avoiding eating situations (r = 0.43–0.60). Separation and generalised anxiety traits both showed similar correlations with the three concern about aversive consequences symptoms (separation 0.37–0.49 vs. generalised anxiety 0.36–0.50). The correlations between the anxiety traits and the lack of interest symptoms were consistent and ranged between 0.22 and 0.31. For the sensory sensitivity symptoms, separation and generalised anxiety traits showed the strongest correlations with sensitivity to the appearance of food (separation r = 0.41 and generalised anxiety r = 0.40; Fig. 1).
The correlations between ARFID symptoms and anxiety traits were largely consistent across males and females (Supplementary Figure S1). However, a few differences are worth noting: social anxiety traits had stronger positive correlations with the symptoms of concern about aversive consequences in males (r = 0.33–0.47) than in females (r = 0.18–0.35). By contrast, in females (r = 0.48–0.52) separation anxiety traits showed a stronger positive correlation with symptoms of concern about aversive consequences than in males (r = 0.27–0.48). Generalised anxiety traits showed a stronger correlation with stopping eating early in females (r = 0.38) than males (r = 0.06).
Driver level results: regressions
Sex differences
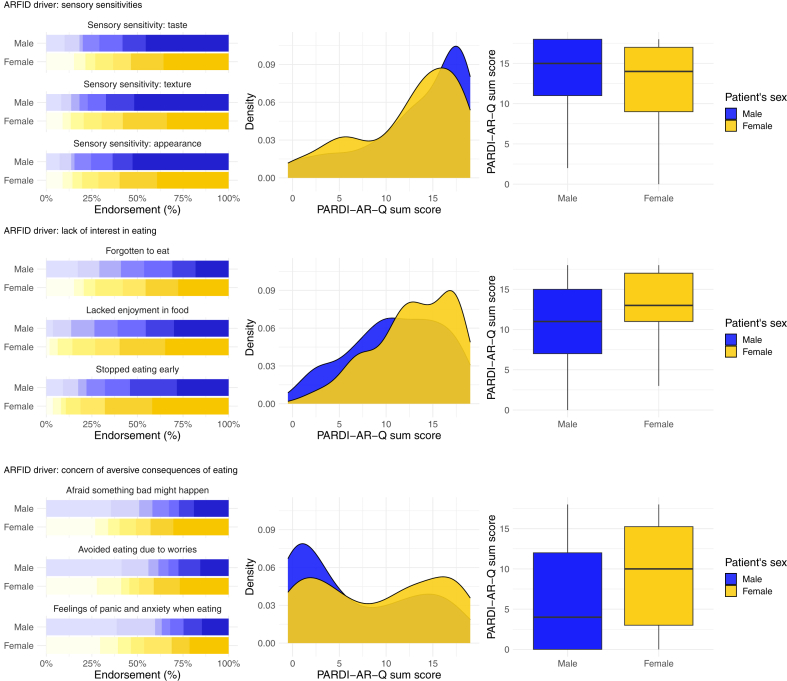
Male and female patients did not statistically significantly differ in the sensory sensitivities (RR = 0.97; 95% CI, 0.85–1.11; P = 0.65), the lack of interest (RR = 1.13; 95% CI, 1.01–1.26; P = 0.03), or the concern about aversive consequences (RR = 1.23; 95% CI, 1.00–1.52; P = 0.05) drivers of ARFID at our predefined alpha threshold (Table 2). ARFID drivers by sex are visualised in Fig. 2.
Table 2.
Associations between the clinical drivers of avoidant restrictive food intake disorder and (1) patient sex whilst adjusting for age and (2) autism spectrum disorder (ASD) diagnosis whilst adjusting for sex and age.
| Explanatory variable | Sensory sensitivities |
Lack of interest in eating |
Concern about aversive consequences of eating |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Rate ratio | CI (95%) | P-valuea | Rate ratio | CI (95%) | P-valuea | Rate ratio | CI (95%) | P-valuea | |
| Negative binomial regressions | |||||||||
| ASD diagnosis: suspectedd | 1.11 | 0.95–1.30 | 0.20 | 1.13 | 0.99–1.29 | 0.08 | 1.32 | 1.02–1.71 | 0.03 |
| ASD diagnosis: ASDd | 1.26 | 1.09–1.46 | 0.001f | 1.18 | 1.04–1.33 | 0.008f | 1.27 | 1.01–1.59 | 0.05 |
| Patient sex: femalee | 0.97 | 0.85–1.11 | 0.65 | 1.13 | 1.01–1.26 | 0.03 | 1.23 | 1.00–1.52 | 0.05 |
| Zero-inflated model | |||||||||
| ASD diagnosis: suspectedd | 1.17 | 0.51–2.66 | 0.72 | ||||||
| ASD diagnosis: ASDd | 0.72 | 0.31–1.65 | 0.43 | ||||||
| Patient sex: femalee | 0.59 | 0.29–1.21 | 0.15 | ||||||
| Observationsb | 222 | 221 | 221 | ||||||
| R2 Nagelkerkec | 0.116 | 0.148 | 0.77/0.76 | ||||||
CI (95%) = 95% confidence intervals; ASD = autism spectrum disorder.
The Bonferroni adjusted significance level is P = 0.008.
Observations are the number of pairwise complete cases per analysis.
R2 Nagelkerke calculated from ‘performance’ package in R.50
The regression models investigating associations between ASD and the ARFID drivers were adjusted for patient sex and age.
The regression models investigating sex differences in the ARFID drivers were adjusted for patient age.
Indicates statistically significant result.
Fig. 2.
The clinical drivers of avoidant restrictive food intake disorder (ARFID) plotted by sex in child and adolescent ARFID outpatients. Left. Bar plots for endorsement of the nine PARDI-AR-Q items measuring the three ARFID drivers (sensory sensitivities, lack of interest, and concern about aversive consequences) on a 0–6-point scale, plotted by patient sex. Middle. Density plots for the sum score of the three ARFID drivers plotted by patient sex. Right. Box plots for the sum score of the three ARFID drivers plotted by patient sex. PARDI-AR-Q = Pica, ARFID, Rumination Disorder Interview—ARFID—Questionnaire.
ASD
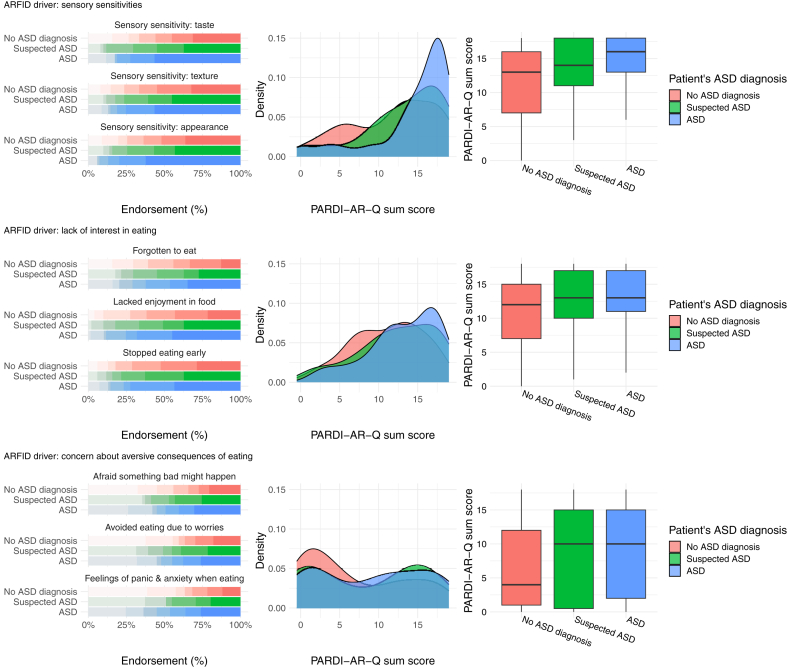
Patients with a comorbid diagnosis of ASD scored on average 26% higher on the sensory sensitivities driver (RR = 1.26; 95% CI, 1.09–1.46; P = 0.001) and 18% higher on the lack of interest driver (RR = 1.18; 95% CI, 1.04–1.33; P = 0.008) in comparison to the non-ASD group (Table 2). Comorbid ASD was not significantly associated with the concern about aversive consequences driver (RR = 1.27; 95% CI, 1.01–1.59; P = 0.05) or the probability of scoring more than zero on this driver (RR = 0.72; 95% CI, 0.31–1.65; P = 0.43). Distributions of the ARFID drivers across ASD groups are visualised in Fig. 3 and additionally by sex in Supplementary Table S4.
Fig. 3.
The clinical drivers of avoidant restrictive food intake disorder (ARFID) plotted across comorbid ASD diagnosis in child and adolescent ARFID outpatients. Left. Bar plots for endorsement of the nine PARDI-AR-Q items measuring the three ARFID drivers (sensory sensitivities, lack of interest, and concern about aversive consequences) on a 0–6-point scale, plotted by patient ASD diagnosis. Middle. Density plots for the sum score of the ARFID drivers, plotted by patient ASD diagnosis. Right. Box plots for the sum score of the ARFID drivers, plotted by patient ASD diagnosis. PARDI-AR-Q = Pica, ARFID, Rumination Disorder Interview—ARFID—Questionnaire.
Discussion
ARFID is a newly introduced eating disorder in the DSM-5 and ICD-11. Our cross-sectional study including 261 ARFID patients aged 2–17 years highlights heterogeneity in the clinical presentation of ARFID. Our correlation analyses showed that patients experience combinations of symptoms across the three main drivers of ARFID: 1) sensory sensitivities, 2) lack of interest in eating and food, and 3) concern about aversive consequences in relation to eating. These three drivers in concert contribute to the patient’s avoidant and restrictive eating (Fig. 1). In our study, the eating problems were associated with a wide range of BMIs, not only underweight. Fear- and distress-based anxiety were most strongly correlated with symptoms of the concern about aversive consequences of eating driver (Fig. 1).
We found that the ARFID drivers are largely independent of patient sex as none of the drivers showed a statistically significant association with sex at our current sample size and predefined alpha threshold (Fig. 2; Table 2), however, larger independent samples may show different results. ARFID can co-occur with psychiatric traits and neurodiversity. Our ARFID patients with ASD experienced all three ARFID drivers (Fig. 3), and they scored statistically significantly higher on the sensory sensitivities and lack of interest drivers than patients without ASD (Table 2).
During the development of the PARDI-AR-Q, the abovementioned three drivers were identified to play a crucial role in the disorder development.37 Our correlations between ARFID symptoms measured on the PARDI-AR-Q were congruent with the three drivers of ARFID: sensory sensitivities (r = 0.73–0.79); a lack of interest in eating and food (r = 0.51–0.63); and concern about aversive consequences of eating (r = 0.77–0.87; Fig. 1). The symptom correlations further showed positive associations amongst symptoms across the different drivers, highlighting that the three ARFID drivers do not occur in isolation and providing supporting evidence for a multi-dimensional disorder model of ARFID.13,44 Therefore, assuming that the clinical presentation of a patient is only driven by one specific driver is an inappropriate reduction of important clinical information. Clinicians should complete a full multidisciplinary assessment to sufficiently understand the drivers of the eating behaviour and associated physical health, nutritional, and psycho-social risk and impact.
Thomas et al.13 theorised a neurobiological model proposing that differences in sensory taste perception, appetite regulation, and attentional bias to fear may be implicated in the aetiology of the three drivers of ARFID. Our patients who had more sensory sensitivities had higher BMI-for-age z-scores, suggesting that these young people may restrict the variety of food consumed rather than the amount.10,45, 46, 47 Contrastingly, patients who lack interest in eating and food may present with chronic low appetite and early satiety resulting in lower BMIs.13,48 This was supported by our findings. In our sample, nutritional deficiencies showed small to moderate positive correlations with each ARFID symptom, although did not reach statistical significance at our alpha threshold.
Social anxiety, panic disorder, separation anxiety (fear-based anxiety), and generalised (distress-based) anxiety correlated positively with all symptoms of ARFID. The strongest correlations between fear- and distress-based anxiety were with the three ARFID symptoms of concern about aversive consequences and with sensory sensitivities to the appearance of food as assessed by the PARDI-AR-Q. This replicates the finding that anxiety disorders are associated with the concern about aversive consequences and the sensory sensitivities drivers,21 and extends it by highlighting specific anxiety traits associated with specific ARFID symptoms and that these traits should be included in clinical assessment.
Our clinical ARFID patient sample had an equal male-to-female ratio consistent with a population-based study.12 Thus, ARFID may be equally common in males and females when considering clinical populations, differing from anorexia nervosa where females more often seek treatment than males.49, 50, 51 These findings require replication in large epidemiological studies with two-stage sampling to clarify if this translates to the general population.
In our sample, patient sex was not significantly associated with any of the three ARFID drivers at our pre-registered alpha threshold. There was a trend for female patients to score higher on average than males on the concern about aversive consequences driver (RR = 1.23), consistent with previous research on retrospective chart reviews.11 In our sample, this finding was largely driven by a greater number of zero scores for males on this driver (Fig. 2). Likewise, a trend was observed with females scoring higher on average than males on the lack of interest driver (RR = 1.13). These trends did not reach statistical significance at our predefined Bonferroni-adjusted alpha threshold and we do not interpret them as clinically meaningful differences in the driver presentation. However, larger samples may show different results.
In a Canadian Paediatric Surveillance Program, males had more sensory sensitivities than females.16 By contrast, in our standardised assessed clinical sample, the sensory sensitivities driver did not discriminate between males and females, consistent with retrospective clinical findings.10,11 The difference in findings may be attributed to our patients attending a specialised ARFID service while the Canadian study sampled from paediatricians nationwide.
In our sample, patients with comorbid ASD showed similar patterns across the three ARFID drivers as patients with suspected ASD or no ASD (Fig. 3). Patients’ comorbid diagnosis of ASD was significantly associated with accentuated sensory sensitivities (RR = 1.26) and greater lack of interest (RR = 1.18; Table 2). Restrictive and repetitive behaviours and interests, including sensory sensitivities, highly-focused special interests, and difficulties with interoceptive awareness may be transdiagnostic traits of ASD and ARFID.27,52,53 These traits may limit the variety of foods consumed and an individual’s interest in eating and food. However, the differences were not striking enough to justify specialised services offering assessment and treatment for patients with ARFID and ASD only.
Our study benefits from several strengths. We collected one of the largest clinical samples of ARFID patients to date. Data were routinely gathered in a standardised manner when patients first presented to our specialist ARFID outpatient service, advancing previous clinical research reliant on retrospective patient chart reviews or small sample sizes. Parents reported ARFID symptoms on a dimensional measure capturing the co-occurrence between the three ARFID symptoms and drivers, emphasising the importance of a multi-dimensional disorder model of ARFID in research and clinical practice. Nevertheless, a few limitations warrant consideration. Our sample is clinical, and we may have sampled from the extremes of population; results may therefore not translate to the wider population and need replication in population samples. ARFID symptoms and anxiety traits were reported by parents and future research could collect data from several informants. Due to Covid-19 restrictions during data collection, for 57% of our patients, parents reported anthropometrics. Moreover, Covid-19 may have impacted patients’ anxiety scores, although some research suggests that this may not be the case.54 Our measure of anxiety (RCADS-P) is only validated for parent completion in youth aged ≥8 years, and therefore, we do not report correlations between anxiety and ARFID symptoms for patients under the age of 8. Furthermore, our study did not consider associations between ARFID presentation and other psychiatric comorbidities, such as depression, specific phobias, obsessive compulsive disorder, and attention deficit hyperactivity disorder which should be included in future studies. Even though our sample was relatively large, statistical power was limited: some findings would have reached statistical significance at a conventional alpha threshold of 0.05 but did not survive multiple testing corrections. Therefore, our research requires replication in larger samples with greater statistical power.
ARFID is a multi-faceted eating disorder and patients present with varying symptom presentations across the three ARFID drivers. Clinicians should consider that the ARFID drivers are rarely seen in isolation and it is important that this is captured during assessment to fully understand the symptom presentation and underlying drivers of avoidant and restrictive eating. Our patients presented with a variety of BMIs in the low and high weight range, challenging the common misconception that eating disorders occur solely in individuals who are underweight. Every patient should be comprehensively assessed for all ARFID diagnostic criteria by a multidisciplinary team to establish the physical health, dietetic, and psycho-social risk and impact and to rule out other conditions. ARFID assessments can benefit from the use of food diaries reviewed by dietitians to consider nutritional status and to identify patients who may be at high weight yet malnourished. Healthcare professionals need to be aware that males can present with eating disorders, especially ARFID. Anxiety and ASD can co-occur with ARFID, and therefore should be regularly screened for during assessment. Patient sex and ASD may influence the strength of some of the drivers of food avoidance and restrictive eating, but they are not associated with clinically meaningful differences in the underlying drivers. Therefore, it is imperative that all ARFID patients should be treated by multidisciplinary teams to address psychological mechanisms, presenting psychiatric symptoms, nutritional and physical health complications. ARFID patients with comorbid ASD should receive the same treatment as those without ASD but may require individualised adaptations in light of neurodiversity. Our study raises several important considerations for clinicians assessing and treating young people with avoidant and restrictive eating.
Contributors
Conceptualization: Rosie Watts, Rachel Loomes, Alfonce Munuve, Charlotte Rhind, Rachel Bryant-Waugh, and Christopher Hübel; Methodology: Rosie Watts, Rachel Bryant-Waugh, and Christopher Hübel; Software: Rosie Watts, Zain Ahmad, and Christopher Hübel; Validation: Rosie Watts, Zain Ahmad, and Christopher Hübel; Formal analysis: Rosie Watts and Christopher Hübel; Investigation: Rosie Watts, Tanith Archibald, Pippa Hembry, Maxine Howard, Cate Kelly, Rachel Loomes, Laura Markham, Harry Moss, Alfonce Munuve, Anca Oros, Amy Siddall, Charlotte Rhind, Mohammed Uddin, Rachel Bryant-Waugh, and Christopher Hübel; Resources: Rachel Bryant-Waugh; Data Curation Management: Rosie Watts, Tanith Archibald, Cate Kelly, Harry Moss, Alfonce Munuve, Mohammed Uddin, Rachel Bryant-Waugh, and Christopher Hübel; Writing–Original Draft: Rosie Watts, Rachel Bryant-Waugh, and Christopher Hübel; Writing–Review & Editing: Rosie Watts, Tanith Archibald, Pippa Hembry, Maxine Howard, Cate Kelly, Rachel Loomes, Laura Markham, Alfonce Munuve, Anca Oros, Amy Siddall, Charlotte Rhind, Mohammed Uddin, Zain Ahmad, Rachel Bryant-Waugh, and Christopher Hübel; Visualization: Rosie Watts and Christopher Hübel; Supervision: Rachel Bryant-Waugh and Christopher Hübel; Project administration: Rachel Bryant-Waugh and Christopher Hübel; Funding acquisition: n.a. All authors have read and revised the work critically for important intellectual content and have provided their final approval of the version to be published. Rosie Watts, Tanith Archibald, Cate Kelly, Harry Moss, Alfonce Munuve, Mohammed Uddin, Rachel Bryant-Waugh, and Christopher Hübel had full access to the data and verified the data.
Data sharing statement
The data was routinely gathered during patient treatment and therefore cannot be shared with researchers outside of the National Health Service Trust. The code is available on the following GitHub repository: https://github.com/topherhuebel/arfid.
Declaration of interests
All authors have no disclosures.
Acknowledgements
We would like to say thank you to all the children and young people and their families who took part in this research.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102190.
Appendix A. Supplementary data
References
- 1.American Psychiatric Association . 5th ed. 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 2.Bryant-Waugh R. Avoidant/restrictive food intake disorder. Child Adolesc Psychiatr Clin. 2019;28(4):557–565. doi: 10.1016/j.chc.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . 2019. ICD-11: international classification of diseases (11th Revision)https://icd.who.int/ [Google Scholar]
- 4.Sharp W.G., Stubbs K.H. Avoidant/restrictive food intake disorder: a diagnosis at the intersection of feeding and eating disorders necessitating subtype differentiation. Int J Eat Disord. 2019;52(4):398–401. doi: 10.1002/eat.22987. [DOI] [PubMed] [Google Scholar]
- 5.Hay P. Current approach to eating disorders: a clinical update. Intern Med J. 2020;50(1):24–29. doi: 10.1111/imj.14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence Guidelines . 2017. Eating disorders: recognition and treatment. [PubMed] [Google Scholar]
- 7.Couturier J., Isserlin L., Norris M., et al. Canadian practice guidelines for the treatment of children and adolescents with eating disorders. J Eat Disord. 2020;8(1):4. doi: 10.1186/s40337-020-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cañas L., Palma C., Molano A.M., et al. Avoidant/restrictive food intake disorder: psychopathological similarities and differences in comparison to anorexia nervosa and the general population. Eur Eat Disord Rev. 2021;29(2):245–256. doi: 10.1002/erv.2815. [DOI] [PubMed] [Google Scholar]
- 9.Norris M.L., Spettigue W.J., Katzman D.K. Update on eating disorders: current perspectives on avoidant/restrictive food intake disorder in children and youth. Neuropsychiatr Dis Treat. 2016;12:213–218. doi: 10.2147/NDT.S82538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norris M.L., Spettigue W., Hammond N.G., et al. Building evidence for the use of descriptive subtypes in youth with avoidant restrictive food intake disorder. Int J Eat Disord. 2018;51(2):170–173. doi: 10.1002/eat.22814. [DOI] [PubMed] [Google Scholar]
- 11.Zickgraf H.F., Lane-Loney S., Essayli J.H., Ornstein R.M. Further support for diagnostically meaningful ARFID symptom presentations in an adolescent medicine partial hospitalization program. Int J Eat Disord. 2019;52(4):402–409. doi: 10.1002/eat.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurz S., van Dyck Z., Dremmel D., Munsch S., Hilbert A. Early-onset restrictive eating disturbances in primary school boys and girls. Eur Child Adolesc Psychiatry. 2015;24(7):779–785. doi: 10.1007/s00787-014-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas J., Lawson E.A., Micali N., Misra M., Deckersbach T., Eddy K.T. Avoidant/restrictive food intake disorder: a three-dimensional model of neurobiology with implications for etiology and treatment. Curr Psychiatry Rep. 2017;19(8):54. doi: 10.1007/s11920-017-0795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reilly E.E., Brown T.A., Gray E.K., Kaye W.H., Menzel J.E. Exploring the cooccurrence of behavioural phenotypes for avoidant/restrictive food intake disorder in a partial hospitalization sample. Eur Eat Disord Rev. 2019;27(4):429–435. doi: 10.1002/erv.2670. [DOI] [PubMed] [Google Scholar]
- 15.Grubb L.K. Avoidant restrictive food intake disorder—what are we missing? What are we waiting for? JAMA Pediatr. 2021;175(12) doi: 10.1001/jamapediatrics.2021.3858. [DOI] [PubMed] [Google Scholar]
- 16.Katzman D.K., Spettigue W., Agostino H., et al. Incidence and age- and sex-specific differences in the clinical presentation of children and adolescents with avoidant restrictive food intake disorder. JAMA Pediatr. 2021;175(12) doi: 10.1001/jamapediatrics.2021.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncombe Lowe K., Barnes T.L., Martell C., et al. Youth with avoidant/restrictive food intake disorder: examining differences by age, weight status, and symptom duration. Nutrients. 2019;11(8):1955. doi: 10.3390/nu11081955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourne L., Bryant-Waugh R., Cook J., Mandy W. Avoidant/restrictive food intake disorder: a systematic scoping review of the current literature. Psychiatry Res. 2020;288 doi: 10.1016/j.psychres.2020.112961. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Cerezo J., Nagularaj L., Gledhill J., Nicholls D. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? A systematic review of the literature. Eur Eat Disord Rev. 2023;31(2):226–246. doi: 10.1002/erv.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher M.M., Rosen D.S., Ornstein R.M., et al. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. J Adolesc Health. 2014;55(1):49–52. doi: 10.1016/j.jadohealth.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Kambanis P.E., Kuhnle M.C., Wons O.B., et al. Prevalence and correlates of psychiatric comorbidities in children and adolescents with full and subthreshold avoidant/restrictive food intake disorder. Int J Eat Disord. 2020;53(2):256–265. doi: 10.1002/eat.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard M., Hembry P., Rhind C., Siddall A., Uddin M.F., Bryant-Waugh R. Cognitive behaviour therapy (CBT) as a psychological intervention in the treatment of ARFID for children and young people. Cogn Behav Ther. 2023;16 doi: 10.1017/S1754470X22000629. [DOI] [Google Scholar]
- 23.Wessing I., Romer G., Junghöfer M. Hypervigilance-avoidance in children with anxiety disorders: magnetoencephalographic evidence. J Child Psychol Psychiatry. 2017;58(1):103–112. doi: 10.1111/jcpp.12617. [DOI] [PubMed] [Google Scholar]
- 24.Farrow C.V., Coulthard H. Relationships between sensory sensitivity, anxiety and selective eating in children. Appetite. 2012;58(3):842–846. doi: 10.1016/j.appet.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Pliner P., Loewen R. The effects of manipulated arousal on children’s willingness to taste novel foods. Physiol Behav. 2002;76(4):551–558. doi: 10.1016/S0031-9384(02)00782-5. [DOI] [PubMed] [Google Scholar]
- 26.Bourne L., Mandy W., Bryant-Waugh R. Avoidant/restrictive food intake disorder and severe food selectivity in children and young people with autism: a scoping review. Dev Med Child Neurol. 2022;64(6):691–700. doi: 10.1111/dmcn.15139. [DOI] [PubMed] [Google Scholar]
- 27.Dovey T.M., Kumari V., Blissett J., Mealtime Hostage Parent Science Gang Eating behaviour, behavioural problems and sensory profiles of children with avoidant/restrictive food intake disorder (ARFID), autistic spectrum disorders or picky eating: same or different? Eur Psychiatry. 2019;61:56–62. doi: 10.1016/j.eurpsy.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Kinnaird E., Norton C., Pimblett C., Stewart C., Tchanturia K. Eating as an autistic adult: an exploratory qualitative study. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0221937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coelho J.S., Norris M.L., Tsai S.C.E., Wu Y.J., Lam P.Y. Health professionals’ familiarity and experience with providing clinical care for pediatric avoidant/restrictive food intake disorder. Int J Eat Disord. 2021;54(4):587–594. doi: 10.1002/eat.23438. [DOI] [PubMed] [Google Scholar]
- 30.Magel C.A., Hewitt K., Dimitropoulos G., von Ranson K.M., McMorris C.A. Who is treating ARFID, and how? The need for training for community clinicians. Eat Weight Disord. 2021;26(4):1279–1280. doi: 10.1007/s40519-020-01007-1. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan I., DeHaven A., Mellor D. Open and reproducible research on open science framework. Curr Protoc Essent Lab Tech. 2019;18(1) doi: 10.1002/cpet.32. [DOI] [Google Scholar]
- 32.Cole T.J., Freeman J.V., Preece M.A. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):25–29. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole T.J., Freeman J.V., Preece M.A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17(4):407–429. doi: 10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Wright C.M., Booth I.W., Buckler J.M.H., et al. Growth reference charts for use in the United Kingdom. Arch Dis Child. 2002;86(1):11–14. doi: 10.1136/adc.86.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel M. 2022. childsds: data and methods around reference values in pediatrics. [Google Scholar]
- 36.Bryant-Waugh R., Loomes R., Munuve A., Rhind C. Towards an evidence-based out-patient care pathway for children and young people with avoidant restrictive food intake disorder. J Behav Cogn Ther. 2021;31(1):15–26. doi: 10.1016/j.jbct.2020.11.001. [DOI] [Google Scholar]
- 37.Bryant-Waugh R., Micali N., Cooke L., Lawson E.A., Eddy K.T., Thomas J.J. Development of the Pica, ARFID, and rumination disorder interview, a multi-informant, semi-structured interview of feeding disorders across the lifespan: a pilot study for ages 10–22. Int J Eat Disord. 2019;52(4):378–387. doi: 10.1002/eat.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chorpita B.F., Ebesutani C., Spence S.H. Vol. 35. 2015. Revised children’s anxiety and depression scale. [Google Scholar]
- 39.Chorpita B.F., Yim L., Moffitt C., Umemoto L.A., Francis S.E. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther. 2000;38(8):835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 40.Jones M., Hopkins K., Kyrke-Smith R., Davies R., Vostanis P., Wolpert M. CAMHS Press; 2013. Current view tool: completion guide. [Google Scholar]
- 41.R: the R project for statistical computing. https://www.r-project.org/
- 42.Fox J. 2022. polycor: polychoric and polyserial correlations. [Google Scholar]
- 43.Harrell F.E., Jr. 2022. Hmisc: Harrell miscellaneous.https://CRAN.R-project.org/package=Hmisc [Google Scholar]
- 44.Pulumo R., Coniglio K., Lawson E.A., et al. 2016. DSM-5 presentations of avoidant/restrictive food intake disorder: are categories mutually exclusive or overlapping. (Poster presentation at the eating disorders research society meeting). New York, NY. [Google Scholar]
- 45.Menzel J.E., Reilly E.E., Luo T.J., Kaye W.H. Conceptualizing the role of disgust in avoidant/restrictive food intake disorder: implications for the etiology and treatment of selective eating. Int J Eat Disord. 2019;52(4):462–465. doi: 10.1002/eat.23006. [DOI] [PubMed] [Google Scholar]
- 46.Zucker N., Copeland W., Franz L., et al. Psychological and psychosocial impairment in preschoolers with selective eating. Pediatrics. 2015;136(3):e582–e590. doi: 10.1542/peds.2014-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chistol L.T., Bandini L.G., Must A., Phillips S., Cermak S.A., Curtin C. Sensory sensitivity and food selectivity in children with autism spectrum disorder. J Autism Dev Disord. 2018;48(2):583–591. doi: 10.1007/s10803-017-3340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zickgraf H.F., Ellis J.M. Initial validation of the nine item avoidant/restrictive food intake disorder screen (NIAS): a measure of three restrictive eating patterns. Appetite. 2018;123:32–42. doi: 10.1016/j.appet.2017.11.111. [DOI] [PubMed] [Google Scholar]
- 49.Hudson J.I., Hiripi E., Pope H.G., Kessler R.C. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaite C., Bühren K., Dahmen B., et al. Clinical characteristics of inpatients with childhood vs. adolescent anorexia nervosa. Nutrients. 2019;11(11):2593. doi: 10.3390/nu11112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinhausen H.C., Jensen C.M. Time trends in lifetime incidence rates of first-time diagnosed anorexia nervosa and bulimia nervosa across 16 years in a Danish nationwide psychiatric registry study. Int J Eat Disord. 2015;48(7):845–850. doi: 10.1002/eat.22402. [DOI] [PubMed] [Google Scholar]
- 52.Farag F., Sims A., Strudwick K., et al. Avoidant/restrictive food intake disorder and autism spectrum disorder: clinical implications for assessment and management. Dev Med Child Neurol. 2022;64(2):176–182. doi: 10.1111/dmcn.14977. [DOI] [PubMed] [Google Scholar]
- 53.Zickgraf H.F., Richard E., Zucker N.L., Wallace G.L. Rigidity and sensory sensitivity: independent contributions to selective eating in children, adolescents, and young adults. J Clin Child Adolesc Psychol. 2022;51(5):675–687. doi: 10.1080/15374416.2020.1738236. [DOI] [PubMed] [Google Scholar]
- 54.Young K.S., Purves K.L., Hübel C., et al. Depression, anxiety and PTSD symptoms before and during the COVID-19 pandemic in the UK. Psychol Med. 2022:1–14. doi: 10.1017/S0033291722002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.