Abstract
Background
COVID-19 accounts for more than half a billion deaths globally. The clinical manifestations may vary in due course. Despite several studies aimed at determining the extent to which the disease's severity and mortality remain high when combined with other comorbidities, more research is required. Therefore, this review aimed to measure the pooled prevalence of coronary artery disease (CAD) among COVID-19 patients, specifically those with a history of CAD. Additionally, we aim to assess the association between mortality due to CAD and the severity of COVID-19 among hospitalized patients.
Method
A comprehensive search in PubMed, Web of Science, the Cochrane Library, and the WHO COVID-19 database was conducted. English studies with original data on CAD, mortality, and ARDS among COVID-19 patients were included. PRISMA guidelines were followed.
Results
Among the 2007 identified articles, 76 studies met the inclusion criteria. The pooled prevalence of CAD among COVID-19 patients was 14.4%(95% CI: 12.7–16.2). The highest prevalence was observed in European studies at 18.2%(95% CI: 13.3–24.2), while the lowest was in Asian studies at 10.4% (95% CI: 6.4–16.3). Participants with concurrent CAD at the time of hospital admission had twice the odds of mortality due to COVID-19 (2.64 [95% CI: 2.30–3.04]) with moderate heterogeneity (I2 = 45%, p < 0.01). Hospitalized COVID-19 patients with CAD had a 50% higher risk of ARDS (95% CI: 0.62–3.66), but this difference was not statistically significant.
Conclusion
Although our analysis revealed evidence for a relationship between concurrent CAD at the time of hospital admission and mortality from COVID-19, however, global variation in health infrastructure, limitations of data reporting, and the effects of emerging variants must be considered in future investigations.
Keywords: Coronary artery disease, CAD, COVID-19, ARDS, Mortality, Prevalence
1. Introduction
The coronavirus disease 2019 (COVID-19) has posed a major threat to healthcare systems worldwide [1]. A total of 651,918,402 people have been infected and 6,656,601 have died due to COVID-19 up to December 22, 2022 [2].
The clinical manifestations of COVID-19 are varied [3]. The condition may proceed to Acute Respiratory Distress Syndrome (ARDS) in some patients, whereas it may be asymptomatic in others [4]. ARDS is a condition characterized by damage to the epithelial and endothelial barriers of the alveoli [5]. The incidence of ARDS is 15–18% of all coronary artery disease (CAD) patients and is associated with a mortality rate of 50% [6]. While the incidence of ARDS among COVID-19 patients was reported to be 33% [7]. An average of 25–50% of COVID-19 deaths appear to be related to the presence of severe ARDS [8].
CAD causes almost 7 million deaths and 129 million disability-adjusted life years (DALYs) lost annually, making it the leading cause of mortality and DALYs loss worldwide [9]. A high proportion of COVID-19 patients have comorbidities [10,11]. Several studies suggest that patients admitted to hospitals with severe COVID-19 have a relatively high prevalence of CAD, and CAD may be associated with a significant risk of mortality due to COVID-19 [[10], [11], [12], [13]]. A meta-analysis concludes that patients who have preexisting CAD and contract COVID-19 face an increased risk of mortality [14].Since CAD causes insufficient oxygen supply to the heart, an infection of the respiratory system may reduce oxygenation capacity, aggravating the heart's oxygen shortage. Recent studies suggest that CAD might lead to severe COVID-19 due to exacerbating hypoxemia [15,16]. Therefore, this review is essential to quantify evidence on the pooled prevalence of CAD among patients suffering from COVID-19, including those with a history of CAD. We further intend to measure the strength of evidence for an association between CAD and COVID-19 severity as measured by ARDS rate and in-hospital mortality.
2. Methodology
2.1. Literature search
The initial literature search was conducted using PubMed, where terms such as “COVID-19”, “Prevalence”, “Mortality”, and “Coronary Artery Disease” were used with Boolean operators to retrieve the first few articles. A subsequent search using all identified keywords and index terms was conducted up to 29th April 2022 in PubMed, Web of Science, Cochrane Central Register of Controlled Trials, and the World Health Organization (WHO) COVID-19 global literature on coronavirus disease (Detailed search strategy in supplementary appendix, Appendix A_ Tables A.1-A.5). Search results from electronic databases were exported into Rayyan software [17] for handling the articles. The protocol of this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO), having a registration number (CRD42022308188), and the article followed PRISMA guidelines.
2.2. Inclusion and exclusion criteria
The study was restricted to hospitalized adult patients who were 18 years or above with a confirmed COVID-19. We confined our review to studies that had reported the prevalence of CAD with a comparison group i.e., patients with and without CAD. The search strategy excluded all non-human studies and all articles that were not written in English. Articles excluded from our search strategy included case reports, case series, letters, systematic reviews, meta-analyses, and those that lacked full texts.
2.3. Data extraction and synthesis
Two authors independently extracted the data from each study into an Excel database, while another two authors arbitrated when discrepancies occurred. Data on the study design, study period, study population, and demographics (sample size, age, gender, and confirmed diagnosis of COVID-19 infection) were extracted from articles meeting inclusion criteria. In addition, we retrieved effect estimates on the association between the severity of COVID-19 and CAD, ARDS, and mortality caused by these conditions.
2.4. Quality assessment of included studies
The Newcastle-Ottawa quality assessment scale (NOS) [18] was used to assess the methodological quality and risk of bias of all included articles. The tool has eight items with a minimum score of zero and a maximum score of nine. The overall risk of bias was defined as low, if the cumulative score on eight items for the respective study ranged between 7 and 9, moderate for a score of 4–6, and the study with a score of 0–3 was classified to have a high risk of bias. To maintain reliability, two reviewers were calibrated on 8 items of quality assessment tools and further assessed the quality of included studies independently. The supplementary appendix contains a summary of the risk of bias for all included articles (Appendix B).
2.5. Statistical analysis
A meta-analysis was performed in RStudio.Version (3.3.0) [19] for all eligible studies (n = 76). For the primary outcome of interest, the proportions of CAD in the study population for respective studies were pooled together and the precise summary estimates were reported as prevalence with 95% Confidence Intervals (CIs). We had pre-specified to use a random-effect model when I2 was higher than 50% [20]. We further evaluated statistical heterogeneity by using the I-squared test and defined considerable heterogeneity at an I2 value greater than 75%, moderate heterogeneity for a value between 25 and 27%, and for an I2 value of less than 25% to categorize a low degree of heterogeneity [21]. For studies that reported similar outcomes of interest, we reported pooled effect size as Odds Ratios (ORs), the degree of uncertainty as 95% CIs, and the strength of evidence for hypothesized associations in the forest plot with weights given to individual studies based on their sample size. We further conducted a pre-specified subgroup analysis to control for heterogeneity in pooled estimates under the assumption that any variations in the sampling of study participants in individual studies might have been caused by geographical and health system variation.
3. Results
3.1. Results of the search
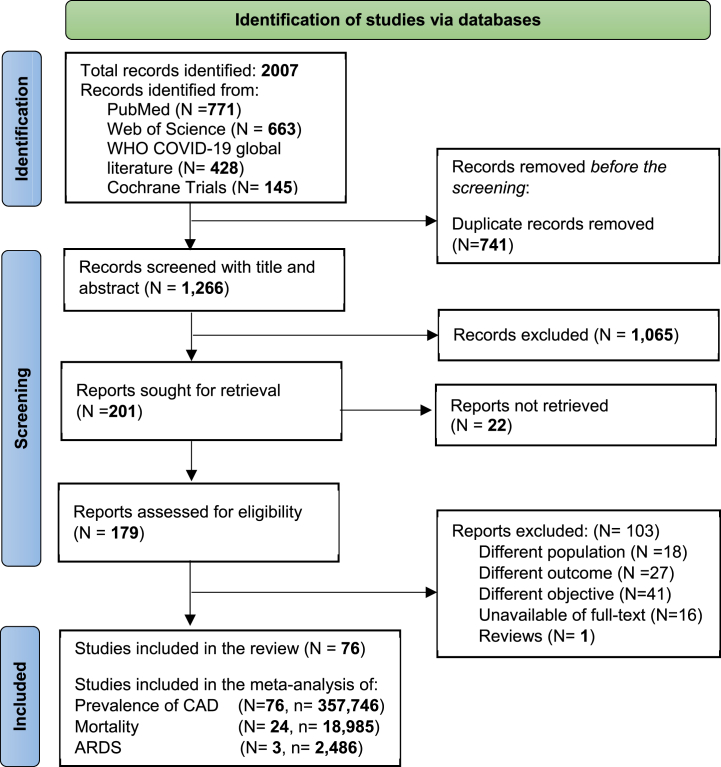
The results of our search are summarized in Fig. 1. Overall, we retrieved 2007 records from different databases. Prior to the screening, 741 records were removed due to duplication, leaving a total of 1266 records to be screened. After screening the title and abstract, 201 reports were kept and sought for retrieval. Then, the full-texts of 103 articles were excluded leaving 76 articles to be included in this analysis (Fig. 1). Reasons for excluding articles after full-text screening were provided in the supplementary appendix (Appendix A_ Tables A.6- A.7).
Fig. 1.
The PRISMA flowchart showing the studies included in the systematic review.
3.2. Participants and study settings
A total of 76 articles with 357,746 participants were included in this systematic review and meta-analysis. Forty-one percent (n = 31) of the included articles were conducted in Europe and 33% (n = 25) in the USA, while the rest were conducted in Asian countries (n = 20). Of the 76 included studies, 13 were cross-sectional, 7 were cohorts, and 1 was case-control, with the remainder being retrospective in design. No single randomized trial met our inclusion criteria. Characteristics of included articles are provided in Table 1.
Table 1.
Characteristics of the included studies (N = 76).
| NO | Author, Year | Country | Study type | Sample Size | Age Mean ± SD/median (IQR) | Male n (%) | SARS-2 method of confirmation |
|---|---|---|---|---|---|---|---|
| 1 | Loffi, 2020 [22] | Italy | Retrospective | 1252 | 64.7 ± 15.5 | 798 (63.70) | RT-PCR |
| 2 | Yuan, 2021 [23] | China | Retrospective | 2886 | 59.1± NR | 1402 (49.1) | NR |
| 3 | Peterson, 2021 [24] | USA | Retrospective | 355 | 66.21 ± 14.21 | 181 (51) | RT-PCR |
| 4 | Park, 2021 [25] | Korea | Retrospective | 2269 | 55.5 ± 20.2 | 814 (35.9) | PCR |
| 5 | Yamada, 2021 [26] | Japan | Cross-sectional | 693 | 68.3 ± 14.9 | 449 (64.8) | PCR |
| 6 | Bonnet, 2021 [27] | France | Cohort study | 2878 | 66.6 ± 17.0 | 1666 (57.8) | RT_PCR or CT |
| 7 | Langnau, 2021 [28] | Germany | Cross-sectional | 122 | 63.5 (47–79) | 122 (100) | RT-PCR |
| 8 | Li, 2020 [29] | China | Retrospective | 83 | 43 (32–64) | 34 (50) | Laboratory-confirmed and CT |
| 9 | Toprak, 2021 [30] | Turkey | Cross-sectional | 298 | 58.33 ± 15.52 | 156 (52.3) | RT-PCR |
| 10 | Gunawardene, 2021 [31] | Germany | Prospective cohort | 414 | 68 ± 18 | 339 (58.24) | Positive for SARSCoV-2 using a reliable test method |
| 11 | Cheng, 2021 [32] | China | Retrospective | 1157 | NR | 586 (50.6) | Laboratory-confirmed COVID-19 according to WHO guidelines |
| 12 | Akıllı, 2021 [33] | Turkey | Cross-sectional | 582 | NR | 339 (58.2) | NR |
| 13 | Inciardi, 2020 [34] | Italy | Cross-sectional | 99 | 67 ± 12 | 80 (80.8) | PCR |
| 14 | Xie, 2020 [35] | China | Cross-sectional | 62 | 66 (53.3–73) | 27 (43.5) | RT-PCR |
| 15 | Jalali, 2021 [36] | Iran | Cohort study | 196 | 65 (52–67) | 104 (53.1) | RT-PCR |
| 16 | Xiong, 2020 [37] | China | Cross-sectional | 116 | 58.5 (47–69) | 80 (69.0) | RT-PCR |
| 17 | Zhang, 2020 [38] | China | Retrospective | 541 | 53.25 ± 16.29 | 183 (33.8) | RT-PCR |
| 18 | Li, 2021 [39] | China | Cohort study | 2954 | 60 (50–68) | 1493 (50.5) | RT-PCR |
| 19 | Aoun, 2021 [40] | Lebanon | Retrospective | 231 | 61.46 ± 13.99 | 128 (55.4) | RT-PCR |
| 20 | Scoccia, 2021 [41] | Italy | Retrospective | 1625 | 69 (58–77) | 1092 (67.2) | laboratory-confirmed SARS-CoV-2 |
| 21 | Gottlieb, 2020 [42] | USA | Retrospective | 1483 | 56 (44–68) | 792 (53.4) | RT-PCR |
| 22 | Birtay, 2021 [43] | Turkey | Retrospective | 124 | 79 (64–91) | 62 (50) | RT_PCR |
| 23 | Angeli, 2020 [44] | Italy | Retrospective | 954 | 72 (59–85) | 543 (57) | RT-PCR |
| 24 | Brojakowska, 2021 [45] | USA | Retrospective | 7032 | NR | NR | RT-PCR |
| 25 | Pezel, 2021 [46] | France | Retrospective | 481 | 68.4 ± 9.6 | 295 (61.3) | RT-PCR |
| 26 | Salinas, 2021 [47] | Spain | Case control | 316 | 68 (58–78) | 224 (70.9) | PCR |
| 27 | Xiong, 2020 [48] | China | Retrospective | 472 | 43 (32–53.5) | 250 (53) | RT-PCR |
| 28 | Caliskan, 2020 [49] | Turkey | Retrospective | 565 | 48.0 ± 19.7 | NR | PCR |
| 29 | Chacko, 2021 [50] | USA | Retrospective | 255 | 65.4 ± 15.2 | 130 (51) | RT-PCR |
| 30 | Khawaja, 2021 [51] | U.K | Retrospective | 498 | 67.4 ± 16.1 | 310 (62.2) | RT-PCR |
| 31 | Barman, 2021 [52] | Turkey | Retrospective | 607 | 62.5 ± 14.3 | 334 (55) | RT-PCR |
| 32 | Mithal, 2021 [53] | India | Cross-sectional | 401 | 54 (19–92) | 276 (68.8) | RT-PCR |
| 33 | Terlecki, 2021 [54] | Poland | Retrospective | 1729 | 63 (50–75) | 886 (51.2) | NR |
| 34 | Cen, 2020 [55] | China | Cohort study | 1007 | 61 (49–68) | 493 (49) | RT-PCR |
| 35 | Keskin, 2021 [56] | Turkey | Cross-sectional | 37 | 66 (27–84) | 22 (59.5) | RT-PCR |
| 36 | Girardin, 2021 [57] | USA | Retrospective | 4446 | 65.7 ± 16.4 | 1166 (26.2) | SARSCoV2 Xpert Xpress assay |
| 37 | Nguyen, 2020 [58] | USA | Retrospective | 689 | 55 (40–68) | 296 (43) | NR |
| 38 | Ko, 2020 [59] | USA | Retrospective | 5416 | NR | 22,847 (53) | laboratory-confirmed COVID-19 |
| 39 | Nicholson, 2021 [60] | USA | Retrospective | 1042 | 64 (53–75) | 592 (56.8) | RT-PCR |
| 40 | Ayten, 2020 [61] | Turkey | Retrospective | 73 | 56.9 ± 13.3 | 47 (64.4) | RT-PCR |
| 41 | Aldabagh, 2021 [62] | USA | Retrospective | 450 | 66.4 ± 13.1 | 271 (60.2) | RT_PCR |
| 42 | Ciceri, 2020 [63] | Italy | Cohort study | 410 | 65 (56–75) | 299 (72.9) | RT-PCR |
| 43 | Kantroo, 2021 [64] | India | Retrospective | 1192 | 50 (35–61) | 832 (70) | RT-PCR |
| 44 | Guarin, 2021 [65] | USA | Retrospective | 275 | 64.69 ± 14.64 | 142 (51.6) | RT-PCR |
| 45 | Wei, 2020 [66] | China | Retrospective | 566 | 61.5 (NR) | 267 (47.2) | laboratory-confirmed COVID-19 |
| 46 | Weizman, 2021 [67] | France | Retrospective | 2878 | 66.9 ± 17 | 1666 (57.9) | RT-PCR |
| 47 | Mousseaux, 2021 [68] | France | Retrospective | 169 | 65.6 ± 18.8 | 118 (69.8) | RT-PCR |
| 48 | Ciprian, 2021 [69] | Italy | Retrospective | 109 | 71 (60–81) | 73 (67) | laboratory-confirmed SARSCoV-2 infection and clinical and radiological signs of COVID-19 |
| 49 | Poterucha, 2020 [70] | USA | Retrospective | 887 | 64.1 ± 17.2 | 513 (57.8) | RT-PCR |
| 50 | Zhang, 2021 [71] | China | Retrospective | 541 | 61.4 ± 13.6 | 255 (47.1) | |
| 51 | Lip, 2021 [72] | USA | Prospective cohort | 280,592 | 72.5 ± 9.9 | 115,629 (41.2) | NR |
| 52 | Russo, 2021 [73] | Italy | Retrospective | 467 | 66.88 ± 14.55 | 294 (63) | RT-PCR |
| 53 | Russo, 2020 [74] | Italy | Cross-sectional | 192 | 67.7 ± 15.2 | 115 (60) | RT-PCR |
| 54 | Gsblom, 2021 [75] | Finland | Retrospective | 585 | 57(46–70) | 316 (54) | RT-PCR |
| 55 | Turagam, 2020 [76] | USA | Retrospective | 140 | 61 (48–74) | 102 (72.9) | RT-PCR |
| 56 | Nanda, 2021 [77] | USA | Retrospective | 1169 | 43.9 ± 17.6 | 575 (49.2) | RT-PCR |
| 57 | Gupta, 2021 [78] | USA | Retrospective | 180 | 68 ± 59-80 | 98 (54.4) | RT-PCR |
| 58 | Adrish, 2020 [79] | USA | Retrospective | 1173 | 63 (53–73) | 720 (61.4) | RT-PCR |
| 59 | Raghavan, 2021 [80] | India | Retrospective | 845 | NR | 553 (65.4) | RT-PCR |
| 60 | Linschoten, 2020 [81] | Netherlands | Retrospective | 3011 | 67 (56–76) | 1890 (62.8) | NR |
| 61 | Koutroumpakis, 2021 [82] | USA | Retrospective | 514 | 59 (48–71) | 246 (47.9) | RT-PCR |
| 62 | Valenzuela, 2020 [83] | USA | Retrospective | 2039 | 52 (38–65) | 1081 (53) | RT-PCR |
| 63 | Gupta, 2021 [84] | USA | Retrospective | 529 | 70 (61–80) | 286 (54.1) | RT-PCR |
| 64 | Violi, 2021 [85] | Italy | Retrospective | 373 | 67.4 ± 16.8 | 228 (61.1) | RT-PCR and CT |
| 65 | Erben, 2021 [86] | USA | Retrospective | 915 | 60.8 ± 17.0 | 520 (56.8) | PCR or serology testing |
| 66 | Lodiqiani, 2020 [87] | Italy | Retrospective | 388 | 66 (57–75) | 264 (68) | laboratory-proven COVID-19 |
| 67 | Guner, 2020 [88] | Turkey | Cross-sectional | 222 | 50.6 ± 16.5 | 132 (59.5) | PCR |
| 68 | Maeda, 2021 [89] | USA | Retrospective | 181 | 64 ± 16.6 | 101 (55.8) | RT-PCR |
| 69 | Lala, 2020 [90] | USA | Retrospective | 2736 | 66.40 ± 15.80 | 1630 (59.6) | laboratory-confirmed SARS-CoV-2 |
| 70 | Kini, 2021 [16] | USA | Retrospective | 4695 | 64 ± 16.5 | 2643 (56.3) | laboratory-confirmed SARS-CoV2 |
| 71 | Gormez, 2021 [91] | Turkey | Retrospective | 103 | 45 (39–52) | 50 (48.5) | RT-PCR |
| 72 | Gupta, 2020 [92] | USA | Cross-sectional | 2215 | 60.5 ± 14.5 | 1436 (64.8) | Laboratory-confirmed COVID-19 (detected by nasopharyngeal or oropharyngeal swab) |
| 73 | Al-Ani, 2022 [93] | Iraq | Cross-sectional | 101 | 53.05 ± 15.16 | 69 (68.3) | RT_PCR |
| 74 | Atlas, 2021 [94] | Turkey | Retrospective | 102 | 69.1 ± 14.3 | 71 (69.6) | NR |
| 75 | Banoei, 2021 [95] | USA | Retrospective | 250 | 69.34 ± 13.69 | 130 (52) | PCR |
| 76 | Akhavizadegan, 2021 [96] | Iran | Retrospective | 1CE12 | 67.55 ± 12.57 | 77 (68.8) | RT_PCR |
CT=Chest computed tomography; IQR=Interquartile range; N=Number of participants; RT-PCR=Real time_polymerase chain reaction; SD=Standard deviation; USA=United States of America; who = World Health Organization.
N, number of studies; n, cohort size; WHO, World Health Organization.
3.3. Quality and risk of bias assessment
Overall, the risk of bias in this meta-analysis of 76 studies was relatively low and only 4% (n = 3) of the studies were reported to have a high risk of bias (Appendix C_ fig. C1).
3.4. Prevalence of CAD among hospitalized COVID-19 patients
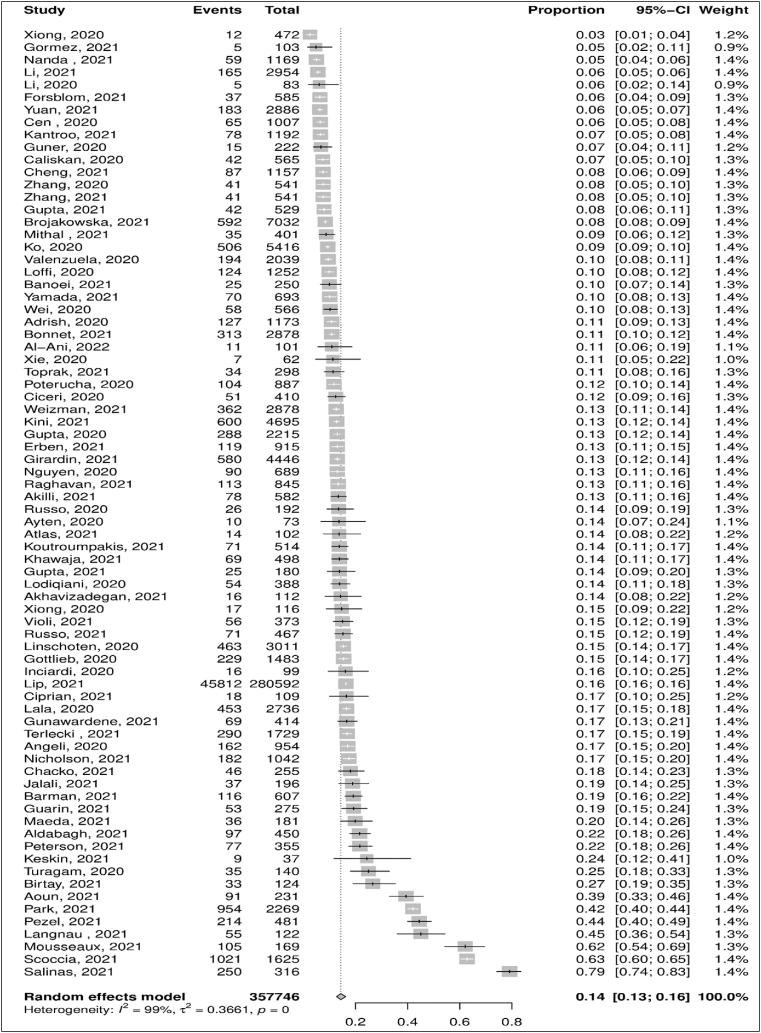
The prevalence of CAD among hospitalized COVID-19 patients was reported in 76 observational studies with a total participant count of 357,746. Using the random-effect model (I2 = 98.7% [95% CI: 98.5–98.8]), the pooled prevalence of CAD was 14.4% [95% CI: 12.7–16.2] (Fig. 2). A sensitivity analysis was performed to investigate further potential sources of heterogeneity in prevalence. Using leave-one-out analysis, three studies were identified as influential studies with an effect of 3.9% [41], 5.6% [47], and 3.6% [68] above the overall estimate of CAD prevalence. The pooled prevalence got adjusted to 13.2% [95% CI: 11.9–14.6] with an I2 of 97.8% [95% CI: 97.5–98.0] after removing these 3 influential studies and studies with a high risk of bias [47,79,93](Appendix C_ fig. C.2 and C.3).
Fig. 2.
Forest plot estimating pooled prevalence of Coronary Artery Disease (CAD) among COVID-19 hospitalized patients.
3.5. Mortality among hospitalized COVID-19 patients with CAD
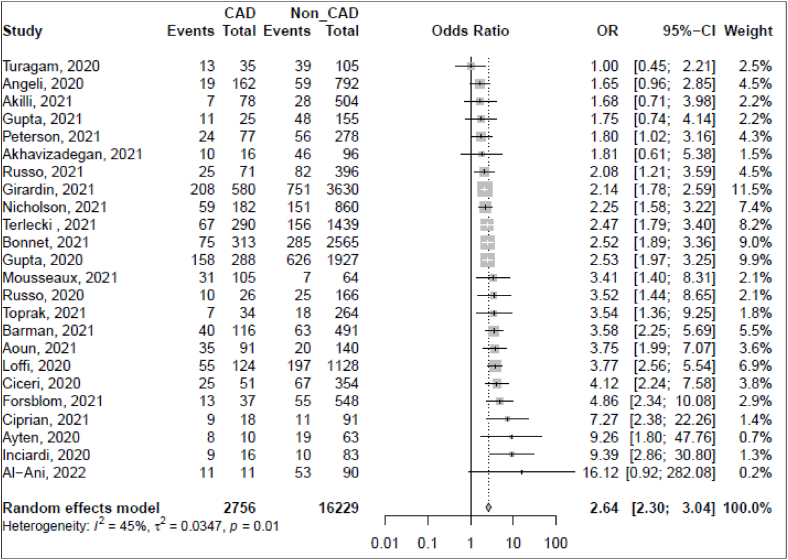
Data on mortality among hospitalized COVID-19 patients with CAD were available in twenty-four observational studies, with a total of 18,985 participants. Using a random-effect model (I2 = 44.7% [95% CI: 10.6–65.9]), the pooled odds ratio (OR) of mortality among hospitalized COVID-19 patients with CAD was 2.64 [95% CI: 2.30–3.04] (Fig. 3). A sensitivity analysis was performed to investigate further potential sources of heterogeneity for the estimated association. Using leave-one-out analysis, the highest estimated OR would be adjusted to 2.72 with an I2 of 42% and the lowest would be adjusted to 2.52 with an I2 of 41% after removing Girardin [57] and Loffi [22] studies, respectively (Appendix C_ fig. C4). Further analysis was performed, removing the high risk of bias study [93]. The adjusted OR was 2.63 [95% CI: 2.29–3.02] with an I2 of 45.0% [95% CI: 10.2–66.3] (Appendix C_ fig. C.5).
Fig. 3.
Forest plot for the association of CAD and mortality among hospitalized COVID-19 patients.
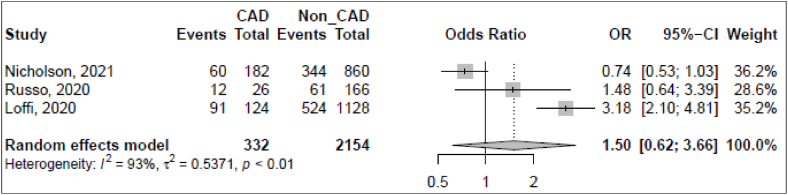
Fig. 4.
Forest plot for the association of CAD and ARDS among hospitalized COVID-19 patients.
3.6. ARDS among hospitalized COVID-19 patients with CAD
Out of 76 studies, we found only three studies reviewing the association between ARDS and CAD among hospitalized COVID-19 patients. With a total of 2486 participants reported for these studies, the pooled estimated association of ARDS with CAD was 1.50 [95% CI: 0.62–3.66). A high heterogeneity was reported among included studies (I2 = 93.0% [95% CI: 83.0–97.1]) (Fig. 4).
3.7. Subgroup analysis
We further evaluated the differences caused by geographical variations as a sub-group analysis. The pooled prevalence of CAD among hospitalized COVID-19 patients in European studies was 18.2% ([95% CI: 13.3–24.2], I2 = 98.9%), 13.5% ([95% CI: 12.0–15.1], I2 = 97.1%) in US studies and about 10.4% ([95% CI: 6.4–16.3], I2 = 99.0%) in Asian studies. The between-group analysis was not significant (χ2 = 4.69, p = 0.10). However, the prevalence was 4.7% higher among the European studies compared to US studies and 7.8% higher compared to the Asian studies (Appendix C_ fig. C.6).
For the association between mortality and CAD among hospitalized COVID-19 patients, the subgroup analysis showed that a higher pooled OR of mortality was among studies conducted in Asian countries (OR = 3.29 [95% CI: 1.88–5.76], I2 = 20%) compared to studies conducted in the US (OR = 2.18 [95% CI: 1.91–2.48], I2 = 14%) and Europe (OR = 3.07 [95% CI: 2.52–3.74], I2 = 40%). The estimated associations for the subgroup analysis were significant (χ2 = 9.16, p = 0.01) (Appendix C_ fig. C.7).
4. Discussion
4.1. Prevalence of CAD among hospitalized COVID-19 patients
Our review of 76 studies estimated a pooled prevalence of CAD in 14% of the patients with COVID-19 hospital admissions which varied by region. In comparison to Europe and the United States of America, despite the limited number of Asian studies, people living in Asia had a lower prevalence of CAD among patients infected with SARS-CoV2. Although the risk for mortality due to COVID-19 was two times higher among those with pre-existing CAD. Despite strong evidence for this relationship, the degree of certainty was relatively low. Likewise, our analysis revealed a 50% higher of ARDS among COVID-19 patients with CAD, although the association did not reach statistical significance.
4.2. CAD and mortality association among hospitalized COVID-19 patients
Numerous studies have reported that COVID-19 patients with CAD are at a higher risk of mortality [[97], [98], [99], [100]]. A high increment in the prevalence of mortality was observed across included studies, with a prevalence rate ranging between 9% [33] and 100% [93]. A possible explanation can be drawn from the fact that the S1 sub-unit of the spike protein of severe acute respiratory syndrome (SARS-CoV-2) binds to the host cells through an angiotensin-converting enzyme (ACE2) which triggers coagulation pathways, vasoconstriction, myocarditis and fibrosis [101]. The release of ACE2 is exacerbated as the cardiac condition progresses to systolic dysfunction and may lead to fatal outcomes, one being mortality. All of these increases the chance of severe outcomes among COVID-19 patients, one of which is mortality [102].
Our analysis revealed that the pooled estimated association of mortality among COVID-19 patients with CAD was high (OR = 2.62). Our finding was in harmony with the findings of a recent systematic review which reported that 22.9% of non-survival COVID-19 patients had CAD [4]. Studies show a higher rate of admission to the intensive unit and a more severe condition were observed among COVID-19 patients with CAD compared to those without CAD [4]. A multicenter cohort study reported that CAD was independently related to the higher risk of 28-days in hospital mortality (OR = 1.47, 95% CI: 1.07–2.02) [92]. Similarly another study reported that the likelihood of COVID-19 mortality was relatively higher at older ages compared to younger ages [57]. The study also suggested that the presence of comorbidities including CAD, diabetes mellitus, and hypertension may be attributed to the higher age-related mortality in COVID-19 patients [57]. Across included studies, the median age of the participants ranged between 57 and 72 years old, which might give justification for the high estimated association that was revealed by our analysis, as suggested by others [13,31,36,42,60,74,103].
Based on the geographical distribution, the subgroup meta-analysis showed the highest pooled estimate of mortality with CAD in Asian studies compared to those conducted in Europe and the US. However, a lower pooled estimate of mortality was observed in Asian studies after removing the study with a high risk of bias [93](OR = 2.99 [1.5524; 5.7895], I2 21.9%). As a result, taking into account the pooled estimate of mortality after removing the influential study could yield a reliable estimate. The subgroup analysis was significant and the possible reason for this variation might be due to the healthcare services disturbed across the globe, which might have serious implications for the prognosis of COVID-19 patients with CAD [104].
4.2.1. CAD and ARDS association among hospitalized COVID-19 patients
COVID-19 patients with prior CAD or heart failure are more vulnerable to developing adverse events or having severe clinical phenotypes [4]. A recent study reported that patients with CAD had an absolute increase of about 27% in the incidence of ARDS among COVID-19 patients during hospitalization [22]. Although, respiratory dysregulation has been causally linked to SARS-CoV-1 and Middle East respiratory syndrome (MERS). However, multifactorial etiology complicates the understanding of physiological links and pathogenesis involved in the development of respiratory changes and mortality associated with SARS-CoV-2 infection [105]. It is likely that genomic similarity between SARS-CoV-1 and SARS-CoV-2 could be a potential area for scientific inquiry and investigation of precise mechanisms and etiological links to the proliferation of inflammatory mediators and cellular pathways leading to the damage of pulmonary vessels and respiratory complications [106]. Despite observing an association between the occurrence of ARDS and CAD, albeit non-significant, our findings indicate a 50% increased likelihood of ARDS among patients with comorbid conditions such as CAD and COVID-19 infection [107]. Yet, no ARDS interventions that are used are effective enough to employ the advantages of early ARDS detection. Due to these considerations, identifying a reliable biomarker for ARDS is a challenge [107]. Hence, further knowledge of the impact of CAD on the occurrence of ARDS among hospitalized COVID-19 patients is needed.
4.3. Limitations
Our review included observational studies which were either cross-sectional in nature or were retrospectively conducted and the element of residual confounding and selection bias cannot be ruled out. Cause and effect link cannot be studied between mortality rate and the existence of CAD among hospitalized COVID-19 patients as none of the included studies were randomized control trial.
In some of the studies, the ascertainment of CAD relied on subjective assessments, which introduces the risk of non-differential misclassification and measurement errors. We would like to acknowledge the limitations associated with diagnosing CAD as a categorical variable. The categorical nature of CAD diagnosis is arbitrary and somewhat restrictive in terms of clinical assumptions.
Furthermore, our review did not evaluate the effects of existing CAD on the length of hospital stay among COVID-19 patients, which could have served as a proxy indicator for disease severity, complications, and its impact on the health system.
Additionally, the studies included could not ascertain the temporal links on whether infectivity with COVID-19 increases the length of hospital stays and adverse health outcomes or whether it is the existing chronic condition or comorbidity that can lead to multi-morbidity and mortality among COVID-19 patients. Despite acknowledging the geographical variations and implications of the prevalence of cardiac morbidities among COVID-19 patients. The analysis might have adjusted for differences in healthcare infrastructure which differs between these regions, however, we could not control for disparities in the severity of diseases caused by different variants of coronavirus infection and how this affects the relationship between mortality due to CAD among COVID-19.
The available data limited our ability to determine if severe outcomes varied based on the type and duration of CAD. Additionally, the majority of the included studies were conducted in Europe and the USA, limiting the generalizability of our findings to other regions. Testing protocols varied across the world and could not be controlled for in this study.
Finally, our literature search was restricted to English-language sources only, which introduces the risk of publication bias and the exclusion of evidence from unpublished papers and non-English language sources.
5. Conclusion
The existence of CAD was found relatively associated with increased severity and mortality in COVID-19 patients in our study. This study revealed a pooled prevalence of 14% of CAD among hospitalized COVID-19 patients. CAD and COVID-19 share the existence of ARDS, which might be the main reason for increased mortality among those patients who suffer from both diseases at once. Further research on determining how CAD affects the likelihood of ARDS in COVID-19 hospitalized patients is needed.
Author contribution statement
Mohammed Merzah: Conceived and designed the experiments; Analyzed and interpret the data; Performed the experiments; Contributed analysis tools; Wrote the paper.
Atiya Abdul Karim: Performed the experiments; Analyzed and interpreted the data; Contributed analysis tools; Wrote the paper.
Dahy Sulaiman: Performed the experiments; Contributed analysis tools; Wrote the paper.
Mazin E. Khalil, Stany Mathew, Yasir Almuzaini, Shima Hashemi, Salina Khatoon, Sabyasachi Gupta, Mohima Benojir Hoquej: Performed the experiments; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We express our sincere gratitude to all the researchers who conducted the studies included in this analysis. Additionally, our sincere thanks go to the LinkedIn group "Public Health and Epidemiology: Scientific Cooperation Forum" https://www.linkedin.com/groups/9156184/, through which we gathered to conduct this review.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19493.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO . World Health Organization; Sep-2022. Coronavirus Disease (COVID-19) Pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Online]. Available: [Google Scholar]
- 2.WHO, “WHO Coronavirus (COVID-19) Dashboard,” World Health Organization. [Online]. Available: https://covid19.who.int/. [Accessed: 23-September-2022].
- 3.da Rosa Mesquita R., et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin. Wochenschr. 2021;133(7–8):377–382. doi: 10.1007/s00508-020-01760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szarpak L., et al. Effect of coronary artery disease on COVID-19—prognosis and risk assessment : a systematic review and meta-analysis. Biology. 2022;11(2):1–11. doi: 10.3390/biology11020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyck D.R., Zylak C.J. Acute respiratory distress in adults. Radiology. 1973;106(3):497–501. doi: 10.1148/106.3.497. [DOI] [PubMed] [Google Scholar]
- 6.Vakili M., Shirani S., Paknejad O., Yousefshahi F. Acute respiratory distress syndrome diagnosis after coronary artery bypass: comparison between diagnostic criteria and clinical picture. Acta Med. Iran. 2015;53(1):51–56. [PubMed] [Google Scholar]
- 7.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit. Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sjoding M.W., et al. Comparing clinical features and outcomes in mechanically ventilated patients with COVID-19 and acute respiratory distress syndrome. Ann. Am. Thorac. Soc. 2021;18(11):1776–1885. doi: 10.1513/AnnalsATS.202008-1076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ralapanawa U., Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J. Epidemiol. Glob. Health. 2021;11(2):169–177. doi: 10.2991/jegh.k.201217.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA, J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji W., et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J. Korean Med. Sci. 2020;35(25):1–15. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F. Clinical course and risk factors for mortality of adult in patients with COVID-19 in wuhan, China: a retrospective cohort study. J. Med. Study Res. 2020;3(1):1–2. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuin M., Rigatelli G., Bilato C., Rigatelli A., Roncon L., Ribichini F. Preexisting coronary artery disease among coronavirus disease 2019 patients: a systematic review and meta-analysis. J. Cardiovasc. Med. 2022;23(8):535–545. doi: 10.2459/JCM.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 15.Qiang X.-L., Xu P., Fang G., Liu W.-B., Kou Z. Using the spike protein feature to predict infection risk and monitor the evolutionary dynamic of coronavirus. Infect. Dis. Poverty. 2020;9(1):33. doi: 10.1186/s40249-020-00649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kini A., et al. Types of myocardial injury and mid-term outcomes in patients with COVID-19. Eur. Hear. J. - Qual. Care Clin. Outcomes. 2021;7(5):438–446. doi: 10.1093/ehjqcco/qcab053. [DOI] [PubMed] [Google Scholar]
- 17.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A., Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):1–11. doi: 10.1186/s13643-016-0384-4. [Internet] Disponível em:Syst. Rev., vol. 5, no. 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells G., et al. Ottawa Hosp.; 2004. The Newcastle–Ottawa Scale (NOQAS) for Assessing the Quality of Non-randomized Studies in Meta-Analysis. [Google Scholar]
- 19.Rs. Team . RStudio, Inc; Boston, MA: 2021. RStudio: Integrated Development for R. [Google Scholar]
- 20.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14(1):1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loffi M., et al. Coronary artery disease in patients hospitalised with Coronavirus disease 2019 (COVID-19) infection. Open Hear. 2020;7(2):1–7. doi: 10.1136/openhrt-2020-001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan S., Chen P., Li H., Chen C., Wang F., Wang D.W. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J. Cell Mol. Med. 2021;25(2):1263–1273. doi: 10.1111/jcmm.16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson E., et al. The relationship between coronary artery disease and clinical outcomes in COVID-19: a single-center retrospective analysis. Coron. Artery Dis. 2021:367–371. doi: 10.1097/MCA.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 25.Park B.E., et al. Impact of cardiovascular risk factors and cardiovascular diseases on outcomes in patients hospitalized with COVID-19 in daegu metropolitan city. J. Korean Med. Sci. 2021;36(e15) doi: 10.3346/jkms.2021.36.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada T., et al. Multiple cardiovascular diseases or risk factors increase the severity of Coronavirus disease 2019. Circ. J. 2021;85(11):2111–2115. doi: 10.1253/circj.CJ-21-0684. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet G., et al. Characteristics and outcomes of patients hospitalized for COVID-19 in France: the Critical COVID-19 France (CCF) study. Arch. Cardiovasc. Dis. 2021;114(5):352–363. doi: 10.1016/j.acvd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langnau C., et al. Platelet activation and plasma levels of furin are associated with prognosis of patients with coronary artery disease and COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021;41(6):2080–2096. doi: 10.1161/ATVBAHA.120.315698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M., et al. Cardiovascular disease potentially contributes to the progression and poor prognosis of COVID-19. Nutr. Metab. Cardiovasc. Dis. 2020;30(7):1061–1067. doi: 10.1016/j.numecd.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toprak İl D., Sağlam S., Aşikoğlu B., Eruzun H., Öksüz S., Altunok E.S. Do age, hypertension, coronary artery disease, ace-i, ARB or beta-blockers therapy increase the risk of mortality in COVID 19 patients? The results of a tertiary center in Turkey. Acta Medica Mediterr. 2021;37(1):547–552. [Google Scholar]
- 31.Gunawardene M.A., et al. Prognostic impact of acute cardiovascular events in covid‐19 hospitalized patients—results from the corona Germany study. J. Clin. Med. 2021;10(17):1–17. doi: 10.3390/jcm10173982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng R., et al. Sex differences in the incidence and risk factors of myocardial injury in COVID-19 patients: a retrospective cohort study. Front. Physiol. 2021;12:1–9. doi: 10.3389/fphys.2021.632123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akıllı I.K., Bilge M. Prognostic performance of the CALL score in hospitalized patients with COVID-19 pneumonia. Med J Bakirkoy. 2021;17:359–366. [Google Scholar]
- 34.Inciardi R.M., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y., et al. Impact of cardiovascular disease on clinical characteristics and outcomes of coronavirus disease 2019 (COVID-19) Circ. J. 2020;84(8):1277–1283. doi: 10.1253/circj.CJ-20-0348. [DOI] [PubMed] [Google Scholar]
- 36.Jalali F., et al. Characteristics and outcomes of hospitalized patients with cardiovascular complications of COVID-19. J. Cardiovasc. Thorac. Res. 2021;13(4):355–363. doi: 10.34172/jcvtr.2021.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong S., et al. Clinical characteristics of 116 hospitalized patients with COVID-19 in Wuhan, China: a single-centered, retrospective, observational study. BMC Infect. Dis. 2020;20(1):1–11. doi: 10.1186/s12879-020-05452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., et al. Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart. 2020;106(15):1148–1153. doi: 10.1136/heartjnl-2020-316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li P., et al. Implications of cardiac markers in risk-stratification and management for COVID-19 patients. Crit. Care. 2021;25(1):1–14. doi: 10.1186/s13054-021-03555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoun M., et al. Age and multimorbidities as poor prognostic factors for COVID-19 in hemodialysis: a Lebanese national study. BMC Nephrol. 2021;22(1):1–10. doi: 10.1186/s12882-021-02270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scoccia A., et al. Impact of clinical and subclinical coronary artery disease as assessed by coronary artery calcium in COVID-19,” Atherosclerosis. 2021;328(2021):136–143. doi: 10.1016/j.atherosclerosis.2021.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottlieb M., Sansom S., Frankenberger C., Ward E., Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad. Emerg. Med. 2020;27(10):963–973. doi: 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birtay T., Bahadir S., Kabacaoglu E., Yetiz O., Demirci M.F., Genctoy G. Prognosis of patients hospitalized with a diagnosis of COVID-19 pneumonia in a tertiary hospital in Turkey. Ann. Saudi Med. 2021;41(6):327–335. doi: 10.5144/0256-4947.2021.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angeli F., et al. Joint effect of heart failure and coronary artery disease on the risk of death during hospitalization for COVID-19. Eur. J. Intern. Med. 2021:81–86. doi: 10.1016/j.ejim.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brojakowska A., et al. Comorbidities, sequelae, blood biomarkers and their associated clinical outcomes in the Mount Sinai Health System COVID-19 patients. PLoS One. 2021;16(7 July):1–17. doi: 10.1371/journal.pone.0253660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pezela T., et al. Prognostic value of pre-hospitalization stress perfusion cardiovascular magnetic resonance to predict death in patients hospitalized for COVID-19. Arch. Cardiovasc. Dis. 2021;114:781–792. doi: 10.1016/j.acvd.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salinas P., et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int. Heart J. 2021;62(2):274–281. doi: 10.1536/ihj.20-574. [DOI] [PubMed] [Google Scholar]
- 48.Xiong T.Y., et al. Hypertension is a risk factor for adverse outcomes in patients with coronavirus disease 2019: a cohort study. Ann. Med. 2020;52(7):361–366. doi: 10.1080/07853890.2020.1802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caliskan T., Saylan B. Smoking and comorbidities are associated with COVID-19 severity and mortality in 565 patients treated in Turkey: a retrospective observational study. Rev. Assoc. Med. Bras. 2020;66(12):1679–1684. doi: 10.1590/1806-9282.66.12.1679. [DOI] [PubMed] [Google Scholar]
- 50.Chacko S.R., et al. Association of pre-admission statin use with reduced in-hospital mortality in COVID-19. Am. J. Med. Sci. 2021;361(6):725–730. doi: 10.1016/j.amjms.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khawaja S.A., et al. COVID-19 and its impact on the cardiovascular system. Open Hear. 2021;8(1) doi: 10.1136/openhrt-2020-001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barman H.A., et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron. Artery Dis. 2021;(2020):359–366. doi: 10.1097/MCA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mithal A., Jevalikar G., Sharma R., Singh A. High prevalence of diabetes and other comorbidities in hospitalized patients with COVID-19 in Delhi, India, and their association with outcomes. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15(1):169–175. doi: 10.1016/j.dsx.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terlecki M., et al. Association between cardiovascular disease, cardiovascular drug therapy, and in-hospital outcomes in patients with COVID-19: data from a large single-center registry in Poland. Kardiol. Pol. 2021;79(8):773–780. doi: 10.33963/KP.15990. [DOI] [PubMed] [Google Scholar]
- 55.Cen Y., et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi-centre observational study. Clin. Microbiol. Infect. 2020;26(9):1242–1247. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keskin G., Uysal A., Hafiz E., Dogan O.F. Urgent percutaneous coronary artery intervention and coronary artery bypass grafting in stemi patients with confirmed covid-19. Heart Surg. Forum. 2021;24(30):E564–E574. doi: 10.1532/hsf.3567. [DOI] [PubMed] [Google Scholar]
- 57.Girardin J.L., et al. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron. Respir. Dis. 2021;18 doi: 10.1177/1479973120986806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen A.B., et al. 2020. Outcomes and Cardiovascular Comorbidities in a Predominantly African-American Population with COVID-19,” medRxiv; p. 2020. 06.28.20141929. [Google Scholar]
- 59.Ko J.Y., et al. Risk factors for coronavirus disease 2019 (COVID-19)-Associated hospitalization: COVID-19-Associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin. Infect. Dis. 2021;72(11):E695–E703. doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicholson C.J., et al. Estimating risk of mechanical ventilation and in-hospital mortality among adult COVID-19 patients admitted to Mass General Brigham: the VICE and DICE scores. EClinicalMedicine. 2021;33 doi: 10.1016/j.eclinm.2021.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayten O., Saylan B. Retrospective analysis of severe COVID-19 pneumonia patients treated with lopinavir/ritonavir: a comparison with survivor and non-survivor patients. South. African J. Infect. Dis. 2020;35(1):1–7. doi: 10.4102/sajid.v35i1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aldabagh M., Wagle S., Cesa M., Yu A., Farooq M., Goldberg Y. Survival of in‐hospital cardiac arrest in covid‐19 infected patients. Healthc. 2021;9(10) doi: 10.3390/healthcare9101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciceri F., et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kantroo V., et al. Mortality and clinical outcomes among patients with COVID-19 and diabetes. Med. Sci. 2021;9(4):65. doi: 10.3390/medsci9040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guarin G., et al. Factors associated with hospital readmissions among patients with COVID-19: a single-center experience. J. Med. Virol. 2021;93(9):5582–5587. doi: 10.1002/jmv.27104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei C., et al. Clinical characteristics and manifestations in older patients with COVID-19. BMC Geriatr. 2020;20(1):1–9. doi: 10.1186/s12877-020-01811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weizman O., et al. Characteristics and impact of cardiovascular comorbidities on coronavirus disease 2019 in women: a multicentre cohort study. Arch. Cardiovasc. Dis. 2021;114(5):394–406. doi: 10.1016/j.acvd.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mousseaux E., Fayol A., Danchin N., Soulat G. Association between coronary artery calcifications and 6-month mortality in hospitalized patients with COVID-19. Diagn. Interv. Imaging. 2021;102(12):717–725. doi: 10.1016/j.diii.2021.06.007. Original. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cipriani A., et al. Cardiac injury and mortality in patients with Coronavirus disease 2019 (COVID-19): insights from a mediation analysis. Intern. Emerg. Med. 2021;16(2):419–427. doi: 10.1007/s11739-020-02495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poterucha T.J., et al. Admission cardiac diagnostic testing with electrocardiography and troponin measurement prognosticates increased 30-day mortality in COVID-19. J. Am. Heart Assoc. 2021;10(1):1–14. doi: 10.1161/JAHA.120.018476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhong L., et al. Effects of hypertension on the outcomes of COVID-19: a multicentre retrospective cohort study. Ann. Med. 2021;53(1):770–776. doi: 10.1080/07853890.2021.1931957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lip G.Y.H., Genaidy A., Tran G., Marroquin P., Estes C. Incident atrial fibrillation and its risk prediction in patients developing COVID-19: a machine learning based algorithm approach. Eur. J. Intern. Med. 2021;91(January):53–58. doi: 10.1016/j.ejim.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.R V., et al. Preadmission statin therapy and clinical outcome in hospitalized patients with COVID-19: an Italian multicenter observational study. J. Cardiovasc. Pharmacol. 2021;78(1):e94–e100. doi: 10.1097/FJC.0000000000001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russo V., et al. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol. Res. 2020;159(May) doi: 10.1016/j.phrs.2020.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forsblom E., et al. Male predominance in disease severity and mortality in a low Covid-19 epidemic and low case-fatality area–a population-based registry study. Infect. Dis. (Auckl). 2021;53(10):789–799. doi: 10.1080/23744235.2021.1936157. [DOI] [PubMed] [Google Scholar]
- 76.Turagam M.K., et al. Malignant arrhythmias in patients with COVID-19: incidence, mechanisms, and outcomes. Circ. Arrhythm. Electrophysiol. 2020;13(11) doi: 10.1161/CIRCEP.120.008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nanda S., et al. A midwest COVID-19 cohort for the evaluation of multimorbidity and adverse outcomes from COVID-19. J. Prim. Care Community Heal. 2021;12 doi: 10.1177/21501327211010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta Y.S., et al. Coronary artery calcification in COVID-19 patients: an imaging biomarker for adverse clinical outcomes. Clin. Imaging. 2021;77:1–8. doi: 10.1016/j.clinimag.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adrish M., et al. Association of smoking status with outcomes in hospitalised patients with COVID-19. BMJ Open Respir. Res. 2020;7(1):1–6. doi: 10.1136/bmjresp-2020-000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raghavan A., Nanditha A., Satheesh K., Susairaj P. Profile and prognosis of patients hospitalized for COVID-19 virus infection with and without diabetes e an observational study from South India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15(4) doi: 10.1016/j.dsx.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Linschoten M., et al. Cardiac complications in patients hospitalised with COVID-19. Eur. Hear. Journal. Acute Cardiovasc. Care. 2020;9(8):817–823. doi: 10.1177/2048872620974605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koutroumpakis E., et al. Geographical differences in cardiovascular comorbidities and outcomes of COVID-19 hospitalized patients in the USA. Cardiol. 2021;146(4):481–488. doi: 10.1159/000515064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valenzuela R.G., et al. Outcomes in hispanics with COVID-19 are similar to those of caucasian patients in suburban New York. Acad. Emerg. Med. 2020;27(12):1260–1269. doi: 10.1111/acem.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta R., et al. Higher comorbidities and early death in hospitalized African-American patients with Covid-19. BMC Infect. Dis. 2021;21(1):1–12. doi: 10.1186/s12879-021-05782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Violi F., et al. Arterial and venous thrombosis in coronavirus 2019 disease (Covid-19): relationship with mortality. Intern. Emerg. Med. 2021;16(5):1231–1237. doi: 10.1007/s11739-020-02621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erben Y., et al. Deep vein thrombosis and pulmonary embolism among hospitalized coronavirus disease 2019–positive patients predicted for higher mortality and prolonged intensive care unit and hospital stays in a multisite healthcare system. J. Vasc. Surg. Venous Lymphat. Disord. 2021;9(6):1361–1370.e1. doi: 10.1016/j.jvsv.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lodigiani C., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Güner R., et al. COVID-19 experience of the major pandemic response center in the capital: results of the pandemic's first month in Turkey. Turkish J. Med. Sci. 2020;50(8):1801–1809. doi: 10.3906/sag-2006-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maeda T., Obata R., Rizk D., Kuno T. Cardiac injury and outcomes of patients with COVID-19 in New York city. Hear. Lung Circ. 2021;30(6):848–853. doi: 10.1016/j.hlc.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lala A., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection Anuradha. J. Am. Coll. Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Görmez S., et al. Comparison of hypertension prevalence and the use of renin-angiotensin-aldosterone system blockers in hospitalized patients with COVID-19 and non-COVID-19 viral pneumonia. Turk Kardiyol. Dern. Ars. 2021;49(4):286–292. doi: 10.5543/tkda.2021.87750. [DOI] [PubMed] [Google Scholar]
- 92.Gupta S., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern. Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Ani A., Ghazzay H.I., Al Shawi A.F., Al-Koubaisy H.N.E., Al-Ani F., Aldouri M. Association of chronic diseases with mortality among hospitalized patients with COVID-19 treated with convalescent plasma: evidence from a single center - Iraq. J. Emerg. Med. Trauma Acute Care. 2022;2022(2) [Google Scholar]
- 94.Atlas A., et al. Neutrophil-to-lymphocyte and fibrinogen-to-albumin ratios may be indicators of worse outcomes in ICU patients with COVID-19. J. Surg. Med. 2021;5(6):623–627. [Google Scholar]
- 95.Banoei M.M., Dinparastisaleh R., Zadeh A.V., Mirsaeidi M. Machine-learning-based COVID-19 mortality prediction model and identification of patients at low and high risk of dying. Crit. Care. 2021;25(1):1–14. doi: 10.1186/s13054-021-03749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akhavizadegan H., et al. Can laboratory tests at the time of admission guide us to the prognosis of patients with COVID-19? J. Prev. Med. Hyg. 2021;62(2):E321–E325. doi: 10.15167/2421-4248/jpmh2021.62.2.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi S., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guzik T.J., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramanathan K., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(20):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hui H., et al. medRxiv; 2020. Clinical and Radiographic Features of Cardiac Injury in Patients with 2019 Novel Coronavirus Pneumonia. [Google Scholar]
- 101.Seeherman S., Suzuki Y.J. Viral infection and cardiovascular disease: implications for the molecular basis of COVID-19 pathogenesis. Int. J. Mol. Sci. 2021;22(4):1–13. doi: 10.3390/ijms22041659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pacurari M., Kafoury R., Tchounwou P.B., Ndebele K. The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflam. 2014;2014 doi: 10.1155/2014/689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheraz M., et al. COVID-19 patients in a tertiary US hospital: assessment of clinical course and predictors of the disease severity. Respir. Med. 2020;172(October) doi: 10.1016/j.rmed.2020.106130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiss P., Carcel C., Hockham C., Peters S.A.E. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur. Hear. J. - Qual. Care Clin. Outcomes. 2021;7(1):18–27. doi: 10.1093/ehjqcco/qcaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baig A.M. Computing the effects of SARS-CoV-2 on respiration regulatory mechanisms in COVID-19. ACS Chem. Neurosci. 2020;11(16):2416–2421. doi: 10.1021/acschemneuro.0c00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020;21(1):1–14. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laffey J.G. Predicting the development of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2013;187(7):671–672. doi: 10.1164/rccm.201301-0168ed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.