Abstract
The forelimbs of hominoid primates (apes) are decidedly more flexible than those of monkeys, especially at the shoulder, elbow and wrist joints. It is tempting to link the greater mobility of these joints to the functional demands of vertical climbing and below-branch suspension, but field-based kinematic studies have found few differences between chimpanzees and monkeys when comparing forelimb excursion angles during vertical ascent (upclimbing). There is, however, a strong theoretical argument for focusing instead on vertical descent (downclimbing), which motivated us to quantify the effects of climbing directionality on the forelimb kinematics of wild chimpanzees (Pan troglodytes) and sooty mangabeys (Cercocebus atys). We found that the shoulders and elbows of chimpanzees and sooty mangabeys subtended larger joint angles during bouts of downclimbing, and that the magnitude of this difference was greatest among chimpanzees. Our results cast new light on the functional importance of downclimbing, while also burnishing functional hypotheses that emphasize the role of vertical climbing during the evolution of apes, including the human lineage.
Keywords: kinematics, descents, ascents, vertical climbing, primates, morphology
1. Introduction
Vertical climbing—defined as the ascent or descent of substrates angled greater than 45° to the horizontal plane [1]—is a widespread behaviour across animal forms, including invertebrates [2,3], squamates [4,5] and mammals [6–10]. Strongly associated with arboreal life, vertical-climbing animals must resist downward slips and the pull of gravity, as falling can result in serious injury, death or predation [7]. It follows that natural selection has favoured anatomical or behavioural traits to mitigate slippage, either by means of claws [6,7], surface adhesion [4] or friction [5]. But with few exceptions, primates are devoid of claws or adhesive capabilities, relying instead on the normal forces generated by their limbs to produce friction grips [6–8].
Vertical climbing is a conspicuous and essential behaviour of hominoid primates, including all great apes [11–14] and some human populations [15,16]. The large body size of most apes, relative to monkeys and other primates, is expected to substantially increase the energetic costs of vertical climbing [17] and risk of falling [18], and it is long hypothesized that the morphologies of hominoid shoulders and elbows evolved to mitigate these twin challenges [19–22]. The retention of these same forelimb traits across early hominin taxa is another topic of enduring debate [23], in part because it suggests that vertical climbing was an essential preadaptation for obligate bipedalism [24–30] (but see [31]).
These discussions have focused almost exclusively on vertical ascent and the functional complexes that reduce the downward torque of gravity by positioning the centre of mass closer to a given support [6]. For instance, extreme dorsiflexion of the ankle—common during chimpanzee ascension [32]—is predicted to improve pedal friction by forcing the forelimbs to subtend large angles, enhancing stability during upward propulsion [6,7]. Captive apes affirm this expectation, exhibiting high degrees of shoulder flexion and elbow extension when climbing flexible ropes [33], yet wild chimpanzees rarely achieve the same joint angles when ascending trees, exhibiting monkey-like forelimb kinematics instead [34,35]. Given these contradictory patterns, some scholars have shifted their attention to postural behaviours, suggesting that the highly mobile forelimbs of apes evolved primarily in response to below-branch, arm-hanging suspensory activities [36–38].
All but ignored in this debate is the challenge of reverse (caudal-first) descent (hereafter, downclimbing). The reason is simple enough—gravitational forces are indifferent to the direction of climbing—but even so, only downclimbing requires controlled braking to counteract gravitational, or passive, acceleration; i.e. eccentric muscular contractions to control the reduction of potential energy and corresponding increases in kinetic energy [39–42]. Studies of chameleons [43], tamarins [42] and humans [44] have shown that steeper arboreal declines reduce vertical force production by the lower-positioned limbs. Consequently, greater support and braking from the higher-positioned limbs may be needed to control descent safely [43]. Downclimbing, then, is expected to increase external loading by the forelimbs on the vertical substrate, which would: (i) generate braking impulse via increasing friction; (ii) improve medio-lateral stability; and (iii) reduce downward toppling moments that compromise safety [45–47].
To exert elevated forces on the vertical substrate, it follows that the shoulders and elbows of primates will subtend larger joint angles during downclimbing [6,7], and that the magnitude of this difference will be greatest at larger body sizes [46]. Thus, differences in forelimb mobility between monkeys and larger-bodied apes are more likely to manifest themselves most strongly during downclimbing. Here, we test this hypothesis by comparing the upper limb kinematics of wild chimpanzees and a species of cercopithecid monkey during vertical climbing.
2. Material and methods
2.1. Study sites and subjects
We observed chimpanzees (Pan troglodytes schweinfurthii; Ngogo community) in Kibale National Park, Uganda during two three-week periods in June 2006 and July–August 2007; and we observed sooty mangabeys (Cercocebus atys) in the primary study grid of the Taï Monkey Project in Taï National Park, Côte d'Ivoire for two two-month spans: August–September 2019 and January–March 2022. Defined as a montane or lowland rainforest, respectively, the sites have similar densities of canopy-level trees (540 and 507 ha−1, respectively) [48,49] and understory poles/saplings (4290 and 3687 ha−1, respectively) [49,50].
Sooty mangabeys are exceptional among cercopithecid monkeys for exhibiting a suite of derived skeletal traits associated with vertical climbing [51,52]. Accordingly, we sought to verify classic monkey–ape morphometric differences in the glenohumeral and humeroulnar joints of our two study species. We accessed osteological collections housed in the Museum of Comparative Zoology, Harvard University and the Department of Anthropology, Ohio State University. We calculated size-standardized measures of glenoid width and depth [53] and olecranon process length [19], following standard procedures with linear caliper measurements.
2.2. Video capture and kinematic analysis
We filmed chimpanzees at 14 fps with a Canon GL2 hand-held digital video recorder, targeting adult males, although juveniles and females were also filmed opportunistically. We filmed mangabeys at 30 fps using a tripod-mounted Canon EOS 7D video camera, targeting adults and subadults, although some older juveniles were also recorded opportunistically. Filming distances varied between 5 and 10 m, but every bout of vertical climbing was recorded in lateral view and limited to the first 2–5 m of ascent/descent to minimize angle-induced errors [15].
Following DeSilva [32], we isolated video stills of climbing bouts that depicted the shoulder joint in lateral view at the points of maximum excursion, defined as the largest visible joint angles during shoulder (glenohumeral) flexion and elbow (humeroulnar) extension. Following Levangie & Norkin [54], we defined shoulder flexion as the anterior movement of the humerus in the sagittal plane around a coronal axis passing through the centre of the humeral head, and elbow extension as the posterior movement of the forearm in the sagittal plane around a coronal axis passing through the humeroulnar joint. Shoulder flexion was measured relative to the bole of the tree, which was parallel to the gravitational vector in most videos (figure 1; electronic supplementary material, figure S1). It is also the most functionally relevant reference point for exploring species-level contrasts in shoulder morphology and mobility [53]. Following similar reasoning [36], elbow extension was measured relative to the position of the upper arm. We preferentially targeted the near side of the animal, using three points to define the excursion angles of the shoulder and elbow joints (electronic supplementary material, figure S1). We calculated excursion angles using the manual angle tools in Kinovea v0.8.15 or ImageJ v2.3.0 [55]. We compared angles during vertical ascent and descent intraspecifically (one-tailed tests) and interspecifically (two-tailed tests) using unpooled two-sample t-tests in JMP 16 (SAS Institute, Cary, NC, USA).
Figure 1.

Bouts of vertical climbing in chimpanzees (a,b) and sooty mangabeys (c,d). Maximum angles of shoulder flexion and elbow extension were greater during downclimbs (b,d) compared to upclimbs (a,c), and the magnitudes of these differences were greatest among chimpanzees. Panels (a) and (b) taken by J.M.D and panels (c) and (d) taken by L.D.F.
To assess intra- and inter-observer reliability, we examined a subset of 10 video stills using the protocol of Guatelli-Steinberg et al. [56]. Briefly, L.D.F. and M.S.J. measured shoulder and elbow excursion angles from each still, replicating the process three days later to produce a total of 40 measures. We calculated inter- and intra-observer error and used the irr package in R (version 4.2.3) to calculate intraclass correlation coefficients (ICCs) [57]. ICCs can be used to evaluate intra- and inter-observer reliability, with values ranging from 0 (no reliability between observers) to 1 (perfect reliability between observers). Average intra-observer measurement error for all joint angles was ±2% (ICCs for both joints = 0.98 for L.D.F. and 0.99 for M.S.J.), whereas average inter-observer error was ±3% for shoulder flexion (ICC: 0.97 (95% CI: 0.89 < ICC < 0.992)) and ±2% for elbow extension (ICC coefficient: 0.98 (95% CI: 0.935 < ICC < 0.995)). Overall, this level of agreement compares favourably to previous kinematic studies of wild primates [32].
Given that substrate diameters could affect forelimb kinematics during climbing [35], we measured the diameter at breast height (DBH; 1.5 m) of most trees associated with climbing bouts. We used the overlapping package in R [58] to determine interspecific overlap in the DBH of trees climbed and used regression to assess the potential effects of DBH on shoulder and elbow excursion.
3. Results
3.1. Intraspecific differences during climbing
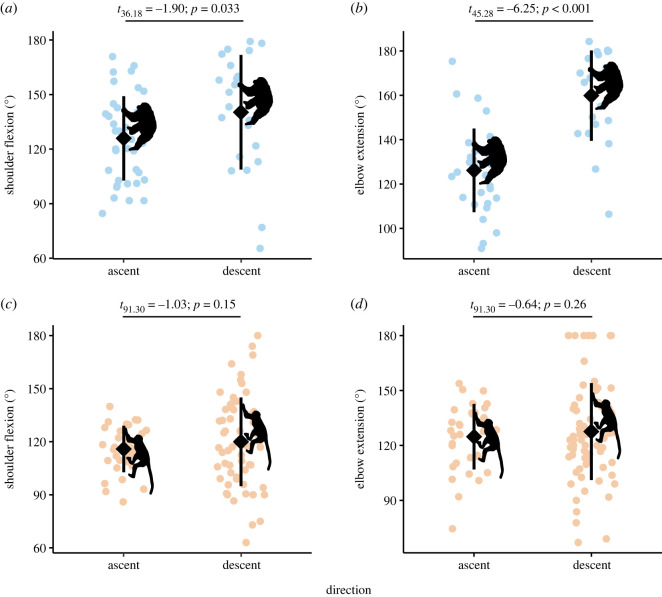
Chimpanzee maximum shoulder flexion was 14° greater during bouts of downclimbing (mean: 140 ± 7° (s.e.); n = 23 bouts) than upclimbing (mean: 126 ± 4°; n = 39 bouts; figure 2a), and maximum elbow extension was 34° greater during bouts of downclimbing (mean: 160 ± 3°; n = 23 bouts) than upclimbing (mean: 126 ± 4°; n = 32 bouts; figure 2b). Among mangabeys, such differences were marginal: maximum shoulder flexion was 4° greater during downclimbing (mean: 120 ± 3°; n = 63) than upclimbing (mean: 116 ± 3°; n = 32; figure 2c), and maximum elbow extension was 3° greater during bouts of downclimbing (mean: 128 ± 3°; n = 71 bouts) than upclimbing (mean: 125 ± 3°; n = 34 bouts; figure 2d).
Figure 2.
The effect of vertical climbing direction on shoulder flexion and elbow extension in chimpanzees (a,b) and sooty mangabeys (c,d). Chimpanzees (light blue) exhibited significantly higher degrees of average shoulder flexion (a) and elbow extension (b) during vertical descent compared to vertical ascent. By contrast, shoulder flexion (c) and elbow extension (d) in sooty mangabeys (light orange) were invariant across climbing directions. Diamonds indicate means and vertical bars are ±1 s.d.
3.2. Interspecific differences during climbing
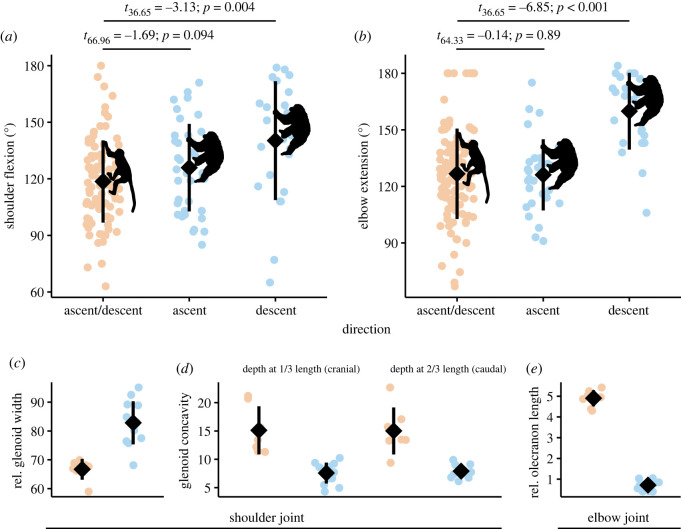
To simplify interspecific comparisons, we compared joint angles during up- and downclimbing by mangabeys and pooled the datasets based on their homogeneity (figure 2c,d). During ascent, the degree of shoulder flexion and elbow extension was similar between the two species (figure 3a,b), but during descent, the degree of shoulder flexion and elbow extension differed greatly, with chimpanzees expressing mean joint angles that were 21° and 33° greater, respectively, than those of mangabeys (figure 3a,b).
Figure 3.
Forelimb vertical climbing kinematic (a,b) and morphological (c–e) contrasts between chimpanzees (light blue) and sooty mangabeys (light orange). Chimpanzees exhibited significantly greater degrees of shoulder flexion (a) and elbow extension (b) than sooty mangabeys during vertical descent, but not vertical ascent. Sooty mangabeys had relatively narrower (c) and craniocaudally curved (d) glenoid cavities, but larger olecranon processes than chimpanzees (e). Diamonds indicate means and vertical bars are ±1 s.d. See electronic supplementary material for definitions of size-standard measurements.
3.3. Comparative morphometrics
Relative to mangabeys, we found that the glenoid cavities of chimpanzees are wider, uniformly shallower, and more ovate (figure 3c,d), and that their olecranon processes are shorter (figure 3e), which is consistent with expected ape–monkey differences in forelimb functional morphology.
3.4. Diameter at breast height and joint angles
The DBH of climbed trees differed between the two species—chimpanzees climbed wider trees (mean DBH = 14 ± 8 cm; n = 166) than those used by mangabeys (mean DBH = 6 ± 3 cm; n = 259), but observed DBHs overlapped by 24%. We found no statistical effect of DBH on shoulder flexion or elbow extension among chimpanzees (electronic supplementary material, figure S2). Variation in DBH explained approximately 10% of variation in mangabey shoulder flexion, but there was no statistical relationship with elbow extension (electronic supplementary material, figure S2).
4. Discussion
We found that the maximum angles of shoulder flexion and elbow extension of chimpanzees and sooty mangabeys were largest during downclimbing, and that the magnitude of these differences was greatest among chimpanzees. The significance of our findings is twofold. First, our data corroborate conclusions drawn from observational categorical data: chimpanzees and cercopithecid monkeys use their forelimbs in kinematically similar ways during upclimbing [34–37]. Second, our findings extend classic [6,7] and more recent [39–41] theoretical discussions of vertical climbing by casting new light on the functional importance of downclimbing during ape evolution.
The evolutionary milestones of ape forelimb mobility are attested by several fossil taxa. For instance, the early Miocene ape Morotopithecus (approx. 21 Ma) possessed an ape-like glenoid cavity, which is interpreted as evidence of ‘forelimb suspensory and forelimb dominated climbing behaviours, including vertical ascension’ (emphasis ours) [53]. The earliest evidence of olecranon reduction emerged later in Danuvius (12 Ma) and Hispanopithecus (10 Ma), and is fully modern with the appearance of Oreopithecus (7 Ma) [59,60]. Authors have interpreted this aspect of elbow morphology as an ‘unmistakable hallmark of below-branch or suspensory behaviour' [60]; or evidence of ‘eclectic climbing and below-branch suspensory behaviours’ [59]. We do not dispute the essential role of these positional behaviours, but we would argue that these same morphologies also speak loudly to the critical importance of controlled downclimbing.
Improved downclimbing among Miocene apes can be viewed as a by-product, or ‘spandrel' [61], of selection favouring below-branch suspension in some large-bodied lineages [62]. But it also raises the possibility of changing arboreal conditions, such as increasing canopy stratification or rugosity [63–65], which would favour more frequent movement in the vertical dimension. Another possibility pivots around increasing terrestrial behaviours. Indeed, there is evidence of Miocene apes exploiting non-arboreal foods, such as fallen fruits [66] as well as aquatic plants and underground storage organs [67]; and further, some habitat reconstructions would appear to favour frequent downclimbing to traverse heterogeneous swamp-woodlands [68] or wooded grasslands [69]. Dual use of terrestrial and arboreal milieus, mediated by frequent downclimbs, may also explain the retention of ancestral, ape-like traits in the forelimbs of early fossil hominins such as Sahelanthropus [70], Ardipithecus [71] and Australopithecus [72]. This perspective begins to reconcile competing hypotheses focused on either postural or locomotor behaviours [36], as downclimbing is necessary to connect both.
Contrary to published predictions [34,35], we found mixed evidence for DBH-mediated effects on joint angles during climbing, but these results are partially underpowered with only 12 DBH associations for chimpanzees. Thus, our limited sample size was insufficient for testing the hypothesized (positive) effect of larger DBH on chimpanzee elbow extension during vertical climbing. Moving forward, it would be instructive to explore more systematically if and how substrate properties (e.g. size, angle, surface texture) affect the forelimb kinematics of downclimbing. Another limitation of our study concerns climbing speed, which we were unable to measure, and its potential effects on the kinematics of downclimbing. Greater travel speed can affect forelimb protraction during knuckle-based quadrupedalism [73], but there is little evidence of it affecting shoulder or elbow excursions during upclimbing [33]. Still, passive acceleration due to gravity could make downclimbing faster and inherently less stable [39], factors that could favour a braking impulse and greater forelimb excursion angles. In such cases, the velocity of descent is expected to be lower than that of ascent, as reported for some primates that descend trees headfirst [74]; but see [42].
Species inviting future comparative study include the larger-bodied forest papionins of West Africa. Wild mandrills (Mandrillus sphinx), for example, attain masses up to 36 kg [75] and it is an open question whether they descend trees in a monkey- or ape-like fashion. Investigating downclimbing in olive baboons (Papio anubis) would also be informative, since they too can attain large masses (approx. 40 kg) while upclimbing in a manner like chimpanzees [11]. Lastly, our functional predictions at the outset drew on deductive logic, arguing that downclimbing increases energetic costs and the risk of falling, factors that are expected to incur relatively high fitness costs [46]. This premise could be further tested among human populations that regularly climb vertical surfaces [76], including large-diameter trees [77].
Acknowledgements
We are grateful for the practical assistance of L. Bele, E. A. Bitty, T. Cannon, S. Fannin, L. Francomacaro, Q. Gallot, J. Lwanga, J. León, J. Traff, B. Kare, P. Kalo, L. MacLatchy, J. Mitani, S. Noel, M. Omura, N. Thompson, D. Watts, G. West, and J. Young. Fieldwork was approved by the village of Poule Oula, Ministère de l'Enseignement Supérieur et de la Recherche Scientifique, Direction Générale de la Recherche Scientifique et de l'Innovation Technologie, the Ministère de l'Environnement, des Eaux et Forêts, Office Ivoirien de Parcs et Reserves (Permit nos. 029-2019 and 198-2022 to L.D.F.), the Uganda Wildlife Authority and Uganda National Council for Science and Technology.
Ethics
Data collected for this study were observational and followed the American Society of Primatologists' principles for the ethical treatment of non-human primates, the guidelines of permit-granting bodies in Côte d'Ivoire and Uganda and IACUC at Ohio State University (protocol 2008A0051-R4).
Data accessibility
Data are accessible in the Dryad Digital Repository: https://doi.org/10.5061/dryad.hqbzkh1m8 [78]. https://datadryad.org/stash/share/qS3yB8WyjeDQePSxUwk552DsZ_HZlyDV4bm6ZLPoDgM
Supplementary figures are provided in the electronic supplementary material [79].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
L.D.F.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, software, visualization, writing—original draft, writing—review and editing; M.S.J.: conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, writing—original draft, writing—review and editing; N.J.D.: conceptualization, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing; W.S.M.: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing—original draft, writing—review and editing; J.M.D.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Funding was received from Dartmouth College (Clare Garber Goodman Fund to L.D.F.; James O. Freedman Presidential Scholars Research Fund to M.S.J.), the Explorer's Club (Mamont Scholars grant to L.D.F.), the Leakey Foundation (to J.M.D.), the National Science Foundation (BCS 0840110, 0921770 and 0922429 to W.S.M.; GRFP 1840344 to L.D.F.) and the Primate Society of Great Britain (research grant to L.D.F.).
References
- 1.Hunt KD, Cant JGH, Gebo DL, Rose MD, Walker SE, Youlatos D. 1996. Standardized descriptions of primate locomotor and postural modes. Primates 37, 363-387. ( 10.1007/BF02381373) [DOI] [Google Scholar]
- 2.Von Hagen H-O. 1977. The tree-climbing crabs of Trinidad. Stud. Fauna Curaço Other Caribb. Isl. 54, 25-59. [Google Scholar]
- 3.Goldman DI, Chen TS, Dudek DM, Full RJ. 2006. Dynamics of rapid vertical climbing in cockroaches reveals a template. J. Exp. Biol. 209, 2990-3000. ( 10.1242/jeb.02322) [DOI] [PubMed] [Google Scholar]
- 4.Autumn K, Hsieh ST, Dudek DM, Chen J, Chitaphan C, Full RJ. 2006. Dynamics of geckos running vertically. J. Exp. Biol. 209, 260-272. ( 10.1242/jeb.01980) [DOI] [PubMed] [Google Scholar]
- 5.Byrnes G, Jayne BC. 2014. Gripping during climbing of arboreal snakes may be safe but not economical. Biol. Lett. 10, 20140434. ( 10.1098/rsbl.2014.0434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartmill M. 1974. Pads and claws in arboreal locomotion. In Primate locomotion (ed. Jenkins FA), pp. 45-83. New York, NY: Academic Press. [Google Scholar]
- 7.Cartmill M. 1985. Climbing. In Functional vertebrate morphology (eds Hildebrand M, Bramble DM, Liem KF, Wake DB), pp. 73-88. Cambridge, MA: Belknap Press. [Google Scholar]
- 8.Hanna JB, Granatosky MC, Rana P, Schmitt D. 2017. The evolution of vertical climbing in primates: evidence from reaction forces. J. Exp. Biol. 220, 3039-3052. ( 10.1242/jeb.157628) [DOI] [PubMed] [Google Scholar]
- 9.Kitchener AC, Van Valkenburgh B, Yamaguchi N. 2010. Felid form and function. In Biology and conservation of wild felids (eds Macdonald DW, Loveridge A), pp. 83-106. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Amanat S, Mayer J, Paracha H, Ali Z, Granatosky MC. 2020. Bear locomotion. In Encyclopedia of animal cognition and behavior (eds Vonk J, Shackelford T), pp. 1-6. Cham, Switzerland: Springer International Publishing. ( 10.1007/978-3-319-47829-6_1707-1) [DOI] [Google Scholar]
- 11.Hunt KD. 1991. Positional behavior in the Hominoidea. Int. J. Primatol. 12, 95-118. ( 10.1007/BF02547576) [DOI] [Google Scholar]
- 12.Doran DM. 1993. Comparative locomotor behavior of chimpanzees and bonobos: the influence of morphology on locomotion. Am. J. Phys. Anthropol. 91, 83-98. ( 10.1002/ajpa.1330910106) [DOI] [PubMed] [Google Scholar]
- 13.Sarringhaus LA, MacLatchy LM, Mitani JC. 2014. Locomotor and postural development of wild chimpanzees. J. Hum. Evol. 66, 29-38. ( 10.1016/j.jhevol.2013.09.006) [DOI] [PubMed] [Google Scholar]
- 14.Neufuss J, Robbins MM, Baeumer J, Humle T, Kivell TL. 2017. Comparison of hand use and forelimb posture during vertical climbing in mountain gorillas (Gorilla beringei beringei) and chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 164, 651-664. ( 10.1002/ajpa.2330) [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman VV, Kraft TS, Dominy NJ. 2013. Tree climbing and human evolution. Proc. Natl Acad. Sci. USA 110, 1237-1242. ( 10.1073/pnas.1208717110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft TS, Venkataraman VV, Dominy NJ. 2014. A natural history of human tree climbing. J. Hum. Evol. 71, 105-118. ( 10.1016/j.jhevol.2014.02.002) [DOI] [PubMed] [Google Scholar]
- 17.Hanna JB, Schmitt D. 2011. Locomotor energetics in primates: gait mechanics and their relationship to the energetics of vertical and horizontal locomotion. Am. J. Phys. Anthropol. 145, 43-54. ( 10.1002/ajpa.21465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pontzer H, Wrangham RW. 2004. Climbing and the daily energy cost of locomotion in wild chimpanzees: implications for hominoid locomotor evolution. J. Hum. Evol. 46, 315-333. ( 10.1016/j.jhevol.2003.12.006) [DOI] [PubMed] [Google Scholar]
- 19.Rose MD. 1988. Another look at the anthropoid elbow. J. Hum. Evol. 17, 193-224. ( 10.1016/0047-2484(88)90054-1) [DOI] [Google Scholar]
- 20.MacLatchy L. 2004. The oldest ape. Evol. Anthropol. 13, 90-103. ( 10.1002/evan.10133) [DOI] [Google Scholar]
- 21.Thompson NE, Rubinstein D, Larson SG. 2018. Great ape thorax and shoulder configuration—an adaptation for arboreality or knuckle-walking? J. Hum. Evol. 125, 15-26. ( 10.1016/j.jhevol.2018.09.005) [DOI] [PubMed] [Google Scholar]
- 22.Arias-Martorell J. 2019. The morphology and evolutionary history of the glenohumeral joint of hominoids: a review. Ecol. Evol. 9, 703-722. ( 10.1002/ece3.4392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cazenave M, Kivell TL. 2023. Challenges and perspectives on functional interpretations of australopith postcrania and the reconstruction of hominin locomotion. J. Hum. Evol. 175, 103304. ( 10.1016/j.jhevol.2022.103304) [DOI] [PubMed] [Google Scholar]
- 24.Prost JH. 1980. Origin of bipedalism. Am. J. Phys. Anthropol. 52, 175-189. ( 10.1002/ajpa.1330520204) [DOI] [PubMed] [Google Scholar]
- 25.Fleagle JG, Stern JT, Jungers WL, Susman RL, Vangor AK, Wells JP. 1981. Climbing: a biomechanical link with brachiation and with bipedalism. Symp. Zool. Soc. Lond. 48, 359-375. [Google Scholar]
- 26.Tuttle RH. 1981. Evolution of hominid bipedalism and prehensile capabilities. Phil. Trans. R. Soc. Lond. B 292, 89-94. ( 10.1098/rstb.1981.0016) [DOI] [Google Scholar]
- 27.Hirasaki E, Kumakura H, Matano S. 1993. Kinesiological characteristics of vertical climbing in Ateles geoffroyi and Macaca fuscata. Folia Primatol. 61, 148-156. ( 10.1159/000156742) [DOI] [PubMed] [Google Scholar]
- 28.Hirasaki E, Kumakura H, Matano S. 2000. Biomechanical analysis of vertical climbing in the spider monkey and the Japanese macaque. Am. J. Phys. Anthropol. 113, 455-472. () [DOI] [PubMed] [Google Scholar]
- 29.Almécija S, Hammond AS, Thompson NE, Pugh KD, Moyà-Solà S, Alba DM. 2021. Fossil apes and human evolution. Science 372, eabb4363. ( 10.1126/science.abb4363) [DOI] [PubMed] [Google Scholar]
- 30.Williams SA, Prang TC, Russo GA, Young NM, Gebo DL. 2023. African apes and the evolutionary history of orthogrady and bipedalism. Am. J. Biol. Anthropol. 181, 58-80. ( 10.1002/ajpa.24684) [DOI] [Google Scholar]
- 31.Lovejoy CO, Suwa G, Simpson SW, Matternes JH, White TD. 2009. The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science 326, 73-106. ( 10.1126/science.1175833) [DOI] [PubMed] [Google Scholar]
- 32.DeSilva JM. 2009. Functional morphology of the ankle and the likelihood of climbing in early hominins. Proc. Natl Acad. Sci. USA 106, 6567-6572. ( 10.1073/pnas.0900270106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isler K. 2005. 3D-kinematics of vertical climbing in hominoids. Am. J. Phys. Anthropol. 126, 66-81. ( 10.1002/ajpa.10419) [DOI] [PubMed] [Google Scholar]
- 34.Hunt KD. 1991. Mechanical implications of chimpanzee positional behavior. Am. J. Phys. Anthropol. 86, 521-536. ( 10.1002/ajpa.1330860408) [DOI] [PubMed] [Google Scholar]
- 35.Hunt KD. 1992. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am. J. Phys. Anthropol. 87, 83-105. ( 10.1002/ajpa.1330870108) [DOI] [PubMed] [Google Scholar]
- 36.Hunt KD. 2016. Why are there apes? Evidence for the co-evolution of ape and monkey ecomorphology. J. Anat. 228, 630-685. ( 10.1111/joa.12454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebo DL. 1996. Climbing, brachiation, and terrestrial quadrupedalism: historical precursors of hominid bipedalism. Am. J. Phys. Anthropol. 101, 55-92. () [DOI] [PubMed] [Google Scholar]
- 38.Temerin LA, Cant JGH. 1983. The evolutionary divergence of Old World monkeys and apes. Am. Nat. 122, 335-351. ( 10.1086/284139) [DOI] [Google Scholar]
- 39.Preuschoft H. 2002. What does ‘arboreal locomotion’ mean exactly and what are the relationships between ‘climbing’, environment and morphology? Z. Morph. Anthropol. 83, 171-188. [PubMed] [Google Scholar]
- 40.Perchalski B. 2021. Headfirst descent behaviors in a comparative sample of strepsirrhine primates. Am. J. Primatol. 83, e23259. ( 10.1002/ajp.23259) [DOI] [PubMed] [Google Scholar]
- 41.Youlatos D, Gasc J-P. 1994. Critical foraging locomotor patterns: head-first vertical descent in the red howler monkey (Alouatta seniculus). Z. Morph. Anthropol. 80, 65-77. [Google Scholar]
- 42.Hesse B, Nyakatura JA, Fischer MS, Schmidt M. 2015. Adjustments of limb mechanics in cotton-top tamarins to moderate and steep support orientations: significance for the understanding of early primate evolution. J. Mamm. Evol. 22, 435-450. ( 10.1007/s10914-014-9283-4) [DOI] [Google Scholar]
- 43.Krause C, Fisher MS. 2013. Biodynamics of climbing: effects of substrate orientation on the locomotion of a highly arboreal lizard (Chamaeleo calyptratus). J. Exp. Biol. 216, 1448-1457. ( 10.1242/jeb.082586) [DOI] [PubMed] [Google Scholar]
- 44.Baláš J, et al. 2014. The effect of climbing ability and slope inclination on vertical foot loading using a novel force sensor instrumentation system. J. Hum. Kinet. 44, 75-81. ( 10.2478/hukin-2014-0112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birn-Jeffery AV, Higham TE. 2014. Geckos significantly alter foot orientation to facilitate adhesion during downhill locomotion. Biol. Lett. 10, 20140456. ( 10.1098/rsbl.2014.0456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birn-Jeffery AV, Higham TE. 2014. The scaling of uphill and downhill locomotion in legged animals. Integr. Comp. Biol. 54, 1159-1172. ( 10.1093/icb/icu015) [DOI] [PubMed] [Google Scholar]
- 47.Lammers AR, Earls KD, Biknevicius AR. 2006. Locomotor kinetics and kinematics on inclines and declines in the gray short-tailed opossum Monodelphis domestica. J. Exp. Biol. 209, 4154-4166. ( 10.1242/jeb.02493) [DOI] [PubMed] [Google Scholar]
- 48.Janmaat KRL, Ban SD, Boesch C. 2013. Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Anim. Behav. 86, 1183-1205. ( 10.1016/j.anbehav.2013.09.021) [DOI] [Google Scholar]
- 49.Massan JM. 1987. The influence of mechanized selective logging, felling intensity and gap-size on the regeneration of a tropical moist forest. PhD thesis, Michigan State University, East Lansing, MI, USA. [Google Scholar]
- 50.McGraw WS. 1996. Cercopithecid locomotion, support use, and support availability in the Tai Forest, Ivory Coast. Am. J. Phys. Anthropol. 100, 507-522. () [DOI] [PubMed] [Google Scholar]
- 51.Fleagle JG, McGraw WS. 1999. Skeletal and dental morphology supports diphyletic origin of baboons and mandrills. Proc. Natl Acad. Sci. USA 96, 1157-1161. ( 10.1073/pnas.96.3.1157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleagle JG, McGraw WS. 2002. Skeletal and dental morphology of African papionins: unmasking a cryptic clade. J. Hum. Evol. 42, 267-292. ( 10.1006/jhev.2001.0526) [DOI] [PubMed] [Google Scholar]
- 53.MacLatchy L, Gebo D, Kityo R, Pilbeam D. 2000. Postcranial functional morphology of Morotopithecus bishopi, with implications for the evolution of modern ape locomotion. J. Hum. Evol. 39, 159-183. ( 10.1006/jhev.2000.0407) [DOI] [PubMed] [Google Scholar]
- 54.Levangie PK, Norkin CC. 2011. Joint structure and function, 5th edn. Philadelphia, PA: F.A. Davis Company. [Google Scholar]
- 55.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guatelli-Steinberg D, Guerrieri T, Kensler TB, Maldonado E, Francis G, Kohn LA, Zhao MQ, Turnquist JE, Wang Q. 2022. Male Cayo Santiago rhesus macaques (Macaca mulatta) tend to have greater molar wear than females at comparable ages: exploring two possible reasons why. Am. J. Biol. Anthropol. 178, 437-447. ( 10.1002/ajpa.24519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koo TK, Li MY. 2016. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155-163. ( 10.1016/j.jcm.2016.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastore M, Calcagnì A. 2019. Measuring distribution similarities between samples: a distribution-free overlapping index. Front. Psychol. 10, 1089. ( 10.3389/fpsyg.2019.01089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alba DM, Almécija S, Casanovas-Vilar I, Méndez JM, Moyà-Solà S. 2012. A partial skeleton of the fossil great ape Hispanopithecus laietanus from Can Feu and the mosaic evolution of crown-hominoid positional behaviors. PLoS ONE 7, e39617. ( 10.1371/journal.pone.0039617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Böhme M, Spassov N, Fuss J, Tröscher A, Deane AS, Prieto J, Kirscher U, Lechner T, Begun DR. 2019. A new Miocene ape and locomotion in the ancestor of great apes and humans. Nature 575, 489-493. ( 10.1038/s41586-019-1731-0) [DOI] [PubMed] [Google Scholar]
- 61.Raichlen D, Shapiro L. 2007. The evolution of mammalian locomotor biomechanics: adaptations or spandrels? J. Morph. 268, 1122. ( 10.1002/jmor.10589) [DOI] [Google Scholar]
- 62.Andrews P, Martin L. 1991. Hominoid dietary evolution. Phil. Trans. R. Soc. Lond. B 334, 199-209. ( 10.1098/rstb.1991.0109) [DOI] [PubMed] [Google Scholar]
- 63.Grand TI. 1972. A mechanical interpretation of terminal branch feeding. J. Mammal. 53, 198-201. ( 10.2307/1378849) [DOI] [Google Scholar]
- 64.Ripley S. 1979. Environmental grain, niche diversification, and positional behavior in Neogene primates: an evolutionary hypothesis. In Environment, behavior, and morphology: dynamic interactions in primates (eds Morbeck ME, Preuschoft H, Gomberg N), pp. 37-74. New York, NY: Gustav Fischer. [Google Scholar]
- 65.Michel LA, et al. 2014. Remnants of an ancient forest provide ecological context for Early Miocene fossil apes. Nat. Commun. 5, 3236. ( 10.1038/ncomms4236) [DOI] [PubMed] [Google Scholar]
- 66.Carrigan MA, Uryasev O, Frye CB, Eckman BL, Myers CR, Hurley TD, Benner SA. 2015. Hominids adapted to metabolize ethanol long before human-directed fermentation. Proc. Natl Acad. Sci. USA 112, 458-463. ( 10.1073/pnas.1404167111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson SV, Rook L. 2016. Isotopic reconstructions of habitat change surrounding the extinction of Oreopithecus, the last European ape. Am. J. Phys. Anthropol. 160, 254-271. ( 10.1002/ajpa.22970) [DOI] [PubMed] [Google Scholar]
- 68.Andrews P. 2020. Last common ancestor of apes and humans: morphology and environment. Folia Primatol. 91, 122-148. ( 10.1159/000501557) [DOI] [PubMed] [Google Scholar]
- 69.MacLatchy LM, et al. 2023. The evolution of hominoid locomotor versatility: evidence from Moroto, a 21 Ma site in Uganda. Science 380, eabq2835. ( 10.1126/science.abq2835) [DOI] [PubMed] [Google Scholar]
- 70.Daver G, Guy F, Mackaye HT, Likius A, Boisserie JR, Moussa A, Pallas L, Vignaud P, Clarisse ND. 2022. Postcranial evidence of late Miocene hominin bipedalism in Chad. Nature 609, 94-100. ( 10.1038/s41586-022-04901-z) [DOI] [PubMed] [Google Scholar]
- 71.Prang TC, Ramirez K, Grabowski M, Williams SA. 2021. Ardipithecus hand provides evidence that humans and chimpanzees evolved from an ancestor with suspensory adaptations. Sci. Adv. 7, eabf2474. ( 10.1126/sciadv.abf2474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunt KD. 1996. The postural feeding hypothesis: an ecological model for the evolution of bipedalism. S. Afr. J. Sci. 92, 77-90. ( 10.10520/AJA00382353_7777) [DOI] [Google Scholar]
- 73.Pontzer H, Raichlen DA, Rodman PS. 2014. Bipedal and quadrupedal locomotion in chimpanzees. J. Hum. Evol. 66, 64-82. ( 10.1016/j.jhevol.2013.10.002) [DOI] [PubMed] [Google Scholar]
- 74.Kivell TL, Schmitt D, Wunderlich RE. 2010. Hand and foot pressures in the aye-aye (Daubentonia madagascariensis) reveal novel biomechanical trade-offs required for walking on gracile digits. J. Exp. Biol. 213, 1549-1557. ( 10.1242/jeb.040014) [DOI] [PubMed] [Google Scholar]
- 75.Setchell JM, Lee PC, Wickings EJ, Dixson AF. 2001. Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx). Am. J. Phys. Anthropol. 115, 349-360. ( 10.1002/ajpa.1091) [DOI] [PubMed] [Google Scholar]
- 76.Kozma EE, Pontzer H. 2021. Determinants of climbing energetic costs in humans. J. Exp. Biol. 224, jeb234567. ( 10.1242/jeb.234567) [DOI] [PubMed] [Google Scholar]
- 77.Demps K, Zorondo-Rodríguez F, García C, Reyes-García V. 2012. Social learning across the life cycle: cultural knowledge acquisition for honey collection among the Jenu Kuruba, India. Evol. Hum. Behav. 33, 460-470. ( 10.1016/j.evolhumbehav.2011.12.008) [DOI] [Google Scholar]
- 78.Fannin LD, Joy MS, Dominy NJ, McGraw WS, DeSilva JM. 2023. Data from: Downclimbing and the evolution of ape forelimb morphologies. Dryad Digital Repository. ( 10.5061/dryad.hqbzkh1m8) [DOI] [PMC free article] [PubMed]
- 79.Fannin LD, Joy MS, Dominy NJ, McGraw WS, DeSilva JM. 2023. Downclimbing and the evolution of ape forelimb morphologies. Figshare. ( 10.6084/m9.figshare.c.6805017) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fannin LD, Joy MS, Dominy NJ, McGraw WS, DeSilva JM. 2023. Data from: Downclimbing and the evolution of ape forelimb morphologies. Dryad Digital Repository. ( 10.5061/dryad.hqbzkh1m8) [DOI] [PMC free article] [PubMed]
- Fannin LD, Joy MS, Dominy NJ, McGraw WS, DeSilva JM. 2023. Downclimbing and the evolution of ape forelimb morphologies. Figshare. ( 10.6084/m9.figshare.c.6805017) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are accessible in the Dryad Digital Repository: https://doi.org/10.5061/dryad.hqbzkh1m8 [78]. https://datadryad.org/stash/share/qS3yB8WyjeDQePSxUwk552DsZ_HZlyDV4bm6ZLPoDgM
Supplementary figures are provided in the electronic supplementary material [79].