Abstract
The human menstrual cycle is characterized by substantial variability both within and between women. Here, we sought to account for such variability by examining whether human menstrual cycle frequency varies as a function of the projected fitness payoffs associated with investment in mating effort. We used structural equation modeling to test the prediction that women whose environmental conditions or life histories favor heavier investment in mating effort would have shorter, more regular cycles. Results supported our hypothesis, revealing that women who project more mating success and have faster life history strategies exhibit greater mating effort and shorter, more regular menstrual cycles. An alternative model that specified cycle frequency as a predictor of mating effort was a poor fit for the data, lending support for the hypothesized directionality of the path between these variables. Together, these results provide some of the first empirical evidence that the length and regularity of the human menstrual cycle may be calibrated to investment in mating effort.
Keywords: life history theory, menstrual cycle, fecundity, mating effort, mating success
Although the human menstrual cycle typically lasts between 21 and 35 days, there is considerable variability in cycle length both between and within women (Chiazze, Brayer, Macisco, Parker, & Duffy, 1968; Creinin, Keverline, & Meyn, 2004). For example, one seminal study tracking 2,316 women over 30,655 total cycles found that healthy women’s menstrual cycles often last anywhere between 15 and 45 days (Chiazze et al., 1968). Others find sizable differences in cycle length even within individual women, with most reporting inter-cycle differences ranging between 7 and 14 days (Creinin et al., 2004).
Why is there so much variability in women’s cycles? Much research addressing this issue has examined the role that lifestyle and environmental factors each play in contributing to the observed variability in women’s cycles (e.g., Fenster et al., 1999; Harlow & Matanoski, 1991; Rowland et al., 2002). For example, longer, more irregular cycles have been associated with higher body mass index (BMI), stress, and disorders such as diabetes (Matteo, 1987; Solomon et al., 2001). Shorter, more regular cycles, on the other hand, have been linked to healthier BMI, nulliparity, and earlier age of menarche (Kato et al., 1999).
Although such research has provided a useful first step in identifying various contributors to menstrual cycle variability, it has been done in isolation of a larger predictive framework that could account for these findings and generate new predictions about contextual effects on cycle length. Here, we redress these gaps using insights from life history theory (LHT), hypothesizing that variation in women’s cycle frequency should reflect the projected fitness payoffs associated with investment in mating effort. Specifically, we predicted that women whose life histories or environmental conditions favor heavier investment in mating effort would have shorter, more regular cycles, whereas women whose life histories or environmental conditions favor lesser investment in mating effort would have longer, less regular cycles.
Factors Influencing the Human Menstrual Cycle
Cycle length and regularity are often closely related, such that women with irregular cycles are more likely to have longer cycles, while women with more regular cycles are more likely to have shorter cycles (e.g., Gleeson et al., 2016; Jensen, Scheike, Keiding, Schaumburg, & Grandjean, 1999; Kolstad et al., 1999). To date, much of the research examining variability in menstrual cycle length and regularity has utilized an epidemiological perspective, focusing on the influence of chronic diseases and lifestyle factors (e.g., Rowland et al., 2002; Saha et al., 2014; Solomon et al., 2001). This research generally finds that disease states predict a shift toward longer, more irregular menstrual cycles. For example, longer cycle lengths and cycle irregularity have both been found in women with chronic medical conditions, such as irritable bowel disease, diabetes, and depression, among other disorders (Rowland et al., 2002; Saha et al., 2014; Solomon et al., 2001). Further, both long cycles (i.e., 36 days or more) and cycle irregularity are each associated with a history of infertility (Rowland et al., 2002). Shorter, more regular cycles, however, may also be related to certain health issues in women, such as having an increased risk of endometriosis (Cramer et al., 1986; Wei, Cheng, Bu, Zhao, & Zhao, 2016), breast cancer (Den Tonkelaar & De Waard, 1996; Terry, Willet, Rich-Edwards, Hunter, & Michels, 2005), and anxiety disorders (Barron, Flick, Cook, Homan, & Campbell, 2008).
Others find that healthy women’s cycles are also subject to variability based on lifestyle factors and transient changes in health and well-being. For example, being overweight, experiencing rapid changes in body composition (Harlow & Matanoski, 1991), and engaging in both high and low levels of physical activity (Gudmundsdottir, Flanders, & Augestad, 2011; Hahn et al., 2013) are associated with longer cycle lengths. Further, aspects of one’s occupation such as working night shifts (Lawson et al., 2011) and experiencing stress in the workplace are each predictive of cycle irregularity (Matteo, 1987). Shorter, more regular cycles, on the other hand, are linked to smoking, alcohol consumption, and an earlier age of menarche (Hahn et al., 2013; Jukic et al., 2007; Kato et al., 1999; Liu, Gold, Lasley, & Johnson, 2004).
Although the epidemiological perspective has offered a number of important insights into the relationship between disease states, health, and menstrual cycle characteristics, this perspective has key limitations. First, the epidemiological perspective fails to account for normal menstrual cycle variability that exists in healthy women (i.e., in the absence of disease states, acute stress, or illness). For example, substantial variability in menstrual cycle length remains even when adjusting for factors related to health outcomes (see, e.g., Fehring, Schneider, & Raviele, 2006). Further, research using the epidemiological perspective lacks a larger predictive framework that explains why certain health and lifestyle factors appear to reliably increase or decrease cycle frequency. Here, we use the theoretical framework of LHT to account for these findings and generate new predictions about some of the specific contexts that should influence cycle length and regularity.
Contextual Variables Predicting Mating Effort
LHT is a framework that yields predictions about how and when organisms will allocate resources toward accomplishing the various tasks necessary for survival and reproduction, including mating, offspring care, foraging, growth, and somatic defense (Kaplan & Gangestad, 2005; Roff, 1992; Stearns, 1992). According to this perspective, the resource allocation strategy “selected” by an organism varies as a function of the expected fitness payoffs associated with investment in each of these domains relative to the others (Ellis, Figueredo, Brumbach, & Schlomer, 2009; Kaplan & Gangestad, 2005; Roff, 1992; Stearns, 1992). When the expected fitness benefits associated with investing effort in one domain—such as mating—is relatively high, energetic investment in that domain is expected to increase. Conversely, when the fitness benefits associated with investing effort in a particular domain are relatively low, energetic investment is expected to decrease.
Much research supports this view. For example, for men, the expected fitness payoffs associated with increased mating effort are higher than they are for women because their initial investment in offspring production is so low (Bateman, 1948; Courtiol, Pettay, Jokela, Rotkirch, & Lummaa, 2012; Symons, 1979; Trivers, 1972). Consistent with predictions from LHT, research finds that men invest more effort in mating than do women (e.g., desire for more sexual variety, Schmitt, 2003; greater frequency of sexual fantasies, Ellis & Symons, 1990; desire for greater number of partners, Buss & Schmitt, 1993). Research also finds support for this general hypothesis in women based on internal cyclic fertility cues. Several studies have found, for example, that women invest more in mating effort (e.g., ornamentation, Durante, Li, & Haselton, 2008; Haselton, Mortezaie, Pillsworth, Bleske-Rechek, & Frederick, 2007; 1 greater sexual desire, Jones et al., 2018; Pillsworth, Haselton, & Buss, 2004) when the probability of conception is high (i.e., near ovulation), particularly for those from environments favoring faster life history strategies (Kim, Bradshaw, Durante, & Hill, 2018). These findings suggest that when the fitness payoffs associated with allocating resources toward mating effort are high, greater investment in this domain follows.
Guided by these insights, we sought to examine whether conditions that favor investment in mating effort predict shorter, more regular cycles. Total fecundity varies, in large part, by the length and frequency of women’s cycles, with shorter, more regular cycles corresponding to increased fecundity (Jensen et al., 1999; Wesselink et al., 2016; Zhang et al., 2017). Such a relationship might exist because greater cycle frequency provides more opportunities for conception. Accordingly, contexts favoring heavier investment in mating effort should calibrate functioning of the hypothalamic–pituitary–gonadal (HPG) axis, leading to shorter, more regular cycles, which would increase potential conceptive opportunities (Belsky, Steinberg, & Draper, 1991; Wood & Weinstein, 1988).
Support for this hypothesis is found in research examining the link between sexual activity and menstrual cycle length and variability. For example, women who are regularly sexually active have less cycle variability than women who are celibate or report only sporadic sexual activity (Burleson, Gregory, & Trevathan, 1991; Cutler, Garcia, & Krieger, 1980). Others find that women who spend more time with men have a significantly higher rate of ovulation than those spending less time around men (Veith, Buck, Getzlaf, Van Dalfsen, & Slade, 1983).
Additional support for our hypothesis is found in research conducted in nonhuman animals. For example, research finds that exposure to desirable males (or their mating calls) advances timing of estrous in a female deer (both the red deer, Cervus elaphus, Komers, Birgersson, & Ekvall, 1999; as well as the fallow deer, Dama dama, McComb, 1987). Similarly, menstrual cycles of the female mandrill, Mandrillus sphinx (Setchell & Wickings, 2004) and chacma baboon, Papio ursinus (Howard-Tripp & Bielert, 1978) become shorter and more regular in contexts that favor increased mating effort (higher dominance rank and contact with male conspecifics, respectively). Research such as this suggests that females’ HPG axis may be tuned to environmental- and individual-based cues that influence the expected payoff associated with increased mating effort, and females respond to these cues by adjusting the frequency of their menstrual cycles.
In humans, one important factor that influences the expected fitness payoffs associated with investment in mating effort is one’s life history strategy. Individuals with “faster” life history strategies (which emerge in the context of early life stress, father absence, and ecological unpredictability) tend to favor heavier investment in mating effort than those with “slower” life history strategies (Belsky et al., 1991; Chua, Lukaszewski, Grant, & Sng, 2016; Ellis et al., 2009; Kaplan & Gangestad, 2005; Szepsenwol et al., 2017). Accordingly, we should find that women with faster life history strategies—which are characterized by relatively greater investment in mating effort—have shorter, more regular cycles than women with slower life history strategies.
A second factor that plays an important role in modulating humans’ mating effort is one’s ability to successfully compete for desirable mates in their current environment (Clark, 2004; Perilloux, Cloud, & Buss, 2013; Weeden & Sabini, 2007). Because availability of partners in the future is not guaranteed, the expected fitness payoff of investment in mating effort increases with one’s projected mating success in the current environment. That is, mating effort should increase in contexts in which one expects a high likelihood of achieving a desired mating outcome. Consistent with this idea, research finds that both men and women report greater mating effort in contexts in which they perceive themselves as being highly desirable to potential mates (Clark, 2004; Perilloux et al., 2013; Weeden & Sabini, 2007). Others find that mating effort increases when individuals are led to believe that mating opportunities are abundant (e.g., Moss & Maner, 2016). Together, this research demonstrates that factors such as individual differences in life history strategies and access to mating opportunities influence investment in mating effort.
The Current Research
In the current research, we tested the impact of two conceptually distinct predictors of increased mating effort in humans—faster life history strategies (Belsky, Schlomer, & Ellis, 2012; Del Giudice, Gangestad, & Kaplan, 2015; Ellis et al., 2009) and one’s ability to achieve a successful mating outcome in their current environment (Durante & Li, 2009; Perilloux et al., 2013)—on women’s cycle length and regularity. We predicted that women whose life histories favor increased mating effort (those with relatively faster life history strategies) and women who perceive themselves as being most able to achieve a successful mating outcome in their current environment would exhibit greater mating effort and shorter, more regular cycles. Because cycle frequency over time is determined by both the length of each individual cycle, as well as the regularity of cycle occurrence, we predicted that both variables would be influenced by investment in mating effort. Finally, we tested an alternative to our hypothesized model in which cycle length and regularity were specified as predictors of mating effort to test the proposed directionality of the path between these variables.
Method
Participants
Participants were 176 female undergraduate students ranging from 17 to 30 years of age (Mage = 19.55, SDage = 2.15) who participated in exchange for partial course credit. All women who participated were (a) nulliparous, (b) naturally cycling (i.e., had not been on hormonal birth control for at least 3 months prior to participating), (c) without chronic health problems, including hormonal disorders of any kind, and (d) nonsmoking.
Materials and Procedure
After determining eligibility using a presurvey, women who indicated that they wished to participate in the study were contacted by the researchers via e-mail and were provided a link to a survey containing the target measures. After providing informed consent, participants completed the survey, were thanked, debriefed, and awarded credit.
Life history strategy
Participants’ life history strategies were measured using the short-form Arizona Life History Battery (the Mini-K; Figueredo et al., 2006). We chose the Mini-K because it is one of the most reliable predictors of life history strategies (Dunkel & Decker, 2010). The Mini-K Scale consists of 20 statements assessing behaviors (e.g., “I am often in social contact with my friends”) and attitudes (e.g., “I can often tell how things will turn out”) related to one’s life history strategy. Ratings are made on 7-point scales (−3 = strongly disagree, 3 = strongly agree) with higher scores indicating slower strategies. Together, the items were formed into a mean composite (α = .69).
Projected mating success
Projected mating success was measured using the Self-Perceived Mating Success Scale (Landolt, Lalumière, & Quinsey, 1995). This 8-item scale assesses an individual’s perceived likelihood of achieving a successful mating outcome in their environment (e.g., “Members of the opposite-sex are attracted to me” and “I can have as many sexual partners as I choose”). Ratings were made on 7-point scales (1 = completely disagree, 7 = completely agree), and all items were formed into a mean composite with higher scores indicating more expected mating success (α = .90).
Mating effort
Mating effort was measured using the revised Sociosexual Orientation Inventory (SOI-R; Penke & Asendorpf, 2008). The SOI-R consists of three subscales that capture unique facets of mating effort: Mating Behavior (e.g., “With how many different partners have you had sex within the past 12 months?”), Sexual Attitudes (e.g., “Sex without love is OK”), and Sexual Desire (“How often do you have fantasies about having sex with someone you are not in a committed romantic relationship with?”). Participants provided answers using 9-point scales with higher scores indicating higher mating effort. Each subscale was formed into a mean composite (behaviors: α = .89, attitudes: α = .87, desire: α = .84). We chose the SOI-R as a proxy measure of current mating effort as this scale has been used in previous research as part of a latent factor of the mating effort construct (e.g., Fernandes, Menie, Hutz, Kruger, & Figueredo, 2016), it is predictive of one’s lifetime number of sexual partners (Ostovich & Sabini, 2004), and it is associated with investment in other mating-related activities (e.g., ornamentation; Kruger, 2017).
Average menstrual cycle length and regularity
Participants provided a whole number representing their average cycle length in response to the question: “How many days long are your menstrual cycles (for most women, the range is between 25 and 35 days)?” We informed participants that menstrual cycle length refers to the number of days between the start of one menstrual period and the start of the next menstrual period. Cycle regularity was assessed with the question: “How well can you predict the date on which you will have another period; that is, how regular is your cycle?” Participants provided answers on a 9-point scale (1 = not at all, 9 = completely), with a higher score indicating more regular cycles.
Alternative explanations
In order to statistically control for the effects of internal and environmental factors previously shown to influence cycle characteristics or mating effort, we collected measures of age, relationship status, BMI, and weekly exercise frequency (Edelstein, Chopik, & Kean, 2011; Harlow & Matanoski, 1991; Meskó, Láng, & Kocsor, 2014). Participants indicated their current relationship status by answering the question: “What is your current relationship status?” using the options single, casually dating someone, in a committed relationship, engaged, or married. Participants also self-reported height and weight from which BMI was calculated (kg/m2). Finally, participants indicated how frequently they exercised by providing an answer to the question: “How many hours of exercise do you do in a typical week?” using a whole number.
Data Analytic Plan
We first examined the data to determine whether all assumptions for accurate estimation using maximum likelihood were met (MPlus Version 7.4 statistical software, Muthén & Muthén, 1998–2012, Los Angeles, CA; Kline, 2016). Three participants reported average cycle lengths greater than 3 standard deviations (SDs) above the mean (45 [+3.67 SDs], 46 [+3.91 SDs], and 54 [+5.81 SDs] days). Because each of these values was within the range of cycles recorded in prior research (Mumford et al., 2012; Wilcox, Dunson, & Baird, 2000), it was determined prior to analyses that these outliers would only be removed if doing so significantly improved model fit. As average cycle length and the SOI-R scales were positively skewed, we used the robust maximum likelihood estimator which is robust to nonnormality in observed variables. We assessed model fit using four fit indices: χ2 test of model fit, the comparative fit index (CFI), the root mean square error of approximation (RMSEA), and the standardized root mean square residual (SRMR). Adequate model fit was indicated by a nonsignificant χ2 value (p > .05), a CFI value > .95, an RMSEA value < .05, and an SRMR statistic < .05.
Results
Confirmatory Factor Analysis (CFA)
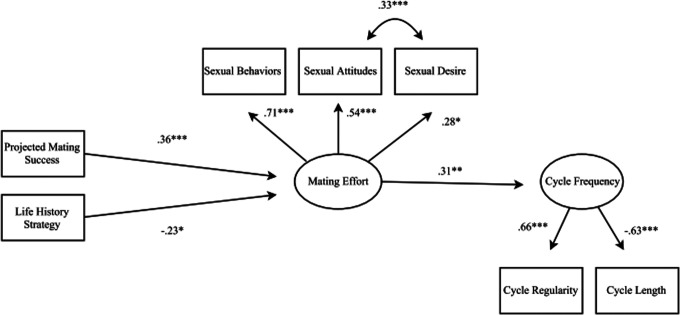
Descriptive statistics for all observed variables are shown in Table 1. Before we estimated the full hypothesized model (see Figure 1 for model), we first conducted a CFA to test the validity of the proposed model factor structure. We tested a two-factor model with each SOI-R subscale indicating a latent factor of mating effort and both cycle length and regularity indicating a latent factor of cycle frequency. Due to a high correlation between the Attitude and Desire subscales of the SOI-R, specifying a correlation between the errors of these scales was necessary for model convergence and adequate model fit (see Table 2 for model fit statistics). Removing cycle length outliers did not improve model fit; these values were thus included in subsequent analyses (see Table 2). All factor loadings were significant (see Table 3).
Table 1.
Descriptive Statistics for Observed Variables.
| Variable | Range | M | SD |
|---|---|---|---|
| Estimated cycle length | 22–54 | 29.57 | 4.20 |
| Estimated cycle regularity | 1–9 | 5.54 | 2.16 |
| SOI behaviors | 1–6.67 | 1.55 | 1.05 |
| SOI attitudes | 1–9 | 3.31 | 2.13 |
| SOI desire | 1–8.67 | 2.67 | 1.60 |
| SPMV | 1–7 | 4.42 | 1.20 |
| Mini-K | 0.21–2.53 | 1.57 | 0.52 |
| Age | 17–30 | 19.55 | 2.15 |
| BMI | 18.36–31.70 | 22.68 | 2.63 |
| Exercise frequency | 0–11 | 4.10 | 2.52 |
Note. SOI = sociosexual orientation inventory; SPMV = self-perceived mate value; BMI = body mass index.
Figure 1.
Final model with standardized estimates. *p < .05. **p < .01. ***p < .001.
Table 2.
Summary of Model Fit Indices.
| Model | χ2(df) | CFI | RMSEA | SRMR |
|---|---|---|---|---|
| CFA model | 3.61(3) | 0.99 | .03 [.00, .14] | .03 |
| CFA excluding outliers | 5.92(3) | 0.97 | .07 [.00, .16] | .04 |
| Hypothesized model | 9.18(11) | 1.00 | .00 [.00, .07] | .04 |
| Covariate model | 7.56(11) | 1.00 | .00 [.00, .06] | .02 |
| Alternative model | 22.42(11)* | 0.88 | .08 [.03, .12] | .06 |
Note. CFA = confirmatory factor analysis; CFI = comparative fit index; RMSEA = root mean square error of approximation; SRMR = standardized root mean square residual.
*p < .05. **p < .01. ***p < .001.
Table 3.
Estimates for Confirmatory Factor Analysis (CFA).
| Variable | Estimate | SE | p | R 2 |
|---|---|---|---|---|
| Mating effort | ||||
| SOI attitudes | .60 | .18 | .001 | .36 |
| SOI behaviors | .65 | .20 | .001 | .42 |
| SOI desire | .26 | .12 | .027 | .07 |
| Cycle frequency | ||||
| Cycle regularity | .61 | .18 | .001 | .37 |
| Cycle length | −.68 | .20 | <.001 | .46 |
Note. Standardized estimated for CFA model, along with standard errors, p values, and R2. SOI = sociosexual orientation inventory.
Test of the Hypothesized Structural Model
All fit statistics indicated adequate model fit (see Table 2). As shown in Figure 1, greater mating effort was independently predicted each by expected mating success, β = .36, SE = .09, t = 3.86, p < .001, and faster life history strategies, β = −.23, SE = .09, t = −2.49, p = .01. Further, greater mating effort predicted shorter, more regular cycles, β = .31, SE = .09, t = 3.29, p = .001. Indirect effects on cycle frequency via increased mating effort were significant for each expected mating success, β = .11, SE = .05, t = 2.34, p = .02, and life history strategy, β = −.07, SE = .04, t = −1.98, p = .047, indicating that each uniquely predicts cycle length/regularity through increased mating effort. Together, the model accounted for 9.4% of the variance in cycle frequency.
Hypothesized Model With Covariates Included
The results of the hypothesized model remained significant when controlling for age, BMI, relationship status, and exercise level. All latent construct indicators were regressed on each of these factors, and the test of the hypothesized model was repeated. Results revealed that direct effects on mating effort remained significant for both life history strategy, β = −.25, SE = .09, t = −2.65, p = .008, and projected mating success, β = .30, SE = .09, t = 3.22, p = .001. Specifically, both a faster life history strategy and a higher projected ability to secure mating opportunities predicted greater mating effort. The direct effect of mating effort on cycle frequency also remained significant, β = .31, SE = .10, t = 3.07, p = .002, indicating that greater mating effort predicted shorter, more regular cycles. No covariates significantly predicted cycle characteristics (ps > .17).
Test of an Alternative Model
We next conducted a test of the fit of an alternative model for which the order of the mediator (mating effort) and outcome variables (cycle frequency) was switched (see Figure 2). This allowed us to statistically test whether cycle frequency is better represented as a mediator of mating effort (rather than as an outcome of mating effort, as our hypothesis specifies). Results revealed poor model fit (see Table 2), supporting the proposed directionality of the path between these variables.
Figure 2.
Alternative model with standardized estimates. Nonsignificant paths denoted by dotted lines. *p < .05. **p < .01. ***p < .001.
Discussion
Past research demonstrates considerable variability exists in the length and regularity of the human menstrual cycle (Chiazze et al., 1968; Creinin et al., 2004). Explanations for this have mostly focused on the proximate role of disease states and health issues, neglecting to consider adaptive reasons for why such variability exists in healthy women. Here, we proposed that variability in the human menstrual cycle may arise as a function of the projected payoffs associated with investment in mating effort. Accordingly, we predicted that women whose life history strategies or environments favored greater mating effort would report shorter, more regular cycles. We hypothesized that both cycle length and regularity would be influenced by mating effort, as these characteristics each determine cycle frequency—and the number of total conception opportunities—over time.
The results of the current research supported our hypothesis. Women with faster life history strategies and those expecting more favorable mating outcomes reported investing more effort in mating. Increased mating effort, in turn, predicted shorter, more regular cycles. Importantly, these results were robust to controlling for covariates that have previously been found to impact cycle length and regularity, such as age, BMI, and exercise. Using model fit as a guide, we also statistically explored the alternative possibility that cycle frequency better represented a predictor, rather than an outcome, of mating effort. This alternative model was a poor fit to the current data, lending support for the hypothesis that cycle frequency is calibrated to mating effort and not vice versa. In sum, our results provide some of the first empirical evidence that human menstrual cycle length and regularity may vary as a function of mating effort.
Prior research investigating the factors that influence human menstrual cycle characteristics has found that longer, more irregular cycles are often associated with predictors of poor health: rapid weight change, stress, and disorders such as diabetes (Kato et al., 1999; Matteo, 1987; Solomon et al., 2001). Although not previously considered in this light, research such as this provides additional support for the hypothesis that the human menstrual cycle changes in response to cues that increase or decrease the projected payoff from investing energetic resources in mating effort. When energetic resources are constrained or the costs of investing in mating are high and the benefits low (e.g., when one is ill), longer, more irregular cycles should result. Conversely, when the costs are relatively low and the benefits high, shorter, more regular cycles should occur. Exceptions to this pattern of health problems predicting longer, more irregular cycles are the incidence of relatively shorter cycles found in women with endometriosis, as well as those with a history of psychiatric disorders, particularly anxiety (Barron et al., 2008; Rowland et al., 2002). These exceptions, however, are not inconsistent with our predictive framework. Endometriosis, for example, is associated with greater exposure to estrogens, which play a critical role in women’s sexual desire (Cappelletti & Wallen, 2016). Additionally, research has identified anxiety as a psychological characteristic of faster life history strategies (e.g., Chua et al., 2016), lending further support for the hypothesis that life history characteristics influence cycle frequency.
Limitations and Future Directions
The current research has several limitations. Although the results of our alternative model supported the proposed directionality of our hypothesized model, the conclusions drawn from the current study are limited by the nature of our cross-sectional data. Future research would benefit from tracking women’s cycles over time, as longitudinal designs are necessary to confirm the causal chain from investment in mating effort to adjustments in cycle frequency. Such studies examining the relationship between these factors might also include measurements of health and somatic effort to examine whether increases in mating effort are accompanied by trade-offs in other domains of energetic investment. It should be noted that all women in our study were nulliparous. Given that nulliparous women are found to have shorter, more regular cycles than women with children (Kato et al., 1999), the present findings may not extend to women who have been pregnant. Longitudinal studies would also be able to examine whether pregnancy influences relationships between mating effort and cycle characteristics.
Next, our study was limited by our use of only one measure of mating effort (i.e., sociosexual orientation). More research is needed to examine relationships between cycle frequency and mating effort across a broader range of variables representative of one’s energetic investment in mating. Although sociosexual orientation has been shown in previous research to be closely related to important facets of mating effort, including one’s lifetime number of sexual partners (Ostovich & Sabini, 2004) and others (Kruger, 2017), future studies should assess whether cycle frequency is also predicted by other aspects of mating effort, such as sex steroid production, reproductive timing, or costly mate attraction displays (e.g., luxury brand signaling, conspicuous consumption; Griskevicius et al., 2007; Sundie et al., 2011).
We only collected one measure of participants’ life history strategies, the Mini-K. While some research has found convergent validity between the Mini-K and other life history measures (e.g., Dunkel & Decker, 2010), it should be noted that others have recently suggested that this scale incompletely captures the life history strategy construct (e.g., Richardson et al., 2017). The results of the current research provide some evidence for the validity of the Mini-K, given that it significantly predicted mating effort—another indicator of one’s life history strategy (Del Giudice et al., 2015). Nonetheless, critiques of the Mini-K should be considered when interpreting the results of the current research.
Additional research is also needed to explore the hormonal shifts underlying changes in mating effort and cycle characteristics. Identifying the hormonal mechanisms involved in these effects would make an important contribution to both researchers and clinicians interested in the biological correlates of sexual behavior, fertility, and overall reproductive health. Ovulation status, in particular, might be of particular interest to future work given that longer cycle lengths are associated with infertility (Rowland et al., 2002).
Finally, it should be noted that we asked women to self-report the length and regularity of their cycles. Although multiple studies have successfully used self-reporting to measure estimated cycle length (e.g., Barron et al., 2008; Rowland et al., 2002; Wesselink et al., 2016), others find that such methods are less accurate for those with very long or very short cycle lengths, as well as those reporting high variability in cycle lengths (Small, Manatunga, & Marcus, 2007). Future research would benefit from validating self-reported cycle characteristics with more objective measures, such as period tracking phone applications or hormone analysis. These studies might also collect a broader range of measures regarding factors that potentially impact cycle length and regularity. Although we accounted for many of these factors in our analyses and recruitment (i.e., health, smoking, BMI, relationship status, age, and exercise), there are others that may influence relationships between mating effort and cycle characteristics (e.g., stress).
Despite these limitations, the current research provides an important first step in establishing a general evolutionary theory that accounts for cycle length and variability observed between and within human females. These findings represent some of the first empirical evidence that human menstrual cycle characteristics are influenced by investment in mating effort. Future work applying this predictive framework may advance our understanding of the human menstrual cycle and its relation to overall health.
Acknowledgment
We thank Randi P. Proffitt Leyva, Marjorie L. Prokosch, and Maggie Kleiser for assistance with participant recruitment.
Note
Recent research has challenged the hypothesis that women experience cyclical shifts in ornamentation (Arslan, Schilling, Gerlach, & Penke, 2018).
Authors’ Note: The data associated with this research are promptly available from corresponding author upon request. This research was approved as ethical by the institutional review board at Texas Christian University.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Science Foundation (NSF 1551201).
References
- Arslan R. C., Schilling K. M., Gerlach T. M., Penke L. (2018). Using 26,000 diary entries to show ovulatory changes in sexual desire and behavior. Journal of Personality and Social Psychology. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Barron M. L., Flick L. H., Cook C. A., Homan S. M., Campbell C. (2008). Associations between psychiatric disorders and menstrual cycle characteristics. Archives of Psychiatric Nursing, 22, 254–265. doi:10.1016/j.apnu.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A. J. (1948). Intra-sexual selection in Drosophila. Heredity, 2, 349–368. [DOI] [PubMed] [Google Scholar]
- Belsky J., Schlomer G. L., Ellis B. J. (2012). Beyond cumulative risk: Distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Developmental Psychology, 48, 662–673. doi:10.1037/a0024454 [DOI] [PubMed] [Google Scholar]
- Belsky J., Steinberg L., Draper P. (1991). Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development, 62, 647–670. [DOI] [PubMed] [Google Scholar]
- Burleson M. H., Gregory W. L., Trevathan W. R. (1991). Heterosexual activity and cycle length variability: Effect of gynecological maturity. Physiology & Behavior, 50, 863–866. doi:10.1016/0031-9384(91)90032-J [DOI] [PubMed] [Google Scholar]
- Buss D. M., Schmitt D. P. (1993). Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review, 100, 204. [DOI] [PubMed] [Google Scholar]
- Cappelletti M., Wallen K. (2016). Increasing women’s sexual desire: The comparative effectiveness of estrogens and androgens. Hormones and Behavior, 78, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiazze L., Brayer F. T., Macisco J. J., Parker M. P., Duffy B. J. (1968). The length and variability of the human menstrual cycle. Journal of the American Medical Association, 203, 377–380. [PubMed] [Google Scholar]
- Chua K. J., Lukaszewski A. W., Grant D. M., Sng O. (2016). Human life history strategies: Calibrated to external or internal cues? Evolutionary Psychology, 15, 1–16. doi:10.1177/1474704916677342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. P. (2004). Self-perceived attractiveness and masculinization predict women’s sociosexuality. Evolution and Human Behavior, 25, 113–124. doi:10.1016/S1090-5138(03)00085-0 [Google Scholar]
- Courtiol A., Pettay J. E., Jokela M., Rotkirch A., Lummaa V. (2012). Natural and sexual selection in a monogamous historical human population. Proceedings of the National Academy of Sciences, 109, 8044–8049. doi:10.1073/pnas.1118174109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer D. W., Wilson E., Stillman R. J., Berger M. J., Belisle S., Schiff I.…Schoenbaum S. C. (1986). The relation of endometriosis to menstrual characteristics, smoking, and exercise. Journal of the American Medical Association, 255, 1904–1908. [PubMed] [Google Scholar]
- Creinin M. D., Keverline S., Meyn L. A. (2004). How regular is regular? An analysis of menstrual cycle regularity. Contraception, 70, 289–292. doi:10.1016/j.contraception.2004.04.012 [DOI] [PubMed] [Google Scholar]
- Cutler W. B., García C. R., Krieger A. M. (1980). Sporadic sexual behavior and menstrual cycle length in women. Hormones and Behavior, 14, 163–172. [DOI] [PubMed] [Google Scholar]
- Del Giudice M., Gangestad S. W., Kaplan H. S. (2015). Life history theory and evolutionary psychology. In Buss D. M. (Ed.), The handbook of evolutionary psychology (Vol. 1, pp. 88–114). New York, NY: Wiley. [Google Scholar]
- Den Tonkelaar I., De Waard F. (1996). Regularity and length of menstrual cycles in women aged 41–46 in relation to breast cancer risk: Results from the DOM-project. Breast Cancer Research and Treatment, 38, 253–258. [DOI] [PubMed] [Google Scholar]
- Dunkel C. S., Decker M. (2010). Convergent validity of measures of life-history strategy. Personality and Individual Differences, 48, 681–684. doi:10.1016/j.paid.2009.12.014 [Google Scholar]
- Durante K. M., Li N. P. (2009). Oestradiol level and opportunistic mating in women. Biology Letters, 5, 179–182. doi:10.1098/rsbl.2008.0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante K. M., Li N. P., Haselton M. G. (2008). Changes in women’s choice of dress across the ovulatory cycle: Naturalistic and laboratory task-based evidence. Personality and Social Psychology Bulletin, 34, 1451–1460. doi:10.1177/0146167208323103 [DOI] [PubMed] [Google Scholar]
- Edelstein R. S., Chopik W. J., Kean E. L. (2011). Sociosexuality moderates the association between testosterone and relationship status in men and women. Hormones and Behavior, 60, 248–255. doi:10.1016/j.yhbeh.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Ellis B. J., Figueredo A. J., Brumbach B. H., Schlomer G. L. (2009). Fundamental dimensions of environmental risk. Human Nature, 20, 204–268. doi:10.1007/s12110-009-9063-7 [DOI] [PubMed] [Google Scholar]
- Ellis B. J., Symons D. (1990). Sex differences in sexual fantasy: An evolutionary psychological approach. Journal of Sex Research, 27, 527–555. doi:10.1080/00224499009551579 [Google Scholar]
- Fehring R. J., Schneider M., Raviele K. (2006). Variability in the phases of the menstrual cycle. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 35, 376–384. doi:10.1111/j.1552-6909.2006.00051.x [DOI] [PubMed] [Google Scholar]
- Fenster L., Waller K., Chen J., Hubbard A. E., Windham G. C., Elkin E., Swan S. (1999). Psychological stress in the workplace and menstrual function. American Journal of Epidemiology, 149, 127–134. doi:10.1093/oxfordjournals.aje.a009777 [DOI] [PubMed] [Google Scholar]
- Fernandes H. B., Menie M. A. W., Hutz C. S., Kruger D. J., Figueredo A. J. (2016). The strength of associations among sexual strategy traits: Variations as a function of life history speed. Personality and Individual Differences, 98, 275–283. [Google Scholar]
- Figueredo A. J., Vásquez G., Brumbach B. H., Schneider S. M., Sefcek J. A., Tal I. R.…Jacobs W. J. (2006). Consilience and life history theory: From genes to brain to reproductive strategy. Developmental Review, 26, 243–275. doi:10.1016/j.dr.2006.02.002 [Google Scholar]
- Gleeson P. C., Worsley R., Gavrilidis E., Nathoo S., Ng E., Lee S., Kulkarni J. (2016). Menstrual cycle characteristics in women with persistent schizophrenia. Australian and New Zealand Journal of Psychiatry, 50, 481–487. [DOI] [PubMed] [Google Scholar]
- Griskevicius V., Tybur J. M., Sundie J. M., Cialdini R. B., Miller G. F., Kenrick D. T. (2007). Blatant benevolence and conspicuous consumption: When romantic motives elicit strategic costly signals. Journal of Personality and Social Psychology, 93, 85. [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir S. L., Flanders W. D., Augestad L. B. (2011). A longitudinal study of physical activity and menstrual cycle characteristics in healthy Norwegian women-the Nord-Trøndelag health study. Norsk Epidemiologi, 20, 163–171. [Google Scholar]
- Hahn K. A., Wise L. A., Riis A. H., Mikkelsen E. M., Rothman K. J., Banholzer K., Hatch E. E. (2013). Correlates of menstrual cycle characteristics among nulliparous Danish women. Journal of Clinical Epidemiology, 5, 311–319. doi:10.2147/CLEP.S46712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow S. D., Matanoski G. M. (1991). The association between weight, physical activity, and stress and variation in the length of the menstrual cycle. American Journal of Epidemiology, 133, 38–49. [DOI] [PubMed] [Google Scholar]
- Haselton M. G., Mortezaie M., Pillsworth E. G., Bleske-Rechek A., Frederick D. A. (2007). Ovulatory shifts in human female ornamentation: Near ovulation, women dress to impress. Hormones and Behavior, 51, 40–45. doi:10.1016/j.yhbeh.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Howard-Tripp M. E., Bielert C. (1978). Social contact influences on the menstrual cycle of the female chacma baboon (Papio ursinus). Journal of the South African Veterinary Association, 49, 191–192. [PubMed] [Google Scholar]
- Jensen T. K., Scheike T., Keiding N., Schaumburg I., Grandjean P. (1999). Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology, 10, 422–428. [DOI] [PubMed] [Google Scholar]
- Jones B. C., Hahn A. C., Fisher C. I., Wang H., Kandrik M., DeBruine L. M. (2018). General sexual desire, but not desire for uncommitted sexual relationships, tracks changes in women’s hormonal status. Psychoneuroendocrinology, 88, 153–157. [DOI] [PubMed] [Google Scholar]
- Jukic A. M. Z., Weinberg C. R., Wilcox A. J., McConnaughey D. R., Hornsby P., Baird D. D. (2007). Accuracy of reporting of menstrual cycle length. American Journal of Epidemiology, 167, 25–33. doi:10.1093/aje/kwm265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S., Gangestad S. W. (2005). Life history theory and evolutionary psychology. In Buss D. M. (Ed.), The handbook of evolutionary psychology (pp. 68–95). Hoboken, NY: Wiley. [Google Scholar]
- Kato I., Toniolo P., Koenig K. L., Shore R. E., Zeleniuch-Jacquotte A., Akhmedkhanov A., Riboli E. (1999). Epidemiologic correlates with menstrual cycle length in middle aged women. European Journal of Epidemiology, 15, 809–814. [DOI] [PubMed] [Google Scholar]
- Kim A., Bradshaw H., Durante K. M., Hill S. E. (2018). Life history, fertility, and short-term mating motivation. Evolutionary Psychology, 16. doi:10.1177/1474704918800062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. B. (2016). Principles and practice of structural equation modeling. New York, NY: Guilford Press. [Google Scholar]
- Kolstad H. A., Bonde J. P., Hjøllund N. H., Jensen T. K., Henriksen T. B., Ernst E.…Olsen J. (1999). Menstrual cycle pattern and fertility: A prospective follow-up study of pregnancy and early embryonal loss in 295 couples who were planning their first pregnancy. Fertility and Sterility, 71, 490–496. [DOI] [PubMed] [Google Scholar]
- Komers P. E., Birgersson B., Ekvall K. (1999). Timing of estrus in fallow deer is adjusted to the age of available mates. The American Naturalist, 153, 431–436. doi:10.1086/303185 [DOI] [PubMed] [Google Scholar]
- Kruger D. J. (2017). Brief self-report scales assessing life history dimensions of mating and parenting effort. Evolutionary Psychology, 15. doi:10.1177/1474704916673840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt M. A., Lalumière M. L., Quinsey V. L. (1995). Sex differences in intra-sex variations in human mating tactics: An evolutionary approach. Ethology and Sociobiology, 16, 3–23. doi:10.1016/0162-3095(94)00012-V [Google Scholar]
- Lawson C. C., Whelan E. A., Hibert E. N. L., Spiegelman D., Schernhammer E. S., Rich-Edwards J. W. (2011). Rotating shift work and menstrual cycle characteristics. Epidemiology, 22, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gold E. B., Lasley B. L., Johnson W. O. (2004). Factors affecting menstrual cycle characteristics. American Journal of Epidemiology, 160, 131–140. doi:10.1093/aje/kwh188 [DOI] [PubMed] [Google Scholar]
- Matteo S. (1987). The effect of job stress and job interdependency on menstrual cycle length, regularity and synchrony. Psychoneuroendocrinology, 12, 467–476. [DOI] [PubMed] [Google Scholar]
- McComb K. (1987). Roaring by red deer stags advances the date of oestrus in hinds. Nature, 330, 648–649. doi:10.1038/330648a0 [DOI] [PubMed] [Google Scholar]
- Meskó N., Láng A., Kocsor F. (2014). The Hungarian version of sociosexual orientation inventory revised (SOI-R): Sex and age differences. Interpersona, 8, 85–99. doi:10.5964/ijpr.v8i1.130 [Google Scholar]
- Moss J. H., Maner J. K. (2016). Biased sex ratios influence fundamental aspects of human mating. Personality and Social Psychology Bulletin, 42, 72–80. doi:10.1177/0146167215612744 [DOI] [PubMed] [Google Scholar]
- Mumford S. L., Steiner A. Z., Pollack A. Z., Perkins N. J., Filiberto A. C., Albert P. S.…Schisterman E. F. (2012). The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. The Journal of Clinical Endocrinology & Metabolism, 97, E1871–E1879. doi:10.1210/jc.2012-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. (1998. –2012). Mplus user’s guide (7th ed.). Los Angeles, CA: Author. [Google Scholar]
- Ostovich J. M., Sabini J. (2004). How are sociosexuality, sex drive, and lifetime number of sexual partners related? Personality and Social Psychology Bulletin, 30, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Penke L., Asendorpf J. B. (2008). Beyond global sociosexual orientations: A more differentiated look at sociosexuality and its effects on courtship and romantic relationships. Journal of Personality and Social Psychology, 95, 1113–1135. doi:10.1037/0022-3514.95.5.1113 [DOI] [PubMed] [Google Scholar]
- Perilloux C., Cloud J. M., Buss D. M. (2013). Women’s physical attractiveness and short-term mating strategies. Personality and Individual Differences, 54, 490–495. doi:10.1016/j.paid.2012.10.028 [Google Scholar]
- Pillsworth E. G., Haselton M. G., Buss D. M. (2004). Ovulatory shifts in female sexual desire. Journal of Sex Research, 41, 55–65. doi:10.1080/00224490409552213 [DOI] [PubMed] [Google Scholar]
- Richardson G. B., Sanning B. K., Lai M. H., Copping L. T., Hardesty P. H., Kruger D. J. (2017). On the psychometric study of human life history strategies: State of the science and evidence of two independent dimensions. Evolutionary Psychology, 15, 1474704916666840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D. A. (1992). The evolution of life histories: Theory and analysis. New York, NY: Chapman & Hall. [Google Scholar]
- Rowland A. S., Baird D. D., Long S., Wegienka G., Harlow S. D., Alavanja M., Sandler D. P. (2002). Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology, 13, 668–674. doi:10.1097/.01.EDE.0000024628.42288.8F [DOI] [PubMed] [Google Scholar]
- Saha S., Zhao Y. Q., Shah S. A., Esposti S. D., Lidofsky S., Salih S.…Sands B. E. (2014). Menstrual cycle changes in women with inflammatory bowel disease: A study from the ocean state Crohn’s and colitis area registry. Inflammatory Bowel Diseases, 20, 534–540. doi:10.1097/01.MIB.0000441347.94451.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt D. P. (2003). Universal sex differences in the desire for sexual variety: Tests from 52 nations, 6 continents, and 13 Islands. Journal of Personality and Social Psychology, 85, 85. doi:10.1037/0022-3514.85.1.85 [DOI] [PubMed] [Google Scholar]
- Setchell J. M., Wickings E. J. (2004). Social and seasonal influences on the reproductive cycle in female mandrills (Mandrillus sphinx). American Journal of Physical Anthropology, 125, 73–84. doi:10.1002/ajpa.10375 [DOI] [PubMed] [Google Scholar]
- Small C. M., Manatunga A. K., Marcus M. (2007). Validity of self-reported menstrual cycle length. Annals of Epidemiology, 17, 163–170. [DOI] [PubMed] [Google Scholar]
- Solomon C. G., Hu F. B., Dunaif A., Rich-Edwards J., Willett W. C., Hunter D. J.…Manson J. E. (2001). Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. Journal of the American Medical Association, 286, 2421–2426. doi:10.1001/jama.286.19.2421 [DOI] [PubMed] [Google Scholar]
- Stearns S. C. (1992). The evolution of life histories. Oxford, England: Oxford University Press. [Google Scholar]
- Sundie J. M., Kenrick D. T., Griskevicius V., Tybur J. M., Vohs K. D., Beal D. J. (2011). Peacocks, Porsches, and Thorstein Veblen: Conspicuous consumption as a sexual signaling system. Journal of Personality and Social Psychology, 100, 664. [DOI] [PubMed] [Google Scholar]
- Symons D. (1979). The evolution of human sexuality. Oxford, England: Oxford University Press. [Google Scholar]
- Szepsenwol O., Griskevicius V., Simpson J. A., Young E. S., Fleck C., Jones R. E. (2017). The effect of predictable early childhood environments on sociosexuality in early adulthood. Evolution Behavioral Sciences, 11, 131. doi:10.1037/ebs0000082 [Google Scholar]
- Terry K. L., Willett W. C., Rich-Edwards J. W., Hunter D. J., Michels K. B. (2005). Menstrual cycle characteristics and incidence of premenopausal breast cancer. Cancer Epidemiology, Biomarkers & Prevention, 14, 1509–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R. (1972). Parental investment and sexual selection. In Campbell B. (Ed.), Sexual selection and the descent of man (pp. 136–179). Chicago, IL: Aldine-Atherton. [Google Scholar]
- Veith J. L., Buck M., Getzlaf S., Van Dalfsen P., Slade S. (1983). Exposure to men influences the occurrence of ovulation in women. Physiology & Behavior, 31, 313–315. [DOI] [PubMed] [Google Scholar]
- Weeden J., Sabini J. (2007). Subjective and objective measures of attractiveness and their relation to sexual behavior and sexual attitudes in university students. Archives of Sexual Behavior, 36, 79–88. doi:10.1007/s10508-006-9075-x [DOI] [PubMed] [Google Scholar]
- Wei M., Cheng Y., Bu H., Zhao Y., Zhao W. (2016). Length of menstrual cycle and risk of endometriosis: A meta-analysis of 11 case–control studies. Medicine, 95, e2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselink A. K., Wise L. A., Hatch E. E., Rothman K. J., Mikkelsen E. M., Stanford J. B.…Mahalingaiah S. (2016). Menstrual cycle characteristics and fecundability in a North American preconception cohort. Annals of Epidemiology, 26, 482–487. doi:10.1016/j.annepidem.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox A. J., Dunson D., Baird D. D. (2000). The timing of the “fertile window” in the menstrual cycle: Day specific estimates from a prospective study. British Medical Journal, 321, 1259–1262. doi:10.1136/bmj.321.7271.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. W., Weinstein M. (1988). A model of age-specific fecundability. Population Studies, 42, 85–113. [Google Scholar]
- Zhang Q., Wang Y. Y., Zhang Y., Zhang H. G., Yang Y., He Y.…Ma X. (2017). The influence of age at menarche, menstrual cycle length and bleeding duration on time to pregnancy: A large prospective cohort study among rural Chinese women. International Journal of Gynecology & Obstetrics, 124, 1654–1662. doi:10.1111/1471-0528.14469 [DOI] [PubMed] [Google Scholar]