Summary
Background
The outcome of non-transplant eligible newly diagnosed multiple myeloma (NDMM) patients is heterogeneous, partly depending on frailty level. The aim of this study was to prospectively investigate the efficacy and safety of Ixazomib-Daratumumab-low-dose dexamethasone (Ixa-Dara-dex) in NDMM intermediate-fit patients.
Methods
In this phase II multicenter HOVON-143 study, IMWG Frailty index based intermediate-fit patients, were treated with 9 induction cycles of Ixa-Dara-dex, followed by maintenance with ID for a maximum of 2 years. The primary endpoint was overall response rate on induction treatment. Patients were included from October 2017 until May 2019. Trial Registration Number: NTR6297.
Findings
Sixty-five patients were included. Induction therapy resulted in an overall response rate of 71%. Early mortality was 1.5%. At a median follow-up of 41.0 months, median progression-free survival (PFS) was 18.2 months and 3-year overall survival 83%. Discontinuation of therapy occurred in 77% of patients, 49% due to progression, 9% due to toxicity, 8% due to incompliance, 3% due to sudden death and 8% due to other reasons. Dose modifications of ixazomib were required frequently (37% and 53% of patients during induction and maintenance, respectively), mainly due to, often low grade, polyneuropathy. During maintenance 23% of patients received daratumumab alone. Global quality of life (QoL) improved significantly and was clinically relevant, which persisted during maintenance treatment.
Interpretation
Ixazomib-Daratumumab-low-dose dexamethasone as first line treatment in intermediate-fit NDMM patients is safe and improves global QoL. However, efficacy was limited, partly explained by ixazomib-induced toxicity, hampering long term tolerability of this 3-drug regimen. This highlights the need for more efficacious and tolerable regimens improving the outcome in vulnerable intermediate-fit patients.
Funding
Janssen Pharmaceuticals, Takeda Pharmaceutical Company Limited.
Keywords: Multiple myeloma, Elderly, Daratumumab, Ixazomib, Intermediate-fit, IMWG frailty index
Research in context.
Evidence before this study
The outcome of older newly diagnosed multiple myeloma (NDMM) patients is heterogeneous. The International Myeloma Working Group (IMWG) developed a frailty index (FI) in a pooled analysis of 869 individual older NDMM patient data from 3 prospective trials. This prognostic score classifies patients as fit, intermediate-fit, or frail based on age, co-morbidities and patient reported (instrumental) activities of daily living. The IMWG-FI predicts mortality and non-haematologic toxicity.
In order to identify prospective clinical studies investigating anti-myeloma treatment in intermediate-fit patients, we performed a Pubmed search, containing “multiple myeloma” AND “intermediate-fit”, in May 2023, and found 29 articles. Only 1/29 described a prospective randomized clinical trial in intermediate-fit patients based on the IMWG frailty index. In this study continuous treatment with lenalidomide (25 mg) and dexamethasone (Rd) was compared with 9 cycles of lenalidomide (25 mg) and dexamethasone followed by lenalidomide (10 mg) without dexamethasone (Rd-R) in 199 NDMM patients. The primary endpoint was event-free survival (EFS), a composite endpoint of grade 4 haematologic adverse events, grade 3 and 4 non-haematologic events, discontinuation of lenalidomide, progression, or death. Dose reduction of lenalidomide and limited duration of dexamethasone therapy was found to improve EFS (Rd-R 10.4 months vs Rd 6.9 months), without compromising progression-free survival (20.2 vs 18.3 months).
In 2 studies a simplified frailty index, using a physician-instead of patient-reported performance status (WHO-PS) was used to categorize patients, which was used in a non-planned post-hoc frailty analyses. In the ALCYONE trial, patients were treated with bortezomib-melphalan-prednisone, with or without daratumumab (D-VMP vs VMP). The addition of daratumumab led to an increase in median PFS independent of frailty level, however the outcome was inferior in frail and intermediate fit as compared to fit; frail patients (32.9 with D-VMP vs 19.5 months with VMP, HR 0.51, 95% CI 0.39–0.68, p < 0.0001), intermediate-fit (40.1 with D-VMP vs 18.3 months with VMP, HR 0.37, 95% CI 0.27–0.50, p < 0.0001) and fit (NR with D-VMP vs 22.2 months with VMP, HR 0.34, 95% CI 0.20–0.57, p < 0.0001). Accordingly, in the MAIA trial, the addition of daratumumab to lenalidomide-dexamethasone, led to a superior PFS, independent of frailty level. Again, the outcome of frail and intermediate patients was still inferior to fit patients; frail patients (NR vs 30.4 months, HR 0.62, 95% CI 0.45–0.85, p = 0.003), intermediate-fit (NR in both arms, HR 0.53, 95% CI 0.35–0.80, p = 0.0024) and fit (NR vs 41.7 months, HR 0.41, 95% CI 0.22–0.75, p = 0.0028). The outcome with lenalidomide-dexamethasone in this post-hoc analysis of intermediate-fit patients of the MAIA trial using a simplified frailty index (projected to be more than 40 months) is considerably higher than with exactly the same regimen in the prospective clinical study in intermediate fit patients according to the IMWG-FI. This indicates that both indexes may identify different levels of frailty.
Added value of this study
Our study is the first study investigating daratumumab as part of first line treatment in a solely intermediate-fit patient population. Furthermore, it is the second prospective study globally to employ the IMWG-FI, the gold standard, in identifying intermediate-fit patients.
Implications of all the available evidence
Newly diagnosed intermediate-fit patients have an inferior PFS compared to fit non-transplant eligible patients. This might be caused by the fact that treatment regimens induce more toxicity in a vulnerable population, leading to early and higher levels of treatment discontinuation. Therefore, in future studies endpoints including feasibility should be used, such as EFS incorporating efficacy and toxicity, and knowing that even low grade toxicity hampers continuation of treatment, early adaptation of therapy could be implemented. Furthermore, the use of the IMWF-FI to identify intermediate-fit patients is encouraged, to enable better cross trial comparisons.
Introduction
The prognosis of non-transplant eligible (NTE) patients with newly diagnosed multiple myeloma (NDMM) has significantly improved over the last decade, with an unprecedented duration of progression-free survival (PFS) and overall survival (OS) with daratumumab, added to either bortezomib-melphalan-prednisone (VMP, ALCYONE trial) or lenalidomide-dexamethasone (Rd, MAIA trial).1,2 However, the question is whether this applies to all NTE patients, irrespective of frailty level. The level of frailty by the International Myeloma Working Group Frailty Index (IMWG-FI), that includes age, comorbidities and patient-reported daily activities was not determined in the ALCYONE and MAIA trials.3 To date, only two prospective trials have specifically examined the outcomes of intermediate-fit and frail patients.4,5 In the HOVON 143 study, IMWG-FI based frail patients were treated with ixazomib, daratumumab and low dose dexamethasone (Ixa-Dara-dex), resulting in a median PFS of 13.8 months and a 12-month overall survival of 78%.4 Larocca and colleagues performed a randomized clinical trial in intermediate fit patients comparing dose-adjusted Rd vs standard Rd, the latter being administered according to the same treatment schedule as in MAIA, allowing a non-head to head comparison. A pronounced difference in PFS was observed; 18.3 months vs 35 months in the MAIA trial.1,5 A post-hoc analysis of the MAIA trial, using a simplified frailty index (SFI) based on physician-reported performance status instead of patient-reported daily activities, showed that 51.7% of the intermediate-fit patients were progression-free at 36 months with Rd treatment.6,7 These data show that in patients, who are defined intermediate-fit using the IMWG-FI, the clinical outcome is inferior as compared to NTE-NDMM and intermediate-fit patients using the S-FI. However, whether the negative impact of being intermediate-fit according to the IMWG-FI would be overcome by daratumumab is currently unknown, as no trials investigating daratumumab in this specific patient population are available. We prospectively investigated the novel daratumumab-containing triplet; Ixa-Dara-dex in intermediate-fit patients according to the IMWG-FI. At the time the study was developed it was known that the toxicity of daratumumab was limited, being mainly infusion-related. The combination with bortezomib-dexamethasone (Dara-Vd) was superior and did not lead to an excess of treatment discontinuation due to toxicity, compared to Vd alone in the relapsed-refractory setting,8,9 therefore we decided to investigate a daratumumab-proteasome inhibitor combination. The oral proteasome inhibitor ixazomib was selected because of inducing less peripheral neuropathy (PNP) than bortezomib, with limited severe PNP only, allowing long-term administration.9,10 We used a low dose of dexamethasone as previous studies observed superior outcomes when sparing steroids.5,11
Methods
Patients
The HOVON-143 (NTR6297) is a multicenter, prospective phase II trial, that included patients with a previously untreated symptomatic MM with measurable disease12 and who were intermediate-fit or frail according to the IMWG-FI (Supplemental Methods). This analysis concerns the intermediate-fit patients, for further details see statistical analysis. Exclusion criteria consisted of non-secretory MM; severe organ dysfunction (cardiac dysfunction NYHA III-IV; COPD with FEV1 <50%; hepatic dysfunction (bilirubin or transaminases ≥3 times normal); renal dysfunction (creatinine clearance <20 ml/min)); neuropathy grade 1 with pain or grade ≥2; active/uncontrolled infections; and an active malignancy (for complete in- and exclusion see Supplemental Methods). All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and principles of good clinical practice and approved by the ethics committees.
Study design and treatment
Patients received nine 28-day induction cycles consisting of ixazomib 4 mg (orally; days 1, 8, 15), daratumumab 16 mg/kg (intravenous administration; cycles 1–2: days 1, 8, 15, 22; cycles 3–6: days 1, 15; cycles 7–9: day 1) and dexamethasone (on the days daratumumab was administered; cycle 1–2: 20 mg; subsequent cycles 10 mg). This was followed by maintenance therapy, consisting of 8-week cycles with ixazomib (days 1, 8, 15, 29, 36, 43) and daratumumab (day 1) until progression for a maximum of 2 years. In accordance with the protocol, dose adaptations for the combination of ixazomib, daratumumab and dexamethasone were performed in case of toxicity. The study protocol recommended antibiotic- and antiviral prophylaxis with trimethoprim/sulfamethoxazole and valaciclovir, and vaccinations were advised according to national guidelines. Myeloid growth factor use was permitted according to local practice.
Study endpoints
The primary endpoint was overall response rate (ORR) on induction treatment, defined as at least a partial response (≥PR).13 Secondary endpoints included PFS, progression-free survival 2 (PFS2; defined as time from registration to date of objective disease progression or death from any cause after second line therapy), overall survival (OS), tolerability (defined as treatment discontinuation), and safety ((severe) adverse events ((S)AEs)). In addition, event-free survival (EFS) was assessed post-hoc, defined as time to treatment discontinuation, progressive disease (PD), death, hematological toxicity grade 4 or non-hematological toxicity grade 3 or 4 (see Supplemental Objectives). Secondary endpoints during maintenance included the improvement of response during maintenance and discontinuation due to treatment-related toxicity of ixazomib and daratumumab. Quality of life, as defined by the EORTC QLQ-C30 was assessed during induction and maintenance treatment. Other secondary endpoints are described in the Supplemental Methods.
Statistical analysis
It was predefined in the protocol that the intermediate-fit and frail patients would be analysed separately, and that the results would not be formerly compared, nor would the results of both populations be pooled together (Supplemental Methods). For sample size calculation, the optimal Simon 2-stage design was used. An ORR of 50% was considered to be insufficient and an ORR of 65% sufficient. With an alpha of 0.1, a power of 80% and taking into account a 10% ineligibility rate, 66 intermediate-fit patients had to be included (Supplemental Methods). There were 2 interim analyses, one for safety and one for efficacy. Patients were analyzed on a modified intention-to-treat basis, i.e. only eligible patients were included in analyses. Time-to-event endpoints were estimated using the Kaplan–Meier method and 95% confidence interval (CI). Global health status/quality of life (GHS) was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC-QLQ-C30) at baseline (T0), after induction cycles 3 and 9 (T1 and T2, respectively), and after 6, 12 and 24 months of maintenance treatment (T3, T4 and T5, respectively) for patients who were still on protocol. Health Related Quality of Life (HRQoL) course over time was assessed by a linear-mixed-effects model and whether cross-sectional changes from baseline were clinically meaningful was evaluated using calculated minimal important difference (MID), of which the definition is described in the Supplemental Methods. Finally, the percentage of patients improving/deteriorating clinically relevant from baseline (i.e. more than the MID) were reported. Statistical analyses were performed by Stata version 15.1 and R version 3.6.1. Observations were censored on June 15, 2022.
Role of the funding source
The study was funded by Janssen Pharmaceuticals and Takeda Pharmaceutical Company Limited. The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Results
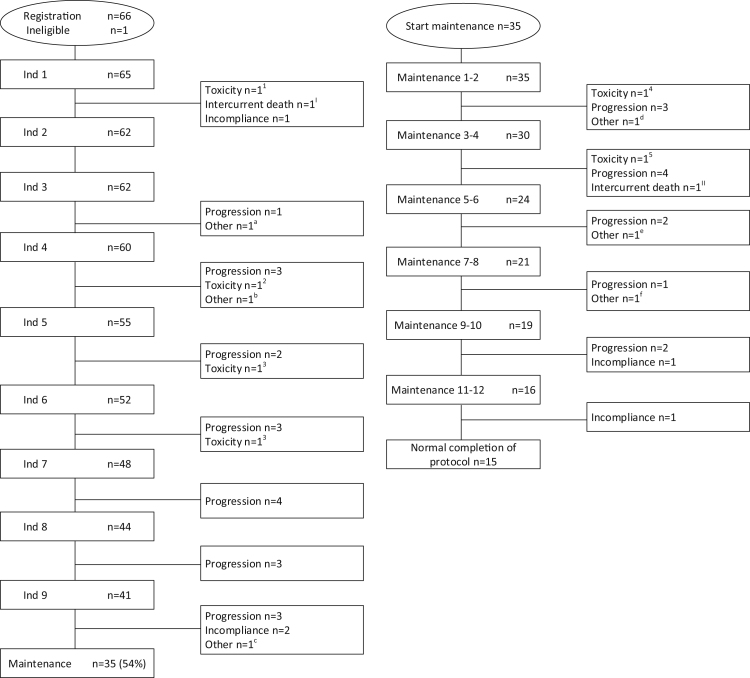
From October 2017 to May 2019, 66 intermediate-fit patients were enrolled of whom 1 was excluded due to ineligibility (Fig. 1, CONSORT diagram). The demographics of the 65 eligible patients are described in Table 1. Median age was 76 years (range 65–80), 14% had a WHO performance status (WHO-PS) ≥2, 18% had ISS3 and 14% had high-risk cytogenetic abnormalities.14,15 Patients were defined intermediate-fit based on age 76–80 years in 57%, based on comorbidities (Charlson Comorbidity Index (CCI) ≥2) in 29%, and due to iADL-dependency (iADL ≤ 5) in 14%. None of the patients were intermediate-fit because of ADL dependency (ADL ≤ 4) (Table 1). Specific details regarding specific iADL-dependencies and comorbidities are defined in Table S1.
Fig. 1.
CONSORT diagram—patient flow and causes of treatment discontinuation. Consort diagram of the number of intermediate-fit patients participating in the HOVON 143 study, flow through the induction and maintenance phase and timing and reason for treatment discontinuation.
Reasons for treatment discontinuation:
– Toxicity: 1cardiac decompensation; 2PNP grade 3 with pain; 3acute renal failure; 4 PNP grade 2; 5 Orthostatic hypotension grade 3
– Intercurrent death: I sudden death; II unknown cause of death
– Other reasons: a hypothyroidism related to amiodarone; b increase in M-protein not formally meeting criteria of progressive disease (PD); c progressive vascular dementia; d Squamous cell carcinoma grade 3; e PNP grade 2 and increase in M-protein (not formally meeting criteria of PD) during COVID19 pandemic; f Physician decision (increasing M-protein not formally meeting criteria of PD).
Table 1.
Demographics at registration of eligible intermediate-fit patients.
| Ixa-Dara-dex | N = 65 |
|---|---|
| Male (%) | 35 (54) |
| Median age (years) [range] | 76 [65–80] |
| ≤75 years | 28 (43) |
| 76–80 years | 37 (57) |
| WHO performance (%) | |
| 0 | 25 (38) |
| 1 | 28 (43) |
| 2 | 6 (9) |
| 3 | 3 (5) |
| Unknown | 3 (5) |
| Charlson Comorbidity Index (CCI) | |
| ≤1 (%) | 46 (71) |
| ≥2 (%) | 19 (29) |
| Activities of Daily Living (ADL) | |
| ≥5 (%) | 65 (100) |
| ≤4 (%) | – |
| Instrumental ADL (iADL) | |
| ≥6 (%) | 56 (86) |
| ≤5 (%) | 9 (14) |
| Type of measurable disease (%) | |
| IgG | 45 (69) |
| IgA | 13 (20) |
| FLC | 7 (11) |
| ISS disease stage (%) | |
| I | 16 (25) |
| II | 37 (57) |
| III | 12 (18) |
| LDH (%) | |
| Normal | 61 (94) |
| Elevated | 3 (5) |
| Unknown | 1 (2) |
| Cytogenetic results by FISH/array (%) | |
| t (4; 14) | 0/60 (0) |
| del (17p) | 5/58 (9) |
| t (14; 16) | 3/58 (5) |
| High risk cytogenetic diseasea | 8/56 (14) |
| R-ISS disease stage (%) | |
| I | 10 (15) |
| II | 49 (75) |
| III | 3 (5) |
| Unknown | 3 (5) |
| Median hemoglobin (g/dl) [range] | 10.3 [8.1–16.0] |
| Median creatinine (mg/dl) [range] | 0.96 [0.53–2.68] |
Efficacy
The ORR during induction treatment was 71% (95% confidence interval (CI) 63–73), including 24 (37%) patients with a very good partial response or better (Table 2). The median time to first response was 2 months (range 1–5) and the median duration of response was 20.8 months (95% CI 12.0–36.7).
Table 2.
Best response on induction and maintenance treatment with Ixa-Dara-dex (n = 65).
| Response status,13 n (%) | During induction | During induction and maintenance |
|---|---|---|
| Overall response rate (≥PR) | 46 (71) | 47 (72) |
| (s)CR | 1 (2) | 8 (12) |
| VGPR | 23 (35) | 20 (31) |
| PR | 22 (34) | 19 (29) |
| MR | 11 (17) | 10 (15) |
| SD | 7 (11) | 7 (11) |
| NE | 1 (2) | 1 (2) |
MR: minimal response; n: number; NE: not evaluable; PR: partial response; SD: stable disease (s) CR: (stringent) complete response; VGPR: very good partial response.
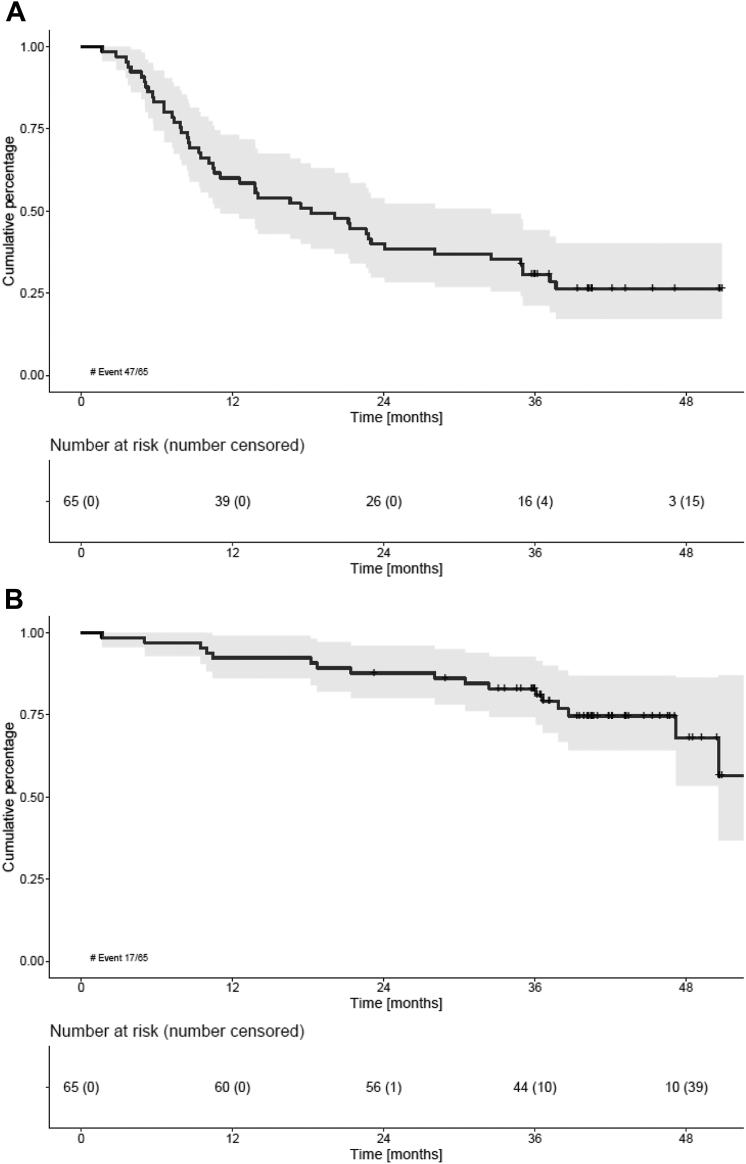
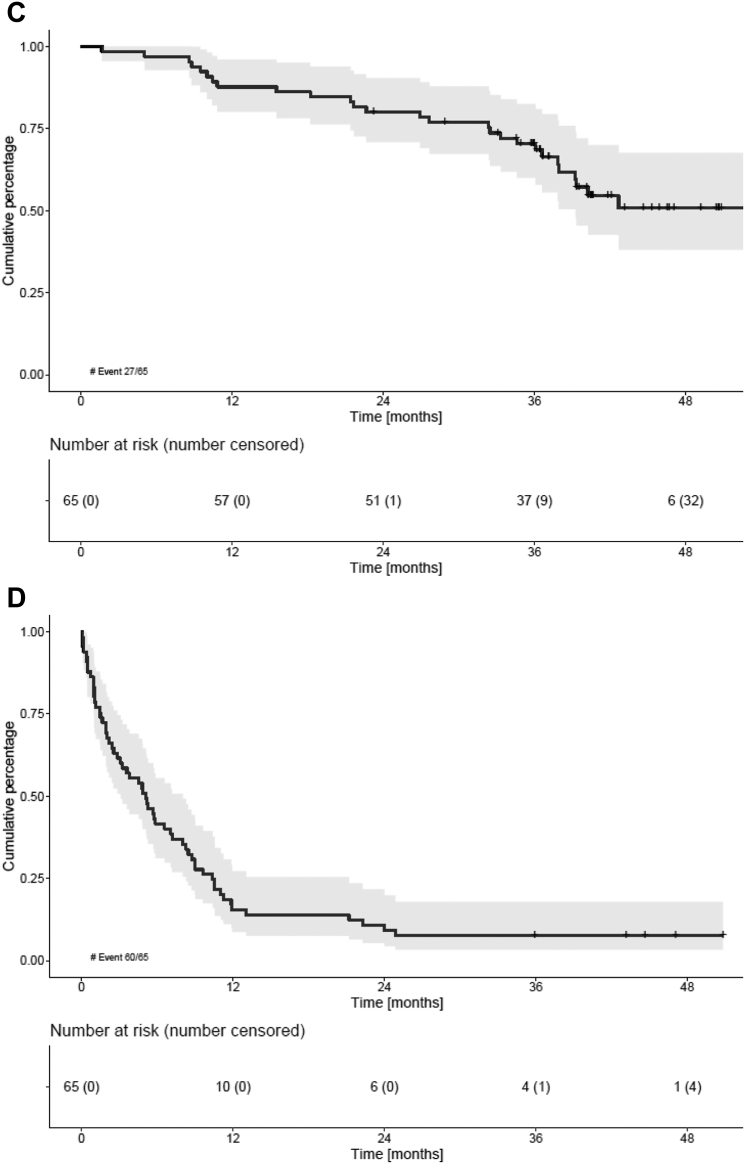
At a median follow-up of 41.0 months (IQR 36–46 months), 47/65 (72.3%) patients had progressed or died. The median PFS was 18.2 months (95% CI 10.5–28.1) (Fig. 2a). Both the median PFS2 and OS were not reached (NR) (95% CI 37.9-NR, Fig. 2c) and (95% CI 47.2-NR, Fig. 2b), respectively). The 36-month OS was 83% (95% CI 71–90%) (Fig. 2b). No differences in PFS, PFS2 or OS were observed between patients defined intermediate-fit based on age 76–80 years vs based on other frailty parameters (either comorbidities or iADL-dependency; Figure S1a and b).
Fig. 2.
Survival outcomes of intermediate-fit patients A) Progression-free survival (PFS), B) overall survival (OS), C) progression-free survival 2 (PFS2) and D) event-free survival (EFS). For further specification of EFS reasons, please be referred to Table S2 in the Supplemental data. No differences in PFS, OS or EFS were observed between patient subgroups defined intermediate-fit based on age 76–80 vs based on another frailty parameter (comorbidities or iADL dependency) (Figure S1).
Relapse-related mortality occurred in 7/65 (11%) patients and non-relapse mortality in 10/65 patients (15%), including 1 patient who died within 60 days of registration (early death rate 1.5%). Reasons for non-relapse mortality were three secondary primary malignancies (SPMs) (small cell lung carcinoma, myelodysplastic syndrome with excessive blasts-2 and mesothelioma), two cardiac events (heart failure and ventricular arrhythmia), two infections and three with an unknown cause of death.
The median EFS was 5.1 months (95% CI 2.8–7.2) (Fig. 2d). At data cut-off, only five patients (8%) were event-free. The most common event was non-hematological AEs grade 3 or 4 (40/65; 62%), followed by PD (14/65; 22%), treatment discontinuation of the whole treatment regimen (3/65; 5%), hematological AEs grade 4 (2/65; 3%) and death (1/65; 2%) (see Table S2 for a detailed description of events). No difference in EFS was observed between patients defined intermediate-fit based on age 76–80 years vs based on other frailty parameters (Figure S1c).
Thirty-five out of 65 (54%) patients completed induction treatment and started with maintenance treatment. The median follow-up of the patients who reached maintenance is 41.3 months (range 34.6–53.8). Improvement of response during maintenance was observed in 12/35 (34%) patients. One patient changed from a minimal response (MR) to a partial response (PR), four patients from a PR to a VGPR and 7 patients from a VGPR to (s)CR (Table 2). The ORR during induction and maintenance treatment was 72%.
Tolerability
Thirty out of 65 (46%) patients did not proceed to maintenance (Fig. 1), of whom 63% (19/30) due to PD, 13% (4/30) due to toxicity, 10% (3/30) due to incompliance, 3% (1/30) due to sudden death and 10% (3/30) due to other reasons (see Fig. 1 for more details).
In addition, dose reductions and/or interruptions occurred during induction therapy (Table S3). Twenty-four out of 65 (37%) patients had at least one dose modification of ixazomib. In 10/65 (15%) the dose of dexamethasone was modified. In 11/65 (17%) patients one or more daratumumab doses were skipped. Six out of 65 (9%) patients discontinued ixazomib, while continuing treatment with daratumumab and dexamethasone. The median relative dose intensity (RDI) (with interquartile range) was 0.96 (0.87–1.00), 0.99 (0.94–1.00) and 1.00 (0.97–1.00), for ixazomib, daratumumab and dexamethasone, respectively. During protocol induction treatment, full doses of ixazomib, daratumumab and dexamethasone, were administered in 37/65 (57%) of patients (Table S3).
Of the 35 patients who started maintenance therapy, 20 (57%) discontinued therapy, the majority (13/20, 65%) due to PD. Other reasons were patient choice (2/20, 10%), toxicity (2/20, 10%), death (1/20, 5%) or other reasons (2/20, 10%; 1 physician decision, 1 squamous cell carcinoma) (Fig. 1, CONSORT diagram). Ixazomib dose modifications during maintenance occurred in 19/36 (53%) patients, of whom 8 patients did not have dose modifications of ixazomib during induction treatment. Six out of 35 (17%) patients skipped one or more doses of daratumumab and 4/35 (11%) of patients received ≥1 dose modification of dexamethasone during maintenance treatment, for all new onset (full dose during the induction treatment). Eight out of 35 (23%) patients discontinued treatment with ixazomib, while continuing with daratumumab and dexamethasone once every eight weeks (Table S3).
Full doses of ixazomib, daratumumab and dexamethasone during maintenance were administered in 13/36 (36%) patients. Six out of 65 patients (9%) completed induction and two year maintenance treatment without any dose modification.
Toxicity
Adverse events (AEs) during induction treatment are described in Table 3. Cumulative hematological toxicity grade ≥3 during induction occurred in 12% of patients, with neutropenia being most commonly reported (6%). Cumulative non-hematological toxicity grade ≥3 occurred in 51% of patients, predominantly grade 3 AEs (91%). The most common non-hematological AEs were gastro-intestinal AEs (14%, mainly diarrhea) and central nervous system AEs (14%), which were diverse (for details see legend Table 3). In addition, a total of 42% of patients developed any grade PNP, including 5 (8%) patients with grade 3 PNP. The occurrence of grade 3 infections was 8%.
Table 3.
Adverse events grade 2–4 during induction treatment.
| CTCAE grade n (%) | 2 | 3 | 4 |
|---|---|---|---|
| Any hematologic AE | 17 (26) | 6 (9) | 2 (3) |
| Anemia | 7 (11) | 2 (3) | – |
| Thrombocytopenia | 8 (12) | 3 (5) | – |
| Neutropenia | 15 (23) | 2 (3) | 2 (3) |
| Any non-hematologic AE | 28 (43) | 30 (46) | 3 (5)b |
| Cardiac | 3 (5) | 1 (2) | 2 (4)a |
| Central nervous system | 7 (11) | 7 (11)d | – |
| Gastro-intestinal | 14 (22) | 9 (14) | – |
| Infections | 18 (28) | 6 (9) | – |
| Infusion related reactions | 2 (3) | 2 (3) | – |
| Pain | 14 (22) | 4 (6) | – |
| Peripheral neuropathyc | 10 (15) | 5 (8) | – |
| Secondary primary malignancy | 3 (5) | 2 (3) | 1 (2)a |
AEs of grade ≥3 that occurred in at least 5% of patients and AEs of special interest are reported.
Including 1 patient with grade 5 AE.
Including 2 patients with grade 5 AE.
Grade 1 peripheral neuropathy was observed in 12 (18%) patients.
2 gait disturbance, 2 syncope, 1 Guillain-Barré syndrome, 1 brain stem infarction, 1 carpal tunnel syndrome AE: adverse event; CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.016; n: number.
During maintenance, 1/35 (3%) patients experienced grade 3 thrombocytopenia. Non-hematologic AEs occurred in 16/35 (46%) patients, of which the most common were gastro-intestinal AEs (3 patients grade 3, 1 grade 4) and infections (3 patients grade 3). There was no new onset grade ≥3 PNP during maintenance (Table 4).
Table 4.
Adverse events grade 2–4 during maintenance treatment.
| CTCAE grade n (%) | 2 | 3 | 4 |
|---|---|---|---|
| Hematologic AE | 5 (14) | 1 (3) | – |
| Anemia | 1 (3) | – | – |
| Thrombocytopenia | 1 (3) | 1 (3) | – |
| Neutropenia | 4 (11) | – | – |
| Non-hematologic AE | 14 (40) | 14 (40) | 2 (6) |
| Cardiac | 2 (6) | – | – |
| Central nervous system | 1 (3) | – | 1 (3) |
| Gastro-intestinal | 6 (17) | 3 (9) | 1 (3) |
| Infections | 8 (23) | 3 (9) | – |
| Infusion related reactions | – | – | – |
| Pain | 8 (23) | 1 (3) | – |
| Peripheral neuropathya | 4 (11) | – | – |
| Secondary primary malignancy | – | 3 (9) | – |
AEs of grade ≥3 that occurred in at least 5% of patients and AEs of special interest are reported.
Grade 1 peripheral neuropathy was observed in 3 (9%) patients.
Seventy-nine SAEs were reported in 41/65 (63%) of patients on protocol, of which the majority (78%) were due to (prolongation of) hospitalization. Four SAEs resulted in death, of which all four were unlikely related to study treatment.
Second line treatment
Of the patients who had progressive disease (either on- or off protocol), second line treatment was started in 40 of 42 patients (95%). This was comparable in patients who went off protocol because of progressive disease (30/31, 97%), premature treatment discontinuation due to toxicity (3/3, 100%), incompliance (3/3, 100%), other reasons (2/2) or after disease progression after normal completion of protocol (2/3, 67%). The remaining 23 patients were still free of progression (18) or died before progressive disease was documented (5).
The most common initiated second-line treatment was lenalidomide-based (35/40, 88%). For a complete overview of second-line regimens please be referred to Table S4.
HRQoL
All 65 patients completed the baseline HRQoL questionnaires and were included in HRQOL analysis. Overall compliance was 60/62 (97%) after 3 cycles, 39/41 (95%) after 9 cycles, 29/30 (97%) after 6 months (C12), 20/23 (87%) after 12 months (C15) and 15/15 (100%) after 24 months (C21) of maintenance treatment. Mean HRQoL score of Global Health Status/QoL (GHS/QoL) at baseline and five follow-up time points during induction and maintenance treatment are presented in Table S5.
During treatment, patients on protocol reported a statistically significant improvement in GHS/QoL, which was clinically relevant (i.e. reached the threshold for MID of 8.49) from 9 induction cycles onwards (Figure S2). At all time points, the number of patients experiencing a clinically relevant (> MID of 8.49 points from baseline) improvement in their GHS/QoL, was numerically higher than whom who reported a deterioration (Figure S3).
Discussion
Frailty levels have a pronounced effect on the outcome of treatment, even in equally aged patient populations with comparable performance status.3 We here report the second prospective study specifically designed for intermediate-fit patients with NDMM. Treatment with ixazomib-daratumumab-low dose dexamethasone resulted in an ORR of 72% and was safe, reflected by a low early mortality of 1.5%. Moreover, patients reported a clinically relevant improvement in GHS/QoL from the end of induction that persisted during maintenance. However, the median PFS of 18.2 months was limited, irrespective of being intermediate-fit based on age or based on having comorbidities or being dependent in iADL.
Our data confirm the inferior outcome of intermediate-fit patients identified as such by the IMWG-FI. Although cross-trial comparisons have inherent limitations due to differences in treatment, trial design and methodology, in the study of Larocca and colleagues, a comparable PFS of 18.3 months was found when using treatment with Rd.5 Treatment with a 3-drug regimen, consisting of ixa-dara-dex, did not result in a superior outcome. A higher level of frailty in our study is not a likely explanation as in both studies intermediate-fit patients according to the IMWG-FI were included and we found no differences in outcome in patients defined intermediate-fit based on age or based on either comorbidities or impairments in iADL. Furthermore, patient populations were comparable with regards to disease characteristics. Both studies show inferior results as compared with a post-hoc frailty analysis of the ALCYONE trial and the MAIA trial, comparing VMP with Dara-VMP and Rd with Dara-Rd respectively.7,17 In both trails the median PFS was considerably longer than in the trial of Larocca et al. and our trial, ranging from 40.1 months to not reached, except for patients treated with VMP; 18.3 months.5,7,17 The longer PFS might be explained by the fact that patients were defined intermediate-fit based on the S-FI instead of the gold standard; the IMWG-FI, using physician-reported vs patient-reported performance.6 The S-FI probably identifies a less vulnerable intermediate-fit patient population, which is not unreasonable to hypothesize as in general more fit patients tend to be included in trials investigating non-registered drugs. We would like to make plea for the incorporation of the IMWG-FI, which is the gold standard, in clinical trial design, in order to enable future cross-trial comparisons.
Almost half of the patients did not proceed to maintenance therapy, of which the majority because of progression, for which there are several explanations. Firstly, the effectiveness of the regimen was undermined by vulnerability of intermediate-fit patients, with almost a third of patients discontinuing induction therapy because of toxicity, sudden death and incompliance. In addition, a third of the patients needed dose modifications of ixazomib during induction treatment, and even more than half of the patients during maintenance, mainly due to neurotoxicity. The occurrence of PNP was higher than expected from earlier studies.10,18 The level of frailty or known risk factors for PNP, such as diabetes of high BMI, might play a role, however cannot be substantiated because the data to make such comparisons are lacking. Importantly, discontinuation due to toxicity occurred even with mild toxicity, indicating that patients prioritize maintaining independence over prolonging their life. This emphasizes the importance of reporting all levels of toxicity and the impact on feasibility, not just severe toxicity.19,20 Secondly, the regimen itself might have limited efficacy. This is supported by several observations. There is less improvement in PFS with ixazomib maintenance therapy following stem cell transplantation than lenalidomide although not head to head compared.21,22 In non-transplant eligible patients there is heterogeneous results on the added value of ixazomib maintenance; in the TOURMALINE-MM04 an improvement was observed, however we found that ixazomib maintenance did not result in an improvement in PFS compared to placebo.18,23 In addition, the addition of ixazomib to lenalidomide was found to significantly increase PFS only in the relapsed setting but not in newly-diagnosed non-transplant eligible patients.10,24
Moreover, the combination of daratumumab with a proteasome inhibitor might be less effective than with an IMiD. This is supported by non-head to head compared regimens (Dara-Rd, Dara-VMP and dara-Vd) both in first line and later lines of therapy.1,2,9 Whether this is caused by the longer duration of lenalidomide treatment vs bortezomib treatment is unknown. By replacing bortezomib with ixazomib we aimed to prolong proteasome inhibitor treatment in combination with daratumumab. However, this was found to be less feasible than expected in a vulnerable population, with limited efficacy as a result. Therefore, 8/35 (23%) patients received only daratumumab once every eight weeks during maintenance due to discontinuation of ixazomib. This might be the third reason for limited efficacy of our regimen, as the added value of daratumumab every 8 weeks in the maintenance phase might be limited. Accordingly, results of the CASSIOPEIA study showed no benefit of daratumumab maintenance every 8 weeks in patients who were treated with daratumumab in induction, like in our study.25 Whether this is caused by the treatment schedule of daratumumab (q8W) or that benefit is lacking irrespective of the density of the maintenance scheme has not been clarified yet.
Taken into account that many older, vulnerable patients may prioritize other outcomes than disease control, study endpoints should not only reflect survival but also tolerability and toxicity.20 Therefore, we investigated the EFS, incorporating the same events as in the study of Larocca et al.; next to progression and death, also hematological toxicity grade 4, non-hematological toxicity grade 3 or 4 or treatment discontinuation.5 We confirmed that the EFS was considerably shorter than the PFS; 5.3 months vs 18.2 months, indicating the importance of endpoints reflecting benefit–risk profiles. In both studies, grade 3–4 non-hematologic toxicities were the most common events. In our study gastro-intestinal complaints and peripheral neuropathy, being attributed to ixazomib, led to a 9% discontinuation of ixazomib during induction treatment, which was even 23% during maintenance treatment. Actually, such patients were treated with daratumumab alone, hampering efficacy. This stresses the need for reducing non-hematologic toxicity, especially as grade ≥3 non-hematologic toxicity (especially cardiac, gastro-intestinal events, and infections) has been linked to reduced overall survival within the first 6 months of its occurrence.26 Secondly, utilizing composite endpoints will be necessary for directing treatment in vulnerable patients, and we advocate for including these in all future clinical trials for non-fit patients.27 Furthermore, in future studies a frailty-adjusted approach should be implemented, which is currently under investigation in the FiTNEss trial, in order to improve outcome.28
Almost all patients with progressive disease were able to receive second line treatment (40/42, 95%). This is in contrast to other studies showing that older patients are less likely to receive subsequent treatment lines.2,7 Therefore, we demonstrate that the combination of daratumumab in first-line treatment, along with carefully monitored dose adjustments of ixazomib and dexamethasone, does not hinder subsequent treatment. Interestingly, the PFS2 (not reached after a median follow-up of 41.0 months) was considerably longer compared to the PFS1 (18.2 months). The majority of patients received a second line regimen including lenalidomide. Probably lenalidomide has a more favourable risk-benefit ratio, compared to a PI in a vulnerable patient population. Additionally, lenalidomide might have enhanced the immunomodulatory effect of daratumumab knowing to remain for several months after discontinuation.29,30
In conclusion, we here show that the outcome of intermediate-fit NDMM patients is limited, even with a 3-drug regimen. Treatment with ixazomib-daratumumab-dexamethasone was found to be safe and enhance overall quality of life. However, feasibility of ixazomib was limited reflected by frequent dose adjustments and discontinuation due to, often low grade, toxicity. We propose to design studies with composite or even co-primary endpoints, that include feasibility and all grade toxicity. In addition, the reasons for adapting and discontinuation of therapy should be investigated in more detail. This will allow the identification of less toxic partner drugs for daratumumab, paving the way for optimization of therapy of this vulnerable patient population.
Contributors
KN, PS, M-DL, and SZ designed the research.
AV, DB and SC collected the data.
KN accessed and verified the data.
KG, CS, KN and SZ analyzed the data.
KG, CS and SZ wrote the paper.
CS, KdH, RvK, RL, NT, MW, KLW, IL, DI, GV, M-CV, NvdD, G-JT, FdB, LT, EvdS, EdW, MS, PS, IN, SK, PY, M-DL, and SZ provided study patients.
All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Data sharing statement
For any data requests please contact the corresponding author.
Declaration of interests
Claudia Stege.
Speaker's Bureau: Sanofi, Celgene/Bristol Myers Squibb, Takeda.
Consulting or Advisory Role: Sanofi, Janssen.
Marie-Christiane Vekemans.
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Amgen, Janssen, Takeda, Bristol Myers Squibb/Celgene.
Consulting or Advisory Role: Amgen, Celgene-Bristol Myers Squibb, Janssen, Takeda, Sanofi, Pfizer, GlaxoSmithKline, Menarini.
Ka-Lung Wu.
Consulting or Advisory Role: Pfizer, Janssen, Bristol Myers Squibb.
Niels W. C. J. van de Donk.
Consulting or Advisory Role: Janssen, Celgene, Bristol Myers Squibb, Novartis, Amgen, Servier, Takeda, Bayer, Roche, Pfizer, Abbvie, Adaptive.
Research Funding: Janssen, Celgene, Amgen, Novartis, Bristol Myers Squibb, Cellectis.
Gert Jan Timmers.
Participation on an Advisory Board: Novartis.
Travel, Accommodations, Expenses: Novartis, Janssen.
Ellen van der Spek.
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Janssen.
Pieter Sonneveld.
Participation on a Data Safety Monitoring Board or Advisory Board: Celgene, Janssen, Amgen, Bristol Myers Squibb, Karyopharm Therapeutics, Pfizer.
Research Funding: Janssen, Amgen, Bristol Myeres Squibb/Celgene, Karyopharm Therapeutics, Pfizer.
Inger S. Nijhof.
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Janssen, Celgene/Bristol Myers Squibb, Sanofi.
Mark-David Levin.
Support for attending meetings and/or travel: Janssen, Takeda.
Paula F. Ypma.
Payment or honoraria for presentations: Janssen.
Support for attending meetings and/or travel: Janssen.
Sonja Zweegman.
Consulting or Advisory Role: Janssen-Cilag, Takeda, Celgene/Bristol Myers Squibb, Sanofi, Oncopeptides (no personal funding).
Research Funding: Janssen, Takeda.
No other potential conflicts of interest were reported.
Acknowledgements
The authors would like to thank all participating patients and centers, the HOVON data center and the Data Safety Monitoring Board (Dr Maria-Victoria Mateos, Dr Philippe Moreau and Dr Jerome Lambert).
Scientific category
multiple myeloma; clinical trials.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102167.
Appendix A. Supplementary data
material
References
- 1.Facon T., Kumar S., Plesner T., et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–2115. doi: 10.1056/NEJMoa1817249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateos M.V., Cavo M., Blade J., et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395(10218):132–141. doi: 10.1016/S0140-6736(19)32956-3. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A., Bringhen S., Mateos M.V., et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stege C.A.M., Nasserinejad K., van der Spek E., et al. Ixazomib, daratumumab, and low-dose dexamethasone in frail patients with newly diagnosed multiple myeloma: the hovon 143 study. J Clin Oncol. 2021;39(25):2758–2767. doi: 10.1200/JCO.20.03143. [DOI] [PubMed] [Google Scholar]
- 5.Larocca A., Bonello F., Gaidano G., et al. Dose/schedule-adjusted Rd-R vs continuous Rd for elderly, intermediate-fit patients with newly diagnosed multiple myeloma. Blood. 2021;137(22):3027–3036. doi: 10.1182/blood.2020009507. [DOI] [PubMed] [Google Scholar]
- 6.Facon T., Dimopoulos M.A., Meuleman N., et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the first (MM-020) trial. Leukemia. 2020;34(1):224–233. doi: 10.1038/s41375-019-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facon T., Cook G., Usmani S.Z., et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066–1077. doi: 10.1038/s41375-021-01488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonneveld P., Chanan-Khan A., Weisel K., et al. Overall survival with daratumumab, bortezomib, and dexamethasone in previously treated multiple myeloma (CASTOR): a randomized, open-label, phase III trial. J Clin Oncol. 2022;41(8):1600–1609. doi: 10.1200/JCO.21.02734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palumbo A., Chanan-Khan A., Weisel K., et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 10.Moreau P., Masszi T., Grzasko N., et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 11.Rajkumar S.V., Jacobus S., Callander N.S., et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajkumar S.V., Dimopoulos M.A., Palumbo A., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S., Paiva B., Anderson K.C., et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 14.Ross F.M., Avet-Loiseau H., Ameye G., et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97(8):1272–1277. doi: 10.3324/haematol.2011.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonneveld P., Avet-Loiseau H., Lonial S., et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services NIoH. National Cancer Institute . 2009. Common Terminology criteria for adverse events (CTCAE) version 4.0. [Google Scholar]
- 17.Mateos M.V., Dimopoulos M.A., Cavo M., et al. Daratumumab plus bortezomib, melphalan, and prednisone versus bortezomib, melphalan, and prednisone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of ALCYONE. Clin Lymphoma Myeloma Leuk. 2021;21(11):785–798. doi: 10.1016/j.clml.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos M.A., Spicka I., Quach H., et al. Ixazomib as postinduction maintenance for patients with newly diagnosed multiple myeloma not undergoing autologous stem cell transplantation: the phase III TOURMALINE-MM4 trial. J Clin Oncol. 2020;38(34):4030–4041. doi: 10.1200/JCO.20.02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpos E., Mikhael J., Hajek R., et al. Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J. 2021;11(2):40. doi: 10.1038/s41408-021-00432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celis E.S.P.D., Li D., Sun C.-L., et al. Patient-defined goals and preferences among older adults with cancer starting chemotherapy (CT) J Clin Oncol. 2018;36(15_suppl):10009. doi: 10.1200/JCO.2018.36.15_suppl.10009. [DOI] [Google Scholar]
- 21.Dimopoulos M.A., Gay F., Schjesvold F., et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;393(10168):253–264. doi: 10.1016/S0140-6736(18)33003-4. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy P.L., Holstein S.A., Petrucci M.T., et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279–3289. doi: 10.1200/JCO.2017.72.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zweegman S., Stege C.A.M., Haukas E., et al. Ixazomib-Thalidomide-low dose dexamethasone induction followed by maintenance therapy with ixazomib or placebo in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplantation; results from the randomized phase II HOVON-126/NMSG 21.13 trial. Haematologica. 2020;105(12):2879–2882. doi: 10.3324/haematol.2019.240374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Facon T., Venner C.P., Bahlis N.J., et al. Oral ixazomib, lenalidomide, and dexamethasone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood. 2021;137(26):3616–3628. doi: 10.1182/blood.2020008787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau P., Hulin C., Perrot A., et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(10):1378–1390. doi: 10.1016/S1470-2045(21)00428-9. [DOI] [PubMed] [Google Scholar]
- 26.Bringhen S., Mateos M.V., Zweegman S., et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6):980–987. doi: 10.3324/haematol.2012.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levit L.A., Singh H., Klepin H.D., Hurria A. Expanding the evidence base in geriatric oncology: action items from an FDA-ASCO workshop. J Natl Cancer Inst. 2018;110(11):1163–1170. doi: 10.1093/jnci/djy169. [DOI] [PubMed] [Google Scholar]
- 28.Coulson A.B., Royle K.L., Pawlyn C., et al. Frailty-adjusted therapy in Transplant Non-Eligible patients with newly diagnosed Multiple Myeloma (FiTNEss (UK-MRA Myeloma XIV Trial)): a study protocol for a randomised phase III trial. BMJ Open. 2022;12(6) doi: 10.1136/bmjopen-2021-056147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krejcik J., Casneuf T., Nijhof I.S., et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Veer M.S., de Weers M., van Kessel B., et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011;96(2):284–290. doi: 10.3324/haematol.2010.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
material