Summary
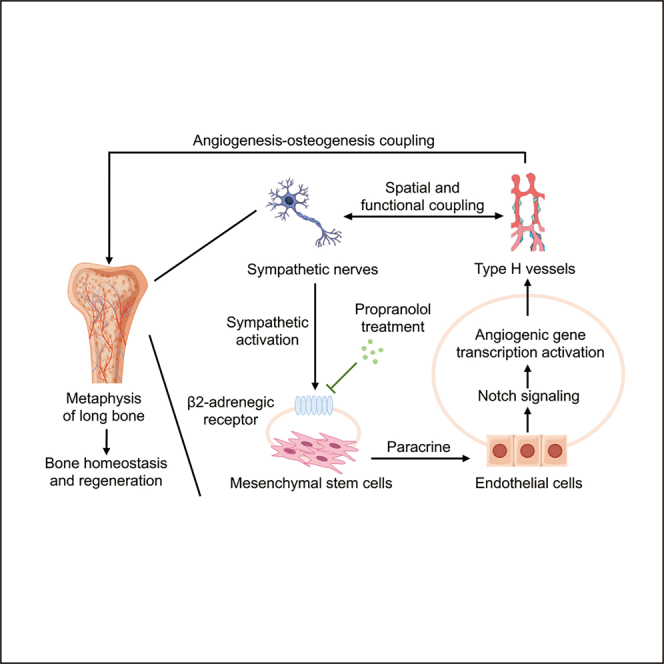
Type H vessels couple angiogenesis with osteogenesis, while sympathetic cues regulate vascular and skeletal function. The crosstalk between sympathetic nerves and type H vessels in bone remains unclear. Here, we first identify close spatial connections between sympathetic nerves and type H vessels in bone, particularly in metaphysis. Sympathoexcitation, mimicked by isoproterenol (ISO) injection, reduces type H vessels and bone mass. Conversely, beta-2-adrenergic receptor (ADRB2) deficiency maintains type H vessels and bone mass in the physiological condition. In vitro experiments reveal indirect sympathetic modulation of angiogenesis via paracrine effects of mesenchymal stem cells (MSCs), which alter the transcription of multiple angiogenic genes in endothelial cells (ECs). Furthermore, Notch signaling in ECs underlies sympathoexcitation-regulated type H vessel formation, impacting osteogenesis and bone mass. Finally, propranolol (PRO) inhibits beta-adrenergic activity and protects type H vessels and bone mass against estrogen deficiency. These findings unravel the specialized neurovascular coupling in bone homeostasis and regeneration.
Subject areas: Vascular anatomy, Orthopedics, Molecular neuroscience
Graphical abstract
Highlights
-
•
Sympathetic nerves spatially and functionally correlate with type H vessels in bone
-
•
Sympathoexcitation regulates type H vessel formation and bone mass maintenance
-
•
Sympathetic cues regulate angiogenic signaling via an MSC-EC paracrine mode
-
•
Pharmacological ADRB inhibition protects type H vessels and bone mass in OVX
Vascular anatomy; Orthopedics; Molecular neuroscience
Introduction
Over the past decades, extensive research has established the intricate connection between the nervous system and the vasculature. It has become evident that the nervous system plays a crucial role in maintaining vascular homeostasis and regulating the physiological function of blood vessels.1,2,3 In the central nervous system (CNS), the neurovascular unit (NVU) comprising neurons, astrocytes, and vascular smooth muscle has emerged as a pivotal regulatory structure for blood vessels. Conversely, in the peripheral nervous system (PNS), neural regulation of blood vessels primarily occurs via the secretion of neurotransmitters.4,5,6 Of particular importance in the PNS is the sympathetic nervous system (SNS), which releases various vasoactive mediators, including norepinephrine/noradrenaline (NE/NA), to regulate vasoconstriction and blood pressure.7,8 This vascular effect of sympathoexcitation is predominantly mediated by adrenergic receptors (ADRs) expressed in smooth muscle cells, the principal cells involved in vascular wall formation.9,10 As another essential component of the vasculature, the endothelium has also been reported to be regulated by the SNS, either indirectly or directly. Indirectly, SNS-induced smooth muscle contractions trigger the release of signaling molecules from ECs, which in turn mediate endothelium-dependent anticontractile responses.11 Alternatively, the SNS directly activates ADRs expressed in ECs, leading to the release of nitric oxide (NO), regulation of vascular contraction, and overall endothelial function.12 Among the various ADRs, the activation of the alpha-adrenergic receptor (ADRA) has been implicated in endothelial dysfunction characterized by increased leukocyte adhesion to ECs, vascular inflammation and vascular permeability.13,14,15 Activation of the beta-1-adrenergic receptor (ADRB1) has been associated with arterial endothelial injury.16 Activation of beta-2-adrenergic receptor (ADRB2) has been shown to promote NO release and vascular contraction which is opposite to that of ADRA.17,18 In addition, the ADRB2 activation has been demonstrated to promote the proliferation, migration, and differentiation of endothelial progenitor cells (EPCs) which is essential for vasculogenesis during embryonic development.19,20 However, in the postnatal tissues, whether and how the mature endothelium responds to sympathoexcitation through ADRB2 activation and regulates angiogenesis remains unclear.
In the mammalian skeletal system, the neural and vascular networks are densely distributed and are crucial in governing bone formation and remodeling.21 Particularly, the autonomic nervous system, through sympathetic and parasympathetic pathways, regulates bone formation, homeostasis, and remodeling.21 The neurotransmitters released from active sympathetic nerves are recognized by ADRBs that have been identified as a critical messenger mediator linking the brain to the skeleton since the early 2000s.22,23,24,25,26 In the context of bone, the three different ADRB receptors exert diverse functions. ADRB1 primarily regulates bone metabolism, exerting an anabolic stimulus during growth and development, and in response to mechanical stimulation.27,28,29 In contrast, ADRB2 signaling predominantly suppresses bone formation, leading to bone pathologies and decreased bone mass.22,27 ADRB3 plays a limited role in bone regulation, primarily influencing hematopoiesis rather than bone remodeling.29,30,31,32 Additionally, the vasculature, as an integral component of the bone remodeling unit, is critical for bone homeostasis by providing nutrient transport, delivering immune cells and supporting hematopoiesis.21,33 Notably, recent evidence has highlighted the remarkable morphological and functional heterogeneity of ECs in local microenvironments.34 Specifically, type H endothelium that can give rise to type L endothelium has been discovered as a specific vessel subtype coupling angiogenesis with osteogenesis in bone.35,36 However, the relationship between sympathetic nerves and type H vessels and its implications for bone physiology remains unclear. Thus, it is of great significance to uncover the effects and underlying mechanisms of the specialized nerve-vessel crosstalk in bone homeostasis and regeneration.
In this study, we demonstrate the close spatial distribution of the sympathetic nerves and type H vessels in bone, especially in the metaphysis region. The sympathetic activation induced by isoproterenol (ISO) injection led to type H vessel injury and bone loss. Conversely, in physiological conditions, deficiency of ADRB2 resulted in the restoration of type H vessels and bone mass. In vitro experiments were performed to evaluate the direct regulatory effect of sympathetic signal on ECs or indirectly through paracrine action of mesenchymal stem cells (MSCs), the latter of which was proved to be critical and induced alterations of expression of multiple angiogenic factors in ECs. Mechanistically, the Notch signaling in ECs was revealed to underlie the sympathoexcitation-governed type H vessel formation, and corresponding effects on osteogenesis contributed to the regulation of bone mass. Moreover, ADRB inhibition by propranolol (PRO) was proved to protect type H vessels and maintain bone mass in the estrogen-deficient pathological condition. Overall, our study confirms the close spatial and functional relationship between sympathetic nerves and type H vessels in bone under both physiological and pathological conditions, emphasizing the significance of specialized neurovascular coupling in maintaining bone homeostasis and supporting bone regeneration.
Results
Sympathetic nerves are spatially coupled with type H vessels in bone
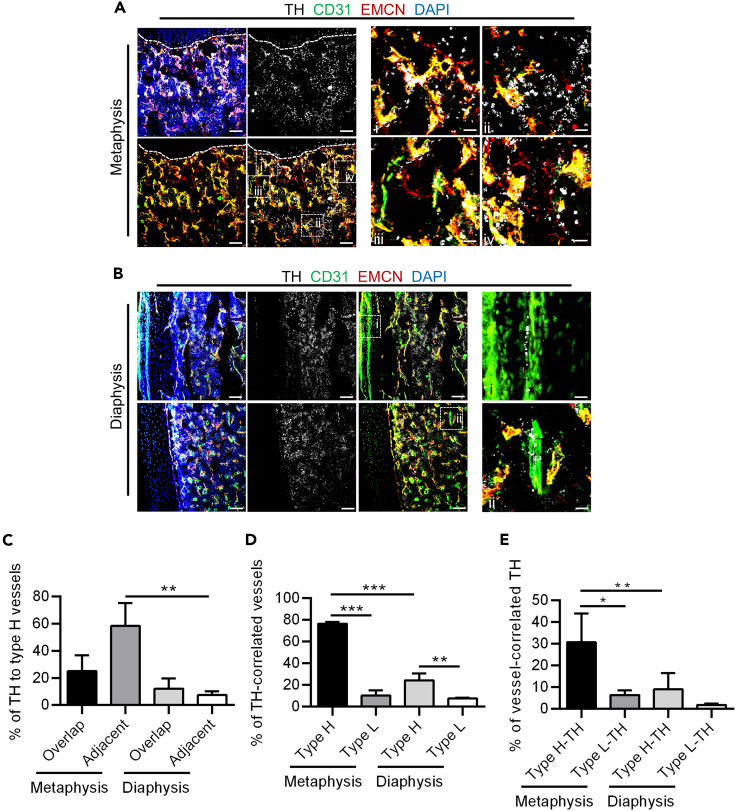
To investigate the relationship between sympathetic nerves and specialized vessels, at the beginning of this study, we particularly investigated the distribution of sympathetic nerves (marked by tyrosine hydroxylase, TH), CD31hiEMCNhi vessels (Type H vessels) and CD31loEMCNlo vessels (Type L vessels) in femurs (Figures 1A and 1B). Immunofluorescent staining revealed a correlation between the distribution of sympathetic nerves and type H vessels rather than single CD31hi or EMCNhi vessels in both metaphysis and diaphysis (Figures 1A and 1B). Our observations align with previous literature, showing the presence of TH+ cells in the metaphysis, diaphysis, and periosteum.37,38 In the metaphysis, TH+ cells predominantly exist as free nerve endings and were demonstrated to be distributed closely with type H vessels, as also shown in regional magnification (Figure 1A). While in the diaphysis and periosteum, sympathetic nerve fibers in addition to free nerve endings were observed around blood vessels marked with CD31 rather than specifically associated with type H vessels, as also shown in regional magnification (Figure 1B). Based on the predominant distribution of type H and L vessels in the metaphysis and diaphysis, we conducted quantitative analyses of TH+ cells in these areas (Figures 1C–1E). The specific criteria for analyzing the regions of interest (ROIs) are illustrated (Figures S1A–S1C). Quantification analysis confirmed that sympathetic nerves were overlapped or adjacent to type H vessels (Figure 1C), and this distribution pattern was particularly prominent in the metaphysis. Although the percentage of sympathetic nerves-correlated type H vessels was higher than type L vessels compared between the Type H group and the Type L group in both metaphysis and diaphysis, this distribution pattern is more pronounced in the metaphysis compared between the Type H group in the metaphysis and the Type H group in the diaphysis (Figure 1D). Moreover, the percentage of type H vessel-correlated sympathetic nerves was also higher than that of type L vessel-correlated sympathetic nerves in the metaphysis (Figure 1E), indicating a close spatial relationship between sympathetic nerves and type H vessels. Notably, as the close association between TH+ sympathetic nerves and type H vessels is especially prominent in the metaphysis, and given the important role of the metaphysis in bone homeostasis maintenance and regeneration,37 we focused on the metaphysis in further experiments.
Figure 1.
Sympathetic nerves are spatially coupled with type H vessels in bone
(A) TH (white), CD31 (green) and EMCN (red) co-immunostaining at metaphysis in femurs, counterstained by DAPI (blue). Dashed lines indicate the margin of growth plates. Scale bars, 100 μm i-iv, enlarged regions. Scale bars, 20 μm.
(B) TH (white), CD31 (green) and EMCN (red) co-immunostaining at diaphysis in femurs, counterstained by DAPI (blue). Scale bars, 100 μm i-ii, enlarged regions. Scale bars, 20 μm.
(C) Quantification of sympathetic nerves overlapped or adjacent to type H vessels (%). N = 3 per group.
(D) Quantification of sympathetic nerves-correlated vessels (%). N = 3 per group.
(E) Quantification of vessel-correlated sympathetic nerves (%). N = 3 per group.
Data represent mean ± SD. Statistical analysis is performed by Student’s t test for two-group comparison or by one-way analysis of variance followed by Newman–Keuls post-hoc tests for multiple comparisons. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
Sympathoexcitation impaired type H vessels and resulted in osteopenia
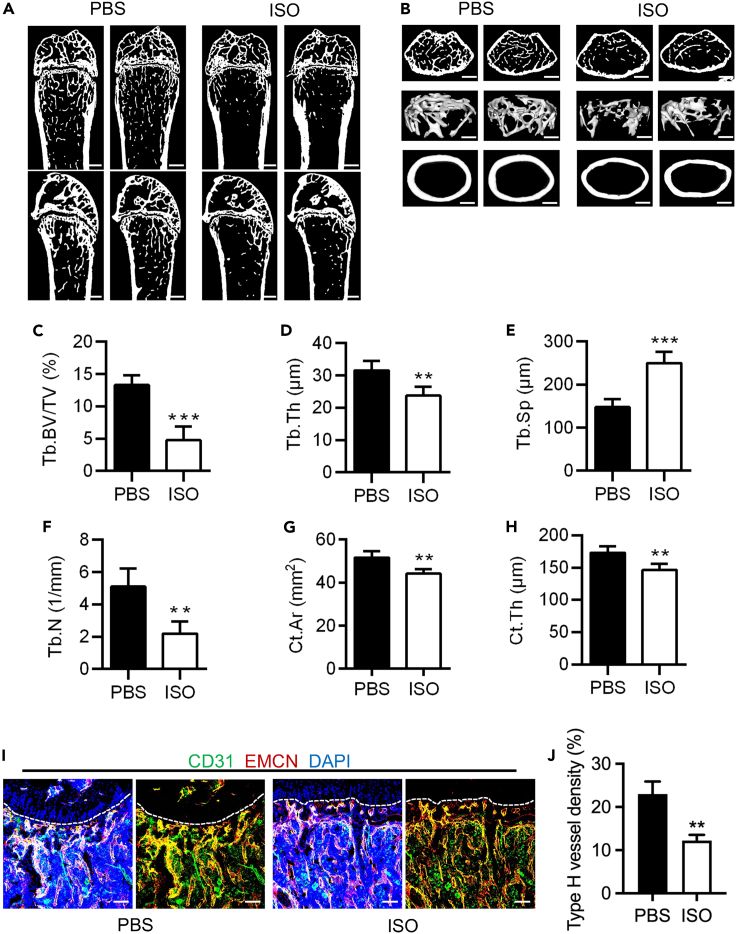
To investigate the effect of sympathetic activation on type H vessels and bone mass, the model of sympathetic activation was established by administering continuous daily intraperitoneal injections of the ADRB agonist ISO for four weeks (Figure S2A). We evaluated the impact of sympathetic activation by examining bone mass and type H vessel density (Figure S2A). Micro-CT technology was used to examine the mouse femurs, and the results showed a significant decrease in both trabecular and cortical bone in the ISO group compared to the PBS group (Figures 2A and 2B). Further analysis of the parameters such as trabecular bone volume over tissue volume (Tb.BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) for the trabecular bone (Figures 2C–2F), as well as the cortical area (Ct.Ar) and cortical thickness (Ct.Th) for the cortical bone (Figures 2G and 2H), confirmed the bone phenotypic changes. Additionally, fluorescent labeling techniques were employed to label type H vessels at the metaphysis, and a comparison of type H vessel density between the ISO group and the PBS group was performed. Fluorescent images and statistical analysis revealed a lower density of type H vessels in the ISO group compared to the PBS group (Figures 2I and 2J). Overall, our findings indicate that the mimic of sympathetic activation through ISO injection leads to type H vessel decrease and bone loss.
Figure 2.
Sympathoexcitation impaired type H vessels and resulted in osteopenia
(A and B) Micro-CT analysis and 3D views of femurs of mice treated with PBS and ISO. ISO was injected intraperitoneally for 4 weeks. Scale bars, 500 μm (A) and 250 μm (B).
(C–F) Parameter analysis of trabecular bone mass. Tb.BV/TV, trabecular bone volume over tissue volume; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number. N = 4 per group.
(G and H) Parameter analysis of cortical bone mass. Ct.Ar, cortical bone area; Ct.Th, cortical bone thickness. N = 4 per group.
(I) CD31 (green) and EMCN (red) co-immunostaining at metaphysis of mice treated with PBS and ISO, counterstained by DAPI (blue). Scale bar, 100 μm.
(J) Quantification of type H vessel density (%). N = 3 per group.
Data represent mean ± SD. Statistical analysis is performed by Student’s t test. ∗∗, p < 0.01; ∗∗∗, p < 0.001.
ADRB2 deficiency maintained type H vessels in bone mass accrual
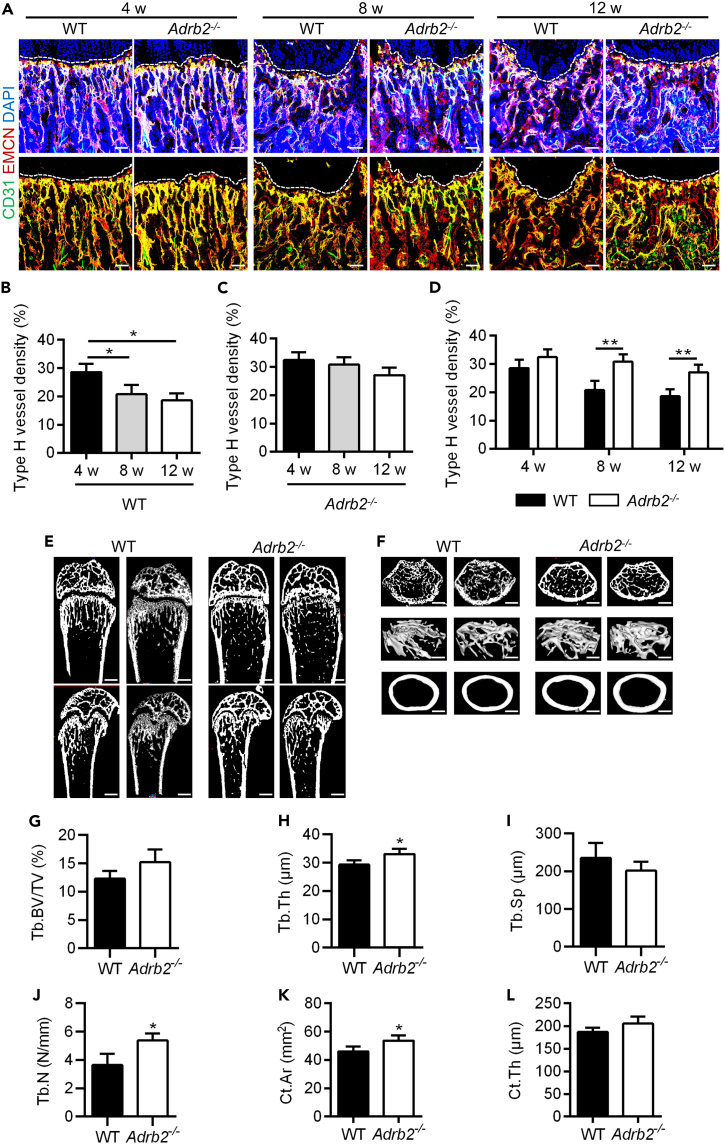
Next, we intended to further confirm the significance of sympathetic regulation of type H vessels in bone physiology. In our previous findings, we showed that type H vessels reduce from mouse adolescence to adulthood.39 Accordingly, we used Adrb2−/− mice as described27 and measured the density of type H vessels and bone mass in the femurs of mice during the physiological bone mass accrual process. Immunofluorescent staining revealed that the type H vessel density of 4, 8, and 12 weeks Adrb2−/− mice was higher than wild-type (WT) mice at paired age (Figure 3A). Further quantitative analysis confirmed that the density of type H vessels in WT mice gradually decreased with age (Figure 3B). Although that type H vessels in Adrb2−/− mice seemed to also decline with age, there was no statistical significance among groups (Figure 3C). Furthermore, 8- and 12-week-old, Adrb2−/− mice demonstrated higher levels of type H vessel density compared to WT mice (Figure 3D). These findings suggest that the deficiency of ADRB2 mitigates the age-related decrease in type H vessels. Micro-CT analysis showed the corresponding bone mass changes with type H vessel maintenance because the bone mass of Adrb2−/− mice was also preserved in 8 weeks compared with age-paired WT mice in both trabecular and cortical bone (Figures 3E and 3F), confirmed by statistical analysis of the corresponding parameters of Tb.Th and Cr.Ar (Figures 3G–3L). These findings provide evidence supporting the pivotal role of ADRB2 in mediating the physiological regulation of type H vessels and bone mass by sympathetic nerves.
Figure 3.
ADRB2 deficiency maintained type H vessels in bone mass accrual
(A) CD31 (green) and EMCN (red) co-immunostaining at metaphysis of WT and Adrb2−/− mice at 4, 8, and 12 weeks, counterstained by DAPI (blue). Scale bar, 100 μm.
(B) Quantification of type H vessels density (%) in WT mice at 4/8/12 w. N = 3 per group.
(C) Quantification of type H vessels density (%) in Adrb2−/− mice at 4/8/12 w. N = 3 per group.
(D) Quantification of type H vessels density (%) in WT mice and Adrb2−/− mice at 4/8/12 w.
(E and F) Micro-CT analysis and 3D views of femurs of WT and Adrb2−/− mice at 8 w. Scale bars, 500 μm (E) and 250 μm (F).
(G–J) Parameter analysis of trabecular bone mass. Tb.BV/TV, trabecular bone volume over tissue volume; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number. N = 4 per group.
(K and L) Parameter analysis of cortical bone mass. Ct.Ar, cortical bone area; Ct.Th, cortical bone thickness. N = 4 per group.
Data represent mean ± SD. Statistical analysis is performed by Student’s t test for two-group comparison, by one-way analysis of variance followed by Newman–Keuls post-hoc tests for multiple comparisons, or by two-way analysis of variance followed by Sidak’s tests for multiple comparisons. ∗, p < 0.05; ∗∗, p < 0.01.
Sympathoexcitation regulated angiogenesis through a paracrine mode and at the gene transcription level
To investigate the mechanism of sympathoexcitation regulating type H vessels, we next conducted in vitro experiments of angiogenesis regulation. It has been reported that capillaries are composed of ECs supported by pericytes, which refer to a collective term for cells located around blood vessels encompassing various cell types, including MSCs with a regulatory role in angiogenesis.40,41 Thus, we included both ECs and MSCs that could potentially contribute to the regulation of EC function in our further experiments. Firstly, considering the close spatial relationship between sympathetic nerves and blood vessels, we detected the protein expression of ADRB1/2/3 in both human- and mouse-derived MSCs and ECs. The results showed the presence of all three beta-adrenergic receptors in both MSCs and ECs in humans and mice (Figures S3A–S3C and S4A–S4C). To mitigate the risk of non-specific antibody binding, we further evaluated the mRNA expression of these ADRBs in MSCs and ECs from both species. The analysis confirmed the expression of all three ADRB mRNAs in the included samples (Figures S3D, S3F, S4D, and S4F), suggesting the possible response to sympathetic stimulation of both MSCs and ECs.
Secondly, we examined whether sympathetic cues directly regulated ECs or indirectly through MSCs. We treated ECs with ISO or with the supernatant of ISO-pretreated MSCs and evaluated their tube forming ability (Figures S5A and S5B). Notably, we used human-derived MSCs and ECs to provide clinical relevance in this section, which is also acceptable in supporting the angiogenic research in vivo.42,43 Results showed that direct treatment of ECs with ISO did not have a significant inhibitory effect on their tube formation, while indirect treatment of ECs with the supernatant of ISO-conditioned MSCs substantially inhibited their tube formation (Figures S5A and S5B). Based on the analysis parameters provided (Figure S6), we have conducted statistical analysis on parameters related to junctions and branches. The quantitative analysis further revealed that direct ISO treatment of ECs did not lead to a decrease in “junction/per field” and “ratio of branching length” compared to the control group (Figures S5C and S5D). In contrast, indirect ISO treatment via MSCs significantly inhibited the formation of junctions and branches of ECs (Figures S5E and S5F). These results suggested that the sympathetic regulation of EC angiogenesis is through the paracrine effect of MSCs rather than the direct response of ECs.
Lately, to further investigate the mechanism by which ISO indirectly regulated EC angiogenesis through MSCs, we performed the analysis at the transcription levels of angiogenesis-related genes (VEGF, BFGF, PDGFB, ANG1, and ANG2) in human-derived MSCs and ECs after different modes of ISO treatment (Figures S7A–S7E). The results revealed a decrease in mRNA levels of VEGF, BFGF, PDGFB, and ANG1 after direct ISO treatment (Figures S7A–S7D). However, there were no significant changes in the mRNA levels of ANG2 after direct ISO treatment (Figure S7E). In contrast, after indirect ISO treatment of ECs through MSCs, all five angiogenesis-related genes exhibited a significant decrease in mRNA levels, and the extent of mRNA level reduction was more remarkable than that in the direct group (Figures S7A–S7E). Furthermore, based on our previous findings indicating that the Notch signaling contributes to the formation of type H vessels,44 we examined the expression of NICD, the active component of the Notch pathway, in ECs (Figure S7F). The results demonstrated a significant reduction in NICD expression after indirect ISO treatment of ECs through MSCs (Figure S7F). These data suggested that under indirect treatment with ISO, the paracrine effects of MSCs importantly regulated the angiogenic gene expression of ECs possibly in related to Notch signaling alterations. These findings reflected the adaptability and responsiveness of ECs under sympathetic cues and implied that ECs undergo genetically programmed changes after indirect treatment with ISO.
Sympathoexcitation regulated type H vessel formation attributing to Notch signaling and governed bone mass through the angiogenesis-osteogenesis coupling
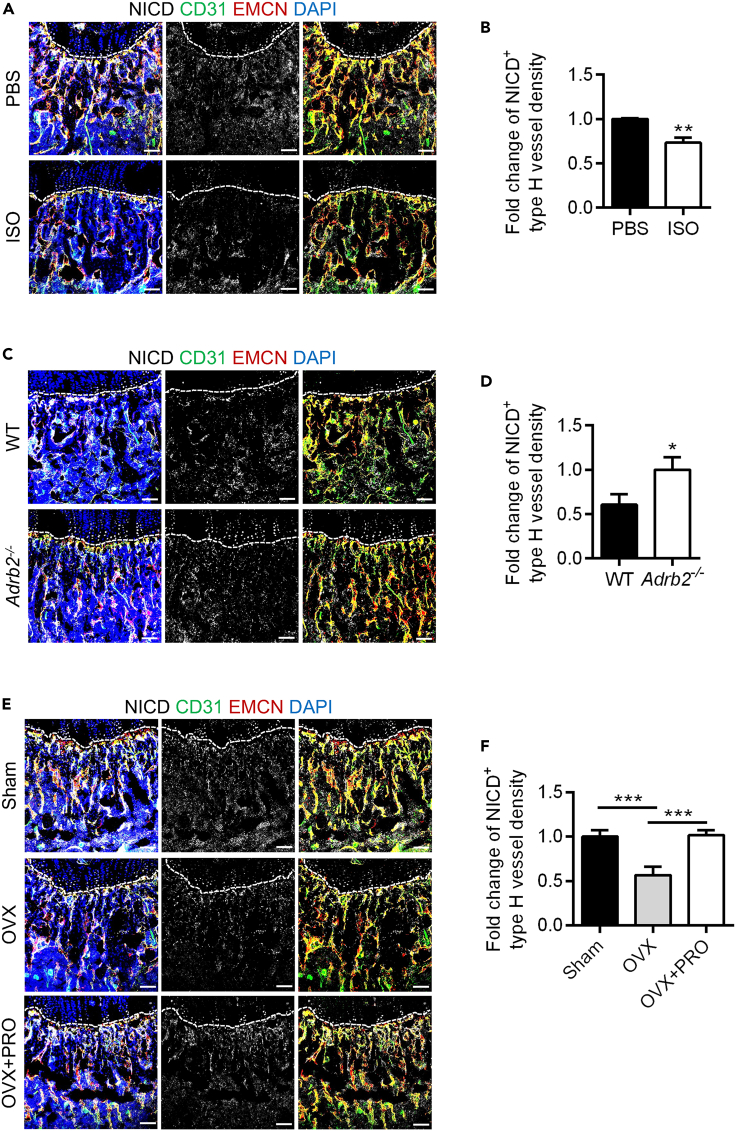
Next, we further investigated the in vivo mechanism underlying sympathetic regulation of the formation and maintenance of type H vessels. Immunofluorescence staining was conducted in the above mouse models, including ISO-mimicked sympathoexcitation and ADRB2 deficiency, to examine NICD+ cell expression in the metaphysis (Figures 4A and 4C). Quantification analyses were further conducted to calculate the NICD+ type H vessel density in the two models, and the specific criteria of ROIs for quantification of NICD+ type H vessels are shown (Figure S1D). Statistical analysis revealed a significant decrease in NICD+ type H vessel density in the ISO group compared to the PBS group (Figure 4B). In the ADRB2 deficiency model, the density of NICD+ type H vessels was noticeably higher compared to WT (Figure 4D). These results indicate that ISO, acting as an ADRB agonist, exerts a negative effect, while the deficiency of ADRB2 provides a protective effect on type H vessels in bone possibly attributing to changes of the Notch pathway.
Figure 4.
Notch signaling contributed to sympathoexcitation-regulated type H formation
(A) NICD (white), CD31 (green) and EMCN (red) co-immunostaining, counterstained by DAPI (blue), at metaphysis in femurs collected after daily intraperitoneal injections of ISO for one week. Dashed lines indicate the margin of growth plates. Scale bars, 100 μm.
(B) Quantification of NICD+ type H vessel density (%) of (A). N = 3 per group.
(C) NICD (white), CD31 (green) and EMCN (red) co-immunostaining, counterstained by DAPI (blue), at metaphysis in femurs of WT and Adrb2−/− mice. Dashed lines indicate the margin of growth plates. Scale bars, 100 μm.
(D) Quantification of NICD+ type H vessel density (%) of (C). N = 3 per group.
(E) NICD (white), CD31 (green) and EMCN (red) co-immunostaining, counterstained by DAPI (blue), at metaphysis in femurs collected after OVX and daily intraperitoneal injections of PRO for one week. Dashed lines indicate the margin of growth plates. Scale bars, 100 μm.
(F) Quantification of NICD+ type H vessel density (%) of (E). N = 3 per group.
Data represent mean ± SD. Statistical analysis is performed by Student’s t test for two-group comparison or by one-way analysis of variance followed by Newman–Keuls post-hoc tests for multiple comparisons. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
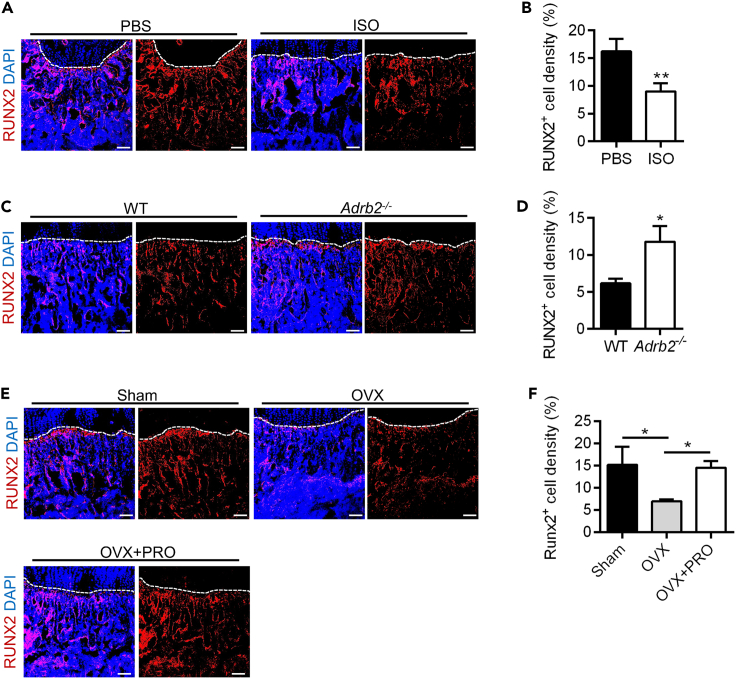
Taking into consideration the coupling of type H vessel formation and osteogenesis, we further conducted immunofluorescence staining to label RUNX2+ cells in the metaphysis of both models of sympathetic regulation (Figures 5A–5D). In the ISO model, a significant decrease in RUNX2+ cell density was observed and quantified in the ISO group compared to the PBS group (Figures 5A and 5B). On the contrary, ADRB2 deficiency maintained a significantly higher density of RUNX2+ cells compared to the WT group (Figures 5C and 5D). These findings suggest that ISO stimulation or ADRB2 deficiency modulates bone mass through the coupling of angiogenesis and osteogenesis.
Figure 5.
Sympathoexcitation regulated bone mass through the angiogenesis-osteogenesis coupling
(A and B) RUNX2 (red) immunostaining counterstained by DAPI (blue) and RUNX2+ cell density (%) quantification at metaphysis in femurs collected after daily intraperitoneal injections of ISO for one week. Dashed lines indicate the margin of growth plates. Scale bars, 100 μm. N = 3 per group.
(C and D) RUNX2 (red) immunostaining counterstained by DAPI (blue) and RUNX2+ cell density (%) quantification at metaphysis in femurs of 8 weeks WT and Adrb2−/− mice. Dashed lines indicate the margin of growth plates. Scale bars, 100 μm. N = 3 per group.
(E and F) RUNX2 (red) immunostaining counterstained by DAPI (blue) and RUNX2+ cell density (%) quantification at metaphysis in femurs collected after OVX and daily intraperitoneal injections of PRO for one week. Dashed lines indicate the margin of growth plates. Scale bars, 100 μm. N = 3 per group.
Data represent mean ± SD. Statistical analysis is performed by Student’s t test for two-group comparison or by one-way analysis of variance followed by Newman–Keuls post-hoc tests for multiple comparisons. ∗, p < 0.05; ∗∗, p < 0.01.
Pharmacological ADRB inhibition protected type H vessels and bone mass in estrogen deficiency
The obtained results prompted us to examine the pathological and therapeutic implications of sympatho-adrenergic regulation of type H vessels. Our previous studies have shown an increase in the release of NE by sympathetic nerves in ovariectomized (OVX) mice,39 indicating sympathetic nerve activation. Furthermore, mouse OVX replicates human estrogen deficiency which represents one major cause of bone loss often with psychological neural stress.45,46 Therefore, we selected this model as a pathological condition of osteoporosis to investigate the regulatory role of sympathetic excitation on type H vessels. One week after the OVX procedure, immunofluorescence staining was performed to label TH+ cells in the metaphysis in both the Sham and OVX groups to confirm the effects of OVX on the sympathetic nerves in mice. Images and quantitative analysis showed no significant changes in the density of TH+ cells in the Sham and OVX groups one week after OVX modeling (Figures S8A and S8B). Nevertheless, by measuring the concentration of NE in the bone marrow of the Sham and OVX groups, we detected a significant increase in OVX mice compared to the Sham group, indicating an elevated level of sympathetic activation (Figure S8C). Collectively, these findings suggest that although the TH+ sympathetic nerve does not alter in early OVX situations, sympathetic excitation occurs, which serves as a basis for pharmacological intervention.
Next, we accordingly administered PRO injections to OVX mice to inhibit potential ADRB activation under OVX conditions (Figure S2B). For mechanistic and target support, we found that the density of NICD+ type H vessels was decreased in the OVX group compared to the Sham group, while after PRO treatment, the density of NICD+ type H vessels was recovered (Figures 4E and 4F). Furthermore, RUNX2+ cell staining and quantitative analysis demonstrated a significant decrease in the density of RUNX2+ cells in the OVX group compared to the Sham group, whereas, after PRO treatment, an increase in the density of RUNX2+ cells was observed (Figures 5E and 5F). These results suggested that ADRB inhibition by PRO counteracted the pathological effect of sympathetic activation after OVX, indicating that PRO may serve as an effective treatment for the decrease of type H vessels and bone mass under estrogen deficiency.
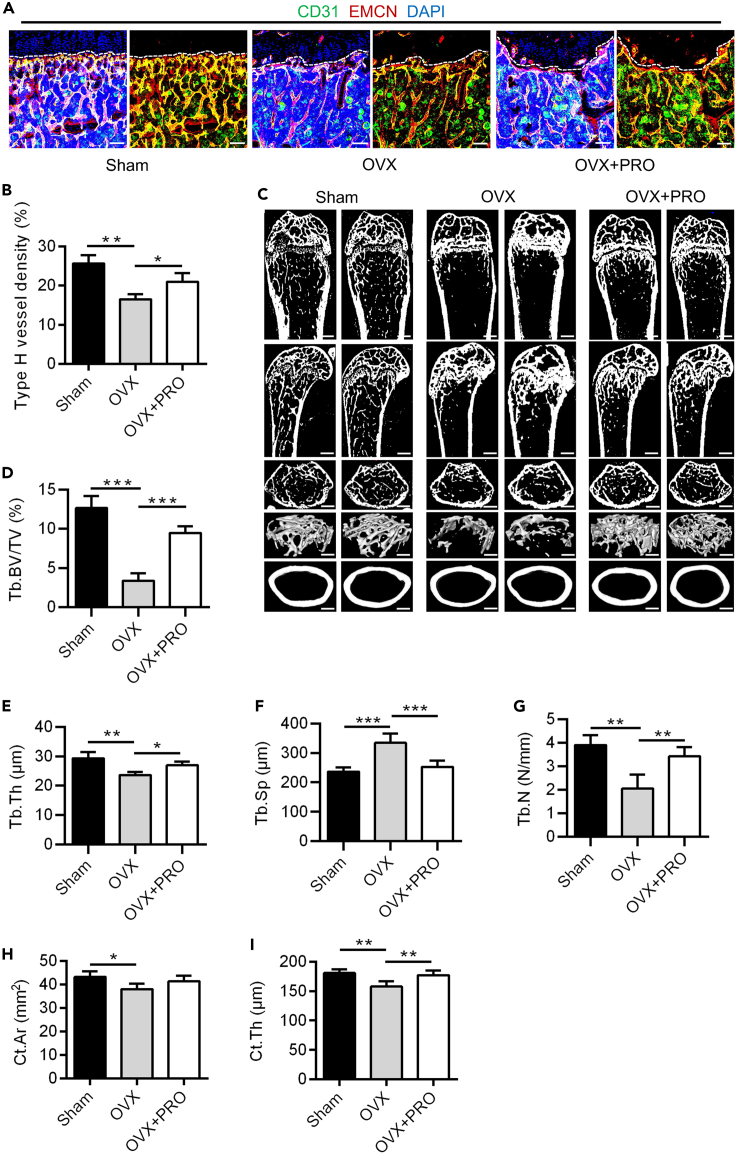
To further validate the therapeutic effect of PRO treatment, in vivo examination of type H vessels and bone mass was conducted. The fluorescence images showed a decrease of type H vessel density in the OVX group compared to the Sham group and remarkable protection of type H vessels in the PRO group compared to the OVX group (Figure 6A), which was confirmed by further quantitative analysis (Figure 6B). Micro-CT analysis further showed that bone mass was significantly decreased in OVX group compared to Sham group and was maintained in the PRO group compared to OVX mice (Figure 6C), which was supported by the quantitative analysis of parameters of both trabecular and cortical bone (Figures 6D–6I). Taken together, the above results show that the pharmacological ADRB inhibition protected type H vessels and maintained bone mass under sympathetic activation in pathological circumstances.
Figure 6.
Pharmacological ADRB inhibition protected type H vessels and bone mass in estrogen deficiency
(A) CD31 (green) and EMCN (red) co-immunostaining, counterstained by DAPI (blue), at metaphysis of sham-operated mice, estrogen-deficient OVX mice and PRO-treated estrogen-deficient OVX mice after daily intraperitoneal injections of PRO for four weeks. Dashed lines indicate the margin of growth plates. Scale bar, 100 μm.
(B) Quantification of type H vessels density (%). N = 3 per group.
(C) Micro-CT analysis and 3D views of femurs of sham-operated mice, OVX mice and PRO-treated OVX mice. Scale bar of the lengthwise section, 500 μm. Scale bar of cross section and 3D views, 250 μm.
(D–G) Parameters analysis of trabecular bone mass. Tb.BV/TV, trabecular bone volume over tissue volume; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number. N = 4 per group.
(H and I) Parameters analysis of cortical bone mass. Ct.Ar, cortical bone area; Ct.Th, cortical bone thickness. N = 4 per group.
Data represent mean ± SD. Statistical analysis is performed by Student’s t test for two-group comparison or by one-way analysis of variance followed by Newman–Keuls post-hoc tests for multiple comparisons. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
Discussion
The mammalian skeleton is a principal structural and endocrine organ that undergoes lifelong remodeling, which relies on niche microenvironments, including blood vessels and autonomic and sensory nerves.21 Recent studies have identified type H vessels, a specialized vasculature subtype with distinct morphological and molecular properties and play a crucial role in formation and regeneration.35,36 While the involvement of ADRBs in the SNS regulation of bone formation is well-established, their roles in angiogenesis and the significance of neurovascular crosstalk in the skeleton remain largely unknown.22,47 In this study, we identified a close spatial association between sympathetic nerves and the type H endothelium, indicating a phenotypic connection. Furthermore, the negative effect on type H vessels exerted by sympathoexcitation was uncovered by ISO injection. Additionally, genetic ablation of ADRB2 was confirmed to maintain type H vessel density associated with preserved bone mass. Mechanistically, the indirect regulation of EC angiogenesis under sympathoexcitation through the paracrine effect of MSCs was unraveled in vitro. Moreover, the Notch signaling in ECs was revealed to underlie the sympathoexcitation-governed type H vessel formation, and corresponding effects on osteogenesis contributed to the regulation of bone mass. Importantly, the pharmacological ADRB inhibition promoted the formation of type H vessels and prevented bone loss in the OVX pathological condition. These findings highlight the marked phenotypic and functional relationship between sympathetic nerves and specialized vasculature in bone physiology and pathology. They also underscore the crucial implications of specific neurovascular coupling in bone homeostasis and regeneration.
Various physiological conditions, such as growth, aging, psychological stress and unloading, activate the SNS, which can disrupt the homeostasis and regeneration of various tissues.39 Among the ADRs that respond to sympathoexcitation, ADRBs are thought to play a crucial role in mediating the effects of sympathetic outflow across the organism, particularly in relation to bone, which has become a focal point of research attention.36 Moreover, it has been verified that bone development, remodeling and regeneration depend on microenvironmental factors,48 among which vascular endothelium transports oxygen and nutrients as well as carries away waste products.36 Specifically, type H vessels are located at the distal end of the bone arterial network, creating a metabolically active microenvironment that has privileged access to oxygen, nutrients, and growth factors released by ECs, maintaining perivascular osteoprogenitors, contributing to bone vasculature growth and coupling angiogenesis with osteogenesis, ultimately promoting the bone generation and regeneration.35,36 Notably, the distribution of nerves and blood vessels in bone is intricately connected. However, whether sympathetic activation affects homeostasis and regeneration in bone through regulating specialized vasculature is still unknown. Our results show that in bone, the spatial distribution of the sympathetic nervous is closely related to type H vessels, suggesting the possibility of sympathetic control of type H vessel function for bone regulation and a new perspective for understanding the role and significance of specialized neurovascular coupling in bone.
To investigate the crosstalk effects of sympathetic nerves and type H vessels in bone homeostasis and regeneration, we conducted experiments using three different models that encompassed both physiological and pathological conditions. Notably, in the investigation of the impact of ADRB2 deficiency on type H vessels in mice, we observed a similar pattern as previously reported, where type H vessels in WT mice decreased with age.36,49 However, in the Adrb2−/− group, there was no significant age-related change in the density of type H vessels. The age-related decrease in type H vessels is a complex phenomenon influenced by various factors, including the coupling with osteogenesis,36,49 environmental changes including oxidative stress50 and hormone levels,51 and intracellular signaling pathways, such as hypoxia-inducing factor-1 alpha (HIF-1α).49 Besides, the increase in sympathetic activation with age also occurs, which might also be correlated with the decline of type H vessels. However, the impact of sympathetic nerve activation on type H vessels remains unclear. Therefore, the present findings of the sympathetic excitation effect on the maintenance of type H vessels in bone add to the current knowledge of regulatory mechanisms of type H vessels. It remains to be investigated whether ADRB2 deficiency influenced the age-related environmental and intracellular factors potentially contributing to the type H vessel alterations.
Additionally, in assessing bone mass, we found slight variations in cortical bone parameters among the different models. We propose two possible explanations for these findings. Firstly, Ct.Ar and Ct.Th represent different aspects of bone structure. Ct.Ar reflects the total area of bone tissue, while Ct.Th measures the thickness of bone tissue in a specific region. Thus, even within the same model, individual sample variations can contribute to differences in the trends of these two parameters. Secondly, based on our review of the relevant literature, we found that the sympatho-adrenergic influence on cortical bone becomes more prominent as the mice age. For example, Bouxsein et al. demonstrated that 16-week-old β-less mice exhibited a more prominent maintenance effect on Ct.Th compared to 6-week-old β-less mice. In this study, we employed 8-week-old and 12-week-old mice for micro-CT assessments, and it is possible that age-related factors contributed to the less prominent changes in cortical values, as well as in the 4-week-old mouse counterparts. Overall, all these findings significantly contribute to our understanding of the complex interplay between the SNS and the specialized vasculature in regulating bone homeostasis and regeneration. Considering that sympathoexcitation often occurs under stress conditions and that psychological stress may lead to bone loss, whether the sympathetic nerve-type H vessel coupling serves as a mechanism underlying stress-induced osteopenia will be interesting to investigate in the future.
In bone homeostasis and the process of regeneration, angiogenesis plays a crucial role in regulating the maintenance and formation of blood vessels, which involves providing essential nutrients and signaling support to cells, promoting tissue structure and functional restoration.35,51 The involvement of the Notch pathway in regulating the formation and maintenance of type H vessels has been established.44 However, it remains unclear whether the Notch pathway is also involved in the regulation of type H vessels under sympathoexcitation. In this study, our results demonstrated responsive changes in the expression of NICD, a key active component of the Notch pathway, under three different types of sympathetic regulation models. This provides the first evidence of the involvement of the Notch pathway in the regulation of type H vessels by sympathetic activation.
Moreover, angiogenesis in tissue regeneration is regulated through both cell-autonomous and non-autonomous mechanisms. The cell-autonomous regulation is achieved through angiogenic response and EC metabolic adaption,52 while the non-autonomous regulation is mediated through the indirect effects of vascular pericytes, including MSCs.53,54 While sympathetic innervation has been reported to regulate MSC function, primarily in terms of proliferation and differentiation rather than endothelial function such as angiogenesis,55 the extent to which MSCs participate in the angiogenic process under sympathetic activity remains unknown. Our results confirmed the expression of ADRB1/2/3 in both human and mouse MSCs and ECs, and that MSCs indirectly regulate EC angiogenesis under sympathoexcitation through paracrine effects. Notably, we used human cells as the cell source for rapid and convenient analysis of the responses of MSCs and ECs to sympathetic regulation and provide clinical relevance, as also seen in published papers.56,57 Although this conclusion has yet to be validated in vivo, our findings open up a new direction for further investigations into the effects of sympathetic activation on the density and function of type H vessels. Besides, although we have discovered the involvement of paracrine factors in the angiogenesis process of sympathetic nerve activation, the specific regulatory components are yet to be fully elucidated. In recent years, there have been increasing studies on extracellular vesicles (EVs) as intercellular communication.58 Exploring whether and how EVs play a role in this regulation is a promising area for future research.
Intriguingly, ADRBs have emerged as potential targets for mitigating bone loss in multiple osteopenic disorders.59,60 Particularly, pathological conditions, such as estrogen deficiency, lead to sympathetic activation and osteopenia.48 The regulation of the sympathetic nerves on bone mass presents a promising target for osteoporosis treatment.39,48 Previous studies, including our own, have demonstrated that the concentration of NE, the neurotransmitter of sympathetic nerve fibers, increases in the serum of OVX mice and that genetic or pharmacological inhibition of ADRB2 can alleviate the bone loss resulting from estrogen deficiency or unloading.39,61 However, the specific role of type H vessel recovery in ADRB intervention to counteract sympathoexcitation-induced osteopenia remains unclear. Notably, in the context of the known impact of castration-mediated sex hormone deficiency, it has a similar impact on osteoporosis development and treatment regardless of sex,62 and we ultimately chose the OVX model to better mimic clinical relevance. Our findings indicated that pharmacological ADRB inhibition leads to a significant increase of type H vessels in OVX mice, along with restored bone mass, providing a new mechanism underlying ADRB antagonist-induced osteopenia alleviation and further supporting the establishment of vascular-targeted anabolic therapy for postmenopausal osteoporosis.
In conclusion, our study has revealed the specialized spatial and functional neurovascular crosstalk, demonstrating that ADRBs, particularly ADRB2, negatively regulate type H vasculature and bone homeostasis. Our results also provide insights into the regulatory mechanism of sympathoexcitation control of angiogenesis, and importantly, ADRB inhibition protects against bone loss induced by estrogen deficiency through type H vessel recovery. These findings uncover a specific form of neurovascular coupling and indicate a neural signaling-based and vasculature-targeted approach for osteoanabolic therapies.
Limitations of the study
When considering these results, it should be noticed that there are certain limitations regarding the sex-specific selection in the three models in this study. However, the selection of the OVX model, which is more often used as an established model for osteoporotic research compared to orchidectomy (ORX), was aimed at better representing the clinical condition of postmenopausal osteoporosis. Additionally, in the ISO and ADRB2-deficient models, male mice were included with relatively stable hormone levels to minimize the potentially interfering factor of hormonal fluctuations present in female animals, which enabled us to explore the relationship between sympathetic cues and type H vessels conveniently.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse/Rat CD31/PECAM-1 Alexa Fluor® 488-conjugated Antibody | R&D Systems | Cat# FAB3628G; RRID: AB_10972784 |
| Endomucin (V.7C7) antibody | Santa Cruz Biotechnology | Cat# sc-65495; RRID: AB_2100037 |
| Anti-Tyrosine Hydroxylase Antibody | Millipore | Cat# ab152; RRID: AB_390204 |
| Cy3-AffiniPure Goat Anti-Rat IgG(H + L) | Yeasen | Cat# 33308ES60 |
| Goat Anti-Rabbit IgG H&L (Cy5 ®) preadsorbed ab6564 | Abcam | Cat# ab6564; RRID: AB_955061 |
| β1-Adrenergic Receptor Rabbit pAb | ABclonal | Cat# A20818 |
| ADRB1 Polyclonal antibody | Proteintech | Cat# 28323-1-AP |
| ADRB2 Rabbit pAb | ABclonal | Cat# A2048 |
| ADRB2 Polyclonal antibody | Proteintech | Cat# 29864 |
| ADRB3 Rabbit pAb | ABclonal | Cat# A8607 |
| GAPDH(Clone:1A6), Mouse mAb | Yeasen | Cat# 30201ES20 |
| β-actin, Mouse mAb | Yeasen | Cat# 30101ES60 |
| Notch1 (D1E11) XP® Rabbit mAb | Cell Signaling Technology | Cat# 3608S |
| RUNX2 (F-2) antibody | Santa Cruz Biotechnology | Cat# sc-390351; RRID: AB_2892645 |
| Chemicals, peptides, and recombinant proteins | ||
| Isoprenaline hydrochloride | MedChemExpress | Cat# HY-B0468 |
| Propranolol | MedChemExpress | Cat# HY-B0573B |
| 4% polyformaldehyde (PFA) | Sigma | Cat# 1004965000 |
| 17% Ethylene Diamine Tetraacetic Acid (EDTA) decalcification solution | Proandy | Cat# 10218-1 |
| Optimal cutting temperature (OCT) compound | Leica | Cat# 3801480 |
| 0.3% Triton X-100 | Sigma | Cat# X100PC-5ML |
| Goat serum | BOSTER | Cat# AR0009 |
| L-glutamine | Invitrogen | Cat# 35050061 |
| Penicillin/streptomycin | Invitrogen | Cat# 15070063 |
| Matrigel | Shanghai Nova Medical Technology | Cat# 0827775 |
| Mounting Medium With DAPI-aqueous fluoroshield | Abcam | Cat# ab104139 |
| Cell Lysis Buffer | Beyotime | Cat# P0013J |
| Protease inhibitor | Roche | Cat# P8215 |
| 5% Bovine Serum Albumin (BSA) | MP Biomedicals | Cat# 0881066-CF |
| Tris-buffered saline-Tween (TBS-T) | Solarbio | Cat# T1081-10 |
| Trizol Reagent | Takara | Cat# T9424 |
| α-MEM | Invitrogen | Cat# 12571-048 |
| Fetal bovine serum (FBS) | Sijiqing | Cat# 11011-8611 |
| Endothelial cell medium (ECM) | Sciencell | Cat# 1001 |
| Critical commercial assays | ||
| BCA Protein Assay Kit | TIANGEN | Cat# PA115-02 |
| Polyvinylidene fluoride membranes | Roche | Cat# GVWP02500 |
| Chemiluminescence kit | 4A Biotech | Cat# 4AW011-100 |
| Abnova™ Epinephrine/Norepinephrine ELISA Kit | Abnova | Cat# KA1877 |
| SYBR Premx Ex Taq ll Kit | Takara | Cat# S5193 |
| Experimental models: Cell lines | ||
| HUVEC | ATCC | Cat# CRL-1730; RRID: CVCL_2959 |
| MUVEC | Otwo Biotech | Cat# HTX3109 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | The Laboratory Animal Center of the Fourth Military Medical University | N/A |
| Mouse: FVB.129-Adrb2tm1Bkk/J | The Jackson Laboratory | Cat#JAX: 031496; RRID: IMSR_JAX:031496 |
| Oligonucleotides | ||
| qRT-PCR primers | Sangon | N/A |
| Software and algorithms | ||
| GraphPad Prism | GraphPad | RRID: SCR_002798 |
| ImageJ 1.47 | National Institute of Health | RRID: SCR_003070 |
| GE Volume Viewer | GE Healthcare | RRID: SCR_023417 |
| VGStudio MAX software | Volume Graphocs | RRID: SCR_017997 |
| FV10-ASW Viewer software | Olympus | RRID: SCR_014215 |
| Gel imaging system | Tanon | N/A |
| Real-Time System | Bio-Rad | RRID: SCR_018057 |
| CFX Manager software | Bio-Rad | RRID: SCR_017251 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Bing-Dong Sui (bingdong@fmmu.edu.cn).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed materials transfer agreement.
Experimental model and participant details
Experimental model
All animal experiments were performed in compliance with the relevant laws and ethical regulations, following the Guidelines of Intramural Animal Use and Care Committees of Fourth Military Medical University, approved by the Ethics Committee of Fourth Military Medical University (Protocol No. 2014-073), and following the ARRIVE guidelines. Male and female C57BL/6 mice at 8 weeks of age, which we have used in previous studies, were purchased from the Laboratory Animal Center of the Fourth Military Medical University.48 Male WT and Adrb2−/− mice at the same ages (4/8/12 w) were obtained from the Jackson Laboratory. All mice were maintained under specific pathogen-free conditions with a temperature of 24°C, 12-h light/dark cycles and 50% humidity, and were given ad libitum access to food and water.
Participant details
In vivo experiments
To induce an ovariectomy (OVX) model, 8-week-old female mice underwent a bilateral OVX by the dorsal approach under general anesthesia and were modeled for 1 month (40). The ISO (MedChemExpress, USA) was dissolved in PBS as 1 mg/mL, stored at −20°C, and intraperitoneally injected at a dose of 10 mg/kg every day during protection from light. The PRO (MedChemExpress, USA) was dissolved in PBS at 2 mg/mL, stored at −20°C, and intraperitoneally injected at a dose of 20 mg/kg every day. The femurs of mice were harvested for the detection of NICD+ and RUNX2+ cells after respective injections of ISO or PRO for 1 week. The femurs of mice were collected after 4 weeks of continuous injection for bone mass analysis and type H vessel assessment.
Micro-CT analysis
For micro-CT analysis, hindlimb bones were collected after sacrificing the mice and then fixed in a 4% polyformaldehyde (PFA) solution (Sigma, USA) overnight at 4°C. The fixed specimens were prepared as 1-cm length segments and scanned using a desktop micro-CT system (eXplore Locus SP, GE Healthcare, USA) with a resolution of 8 μm, voltage of 80 kV, and a current of 80 μA, following previously described methods.63 Following 3-dimensional image reconstruction, the ROI was selected in the medial metaphysis of the femur. Data analysis was conducted using VGStudio MAX software (Volume Graphics, Germany) and the following trabecular bone parameters were measured as recommended: Tb.BV/TV, Tb.Th, Tb.Sp, Tb.N, Ct.Ar and Ct.Th parameters.64
Enzyme-linked immunosorbent assay (ELISA)
To collect bone marrow fluid, the bone marrow cavity was washed multiple times with 200 μL of PBS. The collected fluid was then subjected to centrifugation at 12,000 g for 10 min to collect the supernatant, which was subsequently used for analysis. The concentration of NE (KA1877, Abnova, China) was determined using a commercial ELISA kit.39
Immunofluorescent staining
Immunofluorescent staining was performed according to previously published methods.65 Briefly, fresh femur samples were fixed in 4% PFA (Biosharp, China), decalcified with 17% Ethylene Diamine Tetraacetic Acid (EDTA) decalcification solution (Proandy, China), and dehydrated with 30% sucrose. The femurs were then embedded in an optimal cutting temperature (OCT) compound (Leica, Germany), and 20 μm cryosections were prepared with a cryostat (CM1950, Leica, Germany). The cryosections were permeabilized with 0.3% Triton X-100 (Sigma-Aldrich, USA) for 20 min at room temperature, blocked with goat serum (BOSTER, China) for 30 min at room temperature, and incubated with the primary antibodies overnight at 4°C. The primary antibodies used were anti-CD31 (FAB3628G, R&D Systems, USA; diluted 1:100), anti-EMCN (sc-65495, Santa Cruz Biotechnology, USA; diluted 1:100), anti-TH (ab152, Millipore, USA; diluted 1:100), anti-NICD (3608S, Cell Signaling Technology, USA; diluted 1:100), and anti-RUNX2 (sc-390351, Santa Cruz Biotechnology, USA; diluted 1:100). Following washing with PBS, the cryosections were incubated with fluorescence-conjugated secondary antibodies at room temperature for 1 h. The secondary antibodies used were Cy3-conjugated goat anti-rat IgG (33308ES60, YEASEN, China; diluted 1:200) and Cy5-conjugated goat anti-rabbit IgG (ab6564, Abcam, UK; diluted 1:200). After washing with PBS, the slides were mounted with Mounting Medium With DAPI-aqueous fluoro shield (Abcam, UK).
Confocal laser scanning microscopy (CLSM) (FV1000, Olympus, Japan) was used to capture images, which were analyzed using the ImageJ software (NIH, USA), as we and others previously reported.36,44,49 Z stacks of high magnification images were scanned at high resolution and were processed and reconstructed in three dimensions with the FV10-ASW Viewer software (Olympus, Japan). Quantification of type H vessel (CD31hiEMCNhi) area, type L vessel (CD31loEMCNlo) area, and 20 μm-adjacent areas to type H vessels for TH+ or NICD+ cell quantification were carried out with ImageJ software. Briefly, ImageJ was used to select the desired regions individually and analyze the proportion (the “density” we refer to) of these areas to the total visual area (% total area), or their respective areas to the region of interest (% ROI). We defined the spatial relationship between TH+ cells and vessels as “overlap” when positive staining signals were merged. Furthermore, we defined the spatial relationship between TH+ cells and vessels within a 20-μm range around the vessels, but not merged, as “adjacent”. For NICD+ type H vessel quantification, after absolute quantification of the positive NICD+CD31hiEMCNhi signal area, we performed relative quantification by taking the numerical value of one group as the reference and calculating the relative values of other groups, shown as the fold changes. Each quantification is performed by analyzing three independent regions.
Cell culture and cell experiments
The isolation and culture methods of human umbilical cord-derived MSCs and mouse bone marrow-derived MSCs were previously described.66 MSCs were cultured with alpha-minimum essential medium (α-MEM, Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Sijiqing, China), 2 mM L-glutamine (Invitrogen, USA) and 1% penicillin/streptomycin (Invitrogen, USA) in a humidified atmosphere of 5% CO2 at 37°C. ECs including human umbilical vein endothelial cells and mouse umbilical vein endothelial cells were purchased from the ATCC and Otwo Biotech and cultured in endothelial cell medium (ECM) (Sciencell, USA) and high glucose Dulbecco’s modified Eagle's medium (Hyclone, China) respectively, according to the manufacturer’s instructions. For indirect treatment of ECs, the supernatants from MSCs were filtrated at 0.22 μm and mixed with fresh ECM at a 1:1 ratio. The final concentration of ISO treatment for ECs was 100 μM. To determine the formation of a capillary network, an in vitro tube formation assay was performed on ibidi μ-Slide angiogenesis dishes (ibidi, Germany) coated with Matrigel (Shanghai NovaMedical Technology, China). ECs were seeded at 2×105 cells/mL (50 μL of medium per well) and incubated in the medium without FBS. The specific treatments were the same as described in the migration assay. Tube formation was observed and photographed using an inverted microscope (Leica, Germany) after 5 h. The number of network structures was analyzed by measuring three randomly selected microscopic fields using ImageJ software (NIH, USA). Specific parameters of junctions (points identified as either individual nodes or clusters of fused nodes) and branches (elements delimited by a junction and one extremity) were quantified using the “Angiogenesis Analyzer” for ImageJ software.
Western blot analysis
Western blot analysis was performed following the previously reported protocol.39 In brief, Cell Lysis Buffer (Beyotime, China) containing protease inhibitor (Roche, Switzerland) was used to prepare cell lysates. Protein quantification was performed using a BCA Protein Assay Kit (TIANGEN, China), and 20 μg of extracted proteins were separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Roche, Switzerland). The membranes were blocked with 5% Bovine Serum Albumin (BSA) (MP Biomedicals, USA) for 2 h at room temperature, and then incubated overnight at 4°C with primary antibodies including anti-ADRB1 (A20818, ABclonal, China; diluted 1:1000), anti-ADRB1 (28323-1-AP, Proteintech, China; diluted 1:1000), anti-ADRB2 (A2048, ABclonal, China; diluted 1:1000), anti-ADRB2 (29864, Proteintech, China; diluted 1:1000), anti-ADRB3 (A8607, ABclonal, China; diluted 1:1000), anti-NICD (3608S, Cell Signaling Technology, USA; diluted 1:100), anti-GAPDH (30201ES20, Yeasen, China; diluted 1:1000) and anti-β-Actin (30101ES60, Yeasen, China; diluted 1:1000). Subsequently, the membranes were washed three times with Tris-buffered saline-Tween (TBS-T, Solarbio, China), and then incubated with peroxidase-conjugated secondary antibodies for 1 h at room temperature. The protein bands were visualized using an enhanced chemiluminescence kit (4A Biotech, China) and evaluated using a gel imaging system (4600, Tanon, China). The gray value of protein bands was quantified using ImageJ software (NIH, USA).
mRNA expression analysis
Samples were obtained from cultured cells. Total RNA was extracted from tissues or cells using Trizol Reagent (Takara, Japan) and purified through phenol-chloroform extraction. To synthesize cDNA, RNA was reverse transcribed using the PCR Amplifier (EasyCycler, German). The primer sequences can be found in Table S1. GAPDH and Actin were chosen as reference genes for human ECs and mouse ECs respectively. Quantitative real-time PCR (qRT-PCR) was performed using the SYBR Premix Ex Taq II Kit (Takara, Japan) and detected using a Real-Time System (Bio-Rad, USA). For mRNA analysis, the CFX Manager software (Bio-Rad, USA) was utilized.
Quantification and statistical analysis
Statistical analysis was performed to determine the significance of the data. The mean and standard deviation (SD) were calculated and presented. Statistical analysis is performed by Student’s t test for two-group comparison, by one-way analysis of variance followed by Newman-Keuls post-hoc tests for multiple comparisons, or by two-way analysis of variance followed by Sidak’s tests for multiple comparisons, with p < 0.05 indicating significance (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001). Graphical and statistical analyses were conducted using GraphPad Prism software (GraphPad Software, USA).
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (81930025 to B.D.S., 32000974 to B.D.S., 82071136 to Y.J.C., 82201090 to Y.J.Z.), the Independent Project of State Key Laboratory of Military Stomatology (2019ZA02 to Y.J.C.) and the Special project of National Clinical Research Center for Oral Diseases (LCB202006 to L.K.).
Author contributions
Conceptualization, B.D.S., Y.J.C., and L.K.; Methodology, H.K.X., J.X.L., and C.X.Z.; Software, S.J.X. and C.H.H.; Validation, Q.L.; Formal analysis, H.K.X., J.X.L., and C.X.Z.; Investigation, H.K.X., J.X.L., and C.X.Z.; Resources, Y.J.Z.; Data curation, L.L., C.M., J.Y.T., Y.Y., and Y.C.; Writing—original draft preparation, H.K.X., J.X.L., and C.X.Z.; Writing—review and editing, S.J.X., C.H.H., L.L., C.M., J.Y.T., Y.Y., Y.C., Q.L., Y.J.Z., B.D.S., Y.J.C., and L.K.; Visualization, H.K.X., J.X.L., and C.X.Z.; Supervision, B.D.S., Y.J.C., and L.K.; Project administration, B.D.S., Y.J.C., and L.K.; Funding acquisition, B.D.S., Y.J.C., L.K., and Y.J.Z. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no conflict of interest.
Published: July 22, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107455.
Contributor Information
Liang Kong, Email: liangkong2014@163.com.
Yong-Jin Chen, Email: 13991979822@139.com.
Bing-Dong Sui, Email: bingdong@fmmu.edu.cn.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work is available from the lead contact upon request.
References
- 1.Distler A., Liebau H., Wolff H.P. Action of angiotensin on sympathetic nerve endings in isolated blood vessels. Nature. 1965;207:764–765. doi: 10.1038/207764a0. [DOI] [PubMed] [Google Scholar]
- 2.Makita T. Nerve control of blood vessel patterning. Dev. Cell. 2013;24:340–341. doi: 10.1016/j.devcel.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovach A.G., Takacs L. Response of the vascular system to sympathetic stimuli during shock. Nature. 1953;171:433–434. doi: 10.1038/171433a0. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffer S., Iadecola C. Revisiting the neurovascular unit. Nat. Neurosci. 2021;24:1198–1209. doi: 10.1038/s41593-021-00904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mastorakos P., McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aalkjær C., Nilsson H., De Mey J.G.R. Sympathetic and Sensory-Motor Nerves in Peripheral Small Arteries. Physiol. Rev. 2021;101:495–544. doi: 10.1152/physrev.00007.2020. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong J.M., Blackwell G.J., Flower R.J., McGiff J.C., Mullane K.M., Vane J.R. Genetic hypertension in rats is accompanied by a defect in renal prostaglandin catabolism. Nature. 1976;260:582–586. doi: 10.1038/260582a0. [DOI] [PubMed] [Google Scholar]
- 8.Tarazi R.C. Sympathomimetic agents in the treatment of shock. Ann. Intern. Med. 1974;81:364–371. doi: 10.7326/0003-4819-81-3-364. [DOI] [PubMed] [Google Scholar]
- 9.Sims S.M., Singer J.J., Walsh J.V., Jr. Antagonistic adrenergic-muscarinic regulation of M current in smooth muscle cells. Science. 1988;239:190–193. doi: 10.1126/science.2827305. [DOI] [PubMed] [Google Scholar]
- 10.Scheid C.R., Honeyman T.W., Fay F.S. Mechanism of beta-adrenergic relaxation of smooth muscle. Nature. 1979;277:32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- 11.Cocks T.M., Angus J.A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- 12.Vandecasteele G., Eschenhagen T., Scholz H., Stein B., Verde I., Fischmeister R. Muscarinic and beta-adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat. Med. 1999;5:331–334. doi: 10.1038/6553. [DOI] [PubMed] [Google Scholar]
- 13.Tymko M.M., Lawley J.S., Ainslie P.N., Hansen A.B., Hofstaetter F., Rainer S., Amin S., Moralez G., Gasho C., Vizcardo-Galindo G., et al. Global Reach 2018 Heightened alpha-Adrenergic Signaling Impairs Endothelial Function During Chronic Exposure to Hypobaric Hypoxia. Circ. Res. 2020;127:e1–e13. doi: 10.1161/CIRCRESAHA.119.316053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swenson E.R. Sympathetic Nervous System Activation and Vascular Endothelial Function With Chronic Hypoxia. Circ. Res. 2020;127:247–248. doi: 10.1161/CIRCRESAHA.120.317114. [DOI] [PubMed] [Google Scholar]
- 15.Ungvari Z., Tarantini S., Kiss T., Wren J.D., Giles C.B., Griffin C.T., Murfee W.L., Pacher P., Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H.I., Li H.T., Chen C.C. Physical conditioning decreases norepinephrine-induced vasoconstriction in rabbits. Possible roles of norepinephrine-evoked endothelium-derived relaxing factor. Circulation. 1994;90:970–975. doi: 10.1161/01.cir.90.2.970. [DOI] [PubMed] [Google Scholar]
- 17.Massion P.B., Dessy C., Desjardins F., Pelat M., Havaux X., Belge C., Moulin P., Guiot Y., Feron O., Janssens S., Balligand J.L. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates beta-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation. 2004;110:2666–2672. doi: 10.1161/01.CIR.0000145608.80855.BC. [DOI] [PubMed] [Google Scholar]
- 18.Fukuchi M., Hussain S.N., Giaid A. Heterogeneous expression and activity of endothelial and inducible nitric oxide synthases in end-stage human heart failure: their relation to lesion site and beta-adrenergic receptor therapy. Circulation. 1998;98:132–139. doi: 10.1161/01.cir.98.2.132. [DOI] [PubMed] [Google Scholar]
- 19.Galasso G., De Rosa R., Ciccarelli M., Sorriento D., Del Giudice C., Strisciuglio T., De Biase C., Luciano R., Piccolo R., Pierri A., et al. beta2-Adrenergic receptor stimulation improves endothelial progenitor cell-mediated ischemic neoangiogenesis. Circ. Res. 2013;112:1026–1034. doi: 10.1161/CIRCRESAHA.111.300152. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.J., Kim D.Y., Yun J., Choi S.H., Jung S.Y., Kang S., Park J.H., Kim Y.J., Ha J.S., Ji S.T., et al. Angiotensin II Attenuates the Bioactivities of Human Endothelial Progenitor Cells via Downregulation of beta2-Adrenergic Receptor. Stem Cells Int. 2018;2018 doi: 10.1155/2018/7453161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Q., Lee S., Patel N., Walden K., Gomez-Salazar M., Levi B., James A.W. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 2022;54:1844–1849. doi: 10.1038/s12276-022-00899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elefteriou F. Impact of the Autonomic Nervous System on the Skeleton. Physiol. Rev. 2018;98:1083–1112. doi: 10.1152/physrev.00014.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elefteriou F., Ahn J.D., Takeda S., Starbuck M., Yang X., Liu X., Kondo H., Richards W.G., Bannon T.W., Noda M., et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 24.Fu L., Patel M.S., Bradley A., Wagner E.F., Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Ducy P., Amling M., Takeda S., Priemel M., Schilling A.F., Beil F.T., Shen J., Vinson C., Rueger J.M., Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 26.Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L., Armstrong D., Ducy P., Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 27.Pierroz D.D., Bonnet N., Bianchi E.N., Bouxsein M.L., Baldock P.A., Rizzoli R., Ferrari S.L. Deletion of beta-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J. Bone Miner. Res. 2012;27:1252–1262. doi: 10.1002/jbmr.1594. [DOI] [PubMed] [Google Scholar]
- 28.Bouxsein M.L., Devlin M.J., Glatt V., Dhillon H., Pierroz D.D., Ferrari S.L. Mice lacking beta-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology. 2009;150:144–152. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khosla S., Drake M.T., Volkman T.L., Thicke B.S., Achenbach S.J., Atkinson E.J., Joyner M.J., Rosen C.J., Monroe D.G., Farr J.N. Sympathetic beta1-adrenergic signaling contributes to regulation of human bone metabolism. J. Clin. Invest. 2018;128:4832–4842. doi: 10.1172/JCI122151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maryanovich M., Zahalka A.H., Pierce H., Pinho S., Nakahara F., Asada N., Wei Q., Wang X., Ciero P., Xu J., et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 2018;24:782–791. doi: 10.1038/s41591-018-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraro F., Lymperi S., Méndez-Ferrer S., Saez B., Spencer J.A., Yeap B.Y., Masselli E., Graiani G., Prezioso L., Rizzini E.L., et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci. Transl. Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arranz L., Sánchez-Aguilera A., Martín-Pérez D., Isern J., Langa X., Tzankov A., Lundberg P., Muntión S., Tzeng Y.S., Lai D.M., et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 33.Zhu S., Bennett S., Kuek V., Xiang C., Xu H., Rosen V., Xu J. Endothelial cells produce angiocrine factors to regulate bone and cartilage via versatile mechanisms. Theranostics. 2020;10:5957–5965. doi: 10.7150/thno.45422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalucka J., de Rooij L.P.M.H., Goveia J., Rohlenova K., Dumas S.J., Meta E., Conchinha N.V., Taverna F., Teuwen L.A., Veys K., et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell. 2020;180:764–779.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Peng Y., Wu S., Li Y., Crane J.L. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10:426–436. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wee N.K.Y., Lorenz M.R., Bekirov Y., Jacquin M.F., Scheller E.L. Shared Autonomic Pathways Connect Bone Marrow and Peripheral Adipose Tissues Across the Central Neuraxis. Front. Endocrinol. 2019;10:668. doi: 10.3389/fendo.2019.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q., Liu K., Yang L., Wang H., Yang J. BoneClear: whole-tissue immunolabeling of the intact mouse bones for 3D imaging of neural anatomy and pathology. Cell Res. 2019;29:870–872. doi: 10.1038/s41422-019-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu C.H., Sui B.D., Liu J., Dang L., Chen J., Zheng C.X., Shi S., Zhao N., Dang M.Y., He X.N., et al. Sympathetic Neurostress Drives Osteoblastic Exosomal MiR-21 Transfer to Disrupt Bone Homeostasis and Promote Osteopenia. Small Methods. 2022;6 doi: 10.1002/smtd.202100763. [DOI] [PubMed] [Google Scholar]
- 40.Schwab K.E., Gargett C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 41.Yianni V., Sharpe P.T. Perivascular-Derived Mesenchymal Stem Cells. J. Dent. Res. 2019;98:1066–1072. doi: 10.1177/0022034519862258. [DOI] [PubMed] [Google Scholar]
- 42.Radmanesh F., Sadeghi Abandansari H., Ghanian M.H., Pahlavan S., Varzideh F., Yakhkeshi S., Alikhani M., Moradi S., Braun T., Baharvand H. Hydrogel-mediated delivery of microRNA-92a inhibitor polyplex nanoparticles induces localized angiogenesis. Angiogenesis. 2021;24:657–676. doi: 10.1007/s10456-021-09778-6. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Dong L., Zhong H., Yang L., Li Q., Su C., Gu W., Qian Y. Extracellular Vesicles (EVs) from Lung Adenocarcinoma Cells Promote Human Umbilical Vein Endothelial Cell (HUVEC) Angiogenesis through Yes Kinase-associated Protein (YAP) Transport. Int. J. Biol. Sci. 2019;15:2110–2118. doi: 10.7150/ijbs.31605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L., Zheng C.X., Zhao N., Zhu T., Hu C.B., Zhang N., Chen J., Zhang K.C., Zhang S., Liu J.X., et al. Mesenchymal Stem Cell Aggregation-Released Extracellular Vesicles Induce CD31(+) EMCN(+) Vessels in Skin Regeneration and Improve Diabetic Wound Healing. Adv. Healthc. Mater. 2023 doi: 10.1002/adhm.202300019. [DOI] [PubMed] [Google Scholar]
- 45.Deng Y.X., He W.G., Cai H.J., Jiang J.H., Yang Y.Y., Dan Y.R., Luo H.H., Du Y., Chen L., He B.C. Analysis and Validation of Hub Genes in Blood Monocytes of Postmenopausal Osteoporosis Patients. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.815245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia X., Jia L., Mo L., Yuan S., Zheng X., He J., Chen V., Guo Q., Zheng L., Yuan Q., et al. Berberine Ameliorates Periodontal Bone Loss by Regulating Gut Microbiota. J. Dent. Res. 2019;98:107–116. doi: 10.1177/0022034518797275. [DOI] [PubMed] [Google Scholar]
- 47.Zaidi M. Neural surveillance of skeletal homeostasis. Cell Metab. 2005;1:219–221. doi: 10.1016/j.cmet.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Sui B., Liu J., Zheng C., Dang L., Chen J., Cao Y., Zhang K., Liu L., Dang M., Zhang L., et al. Targeted inhibition of osteoclastogenesis reveals the pathogenesis and therapeutics of bone loss under sympathetic neurostress. Int. J. Oral Sci. 2022;14:39. doi: 10.1038/s41368-022-00193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J., Li M., Liu A.Q., Zheng C.X., Bao L.H., Chen K., Xu X.L., Guan J.T., Bai M., Zhou T., et al. Gli1(+) Cells Couple with Type H Vessels and Are Required for Type H Vessel Formation. Stem Cell Rep. 2020;15:110–124. doi: 10.1016/j.stemcr.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu X.F., Xiang G., Wang T.J., Ma Y.B., Zhang Y., Yan Y.B., Zhao X., Wu Z.X., Feng Y.F., Lei W. Impairment of type H vessels by NOX2-mediated endothelial oxidative stress: critical mechanisms and therapeutic targets for bone fragility in streptozotocin-induced type 1 diabetic mice. Theranostics. 2021;11:3796–3812. doi: 10.7150/thno.50907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang J., Yin H., Rao S.S., Xie P.L., Cao X., Rao T., Liu S.Y., Wang Z.X., Cao J., Hu Y., et al. Harmine enhances type H vessel formation and prevents bone loss in ovariectomized mice. Theranostics. 2018;8:2435–2446. doi: 10.7150/thno.22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 53.Guiducci S., Manetti M., Romano E., Mazzanti B., Ceccarelli C., Dal Pozzo S., Milia A.F., Bellando-Randone S., Fiori G., Conforti M.L., et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann. Rheum. Dis. 2011;70:2011–2021. doi: 10.1136/ard.2011.150607. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L., Jiao G., Ren S., Zhang X., Li C., Wu W., Wang H., Liu H., Zhou H., Chen Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020;11:38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu B., Lv X., Chen H., Xue P., Gao B., Wang X., Zhen G., Crane J.L., Pan D., Liu S., et al. Sensory nerves regulate mesenchymal stromal cell lineage commitment by tuning sympathetic tones. J. Clin. Invest. 2020;130:3483–3498. doi: 10.1172/JCI131554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pérez Piñero C., Bruzzone A., Sarappa M.G., Castillo L.F., Lüthy I.A. Involvement of alpha2- and beta2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012;166:721–736. doi: 10.1111/j.1476-5381.2011.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuvia S., Moses A., Gulayev N., Levin S., Korenstein R. Beta-adrenergic agonists regulate cell membrane fluctuations of human erythrocytes. J. Physiol. 1999;516:781–792. doi: 10.1111/j.1469-7793.1999.0781u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng C., Sui B., Zhang X., Hu J., Chen J., Liu J., Wu D., Ye Q., Xiang L., Qiu X., et al. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baek K., Bloomfield S.A. Beta-adrenergic blockade and leptin replacement effectively mitigate disuse bone loss. J. Bone Miner. Res. 2009;24:792–799. doi: 10.1359/jbmr.081241. [DOI] [PubMed] [Google Scholar]
- 60.Hanyu R., Wehbi V.L., Hayata T., Moriya S., Feinstein T.N., Ezura Y., Nagao M., Saita Y., Hemmi H., Notomi T., et al. Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2012;109:7433–7438. doi: 10.1073/pnas.1109036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He J.Y., Jiang L.S., Dai L.Y. The roles of the sympathetic nervous system in osteoporotic diseases: A review of experimental and clinical studies. Ageing Res. Rev. 2011;10:253–263. doi: 10.1016/j.arr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Sui B.D., Chen J., Zhang X.Y., He T., Zhao P., Zheng C.X., Li M., Hu C.H., Jin Y. Gender-independent efficacy of mesenchymal stem cell therapy in sex hormone-deficient bone loss via immunosuppression and resident stem cell recovery. Exp. Mol. Med. 2018;50:1–14. doi: 10.1038/s12276-018-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sui B.D., Hu C.H., Zheng C.X., Shuai Y., He X.N., Gao P.P., Zhao P., Li M., Zhang X.Y., He T., et al. Recipient Glycemic Micro-environments Govern Therapeutic Effects of Mesenchymal Stem Cell Infusion on Osteopenia. Theranostics. 2017;7:1225–1244. doi: 10.7150/thno.18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 65.Zheng C.X., Chen J., Tian J.Y., Huang X.Y., Jin Y., Sui B.D. Optimized immunofluorescence staining protocol for identifying resident mesenchymal stem cells in bone using LacZ transgenic mice. STAR Protoc. 2022;3 doi: 10.1016/j.xpro.2022.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing S.J., Zhang K.C., Tang S.Y., Liu L., Cao Y., Zheng C.X., Sui B.D., Jin Y. Isolation, Characterization, and Therapeutic Application of Extracellular Vesicles from Cultured Human Mesenchymal Stem Cells. J. Vis. Exp. 2022 doi: 10.3791/64135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work is available from the lead contact upon request.