Abstract
Objective
To compare comorbidities, symptoms and end-of-life (EoL) palliative medication (antisecretories, opioids, antipsychotics and sedatives) use among decedents before and during the COVID-19 pandemic.
Design
In a retrospective cohort study, decedent records in three acute care hospitals were abstracted, generating a prepandemic (November 2019–February 2020) group (pre-COVID) and two intrapandemic (March–August 2020, wave 1) groups, one without (COVID-ve) and one with COVID-19 infection (COVID+ve). Control group decedents were matched 2:1 on age, sex and care service (medicine/intensive care unit (ICU)) with COVID+ve decedents.
Setting
Three regional acute care teaching hospitals in Ottawa, Canada
Participants
Decedents (N=425): COVID+ve (n=85), COVID-ve (n=170) and pre-COVID (n=170).
Main outcome measures
Data were abstracted regarding demographics, admission comorbidities and symptoms, and EoL medication use; opioid doses were standardised to parenteral morphine equivalent daily dose (MEDD), and the predictors of upper quartile MEDD in the last 24 hours of life were examined in multivariable logistic regression with adjusted ORs (aORs) and 95% CIs.
Results
The prevalence of dementia (41% vs 28% and 26%, p=0.03), breathlessness (63.5% vs 42% and 47%, p<0.01), cough (40% vs 27% and 19%, p<0.01) and fever (54% vs 9% and 13.5%) was higher in COVID+ve versus pre-COVID and COVID-ve groups, respectively. The median (IQR) of MEDD over the last 72 hours of life was 16.7 (9–36.5) vs 13.5 (5.7–21.8) and 10.5 (5.3–23.8) for COVID+ve versus pre-COVID and COVID-ve groups, respectively, (p=0.007). Male sex, COVID+ve grouping, ICU death and high-flow nasal cannula use predicted upper quartile MEDD dose, aORs (95% CIs): 1.84 (1.05 to 3.22), 2.62 (1.29 to 5.3), 5.14 (2.47 to 10.7) and 1.93 (1.05 to 3.52), respectively. COVID+ve group decedents used highest lorazepam and propofol doses.
Conclusions
COVID-19 decedents, particularly those in ICU, required higher EoL opioid and sedating medication doses than matched prepandemic or intrapandemic controls. These findings should inform and guide clinical practice.
Keywords: adult palliative care, COVID-19, adult intensive & critical care, pain management
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The decedent cohort was representative of the source population in all adult acute care hospitals in a large urban region, and use of control groups from within and prior to the COVID-19 pandemic facilitated valid and unique comparisons.
This study relates to wave 1 of the pandemic. It is possible that symptom burden, and thus use of symptom control medications, has changed with subsequent waves.
Although rigorous training and accuracy checks were conducted in relation to data abstraction, abstractors were not blinded in relation to the study hypothesis, posing a potential source of bias.
The study’s retrospective design and recording of admission symptom assessment and comorbidity data without similar data, including medication efficacy and side effects, from within the more immediate end-of-life period are obvious limitations.
The generalisability of our study findings is largely limited to end-of-life care for hospitalised decedents, whereas many of the COVID-19-related deaths in wave 1 of the pandemic occurred in nursing homes.
Introduction
Globally, by mid-January 2023, over six million deaths due to COVID-19 are reported to have occurred.1 However, a bigger picture estimate of overall excess mortality due to the COVID-19 pandemic suggests a figure of just over 18 million deaths by the end of 2021.2 These estimates highlight the need for effective integration of specialist palliative care within hospitals,3 4 and adoption of a palliative care approach to ensure end-of-life care provision in the COVID-19 pandemic.5–7 Although the uptake of vaccines has helped to reduce COVID-19 disease severity and mortality,8 the mortality risk remains higher with chronic medical conditions, socioeconomic deprivation and in certain ethnic groups.9 10 Prior to vaccination uptake, earlier in the pandemic, infection with COVID-19 posed a greater risk of hospitalisation, intensive care unit (ICU) admission and subsequent death, particularly for older people, those with frailty and chronic medical comorbidities.11–13
Among those hospitalised with severe COVID-19 infection, dyspnoea, cough, fatigue, delirium, agitation and myalgia are the most prevalent symptoms.14–18 Both pharmacological and respiratory support interventions are often required for symptom control.12 19 20 In caring for those dying of COVID-19 infection, clinicians, particularly those with limited palliative expertise, are often faced with urgent need for information and support,21 22 and are guided in their use of pharmacological interventions by expert publications and specific guidelines.6 7 23 24
Palliative medications used in severe COVID-19 infection include: opioids for pain and dyspnoea; benzodiazepines for anxiety, agitation and dyspnoea; antipsychotics for refractory delirium symptoms; and antisecretory medications for airway secretions.20 Phenobarbitone and propofol are also used for sedation,25 26 the latter mainly in ICU settings. However, higher-level evidence derived directly from COVID-19 infected study populations for the efficacy and safety of pharmacological interventions in targeting symptom control is limited.27 28 Furthermore, guidelines addressing end-of-life symptom management in the COVID-19 context, for example, dyspnoea, are largely informed by primary studies conducted prepandemically in patients with either cancer or COPD,29 raising potential generalisability concerns. There is also a paucity of real world reported data on palliative medication use during the pandemic.30 31 Although most reports suggest that opioid requirements for end-of-life symptom management in COVID-19 infection are similar to other end-of-life conditions,28 30 31 some report higher requirements.32 33 Based on clinical experience, we hypothesised that higher opioid and sedative doses are needed to control symptoms in hospitalised patients dying of COVID-19 infection.
We conducted a study with the primary objective of comparing palliative medication use in the last 72 hours of life among three hospitalised decedent groups: a prepandemic group and two groups from wave 1 of the pandemic, one who died of COVID-19 infection, and the other who died of other causes without COVID-19 infection. Group comparisons of admission comorbidity and symptom prevalence, and respiratory/circulatory support use were additional objectives.
Methods
Study design
As part of a larger project on grief and bereavement in the COVID-19 pandemic,34 35 we conducted a retrospective multicentre matched cohort study of decedents’ documented end-of-life care in acute care hospitals. The study is reported according to the Strengthening the Reporting of Observational studies in Epidemiology criteria.36
Setting
The study population source consisted of inpatients in Ottawa (city and catchment area population 1.4 million), Canada, who died in the city’s three adult acute care hospital sites between 1 November 2019 and 31 August 2020. Site 1, Hôpital Montfort is a tertiary hospital with 289 inpatient beds. Site 2, Queensway-Carleton Hospital is a tertiary hospital with 264 inpatient beds. Site 3, The Ottawa Hospital is a quaternary hospital with 1271 inpatient beds. All sites used established electronic health records (EHR) software systems, Medical Information Technology at sites 1 and 2, and Epic (Epic Systems Corporation) at site 3, in documenting patient care.
Key exposures
Between 1 March 2020 and 31 August 2020, a total of 85 people died of COVID-19 infection in the region’s three acute care hospitals. The study’s key exposures related to COVID-19 infection status during decedents’ last hospital admission and when the admission occurred in relation to the pandemic. Three decedent study groups were identified on the basis of these exposures: a pre-COVID group who died between 1 November 2019 and 29 February 2020; and two groups who died between 1 March 2020 and 31 August 2020, within wave 1 of the pandemic, one who died of COVID-19 infection, and the other, without any record of COVID-19 during their hospital admission, designated COVID+ve and COVID-ve, respectively.
Participants
Adult (≥18 years old) decedents were included if they died in ICU or under the care of internal medicine in the designated study period. Both emergency department decedents and those primarily under surgical care were excluded. The index study group was COVID+ve (n=85), and each of these decedents was included. Using a 2: 1 ratio, the control pre-COVID (n=170) and COVID-ve (n=170) group members were matched with COVID+ve members at each site on the basis of age (±5 years), sex and care service (medicine or ICU) at the time of death.
Data sources/measurement
Anonymised EHR data, including study variables, were abstracted by teams of internal/palliative medicine physicians and two research assistants at each site, and entered into a common electronic study database. All abstractors received training regarding abstraction requirements. A senior study team member conducted a duplicate data abstraction of 154 (35%) of the patient records to confirm accuracy of details.
Variables
Study group designation was based on EHR documentation of COVID-19 infection status, date of death and death certification. Demographic variables included age, sex, admission referral source, acute care site, care service at death and admission duration (days). Based on EHR documentation, comorbidities and symptoms at admission, and respiratory/circulatory support use during admission, were recorded (yes/no) by abstractors (online supplemental table, appendix 1). Abstractors recorded medications prescribed (yes/no) and administered (yes/no) in the last 72 hours of life. Administered doses were totalled for each 24-hour interval (T3: >48 and ≤72 hours, T2: >24 and ≤48 hours, and T1: the last 24 hours of life) within this period, where available, and recorded for the following: opioids (morphine, fentanyl, hydromorphone), antisecretory medications (glycopyrrolate and hyoscine hydrobromide), antipsychotics (haloperidol and methotrimeprazine), benzodiazepines (lorazepam and midazolam), other sedating medication (phenobarbitone and propofol). Opioid doses were recorded in parenteral equivalent using a standard oral to parenteral ratio of 2:1.37
bmjopen-2023-075518supp001.pdf (41.6KB, pdf)
Patient and public involvement
Decedents’ study data were retrospectively acquired and are part of a project involving the prospective evaluation of grief in decedents’ bereaved family members. Although there was no direct patient or public involvement in the project’s retrospective component, the study team engaged with three knowledge user organisations (Bereaved Families of Ontario, Canadian Virtual Hospice and Champlain Hospice Palliative Care Programme), whose representatives collaborated with the study planning team and were co-applicants in funding applications for the overall project.
Bias
Data abstractors were not blinded to the study objectives and consequently there was potential for misclassification bias.
Study size
The sample size (N=425) was predetermined, based on the inclusion of all known wave 1 deaths due to COVID-19 in the index group (COVID+ve, n=85), and subsequent 2:1 matching to generate the other two study groups.
Quantitative variables
The administered opioid doses abstracted for each 24-hour period in the last 72 hours of life were used to calculate the parenteral morphine equivalent daily dose (MEDD) in mg using standard equianalgesic ratios.37
An individual mean total 24-hour medication dose was calculated for palliative medications administered to each patient who had data for one or more of the 24-hour periods in their last 72 hours of life; the median (IQR, Q1–Q3 range) of these individual mean doses was used as an aggregate summary measure in relation to both opioids (MEDD) and non-opioid medications administered in this period. Also, the maximum 24-hour dose of opioid, midazolam and propofol within the last 72 hours of life were determined for study group comparison. Continuous variables were expressed as mean±SD unless otherwise indicated.
Statistical methods
Demographic characteristics, palliative care consultation, comorbidities, symptoms, occurrence of medication use, median group values for individual mean 24-hour doses and MEDD values, and maximum MEDD, midazolam and propofol doses within the last 72 hours of life were compared among study groups, using a χ2 test for categorical variables, and an ANOVA or Kruskal-Wallis test for continuous variables, as appropriate. Subgroup analyses for MEDD at TI were conducted in relation to site and care service at death. The association of variables with the upper quartile of MEDD at T1 was examined in unadjusted bivariable and adjusted multivariable logistic regression analyses, reporting ORs and CIs. Based on clinical relevance and/or having a p<0.25 in bivariable analyses, variables were selected for a forced entry multivariable model with adjusted ORs (aORs). Terms were tested in the model for study group, age, sex and care service interactions. Statistical significance, using Stata (StataCorp. 2015. Stata Statistical Software: Release V.14., StataCorp) for analyses, was set at p<0.05.
Results
Study sample
The derivation of the study groups is summarised in online supplemental figure, appendix 2. Data from all COVID+ve decedents (n=85) and all pre-COVID (N=170) and COVID-ve (n=170) matched groups were used in comparison of admission comorbidity and symptom prevalence, and use of respiratory or circulatory support. To enable valid group comparisons, decedents who died <24 hours of admission (n=14) were excluded in medication analyses. Demographic characteristics are summarised in table 1.
Table 1.
Demographic characteristics of study groups according to COVID-19 status and time periods
| Demographic characteristics | Time periods and designated study groups | P value | ||
| November 2019–February 2020 | March 2020–August 2020 (wave 1) | |||
| Pre-COVID group N=170 (%)* |
COVID-ve group N=170 (%)* |
COVID+ve group N=85 (%)* |
||
| Age | ||||
| Years, mean±SD | 79.5±12.3 | 79.2±12.3 | 78.9±12.2 | 0.942 |
| Sex | ||||
| Male | 100 (58.8) | 100 (58.8) | 50 (58.8) | 1.0 |
| Hospital location | ||||
| Site 1, n=155, (row %) | 62 (40) | 62 (40) | 31 (20) | 1.0 |
| Site 2, n=100, (row %) | 40 (40) | 40 (40) | 20 (20) | |
| Site 3, n=170, (row %) | 68 (40) | 68 (40) | 34 (20) | |
| Care service at death | ||||
| Medicine service/unit | 118 (69.4) | 122 (71.7) | 62 (72.9) | 0.814 |
| Intensive care unit | 52 (30.6) | 48 (28.2) | 23 (27.1) | |
| Admission referral source | ||||
| Home | 99 (58.2) | 109 (64.1) | 31 (36.5) | <0.001 |
| Retirement home | 36 (21.2) | 34 (20.0) | 11 (11.8) | |
| Nursing home | 22 (12.9) | 8 (4.7) | 43 (50.6) | |
| Complex continuing care | 2 (1.2) | 2 (1.2) | 0 (0.0) | |
| Other | 11 (6.5) | 17 (10.0) | 1 (1.2) | |
| Admission duration category | ||||
| <24 hours | 7 (4.1) | 7 (4.1) | 0 (0) | 0.061 |
| ≥24 and <48 hours | 26 (15.3) | 18 (10.6) | 6 (7.1) | |
| ≥48 hours and <72 hours | 16 (9.4) | 8 (4.7) | 5 (5.9) | |
| ≥72 hours | 121 (71.2) | 137 (80.6) | 74 (87.1) | |
| Palliative care involvement | ||||
| Consult requested | 70 (41.2) | 71 (41.8) | 26 (30.6) | 0.184 |
| Consult completed | 67 (39.4) | 67 (39.4) | 25 (29.4) | 0.234 |
| Days from consult completion to death (median, Q1–Q3) | 4 (1–9) | 3 (1–6) | 3 (2–12) | 0.577 |
*Column numbers refer to number of persons (%) in respective study groups unless stated otherwise.
bmjopen-2023-075518supp002.pdf (48.6KB, pdf)
There were no study group differences in age, sex and care service at death, reflecting effective matching across study sites. Referral from nursing homes was highest (50.6%) in the COVID+ve group, compared with 12.9% and 4.7% in the pre-COVID and COVID-ve groups, respectively (p<0.001). Palliative care consultation rates were similar across study groups but lowest (29.4%) in the COVID+ve group.
Clinical characteristics
Admission comorbidities and symptoms in addition to use of respiratory or circulatory support are summarised in online supplemental table, appendix 3. Atrial fibrillation was less prevalent in the COVID+ve group (15.3%) compared with the pre-COVID (26.5%) and COVID-ve (32.4%) groups (p=0.015). However, dementia and miscellaneous other comorbidities occurred more frequently (41.2% and 77.7%, p=0.032 and 0.018, respectively) in the COVID+ve group compared with the pre-COVID (27.7% and 63.5%, respectively) and COVID-ve groups (25.9% and 60.0%, respectively). In the COVID+ve group compared with other groups, pain occurred less frequently (10.6% vs 29.4% and 28.8%, p=0.002), but breathlessness, (63.5% vs 42.4% and 47.1%, p=0.006), cough (40.0% vs 27.1% and 19.4%, p=0.002) and fever (54.1% vs 9.4% and 13.5%, p<0.001) occurred more frequently. High-flow nasal cannula use was more frequent in the COVID+ve group versus pre-COVID and COVID-ve groups (54.1% vs 37.1% and 28.8%, respectively, p<0.001)
bmjopen-2023-075518supp003.pdf (59.1KB, pdf)
Medication use at end of life
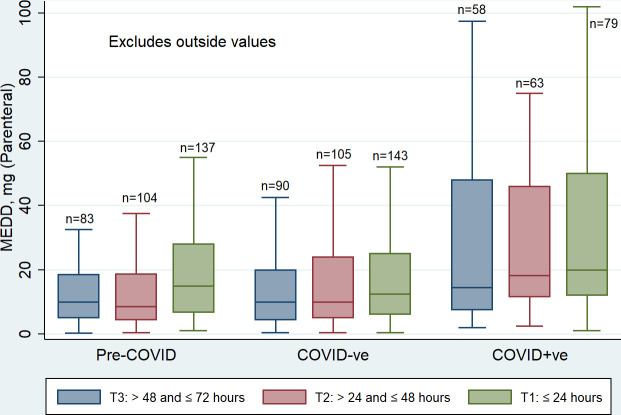
Opioids were prescribed for 92.4%, 91.2% and 95.3% of the pre-COVID, COVID-ve and COVID+ve groups (including those who died <24 hours of admission, respectively. The median and IQR MEDD values for study groups in relation to each 24-hour interval (T3, T2 and T1) in which decedents received an opioid, is presented in figure 1, illustrating a progressive increase according to proximity to death, in both the proportion of decedents receiving opioids and in doses administered. Group comparison of opioid use within the last 72 hours of life is summarised in table 2.
Figure 1.
Median MEDD for consecutive 24-hour periods (T3–T1) within the last 72 hours of life. MEDD, Morphine Equivalent Daily Dose.
Table 2.
Comparative inpatient opioid use within the last 72 hours of life among decedent study groups
| Opioid use in last 72 hours of life | Decedent reference periods and study groups | P value | ||
| November 2019–February 2020 | March 2020–August 2020 (wave 1) | |||
| Pre-COVID group N=163 (%)* |
COVID-ve group N=163 (%)* |
COVID+ve group N=85 (%)* |
||
| Type of opioid administered† | ||||
| Any opioid, n (%) | 145 (89.0) | 146 (89.6) | 81 (95.3) | 0.236 |
| Morphine, n (%) | 63 (38.7) | 65 (39.9) | 40 (47.1) | 0.418 |
| Hydromorphone, n (%) | 92 (56.4) | 93 (57.1) | 52 (61.2) | 0.758 |
| Fentanyl, n (%) | 25 (15.3) | 15 (9.2) | 6 (7.1) | 0.085 |
| Total MEDD‡ for each 24-hour period (T3–T1) within last 72 hours of life§ | ||||
| T3: mg (Q1–Q3) | 10.0 (5.0–18.5) | 10.0 (4.4–20.0) | 14.5 (7.5–48.0) | 0.041 |
| No of decedents: n (%) | 83 (50.9) | 90 (55.2) | 58 (68.2) | 0.032 |
| T2: mg (Q1–Q3) | 8.5 (4.3–18.8) | 10.0 (5.0–24.0) | 18.3 (11.5–46.0) | <0.001 |
| No of decedents: n (%) | 104 (63.8) | 105 (64.4) | 63 (74.1) | 0.220 |
| T1: mg (Q1–Q3) | 15.0 (6.5–29.8) | 12.5 (6.3–25.0) | 20.0 (12.0–50) | 0.011 |
| No of decedents: n (%) | 137 (84.1) | 143 (87.7) | 79 (92.9) | 0.133 |
| T1 MEDD by care service at death | ||||
| Internal Medicine: mg (Q1–Q3) | 12.3 (5.8–24.5) | 10.0 (5.0–20.5) | 14.5 (8.0–26.3) | 0.140 |
| No of decedents: n (subgroup %) | 96/117 (82.1) | 104/119 (87.4) | 56/62 (90.3) | 0.265 |
| Intensive care unit: mg (Q1–Q3) | 25.0 (14.4–49.5) | 23.8 (10.5–45.0) | 52.5 (31.5–80.0) | 0.014 |
| No of decedents: n (row %) | 41/46 (89.1) | 39/44 (88.6) | 23/23 (100) | 0.245 |
| T1 MEDD by hospital site | ||||
| Site 1: mg (Q1–Q3) | 15.0 (9.0–27.5) | 11.3 (5.0–25.0) | 16.5 (10.0–45.0) | 0.199 |
| No of decedents: n (subgroup %) | 55/60 (91.6) | 49/57 (86.0) | 26/31 (83.9) | 0.480 |
| Site 2: mg (Q1–Q3) | 11.0 (5.8–32.5) | 16.8 (8.0–28.4) | 31.7 (12.8–63.8) | 0.019 |
| No of decedents: n (subgroup %) | 32/38 (84.2) | 36/39 (92.3) | 20/20 (100.0) | 0.130 |
| Site 3: mg (Q1–Q3) | 16.5 (8.0–33.8) | 10.5 (6.0–22.5) | 18.0 (9.0–35.0) | 0.105 |
| No of decedents: n (subgroup %) | 50/65 (76.0) | 58/67 (86.6) | 33/34 (97.1) | 0.026 |
| Patient groups for aggregate MEDD summary measures estimation¶ | ||||
| Decedent administered opioid n (%) | 145 (89.0) | 146 (89.6) | 81 (95.3) | 0.236 |
| Internal medicine: n (subgroup %) | 102/117 (87.2) | 105/119 (88.2) | 58 (93.6) | 0.414 |
| Intensive care: n (subgroup %) | 43/46 (93.5) | 41/44 (93.2) | 23/23 (100) | 0.444 |
| Aggregate MEDD measures | ||||
| Maximum MEDD: mg (Q1–Q3) | 16.5 (7.5–30.0) | 15.0 (7.5–30.0) | 21.0 (12.0–54.5) | 0.012 |
| Internal medicine: mg (Q1–Q3) | 13.4 (6.0–27.5) | 11.3 (6.8–22.5) | 15.7 (8.0–30.0) | 0.172 |
| Intensive care: mg (Q1–Q3) | 25.0 (14.4–55.0) | 24 (11.3–54.5) | 59.5 (44.8–120.0) | 0.005 |
| Individual mean MEDD: mg (Q1–Q3) | 13.5 (5.7–21.8) | 10.5 (5.3–23.8) | 16.7 (9.0–36.5) | 0.007 |
| Internal medicine: mg (Q1–Q3) | 10.3 (5.0–17.3) | 9.4 (4.5–15.0) | 13.6 (6.7–24.7) | 0.072 |
| Intensive care: mg (Q1–Q3) | 20.9 (11.5–38.5) | 19.8 (10.0–44.8) | 40.0 (24.9–64.2) | 0.009 |
Bold values were statistically significant
*Column proportions expressed as percentages in parentheses unless otherwise specified.
†Opioid administered to decedents in a minimum of one complete 24-hour admission period within the last 72 hours of life; data were excluded for seven decedents each in the pre-COVID and COVID-ve groups whose admission duration was <24 hours.
‡MEDD: parenteral, mg; summarised as a median (IQR, Q1–Q3) value for each of the three decedent study groups.
§Designation based on hours before death: T3, >48 and ≤72 hours; T2, >24 and ≤48 hours; T1, last 24 hours as an inpatient.
¶Based on exposure to a minimum of one complete inpatient 24-hour admission period (T3, T2 or T1) for opioid dose administration. Aggregate measures are reported as median group values (IQR, Q1–Q3).
MEDD, morphine equivalent daily dose.
Although more COVID+ve group patients (68.2% vs 50.9% and 55.2%, p=0.032) received opioids in the T3 period, there were no other significant study group differences in opioid administration as a binary (yes/no) outcome, specifically in comparisons based on opioid type, T2 or T1 period MEDDs, care service at death, hospital site, or with reference to the 72-hour aggregate summary measures (individual mean and maximum dose). However, the median MEDD in the COVID+ve group at T1 was 20.0 (12.0–50.0) compared with 15.0 (6.5–29.8) and 12.5 (6.3–25.0) in the pre-COVID and COVID-ve groups, respectively (p=0.011). This group difference in MEDD was consistent at each time point (T3-T1) and in relation to 72-hour aggregate summary measures. A site subgroup analysis at T1 revealed higher median MEDD in the COVID+ve group at site 2. An additional subgroup analysis at T1 revealed a higher median MEDD in the COVID+ve group decedents who died in ICU but not in those who died in medicine units/wards; a similar difference was also found in relation to the aggregate measures of opioid administration over the last 72 hours of life. The independent association of variables with MEDD was examined in multivariable logistic regression.
The logistic regression analyses examining the predictors of the T1 MEDD upper quartile (≥30 mg of parenteral morphine) are summarised in table 3.
Table 3.
Logistic regression analyses examining the association of variables with parenteral MEDD≥30 mg (upper quartile) in the last 24 hours of life in those who received opioids (n=359)
| Variables examined | Proportion of patients* (%) | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | ||
| Age of decedent† | … | 0.951 | (0.93 to 0.97) | <0.001 | 0.99 | (0.96 to 1.01) | 0.313 |
| Sex | |||||||
| Female | 31/155 (20.0) | 1 | 1 | ||||
| Male | 64/204 (31.4) | 1.82 | (1.12 to 2.99) | 0.016 | 1.84 | (1.05 to 3.22) | 0.034 |
| Study group | |||||||
| Pre-COVID | 34/137 (24.8) | 1 | 1 | ||||
| COVID-ve | 30/143 (21.0) | 0.804 | (0.46 to 1.41) | 0.445 | 0.95 | (0.51 to 1.76) | 0.866 |
| COVID+ve | 31/79 (39.2) | 1.96 | (1.08 to 3.55) | 0.027 | 2.62 | (1.29 to 5.32) | 0.008 |
| Hospital site | |||||||
| Site 1 | 32/130 (24.6) | 1 | 1 | ||||
| Site 2 | 27/88 (30.7) | 1.36 | (0.74 to 2.48) | 0.323 | 0.83 | (0.40 to 1.72) | 0.617 |
| Site 3 | 36/141 (25.5) | 1.05 | (0.61 to 1.82) | 0.862 | 0.51 | (0.25 to 1.05) | 0.067 |
| Care service at death | |||||||
| Medicine | 45/256 (17.6) | 1 | 1 | ||||
| ICU | 50/103 (48.5) | 4.42 | (2.68 to 7.31) | <0.001 | 5.14 | (2.47 to 10.70) | <0.001 |
| High-flow nasal cannula | |||||||
| No | 46/219 (21.0) | 1 | |||||
| Yes | 49/140 (35.0) | 2.03 | (1.26 to 3.26) | 0.004 | 1.93 | (1.05 to 3.52) | 0.033 |
| Palliative care consult | |||||||
| No | 61/211 (28.9) | 1 | 1 | ||||
| Consult completed | 34/148 (23.0) | 0.733 | (0.45 to 1.19) | 0.210 | 1.51 | (0.80 to 2.86) | 0.205 |
| Admission assessment‡ | |||||||
| Cognitive status | |||||||
| Not impaired | 71/229 (31.0) | 1 | 1 | ||||
| Impaired | 24/130 (18.5) | 0.504 | (0.30 to 0.85) | 0.010 | 0.85 | 0.46 to 1.57 | 0.606 |
| Documented pain | |||||||
| No | 69/264 (26.0) | 1 | 1 | ||||
| Yes | 26/95 (27.4) | 1.07 | (0.63 to 1.81) | 0.815 | 1.48 | (0.80 to 2.74) | 0.209 |
| Active cancer | |||||||
| No | 67/275 (24.4) | 1 | 1 | ||||
| Yes | 28/84 (33.3) | 1.55 | (0.91 to 2.64) | 0.104 | 1.68 | (0.88 to 3.18) | 0.114 |
| Chronic kidney disease | |||||||
| No | 75/283 (26.5) | 1 | |||||
| Yes | 20/76 (26.3) | 0.991 | (0.56 to 1.76) | 0.974 | |||
| Agitation | |||||||
| No | 89/330 (27.0) | 1 | |||||
| Yes | 6/29 (20.7) | 0.706 | (0.28 to 1.79) | 0.464 | |||
Bold values were statistically significant
*Proportion of patients in upper quartile MEDD (≥30 mg of parenteral morphine) for T1 period (last 24 hours of life).
†Treated as a continuous variable or covariate.
‡Documented on admission assessment.
ICU, intensive care unit; MEDD, morphine equivalent daily dose.
In the unadjusted analyses, both older age and cognitive impairment were statistically significant negative predictors of the upper quartile MEDD, whereas male sex, COVID+ve group membership, death in ICU and use of high-flow nasal cannula for oxygen delivery were positive predictors. In the multivariable model, only male sex, COVID+ve group membership, death in ICU and use of high-flow nasal cannula remained statistically significant, all as positive predictors with aORs of 1.84 (95% CI 1.05 to 3.22), 2.62 (95% CI 1.29 to 5.3), 5.14 (95% CI 2.47 to 10.7) and 1.93 (95% CI 1.05 to 3.52), respectively. Potential variable interactions among COVID-19 study group status, age, sex and care service at death were tested in the model, and the interaction terms were not statistically significant.
Comparative non-opioid medication doses (mg) administered within the last 72 hours of life for the study groups are summarised in table 4.
Table 4.
Comparative inpatient use of non-opioid end-of-life medications within the last 72 hours of life among decedent study groups
| Non-opioid medications administered in the last 72 hours of life* | Decedent reference periods and study groups | P value | ||
| November 2019–February 2020 | March 2020–August 2020 (wave 1) | |||
| Pre-COVID group† N=163 (%) |
COVID-ve group† N=163 (%) |
COVID+ve group N=85 (%) |
||
| Antisecretory medications | ||||
| Glycopyrrolate, n (%) | 36 (22.1) | 37 (22.7) | 12 (14.1) | 0.243 |
| Mean 24-hour dose, mg‡ | 0.5 (0.4–0.9) | 0.6 (0.4–1.2) | 0.4 (0.4–0.6) | 0.570 |
| Scopolamine, n (%) | 20 (12.3) | 21 (12.9) | 14 (16.5) | 0.635 |
| Mean 24-hour dose, mg‡ | 0.4 (0.4–0.9) | 0.4 (0.4–0.8) | 0.5 (0.4–1.0) | 0.909 |
| Antipsychotic medications | ||||
| Haloperidol, n (%) | 32 (19.6) | 25 (15.3) | 10 (11.8) | 0.257 |
| Mean 24-hour dose, mg‡ | 1.0 (0.5–1.3) | 1.0 (0.5–1.5) | 1.4 (0.7–4.5) | 0.656 |
| Methotrimeprazine, n (%) | 37 (22.7) | 40 (24.5) | 26 (30.6) | 0.389 |
| Mean 24-hour dose, mg‡ | 10 (6.3–22.5) | 11.7 (6.9–24.4) | 11.3 (5.0–25.0) | 0.947 |
| Benzodiazepines | ||||
| Lorazepam, n (%) | 19 (11.7) | 17 (10.4) | 7 (8.2) | 0.705 |
| Mean 24-hour dose, mg‡ | 1.0 (0.5–1.5) | 1.5 (1.0–2.3) | 3.7 (1.5–25.0) | 0.017 |
| Midazolam, n (%) | 96 (58.9) | 100 (61.4) | 57 (67.1) | 0.454 |
| Mean 24-hour dose, mg‡ | 3.7 (1.5–12.5) | 3.0 (1.5–11.3) | 5.7 (2.0–19.0) | 0.255 |
| Maximum 24-hour dose, mg‡ | 4.3 (2.0–13.5) | 4.0 (1.7–13.0) | 7.0 (2.0–22.0) | 0.199 |
| Other sedating medications | ||||
| Phenobarbitone, n (%) | 4 (2.5) | 6 (3.7) | 5 (5.9) | 0.393 |
| Mean 24-hour dose, mg‡ | 150.0 (90.0–210.0) | 127.5 (90.0–140.0) | 150.0 (75.0–180) | 0.811 |
| Propofol administered, n (%) | 21 (12.9) | 28 (17.2) | 13 (15.3) | 0.555 |
| Mean 24-hour dose, mg‡ | 1078.5 (692.5–1984.0) |
1329.2 (634.0–2811.6) |
1887.5 (1337.5–5527.3) |
0.080 |
| Maximum 24-hour dose, mg‡ |
1444.8 (692.5–2207.0) |
1624.4 (851.0–3491.5) |
2665.6 (2119.4–6304.0) |
0.033 |
Bold values were statistically significant
*Based on exposure to a minimum of at least one full inpatient 24-hour period for mean 24-hour dose determination within the last 72 hours of life.
†Data were excluded for seven decedents in each of the original pre-COVID and COVID-ve groups due to admission duration <24 hours.
‡Individual mean 24-hour doses are summarised for the study group as a median (IQR) value for each of the three study groups.
Although both mean and maximum 24-hour doses of midazolam were higher in the COVID+ve group, the differences were not statistically different. The median lorazepam COVID+ve group dose, 3.7 (1.5–25.0) was higher than that of the pre-COVID and COVID-ve groups, 1.0 (0.5–1.5) and 1.5 (1.0–2.3), respectively (p=017). Similarly, the median of the maximum propofol dose, 2665.6 (2119.4–6304.0) was higher than that of the pre-COVID and COVID-ve groups, 1444.8 (692.5–2207.0) and 1624.4 (851.0–3491.5), respectively (p=0.033).
Discussion
Study findings and putative explanations
Our study found that COVID+ve decedents received significantly higher opioid doses than matched prepandemic or intrapandemic control patients. This finding was moderately robust: it was consistent in each 24-hour time period within the last 72 hours of life, and further bolstered by finding that dying of COVID-19 was independently associated (aOR=2.6) with a parenteral MEDD≥30 mg in the last 24 hours of life. COVID+ve decedents had significantly higher maximum 24-hour propofol use in ICU compared with control group decedents. Also, higher lorazepam and midazolam doses were used in the COVID+ve group than either of the other groups; the difference was only statistically significant in relation to lorazepam. Collectively, these findings regarding opioid and sedative use support our study hypothesis that the requirement for these medications is higher in hospitalised patients dying of COVID-19 infection. In subgroup analyses, COVID+ve ICU decedents had significantly higher opioid use than ICU decedents in either of the control groups, which was evident in the last 24 hours (T1) and over the last 72 hours of life, suggesting that dying in ICU with COVID-19 infection is particularly associated with increased opioid and propofol requirements. These findings warrant a symptom profile evaluation of those dying of COVID-19.
Although our study patients’ comfort in the last 72 hours of life was regularly assessed and documented, there was no formal standardised recording of symptom intensity across sites. For symptom profile comparisons, we used the admission documentation of symptoms, which fell within the last 72 hours of life for approximately 20% of the study sample. The COVID+ve group had significantly higher admission prevalence of breathlessness, cough and fever, and used high-flow nasal cannula oxygen support more frequently during admission. Previous studies have found that breathlessness is a major symptom in patients dying with COVID-19 infection.15 16 31 38–40 Although myalgic pain is reported in those dying of COVID-19 infection,15 among our three study groups, pain was least frequent in COVID+ve decedents at admission, but higher prevalence could have occurred closer to death. High-flow nasal cannula use was independently associated (aOR=1.9) with a parenteral MEDD≥30 mg in the last 24 hours of life. Collectively, our results suggest that respiratory distress mediated higher opioid use in the COVID+ve group, particularly in ICU decedents. Agitation and delirium are reported in patients dying of COVID-19 infection.14 18 31 33 40 Although the admission prevalence of agitation was largely similar across our groups, subsequent group differences in agitation level could have arisen nearer to death. Furthermore, COVID+ve group decedents had a higher admission prevalence of dementia and other comorbidity burden, both risk factors for delirium.41 The higher lorazepam and maximum 24-hour propofol doses in our COVID+ve group were possibly due to COVID-19 related respiratory distress in addition to potential contributions of cognitive dysfunction with agitation, and greater comorbidity-related distress.
Logistical issues associated with the COVID-19 pandemic, particularly the increased healthcare demands that stretched acute care services to and often beyond their limits, also warrant consideration in interpreting our study findings. Fewer COVID+ve group decedents (16.5%) were intubated compared with pre-COVID (26.5%) or COVID-ve (25.3%) decedents, raising the possibility that greater emphasis was placed on the medication management of dyspnoea with opioids and sedatives for some patients rather than mechanical ventilation per se. It is also possible that more rigorous and prompt assessment of those dying of COVID-19 could have been impeded to some extent by isolation requirements and the need for staff to don burdensome personal protective equipment; this could have resulted in greater reliance on opioids and sedatives for symptom management.
Study findings in the context of published data
Although atrial fibrillation is a risk factor for mortality in high-risk COVID-19 patients,42 it was least prevalent in our COVID+ve study group. Meanwhile, the higher COVID+ve group admission prevalence of cognitive impairment and other comorbidities were largely consistent with published data on COVID-19 risk factors.11 17 Similarly, the higher prevalence of respiratory symptoms and fever is consistent with reported end-of-life prevalence in COVID-19 deaths.12 14 17 Literature comparison of palliative medication use in patients dying due to COVID-19 infection is limited by paucity of data, particularly on ICU deaths, and further compromised by differences in type of aggregate dose measures reported, time reference, care setting, regional medication formularies and in the separate reporting of pro re nata (PRN) or ‘as-needed’ medication use in addition to continuous infusional use.28 We reported the total daily medication use which included regularly scheduled and PRN doses, or solely PRN doses in the absence of scheduled dosing. Although antisecretory and antipsychotic medication use was similar across all of our study groups, and comparable to published estimates in COVID-19 deaths,28 30 31 our findings regarding opioid and benzodiazepine use warrant more detailed evaluation in the context of published data.
A systematic review of symptom management in COVID-19-related deaths, which excluded ICU deaths,28 concluded that although a higher proportion of those dying with COVID-19 infection required continuous administration of opioid or midazolam than previously reported in pre-COVID-19 palliative care, doses were relatively low (median of 10–15 mg of parenteral morphine, and 10 mg of midazolam, in the last 24 hours of life, in an aggregate dose summary of 5 of the studies) and in keeping with published guidelines.24 A study of COVID-19 deaths in a hospital palliative care unit in New York reported a median parenteral MEDD (range) of 48 (24–144) mg in the last days of life.33 A Belgian study of hospitalised COVID-19 decedents, excluded ICU deaths, and reported a mean parenteral MEDD of 31.3 (range 2–120) mg, and mean midazolam dose of 20.4 (range 1–100) mg in the last 24 hours of life.32 An Australian study of hospitalised COVID-19 decedents, including 9 (4%) who died in ICU, reported a median (Q1–Q3) oral MEDD of 45 (22.5–75.0) in the last day before death.31 Our study’s higher MEDD findings in the COVID+ve group were comparable to this study; the inclusion of ICU decedents with possibly higher levels of symptom distress in our study could explain the higher opioid and sedative doses than those reported in the systematic review by Heath et al.28 The progressive MEDD increase in the COVID+ve group over the last 72 hours is consistent with a longitudinal study reporting a doubling of median daily opioid use in the last 7 days of life in COVID-19 decedents.31 Our finding of an independent association between male sex and higher opioid dosing is difficult to explain, as larger prepandemic studies have not reported a sex difference in relation to opioid dosing.43 44 Although male sex is a recognised mortality-related risk factor in COVID-19 infection,11 45 a statistically significant interaction between sex and study group status was not detected in the model.
Although 67.1% of the COVID+ve group received midazolam in the last 72 hours of life, the daily midazolam dose estimates in this period were lower than the 10 mg estimate reported in a systematic review.28 Although palliative care involvement was similar across our study groups, the completion of a consult in only 29.4% of the COVID+ve group is below the 39%–51% range reported in other studies of COVID-19 decedents,3 31 and possibly impacted the prescribing patterns of some medications used for end-of-life symptom control.
Study implications and future research
In addition to informing end-of-life guidelines on medication use for symptom management in COVID-19 infection and in future pandemics, our study findings warrant further research, particularly regarding the use of opioids and sedatives in the ICU setting. Moreover, regarding end-of-life comfort assessment, our study highlights the need for standardised symptom assessment measures such as the palliative version of the Richmond Agitation-Sedation Scale,46 which can be used to evaluate medication efficacy and audit quality of care. Specialist palliative care involvement in end-of-life care of hospitalised individuals warrants further study both in relation to predictors and outcomes.
Study strengths and limitations
Our study’s decedent cohort was representative of the source population in all adult acute care hospitals in a large urban region; using matched control groups from within and prior to the COVID-19 pandemic facilitated valid and unique comparisons, which generated some robust findings, particularly regarding opioid use. The retrospective design and use of admission symptom assessment and comorbidity data without similar data, including medication efficacy and side effects, from within the more immediate end-of-life period are obvious limitations. The role of non-pharmacological interventions was not examined. Although rigorous training and accuracy checks were conducted regarding data abstraction, misclassification bias cannot be excluded, and absence of abstractor blinding to the study hypothesis is a potential source of bias. This study was performed during wave 1 of the pandemic, and both symptom burden and medication requirements for symptom control could have changed to some extent with subsequent waves. The generalisability of our study findings is largely limited to end-of-life care for hospitalised decedents, whereas many of the COVID-19 pandemic related deaths in wave 1 of the pandemic occurred in nursing homes.
Conclusions
Overall, our study evidence suggests that in addition to the association of male sex with higher end-of-life opioid requirements, patients dying of COVID-19 infection required higher daily opioid and lorazepam doses than those dying of other causes both before and during the COVID-19 pandemic. Furthermore, patients who died of COVID-19 infection in ICU required higher maximum 24-hour propofol doses than those who died in ICU without COVID-19 infection. Increased breathlessness and agitation due to COVID-19 and higher underlying comorbidity levels may require higher doses of opioids and sedatives for symptom control. These findings warrant consideration in the context of managing ongoing life threatening COVID-19 infection and in anticipatory preparation for future respiratory virus pandemics.
Supplementary Material
Acknowledgments
The authors are grateful to Dong Vo, Ottawa Methods Centre’s Data Management Services and Ottawa Hospital Research Institute for the creation of an electronic study database. We gratefully acknowledge the input of representatives from Bereaved Families of Ontario, Canadian Virtual Hospice and the Champlain Hospice Palliative Care Program. PGL, LC, VG, RM, GW, AB, PE, SHB, PT and JD receive Academic Protected Time Awards from the Department of Medicine, University of Ottawa, Ottawa, Canada.
Footnotes
Twitter: @PeterLawlor20
Contributors: JD conceptualised the project and designed the study with assistance from PL, HP, LC, VG, RM, GW, AB, KW, JL, CW, DB, PE, ID, KB, CD, AI, SHB, SI, PT and BV-W. The study site leads, HP, VG and LC, co-ordinated ethics applications along with PL, JL and DB. Data were abstracted by PL, HP, SRA, EB, LC, RM, GW, AB, KA-M, KW, PE, ID, KB and CD. Data verification was coordinated by PL with the assistance of HP, SRA, EB, LC, RM, GW, AB, PE and KB. Statistical analyses were performed by PL with support from LC and CW. All authors, including MK, CN, BH and KA, assisted with data interpretation. The original version of the manuscript was drafted by PL and LC and critically reviewed by all authors. All authors approved the final manuscript as submitted. PL acts as guarantor and takes full responsibility for the study.
Funding: This work has been funded in part by a grant from the University of Ottawa Faculty of Medicine COVID-19 Pandemic Response Funding Program, and in part by a contribution from Health Canada, Health Care Policy and Strategies Program.
Disclaimer: The views expressed herein do not necessarily represent the views of Health Canada nor the University of Ottawa.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and each hospital’s research ethics board (REB) approved the study: Ottawa Health Science Network-REB (20200653-01H, 18 December 2020); Montfort REB (20-21-10-032, 2 December 2020) and Queensway Carleton Hospital REB (20-06, 1 December 2020). This is a retrospective study of hospitalised decedents.
References
- 1.World health Organisation. 2023. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2023
- 2.Wang H, Paulson KR, Pease SA, et al. COVID-19 excess mortality collaborators. estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022;399:1513–36. 10.1016/S0140-6736(21)02796-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy T, Seaton RA, McKeown A, et al. Hospital specialist palliative care team influence on end-of-life care in Coronavirus disease 2019? A retrospective observational cohort study. Palliat Med Rep 2022;3:235–43. 10.1089/pmr.2022.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wentlandt K, Wolofsky KT, Weiss A, et al. Identifying barriers and Facilitators to palliative care integration in the management of hospitalized patients with COVID-19: A qualitative study. Palliat Med 2022;36:945–54. 10.1177/02692163221087162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arya A, Buchman S, Gagnon B, et al. Pandemic palliative care: beyond ventilators and saving lives. CMAJ 2020;192:E400–4. 10.1503/cmaj.200465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mottiar M, Hendin A, Fischer L, et al. End-of-life care in patients with a highly transmissible respiratory virus: implications for COVID-19. Can J Anaesth 2020;67:1417–23. 10.1007/s12630-020-01699-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting R, Edmonds P, Higginson IJ. n.d. Palliative care for patients with severe COVID-19 [clinical research Ed) 2020;370:M2710]. BMJ:m2710. 10.1136/bmj.m2710 [DOI] [PubMed] [Google Scholar]
- 8.Muhsen K, Maimon N, Mizrahi AY, et al. Association of BNT162b2 vaccine third dose receipt with incidence of SARS-Cov-2 infection, COVID-19-related hospitalization, and death among residents of long-term care facilities. JAMA Netw Open 2022;5:e2219940. 10.1001/jamanetworkopen.2022.19940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal U, Bedston S, McCowan C, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in. Lancet 2022;400:1305–20. 10.1016/S0140-6736(22)01656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of COVID-19 related death and hospital admission in adults after COVID-19 vaccination: national prospective cohort study. BMJ 2021;374:n2244. 10.1136/bmj.n2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty AB, Harrison EM, Green CA, et al. n.d. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical Characterisation protocol: prospective observational cohort study [BMJ (Clinical research ed) 2020;369:m1985]. BMJ:m1985. 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy S, Archambault PM, Atique A, et al. Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study. CMAJ Open 2021;9:E181–8. 10.9778/cmajo.20200250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrilli CM, Jones SA, Yang J, et al. n.d. Factors associated with hospital admission and critical illness among 5279 people with Coronavirus disease 2019 in New York city: prospective cohort study [BMJ (Clinical research ed) 2020;369:m1966]. BMJ:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hetherington L, Johnston B, Kotronoulas G, et al. COVID-19 and hospital palliative care - A service evaluation exploring the symptoms and outcomes of 186 patients and the impact of the pandemic on specialist hospital palliative care. Palliat Med 2020;34:1256–62. 10.1177/0269216320949786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeley P, Buchanan D, Carolan C, et al. Symptom burden and clinical profile of COVID-19 deaths: a rapid systematic review and evidence summary. BMJ Support Palliat Care 2020;10:381–4. 10.1136/bmjspcare-2020-002368 [DOI] [PubMed] [Google Scholar]
- 16.Martinsson L, Bergström J, Hedman C, et al. Symptoms, symptom relief and support in COVID-19 patients dying in hospitals during the first pandemic wave. BMC Palliat Care 2021;20:102. 10.1186/s12904-021-00785-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. The Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcez FB, Aliberti MJR, Poco PCE, et al. Delirium and adverse outcomes in hospitalized patients with COVID-19. J Am Geriatr Soc 2020;68:2440–6. 10.1111/jgs.16803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etkind SN, Bone AE, Lovell N, et al. The role and response of palliative care and Hospice services in epidemics and Pandemics: A rapid review to inform practice during the COVID-19 pandemic. J Pain Symptom Manage 2020;60:e31–40. 10.1016/j.jpainsymman.2020.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oluyase AO, Bajwah S, Sleeman KE, et al. Symptom management in people dying with COVID-19: multinational observational study. BMJ Support Palliat Care 2022;12:439–47. 10.1136/spcare-2022-003799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowman BA, Back AL, Esch AE, et al. Crisis symptom management and patient communication protocols are important tools for all Clinicians responding to COVID-19. J Pain Symptom Manage 2020;60:e98–100. 10.1016/j.jpainsymman.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.deLima Thomas J, Leiter RE, Abrahm JL, et al. Development of a palliative care Toolkit for the COVID-19 pandemic. J Pain Symptom Manage 2020;60:e22–5. 10.1016/j.jpainsymman.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheyne S, Lindley RI, Smallwood N, et al. Care of older people and people requiring palliative care with COVID-19: guidance from the Australian national COVID-19 clinical evidence Taskforce. Med J Aust 2022;216:203–8. 10.5694/mja2.51353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence (NICE) in collaboration with NHS England and NHS Improvement . Managing COVID-19 symptoms (including at the end of life) in the community: summary of NICE guidelines. BMJ 2020;369:m1461. 10.1136/bmj.m1461 [DOI] [PubMed] [Google Scholar]
- 25.Luz M, Brandão Barreto B, de Castro REV, et al. Practices in sedation, analgesia, mobilization, delirium, and sleep deprivation in adult intensive care units (SAMDS-ICU): an international survey before and during the COVID-19 pandemic. Ann Intensive Care 2022;12:9. 10.1186/s13613-022-00985-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim J, Goh WY, Wiryasaputra L, et al. Use of Phenobarbitone for palliative sedation in Dyspneic crises due to COVID-19 pneumonia - A case series. J Pain Palliat Care Pharmacother 2022;36:242–8. 10.1080/15360288.2022.2113596 [DOI] [PubMed] [Google Scholar]
- 27.Andreas M, Piechotta V, Skoetz N, et al. Interventions for palliative symptom control in COVID-19 patients. Cochrane Database Syst Rev 2021;8:CD015061. 10.1002/14651858.CD015061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heath L, Carey M, Lowney AC, et al. Pharmacological strategies used to manage symptoms of patients dying of COVID-19: A rapid systematic review. Palliat Med 2021;35:1099–107. 10.1177/02692163211013255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory Breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev 2016;3:CD011008. 10.1002/14651858.CD011008.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson T, Hobson K, Clare H, et al. End-of-life care in COVID-19: an audit of pharmacological management in hospital Inpatients. Palliat Med 2020;34:1235–40. 10.1177/0269216320935361 [DOI] [PubMed] [Google Scholar]
- 31.Wong AK, Philip J, Wawryk O, et al. A multi-centre COVID-19 study examining symptoms and medication use in the final week of life. J Pain Symptom Manage 2022;64:e139–47. 10.1016/j.jpainsymman.2022.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssens WH, Van Den Noortgate NJ, Piers RD. Erratum to: terminal care in oldest old dying from COVID-19 in the acute hospital: A multicenter study describing pharmacological treatment in the last 24 H. Z Gerontol Geriatr 2022;55:135. 10.1007/s00391-022-02052-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H, Lee J, Meyer BJ, et al. Characteristics and palliative care needs of COVID-19 patients receiving comfort-directed care. J Am Geriatr Soc 2020;68:1162–4. 10.1111/jgs.16507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Downar J, Parsons HA, Cohen L, et al. Bereavement outcomes in family members of those who died in acute care hospitals before and during the first wave of COVID-19: A cohort study. Palliat Med 2022;36:1305–12. 10.1177/02692163221109711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawlor P, Parsons H, Adeli SR, et al. Comparative end-of-life communication and support in hospitalised Decedents before and during the COVID-19 pandemic: a retrospective regional cohort study in Ottawa, Canada. BMJ Open 2022;12:e062937. 10.1136/bmjopen-2022-062937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira J, Lawlor P, Vigano A, et al. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage 2001;22:672–87. 10.1016/s0885-3924(01)00294-9 [DOI] [PubMed] [Google Scholar]
- 38.Alderman B, Webber K, Davies A. An audit of end-of-life symptom control in patients with Corona virus disease 2019 (COVID-19) dying in a hospital in the United Kingdom. Palliat Med 2020;34:1249–55. 10.1177/0269216320947312 [DOI] [PubMed] [Google Scholar]
- 39.Chidiac C, Feuer D, Flatley M, et al. The need for early referral to palliative care especially for black, Asian and minority ethnic groups in a COVID-19 pandemic: findings from a service evaluation. Palliat Med 2020;34:1241–8. 10.1177/0269216320946688 [DOI] [PubMed] [Google Scholar]
- 40.Lovell N, Maddocks M, Etkind SN, et al. Characteristics, symptom management, and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage 2020;60:e77–81. 10.1016/j.jpainsymman.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911–22. 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuin M, Rigatelli G, Bilato C, et al. Pre-existing atrial fibrillation is associated with increased mortality in COVID-19 patients. J Interv Card Electrophysiol 2021;62:231–8. 10.1007/s10840-021-00992-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall S, Gallagher RM, Gracely E, et al. The terminal cancer patient: effects of age, gender, and primary tumor site on opioid dose. Pain Medicine 2003;4:125–34. 10.1046/j.1526-4637.2003.03020.x [DOI] [PubMed] [Google Scholar]
- 44.Yennurajalingam S, Lu Z, Reddy SK, et al. Patterns of opioid prescription, use, and costs among patients with advanced cancer and inpatient palliative care between 2008 and 2014. J Oncol Pract 2019;15:e74–83. 10.1200/JOP.18.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 2021;21:855. 10.1186/s12879-021-06536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bush SH, Grassau PA, Yarmo MN, et al. The Richmond agitation-sedation scale modified for palliative care Inpatients (RASS-PAL): a pilot study exploring validity and feasibility in clinical practice. BMC Palliat Care 2014;13:17. 10.1186/1472-684X-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075518supp001.pdf (41.6KB, pdf)
bmjopen-2023-075518supp002.pdf (48.6KB, pdf)
bmjopen-2023-075518supp003.pdf (59.1KB, pdf)
Data Availability Statement
No data are available.