Abstract
Introduction
Incorrect penicillin allergy records are recognised as an important barrier to the safe treatment of infection and affect an estimated 2.7 million people in England. Penicillin allergy records are associated with worse health outcome and antimicrobial resistance. The ALlergy AntiBiotics And Microbial resistAnce (ALABAMA) trial aims to determine if an intervention package, centred around a penicillin allergy assessment pathway (PAAP) initiated in primary care, is safe and effective in improving patient health outcomes and antibiotic prescribing.
Methods and analysis
The ALABAMA trial is a multicentre, parallel-arm, open-label, randomised pragmatic trial with a nested pilot study. Adults (≥18 years) with a penicillin allergy record and who have received antibiotics in the previous 24 months will be eligible for participation. Between 1592 and 2090 participants will be recruited from participating National Health Service general practices in England. Participants will be randomised to either usual care or intervention to undergo a pre-emptive PAAP using a 1:1 allocation ratio. The primary outcome measure is the percentage of treatment response failures within 28 days of an index prescription. 2090 and 1592 participants are estimated to provide 90% and 80% power, respectively, to detect a clinically important absolute difference of 7.9% in primary outcome at 1 year between groups. The trial includes a mixed-methods process evaluation and cost-effectiveness evaluation.
Ethics and dissemination
This trial has been approved by London Bridge Research Ethics Committee (ref: 19/LO/0176). It will be conducted in compliance with Good Clinical Practice guidelines according to the Declaration of Helsinki. Informed consent will be obtained from all subjects involved in the study. The primary trial results will be submitted for publication to an international, peer-reviewed journal.
Trial registration
Keywords: immunology, infectious diseases, primary care, clinical trial
Strengths and limitations of this study.
This study is a randomised controlled trial of penicillin allergy assessment initiated in primary care assessing patient health outcomes.
The multicentre design recruiting patients from more than 50 primary care sites from across England will support external validity and National Health Service (NHS) implementation.
Penicillin allergy assessment pathway offers efficient, and economical, one-step testing over current ‘gold standard’ testing pathways.
ALlergy AntiBiotics And Microbial resistAnce is a complex intervention with an integrated mixed-methods process evaluation to guide future NHS implementation.
By necessity, the trial is open label and delabelling of participants in the intervention arm may influence clinician behaviour across all participants.
Introduction
A record of penicillin allergy (PEN allergy) in a patient’s health record has a marked effect on antibiotic prescribin; both an increase in total use and a radical change in the agents selected.1–5 In primary care patients, the presence of a PEN allergy record has been associated with higher rates of treatment failure, higher mortality, Clostridioides difficile infection and antimicrobial resistance (AMR) in the form of methicillin resistant (also known as meticillin-resistant) Staphylococcus aureus.4 5 PEN allergy records are common and arise either because of genuine allergy symptoms during a course of treatment or, more often, because side effects and symptoms related to the index infection are mislabelled as allergies. In the UK, PEN allergy prevalence is approximately 6%.5 However, fewer than one in 10 patients with a PEN allergy record are truly allergic after formal assessment.6–8 Consequently, an estimated 2.7 million people in the UK are potentially prevented from accessing highly effective penicillin due to an incorrect PEN allergy record.5
Macrolide, tetracycline, cephalosporin, quinolone and clindamycin prescribing are all more common in primary care patients with a record of PEN allergy compared with those without, and antibiotic prescriptions are almost twice as frequent in patients with a PEN allergy record.4 5 Evidence from USA and elsewhere suggests that antibiotic allergies affect health outcomes, and increase mortality, length of stay and costs.5 8 PEN allergy records are also associated with AMR; evidence from the UK and USA suggests that patients with a penicillin allergy record are more likely to acquire multidrug resistant bacteria, including methicillin-resistant Staphylococcus aureus (MRSA).9–11 Preliminary investigations of 2.3 million adult primary care patients found that a lack of response to treatment and MRSA were significantly more common in patients with a PEN allergy record.5 The 2019 WHO AWaRe classification groups antibiotics into three stewardship categories: ‘Access, Watch and Reserve’, and aims to promote use of Access antibiotics in order to combat AMR.12 Patients with PEN allergy are more likely to be prescribed antibiotics belonging to the Watch and Reserve groups which have a higher propensity to drive AMR.13
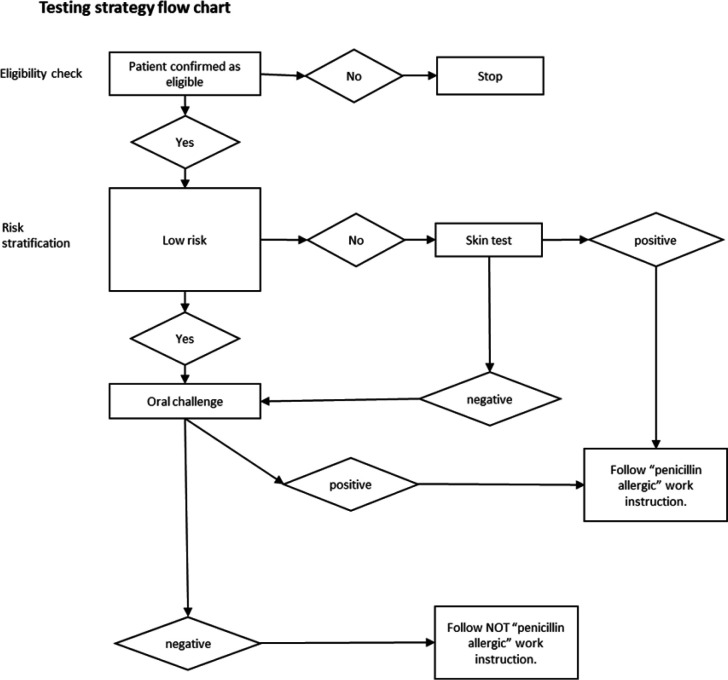
The gold standard test with which to establish tolerance to penicillins is a drug provocation test (which includes oral challenge testing), but previous UK and US guidelines have advised that patients should first be skin tested, using prick or intradermal tests, or both.14 15 The latest US guidelines now recommends for ‘low risk’ historical penicillin allergic patients, direct oral challenge without preceding skin testing.16 Assessment of patients with PEN allergy in specialist clinics is provided within the National Health Service (NHS) and is often performed over at least two clinic visits; the first, to undertake history and perform skin testing; the second to assess reactions and undertake a penicillin oral challenge test, followed by communication of results. Currently, most patients who are eligible to undergo PEN allergy assessment are not offered the service because of a lack of testing capacity.17 One-stop allergy testing offers the potential to improve allergy testing capacity. This currently differs from UK standard and European guidelines in that it offers patients who have been assessed as ‘low risk’ of true allergy an abbreviated test consisting of direct oral challenge, i.e. with no preceding skin tests, and is consistent with more recent guidelines for non-allergists.18 The direct oral challenge approach is already used routinely for children in the UK and several studies have demonstrated safety and efficacy in adults. A recent systematic review has found that direct oral challenge testing by non-allergists is safe and reported an incidence of 1% (95% CI, 0% to 2%) of immediate or delayed reactions in a pooled analysis of 69 studies.19 Patients whose histories are not clearly low risk still need to undergo skin testing, and only proceed to oral challenge if this is negative.
The ALlergy AntiBiotics And Microbial resistance (ALABAMA) trial (full title: penicillin allergy status and its effect on antibiotic prescribing, patient outcomes and AMR) will evaluate participants randomised to either usual care or to receive ‘Penicillin Allergy Assessment Pathway’ (PAAP). PAAP is a complex intervention, incorporating one-stop allergy testing and appropriate delabelling of electronic health records. It will evaluate if PAAP is safe and effective in improving patient health outcomes, influencing antibiotic prescribing and supporting healthcare implementation. ALABAMA is the first randomised controlled trial to our knowledge that looks at adult PEN allergy testing and delabelling with a primary health outcome.
Methods and analysis
Study design
ALABAMA is a multicentre, two parallel-arm, open-label, individually randomised pragmatic trial with a nested pilot study and embedded process evaluation and cost-effectiveness evaluation. The protocol for ALABAMA was developed according to the Standard Protocol Items Recommendations for Interventional Trials guidelines.20 A nested pilot was conducted from December 2018 to July 2020 to determine the safety, feasibility, acceptability and practicality of the ALABAMA trial. This included a ‘stop/go’ assessment criteria which was based on feasibility, recruitment and safety.
The main ALABAMA trial evaluates a complex intervention, designed according to the Medical Research Council guidelines.21 The complex intervention is collectively referred to as the ‘PAAP’. This comprises: (1) an efficient direct referral for a ‘one-stop’ single appointment for an allergy assessment and testing; (2) appropriate guidance for clinicians to refer patients for PEN allergy testing and instruction on how to delabel, that is, update allergy status in participants’ electronic health records appropriately and (3) information for participants to encourage attendance for testing and information pretesting to distinguish side effects (eg, diarrhoea) from true allergic reactions. The development of the physician and participant behavioural intervention component is reported elsewhere.22
Enrolment started at the first general practice (GP) site as part of the feasibility study in October 2019 and recruitment is expected to finalise in 2023.
Participants and eligibility
Between 1592 and 2090 participants will be recruited from participating NHS GPs in England. The inclusion and exclusion criteria are described in table 1. Potential participants who meet the eligibility criteria will be identified during a search of electronic health records at their GP. The electronic search criteria have been developed centrally by the research team in partnership with The Phoenix Partnership (TPP), healthcare technology company, and made available for running locally on SystmOne (an electronic health record system used in primary care that was developed by TPP), thus participating GPs must be using SystmOne. Potentially eligible patients will then be sent an invitation letter.
Table 1.
Inclusion and exclusion criteria for the ALABAMA trial
| Inclusion | Exclusion |
|
|
Note 1, patients with a penicillin allergy record and a recent penicillin prescription would still be eligible because their allergy status will need assessment and records correcting if necessary.
Note 2, patients who have been formally tested for penicillin allergy in the past and been found not to be penicillin allergic but still have a medical record indicating a penicillin allerg are eligible for the trial.
Note 3, Patients who are currently taking medicines with antihistamine properties that cannot be temporarily withheld, or patients with isolated dermographism, may still be eligible to take part but will need to be discussed with the research team prior to consent.
GP, general practice.
Patients interested in taking part will return an expression of interest form to the trial team by post, phone or email or by following a link to add their details to an online secure database. They will then be telephoned and booked into either a face to face or telephone appointment with their GP (or a delegated member of staff) at a time that is convenient to them. The GP, and delegates, will have received full protocol training and the GP will take on the role of Principal Investigator at site. The GP, or a delegated member of staff, will confirm the patient’s eligibility and obtain their consent to participate in the trial (see online supplemental appendices 1 and 2). Participants must meet the inclusion criteria and have none of the exclusion criteria.
bmjopen-2023-072253supp001.pdf (300.6KB, pdf)
bmjopen-2023-072253supp002.pdf (261KB, pdf)
Patient and public involvement
AMR and antimicrobial allergy lack patient groups/hospital networks/local charities to draw on for patient and public involvement and engagement (PPIE), necessitating us building a specific ALABAMA PPIE-Allergy Forum (PPIE-AF) to contribute to the research design, execution and dissemination strategy. The PPIE-AF comprises people with previous PEN allergies, including those whose record has been overturned and can now receive penicillins. It also includes those with self-reported (unsubstantiated) PEN allergy.
Our research adopts a codesign approach where our PPIE-AF contributors input to ensure we designed a trial that is patient-centred with the shared goal to maximise improved NHS care and patient outcomes. Specifically, the trial was designed to be inclusive and to minimise long/multiple hospital visits during the penicillin allergy testing (PAT). This is therefore the first trial designed as a ‘one stop’ efficient allergy assessment for low risk individuals. The guidance to participants about delabelling also facilitates ease of future NHS implementation and patient uptake of PAT.
PPIE-AF members have been engaged in both the nested pilot and main trial—they reviewed and provided input into the protocol development and the ethics submission. They contributed to the design of the qualitative enquiry and its ethics submission, bringing their lived experience to shape the interview topic guide. They guided the need to develop educational material to support patients if their PEN allergy status is changed.
PPIE-AF members have ensured that our inclusion criteria are broad and include patient groups that are high antimicrobial users. The research team incorporated their views that limiting eligibility to a single group of patients (eg, only those with chronic obstructive pulmonary disease) would limit the applicability of findings and thus potential benefit in patients across health conditions and age groups, especially those over 65 years, who probably have the highest rate of inappropriate PEN allergy labels and who may benefit from testing. PPIE-AF members have ensured that the trial material is understandable and appropriate for patients considering participation and that the trial intervention itself is not too onerous and has a clear patient-centred approach. The PPIE-AF have great ambitions for dissemination using a proven Theatre of Debate involvement to make our research findings accessible to all based on our similar award winning application in NIHR COVID and Me.
SystmOne and ALABAMA unit
SystmOne is one of the major electronic health record systems used in primary care in the UK, which was developed by TPP, Leeds, UK, a health technology company. Enrolment of GPs into the ALABAMA trial requires that they use SystmOne as their health record system. Functionality of SystmOne allows the participating GPs to share health records of consented participant and to direct referrals for allergy testing, this sharing functionality is referred to as the ‘ALABAMA unit’. Delegated members of the ALABAMA trial team can gain access to the ALABAMA unit and can then view consented participants’ medical records and monitor antibiotic prescribing activity by running bespoke reports within the ALABAMA unit. Participants’ electronic health records will not be altered by the trial team but selected information, alerts, GP tasks and bespoke data reports can be generated, facilitating trial data capture. For example, the ALABAMA unit allows the GP practice to run a bespoke report of potentially eligible patients, allows the research team to track the delabelling process of ALABAMA participants confirmed as PEN allergy negative and enables the follow-up of participants given an antibiotic in the 12 month period following randomisation.
Randomisation
Randomisation will be performed using Sortition (an online randomisation system developed by the Primary Care Clinical Trials Unit of University of Oxford). Participants will be randomised to either usual care or the intervention arm using an allocation ratio of 1:1. Allocation will be minimised by GP, age, number of antibiotic prescriptions in the 24 months (12 months for participants recruited to nested pilot) prior to randomisation, and number of Quality and Outcomes Framework (QOF) registered diseases to ensure balance of allocation of these baseline covariates. Both the participants and the recruiter will know which arm they have been randomised into. The trial statistician will remain blinded to treatment allocation when performing the final analysis.
Data recording and record keeping
The OpenClinica system will incorporate data entry and validation rules to reduce data entry errors, and management functions to facilitate auditing and data quality assurance. Data protection requirements will be embedded into the design of the web-based system and enforced by best practice trial management procedures. The Clinical Data Manager will oversee the process of electronic data validation and manual listings, sending out Data Clarification Forms when required and following these up until the queries are resolved.
The trial staff will ensure that the participants’ anonymity is maintained. The participants will be identified only by a participant ID number on all trial documents and any electronic database, with the exception of the CRF, where participant initials may be added. All documents will be stored securely and only accessible by trial staff and authorised personnel. The trial will comply with the Data Protection Act 2018, which requires data to be anonymised as soon as it is practical to do so.
Trial outcomes
Primary outcome
The primary objective is to determine whether the intervention package is clinically effective in improving patient health outcomes. This will be measured using ‘treatment response failure’ rate which is defined as: representation with worsening or non-resolving or new symptoms following treatment with an antibiotic up to 28 days after initial antibiotic prescription (including represcription of antibiotic within 28 days of an index prescription) for predefined infections over at least 1 year subsequent to randomisation. These predefined infections are ones managed in the community for which a penicillin would be recommended as first-line therapy (see online supplemental appendix A). Assignment of antibiotic prescriptions as primary events will be checked by clinical members of the research team blinded to both the trial allocation and outcome of the event.
bmjopen-2023-072253supp003.pdf (54.1KB, pdf)
Secondary outcomes
Secondary outcomes are:
Effects of PAAP duration on symptoms rated ‘moderately bad’ or worse by patients after antibiotic treatment.
Effects of PAAP on antibiotic use (total duration, number of courses, defined daily doses and an equivalent analysis by antibiotics class, eg, penicillins).
Effects of PAAP on number of hospital admissions and length of hospital stays.
Effects of PAAP on mortality rates.
Effects of PAAP on number of patients with MRSA infection/colonisation.
Effects of PAAP on number of patients with C. difficile infection.
Cost effectiveness for the PAAP intervention compared with usual care through self-reported health-related quality of life outcomes.
The process evaluation will explore patient and clinician views and experiences of the PAAP, trial procedures and implications on delabelling on subsequent antibiotic prescribing and penicillin use through interviews. We will measure the influences on patient behaviour change through questionnaires.
Trial procedures
Participant screening, eligibility checks, and consent will be carried out by GPs or appropriately trained authorised staff delegated to do this on behalf of the GP. Subsequent trial procedures are carried out by the ALABAMA trial team, who will communicate PAT results to GPs.
Study intervention package
The intervention package includes the PAAP and support materials for clinicians and participants.22
On entry to the study, practices will receive site training and support materials for clinicians to help them in discussing and referring participants to the PAAP. Clinicians will receive an information leaflet (titled Penicillin Allergy Testing: Information for general practice) that includes evidence-based information to increase knowledge about PAT and motivation to refer participants for a PAT and prescribe penicillin after a negative PAT result. They will also receive training in making changes to the electronic health record when a participant receives a negative allergy test result.
The central component of the study intervention package is the PAT which will be carried out in three stages:
Stage 1: in primary care—clinical History.
Stage 2: skin testing in hospital clinic (this may not be needed for all participants, see figure 1 and online supplemental appendix 3)
Stage 3: Oral Challenge Test in hospital clinic/followed by subsequent doses at home, see online supplemental appendix 4)
Figure 1.
The ALlergy AntiBiotics And Microbial resistance (ALABAMA) trial penicillin allergy testing (PAT) strategy.
bmjopen-2023-072253supp004.pdf (648.8KB, pdf)
bmjopen-2023-072253supp005.pdf (503.2KB, pdf)
Stage 2, if needed, and stage 3 are performed together during half-a-day clinic visit. If there is no initial reaction in clinic, the participant will continue the oral challenge test by completing 3 days oral antibiotics at home. Figure 1 shows the PAT flow.
All participants in the intervention arm will be posted a pretest intervention leaflet (titled Penicillin Allergy Testing: going for a test’) prior to their PAT appointment to inform them about incorrect allergy records, how they may benefit from having a PAT and what the test involves.
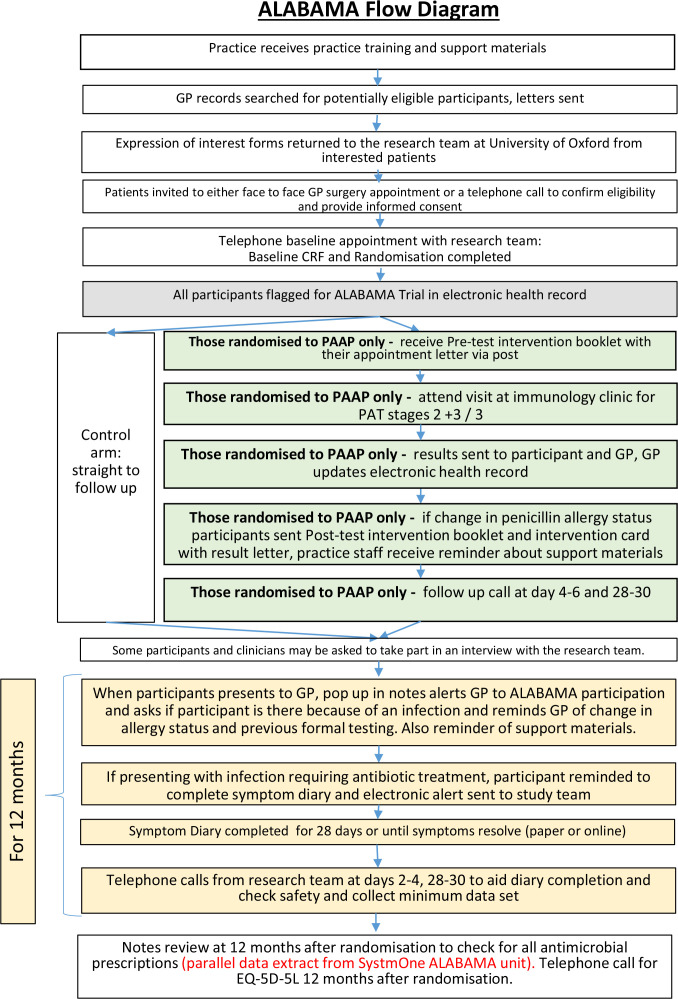
On completion of PAT, practices will be informed of the test result and instructed to update the participant’s electronic health records accordingly. Entry of the PAT result codes into the participant’s electronic health record activates additional behaviour change materials: pop ups that appear when a GP prescribes antibiotics for a trial participant to remind them of a change to PEN allergy records, if appropriate (figure 2)
Figure 2.
ALABAMA flow diagram for penicillin allergy assessment pathway (PAAP). ALABAMA, ALlergy AntiBiotics And Microbial resistAnce; GP, general practice; PAT, penicillin allergy test; CRF, case report form.
Participants will receive an allergy test result letter. If they have tested negative, they will receive a second booklet (titled Penicillin Allergy Testing: a negative test result) and an Intervention Card. The booklet informs participants about the reliability of the test results and consequences of a negative test result. The intervention card is a laminated credit card-sized card that says which test the participant has had and confirms the negative allergy result.
The study comparator is usual care with subsequent monitoring for antibiotic prescriptions and follow-up for trial outcomes as determined by the clinical indication for antibiotics. Usual care in this context means antibiotics prescribed by their general practitioner according to routine clinical practice.
Symptom diary and questionnaires
Symptom diary—participants will be asked to complete a symptom diary when they receive an antibiotic for a predefined list of infections in the 12 month period from randomisation. Information collected will include the predominant presenting symptoms, symptom severity, antibiotic consumption and any side effects. The diary will be completed for 28 days or until the participant’s symptoms are a ‘slight problem’ or less (scoring 2 and below) and they have stopped their course of antibiotics. Participant diaries will either be recorded on paper case report forms (CRFs) or directly into the Research Electronic Data Capture (REDCap) database.
Patient allergy belief questionnaire—participants will be asked to complete this at baseline and if applicable 28–30 days after completing the PAAP.
EQ-5D-5L questionnaire—23participants will be asked to complete this at baseline, 12 months after randomisation and, if applicable, 28–30 days after any GP appointment where an antibiotic was prescribed for one of the predefined infections.
Linkage with NHS Digital
The SystmOne ALABAMA unit will remain in existence for 10 years after the close of the trial to support an evaluation of long-term outcomes. Participants will have their electronic health record interrogated via linkage with NHS Digital for data on hospital admissions (Hospital Episode Statistics, HES data), details of antibiotic prescriptions during their admission (GP notes review and secondary care notes review) and mortality data (Office for National Statistics, ONS data). Participants will be consented for this as part of the current ALABAMA trial consent process.
Safety
PEN allergy testing is routinely carried out in the NHS and is known to carry a very small risk of anaphylaxis and death. To minimise this risk for participants undergoing the pre-emptive PAT, any participant with a prior history suggestive of anaphylaxis or a previous serious reaction to penicillin will be excluded.
Telephone calls by the trial team at 4–6 days and 28–30 days after PAT will collect information on adverse events (AEs) and serious adverse events (SAEs) associated with PAAP.
AEs and SAEs occurring up to 28 days after an antibiotic prescription from their general practitioner for any predefined infections will be captured through the participant diary and telephone calls by the research team 2–4 days and 28–30 days after the start of an antibiotic prescription. We will capture any AEs that result in a change of antibiotic prescription through the safety review telephone calls and/or notes review.
All SAEs identified during the ALABAMA trial will be assessed for their relatedness to PAAP or antibiotic prescriptions for any of the predefined infections. Anaphylaxis to an antibiotic will be considered an SAE as part of the ALABAMA trial.
Participants in the nested pilot study were also be called monthly for 4 months to assess any safety events. If not captured through the telephone calls, we will collect any other SAE by notes review, HES and mortality data, at month 12.
Mixed-methods process evaluation
The mixed-methods process evaluation will include a patient questionnaire (see questionnaires, and semistructured telephone interviews with patients and clinicians). Participants will be asked to complete an allergy belief questionnaire at baseline and, if applicable, 28–30 days after the PAAP.
Purposive sampling will be used to identify a subset of clinicians who will be invited to take part in an interview at the end of the trial to discuss their experiences.
A subset of patient participants will be interviewed once they have completed the PAAP and received their allergy test result to understand their experiences and also for those participants who have received subsequent antibiotic prescriptions following delabelling; this will include those delabelled but refusing penicillin. Participants and clinicians invited to take part in telephone interviews will be provided with patient information sheets and Informed Consent Forms specific to the qualitative component of the process evaluation.
Statistical analysis
Sample size calculation
A total sample size of 2090 or 1592 participants (1045 or 791 per trial arm, respectively) will provide 90% or 80% power, respectively, to detect a clinically important absolute difference of 7.9% in represcription rate (used as surrogate for treatment response failure) at 1 year between groups at 5% level of significance (two-sided). We plan to recruit 2090 but will fall back on 1592 if recruitment is challenging, as recruitment has commenced during the COVID-19 pandemic and will continue in the postpandemic climate. The sample size has been adjusted assuming that only 50% of participants will require at least one prescription within 1 year from randomisation and allowing for 10% dropout. The first 96 participants of the total will comprise the sample for the nested pilot study.
Primary and secondary outcomes
An intention-to-treat analysis will be conducted for the primary outcome and will include all randomised participants irrespective of what treatment they actually receive. Analysis for the primary outcome, that is, ‘treatment response failure’, will be analysed using a generalised linear mixed-effects model specifying a Binomial distribution with a log link function. GP site will be included in the model as a random effect while relevant baseline covariates and other minimisation factors will be treated as fixed effects. A similar approach will be used for other binary secondary outcomes, while continuous outcomes will be analysed using linear mixed-effects models. Appropriate regression models (such as Poisson regression, Hurdle models etc) will be used for the analysis of count outcomes.
All data will be included in the analysis as far as possible, though there will inevitably be the problem of missing data due to withdrawal, loss to follow-up or non-response questionnaire items. Missing data will be reported, with reasons where available, and the missing data mechanism explored. Sensitivity analysis using imputation methods, such as multiple imputation for data missing at random mechanism, will be considered.
Mixed-methods process evaluation analysis
Descriptive statistics (frequencies and percentages) will be used to summarise responses to questionnaire data.
Data from interviews with clinicians and participants will be analysed using thematic analysis taking an inductive approach.24 25 NVivo software will be used to assist with the organisation of data. A thematic framework will be used to chart data across all interviews and will aid comparisons between participants. To further make sense of the data, we will draw in our analysis on behaviour changes theories to facilitate implementation planning.
Cost-effectiveness analysis
A within-trial economic evaluation will estimate the effect on quality of life, costs and incremental cost per quality-adjusted life year (QALY) gained for PAAP versus usual care from the perspective of the NHS and Personal Social Services. The analysis will use trial data collected up to 12 months follow-up post randomisation.
Costs for delivering the PAAP intervention will be measured as part of the trial and the costs of delivering usual care will be calculated based on resource use collected in the trial and unit costs from the published literature. Primary and secondary healthcare service use will be estimated, respectively, from SystmOne electronic records and the linked individual participant HES Health Resource Group (HRG) data. Prescribing data in secondary care will be obtained by the trial team through hand searching of participants’ health records in the lead secondary care centre and other centres when possible or by accessing electronic prescribing systems, if available. Healthcare service costs will be estimated by valuing primary or community care service use using unit costs from published sources,26 use of medications with list prices from the British National Formulary and HRG unit costs from NHS Reference Costs. QALYs will be calculated using area under the curve interpolations between baseline and 12 month EQ-5D-5L utility data collected in the trial and linked ONS mortality data over the first year after randomisation. No discounting will be applied to costs and QALYs and incremental costs per QALY gained as the time horizon will be limited to 12 months.
Costs will be analysed using generalised linear models with a gamma family and log link27 28 to account for skewness, and adjust for GP, age, number of antibiotic prescriptions in the 12 months prior to randomisation and number of QOF registered diseases, as well as baseline EQ-5D-5L score.29 A similar approach will be applied to analyse QALYs, based on parametric survival models and predicted utility differences between trial arms.
Missing data will be imputed using established methods.30 Results will be presented in terms of incremental cost per QALY gained and cost per treatment failure avoided at 12 months. Sampling uncertainty will be analysed using the bootstrap method31 and joint uncertainty in costs and QALYs will be analysed using cost-effectiveness acceptability curves.32 Sensitivity analyses will explore variations in key cost and QALY assumptions, including interpolation of utility scores from baseline to 12 month data collection points, disutilities associated with AEs and joint parametric distributions used to model costs and QALYs.
Ethics and dissemination
This trial is in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice. Research Ethic Committee (REC) approval was granted by the NRES Committee London Bridge (ref: 19/LO/0176). The Investigator will submit and, where necessary, obtain approval from the above parties for all substantial amendments to the original approved documents.
Informed consent will be obtained from all subjects involved in study.
An independent Data Monitoring Committee (DMC) will review efficacy and safety data by treatment allocation, and a Trial Steering Committee will provide oversight of the trial.
The primary trial results will be submitted for publication to an international, peer-reviewed journal, regardless of the nature of the results. Authorship will be determined by the chief investigators in accordance with the ALABAMA Publication Policy developed with the Trial Management Group in accordance with the ICMJE guidelines and other contributors will be acknowledged. Patient and public dissemination is also planned. The data that support the findings of this study will be available on reasonable request.
Discussion
The importance of AMR and the need to reduce its impact is well recognised.31 Penicillins are the most commonly prescribed antibiotics33 and remain first-line therapy for many common infections. However, allergy to penicillin is commonly reported by patients and the presence of a PEN allergy record in a patient’s health record leads to the avoidance of recommended first line penicillin antibiotics. Non-penicillin antibiotics can be less effective, have more side effects and have a greater propensity to drive AMR.
Evidence shows that approximately 5% of patients who have a PEN allergy record are found to have genuine allergy after non-specialist allergy assessment.19 This trial aims to address the large discrepancy between reported and true allergy rates and will determine if introducing ‘pre-emptive’ testing for patients who are more likely to receive antibiotics in the future, could impact on antibiotic prescribing, yield patient benefits, limit AMR/healthcare associated infection and deliver NHS cost savings.
The novel design of the PAAP allows direct oral challenge testing of patient participants deemed to have low risk of a genuine allergic reaction and is intended to make the PAT more efficient. If PAAP is found to be acceptable to patients, this streamlined approach to PAT would enable more patients to be tested within current resources. Additionally, PAAP need not be confined to take place in an immunology clinic and could be undertaken by appropriately trained staff, such as pharmacists, in all units with facilities to deal with any potential severe allergic reaction.
The PAAP is supported by a behavioural package, providing support materials to clinicians and participants to encourage referral to and attendance at PAAP and prescription and use of penicillin following delabelling, where appropriate. These materials were developed with input from stakeholders including PPIE-AF patient public involvement contributors to ensure they address clinician and participants’ needs.
Other strengths of the ALABAMA study include the nested pilot study which ensured the safety of PAAP before transition to the main trial and the multicentre design which allows recruitment of patients from a number of primary care regions across the UK, thus reinforcing the external validity of the trial. In addition, the mixed-methods process evaluation will allow us to understand how the intervention package was used by clinicians and participants, help to interpret the trial findings and provide an insight into optimal implementation. As a result, positive findings from the ALABAMA trial will be readily implementable in the NHS.
This trial has developed unique trial processes utilising SystmOne for data collection which will be discussed elsewhere, however this novel technology can potentially be used to improve trial processes for future primary care research.
The ALABAMA trial is being conducted amidst the COVID-19 pandemic and therefore will provide an insight into the effect of the pandemic on trial processes, in particular on participant recruitment and on how safety procedures for participants and trial staff are implemented.
This trial is the largest randomised trial aiming to pre-emptively address incorrect penicillin allergy records and has potential to significantly impact care by improving patient health outcomes, improving antibiotic prescribing, reducing AMR and overall reducing NHS costs.
A potential limitation is that the trial recruitment period includes the COVID-19 pandemic, which may have influenced antibiotic prescribing rates.
The process evaluation will review delabelling procedures with GPs. As the trial is open label and delabelling of participants in the intervention arm may influence clinician behaviour across all participants, it will be prudent to monitor this impact. Baseline rates of penicillin prescribing practice of those with a PEN allergy are not formally captured in the trial participating sites, although we do know the national average (4%). This will warrant further local audits within SystmOne and/or closer working with NHS-England that are now monitoring this behaviour in some geographic areas of relevance to the trial.
Trial status
Enrolment started at the first GP site as part of the feasibility study in October 2019. The current protocol is version 10.0 03-OCT-2022.
Supplementary Material
Acknowledgments
We acknowledge and thank the hard work and dedication of all the NIHR Clinical Research Networks recruitment teams, general practices and secondary care intervention sites. The authors would like to acknowledge the contribution of the Trial Steering Committee members and the Data Monitoring Committee members. The ALABAMA research team would like to thank the following PPIE-AF members for their valuable contribution—Rosie Woollard, Jenny Boards, Mandy East and Lynne Regent at Anaphylaxis UK, Maureen Jenkins and Amena Warner at Allergy UK.
Footnotes
Twitter: @ChrisColButler
KFA and CEP contributed equally.
Contributors: Conceptualisation, JS, SP, CCB, SS, EB, PH and ST-C; methodology, JS, SP, JB, SA, KFA, CP, MD, JC, EB, PH, RS, RM-M and KC; formal analysis, UG, RMW, L-MY, ST-C, MW and MS; investigation, SS, SA, RS, MW and MS; resources, JS, SP, CCB, SS, EB and ST-C; data curation, UG, L-MY, ST-C, MW, MS and RM-M; writing—original draft preparation, KFA, CP and MD; writing—review and editing, all; supervision, EB, JC, JS and SP; project administration, CP, KFA, MD and KC; funding acquisition, JS, CCB, PH, ST-C, BS and SP. All authors have read and agreed to the published version of the manuscript.
Funding: This work was supported by National Institute for Health and Care Research (NIHR) Programme Grant for Applied Research (PGfAR)—grant number RP-PG-1214-20007.
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498–505. 10.1001/jama.285.19.2498 [DOI] [PubMed] [Google Scholar]
- 2.McLean-Tooke A, Aldridge C, Stroud C, et al. Practical management of antibiotic allergy in adults. J Clin Pathol 2011;64:192–9. 10.1136/jcp.2010.077289 [DOI] [PubMed] [Google Scholar]
- 3.Solensky R, Earl HS, Gruchalla RS. Clinical approach to penicillin-allergic patients: a survey. Ann Allergy Asthma Immunol 2000;84:329–33. 10.1016/S1081-1206(10)62782-2 [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal KG, Kuper K, Schulz LT, et al. Association between penicillin allergy documentation and antibiotic use. JAMA Intern Med 2020;180:1120–2. 10.1001/jamainternmed.2020.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West RM, Smith CJ, Pavitt SH, et al. Warning: allergic to penicillin': association between penicillin allergy status in 2.3 million NHS general practice electronic health records, antibiotic prescribing and health outcomes. J Antimicrob Chemother 2019;74:2075–82. 10.1093/jac/dkz127 [DOI] [PubMed] [Google Scholar]
- 6.NICE . Drug allergy: diagnosis and management of drug allergy in adults, children and young people. NICE clinical guideline 183. 2014. [PubMed] [Google Scholar]
- 7.Borch JE, Andersen KE, Bindslev-Jensen C. The prevalence of suspected and challenge-verified penicillin allergy in a University hospital population. Basic Clin Pharmacol Toxicol 2006;98:357–62. 10.1111/j.1742-7843.2006.pto_230.x [DOI] [PubMed] [Google Scholar]
- 8.Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy 2011;31:742–7. 10.1592/phco.31.8.742 [DOI] [PubMed] [Google Scholar]
- 9.Reddy V, Baman NS, Whitener C, et al. Drug resistant infections with Methicillin-resistant Staphylococcus aureus, Clostridium difficile, and vancomycin resistant Enterococcus are associated with a higher prevalence of penicillin allergy. Journal of Allergy and Clinical Immunology 2013;131:AB170. 10.1016/j.jaci.2012.12.1269 [DOI] [Google Scholar]
- 10.Macy E, Contreras R. “Health care use and serious infection prevalence associated with penicillin "allergy" in hospitalized patients: a cohort study”. J Allergy Clin Immunol 2014;133:790–6. 10.1016/j.jaci.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal KG, Lu N, Zhang Y, et al. Risk of Meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ 2018;361:k2400. 10.1136/bmj.k2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . WHO AWaRe classification database of antibiotics for evaluation and monitoring of use. 2019. [Google Scholar]
- 13.Powell N, West R, Sandoe JAT. The impact of penicillin allergy de-labelling on the WHO aware antibiotic categories: a retrospective cohort study. J Hosp Infect 2021;115:10–6. 10.1016/j.jhin.2021.04.011 [DOI] [PubMed] [Google Scholar]
- 14.Mirakian R, Leech SC, Krishna MT, et al. Management of allergy to penicillins and other beta-Lactams. Clin Exp Allergy 2015;45:300–27. 10.1111/cea.12468 [DOI] [PubMed] [Google Scholar]
- 15.Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105:259–73. 10.1016/j.anai.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 16.Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol 2022;150:1333–93. 10.1016/j.jaci.2022.08.028 [DOI] [PubMed] [Google Scholar]
- 17.Krishna MT, Huissoon AP, Li M, et al. “Enhancing antibiotic stewardship by tackling "spurious" penicillin allergy”. Clin Exp Allergy 2017;47:1362–73. 10.1111/cea.13044 [DOI] [PubMed] [Google Scholar]
- 18.Savic L, Ardern-Jones M, Avery A, et al. BSACI guideline for the set-up of penicillin allergy de-labelling services by non-allergists working in a hospital setting. Clin Exp Allergy 2022;52:1135–41. 10.1111/cea.14217 [DOI] [PubMed] [Google Scholar]
- 19.Powell N, Stephens J, Kohl D, et al. The effectiveness of interventions that support penicillin allergy assessment and delabeling of adult and pediatric patients by nonallergy specialists: a systematic review and meta-analysis. Int J Infect Dis 2023;129:152–61. 10.1016/j.ijid.2022.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ 2021;374:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santillo M, Wanat M, Davoudianfar M, et al. Developing a behavioural intervention package to identify and amend incorrect penicillin allergy records in UK general practice and subsequently change antibiotic use. BMJ Open 2020;10:e035793. 10.1136/bmjopen-2019-035793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 25.Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of Inductive and deductive coding and theme development. International Journal of Qualitative Methods 2006;5:80–92. 10.1177/160940690600500107 [DOI] [Google Scholar]
- 26.Curtis L, Burns A. Unit costs of health and social care 2020. Personal Social Services Research Unit, University of Kent, Canterbury, 2020. [Google Scholar]
- 27.Thompson SG, Nixon RM. How sensitive are cost-effectiveness analyses to choice of parametric distributions Med Decis Making 2005;25:416–23. 10.1177/0272989X05276862 [DOI] [PubMed] [Google Scholar]
- 28.Mihaylova B, Briggs A, O’Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897–916. 10.1002/hec.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial‐based cost‐effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487–96. 10.1002/hec.944 [DOI] [PubMed] [Google Scholar]
- 30.Faria R, Gomes M, Epstein D, et al. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157–70. 10.1007/s40273-014-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost‐effectiveness analysis up by its bootstraps: a non‐parametric approach to confidence interval estimation. Health Econ 1997;6:327–40. [DOI] [PubMed] [Google Scholar]
- 32.Davies SC. Annual report of the chief medical officer, volume two, 2011, infections and the rise of antimicrobial resistance. London: Health Do, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Public Health England . English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) Report 2017. London, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072253supp001.pdf (300.6KB, pdf)
bmjopen-2023-072253supp002.pdf (261KB, pdf)
bmjopen-2023-072253supp003.pdf (54.1KB, pdf)
bmjopen-2023-072253supp004.pdf (648.8KB, pdf)
bmjopen-2023-072253supp005.pdf (503.2KB, pdf)