Abstract
Nearly two-thirds of patients with cirrhosis suffer from malnutrition resulting from multiple contributory factors such as poor intake, accelerated starvation, catabolic milieu, and anabolic resistance. Nutritional assessment and optimization are integral to adequate management of a liver transplant (LT) candidate. A detailed nutritional assessment should be done at baseline in all potential transplant candidates with periodic reassessments. Sarcopenia is defined as a reduction in muscle mass, function, and/or performance. Skeletal muscle index at 3rd lumbar vertebra determined by computed tomography is the most objective tool to assess muscle mass. Hand-grip strength and gait speed are simple tools to gauge muscle strength and performance, respectively. Sarcopenia, sarcopenic obesity, and myosteatosis portend poor outcomes. Sarcopenia contributes greatly to frailty, which is a syndrome of reduced physiological reserve and impaired response to stressors. Dietary interventions must ensure adequate calorie (35–40 kcal/kg/day) and protein (1.2–1.5 gm/kg/day) intake via multiple frequent meals and late-evening calorie-dense snack. Micronutrient supplementation is essential, keeping in mind the etiology of cirrhosis. Individualized, gradually up-titrated exercise prescription consisting of both aerobic and resistance training of 150 min/week is advisable after appropriate risk assessment. Early initiation of enteral nutrition within 12–24 h of LT is recommended. Data with respect to immune-nutrition, monomeric formulas, and hormone replacement remain conflicting at present. A multidisciplinary team comprising of hepatologists, transplant surgeons, intensivists, dieticians, and physiotherapists is vital to improve overall nutrition and outcomes in this vulnerable group.
Keywords: nutrition in liver disease, liver transplantation, sarcopenia, obesity, sarcopenic obesity
Graphical abstract
Adequate nutritional assessment and optimization is a vital but often overlooked part of multidisciplinary management of a prospective adult liver transplant (LT) candidate.1, 2, 3 Dynamic and structured evaluation of nutritional status followed by appropriate interventions are essential components of care in the pre-transplant period.1,2,4, 5, 6 Moreover, continuous reinforcement of such measures must continue during the peri-operative, immediate and late post-transplant period to improve overall outcomes. A sizeable number of LT recipients develop obesity and metabolic syndrome related complications leading to increased adverse cardiovascular events and mandates need of continuous nutritional intervention long after LT.7, 8, 9, 10
Malnutrition refers to excess, deficiency, or imbalance in nutrient or energy intake of a person.1,3 In this review, the term “malnutrition” primarily refers to “undernutrition”. Apart from macronutrients such as carbohydrates, proteins, and fats, especial emphasis must be given to ameliorate micronutrient deficiencies that are frequent in LT candidates and recipients both. Sarcopenia is defined as generalized loss of muscle mass, performance, and function and is one of the most validated and objective components of a detailed assessment of chronic protein energy malnutrition in patients with cirrhosis.11, 12, 13, 14 Malnutrition and sarcopenia negatively impact waitlist survival and post-LT outcomes15 in view of increased risk of infections, need of prolonged mechanical ventilation, longer intensive care unit (ICU) stay, and poorer response to surgical stress.3,5,16 Nearly 60–70% of patients with decompensated cirrhosis/end-stage liver disease (ESLD), which is the most common indication for LT, have malnutrition.1,3,12 With increasing expertise and improvement in critical care hepatology, acute-on-chronic liver failure (ACLF) patients are undergoing LT more frequently and this group of patients is at high risk of malnutrition.5,17,18 Unfortunately, lack of well-defined population specific cut-offs, the absence of standardization of nutritional assessment techniques, the presence of ascites and ACLF gravely limit the capability to adequately assess and manage nutritional status in this highly vulnerable population.1,2
In concert with the global obesity pandemic, non-alcoholic fatty liver disease (NAFLD) is now rapidly becoming the leading etiology of ESLD requiring LT.19 Relatively higher body mass index (BMI) of these patients may give a false impression of adequate nutrition in this cohort. However, one-third of these patients have sarcopenia signifying isolated increase in the harmful fat mass. This confluence of sarcopenia and obesity is known as sarcopenic obesity20, 21, 22 (SaO). Apart from pre-LT implications such as small-for-size grafts and higher prevalence of cardiometabolic co-morbidities, SaO also predisposes to post-LT obesity and metabolic syndrome22, 23, 24, 25 (post-transplant metabolic syndrome [PTMS]). Significant improvement in the long-term survival of LT recipients in the last two decades further reiterates the need of incorporating nutritional and metabolic assessments as the vital part of post-transplant care.
In this review, we will discuss the prevalence, impact, and management of malnutrition in LT candidates focusing primarily on ESLD patients. Further, we will elaborate peri-operative and long-term post-LT nutritional care, with especial emphasis on PTMS and other metabolic complications.
Burden and etiology of malnutrition in LT candidates
Malnutrition is almost universal in wait-listed LT candidates. Depending on the tools used to identify undernutrition, 30%–70% of decompensated cirrhotics4,26 suffer from malnutrition. The prevalence further increases in patients with alcohol-related cirrhosis and more advanced liver disease i.e., Child Turcot Pugh (CTP) class C and ACLF.1, 2, 3
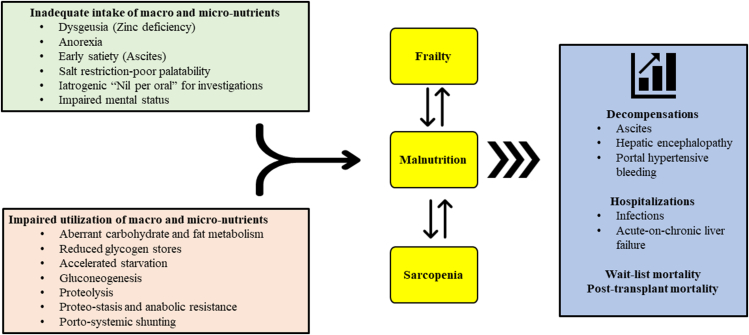
Primary contributors to undernutrition include poor oral intake as a result of dysgeusia (zinc deficiency), ascites, poor performance status, peer/family imposed dietary restrictions, and polypharmacy.1,26, 27, 28 Malabsorption resulting from bowel edema, portal hypertensive gastroenteropathy, impaired bile secretion (especially in cholestatic liver diseases), altered gut motility, bacterial overgrowth, and gut dysbiosis further compound the problem.5,29 Hormonal alterations such as reduced growth hormone and testosterone also likely contribute to loss of muscle mass.30,31 Simultaneously, there is dysregulation of carbohydrate utilization and energy metabolism in ESLD due to altered insulin kinetics, diminished hepatic glycogen stores, and accelerated starvation response.32,33 It has been shown that a state of starvation, increased fatty acid oxidation, protein catabolism, and ketogenesis is reached after mere 10 h of fasting in cirrhotics compared to 3 days in normal individuals.34,35 Rapid depletion of glycogen stores lead to muscle catabolism to release amino acids (both branched chain and aromatic amino acids) for gluconeogenesis.32,35 Muscle can only utilize branched chain amino acids (BCAA) for gluconeogenesis whereas aromatic amino acids released via muscle catabolism contribute to increased ammonia generation and may contribute to recurrent episodes of hepatic encephalopathy (HE) which further impairs adequate nutrition intake.1,26 Moreover, ammonia induces a state of anabolic resistance thus exacerbating lean muscle loss and sarcopenia.26,36,37 This vicious cycle of malnutrition, accelerated starvation, proteolysis, proteostasis, sarcopenia, ammoniagenesis, anabolic resistance, and HE, drives the continuous downhill course. Further, acute events such as portal hypertensive bleeding, infections, sepsis, large volume paracentesis and repeated hospital admissions tremendously aggravate the catabolic state1,2 (Figure 1). To prevent and break this vicious cycle and to improve nitrogen balance, it is advised to minimize fasting periods38 as elaborated later in this review.
Figure 1.
Multifactorial causation of malnutrition and frailty in patients with cirrhosis.
Nutritional evaluation of the LT candidate
All patients with cirrhosis must undergo nutritional screening at baseline followed by a detailed nutritional assessment of at-risk patients. A good nutritional screening tool (NST) should be sensitive, simple, reproducible, and require minimal training so that it can be performed quickly bedside or in an outpatient setting. Patients with dry BMI <18.5 kg/m2 and/or CTP class C can be directly evaluated1, 2, 3 using a detailed nutritional assessment tool which require trained professionals and sophisticated tools.
Initial Nutritional Screening
There are many NST available; however, their validity and prognostic accuracy in ESLD patients remains to be assessed prospectively in larger cohorts. Ney et al.39 recently systematically reviewed and meta-analyzed various nutritional screening and assessment tools in cirrhosis patients. Out of 47 studies (8850 patients), only 3 studies assessed NSTs. More than 32 definitions of malnutrition were utilized across the studies suggesting lack of consensus. Despite extremely limited and heterogenous data on NSTs, a clear association between malnutrition and waitlist mortality was reported.
Malnutrition screening tool, a community screening tool,40 and nutritional risk screening 2002 (NRS-2002), a hospital screening tool,41 are practical scores with good interrater correlation. However, both these scores do not take into account the effect of fluid collections such as ascites which are quite common in ESLD patients. Nutrition risk in critically ill score,42,43 which also incorporates acute physiology and chronic health evaluation (APACHE)-II and sequential organ failure assessment (SOFA) score within itself, has been validated in this population but requires interleukin-6 levels which limits its routine use.
Royal free hospital-nutrition prioritizing tool (RFH-NPT) is a cirrhosis specific tool44 and has been validated against RFH-subjective global assessment.45 RFH-NPT can be done quickly (takes <3 min) with minimal training. It includes variables such as presence of alcoholic hepatitis, need of tube feeding, third space fluid collections, BMI (if no ascites/edema), dietary pattern, recent weight loss, and presence of any acute illness. Score ranges from 0 to 7 with scores between 2 and 7 signifying high risk of malnutrition and need of detailed nutritional assessment and intervention. A prospective study, involving 84 ESLD patients in the study cohort and 64 patients in the validation cohort, compared RFH-NPT with NRS-2002 score.46 RFH-NPT independently correlated with clinical deterioration, severity of disease, and clinical outcomes such as ascites, HE, hepatorenal syndrome, and transplant-free survival. An improvement in RFH-NPT within 500 days was also associated with better survival.46
The liver disease undernutrition screening tool (LDUST) is another cirrhosis-specific tool developed for ambulatory patients.47 LDUST uses six patient directed questions. Booi et al.48 in their 3-phase study showed more than 70% sensitivity and specificity of the tool with 93% positive predictive value to identify risk of malnutrition. However, the low negative predictive value (38%) exposed its inability to reliably rule-out undernutrition and need of further refinement and prospective validation. Wu et al.49 prospectively compared NRS-2002, RFH-NPT, malnutrition universal screening tool, and LDUST in 145 patients with cirrhosis. The RFH-NPT and NRS-2002 demonstrated higher sensitivities (64.8% and 52.4%) and specificities (60% and 70%) and RFH-NPT was the only score which independently predicted mortality. With the available evidence, guidance, and expert opinions, RFH-NPT appears to be the best NST in ESLD patients.1,2
Detailed nutritional assessment of at-risk patients
Dietary Assessment
Inadequate dietary intake is a significant contributor to malnutrition.2,26 A 3-day recall diary is a better tool to assess macronutrient intake than a 24-h recall as covert memory impairments are common in ESLD patients.2,50 Apart from total calorie intake, special attention should be given to relative contribution by various macronutrients, type and quality of protein intake, and possible micronutrient deficiencies. Pattern of food intake (number of meals and gap between meals) and any recent change in amount of food intake must be inquired.3 Frequent day-time meals and a late-evening calorie-dense snack and protein should be ensured.2,50,51 Further any barriers to inadequate dietary intake such as progressive ascites, dysgeusia, covert HE, constipation, self-imposed dietary restrictions, and socio-economic factors should be looked out for and addressed appropriately.1,3,5
Micronutrient deficiencies especially zinc, magnesium, vitamin A, and D are quite frequent in ESLD.52,53 Diuretics, laxatives, and frequent antibiotic use also contribute to these deficiencies apart from dietary insufficiency. Patients with cholestatic etiologies require regular assessment and supplementation with calcium and fat-soluble vitamins. Adequate supplementation and periodic reassessment should form a part of both outpatient and inpatient visits in pre and post-LT setting as well.6,54
Sarcopenia
Sarcopenia is the “central component” of nutritional assessment in cirrhotic population. Unfortunately, standardized consensus-based universal definition of sarcopenia is still lacking. Overall, two-thirds of patients with ESLD have sarcopenia with significantly higher prevalence in males (51%) than females (21%).15,16,55,56 Females tend to lose more fat mass whereas males lose muscle mass more rapidly. Also, prevalence increases from compensated (20%) to decompensated cirrhosis (>50%).57,58 Older age of patients with cirrhosis further contributes to reduction in muscle mass (compound sarcopenia).3 Loss of muscle function usually commences before detectable sarcopenia59 and contributes to functional loss and poorer quality of life suggesting a non-linear relationship between muscle mass and muscle strength.12,60, 61, 62 Consistent with current evidence, European working group on sarcopenia in older people incorporated diminished muscle function and performance also as a component of sarcopenia.13,62 However, most studies assessing the impact of sarcopenia on clinical outcomes in ESLD patients have used a static measure of muscle mass such as skeletal muscle index (SMI).
Sarcopenia has been found to be associated with increased cost of care, higher risk of developing new and progressive decompensations, greater risk of pre as well as post-LT infections, prolonged ICU stay, and mechanical ventilation requirement post-LT.1,2,13, 14, 15, 16,63,64 Sarcopenia also predicts increased risk of HE after trans-jugular intra-hepatic portosystemic shunt (TIPS) and improvement in sarcopenia (>10%) post-TIPS reduces risk of post-TIPS HE.65,66 In a recent meta-analysis,67 the overall prevalence of sarcopenia among ESLD patients was 37.5% with higher prevalence in males, alcohol-related liver disease, and advanced cirrhosis. The cumulative survival was significantly less in sarcopenic patients at 1 year (76.6% vs 93.4%), 3-years (64.3% vs 82%), and 5-years (45.3% vs 74.2%). Every 1 cm2/m2 increase in SMI at L3 and 1 mm/m increase in umbilicus-total psoas muscle thickness (TPMT) was associated with a 3% and 12% reduction in mortality risk, respectively.
Assessment of Muscle Mass
Various direct and indirect tools like anthropometry, dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), ultrasonography (USG), magnetic resonance imaging (MRI), and computed tomography (CT) have been studied to quantify muscle mass in ESLD.39,47,68 Individual muscle and/or muscle groups such as SMI at 3rd lumber vertebra (L3), total psoas area, TPMT, psoas muscle index, and69 total skeletal muscle attenuation have been studied to assess muscle mass and quality.1,2,68
Mid-arm muscle circumference (MAMC), which requires triceps skin fold thickness (TSF), and mid-arm muscular area measurement, is the most frequently used anthropometric tool to assess sarcopenia.1,2,47 These tools are simple, rapid, cheap, unaffected by fluid retention, and suitable for use in routine clinical practice. They show good intra- and inter-observer reproducibility when performed by trained individuals.2 MAMC has shown good correlation with CT SMI-L3 with an area under receiver operator curve of 0.75 for men and 0.84 for women.69,70 Both MAMC and TSF have a shown prognostic significance in ESLD patients with MAMC having an upper hand over TSF.70
Body composition analysis to calculate total fat mass and fat-free mass (FFM) can be accomplished using BIA, air displacement plethysmography, DEXA, and magnetic resonance spectroscopy (MRS).39,47,68 DEXA can additionally measure bone mineral density apart from estimation of the absolute skeletal muscle mass and/or appendicular skeletal muscle mass which can be adjusted for height or weight. DEXA has small-risk of radiation exposure which limits its routine use in community. BIA is portable, non-invasive tool with no radiation risk. However, accuracy of both DEXA and BIA is affected by hydration status, ascites, and presence of edema.1, 2, 3,14
SMI at L3 assessed by CT is the most validated and frequently used tool.1, 2, 3 Total cross-sectional area of psoas, erector spinae, quadratus lumborum, transversus abdominis, external oblique, internal obliques, and rectus abdominis are calculated at L3 using various available and embedded software packages. It is then expressed as the total area of skeletal muscles at L3 vertebra (cm2) normalized to total body surface area (m2). In a North-American study58 done in LT-wait-listed patients with cirrhosis, cut-off values for the diagnosis of sarcopenia were suggested to be <50 cm2/m2 and <39 cm2/m2 for men and women, respectively. The mean muscle mass of Asians is about 15% less than that of Western population, even when corrected for height.71 Hence, it is imperative to establish, standardize, and validate criteria to diagnose sarcopenia in various ethnicities. A Japanese study14 suggested 42 cm2/m2 (men) and 38 cm2/m2 (women) as the optimal cut-off values for CT SMI-L3 to identify sarcopenia with 89% sensitivity and 57% specificity. Sidhu et al.72 assessed normative value of CT SMI-L3 in 3087 non-cirrhotic Indian patients (67% males) without cirrhosis who underwent CT for acute abdomen. They identified mean CT SMI-L3 of 44.33 ± 6.56 and 41.25 ± 4.42 in non-cirrhotic adult males and females, respectively. Another Indian study60 done in 2002 non-cirrhotic individuals (1308 males) suggested cut-off for sarcopenia as 39.59 cm2/m2 and 31.83 cm2/m2 in males and females, respectively. A CT SMI-L3 ≤ 21.2 cm2/m2 was associated with increased 6-month mortality.73 Cut-offs suggested by various societies to diagnose sarcopenia using different assessment tools are summarized in Table 1.
Table 1.
Various Tools and Their Population Specific Cut-Offs to Diagnose Sarcopenia.
| EWGSOP [13,62] | AASLD [3] | AWGS [12] | JSH [14] | INASL [1] | Comments | |
|---|---|---|---|---|---|---|
| Hand-grip strength (kg) | M: 27 F: 16 |
M: 26 F: 18 |
M: 28 F: 18 |
M:28 F: 18 |
M: 27 F: 16 |
Simple, inexpensive tool Can be used in OPD Take average of 3 readings using the non-dominant hand |
| Gait speed (m/s) | ≤0.8 (4 m) | ≤0.8 (4 m) | <1 (6 m) | ≤0.8 (4 m) | ≤0.8 (4 m) | Simple, inexpensive tool Can be used in OPD May not be feasible in very sick hospitalized patients |
| Five Chair stands (sec)) | 15 | – | 12 | – | – | Feasible, inexpensive method Can be used in OPD May not be feasible in very sick hospitalized patients |
| BIA (kg/m2) | – | – | M: <7 F: <5.7 |
M: <7 F: <5.7 |
M: <7 F: <5.7 |
Good precision and accuracy Non-invasive, no radiation Ascites/oedema affects accuracy |
| DEXA (kg/m2) | M: <7 F: <5.5 |
– | M: <7 F: <5.4 |
M: <7 F: <5.4 |
M: <7 F: <5.4 |
Measures appendicular lean mass Good accuracy and reproducible Requires trained radiologist Low dose radiation, cost, and infrastructural issues Ascites/oedema affects accuracy |
| CT SMI-L3 (cm2/m2) | M: <41.6 F: <32 |
M:<50 F: <39 |
M:<40.8 F: <34.9 |
M:<42 F:<38 |
M: <42 F: <38 |
Most objective and validated tool Good accuracy and clinical correlation Unaffected by ascites/oedema Requires trained radiologist High radiation, cost, and infrastructural issues Can be incorporated in surveillance CT |
EWGSOP, European working group on Sarcopenia in older people; AASLD, American association for study of liver disease; AWGS, Asian working group on Sarcopenia; JSH, Japanese society of Hepatology; INASL, Indian National association for the study of liver; BIA, Bioelectrical impedance analysis; DEXA, Dual energy X-ray absorptiometry; CT, Computed tomography; SMI, Skeletal muscle index; L3, 3rd lumbar vertebra; kg/m2, Kilogram per meter square; m/s, meter per second; cm2/m2, centimeter square per meter square; M, Males; F, Females; OPD, Outpatient department.
MRI has also been used to assess muscle mass at various areas; however, data remain scarce and poorly validated.39,47,68 Furthermore, availability, feasibility, and higher costs limit the use of MRI to solely assess sarcopenia. Tandon et al.69 evaluated the thigh muscle index during both compression and no compression at two predetermined points on the thigh via USG. They found that the average feather index (non-compression) was strongly associated with sarcopenia in ESLD patients. This safe and interesting modality requires further validation.75
SaO and Myosteatosis
Baumgartner first defined SaO in the elderly population74 as co-existence of sarcopenia and obesity as measured by DEXA and associated it with decline in physical activity and performance. Clinically inconspicuous alterations in body composition such as increase in body fat with simultaneous reduction in muscle mass have been noted in the elderly population.75 A complex interplay of multiple pathophysiological pathways such as increased inflammatory cytokines milieu and oxidative stress, insulin resistance, hormonal imbalances, and reduced physical activity has been implicated in the development of SaO.21,22 With rising incidence and prevalence of NAFLD worldwide, increasing number of ESLD patients present with obesity.19 Currently available literature in ESLD patients suggests a wide prevalence range (2%–40%) of SaO likely due to heterogeneity in the definitions used.2 To diagnose SaO, sarcopenia is assessed using CT SMI-L3 and obesity is defined by dry BMI of >25 kg/m2 for Asians or >30 kg/m2 for Caucasians. SaO is diagnosed when both sarcopenia and obesity are present concurrently.12,22,23
Apart from reduction in muscle mass, there is simultaneous deterioration of muscle quality even in the apparently healthy elderly population. Muscle quality reduction is further accentuated in patients with chronic diseases such ESLD, malignancies, and so on in the form of pathological accumulation of fat in the skeletal muscle known as myosteatosis.76,77 Aberrant fat accumulation can occur within the muscular fibers (intramyocellular) or within the fascia (intermuscular). Myosteatosis has been linked to insulin resistance and enhanced inflammatory phenotype.76 It can be present even in the absence of sarcopenia and/or obesity. Myosteatosis is usually assessed via radiological tools such as CT, MRI, and MRS. Muscle attenuation on CT scan can indirectly assess muscle fat infiltration. Skeletal muscle is identified and quantified by Hounsfield units (HU) thresholds of −29 to +150.21 Similar to CT SMI-L3, mean muscle attenuation is reported for the entire muscle area at the L3. Using this method, mean muscle attenuation values of <41 HU in patients with a BMI ≤24.9 kg/m2 and <33 HU in patients with a BMI ≥25 has been shown to be associated with increased mortality.76,77 In wait-listed ESLD patients, myosteatosis was documented in >50% cases and was associated with an increased risk of HE independent of liver function.78 In 678 (67% males) patients with cirrhosis, Montano-Loza et al.77 detected sarcopenia, SaO, and myosteatosis in 292 (43%), 135 (20%), and 353 (52%) patients, respectively, and all muscular abnormalities were associated with significantly reduced median survival. Kaibori et al.79 in their study done in patients undergoing hepatectomy for HCC showed reduced 5-year overall survival (46% vs 75%) and disease-free survival (18% vs 38%) in patients with intramuscular adipose tissue content. The presence of myosteatosis has been found to be independently associated with higher mortality (HR: 3.3), allograft failure (HR: 4.1), and longer hospital and ICU stay in LT recipients.76,77 Whether SaO independently exerts negative impact on post-LT outcomes compared to sarcopenia alone remains inconclusive; however, very limited evidence suggests that pre-LT SaO was associated with two times higher mortality at 1-year, 3-year, and 5-year after LT in a meta-analysis which may be attributed to higher incidence of PTMS in these patients.25
Assessment of Muscle Strength and Performance
Muscle strength and physical performance reflect a person's overall functional status and correlate more with overall outcomes than the muscle mass alone.60,80 Simple and cheap tools such as hand-grip strength (HGS), gait speed (GS), chair stand test, short physical performance battery (SPPB), and 6-min walk test (6-MWT) can be used to assess muscle strength and performance.39,47,68,81,82 Apart from sex and ethnicity, measurement protocol and type of dynamometer used also affects HGS cut-off value. An HGS ≤25.3 kg-force was associated with increased mortality in an Asian study.60 The chair stand test assesses the strength of the lower extremities by measuring the time needed by the patient to rise from chair 5 times without using the arms. Short physical performance battery (SPPB) is a composite assessment of muscle strength, performance and frailty and consists of GS, chair stand test, and balance testing.1 Each tool is scored out of 4 with maximal score being 12. A score <10 increases mortality by 2.5 times.5,54 Cardiopulmonary exercise testing (CPET) is an advanced test to assess aerobic capacity but requires expensive equipment and is time consuming.6,28 Also, ESLD/ACLF patients may find it difficult to perform CPET. The cut-offs for above tools to assess muscle quality and performance vary depending on the sex, population, and ethnicity and are elaborated in Table 1.
Assessment of Frailty
Frailty extends beyond sarcopenia and was originally defined in the geriatric population as a distinct biologic syndrome of reduced physiologic reserve along with heightened susceptibility to health stressors.3,16,68 Age-related frailty results from imbalances across multiple physiologic systems whereas liver-specific factors such as encephalopathy, variceal bleeding, sarcopenia, infections, proteostasis, repeated hospitalizations and psycho-social factors drive frailty in ESLD patients.16 Across multiple studies, frailty has been shown to be a strong independent predictor of waitlist drop-outs, repeated admissions, increased length of hospital stays, and post-LT mortality.16,68,83,84
A wide range of tools to assess frailty have shown their prognostic utility especially in ambulatory patients. Activities of daily living (ADLs) and Karnofsky Performance Scale (KPS) have been shown to be valuable in prognosticating in-patients81 whereas most other tools such as 6-MWT, GS, HGS, Liver frailty index85 (LFI), and Clinical Frailty Scale86 (CFS) have been validated primarily in the ambulatory setting.1,3,87 At least one frailty tool should be incorporated at initial evaluation and during longitudinal follow-up. LFI is an objective cirrhosis specific frailty assessment tool.81,85 It is simple and can be done in the outpatient setting and includes HGS, balance testing (assesses neuromuscular function), and chair stands. Formula to calculate LFI based on above parameters is available online at https://liverfrailtyindex.ucsf.edu. LFI score of ≥4.4 indicates frailty whereas prefrail status is indicated by LFI between 3.2-4.3.88 Longitudinal changes in frailty assessed by KPS and LFI scores also predict clinical outcomes in patients with ESLD.89
Nutritional interventions in prospective LT candidates
Ideally, efforts to assess and preserve muscle mass and function should begin as soon as a patient is diagnosed with chronic liver disease.6,28,68 However, most ESLD patients already have sarcopenia at first presentation and efforts should be made to assess severity and reverse it using patient education and individualized nutritional interventions involving a multidisciplinary team.2,6 Reassessment of sarcopenia and frailty should be done at least yearly in patients with stable compensated cirrhosis whereas more frequent re-evaluations (every 2–3 months) should be undertaken in patients with advanced/decompensated cirrhosis using the same tool used for baseline evaluation.1,2,6 Apart from guideline directed individualized management of ESLD and its complications, dietary interventions and exercise prescription form the mainstay of therapy for malnutrition. Involvement of an experienced dietician/nutritionist is vital.
Treating the Primary Disease
Adequate management of primary disease improves all-cause mortality. It slows the progression and minimizes associated pathophysiological alterations that lead to sarcopenia and frailty.1,2,17 Abstinence from alcohol, apart from removing the chronic hepatic insult, also improves alcohol-related myopathy and oral nutrient intake.1 Treatment of chronic hepatitic C has shown reduction in overall systemic inflammation.2 Similarly, interventions for NAFLD improve inflammation and insulin resistance.90 Furthermore, early resolution of HE and improvement in ascites improves oral intake and overall nutritional status.
Dietary Interventions
Developing an individualized nutritional prescription requires calculation of the patient's resting energy expenditure91,92 (REE). Indirect calorimetry is the gold standard for determining actual REE but is limited by its availability.6 Inexpensive handheld calorimeters have now been validated in ESLD patients and can be used bedside.93 Sans indirect calorimetry, predictive equations such as Harris Benedict equation can be used to estimate REE but may be unreliable.91,92,94 Patients with cirrhosis have been found to have REE 1.3–1.5 times that of general population.6,94 Studies have reported a total energy expenditure (TEE) between 28 and 38 kcal/kg/day in patients with cirrhosis.2,38,94 Dry weight or ideal body weight can be used to estimate calories requirement depending on the scenario. Subjective estimation of the dry weight can be done by either considering the post-paracentesis weight or by subtracting 5%, 10%, 15% from actual body weight in patients with mild, moderate, or severe ascites, respectively. An additional 5% should be taken off if there is significant bilateral pedal edema.2 The European Society for Parenteral and Enteral Nutrition (ESPEN) recommends overall calorie intake of 35–40 kcal/kg/day and protein intake of 1.2–1.5 g/kg/day.6 Enteral nutrition via oral route is always preferable. Up to 50–60% calories should come from carbohydrates whereas 20–30% should come from fat in the diet.3,6 Moderate calorie deficit of approximately 500 Kcal per day may be considered in obese patients.2 International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) recommended38 stratification of 24-h energy requirement based on BMI. They suggested targets of 20–25 kcal/kg/day, 25–35 kcal/kg/day, and 35–40 kcal/kg/day for patients with BMI of >40 kg/m2, 30–40 kg/m2, and 20–30 kg/m2, respectively, with the same protein requirement as ESPEN. ISHEN recommends using ideal body weight to calculate total energy and protein requisite.38
Moderate dietary sodium restriction i.e., 2 g of sodium corresponding to 5 g of salt may be recommended1,2 in patients with ascites/oedema but special care should be undertaken to ensure palatability, by educating patient and care-givers so that total caloric and protein requirements are met. Avoidance of fasting of more than 6 h with small frequent meals throughout the day is essential.34,35,95 Ingestion of a carbohydrate dense late-evening snack (at least 50 g of complex carbohydrates and 15 gm of protein) should be reinforced at every follow-up visit.17,51,96 Late-evening or nocturnal calorie-dense snack has been shown to improve aberrant substrate utilization leading to improved total body protein mass, FFM and clinical outcomes.51
In patients with HE, dietary protein restriction should not be done.38 Cordoba et al.97 in their landmark study showed a similar rate of recovery rate from HE in patients on normal protein versus protein restricted diet with increased protein breakdown in the latter group. Further, daily protein intake of <0.8 g/kg/day was an independent predictor of wait-list mortality in a study on more than 600 patients with cirrhosis.98 Vegetable and dairy proteins may have some advantage over animal proteins as they have more fiber and less methionine and aromatic amino acids content.99, 100, 101 Further, BCAA can be supplemented to achieve target in case of protein intolerance.102,103 Therapeutic effects of oral BCAA in the form of earlier HE resolution can also be secured especially in sarcopenic patients at a dose of 30 g per day.38,104 Japanese guidelines14 recommend the use of BCAA granules at a ratio of 1.2:2:1 (l-valine, l-leucine, and l-isoleucine) in ESLD patients to preserve liver function and inhibit carcinogenesis.
Patients presenting with acute variceal bleeding form another subset of nutritionally vulnerable patients. Previous views that early enteral feeding increases the risk of re-bleeding and HE due to increased splanchnic blood flow and protein load, respectively, have been proven wrong.105 Initiation of enteral nutrition within 24-h after variceal banding reduced length of hospital stay without any increased risk of re-bleeding, HE, and mortality compared to delayed enteral nutrition.105,106 Careful placement of an enteral feeding tube after variceal banding has also been found safe and should be considered in patients unable to take orally themselves to meet the calorie and protein requirements.1
Ensuring adequate nutrition in critically ill, hospitalized patients with cirrhosis is quite challenging.6,107 If such patients cannot meet energy targets via volitional oral intake, early enteral nutritional supplementations should be considered via enteral feeding tube to improve outcomes.2,107,108 The presence of esophageal varices does not contraindicate naso or orogastric tube placement.1 A metanalysis comparing early versus delayed enteral nutrition in critically ill patients, which also included ESLD patients, demonstrated a significant reduction in mortality and infections among the former group.109 Benefits of nutritional supplementation have also been shown in patients with severe alcoholic hepatitis.51,96,110 Daily reassessments and dynamic modifications by dieticians and clinicians must be done in these patients. Parenteral nutritional should be reserved for patients in whom enteral nutrition is not feasible or when they are unable to meet protein and energy targets via enteral intake alone.6,107,111
Micronutrient deficiencies are common in ESLD patients,52 especially thiamine and fat-soluble vitamins deficiency in chronic alcoholism and cholestatic liver diseases, respectively. There is high prevalence of vitamin D deficiency and consequent osteodystrophy and requires adequate evaluation and supplementation.1,8,112 Thiamine must be given prior to glucose administration in malnourished chronic alcoholic patients to avoid precipitating Wernicke's encephalopathy.107 Zinc deficiency is frequent in this cohort and zinc supplementation has shown benefit in patients with HE in some studies; however, the data remain unconvincing with respect to dose, duration and beneficial effects.53,113
Exercise
ESLD patients have severe muscular and cardiovascular deconditioning.16,81 Emerging evidence, mostly in compensated cirrhosis, has shown benefit of tailored exercise prescription in cirrhosis patients with respect to improvement in both aerobic capacity and sarcopenia.114, 115, 116 A titrated approach of “start low and go slow” should be used1 after ruling out any contraindications such as HE, cardiopulmonary issues, high risk of fall, and so on. A final target of total 150 min of exercise per week is reasonable.1,2 Both aerobic and resistance training should be combined in various proportions.114,115 Formulation of an exercise prescription using frequency, intensity, type, time format that divides exercise components into resistance, aerobic, and flexibility/balance is recommended.1,3
Interventions Beyond Diet and Exercise
Role of growth hormone,30,117 BCAAs,38,103,104 long chain n-3 polyunsaturated fatty acids118,119 (PUFAs), and testosterone replacement31,120,121 as plausible nutritional interventions beyond diet and exercise have been evaluated in limited studies. Oral leucine supplementation (10 g/day) along with moderate exercise showed improvement in exercise capacity and leg muscle mass.103 Fish oil-derived long chain n-3 PUFAs have shown anti-inflammatory and anti-oxidant properties.122 Lipid emulsions (10%, 2 mL/kg per day) containing n-3 PUFAs given peri-operatively in LT recipients for a week showed reduced infection-related death and shortened length of hospital stay.122,123 Similarly, oral nutritional supplementation with immunonutrient formulas containing omega-3 fatty acids, arginine, or nucleotides in LT-wait-listed patients also showed a lesser number of peri-LT infections in a pilot study.123,124 A meta-analysis of randomized controlled trials125 on various peri-operative immuno-nutrition like glutamine or omega-3 fatty acids by the parenteral or enteral route in LT recipients reported overall improved morbidity and liver function without any significant difference in survival. Intramuscular testosterone showed increase in muscle mass without a definitive effect on muscle function in a recent clinical trial in male ESLD patients with low serum testosterone.31,121 Although encouraging, the potential benefits of these interventions need to be confirmed in larger, multicenter, randomized controlled trials.
Perioperative nutritional care
Treating hepatologists, surgeons, and dieticians should formulate individualized immediate, short-term, and long-term nutritional plans for patients undergoing LT.5, 6, 7,111
Immediate Pre-operative Nutritional Care of LT Candidate
Adoption of ERAS 127 protocols help in minimizing morbidity, length of hospital stays, and improving overall outcomes.6,127,128 ERAS protocol recommends to give carbohydrate containing clear liquids up to 2 h prior to surgery to reduce starvation with early initiation enteral feeding and post-operative mobilization.126 Current evidence suggests utilizing standard nutrition regimens only as supplementation with immune enhancing diets have not shown any definite clinical benefit.6,7,111
Immediate Post-operative Nutritional Care of the LT Recipient
LT recipients usually have energy requisites equivalent to those patients who have underwent a major abdominal surgery.129 Post-operative hypermetabolic state peaks around day 10–124% of the predicted basal metabolic rate. The hypermetabolic state usually subsides between 6 and 12 months after LT.130 Early initiation of enteral nutrition usually within 12 h after the LT is recommended.6 In patients with hepatico-jejunostomy enteral, feeding is usually delayed till day 5 after LT. compared to infusing only fluids and electrolytes, post-LT enteral or parenteral nutrition has shown reduced ventilator time, ICU stay, bacterial infections, and biliary complications.6,54,131,132 Early enteral nutrition was associated with lesser infections and better nitrogen balance than parenteral nutrition.108,133 For early enteral nutrition in adult LT recipients, whole protein formulae with or without probiotics have been used and can be given via nasogastric or nasoduodenal tubes.6,108 In initial the 48 h, it is advisable to start with a lower number of calories i.e., less than 18 kcal/kg/d and proteins i.e., 1–1.5 g/kg/day with dynamic up-titration as per tolerability.29,95,127 Blood glucose levels should be closely monitored and managed the same as in other surgical patients. Immune-enhancing formulae have not shown any edge over standard nutrition supplementation.122,125 Special care should be undertaken while correcting chronic dilutional hyponatremia frequently seen in cirrhosis to avoid pontine myelinolysis. Immunosuppressants drugs-related dyselectrolytemia must be kept in mind.107
Long-term nutritional care of the LT recipient
Alterations in the Body Composition post-LT: PTMS
PTMS encompasses obesity, pre-existing diabetes, or new-onset diabetes after LT, hypertension, and dyslipidemia134,135 and salient management principles8,9,134 are outlined in Table 2. PTMS increases the risk of cardiovascular events by four times, which are the second most common non-hepatic cause of death after LT.136,137 The risk of developing PTMS after LT is nearly 33% at 3 months and 40% at 12 months after LT.138 Presence of metabolic syndrome (MetS) in pre-LT period increases the risk of PTMS.139,140 Aging post-LT population combined with increasing number of NAFLD patients undergoing LT will further increase the incidence of PTMS and cardiovascular morbidity and mortality in future.
Table 2.
Summary of Nutritional Recommendations for Liver Transplant Candidates and Recipients.
| Liver transplant candidate |
|
| Liver transplant recipients |
|
| Perioperative period |
|
| Long-term follow-up |
|
ESLD, End-stage liver disease; RFH-NPT, Royal free hospital-Nutrition prioritizing tool; BMI, Body mass index; SMI, Skeletal muscle index; CT, Computed tomography; REE, Resting energy expenditure; BCAA, Branched chain amino acids; HE, Hepatic encephalopathy; ERAS, Enhanced recovery after surgery; PTMS, Post-transplant metabolic syndrome.
The rapid weight gain in the first year after LT may be attributed to improved oral intake, reversal of catabolic state, and immunosuppressant medications.6,134,140,141 In a prospective cohort study involving malnourished ESLD on the LT-waiting list, further deterioration in nutritional status was seen at 3-months after LT with gradual improvement by 6 and 12 months, however, most gain was in fat mass with insignificant changes in the lean mass.50 A recent study prospectively evaluated CT SMI-L3 at various time-points after LT up to a mean of 19.3 ± 9 months. Out of 66% of sarcopenic patients' pre-LT, only 6% had a reversal of sarcopenia whereas 14 out of 20 non-sarcopenic patients’ pre-LT developed de-novo sarcopenia post-LT. Reversal of lost muscle mass appears challenging in this cohort even after LT.142
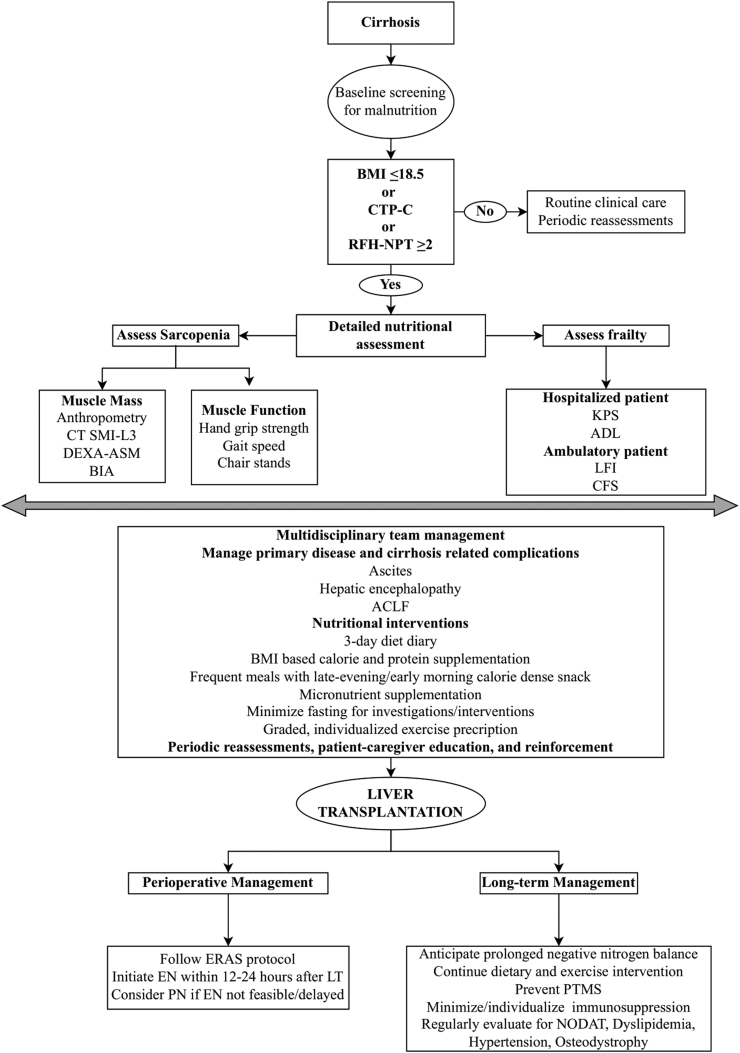
Table 3 summarizes recommendations and Figure 2 suggests a simplified algorithm for pre-operative, peri-operative, and post-LT nutritional management of an ESLD patient.
Table 3.
Salient Features and Management Principles of Various Components of PTMS.
| Obesity | NODAT | Dyslipidemia | Osteodystrophy | |
|---|---|---|---|---|
| Diagnosis |
BMI Asians: ≥25 kg/m2 Caucasians: ≥30 kg/m2 |
Persistent hyperglycemia after >45 days post-LT | Same as normal population cut-offs | BMD by DEXA |
| Incidence | Up to 30–70% Most gain 5 kg in the 1st year and 10 kg within 3 years. |
up to 30% (depending on the definition used) | Immunosuppressant drugs [mTOR inhibitors cause hypertriglyceridemia, CNIs raise LDL (cyclosporin > tacrolimus)], pre-LT MetS or dyslipidemia. | Accelerated bone loss in nearly all LT-recipients in the initial 4 months irrespective of pre-LT BMD |
| Risk factors | Age >50 years Pre-LT obesity Immunosuppressant drugs |
Immunosuppressant drugs (tacrolimus > cyclosporine), hepatitis C, cytomegalovirus infection, and pre-LT MetS. | Immunosuppressant drugs (mTOR inhibitors cause hypertriglyceridemia, CNIs raise LDL (cyclosporin > tacrolimus), pre-LT MetS or dyslipidemia. | Immunosuppressant drugs, pre-existing osteopenia |
| Impact | Cardiovascular adverse events PTMS |
Reduces graft survival, increases risk of cardiovascular events, bacterial infections and renal dysfunction. | Increased cardiovascular morbidity and mortality | Increased risk of low-impact fractures, poor quality of life |
| Management | Lifestyle changes (diet and exercise). Minimize CNI and steroid exposure. Pharmacotherapy (orlistat, GLP-1 analogs) scarce but promising data. Bariatric surgery- sleeve gastrectomy preferred. Concomitant LT and bariatric surgery can be an option in selected patients. | Stepwise approach, involve dietician and endocrinologist, dietary and lifestyle changes, individualize immunosuppression, oral anti-diabetic drugs, metformin reasonable first choice, followed by insulin, avoid sodium-glucose co-transporter-2 inhibitors | Stepwise approach, diet and lifestyle changes, and immunosuppressant minimization or switch. If lifestyle changes fail add statins, preferably pravastatin and fluvastatin (not metabolized by cytochrome P450) For isolated hypertriglyceridemia, fish oils (omega 3 fatty acids) can be used. Fibrates can be used as add-on keeping in mind the risk of rhabdomyolysis with concurrent use of statins. |
Early steroid minimization, calcium (1000–1200 mg per day) and vitamin D supplementation (400–1000 IU/day) supplementation, bisphosphonates can be considered. |
| Follow-up | Monitor weight, obesity related complications periodically and reinforce lifestyle changes on every visit. | Routine fasting glucose and HbA1c examination at 3–6 months, 12 months, and annually afterward in all LT-recipients. HbA1c target <7.0%. Screen annually for retinopathy and proteinuria. |
Fasting lipid profile at 3–6 months, 12 months, and annually afterward, target serum LDL and triglycerides to <100 mg/dl and <250 mg/dl | Regular reassessments and exercise prescription, in the first 5 years after LT, yearly screening by BMD should be done in patients with osteopenia and at every 2–3 years in patients with normal BMD. |
BMI, Body mass index; PTMS, Post-transplant metabolic syndrome; NODAT, New onset diabetes after transplant; BMD, Bone mineral densitometry; DEXA, Dual energy X-ray absorptiometry; mTOR, Mammalian target of rapamycin; CNI, Calcineurin inhibitors; LT, Liver transplantation; MetS, Metabolic syndrome; LDL, Low density lipoprotein; GLP-1, Glucagon like peptides-1.
Figure 2.
Proposed algorithm for nutritional management of liver transplant candidates and recipients. BMI: Body mass index (kg/m2), dry weight should be taken to calculate BMI in patients with ascites/edema. It can be done by either considering the post-paracentesis weight or by subtracting 5%, 10%, 15% from actual body weight in patients with mild, moderate or severe ascites, respectively. An additional 5% should be taken off if there is significant bilateral pedal oedema; CTP, Child Turcot Pugh score; RFT-NPT, Royal free hospital-nutrition prioritizing tool; CT SMI-3, Computed tomography skeletal muscle index at 3rd lumbar vertebra; DEXA, Dual energy X-ray absorptiometry; ASM, Appendicular skeletal mass index; BIA, Bioelectrical impedance analysis; KPS, Karnofsky performance scale; ADL, Activity of daily living; LFI, Liver frailty index; CFS, Clinical frailty score; ACLF, Acute-on-chronic liver failure; ERAS, Enhanced recovery after surgery; EN, Enteral nutrition; PN, Parenteral nutrition; LT, Liver transplantation; PTMS, Post-transplant metabolic syndrome; NODAT, New onset diabetes after transplant.
Almost all patients with ESLD are at-risk of malnutrition which has significant negative impact on waitlist and post-LT morbidity and mortality.3,68 Sarcopenia (muscle mass, function, and performance) and frailty are clinical and objective measures of malnutrition.15,16 There is an unmet need to develop and validate simple, safe, reliable, and population-specific tools that can perform rapid nutrition screening in an out-patient setting. Further, population specific cut-offs, not just to diagnose sarcopenia, but to grade its severity based on its clinical impact need to be derived via larger, randomized, prospective, multicenter studies. Personalized dietary interventions, periodic reassessments, and reinforcements are key to the management.1, 2, 3 Role and effectiveness of remotely monitored exercise interventions need evaluation and prospective validation. Further research to identify and modify molecular pathways responsible for proteostasis and anabolic resistance should be undertaken. In the perioperative period, ERAS protocol recommended early enteral nutrition improves outcomes.126 Immune-enhancing formulas, BCAAs, pre-biotic and probiotics have shown promising results in some studies.118,125 Special care should be given to prevent and manage PTMS and its components during long-term care of the LT recipient.134
Credit authorship contribution statement
Saurabh Mishra and Madhumita Premkumar: Literature review, writing-original draft, writing-review and editing and final approval of manuscript.
Conflicts of interest
The authors have none to declare.
Financial support and grants
None.
Disclosure statement
Nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2023.03.012.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Puri P., Dhiman R.K., Taneja S., et al. Nutrition in chronic liver disease: consensus statement of the Indian national association for study of the liver. J Clin Exp Hepatol. 2021;11:97–143. doi: 10.1016/j.jceh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merli M., Berzigotti A., Zelber-Sagi S., et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai J.C., Tandon P., Bernal W., et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74:1611–1644. doi: 10.1002/hep.32049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramachandran G., Pottakkat B. Nutritional therapy to cirrhotic patients on transplantation waiting lists. J Liver Transplant. 2022;5 doi: 10.1016/j.liver.2021.100060. [DOI] [Google Scholar]
- 5.Mazurak V.C., Tandon P., Montano-Loza A.J. Nutrition and the transplant candidate. Liver Transplant. 2017;23:1451–1464. doi: 10.1002/lt.24848. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff S.C., Bernal W., Dasarathy S., et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. 2020;39:3533–3562. doi: 10.1016/j.clnu.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Miller C.M., Quintini C., Dhawan A., et al. The international liver transplantation society living donor liver transplant recipient guideline. Transplantation. 2017;101:938–944. doi: 10.1097/TP.0000000000001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlton M., Levitsky J., Aqel B., et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102:727–743. doi: 10.1097/TP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary N.S., Saraf N., Saigal S., Soin A.S. Long-term management of the adult liver transplantation recipients. J Clin Exp Hepatol. 2021;11:239–253. doi: 10.1016/j.jceh.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuberger J. Long-term care of the adult liver transplant recipient. J Clin Exp Hepatol. 2022;12:1547–1556. doi: 10.1016/j.jceh.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16. doi: 10.1093/AGEING/AFY169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L.K., Woo J., Assantachai P., et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Bahat G., Tufan A., Tufan F., et al. Cut-off points to identify sarcopenia according to European working group on sarcopenia in older people (EWGSOP) definition. Clin Nutr. 2016;35:1557–1563. doi: 10.1016/j.clnu.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 15.Englesbe M.J., Patel S.P., He K., et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/J.JAMCOLLSURG.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tandon P., Montano-Loza A.J., Lai J.C., Dasarathy S., Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75:S147–S162. doi: 10.1016/j.jhep.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammad A., Kaido T., Aliyev V., Mandato C., Uemoto S. Nutritional therapy in liver transplantation. Nutrients. 2017;9 doi: 10.3390/nu9101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandato C., di Nuzzi A., Vajro P. Nutrition and liver disease. Nutrients. 2018;10 doi: 10.3390/nu10010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong R.J., Singal A.K. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014-2019. JAMA Netw Open. 2020;3 doi: 10.1001/JAMANETWORKOPEN.2019.20294. e1920294-e1920294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moctezuma-Velazquez C., Márquez-Guillén E., Torre A. Obesity in the liver transplant setting. Nutrients. 2019;11 doi: 10.3390/NU11112552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eslamparast T., Montano-Loza A.J., Raman M., Tandon P. Sarcopenic obesity in cirrhosis—the confluence of 2 prognostic titans. Liver Int. 2018;38:1706–1717. doi: 10.1111/liv.13876. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa H., Enomoto H., Nishiguchi S., Iijima H. Sarcopenic obesity in liver cirrhosis: possible mechanism and clinical impact. Int J Mol Sci. 2021;22:1–13. doi: 10.3390/ijms22041917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anastácio L.R., Ferreira L.G., Ribeiro H.S., et al. Sarcopenia, obesity and sarcopenic obesity in liver transplantation: a body composition prospective study. Arquivos Brasileiros de Cirurgia Digestiva. 2019;32 doi: 10.1590/0102-672020190001e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhary N.S., Saigal S., Saraf N., et al. Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant. 2015;29:211–215. doi: 10.1111/ctr.12505. [DOI] [PubMed] [Google Scholar]
- 25.Hegyi P.J., Soós A., Hegyi P., et al. Pre-transplant sarcopenic obesity worsens the survival after liver transplantation: a meta-analysis and a systematic review. Front Med. 2020;7:991. doi: 10.3389/FMED.2020.599434/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand A.C. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol. 2017;7:340–357. doi: 10.1016/j.jceh.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aller de la Fuente R. Nutrition and chronic liver disease. Clin Drug Invest. 2022;42:55–61. doi: 10.1007/s40261-022-01141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tandon P., Raman M., Mourtzakis M., Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044–1057. doi: 10.1002/hep.29003/suppinfo. [DOI] [PubMed] [Google Scholar]
- 29.Raman M., Tandon P., Merli M. Nutrition in liver cirrhosis and transplantation— current state and knowledge gaps. Nutrients. 2020;12 doi: 10.3390/nu12030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi Y. The role of growth hormone and insulin-like growth factor-I in the liver. Int J Mol Sci. 2017;18 doi: 10.3390/IJMS18071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinclair M., Grossmann M., Gow P.J., Angus P.W. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol. 2015;30:244–251. doi: 10.1111/JGH.12695. [DOI] [PubMed] [Google Scholar]
- 32.Oe O., Ve T., Ga R., et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72:1821–1832. doi: 10.1172/JCI111142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nosadini R., Avogaro A., Mollo F., et al. Carbohydrate and lipid metabolism in cirrhosis. Evidence that hepatic uptake of gluconeogenic precursors and of free fatty acids depends on effective hepatic flow. J Clin Endocrinol Metab. 1984;58:1125–1132. doi: 10.1210/jcem-58-6-1125. [DOI] [PubMed] [Google Scholar]
- 34.Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974;53:1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen O.E., Reichle F.A., Mozzoli M.A., et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981;68:240–252. doi: 10.1172/JCI110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebadi M., Bhanji R.A., Mazurak V.C., Montano-Loza A.J. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845–859. doi: 10.1007/S00535-019-01605-6/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasarathy S., Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care. 2018;21:30–36. doi: 10.1097/MCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amodio P., Bemeur C., Butterworth R., et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: international society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology. 2013;58:325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 39.Ney M., Li S., Vandermeer B., et al. Systematic review with meta-analysis: nutritional screening and assessment tools in cirrhosis. Liver Int. 2020;40:664–673. doi: 10.1111/liv.14269. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson M., Capra S., Bauer J., Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15:458–464. doi: 10.1016/S0899-9007(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 41.Kondrup J., Ramussen H.H., Hamberg O., et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/S0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 42.Rahman A., Hasan R.M., Agarwala R., Martin C., Day A.G., Heyland D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. 2016;35:158–162. doi: 10.1016/J.CLNU.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Heyland D.K., Dhaliwal R., Jiang X., Day A.G. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15 doi: 10.1186/CC10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arora S., Mattina C., McAnenny C., et al. The development and validation of a nutritional prioritizing tool for use in patients with chronic liver disease. J Hepatol. 2012;56:S241. doi: 10.1016/s0168-8278(12)60621-7. [DOI] [Google Scholar]
- 45.Morgan M.Y., Madden A.M., Soulsby C.T., Morris R.W. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology. 2006;44:823–835. doi: 10.1002/HEP.21358. [DOI] [PubMed] [Google Scholar]
- 46.Borhofen S.M., Gerner C., Lehmann J., et al. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016;61:1735–1743. doi: 10.1007/S10620-015-4015-Z. [DOI] [PubMed] [Google Scholar]
- 47.McFarlane M., Hammond C., Roper T., et al. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis. Clin Nutr ESPEN. 2018;23:156–161. doi: 10.1016/J.CLNESP.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Booi A.N., Menendez J., Norton H.J., Anderson W.E., Ellis A.C. Validation of a screening tool to identify undernutrition in ambulatory patients with liver cirrhosis. Nutr Clin Pract. 2015;30:683–689. doi: 10.1177/0884533615587537. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y., Zhu Y., Feng Y., et al. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br J Nutr. 2020;124:1293–1302. doi: 10.1017/S0007114520002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmese F., Bolondi I., Giannone F.A., et al. The analysis of food intake in patients with cirrhosis waiting for liver transplantation: a neglected step in the nutritional assessment. Nutrients. 2019;11 doi: 10.3390/NU11102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plank L.D., Gane E.J., Peng S., et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557–566. doi: 10.1002/HEP.22367. [DOI] [PubMed] [Google Scholar]
- 52.Kozeniecki M., Ludke R., Kerner J., Patterson B. Micronutrients in liver disease: roles, risk factors for deficiency, and recommendations for supplementation. Nutr Clin Pract. 2020;35:50–62. doi: 10.1002/NCP.10451. [DOI] [PubMed] [Google Scholar]
- 53.Mohommad M.K., Zhou Z., Cave M., Barve A., McClain C.J. Zinc and liver disease. Nutr Clin Pract. 2012;27:8. doi: 10.1177/0884533611433534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plauth M., Bernal W., Dasarathy S., et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Son S.W., Song D.S., Chang U.I., Yang J.M. Definition of sarcopenia in chronic liver disease. Life. 2021;11 doi: 10.3390/life11040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinclair M. Controversies in diagnosing sarcopenia in cirrhosis—moving from research to clinical practice. Nutrients. 2019;11 doi: 10.3390/nu11102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carey E.J., Lai J.C., Wang C.W., et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017;23:625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong J., Tian Y. Evaluating sarcopenia in patients with cirrhosis: the role of muscle function. J Hepatol. 2022;77:564–565. doi: 10.1016/j.jhep.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Sidhu S.S., Saggar K., Goyal O., et al. Muscle strength and physical performance, rather than muscle mass, correlate with mortality in end-stage liver disease. Eur J Gastroenterol Hepatol. 2021;33:555–564. doi: 10.1097/MEG.0000000000001761. [DOI] [PubMed] [Google Scholar]
- 61.Wu W.Y., Dong J.J., Huang X.C., et al. AWGS2019 vs EWGSOP2 for diagnosing sarcopenia to predict longterm prognosis in Chinese patients with gastric cancer after radical gastrectomy. World J Clin Cases. 2021;9:4668–4680. doi: 10.12998/wjcc.v9.i18.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng X., Shi Z.W., Yu J.J., et al. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. 2021;12:1948–1958. doi: 10.1002/jcsm.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridola L., Gioia S., Faccioli J., Nardelli S., Riggio O. Determinants of prognosis in cirrhosis: a new outlook. Hepatobiliary Surg Nutr. 2022;11:759–761. doi: 10.21037/hbsn-22-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu J., Ma J., Yang C., et al. Sarcopenia in patients with cirrhosis after transjugular intrahepatic portosystemic shunt placement. Radiology. 2022;303:711–719. doi: 10.1148/RADIOL.211172/ASSET/IMAGES/LARGE/RADIOL.211172.VA.JPEG. [DOI] [PubMed] [Google Scholar]
- 66.Tsien C., Shah S.N., Mccullough A.J., Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25:85–93. doi: 10.1097/MEG.0B013E328359A759. [DOI] [PubMed] [Google Scholar]
- 67.Tantai X., Liu Y., Yeo Y.H., et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76:588–599. doi: 10.1016/J.JHEP.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Buchard B., Boirie Y., Cassagnes L., Lamblin G., Coilly A., Abergel A. Assessment of malnutrition, sarcopenia and frailty in patients with cirrhosis: which tools should we use in clinical practice? Nutrients. 2020;12 doi: 10.3390/nu12010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodge G.A., Goenka U., Jajodia S., et al. Psoas muscle index: a simple and reliable method of sarcopenia assessment on computed tomography scan in chronic liver disease and its impact on mortality. J Clin Exp Hepatol. 2023;0 doi: 10.1016/j.jceh.2022.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tandon P., Low G., Mourtzakis M., et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480.e3. doi: 10.1016/J.CGH.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 71.Wu L.W., Lin Y.Y., Kao T.W., et al. Mid-arm muscle circumference as a significant predictor of all-cause mortality in male individuals. PLoS One. 2017;12 doi: 10.1371/JOURNAL.PONE.0171707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rush E.C., Freitas I., Plank L.D. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr. 2009;102:632–641. doi: 10.1017/S0007114508207221. [DOI] [PubMed] [Google Scholar]
- 73.Sidhu S., Saggar K., Goyal O., Kishore H., Sidhu S.S. Indian society of gastroenterology. Indian J Gastroenterol. 2018;37:1–137. doi: 10.1007/S12664-018-0911-4. 2018 37:1. [DOI] [Google Scholar]
- 74.Choudhary S., Wadhawan M., Dhawan S., et al. Normative values of skeletal muscle indices for nutritional assessment and implications on definition of sarcopenia in Indian adult population. Indian J Gastroenterol. 2022;41:69–76. doi: 10.1007/s12664-021-01207-2. [DOI] [PubMed] [Google Scholar]
- 75.Baumgartner R.N. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/J.1749-6632.2000.TB06498.X. [DOI] [PubMed] [Google Scholar]
- 76.St-Onge M.P., Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152. doi: 10.1016/J.NUT.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Irwin N.E.A., Fabian J., Hari K.R., Lorentz L., Mahomed A., Botha J.F. Myosteatosis, the more significant predictor of outcome: an analysis of the impact of myosteatosis, sarcopenia, and sarcopenic obesity on liver transplant outcomes in johannesburg, South Africa. Exp Clin Transplant. 2021;19:948–955. doi: 10.6002/ECT.2021.0083. [DOI] [PubMed] [Google Scholar]
- 78.Montano-Loza A.J., Angulo P., Meza-Junco J., et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ebadi M., Tsien C., Bhanji R.A., et al. Myosteatosis in cirrhosis: a review of diagnosis, pathophysiological mechanisms and potential interventions. Cells. 2022;11 doi: 10.3390/CELLS11071216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaibori M., Ishizaki M., Iida H., et al. Effect of intramuscular adipose tissue content on prognosis in patients undergoing hepatocellular carcinoma resection. J Gastrointest Surg. 2015;19:1315–1323. doi: 10.1007/S11605-015-2838-8/METRICS. [DOI] [PubMed] [Google Scholar]
- 81.Nishikawa H., Shiraki M., Hiramatsu A., et al. Reduced handgrip strength predicts poorer survival in chronic liver diseases: a large multicenter study in Japan. Hepatol Res. 2021;51:957–967. doi: 10.1111/HEPR.13679. [DOI] [PubMed] [Google Scholar]
- 82.Williams F.R., Milliken D., Lai J.C., Armstrong M.J. Assessment of the frail patient with end-stage liver disease: a practical overview of sarcopenia, physical function, and disability. Hepatol Commun. 2021;5:923. doi: 10.1002/HEP4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh S., Taneja S., Roy A., et al. Bedside tests of muscle function are non-inferior to skeletal muscle index in predicting outcomes in patients with cirrhosis and correlate better with health-related quality of life and cognitive function. J Clin Exp Hepatol. 2022;12:S7–S8. doi: 10.1016/j.jceh.2021.10.091. [DOI] [Google Scholar]
- 84.Wang S., Whitlock R., Xu C., et al. Frailty is associated with increased risk of cirrhosis disease progression and death. Hepatology. 2022;75:600–609. doi: 10.1002/HEP.32157. [DOI] [PubMed] [Google Scholar]
- 85.Soto R., Díaz L.A., Rivas V., et al. Frailty and reduced gait speed are independently related to mortality of cirrhotic patients in long-term follow-up. Ann Hepatol. 2021;25 doi: 10.1016/j.aohep.2021.100327. [DOI] [PubMed] [Google Scholar]
- 86.Lai J.C., Covinsky K.E., Dodge J.L., et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66:564–574. doi: 10.1002/HEP.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ (Can Med Assoc J) 2005;173:489–495. doi: 10.1503/CMAJ.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anand A., Saraya A. Assessment of sarcopenia in chronic liver disease: Indian perspective. Clin Liver Dis. 2021;18:164–167. doi: 10.1002/cld.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kardashian A., Ge J., McCulloch C.E., et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology. 2021;73:1132–1139. doi: 10.1002/HEP.31406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu C.Q., Yao F., Mohamad Y., et al. Evaluating the associations between the liver frailty index and Karnofsky performance status with waitlist mortality. Transplant Direct. 2021;7 doi: 10.1097/TXD.0000000000001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cotter T.G., Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–1864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 92.Selberg O., Böttcher J., Tusch G., Pichlmayr R., Henkel E., Müller M.J. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25:652–657. doi: 10.1002/HEP.510250327. [DOI] [PubMed] [Google Scholar]
- 93.Ferreira L.G., Santos L.F., Anastácio L.R., Lima A.S., Correia M.I.T.D. Resting energy expenditure, body composition, and dietary intake: a longitudinal study before and after liver transplantation. Transplantation. 2013;96:579–585. doi: 10.1097/TP.0b013e31829d924e. [DOI] [PubMed] [Google Scholar]
- 94.Glass C., Hipskind P., Cole D., Lopez R., Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract. 2012;27:677. doi: 10.1177/0884533612446195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferreira S., Marroni C.A., Stein J.T., et al. Assessment of resting energy expenditure in patients with cirrhosis. World J Hepatol. 2022;14:802–811. doi: 10.4254/wjh.v14.i4.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Plank L.D., Russell K. Nutrition in liver transplantation: too little or too much? Curr Opin Clin Nutr Metab Care. 2015;18:501–507. doi: 10.1097/MCO.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 97.Vaisman N., Katzman H., Carmiel-Haggai M., Lusthaus M., Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr. 2010;92:137–140. doi: 10.3945/ajcn.2010.29211. [DOI] [PubMed] [Google Scholar]
- 98.Córdoba J., López-HellÍn J., Planas M., et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 99.Ney M., Abraldes J.G., Ma M., et al. Insufficient protein intake is associated with increased mortality in 630 patients with cirrhosis awaiting liver transplantation. Nutr Clin Pract. 2015;30:530–536. doi: 10.1177/0884533614567716. [DOI] [PubMed] [Google Scholar]
- 100.Uribe M., Dibildox M., Malpica S., et al. Beneficial effect of vegetable protein diet supplemented with psyllium plantago in patients with hepatic encephalopathy and diabetes mellitus. Gastroenterology. 1985;88:901–907. doi: 10.1016/s0016-5085(85)80006-8. http://www.ncbi.nlm.nih.gov/pubmed/2982694 [DOI] [PubMed] [Google Scholar]
- 101.Keshavarzian A., Meek J., Sutton C., Emery V.M., Hughes E.A., Hodgson H.J. Dietary protein supplementation from vegetable sources in the management of chronic portal systemic encephalopathy. Am J Gastroenterol. 1984;79:945–949. http://www.ncbi.nlm.nih.gov/pubmed/6391154 [PubMed] [Google Scholar]
- 102.Iqbal U., Jadeja R.N., Khara H.S., Khurana S. A comprehensive review evaluating the impact of protein source (vegetarian vs. meat based) in hepatic encephalopathy. Nutrients. 2021;13:1–15. doi: 10.3390/nu13020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakaya Y., Okita K., Suzuki K., et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113–120. doi: 10.1016/j.nut.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 104.Tsien C., Davuluri G., Singh D., et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gluud L.L., Dam G., Les I., et al. In: Cochrane Database of Systematic Reviews. Gluud L.L., editor. John Wiley & Sons, Ltd; 2015. Branched-chain amino acids for people with hepatic encephalopathy. [DOI] [Google Scholar]
- 106.Sidhu S.S., Goyal O., Singh S., Kishore H., Chhina R.S., Sidhu S.S. Early feeding after esophageal variceal band ligation in cirrhotics is safe: randomized controlled trial. Dig Endosc. 2019;31:646–652. doi: 10.1111/DEN.13423. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H., Wang Y., Sun S., et al. Early enteral nutrition versus delayed enteral nutrition in patients with gastrointestinal bleeding: a PRISMA-compliant meta-analysis. Medicine. 2019;98 doi: 10.1097/MD.0000000000014864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mehtani R., Premkumar M., Kulkarni A.V. Nutrition in critical care hepatology. Curr Hepat Rep. October 5, 2022 doi: 10.1007/s11901-022-00586-0. Published online. [DOI] [Google Scholar]
- 109.McClave S.A., Dibaise J.K., Mullin G.E., Martindale R.G. ACG clinical guideline: nutrition therapy in the adult hospitalized patient. Am J Gastroenterol. 2016;111:315–334. doi: 10.1038/ajg.2016.28. [DOI] [PubMed] [Google Scholar]
- 110.Elke G., van Zanten A.R.H., Lemieux M., et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20:1–14. doi: 10.1186/S13054-016-1298-1/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Puri P., Thursz M. Intensive enteral nutrition in alcoholic hepatitis: more food for thought. Gastroenterology. 2016;150:803. doi: 10.1053/J.GASTRO.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campos-Varela I., Gómez-Gavara C., Augustin S. Recommendations and guidance on nutritional supplementation in the liver transplant setting. Transplantation. 2021;105:2528–2537. doi: 10.1097/TP.0000000000003736. [DOI] [PubMed] [Google Scholar]
- 113.Patel N., Muñoz S.J. Bone disease in cirrhosis. Clin Liver Dis. 2015;6:96–99. doi: 10.1002/CLD.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen Y.C., Chang Y.H., Fang C.J., Lin Y.S. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr J. 2019;18:1–9. doi: 10.1186/S12937-019-0461-3/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duarte-Rojo A., Ruiz-Margáin A., Montaño-Loza A.J., Macías-Rodríguez R.U., Ferrando A., Kim W.R. Exercise and physical activity for patients with end-stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transplant. 2018;24:122–139. doi: 10.1002/lt.24958. [DOI] [PubMed] [Google Scholar]
- 116.Sirisunhirun P., Bandidniyamanon W., Jrerattakon Y., et al. Effect of a 12-week home-based exercise training program on aerobic capacity, muscle mass, liver and spleen stiffness, and quality of life in cirrhotic patients: a randomized controlled clinical trial. BMC Gastroenterol. 2022;22 doi: 10.1186/s12876-022-02147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meena B.L., Taneja S., Tandon P., et al. Home-based intensive nutrition therapy improves frailty and sarcopenia in patients with decompensated cirrhosis: a randomized clinical trial. J Gastroenterol Hepatol. 2022 doi: 10.1111/JGH.16035. Published online November 9. [DOI] [PubMed] [Google Scholar]
- 118.Donaghy A., Ross R., Wicks C., et al. Growth hormone therapy in patients with cirrhosis: a pilot study of efficacy and safety. Gastroenterology. 1997;113:1617–1622. doi: 10.1053/gast.1997.v113.pm9352864. [DOI] [PubMed] [Google Scholar]
- 119.Vielma F.H., Valenzuela R., Videla L.A., Zúñiga-Hernández J. N-3 polyunsaturated fatty acids and their lipid mediators as A potential immune-nutritional intervention: a molecular and clinical view in hepatic disease and other non-communicable illnesses. Nutrients. 2021;13 doi: 10.3390/NU13103384. 3384-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang M., Ma L.J., Yang Y., Xiao Z., Wan J.B. n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: a critical review. Crit Rev Food Sci Nutr. 2019;59(suppl 1):S116–S129. doi: 10.1080/10408398.2018.1544542. [DOI] [PubMed] [Google Scholar]
- 121.Yurci A., Yucesoy M., Unluhizarci K., et al. Effects of testosterone gel treatment in hypogonadal men with liver cirrhosis. Clin Res Hepatol Gastroenterol. 2011;35:845–854. doi: 10.1016/j.clinre.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 122.Sinclair M., Grossmann M., Hoermann R., Angus P.W., Gow P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016;65:906–913. doi: 10.1016/J.JHEP.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 123.Gao B., Luo J., Liu Y., et al. Clinical efficacy of perioperative immunonutrition containing omega-3-fatty acids in patients undergoing hepatectomy: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab. 2020;76:375–386. doi: 10.1159/000509979. [DOI] [PubMed] [Google Scholar]
- 124.Waitzberg D.L., Saito H., Plank L.D., et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30:1592–1604. doi: 10.1007/S00268-005-0657-X. [DOI] [PubMed] [Google Scholar]
- 125.Russell K., Zhang H.G., Gillanders L.K., et al. Preoperative immunonutrition in patients undergoing liver resection: a prospective randomized trial. World J Hepatol. 2019;11:305. doi: 10.4254/WJH.V11.I3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lei Q., Wang X., Zheng H., Bi J., Tan S., Li N. Peri-operative immunonutrition in patients undergoing liver transplantation: a meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2015;24:583–590. doi: 10.6133/APJCN.2015.24.4.20. [DOI] [PubMed] [Google Scholar]
- 127.Brustia R., Monsel A., Skurzak S., et al. Guidelines for perioperative care for liver transplantation. Transplantation. 2021;106:552–561. doi: 10.1097/TP.0000000000003808. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Q.K., Wang M.L. The management of perioperative nutrition in patients with end stage liver disease undergoing liver transplantation. Hepatobiliary Surg Nutr. 2015;4:336–344. doi: 10.3978/j.issn.2304-3881.2014.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hammad A., Kaido T., Uemoto S. Perioperative nutritional therapy in liver transplantation. Surg Today. 2015;45:271–283. doi: 10.1007/s00595-014-0842-3. [DOI] [PubMed] [Google Scholar]
- 130.Silva T.A., Maia F. de CP., Zocrato M.C.A., Mauricio S.F., Correia M.I.T.D., Generoso S. de V. Preoperative and postoperative resting energy expenditure of patients undergoing major abdominal operations. J Parenter Enteral Nutr. 2021;45:152–157. doi: 10.1002/JPEN.1825. [DOI] [PubMed] [Google Scholar]
- 131.Sugihara K., Yamanaka-Okumura H., Teramoto A., et al. Recovery of nutritional metabolism after liver transplantation. Nutrition. 2015;31:105–110. doi: 10.1016/J.NUT.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 132.Hasse J.M., Blue L.S., Liepa G.U., et al. Early enteral nutrition support in patients undergoing liver transplantation. JPEN - J Parenter Enter Nutr. 1995;19:437–443. doi: 10.1177/0148607195019006437. [DOI] [PubMed] [Google Scholar]
- 133.Hill A., Heyland D.K., Ortiz Reyes L.A., et al. Combination of enteral and parenteral nutrition in the acute phase of critical illness: an updated systematic review and meta-analysis. JPEN - J Parenter Enter Nutr. 2022;46:395–410. doi: 10.1002/JPEN.2125. [DOI] [PubMed] [Google Scholar]
- 134.Kim J.M., Joh J.W., Kim H.J., et al. Early enteral feeding after living donor liver transplantation prevents infectious complications. Medicine. 2015;94 doi: 10.1097/MD.0000000000001771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fatourou E.M., Tsochatzis E.A. Management of metabolic syndrome and cardiovascular risk after liver transplantation. Lancet Gastroent Hepatol. 2019;4 doi: 10.1016/S2468-1253(19)30181-5. www.thelancet.com/gastrohep [DOI] [PubMed] [Google Scholar]
- 136.de Andrade A.R.C.F., Bittencourt P.L., Codes L., et al. New onset diabetes and non-alcoholic fatty liver disease after liver transplantation. Ann Hepatol. 2017;16:932–940. doi: 10.5604/01.3001.0010.5285. [DOI] [PubMed] [Google Scholar]
- 137.Madhwal S., Atreja A., Albeldawdi M., Lopez R., Post A., Costa M.A. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transplant. 2012;18:1140–1146. doi: 10.1002/LT.23508. [DOI] [PubMed] [Google Scholar]
- 138.Albeldawi M., Aggarwal A., Madhwal S., et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transplant. 2012;18:370–375. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 139.Becchetti C., Ferrarese A., Zeni N., et al. A prospective longitudinal assessment of de novo metabolic syndrome after liver transplantation. Clin Transplant. 2022;36 doi: 10.1111/CTR.14532. [DOI] [PubMed] [Google Scholar]
- 140.Herreras López J., Puchades L., di Maira T., et al. Metabolic syndrome before liver transplantation: does it have an impact on post liver transplantation outcome? Rev Esp Enferm Dig. 2022;114:586–591. doi: 10.17235/reed.2022.8384/2021. [DOI] [PubMed] [Google Scholar]
- 141.Vida Perez L., Montero Alvarez J.L., Poyato Gonzalez A., et al. Prevalence and predictors of metabolic syndrome after liver transplantation. Transplant Proc. 2016;48:2519–2524. doi: 10.1016/j.transproceed.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 142.Merli M., Giusto M., Riggio O., et al. Improvement of nutritional status in malnourished cirrhotic patients one year after liver transplantation. E Spen Eur E J Clin Nutr Metab. 2011;6:e142–e147. doi: 10.1016/J.ECLNM.2011.02.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.