Abstract
The subtropical fruit known as the loquat is prized for both its flavour and its health benefits. The perishable nature of loquat makes it vulnerable to several biotic and abiotic stressors. During the previous growing season (March–April 2021), loquat in Islamabad showed signs of fruit rot. Loquat fruits bearing fruit rot symptoms were collected, and the pathogen that was causing the disease isolated and identified using its morphology, microscopic visualisation, and rRNA sequence. The pathogen that was isolated was identified as Fusarium oxysporum. Green synthesized metallic iron oxide nanoparticles (Fe2O3 NPs) were employed to treat fruit rot disease. Iron oxide nanoparticles were synthesized using a leaf extract of the Calotropis procera. Characterization of NPs was performed by different modern techniques. Fourier transform infrared spectroscopy (FTIR) determined the existence of stabilizing and reducing compounds like phenol, carbonyl compounds, and nitro compounds, on the surface of Fe2O3 NPs. X-ray diffraction (XRD) explained the crystalline nature and average size (~49 nm) of Fe2O3 NPs. Energy dispersive X-ray (EDX) exhibited Fe and O peaks, and scanning electron microscopy (SEM) confirmed the smaller size and spherical shape of Fe2O3 NPs. Following both in vitro and in vivo approaches, the antifungal potential of Fe2O3 NPs was determined, at different concentrations. The results of both in vitro and in vivo analyses depicted that the maximum fungal growth inhibition was observed at concentration of 1.0 mg/mL of Fe2O3 NPs. Successful mycelial growth inhibition and significantly reduced disease incidence suggest the future application of Fe2O3 NPs as bio fungicides to control fruit rot disease of loquat.
Keywords: Fusarium oxysporum, Fruit rot, Green synthesis, Calotropis procera, Fe2O3 NPs, NPs characterization
Introduction
Loquat (Eriobotrya japonica Lindl.) is a member of family Rosaceae, subfamily Pomoideae, and it is mostly cultivated in subtropical areas [1]. Loquat tree has medicinal and ornamental importance. It is used to treat respiratory disorders, infection, and cancer [2, 3]. Major loquat producing countries are China, Pakistan, Mauritius Island, Japan, Reunion Island, India, and Mediteranean countries [4–6]. Approximate fruit production of loquat is 549,220 tonnes, worldwide. China is the major fruit producing country followed by Spain, Japan, India, and Pakistan [7, 8]. In Pakistan, loquat is grown in Murree, Hasan Abdal, Mardan, Tret, Kalar Kahar, Wah, Haripur, Sargodha, Choa Saiden Shah, Kasur, and Chhattar [9]. In Pakistan, Punjab and Khyber Pakhtunkhwa are the major loquat producing provinces [10].
Fungi are major biotic factors to causes 50% yield losses in fruit crops. Approximately one third of all food crops are destroyed by fungal pathogens [11]. About 248 species of fungi have been reported to cause diseases of E. japonica [12]. Colletotrichum gloeosporioides and Colletotrichum truncatum have been reported to cause pre-harvest and post-harvest diseases of loquat [13, 14]. Previous studies have described fungal diseases of loquat including anthracnose [15], fruit rot by Colletotrichum species [16], fruit rot by Lasiodiplodia theobromae [17, 18], circular brown leaf spot [19], Fusarium fruit rot [17], and scab by Fusicladium eriobotryae [20–22]. In Pakistan, few major fungi including Diplodia seriata [23], Fusarium solani [17], C. gloeosporioides [24], Curvularia lunata [25], and Alternaria mali [26] have been reported to cause diseases on different parts of loquat tree.
Use of chemical fungicide is very common in different parts of the world [27]. In Pakistan, approximately 108 types of insecticides are being used [28]. Fungicides have possible side effects, and they have been reported to affect human health [29]. Due to these health risks, scientists are working on environment-friendly disease control technologies for controlling post-harvest fruit disease [30, 31]. Nowadays, there is too much attention for alternatives of chemical fungicides and development of biological control measures against plant diseases [32]. Due to their biological origin, bio fungicides have minimal toxicity and increased resistance against diseases, and they are cheaper [33].
Nanotechnology is inspiring weapon against many factors that threaten plant health [34]. Green synthesis of nanoparticles involving the use of plants to synthesize metallic nanoparticles is safe and has environment friendly approach. To synthesize nanoparticles, different plant parts such as stem, seeds, leaves, roots, and fruits are being used. All these parts contain phytochemicals that have stabilizing and reducing abilities [35]. The aqueous leaf extract of C. procera has been used to synthesize nanoparticles. Antimicrobial potential of C. procera is well-documented [36]. To overcome drawbacks of classical hazardous disease-control methods, use of plants to synthesize nanoparticles is a novel approach, as the phytochemicals of plants act as capping and reducing agents [37]. Efficient application of Fe2O3 NPs in controlling fruit rot diseases has been reported earlier [38]. Iron oxide nanoparticles synthesized in aqueous leaf extract of Calotropis procera has been reported to control fruit rot of cherry [39]. Iron oxide NPs have also diminished the rot of apple [40].
The goal of this study was to synthesize iron oxide nanoparticles using the aqueous leaf extract of C. procera that could be used to treat post-harvest pathogen of loquat.
Materials and methods
Collection of diseased fruit
During the months of March–April 2021, field surveys were conducted, and diseased loquat samples were collected from the orchards of Quaid-i-Azam University Islamabad. Collected fruits were transferred to the lab in sterile polythene bags for further analyses [39].
Isolation of pathogen
Loquat fruit surface sterilization was done with 1% sodium hypochlorite solution (1 mL of sodium hypochloride was dissolved in 99 mL of distilled water) and then washed with distilled water for 2 min before isolation of causative agent. With sterilized blade, segments of diseased fruits were cut from edges and put on potato dextrose agar (PDA) media plates. Petri plates were sealed using parafilm and were kept in an incubator at 25 ± 2 °C. After 5 to 7 days, fungus morphology was observed by observing the colony growth, color, and its pattern from front and backside of the plate [40].
In vivo pathogenicity test
Pathogenicity was confirmed by following Koch’s postulates. Mycelia of 7-day-old fungus were transferred to Czapek broth media and placed in shaking incubator at 25 ± 2 °C. After achieving desired concentration of conidia suspension (106 conidia/ mL), broth culture was filtered to remove mycelia. To infect healthy loquat fruit, wounded sterile needle was used, and 5 μL conidial suspension was injected into three randomly selected fruit. Control fruits were injected with sterile distilled water. To protect all treated fruits, muslin cloth was used and kept in an incubator at 25 ± 2 °C. After 1 week, symptoms of disease with earlier field symptoms were compared. Pathogen was re-isolated by culturing on PDA media for 5–7 days at 25 ± 2 °C [39].
Microscopic identification
For identification, hyphae along with reproductive structures of isolated fungus were analyzed under light microscope. Slides were prepared using lactophenol blue [41]. Drop of lactophenol blue was placed in the center of slide, and growing mycelium was mounted on slides. By avoiding air bubbles, cover slip was placed, and slide was observed under microscope at ×100 magnification to examine spores, hyphae, and other pathogenic characteristics [42].
Molecular characterization and phylogenetic analysis
For genetic identification, rDNA of isolated fungus was amplified using specific small subunit ribosomal RNA primers. CTAB method was used for the extraction of fungal DNA [43]. The fungal mycelia (50 mg) were scraped from 10-day-old PDA cultures, manually ground in 1.5 mL of microfuge tubes with micro pestle adding 500 μL of pre-warmed (60 °C) TES lysis buffer (100 mM Tris pH 8.0; 10 mM EDTA pH 8.0; 2% SDS) of proteinase K that was added to the ground material, incubated in 60 °C for 60 min. One hundred forty microliter of 5M NaCl and 64 μL of 10% (w/v) of CTAB were added to the suspension incubated at 65 °C for 10 min. DNAs were extracted by adding equal volume of chloroform: isoamylalcohol (24:1) centrifuged at 14000 × g/10 min. DNA was precipitated by adding 0.6 volume of cold isopropanol and 0.1 volume of 3M sodium acetate pH 5.2, centrifuged, and washed twice with 70% ethanol suspended in 100 μL of TE (10 mM Tris pH 8.0; 1 mM EDTA pH 8.0). RNA was digested by adding 10 mg/mL of RNAse A and incubating at 37 °C for 45 min and stored at −20 °C for further use. For the amplification of rRNA, in polymerase chain reaction (PCR), ITS1 forward (TCCGTAGGTGAACCTGCGG) and ITS4 reverse (TCCTCCGCTTATTGATATGC) primers were used [44]. Reaction mixture contains 0.5 μL of dNTPs, 1.5 μL of Taq DNA polymerase, 5 μL of 10× polymerase buffer, 1 μL of genomic DNA, and 1 μL of each primer. PCR reaction temperature was 94 °C for 4 min, followed by 35 cycles of 94 °C for 40 s, 58 °C for 40 s, and 72 °C for 40 s. Sequencing of amplified PCR product was done and subjected to BLAST analysis on NCBI database. MEGA 7 with maximum likelihood method with 1000 bootstrap was used for phylogenetic analysis [45].
Preparation of leaf extract
To prepare extract, fresh leaves of Calotropis procera were washed and shade dried for 1 week. Leaves were ground into fine powder. C. procera extract was prepared by mixing of 30 g of powder into 300 mL of de-ionized water and boiled for 5 to 10 min. Solution was warmed up in water bath at temperature of 80 °C for 30 min. Extract was permissible to cool down and then filtered through muslin cloth. Further filtration was performed using Whatman filter paper and kept at 4 °C for storage [39].
Calotropis procera-mediated synthesis of iron nanoparticles
For the preparation of Fe2O3 NPs, 1 mM FeCl3 solution was prepared and added into C. procera extract in 1:1 ratio. Heating temperature of hot plate for blend was 80 °C for 120 min, until the color was changed. Color change was the indication of reduction process of Fe2O3 NPs. Mixture was centrifuged for 10 min at 6000 rpm, and the pellet was washed with de-ionized water. Pellet was dried in an incubator for 3 to 4 h at 100 °C. Crystalline form of Fe2O3 NPs was obtained by calcination at 500 °C for 2 to 3 h. The synthesized nanoparticles were characterized before their antifungal activity analyses [39].
Characterization of nanoparticles
Fe2O3 NPs were characterized by the different laboratory techniques [46].
FTIR spectroscopy
Fourier transform infrared spectroscopy (FTIR) was used to determine the nature and types of functional groups associated with iron nanoparticles, in the range of 400 to 4000 cm−1 with resolution power of 4cm−10. KBr pellet method was adopted to prepare sample by enveloping 10 mg of nanoparticles in 100 mg of KBr pellet.
XRD
To understand the nature and size of nanoparticles, X-ray diffraction (XRD) spectroscopy was used. Size of synthesized Fe2O3 NPs was assessed by the following Scherrer equation:
D is the crystalline size, shape factor is K, λ is the wavelength of X-ray, ß is the full width at half-maximum of radians, and θ is angle of diffraction.
SEM-EDX characterization
Scanning electron microscopy (SEM) was used to see the morphology of NPs. There was use of energy dispersive X-ray spectroscopy (EDX) to reveal elemental composition of Fe2O3 NPs. Fe and O peaks were observed in X-ray spectra.
In vitro antifungal assay of Fe2O3 NPs
Fe2O3 NP fungicidal activity against F. oxysporum was tested by poisoned food technique [47]. Five concentrations of Fe2O3 NPs (0.1, 0.25, 0.50, 0.75, and 1 mg/ ml) were mixed with PDA media. Fungal discs (5 mm) were positioned in the middle of each Petri plate. One Petri plate with no Fe2O3 NPs was used as a control. Plates were kept in an incubator at 25 °C. Percentage inhibitions and diameters of fungal mycelia were measured at regular intervals in 7 days. Following formula was used to calculate inhibition percentage of fungal growth:
where C is the growth in the control and T is the sample mycelial growth [36].
In vivo antifungal activity analysis of Fe2O3 NPs
For the control of disease on loquat fruit, Fe2O3 NPs were used, following “detached fruit inoculation method” [48]. For this purpose, 18 fruits were surface sterilized, wounded with needle, and inoculated with fungus. After inoculation of fungus, Fe2O3 NPs were applied in five different concentrations (0.1, 0.25, 0.50, 0.75, and 1.0 mg/mL). Each concentration was applied on three inoculated fruit, and all samples were kept at 25 °C in an incubator. Lesions on fruit were observed, and area of lesion was measured in regular intervals of 72 h of treatment.
Statistical analysis
Experimental data was statistically analyzed by following one-way ANOVA. The significance of difference between treatments was tested by least significance difference (LSD) post hoc test (α = 0.05). All data expressed as mean ± standard error.
Results
Morphological and microscopic identification of pathogen
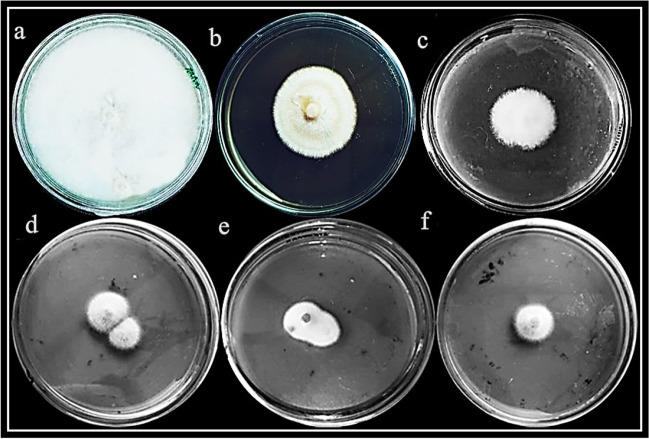
Diseased samples were collected (Fig. 1a), and after isolation from diseased fruit, the color of aerial mycelium was faded white, which later changed to purplish blue color (Fig. 1b). Mycelial color, on the backside of petri plates, was light yellow (Fig. 1c). Macro conidial septation was observed under microscope (Fig. 1d).
Fig. 1.
Fruit rot symptoms were observed on loquat fruit (a). Disease-causing pathogen was isolated and observed from the front side (b) and back side of the Petri plates (c). Microscopic observation of fungus was performed at ×40 magnification (d). Fungus was re-inoculated on healthy fruit, and disease symptoms were observed after 3 days (e) and 5 days post inoculation (f). Pathogen was re-isolated on PDA and observed from front side (g) and back side (h)
Abundant chlamydospores were produced. These all-surface pattern and microscopic examinations demonstrated this pathogen as F. oxysporum [17].
In vivo pathogenicity test
Koch’s postulates validated pathogenicity of isolated F. oxysporum. After 3 days of inoculation of healthy fruit, light brown disease circles were observed (Fig. 1e), which enlarged in size, later (Fig. 1f). These disease symptoms were similar to the symptoms of initially collected diseased fruit. Fungus was re-isolated and showed similar growth pattern to inoculated fungus (Fig. 1g, h). Based on these findings, F. oxysporum was demonstrated as causal agent of loquat fruit rot disease.
Molecular identification and phylogenetic analysis of isolated fungus
Isolated fungus sequence was 100% similar with F. oxysporum strain FO_99 (Accession no. MT649536.1). The sequence was submitted in NCBI database with accession number OL744437. Maximum likelihood method was successfully used to construct phylogenetic tree, which showed that the presence of obtained sequence is the same clade with F. oxysporum (Fig. 2). It also confirmed the evolutionary relationship of isolated fungus with F. oxysporum.
Fig. 2.

Phylogenetic analysis of isolated pathogen with 15 related gene sequences from GenBank
Characterization of green synthesized Fe2O3 NPs
FTIR
FTIR spectra of Fe2O3 NPs showed different absorption band (Fig. 3). Absorption peak at 3414.48 cm−1 represented O-H stretching. Absorption peak at 1739.97 cm−1 showed C=O stretch of aldehyde group. Stretching vibrations at 1653.12 cm−1 described alkene group while 1599.47 cm−1 attributed polyphenols [49]. Stretching vibrations at 1558.83 cm−1 and 1506.56 cm−1 demonstrated nitro compounds (N-O). Peak at 1367.44 cm−1 signified S=O stretching of sulfonamide group. Peak at 1149.97 cm−1 indicated C-O stretching of aliphatic ether. Stretching vibrations of Fe-OFe were observed at 667 cm−1, 512 cm−1, and 433 cm−1 [14].
Fig. 3.
FTIR spectrum of Fe2O3 NPs synthesized in the leaf extract of C. procera
XRD
Crystalline structure of Fe2O3 NPs was described by XRD spectroscopy (Fig. 4). XRD pattern showed five notable peaks at 2θ angle between 100 and 800. Peaks were observed at 110, 15.80, 20.50, 30.70, and 430. The average size of nanoparticle was observed to be 49 nm. Sharp peaks revealed crystalline nature of Fe2O3 NPs. Crystalline nature of NPs is inclined by plant extract [43]. Other than these peaks, there were no other detectable peaks, which indicated the purity of sample. XRD pattern of Fe2O3 NPs was in accordance with previous studies [7].
Fig. 4.
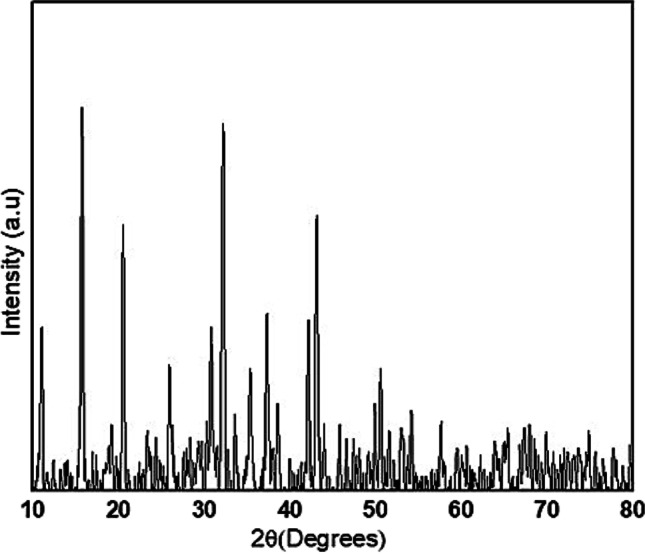
XRD pattern of Fe2O3 NPs synthesized in the leaf extract of C. procera
SEM and EDX analyses
SEM analysis explored the surface of synthesized NPs (Fig. 5). From the micrographs, spherical shape of Fe2O3 NPs was observed. These NPs were present in a size range of 40 to 50 nm and confirmed the size of NPs, estimated from XRD spectra. Fe2O3 NPs were spherical in shape. Peaks of Fe and O in EDX confirmed the formation of NPs. Weight percentage of chlorine, iron, and oxygen was 45.67%, 43.06%, and 20.18%, respectively (Fig. 6).
Fig. 5.

SEM of Fe2O3 NPs synthesized in the leaf extract of C. procera
Fig. 6.

Energy dispersive X-ray (EDX) spectroscopy of Fe2O3 NPs revealed the presence of Fe, Cl, and O
Antifungal activity of Fe2O3 NPs, in vitro
NPs of Fe2O3 showed maximum mycelial growth suppression (72%) at the concentration of 1 mg/mL. While growth inhibition at 0.75, 0.50, 0.25, and 0.1 mg/mL was 64.44%, 60%, 52%, and 37% (Table 1) (Fig. 7).
Table 1.
Mycelial growth inhibition at different concentration of Fe2O3 NPs
| Concentration mg/mL | Inhibition percentage |
| 1.0 | 72.22 ± 1.0 |
| 0.75 | 64.44 ± 1.0 |
| 0.50 | 60.77 ± 0.5 |
| 0.25 | 52.22 ± 0.8 |
| 0.1 | 37.77 ± 1.2 |
Fig. 7.
Inhibitory effect of Fe2O3 NPs on F. oxysporum using five different concentrations of NPs. Fungus growth was observed in control (a) and at five concentrations of NPs including 0.1 mg/mL (b), 0.25 mg/mL (c), 0.50 mg/mL (d), 0.75 mg/mL (e), and 1.0 mg/mL (f)
Antifungal activity of Fe2O3 NPs, in vivo
In vivo antifungal activity analysis revealed the best disease control at concentration of 1.0 mg/mL of Fe2O3 NPs. All concentration of Fe2O3 NPs exhibited variable disease control (Fig. 8).
Fig. 8.
Control of fruit rot disease using Fe2O3 NPs on loquat. Control fruit exhibited maximum disease (a). Variable fruit rot disease was observed at 0.1 mg/mL (b), 0.25 mg/mL (c), 0.50 mg/mL (d), 0.75 mg/mL (e), and 1.0 mg/mL (f) concentration of Fe2O3 NPs
Discussion
Most frequent disease of loquat and other perishable fruits is fruit rot [15]. This work includes a thorough examination of the mycobiota linked to the fruit rot of loquat in Islamabad. To obtain pure colonies, the pathogen was isolated from rotten fruit segments and cultured on Petri plates. Utilizing morphological and molecular traits, fungal isolates were identified. Isolated fungus from loquat was identified as F. oxysporum. Microscopic observation revealed similar morphology, and macro conidial septation has been described earlier [50].
To control plant diseases, biocontrol efforts are being done for last few decades. Nanomaterials have gained popularity in the modern period as a result of their unique features and important applications in the food and agricultural industries [51]. The manufacturing of nanoparticles has advanced greatly, and a wide range of biological agents, including bacteria and plants, are being exploited [52]. Use of plants to synthesize metallic nanoparticles is a more simple method, and many plant metabolites act as stabilizing and reducing agents [49].
In this study, C. procera was successfully used to synthesize iron oxide nanoparticles. Antifungal potential of C. procera-mediated NPs has been described earlier [39]. With the aid of FTIR, XRD, SEM, and EDX, iron oxide nanoparticle characterization was performed. Different functional groups were identified by FTIR spectrum. These functional groups consist of bioactive molecules. While reviewing FTIR spectra, amino groups were present, and they show the presence of proteins all around iron oxide NPs. Results affirmed that protein molecules are present in the extract, and they are able to function as stabilizing and reducing agent by attachment to iron oxide NPs, with the aid of free primary amino group [53]. Carboxylic groups help to bind on Fe surface [54]. XRD affirmed crystal nature and smaller size of NPs. Previous research has demonstrated that small size and crystalline structure of NPs provide them strong antimicrobial capabilities [40, 46].
SEM images revealed spherical shape of iron oxide NPs. Additionally, high surface area to volume ratio was observed that depicted that nanoparticles have a propensity to aggregate in suspension [55]. An EDX study verified the elemental composition of the iron oxide nanoparticles’ elemental makeup. Iron oxide nanoparticles’ EDX spectrum pattern revealed narrow peaks that confirmed the crystalline structure of the iron nanoparticles [56]. The EDX spectra revealed the existence of iron peaks in two distinct regions. The culture filtrate may be responsible for the existence of additional organic substances like O and C [57]. Iron oxide NPs were synthesized using a precursor solution that contained the element chlorine [58].
In this study, different concentrations of iron oxide nanoparticles synthesized using C. procera (0.1, 0.25, 0.5, 0.75, and 1 mg/mg) were utilized to both in vitro and in vivo test to suppress the fungus that causes fruit rot disease in loquat. It was found that the antifungal activity increased with increasing NP concentration. NP application results in membrane disability of fungus, by producing active oxygen radical suppression of transporter genes and genotoxicity [59]. NPs affect morphological properties of fungal membrane like depolarization of membrane and permeability. This breakdown causes leakage of different substances like enzymes, proteins, and DNA that result in cell death. Furthermore, NPs penetrate through the holes of cell wall of microorganisms [60]. NPs also cause lipid peroxidation and depletion fungal membrane ergosterol contents [61]. Higher ratios of surface-to-volume allow NPs to adhere the fungal cell surface, penetrate directly into cell, and cause damage of cell wall [62]. In the past, it has been reported that mycosynthesized iron oxide nanoparticles can prevent apple brown rot [46]. Green iron oxide nanoparticles prevented cherry fruit rot [40]. Citrus brown rot was prevented by bio-fabricated iron oxide nanoparticles [39].
This study has described a simple, predictable, and ecologically secure technique of preventing fruit rot disease of loquat by using green iron oxide nanoparticles. It has been concluded that the synthesized Fe2O3 NPs show effective antifungal properties The outcomes showed that rot disease loquat could be effectively controlled by using the optimal concentration of Fe2O3 NPs (1.0 mg/ml).
Author contribution
F.N. conducted all experiments, collected data, and wrote up the manuscript; M.A. compiled and organized the data; U.H. supervised the isolation of strains; F. and A.K. helped in the characterization of nanoparticles, T.R. and F.A. assisted in antifungal activity analyses, R.N. assisted in field experiments, H.J.C. supervised the write-up, and M.F.H.M. designed and supervised the whole study. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Higher Education Commission (HEC), Pakistan, under NRPU project No: 9739/Federal/NRPU/R&D/HEC/2017. The authors acknowledge the support from Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data availability
Not applicable
Code availability
Not applicable
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Badenes ML, Martinez-Calvo J, Llacer G. Analysis of a germplasm collection of loquat (Eriobotrya japonica Lindl.) Euphytica. 2000;114(3):187–194. [Google Scholar]
- 2.Li JQ, Hou CX, Luo N, Deng QX, Wang YQ. II International Symposium on Loquat. 2006. Direct embyogenesis from anther culture of loquat; pp. 209–214. [Google Scholar]
- 3.Vilanova S, Badenes ML, Martínez-Calvo J, Llácer G. Analysis of loquat germplasm (Eriobotrya japonica Lindl) by RAPD molecular markers. Euphytica. 2001;121(1):25–29. [Google Scholar]
- 4.Razeto B, Reginato G, Rojas S. Chemical thinning of loquat with naphthalene acetic acid. Horttechnology. 2003;13(1):128–132. [Google Scholar]
- 5.Yang Q, Fu Y, Wang Y, Liu L, Li X, Peng S. Identification of 21 novel S-RNase alleles and determination of S-genotypes in 66 loquat (Eriobotrya) accessions. Mol Plant Breed. 2018;389(5):1–13. [Google Scholar]
- 6.Zhang W, Zhao X, Sun C, Li X, Chen K. Phenolic composition from different loquat (Eriobotrya japonica Lindl.) cultivars grown in China and their antioxidant properties. Molecules. 2015;20(1):542–555. doi: 10.3390/molecules20010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin SQ. II International Symposium on Loquat. 2007. World loquat production and research with special reference to China; pp. 37–44. [Google Scholar]
- 8.Lin S, Huang X, Cuevas J, Janick J. Loquat: an ancient fruit crop with a promising future. Chronica Hort. 2007;47(2):12–15. [Google Scholar]
- 9.Hussain A, Abbasi NA, Hafiz IA, Shakoor A, Naqvi SS. Performance of loquat (Eriobotrya japonica) genotypes under agro-ecological conditions of Khyber Pakhtunkhwa province of Pakistan. Int J Agric Biol. 2011;13(5):746–750. [Google Scholar]
- 10.Abbas MF, Naz F, Batool S, Naeem M, Qamar MI. First evidence of Mucor rot infecting loquat (Eriobotrya japonica L.) in Pakistan. Mycopath. 2018;16(2):81–85. [Google Scholar]
- 11.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damm U, Sun YC, Huang CJ. Colletotrichum eriobotryae sp. nov. and Colletotrichum nymphaeae, the anthracnose pathogens of loquat fruit in central Taiwan, and their sensitivity to azoxystrobin. Mycol Prog. 2020;19(4):367–380. [Google Scholar]
- 13.Jeffries P, Dodd J, Jeger MJ, Plumbley RA. The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathol. 1990;39(3):343–366. [Google Scholar]
- 14.Sarkar AK. Anthracnose diseases of some common medicinally important fruit plants. J Med Plants Stud. 2016;4(3):233–236. [Google Scholar]
- 15.Naz F, Abbas MF, Rauf CA, Tariq A, Mumtaz A, Irshad G, Shaheen FA, Hassan I. First report of Colletotrichum gloeosporioides causing anthracnose on loquat in Pakistan. Plant Dis. 2017;101(8):1550–1550. [Google Scholar]
- 16.Cao S, Zheng Y, Yang Z, Tang S, Jin P, Wang K, Wang X. Effect of methyl jasmonate on the inhibition of Colletotrichum acutatum infection in loquat fruit and the possible mechanisms. Postharvest Biol Technol. 2008;49(2):301–307. [Google Scholar]
- 17.Wu D, Zhang DH, Wang CX, Wei Y, Timko MP, Liang GL. First report of Fusarium solani species complex causing root rot of loquat (Eriobotrya japonica) in China. Plant Dis. 2021;105(05):1562. [Google Scholar]
- 18.Shah MD, Verma KS, Singh K, Kaur R. Morphological, pathological and molecular variability in Botryodiplodia theobromae (Botryosphaeriaceae) isolates associated with die-back and bark canker of pear trees in Punjab, India. Genet Mol Res. 2010;9(2):1217–1228. doi: 10.4238/vol9-2gmr812. [DOI] [PubMed] [Google Scholar]
- 19.Tziros GT. Alternaria alternata causes leaf spot and fruit rot on loquat (Eriobotrya japonica) in Greece. Australas Plant Dis Notes. 2013;8(1):123–124. [Google Scholar]
- 20.Abbas MF, Naz F, Rauf CA, Mehmood N, Zhang X, Rosli BH, Gleason ML. First report of Fusarium solani causing fruit rot of loquat (Eriobotrya japonica) in Pakistan. Plant Dis. 2017;101(5):839. [Google Scholar]
- 21.González-Domínguez E, Martins RB, Del Ponte EM, Michereff SJ, García-Jiménez J, Armengol J. Development and validation of a standard area diagram set to aid assessment of severity of loquat scab on fruit. Eur J Plant Pathol. 2014;139(2):419–428. [Google Scholar]
- 22.Sánchez-Torres P, Hinarejos R, Tuset JJ. Characterization and pathogenicity of Fusicladium eriobotryae, the fungal pathogen responsible for loquat scab. Plant Dis. 2009;93(11):1151–1157. doi: 10.1094/PDIS-93-11-1151. [DOI] [PubMed] [Google Scholar]
- 23.Soler E, Martínez-Calvo J, Llácer G, Badenes ML. II International Symposium on Loquat. 2006. Loquat in Spain: production and marketing; pp. 45–48. [Google Scholar]
- 24.Abbas MF, Naz F. First report of Diplodia seriata causing fruit rot of loquat in Pakistan. J Plant Pathol. 2018;100(2):325–325. [Google Scholar]
- 25.Naz F, Abbas MF, Rauf CA, Tariq A, Mumtaz A, Irshad G, Shaheen FA, Hassan I. First report of Colletotrichum gloeosporioides causing anthracnose on loquat in Pakistan. Plant Dis. 2017;101(8):1550–1550. [Google Scholar]
- 26.Abbas MF, Naz F, Tariq A, Mumtaz A, Irshad G, Rauf CA. First report of Curvularia lunata causing leaf spots on loquat from Pakistan. J Plant Pathol. 2017;98(2):374–374. [Google Scholar]
- 27.Abbas MF, Naz F, Rauf CA, Khan MA. Cultural, morphological, pathogenic and molecular characterization of Alternaria mali associated with necrotic leaf spot of loquat. Int J Biosci. 2016;9:271–228. [Google Scholar]
- 28.Nasir M, Iqbal B, Saqib M, Sajjad M, Niaz MZ, Idrees M, Abbas W, Mohy-ud-Din G. Evaluation and standardization of fungicides against plant diseases in Punjab-Pakistan crop production system. J Agric Sci (Lahore) 2016;54(2):233–249. [Google Scholar]
- 29.Ramzy AY, Shakil A, Sabah Z. Pesticide residues in Fish, Karachi-Pakistan: a review. J Agric Environ. 2021;5(1):25–29. [Google Scholar]
- 30.Lo CC. Effect of pesticides on soil microbial community. J Environ Sci Health B. 2010;45(5):348–359. doi: 10.1080/10934520903467873. [DOI] [PubMed] [Google Scholar]
- 31.Conway WS, Leverentz B, Janisiewicz WJ, Blodgett AB, Saftner RA, Camp MJ. Integrating heat treatment, biocontrol and sodium bicarbonate to reduce postharvest decay of apple caused by Colletotrichum acutatum and Penicillium expansum. Postharvest Biol Technol. 2004;34(1):11–20. [Google Scholar]
- 32.Ragsdale NN, Sisler HD. Social and political implications of managing plant diseases with decreased availability of fungicides in the United States. Annu Rev Phytopathol. 1994;32(1):545–557. doi: 10.1146/annurev.py.32.090194.002553. [DOI] [PubMed] [Google Scholar]
- 33.Azizbekyan RR. Use of spore-forming bacteria as biological plant protection products. Biotechnol. 2013;1:69–77. [Google Scholar]
- 34.Khakimov AA, Omonlikov AU, Utaganov SBU. Current status and prospects of the use of biofungicides against plant diseases. GSC Biol Pharm Sci. 2020;13(3):119–126. [Google Scholar]
- 35.Elmer W, White JC. The future of nanotechnology in plant pathology. Annu Rev Phytopathol. 2018;56:111–133. doi: 10.1146/annurev-phyto-080417-050108. [DOI] [PubMed] [Google Scholar]
- 36.Narayanan KB, Sakthivel N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci. 2011;169(2):59–79. doi: 10.1016/j.cis.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Murad MT, Habib A, Ashraf W, Rehman MS, Rafiq F, Murad S, Zeshan MA. Efficacy of desert medicinal plants against postharvest losses caused by Botrytis cineria (Pers.) in strawberry. Plant Prot. 2021;5(1):31–38. [Google Scholar]
- 38.Wang T, Jin X, Chen Z, Megharaj M, Naidu R. Green synthesis of Fe nanoparticles using Eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ. 2014;466:210–213. doi: 10.1016/j.scitotenv.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Ali M, Haroon U, Khizar M, Chaudhary HJ, Munis MFH. Facile single step preparations of phyto-nanoparticles of iron in Calotropis procera leaf extract to evaluate their antifungal potential against Alternaria alternata. Curr Plant Biol. 2020;23:100157. [Google Scholar]
- 40.Zubair MS, Munis MFH, Alsudays IM, Alamer KH, Haroon U, Kamal A, Attia H. First report of fruit rot of cherry and its control using Fe2O3 nanoparticles synthesized in Calotropis procera. Molecules. 2022;27(14):4461. doi: 10.3390/molecules27144461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kour A, Shawl AS, Rehman S, Sultan P, Qazi PH, Suden P, Rk K, Verma V. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J Microbiol Biotechnol. 2008;24(7):1115–1121. [Google Scholar]
- 42.Tafinta IY, Shehu K, Abdulganiyyu H, Rabe AM, Usman A. Isolation and identification of fungi associated with the spoilage of sweet orange (Citrus sinensis) fruits in Sokoto State. Nig J Basic Appl Sci. 2013;21(3):193–196. [Google Scholar]
- 43.Koh RBL, Barbosa CFC, Aquino VM, Galvez LC. Extraction of high molecular weight DNA suitable for next-generation sequencing from the fiber crop abaca. Ind Crops Prod. 2021;161:113194. [Google Scholar]
- 44.White TJ, Bruns T, Lee SJWT, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols. 1990;18(1):315–322. [Google Scholar]
- 45.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akbar M, Haroon U, Ali M, Tahir K, Chaudhary HJ, Munis MFH. Mycosynthesized Fe2O3 nanoparticles diminish brown rot of apple whilst maintaining composition and pertinent organoleptic properties. J Appl Microbiol. 2022;132(5):3735–3745. doi: 10.1111/jam.15483. [DOI] [PubMed] [Google Scholar]
- 47.Yassin MT, Mostafa AAF, Al-Askar AA. In vitro antagonistic activity of Trichoderma harzianum and Trichoderma viride strains compared to carbendazim fungicide against the fungal phytopathogens of Sorghum bicolor (L.) Moench J Biol Pest Control. 2021;31(1):1–9. [Google Scholar]
- 48.Iliger KS, Sofi TA, Bhat NA, Ahanger FA, Sekhar JC, Elhendi AZ, AL-Huqail AA, Khan F (2021) Copper nanoparticles: green synthesis and managing fruit rot disease of chilli caused by Colletotrichum capsici. Saudi J Biol Sci 28(2):1477– 1486. [DOI] [PMC free article] [PubMed]
- 49.Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK. Green chemistry based benign routes for nanoparticle synthesis. J Nanopart. 2014;3:1–14. [Google Scholar]
- 50.Hafizi R, Salleh B, Latiffah Z. Morphological and molecular characterization of Fusarium solani and Fusarium oxysporum associated with crown disease of oil palm. Braz J Microbiol. 2013;44:959–968. doi: 10.1590/s1517-83822013000300047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alghuthaymi MA, Ali AA, Hashim AF, Abd-Elsalam KA. A rapid method for the detection of Ralstonia solanacearum by isolation DNA from infested potato tubers based on magnetic nanotools. Philiph Agric Scientist. 2016;99(1):113–118. [Google Scholar]
- 52.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10(3):507–517. [Google Scholar]
- 53.Ahmed J, Ali M, Sheikh HM, Al-Kattan MO, Haroon U, Safaeishakib M, Munis MFH. Biocontrol of fruit rot of Litchi chinensis using zinc oxide nanoparticles synthesized in Azadirachta indica. Micromach. 2022;13(9):1461. doi: 10.3390/mi13091461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yim HS, Chye FY, Rao V, Low JY, Matanjun P, How SE, Ho CW. Optimization of extraction time and temperature on antioxidant activity of Schizophyllum commune aqueous extract using response surface methodology. J Food Sci Technol. 2013;50(2):275–283. doi: 10.1007/s13197-011-0349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bae E, Lee BC, Kim Y, Choi K, Yi J. Effect of agglomeration of silver nanoparticle on nanotoxicity depression. Kor J Chem Eng. 2013;30(2):364–368. [Google Scholar]
- 56.Mohamed YM, Azzam AM, Amin BH, Safwat NA. Mycosynthesis of iron nanoparticles by Alternaria alternata and its antibacterial activity. Afr J Biotechnol. 2015;14(14):1234–1241. [Google Scholar]
- 57.Zakariya NA, Majeed S, Jusof WHW. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sensors Int. 2022;3:100164. [Google Scholar]
- 58.Bhuiyan MSH, Miah MY, Paul SC, Aka TD, Saha O, Rahaman MM, Ashaduzzaman M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon. 2020;6(8):e04603. doi: 10.1016/j.heliyon.2020.e04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 60.Alghuthaymi MA, Almoammar H, Rai M, Said-Galiev E, Abd-Elsalam KA. Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol Biotechnol Equip. 2015;29(2):221–236. doi: 10.1080/13102818.2015.1008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senthilkumar SR, Sivakumar T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int J Pharm Pharm Sci. 2014;6(6):461–465. [Google Scholar]
- 62.Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against (Campylobacter jejuni) Appl Environ Microbiol. 2011;77(7):2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable
Not applicable