Abstract
Sirtuin 1 (SIRT1) is a key physiological regulator of metabolism and a target of therapeutic interventions for cardiometabolic and ageing-related disorders. Determining the factors and possible mechanisms of acute and adaptive SIRT1 response to exercise is essential for optimising exercise interventions aligned to the prevention and onset of disease. Exercise-induced SIRT1 upregulation has been reported in animals, but, to date, data in humans have been inconsistent. This exploratory systematic review and meta-analysis aims to assess various exercise interventions measuring SIRT1 in healthy participants. A total of 34 studies were included in the meta-analysis (13 single bout exercise, 21 training interventions). Studies were grouped according to tissue sample type (blood, muscle), biomarkers (gene expression, protein content, enzyme level, enzyme activity), and exercise protocols. A single bout of high-intensity or fasted exercise per se increases skeletal muscle SIRT1 gene expression as measured by qPCR or RT-PCR, while repeated resistance training alone increases blood SIRT1 levels measured by ELISA. A limited number of studies also show a propensity for an increase in muscle SIRT1 activity as measured by fluorometric or sirtuin activity assay. In conclusion, exercise acutely upregulates muscle SIRT1 gene expression and chronically increases SIRT1 blood enzyme levels.
Subject terms: Ageing, DNA, Biochemistry, Cell biology
Introduction
Sirtuins, dubbed as cellular ‘watchmen’ and ‘stress sensors’, control cell function by determining cell fate, maintaining energy supply, and preventing DNA damage to maintain genomic integrity. The mammalian sirtuin family of enzymes, composed of sirtuins 1–7 (SIRT1-SIRT7), have received considerable interest due to their role in regulating responses aligned to physiological stress and thus, health and longevity. Sirtuins function as histone deacetylase (HDAC), removing acetyl groups from target proteins and effectively activating or inhibiting these proteins depending on the specific cellular context1. For sirtuins to work efficiently, they use nicotinamide adenine dinucleotide (NAD+), through a reduction and oxidation mechanism which fuels the synthesis of ATP2. NAD+ and its redox couples, NADH and NADP(H), not only control metabolism and sirtuins, but also regulate several redox-sensitive pathways3. Sirtuins, in turn, regulate these redox pathways directly through deacetylation and indirectly by maintaining the NAD+ pool.
SIRT1 is known to deacetylate a range of salient transcription factors and proteins, including: AMP-activated protein kinase (AMPK), a central regulator of energy metabolism that maintains cell ATP concentration; peroxisome proliferator-activated receptor alpha (PPAR-α) involved in lipid metabolism; peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) involved in mitochondrial metabolism; nuclear factor erythroid 2–related factor 2 (Nrf2), an antioxidant transcription factor; nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), the regulator of innate immunity; and Ku70, p53, forkhead box transcription factors (FOXO) that are involved in DNA repair and cell survival in ageing and cancer1,4. In cancer for example, the balance between inhibitory SIRT1 deacetylation of p53 and SIRT1 recruitment of p53 acetylation, can determine whether damaged cells survive or undergo apoptosis5. SIRT1 also enhances cell survival in the heart-brain axis by upregulating brain-derived neurotrophic factor (BDNF) that is crucial for neuronal growth, synaptic plasticity, and vascular endothelial growth factor (VEGF) signalling6.
In addition to transcription factors, SIRT1 deacetylates histones—structures around which DNA wraps—facilitating chromatin compaction and silencing of genes involved in disease. For example, SIRT1 deacetylates histones at H4K16, decreasing its binding with the pro-inflammatory cytokine TNF-α promoter and alleviating inflammation7. Histone deacetylation is also involved in SIRT1’s regulation of circadian genes that control the production of hormones and enzymes, including nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in NAD synthesis8,9. Hence, loss of SIRT1 results in reduced NAD, impaired circadian rhythm, and increased expression of ageing-related genes10.
Ageing is characterised by decreased SIRT1, NAMPT, NAD+, and changes in circadian rhythm9. This regulatory network forms the basis of developing SIRT1 activators and NAD+ precursors for ageing-related disorders. In old mice, supplementation with NAD+ precursors can increase SIRT1 activity, stem cell regeneration, mitochondrial and physical function, and lifespan11. Similarly, SIRT1 overexpression can delay ageing, restore physical fitness, and extend lifespan in old mice12. Exercise-induced SIRT1 upregulation has been shown to decrease inflammation, apoptosis, and metabolic dysfunction in mice13–15. Similarly, in humans, aerobic exercise-induced SIRT1 increases antioxidant capacity (catalase and superoxide dismutase mainly) while decreasing cell senescence in heart failure16,17, and improves overall metabolic profile in type 2 diabetes18.
ATP demand during high-intensity exercise increases the AMP:ATP ratio that is sensed by AMPK, which responds by stimulating cell glucose uptake and fatty acid oxidation to generate more ATP and NAD+, the latter serving as a fuel for SIRT119. Exercise also generates reactive oxygen species (ROS) such as H2O2, a known signalling molecule that activates AMPK as a secondary consequence of mitochondrial ATP production20. However, excessive ROS can also repress SIRT1 activity by post-translational oxidative modification or by modifying intracellular NAD+ levels21. Indeed, high-intensity exercise increases DNA damage, which can recruit other NAD+-consuming repair enzymes such as poly ADP ribose polymerases (PARPs) that compete with and inhibit SIRT121,22. As the SIRT1 response to exercise is complex, it is essential to ascertain how exercise intensity, type, or duration can affect SIRT1 levels, both acutely (single bout) and after repeated training. Therefore, the aim of this exploratory review is to summarise and systematically assess published exercise interventions quantifying SIRT1 (protein content, gene expression, enzyme levels, and enzyme activity) in apparently healthy participants. Specifically, our primary objective is to determine if exercise can increase SIRT1, with a further objective to ascertain the type of exercise that may cause any modification to SIRT1.
Results
Literature search
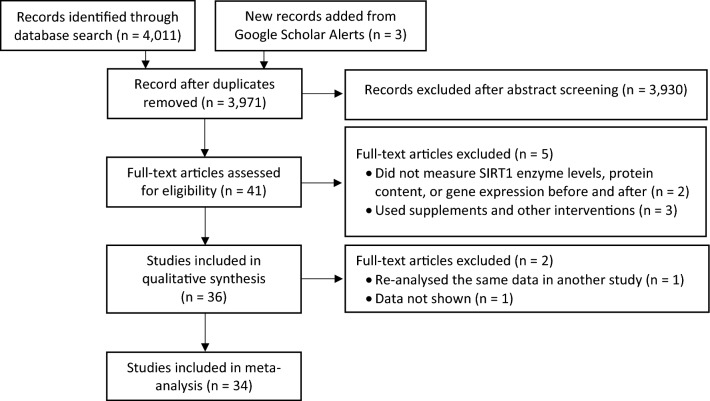
A database search retrieved a total of 3,971 non-duplicate articles, from which 34 were included in the meta-analysis (13 acute response and 21 training interventions). The search and selection process is summarised in Fig. 1.
Figure 1.
PRISMA flow diagram showing the database search and selection process.
Study characteristics
Participants
Participant age ranged from 20–66 years old. Six studies involved seniors, six studies tested overweight/obese participants, and the remaining studies involved young to middle-aged, normal weight participants. Only 10 studies involved women.
Quality assessment of individual studies
Studies scored 6 or higher, which is within the threshold for separating high-quality from low-quality studies based on a validity study of the original 11-item Cochrane Back Group Risk of Bias Tool23.
Biomarkers/analytical techniques
Studies were classified according to tissue and biomarker: SIRT1 gene expression in skeletal muscle (measured via qPCR or RT-PCR), SIRT1 protein content in skeletal muscle (measured via Western blot), SIRT1 enzyme levels in blood (measured via ELISA), and SIRT1 enzyme activity in skeletal muscle (measured via fluorometric or sirtuin activity assay).
Exercise classification
Studies were also grouped based on exercise type, intensity, duration, and feeding status of participants (fasted or fed). “Fasted” was used to accurately describe the exercise protocols that implemented an overnight fast, however, we cannot determine the effects of fasting on exercise per se since the said studies did not include a non-fasted control group. Intermediate and advanced yoga and Pilates interventions were included and classified as resistance training, while meditative yoga interventions consisting of breathing exercises alone were excluded. Table 1 summarises all exercise studies used in the meta-analysis and their abbreviations, while the exact protocols used are summarised in Tables 2 and 3.
Table 1.
Exercise classification table based on type, intensity, duration, tissue sample, biomarker, and feeding status of participants.
| Type, intensity | Feeding status | Tissue | Biomarker | |
|---|---|---|---|---|
| Acute response studies | ||||
| Aird et al., 202125 | SIT | Fasted | Muscle | Gene expression |
| Cho et al., 202226 | MIA, HIA | Fed | Blood | Enzyme levels |
| Dumke et al., 200927 | EnA | Fed | Muscle | Gene expression |
| Edgett et al., 201328 | HIIT | Fed | Muscle | Gene expression |
| Ghasemi et al., 202029 | SIT | Fed | Blood | Enzyme levels |
| Granata et al., 202030 | HIIT | Fed | Muscle | Gene expression |
| Guerra et al., 201031 | SIT | Fasted | Muscle | Protein content |
| Margolis et al., 201732 | EnA | Fasted | Muscle | Gene expression |
| Morales-Alamo et al., 201333 | SIT | Fasted | Muscle | Protein content |
| Mendham et al., 201634 | MIA | Fasted | Muscle | Protein content |
| Potthast et al., 202035 | HIA | Fed | Blood | Enzyme activity |
| Radak et al., 201136 | HIA | Fasted | Muscle | Gene expression |
| Skelly et al., 201737 | SIT | Fed | Muscle | Gene expression |
| Intervention studies | ||||
| Afzalpour et al., 201738 | HIIT | Blood | Enzyme levels | |
| Alfieri et al., 201539 | MIA | Muscle | Gene expression | |
| Amirsasan et al., 201940 | RT | Blood | Enzyme levels | |
| Boyd et al., 201341 | HIIT | Muscle | Protein content | |
| Dimauro et al., 201642 | RT | Blood | Enzyme levels | |
| Ghasemi et al., 202029 | HIIT | Blood | Enzyme levels | |
| Gliemann et al., 201343 | HIIT | Muscle | Protein content | |
| Granata et al., 202030 | HIIT | Muscle | Gene expression | |
| Gray et al., 201844 | SIT | Blood | Gene expression | |
| Gurd et al., 201045 | HIIT | Muscle | Enzyme activity protein content | |
| Gurd et al., 201146 | HIIT | muscle | Enzyme activity protein content | |
| Hooshmand-Moghadam et al., 202047 | RT | Blood | Enzyme levels | |
| Kababi et al., 202248 | RT | Blood | Enzyme levels | |
| Lamb et al., 202049 | RT | Muscle | Enzyme activity protein content | |
| Little et al., 201050 | HIIT | Muscle | Protein content | |
| Ma et al., 201351 | HIIT | Muscle | Protein content | |
| Scribbans et al., 201452 | HIIT | Muscle | Gene expression | |
| Soltani et al., 201853 | HIA | Blood | Enzyme levels | |
| Skleryk et al., 201354 | SIT | Muscle | Protein content | |
| Tolahunase et al., 201755 | RT | Blood | Enzyme levels | |
| Wasserfurth et al., 202156 | RT | Blood | Gene expression enzyme activity | |
EnA endurance aerobic, HIA high-intensity aerobic, HIIT high-intensity interval training, MIA moderate-intensity aerobic, RT resistance training, SIT sprint interval training.
Table 2.
Effects of a single bout of exercise on SIRT1.
| Study | Participants | Exercise protocol | Sample | Biomarker | Technique | Post-exercise SIRT1 (vs rest) |
|---|---|---|---|---|---|---|
| Aird et al., 202125 | Recreationally active males (N = 9, 26.1 ± 5.1 years) | SIT (4 × 30 s all-out cycle sprints against resistance of 75% BM, at > 80% VO2max) on overnight fast | Muscle tissue | SIRT1 mRNA gene expression | Multiplex PCR | ↑ at 3 h |
| Cho et al., 202226 | Young men (MIA N = 10, HIA N = 10, 20.70 ± 1.34 years) | Treadmill MIA (65% VO2max) and HIA (85% VO2max) | Blood | SIRT1 enzyme levels | ELISA | ↑ in both conditions |
| Dumke et al., 200927 | Trained male cyclists (N = 40, 29.1 ± 2.4 years) | 3 h of cycling at ≈57% Wmax | Muscle tissue | SIRT1 mRNA gene expression | qPCR | ↑ at 0 h |
| Edgett et al., 201328 | Recreationally active males (N = 8 in each intensity, 21.9 ± 2.2 years) | HIIT (cycling at 11 × 60 s at 73% WRpeak, 8 × 60 s at 100% WRpeak, or 6 × 60 s at 133% WRpeak) | Muscle tissue | SIRT1 mRNA gene expression | RT-PCR | ↑ at 3 h in all conditions |
| Ghasemi et al., 202029 | Overweight women (trained N = 10, untrained N = 10, 23.58 ± 2.23 years) | Wingate test (4 × 30 s all-out cycling at .075 kg/kg BM) | Blood | Serum SIRT1 | ELISA |
↑ in trained No significant effect in untrained |
| Granata et al., 202030 | Males (trained N = 8, untrained N = 8, 20 ± 2 years) | HIIT (5 × 4 min cycling at ≈107.4% of ẆLT) | Muscle tissue | SIRT1 mRNA gene expression | qPCR | ↓ at 0 h in both conditions |
| Guerra et al., 201031 | Male P.E. students (N = 8, 23.4 ± .6 years) | 30 s Wingate test at 100 rpm, ≈120% VO2max on overnight fast | muscle tissue | SIRT1 protein content | Western blot | ↑ at 2 h |
| Margolis et al., 201732 | Physically fit men and women (cycling group N = 7, treadmill group N = 5, 22 ± 1 years) | 1.5 h of of cycling or loaded treadmill walk at ≈58% VO2peak on overnight fast | Muscle tissue | SIRT1 mRNA gene expression | RT-PCR | ↑ at 0 h and 3 h in both groups |
| Morales-Alamo et al., 2012 | Male P.E. students (N = 10, 25 ± 4 years) | 30 s Wingate test at 100 rpm, ≈120% VO2max on overnight fast | Muscle tissue | SIRT1 protein content | Western blot | No significant effect |
| Morales-Alamo et al., 201333 | Male P.E. students (N = 9, 25 ± 5 years) | 30 s Wingate test at 100 rpm, ≈120% VO2max on overnight fast | Muscle tissue | SIRT1 protein content | Western blot | No significant effect |
| Mendham et al., 201634 | Sedentary, obese, middle-aged men (N = 9 rugby, N = 9 cycling, 48.8 ± 1.7 years) | 40 min of touch rugby or cycling at RPE = 13–14 on overnight fast | Muscle tissue | SIRT1 protein content | Western blot | No significant effect in either condition |
| Potthast et al., 202035 | Recreational runners (N = 25, 27.2 ± 4.1 years) | GXT (16.7 W per minute) until voluntary exhaustion on bicycle | Blood | SIRT1 activity | Sirtuin activity assay | ↑ at 0 h |
| Radak et al., 201136 | Young sedentary (N = 6, 26.0 ± 4.5 years), young physically active (N = 6, 30.2 ± 7.9 years), old sedentary (N = 6, 63.4 ± 4.7 years), and old physically active (N = 6, 62.4 ± 2.9) | 45 min of treadmill run at 70–75% VO2max then increased to 90%VO2max and terminated at exhaustion, on overnight fast | Muscle tissue | SIRT1 mRNA gene expression | RT-PCR |
↑ at 0 h in young sedentary ↑ at 0 h in young active ↑ at 0 h in old sedentary No significant effect in old active |
| Skelly et al., 201737 | Sedentary participants (men N = 8, women N = 8, 22 ± 3 years) | SIT (3 × 20 s all-out cycling at ≈ 500W) | Muscle tissue | SIRT1 mRNA gene expression | RT-PCR |
↑ at 3 h in men ↑ at 3 h in women |
BM body mass, GXT graded exercise test, HIIT high-intensity interval training, HRmax maximum heart rate, RPE rating of perceived exertion using Borg scale, rpm revolutions per minute, SIT sprint interval training, W watts, Wmax maximal power, Wpeak peak power, WLT power at lactate threshold, WRpeak peak work rate, ↑ increase, ↓ decrease.
Table 3.
Effects of repeated exercise training on SIRT1.
| Study | Participants | Training Intervention | Sample | Biomarker | Technique | Resting SIRT1 Post-Training (vs Pre-Training) |
|---|---|---|---|---|---|---|
| Afzalpour et al., 201738 | Overweight women (N = 10, 20–25 years) | HIIT (85–95% HRmax) 3 times/week for 10 weeks | Blood | SIRT1 enzyme levels | ELISA | ↑ |
| Alfieri et al., 201539 | Untrained males (N = 5, 20–43 years) | 1 h of football training for 64 weeks (2.4 times/week for the first 12 weeks, and 1.3 times/week for the following 52 weeks) | Muscle Tissue | SIRT1 gene expression | RT-PCR | ↑ |
| Amirsasan et al., 201940 | Sedentary overweight middle-aged women (N = 12) | Pilates with weights and bands, 3 times/week for 12 weeks | Blood | SIRT1 enzyme levels | ELISA | ↑ |
| Boyd et al., 201341 | Sedentary overweight/obese males (N = 10 moderate-intensity, N = 9 high-intensity, 22.7 ± 3.9 years) | Progressive interval cycling (8–10 × 60 s at either 70% or 100% WRpeak) 3 times/week for 3 weeks | Muscle Tissue | Whole muscle SIRT1 protein content | Western blot | ↑ in both conditions |
| Dimauro et al., 201642 | Senior men and women (N = 10) | Explosive resistance training (70% 1RM) 2 times/week for 12 weeks | Blood | SIRT1 protein levels | Western blot | No significant effect |
| Ghasemi et al., 202029 | Sedentary overweight women (N = 10, 23.58 ± 2.23 years) | HIIT (shuttle run at 90% HRmax) 3 times/week for 10 weeks | Blood | SIRT1 enzyme levels | ELISA | ↑ |
| Gliemann et al., 201343 | Physically inactive senior men (N = 13, 65 ± 1 years) | HIIT on bicycle twice/week, CrossFit once a week, and 5 km walk once a week, for 8 weeks | Muscle Tissue | SIRT1 protein content | Western blot | No significant effect |
| Granata et al., 202030 | Moderately trained males (N = 8, 20 ± 2 years) | Progressive HIIT (5–12 × 4 min or 8–22 × 2 min cycling intervals at up to ≈98.8% of ẆLT) twice a day for 20 days | Muscle Tissue | SIRT1 mRNA gene expression | qPCR | No significant effect |
| Gray et al., 201844 | Recreationally active men and women (N = 20, 22.8 ± 2.8 years) | SIT (4–6 × 30 s maximal cycling sprints at a resistance of 7.5% BM) 3 times/week for 4 weeks | Blood | SIRT1 mRNA gene expression | qPCR, hSIRTNADPlex assay | No significant effect |
| Gurd et al., 201045 | Recreationally active men and women (N = 9, 23.4 ± 1.1 years) | HIIT (∼1 h of 10 × 4 min cycling intervals at 90% VO2peak) 3 times/week for 6 weeks | Muscle Tissue | Total SIRT1 activity, intrinsic activity per SIRT1 protein in muscle, SIRT1 protein content | Western blot, fluorometric assay |
↑ total activity ↑ intrinsic activity ↓ content |
| Gurd et al., 201146 | Recreationally active men and women (N = 7, 23.4 ± 1.1 years) | HIIT (10 × 4 min cycling intervals at 90% VO2peak) 3 times/week for 2 weeks | Muscle Tissue | Whole muscle and nuclear SIRT1 protein content, nuclear SIRT1 activity | Western blot, fluorometric assay, qPCR |
No significant change in whole muscle and nuclear SIRT1 content ↑ nuclear activity |
| Hooshmand-Moghadam et al., 202047 | Untrained senior men (N = 15, 66.33 ± 3.35 years) | Progressive full-body resistance training (4 × 15 tempo repetitions per muscle group at 60% 1RM) 3 times/week for 12 weeks | Blood | SIRT1 enzyme levels | ELISA | ↑ |
| Kababi et al., 202248 | Male athletes (N = 10) | Progressive lower-body resistance training (30–70% 10RM) for 12 weeks | Blood | SIRT1 enzyme levels | ELISA | No significant effect |
| Lamb et al., 202049 | Untrained overweight middle-aged (N = 16) | Full-body resistance training 2 times/week for 10 weeks | Muscle Tissue | SIRT1 protein content, activity |
Western blot, SIRT1 activity assay |
No significant effect in content ↑ activity |
| Little et al., 201050 | Recreationally active men (N = 7, 21 ± 1 years) | Progressive HIIT (8–12 × 60 s cycling intervals at Wpeak (355 ± 10 W) 3 times/week for 2 weeks | Muscle Tissue | SIRT1 protein content | Western blot | ↑ |
| Ma et al., 201351 | Recreationally active men (N = 8, 20.6 ± 1.6 years) | Tabata protocol (8 × 20 s cycling intervals at 170% WRpeak) 4 times/week for 4 weeks | Muscle Tissue | Whole muscle SIRT1 protein content | Western blot | No significant effect |
| Scribbans et al., 201452 | Recreationally active men (N = 8, 21 ± 1 years) | Tabata protocol (8 × 20 s cycling intervals at 170% WRpeak) 3 times/week for 4 weeks | Muscle Tissue | SIRT1 gene expression | qPCR | No significant effect |
| Skleryk et al., 201354 | Sedentary obese men (N = 8 sprint, N = 8 traditional exercise, 37.8 ± 5.8 years) | SIT (6 sessions of 8–12 × 10 s sprints) or traditional exercise (10 sessions of 30 min cycling) at 65% VO2peak in a span of 2 weeks | Muscle Tissue | SIRT1 protein expression | Western blot | No significant effect in either intervention |
| Soltani et al., 201853 | Obese men (N = 11) | Water training (60–80% HRmax) 3 times/week for 8 weeks | Blood | SIRT1 enzyme levels | ELISA | ↑ |
| Tolahunase et al., 201755 | Young, middle-aged, and seniors (N = 94) | Progressive yoga 5 times/week for 12 weeks | Blood | SIRT1 enzyme levels | ELISA | ↑ |
| Wasserfurth et al., 202156 | Untrained seniors (N = 14, 60 ± 6 years) | Progressive strength endurance circuit (full-body strength exercises with machines and 2 × 4 min bouts on bicycle and cross-trainer) at RPE = 15, twice/week for 12 weeks | Blood | SIRT1 gene expression, SIRT1 activity | RT-PCR, fluorometric assay |
No significant effect in expression ↑ activity |
BM body mass, HIIT high-intensity interval training, HRmax maximum heart rate, RPE rating of perceived exertion using Borg scale, RM repetition maximum, SIT sprint interval training, VO2peak peak oxygen consumption, WR work rate, W watts, Wpeak peak power, WRpeak peak work rate, ↑ increase, ↓ decrease.
Acute SIRT1 response to exercise
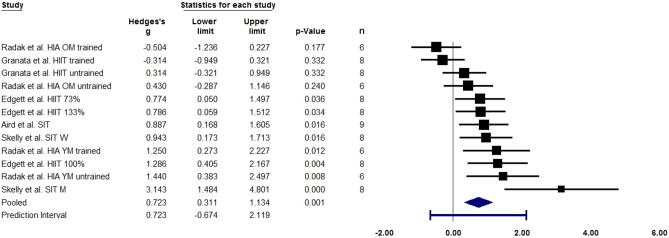
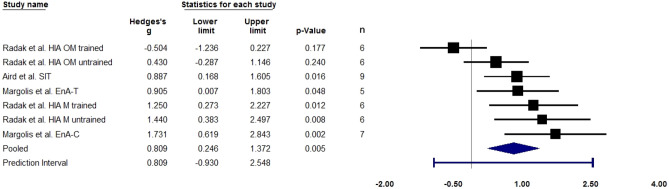
Skeletal muscle SIRT1 gene expression (measured via qPCR or RT-PCR) increased after a single bout of high-intensity exercise or following fasted exercise. Study characteristics are summarized in Figs. 2 and 3.
Figure 2.
Forest plot quantifying skeletal muscle SIRT1 gene expression (measured via qPCR or RT-PCR) following a single bout of high-intensity exercise. Adjusted standardised mean difference (Hedges’ g), relative weight of each acute study response, confidence interval (diamond), and prediction interval (blue line) are also shown. HIA high-intensity aerobic, HIIT high-intensity interval training, OM old men, M men, SIT sprint interval training, W women, YM young men. Percentages denote proportion of exercise intensity. n = sample size.
Figure 3.
Forest plot quantifying skeletal muscle SIRT1 gene expression (measured through qPCR or RT-PCR) following a single bout of fasted exercise (overnight fast). Adjusted standardised mean difference (Hedges’ g), relative weight of each acute study response, confidence interval (diamond), and prediction interval (blue line) are also shown. EnA-C endurance aerobic cycling, EnA-T endurance aerobic treadmill, HIA high-intensity aerobic, OM old men, M men, SIT sprint interval training. n = sample size.
High-intensity exercise
The analysis is based on 12 studies and utilised a random-effects model. The mean effect size adjusted with Hedges’ g is 0.723 with a 95% confidence interval of 0.311–1.134. To test the null hypothesis that the mean effect size is zero, we used the Z-value which is 3.444 with p = 0.001, and using a criterion alpha of 0.050, we reject the null hypothesis and conclude that in the universe of populations comparable to those in the analysis, the mean effect size is not precisely zero. To test the null hypothesis that all studies in the analysis share a common effect size, we used the Q-value which is 35.016 with 11 degrees of freedom and p < 0.001, and using a criterion alpha of 0.100, we reject the null hypothesis that the true effect size is the same in all these studies. The I-squared statistic is 69%, which suggests that some 69% of the variance in observed effects reflects variance in true effects rather than sampling error. Assuming that the true effects are normally distributed, we can estimate that the prediction interval is − 0.674 to 2.119. The true effect size in 95% of all comparable populations falls between this interval.
Fasted exercise
The analysis is based on seven studies and utilised a random-effects model. The mean effect size adjusted with Hedges’ g is 0.809 with a 95% confidence interval of 0.246–1.372. The Z-value is 2.815 with p = 0.005, hence we reject the null hypothesis and conclude that in the universe of populations comparable to those in the analysis, the mean effect size is not precisely zero. The Q-value is 17.807 with 6 degrees of freedom and p = 0.007, thus we reject the null hypothesis that the true effect size is the same in all these studies. The I-squared statistic is 66%, which suggests that some 66% of the variance in observed effects reflects variance in true effects rather than sampling error. Assuming that the true effects are normally distributed, we can estimate that the prediction interval is -0.930 to 2.548. The small number of studies may limit the reliability of the analysis.
Adaptive SIRT1 response to exercise
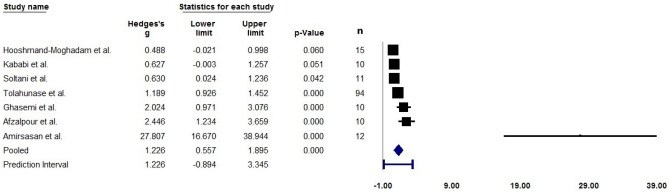
A limited number of studies showed an increase in SIRT1 levels in blood (measured via ELISA) and increased SIRT1 activity in muscle (measured via fluorometric or sirtuin activity assay) after exercise training. The small number of studies may limit the reliability of the meta-analysis. Meanwhile, SIRT1 protein content in skeletal muscle (measured via Western blot) did not reach statistical significance. Figures 4 and 5 summarise the studies.
Figure 4.
Forest plot quantifying SIRT1 levels in blood (measured via ELISA) after exercise training. Adjusted standardised mean difference (Hedges’ g), relative weight of each acute study response, confidence interval (diamond), and prediction interval (blue line) are also shown. n = sample size.
Figure 5.
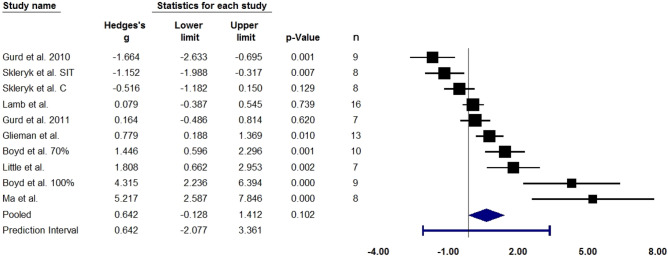
Forest plot quantifying SIRT1 protein content (Western blot) in skeletal muscle after exercise training. Adjusted standardised mean difference (Hedges’ g), relative weight of each acute response study, confidence interval (diamond), and prediction interval (blue line) are also shown. C cycling, SIT sprint interval training. Percentages denote exercise intensity. n = sample size.
Blood SIRT1 levels after training
The analysis is based on seven studies (n = 162 participants) and utilised a random-effects model. The mean effect size adjusted with Hedges’ g is 1.226 with a 95% confidence interval of 0.557 to 1.895. The Z-value is 3.592 with p < 0.001, while the Q-value is 39.873 with 6 degrees of freedom and p < 0.001. The I-squared statistic is 85%, which suggests that some 85% of the variance in observed effects reflects variance in true effects rather than sampling error. Assuming that the true effects are normally distributed, we can estimate that the prediction interval is − 0.894 to 3.345.
With analysing resistance training exercise studies alone (4 studies, n = 131 participants), the mean effect size adjusted with Hedges’ g is 0.987 with a 95% confidence interval of 0.001 to 1.973. The Z-value is 1.962 with p = 0.05, while the Q-value is 29.474 with 3 degrees of freedom and p < 0.001. The I-squared statistic is 90%, and the prediction interval is − 3.221 to 5.195.
Muscle SIRT1 activity after training
There are only three studies (N = 32 participants) quantifying SIRT1 activity in muscle (via fluorometric or a sirtuin activity assay) after exercise training. The mean effect size adjusted with Hedges’ g is 1.476 with a 95% confidence interval of 0.464–2.487. The Z-value is 2.860 with p = 0.004, while the Q-value is 7.262 with 2 degrees of freedom and p = 0.026. The I-squared statistic is 72%, and the prediction interval is -9.990 to 12.941. The small number of studies may exaggerate the range of prediction interval.
Muscle SIRT1 content after training
The analysis is based on ten studies and utilised a random-effects model. The mean effect size adjusted with Hedges’ g is 0.642 with a 95% confidence interval of − 0.128 to 1.412. The Z-value is 1.634 with p = 0.102, hence we accept the null hypothesis. Nine out of 10 studies used high-intensity aerobic training, while one used resistance training. Analysing high-intensity aerobic training studies alone (9 studies) does not reach statistical threshold.
Discussion
The main purpose of this exploratory review was to summarise and systematically assess published exercise interventions measuring SIRT1 (protein content, gene expression, enzyme levels, enzyme activity) in apparently healthy participants. Specifically, we wanted to know if and what type of exercise can increase SIRT1. A single bout of high-intensity or fasted exercise was shown to increase skeletal muscle SIRT1 gene expression as measured by qPCR or RT-PCR, while repeated exercise training enhances blood SIRT1 levels as measured by ELISA. A limited number of studies (3, N = 32 participants) also observed an increase in muscle SIRT1 activity following exercise training. Overall, we determine that exercise acutely upregulates muscle SIRT1 gene expression and chronically increases blood enzyme concentration. To our knowledge, this is the first systematic review and meta-analysis on SIRT1 response to exercise. Based on data from 34 studies (13 acute response, 21 training interventions), we highlight several novel outcomes.
Firstly, high-intensity or fasted exercise immediately upregulates SIRT1 gene expression in human skeletal muscle. The said studies measured SIRT1 expression using qPCR or RT-PCR from 0 to 3 h after a single bout of exercise. Immediate SIRT1 upregulation with PGC-1α deacetylation through protein kinase A (PKA) has been shown in C2C12 myotubes following adrenergic administration57. Although not in an exercise context, this demonstrates that hormonal signals may directly and rapidly modulate SIRT1 deacetylase activity, which can subsequently be sustained through an elevation in AMPK-dependent NAD+58. Cardiac muscle contraction and catecholamines, which are released during intense exercise, can activate PKA, perhaps explaining the immediate SIRT1 activation in high-intensity exercise59. In addition, PKA is activated when plasma glucose concentration falls below 65–70 mg/dL, which may explain the immediate increase in SIRT1 while in the fasted exercise state60.
SIRT1 levels increase with exercise intensity26 but only up to a certain point. One study found that supramaximal exercise (133% peak work rate) resulted in less expression of the SIRT1 target PGC-1α but a larger expression in early growth response 1 (EGR1), which is also known to induce SIRT1 as a response to mechanical stretch in skeletal muscle28. This stretch-induced activation of SIRT1 leads to deacetylation of FOXO4 and upregulation of superoxide dismutase which scavenges excess ROS61. EGR1 and SIRT1 form a negative-feedback loop, which may explain the decreased SIRT1 expression at supramaximal exercise61. Supramaximal exercise also increases DNA damage, which recruits PARPs that use NAD and compete with SIRT122.
High-intensity exercise increases muscle fibre recruitment and ATP turnover, which activates AMPK and SIRT1 activity; the latter by increasing cellular NAD+61. In support, a study in mice observed AMPK-dependent increases in NAD+ and PGC-1α deacetylation 3 h after resistance running, however, SIRT1 was not directly measured62. In young and fit individuals, but not in old and unfit, a single bout of cycling (20 min at 70% VO2max) can release extracellular NAMPT (eNAMPT) into the circulation, which increases NAD and SIRT1 within 1 h when administered to skeletal muscle cells63. Interestingly, injecting eNAMPT into muscle cells from young mice can prolong health and lifespan in old mice64.
Two studies have examined blood SIRT1 levels (as measured via ELISA) after a single bout of exercise (moderate intensity and high-intensity) and both observed an increase following exercise26,29. An additional investigation demonstrated that blood SIRT1 activity (fluorometric assay) is also enhanced following a single bout of exhaustive cycling35. With regards to repetitive exercise training, resting blood SIRT1 levels (ELISA) has also been shown to increase; albeit based on a moderate number of studies (7, n = 162 participants). The aforementioned studies involved resistance training and high-intensity aerobic training, which can both improve body composition, a factor that has been associated with increased circulating SIRT1 in larger long-term studies18,65. Circulating SIRT1 is negatively associated with fat mass, leptin, and insulin resistance, and positively associated with adiponectin, an “anti-obesity” hormone66,67. Interestingly, leptin and adiponectin are regulated partially by SIRT1 through PPAR-α and the circadian cycle8,68.
Low circulating SIRT1 is also associated with ageing-related disorders and has been proposed as a biomarker for Alzheimer’s and Parkinson’s disease69,70. In senior participants, two studies measured blood SIRT1 levels after resistance training, while another measured blood SIRT1 activity after resistance training; all demonstrating an increase post training47,55,56. Acutely, high-intensity aerobic exercise can also increase skeletal muscle SIRT1 expression in seniors36.
SIRT1 levels in skeletal muscle, however, may not always increase after exercise training. It is also possible that SIRT1 muscle protein content does not increase until after several months of training, and several of the observed studies implemented less than 1 month of training. Several post-translational protein modifications may also regulate skeletal muscle SIRT1 adaptation to exercise. One of the studies found increased NAD, NAMPT, global sirtuin activity, and mitochondrial density, but no increase in skeletal muscle SIRT1 protein content after 10 weeks of resistance training, suggesting that it may be NAD or NAMPT levels, rather than SIRT1 protein content, that contributes to increased SIRT1 activity49.
There are only three published studies that quantified skeletal muscle SIRT1 activity through a fluorometric or sirtuin activity assay-based approach after exercise training. Although the meta-analysis reached statistical significance, it is limited by the small sample (n = 32). Only one study measured SIRT1 activity in blood, with significant effects after resistance training observed56. Muscle SIRT1 activity, rather than content, has been associated with mitochondrial biogenesis during exercise46. Meanwhile, blood SIRT1 activity has been positively associated with basal metabolic rate71. Thus, SIRT1 activity may be more responsive to exercise training. It is therefore suggested that any future studies in this exciting domain of investigation should consider measuring SIRT1 activity as well as expression.
Most studies documented in our analysis utilized high-intensity aerobic exercise protocols and young participants, with several using resistance training in aged and overweight/obese participants. A genuine attempt was made for this review to be exhaustive and group all eligible studies according to biomarker, exercise protocol, and participant characteristics. However, there were limited studies on old and overweight/obese groups to reach statistical significance. Hence, all age and weight groups (young, old, overweight, obese, normal) as well as training levels (trained, untrained, athletes, sedentary) were combined. We are aware that ageing and overweight participants may have heightened inflammatory profiles that may affect the exercise response. Moreover, athletes may have a blunted response to exercise due to their enhanced fitness. It is also conceivable that differences due to sex in the exercise-induced SIRT1 response may also exist, particularly aligned to muscle fiber type composition, given that SIRT1 expression is higher in female Type I muscle fibers.
One major limitation of this study is the unclear risk of bias in exercise related studies due to failure of reporting randomisation, concealment, and blinding protocols72. We used the original 11-item Cochrane Back Group Risk of Bias Tool that was partially utilized in a meta-analysis on exercise and DNA damage22. A subsequent validity analysis of the original tool determined that a summary score of 6 is the threshold for the separation of high-quality from low-quality studies23. Hence, and in line with a previous study, lower quality is classified with a score of 0–5, while the 6–11 range confirms higher quality studies24. Studies scoring 6 or higher are thus within the quality threshold. It is however, important to note, that a number of items in the current Cochrane tools repertoire are nearly impossible to implement in exercise focused studies, such as blinding and randomisation (e.g., in a repeated measures design, acute response studies where pre and post-exercise cannot be interchanged). As such, this can be interpretated that in fact all exercise studies are inherently at risk for performance bias. Comprehensive and recent work by Bonafiglia et al. suggests that given the apparent and perceived methodological issues when conducting a meta-analysis, future studies are encouraged to implement bias-reducing methodologies, such as the one used in the current work, and indeed the authors report on the various approaches that should be considered when attempting to mitigate performance bias in exercise studies72.
Another limitation of this study is that most data were extracted from figures rather than raw values using a graph digitizer software. However, to minimise error, highly accurate software was used based on other published work73.
We conclude that high-intensity exercise has a relatively small effect in acutely increasing SIRT1 gene expression in skeletal muscle, while the effect observed in fasted exercise appears to be larger. Exercise training in general, or resistance training alone, has a large effect on resting blood SIRT1 levels, while a limited number of studies provide an evidence base for an increase in resting SIRT1 activity in skeletal muscle following exercise training. Taken together, these results reiterate the potential of exercise in prolonging health, partly due to upregulating SIRT1 acutely and chronically. Training variables such as intensity, adding resistance, or feeding status can be adjusted to maximise the health benefits of exercise. More studies examining SIRT1 activity following exercise and incorporating aged and overweight/obese populations are warranted.
Methods
Search strategy
Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, a comprehensive article search was conducted using the keywords “exercise AND SIRT1” from March 1–31, 2022, using five databases: Scopus, PubMed, Embase, MEDLINE, and Web of Science. Search results were filtered to include only human trials, in peer-reviewed journals, and in English language. New studies published after the said dates were also added. Our search protocol was registered and published on PROSPERO (CRD42023427141).
Inclusion/exclusion criteria
Articles were checked for the following inclusion criteria: (1) full report published in a peer-reviewed journal, (2) involving healthy adults, (3) controlled trial, and (4) with measures of SIRT1 (gene expression, protein content, enzyme levels, enzyme activity) in blood or skeletal muscle before and after exercise in acute response studies, or before and after training in intervention studies.
Studies with independent variables other than exercise (e.g., supplementation, diet, etc.) were included providing they consisted of a control group that received exercise alone, and only data from exercise-only groups were included in the meta-analysis.
“Training intervention studies” were those that implemented repeated exercise for a minimum of 2 weeks, which is the shortest intervention among the included studies. Meanwhile, “acute response studies” were those that conducted a single bout of exercise. “Fed state” suggests normal diet with the recommended macronutrient ratio (45–65% carbohydrates, 20–35% fats, 10–35% protein), while “fasted state” suggests 8–12 h overnight fasting which is a normal practice in exercise related studies. As such, data from participants doing more than 12 h fasting and special diets (e.g., vegan diet, high-carbohydrate diet, etc.) were excluded. Retracted articles were also excluded. The inclusion/exclusion criteria are shown in Table 4.
Table 4.
Inclusion/exclusion criteria.
| Criteria | Include | Exclude |
|---|---|---|
| Participants | Adults with no known disease | Children, adults with disease, animals |
| Exercise protocol | Aerobic and resistance exercise | Stretching, breathing, etc |
| Nutritional status (for acute response studies) | Fed state (normal diet and macronutrient ratio) or fasted state (8–12 h overnight fast) | High-fat, high-carbohydrate, high-protein, vegan diet, fasting for more than 12 h |
| Sample | Blood or skeletal muscle tissue | Neurons, adipocytes, etc |
| Outcome measure | SIRT1 gene expression, protein content, or enzyme levels | All other measures of SIRT1 |
Data extraction
Articles that met the inclusion criteria were printed and summarised in a table following PICO (Patient, Intervention, Comparison, Outcome) guidelines and were grouped according to tissue sample (muscle vs blood), biomarkers of SIRT1 (gene expression, protein content, enzyme levels, enzyme activity), participants’ nutritional status (fed vs fasted), exercise type and intensity (see Table 1). In case of missing data (e.g., nutritional status of participants, average intensity of exercise protocol), authors were contacted via email to clarify information. Means, standard deviations (SD), and standard errors (SE) were extracted from full texts and figures using GetData Graph Digitizer software73.
Quality assessment
Primary outcomes were defined as pre- and post-exercise SIRT1 measures (gene expression, protein content, enzyme levels, or enzyme activity). Risk of bias was assessed using the original 11-item Cochrane Back Group Risk of Bias Tool, namely: randomisation, concealment, baseline differences, patient blinding, care provider blinding, outcome blinding, co-intervention, compliance, dropouts, timing, and intention to treat (i.e., whether all participants were included in the analysis regardless of compliance). This approach was based on a related meta-analysis on exercise-induced DNA damage in healthy participants22. Studies were scored from 1 to 11, with a score of 6 distinguishing low from high quality studies; aligned to a validity study by Van Tulder et al.23. In practice, Paige et al. considered a score of 0–5 as “lower quality”, while a score of 6–11 was considered “higher quality”24. Table 5 summarises the quality assessment for risk of bias.
Table 5.
Quality assessment for risk of bias using the Cochrane back group risk of bias tool.
| Study | Randomisation | Concealment | Baseline similar | Patient blinding | Care provider blinding | Outcome blinding | Co-intervention avoided | Compliance acceptable | Dropout acceptable | Timing similar | Intent to treat | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afzalpour et al., 201738 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Aird et al., 202125 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Alfieri et al., 201539 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Amirsasan et al., 201940 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Boyd et al., 201341 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Cho et al., 202226 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Dimauro et al., 201642 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Dumke et al., 200927 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Edgett et al., 201328 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Ghasemi et al., 202029 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Gliemann et al., 201343 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 |
| Granata et al., 202030 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Gray et al., 201844 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Guerra et al., 201031 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Gurd et al., 201045 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Gurd et al., 201146 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Hooshmand al., 202047 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Kababi et al., 202248 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Lamb et al., 202049 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Little et al., 201050 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Ma et al., 201351 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Margolis et al., 201732 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Morales et al., 201333 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Mendham et al., 201634 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Potthast et al., 202035 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Radak et al., 201136 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Scribbans et al., 201452 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Skelly et al., 201737 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Skleryk et al., 201354 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Soltani et al., 201853 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Tolahunase et al., 201755 | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 6 |
| Wasserfuh et al., 202156 | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
Statistical analysis
Assessment of effect size
The Comprehensive Meta-Analysis (Version 4, NJ: USA: Biostat, Inc.) software was used to calculate random effects from means, standard deviations, and standard errors extracted from each article. Standardized mean difference (SMD) adjusted with Hedges’ g at 95% CI was calculated as the difference in means before and after exercise divided by the pooled standard deviation. SMD was used to express effect size, which was assessed using Cohen’s categories: 0.2–0.5 = small, 0.5–0.8 = medium, and > 0.8 = large. The overall effect size was assessed using Z-values with a significance level of p < 0.05.
Assessment of heterogeneity
The Q-statistic was used to test the null hypothesis that the true effect size is the same in all these studies, where p value ≤ 0.10 was considered significant heterogeneity. The I-squared (I2) statistic was used to express the proportion of the variance in observed effects that reflects variance in true effects rather than sampling error74.
Publication bias
Publication bias was assessed by visually analysing funnel plots, with a caveat that funnel plots may not be appropriate for small studies75. Both observed values and imputed values were plotted whereby imputed studies are perceived to be primarily negative in origin, while actual studies are regarded as being more positive (see Supplementary Materials S1).
Supplementary Information
Author contributions
All authors contributed equally, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work.
Funding
CGJ was supported by grants provided by the British Society for Research on Ageing and the Department of Science and Technology of the Philippines.
Data availability
All data presented in this review were taken from the cited studies which are publicly available. Standardised values are shown in the forest plots, while funnel plots are provided in the Supplementary Materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-38843-x.
References
- 1.Alves-Fernandes DK, Jasiulionis MG. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int. J. Mol. Sci. 2019;20:1. doi: 10.3390/ijms20133153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner S, et al. NAD+ centric mechanisms and molecular determinants of skeletal muscle disease and aging. Mol. Cell Biochem. 2022;477:1829–1848. doi: 10.1007/s11010-022-04408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao W, et al. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid. Redox. Signal. 2018;28:251–272. doi: 10.1089/ars.2017.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radak Z, et al. The systemic role of SIRT1 in exercise mediated adaptation. Redox. Biol. 2020;35:101467. doi: 10.1016/j.redox.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z, Fang D. The roles of SIRT1 in cancer. Cancer. 2013;4:97–104. doi: 10.1177/1947601912475079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maroofi A, et al. Cognitive decline in heart failure: Biomolecular mechanisms and benefits of exercise. Biochim. Biophys. Acta Mol. Basis Dis. 2022 doi: 10.1016/j.bbadis.2022.166511. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Yu W, Chen X. Sirt1 activator represses the transcription of TNF-α in THP-1 cells of a sepsis model via deacetylation of H4K16. Mol. Med. Rep. 2016;14:5544–5550. doi: 10.3892/mmr.2016.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, Sassone-Corsi P. Nutrition, metabolism, and epigenetics: Pathways of circadian reprogramming. EMBO Rep. 2022;23:e52412. doi: 10.15252/embr.202152412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai S, Guarente L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. Aging Mech. Dis. 2016;2:16017. doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang RH, et al. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci. Rep. 2016;6:28633. doi: 10.1038/srep28633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das A, et al. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2019;176:944–945. doi: 10.1016/j.cell.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh A, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell. Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alavi SS, et al. Involvement of sirtuins and klotho in cardioprotective effects of exercise training against waterpipe tobacco smoking-induced heart dysfunction. Front. Physiol. 2021;12:1. doi: 10.3389/fphys.2021.680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donniacuo M, et al. Cardioprotective effect of a moderate and prolonged exercise training involves sirtuin pathway. Life Sci. 2019;222:140–147. doi: 10.1016/j.lfs.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Liu HW, et al. Exercise training upregulates SIRT1 to attenuate inflammation and metabolic dysfunction in kidney and liver of diabetic db/db mice. Nutr. Metab. 2019;16:1–10. doi: 10.1186/s12986-019-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbi G, et al. Cardiac rehabilitation increases SIRT1 activity and β-hydroxybutyrate levels and decreases oxidative stress in patients with HF with preserved ejection fraction. Oxid. Med. Cell Longev. 2019 doi: 10.1155/2019/7049237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russomanno G, et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immun. Ageing. 2017;14:1–9. doi: 10.1186/s12979-017-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gharakhanlou BJ, Bonab SB. The effect of 12 weeks of training in water on serum levels of SIRT1 and FGF-21, glycemic index, and lipid profile in patients with type 2 diabetes. Int. J. Diabetes Dev. Ctries. 2022;1:1–8. [Google Scholar]
- 19.Hardie DG. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinchy EC, et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J. Biol. Chem. 2018;293:17208–17217. doi: 10.1074/jbc.RA118.002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji LL, et al. Maintenance of NAD+ homeostasis in skeletal muscle during aging and exercise. Cells. 2022;11:1. doi: 10.3390/cells11040710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tryfidou DV, et al. DNA damage following acute aerobic exercise: A systematic review and meta-analysis. Sports Med. 2020;50:103–127. doi: 10.1007/s40279-019-01181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Tulder MW, Suttorp M, Morton S, Bouter LM, Shekelle P. Empirical evidence of an association between internal validity and effect size in randomized controlled trials of low-back pain. Spine. 2009;34:1685–1692. doi: 10.1097/BRS.0b013e3181ab6a78. [DOI] [PubMed] [Google Scholar]
- 24.Paige NM, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: Systematic review and meta-analysis. JAMA. 2017;317:1451–1460. doi: 10.1001/jama.2017.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aird TP, et al. Divergent serum metabolomic, skeletal muscle signaling, transcriptomic, and performance adaptations to fasted versus whey protein-fed sprint interval training. Am. J. Physiol. Endocrinol. 2021;321:E802–E820. doi: 10.1152/ajpendo.00265.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho SY, et al. Impact of exercise intensity on systemic oxidative stress, inflammatory responses, and sirtuin levels in healthy male volunteers. Int. J. Environ. Res. Public Health. 2022;19:11292. doi: 10.3390/ijerph191811292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumke CL, et al. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur. J. Appl. Physiol. 2009;107:419–427. doi: 10.1007/s00421-009-1143-1. [DOI] [PubMed] [Google Scholar]
- 28.Edgett BA, et al. Dissociation of increases in PGC-1α and its regulators from exercise intensity and muscle activation following acute exercise. PLoS ONE. 2013;8:e71623. doi: 10.1371/journal.pone.0071623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghasemi E, et al. Combined high-intensity interval training and green tea supplementation enhance metabolic and antioxidant status in response to acute exercise in overweight women. J. Physiol. Sci. 2020;70:31. doi: 10.1186/s12576-020-00756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granata C, et al. Forty high-intensity interval training sessions blunt exercise-induced changes in the nuclear protein content of PGC-1α and p53 in human skeletal muscle. Am. J. Physiol. Endocrinol. 2020;318:E224–E236. doi: 10.1152/ajpendo.00233.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra B, et al. SIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: Influence of glucose ingestion. Eur. J. Appl. Physiol. 2010;109:731–743. doi: 10.1007/s00421-010-1413-y. [DOI] [PubMed] [Google Scholar]
- 32.Margolis LM, et al. Ingesting a combined carbohydrate and essential amino acid supplement compared to a non-nutritive placebo blunts mitochondrial biogenesis-related gene expression after aerobic exercise. Curr. Dev. Nutr. 2017 doi: 10.3945/cdn.117.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morales-Alamo D, et al. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKα phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013;114:566–577. doi: 10.1152/japplphysiol.01246.2012. [DOI] [PubMed] [Google Scholar]
- 34.Mendham AE, et al. Similar mitochondrial signaling responses to a single bout of continuous or small-sided-games-based exercise in sedentary men. J. Appl. Physiol. 2016;121:1326–1334. doi: 10.1152/japplphysiol.00289.2016. [DOI] [PubMed] [Google Scholar]
- 35.Potthast AB, et al. Impact of nutrition on short-term exercise-induced sirtuin regulation: Vegans differ from omnivores and lacto-ovo vegetarians. Nutrients. 2020;12:1004. doi: 10.3390/nu12041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radak Z, et al. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free Radic. Biol. Med. 2011;51:417–423. doi: 10.1016/j.freeradbiomed.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skelly LE, et al. Effect of sex on the acute skeletal muscle response to sprint interval exercise. Exp. Physiol. 2017;102:354–365. doi: 10.1113/EP086118. [DOI] [PubMed] [Google Scholar]
- 38.Afzalpour ME, Ghasemi E, Zarban A. Effects of 10 weeks of high intensity interval training and green tea supplementation on serum levels of Sirtuin-1 and peroxisome proliferator-activated receptor gamma co-activator 1-alpha in overweight women. Sci. Sports. 2017;32:82–90. doi: 10.1016/j.scispo.2016.09.004. [DOI] [Google Scholar]
- 39.Alfieri A, et al. Effects of long-term football training on the expression profile of genes involved in muscle oxidative metabolism. Mol. Cell Probes. 2015;29:43–47. doi: 10.1016/j.mcp.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Amirsasan R, et al. Effects of Pilates training and turmeric supplementation on Sirtuin 1 level and body composition in postmenopausal females with sedentary overweight: A randomized, double-blind, clinical trial. ZJRMS. 2019;21:e81620. doi: 10.5812/zjrms.81620. [DOI] [Google Scholar]
- 41.Boyd JC, et al. Reducing the intensity and volume of interval training diminishes cardiovascular adaptation but not mitochondrial biogenesis in overweight/obese men. PLoS ONE. 2013;8:e68091. doi: 10.1371/journal.pone.0068091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimauro I, et al. Resistance training and redox homeostasis: Correlation with age-associated genomic changes. Redox. Biol. 2016;10:34–44. doi: 10.1016/j.redox.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gliemann L, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray SR, et al. Inter-individual responses to sprint interval training, a pilot study investigating interactions with the sirtuin system. Appl. Physiol. Nutr. Metab. 2018;43:84–93. doi: 10.1139/apnm-2017-0224. [DOI] [PubMed] [Google Scholar]
- 45.Gurd BJ, et al. High-intensity interval training increases SIRT1 activity in human skeletal muscle. Appl. Physiol. Nutr. Metab. 2010;35:350–357. doi: 10.1139/H10-030. [DOI] [PubMed] [Google Scholar]
- 46.Gurd BJ, et al. Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle. Am. J. Physiol. Regul. 2011;301:R67–R75. doi: 10.1152/ajpregu.00417.2010. [DOI] [PubMed] [Google Scholar]
- 47.Hooshmand-Moghadam B, et al. The effect of 12-week resistance exercise training on serum levels of cellular aging process parameters in elderly men. Exp. Gerontol. 2020;141:111090. doi: 10.1016/j.exger.2020.111090. [DOI] [PubMed] [Google Scholar]
- 48.Kababi MH, et al. Effect of resistance training along with electrical muscle stimulation on serum levels of some of the molecular markers of muscle hypertrophy in male athletes after anterior cruciate ligament surgery. J. Bas. Res. Med. Sci. 2022;9:1–8. [Google Scholar]
- 49.Lamb DA, et al. Resistance training increases muscle NAD+ and NADH concentrations as well as NAMPT protein levels and global sirtuin activity in middle-aged, overweight, untrained individuals. Aging. 2020;12:9447–9460. doi: 10.18632/aging.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little JP, et al. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J. Physiol. 2010;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma JK, et al. Extremely low-volume, high-intensity interval training improves exercise capacity and increases mitochondrial protein content in human skeletal muscle. Open J. Mol. Integr. Physiol. 2013 doi: 10.4236/ojmip.2013.34027. [DOI] [Google Scholar]
- 52.Scribbans TD, et al. Resveratrol supplementation does not augment performance adaptations or fibre-type–specific responses to high-intensity interval training in humans. Appl. Physiol. Nutr. Metab. 2014;1:1305–1313. doi: 10.1139/apnm-2014-0070. [DOI] [PubMed] [Google Scholar]
- 53.Soltani M, et al. The effect of eight weeks of water training on SIRT1, PGC -1Α and body fat percentage in obese men. J. Babol. Univ. Med. Sci. 2018;20:55–60. [Google Scholar]
- 54.Skleryk JR, et al. Two weeks of reduced-volume sprint interval or traditional exercise training does not improve metabolic functioning in sedentary obese men. Diabetes Obes. Metab. 2013;15(114):6–53. doi: 10.1111/dom.12150. [DOI] [PubMed] [Google Scholar]
- 55.Tolahunase M, Sagar R, Dada R. Impact of yoga and meditation on cellular aging in apparently healthy individuals: A prospective, open-label single-arm exploratory study. Oxid. Med. Cell Longev. 2017;2017:7928981. doi: 10.1155/2017/7928981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasserfurth P, et al. Impact of dietary modifications on plasma sirtuins 1, 3 and 5 in older overweight individuals undergoing 12-weeks of circuit training. Nutrients. 2021 doi: 10.3390/nu13113824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerhart-Hines Z, et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+) Mol. Cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canto C, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo IY, Ehrlich BE. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015;7:a006023. doi: 10.1101/cshperspect.a006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soundarapandian MM, et al. Activation of Protein Kinase A (PKA) signaling mitigates congenital hyperinsulinism associated hypoglycemia in the Sur1-/- mouse model. PLoS ONE. 2020;15:e0236892. doi: 10.1371/journal.pone.0236892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardo PS, Boriek AM. An autoregulatory loop reverts the mechanosensitive Sirt1 induction by EGR1 in skeletal muscle cells. Aging. 2012;184:456–461. doi: 10.18632/aging.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chong MC, et al. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD+ activity in recipient cells. Aging Cell. 2022;21:e13647. doi: 10.1111/acel.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida M, et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019;30:329–342.e5. doi: 10.1016/j.cmet.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Opstad TB, et al. Effect of intermittent and continuous caloric restriction on Sirtuin1 concentration depends on sex and body mass index. Nutr. Metab. Cardiovasc. Dis. 2021;31:1871–1878. doi: 10.1016/j.numecd.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Mariani S, et al. Blood SIRT1 shows a coherent association with leptin and adiponectin in relation to the degree and distribution of adiposity: A study in obesity, normal weight and anorexia nervosa. Nutrients. 2020;12:3506. doi: 10.3390/nu12113506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahimi M, et al. Comparison of sirtuin 1 level and related blood factors in diabetic and healthy subjects. Pediatr. Endocrinol. Diabetes Metab. 2020;26:17–21. doi: 10.5114/pedm.2020.94392. [DOI] [PubMed] [Google Scholar]
- 68.Majeed Y, et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021;11:8177. doi: 10.1038/s41598-021-87759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Y, et al. Reduced serum SIRT1 levels in patients with Parkinson’s disease: a cross-sectional study in China. Neurol. Sci. 2021;42:1835–1841. doi: 10.1007/s10072-020-04711-z. [DOI] [PubMed] [Google Scholar]
- 70.Kumar, R., et al. Sirtuin1: A promising serum protein marker for early detection of Alzheimer's disease. PLoS One8, e61560. 10.1371/journal.pone.0061560 (2013). [DOI] [PMC free article] [PubMed]
- 71.Lee HJ, Yang SJ. Aging-related correlation between serum sirtuin 1 activities and basal metabolic rate in women, but not in men. Clin. Nutr. Res. 2017;6(1):18–26. doi: 10.7762/cnr.2017.6.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonafiglia JT, Islam H, Preobrazenski N, Gurd BJ. Risk of bias and reporting practices in studies comparing VO2max responses to sprint interval vs. continuous training: A systematic review and meta-analysis. J. Sport Health Sci. 2022;11:552–566. doi: 10.1016/j.jshs.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wojtyniak JG, et al. Data digitizing: Accurate and precise data extraction for quantitative systems pharmacology and physiologically-based pharmacokinetic modeling. CPT Pharmacomet. Syst. Pharmacol. 2020;9:322–331. doi: 10.1002/psp4.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borenstein, M. Common mistakes in meta-analysis and how to avoid them (Biostat, 2019).
- 75.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this review were taken from the cited studies which are publicly available. Standardised values are shown in the forest plots, while funnel plots are provided in the Supplementary Materials.