Abstract
Background
Loss of PTEN function leads to increased PI3Kβ signaling. AZD8186, a selective PI3Kβ/δ inhibitor, has shown anti-tumor activity in PTEN-deficient preclinical models. This phase 1b/2 study was conducted to determine the safety and efficacy of AZD8186 and paclitaxel combination in patients with metastatic or recurrent gastric cancer (MRGC).
Methods
In the phase Ib dose-escalation, subjects with advanced solid tumors received oral AZD8186 (60 mg or 120 mg; twice daily (BID); 5 days on/2 days off) plus intravenous paclitaxel (70 mg/m2 or 80 mg/m2; days 1, 8, and 15) every 4 weeks. In the phase II part, MRGC patients with PTEN loss or PTEN/PIK3CB gene abnormality were enrolled and received recommended phase II dose (RP2D) of AZD8186 plus paclitaxel. Primary endpoints were to determine maximum tolerated dose (MTD) and RP2D in phase Ib and 4-month progression-free survival (PFS) rate in phase II.
Results
In phase Ib, both MTD and RP2D were determined at paclitaxel 80 mg/m2 and AZD8186 120 mg BID. In phase II, 18 patients were enrolled [PTEN loss (n = 18) and PIK3CB mutation (n = 1)]. The 4-month PFS rate was 18.8% (3 of 16 evaluable patients) and further enrollment stopped due to futility.
Conclusion
Although the combination of AZD8186 and paclitaxel was well tolerated, limited clinical efficacy was observed.
ClinicalTrials.gov Identifier: NCT04001569.
Keywords: PTEN, PI3K, PIK3CB, AZD8186, paclitaxel, gastric cancer
This phase Ib/II study was conducted to determine the safety and efficacy of AZD8186 and paclitaxel combination in patients with metastatic or recurrent gastric cancer.
Lessons Learned.
The combination of paclitaxel with AZD8186, a selective PI3Kβ/δ inhibitor, was safe. In phase Ib, both maximum tolerated dose and recommended phase II dose of this combination were determined.
In phase II, AZD8186 plus paclitaxel showed favorable toxicity profiles, but only modest efficacy in patients with advanced gastric cancer with PTEN loss.
A long-term clinical benefit was observed in a patient with PIK3CB mutation. Further studies of AZD8186 in more selected population is warranted.
Discussion
Although treatments for MRGC have improved considerably over the past 30 years, cytotoxic chemotherapy remains the primary treatment, and only drugs targeting HER2 and VEGF receptors have proven effective as targeted therapies.
Hyperactivation of PI3K signaling is a feature of subsets of many different tumor types. The PI3K pathway is negatively regulated by PTEN, a tumor suppressor gene, and inhibition of PIK3β may be used for tumors with PTEN loss/mutation or PIK3CB mutation. Loss of PTEN frequently occurs in a variety of tumors including gastric cancer (GC), and PTEN protein loss by immunohistochemistry is reported in approximately 20% of GC patients.
In this phase Ib/II study, we evaluated the efficacy and safety of AZD8186 and paclitaxel in MRGC with PTEN loss or PTEN/PIK3CB gene abnormality. In phase II, Simon’s minimax 2-stage design was used for sample size calculation, assuming the 4-month PFS rate of AZD8186 plus paclitaxel combination therapy as P1 = 60% andP0 = 35%, with one-sided α = 0.05 and β = 0.2. Target accrual was 18 patients in the first stage and ≥ 7 patients with non-progressive disease at 4 months were required to proceed to second stage.
In the phase Ib part (n = 10), dose-limiting toxicity occurred in one subject (skin rash, grade 3). Other than that, toxicity profiles were favorable, and the most common treatment-related adverse events (TRAEs) was stomatitis (60%). The MTD and RP2D of AZD8186 and paclitaxel were determined as 120 mg BID and 80 mg/m2.
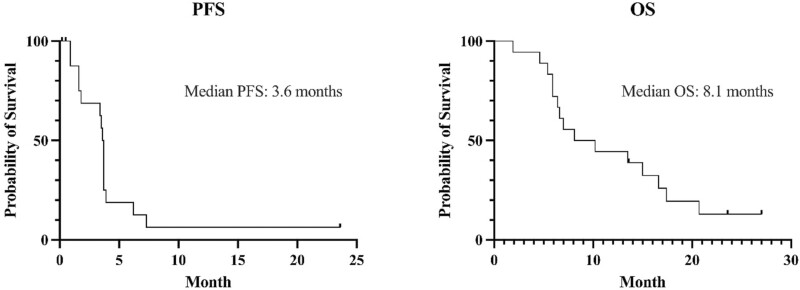
In the phase II part, which enrolled 18 subjects with MRGC, treatment was tolerable with the most common TRAE of neutropenia (44.4%), skin eruption (44.4%), and stomatitis (39.8%). The objective response rate and disease control rate was 18.8% and 68.8%, respectively. Median PFS and overall survival (OS) was 3.6 months and 8.1 months, respectively. The primary endpoint of 4-months PFS rate was 18.8% (3 out of 16 evaluable subjects), and the enrollment stopped due to futility (Table 1). This 4-months PFS rate seemed lower compared with that reported with paclitaxel plus ramucirumab combination (36% of 6-months PFS rate). Median PFS and OS with AZD8186 and paclitaxel were within a similar range to those reported in previous studies with paclitaxel monotherapy (2.9-3.6 months and 7.4-9.5 months). Of note, one patient with PIK3CB E1051K mutation achieved persistent stable disease and was still receiving study treatment at the time of data cutoff (December 1, 2022), with a treatment duration of 23.6 months.
Table 1.
Treatment delivery and outcomes in the phase II cohort (n = 18).
| No. (%) | |
|---|---|
| Number of cycles (range) | 3 (1-20) |
| Mean relative dose intensity, % (range, ±SD) | |
| AZD8186 | 66 (25-100, ±24.1) |
| Paclitaxel | 71 (33-100, ±21.4) |
| Total | 68 (29-100, ±21.5) |
| Objective response rate, % (95% CI)a | 18.8 (0-37.9) |
| Disease control rate, % (95% CI)a | 68.8 (46.0-91.5) |
| 4-months PFS rate, % (95% CI)a | 18.8 (0-37.9) |
| Median PFS, months (95% CI) | 3.6 (1.6-3.7) |
| Median OS, months (95% CI) | 8.1 (5.9-16.6) |
aTwo patients who were withdrawn from this study during cycle 1 (due to consent withdrawal and primary tumor bleeding not related to the study treatment) were not evaluated for tumor response.
Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression-free survival; SD, standard deviation.
In conclusion, the combination of AZD8186 and paclitaxel showed limited efficacy in MRGC. However, the toxicity profile was favorable, and the long-term clinical benefit seen in a subject with PIK3CB mutation suggests that further investigation of AZD8186 in these populations may be warranted.
| Trial Information [Phase II Part] | |
|---|---|
| Disease | Gastric cancer (GC; phase II part) |
| Stage of disease/treatment | Metastatic/recurrent |
| Prior therapy | 1 prior regimen |
| Type of study | Phase II (single arm) |
| Primary endpoint | Profession-free survival (PFS) rate at 4 months |
| Secondary endpoints | PFS, overall survival (OS), objective response rate (ORR), duration of response (DoR), disease control rate (DCR), and safety |
| Investigator’s analysis | Inactive because results did not meet primary endpoint |
Additional Details of Endpoints or Study Design
This was an open-label, multicenter phase Ib/II study at 4 centers in the Republic of Korea. Patients were enrolled between May 2019 and September 2021. The phase Ib part was a dose-escalation study in adult patients with advanced solid tumors and 3+3 design was applied with 4-dose level evaluation planned (Table 2). The starting dose of AZD8186 was 60 mg PO twice-daily (BID; 5 days on/2 days off), with planned escalation to 120 mg PO BID. At the first dose level, paclitaxel was administered intravenously (IV) at 80 mg/m2 on D1, 8, 15 every 4 weeks (Q4W). Dose and schedule of study treatment and definition of dose limiting toxicity (DLT) in phase Ib part are shown in Supplementary Methods. Based on the safety results from the phase Ib part, patients enrolled in the phase II part received recommended phase II dose (PR2D) of AZD8186 plus paclitaxel. The dose of AZD8186 and paclitaxel was adjusted according to the severity of adverse events (AEs) that occurred. Specific details on how to reduce the dose of both agents are described in Supplementary Methods.
Table 2.
Planned dose level of study treatment in phase Ib part.
| Level | Paclitaxel (mg/m2) D1, 8, 15 Q 4 weeks |
AZD8186 (total mg/day) 5 days on/2 days off |
|---|---|---|
| 1 | 80 | 120 (60 mg BID) |
| 2 | 80 | 240 (120 mg BID) |
| −1 | 70 | 240 (120 mg BID) |
| −2 | 70 | 120 (60 mg BID) |
On the phase Ib stage, 4 dose levels were planned. The traditional 3+3 design was applied to these 4 dose levels. The level −1 was planned to be tested if the dose level 2 was deemed to be intolerable (dose-limiting toxicity [DLT] occurs in ≥2 out of 6 people). The level −2 was planned to be tested if the dose level 1 was deemed to be intolerable (DLT occurs in ≥2 out of 6 people).
All patients were aged ≥20 years with a histologically confirmed cancer diagnosis. All patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with adequate bone marrow, renal, and hepatic function at screening. Patients were excluded if they had symptomatic brain metastases, impaired gastrointestinal function or gastrointestinal disorders that could significantly alter the absorption of AZD8186, or any other clinically significant disease. In the phase Ib part, patients with a histologically confirmed metastatic solid tumor that had progressed after approved therapies or for which there was no effective standard therapy were enrolled. Measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) was not required and the existence of an evaluable disease was sufficient for enrollment in the phase Ib part.
In the phase II part, patients with histologically confirmed metastatic or recurrent gastric cancer (MRGC) that have progressed after treatment with first-line fluoropyrimidine-based chemotherapy were enrolled. If the subject received adjuvant chemotherapy after curative gastrectomy, the adjuvant chemotherapy was considered first-line treatment if the disease recurred during or within 6 months after the completion of adjuvant chemotherapy. Measurable disease per RECIST was required in phase II part. Patients should have tumors with PTEN loss identified by immunohistochemistry (IHC) or PTEN/PIK3CB gene abnormalities (deletion or loss of function mutation in PTEN; amplification or gain of function mutation in PIK3CB) which were detected by targeted next-generation sequencing (NGS) tests certified by regulatory authorities. IHC for PTEN was conducted with an antibody against PTEN (138G6, Cell Signaling Technology, Danvers, USA). PTEN expression in the cytoplasm and/or nucleus of tumor cells was examined. Staining intensity was graded (0, negative; 1+, weakly positive; 2+, moderately positive; and 3+, strongly positive) and area of positive cancer cells was measured. Then, PTEN expression level was scored using H-score which incorporates both the staining intensity and a percentage of stained cells at each intensity level. The cut-off for non-expression of PTEN by IHC was H-score < 10 in this study.
The primary endpoint of phase Ib part of this study was to determine maximal tolerated dose (MTD) and RP2D of AZD8186 in combination with paclitaxel. Secondary endpoints included dose-limiting toxicity (DLT), safety, and preliminary antitumor activity of AZD8186 and paclitaxel combination. The primary endpoint of phase II part was PFS rate at 4 months, and secondary endpoints included PFS, OS, ORR according to the RECIST (version 1.1), DoR, DCR, and safety. ORR was defined as the proportion of patients achieving complete response (CR) or partial response (PR). PFS was defined as the period from the starting date of study treatment to disease progression or death from any cause. OS was calculated from the starting date of study treatment to death from any cause.
For phase Ib part, 3+3 design applied and 9-18 DLT-evaluable subjects were expected to be enrolled. For phase II part, Simon’s minimax 2-stage design was used for sample size calculation, with one-sided α = 0.05 and β = 0.2. The number of patients was calculated assuming the 4-month PFS rate of AZD8186 plus paclitaxel combination therapy as P1 = 60% and P0 = 35%. Thirty-five percent was reported as 4-month PFS rate with paclitaxel monotherapy, and 60% was reported as 4-month PFS rate with paclitaxel/ramucirumab combination therapy1; therefore, 35% and 60% were set to P0 and P1, respectively. This calculation required 26 patients. Target accrual was 18 patients in the first stage; 7 or more patients with non-progressive disease at 4 months were required to proceed to second stage. Considering a 15% drop-out rate, a total of 31 patients were planned to be enrolled in phase II part. The Kaplan-Meier method was used for the analysis of PFS and OS. All statistical tests were 2-sided with significance defined as P < .05. All analyses were performed using SPSS for Windows version 23.0 (IBM Corp., Armonk, NY).
The study protocol was reviewed and approved by the institutional review boards of each hospital. The study was conducted in accordance with the tenets of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. Written informed consent was obtained from all the patients before participation. The trial is registered with ClinicalTrials.gov (NCT04001569).
| Drug Information | |
|---|---|
| Drug 1 | |
| Generic/working name | AZD8186 |
| Company name | AstraZeneca (Cambridge, UK) |
| Drug type | Small molecule |
| Drug class | Selective PI3Kβ/δ inhibitor |
| Dose | 120 mg BID [5 days on, 2 days off; every week (phase II part)] |
| Unit | mg |
| Route | Oral (po) |
| Schedule of administration | In the dose-escalation phase Ib part, 3+3 design was applied with 4-dose level evaluation planned (Table 2). The starting dose of AZD8186 was 60 mg PO twice-daily (BID; 5 days on/2 days off), with planned escalation to 120 mg PO BID. AZD8186 was taken orally twice daily at approximately the same time (12 ± 1 h) each day on an empty stomach (water only for at least 2 h prior and 1 h after each dose) for 5 days on, 2 days off every week (days 1-5, 8-12, 15-19, and 22-26). In this dose-escalation part, both MTD and RP2D were determined at AZD8186 120 mg BID and paclitaxel 80 mg/m2. In the phase II part, AZD8186 (120 mg) was taken orally BID each day for 5 days on, 2 days off every week. One cycle of AZD8186 and paclitaxel combination therapy was 28 days, and AZD8186 was administered alone in the 4th week without paclitaxel administration. AZD8186 was provided by AstraZeneca. |
| Drug 2 | |
| Generic/working name | Paclitaxel |
| Company name | (1) Phase Ib part; Paxel (Hanmi Pharmaceutical, Seoul, Korea) (2) Phase II part; Whether it was an original drug or a generic drug, it was used according to the circumstances of each institution. |
| Drug type | Small molecule |
| Drug class | Mitotic—kinetic spindle protein |
| Dose | 80 mg/m2 [On day 1, 8, 15 every 4 weeks (phase II part)] |
| Unit | mg |
| Route | IV |
| Schedule of administration | In the dose-escalation phase Ib part, subjects were planned to receive oral AZD8186 (60 mg or 120 mg; twice-daily; 5 days on/2 days off) plus intravenous paclitaxel (70 mg/m2 or 80 mg/m2; days 1, 8, and 15) every 4 weeks (Table 2). Paclitaxel (Paxel) was provided by Hanmi Pharmaceutical. In the phase Ib part, both MTD and RP2D were determined at AZD8186 120 mg BID and paclitaxel 80 mg/m2. In the phase II part, intravenous paclitaxel (80 mg/m2) was administered on days 1, 8, and 15 every 4 weeks. |
| Patient Characteristics [Dose-Escalation Phase Ib Part] | |
|---|---|
| Number of patients, male | 4 |
| Number of patients, female | 6 |
| Stage | Stage IV (100%) |
| Age: median (range) | 62 (48-67) years |
| Number of prior systemic therapies: median (range) | 2.5 (1-4) |
| Performance status: ECOG | 0: 2 1: 8 2: 0 3: 0 4: 0 |
| Cancer types or histologic subtypes | Stomach cancer (adenocarcinoma): 5 [PTEN (−) by IHC, n = 2; HER2 (+), n = 2] Colorectal cancer (adenocarcinoma): 5 [PTEN (−) by IHC, n = 1] |
| Primary Assessment Method [Dose-Escalation Phase Ib Part] | |
|---|---|
| Title | Efficacy in the phase Ib part |
| Number of patients screened | 10 |
| Number of patients enrolled | 10 |
| Number of patients evaluable for toxicity | 10 |
| Number of patients evaluated for efficacy | 9 |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 2 (22.2%) |
| Response assessment, SD | 1 (11.1%) |
| Response assessment, PD | 6 (66.7%) |
| (Median) duration assessments, PFS | 1.8 months (95% CI: 0.9-5.3) |
| (Median) duration assessments, TTP | 1.8 months (95% CI: 0.9-5.3) |
| (Median) duration assessments, OS | 4.7 months (95% CI: 2.6-21.4) |
| Response duration | 1.5 months and 3.6 months, respectively |
| (Median) duration of treatment | 1.3 months (range: 0.2-5.3 months) |
Outcome Notes
In the phase Ib part, 10 patients were enrolled. In the dose level 1 (AZD8186 60 mg BID and paclitaxel 80 mg/m2; n = 3), no DLT was observed. In the dose level 2 (AZD8186 120 mg BID and paclitaxel 80 mg/m2; n = 7), among initial 3 subjects, one experienced DLT [skin rash (grade 3)]. Four additional subjects were enrolled to this cohort. Among these 4, one subject stopped taking AZD8186 on day 3 because of epigastric discomfort and withdrew his consent to participate in the study because he did not want to take the investigational product anymore. So, this subject was dropped out of this study before DLT evaluation, was replaced by another subject, and was also excluded from the efficacy evaluation. The remaining 3 patients did not develop DLT. Therefore, the investigators declared the dose level 2 (paclitaxel 80 mg/m2 and AZD8186 120 mg BID) as MTD and RP2D.
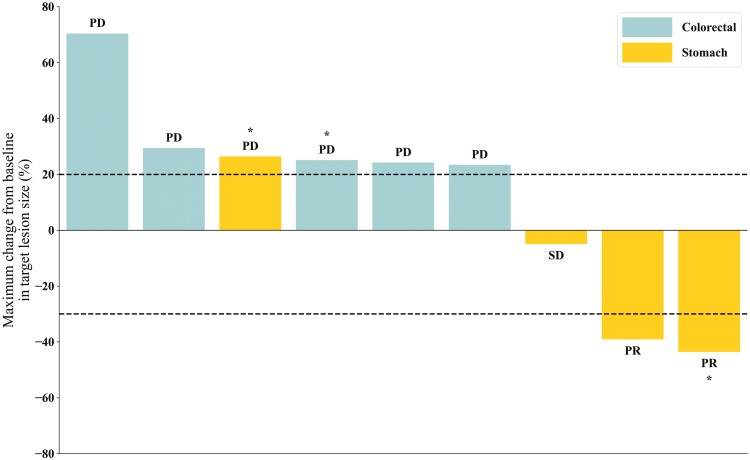
Median follow-up duration in the phase Ib was 5.4 months (range, 2.6-38.4). Tumor response was evaluable in 9 subjects. No CR was observed, and PR was achieved in 2 patients and ORR was 22.2% (2/9) (Fig. 1). One patient had stable disease (SD) (1/9, 11.1%) and 6 patients had progressive disease [PD; 6/9 (66.7%)]. DCR was 33.3% (3/9).
Figure 1.
Waterfall plots of best percentage changes in the sum of the longest diameters of target lesions in phase Ib cohort. Abbreviations: PD, progressive disease; PR, partial response; SD, stable disease. *Phosphatase and tensin homolog (PTEN) negative.
One patient with HER2+ GC in the dose level 1 had a confirmed PR, with a DoR of 3.6 months. PTEN IHC was negative in this patient. The other patient with HER2−, PTEN IHC+ GC in the dose level 2 had a confirmed PR with a DoR of 1.5 months. Among 5 patients with metastatic colorectal cancer (CRC), no response was observed. In the phase Ib part, median PFS was 1.8 months (95% CI, 0.9-5.3), and median OS was 4.7 months (95% CI, 2.6-21.4).
| Patient Characteristics [Phase II Part] | |
|---|---|
| Number of patients, male | 15 |
| Number of patients, female | 3 |
| Stage | Stage IV (100%) |
| Age: median (range) | 67 (56-84) |
| Number of prior systemic therapies: median (range) | 1 |
| Performance status: ECOG | 0: 7 1: 11 2: 0 3: 0 4: 0 |
| Cancer types or histologic subtypes | Stomach cancer (adenocarcinoma): 18 [PTEN (−) by IHC, n = 18; PIK3CB mutation, n = 1] |
| Primary Assessment Method [Phase II Part] | |
|---|---|
| Title | Efficacy in the phase II part |
| Number of patients screened | 42 |
| Number of patients enrolled | 18 |
| Number of patients evaluable for toxicity | 18 |
| Number of patients evaluated for efficacy | 16 |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 3 (18.8%) |
| Response assessment, SD | 8 (50.0%) |
| Response assessment, PD | 5 (31.2%) |
| (Median) duration assessments, PFS | 3.6 months (95% CI: 1.6-3.7) |
| (Median) duration assessments, TTP | 3.6 months (95% CI: 1.6-3.7) |
| (Median) duration assessments, OS | 8.1 months (95% CI: 5.9-16.6) |
| (Median) response duration | 1.8 months (range: 1.3-4.2) |
| (Median) duration of treatment | 2.3 months (range: 0.0-18.7) |
Outcome Notes
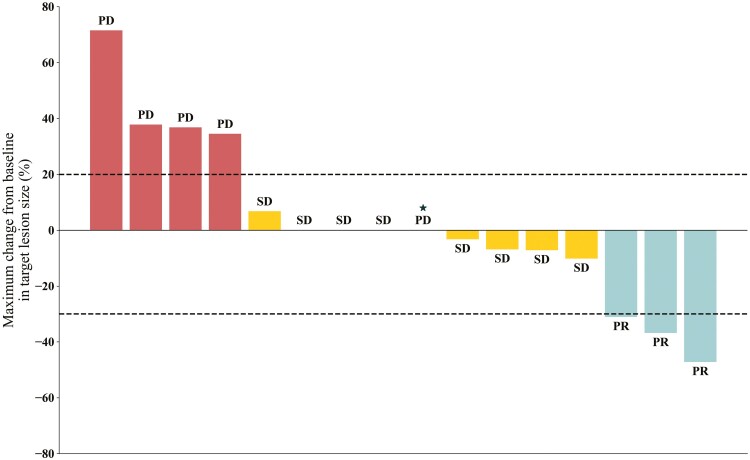
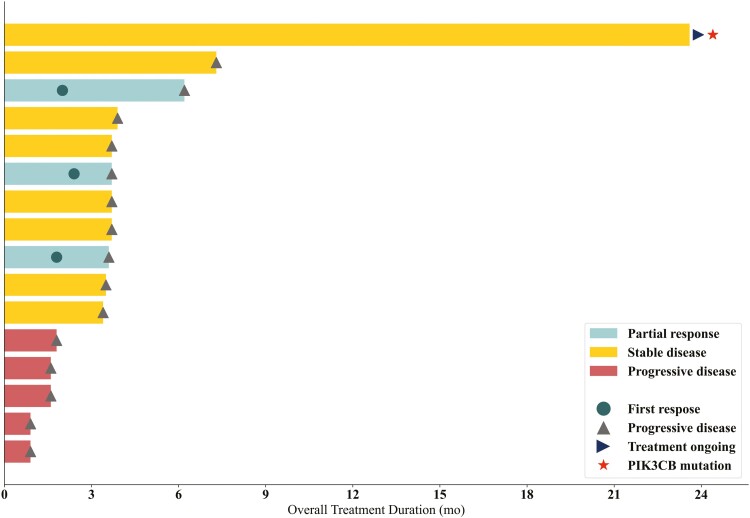
In phase II, 18 patients were enrolled. Detailed patient characteristics are presented in Table 3. All patients had PTEN-negative MRGC by IHC. One patient had PIK3CB-mutated tumor (PIK3CB E1051K), which was likely oncogenic.2 Of the 18 patients enrolled, tumor response was in-evaluable in 2 patients. One patient developed hematemesis on day 7 of Cycle 1, which the site investigator attributed to primary tumor bleeding unrelated to study drug. The patient began receiving palliative radiation therapy to the stomach and were determined to be withdrawn from the study. The other patient withdrew consent on day 2 of Cycle 1, in the absence of any AEs. Therefore, 16 patients were eligible for response-evaluation. Of these 16, 3 patients (18.8%) achieved PR and 8 patients (50%) achieved SD (Fig. 2). DoR for 3 patients with PR was 1.3 months, 1.8 months, and 4.2months, respectively (Fig. 3). ORR and DCR was 18.8% (3/16) and 68.8% (11/16), respectively. The primary endpoint of 4-month PFS rate was 18.8% (3/16), and further enrollment was stopped due to futility (Table 1). Median PFS was 3.6 months (95% CI, 1.6-3.7), and median OS was 8.1 months (95% CI, 5.9-16.6) (Fig. 4).
Table 3.
Patient characteristics (phase II cohort, n = 18).
| No. (%) | |
|---|---|
| Age (years) | |
| Median (range) | 67 (56-84) |
| <70 | 10 (55.6) |
| ≥70 | 8 (44.4) |
| Sex | |
| Male | 15 (83.3) |
| Female | 3 (16.7) |
| ECOG PS | |
| 0 | 7 (38.9) |
| 1 | 11 (61.1) |
| Disease status | |
| Initially metastatic | 12 (66.7) |
| Recurrent | 6 (33.3) |
| Time to PD on first-line therapy | |
| <6 months | 4 (22.2) |
| ≥6 months | 14 (77.8) |
| Tumor grade | |
| Well differentiated | 1 (5.6) |
| Moderately differentiated | 9 (50.0) |
| Poorly differentiated | 8 (44.4) |
| Histologic subtype | |
| Intestinal | 6 (33.3) |
| Diffuse | 2 (11.1) |
| Unknown or not available | 10 (55.6) |
| Previous surgery for gastric cancer | |
| No | 10 (55.6) |
| Yes | 8 (44.4) |
| Total gastrectomy | 4 (22.2) |
| Partial gastrectomy | 3 (16.7) |
| Palliative gastro-jejunostomy | 1 (5.6) |
| Adjuvant chemotherapy | |
| No | 15 (83.3) |
| Yes | 3 (16.7) |
| TS-1 | 2 (11.1) |
| XP + Pembrolizumab (or Placebo) | 1 (5.6) |
| Site of metastasis | |
| Lymph node | 10 (55.6) |
| Liver | 9 (50.0) |
| Peritoneal seeding | 9 (50.0) |
| Pleural seeding | 1 (5.6) |
| Lung | 1 (5.6) |
| Number of metastatic organs | |
| 1 | 9 (50.0) |
| 2 | 7 (38.9) |
| 3 | 1 (5.6) |
| 4 | 1 (5.6) |
| Prior therapy for metastatic disease | |
| Oxaliplatin based chemotherapy | 14 (77.8) |
| Cisplatin based chemotherapy | 3 (16.7) |
| XP + Trastuzumab | 1 (5.6) |
| Time from the start of first-line therapy to thestart of AZD8186 trial (median, range) | 10.5 (2.4-47.3) |
| PTEN IHC | |
| Positive | 0 |
| Negative | 18 (100.0) |
| PIK3CB gene abnormality | |
| Positive | 1 (5.6) |
| Negative | 11 (61.1) |
| Unknown or not available | 6 (33.3) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IHC, immunohistochemistry; PD, progressive disease; PIK3CB, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta; PTEN, phosphatase and tensin homolog; XP, capecitabine + cisplatin
Figure 2.
Waterfall plots of best percentage changes in the sum of the longest diameters of target lesions in phase II cohort (PD, progressive disease; PR, partial response; SD, stable disease). *In this case, there was no change in the target lesion, but a new lesion occurred, so it was judged as PD in the overall response evaluation.
Figure 3.
Swimmer plot of duration of response and clinical outcomes.
Figure 4.
Progression-free survival (PFS) and overall survival (OS) in phase II cohort (N = 18).
Interestingly, there was one patient who showed long-term tumor suppression by this study treatment. A 62-year-old male patient who had HER2-negative GC with PIK3CB E1051K mutation had peritoneal metastasis and received palliative first-line chemotherapy with capecitabine plus oxaliplatin. After 9 months of treatment, the patient underwent total gastrectomy due to radiographic CR findings. The postoperative stage was ypT4bN2M0. Chemotherapy was continued for 14 months after surgery, after which chemotherapy was discontinued since there was no evidence of residual tumor. However, 8 months after cessation of chemotherapy, new peritoneal metastases developed around the rectum, and the patient participated in this clinical trial and received AZD8186 and paclitaxel. He achieved SD (maximal change of −6.7% in a target lesion compared with baseline) and was still receiving study treatment at the time of data cutoff (December 1, 2022), with a treatment duration of 23.6 months.
Assessment, Analysis, and Discussion
| Completion | Study Completed |
|---|---|
| Investigator’s assessment | Inactive because results did not meet primary endpoint |
Gastric cancer (GC) is the third cause of cancer death in 2018.3 In the past 30 years, chemotherapy for MRGC has developed considerably, but OS for patients with MRGC is only slightly over 12 months,4,5 and the targeted therapies have not proved efficacy except trastuzumab, ramucirumab, and recently, trastuzumab deruxtecan.1,6,7 Therefore, there are many unmet needs.
Hyperactivation of PI3K signaling is a feature of subsets of many tumor types. Activation of the PI3K pathway is negatively regulated by the lipid phosphatase PTEN (phosphatase and tension homolog deleted on chromosome 10), a tumor suppressor that controls the levels of intracellular PIP3 by dephosphorylating PIP3 to PIP2.8 In tumors with PI3Kβ (=PIK3CB) mutation or PTEN loss/PTEN mutation, PI3Kβ inhibitors may be useful. Downregulation of the PIK3CB resulted in pathway inactivation and growth inhibition in PTEN-deficient cancer cells in both cell-based and in vivo settings.9 PTEN loss frequently occurs in various tumors, including prostate, breast, stomach, endometrial cancer, etc.10 In GC, PTEN protein loss by IHC was reported in approximately 20% of patients,11 and pathogenic PTEN mutation was reported in 10.6% by NGS.12 Inhibition of PI3Kβ may hold the potential for personalized therapy for these patients.
AZD8186 is a selective PI3Kβ/δ inhibitor and has shown anti-tumor activity in various PTEN-deficient preclinical tumor models. In addition, when AZD8186 was combined with docetaxel, the antitumor effect was significantly increased compared to docetaxel or AZD8186 alone.13 Thus, we aimed to evaluate the safety and efficacy of AZD8186 in combination with paclitaxel as a second-line treatment in MRGC.
In our study, 10 patients were enrolled in phase Ib part (MRGC, n = 5; CRC, n = 5). Three patients received AZD8186 60 mg BID plus paclitaxel 80 mg/m2 (dose level 1), and no DLT was observed. In the dose level 2 (AZD8186 120 mg BID and paclitaxel 80 mg/m2; n = 7), DLT was not evaluable in one patient and only one out of the remaining 6 patients experienced a DLT [skin rash (grade 3)]. Therefore, the dose level 2 was declared as MTD and RP2D. Treatment-related adverse events (TRAEs) during the DLT evaluation period included stomatitis (60%), cytopenia [leukopenia (60%), neutropenia (40%), anemia (40%)], and skin toxicities (40%) (Table 4). Treatment discontinuations occurred in all patients, most commonly due to PD [3 in dose level 1 and 5 in dose level 2, respectively]. Two patients in dose level 2 discontinued due to AEs; grade 3 skin eruption (n = 1), and hematochezia (due to tumor bleeding and not treatment-related; n = 1). TRAEs observed during all cycles in the phase Ib part are shown in Table 4.
Table 4.
Adverse events (phase Ib cohort, n = 10).
| Per person (n = 10) | Cycle 1 | All cycles | ||||||
|---|---|---|---|---|---|---|---|---|
| All grades | Grade 1 | Grade 2 | Grade 3 | All grades | Grade 1 | Grade 2 | Grade 3 | |
| Stomatitis | 6 (60.0) | 4 (40.0) | 2 (20.0) | 0 | 6 (60.0) | 3 (30.0) | 3 (30.0) | 0 |
| Leukopenia | 6 (60.0) | 2 (20.0) | 3 (30.0) | 1 (10.0) | 6 (60.0) | 2 (20.0) | 3 (30.0) | 1 (10.0) |
| Neutropenia | 4 (40.0) | 2 (20.0) | 1 (10.0) | 1 (10.0) | 4 (40.0) | 2 (20.0) | 1 (10.0) | 1 (10.0) |
| Anemia | 4 (40.0) | 4 (40.0) | 0 | 0 | 5 (50.0) | 3 (30.0) | 1 (10.0) | 1 (10.0) |
| Skin eruption | 4 (40.0) | 1 (10.0) | 1 (10.0) | 2 (20.0) | 5 (50.0) | 1 (10.0) | 2 (20.0) | 2 (20.0) |
| Anorexia | 3 (30.0) | 2 (20.0) | 1 (10.0) | 0 | 5 (50.0) | 2 (20.0) | 3 (30.0) | 0 |
| Bilirubin elevation | 3 (30.0) | 2 (20.0) | 1 (10.0) | 0 | 3 (30.0) | 2 (20.0) | 1 (10.0) | 0 |
| AST/ALT elevation | 3 (30.0) | 2 (20.0) | 0 | 1 (10.0) | 3 (30.0) | 2 (20.0) | 0 | 1 (10.0) |
| Epigastric pain | 2 (20.0) | 2 (20.0) | 0 | 0 | 2 (20.0) | 2 (20.0) | 0 | 0 |
| Constipation | 2 (20.0) | 2 (20.0) | 0 | 0 | 2 (20.0) | 2 (20.0) | 0 | 0 |
| Fatigue | 2 (20.0) | 1 (10.0) | 1 (10.0) | 0 | 4 (40.0) | 1 (10.0) | 3 (30.0) | 0 |
| Myalgia | 2 (20.0) | 2 (20.0) | 0 | 0 | 3 (30.0) | 3 (30.0) | 0 | 0 |
| Nausea | 1 (10.0) | 1 (10.0) | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 |
| Dyspepsia | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 |
| Diarrhea | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 |
| Fever | 1 (10.0) | 1 (10.0) | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 |
| Peripheral neuropathy | 1 (10.0) | 1 (10.0) | 0 | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 |
| Weight loss | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 |
| Lip edema | 1 (10.0) | 1 (10.0) | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 |
| Throat discomfort | 0 | 0 | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 |
Data are presented as no. (%).
There was no treatment-related grade 4 adverse event.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In phase II, 18 patients were enrolled. All patients received RP2D of AZD8186 plus paclitaxel. Fourteen patients (77.7%) and 9 patients (50.0%) had ≥ 1 dose reduction and dose interruption of paclitaxel, respectively. Dose reduction and interruption of AZD8186 occurred in 8 (44.4%) and 9 (50.0%) patients, respectively. All were due to AEs. Excluding 2 patients who were withdrawn from the study on days 2 and 7 of Cycle 1 due to consent withdrawal and tumor bleeding, 16 underwent tumor response evaluation. Treatment discontinuations occurred in 15 patients (93.8%) mainly due to PD [14 (93.3%)]. Treatment was discontinued in one patient due to TRAE (pneumonitis). TRAEs that occurred during all treatment cycles are shown in Table 5, and frequently observed TRAE included neutropenia, skin eruption, stomatitis, peripheral neuropathy, fatigue, anorexia, etc. Efficacy was modest, with ORR and DCR of 18.8% (3/16) and 68.8% (11/16), respectively (Table 1; Fig. 2). The 4-month PFS rate was 18.8%, and further enrollment stopped due to futility (Table 1; Fig. 4). There was no treatment-related death in both phase Ib and II parts.
Table 5.
Adverse events (phase II cohort, n = 18)
| Per person (n = 18) | All grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Neutropenia | 8 (44.4) | 0 | 2 (11.1) | 2 (11.1) | 4 (22.2) |
| Skin eruption | 8 (44.4) | 3 (16.7) | 4 (22.2) | 1 (5.6) | 0 |
| Stomatitis | 7 (38.9) | 2 (11.1) | 4 (22.2) | 1 (5.6) | 0 |
| Peripheral neuropathy | 7 (38.9) | 2 (11.1) | 5 | 0 | 0 |
| Fatigue | 7 (38.9) | 3 (16.7) | 4 (22.2) | 0 | 0 |
| Anorexia | 6 (33.3) | 1 (5.6) | 5 | 0 | 0 |
| AST/ALT elevation | 5 (27.8) | 3 (16.7) | 0 | 2 | 0 |
| Diarrhea | 4 (22.2) | 3 (16.7) | 1 (5.6) | 0 | 0 |
| Fever | 4 (22.2) | 3 (16.7) | 1 (5.6) | 0 | 0 |
| Myalgia | 3 (16.7) | 0 | 3 (16.7) | 0 | 0 |
| Febrile neutropenia | 3 (16.7) | 0 | 0 | 3 | 0 |
| Bilirubin elevation | 2 (11.1) | 0 | 2 (11.1) | 0 | 0 |
| Epigastric pain | 2 (11.1) | 1 (5.6) | 1 (5.6) | 0 | 0 |
| Nausea | 2 (11.1) | 1 (5.6) | 1 (5.6) | 0 | 0 |
| Skin itching | 2 (11.1) | 1 (5.6) | 1 (5.6) | 0 | 0 |
| Vomiting | 1 (5.6) | 0 | 1 (5.6) | 0 | 0 |
| Constipation | 1 (5.6) | 1 (5.6) | 0 | 0 | 0 |
| Anemia | 1 (5.6) | 0 | 1 (5.6) | 0 | 0 |
| QT prolongation | 1 (5.6) | 0 | 1 (5.6) | 0 | 0 |
| Pneumonitis | 1 (5.6) | 0 | 0 | 1 (5.6) | 0 |
| Skin infection | 1 (5.6) | 0 | 1 (5.6) | 0 | 0 |
| Hand-foot syndrome | 1 (5.6) | 0 | 1 (5.6) | 0 | 0 |
| Hypokalemia | 1 (5.6) | 0 | 1 (5.6) | 0 | 0 |
| Abdominal pain | 1 (5.6) | 1 (5.6) | 0 | 0 | 0 |
Data are presented as no. (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase;.
Despite the preclinical evidence of anticancer activity of AZD8186 in multiple tumor types, additional benefit of AZD8186 in combination with paclitaxel was not observed. Several hypotheses explaining this result may exist. Tumor heterogeneity is a well-known feature of GC, and in a study using multiregional whole-exome sequencing revealed intratumoral heterogeneity of genetic alterations in all tumors of the discovery cohort, and single-sample analysis of the primary tumor may miss 53.2%-91.3% of the mutations present.14 Patient selection based on data obtained from a single sample (such as primary tumor sample) may have contributed to the limited efficacy in our study. Also, MRGC patients with PTEN loss were all identified by IHC in this study. Although PTEN IHC assays has been studied in various tumor types and is considered a reliable method for detecting PTEN loss,15,16 some tumors with intact PTEN may also have PTEN loss by IHC. In prostate cancer, 15% of cases with homozygous PTEN deletion by fluorescence in situ hybridization (FISH) had intact PTEN by IHC, and intratumoral heterogeneity in PTEN loss was seen in approximately 50% of primary prostate tumors.17 Using both PTEN IHC and FISH as complementary screening tools for PTEN loss, or inactivating mutation of PTEN identified by NGS might be better way of patient selection.
Activating PIK3CB mutation has the potential to be a biomarker that predicts susceptibility to AZD8186. In preclinical studies, 48% of AZD8186-sensitive cell lines were PTEN wild type, suggesting that PTEN deficiency is not the only mechanism by which cells are dependent on PI3Kβ.13 PIK3CB E633K mutation that activate the PI3K/ATK pathway was found in patient-derived breast tumors, and the missense mutation, copy number gain or translocations of PIK3CB have been reported in prostate cancer. In our study, one patient had PIK3CB E1051K mutation. PIK3CB E1051K is a gain-of-function mutation, and tumor cells expressing p110βE1051K were sensitive to p110β inhibition.2 The patient with PIK3CB E1051K achieved SD with ongoing PFS > 23.6 months, highlighting the need for patient selection based on PIK3CB mutation for future studies.
In conclusion, AZD8186 plus paclitaxel showed limited efficacy in MRGC. Considering favorable toxicity profiles and a long-term clinical benefit seen in a subject with PIK3CB mutation, further investigation of AZD8186 in more selected patient population is warranted.
Supplementary Material
Acknowledgments
This research was supported by the National Research and Development Program for Cancer Control, Ministry of Health and Welfare (HA17C0054), and partly funded by Seoul National University Bundang Hospital Research Fund (number: 14-2020-0035). This was also supported in part by the KCSG data center (study number: KCSG ST18-20). AstraZeneca, Cambridge, Unite Kingdom, provided the AZD8186 supply and administrative support for this study. Hanmi Pharmaceutical, Seoul, Korea, provided paclitaxel (Paxel) in the phase Ib part. We are also grateful to Yong Min Shin and Onah Kim (Seoul National University Bundang Hospital) for their support as clinical research coordinators
Contributor Information
Koung Jin Suh, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Gyeonggi-do, Republic of Korea.
Min-Hee Ryu, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Dae Young Zang, Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Anyang, Gyeonggi-do, Republic of Korea.
Woo Kyun Bae, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Republic of Korea.
Hye Seung Lee, Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea.
Hyeon Jeong Oh, Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Gyeonggi-do, Republic of Korea.
Minsu Kang, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Gyeonggi-do, Republic of Korea.
Ji-Won Kim, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Gyeonggi-do, Republic of Korea.
Bum Jun Kim, Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Anyang, Gyeonggi-do, Republic of Korea.
Peter G S Mortimer, Oncology R&D, AstraZeneca, Cambridge, UK.
Hee Jung Kim, Oncology R&D, AstraZeneca, Seoul, Korea.
Keun-Wook Lee, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Gyeonggi-do, Republic of Korea.
Conflict of Interest
Peter G.S. Mortimer is an employee of AstraZeneca. Hee Jung Kim is an employee of AstraZeneca. Keun-Wook Lee reported research funding (to his institution for conducting clinical trials outside this work) from AstraZeneca, Ono Pharmaceutical, Merck, Sharp, and Dohme, Merck KGaA, Pfizer, BeiGene, Zymeworks, ALX Oncology, Astellas, Macrogenics, Five Prime Therapeutics, Seagen, Bolt Therapeutics, Trishula Therapeutics, Oncologie, Pharmacyclics, Daiichi Sankyo, Taiho Pharmaceutical, InventisBio, Leap Therapeutics, MedPacto, LSK BioPharma, Green Cross Corp, ABLBIO, Y-BIOLOGICS, Genexine, Metafines; consulting fees (outside the submitted work) from Metafines, Bayer, Daiichi Sankyo, Merck, Sharp, and Dohme, Bristol Myers Squibb, and Vifor Pharma; and honoraria from Ono Pharmaceutical and Boryung (outside the submitted work). The other authors indicated no financial relationships.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. https://doi.org/ 10.1016/s1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 2. Whale AD, Colman L, Lensun L, et al. Functional characterization of a novel somatic oncogenic mutation of PIK3CB. Sig Transduct Target Ther. 2017;2:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rawla P, Barsouk A.. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. https://doi.org/ 10.1016/s0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee K-W, Chung I-J, Ryu M-H, et al. Multicenter phase III trial of S-1 and cisplatin versus S-1 and oxaliplatin combination chemotherapy for first-line treatment of advanced gastric cancer (SOPP trial). Gastric Cancer. 2021;24:156-167. [DOI] [PubMed] [Google Scholar]
- 6. Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. https://doi.org/ 10.1016/s0140-6736(10)61121-x [DOI] [PubMed] [Google Scholar]
- 7. Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419-2430. https://doi.org/ 10.1056/nejmoa2004413 [DOI] [PubMed] [Google Scholar]
- 8. Cantley LC, Neel BG.. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96(8):4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wee S, Wiederschain D, Maira S-M, et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA. 2008;105:13057-13062. https://doi.org/ 10.1073/pnas.0802655105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salmena L, Carracedo A, Pandolfi PP.. Tenets of PTEN tumor suppression. Cell. 2008;133:403-414. https://doi.org/ 10.1016/j.cell.2008.04.013 [DOI] [PubMed] [Google Scholar]
- 11. Kim HS, Shin S-J, Beom S-H, et al. Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: implications for individualized therapy. Oncotarget. 2016;7:44608-44620. https://doi.org/ 10.18632/oncotarget.10115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim B, Kang SY, Kim D, et al. PTEN protein loss and loss-of-function mutations in gastric cancers: the relationship with microsatellite instability, EBV, HER2, and PD-L1 expression. Cancers. 2020;12(7):1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hancox U, Cosulich S, Hanson L, et al. Inhibition of PI3Kβ signaling with AZD8186 inhibits growth of PTEN-deficient breast and prostate tumors alone and in combination with docetaxel. Mol Cancer Ther. 2015;14:48-58. https://doi.org/ 10.1158/1535-7163.mct-14-0406 [DOI] [PubMed] [Google Scholar]
- 14. Röcken C, Amallraja A, Halske C, et al. Multiscale heterogeneity in gastric adenocarcinoma evolution is an obstacle to precision medicine. Genome Med. 2021;13(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sangale Z, Prass C, Carlson A, et al. A robust immunohistochemical assay for detecting PTEN expression in human tumors. Appl Immunohistochem Mol Morphol. 2011;19:173-183. https://doi.org/ 10.1097/pai.0b013e3181f1da13 [DOI] [PubMed] [Google Scholar]
- 16. Yoshimoto M, Cutz J-C, Nuin PAS, et al. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet. 2006;169(2):128-137. [DOI] [PubMed] [Google Scholar]
- 17. Lotan TL, Heumann A, Rico SD, et al. PTEN loss detection in prostate cancer: comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget. 2017;8:65566-65576. https://doi.org/ 10.18632/oncotarget.19217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.