Abstract
Background/Purpose:
Hispanic/Latinos in the US are at increased risk for type 2 diabetes (T2D). Data suggest that avocado intake is associated with better glycemic control, but whether this translates to protection from T2D has not been studied. The goal of the current analyses was to examine whether consuming avocados at baseline is associated with lower incident T2D over a six-year period, compared to not consuming avocados at baseline.
Subjects/Methods:
Using data from a large population of US adults with Hispanic ancestry, without known or unknown T2D at baseline (N=6,159), participants were classified as avocado consumers (N=983) or non-consumers (N=5,176) based on the mean of two 24-hour dietary recalls. Cox proportional hazard models estimated the association of avocado consumption with incident T2D (N=656 cases) over a six-year follow-up period, in the population as a whole, and separately in those with normoglycemia vs. prediabetes at baseline. A set of three sequential models were run: the first controlling only for sociodemographic factors (“minimally adjusted” models), the second for these and health behaviors (“fully adjusted” models), and a third for both sets of covariates and also body mass index (BMI; “fully adjusted + BMI” models).
Results:
In the population as a whole, avocado intake at baseline was associated with reduced incident T2D in both the minimally adjusted (hazard ratio [HR] (+/− 95% confidence intervals [CIs]): 0.70 (0.52 – 0.94), P=.04) and the fully adjusted models (HR: 0.72 (0.54–0.97), P=.03). This association was observed in both those with prediabetes and with normoglycemia at baseline, but only reached significance in those with prediabetes (minimally adjusted model: HR: 0.68 (0.48–0.97), P=.03; fully adjusted model: HR: 0.69 (0.48–0.98), P=.04), not in those with normoglycemia (minimally adjusted model: HR: 0.86 (0.45–1.65), P=.65; fully adjusted model: HR: 0.80 (0.41–1.55), P=.50). In models which additionally controlled for BMI (“fully adjusted + BMI model”), the associations were slightly attenuated (overall population: HR: 0.79 (0.59–1.06), P=.60; normoglycemia: HR: 0.83 (0.42–1.64), P=.60; prediabetes: HR= 0.75 (0.54 – 1.05), P=0.09).
Conclusions:
In our longitudinal analyses, adults with Hispanic / Latino ancestry who consumed avocado were less likely to develop T2D than those who did not consume avocado at baseline, especially if they had prediabetes at baseline.
Keywords: avocado, diet, type 2 diabetes, dysglycemia, monounsaturated fats
1. INTRODUCTION
Over 22 million U.S. adults have a diagnosis of T2D [1], and the prevalence is ~66% higher in Americans with Hispanic ancestry (Hispanic/Latinos) when compared to non-Hispanic whites [2]. Diet remains one of the main modifiable risk factors for T2D. In addition to reducing overall energy intake and increasing whole grain consumption [3, 4], some (but not universal) evidence supports a role for limited amounts of monounsaturated fatty acids (MUFAs) in supporting glycemic control [5–9], although any beneficial effects may occur only in those with dysglycemia, and most strongly in those meeting criteria for T2D [10–12].
The relatively high MUFA content of avocado (~6.65g per USDA serving) suggests this fruit may offer protection from T2D, and is thought to be one reason why data have observed a lower incidence of incident cardiovascular disease (CVD) in avocado consumers vs. non consumers [13]. However, despite two intervention studies demonstrating an effect of avocado intake on postprandial glucose control [14, 15], and data from one observational study showing avocado consumption is associated with a lower prevalence of the metabolic syndrome [16], and from our own study showing an association with better glycemic control in those with T2D (submitted for publication and available upon request), research has not examined whether the same protection has been observed for incident T2D.
To address this knowledge gap, the goal of the current analyses were to examine the association of consuming avocado intake with incident T2D, in a large, geographically diverse sample of Hispanic/Latinos.
2. METHODS
The data included in the present study were downloaded from NHLBI Biological Specimen and Data Repository Information Coordinating Center (BioLINCC; https://biolincc.nhlbi.nih.gov/home/). The corresponding author had full access to the data and assumed responsibility for the integrity of the analyses and findings as reported.
2.2. Participants and procedure
HCHS/SOL is a community based prospective cohort study of US Hispanic/Latinos[17, 18]. HCHS/SOL recruited 16,415 self-identified Hispanic/Latino persons between 2008 and 2011, aged 18–74 years at baseline, across four US field centers (Chicago, IL; Miami, FL; Bronx, NY; San Diego, CA), who participated in an in-person clinic visit in (2008–2011), and again at an average of six years later in (2014–2017).
We included all participants who consented for their data to be included in NHLBI Biological Specimen and Data Repository Information Coordinating Center (BioLINCC) (N=12,647; Figure 1), who were free from T2D at baseline (N= 9,794; Figure 1), had complete dietary intake data and covariate data at baseline (N=8,690; Figure 1), and who completed the follow-up visit when information on incident T2D was obtained (N=6,159; Figure 1).
Figure 1:
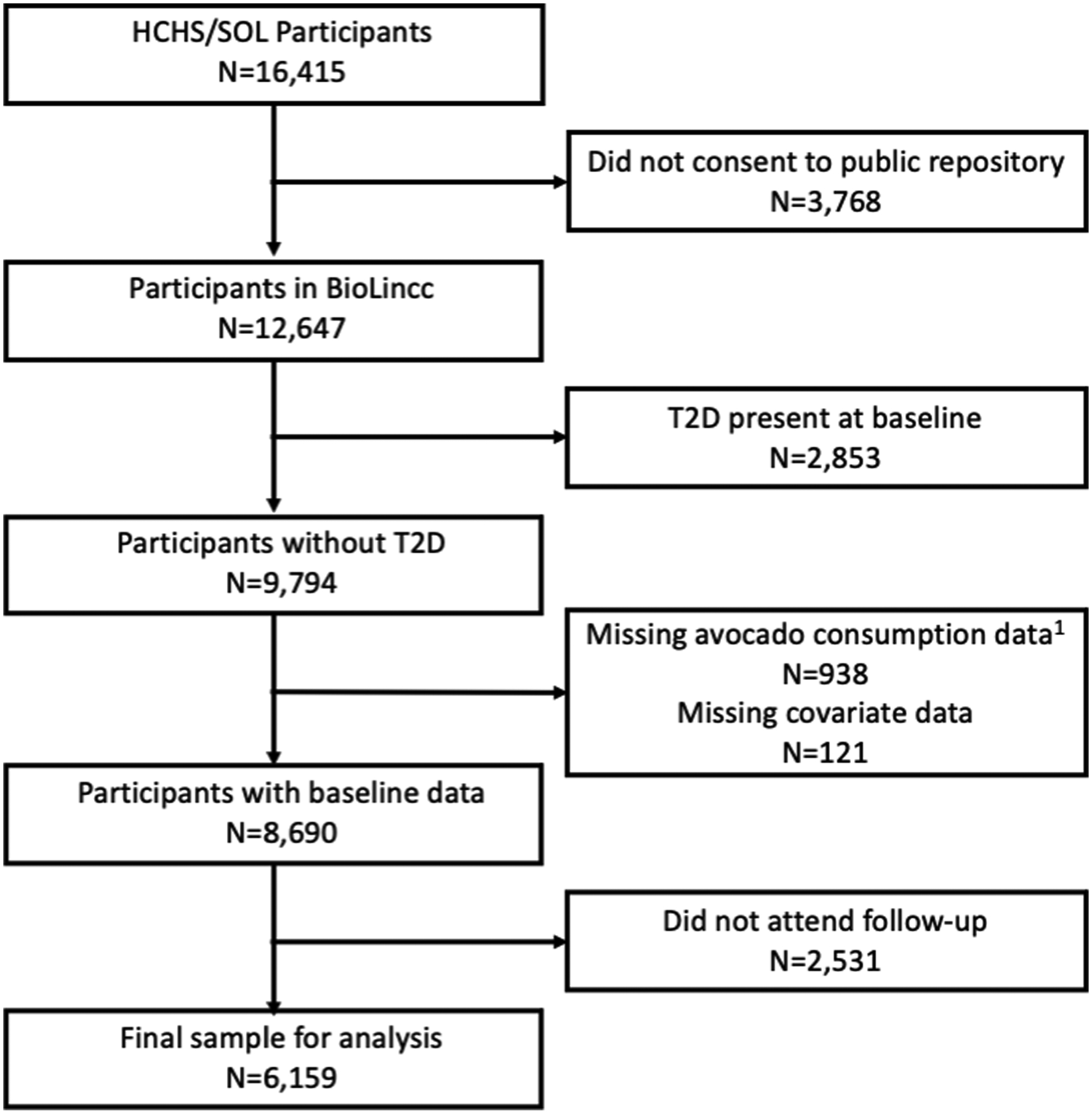
Participant Flow Diagram
1Data on two 24-hour dietary recalls needed for inclusion
All human data were collected in conjunction with the Declaration of Helsinki. The current analyses were approved by the Institutional Review Board at Baylor College of Medicine under exempt status (protocol no: H-49021).
2.3. Sampling Design
The study design for HCHS/SOL is described in detail elsewhere [17]. In brief, a stratified two-stage area probability sample of household addresses was selected in each of the four field centers. The first sampling stage randomly selected census block groups with stratification based on Hispanic/Latino concentration and proportion of high/low socio-economic status. The second sampling stage randomly selected households, with stratification, from US Postal Service registries that covered the randomly selected census block groups. Both stages oversampled certain strata to increase the likelihood that a selected address yielded a Hispanic/Latino household. After households were sampled, in-person or telephone contacts were made to screen eligible households and to roster its members. Lastly, the study oversampled the 45–74 age group (n=9,714, 59.2%) to facilitate examination of target outcomes. As a result, participants included in HCHS/SOL cohort were selected with unequal probabilities of selection, and these probabilities were considered during data analysis to appropriately represent the target population.
HCHS/SOL sampling weights are the product of a “base weight” (reciprocal of the probability of selection) and three adjustments: 1) non-response adjustments made relative to the sampling frame, 2) trimming to handle extreme values (to avoid a few weights with extreme values being overly influential in the analyses), and 3) calibration of weights to the 2010 US Census according to age, sex, and Hispanic heritage [17], and included in all analyses using inferential statistics.
2.4. Measures
The baseline in-person clinic visit included comprehensive assessment of biological (e.g., anthropometrics, blood draw, OGTT, ankle brachial pressure index, electrocardiogram), behavioral (e.g., dietary intake assessed with two 24-hour recalls, physical activity assessment by accelerometer and self-report, overnight sleep exam for apneic events, tobacco and alcohol assessed by self-report), and sociodemographic (e.g., socioeconomic status, migration history) factors [17, 18].
Glycemia status:
T2D status was determined based on American Diabetes Association criteria of: fasting glucose ≥ 126 mg/dL, glucose ≥ 200 mg/dL 2-hours after a glucose challenge as part of an oral glucose tolerance test (OGTT), or hemoglobin A1c (HbA1c) ≥ 6.5%), or if participants self- reported a previous diagnosis of T2D or medication usage for T2D[19]. Prediabetes was defined as fasting glucose range 100–125 mg/dL, or2-h post-OGTT glucose in range 140–199 mg/dL, or 5.7% ≤ A1C<6.5%. Normoglycemic was defined fasting glucose ≤ 100 mg/dL and 2-hour post-OGTT glucose ≤ 140 mg/dL and HbA1c ≤ 5.7%.
Incident diabetes was defined as participants who were free of diabetes at the baseline visit and were identified as having diabetes during the follow-up period. The date of an incident diabetes event was defined as time of the first self-report of diabetes diagnosis during an annual follow up interview or the time of in-person follow-up examination for incident diabetes based on fasting glucose / insulin / HbA1c.
2.4.1. Dietary intake:
Participants completed two 24-hour dietary recalls, using a multi-pass technique, at an interval of 5–45 days apart. The first diet recall covered the 24-hour period prior initiating a fast for the baseline clinic visit during which time the blood draw was collected Although 24-hour recalls are subject to self-report biases and error inherent in all self-reported nutrition data, validity coefficients suggest these are not higher in Hispanic/Latino data, including that from HCHS/SOL, than expected in other US populations [20, 21]. Nutrient and food group values were derived in the Nutrition Data System for Research (NDSR [22]). To minimize for the known biases in self-reported 24-hour recall data, nutrient intake was predicted as specified by the NCI method [23], using single component SAS macros developed at NCI (available at: http://riskfactor.cancer.gov/diet/usualintakes/macros.html). This method estimates the within and between person components and corrects for the high intra-individual variation intrinsic to 24-h recalls given that individuals do not eat the same foods every day [23].
Avocado intake in servings/day and total energy intake (kcal/day) were derived from an average of the two 24-hour recalls (as avocado is an episodically consumed food, participants with only one 24-hour dietary recall were excluded).
A dietary quality score (used as a covariate) was assessed via scores on the Alternative Healthy Eating Index-2010 (AHEI-2010), which sums decile scores for typical consumption across 11 dietary components (i.e., (1) vegetables without potatoes, (2) whole fruits, (3) whole grains, (4) long-chain (n-3) fats (EPA +DHA), (5) polyunsaturated fatty acids (PUFA), and (6) nuts and legumes (which are all positively scored), and (7) sugar sweetened beverages and fruit juices, (8) red/processed meat, (9) trans fats, (10) sodium, and (11) alcohol (which are all negatively scored).
2.4.2. Demographics:
Age, sex, education level, smoking status, Hispanic/Latino heritage / nativity, and preferred language were obtained through in-person interview with trained assessors.
2.4.3. Acculturation:
Following previous epidemiological studies that include Hispanic/Latinos living in the US [24], an acculturation score was constructed from three proxy measures: nativity, years living in the US and preferred language. US Nativity and years in the US were combined and scored as US-born (3 points), foreign-born and lived in the US at least 20 years (2 points), foreign-born and lived in the US 10–19 years (1 point), or foreign-born and lived in the US less than 10 years (0 points). A separate score was given for language spoken at home: English (2 points), English and Spanish (1 point), or non-English language (0 points). The two scores were summed to obtain an acculturation score from 0 (least acculturated) to 5 (most acculturated) [25].
2.4.5. Physical Activity:
Physical activity was measured in metabolic equivalent of task units (METs) of moderate and vigorous physical activity, as self-reported on an adapted version of the World Health Organization (WHO) Global Physical Activity Questionnaire [26], which asks participants to report the time spent in physical activity, and the type of activity conducted during an average week, across three life domains (work-related, transportation, and leisure/recreational).
2.4.6. Anthropometric measures:
Height and weight were measured by trained study staff. BMI was calculated as weight in kilograms (kg) divided by height in meters (m) squared (kg/m2).
2.5. Analyses
All analyses were conducted using the latest version of R software (version 4.0.5) [27], and all values and parameter estimates are adjusted for the sampling strategy using the “survey” package in R[28], with the exception of sample sizes (N and %) for participant characteristics, which are presented as unweighted.
2.5.1. Participant characteristics.
Demographic information stratified by avocado intake (non-consumer vs. consumer) were calculated as a weighted mean (+/− standard error; SE) for continuous variables, or unweighted total number (N) and weighted percentage (%) for categorical or ordinal variables. Differences by avocado consumption, without controlling for covariates, were conducted using weighed t-tests for continuous variables (transformed where necessary) and chi-squared tests of difference for categorical variables. Although unweighted counts are provided for the number of participants with each measure, weighted degrees of freedom (df) from tests of difference corrected for sampling strategy are presented throughout.
2.5.2. Association of avocado intake with incident diabetes.
The association of avocado intake with incident T2D was examined via cox-proportional hazards models, corrected for the complex sampling design with sample weights, which accounted for the reduction in variance from stratified sampling and the increase in variance from having only a small number of clusters. The hazard ratio for T2D associated with being an avocado consumer vs. non was calculated, using time at risk until the first diagnosis, or the last follow-up (if no T2D occurred).
The association between avocado intake and time to T2D was examined in three sets of models: in all models, avocado intake was specified as categorical variable (non-consumer vs. consumer). Model 1 specified age, sex, education level, total energy intake (kilocalories/day), and heritage, and acculturation as covariates (“minimally adjusted model”). Model 2 included model 1 covariates with the addition of alcohol, physical activity, smoking, and dietary quality (“fully adjusted model”). Due to the possibility that BMI mediates our associations of interest; the fully adjusted models did not control for BMI. However, recognizing the possibility that BMI could be a confounder, not a mediator, a final set of models were run which included the same covariates as the fully adjusted models with the addition of BMI (“fully adjusted + BMI” models). Hazard ratios (HR) and 95% confidence intervals (CIs) were reported for each model.
3. RESULTS
3.1. Participant characteristics
Sociodemographic factors, health behaviors and BMI, stratified by baseline avocado consumption (consumer vs. non) are presented in Table 1. Non-consumers did not differ from consumers in age, gender education level, acculturation, physical activity, alcohol intake alcohol consumption, smoking status, nor BMI. Avocado consumers tended to have a better overall dietary quality as measured by the AHEI2010 (t = 6.5, df = 609, P<0.001; Table 1), a lower BMI (t = 4.3, df = 609, P<0.001; Table 1) and have a different distribution of Hispanic heritage (χ2 =7.5, df = 5.1, P<0.001; Table 1) compared to non-consumers.
Table 1:
Weighted Mean +/− standard deviation (SD) or Unweighted Frequency (N) and Percentage (%) for Sociodemographic Factors, Health Behaviors, and Clinical Characteristics for the HCHS/SOL population, Stratified by Avocado Status (Consumers vs. Non-Consumers)
| Avocado non-consumers N1=5,176 |
Avocado consumers N1=983 |
|
|---|---|---|
| Sociodemographics | ||
| Age, y | 39.5 (0.32) | 38.8 (0.68) |
| Gender | ||
| Female, N1 (%) | 3,197 (61.77%) | 624 (63.48%) |
| Male, N1 (%) | 1,979 (38.23%) | 359 (36.52%) |
| Hispanic/Latino Heritage* | ||
| Dominican, N1 (%) | 511 (9.87%) | 73 (7.43%) |
| Central American, N1 (%) | 569 (10.99%) | 95 (9.66%) |
| Cuban, N1 (%) | 913 (17.64%) | 140 (14.24%) |
| Mexican, N1 (%) | 1,798 (34.74%) | 480 (48.83%) |
| Puerto Rican, N1 (%) | 831 (16.05%) | 97 (19.87%) |
| South American, N1 (%) | 398 (7.69%) | 70 (7.12%) |
| More than one heritage / Other, N1 (%) | 156 (3.01%) | 28 (2.85%) |
| Education level | ||
| No high school diploma / GED, N1 (%) | 1,714 (33.11%) | 276 (28.08%) |
| High school diploma / GED, N1 (%) | 1,375 (26.56%) | 255 (25.94%) |
| Greater than high school / GED, N1 (%) | 2,087 (40.32%) | 452 (43.23%) |
| Acculturation score | 1.91 (0.05) | 1.80 (0.09) |
| Health Behaviors | ||
| Physical Activity, MET-min/day | 726 (28.0) | 817 (58.9) |
| Alcohol intake, g/day | 0.29 (0.01) | 0.34 (0.03) |
| Diet quality, aHEI total score* | 46.5 (0.18) | 49.00 (0.36) |
| Smoking status | ||
| Non-smoker, N1 (%) | 4,208 (81.30%) | 828 (84.23%) |
| Current smoker, N1 (%) | 968 (18.70%) | 155 (15.77%) |
| Clinical characteristics | ||
| BMI, kg/m2 * | 29.1 (0.12) | 27.9 (0.25) |
P<.05 for differences between avocado consumers vs. non-consumers
Abbreviations: aHEI: alternative Healthy Eating Index; HCHS/SOL: Hispanic Community Health Study / Study of Latinos; MET-min: Metabolic equivalent minutes
Unweighted frequency
3.2. Association of avocado intake with incident diabetes
During a (weighted) mean of 6.09 (+/− 0.02) years of follow up, there were 656 incident events of diabetes (10.65% of the sample), with 84 occurring in those with normoglycemia at baseline and 572 in those with prediabetes at baseline.
Across the overall population, when adjusting for sociodemographic factors and health behaviors (“fully adjusted” model), consuming avocado at baseline was associated with a lower incident of T2D, with a reduction in risk of 25% (HR: 0.75 (0.54–0.95), P=.03; Table 2). When stratified by baseline dysglycemia, there was a significant inverse association in those with prediabetes, who saw a reduction in risk of 31% (HR: 0.69 (0.48–0.98), P=.04; Table 2, Figure 2). While an effect was seen in those with normoglycemia (20% reduction in risk), the inverse association between avocado consumption and risk of T2D was not significant in this group (HR: 0.80 (0.41–1.55, P=.50; Table 2, Figure 2).
Table 2:
Parameter Estimates from Cox-Proportional Hazards Models (Corrected for the Complex Survey Design with Sample Weights) Examining the Association of Avocado Intake Status (Consumer vs. Non) in HCHS/SOL with Incident Type 2 Diabetes, for the Whole Population and Stratified by Baseline Dysglycemia
| Model | Overall population (N=6,159; 656 incident cases1) |
Normoglycemic (N=3,084, 84 incident cases1) |
Prediabetic (N=3,075; 572 incident cases1) |
|||
|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | |
| 1 |
0.70
(0.52–0.94) |
.02 | 0.86 (0.45–1.65) |
.65 |
0.68
(0.48 – 0.97) |
.03 |
| 2 |
0.72
(0.54–0.97) |
.03 | 0.80 (0.41–1.55) |
.50 |
0.69
(0.48–0.98) |
.04 |
| 3 | 0.79 (0.59 – 1.06 |
.11 | 0.83 (0.42–1.64) |
.60 | 0.75 (0.54 – 1.05) |
.09 |
Unweighted frequency
Note: Significant results (P<.05) in bold
Abbreviations: HCHS/SOL: Hispanic Community Health Study / Study of Latinos; HR: Hazard ratio; N= unweighted frequency
Model 1 includes age, gender, energy intake (kilocalories / day), education level, acculturation, and Hispanic/Latino heritage as covariates (minimally adjusted model).
Model 2 includes the covariates from model 1, with the addition of alcohol intake, physical activity (metabolic equivalent minutes / day), diet quality (alternative Healthy Eating Index [aHEI] score) and smoking status (fully adjusted model)
Model 3 includes the covariates from model 2, with the addition body mass index (BMI; fully adjusted model+ BMI)
Figure 2:
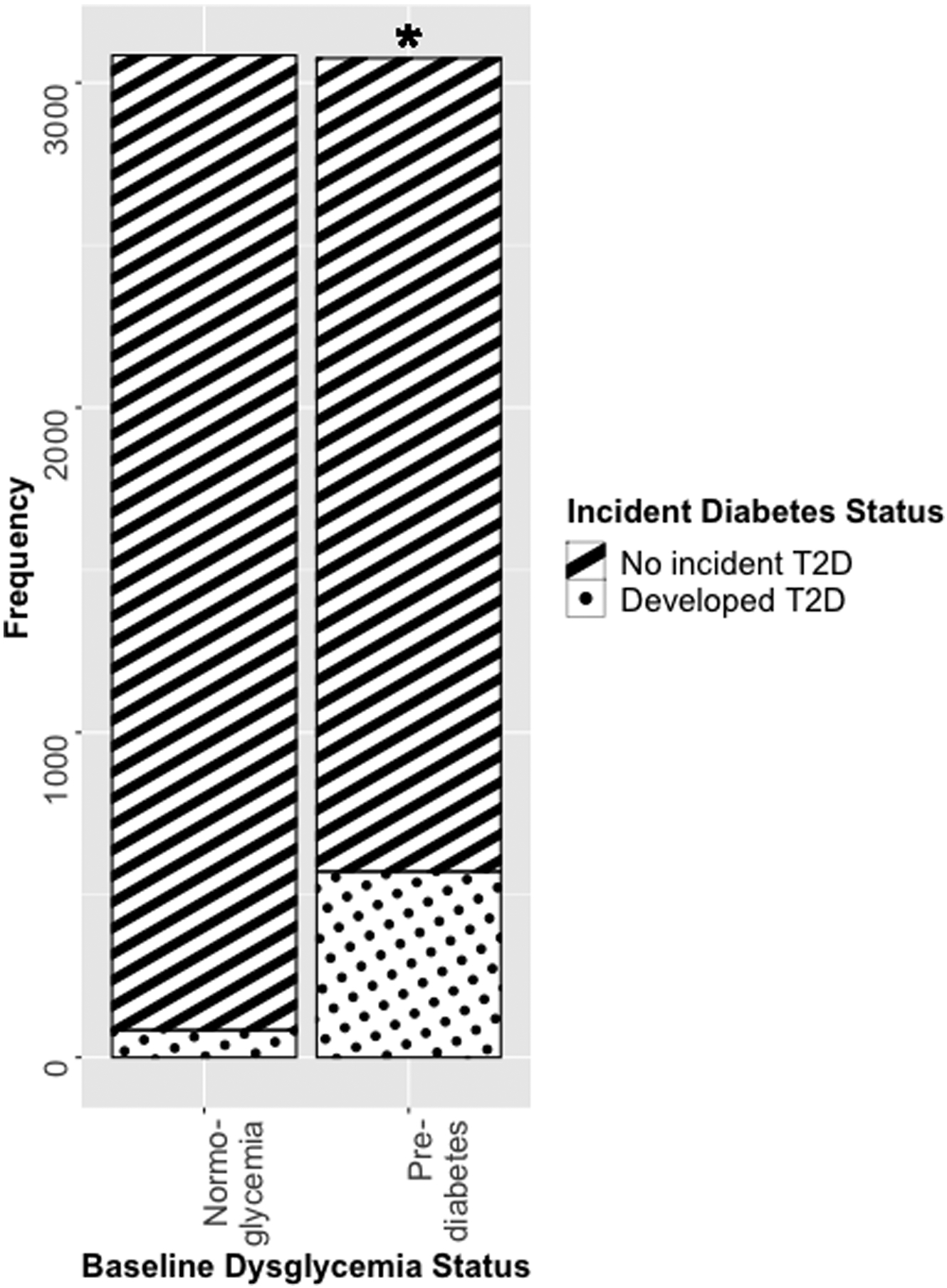
Incident Type 2 Diabetes Over a Six-Year Period, Stratified by Baseline Dysglycemia
*P<.05 for incident type 2 diabetes when comparing avocado consumer at baseline vs. non-consumers, in cox-proportional hazard models which correct for the complex survey design and control for age, gender, energy intake (kilocalories / day), education level, acculturation, Hispanic/Latino heritage, alcohol intake, physical activity (metabolic equivalent minutes / day), diet quality (alternative Healthy Eating Index [aHEI] score) and smoking status as covariates (fully adjusted model).
Although additionally controlling for BMI only resulted a small attenuation of the inverse avocado-T2D association, nonetheless the relationship was no longer significant in the population as a whole (HR: 0.79 (0.59 – 1.06; Table 2), the normoglycemics (HR: 0.83 (0.42–1.64, P=.60) nor those with baseline prediabetes (HR: 0.75 (0.54–1.05), P=.09).
4. DISCUSSION
Using data from HCHS/SOL, a large, population-based cohort of geographically diverse Hispanic/Latinos, the current analyses revealed, for the first time, that consuming avocado at baseline was associated with lower incident T2D over a 6-year follow up period, a relationship that was significant in individuals with prediabetes at baseline, and present but not significant in normoglycemics.
While our study is the first to examine whether avocado is associated with a lower risk of incident T2D, the findings are concordant with previous data from both intervention and observational studies showing an association between avocado intake (or consumption) and better glycemic control [14, 15, 29]. Identifying the reasons underlying this protective relationship are beyond the scope of the current investigation. Previous investigations have concluded that MUFA improves insulin sensitivity via a conserved IRS-1/PI3 kinase insulin signalling pathway [30]. Although the relatively high MUFA content of avocados drove our rationale for conducting these analyses, the nutrient profile of avocado contains multiple elements that could convey protection, such as (in a typical USDA serving): 345 mg of potassium (a nutrient necessary for glucose control) [31], 19.5 mg of magnesium (thought to improve glucose tolerance [32] and offer protection against the incidence of T2D), and 1.14g of linoleic acid -- a polyunsaturated fat (PUFA) which may improve insulin sensitivity [33]. Thus, it is not possible to tease out a single component responsible for the lower incidence of T2D, nor whether the relationship is due to the independent action of one or more micro- / macronutrient, or their synergistic effects.
Avocado was not significantly associated with lower incident T2D in those with normoglycemia, only in those with prediabetes, although the magnitude of effect in normoglycemics was similar to that in prediabetics (conveying 20% vs. 31% protection over a six-year period). Given the lower incidence of T2D in the former group (1.16% vs. 18.58%) these differential levels of significance could reflect a power issue. Nonetheless, previous meta-analyses have shown that the associations of MUFAs and olive oil (an oil rich in MUFAs) with better glucose control are stronger in those with dysglycemia than normoglycemics, and stronger still in those with T2D [10, 12, 34]. This highlights the importance of tailoring dietary advice to whether the goal is primary vs. secondary prevention, and the importance of considering individual’s different responses to dietary intake to long-term health (“personalized nutrition”).
Our study had numerous strengths, including the large number of participants, and the geographical diversity underlying their heritage. We also assessed avocado intake via the average of two 24-hour dietary recalls, which has the advantage of including all foods reported by our ancestrally diverse population with differing acculturation statuses. However, although our estimated avocado consumption was similar to previous estimates of US Hispanic/Latino avocado intake [35, 36], the use of 24-hour recall data on may still have misclassified some consumers as non-consumers introducing a source of error and over above the expected errors when using self-reported dietary data [37]. Finally, as with all observational studies, it is not possible to ensure that all confounders (including both measured and unmeasured factors) were removed or accounted for, which precludes causal inferences and leaves open the possibility that aspects avocado consumers’ overall lifestyle are responsible for their lower incidence of T2D.
Nonetheless, Hispanic/Latinos are at a higher risk of T2D than non-Hispanic white counterparts [38], and preventing the onset of chronic diseases in this population has been noted as a priority research area by the National Institutes of Health [39]. Our large-scale, longitudinal study provides additional support that avocados could form part of a diet aimed at supporting good glucose control, and highlights the need for future studies which research which examine whether the most efficacious dietary strategies are those tailored to an individual’s sensitive overall glucose homeostasis and metabolic functioning. In this way, dietary approaches to prevent T2D may be made more efficacious.
ACKNOWLEDGMENTS
This manuscript was prepared using HCHS/SOL research materials obtained from the NHLBI Biologic Specimen and Data Repository Coordinating Center and does not necessarily reflect the opinions or views of the HCHS/SOL nor the NHLBI.
SOURCES OF FUNDING
This study was funded by Hass Avocado Nutrition Board. Hass Avocado Board did not have input into the study design, analysis nor interpretation of results. Dr. Wood and Ms. Senn are also supported, in part, by USDA/ARS (Cooperative Agreement 58-3092-5-001). The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement from the US government.
Dr. Rotter’s roles in the current study were funded, in part, by the National Institutes of Health grants from the National Institute of Diabetes and Digestive and Kidney Disease (DK063491), from the National Center for Advancing Translational Sciences (UL1TR001881) and the CHARGE Consortium, and the National Heart, Lung, and Blood Institute (NHLBI) (R01HL105756).
DISCLOSURES
This study was funded by Hass Avocado Nutrition Board. Hass Avocado Board did not have input into the study design, analysis nor interpretation of results. Dr. Wood has received funding from The National Cattleman’s Beef Association and Ionis Pharmaceuticals for studies unrelated to the current analyses.
DATA AVAILABILITY STATEMENT:
All data included in the present analyses are available from the NHLBI Biological Specimen and Data Repository Information Coordinating Center (BioLINCC; https://biolincc.nhlbi.nih.gov/home/)
REFERENCES
- [1].Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, et al. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ennis SR, Ríos-Vargas M, Albert NG. The hispanic population: 2010: US Department of Commerce, Economics and Statistics Administration, US: …; 2011. [Google Scholar]
- [3].Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, et al. Nutrition principles and recommendations in diabetes. Diabetes Care. 2004;27 Suppl 1:S36–46. [DOI] [PubMed] [Google Scholar]
- [4].Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes care. 2008;31:S61–S78. [DOI] [PubMed] [Google Scholar]
- [5].Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS medicine. 2016;13:e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rasmussen OW, Thomsen C, Hansen KW, Vesterlund M, Winther E, Hermansen K. Effects on blood pressure, glucose, and lipid levels of high-monounsaturated fat diet compared with a high-carbohydrate diet in NIDDM subjects. Diabetes Care. 1993;16:1565–71. [DOI] [PubMed] [Google Scholar]
- [7].Parillo M, Rivellese A, Ciardullo A, Capaldo B, Giacco A, Genovese S, et al. A high-monounsaturated-fat/low-carbohydrate diet improves peripheral insulin sensitivity in non-insulin-dependent diabetic patients. Metabolism. 1992;41:1373–8. [DOI] [PubMed] [Google Scholar]
- [8].Luscombe N, Noakes M, Clifton P. Diets high and low in glycemic index versus high monounsaturated fat diets: effects on glucose and lipid metabolism in NIDDM. European journal of clinical nutrition. 1999;53:473–8. [DOI] [PubMed] [Google Scholar]
- [9].de Barros CR, Cezaretto A, Curti MLR, Pires MM, Folchetti LD, Siqueira-Catania A, et al. Realistic changes in monounsaturated fatty acids and soluble fibers are able to improve glucose metabolism. Diabetology & Metabolic Syndrome. 2014;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. Annals of Nutrition and Metabolism. 2011;58:290–6. [DOI] [PubMed] [Google Scholar]
- [11].Schwingshackl L, Christoph M, Hoffmann G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function-A Systematic Review and Meta-Analysis. Nutrients. 2015;7:7651–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schwingshackl L, Strasser B. High-MUFA diets reduce fasting glucose in patients with type 2 diabetes. Annals of Nutrition & Metabolism. 2012;60:33. [DOI] [PubMed] [Google Scholar]
- [13].Pacheco LS, Li Y, Rimm EB, Manson JE, Sun Q, Rexrode K, et al. Avocado Consumption and Risk of Cardiovascular Disease in US Adults. J Am Heart Assoc. 2022;11:e024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park E, Edirisinghe I, Burton-Freeman B. Avocado Fruit on Postprandial Markers of Cardio-Metabolic Risk: A Randomized Controlled Dose Response Trial in Overweight and Obese Men and Women. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wien M, Haddad E, Oda K, Sabate J. A randomized 3×3 crossover study to evaluate the effect of Hass avocado intake on post-ingestive satiety, glucose and insulin levels, and subsequent energy intake in overweight adults. Nutr J. 2013;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fulgoni VL, Dreher M, Davenport AJ. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Nutrition journal. 2013;12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Association AD. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3:A77. [PMC free article] [PubMed] [Google Scholar]
- [21].Mossavar-Rahmani Y, Sotres-Alvarez D, Wong WW, Loria CM, Gellman MD, Van Horn L, et al. Applying recovery biomarkers to calibrate self-report measures of sodium and potassium in the hispanic community health study/study of latinos. J Hum Hypertens. 2017;31:860. [DOI] [PubMed] [Google Scholar]
- [22].Harnack L Nutrition Data System for Research (NDSR). In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York, NY: Springer New York; 2013. p. 1348–50. [Google Scholar]
- [23].Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PM, et al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med. 2010;29:2857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Diep CS, Lemaitre RN, Chen TA, Baranowski T, Lutsey PL, Manichaikul AW, et al. Acculturation and Plasma Fatty Acid Concentrations in Hispanic and Chinese-American Adults: The Multi-Ethnic Study of Atherosclerosis. PLoS One. 2016;11:e0149267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kandula NR, Diez-Roux AV, Chan C, Daviglus ML, Jackson SA, Ni H, et al. Association of acculturation levels and prevalence of diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. 2008;31:1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6:790–804. [DOI] [PubMed] [Google Scholar]
- [27].Computing R. R: A language and environment for statistical computing. Vienna: R Core Team. 2013. [Google Scholar]
- [28].Lumley T Package ‘survey’. R package version. 2015;3:3–30. [Google Scholar]
- [29].Fulgoni VL 3rd, Dreher M, Davenport AJ. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Nutr J. 2013;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sabin M, Moon J, Lee J, Kang S, Park J, Lee B, et al. Dietary monounsaturated fatty acids preserve the insulin signaling pathway via IRS-1/PI3K in rat skeletal muscle. Lipids. 2010;45:1109–16. [DOI] [PubMed] [Google Scholar]
- [31].Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–24. [DOI] [PubMed] [Google Scholar]
- [32].Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, et al. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med. 2003;24:39–52. [DOI] [PubMed] [Google Scholar]
- [33].Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schwingshackl L, Lampousi A, Portillo M, Romaguera D, Hoffmann G, Boeing H. Olive oil in the prevention and management of type 2 diabetes mellitus: a systematic review and meta-analysis of cohort studies and intervention trials. Nutrition & diabetes. 2017;7:e262–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pareo-Tubbeh SL, Romero LJ, Baumgartner RN, Garry PJ, D LINDEMAN R, Koehler KM. Comparison of energy and nutrient sources of elderly Hispanics and non-Hispanic whites in New Mexico. J Am Diet Assoc. 1999;99:572–82. [DOI] [PubMed] [Google Scholar]
- [36].Bartholomew A, Young E, Martin H, Hazuda HP. Food frequency intakes and sociodemographic factors of elderly Mexican Americans and non-Hispanic whites. J Am Diet Assoc. 1990;90:1693–6. [PubMed] [Google Scholar]
- [37].Willett W Nutritional epidemiology: issues and challenges. Int J Epidemiol. 1987;16:312–7. [DOI] [PubMed] [Google Scholar]
- [38].Control CfD, Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: US department of health and human services, centers for disease control and prevention. 2011;201:2568–9. [Google Scholar]
- [39].Aviles-Santa ML, Heintzman J, Lindberg NM, Guerrero-Preston R, Ramos K, Abraido-Lanza AL, et al. Personalized medicine and Hispanic health: improving health outcomes and reducing health disparities - a National Heart, Lung, and Blood Institute workshop report. BMC Proc. 2017;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in the present analyses are available from the NHLBI Biological Specimen and Data Repository Information Coordinating Center (BioLINCC; https://biolincc.nhlbi.nih.gov/home/)


