Abstract
Irritable bowel syndrome (IBS) is a common gastrointestinal disease. The efficacy of different probiotics in treating IBS remains controversial. This network meta-analysis aimed to compare and rank the outcome-specific efficacy of different probiotic strains or combinations in adults with IBS. We searched the literature up to June 2023. Randomized controlled trials (RCTs) that evaluated the efficacy of probiotics in IBS were included. A frequentist framework was used to perform this study. In total, 9253 participants from 81 RCTs were included in the study. Four probiotic strains and five mixtures were significantly superior to placebo in improving IBS Symptom Severity Scale, among which Lactobacillus acidophilus DDS-1 ranked first (surface under the cumulative ranking, SUCRA, 92.9%). A mixture containing five probiotics (SUCRA, 100%) ranked first in improving the IBS-Quality of life. Bacillus coagulans MTCC 5856 (SUCRA, 96.9%) and Bacillus coagulans Unique IS2 (SUCRA, 92.6%) were among the most effective probiotics for improving abdominal pain. Three probiotic strains and two mixtures were effective in alleviating abdominal bloating. Four probiotic strains and a mixture were significantly superior to placebo in reducing the bowel movement frequency in diarrhea-predominant IBS (IBS-D). Bacillus coagulans MTCC 5856 (SUCRA, 99.6%) and Saccharomyces cerevisiae CNCM I-3856 (SUCRA, 89.7%) were among the most effective probiotics for improving the Bristol stool form scale of IBS-D. Only some probiotics are effective for particular outcomes in IBS patients. This study provided the first ranking of outcome-specific efficacy of different probiotic strains and combinations in IBS. Further studies are needed to confirm these results.
Keywords: irritable bowel syndrome, probiotic, network meta-analysis, outcome, efficacy
1. Introduction
Irritable bowel syndrome (IBS) is a common functional bowel disease that is induced by disorders of gut–brain interactions. Typical symptoms of IBS include recurrent abdominal pain associated with changes in stool form or frequency [1]. The prevalence of IBS is approximately 9.2% globally, but varies from 1.1% to 35.5% according to the region and diagnostic criteria [2,3]. The annual healthcare cost estimates of IBS are substantial: CNY 123 billion in China, USD 10 billion in the USA, and GBP 2 billion in the UK [4,5,6,7]. IBS exerts a great impact on quality of life and productivity for individuals [8]. Patients with IBS experience troublesome and unpredictable symptoms, which cause frequent medical visits and absenteeism [9,10]. Consistent health worries and a lack of understanding by family may lead to psychological problems in patients, such as anxiety and depression [10]. IBS imposes a huge burden on individuals and society.
The gut microbiota, which is considered the ecologic system of various microorganisms in the gastrointestinal tract, plays a critical role in the pathogenesis of IBS through the gut–brain axis [11]. Altering the composition of gut microbiota toward a healthy community has become a potential strategy for IBS treatment [12,13]. One of the representative choices of this strategy is probiotics, which are live microorganisms that confer a health benefit on the host when administered in adequate amounts [14]. The efficacy of particular species of probiotics in IBS has been reported in randomized controlled trials (RCTs) and meta-analyses. A recent RCT confirmed that Bifidobacterium quadruple viable tablets effectively alleviated abdominal pain and diarrhea for patients with diarrhea-predominant IBS (IBS-D) [15]. Two meta-analyses indicated that probiotics had beneficial effects on abdominal pain and bloating [16,17].
However, it is still unclear which strain or combination of probiotics are effective in global IBS symptoms. It is even harder for physicians to select appropriate probiotics for IBS patients with various symptoms. Numerous single-strain probiotics and multistrain combination products have been developed for IBS every year. Instead of comprehensive evaluations from a standard system, reports from some clinical trials have focused on significant efficacy and specific outcomes in endorsements of particular probiotics [18]. The profusion of data regarding different strains or combinations of probiotics, different IBS subtypes, and different end points, outcomes, and study quality has resulted in a complex evidence network that is difficult to interpret [19]. Hence, a previous meta-analysis made conservative and cautious estimates of the efficacy of probiotics [20]. Relevant guidelines also present different attitudes toward probiotics. The British Society of Gastroenterology (BSG) guidelines set probiotics as first-line treatments but have not yet recommended a specific species or strain [12]. However, the American College of Gastroenterology (ACG) guidelines suggest against probiotics for the treatment of global IBS symptoms [18]. More detailed evidence of probiotic efficacy in IBS is needed.
In this systematic review and network meta-analysis (NMA), we aimed to evaluate the comparative efficacy of different probiotic strains and mixtures based on global conditions, mental health levels, and specific gastrointestinal symptoms.
2. Materials and Methods
This NMA was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis Statement (the PRISMA 2020 statement and the PRISMA Extension Statement of NMA) [21,22]. We registered a protocol (CRD42023387351) on the International Prospective Register of Systematic Reviews. Alterations of the original protocol were presented in Supplementary Text S1.
2.1. Search Strategy and Eligibility Criteria
We performed comprehensive literature searches of five databases from their inception to 1 June 2023 (Web of Science, PubMed, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials, and Embase). No language restrictions were applied. The basic search strategies were as follows: ((((irritable bowel syndrome [Title/Abstract]) OR (IBS [Title/Abstract])) OR (irritable bowel syndrome [MeSH Terms])) AND (((((((((Probiotic* [Title/Abstract]) OR (Probiotic [MeSH Terms])) OR (Lactobacillus [Title/Abstract])) OR (Saccharomyces [Title/Abstract])) OR (Bacillus [Title/Abstract])) OR (Bifidobacterium [Title/Abstract])) OR (Clostridium [Title/Abstract])) OR (Streptococcus [Title/Abstract])) OR (Enterococcus [Title/Abstract]))) AND ((((((clinical trial [Title/Abstract]) OR (clinical trials [MeSH Terms])) OR (clinical trial [Publication Type])) OR (random* [Title/Abstract])) OR (random allocation [MeSH Terms])) OR (therapeutic use [MeSH Subheading])). Supplementary Text S2 presents the detailed search strategies in different databases. We also screened meta-analyses about IBS treatments published in the last five years for eligible clinical trials. The official websites of academic conferences (Digestive Disease Week, American College of Gastroenterology, Asian Pacific Digestive Week and the United European Gastroenterology Week) and probiotic companies were searched for potential ongoing studies and grey literature. These three approaches constitute the literature source for the NMA.
The inclusion criteria were as follows:
RCTs that compared the efficacy of probiotics with placebo or different probiotics for IBS;
Participants were diagnosed with IBS based on Rome I, II, III, or IV criteria, Manning criteria, or physician’s opinion;
Patients in the test group received single or multistrain probiotics;
Patients in the control group received placebo or another probiotic;
RCTs should report at least one of the targeted outcomes (see 2.2 Outcome assessment). Outcomes should be reported as data at baseline and endpoint, or as absolute changes during the study;
The treatment duration was at least two weeks.
The exclusion criteria were as follows:
Open-label trials, single-arm studies, nonrandomized trials, reviews, protocols, and letters. Crossover RCTs that did not report data from the first stage were excluded;
Duplicate study;
Studies involving pregnant or lactating mothers, patients with a history of gastrointestinal surgery, and patients aged < 18 years;
Studies involving patients who received combined treatments, such as synbiotics, antibiotics, antidepressants, and psychological therapy.
2.2. Outcome Assessment
We selected seven outcomes to evaluate the global condition, mental health condition, and core gastrointestinal symptoms of patients with IBS. All data on these outcomes were recorded as the change from baseline to therapy completion.
The global condition of IBS was evaluated based on the following outcomes. (1) Change in IBS Symptom Severity Scale (IBS-SSS) from baseline [23]. The five domains of the IBS-SSS generate a total score from 0 to 500 (no symptoms to very severe). (2) The change in IBS-Quality of Life (IBS-QOL) from baseline [24]. The total score of 34 items in the IBS-QOL generates a total score from 0 to 100. Higher scores indicate a better IBS quality of life.
(3) The mental health condition of patients with IBS was evaluated by the change in the Hospital Anxiety and Depression Scale (HADS) from baseline [25]. The HADS-total, anxiety, and depression scores were collected individually.
Core gastrointestinal symptoms of IBS: (4) Abdominal pain score, reflecting the degree of abdominal pain severity. (5) The abdominal bloating score reflects the severity of abdominal bloating. This outcome was defined as a feeling of abdominal swelling or gas accumulation. The terms “bloating” or “distension” were used in related questionnaires. (6) Bowel movement frequency (per week) in IBS-D or IBS with predominant constipation (IBS-C). (7) Bristol stool form scale in IBS-D or IBS-C. Data on adverse events in each study were collected.
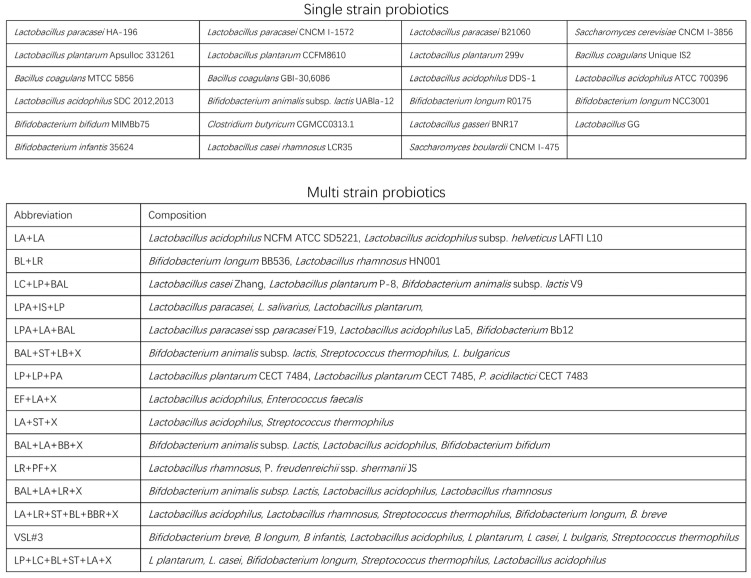
Single-strain probiotics were classified at the strain level. The classification of multistrain probiotics consists of several cornerstone probiotics and “X” probiotics. X can be absent or presented in other probiotics, usually fewer than two strains. Alterations in the gut microbiota after probiotic administration were summarized for a systematic review, including changes in microbiota abundance and diversity.
2.3. Data Extraction
Pairs of reviewers independently screened titles and abstracts of the search results. The full texts and study protocols of potentially eligible articles were examined based on eligibility criteria. Two reviewers used a piloted electronic form to extract the data independently and in duplicate (Supplementary Text S3). Data extraction included the following items: RCT general information (article title, first author, publication year, trial location, trial design, inclusion and exclusion criteria, sample size, composition and dosage of probiotics, and follow-up period), population characteristics (IBS diagnosis criteria, IBS duration and severity, sex, age, and body mass index), and outcomes of interest. Intention-to-treat analyses were performed for data collection. Disagreements were resolved by consensus with a senior investigator. Corresponding authors were queried for original data by e-mail if the outcome data were not reported in full text.
2.4. Risk of Bias and Evidence Quality
The Cochrane risk-of-bias tool version 2 (RoB 2) was used to assess the risk of bias for each outcome in the selected studies [26]. The tool comprises several questions and algorithms that map responses to signaling questions to a proposed risk-of-bias judgment. Two authors independently assessed risk of bias. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was applied to assess the quality of this systematic review and NMA for each outcome [27]. Disagreements were resolved by consensus.
2.5. Statistical Analysis
Traditional pairwise meta-analyses were conducted using a random-effects model [28]. The mean difference (MD) with 95% confidence intervals (CIs) was calculated to measure the treatment effects of continuous data. Standardized mean difference (SMD) was used for abdominal pain and bloating scores. Heterogeneity was described using the I2 statistic [29].
Random-effects NMA was conducted by using the frequentist framework. The MD or SMD with 95% CIs for outcomes was calculated [30]. Heterogeneity among the included studies was assessed using a prediction interval plot. It showed the influence of heterogeneity by providing a predicted range for the true treatment effect in 95% of individual studies [31]. A fundamental assumption of NMA is transitivity, which requires study sets to be similar enough in critical clinical characteristics. Regarding this NMA, the characteristics included the proportion of females and the average age. These effect modifiers were presented using box plots. The consistency was evaluated when a loop was presented in the evidence network. We evaluated local inconsistency using node-splitting analysis and loop-specific analysis [30,32]. Global inconsistency was assessed by an inconsistency model of design-by-treatment interaction [33].
Network plots were constructed to visualize the treatment network at the outcome level. The surface under the cumulative ranking curve (SUCRA) values were calculated for efficacy ranks. The SUCRA value of a treatment indicates the chance of this treatment to be the best [30]. Publication bias was assessed by funnel plots [30].
The above analyses used code packages (mvmeta, network, and network graphs) based on STATA (Stata Corp, College Station, TX, USA) [34,35].
2.6. Efficacy Classification
We referred to a novel and succinct approach to efficacy classification proposed by Morgan et al., and the interventions were categorized into three levels as follows [36].
Level A (among the most effective): probiotics that are significantly superior to placebo and at least one probiotic at Level B;
Level B: probiotics that are more effective than placebo, but not superior to any other probiotic(s) superior to placebo;
Level C (among the least effective): probiotics with no significant difference compared with placebo.
3. Results
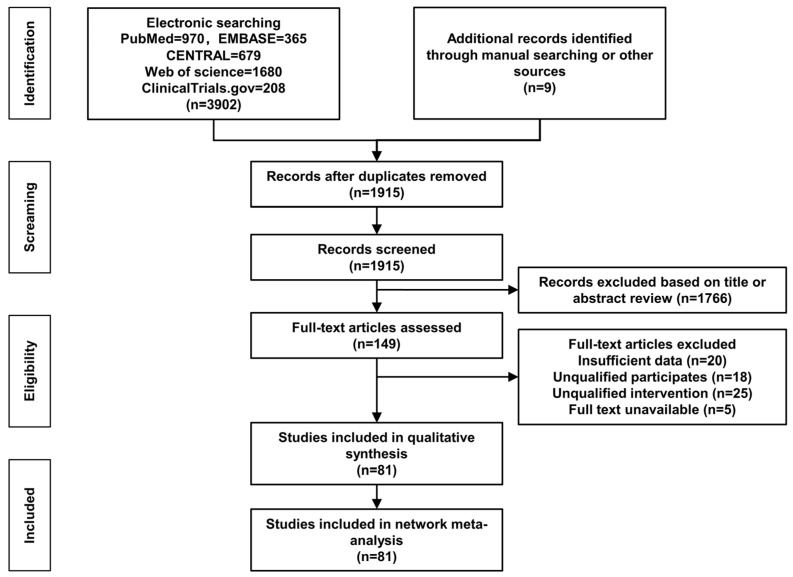
The literature search and refinement procedure are illustrated in Figure 1. Electronic and manual searches yielded 3903 initial records. After removing duplicates and reviewing the titles and abstracts, we evaluated the full texts of 149 articles. Ultimately, the qualitative synthesis and network meta-analysis included 81 RCTs. The references to the RCTs included in this NMA are listed in Supplementary Text S4.
Figure 1.
Flow chart illustrating the procedures of literature search and refinement.
3.1. Characteristics of Included Studies
The characteristics of the included RCTs are summarized in Tables S1 and S2. This NMA included 9253 participants from 81 RCTs [15,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116]. Participants in this study came from 25 countries ranging across Europe, North America, Asia, and Africa. The sample size ranged from 19 to 456 patients. The average age ranged from 21.8 to 63 years. Most studies (n = 53) involved recruiting all subtypes of IBS, while 18 studies focused on IBS-D and five on IBS-C. The distribution of effect modifiers is summarized in Figure S1.The classification of the included probiotics is summarized in Figure 2.
Figure 2.
Single strain and multistrain probiotics included in network meta-analyses.
3.2. Risk of Bias within Studies
Twenty-four trials were evaluated as “some concern” on bias arising from the randomization process. Thirty-two trials did not provide sufficient information about deviations from the intended interventions. Three trials were evaluated as “some concern” for bias due to missing outcome data. Two trials were evaluated as “some concern” on bias in measurement of the outcome. Twenty-two trials did not provide details on the selection of reported results. Notably, 45 RCTs in this NMA were funded by commercial companies. The ROB 2 figures for each outcome are shown in Figure S2.
3.3. Critical Results of Network Meta-Analysis
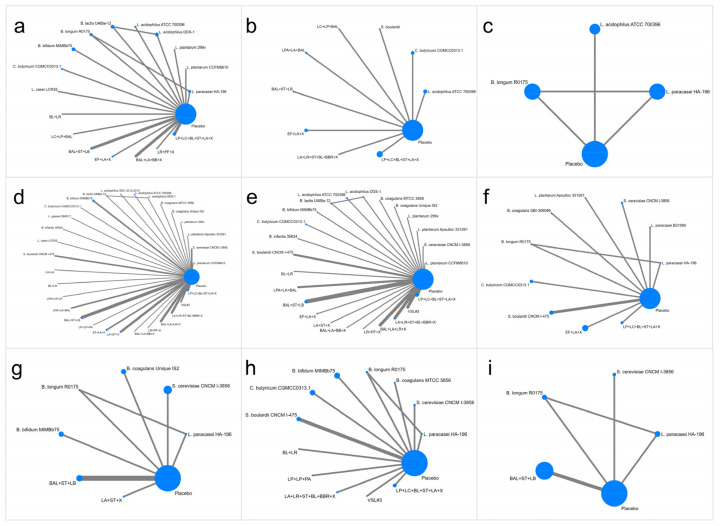
The results of the pairwise meta-analysis are summarized in Table S3. League tables for the different outcomes are summarized in Table S4. Network plots are shown in Figure S3. SUCRAs are summarized in Figure S4 and Table S5.
3.3.1. IBS-SSS
The evidence network was constructed with ten single-strain probiotics and seven multistrain groups from 18 RCTs (2628 patients, Figure 3). Moderate-certainty evidence indicated that Lactobacillus acidophilus DDS-1 (MD, −77.70; 95% CI, −101.72 to −53.68), BL + LR (MD, −80.99; 95% CI, −130.73 to −31.26), LC + LP + BAL (MD, −76.42; 95% CI, −114.90 to −37.95), LP + LC + BL + ST + LA + X (MD, −63.96; 95% CI, −78.66 to −49.26) and Bifidobacterium animalis subsp. lactis UABla-12 (MD, −48.80; 95% CI, −73.00 to −24.60) were classified as efficacy level A (Table 1). EF + LA + X, Bifidobacterium bifidum MIMBb75, Clostridium butyricum CGMCC0313.1, and BAL + LA + BB + X were also significantly superior to the placebo in improving IBS-SSS (efficacy level B, Table 1). The top three treatments based on SUCRAs were Lactobacillus acidophilus DDS-1 (92.9%), BL + LR (91.6%), and LC + LP + BAL (90.9%) (moderate certainty).
Figure 3.
Network plots for different outcomes. (a). IBS Symptom Severity Scale; (b). IBS-Quality of Life Measure; (c). the Hospital Anxiety and Depression Scale; (d). abdominal pain score; (e). abdominal bloating score; (f). bowel movement frequency (per week) in IBS-D; (g). bowel movement frequency (per week) in IBS-C; (h). Bristol stool form scale in IBS-D; (i). Bristol stool form scale in IBS-C. Different probiotics are represented by nodes. The size of each node is proportional to the number of patients. The width of the edges represents the number of RCTs.
Table 1.
Critical results of network meta-analysis for the global condition and mental health condition in patients with IBS.
| Outcome | Efficacy Level A 1 | Efficacy Level B | Efficacy Level C | ||||
|---|---|---|---|---|---|---|---|
| Probiotic | NMA 2 | GRADE | Probiotic | NMA | GRADE | Probiotic | |
| IBS-SSS | L. acidophilus DDS-1 | −77.70 (−101.72, −53.68) |
M | EF + LA + X | −35.00 (−60.44, −9.56) |
M | L. acidophilus ATCC 700396, L. plantarum 299v, L. plantarum CCFM8610, L. casei LCR35, L. paracasei HA-196, B. longum R0175, BAL + ST + LB, LR + PF + X |
| BL + LR | −80.99 (−130.73, −31.26) |
M | B. bifidum MIMBb75 | −29.83 (−48.24, −11.42) |
H | ||
| LC + LP + BAL | −76.42 (−114.90, −37.95) |
M | C. butyricum CGMCC0313.1 | −21.38 (−40.47, −2.29) |
H | ||
| LP + LC + BL + ST + LA + X | −63.96 (−78.66, −49.26) |
M | BAL + LA + BB + X | −18.86 (−25.88, −11.85) |
M | ||
|
B. lactis UABla-12 |
−48.80 (−73.00, −24.60) |
M | |||||
| IBS-QOL | LP + LC + BL + ST + LA + X | 24.80 (20.65, 28.95) |
M | C. butyricum CGMCC0313.1 | 4.07 (0.50, 7.65) |
H | LP + LC + BL + ST + LA + X, S. boulardii CNCM I-475, LC + LP + BAL, LA + LR + ST + BL + BBR + X, EF + LA + X, BAL + ST + LB, LPA + LA + BAL, L. acidophilus ATCC 700396 |
| HADS-total score | B. longum R0175 | −0.34 (−0.48, −0.20) |
H | None | L. paracasei HA-196, L. acidophilus ATCC 700396 | ||
| HADS-anxiety | None | None | B. longum NCC3001, L. acidophilus ATCC 700396, LPA + LA + BAL | ||||
| HADS-depression | B. longum NCC3001 | −3.00 (−4.92, −1.08) |
H | None | L. paracasei CNCM I-1572, L. acidophilus ATCC 700396, LPA + LA + BAL | ||
1 Efficacy level A (among the most effective): probiotics that are significantly superior to placebo and at least 1 probiotic in level B. Efficacy level B: probiotics that are more effective than placebo, but not superior to any other of the probiotic(s) superior to placebo. Efficacy level C (among the least effective): probiotics with no significant difference compared to placebo. 2 The network meta-analysis results were the efficacy of probiotics compared to placebo. Mean difference with 95% confidence intervals in parentheses. Abbreviations: IBS-SSS, IBS Symptom Severity Scale; IBS-QOL, IBS-Quality of Life Measure; HADS, the Hospital Anxiety and Depression Scale; GRADE, The Grading of Recommendations, Assessment, Development and Evaluation system; H, high; M, moderate; L, low.
3.3.2. IBS-QOL
Nine RCTs involving three single-strain probiotics and six multistrain groups reported IBS-QOL (1323 patients, Figure 3). The NMA indicated that LP + LC + BL + ST + LA + X (MD, 15.35; 95% CI, 4.45 to 26.26; moderate certainty) was considered efficacy level A (Table 1). Clostridium butyricum CGMCC0313.1 (MD, 4.07; 95% CI, 0.50 to 7.65) was also significantly superior to the placebo in improving IBS-QOL (Table 1).
3.3.3. HADS Score
Evidence networks were constructed with five single-strain probiotics and a multistrain group from five RCTs (622 patients, Figure 3). Bifidobacterium longum R0175 significantly reduced the HADS total score (MD, −0.34; 95% CI, −0.48 to −0.20; high certainty). Bifidobacterium longum NCC3001 significantly reduced the HADS-depression score (MD, −3.0; 95% CI, −4.92 to −1.08; high certainty) (Table 1). No significant improvement was found in the HADS-anxiety score among the included studies.
3.3.4. Abdominal Pain Score
The abdominal pain score was reported in 47 RCTs (4680 patients) that involved 16 single-strain probiotics and 14 multistrain groups (Figure 3). NMA indicated that Bacillus coagulans MTCC 5856 (SMD, −41.80; 95% CI, −61.59 to −22.00; Moderate certainty) and Bacillus coagulans Unique IS2 (SMD, −32.00; 95% CI, −45.35 to −18.65; Moderate certainty) were classified as efficacy level A (Table 2). Lactobacillus gasseri BNR17, Lactobacillus plantarum Apsulloc 331261, Lactobacillus acidophilus DDS-1, LPA + LS + LP, Saccharomyces cerevisiae CNCM I-3856, VSL#3, EF + LA + X, and LA + ST + X were significantly superior to the placebo in reducing abdominal pain score (Table 2). The top three treatments based on SUCRAs were Bacillus coagulans MTCC 5856 (96.9%), Bacillus coagulans Unique IS2 (92.6%), and Lactobacillus gasseri BNR17 (91.3%).
Table 2.
Critical results of network meta-analysis for abdominal pain and bloating in patients with IBS.
| Outcome | Efficacy Level A 1 | Efficacy Level B | Efficacy Level C | ||||
|---|---|---|---|---|---|---|---|
| Probiotic | NMA 2 | GRADE | Probiotic | NMA | GRADE | Probiotic | |
| Abdominal pain score | B. coagulans MTCC 5856 | −41.80 (−61.59, −22.00) |
M | L. gasseri BNR17 | −36.10 (−64.53, −7.67) |
M | BL + LR, L. acidophilus SDC 2012,2013, B. lactis UABla-12, BAL + LA + BB + X, LR + PF + X, LA + LR + ST + BL + BBR + X, B. bifidum MIMBb75, LP + LP + PA, LP + LC + BL + ST + LA + X, LA + LA, C. butyricum CGMCC0313.1, L. plantarum 299v, L. acidophilus ATCC 700396, L. casei LCR35, BAL + ST + LB, LPA + LA + BAL, BAL + LA + LR + X, S. boulardii CNCM I-475, B. infantis 35624, L. plantarum CCFM8610 |
| B. coagulans Unique IS2 | −32.00 (−45.35, −18.65) |
M |
L. plantarum Apsulloc 331261 |
−26.59 (−47.07, −6.11) |
M | ||
| L. acidophilus DDS-1 | −19.53 (−33.49, −5.57) |
M | |||||
| LPA + LS + LP | −20.00 (−35.97, −4.03) |
L | |||||
| S. cerevisiae CNCM I-3856 | −15.24 (−24.62, −5.87) |
M | |||||
| VSL#3 | −12.93 (−25.59, −0.26) |
L | |||||
| EF + LA + X | −11.37 (−21.68, −1.06) |
M | |||||
| LA + ST + X | −8.14 (−15.54, −0.75) |
M | |||||
| Abdominal bloating score | None | BL + LR | −34.00 (−56.94, −11.06) |
M | B. coagulans MTCC 5856, L. plantarum Apsulloc 331261, L. acidophilus DDS-1, LR + PF + X, LA + ST + X, B. coagulans Unique IS2, S. cerevisiae CNCM I-3856, BAL + LA + BB + X, B. lactis UABla-12, LP + LC + BL + ST + LA + X, EF + LA + X, LA + LR + ST + BL + BBR + X, L. acidophilus ATCC 700396, BAL + LA + LR + X, S. boulardii CNCM I-475, C. butyricum CGMCC0313.1, BAL + ST + LB, B. infantis 35624, LPA + LA + BAL | ||
| L. plantarum CCFM8610 | −19.92 (−34.91, −4.94) |
M | |||||
| L. plantarum 299v | −14.79 (−29.11, −0.48) |
M | |||||
| VSL#3 | −13.71 (−22.12, −5.30) |
L | |||||
| B. bifidum MIMBb75 | −11.83 (−22.93, −0.74) |
M | |||||
1 Efficacy level A (among the most effective): probiotics that are significantly superior to placebo and at least 1 probiotic in level B. Efficacy level B: probiotics that are more effective than placebo, but not superior to any other of the probiotic(s) superior to placebo. Efficacy level C (among the least effective): probiotics with no significant difference compared to placebo. 2 The network meta-analysis results were the efficacy of probiotics compared to placebo. Mean difference with 95% confidence intervals in parentheses. Abbreviations: GRADE, The Grading of Recommendations, Assessment, Development and Evaluation system; H, high; M, moderate; L, low.
3.3.5. Abdominal Bloating Score
Thirty-nine studies involving 13 single-strain probiotics and 11 multistrain groups found improvements in abdominal bloating scores (3383 patients, Figure 3). NMA indicated that BL + LR, Lactobacillus plantarum CCFM8610, Lactobacillus plantarum 299v, VSL#3, Bifidobacterium bifidum MIMBb75 were significantly superior to placebo in reducing the abdominal bloating score (Table 2). The top three treatments based on SUCRAs were BL + LR (SMD, −34.00; 95% CI, −56.94 to −11.06; SUCRA 94.5%), Bacillus coagulans MTCC 5856 (SMD, −19.92; 95% CI, −34.91 to −4.91; SUCRA 85%), and Lactobacillus plantarum CCFM8610 (SMD, −14.79; 95% CI, −29.11 to −0.48; 82.6%).
3.3.6. Bowel Movement Frequency (Per Week) in the IBS-D and IBS-C Groups
The IBS-D evidence network was constructed using eight single-strain probiotics and two multistrain groups from 10 RCTs (877 patients, Figure 3). The NMA indicated that EF + LA + X (MD, −3.95; 95% CI, −5.02 to −2.88; Moderate certainty) were classed as efficacy level A (Table 3). Lactobacillus paracasei B21060, Lactobacillus paracasei HA-196, Bacillus coagulans GBI-306086, and Bifidobacterium longum R0175 significantly reduced bowel movement frequency in patients with IBS-D compared to placebo (Efficacy level B, Table 3). The top three treatments based on SUCRAs were EF + LA + X (89.7%), Lactobacillus paracasei B21060 (89.2%), and Lactobacillus paracasei HA-196 (78.7%).
Table 3.
Critical results of network meta-analysis for bowel movement frequency and Bristol stool form scale in patients with IBS.
| Outcome | Efficacy Level A 1 | Efficacy Level B | Efficacy Level C | ||||
|---|---|---|---|---|---|---|---|
| Probiotic | Network Meta-Analysis 2 | GRADE | Probiotic | Network Meta-Analysis | GRADE | Probiotic | |
| Bowel movement frequency (IBS-D) | EF + LA + X | −3.95 (−5.02, −2.88) |
M | L. paracasei B21060 | −5.11 (−9.98, −0.24) |
H | L. plantarum Apsulloc 331261, LP + LC + BL + ST + LA + X, C. butyricum CGMCC0313.1, S. cerevisiae CNCM I-3856, S. boulardii CNCM I-475 |
| L. paracasei HA-196 | −3.13 (−4.63, −1.62) |
H | |||||
| B. coagulans GBI-306086 | −2.11 (−3.00, −1.23) |
M | |||||
| B. longum R0175 | −1.95 (−3.45, −0.45) |
H | |||||
| Bowel movement frequency (IBS-C) | None | None | B. bifidum MIMBb75, LA + ST + X, S. cerevisiae CNCM I-3856, L. paracasei HA-196, B. coagulans Unique IS2, B. longum R0175, BAL + ST + LB | ||||
| Bristol stool form scale (IBS-D) |
B. coagulans MTCC 5856 | −3.28 (−5.21, −1.34) |
L | BL + LR | −0.80 (−1.57, −0.03) |
L | L. paracasei HA-196, B. bifidum MIMBb75, VSL#3, C. butyricum CGMCC0313.1, B. longum R0175, LP + LC + BL + ST + LA + X, S. boulardii CNCM I-475 |
| S. cerevisiae CNCM I-3856 | −1.24 (−1.63, −0.86) |
L | LA + LR + ST + BL + BBR + X | −0.70 (−1.32, −0.08) |
M | ||
| LP + LP + PA | −0.50 (−0.76, −0.24) |
L | |||||
| Bristol stool form scale (IBS-C) |
None | None | L. paracasei HA-196, S. cerevisiae CNCM I-3856, B. longum R0175, BAL + ST + LB | ||||
1 Efficacy level A (among the most effective): probiotics that are significantly superior to placebo and at least 1 probiotic in level B. Efficacy level B: probiotics that are more effective than placebo, but not superior to any other of the probiotic(s) superior to placebo. Efficacy level C (among the least effective): probiotics with no significant difference compared to placebo. 2 The network meta-analysis results were the efficacy of probiotics compared to placebo. Mean difference with 95% confidence intervals in parentheses. Abbreviations: IBS-D, IBS with predominant diarrhea; IBS-C, IBS with predominant constipation; GRADE, The Grading of Recommendations, Assessment, Development and Evaluation system; H, high; M, moderate; L, low.
The IBS-C evidence network was constructed using five single-strain probiotics and two multistrain groups from eight RCTs (585 patients, Figure 3). However, no significant difference was found between the pooled probiotic and placebo groups (Table 3).
3.3.7. Bristol Stool form Scale in IBS-D and IBS-C
The IBS-D evidence network was constructed with seven single-strain probiotics and five multistrain groups from 12 RCTs (833 patients, Figure 3). NMA indicated that Bacillus coagulans MTCC 5856 (MD, −3.28; 95% CI, −5.21 to −1.34) and Saccharomyces cerevisiae CNCM I-3856 (MD, −1.24; 95% CI, −1.63 to −0.86) were classified as efficacy level A (Table 3). NMA indicated that BL + LR, LA + LR + ST + BL + BBR + X, and LP + LP + PA were significantly superior to placebo in improving the Bristol stool form score (efficacy level B, Table 3). The top three treatments based on SUCRAs were Bacillus coagulans MTCC 5856 (99.6%), Saccharomyces cerevisiae CNCM I-3856 (89.7%), and BL + LR (75%).
The IBS-C evidence network was constructed with three single-strain probiotics and one multistrain group from four RCTs (161 patients, Figure 3). No significant difference was found between pooled probiotics and placebo (Table 3).
3.4. Adverse Events
Most studies did not assess causality between intervention and adverse events. In some studies, abdominal symptoms (pain, bloating, or diarrhea) were recorded as adverse events. But there was no identification of whether these symptoms were attributed to a flare-up of IBS. Hence, there may be inherent heterogeneity among data on adverse events, and we did not conduct a meta-analysis. The overall rate of adverse events was 11.62% (452/3891) in the probiotic group and 10.61% (379/3572) in the placebo group. The rate of serious adverse events was 0.15% (6/4081) in the probiotics group and 0.22% (8/3679) in the placebo group.
3.5. Heterogeneity and Inconsistency
Prediction interval plots are presented in Figure S5. No significant inconsistency between direct and indirect evidence was observed in the abdominal pain score, abdominal bloating score, or bowel movement frequency in IBS-D. The treatment loops of five outcomes (IBS-SSS, HADS-total score, abdominal pain score, abdominal bloating score, Bowel movement frequency, and Bristol stool form scale) were formed only by triple-arm trials; therefore, the NMA was consistent by definition. Inconsistency could not be assessed in the remaining five outcomes because there were no loops in their evidence networks (Table S6). Funnel plots are shown in Figure S6.
3.6. Quality of Evidence
The quality of efficacy rankings was high in one outcome, moderate in eight outcomes, and low in two outcomes (Table S7).
3.7. Alteration of Gut Microbiota
The alterations of bacterial abundance and diversity in the probiotic group are presented in Tables S8 and S9. Six studies calculated the gut microbiota diversity before and after the intervention. In five studies, the differences between probiotics and placebo in modulating alpha diversity were reported as not being significant. Regarding beta diversity, three studies revealed significant differences between the two groups.
4. Discussion
4.1. Key Findings
In this systematic review and network meta-analysis, we compared and ranked the efficacy of different probiotic strains or mixtures based on symptom-specific outcomes. We found that only some probiotic strains and combinations were more effective than the placebo for each specific outcome of IBS. This NMA provides an initial indication of promising probiotic strains or mixtures that can be used in the treatment of IBS patients.
For the global condition and mental health stage of IBS, moderate-certainty evidence indicates that Lactobacillus acidophilus DDS-1, BL + LR, LC + LP + BAL, LP + LC + BL + ST + LA + X, and Bifidobacterium animalis subsp. lactis UABla-12 are among the most effective probiotics for improving IBS-SSS. The mixture (LP + LC + BL + ST + LA + X) was the most effective probiotic for improving IBS-QOL (moderate certainty). High-certainty evidence indicates that Bifidobacterium longum R0175 and Bifidobacterium longum NCC3001 are the most effective probiotics for improving HADS-total and HADS-depression scores, respectively.
In terms of the core and specific gastrointestinal symptoms of IBS, moderate-certainty evidence indicates that Bacillus coagulans MTCC 5856 and Bacillus coagulans Unique IS2 are among the most effective probiotics for improving abdominal pain. BL + LR, Lactobacillus plantarum CCFM8610, Lactobacillus plantarum 299v, VSL#3, Bifidobacterium bifidum MIMBb75 were significantly superior to placebo in alleviating abdominal bloating. EF + LA + X was most effective in reducing bowel movement frequency in patients with IBS-D (moderate certainty). Bacillus coagulans MTCC 5856 and Saccharomyces cerevisiae CNCM I-3856 were among the most effective probiotics for improving the Bristol stool form scale in IBS-D. No significant difference was found among the pooled probiotics and placebo regarding bowel movement frequency and Bristol stool form scale in the IBS-C group.
4.2. Associations with Current Studies
The three current guidelines (BSG, ACG, and The American Gastroenterological Association) still have reservations about the use of probiotics in IBS due to the concern of between-study heterogeneity [12,18,19]. The main challenges in interpreting the existing evidence regarding probiotics in IBS treatments were the numerous strains and combinations of probiotics, different doses, the lack of standard outcomes, and the inconsistency of results [18]. In terms of probiotic taxonomy, the ideal NMA of probiotics in IBS treatment should be analyzed based on the strain level [19,117]. McFarland et al. conducted the first meta-analysis to evaluate the strain- and outcome-specific efficacy of probiotics for IBS [118]. This study included only 14 probiotic formulas because the inclusion criteria required at least two trials within each type of probiotic. The comparative efficacy of different probiotics was also lacking in McFarland’s work owing to the limitations of a traditional pairwise meta-analysis. Although some probiotics were confirmed by a single RCT, we advocate that clinical trials with large sample sizes and high quality should not be excluded from related meta-analyses. Additionally, the efficacy hierarchy is beneficial for probiotic selection in clinical practice. Given the numerous types of probiotics, NMA is ideal for comparing the efficacy.
A standard system for efficacy evaluation is needed in IBS clinical trials. The system should be composed of endpoints that can precisely reflect the change in the core signs and symptoms of IBS. In this NMA, we chose the abdominal pain score, bowel movement frequency (per week), and Bristol stool form scale to assess the most important symptoms of IBS in accordance with the diagnostic elements in ROME IV and the guidance of the US Food and Drug Administration [119,120]. The global condition of IBS was evaluated by the IBS-SSS and IBS-QOL. The IBS-SSS is widely used in IBS symptom severity assessment [121]. IBS-QOL is a valid scoring system for assessing the physical and mental condition of IBS patients [122]. The evaluation of psychological conditions is indispensable for IBS assessments. An increasing number of psychological problems have been associated with worse gastrointestinal symptoms in IBS [123]. Hence, the HADS was also included in this NMA. The above seven outcomes present a panorama of efficacy evaluation in IBS treatment, including disease-defined core symptoms, global conditions, psychological conditions, and subtype-specific symptoms. The binary outcomes of IBS were not included in this NMA because related RCTs used various definitions of “improvement/response rate”, which incur potential heterogeneity.
ROME IV indicates that identifying the main and/or most troubling symptoms is the first step in the treatment of patients with IBS [119]. This was reflected in the different pharmacological treatments of the guidelines, such as antispasmodics, guanylate cyclase-C agonists, and antidepressants [12,124,125]. These drugs have definite pharmacological effects targeting particular IBS symptoms. However, the effects of probiotics on specific symptoms of IBS are still unclear. Ford et al. conducted a comprehensive and rigorous meta-analysis to evaluate the efficacy of probiotics in IBS patients. The results indicated that combinations of probiotics were associated with significant improvements in IBS symptom and flatulence scores as well as a trend of decreasing bloating scores. However, these benefits were not observed when specific combinations or strains were analyzed. The results supported the use of combinations of probiotics as a group [20]. In other words, the use of probiotic combinations may be beneficial from the perspective of the entire IBS population. On the other hand, identifying the comparative efficacy of specific probiotics was also significant for individual patients. Zhang et al. performed a large NMA that calculated the relative ranking of 12 different probiotics on 7 outcomes. The results showed that, based on SUCRA analysis, Bacillus coagulans was ranked first in improving the symptom relief rate, global symptoms, abdominal pain, bloating, and straining [126]. Our NMA yielded results similar to those of the above two studies. Lactobacillus acidophilus DDS-1, Bifidobacterium animalis subsp. lactis UABla-12, and the three probiotic mixtures may improve the global condition of IBS. The two strains of Bacillus coagulans may improve the abdominal pain score of patients with IBS. We used a novel and succinct approach to classify efficacy. Seven probiotic strains and five mixtures were evaluated as level A (among the most effective) for different outcomes. A list of probiotics that may be effective for each outcome is also provided. Different probiotics should be selected according to the specific symptoms of IBS patients.
4.3. Study Merits and Limitations
A plethora of meta-analyses have been published on this topic, but our NMA has several merits. To our knowledge, this study is the first NMA to compare the outcome-specific and strain-level efficacies of different probiotics in IBS. To date, it is the most comprehensive systematic review of the probiotic efficacy in IBS. We included 81 RCTs and 9253 patients, which was attributed to a rigorous literature search and the inclusion of probiotic combinations. This NMA used seven outcomes to conduct full-scale evaluations of probiotics in IBS treatments, including disease-defined core symptoms, global conditions, psychological conditions, and subtype-specific symptoms.
Our study had several limitations. First, there was an inherent heterogeneity among the included RCTs. The study regions, diagnostic criteria, treatment durations, and probiotic doses varied in the pooled studies, undermining the reliability of the results. The classification of probiotic combinations was partly based on the strain level, which resulted in inherent heterogeneity. A more rational classification of probiotic combinations in NMA with less heterogeneity will enhance the quality of evidence in this field. In future studies, the above factors should be considered in NMA, which will help evaluate the efficacy of probiotics in the treatment of IBS. Second, the robustness and complexity of the evidence network are unsatisfactory. The comparative efficacy and ranking of some probiotics, especially single-strain products, are often based on single studies. The scarcity of RCTs has a negative impact on the certainty of the NMA results. On the other hand, the evidence network of the outcomes was “star-shaped”. Most probiotics have been directly compared with placebo. Only three three-arm RCTs provided direct comparisons between different probiotics. This prevents the use of NMA for evaluating the efficacy of different probiotics by combining direct and indirect comparisons. Third, the evaluation of long-term efficacy was not available because few studies reported long-term results with more than one year of follow-up.
The results of this study only reflect the relative efficacy of probiotics assessed by meta-analyses based on the available studies. Further studies are required to confirm the efficacy of probiotics in the real world. A standard evaluation system of efficacy, safety, and gut microbiota alteration is needed in future clinical trials of IBS. Multicenter RCTs are necessary to evaluate the efficacy of strain-specific probiotics in IBS treatment.
5. Conclusions
In conclusion, this NMA provides the first efficacy ranking of different probiotic strains and combinations for specific IBS outcomes. Lactobacillus acidophilus DDS-1, Bifidobacterium animalis subsp. Lactis UABla-12, Bifidobacterium longum R0175, Bifidobacterium longum NCC3001, Bacillus coagulans MTCC 5856, Bacillus coagulans Unique IS2, Saccharomyces cerevisiae CNCM I-3856, and four mixtures may be the most promising probiotics. Probiotics should be selected according to the specific symptoms of IBS patients. Due to the inherent heterogeneity, this evidence should be interpreted with caution. Further studies are needed to confirm these results.
Acknowledgments
We are grateful to the library staff at Sun Yat-Sen University for their advice on the literature search strategy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15173856/s1. Text S1. Supplements and alterations of the original protocol; Text S2. Search strategy; Text S3. Data collection process; Text S4. References to RCTs included in this network meta-analysis; Figure S1. The distribution of effect modifiers; Figure S2. Risk of bias 2 evaluations for each outcome; Figure S3. Network plots; Figure S4. Cumulative ranking curves for each outcome; Figure S5. Predictive interval plots for each pair of interventions; Figure S6. Funnel plots for each outcome; Table S1. Characteristics of included randomized controlled trials (1); Table S2. Characteristics of included randomized controlled trials (2); Table S3. Critical results of pairwise meta-analysis; Table S4. League table demonstrating the relative efficacy for each pair of comparison in the NMA; Table S5. The surface under the cumulative ranking curve (SUCRA) values; Table S6. Results of loop specific analysis, node-splitting method, and design-by-treatment interaction inconsistency model; Table S7. GRADE-based assessment of the quality of evidence for each outcome; Table S8. Alterations of gut microbiota abundance; Table S9. The alteration of gut microbiota diversity before and after intervention.
Author Contributions
Conceptualization, P.X. and L.X.; methodology, P.X.; software, P.X.; validation, P.X., M.L., X.D., J.F. and L.X.; formal analysis, P.X. and M.L.; investigation, P.X., M.L., X.D. and J.F.; resources, L.X.; data curation, P.X.; writing—original draft preparation, P.X.; writing—review and editing, P.X., M.L., X.D., J.F. and L.X.; visualization, P.X.; supervision, L.X.; project administration, L.X.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 81970471.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ford A.C., Sperber A.D., Corsetti M., Camilleri M. Functional Gastrointestinal Disorders 2 Irritable bowel syndrome. Lancet. 2020;396:1675–1688. doi: 10.1016/S0140-6736(20)31548-8. [DOI] [PubMed] [Google Scholar]
- 2.Oka P., Parr H., Barberio B., Black C.J., Savarino E.V., Ford A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020;5:908–917. doi: 10.1016/S2468-1253(20)30217-X. [DOI] [PubMed] [Google Scholar]
- 3.Sperber A.D., Dumitrascu D., Fukudo S., Gerson C., Ghoshal U.C., Gwee K.A., Hungin A.P.S., Kang J.-Y., Minhu C., Schmulson M., et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut. 2017;66:1075–1082. doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 4.Goodoory V.C., Ng C.E., Black C.J., Ford A.C. Direct healthcare costs of Rome IV or Rome III-defined irritable bowel syndrome in the United Kingdom. Aliment. Pharmacol. Ther. 2022;56:110–120. doi: 10.1111/apt.16939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peery A.F., Crockett S.D., Murphy C.C., Lund J.L., Dellon E.S., Williams J.L., Jensen E.T., Shaheen N.J., Barritt A.S., Lieber S.R., et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254–272. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang F., Xiang W., Li C.Y., Li S.C. Economic burden of irritable bowel syndrome in China. World J. Gastroenterol. 2016;22:10450–10460. doi: 10.3748/wjg.v22.i47.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canavan C., West J., Card T. Review article: The economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014;40:1023–1034. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 8.Black C.J., Ford A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020;17:473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- 9.Frandemark A., Tornblom H., Jakobsson S., Simren M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Am. J. Gastroenterol. 2018;113:1540–1549. doi: 10.1038/s41395-018-0262-x. [DOI] [PubMed] [Google Scholar]
- 10.Drossman D.A., Chang L., Schneck S., Blackman C., Norton W.F., Norton N.J. A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Dig. Dis. Sci. 2009;54:1532–1541. doi: 10.1007/s10620-009-0792-6. [DOI] [PubMed] [Google Scholar]
- 11.Raskov H., Burcharth J., Pommergaard H.-C., Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7:365–383. doi: 10.1080/19490976.2016.1218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasant D.H., Paine P.A., Black C.J., Houghton L.A., Everitt H.A., Corsetti M., Agrawal A., Aziz I., Farmer A.D., Eugenicos M.P., et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70:1214–1240. doi: 10.1136/gutjnl-2021-324598. [DOI] [PubMed] [Google Scholar]
- 13.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 14.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 15.Bai T., Xu Z., Xia P., Feng Y., Liu B., Liu H., Chen Y., Yan G., Lv B., Yan Z., et al. The Short-Term Efficacy of Bifidobacterium Quadruple Viable Tablet in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: Potentially Mediated by Metabolism Rather Than Diversity Regulation. Am. J. Gastroenterol. 2022;118:1256–1267. doi: 10.14309/ajg.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 16.Konstantis G., Efstathiou S., Pourzitaki C., Kitsikidou E., Germanidis G., Chourdakis M. Efficacy and safety of probiotics in the treatment of irritable bowel syndrome: A systematic review and meta-analysis of randomised clinical trials using ROME IV criteria. Clin. Nutr. 2023;42:800–809. doi: 10.1016/j.clnu.2023.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Ford A.C., Quigley E.M.M., Lacy B.E., Lembo A.J., Saito Y.A., Schiller L.R., Soffer E.E., Spiegel B.M.R., Moayyedi P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014;109:1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 18.Lacy B.E., Pimentel M., Brenner D.M., Chey W.D., Keefer L.A., Long M.D., Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021;116:17–44. doi: 10.14309/ajg.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 19.Su G.L., Ko C.W., Bercik P., Falck-Ytter Y., Sultan S., Weizman A.V., Morgan R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159:697–705. doi: 10.1053/j.gastro.2020.05.059. [DOI] [PubMed] [Google Scholar]
- 20.Ford A.C., Harris L.A., Lacy B.E., Quigley E.M.M., Moayyedi P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018;48:1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 21.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P.A., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 23.Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 24.Patrick D.L., Drossman D.A., Frederick I.O., Dicesare J., Puder K.L. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig. Dis. Sci. 1998;43:400–411. doi: 10.1023/A:1018831127942. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 27.Salanti G., Del Giovane C., Chaimani A., Caldwell D.M., Higgins J.P.T. Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS ONE. 2014;9:e99682. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Chaimani A., Higgins J.P.T., Mavridis D., Spyridonos P., Salanti G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley R.D., Higgins J.P.T., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 32.Dias S., Welton N.J., Caldwell D.M., Ades A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 33.Higgins J.P.T., Jackson D., Barrett J.K., Lu G., Ades A.E., White I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White I.R. Network meta-analysis. Stata J. 2015;15:951–985. doi: 10.1177/1536867X1501500403. [DOI] [Google Scholar]
- 35.Chaimani A., Salanti G. Visualizing assumptions and results in network meta-analysis: The network graphs package. Stata J. 2015;15:905–950. doi: 10.1177/1536867X1501500402. [DOI] [Google Scholar]
- 36.Morgan R.L., Preidis G.A., Kashyap P.C., Weizman A.V., Sadeghirad B., McMaster Probiotic Prebiotic S. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology. 2020;159:467–480. doi: 10.1053/j.gastro.2020.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mourey F., Decherf A., Jeanne J.-F., Clement-Ziza M., Grisoni M.-L., Machuron F., Legrain-Raspaud S., Bourreille A., Desreumaux P. Saccharomyces cerevisiae I-3856 in irritable bowel syndrome with predominant constipation. World J. Gastroenterol. 2022;28:2509–2522. doi: 10.3748/wjg.v28.i22.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung K., Kim A., Lee J.-H., Cho D., Seo J., Jung E.S., Kang H.-j., Roh J., Kim W. Effect of Oral Intake of Lactiplantibacillus plantarum APsulloc 331261 (GTB1 (TM)) on Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2022;14:2015. doi: 10.3390/nu14102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H., Ma C., Zhao F., Chen P., Liu Y., Sun Z., Cui L., Kwok L.-Y., Zhang H. Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur. J. Nutr. 2021;60:2553–2565. doi: 10.1007/s00394-020-02437-4. [DOI] [PubMed] [Google Scholar]
- 40.Skrzydlo-Radomanska B., Prozorow-Krol B., Cichoz-Lach H., Majsiak E., Bierla J.B., Kanarek E., Sowniska A., Cukrowska B. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients. 2021;13:756. doi: 10.3390/nu13030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Yu X., Yu L., Tian F., Zhao J., Zhang H., Qian L., Wang Q., Xue Z., Zhai Q., et al. Lactobacillus plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering. 2021;7:376–385. doi: 10.1016/j.eng.2020.06.026. [DOI] [Google Scholar]
- 42.Gupta A.K., Maity C. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome A prospective, interventional, randomized, double-blind, placebo-controlled clinical study CONSORT Compliant. Medicine. 2021;100:e23641. doi: 10.1097/MD.0000000000023641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barraza-Ortiz D.A., Perez-Lopez N., Medina-Lopez V.M., Minero-Alfaro J.I., Zamarripa-Dorsey F., Fernandez-Martinez N.d.C., Llorente-Ramon A., Ramos-Aguilar G.A. Combination of a Probiotic and an Antispasmodic Increases Quality of Life and Reduces Symptoms in Patients with Irritable Bowel Syndrome: A Pilot Study. Dig. Dis. 2021;39:294–300. doi: 10.1159/000510950. [DOI] [PubMed] [Google Scholar]
- 44.Sadrin S., Sennoune S., Gout B., Marque S., Moreau J., Zinoune K., Grillasca J.-P., Pons O., Maixent J.-M. A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome: A placebo-controlled randomized clinical trial. Dig. Liver Dis. 2020;52:534–540. doi: 10.1016/j.dld.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Martoni C.J., Srivastava S., Leyer G.J. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients. 2020;12:363. doi: 10.3390/nu12020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis E.D., Antony J.M., Crowley D.C., Piano A., Bhardwaj R., Tompkins T.A., Evans M. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients. 2020;12:1159. doi: 10.3390/nu12041159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J., Cho K., Kim J.S., Jung H.C., Kim B., Park M.S., Ji G.E., Cho J.-Y., Hong K.S. Probiotic treatment induced change of inflammation related metabolites in IBS-D patients/double-blind, randomized, placebo-controlled trial. Food Sci. Biotechnol. 2020;29:837–844. doi: 10.1007/s10068-019-00717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gayathri R., Aruna T., Malar S., Shilpa B., Dhanasekar K.R. Efficacy of Saccharomyces cerevisiae CNCM I-3856 as an add-on therapy for irritable bowel syndrome. Int. J. Color. Dis. 2020;35:139–145. doi: 10.1007/s00384-019-03462-4. [DOI] [PubMed] [Google Scholar]
- 49.Bonfrate L., Di Palo D.M., Celano G., Albert A., Vitellio P., De Angelis M., Gobbetti M., Portincasa P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020;50:e13201. doi: 10.1111/eci.13201. [DOI] [PubMed] [Google Scholar]
- 50.Andresen V., Gschossmann J., Layer P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 2020;5:658–666. doi: 10.1016/S2468-1253(20)30056-X. [DOI] [PubMed] [Google Scholar]
- 51.Oh J.H., Jang Y.S., Kang D., Chang D.K., Min Y.W. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019;11:2887. doi: 10.3390/nu11122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madempudi R.S., Ahire J.J., Neelamraju J., Tripathi A., Nanal S. Randomized clinical trial: The effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci. Rep. 2019;9:12210. doi: 10.1038/s41598-019-48554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashtiani S.Y., Amery M. Effect of Multispecies Probiotic Supplementation on Irritable Bowel Syndrome. J. Pharm. Res. Int. 2019;28:1–9. doi: 10.9734/jpri/2019/v28i630221. [DOI] [Google Scholar]
- 54.Sun Y.-Y., Li M., Li Y.-Y., Li L.-X., Zhai W.-Z., Wang P., Yang X.-X., Gu X., Song L.-J., Li Z., et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018;8:2964. doi: 10.1038/s41598-018-21241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin S.P., Choi Y.M., Kim W.H., Hong S.P., Park J.-M., Kim J., Kwon O., Lee E.H., Hahm K.B. A double blind, placebo-controlled, randomized clinical trial that breast milk derived Lactobacillus gasseri BNR17 mitigated diarrhea-dominant irritable bowel syndrome. J. Clin. Biochem. Nutr. 2018;62:179–186. doi: 10.3164/jcbn.17-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preston K., Krumian R., Hattner J., de Montigny D., Stewart M., Gaddam S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: A double-blind, randomised, placebo-controlled study. Benef. Microbes. 2018;9:697–706. doi: 10.3920/BM2017.0105. [DOI] [PubMed] [Google Scholar]
- 57.Kim J.Y., Park Y.J., Lee H.J., Park M.Y., Kwon O. Effect of Lactobacillus gasseri BNR17 on irritable bowel syndrome: A randomized, double-blind, placebo-controlled, dose-finding trial. Food Sci. Biotechnol. 2018;27:853–857. doi: 10.1007/s10068-017-0296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishaque S.M., Khosruzzaman S.M., Ahmed D.S., Sah M.P. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult (R)) in the management of diarrhea- predominant irritable bowel syndrome. BMC Gastroenterol. 2018;18:71. doi: 10.1186/s12876-018-0788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cremon C., Guglielmetti S., Gargari G., Taverniti V., Castellazzi A.M., Valsecchi C., Tagliacarne C., Fiore W., Bellini M., Bertani L., et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018;6:604–613. doi: 10.1177/2050640617736478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.-P., Cominetti O., Welsh C., Rieder A., et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. 2017;153:448–459. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Nobutani K., Sawada D., Fujiwara S., Kuwano Y., Nishida K., Nakayama J., Kutsumi H., Azuma T., Rokutan K. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J. Appl. Microbiol. 2017;122:212–224. doi: 10.1111/jam.13329. [DOI] [PubMed] [Google Scholar]
- 62.Hod K., Sperber A.D., Ron Y., Boaz M., Dickman R., Berliner S., Halpern Z., Maharshak N., Dekel R. A double-blind, placebo-controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea-predominant IBS. Neurogastroenterol. Motil. 2017;29:e13037. doi: 10.1111/nmo.13037. [DOI] [PubMed] [Google Scholar]
- 63.Thijssen A.Y., Clemens C.H.M., Vankerckhoven V., Goossens H., Jonkers D.M.A.E., Masclee A.A.M. Efficacy of Lactobacillus casei Shirota for patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2016;28:8–14. doi: 10.1097/MEG.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 64.Spiller R., Pelerin F., Decherf A.C., Maudet C., Housez B., Cazaubiel M., Justen P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United Eur. Gastroenterol. J. 2016;4:353–362. doi: 10.1177/2050640615602571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mezzasalma V., Manfrini E., Ferri E., Sandionigi A., La Ferla B., Schiano I., Michelotti A., Nobile V., Labra M., Di Gennaro P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. BioMed Res. Int. 2016;2016:4740907. doi: 10.1155/2016/4740907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majeed M., Nagabhushanam K., Natarajan S., Sivakumar A., Ali F., Pande A., Majeed S., Karri S.K. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: A double blind randomized placebo controlled pilot clinical study. Nutr. J. 2016;21:15–25. doi: 10.1186/s12937-016-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyra A., Hillila M., Huttunen T., Mannikko S., Taalikka M., Tennila J., Tarpila A., Lahtinen S., Ouwehand A.C., Veijola L. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J. Gastroenterol. 2016;22:10631–10642. doi: 10.3748/wjg.v22.i48.10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoon H., Park Y.S., Lee D.H., Seo J.-G., Shin C.M., Kim N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2015;57:129–134. doi: 10.3164/jcbn.15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong R.K., Yang C., Song G.-H., Wong J., Ho K.-Y. Melatonin Regulation as a Possible Mechanism for Probiotic (VSL#3) in Irritable Bowel Syndrome: A Randomized Double-Blinded Placebo Study. Dig. Dis. Sci. 2015;60:186–194. doi: 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- 70.de Chambrun G.P., Neut C., Chau A., Cazaubiel M., Pelerin F., Justen P., Desreumaux P. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig. Liver Dis. 2015;47:119–124. doi: 10.1016/j.dld.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Yoon J.S., Sohn W., Lee O.Y., Lee S.P., Lee K.N., Jun D.W., Lee H.L., Yoon B.C., Choi H.S., Chung W.-S., et al. Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014;29:52–59. doi: 10.1111/jgh.12322. [DOI] [PubMed] [Google Scholar]
- 72.Stevenson C., Blaauw R., Fredericks E., Visser J., Roux S. Randomized clinical trial: Effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition. 2014;30:1151–1157. doi: 10.1016/j.nut.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Sisson G., Ayis S., Sherwood R.A., Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome—A 12 week double-blind study. Aliment. Pharmacol. Ther. 2014;40:51–62. doi: 10.1111/apt.12787. [DOI] [PubMed] [Google Scholar]
- 74.Shavakhi A., Minakari M., Farzamnia S., Peykar M.S., Taghipour G., Tayebi A., Hashemi H., Shavakhi S. The effects of multi-strain probiotic compound on symptoms and quality-of-life in patients with irritable bowel syndrome: A randomized placebo-controlled trial. Adv. Biomed. Res. 2014;3:140. doi: 10.4103/2277-9175.135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ludidi S., Jonkers D.M., Koning C.J., Kruimel J.W., Mulder L., Van der Vaart I.B., Conchillo J.M., Masclee A.A.M. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol. Motil. 2014;26:705–714. doi: 10.1111/nmo.12320. [DOI] [PubMed] [Google Scholar]
- 76.Lorenzo-Zuniga V., Llop E., Suarez C., Alvarez B., Abreu L., Espadaler J., Serra J.I. 31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J. Gastroenterol. 2014;20:8709–8716. doi: 10.3748/wjg.v20.i26.8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jafari E., Vahedi H., Merat S., Momtahen S., Riahi A. Therapeutic Effects, Tolerability and Safety of a Multi-strain Probiotic in Iranian Adults with Irritable Bowel Syndrome and Bloating. Arch. Iran. Med. 2014;17:466–470. [PubMed] [Google Scholar]
- 78.Abbas Z., Yakoob J., Jafri W., Ahmad Z., Azam Z., Usman M.W., Shamim S., Islam M. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: A randomized trial. Eur. J. Gastroenterol. Hepatol. 2014;26:630–639. doi: 10.1097/MEG.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 79.Roberts L.M., McCahon D., Holder R., Wilson S., Hobbs F.D.R. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J., Rheem S., Yun B., Ahn Y., Joung J., Lee S.J., Oh S., Chun T., Rheem I., Yea H.S., et al. Effects of probiotic yoghurt on symptoms and intestinal microbiota in patients with irritable bowel syndrome. Int. J. Dairy Technol. 2013;66:243–255. doi: 10.1111/1471-0307.12028. [DOI] [Google Scholar]
- 81.Charbonneau D., Gibb R.D., Quigley E.M.M. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes. 2013;4:201–211. doi: 10.4161/gmic.24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cappello C., Tremolaterra F., Pascariello A., Ciacci C., Iovino P. A randomised clinical trial (RCT) of a symbiotic mixture in patients with irritable bowel syndrome (IBS): Effects on symptoms, colonic transit and quality of life. Int. J. Color. Dis. 2013;28:349–358. doi: 10.1007/s00384-012-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Begtrup L.M., de Muckadell O.B.S., Kjeldsen J., Christensen R.d., Jarbol D.E. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome—A randomised, double-blind, placebo controlled trial. Scand. J. Gastroenterol. 2013;48:1127–1135. doi: 10.3109/00365521.2013.825314. [DOI] [PubMed] [Google Scholar]
- 84.Amirimani B., Nikfam S., Albaji M., Vahedi S., Nasseri-Moghaddam S., Sharafkhah M., Ansari R., Vahedi H. Probiotic vs. Placebo in Irritable Bowel Syndrome:A Randomized Controlled Trial. Middle East J. Dig. Dis. 2013;5:98–102. [PMC free article] [PubMed] [Google Scholar]
- 85.Kruis W., Chrubasik S., Boehm S., Stange C., Schulze J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int. J. Color. Dis. 2012;27:467–474. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ducrotte P., Sawant P., Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dapoigny M., Piche T., Ducrotte P., Lunaud B., Cardot J.-M., Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: A randomized, double-blind study. World J. Gastroenterol. 2012;18:2067–2075. doi: 10.3748/wjg.v18.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui S., Hu Y. Multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Int. J. Clin. Exp. Med. 2012;5:238–244. [PMC free article] [PubMed] [Google Scholar]
- 89.Cha B.K., Jung S.M., Choi C.H., Song I.-D., Lee H.W., Kim H.J., Do J.H., Chang S.K., Kim K., Chung W.-S., et al. The Effect of a Multispecies Probiotic Mixture on the Symptoms and Fecal Microbiota in Diarrhea-dominant Irritable Bowel Syndrome A Randomized, Double-blind, Placebo-controlled Trial. J. Clin. Gastroenterol. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 90.Sondergaard B., Olsson J., Ohlson K., Svensson U., Bytzer P., Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: A randomized, placebo-controlled trial. Scand. J. Gastroenterol. 2011;46:663–672. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 91.Michail S., Kenche H. Gut Microbiota is Not Modified by Randomized, Double-Blind, Placebo-Controlled Trial of VSL#3 in Diarrhea-Predominant Irritable Bowel Syndrome. Probiotics Antimicrob. Proteins. 2011;3:1–7. doi: 10.1007/s12602-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guglielmetti S., Mora D., Gschwender M., Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—A double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 93.Choi C.H., Jo S.Y., Park H.J., Chang S.K., Byeon J.-S., Myung S.-J. A Randomized, Double-blind, Placebo-controlled Multicenter Trial of Saccharomyces boulardii in Irritable Bowel Syndrome Effect on Quality of Life. J. Clin. Gastroenterol. 2011;45:679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 94.Simren M., Ohman L., Olsson J., Svensson U., Ohlson K., Posserud I., Strid H. Clinical trial: The effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome—A randomized, double-blind, controlled study. Aliment. Pharmacol. Ther. 2010;31:218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 95.Ligaarden S.C., Axelsson L., Naterstad K., Lydersen S., Farup P.G. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: A randomised controlled trial. BMC Gastroenterol. 2010;10:16. doi: 10.1186/1471-230X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hun L. Original Research: Bacillus coagulans Significantly Improved Abdominal Pain and Bloating in Patients with IBS. Postgrad. Med. 2009;121:119–124. doi: 10.3810/pgm.2009.03.1984. [DOI] [PubMed] [Google Scholar]
- 97.Hong K.S., Kang H.W., Im J.P., Ji G.E., Kim S.G., Jung H.C., Song I.S., Kim J.S. Effect of Probiotics on Symptoms in Korean Adults with Irritable Bowel Syndrome. Gut Liver. 2009;3:101–107. doi: 10.5009/gnl.2009.3.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enck P., Zimmermann K., Menke G., Klosterhalfen S. Randomized Controlled Treatment Trial of Irritable Bowel Syndrome with a Probiotic E.-coli Preparation (DSM17252) Compared to Placebo. Z. Für Gastroenterol. 2009;47:209–214. doi: 10.1055/s-2008-1027702. [DOI] [PubMed] [Google Scholar]
- 99.Dolin B.J. Effects of a proprietary bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find. Exp. Clin. Pharmacol. 2009;31:655–659. doi: 10.1358/mf.2009.31.10.1441078. [DOI] [PubMed] [Google Scholar]
- 100.Zeng J., Li Y.Q., Zuo X.L., Zhen Y.B., Yang J., Liu C.H. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 101.Williams E.A., Stimpson J., Wang D., Plummer S., Garaiova I., Barker M.E., Corfe B.M. Clinical trial: A multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2008;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 102.Sinn D.H., Song J.H., Kim H.J., Lee J.H., Son H.J., Chang D.K., Kim Y.-H., Kim J.J., Rhee J.C., Rhee P.-L. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig. Dis. Sci. 2008;53:2714–2718. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 103.Kajander K., Myllyluoma E., Rajilic-Stojanovic M., Kyronpalo S., Rasmussen M., Jarvenpaa S., Zoetendal E.G., De Vos W.M., Vapaatalo H., Korpela R. Clinical trial: Multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment. Pharmacol. Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 104.Enck P., Zimmermann K., Menke G., Mueller-Lissner S., Martens U., Klosterhalfen S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome—A randomized controlled trial with primary care physicians. Neurogastroenterol. Motil. 2008;20:1103–1109. doi: 10.1111/j.1365-2982.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 105.Drouault-Holowacz S., Bieuvelet S., Burckel A., Cazaubiel M., Dray X., Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol. Clin. Et Biol. 2008;32:147–152. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Andriulli A., Neri M., Loguercio C., Terreni N., Merla A., Cardarella M.P., Federico A., Chilovi F., Milandri G.L., De Bona M., et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome—A multicenter, randomized study. J. Clin. Gastroenterol. 2008;42:S218–S223. doi: 10.1097/MCG.0b013e31817fadd6. [DOI] [PubMed] [Google Scholar]
- 107.Agrawal A., Houghton L.A., Morris J., Reilly B., Guyonnet D., Feuillerat N.G., Schlumberger A., Jakob S., Whorwell P.J. Clinical trial: The effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2008;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 108.Guyonnet D., Chassany O., Ducrotte P., Picard C., Mouret M., Mercier C.H., Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: A multicentre, randomized, double-blind, controlled trial. Aliment. Pharmacol. Ther. 2007;26:475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 109.Whorwell P.J., Altringer L., Morel J., Bond Y., Charbonneau D., O’Mahony L., Kiely B., Shanahan F., Quigley E.M.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 110.O’Mahony L., McCarthy J., Kelly P., Hurley G., Luo F.Y., Chen K.S., O’Sullivan G.C., Kiely B., Collins J.K., Shanahan F., et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 111.Niv E., Naftali T., Hallak R., Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome—A double blind, placebo-controlled, randomized study. Clin. Nutr. 2005;24:925–931. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 112.Kim H.J., Roque M.I.V., Camilleri M., Stephens D., Burton D.D., Baxter K., Thomforde G., Zinsmeister A.R. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol. Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 113.Kajander K., Hatakka K., Poussa T., Markkila M., Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: A controlled 6-month intervention. Aliment. Pharmacol. Ther. 2005;22:387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 114.Kim H.J., Camilleri M., McKinzie S., Lempke M.B., Burton D.D., Thomforde G.M., Zinsmeister A.R. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 115.Niedzielin K., Kordecki H., Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2001;13:1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Nobaek S., Johansson M.L., Molin G., Ahrne S., Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 117.Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019;25:716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 118.McFarland L.V., Karakan T., Karatas A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Eclinicalmedicine. 2021;41:101154. doi: 10.1016/j.eclinm.2021.101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Drossman D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology. 2016;150:1262–1279. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 120.US Department of Health and Human Services Food and Drug Administration Center for Drug Evalution and Research (CDER) Guidance for industry: Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment. [(accessed on 15 August 2023)];2012 Available online: https://www.fda.gov/media/78622/download.
- 121.Bijkerk C.J., de Wit N.J., Muris J.W.M., Jones R.H., Knottnerus J.A., Hoes A.W. Outcome measures in irritable bowel syndrome: Comparison of psychometric and methodological characteristics. Am. J. Gastroenterol. 2003;98:122–127. doi: 10.1111/j.1572-0241.2003.07158.x. [DOI] [PubMed] [Google Scholar]
- 122.Andrae D.A., Patrick D.L., Drossman D.A., Covington P.S. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual. Life Outcomes. 2013;11:208. doi: 10.1186/1477-7525-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Midenfjord I., Borg A., Tornblom H., Simren M. Cumulative Effect of Psychological Alterations on Gastrointestinal Symptom Severity in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021;116:769–779. doi: 10.14309/ajg.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 124.Lembo A., Sultan S., Chang L., Heidelbaugh J.J., Smalley W., Verne G.N. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome with Diarrhea. Gastroenterology. 2022;163:137–153. doi: 10.1053/j.gastro.2022.04.017. [DOI] [PubMed] [Google Scholar]
- 125.Chang L., Sultan S., Lembo A., Verne G.N., Smalley W., Heidelbaugh J.J. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome with Constipation. Gastroenterology. 2022;163:118–136. doi: 10.1053/j.gastro.2022.04.016. [DOI] [PubMed] [Google Scholar]
- 126.Zhang T., Zhang C.Z., Zhang J.D., Sun F., Duan L.P. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell. Infect. Microbiol. 2022;12:349. doi: 10.3389/fcimb.2022.859967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.