Abstract
Melatonin is a functionally conserved broad-spectrum physiological regulator found in most biological organisms in nature. Enrichment of tomato fruit with melatonin not only enhances its agronomic traits but also provides extra health benefits. In this study, we elucidate the full melatonin biosynthesis pathway in tomato fruit by identifying biosynthesis-related genes that encode caffeic acid O-methyltransferase 2 (SlCOMT2) and N-acetyl-5-hydroxytryptamine-methyltransferases 5/7 (SlASMT5/7). We further reveal that red light supplementation significantly enhances the melatonin content in tomato fruit. This induction relies on the “serotonin—N-acetylserotonin—melatonin” biosynthesis route via the SlphyB2-SlPIF4-SlCOMT2 module. Based on the regulatory mechanism, we design a gene-editing strategy to target the binding motif of SlPIF4 in the promoter of SlCOMT2, which significantly enhances the production of melatonin in tomato fruit. Our study provides a good example of how the understanding of plant metabolic pathways responding to environmental factors can guide the engineering of health-promoting foods.
Subject terms: Secondary metabolism, Light responses, Molecular engineering in plants
Melatonin is a physiological regulator in many organisms including plants. Here, the authors demonstrate a molecular mechanism of red light-induced melatonin biosynthesis in tomato fruit which could guide the engineering of melatonin-enriched tomatoes.
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine compound found in all organisms from plants to animals. It was first discovered in the pineal gland of cattle in 1958 and is also known as epiphysin1,2, and it has been shown to be the most powerful endogenous free radical scavenger known at present3,4. In animals and humans, melatonin has the functions of improving sleep, delaying aging, alleviating allergic symptoms, and regulating the immune system5,6. Some studies have also shown the oncostatic property of melatonin on different types of tumors, as well as reducing the damage resulting from inflammation7,8.
In plants, melatonin mainly functions as a growth promoter and antioxidant9. It has the activities of delaying senescence, enhancing photosynthesis, regulating the photoperiod, affecting seed germination and root morphogenesis, regulating flowering and fruit ripening, removing free radicals, and alleviating stress damage, and it can give plants the ability to resist adverse environments, which is conducive to plant survival and reproduction9,10.
As the world’s favorite fruit, tomato is the ideal target for plant metabolic engineering11. Synthetic strategies have been successfully applied to tomato metabolic engineering. Fruit-specific expression of the transcription factors AmDel and AmRos 1 leads to the upregulation of genes required for anthocyanin biosynthesis and results in increased anthocyanin levels and higher total antioxidant capacity12,13. The fruit-specific expression of AtMYB12 could be used to enhance the demand for aromatic amino acid biosynthesis, and it can be applied as an effective tool to engineer palpable levels of phenylpropanoids in tomato14,15. During the past several years, the rapid development of genome-editing technology has provided useful tools for creating good tomato germplasm. The accumulation of provitamin D3 in tomatoes was engineered by genome editing, which provides a biofortified food with the added possibility of supplemental production from waste material16. By inducing mutations at the C-terminal region of GAD genes utilizing the CRISPR/Cas9 system, the content of γ-aminobutyric acid (GABA) was greatly increased in tomato leaves and red-stage fruits17. In 2021, the world’s first GABA-enhanced genome-edited tomato, ‘Sicilian Rouge’, made with CRISPR‒Cas9 technology was launched into the open market18.
Previously, tomato fruit treated with exogenous melatonin was found to show higher levels of nutrients (such as carotenoids, flavor, etc.) with better fruit yields compared to nontreated plants19,20. Moreover, melatonin treatments effectively promote fruit ripening while maintaining the sensory and nutritional attributes of fruit by enhancing antioxidant capacity in ripening fruit, which refers to delaying fruit senescence and extending shelf life19–21. Therefore, increasing the content of melatonin in tomato fruit may improve both nutrition and agronomic traits.
Previous studies have shown that the synthesis of melatonin in plants starts from the synthesis of tryptophan, which requires four consecutive enzymatic reactions. Tryptophan decarboxylase (TDC) and tryptophan-5-hydroxylase (T5H) are key enzymes in the first two steps of melatonin synthesis, catalyzing the production of serotonin (5-hydroxytryptamine), 5-hydroxytryptamine-N-acetyltransferase (SNAT) and n-acetyl-5-hydroxytryptamine-methyltransferase (ASMT)/caffeic acid-o-methyltransferase (COMT), catalyzing the final formation of melatonin from serotonin. Studies have found that there are at least four possible melatonin synthesis routes in plants, and TDC and SNAT may be the rate-limiting enzymes in the process of melatonin synthesis22,23. However, it has also been suggested that ASMT may be the rate-limiting enzyme in the process of melatonin synthesis24–27. On the other hand, COMT can effectively catalyse the production of melatonin, showing strong ASMT activity. A previous study showed that melatonin contents were significantly reduced in Arabidopsis comt knockout mutants28.
To date, it has been found that there are at least five TDC candidate genes in tomato, of which SlTDC3 is expressed in almost all tissues, and SlTDC1 and SlTDC2 are only expressed in tomato fruits and leaves, respectively, indicating that the expression of TDC genes may be tissue-specific, and the expression of these genes may play different roles in plant growth and development or resistance29,30. However, the full melatonin biosynthesis pathway, especially in tomato fruit, has yet to be elucidated.
The synthesis and signal transmission of melatonin in plants is significantly affected by environmental factors (such as light and temperature)31,32. The regulation of melatonin synthesis by light signals has been well-studied in animals. Studies in mice have shown that melatonin synthesis depends on the rhythm clock and the core regulator cry1/2 of the light-sensing signal33. However, research on plants is lagging behind. How different light signals coordinate the synthesis and metabolism of melatonin has been unclear.
Here, the melatonin synthesis pathway in tomato fruit was completely elucidated. The functions of biosynthesis-related genes (SlSNAT, SlASMT5, SlASMT7, and SlCOMT2) were identified. We also found that red light treatment significantly promoted melatonin synthesis in tomato fruit via the SlphyB2-SlPIF4-SlCOMT2 module. Based on the regulatory mechanism, we targeted the binding motif of PIF4 in the promoter of SlCOMT2 to design a gene-editing strategy and significantly enhanced the production of melatonin in tomato fruit. Our data not only expand our current knowledge of how environmental factors affect the biosynthesis of key metabolites but also provide a good example of how to use the regulatory mechanism to guide the breeding of crops with enhanced nutrition.
Results
Screening of melatonin biosynthesis-related genes in tomato fruit
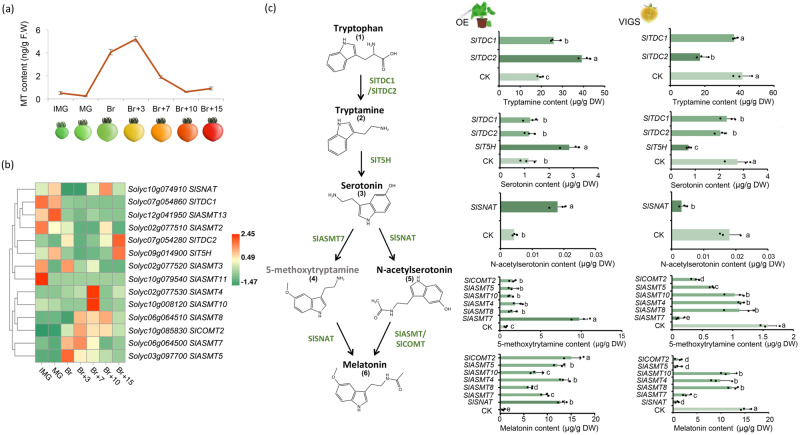
To elucidate the full melatonin biosynthesis pathway in tomato, we performed a BLAST search of the tomato genome for genes homologous to known melatonin biosynthesis-related genes: TDC, T5H, SNAT, ASMT, and COMT. In total, 17 candidates were identified (Fig. S1a). During the tomato fruit development process, the content of melatonin increases significantly at the breaker stage (Fig. 1a). Using the transcriptome data from the MicroTom Metabolic Network (MMN)34, we further conducted correlation (Figs. S1b, 1b) and quantitative (Fig. S1c) analyses to narrow down 10 candidate genes with reasonable expression levels in tomato fruit (Fig. 1b).
Fig. 1. Preliminary screening of key structural genes for melatonin biosynthesis in tomato fruit.
a Determination of melatonin content in tomato fruit at different development stages. IMG (immature green), MG (mature green), Br (breaker), Br+n (breaker plus n days). b The expression analysis heat map of the melatonin biosynthetic genes was obtained by screening from the MMN Database. c Melatonin biosynthetic gene obtained by instantaneous verification screening. ‘OE’ indicates gene transient overexpression (injection into tobacco leaves); ‘VIGS’ indicates gene transient silencing (injection into tomato fruits). CK indicates the determination result after injection of the corresponding empty carrier. 10–12 individual leaves (for transient expression) or 10–12 individual tomato fruits (for VIGS) with uniform sizes were pooled as one biological replicate. Data are represented as Mean ± SEM (n = 3). The P values indicate the results from pairwise comparisons of one-way ANOVA tests. Different letters represent a significant difference at P < 0.05.
All 10 candidate genes were then verified by transient overexpression in tobacco (Nicotiana Benthamiana) leaves and silencing in tomato fruits. We found that Solyc07g054280, which encodes SlTDC2, is responsible for the first step from tryptophan (1) to tryptamine (2) in melatonin biosynthesis. Solyc09g014900, which encodes SlT5H, catalyses the next step from tryptamine (2) to serotonin (3) (Fig. 1c, Fig. S2, S3, S4, S5, and S6).
Previous studies indicate that from serotonin (3), melatonin (6) biosynthesis might have alternative routes, which require the participation of SNAT, ASMT, or COMT9,22,23. We transiently overexpressed/silenced the remaining 7 genes to check the contents of melatonin. Transient overexpression of SlASMT5 (Solyc03g097700), SlASMT7 (Solyc06g064500), SlCOMT2 (Solyc10g85830), and SlSNAT (Solyc10g074910) can significantly induce the production of melatonin in tobacco leaves. Silencing of these genes significantly reduced the melatonin content in tomato fruit (Fig. 1c, Figs. S2, S3, S4, S5, and S6). Notably, the expression level of SlASMT7 was found to be associated with the 5-methoxytryptamine (4) route of melatonin biosynthesis (Fig. 1c). All these data indicate that SlSNAT, SlCOMT2, SlASMT5, and SlASMT7 are involved in the biosynthesis of melatonin.
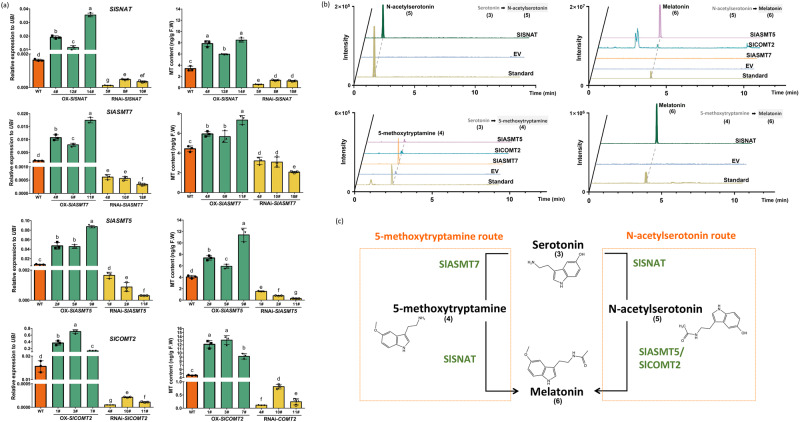
Functional verification of the roles of SlSNAT, SlCOMT2, and SlASMT5/7 in melatonin biosynthesis
The expression levels of SlASMT5, SlASMT7, SlCOMT2, and SlSNAT in different tissues were measured quantitatively. While SlSNAT was expressed in all tested tissues, SlASMT7, SlASMT5, and SlCOMT2 were mainly expressed in fruits after the breaker stage (Fig. S7). The expression levels of these genes matched previous transcriptome data (Fig. S1a). Figure S8 shows the expression of these four genes in the Tomato Expression Atlas database35 and MMN database34. This is consistent with the melatonin content (Fig. 1a). The localization experiment using Arabidopsis protoplasts showed that SlSNAT was localized in the chloroplast, while SlASMT5, SlASMT7, and SlCOMT2 were in the nucleus and cytoplasm (Fig. S9). This is consistent with previous reports22,23,30.
We then generated stable overexpression and RNAi lines for SlASMT5, SlASMT7, SlCOMT2, and SlSNAT (Figs. S10, S11). Compared to WT fruit, overexpression of these four genes individually significantly enhanced the production of melatonin, while silencing of these genes reduced the content of melatonin (Fig. 2a, Fig. S12).
Fig. 2. In vivo and in vitro verification of melatonin biosynthetic genes.
a Determination of gene expression and melatonin content in stable transgenic tomato. OX-SlGENE represents the overexpression lines, and RNAi-SlGENE represents the silencing lines. 10–12 individual tomato fruits at the Br+3 stage were pooled as one biological replicate. Data are represented as Mean ± SEM (n = 3). The P values indicate the results from pairwise comparisons of one-way ANOVA tests. Different letters represent a significant difference at P < 0.05. Source data are provided as a Source Data file. b In vitro enzyme activity verification of key structural genes. Different proteins were incubated with different substrates (serotonin (3), N-acetylserotonin (5) and 5-methoxytryptamine (4), respectively) to detect the production of N-acetylserotonin (5), 5-methoxytryptamine (4), and melatonin (6), respectively. ‘EV’ indicates the empty vector for negative control. c The roles of SlSNAT, SlCOMT2, and SlASMT5/7 in melatonin biosynthesis.
In vitro enzyme assays using recombinant proteins from E. coli confirmed that SlSNAT can catalyse the production of N-acetylserotonin (5) from serotonin (3), as well as the synthesis of melatonin from 5-methoxytryptamine (4) (Fig. 2b and Fig. S13). This indicates that the biosynthesis of melatonin in tomato fruit may have two possible routes: one is through the “serotonin(3)—N-acetylserotonin (5)—melatonin (6)” route, and the other is the “serotonin (3)—5-methoxytryptamine (4)—melatonin (6)” route. We then incubated the recombinant SlASMT5, SlASMT7, and SlCOMT2 proteins with either serotonin or N-acetylserotonin. The recombinant SlASMT7 can catalyse the formation of 5-methoxytryptamine from serotonin but failed to produce melatonin from N-acetylserotonin (Fig. 2b and Fig. S13). This indicates that SlASMT7 is involved in the 5-methoxytryptamine route of MT biosynthesis. On the other hand, both recombinant SlASMT5 and SlCOMT2 can catalyse the production of melatonin from N-acetylserotonin while failing to produce 5-methoxytryptamine from serotonin (Fig. 2b and Fig. S13). We further verified the function of SlASMT5, SlASMT7, and SlCOMT2 in vivo by RNAi and found that only silencing SlASMT7 significantly reduced the contents of 5-methoxytryptamine (Fig. S14). These results indicate that SlASMT5 and SlCOMT2 catalyse the N-acetylserotonin route of MT biosynthesis, while SlASMT7 is involved in the 5-methoxytryptamine route (Fig. 2c).
Melatonin biosynthesis in tomato fruit is significantly induced by red light treatment
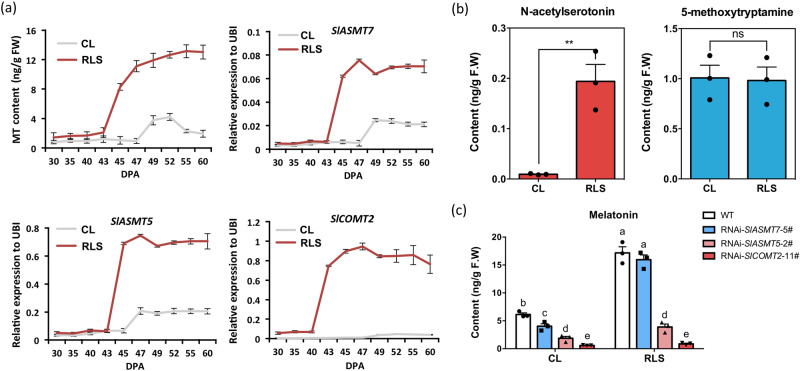
Red light supplementation was reported to introduce excellent characteristics such as early ripening, enhanced nutrients, and delayed senescence to tomato fruit36–39. We found that when tomato plants were provided with red light supplementation, the expression of SlASMT7, SlASMT5, and SlCOMT2 was induced, and the content of melatonin was significantly increased during the ripening process (Fig. 3a, S15). We also found that red light supplementation significantly increased the content of N-acetylserotonin but not 5-methoxytryptamine (Fig. 3b, S16). It seems that red light-induced melatonin biosynthesis relies on the activation of the N-acetylserotonin route via SlCOMT2 and SlASMT5.
Fig. 3. Red light supplement-induced melatonin biosynthesis in tomato fruit.
a Red light can significantly induce melatonin synthesis in tomato fruit. Determination of melatonin content and quantification of biosynthetic genes in tomato fruit at 10 different development stages under control light (CL) and red light supplement (RLS). Data are represented as mean ± SD (n = 3). In which 10–12 individual fruits were pooled as one biological replicate. b Content of N-acetylserotonin (5) and 5-methoxytryptamine (4) in wild-type tomato fruit after red light treatment. Data are represented as Mean ± SD (n = 3). In which 10–12 individual fruit were pooled as one biological replicate. * indicates a significant difference from control light (CL) analyzed by two-sided Student’s t test. (**P = 0.0054), ns (P = 0.8949), ns indicates not significant (P > 0.05). Source data are provided as a Source Data file. c Melatonin content in fruit of transgenic tomato lines after red light treatment. Data are represented as Mean ± SEM (n = 3). In which 10–12 individual fruits were pooled as one biological replicate. The P values indicate the results from pairwise comparisons of one-way ANOVA tests. Different letters represent a significant difference at P < 0.05. Source data are provided as a Source Data file.
To test this hypothesis, we repeated the red light supplementation experiment for the SlASMT7, SlASMT5, and SlCOMT2 RNAi lines. Compared to WT, the fruit of RNAi-SlASMT5 and RNAi-SlCOMT2 lines contained significantly lower melatonin under red light supplementation, while the RNAi-SlASMT7 line still responded well to red light treatment (Fig. 3c). All these data indicate that red light supplementation enhances melatonin biosynthesis in tomato fruit via activation of the expression levels of SlASMT5 and SlCOMT2.
SlPIF4 directly inhibits the expression of SlCOMT2 to suppress melatonin biosynthesis in tomato fruit
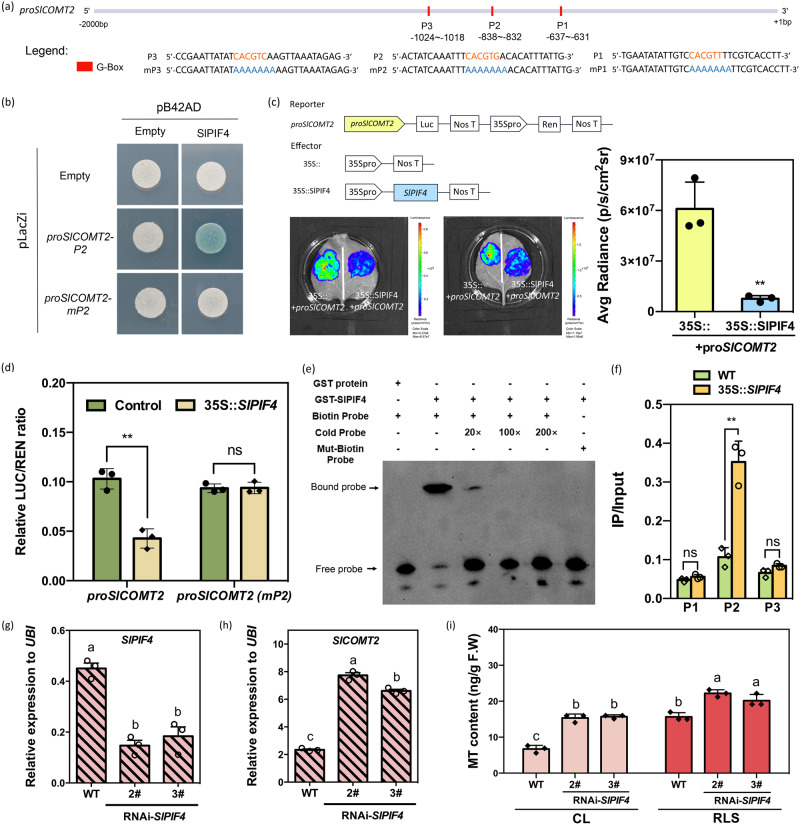
To investigate the molecular mechanism of red light-induced melatonin biosynthesis, we scanned the promoter regions of SlASMT5 and SlCOMT2. A series of light signal-related G-box elements were found in both promoters (Figs. 4a, S17a). On the other hand, proSlASMT5 and proSlCOMT2 were used as baits to screen yeast one-hybrid libraries. A cDNA fragment showing homology to phytochrome-interacting factors 4 (SlPIF4) was identified to bind to both proSlCOMT2 and proSlASMT5 (Supplementary Data 1). PIF4 has been reported to play vital roles in the light response and is capable of binding to the G-box domain40. Therefore, we hypothesize that SlPIF4 is a potential regulator of MT biosynthesis.
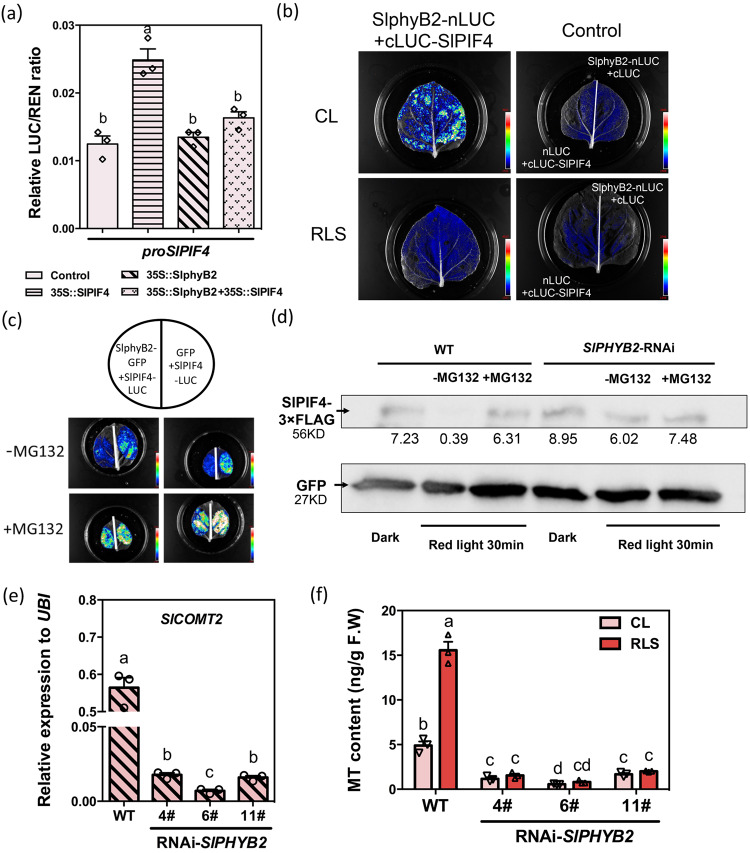
Fig. 4. SlPIF4 directly binds to the G-Box domain (P2) of proSlCOMT2 to inhibit its expression.
a Schematic diagrams showing the SlASMT5 and SlCOMT2 genomic regions. The position of G-BOX is indicated by a red BOX. b Interactions between SlPIF4 proteins and SlCOMT2 promoters with P2 and P2 mutation (mP2) in yeast cells. A blue plaque indicates binding. c Interactions of SlPIF4 protein and the promoters of SlCOMT2 confirmed with dual-luciferase reporter assays in Nicotiana benthamiana leaves. 35 S::+proSlCOMT2 were used as controls. The right column chart shows the quantitative fluorescence intensity. (**P = 0.0045). * indicates significant difference from 35 S:: empty vector analyzed by two-sided Student’s t test. d SlPIF4 binding to the regions of proSlCOMT2 in the wild-type (WT) and transgenic lines of 35 S::SlPIF4. ‘proSlCOMT2(mP2)’ is a 2000 bp promoter sequence with a mutation in the P2 domain. LUC/REN is the average ratio of the bioluminescence of firefly luciferase to that of Renilla luciferase. (**P = 0.0018), ns (P = 0.9387), ns (P = 0.0505, P2), ns indicates not significant (P > 0.05). * indicates significant difference from control analyzed by two-sided Student’s t test. e EMSA of SlPIF4 binding to the P2/mP2 fragment. SlPIF4 binds to the P2 fragment of proSlCOMT2, while the mutant of P2 (mP2) does not present binding. ‘+’ indicates presence; and ‘-’ indicates absence. f ChIP analysis of SlPIF4 binding to the regions of SlCOMT2 in the WT and transgenic lines of 35 S::SlPIF4. (**P = 0.0020), ns (P = 0.1222, P1), ns (P = 0.0505, P2), ns indicates not significant (P > 0.05). * indicates significant difference from WT analyzed by two-sided Student’s t test. This experiment was repeated independently two times with similar results. Data in c, d, f are represented as Mean ± SD (n = 3), Student’s t test. g Transcript level of SlPIF4 in the fruit of RNAi-SlPIF4 transgenic lines as well as WT. h Transcript level of SlCOMT2 in the fruit of RNAi-SlPIF4 transgenic lines as well as WT. i Melatonin content in fruit of RNAi-SlPIF4 transgenic lines as well as WT after red light treatment. Data in g, h, and i are represented as mean ± SEM (n = 3). In which 10–12 individual tomato fruits at the Br+ 3 stage were pooled as one biological replicate. The P values indicate the results from pairwise comparisons of one-way ANOVA tests. Different letters represent a significant difference at P < 0.05. Source data are provided as a Source Data file.
To investigate whether SlPIF4 can directly bind to proSlCOMT2 and proSlASMT5, we first performed a yeast one-hybrid (Y1H) assay. Three G-box elements of the SlCOMT2 genome sequence were selected as possible binding sites (P1-P3) (Fig. 4a). The results showed that SlPIF4 could bind to the P2 element of the SlCOMT2 promoter (Fig. 4b). A G-Box was also predicted on the promoter of SlASMT5, but it could not be bound by SlPIF4 (Fig. S17).
Using the Dual-Luc system in both tobacco leaves (Fig. 4c) and tomato protoplasts (Fig. 4d), we further confirmed that SlPIF4 could repress the activity of the SlCOMT2 promoter. When the P2 motif was mutated, the inhibition of proCOMT2 by SlPIF4 was released (Fig. 4f). EMSA with normal and mutated probes with the CArG motif in the promoter (P2 and mP2) of proSlCOMT2 also suggested that SlPIF4 directly binds to the SlCOMT2 promoter (Fig. 4e).
To further examine the direct binding of SlPIF4 to proSlCOMT2 in vivo, we generated FLAG-tagged SlPIF4-overexpressing tomato lines (Figs. S18, S19). By ChIP‒qPCR, we found that SlPIF4 directly binds to the G-Box element in the P2 site of proCOMT2, while P1 and P3 are invalid sites for SlPIF4 binding, which is consistent with the results found above (Fig. 4f).
Together, these data suggest that SlPIF4 can suppress the expression of SlCOMT2 through interaction with the P2 site of proSlCOMT2. Under normal growth conditions, the expression of SlCOMT2 was significantly upregulated in the RNAi-SlPIF4 lines, together with significant induction of the MT content (Fig. 4g–i). After red light supplementation, although there was still significant induction of MT contents in the transgenic lines (27% and 16% increase, respectively), their MT content enhancement ratios were significantly lower than that of the WT fruit (63%) (Fig. 4i, S20). These data indicate that SlPIF4 is a negative regulator of MT biosynthesis and is involved in the red light-mediated regulation of MT biosynthesis.
The SlphyB2-SlPIF4-SlCOMT2 module mediates red light-induced melatonin biosynthesis in tomato fruit
As one of the key plant phytochrome photoreceptors, phytochrome B2 (phyB2) plays an important role in red light response signaling41–43. In the MMN database34, the expression of SlPHYB2 in different developmental stages of tomato fruits is highly consistent with that of melatonin, while SlPHYB2 and SlPIF4 show opposite trends (Fig. S21). We first checked whether SlphyB2 can inhibit the expression of SlPIF4. The Dual-Luc assay using tomato protoplasts indicated that SlPIF4 could bind to its own promoter to achieve self-activation. Although SlphyB2 alone did not inhibit the activity of proSlPIF4, it inhibited the self-activation of SlPIF4 (Fig. 5a).
Fig. 5. Red light response of melatonin mediated by SlphyB2-SlPIF4-SlCOMT2.
a The transcriptional regulation relationship between SlphyB2 and SlPIF4. The dual-LUC experiment proves that SlphyB2 can inhibit the self-activation of SlPIF4 on its own promotor. Data are represented as Mean ± SEM (n = 3). b Quantitative analysis of luminescence intensity showing the interaction between SlphyB2 and SlPIF4 in Nicotiana benthamiana leaves. SlphyB2 interacts with the SlPIF4 protein, but the interaction disappears under red light. c SlphyB2 can ubiquitously degrade SlPIF4, and MG132 prevents the degradation. d Western blot detection of ubiquitination degradation of SlPIF4 mediated by SlphyB2. The addition of MG132 will inhibit the degradation of SlPIF4 by red light in WT, while in the interference strains of SlPHYB2, the bands of SlPIF4 are not different. GFP acts as an actin ensure consistent protein levels. This experiment was repeated independently two times with similar results. e Gene expression of SlCOMT2 in the SlPHYB2 interference lines. Samples were collected at Br+3. f Silence of SlPHYB2 makes the plant no longer be induced by the red light to produce more melatonin. The content of melatonin in wild tomato fruit was induced and accumulated by red light, but decreased in RNAi-SlPHYB2 lines, and was no longer induced by red light. Samples were collected at Br+3. Data in e, f are represented as Mean ± SEM (n = 3). In which 10–12 fruit collected from the same seedling were pooled as one biological replicate. For a, e, and f, the P values indicate the results from pairwise comparisons of one-way ANOVA tests. Different letters represent a significant difference at P < 0.05. Source data are provided as a Source Data file.
Previous studies suggest that phyB2 activates the thermoresponse by regulating PIF4 stability44,45. Firefly luciferase complementation imaging assays were performed to elucidate the interaction between SlphyB2 and SlPIF4 (Fig. 5b, S22). When SlPIF4-LUC was expressed together with SlPHYB2 in tobacco leaves, the luciferase signal was significantly decreased. This inhibition can be removed by adding the proteasome inhibitor MG132 (Fig. 5c). This indicates that SlphyB2 might facilitate the degradation of SlPIF4.
To verify that SlphyB2 can regulate SlPIF4 at the protein level in vivo, we transiently overexpressed FLAG-tagged SlPIF4 in both WT and RNAi-SlPHYB2 tomato fruit. In the WT fruit, compared to fruit stored in the dark, the SlPIF4 protein content in agroinfiltrated fruit under light was significantly reduced. This phenotype can be effectively blocked by infiltrating the proteasome inhibitor MG132 into fruit (Figs. 5d, S23, S24). In the RNAi-SlPHYB2 tomato fruit, however, the degradation of SlPIF4 under red light supplementation was effectively inhibited. These data indicate that SlphyB2 can regulate SlPIF4 stability via the 26 S proteasome pathway. Consequently, in the RNAi-SlPHYB2 lines (Fig. S25), the expression level of SlCOMT2 was inhibited, the melatonin content was significantly decreased, and the red light treatment was no longer effective (Fig. 5e, f, S26).
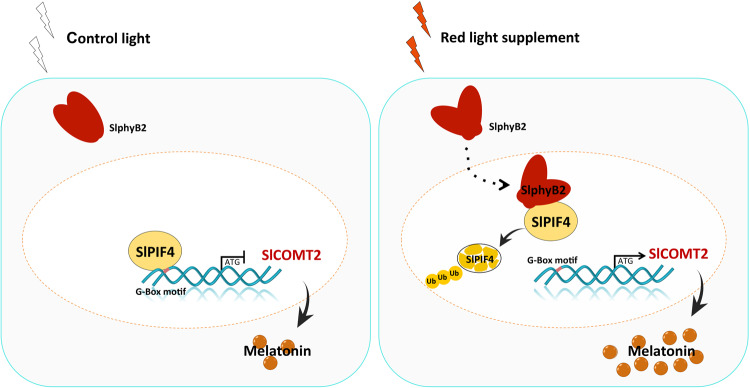
In summary, SlPIF4 negatively regulates melatonin biosynthesis in tomato fruit via direct inhibition of SlCOMT2 expression. Under red light supplementation, the activation of SlphyB2 facilitates the degradation of SlPIF4 via the 26 S proteasome pathway. Therefore, the inhibition of SlCOMT2 expression was released, and the biosynthesis of melatonin was enhanced (Fig. 6).
Fig. 6. Schematic representation of the molecular mechanism of red light-induced melatonin biosynthesis in tomato fruit.
SlphyB2 is activated under the red light supplement and can facilitate the degradation of SlPIF4 through the 26 S proteasome pathway, thus removing the inhibition of SlCOMT2 by SlPIF4, leading to the accumulation of melatonin.
Engineering melatonin-enriched tomatoes
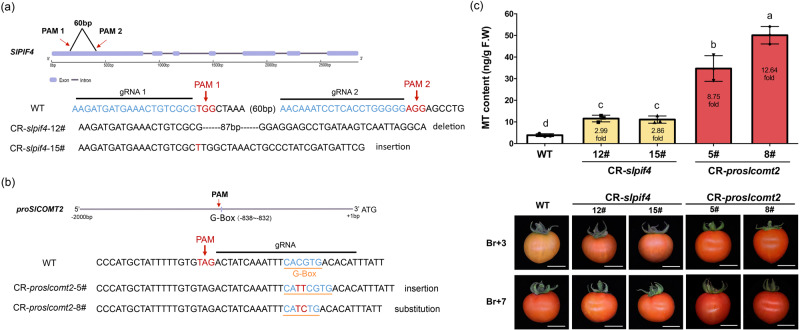
To test whether this regulatory mechanism can be used for breeding tomato varieties with enhanced melatonin production, two gene-editing strategies were designed. One strategy is to directly knockout SlPIF4 (Fig. 7a), and the other is to mutate the SlPIF4 recognition site on proSlCOMT2 (Fig. 7b). Both methods significantly enhanced the production of melatonin (Fig. 7c). However, gene-editing targeting the SlPIF4 recognition site on proSlCOMT2 can induce much stronger melatonin accumulation under normal growth conditions: Compared with WT, the melatonin content of the two CR-slpif4 strains (12# and 15#) increased by approximately threefold. However, the melatonin content in the two CR-proslcomt2 strains (5# and 8#) increased by 8.75- and 12.64-fold, respectively (Fig. 7c, S27).
Fig. 7. Engineering of tomato germplasm with high melatonin content.
a CRISPR/Cas9 target site design and sequencing results of gene editing for SlPIF4. b CRISPR/Cas9 target site design and sequencing results of gene editing for the promoter of SlCOMT2. Cas9 recognize NG-PAM sequence. c Melatonin content of WT and T2 CR fruits at the Br+3 stage. Data is represented as Mean ± SEM (n = 3). 10–12 tomato fruits from the same seedling were pooled as one biological replicate. The P values indicate the results from pairwise comparisons of one-way ANOVA tests. Different letters represent a significant difference at P < 0.05. Fruit phenotype of WT and CR fruits at the Br+3 and the Br+7 stage were also presented. Source data are provided as a Source Data file.
Discussion
Melatonin is an indoleamine compound found in all organisms from plants to animals32,46. Unlike animals, whose melatonin biosynthesis pathway has been thoroughly investigated47,48, the melatonin biosynthesis pathway in most plants remains uncharacterized9,49. In this study, the biosynthetic pathway of melatonin in tomato was fully elucidated. We found that alternative melatonin biosynthesis routes coexist in tomato. One is through the “serotonin (3)—N-acetylserotonin (5)—melatonin (6)” route, in which SlASMT5 and SlCOMT2 are the key enzymes (Fig. 1c). The other route is the “serotonin (3)—5-methoxytryptamine (4)—melatonin (6)” route, in which SlASMT7 is the core enzyme (Fig. 1c).
As sessile photoautotrophic organisms, plants are constantly challenged by diverse external environmental conditions. To develop resistance capacity, plants produce various environment-induced metabolites, such as nutrients, antinutrients, and phytohormones50,51. In this study, we found that red light treatment at the fruit development stage can effectively induce the synthesis of melatonin in tomato fruit. Although there are alternative routes for melatonin biosynthesis in tomato (Fig. 1c), the red light-induced melatonin enhancement mainly relies on the “serotonin (3)—N-acetylserotonin (5)—melatonin (6)” route (Fig. 3b) via the activation of SlCOMT2 and SlASMT5 (Fig. 3a,c).
We further found that SlPIF4 can directly inhibit the expression of SlCOMT2. Under red light supplementation, the activation of SlphyB2 facilitates the degradation of SlPIF4. Therefore, the inhibition of SlCOMT2 was released (Figs. 3–6). Previous studies have shown that PIFs are bHLH family transcription factors that can bind to photoreceptor phytochrome proteins (PHYs), and phytochrome can accelerate the degradation of the PIF-dependent 26 S proteasome by promoting the phosphorylation of PIFs under red light41–43. Studies in Arabidopsis show that PIF4 is a negative regulator of plant light signal transduction and can antagonize and regulate plant signal transduction52,53. The tomato phytochrome interaction factor PIF4 regulates tomato plant responses to temperature stress by integrating light and temperature hormone signals54. SlPIF4 has close homology with Arabidopsis AtPIF4, while AtPIF4 is not only a transcription factor necessary for the process of light signaling but can also positively regulate the synthesis of anthocyanins55,56. The joint cross-response of multiple environmental factors is the general trend of future research on plant growth and development and quality formation. Light and temperature often act on plants together, and PIF4, as an important transcription factor of light and temperature signals, may be a link for further exploration of other regulatory genes and pathways. Indeed, we did find some other TFs, including members of the bHLH, bZIP, WRKY, and MYB families, in the Y1H screen library (Supplementary Data 1), which we will further investigate in subsequent studies. It has been reported that HsfA1a in tomato plants can promote the synthesis of melatonin to confer cadmium tolerance57. In cassava, MeHsf20, MeWRKY79 and MeRAV1/2 are able to induce melatonin production by binding to the promoters of melatonin biosynthesis genes58. However, most studies on the involved TFs are related to the stress response, and more TFs affecting melatonin synthesis need to be identified32.
Recent studies have revealed that effective tomato metabolic engineering can be achieved by gene-editing targeting key biosynthesis-related genes16,17. Our data indicate that silencing and knocking out SlPIF4 can significantly enhance the production of melatonin in tomato fruit (Fig. 4g–i), even under normal growth conditions. However, due to the vital functions of SlPIF4 in various signaling pathways56,59, it is not wise to simply knock down/out this master regulator. Alternatively, we targeted the SlPIF4 recognition site in proSlCOMT2 to design gene-editing strategies. By doing so, we can also significantly enhance melatonin production in tomato fruit. Previous studies have indicated that during tomato fruit ripening, DNA methylation is the key regulatory component60,61. The DNA methylation rate of SlCOMT2 was checked from the green stages to the ripening stages in our unpublished database. We found that at the green stage, proSlCOMT2 was highly methylated (Fig. S28). Therefore, even without the inhibition of SlPIF4, the expression of SlCOMT2 is low during green fruit stages in slpif4 or proslcomt2 mutants. In fact, this is the key advantage of gene editing for proSlCOMT2, which only removed SlPIF4 inhibition during the ripening stages without changing its expression pattern during other stages. Notably, compared to directly knocking out SlPIF4, the proslcomt2 mutants had significantly higher melatonin production than the slpif4 mutants (Fig. 7c), possibly due to other unknown TFs (Supplementary Data 1) interacting with the mutated G-box motif.
In summary, this study elucidated the full melatonin biosynthesis pathway in tomato fruit. We also uncovered the mechanism of red light induction of melatonin biosynthesis and successfully developed melatonin-enriched tomato varieties through gene editing. Our findings demonstrate that understanding the mechanisms by which environmental factors regulate key metabolic processes can be used to create nutrient-enriched crops.
Methods
Plant materials, growth conditions, and light treatments
Tomato (Solanum lycopersicum L. cv. MicroTom) seeds (purchased from Pan American Seed, Inc., Hillsborough, FL, USA) were grown in a standard greenhouse under a 16 h photoperiod (16 h light/8 h dark at 23 °C, relative humidity 70%). The light intensity indicated as PPFD (photosynthetic photon flux density), was set at 250 μmol m−2 s−1 above the plant canopy and maintained by adjusting the distance of 15 cm from the LEDs to the canopies. Red light refers to replacing 30% white light with red light, i.e., 30% red light at a wavelength of 657 nm and 70% white multiwavelength light, with white light as a control. The collected tissues were frozen in liquid nitrogen and stored at −80 °C until further investigation. Three biological replicates, each of which was a pooled sample of 10–12 individual fruits, were analyzed.
Melatonin and metabolic intermediate extraction and analysis
The tomato tissues from three independent biological samples were ground into a fine powder and used for melatonin and metabolic intermediate measurements based on the AB Sciex QTRAP 6500 LC‒MS/MS platform. In short, 200 mg tomato powder was extracted with 1.0 mL 80% aqueous methanol by ultrasonication for 20 min at 4 °C. The supernatants were transferred into new Agilent tubes after 10 min of centrifugation at 10,000 × g for LC‒MS/MS analysis. The LC analytical conditions were as follows: samples were separated using a Hypersil Gold C18 column (100 × 2.1 mm, 1.9 μm; Thermo Fisher Scientific, USA) and the column temperature was set at 40 °C. The flow rate was 0.4 mL/min. The mobile phases were 0.1% formic acid (A) and acetonitrile (B), and the gradient was as follows: 5% B for 0.5 min; 5–95% B for 8 min; 95% B for 2 min followed by a decrease to 5% B for 0.1 min and re-equilibration of the column for 2.9 min with 5% B. The injection volume was 1.0 μL and the sampler temperature was set at 15 °C. Mass spectrometry was performed using an electrospray ionization (ESI) source. The source parameters in positive polarity were as follows: ion source gas 1: 25 psi; ion source gas 2: 60 psi; curtain gas: 40 psi; CAD gas: medium; temperature: 450 C; spray voltage: 5500 V. Fragment XICs were extracted using SCIEX OS software (version 1.7). The same method was used for calibrating and quantifying the mass spectrum peaks of melatonin. Source data are provided as a Source Data file.
Coexpression/coregulation identification and analysis
The Tomato Expression Atlas database35 and the MMN database34 were used for the preliminary identification of melatonin biosynthesis-related genes according to coexpression/coregulation analysis. Heatmaps created by R (v3.6.0) displayed for high-throughput analysis of the expression levels of the coexpressed genes.
Plasmid construction and generation of transgenic lines
The subject sequence was introduced into the relevant vector by a homologous recombination system (ClonExpress® II One-Step Cloning Kit, C211, Vazyme) or restriction endonuclease reaction. pEAQ (for overexpression) and pTRV (pTRV1 and pTRV2 vectors, for virus-induced gene silencing, VIGS) were used for transient transformation. pCAMBIA1306 (35 S::3×FLAG) was used for constitutive expression, and pBWA(V)HS-RNAi was used for RNA interference construction. pHSbdcas9i (for SlPIF4) and pKSE401 (for proSlCOMT2) were used as the vector backbone for a one-step CRISPR/Cas9 binary constitutive system. A plasmid with the correct insertion was introduced into Agrobacterium tumefaciens strain EHA105. Source data are provided as a Source Data file.
In vitro enzyme activity verification
The assay was performed according to the method described by Fu et al.62,63. The subject sequence was introduced into the pDEST17 vector by the Gateway system. Methyltransferase and acetyltransferase were selected for enzyme activity verification, and heat shock transformation was carried out with Escherichia coli BL21 (DE3). Single colonies were selected from LB solid medium (50 μg/mL. ampicillin), and cultivated in 200 mL LB liquid medium with corresponding resistance at low speed for 3–5 h at 37 °C. The positive strains were obtained by polymerase chain reaction. Subsequently, 20 μL of the bacterial solution was added to LB medium containing antibiotics and incubated overnight at 37 °C until the OD600 reached 0.5~1.0. Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to a final concentration of 0.5~1.0 mM and induced at 28 °C for 8 h. SDS polyacrylamide gel electrophoresis (SDS‒PAGE) was performed to determine whether the protein was expressed.
The Escherichia coli liquid with the target protein was centrifuged at 4 °C at 5000 × g for 10 min. Cells were re-suspended in lysis buffer (25 mM Tris-HCl 8.0, 150 mM NaCl, 0.5 mM tris-phosphine) with 1 mM ATP and 1 mM PMSF. The cells were broken by a disruptor and centrifuged at 4 °C at 20,000 × g for 30 min. The supernatant was collected and proteins were purified with Ni2+-NTA column (Qiagen, Germany) according to the manufacturer’s guidance. The concentration of purified protein was determined by the BCA Protein Assay Kit (Sangon Biotech, China). The enzyme assay was performed using a 100 μL reaction system containing 1 μg of purified enzyme, 20 μM of each substrate in 1× PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 2 mM KH2PO4, pH = 7). The mixture was incubated at 30 °C for 60 min and the reaction was stopped by adding 400 μL of methanol. Then, the samples were centrifuged at 4 °C at 20,000 × g for 10 min, and the supernatant was used for mass spectrometry.
Subcellular localization
The full-length coding region without the termination codon was amplified with 35 S::GFP (pCAMBIA1302). It was then transformed into Arabidopsis protoplasts after incubation for 12 h at 28 °C. Arabidopsis leaves (3 weeks) were used for protoplast separation. A vacuum was applied for 20 min after enzymatic hydrolysis, followed by the addition of W5 and resuspension in an ice bath. Then, 200 μL of protoplast was added to the target plasmid. The protoplasts were placed in the dark at 24 °C for 20 h after 40% PEG-mediated transformation. A confocal laser scanning microscope (LSM800, Zeiss) was used for GFP fluorescence detection.
Construction and screening of the yeast library
Five hundred micrograms of high-quality total RNA were extracted from tomato tissues, and Gateway technology was used for yeast library construction. The cDNA library was prepared by Yuanbao Biotech (Nanjing, China). SD/-His-Leu-Trp dropout 3AT culture screening plates and the Y187 yeast strain were used to screen the yeast library. Each obtained more than 600 clones, and high-throughput sequencing was performed after colony collection. The raw data was transferred to fasta using fq2fa and then aligned to the tomato iTAG4.0, the paraments were outfmt 6, e value was 1e−3.
Yeast one-hybrid assays
The promoter fragments were amplified, cloned, and inserted into the pLacZi vector, and the CDS of SlPIF4 was fused to pB42AD. The constructs were then transformed into the yeast strain EGY48, and yeast cells were inoculated on a selective medium for 3 days at 28 °C and transferred to SD/-Ura-Trp medium. Yeast colonies turned blue with X-gal if there was an interaction between the factors.
Transient dual-luciferase reporter assay
The fragment of the SlCOMT2, SlASMT5 or SlPIF4 promoter was cloned and inserted into the pGreenII 0800-LUC vector. Agrobacterium tumefaciens strain GV3101 harboring targeted fragments was grown in infiltration medium (2 mM Na3PO4, 50 mM MES, and 100 mM acetosyringone) to an OD600 of 0.5 and then introduced via a syringe into the leaves of a 4–5-week-old Nicotiana benthamiana plant. After 48–96 h, a CCD camera was used to observe luciferase activity. Tomato leaves (2–3 weeks) were used for protoplast separation. After enzymatic hydrolysis, a vacuum was applied for 30 min, followed by the addition of W5 and resuspension in an ice bath. Then, 200 μL of protoplast was added to the target plasmid. After 40% PEG-mediated transformation, the protoplasts were placed in a dark environment at 24 °C for 20 h. The Dual-Luciferase Reporter Assay System E1960 (Promega, cat. #e1910, Madison, USA) was used to measure the fluorescence intensity of luciferase and renilla (REN). The relative LUC/REN ratios were used to represent the activity of the promoters.
ChIP‒qPCR assay
The transgenic Line 35 S::FLAG-SlPIF4 was assessed by ChIP‒qPCR assays. Six grams of the breaker stage fruit tissues were sliced into small pieces and immersed in the crosslinking buffer (0.4 M Sucrose, 10 mM Tris-HCl pH 8.0, 0.1% β-mercaptoethanol, 100 mM PMSF, 1% formaldehyde and 1× protease inhibitor cocktail (Roche) and vacuum infiltrated three times, for 10 min each time. Glycine was added to a final concentration of 0.125 M and samples were vacuum infiltrated for an additional 5 min. Samples were washed and frozen by liquid Nitrogen and ground into fine powder. Chromatin isolation was performed using the Honda buffer (0.44 M sucrose, 1.25% (wt/vol) Ficoll, 2.5% (wt/vol) Dextran T40, 20 mM HEPES (pH 7.4), 10 mM MgCl2, 0.5% (vol/vol) Triton X-100, 1 mM DTT, 1× protease inhibitor cocktail). Samples were re-suspended with 25 ml Honda buffer on ice for 5 min and filtered through two layers of Miracloth. Pellets were collected after centrifugation at 2000 × g at 4 for 10 min and re-suspended again with 1 mL Honda buffer, repeating the centrifugation and resuspension at least three times. Nuclei were isolated in Nuclei Lysis Buffer (50 mM Tris-HCl pH 8, 10 mM EDTA, 1% SDS, 1 mM PMSF, and 1× Protease Inhibitors) and DNA was sheared into ~250–750 bp via sonication. ChIP was performed using monoclonal anti-FLAG protein antibody (Sigma-Aldrich, F1804). The immunoprecipitation and DNA isolation/purification were done using the EpiTect ChIP OneDay Kit (Qiagen, Germany) according to the manufacturer’s instructions. The primers used for the qPCR assay are listed in Table S3, and each was repeated at least three times.
Electrophoretic mobility shift assay (EMSA)
The fusion proteins of SlPIF4 were generated through prokaryotic expression in vitro. The CDSs of SlPIF4 were cloned and inserted into the PGEX-5T vector containing a GST target and expressed in Escherichia coli strain BL21. IPTG was used to induce protein production. The MagneGSTTM Pull-Down System (Promega, USA) was used for protein purification, and the LightShiftTM Chemiluminescent EMSA Kit (Thermo Fisher, USA) was used for the subsequent EMSAs. Unlabeled probes were used for probe competition. Then, it was loaded onto a prerun native 6.5% polyacrylamide gel with TBE buffer as the electrolyte. After electroblotting onto a nylon membrane (Millipore, Germany) and UV crosslinking (2000 J for 5 min), the membrane was incubated in blocking buffer for 30 min and rinsed in washing buffer. A CCD camera was used to visualize the chemiluminescent signal.
Floated-leaf luciferase complementation imaging assay
To investigate whether SlphyB2 interacts with SlPIF4 in vivo, we used the pCAMBIA1300-cLUC and pCAMBIA1300-nLUC vectors for the FLuCI assay. SlphyB2 was fused to the C-terminal fragment of luciferase (cLUC), while SlPIF4 was fused to the N-terminal fragment of luciferase (nLUC). The interactions between nLUC and SlphyB2-cLUC as well as SlPIF4-nLUC and cLUC were used as negative controls. The final constructs were transformed into Agrobacterium tumefaciens strain GV3101, and different combinations of plasmids were co-infiltrated into Nicotiana benthamiana leaves. After incubation in the dark for 12–14 h and then in light for 48 h, the tobacco leaves were sprayed with 100 mM d-luciferin and kept in the dark for 5–10 min, then photographed with a CCD camera.
Validation of ubiquitination degradation
The ubiquitination and degradation of SlPIF4 by SlphyB2 were validated with the help of the proteasome inhibitor MG132 (Beyotime, S1748), which can effectively block the proteolytic activity of the 26 S proteasome complex. 1 mL 80 μM MG132 (10 mM MgCl2, 80 mM MG132) and its reference solution were injected 6 h before collection. SlPHYB2 was cloned and inserted into 35 S::GFP (pCAMBIA1302), while SlPIF4 was constructed with a luciferase vector. A CCD camera was used to observe luciferase activity in tobacco leaves. A plasmid containing both FLAG and GFP labels for SlPIF4 was used for detecting co-infiltrating control protein to make sure the degradation. Anti-GFP protein antibody bought from Abcam (ab290). For western blotting, 30 DPA tomato fruits were selected from RNAi-SlPHYB2 and wild-type plants, and infection solution was injected from the bottom of the fruit until liquid leached at the stem. The infected fruits were incubated in the dark for 24 h, followed by 3 days of dark cultivation. Half of the plants injected with MG132 or its reference solution were treated with red light for 30 min. BCA was used to determine the total protein concentration. SDS‒PAGE electrophoresis was performed with consistent protein content in each sample. Source data are provided as a Source Data file.
Total RNA isolation and qRT‒PCR analyses
Samples were harvested and ground into a fine powder using liquid nitrogen. Total RNA was extracted using RNAiso reagent (BIOFIT, RN33050) as recommended by the manufacturer. One microgram of RNA was used for first-strand cDNA by the PrimeScriptTM RT reagent Kit containing gDNA eraser (Takara, Kusatsu, Japan). qRT‒PCR was performed using the Bio-Rad CFX384 Real-Time System according to the manufacturer’s instructions. The relative expression level of each gene was calculated using the ΔCt method as described previously34, and SlUBI was used as an internal control. Average values were calculated by three biological replicates (n = 3). One biological replicate is the pool of 10–12 samples.
Statistical analysis
At least three biological replicates were included in the data, and the statistical significance of differences was determined by ANOVA followed by post hoc Tukey’s test or Student’s t test (GraphPad Prism version 8).
Accession numbers
The accession numbers of genes are as follows: SlTDC1 (Solyc07g054860, https://solgenomics.net/locus/30030/view), SlTDC2 (Solyc07g054280, https://solgenomics.net/locus/29973/view), SlT5H (Solyc09g014900, https://solgenomics.net/locus/33811/view), SlSNAT (Solyc10g074910), SlASMT7 (Solyc06g064500, https://solgenomics.net/locus/37357/view), SlASMT5 (Solyc03g097700, https://solgenomics.net/locus/18964/view), SlCOMT2 (Solyc10g085830, https://solgenomics.net/locus/38127/view), SlPHYB2 (Solyc05g053410, https://solgenomics.net/locus/25083/view), SlPIF4 (Solyc07g043580, https://solgenomics.net/locus/29516/view).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was financially supported by grants from the National Key Research and Development Program of China (2022YFF1001900, Y.Z.), the National Natural Science Foundation of China (32200260, Z.X.Z.), the Natural Science Foundation of Sichuan Province, China (2023NSFSC1991, Y.Z.), the China Postdoctoral Science Foundation Funded Project (2020M673207, Z.X.Z.) and the Sichuan University Postdoctoral Science Foundation Funded Project, China (2020SCU12061, Z.X.Z.). We acknowledge the Special Fund for Fundamental Research Funds for the Central Universities (2023SCUD0003, Y.Z.). We acknowledge Dr. Hsihua Wang from the Center of Metabolomics and Proteomics in the College of Life Science, Sichuan University, for technical support in metabolic analysis.
Author contributions
Y.Z. and Z.X.Z. conceived and designed the experiments; Z.X.Z., X.Z., Y.T.C., and W.Q.J. performed most of the experiments; J.Z., J.Y.W. and Y.J.W. provided technical support; X.Y. provided technical support on light treatment; S.C.W. and M.C.L. provided conceptual advice; W.Q.J. and X.Z. contributed to plant transformation; Z.X.Z., X.Z. and Y.Z. analyzed the data and wrote the manuscript with inputs from all authors.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this Article is available as a Supplementary information file. The datasets and plant materials generated and analyzed during the current study are available from the corresponding author upon request. The source data underlying Figs. 1d, 2d–g, 3c, d, 4a, b, and 6, Figs. 2a, 3b, c, 4i, and 5f, 7c, as well as Supplementary Figs. S12, S13, S15, S16, S19a, S20, S23, S24a, S25a, S26, S27 are provided as a Source Data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-41307-5.
References
- 1.Lerner AB, Case JD, Heinzelman RV. Structure of melatonin. J. Am. Chem. Soc. 1959;81:6084–6085. doi: 10.1021/ja01531a060. [DOI] [Google Scholar]
- 2.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958;80:2587–2587. doi: 10.1021/ja01543a060. [DOI] [Google Scholar]
- 3.Arnao MB, Hernández-Ruiz J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020;33:77–87. doi: 10.1007/s00497-020-00388-8. [DOI] [PubMed] [Google Scholar]
- 4.Russel R, et al. Phytomelatonin: assisting plants to survive and thrive. Molecules. 2015;20:7396–7437. doi: 10.3390/molecules20047396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rui GM. Roneilg. Melatonin, sleep, and allergy. Allergy Sleep. 2019;7:367–384. [Google Scholar]
- 6.Slominski AT, et al. Melatonin: a cutaneous perspective on its production, metabolism, and functions. J. Invest. Dermatol. 2018;138:490–499. doi: 10.1016/j.jid.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill SM, et al. Melatonin: an inhibitor of breast cancer. Endocr. Relat. Cancer. 2015;22:183–204. doi: 10.1530/ERC-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter RJ, Calvo JR, Karbownik M, Wenbo QI, Tan DX. Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. 2010;917:376–386. doi: 10.1111/j.1749-6632.2000.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZX, Zhang Y. Melatonin in plants: what we know and what we don’t. Food Qual. Saf. 2021;5:1–9. [Google Scholar]
- 10.Arnao MB, Hernández-Ruiz J. Functions of melatonin in plants: a review. J. Pineal Res. 2015;59:133–150. doi: 10.1111/jpi.12253. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Wang H, Zhang Y, Martin C. Can the world’s favorite fruit, tomato, provide an effective biosynthetic chassis for high-value metabolites? Plant Cell Rep. 2018;37:1443–1450. doi: 10.1007/s00299-018-2283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butelli E, et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008;26:1301–1308. doi: 10.1038/nbt.1506. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013;23:1094–1100. doi: 10.1016/j.cub.2013.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Multi-level engineering facilitates the accumulation of bioactive compounds in tomato. Nat. Commun. 2015;6:8635. doi: 10.1038/ncomms9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jie L, et al. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008;56:316–326. doi: 10.1111/j.1365-313X.2008.03597.x. [DOI] [PubMed] [Google Scholar]
- 16.Li J, et al. Biofortified tomatoes provide a new route to vitamin D sufficiency. Nat. Plants. 2022;8:611–616. doi: 10.1038/s41477-022-01154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nonaka S, Arai C, Takayama M, Matsukura C, Ezura H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. UK. 2017;7:7057. doi: 10.1038/s41598-017-06400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waltz E. GABA-enriched tomato is first CRISPR-edited food to enter market. Nat. Biotechnol. 2022;40:9–11. doi: 10.1038/d41587-021-00026-2. [DOI] [PubMed] [Google Scholar]
- 19.Sun CL, et al. Melatonin: a master regulator of plant development and stress responses. J. Integr. Plant Biol. 2020;63:126–145. doi: 10.1111/jipb.12993. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, et al. A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. J. Pineal res. 2016;61:138–153. doi: 10.1111/jpi.12315. [DOI] [PubMed] [Google Scholar]
- 21.Sun Q, et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015;66:657–668. doi: 10.1093/jxb/eru332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan DX, Lucien M, Eduardo EZ, Zhou Z, Russel R. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20:18886–18906. doi: 10.3390/molecules201018886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye T, et al. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography‐mass spectrometry. J. Pineal Res. 2019;66:e12531. doi: 10.1111/jpi.12531. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara T, et al. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice. J. Biol. Chem. 2010;285:11308–11313. doi: 10.1074/jbc.M109.091371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang K, Kang S, Lee K, Park M, Back K. Enzymatic features of serotonin biosynthetic enzymes and serotonin biosynthesis in plants. Plant Signal. Behav. 2008;3:389–390. doi: 10.4161/psb.3.6.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S, Kang K, Lee K, Back K. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta. 2007;227:263–272. doi: 10.1007/s00425-007-0614-z. [DOI] [PubMed] [Google Scholar]
- 27.Kang K, et al. Molecular cloning of a plant N-acetylserotonin methyltransferase and its expression characteristics in rice. J. Pineal Res. 2011;50:304–309. doi: 10.1111/j.1600-079X.2010.00841.x. [DOI] [PubMed] [Google Scholar]
- 28.Byeon Y, Lee HY, Lee K, Back K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 2014;57:219–227. doi: 10.1111/jpi.12160. [DOI] [PubMed] [Google Scholar]
- 29.Commisso M, et al. Indolamine accumulation and TDC/T5H expression profiles reveal the complex and dynamic regulation of serotonin biosynthesis in tomato (Solanum lycopersicum L.) Front. Plant Sci. 2022;13:975434. doi: 10.3389/fpls.2022.975434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang X, et al. The tryptophan decarboxylase in Solanum lycopersicum. Molecules. 2015;23:998. doi: 10.3390/molecules23050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnao MB, Hernández-Ruiz J. Growth conditions influence the melatonin content of tomato plants. Food Chem. 2013;138:1212–1214. doi: 10.1016/j.foodchem.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 32.Liu GF, et al. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022;73:5818–5827. doi: 10.1093/jxb/erac196. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka Y, et al. Loss of circadian rhythm and light-induced suppression of pineal melatonin levels in Cry1 and Cry2 double-deficient mice. Genes Cells. 2010;15:1063–1071. doi: 10.1111/j.1365-2443.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Chen Y, Zhou L, You SJ, Zhang Y. MicroTom metabolic network: rewiring tomato metabolic regulatory network throughout the growth cycle. Mol. Plant. 2020;13:1203–1218. doi: 10.1016/j.molp.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Shinozaki Y, et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018;9:364. doi: 10.1038/s41467-017-02782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong F, Wang C, Sun X, Zhang X, Liu S. Studies on the sugar metabolism mechanism of tomato fruit under red light revealed by proteomic analysis. Acta Hortic. Sin. 2018;45:1941–1951. [Google Scholar]
- 37.Panjai L, Noga G, Hunsche M, Fiebig A. Optimal red light irradiation time to increase health-promoting compounds in tomato fruit postharvest. Sci. Hortic. 2019;251:189–196. doi: 10.1016/j.scienta.2019.03.019. [DOI] [Google Scholar]
- 38.Schofield A, Paliyath G. Phytochrome regulation of carotenoid biosynthesis during ripening of tomato fruit. HortScience. 2004;39:846–847. doi: 10.21273/HORTSCI.39.4.846E. [DOI] [PubMed] [Google Scholar]
- 39.Tang J, et al. Integrated transcriptomics and metabolomics analyses reveal the molecular mechanisms of red-light on carotenoids biosynthesis in tomato fruit. Food Qual. Saf. 2022;6:1–12. [Google Scholar]
- 40.Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han X, Huang X, Deng XW. The photomorphogenic central repressor COP1: conservation and functional diversification during evolution. Plant Commun. 2020;1:100044. doi: 10.1016/j.xplc.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heng Y, et al. BBX4, a phyB-interacting and modulated regulator, directly interacts with PIF3 to fine tune red light-mediated photomorphogenesis (vol 116, pg 26049, 2019) PNAS. 2020;8:26049–26056. doi: 10.1073/pnas.1915149116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu, X. D. et al. Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in arabidopsis. Mol. Plant8, 467–478 (2015). [DOI] [PubMed]
- 44.Huq E, Quail PH. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 2002;21:2441–2450. doi: 10.1093/emboj/21.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, et al. The epidermis coordinates thermoresponsive growth through the phyB-PIF4-auxin pathway. Nat. Commun. 2020;11:1053. doi: 10.1038/s41467-020-14905-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnao MB, Hernández-Ruiz J. Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019;24:38–48. doi: 10.1016/j.tplants.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Susanne MG, et al. Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae. Biotechnol. J. 2016;11:717–724. doi: 10.1002/biot.201500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan DX, Reiter RJ. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020;71:4677–4689. doi: 10.1093/jxb/eraa235. [DOI] [PubMed] [Google Scholar]
- 49.Back K, Tan DX, Reiter RJ. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016;61:426–437. doi: 10.1111/jpi.12364. [DOI] [PubMed] [Google Scholar]
- 50.Chen, et al. Formation and change of chloroplast-located plant metabolites in response to light conditions. Int. J. Mol. Sci. 2018;19:654–657. doi: 10.3390/ijms19030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shelford T, Wallace C, Both AJ. Calculating and reporting key light ratios for plant research. Acta Hortic. 2020;1296:559–566. doi: 10.17660/ActaHortic.2020.1296.72. [DOI] [Google Scholar]
- 52.Llorente B, D’Andrea L, Rodríguez-Concepción M. Evolutionary recycling of light signaling components in fleshy fruits: new insights on the role of pigments to monitor ripening. Front. Plant Sci. 2016;7:263. doi: 10.3389/fpls.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi H, Oh E. PIF4 integrates multiple environmental and hormonal signals for plant growth regulation in arabidopsis. Mol. Cells. 2016;39:587–593. doi: 10.14348/molcells.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F, et al. Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol. J. 2020;18:1–15. doi: 10.1111/pbi.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni M, Tepperman JM, Quail PH. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature. 1999;400:781–784. doi: 10.1038/23500. [DOI] [PubMed] [Google Scholar]
- 56.Daniele R, Gramegna G, Cruz A, et al. Phytochrome interacting factors (PIFs) in a Solanum lycopersicum: diversity, evolutionary history and expression profiling during different developmental processes. PLos One. 2016;11:e0165929. doi: 10.1371/journal.pone.0165929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai SY, et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017;62:e12387. doi: 10.1111/jpi.12387. [DOI] [PubMed] [Google Scholar]
- 58.Wei Y, et al. RAV transcription factors are essential for disease resistance against cassava bacterial blight via activation of melatonin biosynthesis genes. J. Pineal Res. 2018;63:e12454. doi: 10.1111/jpi.12454. [DOI] [PubMed] [Google Scholar]
- 59.Rosado D, et al. Downregulation of PHYTOCHROME-INTERACTING FACTOR 4 influences plant development and fruit production 1. Plant Physiol. 2019;181:1360–1370. doi: 10.1104/pp.19.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong SL, et al. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat. Biotechnol. 2013;31:2462. doi: 10.1038/nbt.2462. [DOI] [PubMed] [Google Scholar]
- 61.Lü P, et al. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants. 2018;4:784–791. doi: 10.1038/s41477-018-0249-z. [DOI] [PubMed] [Google Scholar]
- 62.Fu R, Zhang P, Jin G, Wang L, Zhang Y. Versatility in acyltransferase activity completes chicoric acid biosynthesis in purple coneflower. Nat. Commun. 2021;12:1563. doi: 10.1038/s41467-021-21853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu R, et al. Substrate promiscuity of acyltransferases contributes to the diversity of hydroxycinnamic acid derivatives in purple coneflower. Plant J. 2022;118:802–813. doi: 10.1111/tpj.15704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this Article is available as a Supplementary information file. The datasets and plant materials generated and analyzed during the current study are available from the corresponding author upon request. The source data underlying Figs. 1d, 2d–g, 3c, d, 4a, b, and 6, Figs. 2a, 3b, c, 4i, and 5f, 7c, as well as Supplementary Figs. S12, S13, S15, S16, S19a, S20, S23, S24a, S25a, S26, S27 are provided as a Source Data file. Source data are provided with this paper.