Abstract
Filamentous fungi Aspergillus and Fusarium species are major causes of visual impairment and blindness in immune competent individuals. Once conidia penetrate the corneal epithelium and enter the stroma, they undergo germination, and exposure of cell wall components induces a pronounced neutrophil-rich cellular infiltrate. In this review, we discuss Aspergillus and novel Fusarium virulence factors that are required for corneal infection, and describe the multiple functions of neutrophils in limiting hyphal growth in the cornea. This review will also discuss the role of neutrophils as an important source of cytokines in fungal keratitis, and highlight recent studies identifying unique characteristics of neutrophil secretion of IL-1α and IL-1β.
Introduction — the global impact of fungal keratitis
Fungal infections of the cornea (keratitis) came to the attention of the public in the United States, Britain and Europe in 2005, when a lens care product failed to control the growth of the environmental mold Fusarium on contact lenses and lens cases, leading to an outbreak of painful and sight-threatening corneal infections in contact lens wearers [1]. Although the outbreak ended after the product was removed from the market, Fusarium and Aspergillus species remain important causes of fungal keratitis in industrialized countries, especially in hotter and more humid regions. Candida species can also cause corneal infections, but these are relatively rare and occur mostly as a consequence of contamination of donor corneas used for transplantation, or following ocular surgery [2–5].
In contrast to industrialized countries, it is in developing countries where fungal keratitis has the greatest medical and socioeconomic impact [6]. Of an estimated 1 million cases occurring annually worldwide, >80% are in developing countries [7], leading to a call to the World Health Organization to designate microbial keratitis as a neglected tropical disease in order to obtain more resources for managing this disease [8]. Corneal infections are initiated following trauma to the corneal epithelium, especially by plant material containing fungal spores; hence, the incidence of fungal keratitis is higher in agricultural regions and during harvest seasons when there is an increased likelihood of ocular trauma from airborne plant material. Fungal keratitis is most prevalent in tropical and subtropical climates, with the highest rates in Asia and Africa [9]. Studies in India and China reported that most culture-positive cases of microbial keratitis were of fungal rather than bacterial etiology, and that Aspergillus and Fusariumspecies, specifically Aspergillus fumigatus and Aspergillus flavus, and Fusarium oxysporum and Fusarium solani, were the most common causes of corneal infections [10–12].
Etiology — corneal structure and development of corneal opacification and loss of vision following fungal infection
The mammalian cornea is a unique structure which has evolved to maintain transparency in order to enable uninterrupted light passage through the lens to the retina (Figure 1a). Each of the cellular layers of the cornea have an important role in maintaining an 80% hydration level in the stroma (Figure 1b). The stratified epithelium forms tight junctions that prevent loss of water by evaporation, and the single layer of corneal endothelial cells contains a molecular pump that regulates transport of water and anions from the stroma to the anterior chamber. This hydration level is essential to maintain the anti-parallel organization of the collagen fibrils in the stroma, which ensures passage of light without diffraction. For comparison, the adjacent sclera (white) of the eye is also comprised of collagen fibrils, but in the absence corneal epithelial and endothelial cells, scleral collagens are randomly organized resulting in scleral opacity. The cornea is also avascular, and resident cells in the healthy corneal stroma comprise fibroblast-like keratocytes that produce collagen and proteoglycans, and sentinel macrophages.
Figure 1. Neutrophils are the predominant cells in corneal ulcers caused by Aspergillus.
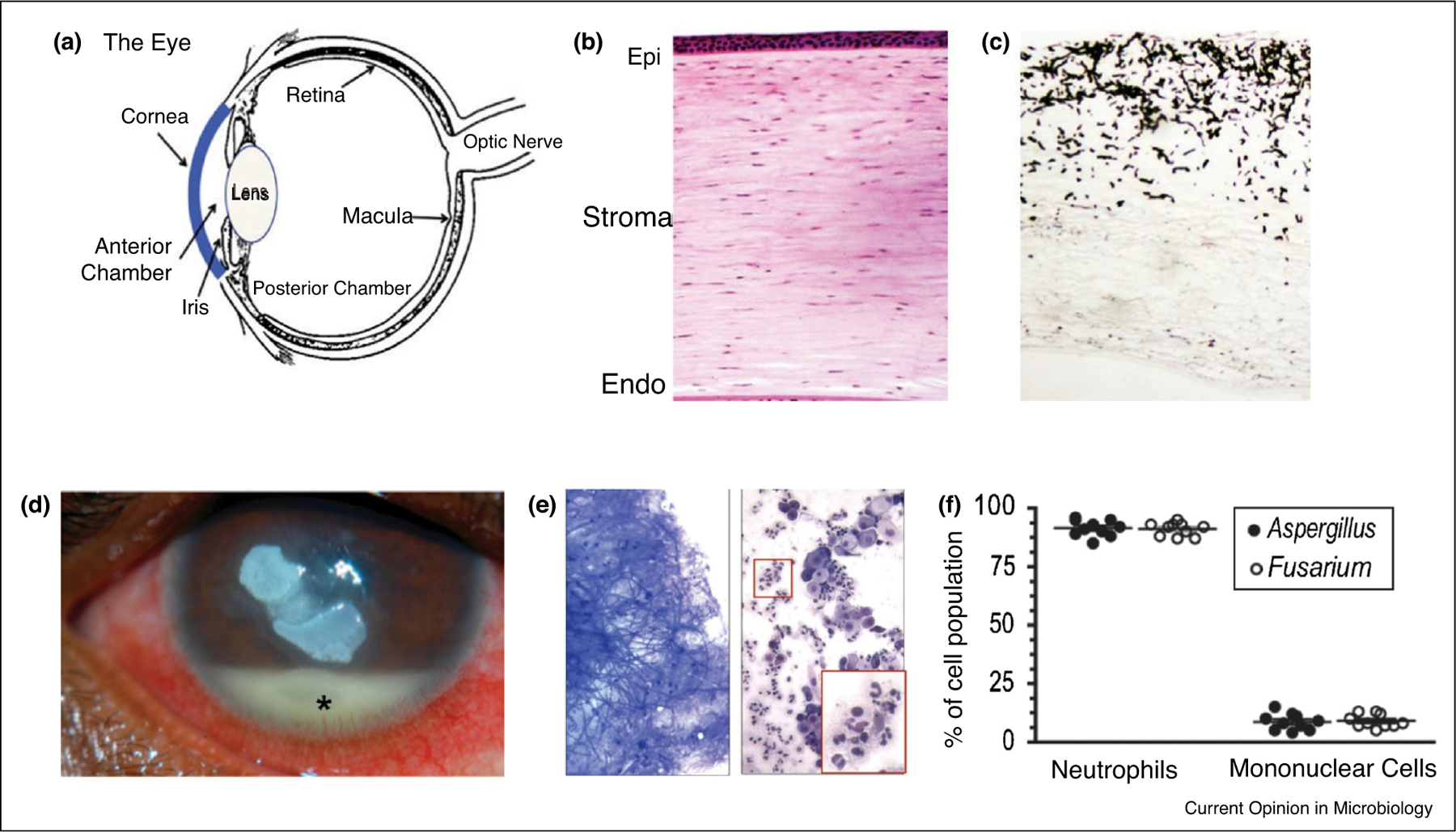
(a) The human eye.
(b) Hematoxylin & eosin (H&E)-stained section of a normal, healthy human cornea showing the epithelial, stromal, and endothelial layers that work together to maintain corneal transparency and clear vision.
(c) Gomori methenamine silver (GMS)-stained cornea from a patient infected with A. flavus keratitis following corneal transplantation.
(d) Fusarium keratitis patient showing central corneal opacification and a hypopyon in the anterior chamber, which is comprised primarily of neutrophils.
(e) Scraping from a corneal ulcer showing A. flavus hyphae (left panel) and Wrights-Giemsa stained cells (right panel). Highlighted areas show neutrophils.
(f) Percentage of neutrophils and mononuclear cells in corneal ulcers caused by Fusarium and Aspergillus (data points represent individual patients).
(a–c) reprinted from Leal and Pearlman [65], Copyright © 2012 Elsevier Inc. with permission from Elsevier. (d) Stock photo from Google Images. (e) and (f) reproduced with permission from Karthikeyan et al. [13].
While the corneal epithelium contributes to transparency, these cells are also a highly effective barrier to infection; therefore, bacterial or fungal infection of the stroma is primarily initiated when conidia penetrate the corneal epithelium and enter the stroma, most often as a consequence of ocular trauma. Following germination, hyphae invade the posterior stroma (Figure 1c), and induce inflammatory cell recruitment from peripheral, limbal capillaries to the growing hyphae.
Both hyphal growth and neutrophil infiltration to the stroma contribute to loss of corneal transparency, resulting in visual impairment and possible blindness. In contrast to systemic fungal infections, fungal keratitis patients are not immune compromised, and experimental models of disease do not require animals to be immune suppressed. Figure 1d shows corneal ulcers in a patient with Fusarium keratitis. Neutrophils comprise over 90% of the total cellular infiltrate (Figure 1e and f), although macrophages and T cells are present in later stage disease as shown in examining post-transplant corneas [13]. As collagen comprises a major component of the corneal stromal matrix, secretion of proteases from neutrophil primary granules, including MMP8/collagenase, digests the corneal stromal matrix, resulting in tissue damage and loss of corneal transparency.
Clinical — treatment of fungal ocular infections
A recent review discusses clinical aspects of disease and treatment strategies for fungal keratitis [14]. The first line of treatment for fungal keratitis is topical natamycin, which is followed by corticosteroids to suppress inflammation. While natamycin is the only antifungal medicine approved by the Food and Drug Administration (as a 5% solution), it has low water solubility and is limited in corneal penetration [14]. Other drugs that are used topically include voriconazole and amphotericin B; however, all are fungistatic and require repeated and frequent application.
Incomplete clearance of fungi before administration of corticosteroids results in rapid hyphal growth in the absence of an immune response. Despite the risk, corticosteroids are still necessary to limit the excessive inflammation that cause tissue damage. Infections that are not resolved using anti-fungal agents are treated by corneal transplantation (keratoplasty). Failure to control the infection can also result in hyphal penetration invasion of the anterior chamber and the posterior of the eye. The resulting endophthalmitis is treated with systemic anti-fungal agents, but if unsuccessful, may require enucleation.
Because of the importance of balancing fungal killing with limiting tissue damage, there is an unmet need for a better understanding of the inflammatory responses in the corneas, which will enable development of alternate approaches to treat fungal keratitis.
Pathogenesis
The dual role of neutrophils in fungal keratitis
Disease outcomes in fungal infections depend on both the virulence of the organisms and on the host immune response, and this section of the review will discuss Aspergillus and Fusarium virulence factors in relation to corneal disease. In terms of the host innate immune response fungal keratitis, we showed that resident corneal macrophages have an important role in initial recognition of hyphae in the cornea, resulting in chemokine secretion that recruits neutrophils [15]. However, neutrophils are the critical cells that are required for fungal killing in the cornea, and release of granule proteases leads to collagen digestion. Neutrophil recruitment to infected corneas requires production of CXC chemokines and expression of CD11b/CD18 (CR3, Mac1) for transendothelial migration, and neutrophils deficient in CXCR2 or CD18−/− are unable to infiltrate the corneal stroma, resulting in impaired fungal clearance and at early stages of disease, decreased corneal opacity [15].
The role of pathogen recognition receptors in fungal keratitis
C-type lectin receptors (CLR) and Toll like receptors (TLR)
Immune cell recognition of invasive fungi by TLRs and CLRs is a critical first step required for fungal clearance, (recently reviewed in Refs. [16,17]). C-type lectin receptors (CLR) on macrophages recognize fungal cell wall components, as Dectin-1 (Clec7a) recognizes β-glucan, and Dectin-2 (Clec6a in humans, Clec4n in mice), which functions as a heterodimer with Dectin-3 (Clec4d) to recognize and respond to cell wall α-mannan [17–19]. Patients with mutations in Dectin-1 or CARD9 exhibit increased susceptibility to mucocutaneous candidiasis (reviewed in Ref. [20]). Dectin-1 is also required for neutrophil recruitment and fungal killing in A. fumigatus infected corneas, and is expressed on resident corneal macrophages [15]. However, neutrophil recognition of β-glucan in the fungal cell wall of Candida albicans appears to depend on the lectin binding site of CR3 (CD11a/CD18) rather than Dectin-1 [21●]. In response to A. fumigatus or isolated β-glucan (curdlan), CR3 was also required to induce ROS production, neutrophil extracellular trap formation (NETosis), and hyphal killing [22●,23].
In addition to CLRs, Toll Like Receptor (TLR)-2 and TLR4 recognize fungal cell wall components and regulate the host response to Candida and Aspergillus. TLR2 and TLR4 gene expression was also elevated in patients with corneal ulcers caused by Fusarium and Aspergillus [13]; however, using murine models of fungal keratitis, we demonstrated that TLR4, but not TLR2 contributed to the hyphal killing and corneal opacification [24,25]. TLR4 is also important for fungal recognition by resident stromal macrophages, which secrete chemotactic and pro-inflammatory cytokines and recruit neutrophils to the site of infection [15].
Masking of CLR recognition by conidia hydrophobin
The hydrophobin rodlet (RodA) protein of conidia is also an important virulence factor in filamentous fungi as it provides a protective covering from environmental factors. Latge and colleagues generated a ΔrodA mutant strain of A. fumigatus, and showed that in the absence of this protein, conidia cell wall components are recognized by dendritic cells in the lungs, leading to more rapid clearance of the organisms and less disease [26]. Using the ΔrodA A. fumigatus mutant or hydrofluoric acid treated A. fumigatus and F. oxysporum, we found that the rodlet protein was essential for recognition by Dectin-1 and Dectin-2 on macrophages [27]. Corneas infected with the ΔrodA mutant induced a more rapid neutrophil infiltration and enhanced clearance of hyphae, which was also dependent on these CLRs. These studies therefore identified a role for the RodA hydrophobin in masking recognition of A. fumigatus conidia and delaying the host response required for Dectin-1 and Dectin-2 dependent fungal killing in infected corneas.
Oxidative and non-oxidative neutrophil inhibitors of hyphal growth in infected corneas
Following recognition of cell wall components, neutrophils employ multiple oxidative and non-oxidative anti-microbial mechanisms that limit hyphal growth and subsequent disease severity in infected corneas. Conversely, Aspergillus and Fusarium express proteins and small molecules that function as virulence factors that counter the host response. In this section, we will describe the role of neutrophil mediators and fungal virulence factors in relation to corneal infections. NADPH-oxidase dependent anti-microbial activity by neutrophils has been well documented; however, neutrophils also have multiple non-oxidative mechanisms that limit microbial growth. These include production of mediators that compete with microbes to acquire essential metal ions including iron, zinc and manganese, and is also termed nutritional immunity [28]. The role of Aspergillus chitinases and the rodlet hydophobin are also discussed.
Neutrophil production of reactive oxygen species and fungal antioxidant factors
Reactive oxygen species (ROS) generated by neutrophils are countered by antioxidants produced by the fungi. The neutrophil NADPH oxidase system generates singlet oxygen O2−, hydrogen peroxide H2O2, and hypochlorous acid (HOCl, following activation of myeloperoxidase, recently reviewed in Refs. [29]). Patients with mutations in NADPH oxidase subunits (especially GP91) exhibit chronic granulomatous disease (CGD) as a consequence of impaired ROS generation by neutrophils, with one of hallmarks being recurrent mucocutaneous fungal infections [30]. Although there are no peer reviewed studies examining fungal keratitis in CGD patients, CGD (GP91−/−) mice exhibit impaired NADPH oxidase activity and reduced ROS production leading increased hyphal growth in the cornea and development of more severe disease [22●]. That study also found no contribution of reactive nitrogen species or myeloperoxidase.
A. fumigatus counters neutrophil ROS by producing catalases and superoxide dismutase that are regulated by the Yap1 transcription factor. We reported that Δyap1 and Δsod1/2/3 mutants were more susceptible to neutrophil killing in vitro and in infected corneas [22●], demonstrating the importance of these antioxidant mediators in regulating hyphal growth in the cornea. In contrast, whereas mycotoxins including gliotoxin also have antioxidant activity, mycotoxin mutants caused the same level of disease as wild type Aspergillus and Fusarium [22●], indicating that the antioxidant activity of mycotoxins is not required for survival in this model of fungal infection.
In addition to Yap1, Latge et al. reported that Protein phosphatase Z (Ppz), which has an important role in tolerance of oxidative stress in yeast and in filamentous fungi [31], and Δppz mutants have reduced A. fumigatus survival and growth in infected corneas [32].
Nutritional immunity — targeting iron and zinc acquisition in A. fumigatus keratitis
Iron acquisition is essential for the redox reactions associated with the thioredoxin pathway, and iron uptake by hyphae is mediated by siderophores. Iron acquisition in A. fumigatus involves activation of the HapX transcription factor that is activated under low iron conditions to activate the SidA-F siderophore genes. Neutrophils secrete the iron binding proteins transferrin, lipocalin and lactoferrin, and we reported that mutants of HapX and sidF, which regulate production of Aspergillus and Fusarium extracellular siderophores, were more effectively killed by neutrophils and exhibited reduced growth in the cornea, thereby implicating an important role for extracellular rather than intracellular siderophores [33●●]. That study also showed that Aspergillus and Fusarium hyphal growth in the cornea is impaired following topical administration of the iron scavengers simvastatin or deferiprone; conversely, topical application of deferoxamine as a source of free iron resulted in enhanced hyphal growth in the cornea. Collectively these studies demonstrated that hyphal growth in infected corneas can be regulated manipulating the amount of free iron at the site of hyphal growth [33●●].
In addition to iron, zinc is also required for hyphal growth as it is a critical component of multiple enzymes and transcription factors. Zinc uptake by A. fumigatus is regulated by ZafA, which encodes a series of zinc transporters required for hyphal growth [34]. We also reported that ZafA mutants exhibited impaired hyphal growth in the cornea and consequently caused less disease [35●]. To compete with microbial zinc transporters, neutrophils secrete the heterodimeric protein S100A8/A9 (calprotectin), which binds zinc and manganese and comprises 40% of total protein in the neutrophil cytosol [36]. A. fumigatus ZafA mutants exhibited impaired hyphal growth and less corneal disease; consistent with the requirement for neutrophils to compete for zinc, infected mice deficient in S100A9 were unable to regulate hyphal growth in infected corneas [35●]. Targeting this pathway using recombinant calprotectin or atovaquone successfully inhibited hyphal growth in the cornea [35●,37]. However, there was no apparent role for S100A8/A9 in a murine model of pulmonary aspergillosis, indicative of either a tissue-specific response or a selective role for S100A8/A9 on hyphae compared with conidia [35●].
There are fewer reports of Fusarium virulence factors compared with Aspergillus, but Ma et al. identified novel mobile, lineage-specific chromosomes in F. oxysporum that are transferred horizontally and which convey pathogenicity in plants [38●●]. More recently, these lineage-specific chromosomes were found in clinical isolates from a cancer patient with a systemic Fusarium infection, and from a fungal keratitis isolate [39]. Among the genes identified in these chromosomes are metal ion transporters, which are likely important in evading nutritional immunity.
Neutrophil acidic mammalian chitinase regulates A. fumigatus keratitis
Another anti-fungal mechanism of neutrophils is acidic mammalian chitinase (AMCase). Although mammalians do not produce chitin, macrophages are a source of AMCase in the lungs asthma [40]; however, we and others demonstrated that it is also produced by neutrophils [41,42], and we showed that AMCase−/− neutrophils were less able to kill A. fumigatus in vitro; consistent with this finding, AMCase−/− mice exhibited an impaired ability to clear hyphae in infected corneas [42]. A. fumigatus expresses eight chitin synthase genes (CSM A-G) with 4 genes in each of the two families that are required for chitin synthesis [43]. Using mutants with deletions in each of the chitin synthase families, hyphal growth in the cornea was found to depend on one family (CSMA/B/D/F) [42].
Neutrophil extracellular traps (NETs) are generated in A. fumigatus infected corneas, but are not required for fungal killing
Neutrophil extracellular trap formation is a coordinated form of neutrophil cell death, first described by Zychlinsky and colleagues, in which genomic DNA is released from neutrophils to trap and mediate killing of pathogenic bacteria [44●●]. The molecular events in this process involve production of reactive oxygen species and mobilization of neutrophil elastase into the nucleus, which leads to chromatin decondensation. NETosis also involves activation of protein deiminase 4 (PAD4) in the nucleus, which converts positively charged arginine residues to neutral citrullines, thereby regulating histone citrullination and contributing to chromatin decondensation and nuclear swelling [45,46]. Candida, Aspergillus, and Fusarium species also induce NETosis, and proteomic analysis revealed that NETs induced by Candida contain histones, calprotectin S100A12, and granule proteins [47,48]. Therefore, in addition to having a role in microbial killing, cytosolic and nuclear products that are present in NETs also cause tissue damage.
NETosis is also induced by A. fumigatus and by particulate β-glucan (curdlan); further, NETosis of murine and human neutrophils was mediated by CR3 rather than Dectin-1, and was completely dependent on production of ROS. PAD4 was required for NETosis as shown using either PAD4 inhibitors or neutrophils from PAD4−/− mice [23], thereby demonstrating a requirement for PAD4 in NET formation. NETs were also present in A. fumigatus infected corneas; however, there was no significant difference between infected PAD4−/− and WT mice in either hyphal killing, cellular infiltration to the cornea, or the severity of corneal opacification [23]. The authors concluded that PAD4 — dependent NETosis does not regulate hyphal growth in the cornea, and that other neutrophil effector mechanisms can compensate for the role of NETs in these experimental conditions.
Cytokine secretion by neutrophils in fungal keratitis
Neutrophils are not traditionally considered a major source of cytokines as they are perceived to be short-lived effector cells. However, pulse-chase experiments revealed that the human neutrophil lifespan in circulation is approximately five days [49]. Further, multiple studies have demonstrated that neutrophils produce high levels of pro-inflammatory and chemotactic cytokines (reviewed in Ref. [50]). Although macrophages produce more cytokines in vitro than neutrophils on a per cell basis, neutrophils are recruited to acutely infected tissues at significantly higher numbers, and are therefore an important source of cytokines, especially in the acute phase of infections when neutrophils greatly outnumber other myeloid cells in infected tissues [13,51].
Neutrophils as a source of pro-inflammatory and chemotactic cytokines in corneal ulcers of infected patients
Studies from the Aravind Eye Hospital in southern India demonstrated that the predominant cells in corneal ulcers caused by Aspergillus and Fusarium comprised >90% neutrophils and ~10% mononuclear cells ([13], Figure 1e and f). Similar results were found in patients with corneal ulcers caused by Pseudomonas aeruginosa or Streptococcus pneumoniae [52]. Quantitative PCR analysis of corneal ulcers showed greatly elevated expression of transcripts for IL-1β, IL-8 and IL-17A (IL-17) compared with post-autopsy corneas from individuals with no ocular diseases. Given that neutrophils are the predominant cell-type in early stage corneal ulcers, they are likely the major source of the cytokine transcripts in corneal ulcers. IL-1α transcripts were also detected, although they were not elevated compared with healthy corneas [10,52], which is likely because IL-1α is an alarmin that is constitutively produced by corneal epithelial cells . There was also no difference in cellular infiltration or cytokine expression in corneal ulcer patients infected with Aspergillus compared with Fusarium species, indicating that both pathogens induce similar innate immune responses.
IL-1β processing and secretion by neutrophils compared with macrophages
IL-1 family members are not secreted through the canonical Golgi-ER pathway, and given the importance of these cytokines in inflammation and infections, identifying the mechanisms underlying secretion of these cytokines continues to be an important area of research. Regulation and secretion of IL-1β by murine macrophages has been described as a two-step process that requires initial priming through PRRs such as TLR4, leading to the production of the 31 kD pro-IL-1β, which is not bioactive (Figure 2, left panel). In this canonical pathway, a second signal such as ATP induces K+ release through P2X7R and mediates assembly of the multi-protein NLRP3 inflammasome complex, resulting in caspase-1 activation and cleavage of pro-IL-1β to the bioactive IL-1β . Caspase-1 also cleaves the pore forming protein Gasdermin D (GSDMD) to generate N-GSDMD, which inserts into the inner leaflet of the plasma membrane and forms pores through which bioactive IL-1β is secreted following pyroptotic cell death (reviewed in Ref. [53]).
Figure 2.
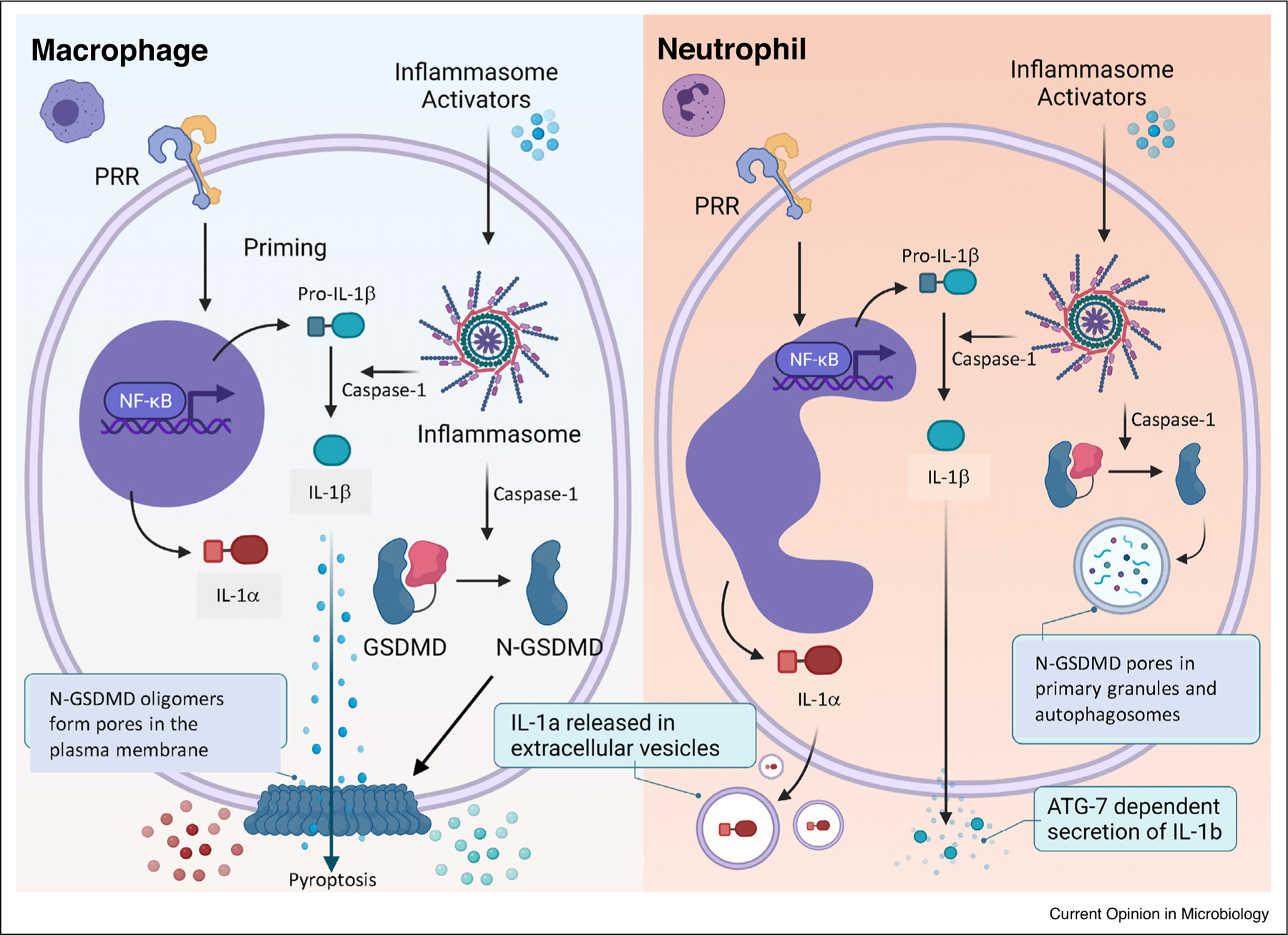
IL-1α and IL-1β secretion by neutrophils compared with macrophages. See text for details.
In contrast to macrophages, IL-1β processing and secretion by neutrophils does not result in pyroptosis primarily because N-GSDMD does not insert into the plasma membrane, but instead is detected in the membranes of neutrophil granules and autophagosomes, which therefore act as a GSDMD sink so there is not enough N-GSDMD to also reach the plasma membrane (Figure 2, right panel) [54●●]. Also, pore formation in primary granules releases neutrophil elastase into the cytosol, which also cleaves pro-GSDMD to a pore-forming 28 kD N-GSDMD in a feed forward mechanism [54●●]. That study also reported that the autophagy protein ATG7 is required for IL-1β secretion by neutrophils, although the mechanism has yet to be determined.
IL-1β processing by neutrophils in fungal keratitis
Our earlier studies showed that IL-1β is elevated during A. fumigatus and F. oxysporum keratitis, and that IL-1R1 deficient mice exhibit an impaired ability to clear the infection [15,24]. More recently, we reported significantly higher fungal load in infected IL-1β deficient compared with wild type mice [55]. To determine if neutrophils are a source of IL-1β, corneas from A. fumigatus infected mice were digested, and total cells were examined by flow cytometry. Neutrophils comprise >80% total cellular infiltrate in the early stage of infection with A. fumigatus (monocytes comprise ~20%), and were the predominant source of IL-1β [55].
Although the canonical pathway of IL-1β cleavage in macrophages involves two steps as shown in Figure 2, macrophages incubated with C. albicans yeast or A. fumigatus conidia in the absence of a separate priming stimulus produced cleaved IL-1β, indicating that yeast and conidia provide both the priming and inflammasome assembly signals [56,57]. Those reports also showed that downstream of Dectin-1, caspase-8 rather than caspase-1 mediates IL-1β processing. Our studies also found that priming was not required for IL-1β cleavage and secretion by neutrophils incubated with live A. fumigatus conidia; however, in addition to identified a role for NLRP3 and caspase-1 in IL-1β cleavage and corneal disease, there was also an unexpected role for caspase-11 in neutrophil IL-1β processing and in hyphal growth in infected corneas [55].
There are very few studies on the role of GSDMD in response to pathogenic fungi. Kanneganti et al. showed that C. albicans and A. fumigatus induce GSDMD cleavage in macrophages from immunocompetent C57BL/6, but not from caspase-1/11 mice, and that GSDMD processing is associated with IL-18 secretion [58]. We also reported that fungal cell wall β -glucan induces GSDMD cleavage in macrophages, dendritic cells and neutrophils, and that IL-1β secretion by neutrophils is inhibited in the absence of GSDMD [59]. The role of GSDMD in neutrophil regulation of fungal keratitis has yet to be determined.
Neutrophil secretion of IL-1α in response to A. fumigatus
Although IL-1α and IL-1β both function by activating IL-1R1, IL-1α has non-redundant activity as IL-1α depletion or genetic deletion results in impaired neutrophil recruitment in murine models of A. fumigatus induced peritonitis, and in pulmonary aspergillosis, especially following infection with more virulent strains of Aspergillus fumigatus [59–61].
In contrast to IL-1β, IL-1α cleavage is not required for bioactivity; thus secretion of either the pro- or the calpain - cleaved form leads to IL-1R1 activation [62]. IL-1α functions as an alarmin that is released by corneal epithelial cells and fibroblasts during sterile inflammation, and is also produced by dendritic cells and macrophages following stimulation with microbial products [63,64]. We recently demonstrated that IL-1α secretion by macrophages and dendritic cells in response to LPS/ATP was dependent on NLRP3 and GSDMD, indicating that as with IL-1β, IL-1α secretion by these cells occurs following pyroptosis.
In that study, we reported that neutrophils are also a source of IL-1α following stimulation with β-glucan, that secretion was independent of NLRP3 and GSDMD, and that neutrophils do not undergo pyroptotic cell death [59]. Instead, neutrophils were found to secrete bioactive IL-1α through release of extracellular vesicles, specifically exosomes (Figure 2). One implication of these findings is that in the absence of pyroptotic cell death, neutrophils at the site of fungal infection will continuously secrete IL-1α and IL-1β, thereby playing an important regulatory role in the outcome of infection.
Conclusions
There have been multiple advances in our understanding of the role of virulence factors and in innate immune responses to these pathogenic fungi in blinding corneal infections. A unique aspect of fungal keratitis is that Fusarium is a major cause of this disease. Although the host response to Fusarium appears to be similar to the immune response to Aspergillus, future studies will identify novel Fusarium virulence factors, including those associated with lineage-specific chromosomes. Advances described in this review also include identifying unappreciated immune regulatory activities of neutrophils as a source of the pro-inflammatory cytokines IL-1α and IL-1β. Taken together, the field is well positioned to identify novel topical anti-fungal and anti-inflammatory agents that will inhibit hyphal growth and disease severity in the absence of systemic complications.
Acknowledgements
The authors thank our many colleagues involved in the studies described in this review, especially Dr Lalitha Prajna at the Aravind Eye Institute in India, and our long-term collaborator Dr Jean-Paul Latge at the Institut Pasteur, who generated and sent us mutant strains of A. fumigatus.
This work was supported by R01 EY18612 (EP) andF31 EY032312 (BR). The authors acknowledge departmental support from an unrestricted grant to the Department of Ophthalmology from the Research to Prevent Blindness Foundation, New York, NY.
Footnotes
Conflict of interest statement
The authors declare that there is no conflict of interest with the studies presented in this review. BR and EP contributed equally to the writing.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
- 1.Chang DC, Grant GB, O’Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, Jacobson LM, Crowell CS, Sneed RS, Lewis FMT et al. : Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 2006, 296:953–963. [DOI] [PubMed] [Google Scholar]
- 2.McElnea E, Power B, Murphy C: Interface fungal keratitis after Descemet stripping automated endothelial keratoplasty: a review of the literature with a focus on outcomes. Cornea 2018, 37:1204–1211. [DOI] [PubMed] [Google Scholar]
- 3.Doshi H, Pabon S, Price MO, Feng MT, Price FW: Overview of systemic Candida infections in hospital settings and report of Candida after DMEK successfully treated with antifungals and partial graft excision. Cornea 2018, 37:1071–1074. [DOI] [PubMed] [Google Scholar]
- 4.Hajjar Sesé A, Lindegaard J, Julian HO, Højgaard-Olsen K, Møller NF, Heegaard S: A presentation of culture-positive corneal donors and the effect on clinical outcomes. Graefes Arch Clin Exp Ophthalmol 2019, 257:135–141. [DOI] [PubMed] [Google Scholar]
- 5.Watson SL, Cabrera-Aguas M, Keay L, Khoo P, McCall D, Lahra MM: The clinical and microbiological features and outcomes of fungal keratitis over 9 years in Sydney, Australia. Mycoses 2020, 63:43–51. [DOI] [PubMed] [Google Scholar]
- 6.Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J: The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol 2019, 64:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongomin F, Gago S, Oladele RO, Denning DW: Global and multinational prevalence of fungal diseases—estimate precision. J Fungi (Basel) 2017, 3. [DOI] [PMC free article] [PubMed]
- 8.Ung L, Acharya NR, Agarwal T, Alfonso EC, Bagga B, Bispo PJ, Burton MJ, Dart JK, Doan T, Fleiszig SM et al. : Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull World Health Organ 2019, 97:854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown L, Burton MJ, Gichangi M, Denning D: The Global Incidence of Fungal Keratitis Social Science Research Network; 2019. [Google Scholar]
- 10.Lalitha P, Prajna NV, Manoharan G, Srinivasan M, Mascarenhas J, Das M, D’Silva SS, Porco TC, Keenan JD: Trends in bacterial and fungal keratitis in South India, 2002–2012. Br J Ophthalmol 2015, 99:192–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Sun S, Jing Y, Han L, Zhang H, Yue J: Spectrum of fungal keratitis in central China. Clin Exp Ophthalmol 2009, 37:763–771. [DOI] [PubMed] [Google Scholar]
- 12.Manikandan P, Abdel-hadi A, Randhir Babu Singh Y, Revathi R, Anita R, Banawas S, Bin Dukhyil AA, Alshehri B, Shobana CS, Panneer Selvam K et al. : Fungal keratitis: epidemiology, rapid detection, and antifungal susceptibilities of fusarium and aspergillus isolates from corneal scrapings. BioMed Res Int 2019, 2019:e6395840. [DOI] [PMC free article] [PubMed]
- 13.Karthikeyan RS, Leal SM, Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, Lalitha P: Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis 2011, 204:942–950 10.1093/infdis/jir426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castano G, Elnahry AG, Mada PK: Fungal keratitis. StatPearls StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 15.Leal SM, Cowden S, Hsia Y-C, Ghannoum MA, Momany M, Pearlman E: Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog 2010, 6:e1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward RA, Vyas JM: The first line of defense: effector pathways of anti-fungal innate immunity. Curr Opin Microbiol 2020, 58:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obar JJ: Sensing the threat posed by Aspergillus infection. Curr Opin Microbiol 2020, 58:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L-L, Zhao X-Q, Jiang C, You Y, Chen X-P, Jiang Y-Y, Jia X-M, Lin X: C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 2013, 39:324–334. [DOI] [PubMed] [Google Scholar]
- 19.Hardison SE, Brown GD: C-type lectin receptors orchestrate anti-fungal immunity. Nat Immunol 2012, 13:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Underhill DM, Pearlman E: Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 2015, 43:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien XM, Heflin KE, Lavigne LM, Yu K, Kim M, Salomon AR, Reichner JS: Lectin site ligation of CR3 induces conformational changes and signaling. J Biol Chem 2012, 287:3337–3348 The first report showing that the CR3 integrin is a receptor for β-glucan, and was subsequently found to be a major receptor on neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leal SM, Vareechon C, Cowden S, Cobb BA, Latgé J-P, Momany M, Pearlman E: Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest 2012, 122:2482–2498 This report identifies antioxidant pathways of Fusarium and Aspergillus in the context of fungal infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark HL, Abbondante S, Minns MS, Greenberg EN, Sun Y, Pearlman E: Protein deiminase 4 and CR3 regulate Aspergillus fumigatus and β-glucan-induced neutrophil extracellular trap formation, but hyphal killing is dependent only on CR3. Front Immunol 2018, 9. [DOI] [PMC free article] [PubMed]
- 24.Tarabishy AB, Aldabagh B, Sun Y, Imamura Y, Mukherjee PK, Lass JH, Ghannoum MA, Pearlman E: MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. J Immunol 2008, 181:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Chandra J, Mukherjee P, Szczotka-Flynn L, Ghannoum MA, Pearlman E: A murine model of contact lens-associated fusarium keratitis. Invest Ophthalmol Vis Sci 2010, 51:1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV et al. : Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460:1117–1121. [DOI] [PubMed] [Google Scholar]
- 27.de Jesus Carrion S, Leal SM, Ghannoum MA, Aimanianda V, Latgé J-P, Pearlman E: The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol 2013, 191:2581–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hood MI, Skaar EP: Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 2012, 10:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban CF, Backman E: Eradicating, retaining, balancing, swarming, shuttling and dumping: a myriad of tasks for neutrophils during fungal infection. Curr Opin Microbiol 2020, 58:106–115. [DOI] [PubMed] [Google Scholar]
- 30.Barese CN, Goebel WS, Dinauer MC: Gene therapy for chronic granulomatous disease. Expert Opin Biol Ther 2004, 4:1423-1434. [DOI] [PubMed] [Google Scholar]
- 31.Leiter É, González A, Erdei É, Casado C, Kovács L, Ádám C, Oláh J, Miskei M, Molnar M, Farkas I et al. : Protein phosphatase Z modulates oxidative stress response in fungi. Fungal Genet Biol 2012, 49:708–716. [DOI] [PubMed] [Google Scholar]
- 32.Muszkieta L, de Jesus Carrion S, Robinet P, Beau R, Elbim C, Pearlman E, Latgé J-P: The protein phosphatase PhzA of A. fumigatus is involved in oxidative stress tolerance and fungal virulence. Fungal Genet Biol 2014, 66:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leal SM, Roy S, Vareechon C, de Jesus Carrion S, Clark H, Lopez-Berges MS, Di Pietro A, diPietro A, Schrettl M, Beckmann N et al. : Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog 2013, 9:e1003436 This report identifies which siderophores are required to compete with neutrophils for iron, and demonstrates that the level of free iron in the cornea regulates hyphal growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno MA, Ibrahim-Granet O, Vicentefranqueira R, Amich J, Ave P, Leal F, Latgé J-P, Calera JA: The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol Microbiol 2007, 64:1182–1197. [DOI] [PubMed] [Google Scholar]
- 35.Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E: Zinc and manganese chelation by neutrophil S100A8/A9 (Calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J Immunol 2016, 196:336–344 This report identifies differences in the role of calprotectin in A. fumigatus infected corneas and lungs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zackular JP, Chazin WJ, Skaar EP: Nutritional immunity: S100 proteins at the host-pathogen interface. J Biol Chem 2015, 290:18991–18998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark HL, Minns MS, Sun Y, de Jesus T, Ghannoum MG, Pearlman E: Atovaquone impairs growth of aspergillus and fusarium keratitis isolates by modulating mitochondrial function and zinc homeostasis. Invest Ophthalmol Vis Sci 2018, 59:1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L-J, van der Does HC, Borkovich KA, Coleman JJ, Daboussi M-J, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B et al. : Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464:367–373 In this report, Li-Jun Ma identified novel Fusarium chromosomes that confer virulence in plants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Yang H, Turra D, Zhou S, Ayhan DH, DeIulio GA, Guo L, Broz K, Wiederhold N, Coleman JJ et al. : The genome of opportunistic fungal pathogen Fusarium oxysporum carries a unique set of lineage-specific chromosomes. Commun Biol 2020, 3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Z, Zheng T, Homer RJ, Kim Y-K, Chen NY, Cohn L, Hamid Q, Elias JA: Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304:1678–1682. [DOI] [PubMed] [Google Scholar]
- 41.Żurawska-Płaksej E, Ługowska A, Hetmańczyk K, Knapik-Kordecka M, Piwowar A: Neutrophils as a source of chitinases and chitinase-like proteins in type 2 diabetes. PLoS One 2015, 10:e0141730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jesus Carrion S, Abbondante S, Clark HL, Marshall ME, Mouyna I, Beauvais A, Sun Y, Taylor PR, Leal SM, Armstrong B et al. : Aspergillus fumigatus corneal infection is regulated by chitin synthases and by neutrophil-derived acidic mammalian chitinase. Eur J Immunol 2019, 49:918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latgé J-P, Beauvais A, Chamilos G: The cell wall of the human fungal pathogen Aspergillus fumigatus: biosynthesis, organization, immune response, and virulence. Annu Rev Microbiol 2017, 71:99–116. [DOI] [PubMed] [Google Scholar]
- 44.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A: Neutrophil extracellular traps kill bacteria. Science 2004, 303:1532–1535 This is the first report describing neutrophil extracellular traps. [DOI] [PubMed] [Google Scholar]
- 45.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y: PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 2010, 207:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A: Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007, 176:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A: Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 2009, 5:e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urban CF, Nett JE: Neutrophil extracellular traps in fungal infection. Semin Cell Dev Biol 2019, 89:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolaczkowska E, Kubes P: Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013, 13:159–175. [DOI] [PubMed] [Google Scholar]
- 50.Tecchio C, Micheletti A, Cassatella MA: Neutrophil-derived cytokines: facts beyond expression. Front Immunol 2014, 5. [DOI] [PMC free article] [PubMed]
- 51.Zhang Q, Zhang J, Gong M, Pan R, Liu Y, Tao L, He K: Transcriptome analysis of the gene expression profiles associated with fungal keratitis in mice based on RNA-seq. Invest Ophthalmol Vis Sci 2020, 61:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karthikeyan RS, Priya JL, Leal SM, Toska J, Rietsch A, Prajna V, Pearlman E, Lalitha P: Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS One 2013, 8: e64867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broz P, Dixit VM: Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016, 16:407–420. [DOI] [PubMed] [Google Scholar]
- 54.Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, Rathkey JK, Abbott DW, Wang K, Shao F et al. : N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun 2020, 11:1–14 This report demonstrated distinct processing of GSDMD in neutrophils compared with macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, Abbondante S, Karmakar M, de Jesus Carrion S, Che C, Hise AG, Pearlman E: Neutrophil caspase-11 is required for cleavage of caspase-1 and secretion of IL-1β in Aspergillus fumigatus infection. J Immunol 2018, 201:2767–2775 10.4049/jimmunol.1701195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TBH: Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol 2012, 13:246–254. [DOI] [PubMed] [Google Scholar]
- 57.Ganesan S, Rathinam VAK, Bossaller L, Army K, Kaiser WJ, Mocarski ES, Dillon CP, Green DR, Mayadas TN, Levitz SM et al. : Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J Immunol 2014, 193:2519–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, Kesavardhana S, Burton A, Kanneganti T-D: ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J Biol Chem 2020, 295:18276–18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ratitong B, Marshall M, Pearlman E: β-Glucan-stimulated neutrophil secretion of IL-1α is independent of GSDMD and mediated through extracellular vesicles. Cell Rep 2021, 35:109139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A et al. : IL-1α signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog 2015, 11:e1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caffrey-Carr AK, Kowalski CH, Beattie SR, Blaseg NA, Upshaw CR, Thammahong A, Lust HE, Tang Y-W, Hohl TM, Cramer RA et al. : Interleukin 1a is critical for resistance against highly virulent Aspergillus fumigatus isolates. Infect Immun 2017, 85:e00661–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Paolo NC, Shayakhmetov DM: Interleukin 1a and the inflammatory process. Nat Immunol 2016, 17:906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griffiths JS, Camilli G, Kotowicz NK, Ho J, Richardson JP, Naglik JR: Role for IL-1 family cytokines in fungal infections. Front Microbiol 2021, 12:633047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukuda K, Ishida W, Miura Y, Kishimoto T, Fukushima A: Cytokine expression and barrier disruption in human corneal epithelial cells induced by alarmin released from necrotic cells. Jpn J Ophthalmol 2017, 61:415–422. [DOI] [PubMed] [Google Scholar]
- 65.Leal SM Jr, Pearlman E: The role of cytokines and pathogen recognition molecules in fungal keratitis — insights from human disease and animal models. Cytokine 2012, 58:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


