Abstract
The thalamus is a critical relay center for neural pathways involving sensory, motor, and cognitive functions, including cortico-striato-thalamo-cortical and cortico-ponto-cerebello-thalamo-cortical loops. Despite the importance of these circuits, their development has been understudied. One way to investigate these pathways in human development in vivo is with functional connectivity MRI, yet few studies have examined thalamo-cortical and cerebello-cortical functional connectivity in development. Here, we used resting-state functional connectivity to measure functional connectivity in the thalamus and cerebellum with previously defined cortical functional networks in 2 separate data sets of children (7–12 years old) and adults (19–40 years old). In both data sets, we found stronger functional connectivity between the ventral thalamus and the somatomotor face cortical functional network in children compared with adults, extending previous cortico-striatal functional connectivity findings. In addition, there was more cortical network integration (i.e. strongest functional connectivity with multiple networks) in the thalamus in children than in adults. We found no developmental differences in cerebello-cortical functional connectivity. Together, these results suggest different maturation patterns in cortico-striato-thalamo-cortical and cortico-ponto-cerebellar-thalamo-cortical pathways.
Keywords: brain networks, cerebellum, functional MRI, neurodevelopment, thalamus
Introduction
The thalamus is a relay and integrative center for cognitive, motor, and sensory circuitry with afferent and efferent projections to and from the cerebral cortex, other subcortical structures, and the cerebellum. In particular, the thalamus is a key node in cortico-striato-thalamo-cortical and cortico-ponto-cerebello-thalamo-cortical loops. This thalamic connectivity has been shown to be important for a wide range of cognitive, motor, and sensory behavioral functions, including regulating the planning and initiation of coordinated movements (Houk and Wise 1995; Bradshaw and Sheppard 2000), higher-level cognitive control (Botvinick and Braver 2015), lower-level motor control (Thach 2014; Peterburs and Desmond 2016), and emotional processing (Lane et al. 1997; Adamaszek et al. 2017).
Cortico-striato-thalamo-cortical loops are topographically and functionally organized, such that “motor” loops include projections between cortical regions and striatal/thalamic nuclei involved in motor functions (e.g. precentral gyrus, dorsal and lateral caudate nuclei, and ventral lateral thalamic nuclei), whereas “cognitive” loops include projections between cortical regions and striatal/thalamic nuclei involved in cognitive functions (e.g. dorsolateral prefrontal cortex, rostral and dorsal caudate nuclei, and dorsomedial nucleus of the thalamus; Alexander et al. 1986). Adjacent loops are sometimes described as separable, yet there is evidence for integration between them through various mechanisms in the thalamus and striatum (Haber et al. 2006; Haber and Calzavara 2009; Cho et al. 2015; Haber 2016; Greene et al. 2020). Functionally, integration would allow the thalamus to facilitate the transfer of information between different cortical regions and thus facilitate interactions between functional networks (Hwang et al. 2017). The cerebellum also has anatomical projections to the cortex via the thalamus (Palesi et al. 2017a, 2017b), with cortico-ponto-cerebello-thalamo-cortical circuitry that defines motor and cognitive loops (Middleton and Strick 2002; Palesi et al. 2017a, 2017b). While previously believed to run strictly parallel to the cortico-striato-thalamo-cortical loops, converging only at the cortical level, recent work supports the overlap between these cortico-ponto-cerebello-thalamo-cortical and cortico-striato-thalamo-cortical pathways at the thalamic level (Hintzen et al. 2018).
Proper development of the thalamic components of cortico-striato-thalamo-cortical and cortico-ponto-cerebello-thalamo-cortical circuits may be critical for normative cognitive and behavioral maturation. Dysfunction in thalamic pathways has been implicated in a number of neurodevelopmental disorders, such as autism spectrum disorder (Nair et al. 2013; Verly et al. 2014; Khan et al. 2015; Li and Pozzo-Miller 2020), Tourette syndrome (Makki et al. 2009; Plessen et al. 2009; Greene et al. 2017; Coulombe et al. 2018; Ramkiran et al. 2019), obsessive–compulsive disorder (Gilbert et al. 2000; Rosenberg et al. 2000; Friedlander and Desrocher 2006; Xu et al. 2019), and schizophrenia (Frazier et al. 1996; James et al. 2004; Huang et al. 2021; Weng et al. 2022). However, typical maturation of cortico-striato-thalamo-cortical and cortico-ponto-cerebello-thalamo-cortical circuits has been significantly less well studied. Research involving cortico-striato-thalamo-cortical loop maturation has focused mostly on cortico-striatal connectivity (Christakou et al. 2011; Greene et al. 2014; Walhovd et al. 2015; Insel et al. 2017; Li and Pozzo-Miller 2020). Findings from human neuroimaging studies investigating the development of thalamo-cortical connections are inconsistent, reporting both age-related increases and decreases in connectivity between the thalamus and frontal and sensorimotor regions in infancy, childhood, and adolescence (Fair et al. 2010; Alkonyi et al. 2011; Alcauter et al. 2014; Steiner et al. 2020; Huang et al. 2021). Furthermore, while studies in adults have identified regions within the thalamus where multiple cortical networks converge and may integrate (measured as spatial overlap of connectivity at the voxel level; Yuan et al. 2016; Hwang et al. 2017; Greene et al. 2020), the development of this integration has not yet been investigated. The typical development of cerebello-cortical connectivity is even less well investigated. There is evidence that the topography of cerebello-cortical connectivity is present in infants and young children (Wang et al. 2016; Herzmann et al. 2019), and for developmental change in cerebello-prefrontal connectivity over 1 year in adolescents and young adults (Bernard et al. 2016). However, studies of cerebello-cortical connectivity in the context of cortical functional network maturation are still lacking.
Previously, our lab investigated the development of cortico-striatal connectivity using resting-state functional connectivity (RSFC) MRI and a network-level approach (Greene et al. 2014). While RSFC patterns were broadly similar in children and adults, we observed stronger functional connectivity (FC) between the somatomotor face (SMF) network and the posterior putamen in children than in adults, which decreased with age and stabilized by adulthood. However, it is not clear if these age-related changes also propagate through the thalamo-cortical elements of the cortico-striato-thalamo-cortical circuits, or whether similar effects may also be observed in cortico-ponto-cerebello-thalamo-cortical circuits. In addition, how cortico-subcortical development leads to mature integration of multiple cortical networks in the thalamus is unknown.
Here, we used RSFC and a functional network approach to investigate the development of FC between the thalamus and the cortex and between the cerebellum and the cortex in 2 separate data sets collected at Washington University in St. Louis and Oregon Health and Science University. Additionally, we investigated how developmental differences in FC might interact with network integration in the thalamus. To preview our results, we found similar functional network topography in children and adults in both the thalamus and the cerebellum. In the thalamus, we found stronger SMF network FC in children compared with adults, which decreased throughout childhood and stabilized in adulthood. Additionally, we found evidence of increased thalamic integration involving the SMF network in children compared with adults. However, in the cerebellum, we found no developmental changes in functional network connectivity. Together, these results suggest a pervasive developmental change in the SMF motor pathway specifically within the cortico-striato-thalamo-cortical pathway that does not extend to the SMF network cortico-ponto-cerebello-thalamo-cortical loop.
Materials and methods
Participants
Two data sets were used for the present analyses. The “WashU” data set, collected at Washington University in St. Louis School of Medicine and previously described (Greene et al. 2014), included 120 typical young adults (19–31 years old; 60 males) and 60 typically developing children (7–12 years old; 33 males). The “OHSU” data set, collected at Oregon Health & Science University, included 57 typical young adults (19–40 years old; 19 males) and 110 typically developing children (7–11 years old; 52 males) who participated as control subjects in ADHD-focused studies (Karalunas et al. 2014; Cary et al. 2016).
All participants were native English speakers and right-handed. Participants reported no history of neurological or psychiatric disease and were not taking psychoactive medications, as assessed by self-report for adults and parental report for children. All adults and a parent/guardian for each child gave informed consent, and all children assented. The Washington University Human Research Protection Office (WashU data set) and Oregon Health & Science University Institutional Review Board (OHSU data set) approved all studies.
Image acquisition
WashU data set
MRI data were acquired on a Siemens 3T Trio scanner with a Siemens 12-channel Head Matrix Coil located in the Mallinckrodt Institute of Radiology at Washington University School of Medicine. A T1-weighted sagittal MP-RAGE structural image (slice time echo 3.06 ms; TR 2.4 s, inversion time 1 s, flip angle 8 degrees, 127 slices, 1 mm isotropic voxels) was acquired from each participant, while they watched a movie or listened to music of their choice. Resting-state functional images were acquired using a BOLD contrast-sensitive echo planar sequence (TE 27 ms, flip angle 90 degrees, in-plane resolution 4 × 4 mm, 32 contiguous interleaved 4 mm axial slices, TR 2.5 s for 177 participants; TR 2.2 s for 3 child participants). A total of 336 ± 121 functional volumes (range 184–724) were acquired from the adults, and 377 ± 166 functional volumes (range 260–780) were acquired from the children. Of these volumes, after motion censoring (see below), 278 ± 10 functional volumes (range 151–719) were included from the adults, and 266 ± 13 functional volumes (range 137–623) were included from the children. During the resting-state scans, participants were asked to relax and hold as still as possible while viewing a white crosshair in the center of the screen on a black background.
OHSU data set
MRI data were acquired on a Siemens 3T Trio scanner with a Siemens 12-channel Head Matrix Coil, located at OHSU’s Advanced Imaging Research Center. A T1-weighted sagittal MP-RAGE structural image (slice time echo 3.58 ms; TR 2.3 s, inversion time 0.9 s, flip angle 10 degrees, 160 slices, 1 mm isotropic voxels) was acquired from each participant, during which they watched a movie or listened to music of their choice. Resting-state functional images were acquired using a BOLD contrast-sensitive echo planar sequence (TE 30 ms, flip angle 90 degrees, resolution 3.75 × 3.75 × 3.8 mm, 36 contiguous interleaved 3.8 mm axial slices, TR 2.5 s). A total of 464 ± 17 functional volumes (range 300–600) were acquired from the adults, and 337 ± 4 functional volumes (range 246–360) were acquired from the children. Of these volumes, after motion censoring, 352 ± 22 functional volumes (range 23–594) were included from the adults, and 248 ± 6 functional volumes (range 121–354) were included from the children. During the resting-state scans, participants were asked to relax and hold as still as possible while viewing white crosshair in the center of the screen on a black background.
Image preprocessing
Functional data from participants from both data sets were preprocessed using methods described in Shulman et al. (2010) and Miezin et al. (2000) to reduce artifacts. Image preprocessing included: (i) sinc interpolation of all slices to the temporal midpoint of the first slice, accounting for differences in the acquisition time of each slice, (ii) correction for head movement within and across runs, and (iii) intensity normalization to a whole-brain mode value (across voxels and TRs) of 1,000 for each run. Atlas transformation of the functional images was computed for each participant via the MP-RAGE T1-weighted scan. Each run was resampled in atlas space on an isotropic 3 mm grid combining movement correction and atlas transformation in a single interpolation. The atlas used in this study (TRIO_KY_NDC) was generated from MP-RAGE T1-weighted images from 13 7–9-year-old children (7 male) and 13 21–30-year-old adults (6 male), and made to conform to the Talairach atlas space as outlined in previous work (Lancaster et al. 1995; Burgund et al. 2002; Black et al. 2004; Greene et al. 2014). The images used to generate the atlas were collected on the same MRI scanner as the WashU data set included in this study.
FC preprocessing
Additional preprocessing for RSFC data was conducted on each of the data sets following the procedures in Greene et al. (2014). These steps included: (i) demeaning and detrending, (ii) multiple regression of nuisance variables from the BOLD data, including motion regressors individualized ventricular and white matter signals, and the derivatives of these signals, and (iii) temporal band-pass filtering (0.009 Hz < f < 0.08 Hz). Consistent with Greene et al. (2014), the global signal was not included as a nuisance regressor because the partial correlation approach used in our analyses (outlined below) accounts for the shared variance among cortical networks.
Artifacts because of head motion were accounted for using the volume censoring procedure described in (Power et al. 2012, 2014). Briefly, high motion volumes were flagged for censoring based on frame-wise displacement greater than a threshold of 0.2 mm. Volumes were included (not censored) only in temporally contiguous sets of at least 5 volumes, and BOLD runs were included only if they retained a minimum of 30 such volumes. The flagged data were then processed following the same procedure previously described (Greene et al. 2014).
Anatomical definitions
Thalamus and basal ganglia segmentation was performed using FSL 4.1.8 [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library]. FMRIB-integrated registration and segmentation tool was applied to the T1 structural images from the WashU data set, delineating the caudate nucleus, putamen, pallidum, nucleus accumbens, and thalamus. For each structure, voxels labeled as belonging to that structure in 75% of the participants were included in a group mask of the structure, as in Greene et al. (2014), as this mask best overlaps with the boundaries of the subcortical structures on the anatomical atlas used for registration. All correlation analyses were conducted within these voxels.
To create a cerebellum mask, we identified voxels that contained BOLD data and overlapped with a previously defined mask (Buckner et al. 2011). Participants were selected on the basis of having BOLD data coverage of the cerebellum above z = −43 (WashU: adults, n = 41; children, n = 41; OHSU: adults, n = 57; children, n = 110).
Cortical networks were defined as in Greene et al. (2014) to encompass most cortical regions with known/suspected anatomical connections to the subcortex and cerebellum (Alexander et al. 1986; Haber and Knutson 2010), based on Power et al. (2011) and (2010). Eleven cortical networks were included: sensorimotor networks: somatomotor hand (SMH), SMF, auditory (AUD), and visual (VIS); attention/control networks: dorsal attention (DAN), ventral attention (VAN), cingulo-opercular (CON), salience (SAL), and frontoparietal (FPN); the default mode network (DMN); and the orbitofrontal network (OFN; Supplementary Fig. 1).
FC analyses
Partial correlation analyses
In each of the structures (thalamus, basal ganglia, and cerebellum), partial correlations were calculated between the BOLD time series from each voxel within that structure and the mean BOLD time series from each of the 11 cortical networks (i.e. a residual correlation was computed after partialling out the signal from the other cortical networks). The partial correlations were then normalized using Fisher’s r-to-z transform, and averaged across the participants in each group (e.g. 120 adults and 60 children from the WashU data set; 57 adults and 110 children from the OHSU data set). Only positive correlations were used for subsequent group and winner-take-all analyses. To identify voxels with significant correlations to a given network, we computed 1 sample t-tests for each voxel and converted the t statistics to z-scores. We also quantified the similarity between children and adults by computing the Spearman correlation between the partial correlation values for each thalamic and cerebellar voxel in children and adults, separately for each network.
Regressing out cortical signal adjacent to the structure of interest
To account for possible signal bleed between subcortical or cerebellar structures and the cortical networks, we regressed out proximal cortical signal (as in Buckner et al. 2011; Choi et al. 2012; Greene et al. 2014). We identified voxels in the cortical networks of interest that were spatially located within 5 mm of the subcortical structure mask and the cerebellum mask, and regressed out the mean signal of those overlapping voxels (i.e. cortical voxels proximal to the subcortical structure).
Group comparisons
To compare the adults and children in each data set, 2-sample t-tests (accounting for unequal group variance) were computed on the Fisher z-transformed r-values for each cortical network within the thalamus and cerebellum, correcting for multiple comparisons across networks using false discovery rate (FDR; P 0.05) and a minimum cluster size of 10 voxels.
Network specificity and integration
We implemented a modified winner-take-all approach to characterize the functional organization of the thalamus, as in Greene et al. (2020). This analysis was not conducted in the cerebellum given the high degree of network specificity previously demonstrated (Marek et al. 2018). We averaged the partial correlation values between each thalamic voxel and each cortical network across the participants in the 4 groups (WashU adults, WashU children, OHSU adults, and OHSU children). We then identified the cortical network with which each thalamic voxel was most highly correlated based on the averaged partial correlation values, and designated that network as the “winning” network. Next, we compared the partial correlations of the winning network with the 10 remaining networks in each voxel. If the partial correlation value of at least 1 other network was within 2/3 (66.7%) of the winning network’s partial correlation value, that voxel was categorized as “integrative,” having its strongest correlations with multiple networks. If the partial correlation values with the remaining 10 networks were all <2/3 of the winning network’s correlation, that voxel was categorized as “network-specific,” having its strongest correlation with a single network. This procedure determined integrative voxels affiliated with multiple networks (minimum 2 networks; maximum 3 networks) and network-specific voxels affiliated with only 1 network. We previously validated this procedure with alternative procedures (e.g. effect sizes) and thresholds for labeling voxels as integrative or network-specific (Greene et al. 2020). Modified winner-take-all maps were generated by color coding the voxels within the thalamus according to the winning network(s), with integrative voxels denoted by colored stripes representing the multiple networks.
Age-related differences in integration
To determine whether integration varied across development, we used 2-sample t-tests to compare the number of integrative voxels between child and adult subjects in each data set. To preview results, we observed significant decreases in both SMF FC strength (as calculated above) and integration in adults compared with children. Given that the determination of a voxel as integrative depended in part on the strength of SMF FC, we tested whether the difference in integration was explained by SMF FC by entering categorical age group and SMF FC into an ANCOVA model explaining the number of integrative voxels.
Results
Children and adults show similar topography in cortico-thalamic FC
Group-averaged partial correlation maps for each of the 11 cortical networks from each data set are displayed in Fig. 1. Qualitatively, children and adults showed similar spatial patterns of FC. Quantitatively, the average Spearman correlation in thalamic partial correlation values between children and adults across all networks was 0.66 (WashU data set) and 0.70 (OHSU data set; correlations by network are listed in Supplementary Table 1).
Fig. 1.
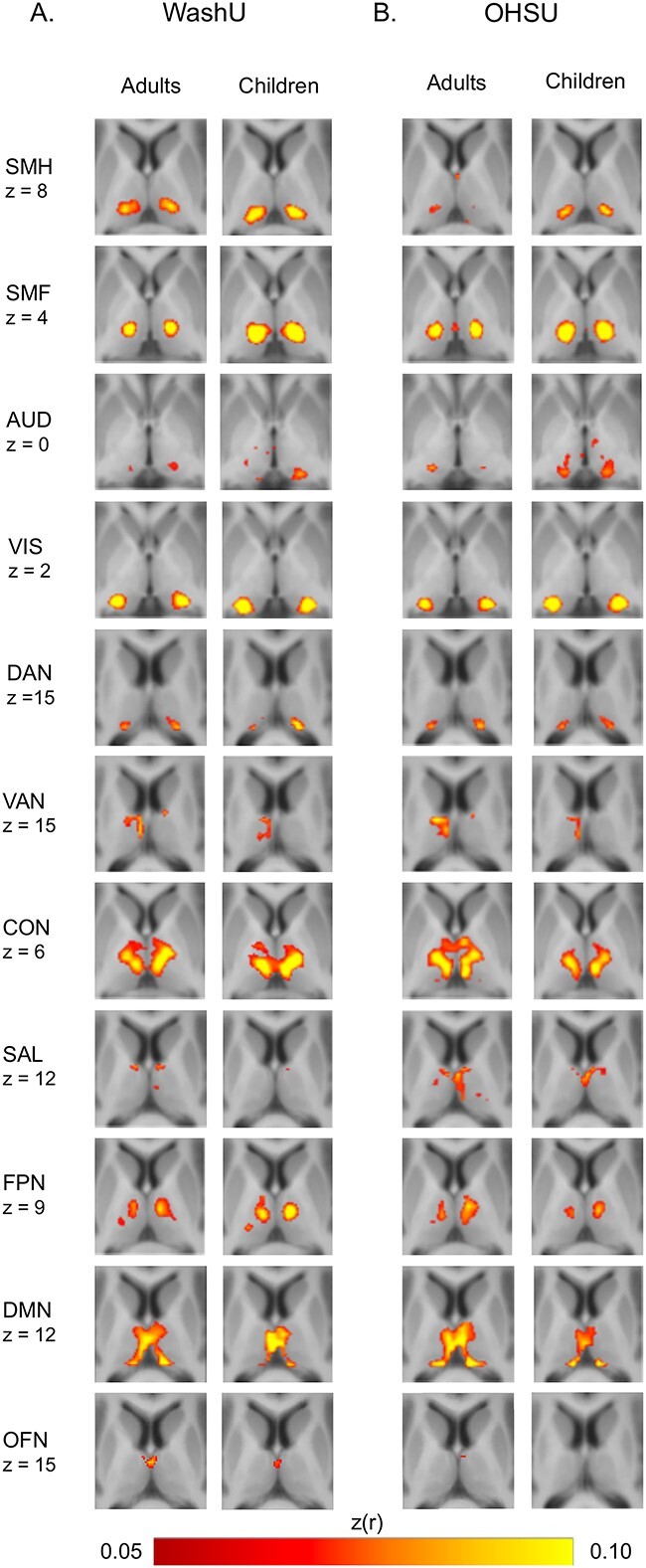
FC maps in the thalamus for 11 cortical networks for the WashU data set A) and for the OHSU data set B) based on peak z-transformed partial correlation values between thalamic voxels and each cortical system. Network labels: SMH, SMF, AUD, VIS, DAN, VAN, CON, SAL, FPN, DMN, and OFN.
The SMH and SMF networks were correlated with the ventral and lateral portions of the thalamus, corresponding to the locations of the ventral lateral and ventral posterior nuclei. The AUD network correlated with the ventral thalamus, corresponding to locations of the medial geniculate nucleus. The VIS and DAN networks were correlated with the lateral thalamus, corresponding to the locations of the pulvinar and lateral geniculate nuclei. The VAN correlated with the medial thalamus, with a larger effect in the left than in the right hemisphere. The CON correlated with the ventral thalamus. The SAL network, FPN, DMN, and OFN correlated with the medial thalamus. The OFN network is included here for completeness, though its cortical location (Supplementary Fig. 1) potentially overlaps with known fMRI susceptibility artifact, and its thalamic (Fig. 1) and particularly its cerebellar (Fig. 4) representations suggest it may reflect primarily non-neuronal artifact.
Fig. 4.
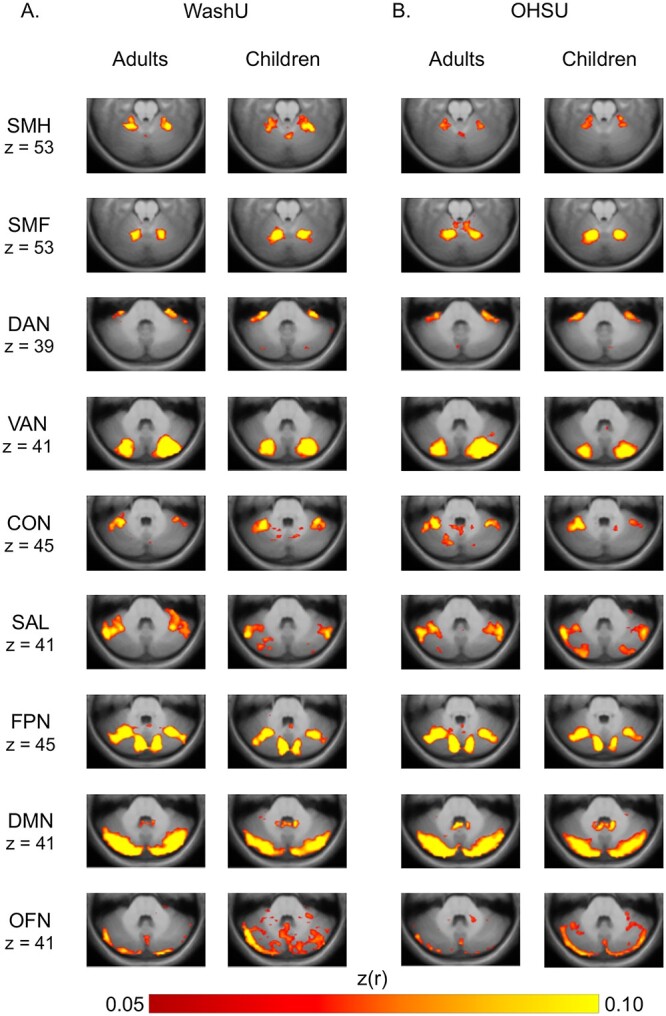
FC maps in the cerebellum for 9 cortical networks for the WashU data set A) and for the OHSU data set B) based on peak z-transformed partial correlation values between cerebellar voxels and each cortical network. Network labels: SMH, SMF, AUD, VIS, DAN, VAN, CON, SAL, FPN, DMN, and OFN.
Children have stronger SMF FC in the thalamus than adults
Direct group comparisons revealed significant differences between children and adults in FC between the SMF network and the ventrolateral parts of the thalamus, corresponding to known locations of motor nuclei (ventral lateral and ventral posterior nuclei; Morel et al. 1997). Specifically, FC was stronger in the children than in the adults in both data sets (FDR corrected P < 0.05; Fig. 2A and B). The SMH network and OFN also showed stronger correlations in ventral lateral and medial thalamus, respectively, in children compared with adults in the WashU data set, whereas the AUD and VIS networks showed stronger correlations in ventral and lateral thalamus, respectively, in children compared with adults in the OHSU data set (not shown because group differences did not replicate across data sets). However, the only differences that replicated across data sets involved the SMF. Group comparisons were not significant for any of the other functional networks.
Fig. 2.
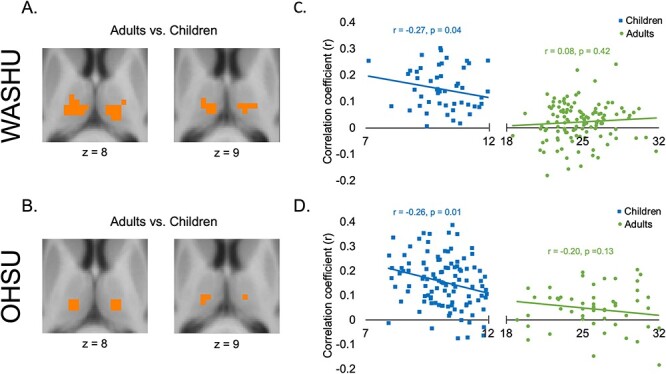
Developmental differences in thalamic-SMF network FC. Voxels with a significant difference between children and adults in the WashU data set A) and the OHSU data set B). Within these voxels, FC with the SMF network decreased with age in both data sets C, D).
Given the consistent effect with the SMF network across both data sets, and our previous finding of an age-related decrease in SMF FC in the putamen (Greene et al. 2014), we tested the relationship between SMF network-thalamus FC and age. Within those voxels in the ventrolateral thalamus that showed a significant group difference (Fig. 2A and B), we computed the Pearson correlation between age and the mean partial correlation coefficient—representing FC between the SMF network and the thalamus—separately for the child and adult groups from each data set. We found a significant negative correlation with age within the children in the WashU (r = −0.27, P = 0.04) and OHSU (r = −0.26, P = 0.01) data sets, and no significant correlation within the adults in the WashU (r = 0.08, P = 0.42) or OHSU (r = −0.20, P = 0.13) data sets. Figure 2C and D shows that FC between the SMF network and the thalamus decreased with age during childhood but did not change during adulthood.
This specific developmental effect in the thalamus with the SMF network extends our previous findings in the basal ganglia, in which we found stronger FC between the putamen and the SMF network in children compared with adults in the WashU data set (Greene et al. 2014). Here, we further replicated this putamen effect in the OHSU data set (Supplementary Fig. 2). Together, these results support the idea of developmental changes in SMF FC within the striatal and thalamic components of the cortico-striato-thalamo-cortical loop.
Children have increased SMF network integration in the thalamus despite overall similar functional network organization to adults
The modified winner-take-all analysis generated thalamus maps showing regions with network specificity (i.e. FC with primarily 1 network) and regions with network integration (i.e. FC with multiple networks) for children and adults in each data set (Fig. 3). For visualization, network-specific voxels were colored according to their affiliated network, and integrative voxels were colored with stripes according to the multiple affiliated networks.
Fig. 3.
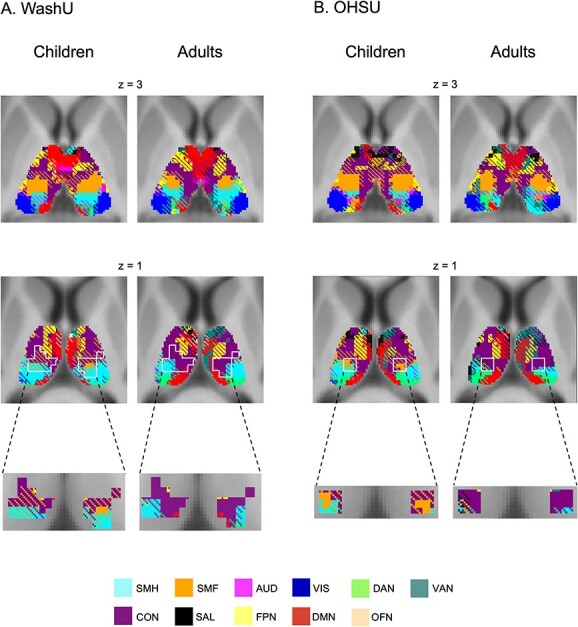
Functional network organization in the thalamus in children and adults in the WashU data set A) and in the OHSU data set B). Network-specific voxels (preferential FC to 1 network) shown in solid colors corresponding to the network; integrative voxels (preferential FC to multiple networks) shown with cross-hatching of the colors corresponding to those networks. Voxels in which there was a significant difference between children and adults, defined by the white outlines (see Fig. 2), are enlarged for clarity. Network labels: SMH, SMF, AUD, VIS, DAN, VAN, CON, SAL, FPN, DMN, and OFN.
Qualitatively, the modified winner-take-all maps looked similar across adults and children both within and between the different data sets (Fig. 3). As in Greene et al. (2020), there were integrative voxels in multiple regions of thalamus. In the ventral lateral thalamus, the strongest correlations were with the CON, SMF, and SMH networks, consistent with the motor integration zone described in individual adults (Greene et al. 2020). In the dorsal thalamus, the strongest correlations were with the DMN, FPN, VAN, and SAL network, consistent with the cognitive integration zone previously described. Finally, in the posterior thalamus, the strongest correlations were with the DAN and VIS network, consistent with the visual attention integration zone also previously described.
The ventrolateral part of the thalamus includes the motor integration zone as well as voxels in which we observed a developmental difference in SMF network FC. Visually, adults appeared to have fewer integrative SMF network voxels than children in this developmental region (Fig. 2A and B). Statistical comparisons between the number of integrative voxels within this region verified significant differences between children and adults. Children exhibited more integrative voxels (specifically with convergence of the CON and SMF network) than adults in both the WashU (t(178) = 6.78, P < 0.001) and OHSU data sets (t(165) = 4.61, P < 0.001).
In the children, the number of integrative voxels within this region was significantly correlated with SMF FC in both data sets (WashU: r = 0.49, P < 0.001; OHSU: r = 0.34, P < 0.001), suggesting that age-related changes in SMF FC may drive age-related changes in network integration. Indeed, the ANCOVA model showed that SMF FC predicted integration (number of integrative voxels) when controlling for effects of age group (Ps < 0.001), but that age group no longer predicted integration when controlling for SMF FC (WashU: P = 0.42; OHSU: P = 0.07). Thus, age-related decreases in integration between CON and SMF networks in the ventrolateral thalamus were driven by age-related decreases in SMF FC.
Children and adults show similar topography in cerebello-cortical FC
The cerebellum is also connected to cortical networks via cortico-ponto-cerebello-thalamo-cortical loops. To test the specificity of the developmental effects found in the nodes of cortico-striato-thalamo-cortical circuitry (i.e. current results in the ventral thalamus and previous results in the putamen), we performed the same group comparisons in cerebellum FC. Partial correlations we computed between each voxel in the cerebellum and the 11 cortical networks of interest in both the WashU and OHSU data sets (AUD and VIS networks are not shown because no consistent network correlations were present; Fig. 4A and B).
Adults and children, across both data sets, showed qualitatively similar FC patterns in the cerebellum. Quantitatively, the average Spearman correlation in cerebellar partial correlation values between children and adults across all networks was 0.47 (WashU data set) and 0.60 (OHSU data set; correlations by network are listed in Supplementary Table 2).
The SMF and SMH networks were correlated with the anterior cerebellum, with the SMF network correlations being located slightly posterior to the SMH, consistent with known organization of motor connections. The DAN and VAN were correlated with the medial parts of the cerebellum, with some differences along the anterior–posterior axis: the DAN was correlated with the anterior cerebellum and the VAN was correlated with the posterior cerebellum. The CON and SAL network were correlated with lateral portions of the anterior cerebellum, and the FPN was correlated with medial and lateral areas. The DMN and OFN were correlated with the posterior cerebellum along the medial-lateral axis. A winner-take-all analysis generated cerebellum maps displaying the networks with the strongest correlations in different regions of the cerebellum (Supplementary Fig. 3). Network integration was not investigated since previous work in adults shows increased network specificity in the cerebellum (Marek et al. 2018).
Unlike our findings in the basal ganglia and thalamus, there were no significant differences (FDR corrected P < 0.05) between children and adults in FC between any cortical networks and cerebellar voxels, in either data set.
Discussion
In this study, we found stronger thalamo-cortical SMF network FC in children compared with adults, which decreased continuously with age before plateauing in adulthood. These FC decreases drove developmental differences in network integration within the thalamus, such that children exhibited greater integration between the CON and SMF network than adults. We did not observe any developmental differences in cortico-cerebellar connectivity. All results were replicated in a separate data set of children and adults. These findings, along with our previous results of stronger cortico-striatal SMF network FC in children compared with adults (Greene et al. 2014), indicate that the maturation of FC in somatomotor pathways continues throughout childhood and adolescence within cortico-striatal-thalamo-cortical circuits. Notably, this effect does not seem extend to cortico-ponto-cerebello-thalamo-cortical circuits within the age ranges studied here.
The brain undergoes highly specific architectural changes through pruning of axonal projections (Cowan et al. 1984; Kantor and Kolodkin 2003; Luo and O’Leary 2005; Low and Cheng 2006) and dendritic projections (Brown et al. 1995), including thalamo-cortical projections, as part of typical development and learning. Given the correlations between anatomical connectivity and FC (Honey et al. 2009; Zhang et al. 2010), and previous work demonstrating developmental changes in thalamic FC that mirror anatomical maturation (Fair et al. 2010), the decrease in FC between the ventrolateral thalamus and the SMF network could reflect the removal of unneeded connections and increased specificity of neural tuning curves.
Parallel with our previous findings on cortical FC with the putamen (Greene et al. 2014), we found an age-related decrease in FC in the thalamus that was specific to the SMF network. This effect was localized to the ventral part of the thalamus, consistent with the location of motor nuclei, namely, the ventral lateral and ventral posterolateral nuclei. Recent work has shown largely consistent results, reporting decreased thalamo-cortical FC specific to the ventral posterolateral nucleus of the thalamus and the somatosensory cortex (part of which falls in the SMF network) from childhood to adulthood (Huang et al. 2021). This previous study also found age-related decreases in FC between the occipital cortex and lateral geniculate nucleus. While we did not find this additional developmental difference here, it is possible that our methods were not sensitive enough to detect more subtle effects given our use of stringent multiple comparisons correction. Still, we show that the effect in thalamo-SMF network FC is particularly strong, and that it relates to age. Indeed, recent demonstrations that reliable brain-phenotype correlations require very large samples (Marek et al. 2022) raises the question of whether or not we have sufficient power for a reliable measure of relationships between FC and age. However, it has been noted that out-of-sample replication is an appropriate way to validate brain–behavior effects in moderate sample sizes (e.g. Gratton et al. 2022). Therefore, the replication of the FC-age correlations in an independent data set and similar effect sizes lend support to the reliability of our findings.
Our results also converge with findings in very early stages of development showing attenuation of sensorimotor thalamo-cortical FC by age 1 year, with further thalamic definition of FC at age 2 years (Alcauter et al. 2014). Our results show continued reduction in this connectivity from age 7 years to adulthood. This reduced connectivity may reflect developmental specialization of thalamic regions that begins in infancy and stabilizes only in early adulthood. Further work filling in the ages of early childhood (ages 2–6 years) would allow us to understand the complete trajectory of thalamo-somatomotor (as well as cerebello-somatomotor) connectivity maturation.
Other prior work has reported increasing thalamo-motor/premotor FC with age (Fair et al. 2010), or no developmental differences in thalamo-motor network connectivity (Steiner et al. 2020), inconsistent with our findings. However, Fair et al. (2010) defined cortical regions of interest as large anatomical divisions, and the motor network used by Steiner et al. (2020) encompassed all somatomotor regions. Here, we used well-characterized functional networks (Power et al. 2011) that separate somatomotor regions subserving facial movements and sensations and those subserving movements and sensations in the rest of the body (Meier et al. 2008; Power et al. 2011). It is possible that this delineation allowed us to uncover a selective developmental effect in FC with the SMF network.
The present results also further our understanding of the differential maturational trajectories of functional circuits involving the thalamus, including cortico-striato-thalamo-cortical and cortico-ponto-cerebello-thalamo-cortical loops. Together with our previous work showing age-related attenuation of FC between the putamen and the SMF network (Greene et al. 2014), we illustrate that this reduced FC with age is present in both the thalamic and striatal components of the cortico-striato-thalamo-cortical motor circuit. Interestingly, we did not find age-related FC differences in the somatomotor circuits that involve the cerebellum for the measured age ranges. It is possible that this developmental change is not pervasive through the entire somatomotor system, but is selective to particular cortico-subcortical circuits. Previous work has shown that cortico-striatal-thalamo-cortical and cortico-ponto-cerebello-thalamo-cortical loops have areas of overlap as well as divergence in function and topography (Hoshi et al. 2005; Kuramoto et al. 2009; Hintzen et al. 2018). For example, while the basal ganglia and the cerebellum were both involved in motor learning tasks, the cerebellum was selectively activated for tasks associated with passive movements and the basal ganglia was selectively activated for movement selection (Jueptner 1998). This work suggests that the cerebellum may play a role in monitoring or the sensory component of movements, whereas the basal ganglia play a role in active movement. Perhaps, this theory speaks to different developmental trajectories, such that the cerebellum has pathways that are established earlier in maturation.
The behavioral significance of decreasing SMF network-thalamus FC during development is unclear. One intriguing possibility relates to what we know about motor development during childhood and adolescence. While many motor functions are adult-like by age 7 years (e.g. balance and gait), subtle motor deficits are still present and show gradual improvement with maturation throughout later childhood and adolescence (Largo et al. 2003; Gidley Larson et al. 2007). One example is “overflow,” referring to extraneous movements in which body parts not needed for the current task move along with those body parts that are needed (Denckla 1985). Overflow movements can involve extraneous orofacial, proximal, or mirror movements and follow a similar decreasing developmental trajectory from age 7 to 14 years as the SMF network-thalamus FC reported here (Gidley Larson et al. 2007). Given the cortico-striato-thalamo-cortical circuitry involved in motor control, it is possible that overflow movements in children may be facilitated by SMF network-thalamic FC. Hence, age-related reductions in overflow movements, especially orofacial overflow movements, may relate to attenuating SMF network-thalamic FC, representing normative refinement of motor control during childhood and adolescence.
Understanding the typical development of somatomotor thalamo-cortical circuits provides context for understanding developmental disorders in which these pathways are disrupted. Given our result of decreasing SMF network-thalamic FC during typical development, disorders in which symptoms emerge or reach heightened severity during childhood, adolescence, or young adulthood may reflect a failure of this normative cortico-subcortical maturation. For example, there is evidence that youth with psychosis have slightly reduced FC between motor cortex and thalamic motor regions compared with healthy controls (Huang et al. 2021), whereas adults with psychosis show increased FC between these regions (Woodward et al. 2012; Anticevic et al. 2015). Tic disorders, which have childhood onset and peak symptom severity around 10–12 years of age, involve atypical subcortical volumes and cortico-striato-thalamo-cortical structural connectivity (Miller et al. 2010; Worbe et al. 2015; Greene et al. 2017). In childhood dyslexia, there is evidence of stronger thalamo-somatomotor cortex structural connectivity compared with controls. In fact, this altered connectivity was located specifically in areas of the thalamus corresponding to the ventral lateral and ventral posterolateral nuclei, similar to the present study. Again, this dyslexia-related increase in connectivity may represent a failure of the normative attenuation we observed in typical development. Moreover, the specificity of this developmental effect with the SMF network is particularly intriguing, given that the refinement of these circuits is important for articulation-related aspects of reading (Fan et al. 2014). Increased connectivity in dyslexia may indicate a prolonged learning phase in which the stronger connectivity required for initially acquiring a skill is not attenuated as seen in typically developing children. Generally, relating these findings in neuropsychiatric disorders to the typical trajectory of cortico-thalamic connectivity could provide further insight into how deviations in brain structure and function relate to clinical phenotypes.
While cortico-striato-thalamo-cortical loops have been previously described as parallel and segregated, there is accumulating evidence for cortical network integration at the level of the striatum and the thalamus (Haber et al. 2006; Cho et al. 2015; Haber 2016; Greene et al. 2020). Human neuroimaging studies have examined integration in the thalamus by identifying regions with strong FC to multiple cortical networks (Hwang et al. 2017; Greene et al. 2020). The current study replicated the presence of these regions, notably the motor integration zone previously observed in the adult thalamus, in which FC was strongest with the CON and somatomotor networks (Greene et al. 2020). This finding corroborates other recent work demonstrating a link between the CON and somatomotor regions (Gratton et al. 2018; Rinne et al. 2018; Newbold et al. 2021).
Here, we demonstrated that integration in particular regions of the thalamus is also present in children. Moreover, we found greater integration between the CON and SMF network in children compared to adults. Integration of these cortico-striato-thalamo-cortical circuits is argued to be important for learning, coordination, and modification of behavior (Haber 2003, 2016; Haber and Calzavara 2009), key aspects of development. Therefore, greater integration in the children may be associated with a greater need to impose top-down control over the motor system in childhood, which declines to some extent with age as fine motor precision develops and becomes more automatized. However, given the resolution limitations of BOLD imaging, we must also consider alternative mechanistic explanations for our measures of integration and the differences between adult and children. For example, increased integration in childhood could represent incomplete differentiation between networks in children that become refined into adulthood.
Previously, we posited that the motor integration zone may be clinically relevant for movement disorders, as it coincides with deep brain stimulation (DBS) sites for treating essential tremor (Perlmutter and Mink 2006; Greene et al. 2020). Our current observation of greater motor integration in the thalamus in children suggests that treatment could extend to pediatric disorders such as cerebral palsy (Wolf et al. 2019), epilepsy (Valentín et al. 2017), and Tourette syndrome (Xu et al. 2020). For example, DBS targeting the palladium and thalamus has shown promise for treating pediatric dystonia (Olaya et al. 2013; Cif and Coubes 2017; Luciano et al. 2020). However, individual response rates are unpredictable and vary, with some patients experiencing minimal or no improvement (Krause et al. 2016; Luciano et al. 2020). This variability in treatment efficacy may be because of a lack of validated treatment sites that could be addressed with a greater understanding of pediatric thalamic motor integration hubs. Additionally, targeting stable integration zones that are consistent between adult and children may help increase treatment efficacy with increased specificity of target.
Author contributions
Carolina B. D'Andrea (Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing), Scott Marek (Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing), Andrew N. Van (Data curation, Formal analysis, Writing—review & editing), Ryland L. Miller (Formal analysis, Writing—review & editing), Eric A. Earl (Data curation, Writing—review & editing), Stephanie B. Stewart (Data curation, Formal analysis, Writing—review & editing), Nico U.F. Dosenbach (Writing—review & editing), Bradley L. Schlaggar (Funding acquisition, Writing—review & editing), Timothy O. Laumann (Formal analysis, Writing—review & editing), Damien A. Fair (Funding acquisition, Writing—review & editing), Evan M. Gordon (Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing—original draft, Writing—review & editing), and Deanna J. Greene (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing—original draft, Writing—review & editing)
Funding
The National Institutes of Mental Health (NIMH—K01MH104592 to DJG, MH100019 to TOL, MH129616 to TOL); the National Institutes of Health (NIH—K99MH121518 to SM, R21MH091512 to BLS, R01HD057076 to BLS, R01MH096773 to DAF, R00MH091238 to DAF); the McDonnell Center for Systems Neuroscience (to DJG).
Conflict of interest statement: None declared.
Data availability
Data from the adults in the WashU dataset are available in the openneuro repository (title: Washington University 120). A subset of the OHSU dataset is available on the NIMH Data Archive (title: Brain trajectories in ADHD). All other data are available upon request.
Supplementary Material
Contributor Information
Carolina Badke D’Andrea, Department of Cognitive Science, University of California San Diego, La Jolla, CA 92093, United States; Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, United States; Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, United States.
Scott Marek, Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, United States.
Andrew N Van, Department of Neurology, Washington University School of Medicine, St. Louis, MO 63110, United States; Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63130, United States.
Ryland L Miller, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, United States; Department of Neurology, Washington University School of Medicine, St. Louis, MO 63110, United States.
Eric A Earl, Data Science and Sharing Team, National Institute of Mental Health, NIH, DHHS, Bethesda, MD 20899, United States.
Stephanie B Stewart, Department of Psychiatry, University of Colorado School of Medicine, Aurora, CO 80045, United States.
Nico U F Dosenbach, Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, United States; Department of Neurology, Washington University School of Medicine, St. Louis, MO 63110, United States; Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63130, United States; Department of Pediatrics, Washington University School of Medicine, St. Louis, MO 63110, United States; Program in Occupational Therapy, Washington University School of Medicine, St. Louis, MO 63110, United States.
Bradley L Schlaggar, Kennedy Krieger Institute, Baltimore, MD 21205, United States.
Timothy O Laumann, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110, United States.
Damien A Fair, Institute of Child Development, College of Education and Human Development, University of Minnesota, Minneapolis, MN 55455, United States; Department of Pediatrics, University of Minnesota Medical School, Minneapolis, MN 55455, United States; Masonic Institute for the Developing Brain, University of Minnesota, Minneapolis, MN 55455, United States.
Evan M Gordon, Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, United States.
Deanna J Greene, Department of Cognitive Science, University of California San Diego, La Jolla, CA 92093, United States.
References
- Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Mariën P, Molinari M, Moulton E, et al. Consensus paper: cerebellum and emotion. Cerebellum. 2017:16(2):552–576. 10.1007/s12311-016-0815-8. [DOI] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci. 2014:34(27):9067–9075. 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986:9(1):357–381. 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alkonyi B, Juhász C, Muzik O, Behen ME, Jeong J-W, Chugani HT. Thalamocortical connectivity in healthy children: asymmetries and robust developmental changes between ages 8 and 17 years. Am J Neuroradiol. 2011:32(5):962–969. 10.3174/ajnr.A2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, et al. Association of Thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015:72(9):882–891. 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA. Differential motor and prefrontal cerebello-cortical network development: evidence from multimodal neuroimaging. NeuroImage. 2016:124(Pt A):591–601. 10.1016/j.neuroimage.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KJ, Koller JM, Snyder AZ, Perlmutter JS. Atlas template images for nonhuman primate neuroimaging: baboon and macaque. Methods Enzymol. 2004:385:91–102. 10.1016/S0076-6879(04)85006-7. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol. 2015:66(1):83–113. 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Sheppard DM. The neurodevelopmental frontostriatal disorders: evolutionary adaptiveness and anomalous lateralization. Brain Lang. 2000:73(2):297–320. 10.1006/brln.2000.2308. [DOI] [PubMed] [Google Scholar]
- Brown KE, Arends JJA, Wasserstrom SP, Zantua JB, Jacquin MF, Woolsey TA. Developmental transformation of dendritic arbors in mouse whisker thalamus. Dev Brain Res. 1995:86(1):335–339. 10.1016/0165-3806(94)00210-Q. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011:106(5):2322–2345. 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002:17(1):184–200. [DOI] [PubMed] [Google Scholar]
- Cary RP, Ray S, Grayson DS, Painter J, Carpenter S, Maron L, Sporns O, Stevens AA, Nigg JT, Fair DA. Network structure among brain Systems in Adult ADHD is uniquely modified by stimulant administration. Cereb Cortex. 2016:27(8):3970–3979. 10.1093/cercor/bhw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Z-H, Law M, Chi J-G, Choi S-H, Park S-Y, Kammen A, Park C-W, Oh S-H, Kim Y-B. An anatomic review of thalamolimbic fiber tractography: ultra-high resolution direct visualization of thalamolimbic fibers anterior thalamic radiation, superolateral and inferomedial medial forebrain bundles, and newly identified septum pellucidum tract. World Neurosurg. 2015:83(1):54–61.e32. 10.1016/j.wneu.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012:108(8):2242–2263. 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. NeuroImage. 2011:54(2):1344–1354. 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Cif L, Coubes P. Historical developments in children’s deep brain stimulation. Eur J Paediatr Neurol. 2017:21(1):109–117. 10.1016/j.ejpn.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Coulombe M-A, Elkaim LM, Alotaibi NM, Gorman DA, Weil AG, Fallah A, Kalia SK, Lipsman N, Lozano AM, Ibrahim GM. Deep brain stimulation for Gilles de la Tourette syndrome in children and youth: a meta-analysis with individual participant data. J Neurosurg Pediatr. 2018:23(2):236–246. 10.3171/2018.7.PEDS18300. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Fawcett JW, O’Leary DDM, Stanfield BB. Regressive events in neurogenesis. Science. 1984:225(4668):1258–1265. 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs (1985). Psychopharmacol Bull. 1985:21(4):773–800. [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TGC, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JT, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010:4:10. 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Davis N, Anderson AW, Cutting LE. Thalamo-cortical connectivity: what can diffusion tractography tell us about reading difficulties in children? Brain Connect. 2014:4(6):428–439. 10.1089/brain.2013.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, et al. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry. 1996:53(7):617–624. 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive–compulsive disorder in adults and children. Clin Psychol Rev. 2006:26(1):32–49. 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB, Mahone EM. Effects of gender and age on motor exam in typically developing children. Dev Neuropsychol. 2007:32(1):543–562. 10.1080/87565640701361013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AR, Moore GJ, Keshavan MS, Paulson LAD, Narula V, Mac Master FP, Stewart CM, Rosenberg DR. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Arch Gen Psychiatry. 2000:57(5):449–456. 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- Gratton C, Sun H, Petersen SE. Control networks and hubs. Psychophysiology. 2018:55:e13032. 10.1111/psyp.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Nelson SM, Gordon EM. Brain-behavior correlations: two paths toward reliability. Neuron. 2022:110(9):1446–1449. 10.1016/j.neuron.2022.04.018. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Laumann TO, Dubis JW, Ihnen SK, Neta M, Power JD, Pruett JR, Black KJ, Schlaggar BL. Developmental changes in the organization of functional connections between the basal ganglia and cerebral cortex. J Neurosci. 2014:34(17):5842–5854. 10.1523/JNEUROSCI.3069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Williams AC III, Koller JM, Schlaggar BL, Black KJ. Brain structure in pediatric Tourette syndrome. Mol Psychiatry. 2017:22(7):972–980. 10.1038/mp.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Marek S, Gordon EM, Siegel JS, Gratton C, Laumann TO, Gilmore AW, Berg JJ, Nguyen AL, Dierker D, et al. Integrative and network-specific connectivity of the basal ganglia and thalamus defined in individuals. Neuron. 2020:105(4):742–758.e6. 10.1016/j.neuron.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003:26(4):317–330. 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016:18(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009:78(2):69–74. 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010:35(1):4–26. 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim K-S, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that Interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006:26(32):8368–8376. 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzmann CS, Snyder AZ, Kenley JK, Rogers CE, Shimony JS, Smyser CD. Cerebellar functional connectivity in term- and very preterm-born infants. Cereb Cortex. 2019:29(3):1174–1184. 10.1093/cercor/bhy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzen A, Pelzer EA, Tittgemeyer M. Thalamic interactions of cerebellum and basal ganglia. Brain Struct Funct. 2018:223(2):569–587. 10.1007/s00429-017-1584-y. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci. 2009:106(6):2035–2040. 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005:8(11):1491–1493. 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Feature article: distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex. 1995:5(2):95–110. 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Huang AS, Rogers BP, Sheffield JM, Vandekar S, Anticevic A, Woodward ND. Characterizing effects of age, sex and psychosis symptoms on thalamocortical functional connectivity in youth. NeuroImage. 2021:243:118562. 10.1016/j.neuroimage.2021.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Bertolero MA, Liu WB, D’Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017:37(23):5594–5607. 10.1523/JNEUROSCI.0067-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel C, Kastman EK, Glenn CR, Somerville LH. Development of corticostriatal connectivity constrains goal-directed behavior during adolescence. Nat Commun. 2017:8(1):1605. 10.1038/s41467-017-01369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AC, James S, Smith DM, Javaloyes A. Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent-onset schizophrenia. Am J Psychiatry. 2004:161(6):1023–1029. 10.1176/appi.ajp.161.6.1023. [DOI] [PubMed] [Google Scholar]
- Jueptner M. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998:121(8):1437–1449. 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Kolodkin AL. Curbing the excesses of youth: molecular insights into axonal pruning. Neuron. 2003:38(6):849–852. 10.1016/S0896-6273(03)00364-7. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT. Annual research review: reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J Child Psychol Psychiatry. 2014:55(6):685–710. 10.1111/jcpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AJ, Nair A, Keown CL, Datko MC, Lincoln AJ, Müller R-A. Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol Psychiatry. 2015:78(9):625–634. 10.1016/j.biopsych.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P, Lauritsch K, Lipp A, Horn A, Weschke B, Kupsch A, Kiening KL, Schneider G-H, Kühn AA. Long-term results of deep brain stimulation in a cohort of eight children with isolated dystonia. J Neurol. 2016:263(11):2319–2326. 10.1007/s00415-016-8253-6. [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T. Two types of Thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb Cortex. 2009:19(9):2065–2077. 10.1093/cercor/bhn231. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp. 1995:3(3):209–223. 10.1002/hbm.460030305. [DOI] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997:35(11):1437–1444. 10.1016/S0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Largo RH, Fischer J, Rousson V. Neuromotor development from kindergarten age to adolescence: developmental course and variability. Swiss Med Wkly. 2003:133:193–199. 10.5167/uzh-34720. [DOI] [PubMed] [Google Scholar]
- Li W, Pozzo-Miller L. Dysfunction of the corticostriatal pathway in autism spectrum disorders. J Neurosci Res. 2020:98(11):2130–2147. 10.1002/jnr.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Cheng H-J. Axon pruning: an essential step underlying the developmental plasticity of neuronal connections. Philos Trans R Soc B Biol Sci. 2006:361(1473):1531–1544. 10.1098/rstb.2006.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano MS, Robichaux-Viehoever A, Dodenhoff KA, Gittings ML, Viser AC, Racine CA, Bledsoe IO, Pereira CW, Wang SS, Starr PA, et al. Thalamic deep brain stimulation for acquired dystonia in children and young adults: a phase 1 clinical trial. J Neurosurg Pediatr. 2020:27(2):203–212. 10.3171/2020.7.PEDS20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O’Leary DDM. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005:28(1):127–156. 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Makki MI, Munian Govindan R, Wilson BJ, Behen ME, Chugani HT. Altered fronto-striato-thalamic connectivity in children with Tourette syndrome assessed with diffusion tensor MRI and probabilistic fiber tracking. J Child Neurol. 2009:24(6):669–678. 10.1177/0883073808327838. [DOI] [PubMed] [Google Scholar]
- Marek S, Siegel JS, Gordon EM, Raut RV, Gratton C, Newbold DJ, Ortega M, Laumann TO, Adeyemo B, Miller DB, et al. Spatial and temporal Organization of the individual human cerebellum. Neuron. 2018:100(4):977–993.e7. 10.1016/j.neuron.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022:603:654–660. 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JD, Aflalo TN, Kastner S, Graziano MSA. Complex organization of human primary motor cortex: a high-resolution fMRI study. J Neurophysiol. 2008:100(4):1800–1812. 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia “projections” to the prefrontal cortex of the primate. Cereb Cortex. 2002:12(9):926–935. 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000:11(6):735–759. 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller AM, Bansal R, Hao X, Sanchez-Pena JP, Sobel LJ, Liu J, Xu D, Zhu H, Chakravarty MM, Durkin K, et al. Enlargement of thalamic nuclei in Tourette syndrome. Arch Gen Psychiatry. 2010:67(9):955–964. 10.1001/archgenpsychiatry.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997:387(4):588–630. . [DOI] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013:136(6):1942–1955. 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold DJ, Gordon EM, Laumann TO, Seider NA, Montez DF, Gross SJ, Zheng A, Nielsen AN, Hoyt CR, Hampton JM, et al. Cingulo-opercular control network and disused motor circuits joined in standby mode. Proc Natl Acad Sci. 2021:118(13):Article 13. 10.1073/pnas.2019128118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya JE, Christian E, Ferman D, Luc Q, Krieger MD, Sanger TD, Liker MA. Deep brain stimulation in children and young adults with secondary dystonia: the Children’s Hospital Los Angeles experience. Neurosurg Focus. 2013:35(5):E7. 10.3171/2013.8.FOCUS13300. [DOI] [PubMed] [Google Scholar]
- Palesi F, De Rinaldis A, Castellazzi G, Calamante F, Muhlert N, Chard D, Tournier JD, Magenes G, D’Angelo E, Gandini Wheeler-Kingshott CAM. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep. 2017a:7(1):Article 1. 10.1038/s41598-017-13079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesi F, Tournier J-D, Calamante F, Muhlert N, Castellazzi G, Chard D, D’Angelo E, Wheeler-Kingshott CG. Reconstructing contralateral fiber tracts: methodological aspects of cerebello-thalamo-cortical pathway reconstruction. Funct Neurol. 2017b:31(4):229–238. 10.11138/FNeur/2016.31.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006:29(1):229–257. 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs J, Desmond JE. The role of the human cerebellum in performance monitoring. Curr Opin Neurobiol. 2016:40:38–44. 10.1016/j.conb.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Peterson BS. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J Psychosom Res. 2009:67(6):559–573. 10.1016/j.jpsychores.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010:67(5):735–748. 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011:72(4):665–678. 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012:59(3):2142–2154. 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014:84:320–341. 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkiran S, Heidemeyer L, Gaebler A, Shah NJ, Neuner I. Alterations in basal ganglia-cerebello-thalamo-cortical connectivity and whole brain functional network topology in Tourette’s syndrome. NeuroImage Clin. 2019:24:101998. 10.1016/j.nicl.2019.101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne P, Hassan M, Fernandes C, Han E, Hennessy E, Waldman A, Sharma P, Soto D, Leech R, Malhotra PA, et al. Motor dexterity and strength depend upon integrity of the attention-control system. Proc Natl Acad Sci U S A. 2018:115(3):E536–E545. 10.1073/pnas.1715617115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Benazon NR, Gilbert A, Sullivan A, Moore GJ. Thalamic volume in pediatric obsessive–compulsive disorder patients before and after cognitive behavioral therapy. Biol Psychiatry. 2000:48(4):294–300. 10.1016/S0006-3223(00)00902-1. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DLW, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010:30(10):3640–3651. 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L, Federspiel A, Slavova N, Wiest R, Grunt S, Steinlin M, Everts R. Functional topography of the thalamo-cortical system during development and its relation to cognition. NeuroImage. 2020:223:117361. 10.1016/j.neuroimage.2020.117361. [DOI] [PubMed] [Google Scholar]
- Thach WT. Does the cerebellum initiate movement? Cerebellum. 2014:13(1):139–150. 10.1007/s12311-013-0506-7. [DOI] [PubMed] [Google Scholar]
- Valentín A, Selway RP, Amarouche M, Mundil N, Ughratdar I, Ayoubian L, Martín-López D, Kazi F, Dar T, Jiménez-Jiménez D, et al. Intracranial stimulation for children with epilepsy. Eur J Paediatr Neurol. 2017:21(1):223–231. 10.1016/j.ejpn.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, Emsell L, Boets B, Noens I, Steyaert J, et al. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. NeuroImage Clin. 2014:4:374–382. 10.1016/j.nicl.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Tamnes CK, Bjornerud A, Due-Tonnessen P, Holland D, Dale AM, Fjell AM. Maturation of cortico-subcortical structural networks—segregation and overlap of medial temporal and fronto-striatal systems in development. Cereb Cortex. 2015:25(7):1835–1841. 10.1093/cercor/bht424. [DOI] [PubMed] [Google Scholar]
- Wang C, Kipping J, Bao C, Ji H, Qiu A. Cerebellar functional parcellation using sparse dictionary learning clustering. Front Neurosci. 2016:10:1–12. 10.3389/fnins.2016.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y, Lin J, Ahorsu DK, Tsang HWH. Neuropathways of theory of mind in schizophrenia: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022:137:104625. 10.1016/j.neubiorev.2022.104625. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Blahak C, Saryyeva A, Schrader C, Krauss JK. Deep brain stimulation for dystonia-choreoathetosis in cerebral palsy: pallidal versus thalamic stimulation. Parkinsonism Relat Disord. 2019:63:209–212. 10.1016/j.parkreldis.2019.01.029. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012:169(10):1092–1099. 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Marrakchi-Kacem L, Lecomte S, Valabregue R, Poupon F, Guevara P, Tucholka A, Mangin J-F, Vidailhet M, Lehericy S, et al. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain J Neurol. 2015:138(Pt 2):472–482. 10.1093/brain/awu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Zhao Q, Wang P, Fan Q, Chen J, Zhang H, Yang Z, Stein DJ, Wang Z. Altered resting-state cerebellar-cerebral functional connectivity in obsessive-compulsive disorder. Psychol Med. 2019:49(7):1156–1165. 10.1017/S0033291718001915. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhang C, Deeb W, Patel B, Wu Y, Voon V, Okun MS, Sun B. Deep brain stimulation for Tourette’s syndrome. Transl Neurodegener. 2020:9(1):4. 10.1186/s40035-020-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Di X, Taylor PA, Gohel S, Tsai Y-H, Biswal BB. Functional topography of the thalamocortical system in human. Brain Struct Funct. 2016:221(4):1971–1984. 10.1007/s00429-015-1018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010:20(5):1187–1194. 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the adults in the WashU dataset are available in the openneuro repository (title: Washington University 120). A subset of the OHSU dataset is available on the NIMH Data Archive (title: Brain trajectories in ADHD). All other data are available upon request.


