Abstract
Obesity is a complex disease that relapses frequently and associates with multiple complications that comprise a worldwide health priority because of its rising prevalence and association with numerous complications, including metabolic disorders, mechanic pathologies, and cancer, among others. Noteworthy, excess adiposity is accompanied by chronic inflammation, oxidative stress, insulin resistance, and subsequent organ dysfunction. This dysfunctional adipose tissue is initially stored in the visceral depot, overflowing subsequently to produce lipotoxicity in ectopic depots like liver, heart, muscle, and pancreas, among others. People living with obesity need a diagnostic approach that considers an exhaustive pathophysiology and complications assessment. Thus, it is essential to warrant a holistic diagnosis and management that guarantees an adequate health status, and quality of life. The present review summarizes the different complications associated with obesity, at the same time, we aim to fostering a novel framework that enhances a patient-centered approach to obesity management in the precision medicine era.
Keywords: Obesity, Visceral adipose tissue, Cardiovascular disease, Adiposity-based chronic disease, Obesity-related adipose tissue disease, Adiposopathy
Introduction
In the last years, efforts have focused on addressing obesity beyond a body mass index (BMI) perspective, since dysfunctional adiposity promotes several diseases [1]. People living with obesity (PlwO), have a higher risk mortality from all causes, with cardiovascular disease (CVD) together with cancer as standing out [2, 3]. The adiposity-based chronic disease (ABCD) concept has been proposed to improve the diagnosis of obesity based on the dimensions of etiology, severity of adiposity excess, and assessment of health risks [1]. This novel diagnostic framework aims to promote an accurate comorbidity screening in a systematic manner leading to an enriched patient care. Recently, the term “obesity-related adipose tissue disease” (OrAD) has been proposed to collectively englobe the diverse pathologies related to “adiposopathy”, which include hypertrophy, inflammation and fibrosis of the adipose tissue (AT) [4]. The present review fosters a novel framework based on dysfunctional adiposity recommending a holistic view with a patient-centered approach in the precision medicine era.
Common pathophysiology in obesity-related diseases
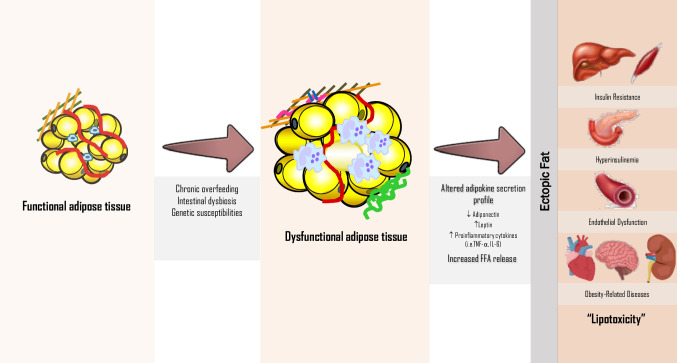
Obesity-related diseases are predominantly determined by physical (i.e. hypoventilation, osteoarthritis) and metabolic features [1]. AT produces a variety of molecules called adipokines to maintain homeostasis (i.e. thermoregulation, energy storage, insulin sensitivity, and immunity, among others) [5]. AT dysfunction underlies the mechanisms linking obesity and the development of metabolic comorbidities [5–7]. AT dysfunction typically appears due to the pathological enlargement of fat mass (hypertrophy and hyperplasia of adipocytes) [7, 8], with subsequent hypoxia as blood supply becomes insufficient. The increased recruitment of macrophages, dendritic cells, and lymphocytes leads to an adiponectin expression downregulation, along with release of pro-inflammatory adipokines via metabolic signaling pathways activation [9, 10]. This increases oxidative stress, insulin resistance (IR), dyslipidemia and incites progressive accumulation of ectopic fat [11–13]. Ectopic fat intensifies the pro-inflammatory cytokine activity favoring the development of lipotoxicity via oxidative stress, activation of platelets, elevated renin–angiotensin–aldosterone system activity, cellular senescence, and dysfunction of the endothelium, eventually underlying obesity-related diseases [13–15]. The different phenotypes of obesity have inflammatory cytokines levels that reflect the dysfunctional AT continuum implicated in the systemic inflammation [16]. Figure 1 summarizes the common pathophysiology in obesity-related diseases. Current trends attempt to foster a more personalized diagnostic and treatment approach of obesity based on adiposopathy [17].
Fig. 1.
Dysfunctional adipose tissue enlargement underlies ectopic fat accumulation
Diverse factors may influence the appearance of a dysfunctional adipose tissue, which through a continuum of altered adipokine secretion and increased FFA release promotes ectopic fat accumulation
FFA: free fatty acids
Organ systems approach in relation to dysfunctional adiposity
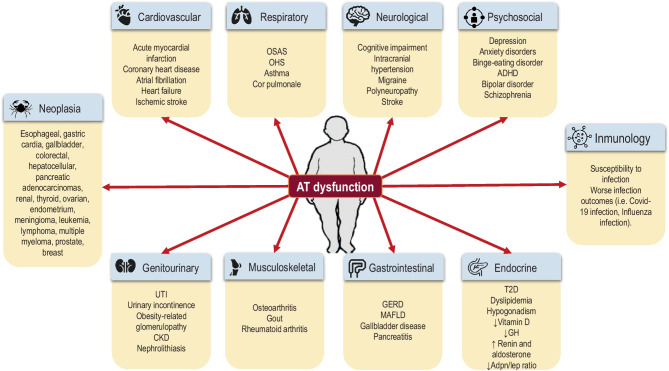
Figure 2 summarizes the obesity-related diseases.
Fig. 2.
Obesity-related diseases. Obesity-related diseases derived from adipose tissue dysfunction ADHD: attention deficit hyperactivity disorder; Adpn/Lep: adiponectin/leptin; AT: adipose tissue; CKD: chronic kidney disease; GERD: gastroesophageal reflux disease; GH: growth hormone; MAFLD: metabolic associated dysfunction fatty liver disease; OSAS: obstructive sleep apnea syndrome; OHS: obesity hypoventilation syndrome; T2D: type 2 diabetes mellitus; UTI: urinary tract infection
Cardiovascular diseases
As a major independent ischemic heart disease risk factor, obesity also directly contributes to incident cardiometabolic risk like type 2 diabetes (T2D), dyslipidemia, sleep disorders, and hypertension [18]. Visceral adipose tissue (VAT) is frequently accompanied by collection of fat in physiologically lean tissues (liver, heart, skeletal muscle), which gradually promotes chronic inflammation, that enhances endothelial cell dysfunction and atherosclerosis, including acute thrombosis, associated with a higher CVD risk [19]. An international multicenter case–control study, involving more than 27,000 participants, reported the waist-height ratio (WHtR) as the strongest myocardial infarction predictor, independently of gender, age, smoking status, ethnicity, hypertension, diabetes, and dyslipidemia [20]. Regarding cardiac arrhythmias, obesity may account for one-fifth of the patients with atrial fibrillation [18]. Additionally, a dose–response meta-analysis and systematic review have evidenced that a BMI > 25 kg/m2 together with abdominal adiposity are related with an elevated heart failure risk [3]. The classical major adverse cardiovascular events (MACE) comprise nonfatal stroke, nonfatal myocardial infarction, and cardiovascular death. The elevated ischemic stroke risk is also related to obesity [21], as expected, although it seems to depend more on the metabolic consequences of obesity [22]. Interestingly, physical activity and weight loss attenuate the detrimental effects of obesity on CVD [23].
Respiratory diseases
PlwO may have a mechanical compression of the chest cavity on the diaphragm, which may induce an increased pulmonary resistance, and reduced respiratory muscle strength, which may eventually lead to cor pulmonale [24]. Lung function and body fat distribution have a robust correlation, especially when fat accumulates in the thorax and in the abdomen [24]. In this line, asthma prevalence and severity are associated with excess total body weight, its incidence increases by 50% in patients with overweightness/obesity [25]. Likewise, the prevalence of pulmonary embolism is higher in PlwO than in people without overweightness [26]. Furthermore, overweightness is considered the most common risk factor of obstructive sleep apnea (OSA) [27]. OSA is traditionally related to an incremented cardiovascular risk and a reduced quality of life with mechanical and metabolic factors playing an important role in its etiology. In PlwO, OSA prevalence is estimated to be 19–31%. The coexistence of excess weight, daytime hypercapnia (pCO2 > 6 kPa) together with disrupted sleep breathing pattern characterizes the obesity hypoventilation syndrome (OHS) [24].
Gastrointestinal diseases
The accumulation of intracellular fat in the liver characterizes metabolic associated fatty liver disease (MAFLD) [28]. Its prevalence worldwide is rising, especially in PlwO or T2D. A meta-analysis involving 8.5 million individuals reported that more than 80% of patients with fatty liver disease had overweightness, 72% had dyslipidemia, and 44% had T2D [29]. IR and visceral fat are the central mechanisms linking both entities [30]. There is evidence to consider MAFLD as an additional independent risk factor for CVD [31]. Moreover, MAFLD will become the first cause of liver transplantation [32]. The presence of fibrosis and its severity are the factors related to the increased all-cause mortality, however due to CVD mainly [33]. Obesity is also associated with esophageal disorders, specially gastroesophageal reflux disease (GERD) [34], which may lead to esophagitis, Barrett’s esophagus, or adenocarcinoma. Regarding other gastrointestinal disorders, PlwO have a higher risk for gallbladder disease [35]. Gallbladder dysmotility is the suggested mechanism to explain this association [36]. Likewise, an association between obesity and increased risk of acute pancreatitis has been reported [37].
Endocrinological diseases
Obesity may have an impact on numerous endocrine organs, encompassing the hypothalamic-pituitary axis, vitamin D alterations and sex steroids disarrangements, among others [38]. IR is responsible for many endocrine abnormalities. In the presence of excess adiposity, increased plasma free fatty acids (FFA) concentrations are observed [30]. Mitochondrial fatty acid β-oxidation mediates lipid removal of the liver, subsequently, triacylglycerols reach the bloodstream as VLDL or can be accumulated as liver lipid droplets. When AT is overwhelmed with FFA, deposition of fat occurs in beta cells of the pancreas as well as in the liver and skeletal muscle [38]. Hepatic triacylglycerol deposition stimulates IR leading to a compensatory hyperinsulinemia that reduces the synthesis of glycogen, elevates uptake of liver FFA at the same time as inhibiting hepatic β-oxidation [15]. Moreover, hyperinsulinemia diminishes the hepatic hormone-binding proteins, frequently related to endocrine dysregulation [38]. All these alterations lead to an increased proinflammatory profile as described above.
The most common endocrinopathy in obesity is T2D [39]. Regarding other endocrinopathies [38], obesity is associated to hypogonadism through the reduction in the release of gonadotropin releasing hormone, the enhancement of aromatase (promoting free testosterone conversion to estrogen), and the decrease of sex hormone-binding globulin (SHBG) mediated through IR. The GH axis may also be altered in PlwO; GH levels may be lower due to an increase in the GH-binding protein and a GHRH central activation decrease. Serum IGF-1, however, is not altered in PlwO. TSH levels may also be altered due to IR and higher leptin levels (which stimulates TSH secretion). Vitamin D is a fat-soluble vitamin, thus AT vitamin D sequestration may decrease its bioavailability. Renin and aldosterone levels may be elevated through RAS activation in the low-grade inflammation setting [40]. Finally, adiponectin, leptin and ghrelin levels are altered in PlwO [41, 42]. Adiponectin/Leptin ratio (Adpn/Lep) is a suitable indicator of AT dysfunction, thus it may be a useful estimator of cardiometabolic risk [42].
Renal and genitourinary diseases
PlwO have a higher risk for urinary tract infection [43]. Likewise, obesity and visceral fat are associated with overactive bladder syndrome and urinary incontinence [44]. Moreover, obesity markedly increases the risk of benign prostatic hyperplasia [45]. PlwO may have an increased risk of kidney stones [46]. Furthermore, IR may damage the acid–base kidney metabolism leading to a lower urinary pH together with an elevated uric acid stone disease risk. Besides, refined sugars intake, purine-rich foods, and low fluid intake may contribute to the development of renal lithiasis. Furthermore, Roux-en-Y bypass surgery may in addition augment the kidney stone risk in relation to the elevated hyperoxaluria [47].
Additionally, obesity represents a further risk factor for CKD development [48], even after additional adjustments for blood pressure and T2D. Diabetic kidney disease and obesity-related glomerulopathy are the two main drivers of CKD in PlwO [49]. Obesity-related glomerulopathy, characterized by proteinuria, hypertrophy, and adaptive focal segmental glomerulosclerosis, can subsequently lead to a reduction of the renal function. The hemodynamic, adipose tissue-related, IR common pathophysiology may explain this relationship [50]. VAT, and not subcutaneous adipose tissue (SCAT), assessed by imaging techniques, is associated with a higher albuminuria prevalence [51], suggesting a key role of visceral adiposity in this relation [52]. In this line, studies have also evidenced that prompt identification and management of MAFLD may decrease the CKD burden [53]. However, there is a need of further studies examining the effects of obesity on kidney disease progression.
Musculoskeletal disease
Obesity can independently lead to loss of muscle mass and function, due to oxidative stress, inflammation and IR [54, 55]. Sedentary lifestyle is both a cause and a consequence of sarcopenia and obesity. Additionally, body fat is associated with widespread and single-site joint pain [56]. Knee osteoarthritis is the most common musculoskeletal comorbidity in PlwO [57]. This comorbid association reduces mobility, which can further increase weight. A recent study showed that living with obesity elevates rheumatoid arthritis risk in women by 40–70% depending on serologic status and age [58]. As expected, weight loss of at least 10% has been associated with an improvement of pain [57]. Moreover, gout, an inflammatory arthritis caused by crystal-deposition subsequent to uric acid serum elevation, is common in PlwO [59]. In all the entities described, weight loss may improve symptoms, nonetheless, gout attacks might occur in the weight loss period [60].
Neurological diseases
Mounting amount of evidence shows the effects of obesity on the central nervous system [61–63]. In a recent prospective cohort study aiming to clarify the relation between life time adiposity and cognitive impairment, a higher dementia risk was evidenced in people with less fat-free mass and more fat distribution on arms [61]. Neuroimaging studies in PlwO highlight a relation with brain structural abnormalities, mainly temporal and frontal lobes atrophy, corresponding to the executive and memory dysfunctions presented by these patients [62, 63]. A chronic low grade systemic inflammation, oxidative stress, the accumulation of senescence cells in the brain that escalates the neuroinflammation, changes in blood barrier permeability and glial activation have been proposed as responsible for the synaptic remodelling and neuronal apoptosis that has been associated with cognitive impairment in PlwO [64–67]. In PlwO, the etiological implications of vascular pathology in cognitive impairment should not be neglected [68]. Obesity is also connected to idiopathic intracranial hypertension and migraine [69]. At the pathophysiological level, the overlap between migraine and both, central and peripheral pathways, regulating feeding, involving serotonin, adiponectin and leptin is expected [70]. The relation between obesity and peripheral nervous system affects both the somatic nerves causing polyneuropathy [71] and the autonomic nervous system, with an autonomic neuropathy inducing a chronic activation of the sympathetic nervous system [72].
Psychosocial disorders
One of the most common forms of discrimination in modern societies is weight discrimination [73]. Impressively, negative attitudes about obesity have been evidenced in some healthcare professionals, consequently disturbing patient care [74]. Weight stigma is associated with adverse physiological and psychological outcomes [75]. Obesity stigmatization starts in schools, therefore, children and adolescents living with obesity experience high proportions of bullying and are at an increased risk for social isolation [76]. Later in life, weight-based stigma weakens opportunities for career development and employment. Body dissatisfaction has being identified as a strong correlate with unfavorable obesity-related behavior among PlwO and specially among women [77]. Depression, anxiety disorders, attention deficit hyperactivity disorder, substance abuse, binge-eating, trauma, bipolar disorder, and schizophrenia are the most frequent psychiatric disorders associated with obesity [78].
Cancer
After smoking obesity accounts for the second cause of cancer that can be prevented [79, 80]. The association of obesity with an elevated cancer risk is observed for esophageal, gastric cardia, gallbladder, colorectal, hepatocellular and pancreatic adenocarcinomas, renal cancer, thyroid cancer, ovarian and endometrium cancer, meningioma, hematological cancer (leukemia, lymphoma, multiple myeloma), prostate, and breast cancer in postmenopausal women [6, 79, 80]. The main pathways linking both entities include hyperinsulinemia, IR, abnormalities of the IGF-1 signaling, low-grade inflammation, oxidative stress, altered intestinal microbiome, and mechanical forces, as elucidated in the common pathophysiology [81, 82].
Towards a novel diagnostic framework
Precision medicine allows applying more intensified measures for primary prevention of metabolic abnormalities. Figure 3 summarizes the holistic syndemic approach of PlwO. In the decades to come, it is expected that a broader range of elements that better reflect the complexity of obesity (i.e. genotype, adipotype, microbiome, and exposome) may be evaluated [17, 83].
Fig. 3.
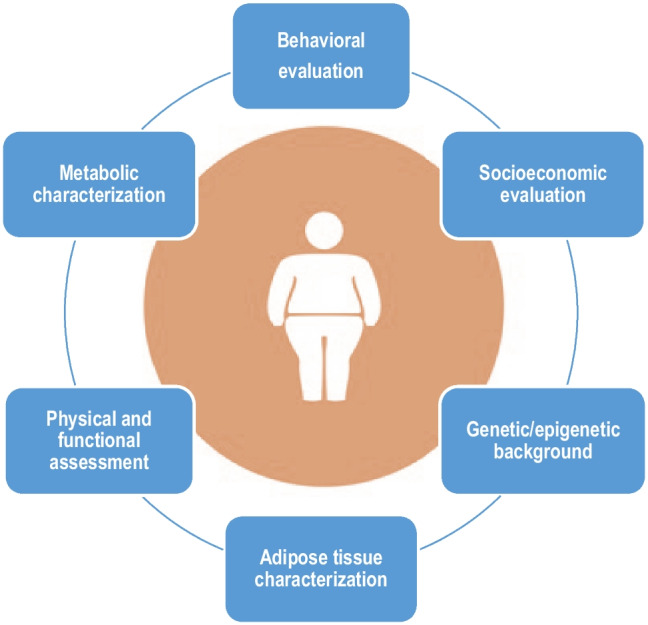
Holistic syndemic approach of people living with obesity
Diverse factors influence the phenotype of people living with obesity. Addressing genetics, epigenetics, metabolic, social, lifestyle, and behavioral aspects may help enhancing a better diagnosis and management
AT amount and distribution are key features of obesity-related diseases. On the last decade, translational studies have provided evidence that VAT has a strong correlation with metabolic diseases [84, 85]. Therefore, clinicians need to properly evaluate PlwO in a dynamic framework. Anthropometric measures of central adiposity like waist circumference [86–88], WHtR [89–91], and more specifically: VAT [92], VAT to SCAT ratio [93], liver steatosis [94], and epicardial adipose tissue [95], among others, have a central role in the development of impaired metabolic disease. In this line, some normal weight individuals may have excess of VAT and a high cardiovascular risk, exposing the limitations of BMI for health evaluation in the general population [87]. Fatty liver index, abdominal ultrasound or Fibroscan must be performed to rule out MAFLD. Morphofunctional assessment has also shown to provide very useful clinical information. A thorough assessment of all potential obesity-associated alterations should be analyzed in a systematic and holistic way.
Several aspects may be considered in the precision medicine era for the diagnostic approach of PlwO with a wide perspective, thereby including quite diverse spheres. The Edmonton Obesity Staging System has proposed the use of a mnemonic consisting of four Ms to help the hard-working practitioner navigate through an exhaustive and careful assessment of PlwO [96]. Figure 4 summarizes the mnemonic of the four Ms standing for: mental, mechanical, metabolic and monetary, to assess the drivers and complications of obesity.
Fig. 4.
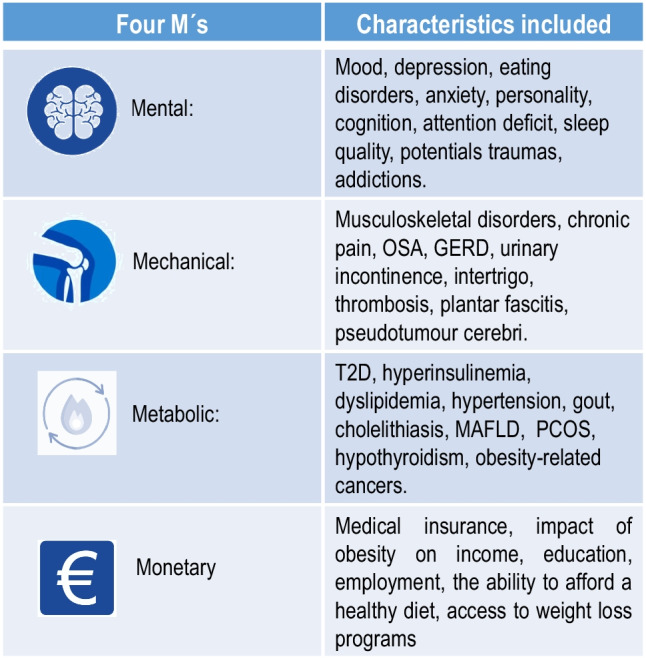
The 4 M’s Mnemonic Framework to assess drivers and complications of obesity [96]
Mnemonic framework proposed by the Edmonton Obesity Staging System for the assessment of the patient living with obesity GERD: gastroesophageal reflux disease; MAFLD: metabolic dysfunction-associated fatty liver disease; OSA: obstructive sleep apnea; T2D: Type 2 diabetes mellitus, PCOS: polycystic ovary syndrome
Psychosocial evaluation is essential in obesity management with the purpose of identifying potential road blocks and challenges that facilitate behavioral changes aimed at enhancing long-term weight management [73]. Not recognizing mental health issues is likely to result in poor compliance as well as high rates of weight regain [96]. The psychosocial profile is also helpful in identifying potential contraindications for undergoing bariatric surgery (i.e. substance abuse, poorly controlled depression).
Beside the behavioral assessment, socioeconomical evaluation, mechanical, and metabolic comorbidities evaluation through AT dysfunction assessment, serum markers and histopathological features, among others features, should be included in the holistic approach of obesity.
Inflammation markers
AT synthesizes and releases a number of factors collectively called adipokines, like adiponectin and leptin, closely related to cardiometabolic risk [97–99]. Leptin is predominantly secreted by AT proportionally to AT amount, being directly implicated in food intake control and energy regulation [99]. Adiponectin is known for its anti-inflammatory effect and decreases in PlwO [42]. The Adpn/Lep ratio is reportedly better related with IR than with each of the adipokines alone [98]. In epidemiological studies, an increase in this ratio has been related with a decreased risk of atherosclerosis and some cancer types [100]. An Adpn/Lep ratio ≥ 1.0 can be considered normal, a ratio ≥ 0.5 and < 1.0 indicates moderate to medium increased risk, while a ratio < 0.5 suggests a severe elevation in cardiometabolic risk [42]. Other adipokines [101], like osteopontin, calprotectin [102], pigment-epithelium derived factor [103], ghrelin [104], and adipocyte-derived lipopolysaccharide binding protein [10], are also involved in inflammation and insulin resistance, as is the case with aquaporins [105] and caveolins [106].
In MAFLD, transient elastography [107] and non-invasive markers of fibrosis (e.g. NAFLD Fibrosis Score [NFS] [108] and Fibrosis 4 Score [FIB-4] [109]) have reportedly provided high diagnostic precision in advanced stages of hepatic fibrosis (F3–F4) and associate with MACE [110] and subclinical cardiovascular disease [111]. If available, the enhanced liver fibrosis test (ELF-Test) can be determined as it reflects the liver extracellular matrix metabolism, it measures the levels of amino-terminal propeptide of type III procollagen, tissue inhibitor of metalloproteinases 1, and hyaluronic acid [32, 112].
Histopathological features
Whenever possible, the histological analysis of AT should be pursued. Histopathological features of AT may predict the possibility of developing diseases associated to obesity or the potential therapeutic response to intervention (i.e. bariatric surgery) [4]. Sampling abdominal subcutaneous and omental AT should be a standard care procedure for PlwO undergoing bariatric surgery. A high fibrosis score in subcutaneous fat [113], a low omental fat mast cell count [114], and a high adipocyte cell size [115], can predict a reduced postoperative weight-loss after bariatric surgery. The balance between lipolysis and lipogenesis is a further relevant aspect given the involvement of adipokines in lipid metabolism regulation and cardiometabolic risk [116–119].
The term “metabolically healthy obesity (MHO)” and “metabolically unhealthy obesity (MUHO)” have been proposed to phenotype and establish risk in PlwO [120]. The MHO definition is still a matter of debate, nonetheless, research has reportedly shown proven risk of CVD not only in MUHO but also in MHO [121–124]. Evaluating subcutaneous adipocyte size in patients with obesity without any comorbid pathology (or “MHO”), may anticipate glycemic control deterioration in patients with even normal glucose tolerance [125, 126], thus metabolic health represents a dynamic marker of elevated risk for progression to unhealthy phenotypes [127]. Inflammatory cytokines concentrations in the diverse obesity phenotypes [5], also support the AT dysfunction continuum gradually leading to the unhealthy phenotype conversion [19, 128].
Molecular features
In the last decade, studies have identified molecular patterns that could theoretically aid in personalizing obesity care; for example, subcutaneous microRNA expression may be related to the magnitude of weight loss [129, 130]. A higher visceral AT miRNA-122 expression anticipates the magnitude of weight loss following bariatric surgery [131]. Moreover, modern ‘omics’ technologies, single-cell RNA-sequencing of stromovascular fat cells, or single-nucleus RNA-sequencing are potential tools to define specific phenotypes in response to weight loss change based on the underlying complexity of energy homeostasis control and, therefore, may predict response to the diverse therapeutic approaches [4, 17]. In this line, recent studies, have also evidenced that environmental influences affect the epigenetic state, phenotype, and susceptibility to different diseases of next generations [17].
Addressing innovative therapeutic approaches
In the last years, substantial knowledge related to the biology of obesity has been gained. Unfortunately, comprehension has had little impact on obesity prevalence [84]. The clinical phenotype of PlwO is complex, thereby reflecting the interconnection between environmental, genetic, epigenetic, and lifestyle factors [17]. To appropriately approach the burden of obesity, a paradigm change is needed [83]. Management of obesity requires long-term follow-up to monitor treatment goals, regarding lifestyle changes and comorbidities [132]. Treatment instauration and goals must be personalized based also on the amount and distribution of fat, beyond BMI. Biological, psychosocial, and economic factors influencing health must be considered, individually and globally. Conventionally, approaches are stepwise, lifestyle interventions represent the first step being followed by the application of anti-obesity drugs, endoscopical procedures (e.i. endoscopic gastroplasty, gastric balloon), and consideration of bariatric surgery [32, 133]. However, currently a multimodal approach seems to be better. After bariatric surgery, pharmacological treatment [134, 135] or endoscopic procedures [136] may be further considered for weight regain.
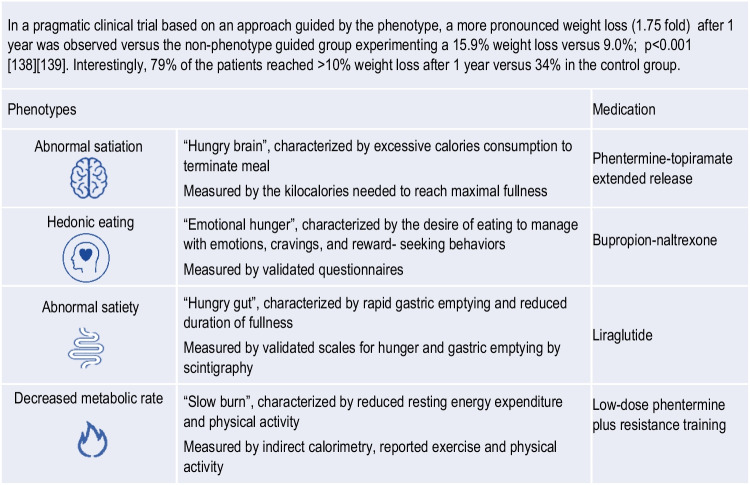
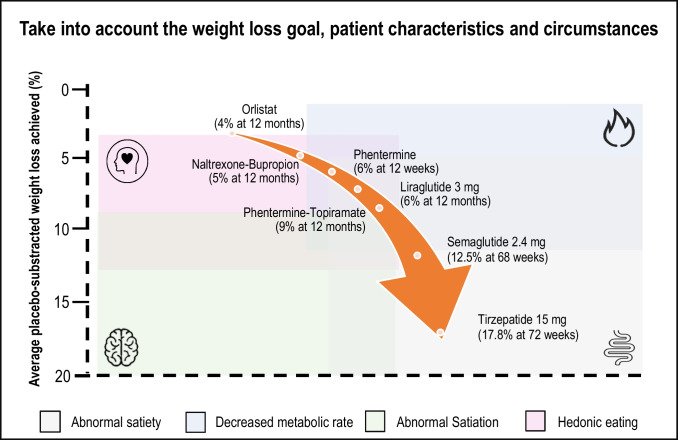
Patient circumstances, preferences, availability, costs, and comorbidities must be considered in the selection of treatment [137]. Acosta et al. have proposed the selection of antiobesity medications based on energy balance phenotypes [138]. Interestingly, two or more phenotypes were identified in 27% of PlwO whereas in 15% of the participants, a specific biological phenotype was not identified. Food intake depends on hunger, satiation, gastric emptying, satiety, and emotional eating; and expenditure depends on resting energy expenditure, physical activity, and exercise. In brief, four distinct profiles were identified by these main characteristics: i) hungry brain, ii) emotional hunger, iii) hungry gut and iv) slow brain. Table 1 describes the phenotypes described by Acosta et al. Figure 5 considers energy balance phenotypes and available antiobesity medications. This therapeutic approach has evidenced a more pronounced weight loss as compared to the use of standard care antiobesity pharmacotherapy. Nonetheless, the medication prescribed for each phenotype may be a matter of debate, as GLP-1 receptor agonists may act on different levels, for instance, on abnormal satiation and satiety.
Table 1.
Biological and behavioural phenotype-guided pharmacotherapy to optimize obesity therapy in a precision medicine context
Fig. 5.
Schematic illustration of plausible phenotype-guided pharmacotherapy
Selection of anti-obesity medications centered on energy balance phenotypes (based on Acosta et al) [138]
Conclusions
Obesity is a complex disease affecting almost every organ and system of the body. Clinicians and politicians need to collaborate in the paradigm change characterized by an holistic approach. Future perspectives on adipobiology with innovative novel molecular and histopathological findings may help us predict which patients will respond better to medical, endoscopic, surgical, or mixed treatment. Whilst precision medicine has advanced remarkably in some specialties like oncology, in the field of obesity, progress has been hampered by old-fashioned views of the disease itself, the applied technology for its diagnosis and the scarcity of treatment tools. A long-term comprehensive strategy with multidimensional initiatives focusing on sustainable changes aimed at improving health and well-being rather than achieving a specific weight target should be pursued. Noteworthy, success can be different for every individual ranging from a better quality of life to greater self-esteem, a 5% weight loss, a decrease in cardiometabolic risk factors, prevention of weight regain, among others.
Abbreviations
- ABCD
Adiposity-based chronic disease
- Adpn/Lep
Adiponectin/leptin ratio
- AT
Adipose tissue
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- ELF-Test
Enhanced liver fibrosis test
- FFA
Free fatty acids
- GERD
Gastroesophageal reflux disease
- IR
Insulin resistance
- MACE
Major adverse cardiovascular events
- MHO
Metabolically healthy obesity
- MUHO
Metabolically unhealthy obesity
- MAFLD
Metabolic associated fatty liver disease
- OHS
Obesity hypoventilation syndrome
- OrAD
Obesity-related adipose tissue disease
- OSA
Obstructive sleep apnea
- PlwO
People living with obesity
- RAS
Renin-angiotensin-aldosterone system
- SCAT
Subcutaneous adipose tissue
- SHBG
Sex hormone-binding globulin
- T2D
Type 2 diabetes
- VAT
Visceral adipose tissue
- WHtR
Waist to height ratio
Author contribution
Each author contributed to the part of their main expertise area, which was then critically revised by all authors until reaching the final version.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Spanish Institute of Health ISCIII (Subdirección General de Evaluación and Fondos FEDER project PI22/00745) and CIBEROBN.
Declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Financial or non-financial interests
DD reports speaker and Advisory fee from: Novonordisk, Lilly, BI, Astra Zenca. CMP reports speaker fees from Novo Nordisk. GF reports research grants paid to her Institution from the Carlos III Health Institute. GF reports payment or honoraria for attendance to the 2022 Lilly Diabetes Global Medical Affairs Portfolio Advisory Board. GF is member of the OPEN Spain Initiative. GF is a co-chair of the Scientific Advisory Board of the European Association for the Study of Obesity—it is an unpaid position.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: An EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12:131–136. doi: 10.1159/000497124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskaran K, dos-Santos-Silva I, Leon DA,, et al. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, Romundstad P, Vatten LJ. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;4(353):i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pincu Y, Yoel U, Haim Y, et al. Assessing obesity-related adipose tissue disease (OrAD) to improve precision medicine for patients living with obesity. Front Endocrinol (Lausanne) 2022;13:1–17. doi: 10.3389/fendo.2022.860799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamjane N, Benyahya F, Nourouti NG, et al. Cardiovascular diseases and metabolic abnormalities associated with obesity: What is the role of inflammatory responses? A systematic review. Microvasc Res. 2020;131. 10.1016/j.mvr.2020.104023. [DOI] [PubMed]
- 6.Cypess AM. Reassessing Human Adipose Tissue. N Engl J Med. 2022;386:768–779. doi: 10.1056/nejmra2032804. [DOI] [PubMed] [Google Scholar]
- 7.Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185:419–446. doi: 10.1016/j.cell.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez A, Ezquerro S, Méndez-Giménez L, et al. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol - Endocrinol Metab. 2015;309:E691–714. doi: 10.1152/ajpendo.00297.2015. [DOI] [PubMed] [Google Scholar]
- 9.Liang W, Qi Y, Yi H, et al. The roles of adipose tissue macrophages in human disease. Front Immunol. 2022;13:1–11. 10.3389/fimmu.2022.908749. [DOI] [PMC free article] [PubMed]
- 10.Moreno-Navarrete JM, Escoté X, Ortega F, et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia. 2013;56:2524–2537. doi: 10.1007/s00125-013-3015-9. [DOI] [PubMed] [Google Scholar]
- 11.Unamuno X, Gómez-Ambrosi J, Rodríguez A, et al. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48:1–11. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- 12.Lipke K, Kubis-Kubiak A, Piwowar A. Molecular mechanism of lipotoxicity as an interesting aspect in the development of pathological states—current view of knowledge. Cells. 2022;11. 10.3390/cells11050844. [DOI] [PMC free article] [PubMed]
- 13.De Fano M, Bartolini D, Tortoioli C, et al. Adipose tissue plasticity in response to pathophysiological cues: A connecting link between obesity and its associated comorbidities. Int J Mol Sci. 2022;23. 10.3390/ijms23105511. [DOI] [PMC free article] [PubMed]
- 14.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants Redox Signal. 2013;19:1110–1120. 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed]
- 15.Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11:1–25. doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira FG, Reitz LK, Valmorbida A, et al. Metabolically unhealthy and overweight phenotypes are associated with increased levels of inflammatory cytokines: a population-based study. Nutrition. 2022;96:111590. 10.1016/j.nut.2022.111590. [DOI] [PubMed]
- 17.Frühbeck G, Kiortsis DN, Catalán V. Precision medicine: diagnosis and management of obesity. Lancet Diabetes Endocrinol. 2018;6:164–166. doi: 10.1016/S2213-8587(17)30312-1. [DOI] [PubMed] [Google Scholar]
- 18.Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease a scientific statement from the American heart association. Circulation. 2021;143:E984–1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel N, Li Y, Kuxhaus O, et al. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714–724. doi: 10.1016/S2213-8587(18)30137-2. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 21.Strazzullo P, D’Elia L, Cairella G, et al. Excess body weight and incidence of stroke: Meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:418–426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 22.Horn JW, Feng T, Morkedal B, et al. Obesity and risk for first ischemic stroke depends on metabolic syndrome: The HUNT study. Stroke. 2021;52:3555–3561. doi: 10.1161/STROKEAHA.120.033016. [DOI] [PubMed] [Google Scholar]
- 23.Katta N, Loethen T, Lavie CJ, Alpert MA. Obesity and coronary heart disease: Epidemiology, pathology, and coronary artery imaging. Curr Probl Cardiol. 2021;46:11–20. doi: 10.1016/j.cpcardiol.2020.100655. [DOI] [PubMed] [Google Scholar]
- 24.Mafort TT, Rufino R, Costa CH, Lopes AJ. Obesity: Systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip Respir Med. 2016;11:1–11. doi: 10.1186/s40248-016-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein PD, Matta F, Goldman J. Obesity and pulmonary embolism: The mounting evidence of risk and the mortality paradox. Thromb Res. 2011;128:518–523. doi: 10.1016/j.thromres.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Bonsignore MR, Baiamonte P, Mazzuca E, et al. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip Respir Med. 2019;14:1–12. doi: 10.1186/s40248-019-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinella ME. Nonalcoholic fatty liver disease a systematic review. J Am Med Assoc. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 29.Abenavoli L, Milic N, Di Renzo L, et al. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22(31):7006–7016. doi: 10.3748/wjg.v22.i31.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budd J, Cusi K. Non-alcoholic fatty liver disease: What does the primary care physician need to know? Am J Med. 2020;133(5):536–543. doi: 10.1016/j.amjmed.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Cusi K, Isaacs S, Barb D, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical setting. Endocr Pract. 2022;28:528–562. doi: 10.1016/j.eprac.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: A systematic review and meta-analysis. Am J Gastroenterol. 2006;101:2619–2628. doi: 10.1111/j.1572-0241.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 35.Aune D, Norat T, Vatten LJ. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol. 2015;30:1009–1019. doi: 10.1007/s10654-015-0081-y. [DOI] [PubMed] [Google Scholar]
- 36.Fraquelli M, Pagliarulo M, Colucci A, et al. Gallbladder motility in obesity, diabetes mellitus and coeliac disease. Dig Liver Dis. 2003;35:12–16. doi: 10.1016/S1590-8658(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 37.Aune D, Mahamat-Saleh Y, Norat T, Riboli E. High body mass index and central adiposity is associated with increased risk of acute pancreatitis: a meta-analysis. Dig Dis Sci. 2021;66:1249–1267. doi: 10.1007/s10620-020-06275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poddar M, Chetty Y, Chetty VT. How does obesity affect the endocrine system? A narrative review Clin Obes. 2017;7:136–144. doi: 10.1111/cob.12184. [DOI] [PubMed] [Google Scholar]
- 39.Taylor R. Calorie restriction for long-term remission of type 2 diabetes. Clin Med J R Coll Physicians London. 2019;19:37–42. doi: 10.7861/clinmedicine.19-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaps L, Labenz C, Galle PR, Weinmann-menke J, Kostev K. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. 2020;8(8):942–948. doi: 10.1177/2050640620944098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuero C, Valenti V, Rotellar F, et al. Revisiting the ghrelin changes following bariatric and metabolic surgery. Obes Surg. 2020;30:2763–2780. doi: 10.1007/s11695-020-04601-5. [DOI] [PubMed] [Google Scholar]
- 42.Frühbeck G, Catalán V, Rodríguez A, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. 2019;11:1–13. doi: 10.3390/nu11020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semins MJ, Shore AD, Makary MA, et al. The impact of obesity on urinary tract infection risk. Urology. 2012;79:266–269. doi: 10.1016/j.urology.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 44.Lai HH, Helmuth ME, Smith AR. Relationship between central obesity, general obesity, overactive bladder syndrome and urinary incontinence among male and female patients seeking care for their lower urinary tract symptoms. Urology. 2019;123:34–43. doi: 10.1016/j.urology.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: Clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189:102–106. doi: 10.1016/j.juro.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed MH, Ahmed HT, Khalil AA. Renal stone disease and obesity: What is important for urologists and nephrologists? Ren Fail. 2012;34:1348–1354. doi: 10.3109/0886022X.2012.723777. [DOI] [PubMed] [Google Scholar]
- 47.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 48.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 49.Martin WP, Bauer J, Coleman J, et al. Obesity is common in chronic kidney disease and associates with greater antihypertensive usage and proteinuria: evidence from a cross-sectional study in a tertiary nephrology centre. Clin Obes. 2020;10:1–12. doi: 10.1111/cob.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.García-Carro C, Vergara A, Bermejo S, et al. A nephrologist perspective on obesity: from kidney injury to clinical management. Front Med. 2021;8:655871. doi: 10.3389/fmed.2021.655871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster MC, Hwang SJ, Massaro JM, et al. Association of subcutaneous and visceral adiposity with albuminuria: The framingham heart study. Obesity. 2011;19:1284–1289. doi: 10.1038/oby.2010.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovesdy CP, Furth SL, Zoccali C. Obesity and kidney disease: Hidden consequences of the epidemic. Am J Nephrol. 2017;45:283–291. doi: 10.1159/000458467. [DOI] [PubMed] [Google Scholar]
- 53.Deng Y, Zhao Q, Gong R. Association between metabolic associated fatty liver disease and chronic kidney disease: A cross-sectional study from NHANES 2017–2018. Diabetes, Metab Syndr Obes Targets Ther. 2021;14:1751–1761. doi: 10.2147/DMSO.S292926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong SH, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. 2020;21(2):494. doi: 10.3390/ijms21020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15:321–335. doi: 10.1159/000521241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh TP, Arnold JB, Evans AM, et al. The association between body fat and musculoskeletal pain: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2018;19:1–13. doi: 10.1186/s12891-018-2137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: Evidence, hypotheses and horizons - a scoping review. Obes Rev. 2014;15:578–586. doi: 10.1111/obr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu B, Hiraki LT, Sparks JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann Rheum Dis. 2014;73:1914–1922. doi: 10.1136/annrheumdis-2014-205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeMarco MAMA, Maynard JW, Huizinga MM, et al. Obesity and younger age at gout onset in a community-based cohort. Arthritis Care Res. 2011;63:1108–1114. doi: 10.1002/acr.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nielsen SM, Bartels EM, Henriksen M, et al. Weight loss for overweight and obese individuals with gout: A systematic review of longitudinal studies. Ann Rheum Dis. 2017;76:1870–1882. doi: 10.1136/annrheumdis-2017-211472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng Y-T, Li Y-Z, Huang S-Y, et al. Association of life course adiposity with risk of incident dementia: a prospective cohort study of 322,336 participants. Mol Psychiatry. 2022;27:3385–3395. doi: 10.1038/s41380-022-01604-9. [DOI] [PubMed] [Google Scholar]
- 62.Favieri F, Forte G, Casagrande M. The Executive Functions in Overweight and Obesity: A Systematic Review of Neuropsychological Cross-Sectional and Longitudinal Studies. Front Psychol. 2019;10:2126. doi: 10.3389/fpsyg.2019.02126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrmann MJ, Tesar A, Beier J, et al. Grey matter alterations in obesity: A meta-analysis of whole-brain studies. Obes Rev. 2019;20:464–471. doi: 10.1111/obr.12799. [DOI] [PubMed] [Google Scholar]
- 64.Miller AA, Spencer SJ. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Li J-W, Zong Y, Cao X-P, et al. Microglial priming in Alzheimer’s disease. Ann Transl Med. 2018;6:176. doi: 10.21037/atm.2018.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Mao L, Xu P, Wang Y. Effects of epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients. 2018;10:1681. doi: 10.3390/nu10111681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salas-Venegas V, Flores-Torres RP, Rodríguez-Cortés YM, et al. The obese brain: Mechanisms of systemic and local inflammation, and interventions to reverse the cognitive deficit. Front Integr Neurosci. 2022;16:1–19. doi: 10.3389/fnint.2022.798995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moretti R, Caruso P. Small vessel disease: Ancient description, novel biomarkers. Int J Mol Sci. 2022;23:3508. doi: 10.3390/ijms23073508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westgate CSJ, Israelsen IME, Jensen RH, Eftekhari S. Understanding the link between obesity and headache- with focus on migraine and idiopathic intracranial hypertension. J Headache Pain. 2021;22:123. doi: 10.1186/s10194-021-01337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: Epidemiology, mechanisms, and implications. Headache J Head Face Pain. 2010;50:631–648. doi: 10.1111/j.1526-4610.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol. 2016;73:1468. doi: 10.1001/jamaneurol.2016.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lambert E, Sari CI, Dawood T, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56:351–358. doi: 10.1161/HYPERTENSIONAHA.110.155663. [DOI] [PubMed] [Google Scholar]
- 73.Sarwer DB, Polonsky HM. The psychosocial burden of obesity. Endocrinol Metab Clin North Am. 2016;45:677–688. doi: 10.1016/j.ecl.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubino F, Logue J, Bøgelund M, et al. Attitudes about the treatment of obesity among healthcare providers involved in the care of obesity-related diseases: A survey across medical specialties in multiple European countries. Obes Sci Pract. 2021;7:659–668. doi: 10.1002/osp4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu YK, Berry DC. Impact of weight stigma on physiological and psychological health outcomes for overweight and obese adults: A systematic review. J Adv Nurs. 2018;74:1030–1042. doi: 10.1111/jan.13511. [DOI] [PubMed] [Google Scholar]
- 76.Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26:485–497. doi: 10.1038/s41591-020-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weinberger NA, Kersting A, Riedel-Heller SG, Luck-Sikorski C. Body dissatisfaction in individuals with obesity compared to normal-weight individuals: A systematic review and meta-analysis. Obes Facts. 2017;9:424–441. doi: 10.1159/000454837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perry C, Guillory TS, Dilks SS. Obesity and psychiatric disorders. Nurs Clin North Am. 2021;56(4):553–563. doi: 10.1016/j.cnur.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 79.WHO European Regional Obesity Report 2022. Copenhagen: WHO Regional Office for Europe. 2022. Licence: CC BY-NC-SA 3.0 IGO.
- 80.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Catalán V, Gómez-Ambrosi J, Rodríguez A, Frühbeck G. Adipose tissue immunity and cancer. Front Physiol. 2013;1–13. 10.3389/fphys.2013.00275. [DOI] [PMC free article] [PubMed]
- 82.Pérez-Hernández AI, Catalán V, Gómez-Ambrosi J, et al. Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol (Lausanne) 2014;5:1–17. doi: 10.3389/fendo.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yárnoz-Esquiroz P, Olazarán L, Aguas-Ayesa M, et al. ‘Obesities’: Position statement on a complex disease entity with multifaceted drivers. Eur J Clin Invest. 2022;52. 10.1111/eci.13811. [DOI] [PMC free article] [PubMed]
- 84.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020:1477–1500. 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed]
- 85.Neeland IJ, Poirier P, Després J-P. Cardiovascular and metabolic heterogeneity of obesity. Circulation. 2018;137:1391–1406. doi: 10.1161/circulationaha.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cameron AJ, Magliano DJ, Söderberg S. A systematic review of the impact of including both waist and hip circumference in risk models for cardiovascular diseases, diabetes and mortality. Obes Rev. 2013;14:86–94. doi: 10.1111/j.1467-789X.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 87.Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16:177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diemer FS, Brewster LM, Haan YC, Oehlers GP, van Montfrans GA, Nahar-van Venrooij LMW. Body composition measures and cardiovascular risk in high-risk ethnic groups. Clin Nutr. 2019;38:450–456. doi: 10.1016/j.clnu.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 89.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 90.Gibson S, Ashwell M. A simple cut-off for waist-to-height ratio (0·5) can act as an indicator for cardiometabolic risk: Recent data from adults in the Health Survey for England. Br J Nutr. 2020;123:681–690. doi: 10.1017/S0007114519003301. [DOI] [PubMed] [Google Scholar]
- 91.Chen N, Hu LK, Sun Y, et al. Associations of waist-to-height ratio with the incidence of type 2 diabetes and mediation analysis: Two independent cohort studies. Obes Res Clin Pract. 2023:1–7. 10.1016/j.orcp.2022.12.005. [DOI] [PubMed]
- 92.Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:1–41. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaess BM, Pedley A, Massaro JM, et al. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55:2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aneni EC, Bittencourt MS, Teng C, et al. The risk of cardiometabolic disorders in lean non-alcoholic fatty liver disease: A longitudinal study. Am J Prev Cardiol. 2020;4:100097. doi: 10.1016/j.ajpc.2020.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cosson E, Nguyen MT, Rezgani I, et al. Epicardial adipose tissue volume and myocardial ischemia in asymptomatic people living with diabetes: a cross-sectional study. Cardiovasc Diabetol. 2021;20:1–10. doi: 10.1186/s12933-021-01420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharma AM. M, M, M & M: A mnemonic for assessing obesity. Obes Rev. 2010;11:808–809. doi: 10.1111/j.1467-789X.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 97.Gómez-Ambrosi J, Salvador J, Páramo JA, et al. Involvement of leptin in the association between percentage of body fat and cardiovascular risk factors. Clin Biochem. 2002;35:315–320. doi: 10.1016/S0009-9120(02)00320-X. [DOI] [PubMed] [Google Scholar]
- 98.Frühbeck G, Catalán V, Rodríguez A, et al. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-06997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghantous CM, Azrak Z, Hanache S, et al. Differential role of leptin and adiponectin in cardiovascular system. Int J Endocrinol. 2015;534320. 10.1155/2015/534320. [DOI] [PMC free article] [PubMed]
- 100.Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. doi: 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Landecho MF, Tuero C, Valentí V, et al. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients. 2019;11:1–16. doi: 10.3390/nu11112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Increased levels of calprotectin in obesity are related to macrophage content: Impact on inflammation and effect of weight loss. Mol Med. 2011;17:1157–1167. doi: 10.2119/molmed.2011.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sabater M, Moreno-Navarrete JM, Ortega FJ, et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J Clin Endocrinol Metab. 2010;95:4720–4728. doi: 10.1210/jc.2010-0630. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez A, Gómez-Ambrosi J, Catalán V, et al. The ghrelin O-Acyltransferase-Ghrelin system reduces TNF-α-Induced apoptosis and autophagy in human visceral adipocytes. Diabetologia. 2012;55:3038–3050. doi: 10.1007/s00125-012-2671-5. [DOI] [PubMed] [Google Scholar]
- 105.Obesity FG. Aquaporin enters the picture. Nature. 2005;438:436–437. doi: 10.1038/438436b. [DOI] [PubMed] [Google Scholar]
- 106.Méndez-Giménez L, Rodríguez A, Balaguer I, Frühbeck G. Role of aquaglyceroporins and caveolins in energy and metabolic homeostasis. Mol Cell Endocrinol. 2014;397:78–92. doi: 10.1016/j.mce.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 107.Lombardi R, Airaghi L, Targher G, et al. Liver fibrosis by FibroScan independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020:347–54. 10.1111/liv.14274. [DOI] [PubMed]
- 108.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005:1313–21. 10.1002/hep.20701. [DOI] [PubMed]
- 109.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 110.Targher G, Byrne CD, Lonardo A, et al. Nonalcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis of observational studies. J Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 111.Perdomo CM, Ezponda A, Córdoba JMN, Herrero JI, Bastarrika G, Frühbeck G, et al. Transient elastography and serum markers of liver fibrosis associate with epicardial adipose tissue and coronary artery calcium in NAFLD. Sci Rep. 2022:1–10. 10.1038/s41598-022-10487-3. [DOI] [PMC free article] [PubMed]
- 112.Xie Q, Zhou X, Huang P, et al. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: A meta-analysis. PLoS One. 2014;9(4):e92772. doi: 10.1371/journal.pone.0092772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lassen PB, Charlotte F, Liu Y, et al. The fat score, a fibrosis score of adipose tissue: Predicting weight-loss outcome after gastric bypass. J Clin Endocrinol Metab. 2017;102:2443–2453. doi: 10.1210/jc.2017-00138. [DOI] [PubMed] [Google Scholar]
- 114.Goldstein N, Kezerle Y, Gepner Y, et al. Higher mast cell accumulation in human adipose tissues defines clinically favorable obesity sub-phenotypes. Cells. 2020;9(6):1508. doi: 10.3390/cells9061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cotillard A, Poitou C, Torcivia A, et al. Adipocyte size threshold matters: Link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99:1466–1470. doi: 10.1210/jc.2014-1074. [DOI] [PubMed] [Google Scholar]
- 116.Frühbeck G, Gómez-Ambrosi J, Salvador J. Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J. 2001;15:333–340. doi: 10.1096/fj.00-0249com. [DOI] [PubMed] [Google Scholar]
- 117.Frühbeck G, Gómez-Ambrosi J. Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell Signal. 2001;13:827–833. doi: 10.1016/S0898-6568(01)00211-X. [DOI] [PubMed] [Google Scholar]
- 118.Pulido MR, Diaz-Ruiz A, Jiménez-Gómez Y, et al. Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity. PLoS One. 2011;6. 10.1371/journal.pone.0022931. [DOI] [PMC free article] [PubMed]
- 119.Poulain-Godefroy O, Lecoeur C, Pattou F, et al. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol. 2008;295:1–7. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]
- 120.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 121.Zhou Z, Macpherson J, Gray SR, Gill JMR, Welsh P, Celis-Morales C, et al. Are people with metabolically healthy obesity really healthy? A prospective cohort study of 381,363 UK Biobank participants. Diabetologia. 2021;64:1963–1972. doi: 10.1007/s00125-021-05484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perdomo CM, Núñez-Córdoba JM, Ezponda A, et al. Cardiometabolic characterization in metabolic dysfunction-associated fatty liver disease. Front Med (Lausanne) 2022;20(9):1023583. doi: 10.3389/fmed.2022.1023583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gómez-Ambrosi J, Catalán V, Rodríguez A, et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37:2813–2821. doi: 10.2337/dc14-0937. [DOI] [PubMed] [Google Scholar]
- 124.Chang Y, Ryu S, Suh BS, et al. Impact of BMI on the incidence of metabolic abnormalities in metabolically healthy men. Int J Obes. 2012;36:1187–1194. doi: 10.1038/ijo.2011.247. [DOI] [PubMed] [Google Scholar]
- 125.Lönn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24:326–331. doi: 10.1096/fj.09-133058. [DOI] [PubMed] [Google Scholar]
- 126.Weyer C, Foley JE, Borgadus C, et al. Enlarged subcutaneous adbominal adipocyte size, but not obesity itself, predicts Type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 127.Elías-López D, Vargas-Vázquez A, Mehta R, et al. Natural course of metabolically healthy phenotype and risk of developing Cardiometabolic diseases: a three years follow-up study. BMC Endocr Disord. 2021;21:1–12. doi: 10.1186/s12902-021-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hosseinpanah F, Tasdighi E, Barzin M, et al. The association between transition from metabolically healthy obesity to metabolic syndrome, and incidence of cardiovascular disease: Tehran lipid and glucose study. PLoS One. 2020;15:1–13. doi: 10.1371/journal.pone.0239164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giardina S, Hernández-Alonso P, Salas-Salvadó J, et al. Modulation of human subcutaneous adipose tissue MicroRNA profile associated with changes in adiposity-related parameters. Mol Nutr Food Res. 2018;62:1–24. doi: 10.1002/mnfr.201700594. [DOI] [PubMed] [Google Scholar]
- 130.Ortega FJ, Mercader JM, Catalán V, et al. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- 131.Liao CH, Wang CY, Liu KH, et al. MiR-122 marks the differences between subcutaneous and visceral adipose tissues and associates with the outcome of bariatric surgery. Obes Res Clin Pract. 2018;12:570–577. doi: 10.1016/j.orcp.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 132.van Baak MA, Mariman ECM. Mechanisms of weight regain after weight loss — the role of adipose tissue. Nat Rev Endocrinol. 2019;15:274–287. doi: 10.1038/s41574-018-0148-4. [DOI] [PubMed] [Google Scholar]
- 133.Perdomo CM, Cohen RV, Sumithran P, Clément K, Frühbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023;401:1116–1130. doi: 10.1016/S0140-6736(22)02403-5. [DOI] [PubMed] [Google Scholar]
- 134.Elhag W, El Ansari W. Effectiveness and safety of liraglutide in managing inadequate weight loss and weight regain after primary and revisional bariatric surgery: anthropometric and cardiometabolic outcomes. Obes Surg. 2022;32:1005–1015. doi: 10.1007/s11695-021-05884-y. [DOI] [PubMed] [Google Scholar]
- 135.Badurdeen D, Hoff AC, Hedjoudje A, et al. Endoscopic sleeve gastroplasty plus liraglutide versus endoscopic sleeve gastroplasty alone for weight loss. Gastrointest Endosc. 2021;93:1316–1324.e1. doi: 10.1016/j.gie.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 136.Brunaldi VO, Jirapinyo P, de Moura DTH, et al. Endoscopic treatment of weight regain following roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Surg. 2018;28:266–276. doi: 10.1007/s11695-017-2986-x. [DOI] [PubMed] [Google Scholar]
- 137.Semlitsch T, Stigler FL, Jeitler K, et al. Management of overweight and obesity in primary care—A systematic overview of international evidence-based guidelines. Obes Rev. 2019;20:1218–1230. doi: 10.1111/obr.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Acosta A, Camilleri M, Abu Dayyeh B, et al. Selection of antiobesity medications based on phenotypes enhances weight loss: A pragmatic trial in an obesity clinic. Obesity. 2021;29:662–671. doi: 10.1002/oby.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]