Nearly every human malady, be it injury, infection, chronic disease, or degenerative disease, damages tissues (1). Moreover, 45% of all deaths can be traced to inflammation- and fibrosis-related regenerative failures (1). Restoring health after damage requires the answer to a key question: How can human tissues be coaxed to regenerate? Identifying instructive cues that direct refractory tissues down a regenerative path remains a critical yet elusive goal. Nonetheless, approaches to target roadblocks that impede regeneration, including insufficient and/or functionally inadequate progenitor cells, fibrosis, and chronic inflammation, are continuing to progress from bench to bedside. Pivotal advances have been made to overcome these hurdles using cell therapy, in vivo reprogramming, synthetic biology, and antifibrotic and anti-inflammatory therapies, but many challenges remain and knowledge gaps must be addressed to make regeneration a mainstay of modern medicine (1).
The most conspicuous requirement for regenerative therapies is to replace the components of tissues that were lost or compromised by disease. Invigorating endogenous stem cells is an appealing strategy, but, to date, the greatest benefits have emerged from cell therapies. Adult stem cell–based regenerative therapies have shown clinical benefit to treat hematological malignancies, burn wounds, and ocular degeneration. These therapeutic modalities also lend themselves to gene editing to correct monogenic perturbations. For instance, a young boy suffering from junctional epidermolysis bullosa, a lethal skin disease caused by mutations in the laminin-332 gene, was treated with an autologous (patient’s own) skin transplant (2). A millimeter-sized sample of the boy’s skin was collected and transduced with a retroviral vector expressing the wild-type laminin 332 cDNA; the tissue was then expanded ex vivo before being grafted to restore 80% of the body surface area.
Human pluripotent stem cell (hPSC)–based therapies have also shown promise and have entered clinical trials in the United States for type 1 diabetes (T1D; clinical trial NCT04786262), Parkinson’s disease (PD; NCT02452723 and NCT03119636), and age-related macular degeneration (AMD; NCT04339764) (3). These three diseases are particularly amenable to stem cell–based therapies because they are associated with deficiency of a defined cell type: insulin producing pancreatic islets in T1D, midbrain dopaminergic (mDA) neurons in PD, and retinal pigment epithelial cells in AMD. Moreover, transplantation sites are surgically accessible. Lab-generated hPSCs are being assessed for safety and efficacy in reducing disease symptoms. A case report of a PD patient who received midbrain transplant of autologous hPSC-derived mDA neurons showed stable grafting without immune reaction and exhibited improved motor function 2 years after implantation (4).
Despite these early glimpses of success, cell therapies are hampered by many biological and technical hurdles. Autologous hPSC-based therapies derived from induced pluripotent stem cells (iPSCs) avoid immune aggravation, but this cost- and labor-intensive strategy requires safety testing for each use. A major concern with nonautologous cell therapy is immune rejection of the graft. Thus, “off-the-shelf” allogeneic therapies must be coupled to strategies that allow them to avoid rejection, such as systemic immunosuppression in T1D hPSC therapy (NCT04786262). An alternative strategy is to encapsulate the graft within a selectively permeable barrier to allow for diffusion of nutrients and the therapeutic molecules (e.g., insulin) while excluding immune components that would reject the engrafted cells. To avoid fibrotic foreign body responses, recent strategies for chemically modified alginate encapsulation showed promise in a nonhuman primate model. However, a phase 1 clinical trial that used a similar approach to deliver cells expressing factor VIII for the treatment of hemophilia A (NCT04541628) was halted after a patient experienced a serious adverse immune event and, subsequently, the implanted capsules were found to have fibrosed. Nonetheless, encapsulation strategies are moving forward in other contexts, including with recent US Food and Drug Administration (FDA) clearance for human trials of encapsulated stem cell–derived pancreatic islets (NCT05791201). In addition, ongoing work is investigating other strategies to reduce immune rejection, including genetic engineering of grafted cells to evade immune recognition or to secrete immune modulatory cytokines that induce localized immune tolerance.
Producing sufficient numbers of cells for engraftment has been successful in the skin (2) but poses a major challenge for regenerating tissue in other organs, particularly if only a small proportion of cells survive after transplant. For example, the survival rates of hPSC-derived mDA neurons is ~0.5 to 10%, and efforts are ongoing to identify the underlying mechanisms of graft loss. In addition, generating appropriate grafts when complex cell mixtures are required, such as in the lung to enable gas exchange, remains a challenge. Preclinical studies that leverage tissue self-assembly and organoid formation to transfer complex tissue units are underway.
Tissue overgrowth is a major concern of stem cell therapies because transferred cells can give rise to teratomas or other tumors. Growth-stop signals, particularly in the case of cell therapy, are not well understood, and molecular “off switches” will be vital to incorporate when deploying regenerative therapies. The Hippo signaling pathway was long considered a master developmental regulator of organ size because inactivating mutations in this pathway produced overgrowth phenotypes. Yet recent studies indicate that rather than engaging normal programs of development, Hippo pathway activation induces ectopic expression of aberrant growth pathways in Drosophila melanogaster (5). Whether developmental pathways are reengaged to rebuild adult tissues or adult regeneration requires a different set of regulators, as suggested by the study of Hippo signaling, is unclear. Thus, it will be necessary to carefully define microenvironmental and cell-intrinsic growth-restriction signals in age-appropriate and organ-specific models to restrain the growth of transplanted cells.
Instructive microenvironmental signals are often necessary for optimal cellular function. The success of stem cell therapies, which may be delivered intravenously, therefore depends on migration and integration into appropriate tissue niches. Cell or tissue grafts must also connect with the host’s nervous and vascular machinery, and the signals involved remain poorly understood (6). An alternative approach to overcome the challenge of directing and integrating grafts in hard-to-reach internal organs is to repurpose cells that are present at the damage site by reprogramming them in situ with specific transcription factors (6). Reprogramming has been a particularly attractive strategy for the adult heart, which, unlike the embryonic heart, lacks bona fide progenitors. A subset of in vivo cellular reprogramming efforts are aimed at converting fibroblasts into cardiomyocytes to maintain cardiac function (6). Alternatively, transient expression of pluripotent transcription factors in adult cardiomyocytes induced proliferation, resulting in improved outcomes in adult mice after myocardial infarction (7). However, prolonged expression of these factors generated tumors. To overcome this substantial safety challenge, efforts to use enhancer elements first identified in highly regenerative zebrafish models to express reprogramming factors only in injury conditions are underway (8). Knowledge of which cells are reprogrammed, if and how they survive, what they become, and precisely how they contribute to clinical outcomes will be crucial to forecast treatment efficacy.
In addition to replacing lost tissue with cell therapies and in vivo reprogramming, many injurious and complex disease states evoke inflammatory responses that must also be suppressed (see the figure). The state of the recipient tissue, often diseased, remains a challenging facet of applying cell therapies. Cells or tissues may fail to engraft in inhospitable environments, or the underlying pathology that destroyed the original tissue may also damage the graft (9). For example, inflammatory bowel diseases (IBDs) are driven by aberrant immune responses, defective epithelial repair, loss of normal intestinal microbial communities, and translocation of bacteria into underlying tissue. Although supplying intestinal epithelial cells to the damaged intestines of an IBD patient may serve as a temporary patch, without mitigating inflammation or translocating microbes, these approaches face substantial hurdles to achieving long-term efficacy.
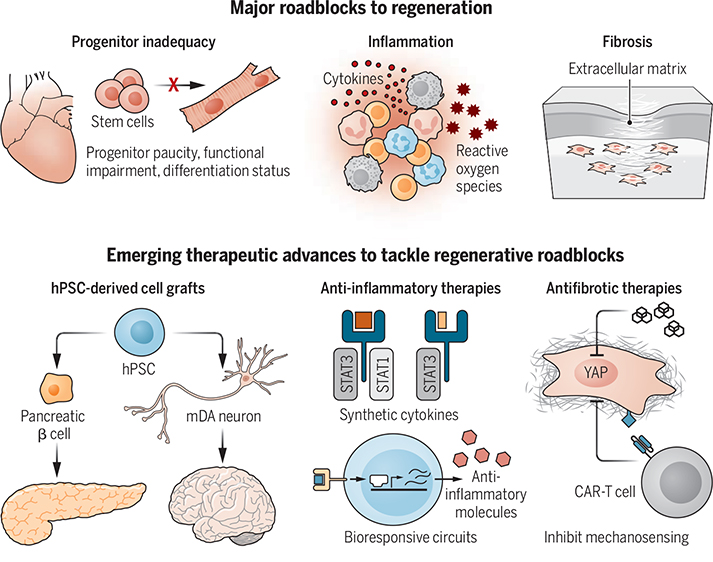
Therapeutic frontiers fueling advances in regenerative medicine.
The three major roadblocks to regeneration are functional inadequacy of progenitors, chronic inflammation, and fibrosis. Ongoing clinical trials are testing the efficacy of hPSC-derived β cells and mDA neurons to treat type 1 diabetes and Parkinson’s disease, respectively. Synthetic cytokines and bioresponsive systems are being developed to limit inflammation. Inhibiting mechanosensing pathways in fibroblasts and fibroblast antigen–specific CAR-T cells are being used to limit fibrosis.
CAR, chimeric antigen receptor; hPSC, human pluripotent stem cell; mDA, midbrain dopaminergic; STAT, signal transducer and activator of transcription; YAP, Yes-associated protein.
New approaches to modulate inflammation include engineering stem cells with bioresponsive gene circuits that can sense inflammatory factors such as cytokines or reactive oxygen species and, in turn, induce the production of anti-inflammatory factors, allowing endogenous progenitors or transplanted cells to repair damage (9). Indeed, the beneficial effects of some stem cell therapies have been traced to immune-modulatory effects of the transplant, rather than the tissue-generating properties of the stem cells themselves. In a mouse model of cardiac ischemic injury, transplanting stem cells augmented cardiac function, not by production of cardiomyocytes but by activating macrophages that limit extracellular matrix (ECM) deposition and modulate the mechanical properties of an injured area to rejuvenate the heart (10).
Tempering the immune system presents a formidable challenge because the same factor can promote repair or inflict damage through inflammatory effects, depending on context (11). For instance, early in wounding, interleukin-17 (IL-17) enables hypoxia adaptation of damaged epithelium, but persistent IL-17 signaling potentiates pathology by recruiting damage-causing neutrophils (11). Synthetic biology provides opportunities to divorce the damage-causing effects of inflammation from those involved in repair. IL-22 induces the expression of proregenerative signal transducer and activator of transcription 3 (STAT3) and pro-inflammatory STAT1 transcription factors. Engineering IL-22 with altered receptor binding to only induce regenerative STAT3 boosted intestinal stem cell proliferation in a mouse model of radiation injury, without driving STAT1-mediated inflammatory outcomes (11).
A critical hurdle to regeneration is fibrosis, which rapidly plugs damaged tissue by haphazardly depositing ECM. Fibrosis profoundly compromises tissue mechanics and cellular interactions and physically obstructs organ function (1, 12). Antiscarring therapies have been notoriously hard to achieve because profibrotic factors such as transforming growth factor–β (TGFβ) also have important functions in maintaining health. Alternatively, modulating mechanical signaling by inhibiting fibroblast Yes-associated protein (YAP), a mechanosensory transcription coregulator, prevented scarring during skin repair in mice (12). Notably, opposing fibrosis in this manner was sufficient to restore the skin’s architecture and tensile strength. Similarly, using engineered chimeric antigen receptor (CAR) T cells to target conserved antigens on ECM-generating cardiac fibroblasts reduced fibrosis and revived heart function after ischemic injury (13). These studies indicate that adult organs may still possess molecular roadmaps to activate regenerative responses. A comparison of fibroblasts from regenerating reindeer antler skin and scarring back skin uncovered that inflammatory priming distinguishes the profibrotic state (14). Thus, precisely targeting inflammation may also ameliorate fibrosis and unlock latent regenerative capacity (1).
What was once considered the future of medicine is now becoming reality. But there is no magic pill for regeneration (yet). In addition to scientific and technological innovation, there are also practical considerations of cost and production. Innovations in regenerative therapies for complex diseases or damage involving multiple cell types have been hampered by the lack of appropriate preclinical models and a paucity of fundamental information on instructive signals to build tissues. Accordingly, efforts to systematically chart tissue repair over time, in different model systems, and after different types of damage are now underway (14). Rather than limiting therapies to the rules of mammalian physiology, radical strategies from non-vertebrate species and even the plant kingdom are surfacing. For example, nanosized plant photosynthetic systems that augment chondrocyte anabolism could limit cartilage degradation and osteoarthritis in mice (15). Finally, achieving regeneration in humans will require a rapid transition from rodent models to clinically relevant large animal and human studies. Ascending the summit of human regeneration demands an interdisciplinary effort that brings together biologists, biomedical engineers, and clinicians. The view from the top will reveal a transformed medical landscape that is able to seamlessly rejuvenate organs, ultimately extending human life span and health span.
ACKNOWLEDGMENTS
The authors thank the participants of the Janelia Research Campus “R3 – Replace, Repair, Regenerate” workshop (2021) for inspiring this perspective. S.N. is a New York Stem Cell Foundation Robertson Stem Cell investigator and is funded by National Institutes of Health (NIH) grants 1DP2AR079173-01 and R01-AI168462. K.L.M. is a New York Stem Cell Foundation Robertson Stem Cell investigator and is funded by NIH grants DP2HD111708 and R00HD101021. M.T.L. is supported by NIH grants R01-GM116892, R01-GM136659, and U24-DE02946. S.N. is on the scientific advisory board of Seed, Inc.; is a consultant for BiomX; and receives research funding from Takeda Pharmaceuticals.
REFERENCES AND NOTES
- 1.Henderson NC et al. , Nature 587, 555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch T et al. , Nature 551, 327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim TW et al. , Front. Cell Dev. Biol. 8, 729 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schweitzer JS et al. , N. Engl. J. Med. 382, 1926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowalczyk W et al. , Science 378, eabg3679 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Srivastava D, DeWitt N, Cell 166, 1386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y et al. , Science 373, 1537 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Yan R et al. , Cell Stem Cell 30, 96 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilak F et al. , J. Orthop. Res. 37, 1287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vagnozzi RJ et al. , Nature 577, 405 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guenin-Mace L et al. , Annu. Rev. Immunol. 41, 207 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbott HE et al. , Cell Stem Cell 29, 1161 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rurik JG et al. , Science 375, 91 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha S et al. , Cell 185, 4717 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P et al. , Nature 612, 546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]