Abstract
A widespread degenerative condition of the aorta, abdominal aortic aneurysm (AAA), severely endangers the health of middle-aged and elderly people. SPARC related modular calcium binding2 (SMOC2) is upregulated in the carotid arteries of rats with atherosclerotic lesions, but its function in AAA is still unknown. Therefore, the aim of this research was to evaluate the function of SMOC2 in AAA. The results showed that in the AAA tissues, SMOC2 expression was upregulated compared with healthy controls. Overexpression of SMOC2 promoted vascular smooth muscle cells (VSMCs) proliferation, migration, and extracellular matrix (ECM) degradation. In contrast, silence of SMOC2 inhibited VSMCs proliferation, migration, and ECM degradation. Overexpression of SMOC2 promoted BMP and TGF-β1 expression and silence of SMOC2 had an opposite effect. Besides, inhibition of BMP or TGF-β1 suppressed VSMCs cell proliferation, migration, and ECM degradation. Moreover, inhibition BMP or TGF-β1 reversed the promotive effects of SMOC2 overexpression on VSMCs proliferation, migration, and ECM degradation. SMOC2 may affecte the formation of AAA by upregulating BMP and TGF-β1 to regulate the proliferation, migration, and ECM degradation of VSMCs.
Keywords: SMOC2, TGF-β1, proliferation, migration, abdominal aortic aneurysm
Introduction
Abdominal aortic aneurysm (AAA) is a widespread chronic aortic disease with high morbidity and mortality, which poses a great risk to middle-aged and old people’s lives and health. However, There is still a lack of suitable drugs to interrupt the development of AAA.(1) Therefore, the basic research to identify the molecular mechanism of AAA is particularly important to discover new biomarkers and therapeutic targets. Clinical studies have shown that irregular aortic vascular smooth muscle cells (VSMCs) proliferation, macrophage infiltration, and degradation of the extracellular matrix (ECM) are closely linked to the incidence and development of AAA.(2) Therefore, targeting VSMCS proliferation, migration, and ECM degradation may be a potential treatment strategy for AAA.
SPARC related modular calcium binding 2 (SMOC2) encodes an ECM non-structurally secreted modular calcium binding protein 2, which contains 457 amino acids and was originally identified from the extracellular extract of articular cartilage.(3) It belongs to the SPARC (Secreted Protein Acidic and Rich in Cysteines) secreted protein family, which have the function of regulating the interaction between cells and matrix.(4) SMOC2 is a stromal cell protein expressed in a range of tissues, including the adult lungs and aorta. There is evidence that SMOC2 is upregulated in the injured aortic vessel wall, which suggests that it plays a role in tissue remodeling.(5) Besides, SMOC2 is up-regulated in the carotid artery of rats with atherosclerotic lesions.(6) These data indicate that SMOC2 may be related to arterial vascular disease. However, the function and mechanism of SMOC2 in aortic aneurysms, especially AAA, are still unclear. The transformation of transforming growth factor-β1 (TGF-β1) and its downstream effector molecules is important for ECM reshaping, cell proliferation and migration.(7) TGF-β1 has been confirmed to be related to the pathogenesis of AAA.(8) A recent research have shown that SMOC2 can interact with TGF-β1 and that SMOC2 overexpression promoted the expression of TGF-β1 in primary mouse liver cells.(9)
The aim of the current study was to investigate the role and mechanisms of SMOC2 in VSMCs proliferation, migration, and ECM degradation, and to provide a valid theoretical basis for clinical treatment of abdominal aortic aneurysms.
Materials and Methods
Patients and tissue samples
The 25 AAA patients were from Weihai Municipal Hospital. AAA tissue and normal abdominal aortic tissue were obtained from AAA patients through surgery. Immediately after resection, the AAA tissue and normal aortic tissue of each participants were quick-frozen in liquid nitrogen and stored at −80°C. The study was approved by Weihai Municipal Hospital, and each participant’s written informed consent was obtained.
RNA isolation and quantitative real-time reverse transcription PCR
The total RNA of tissues or VSMCs were extracted with TRIzol reagent (Invitrogen, Carlsbad, CA), cDNA was synthesized by reverse transcription with First strand cDNA synthesis kit (VWR International, Leuven, Belgium). Quantitative real-time reverse transcription PCR (qRT-PCR) was performed with the DNA Engine OpticonTM real-time PCR system (MJ Research, Hercules, CA). The PCR program is as follows: 94°C for 2 min, then 94°C for 30 s, 56°C for 30 s, and 72°C for 60 s. The data were analyzed by the 2−ΔΔCT method. The primer sequences are as follows: SMOC2, forward 5'-CCCAAGCTCCCCTCAGAAG-3', and reverse 5'-GCCACACACCTGGACACAT-3', GAPDH, forward 5'-AGGTCGTGTTGAACGGATTTG-3', and reverse 5'-TGTAGACCATGTAGTTGAGGTCA-3'.
Cell culture and treatment
VSMCs of human was purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were incubated in RPMI-1640 (Sigma, St. Louis, MO) medium containing 10% Fetal bovine serum (FBS; Gibco, Rockville, MD) in a humidified incubator (37°C, 5% CO2). VSMCs were treated with 1 μM Ang II for different times (0, 12, 24, and 48 h).
Cell transfection
SMOC2 overexpression plasmid (ov-SMOC2) and negative control (ov-NC), SMOC2 interference plasmid (si-SMOC2) and negative control (si-NC) were purchased from Shanghai GenePharma Inc. (Shanghai, China). Lipofectamine 3000 (Invitrogen) was utilized for plasmid transfection. Si-NC sequences: sense 5'-UUCUCCGAACGUGUCACGUTT-3' and antisense 5'-ACGUGACACGUUCGGAGAATT-3'; si-SMOC2 sequences: sense 5'-GCGACAUGAACAAUGACAATT-3' and antisense 5'-UUGUCAUUGUUCAUGUCGCTT-3'.
CCK-8 assay
The CCK-8 kit was utilized to detect VSMCs proliferation. VSMCs (5 × 103 cells/well) were seeded in 96-well culture plates and treated separately according to groups. Then 10 μl of CCK-8 solution was added. Incubation was continued for 4 h. The absorbance value was measured at 450 nm using an enzyme meter.
Transwell assay
Using Transwell migration chambers (8-μm pore size; Millipore, Boston, MA), cell migration was assessed. In the upper compartment of the invasion chamber filled with FBS-free culture medium, cells were seeded into the lower chamber containing 700 μl RPMI-1640 medium with 10% FBS as a chemoattractant. The cells remaining in the upper compartment were swabbed off by cotton swabs after incubation at 37°C for 48 h. Migrated cells were washed with PBS, stained with 0.1% crystal violet, fixed with 4% paraformaldehyde, and counted by light microscopy.
Enzyme-linked immunosorbent assay
Human matrix metalloproteinase (MMP)-2 enzyme-linked immunosorbent assay (ELISA) Kit and Human MMP-9 ELISA Kit (Elabscience, Wuhan, China) were used to detect the contents of MMP-2 and MMP-9 in VSMCs. The cell lysis supernatant was added to the enzyme labeled plates coated with MMP-2 or MMP-9 antibodies. After washing, biotinylated secondary antibodies and horseradish peroxidase-labeled affin were added sequentially. After washing the plate, add the chromogenic substrate (TMB). The OD value was measured at 450 nm using an enzyme marker. The contents of MMP-2 or MMP-9 in the samples were calculated by plotting the standard curve.
Western blot
VSMCS were twice washed with PBS at 4°C, after which the whole proteins was extracted by RIPA buffer. The proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). Then the primary antibody against Alpha-smooth muscle actin (α-SMA) (ab5694; Abcam, Cambridge, UK), SMOC2 (MA5-24301; Invitrogen), TGF-β1 (ab215715; Abcam), tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) (MS608PABX; Invitrogen), osteopontin (OPN) (PA5-34579; Invitrogen), GAPDH (ab8245; Abcam), bone morphogenetic protein 2 (BMP-2) (PA5-85956; Invitrogen), p-Smad1/5 (PA5-105003; Invitrogen), p-Smad2 (40-0800; Invitrogen), and p-Smad3 (44-246G; Invitrogen), was hybridized at 37°C for 2 h. These membranes were then incubated with secondary antibodies for 1.5 h at 37°C. The protein bands were detected with chemiluminescence reagents (Abcam).
Statistical analysis
GraphPad Prism 6 software was used to analyze the data. All data was expressed as mean ± SD. Statistical analysis was performed using one-way or two-way analysis of variance (ANOVA) and t test. P<0.05 indicates that the difference is statistically significant.
Results
SMOC2 expression was increased in the abdominal aorta of AAA patients and Ang II-treated VSMCs
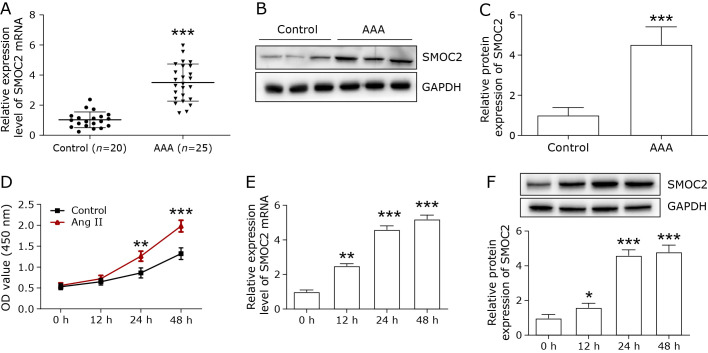
The mRNA and protein expression of SMOC2 in AAA tissue and normal abdominal aortic tissue were examined by qPCR and Western blot. The SMOC2 mRNA level was remarkably increased in AAA tissue compared with normal abdominal aortic tissue (control) (Fig. 1A). Besides, the protein level of SMOC2 in AAA tissue was markedly higher than that in control group (Fig. 1B and C).
Fig. 1.
The expression of SMOC2 in the abdominal aorta of AAA patients and Ang II-treated VSMCs. (A) The mRNA expression of SMOC2 in the abdominal aorta of AAA patients was evaluated by qPCR. (B, C) Western blot was used to detect the protein expression of SMOC2 in the abdominal aorta of AAA patients. The t test was used for statistical analysis. (D) CCK-8 was used to detect the proliferation and migration of Ang II-treated VSMCs at different time points (0, 12, 24, and 48 h). Two-way ANOVA was used for statistical analysis. (E, F) qPCR and Western blot were employed to detect SMOC2 expression in Ang II-treated VSMCs at different time points (0, 12, 24, and 48 h). One-way ANOVA was used for statistical analysis. *p<0.05 compared to 0 h; **p<0.01 compared to 0 h; ***p<0.001 compared to 0 h or control group.
Next, we investigate the effects of Ang II on SMOC2 expression in VSMCs. VSMCs were treated with Ang II for 48 h. CCK-8 assay was performed to detect the proliferation at different times (0, 12, 24, and 48 h). Ang II remarkably promoted cell proliferation in a time-dependent manner (Fig. 1D). The expression of SMOC2 mRNA and protein were increased in a time-dependent manner by Ang II treatment (Fig. 1E and F).
SMOC2 promoted VSMCs proliferation and migration
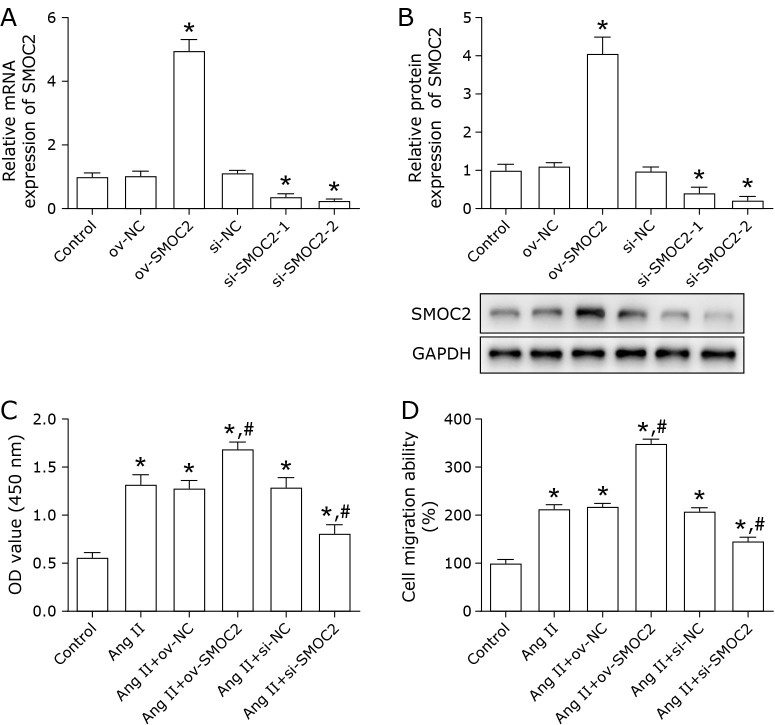
To evaluate the influence of SMOC2 on VSMCs proliferation and migration, overexpression or silencing of SMOC2 was achieved in VSMCs by transfection with ov-SMOC2, si-SMOC2. Transfection efficiency was validated using qPCR and Western blot (Fig. 2A and B). The results suggested that silencing of SMOC2 markedly decreased Ang II-induced VSMCs proliferation (Fig. 2C), and inhibited Ang II-treated VSMCs migration (Fig. 2D), while overexpression of SMOC2 showed the opposite effects (Fig. 2C and D).
Fig. 2.
SMOC2 promotes Ang II-treated VSMCs proliferation and migration. Ang II-treated VSMCs were transfected with ov-SMOC2 or si-SMOC2. (A, B) the transfect efficiency was evaluated using qPCR and Western blot. *p<0.05 compared to control group. (C, D) CCK-8 and Transwell assays were used to detect the proliferation and migration of Ang II-treated VSMCs. One-way ANOVA was used for statistical analysis. *p<0.05 compared to control group; #p<0.05 compared to Ang II group.
SMOC2 facilitated ECM degradation
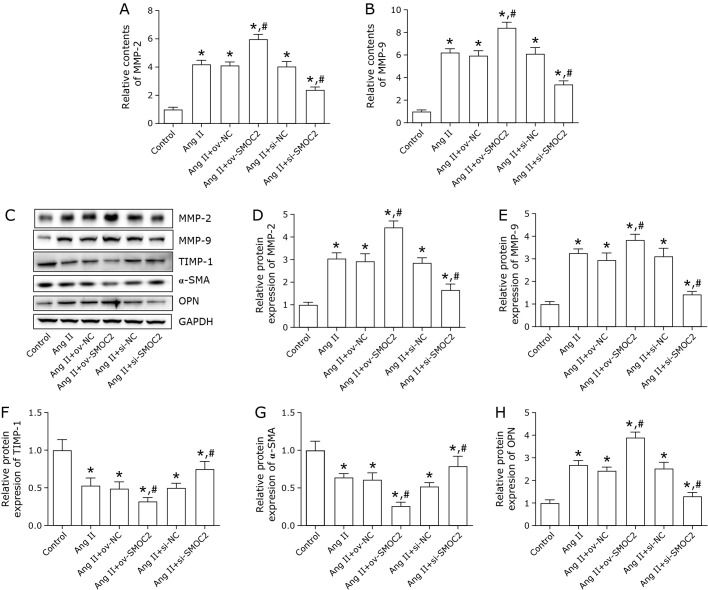
We also tested the influence of SMOC2 on Ang II-treated VSMCs ECM degradation. The results showed the content and protein expression of MMP-2 and MMP-9 were significantly elevated, and TIMP-1 protein expression was reduced by SMOC2 overexpression (Fig. 3A–F). SMOC2 inhibition had an opposite effect as evidenced by decreased MMP-2 and MMP-9 expression and increased TIMP-1 expression (Fig. 3A–F). Besides, The protein expression level of α-SMA was down-regulated by SMOC2 overexpression and up-regulated by SMOC2 silencing (Fig. 3G). OPN expression was increased after transfection ov-SMOC2 and decreased after transfection si-SMOC2 (Fig. 3H).
Fig. 3.
SMOC2 promotes Ang II-treated VSMCs ECM degradation. (A, B) The contents of MMP-2 and MMP-9 were evaluated by ELISA assay. (C–H) Western blot was employed to detect the protein expression of MMP-2, MMP-9, TIMP-1, α-SMA, and OPN. One-way ANOVA was used for statistical analysis. *p<0.05 compared to control group; #p<0.05 compared to Ang II group.
Effect of SMOC2 on BMP/TGF-β1 signaling
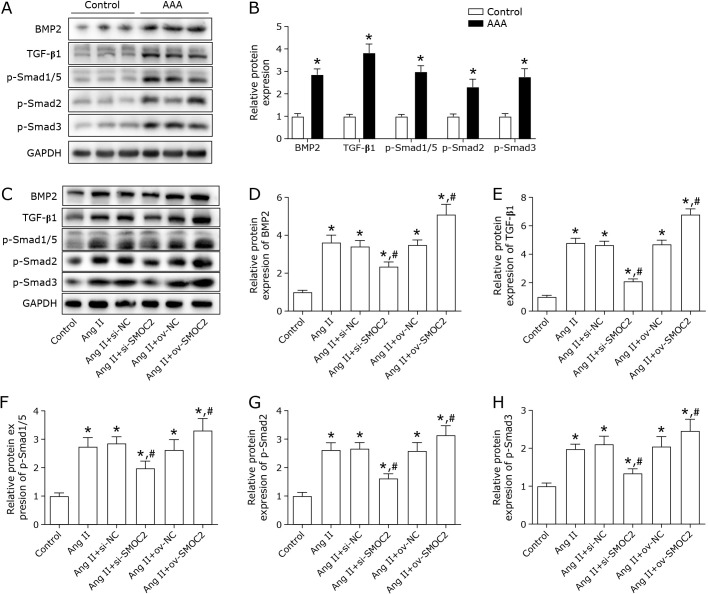
In order to further investigate the mechanisms that involved in the effect of SMOC2, the BMP/TGF-β1 signaling was explored. First, we measured the expression of BMP/TGF-β1 signaling proteins in AAA tissues. The results showed that the protein levels of BMP-2, TGF-β1, p-Smad1/5, p-Smad2, and p-Smad3 were increased in AAA tissues (Fig. 4A and B). In addition, Ang II treatment enhanced the protein expression levels of BMP-2, TGF-β1, p-smad1/5, p-smad2, and p-smad3, which were remarkably decreased by silence of SMOC2 and increased by SMOC2 overexpression (Fig. 4C–H).
Fig. 4.
Effect of SMOC2 on BMP/TGF-β1 signaling. (A, B) Western blot was performed to determine the protein expression of BMP/TGF-β1 signaling related molecular in AAA tissue. The t test was used for statistical analysis. (C–H) BMP/TGF-β1 signaling related proteins were detected in VSMCs by Western blot. One-way ANOVA was used for statistical analysis. *p<0.05 compared to control group; #p<0.05 compared to Ang II group.
Role of BMP/TGF-β1 signaling in the regulation of Ang II-induced VSMCs by SMOC2
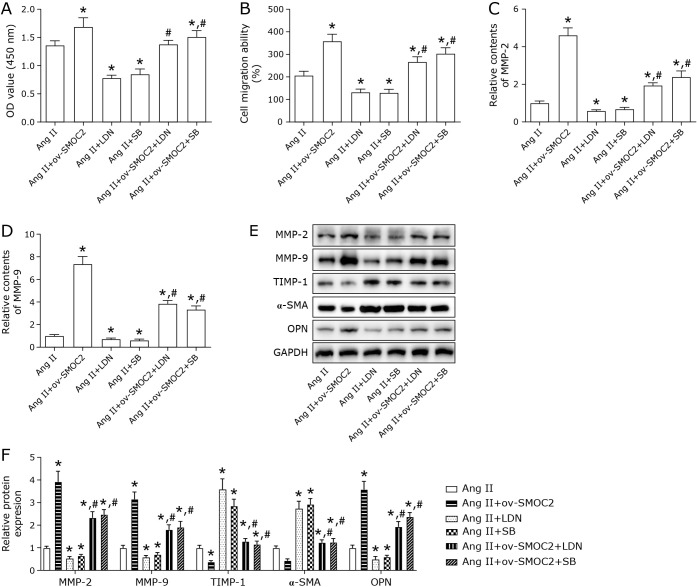
To explore the involvement of BMP/TGF-β1 signaling in SMOC2 modulation of Ang II-induced VSMCs, the BMP receptor inhibitor LDN193189 (LDN), and TGF-β1 receptor inhibitor SB431542 (SB) were used to treat Ang II-injured VSMCs. As indicated in Fig. 5A and B, LDN and SB inhibited the Ang II-treated VSMCs proliferation and migration. LDN and SB decreased the contents of MMP-2 and MMP-9 (Fig. 5C and D). Further, LDN and SB facilitated TIMP-1 and α-SMA expression and reduced OPN expression in Ang II-induced VSMCs (Fig. 5E and F). Subsequently, the rescue experiments were performed to confirm whether SMOC2 regulated Ang II-induced VSMCs proliferation, migration, and ECM degradation via BMP/TGF-β1 pathway. Compared with Ang II + ov-SMOC2 goup, Ang II + ov-SMOC2 + LDN group and Ang II + ov-SMOC2 + SB group showed lower proliferation and migration capacity (Fig. 5A and B), less MMP-2 and MMP-9 contents and protein expression (Fig. 5C–F), higher TIMP-1 and α-SMA level, as well as lower OPN level (Fig. 5E and F). What’s more, we found that LDN and SB effectively inhibited the expression of p-Smad1/5, p-Smad2, and p-Smad3 in the downstream of BMP/TGF-β1 pathway (Supplemental Fig. 1*). These results demonstreated that inhibiting BMP/TGF-β1 signaling partially reversed the influences of SMOC2 on Ang II-treated VSMCs proliferation, migration, and ECM degradation.
Fig. 5.
Inhibiting BMP/TGF-β1 signaling reversed the effects of SMOC2 on Ang II-induced VSMCs proliferation, migration and ECM degradation. (A, B) CCK-8 and Transwell assays were used to detect proliferation and migration. (C, D) The contents of MMP-2 and MMP-9 were evaluated by ELISA assay. (E, F) Western blot was employed to detect the protein expression of MMP-2, MMP-9, TIMP-1, α-SMA, OPN. One-way ANOVA was used for statistical analysis. *p<0.05 compared to Ang II group. #p<0.05 compared to Ang II + ov-SMOC2 group.
Discussion
AAA is a common advanced vascular disease with high mortality and has a major impact on the health of the elderly.(10) Earlier findings found that SMOC2 in atherosclerotic rats’ carotid artery is elevated.(6) However, whether SMOC2 affects the development of AAA is still unknown. In this study, the SMOC2 expression in AAA tissues and Ang II-induced VSMCs was significantly increased. These data indicated that SMOC2 may affect the pathogenesis of AAA.
The specific pathogenesis of AAA is still unclear and its causes are complex. It has been reported that migration and proliferation of smooth muscle cells are key steps in the formation of vascular atherosclerosis and luminal stenosis, which is controlled through coordinated interactions between cells and ECM.(11) Atherosclerosis is traditionally believed to be the cause of AAA.(12) Thus, abnormal proliferation and migration of VSMCs are partly responsible for the formation of abdominal aortic aneurysms. Our study found a significant increase in proliferation and migration in Ang II-treated VSMCs, which is consistent with the findings of Wang et al.,(13) who demontreated that Ang II promoted the proliferation and migration of VSMCs. It is well studied that inhibiting the proliferation and migration of VSMCs could effectively inhibit the formation of AAA.(14,15) We found that silence of SMOC2 inhibited proliferation and migration of Ang II-treated VSMCs, whereas overexpression of SMOC2 had the opposite effect. These data demonstrated that SMOC2 may regulate the AAA developmental process by affecting the proliferation and migration of VSMCs.
More and more studies have confirmed that excessive degradation of ECM and metabolic imbalance are crucial for the development of AAA.(16,17) MMP is one of the major enzyme that cause ECM degradation in smooth muscle cells and is now recognized to play an essential role in the development of AAA. MMP is mainly involved in the degradation of ECM, including elastin, collagen fibers, laminin, and fibronectin.(18) Most members of the MMP family have a role in this process, with MMP-2 and MMP-9 being the most critical. Mice defective in MMP-2 or MMP-9 did not form AAA after CaCl2 perfusion injury.(19) In patients with aneurysms, compared to patients with unruptured aneurysms, there was increased expression and activity of MMP-2 and MMP-9 and higher elastase activity within the arterial wall of the ruptured aneurysm.(20) TIMP-1 is another important regulator of ECM degradation. In AAA, elevated MMP is closely associated with reduced TIMP-1.(21,22) Here, we found that silencing of SMOC2 inhibited MMP-2 and MMP-9 expression and promoted TIMP-1 expression, whereas overexpressing SMOC2 promoted MMP-2 and MMP-9 expression and inhibited TIMP-1 expression in Ang II-induced VSMCs. These data suggested that SMOC2 may modulate the AAA developmental process by regulating ECM degradation.
TGF-β is involved in the pathogenesis of AAA, but the role of TGF-β in the pathogenesis of AAA is controversial.(23,24) For example, overexpression of TGF-β1 in the aortic wall restricts AAA expansion in rats, thereby supporting the protective role of TGF-β in AAA tissues.(25,26) Further, it has been shown that elevated TGF-β concentration is closely associated with inhibition of VSMCs proliferation.(27–31) However, other studies support a different view, DiRenzo et al.(32) reported that the TGF-β signaling pathway prevented the proliferation of VSMCs. Here, we found that inhibition of TGF-β1 promoted Ang II-induced VSMCs proliferation, migration, and ECM degradation. BMP-2, a member of TGF-β superfamily is a key factor for vascular calcification via inducing the osteogenic differentiation of VSMCs.(33) The expression of BMP-2 was significantly increased and associated with the inflamation response in ox-LDL treated coronary artery endothelial cells.(34) Besides, high expression of BMP-2 was observed in hypoxia-induced VSMCs, hrBMP-2 recombinant protein promoted the proliferation and migration, as well as upregulated the activity of MMP-2.(35) Similarly, our study observed that BMP-2 was upregulated by Ang II treatment, and its inhibitor impeded Ang II-induced VSMCs proliferation, migration, and ECM degradation. Thus BMP-2 and TGF-β1 were involved in the Ang II- induced VSMCs injury.
Overexpression of SMOC2 was reported to upregulate the expression of TGF-β1. Recently study indicated that BMP signaling was inhibited by mutant SMOC2.(36) While another ealier study reported SMOC2 depletion in zebrafish inhibited the expression of BMP-2.(37) We found that SMOC2 positively regulte the BMP/TGF-β1 signaling in Ang II-induced VSMCs. Inhibition of BMP/TGF-β1 signaling reversed the facilitative effects of SMOC2 on proliferation, migration, and ECM degradation of VSMCs, suggesting that SMOC2 regulated AAA development through the BMP/TGF-β signaling pathway. These data demonstrated the complexity of the role of BMP/TGF-β1 in controlling the proliferation of VSMCs.
In conclusion, this study showed that SMOC2 is upregulated in AAA tissues and in vitro cell models. The findings demonstrated that SMOC2 may be involved in the pathogenesis of AAA by upregulating BMP/TGF-β1 pathway, and thus promoting the proliferation, migration, and ECM degradation of VSMCs.
Author Contributions
HZ designed the experiments. MW, ZZ, HZ, XZ, GS, QZ, and XW performed the experiments. XW analyzed the data and wrote the manuscript.
Acknowledgments
Not applicable.
Conflict of Interest
No potential conflicts of interest were disclosed.
Availability of Data and Material
All datasets for this study are included in the manuscript/supplementary files.
Supplementary Material
References
- 1.Zhou Y, Wang J, Xue Y, et al. Microarray analysis reveals a potential role of lncRNA expression in 3,4-benzopyrene/angiotensin II-activated macrophage in abdominal aortic aneurysm. Stem Cells Int 2017; 2017: 9495739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legg JS, Legg LM. Abdominal aortic aneurysms. Radiol Technol 2016; 88: 145–163. [PubMed] [Google Scholar]
- 3.Peeters T, Monteagudo S, Tylzanowski P, Luyten FP, Lories R, Cailotto F. SMOC2 inhibits calcification of osteoprogenitor and endothelial cells. PLoS One 2018; 13: e0198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abouzeid H, Boisset G, Favez T, et al. Mutations in the SPARC-related modular calcium-binding protein 1 gene, SMOC1, cause waardenburg anophthalmia syndrome. Am J Hum Genet 2011; 88: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009; 6: 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C. The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem 2006; 281: 22855–22864. [DOI] [PubMed] [Google Scholar]
- 7.Forte A, Galderisi U, Cipollaro M, De Feo M, Della Corte A. Epigenetic regulation of TGF-β1 signalling in dilative aortopathy of the thoracic ascending aorta. Clin Sci (Lond) 2016; 130: 1389–1405. [DOI] [PubMed] [Google Scholar]
- 8.Angelov SN, Hu JH, Wei H, Airhart N, Shi M, Dichek DA. TGF-β (transforming growth factor-β) signaling protects the thoracic and abdominal aorta from angiotensin II-induced pathology by distinct mechanisms. Arterioscler Thromb Vasc Biol 2017; 37: 2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuting Y, Lifeng F, Qiwei H. Secreted modular calcium-binding protein 2 promotes high fat diet (HFD)-induced hepatic steatosis through enhancing lipid deposition, fibrosis and inflammation via targeting TGF-β1. Biochem Biophys Res Commun 2019; 509: 48–55. [DOI] [PubMed] [Google Scholar]
- 10.Lovegrove RE, Javid M, Magee TR, Galland RB. A meta-analysis of 21,178 patients undergoing open or endovascular repair of abdominal aortic aneurysm. Br J Surg 2008; 95: 677–684. [DOI] [PubMed] [Google Scholar]
- 11.Bendeck MP, Irvin C, Reidy M, et al. Smooth muscle cell matrix metalloproteinase production is stimulated via αvβ3 integrin. Arterioscler Thromb Vasc Biol 2000; 20: 1467–1472. [DOI] [PubMed] [Google Scholar]
- 12.Nyberg A, Skagius E, Nilsson I, Ljungh A, Henriksson AE. Abdominal aortic aneurysm and cytomegalovirus infection. J Med Virol 2008; 80: 667–669. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang X, Gao L, et al. Cortistatin exerts antiproliferation and antimigration effects in vascular smooth muscle cells stimulated by Ang II through suppressing ERK1/2, p38 MAPK, JNK and ERK5 signaling pathways. Ann Transl Med 2019; 7: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, Chen G, Wang H. Gadd153 deficiency attenuates abdominal aortic aneurysm formation in mice. Int J Clin Exp Pathol 2018; 11: 169–178. [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Cui L, Yang H, et al. Lysozyme improves the inhibitory effects of Panax notoginseng saponins on phenotype transformation of vascular smooth muscle cells by binding to Ginsenoside Re. Front Nutr 2021; 8: 795888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong LH, Wen JK, Liu G, et al. Blockade of the Ras-extracellular signal-regulated kinase 1/2 pathway is involved in smooth muscle 22α-mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscler Thromb Vasc Biol 2010; 30: 683–691. [DOI] [PubMed] [Google Scholar]
- 17.Lv P, Miao SB, Shu YN, et al. Phosphorylation of smooth muscle 22α facilitates angiotensin II-induced ROS production via activation of the PKCδ-P47phox axis through release of PKCδ and actin dynamics and is associated with hypertrophy and hyperplasia of vascular smooth muscle cells in vitro and in vivo. Circ Res 2012; 111: 697–707. [DOI] [PubMed] [Google Scholar]
- 18.Maguire EM, Pearce SWA, Xiao R, Oo AY, Xiao Q. Matrix metalloproteinase in abdominal aortic aneurysm and aortic dissection. Pharmaceuticals (Basel) 2019; 12: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 2002; 110: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno G, Todor R, Lewis I, Chyatte D. Vascular extracellular matrix remodeling in cerebral aneurysms. J Neurosurg 1998; 89: 431–440. [DOI] [PubMed] [Google Scholar]
- 21.Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci 2014; 71: 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Maegdefessel L. Non-coding RNA contribution to thoracic and abdominal aortic aneurysm disease development and progression. Front Physiol 2017; 8: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin F, Yang X. TGF-β signaling in aortic aneurysm: another round of controversy. J Genet Genomics 2010; 37: 583–591. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Lu H, Rateri DL, Cassis LA, Daugherty A. Conundrum of angiotensin II and TGF-β interactions in aortic aneurysms. Curr Opin Pharmacol 2013; 13: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai J, Losy F, Guinault AM, et al. Overexpression of transforming growth factor-β1 stabilizes already-formed aortic aneurysms: a first approach to induction of functional healing by endovascular gene therapy. Circulation 2005; 112: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Ait-Oufella H, Herbin O, et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest 2010; 120: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moses HL, Yang EY, Pietenpol JA. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 1990; 63: 245–247. [DOI] [PubMed] [Google Scholar]
- 28.Sakakibara K, Kubota K, Worku B, et al. TGF-β inhibits vascular smooth muscle cell proliferation through down regulation of cyclin A. J Surg Res 2003; 114: 283. [Google Scholar]
- 29.Hu W, Wang Z, Li Q, Wang J, Li L, Jiang G. Upregulation of lincRNA-p21 in thoracic aortic aneurysms is involved in the regulation of proliferation and apoptosis of vascular smooth muscle cells by activating TGF-β1 signaling pathway. J Cell Biochem 2019; 120: 4113–4120. [DOI] [PubMed] [Google Scholar]
- 30.Man H, Bi W. Expression of a novel long noncoding RNA (lncRNA), GASL1, is downregulated in patients with intracranial aneurysms and regulates the proliferation of vascular smooth muscle cells in vitro. Med Sci Monit 2019; 25: 1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Zhang Y, Chu L, Chen W, Du Y, Gu J. Long non-coding RNA HIF1A-AS1 is upregulated in intracranial aneurysms and participates in the regulation of proliferation of vascular smooth muscle cells by upregulating TGF-β1. Exp Ther Med 2019; 17: 1797–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiRenzo DM, Chaudhary MA, Shi X, et al. A crosstalk between TGF-β/Smad3 and Wnt/β-catenin pathways promotes vascular smooth muscle cell proliferation. Cell Signal 2016; 28: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Wu P, Shao J, Ke Z, Li D, Wu J. Losartan inhibits vascular calcification by suppressing the BMP2 and Runx2 expression in rats in vivo. Cardiovasc Toxicol 2016; 16: 172–181. [DOI] [PubMed] [Google Scholar]
- 34.Su X, Ao L, Shi Y, Johnson TR, Fullerton DA, Meng X. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J Biol Chem 2011; 286: 12213–12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, Fan Z, Wang F, et al. BMP-2 enhances the migration and proliferation of hypoxia-induced VSMCs via actin cytoskeleton, CD44 and matrix metalloproteinase linkage. Exp Cell Res 2018; 368: 248–257. [DOI] [PubMed] [Google Scholar]
- 36.Long F, Shi H, Li P, et al. A SMOC2 variant inhibits BMP signaling by competitively binding to BMPR1B and causes growth plate defects. Bone 2021; 142: 115686. [DOI] [PubMed] [Google Scholar]
- 37.Bloch-Zupan A, Jamet X, Etard C, et al. Homozygosity mapping and candidate prioritization identify mutations, missed by whole-exome sequencing, in SMOC2, causing major dental developmental defects. Am J Hum Genet 2011; 89: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets for this study are included in the manuscript/supplementary files.