Abstract
Background
Robot-assisted thoracic surgery (RATS) for intrathoracic pathology and especially for mediastinal mass resection has been increasingly accepted as an alternative method to open sternotomy and video-assisted thoracic surgery (VATS). However, the utilization of this approach for complex and advanced in size cases needs more clinical evidence. We are presenting a series of 4 patients who had resection of >10 cm mediastinal masses via RATS.
Cases Description
The mean age was 76.25±10.3 years and 3 were males (75%). All masses were positron emission tomography (PET) positive, and 1 patient had positive Acetyl-cholinesterase antibodies and myasthenia gravis (MG). All patients underwent RATS resection via DaVinci® X system. The dissections were conducted with spatula and/or Maryland bipolar forceps. In 2 cases, the resection was done with bilateral docking, and in 1 case, a drain was not inserted at the end. In 1 patient, pericardial resection was necessitated. All masses were thymomas with 1 dimension measured >10 cm on pathology. All patients were discharged on day 1 or 2 postoperatively with uneventful recoveries. There was no in-hospital, 30- or 90-day mortality. All patients were found to be without issues on follow-up.
Conclusions
This report shows that RATS is safe and can be offered in the management of >10 cm anterior mediastinal masses. The previous size limit of the tumor for minimally invasive and especially RATS approach of 5 cm should be challenged.
Keywords: Robotic, thymoma, thymectomy, case series
Highlight box.
Key findings
• Anterior mediastinal masses >10 cm are successfully resectable with robotic surgery.
What is known and what is new?
• Surgeons treating patients with mediastinal masses >5 cm usually avoid minimally invasive surgery and perform sternotomy.
• Robotic systems allow the resection of mediastinal masses >10 cm, resulting in better postoperative outcomes, quicker recovery and similar to sternotomy oncological results.
What is the implication, and what should change now?
• Robotic systems allow the resection of increased size mediastinal masses via minimally invasive approach, previously not feasible with traditional thoracoscopic techniques. Patients with such masses should be offered robotic resection over sternotomy.
Introduction
Surgical resection is the treatment modality of choice for a significant number for anterior mediastinal tumours (1). Although imaging plays a crucial role in staging and in the establishment of the local invasion, on many occasions this is not possible to be totally clarified preoperatively (2). Moreover, these lesions are mostly symptomatic when they become large enough to compress or invade other organs (3).
Since the introduction of the minimally invasive approaches to the management of the anterior mediastinal tumours, there has been increasing volume of data showing better surgical results, and comparable oncological outcomes in the short and long-term, when compared with open surgery (4-8). Robotic surgery more specifically has been implemented in the armamentarium of the minimally invasive procedures the last decade and has been shown to provide comparable outcomes with the traditional video-assisted thoracic surgery (VATS) (9,10).
In complicated anterior mediastinal masses cases in which either the mass is bigger than 5 cm, or it is infiltrating the pericardium or other structures, most surgeons would prefer to proceed to a sternotomy rather than pursue a VATS or a robotic-assisted thoracic surgery (RATS) resection in order to ensure safe and complete resection (11,12).
Herein, we report a case series of >10 cm and complicated anterior mediastinal masses, which were resected successfully and safely via RATS. We present this article in accordance with the AME Case Series reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-41/rc).
Case presentation
Study design
The present study is a case series of RATS resection of >10 cm and complicated anterior mediastinal masses performed in our institute. The study is retrospective, single-center including non-consecutive cases.
Setting and time-frame
The case series were performed at a tertiary university hospital with a high volume referral Thoracic Surgery department. The time-frame of the cases performed was over a period of 1.5 years (January 2021–June 2022).
Follow-up of patients ranges from 2–17 months and was done for all of them face to face at the outpatient department.
Patients
Four patients are herein reported. The mean age was 76.25±10.3 years and 3 were males (75%). Patients were referred to our department after diagnosing the lesions. Two of the patients were investigated initially for cough and 1 because of myasthenia gravis (MG). In 1 case the mass was found incidentally during a chest X-ray (CXR) for a usual check-up. The demographics of each of the patients and their co-morbidities are reported in Table 1. All patients had a positron emission tomography (PET) scan performed before surgery and all masses were found to be PET positive [mean standardized uptake value (SUV): 3.45±1.23, range SUV 3.2–3.7, Figure 1A,1B]. They also had their acetyl-cholinesterase receptor antibodies determined which were positive in 1 case with MG (Table 1).
Table 1. Demographics and preoperational data of N=4 patients.
| Case | Age (years) | Gender | Co-morbidities | CT mass size (mm) | MG and treatment | CT findings of invasion | SUV | Ab (nmol/L)† |
|---|---|---|---|---|---|---|---|---|
| 1 | 68 | Male | Asthma, IDDM, HT | 105×40×36 | Yes, steroids, pyridostigmine, ICU stay | Pericardium | 3.4 | >20 |
| 2 | 81 | Male | NIDDM, HT | 102×63×76 | No, none | None | 3.2 | negative |
| 3 | 82 | Male | COPD, CAD | 80×56×101 | No, none | None | 3.5 | negative |
| 4 | 74 | Female | Diarrhea | 130×101×57 | No, none | Pericardium, multilobulated | 3.7 | negative |
†, negative if <0.5 nmol/L as per our institute’s laboratory. CT, computed tomography; MG, myasthenia gravis; SUV, standardized uptake value; Ab, acetyl-cholinesterase receptor antibodies; IDDM, insulin dependent diabetes mellitus; HT, hypertension; ICU, intensive care unit; NIDDM, non-insulin dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease.
Figure 1.
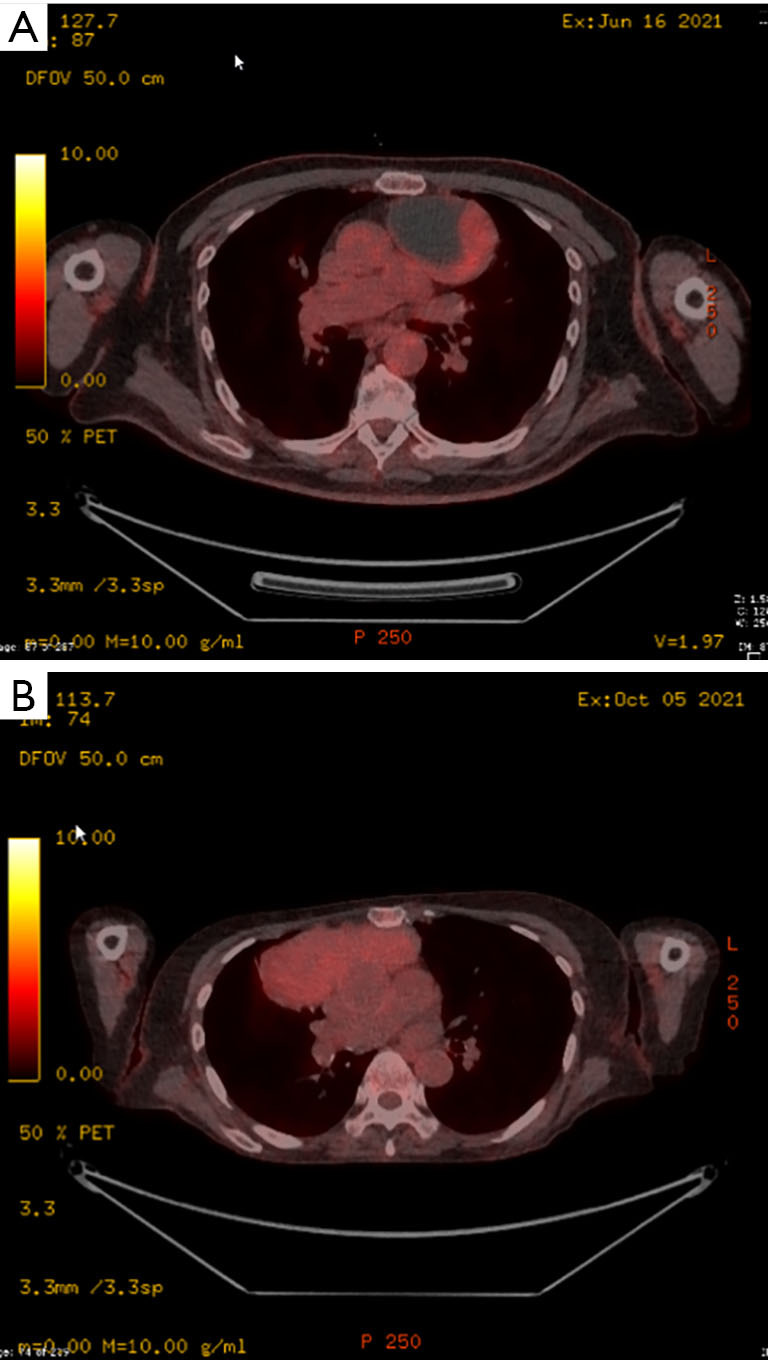
PET imaging of patients undergoing surgery. (A) PET scan from patient with a mass measuring 102×63×76 showing a partial cystic photopenic component and a solid lateral PET positive component. (B) PET scan from patient with a mass measuring 130×101×57 and which was homogenously PET positive. PET, positron emission tomography.
The measured computed tomography (CT) scan size of the masses was found to be above 10 cm at one of the measured axis (Table 1, Figure 2A,2B).
Figure 2.
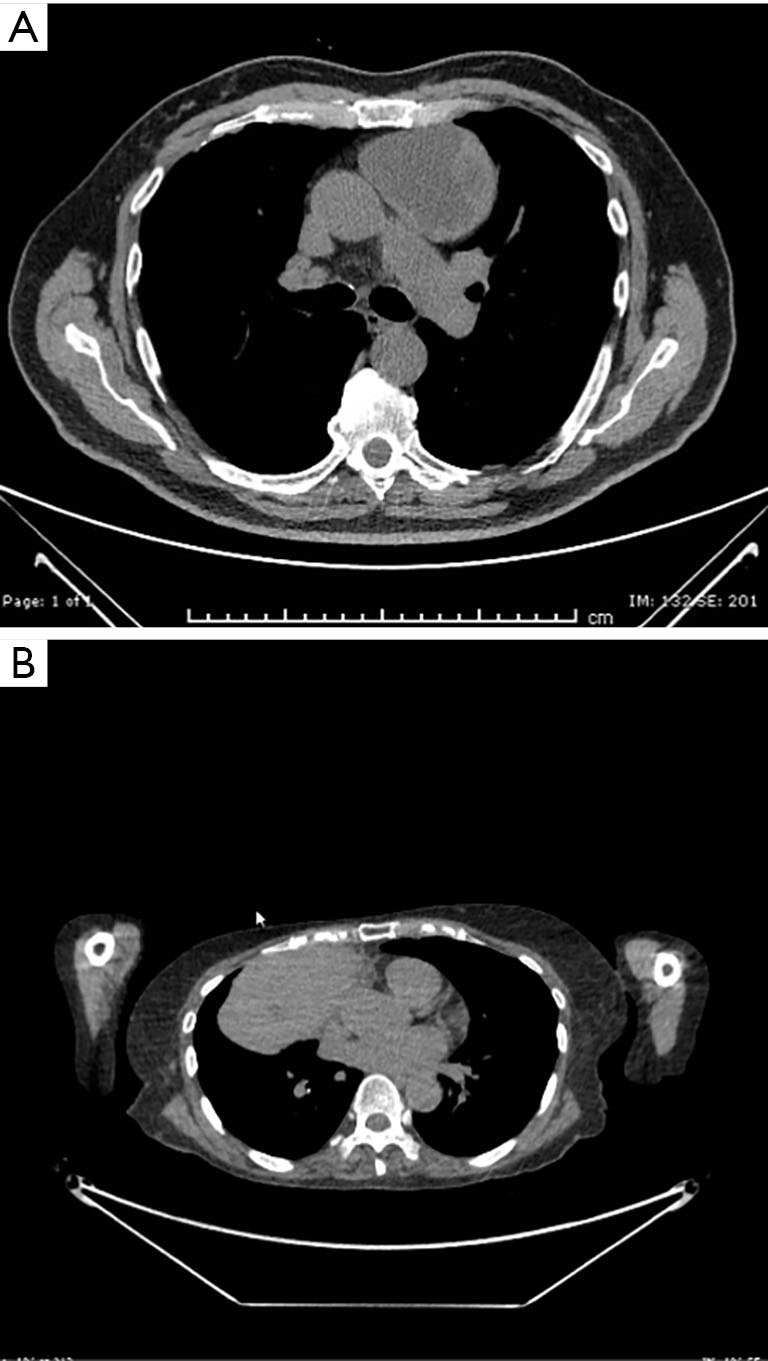
CT scans from the same patients showing the masses in the anterior mediastinum. (A) The mass is depicted to be cystic with a lateral solid part. (B) The mass is multilobulated and was considered to be involving the pericardium. CT, computed tomography.
Anonymity of patients was ensured by using anonymized images and hospital numbers and age in years rather than names of the patients and dates of birth.
Surgical technique
Procedures were performed by 1 board certified surgeon, with adequate experience in robotic surgery previously signed off by the proctors as per the Intuitive™ training schedule.
Single lung ventilation was achieved with a double lumen tube while an arterial line was used in all cases for invasive monitoring during the procedure. A urine catheter was inserted in 2 cases.
All patients were placed with 30-degree right chest up, with the right arm tacked adducted below the body level as all procedures were started from the right aspect of the mediastinum. Draping included both sides for possible double docking or conversion to open.
All procedures were performed with the DaVinci X Intuitive® system either with single docking using 3 ports on the right (2×8 mm: 4th intercostal space mid-axillary line, 6th intercostal space anterior axillary line and 1×12 mm parasternally 5th intercostal space) or double docking with 2 extra ports on the left (2×8 mm: 6th intercostal space anterior axillary line and 4th intercostal space mid-axillary line) to achieve visualization of the left phrenic nerve, skeletonization of the left side of the mediastinum and for retraction of the specimen.
The CO2 insufflation pressure range used was 8–10 mmHg with a flow of 5–20 mLs/min.
Dissection usually started from the diaphragmatic area (Figure 3A) towards the phrenic nerve and along it, dissecting the mediastinal fat/mass off it with Maryland bipolar forceps, in order to avoid damaging the nerve. Dissection with a spatula continued towards the left pleura medially to the internal mammary vessels which were preserved. The left pleura at this point was usually opened. Dissection then continued along the superior vena cava medially to the phrenic nerve towards the confluence with the right innominate vein. At this point, the left innominate vein was identified, and mediastinal fat/thymus/mass was dissected off it with sharp dissection either with spatula or with Maryland forceps. In all cases full thymectomy was performed (in 1 case was additionally necessitated because of MG) apart from resection of the mass. Ligation of thymic tributaries was performed using hem-o-lok clips. In 1 case, pericardial resection was necessitated (Figure 3B) of a 4×4 cm area superiorly and above the ascending aorta, without the need of a reconstruction.
Figure 3.
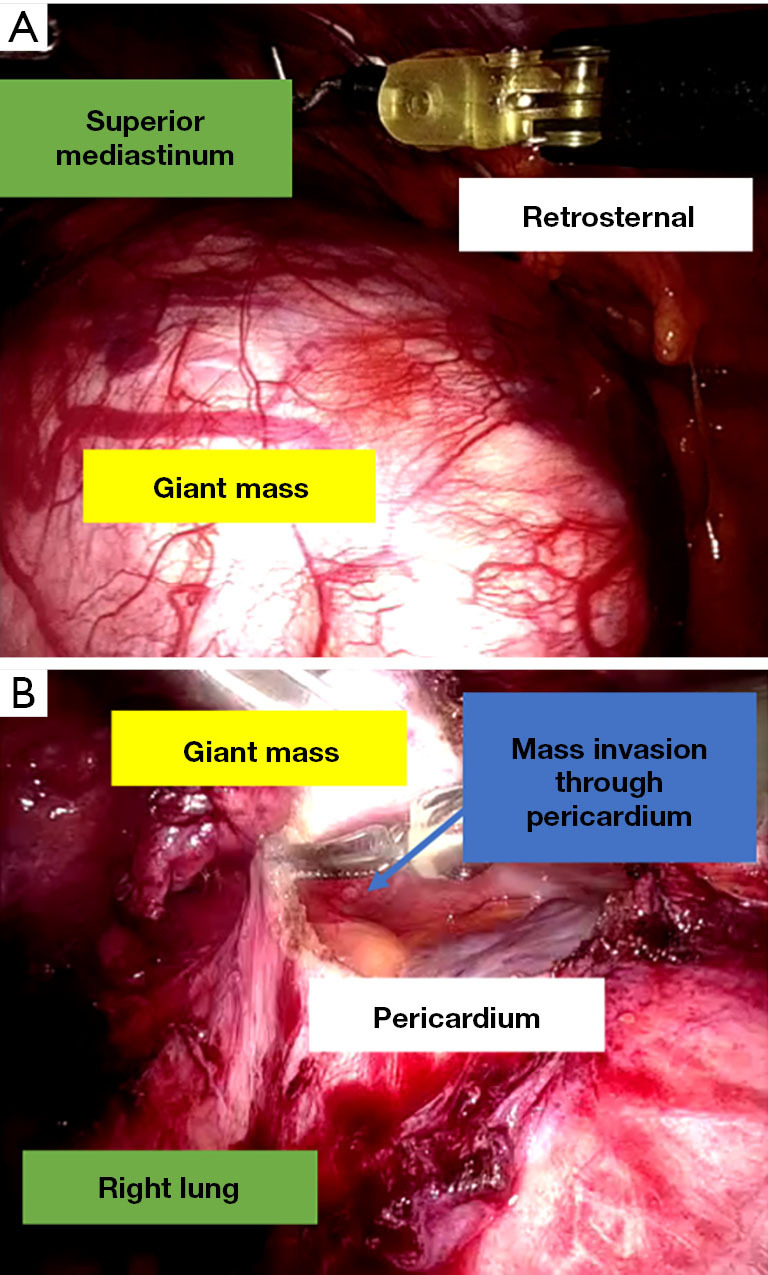
Pictures taken during surgery. (A) 15 cm anterior mediastinal mass. (B) Mass is pushed superiorly, the pericardium has been opened and lesions are found to be infiltrating through the pericardium.
The specimens were removed into an endobag through the 12 mm port which was accordingly extended up to 4 cm laterally as was needed for instance in the case of the 15 cm mass (Figure 4A). In 1 case and because the mass was partially cystic in nature, the mass was drained with the suction inside the endobag and was removed inside it intact.
Figure 4.
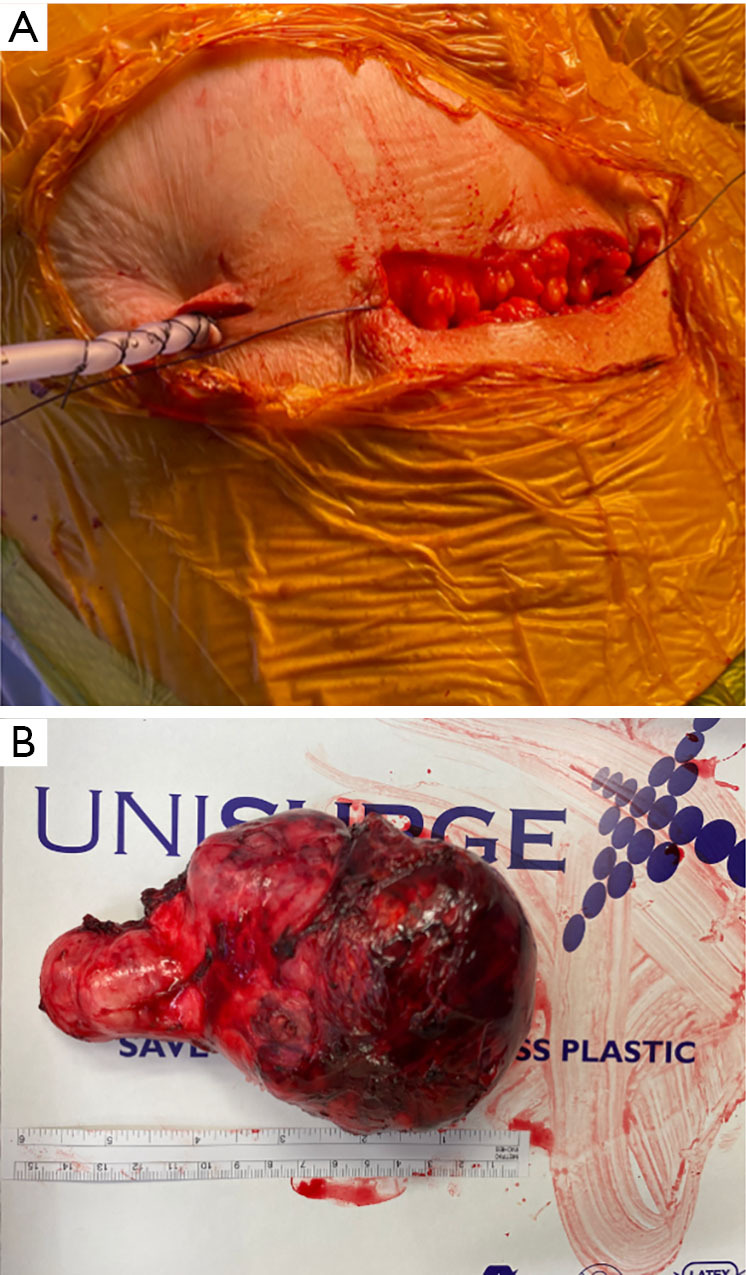
Pictures taken from the resection of 15 cm anterior mediastinal mass. (A) The 12 mm port was elongated up to 4 cm in order to facilitate the removal of the mass and this was used in all cases up to an extend. (B) One of the masses measured bigger than anticipated from imaging i.e., 15 cm in this case.
A single chest tube was inserted in all but 1 case, from the right anterior axillary port site heading towards the superior mediastinum.
All patients were extubated after surgery and were admitted to High Dependency Unit for observation under the fear of myasthenic crisis.
The intraoperative details are summarized in Table 2.
Table 2. Intraoperative data of N=4 patients.
| Case | Docking | Duration of surgery (min) | Blood loss (mL) | Intraoperative events | No. of 8 mm ports | No. of 12 mm ports | Chest drain insertion | Thymectomy |
|---|---|---|---|---|---|---|---|---|
| 1 | Double | 345 | 50 | Resection of pericardium 4×4 cm | 4 | 1 | 20 Fr | Yes |
| 2 | Single left | 90 | 50 | None | 2 | 1 | No | Yes |
| 3 | Single right | 120 | 50 | None | 2 | 1 | 20 Fr | Yes |
| 4 | Double | 120 | 100 | Fibrotic tissues, pericardium not involved | 4 | 1 | 20 Fr | Yes |
Fr, French.
Outcomes
All patients had satisfactory CXR after surgery and the chest drain was removed on postoperative day (POD) 1. Recovery was uneventful for all patients and 3 of them were discharged home on POD 2 with one discharged late on POD 1 after being discharged from high dependency unit (HDU). There was no in-hospital, 30- or 90-day mortality.
All masses were diagnosed by histopathology to represent thymomas, as summarized in Table 3, all measuring above 10 cm with the biggest measuring 15 cm in the long axis (Figure 4B). R0 resection was achieved in all cases. The cases were discussed at the multidisciplinary team (MDT) meeting and the oncologist present decided to offer adjuvant radiotherapy because of the size of the tumor under the fear of recurrence despite the R0 resection and the absence of capsular invasion (Table 3).
Table 3. Postoperative and histology data of N=4 patients.
| Case | Size (mm) | Pathology of mass | Stage | R0 | Drain stay (days) | LOS (days) | Morbidity | In-hospital/30-day mortality |
|---|---|---|---|---|---|---|---|---|
| 1 | 138×50 | Thymoma | B2 | Yes | 1 | 2 | No | No |
| 2 | 100×60 | Thymoma | AB | Yes | N/A | 2 | No | No |
| 3 | 110×45 | Thymoma | AB | Yes | 1 | 1 | No | No |
| 4 | 150×70 | Thymoma | AB | Yes | 1 | 2 | No | No |
R0, resection status; LOS, length of stay; N/A, not available.
On follow-up, all patients reported no important issues from surgery and are under follow-up with CT scan without any reported recurrences up to now.
This case series has been reported in line with the AME Case Series Guideline (13).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We herein present a series of 4 cases, all above 10 cm, with one of them additionally requiring pericardial resection, which were all successfully removed via RATS. All patients recovered quickly from their procedure without adverse events and are well on follow-up. The size of an anterior mediastinal mass should not preclude pursuing minimally invasive surgery and especially RATS resection.
The anatomic boundaries of the narrow mediastinum with its various vital structures, makes it a challenging place to access and to safely dissect and/or resect tumours, especially the bigger in size ones. The open surgical approach i.e., sternotomy or thoracotomy, was notoriously the standard approach for surgical interventions to this region. When facing cases which necessitate resection of big masses, most surgeons would prefer to proceed to an open procedure or convert to one, in order to resect them safely and completely (11,12). It has been reported that a mass is perceived as “big” if it is bigger than 5 cm (14-16). This study shows that perseverance with RATS resection in cases of masses >10 cm can be easily and safely performed, without the necessity of a sternotomy or a thoracotomy. The most important tip for a safe and complete resection is patience and perseverance. Also, bilateral docking is very helpful to achieve more angles and safely identify the important structures in the mediastinum for example big vessels, the phrenic nerves etc. Finally, we used contralateral ports for traction inferiorly of the mass which will allow better access to the superior mediastinum structures for example the left innominate vein, the thymic horns etc.
Minimally invasive approaches are shown to be better in terms of recovery from the open ones offering amongst others shorter duration of surgery, shorter postoperative drainage and decreased blood loss, with comparable oncological outcomes (4,9,17,18). RATS resections and/or thymectomies are specifically shown to have superior outcomes in terms of recovery and safety versus transsternal ones and more specifically are shown to produce less intraoperative blood loss, lower incidence of postoperative complications, less risk of wound infection, fewer transfusions, shorter length of stay (LOS), and better quality of life after surgery, without compromising the oncological outcomes (6,8,10). All these were shown in our case series as all patients had minimal blood loss during surgery, had their drains removed the next day (in 1 case the surgeon decided not even to leave a drain in the chest) and all were discharged home within 2 days without issues. Despite all these, however, RATS resection of such big masses should not be offered at the beginning of the robotic learning curve because in inexperienced hands it could lead to harm, for example from injury to adjacent structures such as great vessels, or to oncological outcome issues, for example R1 resection or capsular rupture before removal etc.
Apart from these, in some cases, avoiding an open approach is very crucial as for example in one of our cases in which the patient had insulin dependent diabetes mellitus and difficult to control MG with high doses of steroids. In this specific case, proceeding to a sternotomy would cause additional issues to the recovery of the patient for example prolonged hospitalization and stay in intensive care unit, wound issues and others.
Comparing RATS with conventional VATS, these were shown to be comparable (9,18), but VATS is technically more challenging, due to its limited handling angles, and the suboptimal 2D vision screen, which makes it difficult to be used for giant masses resection. This case series shows that RATS can provide a wider range of maneuverability of instruments and 3D vision, which make the resection of masses in the mediastinum >10 cm, both safe and successful.
As it would make sense that size does matter in choosing the proper approach of surgery, the biggest size of an anterior mediastinal mass to our knowledge is reported via VATS to be 14 cm (14), although in 1 of our cases the resected mass was 15 cm. Literature is scanty with regards to this and very few cases of big masses have been reported to have been resected via minimally invasive approaches and RATS more specifically. To our knowledge, 9 cm is the biggest mass resected via RATS that has been reported (19).
Apart from the size, another important factor which needs careful consideration is the complexity of the mass to be resected i.e., the need for pericardial resection, invasion of big vessels or proximity to them, resection of lung parenchyma etc. (20). Resection of pericardium is the most common complexity a surgeon can face and there are reports showing efficiency of minimally invasive surgery and RATS in resecting such masses, en block with the pericardium, followed by pericardial patch reconstruction (21). This however was not necessitated in our case because the pericardium resected was on top of the aortic root towards the superior mediastinum.
Another consideration faced by the surgeons is the removal of the big specimen. In the cases of cystic components once the specimen is within the endobag then it can be punctured and hence removed easily via one of the ports which will need to be slightly enlarged (14). In solid masses though, extraction of the specimen is an issue, and consequently this can lead to a mini thoracotomy. In our cases though, we tackled this issue by enlarging the 12 mm port to 4 cm and with slight spreading of the ribs with retractors, the masses, including the 15 cm one, were removed without issues.
Conclusions
In conclusion, we report a series of 4 cases of >10 cm anterior mediastinal masses, with 1 needing pericardial resection, which were all resected successfully and uneventfully via RATS, irrespectively of their size or complexity. Although RATS is not offered routinely by all institutes, its use in cases of >10 cm anterior mediastinal masses is safe and beneficial for the patients and as such they should be considered and attempted should this technology be available.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-41/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-41/coif). VK serves as an unpaid editorial board member of Mediastinum from August 2022 to July 2024. The other authors have no conflicts of interest to declare.
References
- 1.Almeida PT, Heller D. Anterior Mediastinal Mass. [Updated 2021 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546608/ [PubMed] [Google Scholar]
- 2.Juanpere S, Cañete N, Ortuño P, et al. A diagnostic approach to the mediastinal masses. Insights Imaging 2013;4:29-52. 10.1007/s13244-012-0201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. 10.1378/chest.128.4.2893 [DOI] [PubMed] [Google Scholar]
- 4.Chetty GK, Khan OA, Onyeaka CV, et al. Experience with video-assisted surgery for suspected mediastinal tumours. Eur J Surg Oncol 2004;30:776-80. 10.1016/j.ejso.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 5.Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. 10.1093/ejcts/ezr112 [DOI] [PubMed] [Google Scholar]
- 6.Azenha LF, Deckarm R, Minervini F, et al. Robotic vs. Transsternal Thymectomy: A Single Center Experience over 10 Years. J Clin Med 2021;10:4991. 10.3390/jcm10214991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang CH, Na KJ, Park S, et al. Long-Term Outcomes of Robotic Thymectomy in Patients With Thymic Epithelial Tumors. Ann Thorac Surg 2021;112:430-5. 10.1016/j.athoracsur.2020.09.018 [DOI] [PubMed] [Google Scholar]
- 8.Kang CH, Hwang Y, Lee HJ, et al. Robotic Thymectomy in Anterior Mediastinal Mass: Propensity Score Matching Study With Transsternal Thymectomy. Ann Thorac Surg 2016;102:895-901. 10.1016/j.athoracsur.2016.03.084 [DOI] [PubMed] [Google Scholar]
- 9.O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg 2019;8:174-93. 10.21037/acs.2019.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. 10.1016/j.ejcts.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 11.Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. 10.1016/j.jtho.2016.08.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CJ, Hurd J, Shah SA, et al. A national analysis of open versus minimally invasive thymectomy for stage I to III thymoma. J Thorac Cardiovasc Surg 2020;160:555-67.e15. 10.1016/j.jtcvs.2019.11.114 [DOI] [PubMed] [Google Scholar]
- 13.AME Case Series checklist 2020. Available online: https://cdn.amegroups.cn/static/public/20-AME-Case-Series-Checklist.pdf
- 14.Hughes BD, Okereke IC. Giant mediastinal mass: one port video assisted thoracoscopic surgery. J Surg Case Rep 2017;2017:rjx178. 10.1093/jscr/rjx178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess NR, Sarkaria IS, Pennathur A, et al. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg 2016;5:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gossot D, Izquierdo RR, Girard P, et al. Thoracoscopic resection of bulky intrathoracic benign lesions. Eur J Cardiothorac Surg 2007;32:848-51. 10.1016/j.ejcts.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 17.Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early stage thymoma: video assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. 10.1016/j.jtcvs.2013.10.053 [DOI] [PubMed] [Google Scholar]
- 18.Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. 10.1093/ejcts/ezu077 [DOI] [PubMed] [Google Scholar]
- 19.Kodia K, Nguyen DM, Villamizar NR. A. 9 cm robotic thymectomy and pericardial repair case report. Mediastinum 2020;4:38. 10.21037/med-20-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo SW, Huang PM, Lin MW, et al. Robot-assisted thoracic surgery for complex procedures. J Thorac Dis 2017;9:3105-13. 10.21037/jtd.2017.08.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang HC, Coyan G, Vercauteren M, et al. Robot-assisted en bloc anterior mediastinal mass excision with pericardium and adjacent lung for locally advanced thymic carcinoma. J Vis Surg 2018;4:115. 10.21037/jovs.2018.05.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


