Abstract
Colorectal cancer (CRC) has the third highest incidence and the second highest mortality in the world, which seriously affects human health, while current treatments methods for CRC, including systemic therapy, preoperative radiotherapy, and surgical local excision, still have poor survival rates for patients with metastatic disease, making it critical to develop new strategies for treating CRC. In this article, we found that the gut microbiota can modulate the signaling pathways of cancer cells through direct contact with tumor cells, generate inflammatory responses and oxidative stress through interactions between the innate and adaptive immune systems, and produce diverse metabolic combinations to trigger specific immune responses and promote the initiation of systemic type I interferon (IFN-I) and anti-viral immunity. In addition, oncolytic virus-mediated immunotherapy for regulating oncolytic virus can directly lyse tumor cells, induce the immune activity of the body, interact with interferon, inhibit the anti-viral effect of IFN-I, and enhance the anti-tumor effect of IFN-II. Interferon plays an important role in the anti-tumor process. We put forward that exploring the effects of intestinal flora and oncolytic virus on interferon to treat CRC is a promising therapeutic option.
Keywords: colorectal cancer, intestinal flora, oncolytic virus, interferon, interaction, basic mechanism
Graphical abstract
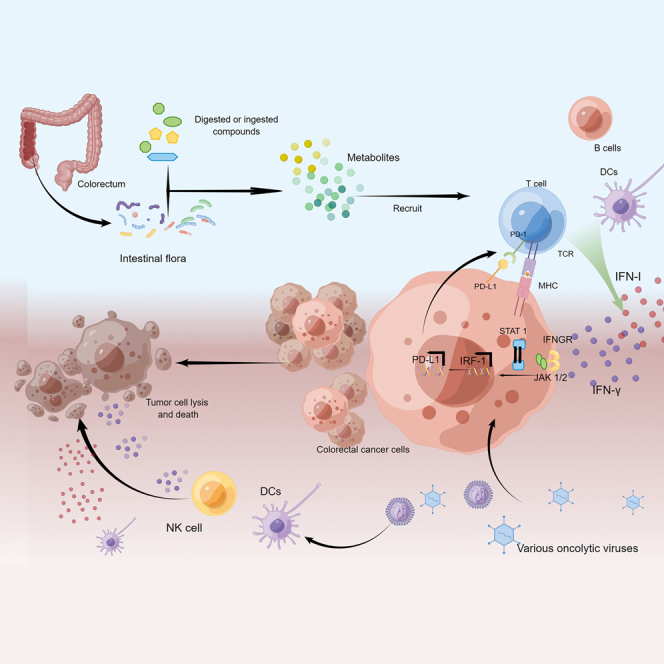
Kong and colleagues discuss in detail the pathogenic mechanisms and potential therapeutic approaches for colorectal cancer from two significant aspects, intestinal flora and oncolytic virus, which suggest that it is a promising treatment choice to explore the effect of intestinal flora and oncolytic virus on interferon in treating colorectal cancer.
Introduction
Colorectal cancer (CRC) ranks third in incidence and second in mortality worldwide. There are expected to be 2.2 million new cases of CRC worldwide and 1.1 million deaths due to CRC by 2030, while China is expected to have more than 191,000 deaths and 376,000 new cases per year.1 About half of all humans will develop benign colorectal tumors, and about 3% of these patients will progress to CRC. The treatment of CRC includes systemic therapy and preoperative radiotherapy, endoscopic and surgical local excision surgery, extensive local and metastatic CRC surgery, local ablation therapy for metastatic CRC, specifically targeted therapy, and immunotherapy. However, the survival of patients with metastatic CRC disease remains poor. Therefore, investigating new strategies for treating CRC is of great interest.
In addition to genetic factors, colonic inflammation is also a significant cause of CRC, regulated by various factors such as microbiota, immune cells, and cytokines. The relationship between gut microbiota and CRC has become one of the main directions for research and treatment of CRC. Microflora can regulate the activation of diverse immune cells through the interaction between signaling, such as nuclear factor kappa B (NF-κB), inflammatory body, and type I interferon (IFN-I), and then affect inflammation and CRC. Therefore, studying the microflora mechanism helps formulate effective CRC prevention and treatment strategies. Immunotherapy is one of the crucial options for CRC treatment. This strategy harnesses the patient’s immune system against tumor cells and effectively improves the specificity of the treatment plan for cancer cells.1 The colonization of intestinal flora at the tumor site can reinforce the anti-tumor effect of immunotherapy. Therefore, in vivo, using specific bacteria or their engineered progeny strains may play an influential therapeutic role in enhancing anti-tumor immunotherapy and may serve as a powerful complement to therapeutic approaches such as surgery and radiotherapy. Meanwhile, the oncolytic virus (OV) uses a natural or modified virus as a vector to activate anti-tumor immune reaction and induce cell apoptosis, which is a new tumor treatment method. Interferon (IFN) is an effective immunostimulatory that is important for immune reaction. It is the first line of defense against pathogens and can even act as an effective mediator to strengthen the immune system. Changes in IFN signaling can support tumor resistance to immune checkpoint blockade (ICB). Gut microbiota and OVs can interfere with the IFN signaling pathway to treat CRC. Therefore, it is necessary to explore the effect of intestinal flora and OVs on IFN to better examine the mechanisms of immunotherapy resistance in CRC.
Interaction between IFN and CRC
IFN-I (IFN-α, IFN-β) and IFN-II-γ jointly regulate the immune response in tumors,2 and IFN signaling plays a dual role in promoting tumor development and immune evasion. IFN-α and IFN-β are effective anti-viral cytokines. At the same time, IFN-II also showed its anti-tumor effect through various mechanisms.
IFN promotes the development of CRC
IFNs have many different pathways of action in CRC. It has been reported that IFN-I upregulates the expression of the tumor suppressor protein p53.3 IFN-β was found to inhibit the transformation of wild-type mouse embryonic fibroblasts in vitro. In addition, ribonuclease L (RNase L), an endoribonuclease regulated by IFN-I, can function as a tumor suppressor in human cells.4 Therefore, IFN-I provides cells with greater potential to control the transformation process. Endogenous IFN-I and IFN-γ do not function in a completely overlapping manner, but they play a role in different host cell populations. In the study of tumor transplantation and monochloroacetic acid (MCA)-induced carcinogenicity, it was found that mice that received plasmids encoding autoantigens and IFN-γ developed fewer tumors and produced weaker regulatory T (Treg) cell responses than mice that received plasmids encoding only autoantigens expressed by tumors. These results suggest that the early production of IFN-γ blocks the development and immunosuppression of Treg cells.2 Furthermore, dysregulation of IFN-γ production and IFNγ-mediated signaling has been shown to promote spontaneous oncogenicity in the colon of mice expressing SOCS1 only in T and B cells.5 IFN-γ can inhibit tumor immune response. For instance, IFN-γ can promote the production of B7-H1 (programmed death-ligand 1 [PD-L1]), arginase (Arg), indoleamine 2,3-dioxygenase (IDO), and other immunosuppressive molecules in the tumor microenvironment (TME).6,7 IDO contributes to immune tolerance of TME, is the pathogenic driver of CRC progress, and is related to the poor prognosis of CRC. IFN-γ is an effective inducer of IDO expression. IFN-γ strongly stimulates the expression of programmed cell death ligand 1 (PD-L1) in the TME, thereby hampering anti-tumor immunity and ICB therapy. The immunogenic effect of IFN-γ may inevitably be accompanied by the enhancement of the immune escape mechanism in TME, and the tumor immune escape effect can be overcome by specific combination therapy. For example, breaking the restriction of single targeted drugs can improve the combined efficacy of ICB, one of which is to interfere with the aromatic hydrocarbon receptor pathway in tumors expressing IDO.8 Many cells produce IFN-γ, such as natural killer (NK) cells,9 helper T (Th) 17 cells, antigen-presenting cells (APCs), and innate lymphoid cells (ILCs). IFN-γ binds to IFN-γ receptors (IFNGRs) and stimulates Janus kinase (JAK)-signal transduction and transcription (STAT) signaling, which activates the transcriptional program of IFN-stimulated genes (ISGs) and regulates immune responses. In contrast, cytokine signaling (SOCS) suppressors, mainly SOCS3 and SOCS1, are negative regulators of IFN-γ signaling.10 In conclusion, IFN-γ can lead to anti-tumor immunosuppression in vivo by activating SOCS family proteins and inducing the expression of immunosuppressive molecules in various ways, limiting the treatment of CRC.
IFN-I and IFN-II participate in many mechanisms for regulating immune response in cancer, thus balancing immune escape and surveillance.11 The action pathways of these IFNs provide therapeutic ideas in the clinical practice of CRC and explain the mechanism of IFN promoting the development of CRC, which consists of three stages: first, elimination, which is based on the recognition of tumor cells and their killing by the innate or adaptive immune system; second, persistence, based on the failure of the elimination step, resulting in a balance between the growing cancer cells and the stress of the immune system; third, escape, when the growth of cancer cells can overcome the protective effect of the immune system, and escape begins due to immune inhibition or generation of resistant cancer cell clones.
Regulate IFN to treat CRC
IFNs have emerged as central orchestrators of tumor-immune system interactions. Studies have shown that hosts that do not respond to IFN show increased tumor incidence, and tumors produced in IFN-unresponsive environments are highly antigenic. IFN-I has broad immunomodulatory effects, and many cell types may be physiologically relevant targets for their anti-tumor activity. For example, IFN-I has been found to activate dendritic cells (DCs), increase the cytolytic activity of macrophages and NK cells, induce interleukin (IL)-15 production, increase T cells' survival, and produce stromal-derived vascular inhibitory molecules.12 It is also clear that IFN-γ, but not IFN-α and IFN-β, controls tumor cell immunogenicity, possibly as a result of selective production of IFN-γ in the TME. Tumors need to respond to IFN-γ to be immunogenic. The ability of IFN-γ to promote tumor rejection is mediated at least in part by upregulating major histocompatibility complex (MHC)-like pathways for antigen processing and presentation in tumor cells.2 This suggests that the effect of IFN-γ on tumor cells may coordinate the progression of the immune response to growing tumors, allowing early recognition or clearance of cancer cells by the innate immune system to be transformed into an immune attack by the adaptive immune system.
Studies have shown that IFN-γ stimulation leads to nuclear translocation and phosphorylation of STAT1, resulting in STAT1-TET2 binding, and the regulation of this mechanism may enhance the body’s anti-tumor immunity. Multiple IFN-γ-responsive genes, including PD-L1, CXCL9 (MIG), CXCL10 (IP-10), and CXCL11 (I-TAC), were found to result in gene silencing of DNA methylation. At the same time, DNA demethylation involving Tet2 can increase the level of 5hmC on the promoters of IFN-γ-responsive genes, thereby improving the immune response.13 Therefore, modulation of the IFN-γ epigenetic signaling pathway can affect tumor immunotherapy. IFN-γ increases the production of MHC-I and MHC-II in cancer cells, increases the production of IL-12 in APCs, promotes Th1 polarization, and promotes tumor translocation of immune cells through the secretion of Th1-type chemokines. In addition, IFN-γ has direct anti-cancer effects on cell replication14 and induces apoptosis15 and necrosis16 in cancer cells. Notably, IFN-γ can result in cancer cell apoptosis, necrosis, or ferroptosis.17 Clinical studies have found that the loss of IFN-γ gene expression is frequently observed in CRC patients, thus enhancing the IFN-γ signaling cascade pathway is a reasonable and novel approach to treat CRC patients.18
Currently, researchers are using these cytokines to arm OV.19 The rationale for using IFN-I as transgenes in OVs is that, in addition to their anti-viral function, they also have anti-tumor potential due to their role in the maturation of DCs and cytotoxic T cells. An oncolytic adenovirus armed with IFN-α produced modest increases in anti-tumor efficacy in a mouse model of pancreatic cancer when combined with radiation. Similarly, after arming OV vesicular stomatitis virus (VSV)20 and measles with IFN-β,21 it was observed that their anti-tumor efficacy was improved to some extent.
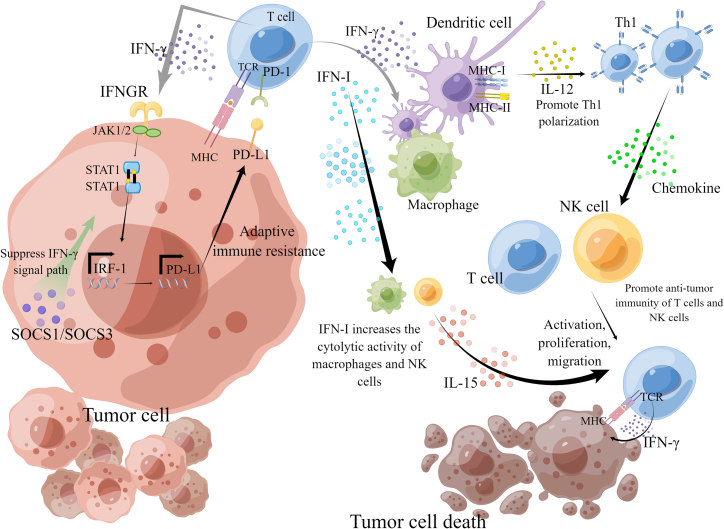
Therefore, it can play a role in treating CRC by regulating IFNs to play a variety of mechanisms. It is clear from many studies that IFN-I can promote many immune functions, but further work using appropriate in vivo tumor models is needed to reveal which immune functions are physiologically relevant to anti-tumor immunity. IFN-γ regulates various biological programs that may be a priori involved in suppressing tumor growth, so additional experimental studies are needed to determine the full induction of IFN-γ on tumors that contributes to tumor growth suppression and is useful for the treatment of CRC (Figure 1).
Figure 1.
The main action pathway of IFN in inhibiting tumor cells
IFN-I can activate DCs, increase the cytolytic activity of macrophages and NK cells, induce the production of IL-15, activate T cells, and increase the survival of T cells. The IFN-γ signal cascade pathway is the main anti-tumor pathway of IFN-II. IFN-γ binds to IFN-γ receptors (IFNGRs) on tumor cells and stimulates Janus kinase (JAK) signal transduction and transcription (STAT) activator signaling pathway, which stimulates phosphorylation and nuclear translocation of STAT1, thus activating the IFN-stimulated gene transcription program and regulating the immune response. IFN-γ enhances the production of MHC-I and MHC-II in cancer cells and IL-12 in APC, promotes Th1 polarization, and promotes tumor transport of immune cells through the secretion of Th1 chemokines, thus achieving the anti-tumor purpose.
Regulate intestinal Flora and IFN to Treat CRC
As an essential participant in the intestinal environment, intestinal microorganisms are nearly connected with the occurrence and development of CRC. Some scholars have pointed out that intestinal microflora preferentially colonizes the tumor site and promotes immunotherapy through the signal of stimulators of IFN genes (STING) in the tumor site. Living bacteria will migrate on their initiative and continuously generate secondary metabolites, thus triggering the STING pathway in DCs.22 Therefore, intestinal flora and IFN are closely associated with CRC.
The basic mechanism of intestinal flora
Under normal conditions, intestinal bacteria maintain a stable flora, which is vital in nutrient intake, regulating intestinal immunity, and maintaining the intestinal mucosal barrier. If the intestinal balance is broken, it will lead to the secretion of inflammatory cytokines, inflammatory mediators, proteases, and oxygen free radicals, which will not only aggravate the mechanical barrier damage of intestinal mucosa but also cause bacterial translocation and the activation of immune regulation of digestive tract, triggering the occurrence, progress, and even deterioration of CRC.23,24
Direct contact with tumor cells
The human gut microbiota contains 1,013–1,014 microorganisms, of which the genes in gut bacteria are over 100 times that of the human genome.25 In the healthy human body, intestinal flora can shape the intestinal epithelium,26 collect the energy needed by the body,27 produce an immune response, etc,28 which plays a huge role.29 Many symbiotic bacteria live in the intestine, including a few tumor-promoting species, such as Fusobacterium nucleatum, Enterococcus faecalis, Escherichia coli, enterotoxigenic Bacteroides fragilis (ETBF), Peptostreptococcus (Peptostreptococcus anaerobius), Salmonella enteritidis (SE), Campylobacter jejuni (CJ).30 Pathogenic bacteria play a role by directly contacting tumor cells through their virulence factors. F. nucleatum selectively binds to E-cadherin through Fusobacterium adhesin A (FadA), thereby triggering the intracellular β-catenin signaling pathway, inducing carcinogenesis and inflammation.31 F. nucleatum can also induce the sharp decrease of m6A modification in tumor cells and patient-derived xenotransplantation (PDX) tissues by downregulating m6A methyltransferase like 3 (METTL3), which helps generate CRC invasion.32 E. faecalis has been proved to produce metalloproteinases, which can directly destroy the intestinal epithelial barrier and cause inflammation.33 E. coli produced the gene toxin E. coli through the hybrid gene cluster34 of polyketide synthetase (PKS) and non-ribosomal peptide synthetase (NRPS). ETBF is similar to E. coli and produces metalloproteinase toxin BFT (bacteroides fragilis toxin), which can damage intestinal mucosa, induce cancer-promoting signal cascade, and trigger myeloid-dependent colon tumors because of its proteolytic activity.35,36 P. anaerobius combines with intestinal epithelial cell (IEC) receptor integrin a2/b1 through the putative cell wall-binding repeat 2 (PCWBR2), which enhances the carcinogenic phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt) signaling, thus promoting the proliferation of cancer cells.37 AvrA secreted by SE can activate STAT3 signaling in CRC cells.38 CJ generates a gene toxin, cytolethal distending toxin (CDT), which results in DNA breaks39 and promotes the occurrence of CRC. Tumor-related flora can directly regulate the signal pathway of tumor cells, and various flora can activate the β-catenin signal pathway related to tumor dryness and proliferation, thus achieving the goal of promoting tumor (Table 1).
Table 1.
Summary of the mechanisms of different intestinal microflora in the initiation, development, and metastasis of CRC
| Enteric microorganisms | Mechanism of action | Authors | Year |
|---|---|---|---|
| F. nucleatum | through FadA, it selectively binds to E-cadherin on the surface of CRC and activates the intracellular β-catenin signaling pathway | Hashemi Goradel et al.30 | 2019 |
| E. faecalis | metalloproteinase is produced, directly destroying the intestinal epithelial barrier and inducing inflammation | de Almeida et al.33 | 2018 |
| E. coli | gene toxin E. coli was produced by the NRPS-PKS hybrid gene cluster | Vizcaino et al.34 | 2015 |
| ETBF | produces BFT, which has proteolytic activity, can damage intestinal mucosa, induces cancer-promoting signal cascade, and triggers medullary cell-dependent colon tumors | Chung et al.35,36 | 2018 |
| P. anaerobius | its surface protein PCWBR2 directly combines with IEC receptor integrin a2/b1 to activate the carcinogenic PI3K-Akt signal pathway, thus promoting the proliferation of tumor cells | Belkaid et al.37 | 2014 |
| SE | secretion of AvrA activates the STAT3 signaling pathway in colon cancer cells | Duijster et al.38 | 2021 |
| CJ | CDT production leads to DNA double-strand break | Lara-Tejero et al.39 | 2000 |
Produce inflammatory reaction
Microbe and host immune systems interact in regulating the development of CRC. As a significant part of the TME, various immune cell groups are of great significance in inhibiting or promoting the growth of CRC. Intestinal microflora closely interacts with the immune system. Immune reactions stimulated by bacteria can cause persistent low-grade inflammation, leading to tumors. On the contrary, inflammation cannot induce CRC without compounds derived from microorganisms or bacteria.40 The formation of inflammatory cells and their related mediators may cause intestinal epithelial damage, which is beneficial to the change of the microenvironment of CRC.
The innate immune system is crucial in mediating inflammatory responses in CRC patients.41 It has been confirmed that microbial nucleic acids are among the main targets of innate immune recognition. Microbial antigens and potential pathogens will also be perceived by pattern recognition receptors (PRRs) encoded by host strains, which recognize specific pathogen-associated molecular patterns (PAMPs). In the innate immune response, Toll-like receptors (TLRs) are specific PRRs that can recognize pathogens and thus enable the innate immune response to proceed smoothly.42 Dense microbial communities in the intestine can produce a variety of molecules identified by TLRs,43 resulting in the excitation of the NF-κB signal and the transcription of multiple cytokines.41 Microorganisms can generate molecular patterns that activate innate immunity. However, symbiotic bacteria also seem to change DNA repair pathways and induce microbe-associated molecular patterns (DAMPs) that trigger innate epithelial immunity.44
In addition, symbiotic microorganisms can modulate the adaptive immune system in various ways, which include stimulating and directing the differentiation of T cell groups in the gut, especially Treg cells and Th17 cells.45 Microorganisms strictly control Treg cells in the intestine, and Treg groups exert immunosuppressive functions on various immune cell groups through transforming growth factor (TGF)-β and IL-10.46,47 Th17 cells may play a role in tumor initiation by changing signaling pathways, such as STAT3, presenting different sites between intact epithelial cells and developed tumor cells.48 In contrast to non-pathogenic Th17 cells induced by TGF-β and IL-6, Th17 cells can acquire a pathogenic pro-inflammatory phenotype in response to serum amyloid protein (SAP) or IL-2349,50 and promote the release of IL-17 (Figure 2). In addition, the microbiota controls the efficacy of tumor immunotherapy by modulating cancer cell-killing functions of cytotoxic T cells.51,52,53,54 In particular, fungi account for a large proportion of intestinal microflora, and it has been proved that fungi can induce the production of inflammasomes. Fungal recognition receptors enable downstream signals through kinase SYK and the common adaptive protein CARD9. In addition, the lack of caspase recruitment domain 9 (CARD9) or C lectin receptors (CLRs) Dectin-1 and Dectin-3 has been shown to exacerbate DSS-induced colitis in mice.
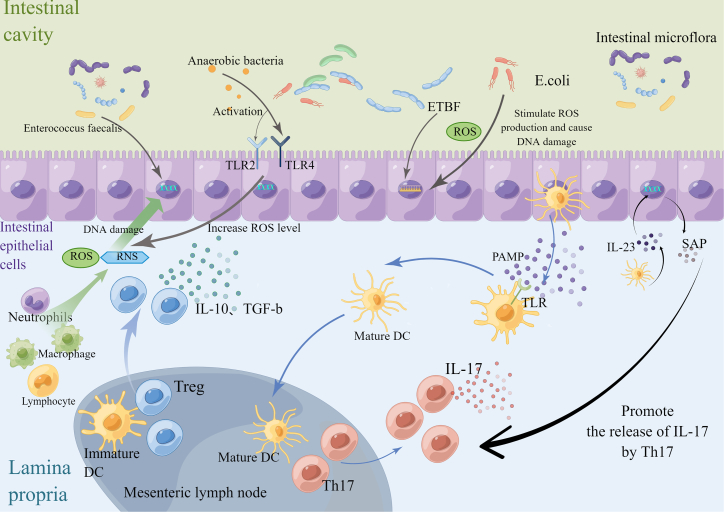
Figure 2.
The mechanism of inflammatory reaction and oxidative stress reaction of intestinal flora
In inflammatory reactions, symbiotic microorganisms can regulate the adaptive immune system in various ways. Symbiotic microorganisms produce adaptive immune responses by stimulating the differentiation of Th17 cells and Treg cells, among which Treg cells exert immunosuppressive function on various immune cells through IL-10 and TGF-β. Th17 cells acquire pathogenic inflammatory phenotype with IL-23 and SAP, and they promote the release of IL-17. In the oxidative stress reaction, inflammatory cells, including neutrophils, macrophages, and lymphocytes, produce a large number of ROS and RNS under the stimulation of inflammation, which leads to DNA damage. Anaerobic bacteria activated TLR2/TLR4 on intestinal epithelial cells (IECs) and increased the level of ROS in cells. E. coli and ETBF also stimulate ROS production and cause DNA damage. Inflammation and oxidative stress promote each other, which further leads to the occurrence of CRC.
With the change of intestinal flora on TME and the deepening understanding of the immune microenvironment of CRC, microsatellite classification (dMMR/MSI-H and pMMR/MSS) has become a key biomarker for the diagnosis and treatment of CRC patients, so it has important clinical value.55 Microsatellite instability (MSI) results from nucleotide insertion or deletion accumulation in the genome. MSI can be divided into MSI-high (MSI-H), MSI-low (MSI-L), and microsatellite stability (MSS).56 The composition and distribution of immune cells and cytokines in TMEs of CRC patients with different MSI types of tumor-infiltrating lymphocytes are different. MSI-H tumor significantly increased the recruitment of tumor-infiltrating lymphocytes, including activated cytotoxic T lymphocytes (CTLs), Th1 cells, and CD4+T cells, as well as NK cells and macrophages. In addition, tumor necrosis factor (TNF), IL-1, IL-6, and IFN-γ in TME, and other related cytokines,57 are added to MSI-H tumor. These cytokines regulate the activation or inhibition state of TME immunity, and different cytokines have different mechanisms and sources. Please refer to Table 2 for details.
Table 2.
Cytokine profile associated with CRC
| Classification | Function/Effect | Source | Reference | ||
|---|---|---|---|---|---|
| IFN | IFN-I | IFN-α | inhibit virus replication, and enhance the role of MHC-II molecules. Activate DCs and increase the cytolytic activity of macrophages and NK cells, induce IL-15 production, increase T cell survival, and increase the production of stromal-derived vascular inhibitory molecules | activated macrophages, monocytes, and activated T cells | Dunn et al. and Di Franco et al.2,11 |
| IFN-β | Fibroblasts | Dunn et al. and Di Franco et al.2,11 | |||
| IFN-II | IFN-γ | inhibit virus replication, increase the production of MHC-I and MHC-II in cancer cells, increase the production of IL-12 in APC, promote Th1 polarization, and activate macrophages. IFN-γ stimulation leads to nuclear translocation and phosphorylation of STAT1, leading to STAT1-TET2 binding. IFN-γ is an effective inducer of IDO expression and a pathogenic driver of CRC progression | Th1,Tc1,NK cells | Dunn et al., Wang et al., and Du et al.2,14,17 | |
| Interleukin | IL-1F | IL-1α, IL-1β, | enhance the expression of cytokines (such as IL-2 and its receptors) to stimulate T cell activation, enhance B cell proliferation and maturation, NK cytotoxicity, induce the expression of IL-1, IL-6, IL-8, TNF, GM-CSF, and PGE2 in macrophages and the expression of chemokines to play a pro-inflammatory role | monocytes, macrophages, DCs, NK, B cells, endothelial cells | Li et al., Kasprzak, Sharma and Kanneganti195,196,197 |
| IL-18 | induce T cells to produce IFN-γ and enhance NK cytotoxicity | macrophages and DCs | Li et al. and Sharma and Kanneganti195,197 | ||
| IL-36α, IL-36β, IL-36γ | activated monocytes, macrophages, and keratinocytes produce a variety of pro-inflammatory factors to stimulate T cells together | keratinocytes, other barrier tissues, neutrophils | Li et al., Li et al., and Xu et al.195,198,199 | ||
| IL-37 | anti-inflammatory effect | monocytes, macrophages, epithelial cells, activated B cells | Li et al.195 | ||
| IL-38 | N terminus of apoptotic cells | Li et al.195 | |||
| IL-2 family (γc family) | IL-2 | induce the proliferation of activated T cells and activated B cells, enhance NK cells' cytotoxicity, and the monocytes and macrophages' ability to kill tumor cells and bacteria | Th1 | Li et al. and Heys et al.195,200 | |
| IL-4 | induce Th2 cells, stimulate the proliferation of B, T, and mast cells, upregulate B cells, macrophage MHC-II molecules and CD23 of B cells, downregulate IL-12 production to inhibit Th1 differentiation, and enhance macrophage endocytosis, mediates tumor cell proliferation, survival, and metastasis in colon cancer | Th2, Tc2, NK, NKT, γδT, and mast cells | Li et al. and Song et al.195,201 | ||
| IL-13 | inhibit macrophage activation and cytokine secretion, stimulate B cell proliferation, upregulate B cell and monocyte MHC-II class molecules and CD23 expression, and mediate tumor cell proliferation, survival and metastasis of colon cancer | Th2 and mast cells | Song et al.201 | ||
| IL-15 | induce the proliferation of T, NK, and activated B cells and the production of NK and CD8+ T cell factors | T, NK, monocytes, macrophages, DCs, and B cells | Li et al. and Zarogoulidis et al.195,202 | ||
| IL-21 | NK differentiation, B cell activation, stimulation of T cell, Follicular helper T cells (Tfh) directed differentiation and survival | Th | Li et al.195 | ||
| chemokine family | IL-3 | promote growth and differentiation of hematopoietic cell precursors, mast cell growth | T, NK, and mast cells | Li et al.195 | |
| IL-8 | mediate chemotaxis and activation of neutrophils IL-8 modulates not only stemness maintenance but also stemness promotion, such as epithelial-mesenchymal transition |
monocytes, macrophages, and endothelial cells | Kasprzak and Conciatori et al.196,203 | ||
| IL-12/IL-6 family | IL-6 | promote the differentiation of myeloid stem cells, and the differentiation of B cells into plasma cells, induce the expression of acute phase proteins, enhance the proliferation of T cells, and it is vital for Th17 and Tfh-directed differentiation | Th2, monocytes, macrophages, DCs, and bone marrow stromal cells | Kasprzak and Taniguchi and Karin196,204 | |
| IL-12 | induce the proliferation of Th1, CD8+T, γδT, NK and the production of IFN-γ and enhance NK cell and CD8+ T cell cytotoxicity | monocytes, macrophages, DCs, and B cells | Taniguchi and Karin and Engel and Neurath204,205 | ||
| IL-23 | induce Th1 proliferation and IFN-γ production, induce Th17 expansion and survival, induce macrophages to express pro-inflammatory factors (such as IL-1, IL-6, TNF), and inhibit intestinal inflammation | DCs | Taniguchi and Karin and Neurath204,206 | ||
| IL-27 (IL-30) | induce Th1 responses, enhance IFN-γ production | DCs and monocytes | Taniguchi and Karin and Engel and Neurath204,205 | ||
| IL-35 | produce immunosuppressive effects on Th1, Th2, and Th17, stimulating Treg proliferation | Treg | Taniguchi and Karin204 | ||
| IL-10 family | IL-10 | suppress IFN-γ secretion and IL-2 secretion, downregulation of MHC-II molecules and cytokines such as IL-2 production, inhibition of Th1 differentiation, inhibition of T cell proliferation, and enhancement of B cell differentiation | Th, Tc, B cells, monocytes, and macrophages | Li et al. and Landskron et al.195,207 | |
| IL-19 | regulate Th1 activity | monocyte | Li et al.195 | ||
| IL-20 | regulate skin inflammation | monocyte and keratinocyte | Li et al.195 | ||
| IL-22 (IL-TIF) | inhibition of Th2 and secretion of IL-4 | T cells | Li et al. and Wei et al.195,208 | ||
| IL-24 (MDA-7) | induce TNF, IL-1, IL-6 expression and anti-tumor activity | Th2, monocytes, and macrophages | Li et al.195 | ||
| IL-26 | enhance epithelial cell production of IL-8 and IL-10 | T cells and NK cells | Li et al. and Niess et al.195,209 | ||
| IL-17 family | IL-17 | pro-inflammatory effect, stimulate the expression of TNF, IL-1β, IL-6, IL-8, G-CSF and other factors, inhibit intestinal inflammation | T cells | Hurtado et al. and Wu et al.210,211 | |
| IL-25 | induce IL-4, IL-5, IL-13 expression and Th2 related pathological changes | Th1, macrophages, and mast cell | Li et al. and Jou et al.195,212 | ||
| other interleukins | IL-7 | induce differentiation of lymphocytic stem cells into T and B lineage progenitor cells, and activate mature T cells | bone marrow and thymic stromal cells | Zarogoulidis et al.202 | |
| IL-33 | promote inflammatory response and induce Th2-mediated innate and adaptive immune responses | macrophages, DCs, fibroblasts, and mast cell | Jou et al.212 | ||
| growth factor | GM-CFS | stimulate the growth of monocytes, neutrophils, eosinophils, and basophils, and activate macrophages | Th, macrophages, fibroblasts, mast cells, and endothelial cells | Aliper et al.213 | |
| VEGF | promote tumor angiogenesis, and it is closely related to the invasion and metastasis of CRC | endothelial cells, smooth muscle cells, some mesenchymal and stromal cells | Maryam et al.214 | ||
| TGF | TGF | TGF-α | associated with tumor metastasis and invasion | macrophages, brain cells, and keratinocytes | Lee et al.215 |
| TGF-β | pro-inflammatory effects include induction of monocyte and macrophage chemotaxis; anti-inflammatory effects, such as inhibition of lymphocyte proliferation | Th, B cells, macrophages, and mast cells | Lee et al.215 | ||
| TNF | TNF | TNF-α | cause cachexia and tumor cytotoxicity, induce cytokine secretion, and activate macrophages | Th, monocytes, macrophages, DCs, mast cells, NK cells, and B cells | Kasprzak and Landskron et al.196,207 |
| TNF-β | cause tumor cytotoxicity, enhance the endocytosis of neutrophils and macrophages, and participate in the development of lymphoid organs | Th1, Tc | Maryam et al.214 | ||
Among them, it has been demonstrated that increased IFN expression correlates with better prognosis and induces the secretion of chemokines and adaptive immune responses with a microenvironment enriched in IFN-I compared with other CRCs. At the same time, various inflammatory mediators infiltrate to form an inflammatory TME, and continuous inflammatory stimulation leads to the exhaustion of T lymphocytes, thereby upregulating inhibitory receptors, such as PD-1.58 The binding of these immunosuppressive receptors to corresponding ligands in the TME regulates anti-tumor immune responses. Activated T cells can express PD-1 on their membranes after antigen recognition and can also produce IFNs that can induce the expression of PD-L1 in various tissues. The binding between PD-1 and its ligand limits T cell activity and extreme immune responses. This immune mechanism controls immune tolerance to self-antigens through the negative regulation of the immune response.59 Currently, MSI is considered a well-known biomarker of PD-1 blockade. Studies have shown that specific subpopulations of CRC can be better treated. Deciphering the mechanisms behind these inconsistencies may provide new insights into identifying advanced treatments and cures for CRC.
More and more evidence shows that the immune system controls the carcinogenicity of the intestine. The communication of microbial communities is connected to the immune system by diverse mechanisms, including inflammatory body induction60 and TLR signaling.61 Among them, NOD-like receptors (NLRs) and TLRs are associated with the incidence of CRC.62 According to data, 20%–30% of CRC patients have inflammation. The inflammation mechanism is recognized as the driving factor of tumor occurrence, which is also reflected in many CRC risks of patients with IBD.43,63,64 Microbes can form an inflammatory microenvironment, which in turn may affect the composition of microbes in the gut. The occurrence of CRC is related to intestinal microbes, inflammatory microenvironment, and immune regulation of the intestinal tract.24
Induce oxidative stress reaction
Chronic inflammation caused by gut microbiota is common in CRC. Due to the irritation of inflammation, inflammatory cells will release a lot of reactive nitrogen species (RNS) and reactive oxygen species (ROS), which will result in DNA damage and activate oncogenes or inhibit oncogenes, which is conducive to the further development of CRC. Gut microbiota can also directly release ROS, such as superoxide production induced by E. faecalis, which damages the DNA of epithelial cells by bystander effects.65,66 In vivo and in vitro studies have shown that E. faecalis can produce a potent mutagen called hydroxyl radical,67 which can cause point mutations, DNA breakage, and protein-DNA cross-linking, leading to chromosome instability of IECs and thus the risk of CRC68 in addition to E. faecalis activating the epidermal growth factor receptor and promoting cell proliferation through the production of hydrogen peroxide. In contrast, anaerobic bacteria start TLR2 or TLR4 on IECs (intestinal epithelial cells) to increase ROS levels inside cells and promote cell proliferation and cholesterol synthesis.69 ETBF and E. coli in the intestinal microenvironment are also capable of causing ROS generation in the IECs.70 ETBF36 causes colitis and increases the activity of the spermine oxidase (SMO) gene in the colon, stimulating ROS production and causing DNA damage.71 The findings suggest that oxidative stress when acting as a metabolic regulator can promote STING-mediated immune response in DCs and emphasize the role of small ubiquitin-like modifier proteins specific protease 3 (SENP3) as a STING signaling-induced overflow in the TME with metabolic abnormalities.72 In summary, intestinal flora not only have pro-tumorigenic effects on their own but they can also further contribute to colorectal carcinogenesis by producing ROS and RNS (Figure 2).
Generation of metabolites
Microbiota produce metabolites of host immunity and CRC and, in addition to direct interactions, the gut microbiota produces diverse combinations of metabolites to generate specific immune responses that might immediately benefit or harm the host. In healthy individuals, the normal metabolism of colonic cells can maintain the balance of commensal communities and the anaerobic state in the human gut.73 Diet, disease, or other injuries can cause transformation and abnormal metabolism of colonic cells, which can lead to disturbance of the host-commensal bacterial symbiosis and dysregulation of the intestinal flora.73,74 Bacterial metabolites, including hydrogen sulfide, secondary bile acids, tryptophan catabolites, polyamines, and short-chain fatty acids, can synergize with other T cell-targeted immunotherapies as well.22 For example, short-chain fatty acids (SCFAs) are mainly composed of propionate, acetate, and butyrate.74 SCFAs, especially butyrate, can inhibit DNA damage and pathogen proliferation by significantly reducing the pH of fecal matter in the gut.75 Extracellular SCFAs also interact with cell surface receptors, such as G protein-coupled receptors (GPCRs), and take part in regulating intestinal immune reactions.76 Butyrate plays a vital part in the cell as a histone deacetylase inhibitor, downregulates IL-6 to attenuate intestinal inflammatory responses,77 induces ROS production,78 and inhibits lipopolysaccharide-induced pro-inflammatory mediators in DCs and macrophages.79 In the DSS-induced colitis model, butyrate can alleviate inflammatory reactions by promoting M2 macrophage polarization.80 Besides, SCFAs can accelerate the generation of Treg cells and rapidly release Th1 cytokines such as TNF and IFN-γ to enhance immune reaction.81 Metabolites produced by intestinal flora can cause a robust adaptive immune response, so bacterial metabolites have the potential to produce synergistic effects in immunotherapy.
Intestinal flora affects IFN in the treatment of CRC
Most research focuses on how gut microbiota activate gut immunity to influence tumor immunotherapy. Therapies related to the innate immune response, including CD47 blockade, rely on the rapid immune reaction in the TME.22 The IFN pathway is significant for activating cytotoxic T cells, which is the core of tumor immune monitoring and immunotherapy. Studies have shown intestinal flora can regulate the human immune mechanism by affecting IFN release.
It was shown that systemic administration of Bifidobacterium could cause a mass of accumulation in tumors, and non-responsive mice were endowed with anti-CD47 immunotherapy responses in an IFN-dependent manner and IFN gene stimulator (STING). After anti-CD47 treatment, local delivery of bifidobacteria effectively promoted the cross-infiltration of DCs and activated STING signaling. This study identified the gut microbiome as a priority to colonize tumor sites and promote immunotherapy through STING signaling.22 At the same time, some studies have found that the modulation of gut microbiota can affect the host response to myriad types of cancer treatment, especially immunotherapy.82,83,84 Oral bifidobacteria failed to promote CD47 blockade in mice conditionally knocked out in DCs. This finding suggests that IFN-I signaling in DCs plays an important role in treating Bifidobacterium-promoting CD47 blockade. IFN-I signaling can promote cross-stimulatory responses of DCs, thereby enabling adaptive immune reactions.85,86 Meanwhile, it was shown that Bifidobacterium bifidum promotes CD47-founded immunotherapy in a T cell-dependent and IFN-β-dependent method, and its anti-tumor function depends on STING signaling within DCs. The STING pathway adjusts the expression of IFN-I in anti-CD47-mediated anti-tumor effects. STING agonists targeting tumor tissue may be effective.22 Active migration and continuous production of metabolites by live bacteria in the gut would activate the STING path in DCs. The synergetic effects of STING activators and the cyclic guanosine monophosphate-adenosine monophosphate (cGAS) product cGAMP on STING activation must be further explored.44
Modulation of the microbiome may become a new immunotherapeutic strategy.87 Naive Th cells (Th0) usually mature and differentiate into Treg, Th1, Th2, or Th17 lineages. Among the classic Th1 cytokines, IFN-γ plays an anti-tumor role in TME, while Treg or Th2-related cytokines, including IL-4, IL-5, and IL-10, can promote tumors in the TME. Depletion of the gut microbiome led to marked growth in the number of T cells secreting anti-tumor IFN-γ and a corresponding reduction in the amount of pro-tumor IL-17a and Tc1 and IL-10-secreting immune populations. Symbiotic intestinal fungi promote activation of the inflammasome and maturation of IL-18 during colitis,44 thus facilitating the secretion of IFN-γ by CD8+ T cells and the restoration of the epithelial barrier. Some fecal strains from healthy providers have been shown to promote the proliferation of IFN-γ+ CD8+ T cells in the gut and to heighten ICB efficacy in mice with CRC.54 In the CD4+ Th cell subset, although Th1 cells are the main cells producing IFN-γ, Th1 cells may alter function in the intestinal TME.88 Aerobic glycolysis can regulate the function of CD4+ T cells and affect the secretion of IFN-γ.17 An experiment89 analyzed the gastrointestinal tract secretion of human BCL-G in healthy and unhealthy conditions; this in vitro study had shown that TNF-α and IFN-γ act synergistically to increase BCL-GS/L and generate apoptosis in primary human colonic-like organs and colonic epithelial cell lines. IFN-I signaling is initiated by a nucleic acid sensor, which is the primary immune signal to detect invading pathogens. In the TME of CRC patients, aerobic glycolysis impairment often occurs, promoting further strengthening of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) binding to IFN-γ AU-rich elements (IFN-γAREs), thus reducing IFN-γ secretion.90 Studies have shown that Lactobacillus (LAB) double-stranded RNA can activate intestinal DCs to produce TLR3-mediated IFN-β, thereby preventing infection and colitis.91 In addition, LAB can also induce IFN-I production via cGAS and RLR (RIG-I-like receptors).92 The IFN-I signaling is initiated by the activation of cyclic GMP-AMP synthase-stimulator of interferon genes (cGASSTING), which is indispensable for innate resistance to RNA and DNA viruses. In-depth studies have shown that stimulatory activation of cGASSTING signaling results from membrane vesicle-mediated DNA scheduling in bacteria93 and is supposed to associate the immune response with DNA sensing.94 Hence, special attention must be paid to controlling the activation of the IFN-I system initiated by RNA and DNA sensors to maintain a steady state of the intestinal mucosa in response to colitis and pathogen infection.
In summary, the gut microbiome mediates the inflammatory response and oxidative stress in the intestine. At the same time, the metabolites produced by microorganisms can affect the immune status of the intestine, which might be advantageous or detrimental to treating CRC. Regulating the composition, distribution, and quantity of the intestinal microbiome and thus modulating the IFN cascade signaling to adjust the intestinal microecological and immune status for treating CRC is a direction worth further exploration.
Use of OV and IFN tampering in the treatment of CRC
OVs are a fresh therapeutic drug that promotes anti-tumor response through the dual mechanism of selective killing of tumor cells and induction of systemic anti-tumor immunity.95,96 Compared with other drugs, OVs have unique advantages, such as specific infection of cancer cells and improvement of TME. OVs can specifically infect cancer cells without affecting normal cells. On the one hand, OVs can naturally identify cancer cells by the characteristics of cancer cell biomarkers97 or the properties of gene and protein expression.98 On the other hand, genetic engineering has further enhanced the specificity of OVs. Virus infection can cause cancer cells to produce soluble antigens and danger signals, release IFN, and improve the TME.99,100,101 In different subtypes of CRC, MSI leads to neoantigens that are more likely to be recognized by the immune system in the tumor, and more cytokines are accumulated here. Hence, CRC patients with MSI type have an ideal response to OV therapy.102
The primary mechanism of OVs
OVs selectively infect and kill cancer cells while enhancing the immune reaction of the body against the tumor.103 The ideal curative effect of OVs depends on the specific recognition of cancer cells by OVs and the rapid proliferation of cancer cells. First, the virus must combine with virus-specific receptors on the cancer cell surface. Some studies have improved the tumor-specific recognition ability of the OV to enhance the tumor-killing effect.104,105 Second, the rapid proliferation ability of cancer cells can promote the replication and amplification of viruses, and the genetic mutation in cancer cells can specifically improve the selective replication ability of viruses in tumor cells.106
Direct lysis of tumor cells
OV replication results in CRC cell apoptosis through a variety of mechanisms, such as activating apoptotic genes and related proteins, especially p53 and caspase genes. Studies have shown that VSV can activate the transcription and translation of the p53 gene in colon cancer CT608 cell line and mediate apoptosis of colon cancer cells.107 Another study showed that late high expression of the p53 gene could effectively enhance cancer cell killing.108 In addition, reovirus induces apoptosis by upregulating the expression of caspase and other genes in CT608 cell line.109 OV can also be used as a genetic engineering vector to treat CRC. A study has shown that oncolytic adenovirus ZD55 carrying multisubstrate deoxyribonucleoside kinase of drosophila melanogaster (DM-DNK) induces high and sustained expression of suicide genes in CRC cells and activates nucleoside analogues (NAs), thus achieving an anti-tumor effect.110
OVs can also induce apoptosis of cancer cells by activating the apoptosis pathway of CRC cells, such as PI3K, mitogen-activated protein kinase (MAPK), and the NF-κB signal pathway. Studies have shown that oncolytic adenovirus induces apoptosis in CRC cell line SW620 by activating the PI3K/Akt pathway.111 Another study shows that adenovirus can provide a more powerful killing effect on CRC cells by activating MAPK signal.112 In addition, reovirus can activate the NF-κB signal pathway and induce apoptosis of CRC cells.113
What is more, the daughter viruses released after cancer cell lysis can infect surrounding cells and generate a cascade response to expand the lysis. The killing effect of lysing viruses on cancer cells increases with time.114,115
Besides direct lysis of cancer cells, OVs attack tumor vasculature and eventually lead to thrombosis and vascular atrophy, leading to ischemia and hypoxia in cancer cells and reducing subsequent viral spread within tumor tissue.116 The strategy of lyssavirus attack on the tumor vascular system is to target vascular endothelial cells within the tumor while selectively enhancing tumor vascular permeability.117 An experimental study showed that oncolytic adenovirus can infect, replicate, and expand in tumor-associated endothelial cells, causing inhibition of tumor angiogenesis.118,119 In contrast, in clinical trials, the tumor lysing virus JX-594 was found to cause acute tumor vascular disruption, causing a significant decrease in blood perfusion at tumor sites.120,121 In addition, lysing viruses can inhibit angiogenesis. Vascular epithelial growth factor (VEGF), a classical angiogenic factor, was found to be significantly reduced by cancer cells infected with lysing viruses.122,123 At the same time, the secretion capacity of VEGF was also reduced in surrounding uninfected cells in the presence of relevant cytokines.124
Induction of organismal immune activity
Compared with directly cracking cancer cells and attacking tumor blood vessels, it is more important to induce immune activity.125 OV therapy interacts with various constituent parts of the immune system in different forms and methods, improving the TME, making it favorable for fuller contact with immune cells, and activating tumor-specific cell reactions.126
OV directly acts on innate immune cells and enhances the immunogenicity of the TME. In the virus replication process, the virus’s structural protein is often expressed on the surface of cancer cells. The host’s anti-viral immune response can recognize these antigens expressed on the surface of cancer cells and kill tumor cells.127 In the process of viral oncolysis, cancer cells also produce molecules such as high-mobility group protein B1 (HMGB1), calreticulin (CRT),128,129 and heat shock proteins (HSPs),130,131 which activate the anti-tumor immune reaction.
OV infection can activate the TLR signaling pathway in the tumor site and cause acute inflammatory reaction,132 which results in the production of IL-2, chemokines, cytokines, IFN-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF),118,133,134,135,136 which can directly kill cancer cells, induce chemotactic immune cells to infiltrate into the cancer location, and enhance immune reaction. The infiltration of immune cells caused by virus infection and the production of cytokines secreted by immune cells improve the effect of infiltrating immune cells, resist tumor-induced immunosuppression, and benefit the activation of immune reaction. For instance, GM-CSF can significantly strengthen the anti-tumor immune effect. GM-CSF is an inducing factor to stimulate the replication of hematopoietic stem cells,137,138 and it can promote the production of tumor-specific cytotoxic T lymphocytes (CTLs) as well, which can directly kill tumors.139 Besides, TNF-α and IL-2 have also been proved to have anti-tumor effects.140,141 When IL-2 is expressed by oncolytic VSV, regulatory T cells in the neovascular system and tumor tissues can be reduced,142,143 and T cell-mediated immune reaction could be induced in the immune cancer stem cell model.
Besides infiltration of inflammatory cells and secretion of cytokines, the OV itself will release viral death signals. Infected cells will also release cell death signals, tumor-associated antigens (TAAs), endogenous danger signals, tumor-derived cytokines, and even immunomodulatory factors144,145 to activate DCs, enhance their antigen presentation ability, activate innate immune responses, and activate DCs, neutrophils (NEs), macrophages (mø), NK cells, and other immune cells, enhancing the body’s innate immunity.146,147
After OV infects tumor cells, it is easy to cross-present tumor antigens, activate acquired immune effects, and cause immunogenic cell death (ICD). At the same time, it leads to the release of PAMP and TAA, and finally activates DAMP and leads to the release of cytokines. First of all, OV promotes the expression of MHC-I and stimulates CD8+ T cells. In addition, the infection of the OV provides inflammatory stimulation for the body, thus promoting antigen processing and the formation of the MHC-II complex and enhancing the adaptive immunity of the body.148,149,150 In addition, OV infection can induce ICD of tumor cells, including cell death, apoptosis, and autophagy.151 Immunogenic death leads to the exposure or release of PAMPs152 and TAAs153 as danger signals. PAMPs activate a series of signals, stimulate inflammatory corpuscles, activate different transcription factors, and finally produce pro-inflammatory cytokines and DAMP.154 The release of pro-inflammatory cytokines reversed the balance between pro-inflammatory factors and anti-inflammatory factors in the TME, thus breaking the immunosuppression state of the tumor. In addition to breaking tumor-induced immunosuppression, immune cells that release cytokines can also be recruited to enhance the host’s anti-tumor immunity155 (Figure 3).
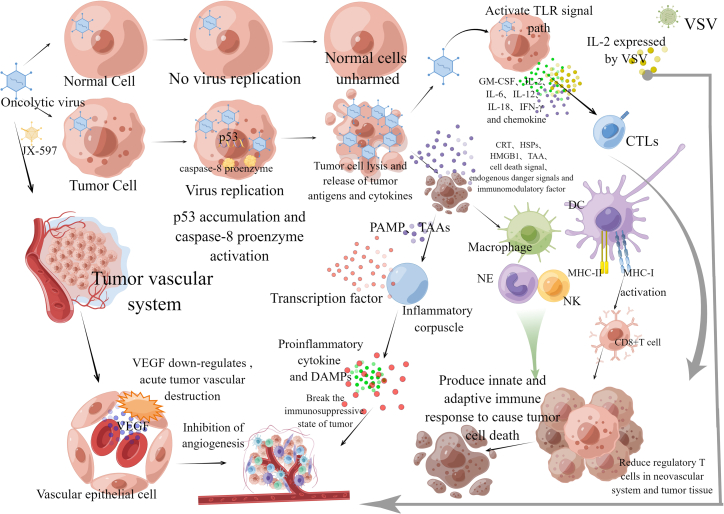
Figure 3.
OV is a new therapeutic drug that promotes anti-tumor response through the dual mechanism of selective tumor cell killing and inducing systemic anti-tumor immunity
OV selectively infects and kills tumor cells, but virus replication does not occur in normal cells of the human body, so normal cells continue to survive. However, when the OV infects tumor cells, viral replication leads to the accumulation of p53 and activation of caspase-8 proenzyme, leading to tumor cell apoptosis and lysis. Besides directly cracking cancer cells, the OV also attacks tumor blood vessels and causes vascular atrophy, among which JX-594 can cause acute tumor blood vessel destruction, and, at the same time, the OV can downregulate the secretion of VEGF by tumor cells and surrounding uninfected cells and inhibit the formation of tumor vascular system. In addition, IL-2 expressed by VSV can reduce regulatory T cells in the neovascular system and tumor tissues. Compared with directly cracking cancer cells and attacking tumor blood vessels, inducing immune activity is more important. In the process of virus oncolysis, OVs replicated in tumor cells are released, as well as CRT, HSPs, HMGB1, TAA, cell death signals, endogenous risk signals, and tumor-derived cytokines. Among them, immunogenic death leads to the exposure or release of PAMPs and TAAs as danger signals. PAMPs activate a series of signals, stimulate inflammatory corpuscles, and release pro-inflammatory cytokines and DAMPs, thus breaking the immunosuppressive state of tumors. In addition, virus oncolysis can also recruit immune cells that release cytokines. OV infection can activate the TLR signaling pathway in the tumor site, resulting in the release of cytokines GM-CSF, IL-2, IL-6, IL-12, IL-18, and IFN-γ, and chemokines, which can directly have toxic effects on tumor cells, stimulate chemotactic cells to infiltrate into the tumor site, and induce anti-tumor immunity. At the same time, cell death signals, TAA, endogenous danger signals, tumor-derived cytokines, and immunoregulatory factors released in the process of oncolysis can activate immune cells such as DCs, NK cells, macrophages, and neutrophils, and activate the innate immune response of the body. At the same time, after the OV infects tumor cells, it is easy to start adaptive immune effect, promote MHC-I expression, activate CD8+T cells, and promote the formation of MHC-II.
Interaction between IFN and OV
IFN has many functions, such as anti-virus, inhibiting cell proliferation, regulating immunity, and anti-tumor. Numerous cells have a complete IFN pathway, have immune clearance against OVs, and cannot carry on playing a therapy role in cancer cells.156,157 Therefore, if the OV is to proliferate rapidly in the body, it is necessary to inhibit the IFN response. On the other hand, the secretion of IFN stimulates the immune response in various ways, thus the immunosuppressive response of TME can be broken, which is very beneficial to tumor treatment.96,158,159 Therefore, some OVs are designed to express IFN, to play a more powerful anti-tumor role.
Inhibition of the anti-viral effect of IFN-I
An important aspect of the interaction between OVs and IFN is that OVs can inhibit the anti-viral response of IFN through several important pathways, such as inhibiting the signal pathway of IFN-I, inhibiting the synthesis of IFN proteins, and producing bait proteins that combine with IFN to inhibit the anti-viral effects of IFN.
One strategy of OVs to inhibit the anti-viral response of the IFN cascade is to interfere with the downstream signal that produces IFN-I.120 This pathway is mainly achieved by blocking the key steps of IFN response and can also be achieved by blocking the function of ISG. In addition, part of the protein shell of OVs can directly inhibit the initiation of IFN. The protein encoded by some OVs can block the key steps of the IFN-I signaling and inhibit the initiation of the IFN-I signaling pathway. For example, herpes simplex virus (HSV) encodes asparagine deaminase UL37, modifies and inactivates retinoic acid-induced gene protein (IRIG-I),160 and inhibits the production of IFN. The E1A protein of oncolytic adenovirus downregulates the STAT activator protein-1 (STAT1), which decreases the signal transduction when IFN-I combines with its receptor, stops the production of immune serum globulin 3(ISG3), and also blocks the interaction between peripheral blood signal transducer and transcription activator-1 (STAT1) and IFN regulatory factor (IRF1), thereby avoiding the induction of transcription of ISGs.161 The C6 protein encoded by an oncolytic poxvirus (VV) binds to STAT2, and paramyxovirus encodes V and W proteins interacting with STAT1 and STAT2,162 which inhibits the signal transduction of IFN-I. At the same time, HSV can also block cyclic GMP-AMP synthetase (cGAS) and the downstream stimuli STING,163 which leads to preventing IFN-I production. Moreover, the oncolytic measles virus (MV) can bind and inactivate phosphatase PP1, thus preventing the activation and dephosphorylation of RIG-I and MDA5164 and also preventing the initiation of type III IFN signaling cascade. Besides perturbing the secretion or signal of IFN-I, a subset of viruses directly intercepts ISG and its multiple features. For instance, the E1A protein of adenovirus combines with the nuclear complex to avoid histone ubiquitination, thus inhibiting chromatin opening and repressing ISG transcription.165 In addition, part of the OV protein coat can block IFN-I response through various pathways, one of which is to avoid the start of the IFN-I signal cascade by finding that virus protein binds to viral RNA and RIG-I.166 Some OV proteins can also directly prevent the stimulation of NF-κB and IRF3, thus preventing the transcription of IFN mRNA167,168 and inhibiting the activation of IFN. Similarly, experiments have confirmed that herpes simplex virus169 and reovirus120 also have the above mechanisms, and they can also block the production of type III IFN.
Another way to inhibit the production of IFN-I is to inhibit its transformation into protein. VSV uses this strategy, and its matrix protein interacts with the nuclear pore, preventing mRNA export to the cytoplasm.170 The effect of inhibition is not only aimed at IFN mRNA but also affects many genes, leading to the widespread blocking of protein production. An animal experiment confirmed that an oncolytic VSV deleted its matrix protein in methionine 51, which inactivated the feature, thus enhancing the specificity for tumor cells with IFN pathway deficiency.171
Another way to inhibit IFN-I is to produce bait protein to bind specifically with IFN, to prevent IFN from binding with cell receptors and starting a signal cascade reaction. For example, an oncolytic pox virus (VV) encodes B18R,172 a king of soluble IFN-I receptors on infected cells, thus preventing IFN from combining with cell receptors. The protein B18R of Wyeth strain OVJx594 of oncolytic pox virus is naturally truncated,173 which seriously reduces its affinity for IFN.
Enhance the anti-tumor effect of IFN-II
Another important aspect of the interaction between OVs and IFN is to promote the anti-tumor effect of IFN. It is mainly realized by promoting the activation of the IFN-II signal pathway and designing OVs into a vector that can express IFN by genetic engineering.
Some OVs can promote the body to produce IFN. For instance, HSV-1 irritates NK cells to produce IFN-II through TLR2/NF-κB signaling pathway and mobilizes DCs, macrophages, and other types of immune cells.174,175 The accumulation of these cancer cells improves the TME in the gut and enhances the lethal effect on cancer cells. Oncolytic virus has a similar effect. It can induce a high level of IFN-II expression by facilitating the transcription activity of IFN regulatory factor 3 (IRF-3)176 and enhancing the anti-tumor effect of OVs.
In addition, modification of the OV to make it a carrier for IFN expression can also enhance the killing effect on cancer cells. An in vitro experiment proved that a VVB18R deletion mutant that also overexpressed IFN-II only replicated in tumors, and its killing effect on cancer cells was significantly increased.177 Similarly, another experiment also proved that, compared with the parent virus, the oncolytic MV encoding IFN-II is more effective in controlling the killing of cancer cells and the spread of tumors.178 For adenovirus, with the adenovirus variant encoding IFN-γ,179 it was found that the virus not only prevented cancer vascularization180 but also improved cancer-killing and immune stimulation ability. In addition, the oncolytic VSV was also designed to express IFN. An experiment confirmed that the variant expressing IFN-γ from oncolytic VSV can preferably control the tumor growth in multifarious CRC models via strengthening the anti-tumor immune response.181 Similarly, research tested a IFN-II-engineered VSV variant and found that, compared with VSV, the virus can inhibit cancer and improve immune reaction by decreasing tumor infiltration of Tregs and enhancing tumor infiltration of CTLS.182 It is worth noting that oncolytic VSV expressing IFN-II is presently in clinical tests on solid tumor,183 which has broad development prospects (Table 3).
Table 3.
Summary of the basic mechanism of OV regulating IFN
| Effect on IFN | Oncolytic virus | Mechanism of action | Authors | Year |
|---|---|---|---|---|
| Anti-viral effect of inhibiting IFN-I | herpes simplex virus | encoding asparagine deaminase UL37 | Watanabe et al.192 | 2018 |
| modify and inactivate IRIG-1 | Ishino et al.191 | 2021 | ||
| blocking the synthesis of cGAS by cyclic GMP-AMP and its downstream effector STING | Glorioso et al.163 | 2021 | ||
| the combination of viral protein with viral RNA and RIG-I prevents the initiation of IFN-I signal cascade | Linder et al.166 | 2021 | ||
| direct connection of virus protein inhibits the activation of NF-κB and IRF3 downstream and prevents IFN mRNA transcription | Ottolino-Perry et al.169 | 2015 | ||
| oncolytic adenovirus | E1A protein downregulates signal transduction and STAT1 and reduces the signal transmitted when IFN-I binds to its receptor | Delwar et al.193 | 2018 | |
| prevents the formation of ISG3 and blocks the interaction between peripheral blood signal transducer and STAT1 and IRF1 | Komatsu et al.161 | 2016 | ||
| preventing the induction of ISG transcription | Hu et al.194 | 2018 | ||
| the binding of the E1A protein to the nuclear complex prevents histone ubiquitination, chromatin opening, and ISG transcription inhibition | Lipatova et al.165 | 2021 | ||
| Paramyxovirus | encoding v and w proteins interacting with STAT1 and STAT2 | Danziger et al.162 | 2018 | |
| reovirus | viral proteins combine with viral RNA and RIG-I to avoid the start of the IFN-I signaling cascade. Directly inhibit the activation of NF-κB and IRF3 downstream, thus preventing the transcription of IFN mRNA and inhibiting the activation of IFN | Lee et al.120 | 2020 | |
| oncolytic MV | inactivated PP1 was combined to prevent dephosphorylation and activation of RIG-I and MDA5 | Walton et al.164 | 2018 | |
| VSV | matrix proteins interact with nuclear pores, preventing mRNA from being exported to the cytoplasm | Means et al.170 | 2020 | |
| VSV δ 51 has lost its matrix protein in methionine 51 | Velazquez-Salinas et al.171 | 2017 | ||
| oncolytic pox virus | VV encodes B18R, which prevents IFN from binding to the fine-cell receptor | Stewart et al.172 | 2021 | |
| the B18R protein of OV Jx594 is naturally truncated, reducing its affinity for IFN | Sun et al.173 | 2019 | ||
| Enhance the anti-tumor effect of IFN-II | herpes simplex virus | HSV-1 stimulates NK cells to release IFN-II through TLR2/NF-κB signaling pathway | Wang et al.174,175 | 2022 |
| oncolytic poxvirus | Recruits macrophages, DCs, and other immune cells to improve the tumor microenvironment and promote IRF-3 transcription activity | Fox et al.176 | 2019 | |
| oncolytic MV | codes IFN-II to kill cancer cells and control tumor proliferation | Robinson et al.178 | 2017 | |
| oncolytic adenovirus | code IFN-γ adenoviral variants to enhance the killing and immune stimulation ability of cancer | Bah et al.179 | 2020 | |
| oncolytic VSV | expresses IFN-γ variants and enhances anti-tumor immunity | Urbiola et al.181 | 2018 | |
| reduces and increases tumor infiltration, Tregs, and CTL respectively, and improves anti-tumor immunity | Ayala Breton et al.182 | 2015 |
Interaction between OV, intestinal flora, and IFN
Cancer is associated with genetic mutations that activate proto-oncogenes and cause diseases related to cell growth. In the process of gene mutation, there will be the non-genetic or genetic inactivation or activation of specific genes that inhibit or irritate tumor metastasis and proliferation. Tumor immunotherapy is prospective and has been considered an important direction for treating tumors in the past 3 to 5 years. It is considered to be an effective alternative to radiotherapy, chemotherapy, and surgery.184 The OV and intestinal flora are promising in treating CRC by controlling IFN changes during immunotherapy.
OV-mediated immunological therapy is a new cancer treatment method using natural or genetically modified viruses. OVs are mainly used as gene vectors carrying specific checkpoint antibodies, which regulate immune checkpoints in the TME through the synergistic effects of cytokine and chemokine secretion, oncolysis, and so on. Genetically modified OVs can forthrightly produce checkpoint antibodies to destroy cancer cells in the TME. For instance, a novel recombinant myxoma virus (vPD-1) infects cells and produces soluble PD-1, which induces and maintains anti-tumor reactions and is more secure and more efficient than αPD-1 antibodies.185 NK cells and CD8+ T cells require OV-mediated immune activation to enhance the efficacy of antibodies that block PD-1/PD-L1- or cytotoxic T lymphocyte-associated protein 4 (CTLA4)/B7-mediated responses.186 OV encoding specific protein gene-IFN-β can significantly enhance checkpoint-blocking therapy. Vaccines made from OVs, such as adenovirus and vaccinia virus, show high oncolytic properties and significantly induce immune reactions against cancer patients with less lymphocyte distribution in the TME. Intestinal microbiota mediates the regulation of more than 100 trillion microorganisms in human intestines. A great deal of evidence shows that the changes in intestinal microflora are related to various cancers. Different microbiota or metabolites can contribute to changes in the immune reaction, such as the activation of Th17 cells and Tregs. Recent studies suggested that the immunocompetence and anti-tumor effect of checkpoint-blocking cures are related to different microbial species of different tumor types. Specific gut microbiota contributes to the efficacy of anti-CTLA-4 and anti-PD-1 treatment by diverse mechanisms.187 Bifidobacterium fragilis promotes the activation of CTLs and DCs in tumor-draining lymph nodes, therefore actuating immune reactions. Interestingly, the reinforcement has nothing to do with the transfer of microorganisms to the external position of the intestine. The symbiotic derivative factors may activate DCs, while soluble systemic chemokines promote DC transfer to TME. Furthermore, during CTLA-4 blockade, the memory T cell response of mice with specific immunomodulatory species was enhanced. Preceding studies have discovered that IFN-γ production and Th1 response are memorized in mice specific to B. fragilis treated with CTLA-4 monoclonal antibody (mAb),188 so it may be very important to study the key mechanism of intestinal flora as an immunotherapy adjuvant to enhance checkpoint-blocking therapy. Implanting immunogenic intestinal microflora (B. fragilis, etc.) into selected cancer patients can prepare living annotations, restore the anti-cancer T cell responses to confront cancer cells, and promote the anti-checkpoint effect.189
The OV and intestinal microbiota jointly regulate the immune checkpoint in TME. Both intestinal flora and the OVs can strengthen the treatment of CRC by anti-CTLA-4 and anti-PD-1 by various mechanisms.184 Intestinal microbial antigens, potential pathogens, and OVs lead to PAMPs. PAMPs activate a series of signals, stimulate inflammatory bodies, activate different transcription factors, and ultimately release pro-inflammatory cytokines and DAMPs.190 At the same time, the dense microbiota and OV infection in the intestine can produce all kinds of molecules identified by TLRs, thus causing inflammatory reactions and releasing IFN, other cytokines, and chemokines. Some intestinal microorganisms, such as Bifidobacterium, can produce immune responses through IFN gene stimulators (STING) and IFN dependence. The OV, such as HSV,163 can block the cGAS and the downstream effector STING, which results in a block of IFN secretion. At this time, OVs can inhibit the immune inflammatory reaction of intestinal bacteria by inhibiting STING and reducing IFN secretion. The OV itself has the effect of promoting the body to produce IFN. For instance, HSV-1191 stimulates pDC to produce IFN-I and can also stimulate NK cells to release IFN-II174,175 by the TLR2/NF-κB signal pathway. For intestinal flora, the IFN-I signaling in DCs is crucial to treating Bifidobacterium-promoting CD47 blocking. IFN-I signal can drive the cross-stimulation of the DCs and further promote adaptive immune reactions,85,86 which indicates that the OV can promote the immunotherapy of intestinal bacteria against CRC by stimulating IFN secretion. To sum up, there is a close relationship between OV, intestinal flora, and IFN. We need to explore the relationship to achieve the goal of treating CRC.
Summary and prospects
From the above review, we can understand that CRC is a disease of the intestinal epithelium that is heterogeneous. Although CRC is generally viewed as a disease associated with environmental and genetic factors, more and more research shows that the gut microbiota plays a significant part in accelerating cancer growth and proliferation and forming an inflammatory environment. The biofilm of intestinal bacteria, toxic metabolites, the destruction of intestinal microbiota to the steady state of the microenvironment, and the immune reaction produced are critical to the treatment of CRC. Identifying the composition of gut microbiota in healthy people is an important basis for assessing the appearance of CRC and plays a critical part in the early diagnosis of CRC. The studies have suggested that observing the variations of intestinal bacteria and fecal metabolites can prevent CRC, and a part of the intestinal flora has active anti-tumor functions in chemotherapy and immunotherapy. In addition, the OVs are also very promising in treating CRC, which can be effectively integrated into tumor immunotherapy. The OVs directly cleave cancer cells, releasing danger signals, soluble antigens, and IFN-I, thus driving anti-tumor immune responses. Besides, some OVs can be modified to convey therapeutic genes or improve the function of tumor-related endothelial cells to strengthen the recruitment of T cells into the TME of immune rejection or rejection. These characteristics make the OV an attractive drug for optimizing the combination strategy of cancer immunotherapy.
IFN is the first recombinant cytokine used to treat cancer. IFN has multiple functions, such as anti-viral, immune regulation, and anti-tumor functions. It exerts an anti-viral effect by inhibiting the synthesis of viral RNA and DNA and activates cytotoxic T cells to enhance the immune reaction while preventing the division of cancer cells from exerting an anti-tumor effect. IFN-induced proteins are vital to cell apoptosis. These proteins have the function of promoting apoptosis, while homozygous deletion gives cell apoptosis resistance. Therefore, IFN has dual functions. In addition to its pro-apoptotic effect, IFN also has an anti-apoptotic effect. The interaction between IFN and cell receptors produces a variety of cell signal pathways, and the anti-viral effect of IFN is usually mediated by the JAK-STAT signal pathway. Therefore, more in-depth research on the IFN-grade pathway will be carried out to deeply understand the interaction between intestinal flora and OV and IFN in CRC, which will help to make greater use of the immune system to treat cancer.
Intestinal flora and OV take part in treating CRC by influencing IFN. Intestinal microbiota promotes the stimulation of systemic IFN-I and anti-viral immunity, while the cancer specificity of biotherapy of OV usually depends on the defective IFN response often observed in cancer cells, so, compared with healthy cells, cancer cells increase their vulnerability to viruses. At present, OVs have been designed to better facilitate the IFN response, but, because of the over-activation of this IFN, the immune system can clear the virus faster, thus limiting direct tumor lysis. Therefore, intestinal flora takes part in controlling the direct oncolysis of OVs by promoting the production of IFN. In addition, OVs can promote the immunotherapy of intestinal bacteria against CRC by stimulating the secretion of IFN. These findings indicate that balancing the connection between enteric microorganisms, OV, and IFN is vital in treating CRC.
In addition, to make IFN effective in clinical use, three conditions need to be fully met: first, the need for the tumor itself to produce high-level IFN; second, the tumors themselves are susceptive to IFN; third, we need to overcome the immunosuppressive activity of the tumor. The intestinal microbiota is preferentially colonized at the tumor site, and its metabolites enhance the immune reaction and promote the secretion of IFN. Meanwhile, OVs encoded by special genes can lift the sensitivity of cancer cells to IFN. Intestinal symbiotic microorganisms can adjust the polarization of T cells, induce DAMPs, and do other things to promote immune activity. Furthermore, some OVs have immunostimulatory genes, and their lysed tumor cell death has high immunogenicity, which can create a pro-inflammatory environment to better control the immunosuppressive activity of tumors. Therefore, intestinal flora combined with OVs can better meet the above three conditions to promote IFN to play an anti-tumor role. In this paper, the pathogenic mechanism and potential therapeutic methods of CRC are discussed in detail from two aspects of intestinal flora and OV, and it is proposed that the effect of intestinal flora and OV on IFN is a prospective therapeutic option for treating CRC.
Acknowledgments
We thank all the authors of the original work and reviewers for their time and kindness in reviewing this paper. This research was funded by the National Natural Science Foundation of China Youth Program (82204962) and the Science and Technology Program of Tianjin, China (22JCQNJC00490). The figures were drawn with Figdraw (https://www.figdraw.com/static/index.html#/).
Author contributions
J.Y. conceived the study and conceptualized and wrote the first manuscript. J.Y. prepared figures. P.Z.L. prepared tables. P.Z.L., Q.B.L., and A.Z. helped with the further conceptualization of the manuscript. X.B.K. wrote the first manuscript and revised the final manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Johdi N.A., Sukor N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn G.P., Koebel C.M., Schreiber R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 3.Takaoka A., Hayakawa S., Yanai H., Stoiber D., Negishi H., Kikuchi H., Sasaki S., Imai K., Shibue T., Honda K., Taniguchi T. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 4.Silverman R.H. Implications for RNase L in prostate cancer biology. Biochemistry. 2003;42:1805–1812. doi: 10.1021/bi027147i. [DOI] [PubMed] [Google Scholar]
- 5.Hanada T., Kobayashi T., Chinen T., Saeki K., Takaki H., Koga K., Minoda Y., Sanada T., Yoshioka T., Mimata H., et al. IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J. Exp. Med. 2006;203:1391–1397. doi: 10.1084/jem.20060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin H., Wei S., Hurt E.M., Green M.D., Zhao L., Vatan L., Szeliga W., Herbst R., Harms P.W., Fecher L.A., et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Invest. 2018;128:1708–1815. doi: 10.1172/JCI120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diskin B., Adam S., Cassini M.F., Sanchez G., Liria M., Aykut B., Buttar C., Li E., Sundberg B., Salas R.D., et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 2020;21:442–454. doi: 10.1038/s41590-020-0620-x. [DOI] [PubMed] [Google Scholar]
- 8.Campesato L.F., Budhu S., Tchaicha J., Weng C.H., Gigoux M., Cohen I.J., Redmond D., Mangarin L., Pourpe S., Liu C., et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat. Commun. 2020;11:4011. doi: 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuang X., Veltri D.P., Long E.O. Genome-Wide CRISPR Screen Reveals Cancer Cell Resistance to NK Cells Induced by NK-Derived IFN-γ. Front. Immunol. 2019;10:2879. doi: 10.3389/fimmu.2019.02879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liau N.P.D., Laktyushin A., Lucet I.S., Murphy J.M., Yao S., Whitlock E., Callaghan K., Nicola N.A., Kershaw N.J., Babon J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018;9:1558. doi: 10.1038/s41467-018-04013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Franco S., Turdo A., Todaro M., Stassi G. Role of Type I and II Interferons in Colorectal Cancer and Melanoma. Front. Immunol. 2017;8:878. doi: 10.3389/fimmu.2017.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdan C., Mattner J., Schleicher U. The role of type I interferons in non-viral infections. Immunol. Rev. 2004;202:33–48. doi: 10.1111/j.0105-2896.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y.P., Lv L., Liu Y., Smith M.D., Li W.C., Tan X.M., Cheng M., Li Z., Bovino M., Aubé J., Xiong Y. Tumor suppressor TET2 promotes cancer immunity and immunotherapy efficacy. J. Clin. Invest. 2019;129:4316–4331. doi: 10.1172/JCI129317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., Wang Y., Song Z., Chu J., Qu X. Deficiency of interferon-gamma or its receptor promotes colorectal cancer development. J. Interferon Cytokine Res. 2015;35:273–280. doi: 10.1089/jir.2014.0132. [DOI] [PubMed] [Google Scholar]
- 15.Chawla-Sarkar M., Lindner D.J., Liu Y.F., Williams B.R., Sen G.C., Silverman R.H., Borden E.C. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 16.Thapa R.J., Basagoudanavar S.H., Nogusa S., Irrinki K., Mallilankaraman K., Slifker M.J., Beg A.A., Madesh M., Balachandran S. NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol. Cell. Biol. 2011;31:2934–2946. doi: 10.1128/MCB.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du W., Frankel T.L., Green M., Zou W. IFNγ signaling integrity in colorectal cancer immunity and immunotherapy. Cell. Mol. Immunol. 2022;19:23–32. doi: 10.1038/s41423-021-00735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemeny N., Younes A., Seiter K., Kelsen D., Sammarco P., Adams L., Derby S., Murray P., Houston C. Interferon alpha-2a and 5-fluorouracil for advanced colorectal carcinoma. Assessment of activity and toxicity. Cancer. 1990;66:2470–2475. doi: 10.1002/1097-0142(19901215)66:12<2470::aid-cncr2820661205>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Chaurasiya S., Fong Y., Warner S.G. Optimizing Oncolytic Viral Design to Enhance Antitumor Efficacy: Progress and Challenges. Cancers (Basel) 2020;12 doi: 10.3390/cancers12061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro F., Cardoso A.P., Gonçalves R.M., Serre K., Oliveira M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., Peng K.W., Dingli D., Kratzke R.A., Russell S.J. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17:550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y., Zheng W., Yang K., Harris K.G., Ni K., Xue L., Lin W., Chang E.B., Weichselbaum R.R., Fu Y.X. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J. Exp. Med. 2020;217 doi: 10.1084/jem.20192282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jobin C. Colorectal cancer: looking for answers in the microbiota. Cancer Discov. 2013;3:384–387. doi: 10.1158/2159-8290.CD-13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tlaskalova-Hogenova H., Vannucci L., Klimesova K., Stepankova R., Krizan J., Kverka M. Microbiome and colorectal carcinoma: insights from germ-free and conventional animal models. Cancer J. 2014;20:217–224. doi: 10.1097/PPO.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 25.Tsai Y.L., Lin T.L., Chang C.J., Wu T.R., Lai W.F., Lu C.C., Lai H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019;26:3. doi: 10.1186/s12929-018-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natividad J.M.M., Verdu E.F. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol. Res. 2013;69:42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Bäumler A.J., Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gensollen T., Iyer S.S., Kasper D.L., Blumberg R.S. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashemi Goradel N., Heidarzadeh S., Jahangiri S., Farhood B., Mortezaee K., Khanlarkhani N., Negahdari B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J. Cell. Physiol. 2019;234:2337–2344. doi: 10.1002/jcp.27250. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi A., Sato T., Kamada N., Mikami Y., Matsuoka K., Hisamatsu T., Hibi T., Roers A., Yagita H., Ohteki T., et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Chen S., Zhang L., Li M., Zhang Y., Sun M., Wang L., Lin J., Cui Y., Chen Q., Jin C., et al. Fusobacterium nucleatum reduces METTL3-mediated m(6)A modification and contributes to colorectal cancer metastasis. Nat. Commun. 2022;13:1248. doi: 10.1038/s41467-022-28913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Almeida C.V., Taddei A., Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap. Adv. Gastroenterol. 2018;11 doi: 10.1177/1756284818783606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vizcaino M.I., Crawford J.M. The colibactin warhead crosslinks DNA. Nat. Chem. 2015;7:411–417. doi: 10.1038/nchem.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung L., Orberg E.T., et al. Geis A.L., Chan J.L., Fu K., DeStefano Shields C.E., Dejea C.M., Fathi P., Chen J., et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe. 2018;23:421. doi: 10.1016/j.chom.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boleij A., Hechenbleikner E.M., Goodwin A.C., Badani R., Stein E.M., Lazarev M.G., Ellis B., Carroll K.C., Albesiano E., Wick E.C., et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015;60:208–215. doi: 10.1093/cid/ciu787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duijster J.W., Hansen J.V., Franz E., Neefjes J.J.C., Frisch M., Mughini-Gras L., Ethelberg S. Association between Salmonella infection and colon cancer: a nationwide registry-based cohort study. Epidemiol. Infect. 2021;149:e56. doi: 10.1017/S0950268821000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara-Tejero M., Galán J.E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 40.Arthur J.C., Perez-Chanona E., Mühlbauer M., Tomkovich S., Uronis J.M., Fan T.J., Campbell B.J., Abujamel T., Dogan B., Rogers A.B., et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semin I., Ninnemann J., Bondareva M., Gimaev I., Kruglov A.A. Interplay Between Microbiota, Toll-Like Receptors and Cytokines for the Maintenance of Epithelial Barrier Integrity. Front. Med. 2021;8 doi: 10.3389/fmed.2021.644333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan T., Du Y., Xing C., Wang H.Y., Wang R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.812774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasry A., Zinger A., Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016;17:230–240. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 44.Malik A., Sharma D., Malireddi R.K.S., Guy C.S., Chang T.C., Olsen S.R., Neale G., Vogel P., Kanneganti T.D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity. 2018;49:515–530.e5. doi: 10.1016/j.immuni.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiechers C., Zou M., Galvez E., Beckstette M., Ebel M., Strowig T., Huehn J., Pezoldt J. The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell. Mol. Immunol. 2021;18:1211–1221. doi: 10.1038/s41423-021-00647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 47.Huber S., Schramm C., Lehr H.A., Mann A., Schmitt S., Becker C., Protschka M., Galle P.R., Neurath M.F., Blessing M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J. Immunol. 2004;173:6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 48.De Simone V., Pallone F., Monteleone G., Stolfi C. Role of T(H)17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2 doi: 10.4161/onci.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J.Y., Hall J.A., Kroehling L., Wu L., Najar T., Nguyen H.H., Lin W.Y., Yeung S.T., Silva H.M., Li D., et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell. 2020;183:2036–2039. doi: 10.1016/j.cell.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghoreschi K., Laurence A., Yang X.P., Tato C.M., McGeachy M.J., Konkel J.E., Ramos H.L., Wei L., Davidson T.S., Bouladoux N., et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.p.m., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daillère R., Vétizou M., Waldschmitt N., Yamazaki T., Isnard C., Poirier-Colame V., Duong C.P.M., Flament C., Lepage P., Roberti M.P., et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Tanoue T., Morita S., Plichta D.R., Skelly A.N., Suda W., Sugiura Y., Narushima S., Vlamakis H., Motoo I., Sugita K., et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 55.Bai J., Chen H., Bai X. Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J. Clin. Lab. Anal. 2021;35 doi: 10.1002/jcla.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., Jemal A. Colorectal cancer statistics, 2017. CA. Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 57.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A., Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui G. The Mechanisms Leading to Distinct Responses to PD-1/PD-L1 Blockades in Colorectal Cancers With Different MSI Statuses. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.573547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Payandeh Z., Khalili S., Somi M.H., Mard-Soltani M., Baghbanzadeh A., Hajiasgharzadeh K., Samadi N., Baradaran B. PD-1/PD-L1-dependent immune response in colorectal cancer. J. Cell. Physiol. 2020;235:5461–5475. doi: 10.1002/jcp.29494. [DOI] [PubMed] [Google Scholar]
- 60.Luddy K.A., Robertson-Tessi M., Tafreshi N.K., Soliman H., Morse D.L. The role of toll-like receptors in colorectal cancer progression: evidence for epithelial to leucocytic transition. Front. Immunol. 2014;5:429. doi: 10.3389/fimmu.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zmora N., Levy M., Pevsner-Fishcer M., Elinav E. Inflammasomes and intestinal inflammation. Mucosal Immunol. 2017;10:865–883. doi: 10.1038/mi.2017.19. [DOI] [PubMed] [Google Scholar]
- 62.Tilg H., Adolph T.E., Gerner R.R., Moschen A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Brennan C.A., Garrett W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 65.Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 66.Kostic A.D., Gevers D., Pedamallu C.S., Michaud M., Duke F., Earl A.M., Ojesina A.I., Jung J., Bass A.J., Tabernero J., et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mima K., Sukawa Y., Nishihara R., Qian Z.R., Yamauchi M., Inamura K., Kim S.A., Masuda A., Nowak J.A., Nosho K., et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brennan C.A., Garrett W.S. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019;17:156–166. doi: 10.1038/s41579-018-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang H., Wang W., Romano K.A., Gu M., Sanidad K.Z., Kim D., Yang J., Schmidt B., Panigrahy D., Pei R., et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abed J., Emgård J.E.M., Zamir G., Faroja M., Almogy G., Grenov A., Sol A., Naor R., Pikarsky E., Atlan K.A., et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215–225. doi: 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sears C.L., Pardoll D.M. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J. Infect. Dis. 2011;203:306–311. doi: 10.1093/jinfdis/jiq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boleij A., van Gelder M.M.H.J., Swinkels D.W., Tjalsma H. Clinical Importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and meta-analysis. Clin. Infect. Dis. 2011;53:870–878. doi: 10.1093/cid/cir609. [DOI] [PubMed] [Google Scholar]
- 73.Litvak Y., Byndloss M.X., Bäumler A.J. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362 doi: 10.1126/science.aat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xing C., Du Y., Duan T., Nim K., Chu J., Wang H.Y., Wang R.F. Interaction between microbiota and immunity and its implication in colorectal cancer. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Obiso R.J., Jr., Lyerly D.M., Van Tassell R.L., Wilkins T.D. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect. Immun. 1995;63:3820–3826. doi: 10.1128/iai.63.10.3820-3826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Athan E., Cabiltes I., Coghill S., Bowe S.J. Silent but deadly: patients with enterococcal bacteraemia should be assessed for colorectal neoplasia. Med. J. Aust. 2019;210:86. doi: 10.5694/mja2.12027. [DOI] [PubMed] [Google Scholar]
- 77.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W., Fang D., Zhang H., Xue J., Wangchuk D., Du J., Jiang L. Sodium Butyrate Selectively Kills Cancer Cells and Inhibits Migration in Colorectal Cancer by Targeting Thioredoxin-1. Onco. Targets Ther. 2020;13:4691–4704. doi: 10.2147/OTT.S235575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nastasi C., Candela M., Bonefeld C.M., Geisler C., Hansen M., Krejsgaard T., Biagi E., Andersen M.H., Brigidi P., Ødum N., et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015;5 doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji J., Shu D., Zheng M., Wang J., Luo C., Wang Y., Guo F., Zou X., Lv X., Li Y., et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016;6 doi: 10.1038/srep24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bullman S., Pedamallu C.S., Sicinska E., Clancy T.E., Zhang X., Cai D., Neuberg D., Huang K., Guevara F., Nelson T., et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geller L.T., Barzily-Rokni M., Danino T., Jonas O.H., Shental N., Nejman D., Gavert N., Zwang Y., Cooper Z.A., Shee K., et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuchtey J., Chefalo P.J., Gray R.C., Ramachandra L., Harding C.V. Enhancement of dendritic cell antigen cross-presentation by CpG DNA involves type I IFN and stabilization of class I MHC mRNA. J. Immunol. 2005;175:2244–2251. doi: 10.4049/jimmunol.175.4.2244. [DOI] [PubMed] [Google Scholar]
- 86.Le Bon A., Etchart N., Rossmann C., Ashton M., Hou S., Gewert D., Borrow P., Tough D.F. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 87.Sethi V., Kurtom S., Tarique M., Lavania S., Malchiodi Z., Hellmund L., Zhang L., Sharma U., Giri B., Garg B., et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology. 2018;155:33–37.e6. doi: 10.1053/j.gastro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schoenborn J.R., Wilson C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 89.Woznicki J.A., Flood P., Bustamante-Garrido M., Stamou P., Moloney G., Fanning A., Zulquernain S.A., McCarthy J., Shanahan F., Melgar S., Nally K. Human BCL-G regulates secretion of inflammatory chemokines but is dispensable for induction of apoptosis by IFN-γ and TNF-α in intestinal epithelial cells. Cell Death Dis. 2020;11:68. doi: 10.1038/s41419-020-2263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang C.H., Curtis J.D., Maggi L.B., Jr., Faubert B., Villarino A.V., O'Sullivan D., Huang S.C.C., van der Windt G.J.W., Blagih J., Qiu J., et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawashima T., Kosaka A., Yan H., Guo Z., Uchiyama R., Fukui R., Kaneko D., Kumagai Y., You D.J., Carreras J., et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity. 2013;38:1187–1197. doi: 10.1016/j.immuni.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 92.Wirusanti N.I., Baldridge M.T., Harris V.C. Microbiota regulation of viral infections through interferon signaling. Trends Microbiol. 2022;30:778–792. doi: 10.1016/j.tim.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Erttmann S.F., Swacha P., Aung K.M., Brindefalk B., Jiang H., Härtlova A., Uhlin B.E., Wai S.N., Gekara N.O. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. 2022;55:847–861.e10. doi: 10.1016/j.immuni.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Du Y., Luo Y., Hu Z., Lu J., Liu X., Xing C., Wu J., Duan T., Chu J., Wang H.Y., et al. Activation of cGAS-STING by Lethal Malaria N67C Dictates Immunity and Mortality through Induction of CD11b(+) Ly6C(hi) Proinflammatory Monocytes. Adv. Sci. 2022;9 doi: 10.1002/advs.202103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kana S.I., Essani K. Immuno-Oncolytic Viruses: Emerging Options in the Treatment of Colorectal Cancer. Mol. Diagn. Ther. 2021;25:301–313. doi: 10.1007/s40291-021-00517-7. [DOI] [PubMed] [Google Scholar]
- 96.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bommareddy P.K., Shettigar M., Kaufman H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018;18:498–513. doi: 10.1038/s41577-018-0014-6. [DOI] [PubMed] [Google Scholar]
- 98.Kim S.I., Chaurasiya S., Park A.K., Kang S., Lu J., Woo Y., Yin H.H., Yin Z., Fong Y., Warner S.G. Vitamin D as a Primer for Oncolytic Viral Therapy in Colon Cancer Models. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21197326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ren Y., Miao J.M., Wang Y.Y., Fan Z., Kong X.B., Yang L., Cheng G. Oncolytic viruses combined with immune checkpoint therapy for colorectal cancer is a promising treatment option. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.961796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oncolytic viruses successfully delivered intravenously. Hum. Vaccin. Immunother. 2012;8:1014–1015. [PubMed] [Google Scholar]
- 101.Engeland C.E. Re: "Oncolytic Measles Virus Encoding Interleukin-12 Mediated Antitumor Activity and Immunologic Control of Colon Cancer In Vivo and Ex Vivo" by Wang et al. Cancer Biother. Radiopharm. 2021;36:106–107. doi: 10.1089/cbr.2020.4566. [DOI] [PubMed] [Google Scholar]
- 102.He R., Lao Y., Yu W., Zhang X., Jiang M., Zhu C. Progress in the Application of Immune Checkpoint Inhibitor-Based Immunotherapy for Targeting Different Types of Colorectal Cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.764618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu D., Ma J., Ding B., Zhou H. [Oncolytic vaccinia virus expressing CD40L (CD40L-VV) inhibits colorectal cancer cell growth and enhances anti-tumor activity of T cells in tumor-bearing mice] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021;37:602–607. [PubMed] [Google Scholar]
- 104.Carpenter S.G., Carson J., Fong Y. Regional liver therapy using oncolytic virus to target hepatic colorectal metastases. Semin. Oncol. 2010;37:160–169. doi: 10.1053/j.seminoncol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vigil A., Park M.S., Martinez O., Chua M.A., Xiao S., Cros J.F., Martínez-Sobrido L., Woo S.L.C., García-Sastre A. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 106.Scheubeck G., Berchtold S., Smirnow I., Schenk A., Beil J., Lauer U.M. Starvation-Induced Differential Virotherapy Using an Oncolytic Measles Vaccine Virus. Viruses. 2019;11 doi: 10.3390/v11070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garijo R., Hernández-Alonso P., Rivas C., Diallo J.S., Sanjuán R. Experimental evolution of an oncolytic vesicular stomatitis virus with increased selectivity for p53-deficient cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho I.R., Koh S.S., Min H.J., Park E.H., Ratakorn S., Jhun B.H., Jeong S.H., Yoo Y.H., Youn H.D., Johnston R.N., Chung Y.H. Down-regulation of HIF-1alpha by oncolytic reovirus infection independently of VHL and p53. Cancer Gene Ther. 2010;17:365–372. doi: 10.1038/cgt.2009.84. [DOI] [PubMed] [Google Scholar]
- 109.Babaei A., Soleimanjahi H., Soleimani M., Arefian E. The synergistic anticancer effects of ReoT3D, CPT-11, and BBI608 on murine colorectal cancer cells. Daru. 2020;28:555–565. doi: 10.1007/s40199-020-00361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma S., Qu W., Mao L., Zhu Z., Jia L., Zhao L., Zheng X. Antitumor effects of oncolytic adenovirus armed with Drosophila melanogaster deoxyribonucleoside kinase in colorectal cancer. Oncol. Rep. 2012;27:1443–1450. doi: 10.3892/or.2012.1665. [DOI] [PubMed] [Google Scholar]
- 111.Yuan S., Wu Y., Wang Y., Chen J., Chu L. An Oncolytic Adenovirus Expressing SNORD44 and GAS5 Exhibits Antitumor Effect in Colorectal Cancer Cells. Hum. Gene Ther. 2017;28:690–700. doi: 10.1089/hum.2017.041. [DOI] [PubMed] [Google Scholar]
- 112.Chia S.L., Lei J., Ferguson D.J.P., Dyer A., Fisher K.D., Seymour L.W. Group B adenovirus enadenotucirev infects polarised colorectal cancer cells efficiently from the basolateral surface expected to be encountered during intravenous delivery to treat disseminated cancer. Virology. 2017;505:162–171. doi: 10.1016/j.virol.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 113.Rezazadeh A., Soleimanjahi H., Soudi S., Habibian A. Comparison of the Effect of Adipose Mesenchymal Stem Cells-Derived Secretome with and without Reovirus in CT26 Cells. Arch. Razi Inst. 2022;77:615–622. doi: 10.22092/ARI.2021.353845.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tedcastle A., Illingworth S., Brown A., Seymour L.W., Fisher K.D. Actin-resistant DNAse I Expression From Oncolytic Adenovirus Enadenotucirev Enhances Its Intratumoral Spread and Reduces Tumor Growth. Mol. Ther. 2016;24:796–804. doi: 10.1038/mt.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu T., Xiang Y., Liu T., Wang X., Ren X., Ye T., Li G. Oncolytic Vaccinia Virus Expressing Aphrocallistes vastus Lectin as a Cancer Therapeutic Agent. Mar. Drugs. 2019;17 doi: 10.3390/md17060363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu J., Chen C., Lu R., Zhang Y., Wang Y., Hu Q., Li W., Wang S., Jing O., Yi H., et al. β-Adrenergic Receptor Inhibitor and Oncolytic Herpesvirus Combination Therapy Shows Enhanced Antitumoral and Antiangiogenic Effects on Colorectal Cancer. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.735278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arulanandam R., Batenchuk C., Angarita F.A., Ottolino-Perry K., Cousineau S., Mottashed A., Burgess E., Falls T.J., De Silva N., Tsang J., Howe G.A., Bourgeois-Daigneault M.C., Conrad D.P., Daneshmand M., Breitbach C.J., Kirn D.H., Raptis L., Sad S., Atkins H., Huh M.S., Diallo J.S., Lichty B.D., Ilkow C.S., Le Boeuf F., Addison C.L., McCart J.A., Bell J.C. VEGF-Mediated Induction of PRD1-BF1/Blimp1 Expression Sensitizes Tumor Vasculature to Oncolytic Virus Infection. Cancer Cell. 2015;28:210–224. doi: 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 118.Li S., Zhang Y., Wang J., Zhao Y., Ji T., Zhao X., Ding Y., Zhao X., Zhao R., Li F., Yang X., Liu S., Liu Z., Lai J., Whittaker A.K., Anderson G.J., Wei J., Nie G. An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation. Hum. Gene Ther. 2017;1:667–679. doi: 10.1089/hum.2017.033. [DOI] [PubMed] [Google Scholar]
- 119.Tysome J.R., Lemoine N.R., Wang Y. Combination of anti-angiogenic therapy and virotherapy: arming oncolytic viruses with anti-angiogenic genes. Curr. Opin. Mol. Ther. 2009;11:664–669. [PubMed] [Google Scholar]
- 120.Lee Y.S., Lee W.S., Kim C.W., Lee S.J., Yang H., Kong S.J., Ning J., Yang K.M., Kang B., Kim W.R., et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heo J., Reid T., Ruo L., Breitbach C.J., Rose S., Bloomston M., Cho M., Lim H.Y., Chung H.C., Kim C.W., et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen D., Wang R., Long M., Li W., Xiao B., Deng H., Weng K., Gong D., Liu F., Luo S., Hao W. Identification of in vitro and in vivo oncolytic effect in colorectal cancer cells by Orf virus strain NA1/11. Oncol. Rep. 2021;45:535–546. doi: 10.3892/or.2020.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Currier M.A., Eshun F.K., Sholl A., Chernoguz A., Crawford K., Divanovic S., Boon L., Goins W.F., Frischer J.S., Collins M.H., et al. VEGF blockade enables oncolytic cancer virotherapy in part by modulating intratumoral myeloid cells. Mol. Ther. 2013;21:1014–1023. doi: 10.1038/mt.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoo J.Y., Kim J.H., Kwon Y.G., Kim E.C., Kim N.K., Choi H.J., Yun C.O. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol. Ther. 2007;15:295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 125.Yoo S.Y., Bang S.Y., Jeong S.N., Kang D.H., Heo J. A cancer-favoring oncolytic vaccinia virus shows enhanced suppression of stem-cell like colon cancer. Oncotarget. 2016;7:16479–16489. doi: 10.18632/oncotarget.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parakrama R., Fogel E., Chandy C., Augustine T., Coffey M., Tesfa L., Goel S., Maitra R. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer. 2020;20:569. doi: 10.1186/s12885-020-07038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jing Y., Tong C., Zhang J., Nakamura T., Iankov I., Russell S.J., Merchan J.R. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009;69:1459–1468. doi: 10.1158/0008-5472.CAN-08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liikanen I., Ahtiainen L., Hirvinen M.L.M., Bramante S., Cerullo V., Nokisalmi P., Hemminki O., Diaconu I., Pesonen S., Koski A., et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol. Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guerrero R., Guerrero C., Acosta O. Induction of Cell Death in the Human Acute Lymphoblastic Leukemia Cell Line Reh by Infection with Rotavirus Isolate Wt1-5. Biomedicines. 2020;8 doi: 10.3390/biomedicines8080242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conrad S.J., El-Aswad M., Kurban E., Jeng D., Tripp B.C., Nutting C., Eversole R., Mackenzie C., Essani K. Oncolytic tanapoxvirus expressing FliC causes regression of human colorectal cancer xenografts in nude mice. J. Exp. Clin. Cancer Res. 2015;34:19. doi: 10.1186/s13046-015-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liikanen I., Koski A., Merisalo-Soikkeli M., Hemminki O., Oksanen M., Kairemo K., Joensuu T., Kanerva A., Hemminki A. Serum HMGB1 is a predictive and prognostic biomarker for oncolytic immunotherapy. Oncoimmunology. 2015;4 doi: 10.4161/2162402X.2014.989771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rommelfanger D.M., Compte M., Grau M.C., Diaz R.M., Ilett E., Alvarez-Vallina L., Thompson J.M., Kottke T.J., Melcher A., Vile R.G. The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice. Mol. Ther. 2013;21:348–357. doi: 10.1038/mt.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deng L., Yang X., Fan J., Ding Y., Peng Y., Xu D., Huang B., Hu Z. IL-24-Armed Oncolytic Vaccinia Virus Exerts Potent Antitumor Effects via Multiple Pathways in Colorectal Cancer. Oncol. Res. 2021;28:579–590. doi: 10.3727/096504020X15942028641011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakao S., Arai Y., Tasaki M., Yamashita M., Murakami R., Kawase T., Amino N., Nakatake M., Kurosaki H., Mori M., et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aax7992. [DOI] [PubMed] [Google Scholar]
- 135.Choi I.K., Li Y., Oh E., Kim J., Yun C.O. Oncolytic adenovirus expressing IL-23 and p35 elicits IFN-γ- and TNF-α-co-producing T cell-mediated antitumor immunity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim K.J., Moon D., Kong S.J., Lee Y.S., Yoo Y., Kim S., Kim C., Chon H.J., Kim J.H., Choi K.J. Antitumor effects of IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1. Gene Ther. 2021;28:186–198. doi: 10.1038/s41434-020-00205-x. [DOI] [PubMed] [Google Scholar]
- 137.Grossardt C., Engeland C.E., Bossow S., Halama N., Zaoui K., Leber M.F., Springfeld C., Jaeger D., von Kalle C., Ungerechts G. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum. Gene Ther. 2013;24:644–654. doi: 10.1089/hum.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yan Y., Li S., Jia T., Du X., Xu Y., Zhao Y., Li L., Liang K., Liang W., Sun H., Li R. Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity. Tumour Biol. 2015;36:4535–4543. doi: 10.1007/s13277-015-3098-7. [DOI] [PubMed] [Google Scholar]
- 139.Jennings V.A., Scott G.B., Rose A.M.S., Scott K.J., Migneco G., Keller B., Reilly K., Donnelly O., Peach H., Dewar D., et al. Potentiating Oncolytic Virus-Induced Immune-Mediated Tumor Cell Killing Using Histone Deacetylase Inhibition. Mol. Ther. 2019;27:1139–1152. doi: 10.1016/j.ymthe.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiao Z., Nie K., Han T., Cheng L., Zhang Z., Peng W., Shi D. Development and Validation of a TNF Family-Based Signature for Predicting Prognosis, Tumor Immune Characteristics, and Immunotherapy Response in Colorectal Cancer Patients. J. Immunol. Res. 2021;2021 doi: 10.1155/2021/6439975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cervera-Carrascon V., Siurala M., Santos J.M., Havunen R., Tähtinen S., Karell P., Sorsa S., Kanerva A., Hemminki A. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2017.1412902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stephenson K.B., Barra N.G., Davies E., Ashkar A.A., Lichty B.D. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012;19:238–246. doi: 10.1038/cgt.2011.81. [DOI] [PubMed] [Google Scholar]
- 143.Sobhanimonfared F., Bamdad T., Sadigh Z.A., Nikoo H.R., Choobin H. Combination of virotherapy with VSV and tumor vaccination significantly enhances the efficacy of antitumor therapy. Acta Virol. 2018;62:394–400. doi: 10.4149/av_2018_407. [DOI] [PubMed] [Google Scholar]
- 144.Dave R.V., Jebar A.H.S., Jennings V.A., Adair R.A., West E.J., Errington-Mais F., Toogood G.J., Melcher A.A. Viral warfare! Front-line defence and arming the immune system against cancer using oncolytic vaccinia and other viruses. Surgeon. 2014;12:210–220. doi: 10.1016/j.surge.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 145.Adair R.A., Roulstone V., Scott K.J., Morgan R., Nuovo G.J., Fuller M., Beirne D., West E.J., Jennings V.A., Rose A., et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jonker D.J., Tang P.A., Kennecke H., Welch S.A., Cripps M.C., Asmis T., Chalchal H., Tomiak A., Lim H., Ko Y.J., et al. A Randomized Phase II Study of FOLFOX6/Bevacizumab With or Without Pelareorep in Patients With Metastatic Colorectal Cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin. Colorectal Cancer. 2018;17:231–239.e7. doi: 10.1016/j.clcc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Sze D.Y., Iagaru A.H., Gambhir S.S., De Haan H.A., Reid T.R. Response to intra-arterial oncolytic virotherapy with the herpes virus NV1020 evaluated by [18F]fluorodeoxyglucose positron emission tomography and computed tomography. Hum. Gene Ther. 2012;23:91–97. doi: 10.1089/hum.2011.141. [DOI] [PubMed] [Google Scholar]
- 148.Kim S.I., Park A.K., Chaurasiya S., Kang S., Lu J., Yang A., Sivanandam V., Zhang Z., Woo Y., Priceman S.J., et al. Recombinant Orthopoxvirus Primes Colon Cancer for Checkpoint Inhibitor and Cross-Primes T Cells for Antitumor and Antiviral Immunity. Mol. Cancer Ther. 2021;20:173–182. doi: 10.1158/1535-7163.MCT-20-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Edukulla R., Woller N., Mundt B., Knocke S., Gürlevik E., Saborowski M., Malek N., Manns M.P., Wirth T., Kühnel F., Kubicka S. Antitumoral immune response by recruitment and expansion of dendritic cells in tumors infected with telomerase-dependent oncolytic viruses. Cancer Res. 2009;69:1448–1458. doi: 10.1158/0008-5472.CAN-08-1160. [DOI] [PubMed] [Google Scholar]
- 150.Nair S., Mazzoccoli L., Jash A., Govero J., Bais S.S., Hu T., Fontes-Garfias C.R., Shan C., Okada H., Shresta S., et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight. 2021;6 doi: 10.1172/jci.insight.144619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bartlett D.L., Liu Z., Sathaiah M., Ravindranathan R., Guo Z., He Y., Guo Z.S. Oncolytic viruses as therapeutic cancer vaccines. Mol. Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heiniö C., Havunen R., Santos J., de Lint K., Cervera-Carrascon V., Kanerva A., Hemminki A. TNFa and IL2 Encoding Oncolytic Adenovirus Activates Pathogen and Danger-Associated Immunological Signaling. Cells. 2020;9 doi: 10.3390/cells9040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Minev B.R., Lander E., Feller J.F., Berman M., Greenwood B.M., Minev I., Santidrian A.F., Nguyen D., Draganov D., Killinc M.O., et al. First-in-human study of TK-positive oncolytic vaccinia virus delivered by adipose stromal vascular fraction cells. J. Transl. Med. 2019;17:271. doi: 10.1186/s12967-019-2011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo Z.S., Liu Z., Bartlett D.L., Tang D., Lotze M.T. Life after death: targeting high mobility group box 1 in emergent cancer therapies. Am. J. Cancer Res. 2013;3:1–20. [PMC free article] [PubMed] [Google Scholar]
- 155.Delpeut S., Sisson G., Black K.M., Richardson C.D. Measles Virus Enters Breast and Colon Cancer Cell Lines through a PVRL4-Mediated Macropinocytosis Pathway. J. Virol. 2017;91 doi: 10.1128/JVI.02191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Geoffroy K., Bourgeois-Daigneault M.C. The pros and cons of interferons for oncolytic virotherapy. Cytokine Growth Factor Rev. 2020;56:49–58. doi: 10.1016/j.cytogfr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 157.Ebrahimi S., Ghorbani E., Khazaei M., Avan A., Ryzhikov M., Azadmanesh K., Hassanian S.M. Interferon-Mediated Tumor Resistance to Oncolytic Virotherapy. J. Cell. Biochem. 2017;118:1994–1999. doi: 10.1002/jcb.25917. [DOI] [PubMed] [Google Scholar]
- 158.Li Q., Tan F., Wang Y., Liu X., Kong X., Meng J., Yang L., Cen S. The gamble between oncolytic virus therapy and IFN. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.971674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsun A., Miao X.N., Wang C.M., Yu D.C. Oncolytic Immunotherapy for Treatment of Cancer. Adv. Exp. Med. Biol. 2016;909:241–283. doi: 10.1007/978-94-017-7555-7_5. [DOI] [PubMed] [Google Scholar]
- 160.Huang H., Zhao J., Wang T.Y., Zhang S., Zhou Y., Rao Y., Qin C., Liu Y., Chen Y., Xia Z., Feng P. Species-Specific Deamidation of RIG-I Reveals Collaborative Action between Viral and Cellular Deamidases in HSV-1 Lytic Replication. mBio. 2021;12 doi: 10.1128/mBio.00115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Komatsu Y., Derwish L., Hirasawa K. IRF1 Downregulation by Ras/MEK Is Independent of Translational Control of IRF1 mRNA. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Danziger O., Pupko T., Bacharach E., Ehrlich M. Interleukin-6 and Interferon-α Signaling via JAK1-STAT Differentially Regulate Oncolytic versus Cytoprotective Antiviral States. Front. Immunol. 2018;9:94. doi: 10.3389/fimmu.2018.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Glorioso J.C., Cohen J.B., Goins W.F., Hall B., Jackson J.W., Kohanbash G., Amankulor N., Kaur B., Caligiuri M.A., Chiocca E.A., et al. Oncolytic HSV Vectors and Anti-Tumor Immunity. Curr. Issues Mol. Biol. 2021;41:381–468. doi: 10.21775/cimb.041.381. [DOI] [PubMed] [Google Scholar]
- 164.Walton R.W., Brown M.C., Sacco M.T., Gromeier M. Engineered Oncolytic Poliovirus PVSRIPO Subverts MDA5-Dependent Innate Immune Responses in Cancer Cells. J. Virol. 2018;92 doi: 10.1128/JVI.00879-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lipatova A.V., Soboleva A.V., Gorshkov V.A., Bubis J.A., Solovyeva E.M., Krasnov G.S., Kochetkov D.V., Vorobyev P.O., Ilina I.Y., Moshkovskii S.A., et al. Multi-Omics Analysis of Glioblastoma Cells' Sensitivity to Oncolytic Viruses. Cancers (Basel) 2021;13 doi: 10.3390/cancers13215268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Linder A., Bothe V., Linder N., Schwarzlmueller P., Dahlström F., Bartenhagen C., Dugas M., Pandey D., Thorn-Seshold J., Boehmer D.F.R., Koenig L.M., Kobold S., Schnurr M., Raedler J., Spielmann G., Karimzadeh H., Schmidt A., Endres S., Rothenfusser S. Defective Interfering Genomes and the Full-Length Viral Genome Trigger RIG-I After Infection With Vesicular Stomatitis Virus in a Replication Dependent Manner. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.595390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Struzik J., Szulc-Dąbrowska L. NF-κB Signaling in Targeting Tumor Cells by Oncolytic Viruses-Therapeutic Perspectives. Cancers (Basel) 2018;10 doi: 10.3390/cancers10110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arwert E.N., Milford E.L., Rullan A., Derzsi S., Hooper S., Kato T., Mansfield D., Melcher A., Harrington K.J., Sahai E. STING and IRF3 in stromal fibroblasts enable sensing of genomic stress in cancer cells to undermine oncolytic viral therapy. Nat. Cell Biol. 2020;22:758–766. doi: 10.1038/s41556-020-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ottolino-Perry K., Acuna S.A., Angarita F.A., Sellers C., Zerhouni S., Tang N., McCart J.A. Oncolytic vaccinia virus synergizes with irinotecan in colorectal cancer. Mol. Oncol. 2015;9:1539–1552. doi: 10.1016/j.molonc.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Means R.E., Roy S.G., Katz S.G. The Propagation and Quantification of Two Emerging Oncolytic Viruses: Vesicular Stomatitis (VSV) and Zika (ZIKV) Methods Mol. Biol. 2020;2097:253–263. doi: 10.1007/978-1-0716-0203-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Velazquez-Salinas L., Naik S., Pauszek S.J., Peng K.W., Russell S.J., Rodriguez L.L. Oncolytic Recombinant Vesicular Stomatitis Virus (VSV) Is Nonpathogenic and Nontransmissible in Pigs, a Natural Host of VSV. Hum. Gene Ther. Clin. Dev. 2017;28:108–115. doi: 10.1089/humc.2017.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stewart G., Chantry A., Lawson M. The Use of Oncolytic Viruses in the Treatment of Multiple Myeloma. Cancers (Basel) 2021;13 doi: 10.3390/cancers13225687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun T., Luo Y., Wang M., Xie T., Yan H. Recombinant Oncolytic Vaccinia Viruses Expressing Human β-Defensin 2 Enhance Anti-tumor Immunity. Mol. Ther. Oncolytics. 2019;13:49–57. doi: 10.1016/j.omto.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Panagioti E., Kurokawa C., Viker K., Ammayappan A., Anderson S.K., Sotiriou S., Chatzopoulos K., Ayasoufi K., Johnson A.J., Iankov I.D., Galanis E. Immunostimulatory bacterial antigen-armed oncolytic measles virotherapy significantly increases the potency of anti-PD1 checkpoint therapy. J. Clin. Invest. 2021;131 doi: 10.1172/JCI141614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Y., Jin J., Li Y., Zhou Q., Yao R., Wu Z., Hu H., Fang Z., Dong S., Cai Q., et al. NK cell tumor therapy modulated by UV-inactivated oncolytic herpes simplex virus type 2 and checkpoint inhibitors. Transl. Res. 2022;240:64–86. doi: 10.1016/j.trsl.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 176.Fox C.R., Parks G.D. Histone Deacetylase Inhibitors Enhance Cell Killing and Block Interferon-Beta Synthesis Elicited by Infection with an Oncolytic Parainfluenza Virus. Viruses. 2019;11 doi: 10.3390/v11050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Inoue T., Byrne T., Inoue M., Tait M.E., Wall P., Wang A., Dermyer M.R., Laklai H., Binder J.J., Lees C., et al. Oncolytic Vaccinia Virus Gene Modification and Cytokine Expression Effects on Tumor Infection, Immune Response, and Killing. Mol. Cancer Ther. 2021;20:1481–1494. doi: 10.1158/1535-7163.MCT-20-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Robinson S., Galanis E. Potential and clinical translation of oncolytic measles viruses. Expert Opin. Biol. Ther. 2017;17:353–363. doi: 10.1080/14712598.2017.1288713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bah E.S., Nace R.A., Peng K.W., Muñoz-Alía M.Á., Russell S.J. Retargeted and Stealth-Modified Oncolytic Measles Viruses for Systemic Cancer Therapy in Measles Immune Patients. Mol. Cancer Ther. 2020;19:2057–2067. doi: 10.1158/1535-7163.MCT-20-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Antoszczyk S., Rabkin S.D. Prospect and progress of oncolytic viruses for treating peripheral nerve sheath tumors. Expert Opin. Orphan Drugs. 2016;4:129–138. doi: 10.1517/21678707.2016.1128322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Urbiola C., Santer F.R., Petersson M., van der Pluijm G., Horninger W., Erlmann P., Wollmann G., Kimpel J., Culig Z., von Laer D. Oncolytic activity of the rhabdovirus VSV-GP against prostate cancer. Int. J. Cancer. 2018;143:1786–1796. doi: 10.1002/ijc.31556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ayala Breton C., Wikan N., Abbuhl A., Smith D.R., Russell S.J., Peng K.W. Oncolytic potency of HER-2 retargeted VSV-FH hybrid viruses: the role of receptor ligand affinity. Mol. Ther. Oncolytics. 2015;2 doi: 10.1038/mto.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gautam S., Xin D., Garcia A.P., Spiesschaert B. Single-step rapid chromatographic purification and characterization of clinical stage oncolytic VSV-GP. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.992069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shi T., Ma Y., Yu L., Jiang J., Shen S., Hou Y., Wang T. Cancer Immunotherapy: A Focus on the Regulation of Immune Checkpoints. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19051389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bartee M.Y., Dunlap K.M., Bartee E. Tumor-Localized Secretion of Soluble PD1 Enhances Oncolytic Virotherapy. Cancer Res. 2017;77:2952–2963. doi: 10.1158/0008-5472.CAN-16-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rojas J.J., Sampath P., Hou W., Thorne S.H. Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy. Clin. Cancer Res. 2015;21:5543–5551. doi: 10.1158/1078-0432.CCR-14-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ott P.A., Hodi F.S., Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 188.Belkaid Y., Bouladoux N., Hand T.W. Effector and memory T cell responses to commensal bacteria. Trends Immunol. 2013;34:299–306. doi: 10.1016/j.it.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillère R., Hannani D., Enot D.P., Pfirschke C., Engblom C., Pittet M.J., et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lamas B., Richard M.L., Leducq V., Pham H.P., Michel M.L., Da Costa G., Bridonneau C., Jegou S., Hoffmann T.W., Natividad J.M., et al. CARD9 affects colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ishino R., Kawase Y., Kitawaki T., Sugimoto N., Oku M., Uchida S., Imataki O., Matsuoka A., Taoka T., Kawakami K., et al. Oncolytic Virus Therapy with HSV-1 for Hematological Malignancies. Mol. Ther. 2021;29:762–774. doi: 10.1016/j.ymthe.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Watanabe D., Goshima F. Oncolytic Virotherapy by HSV. Adv. Exp. Med. Biol. 2018;1045:63–84. doi: 10.1007/978-981-10-7230-7_4. [DOI] [PubMed] [Google Scholar]
- 193.Delwar Z.M., Kuo Y., Wen Y.H., Rennie P.S., Jia W. Oncolytic Virotherapy Blockade by Microglia and Macrophages Requires STATone-third. Cancer Res. 2018;78:718–730. doi: 10.1158/0008-5472.CAN-17-0599. [DOI] [PubMed] [Google Scholar]
- 194.Hu C., Liu Y., Lin Y., Liang J.K., Zhong W.W., Li K., Huang W.T., Wang D.J., Yan G.M., Zhu W.B., et al. Intravenous injections of the oncolytic virus M1 as a novel therapy for muscle-invasive bladder cancer. Cell Death Dis. 2018;9:274. doi: 10.1038/s41419-018-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li J., Huang L., Zhao H., Yan Y., Lu J. The Role of Interleukins in Colorectal Cancer. Int. J. Biol. Sci. 2020;16:2323–2339. doi: 10.7150/ijbs.46651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kasprzak A. The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22041565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharma B.R., Kanneganti T.D. Inflammasome signaling in colorectal cancer. Transl. Res. 2023;252:45–52. doi: 10.1016/j.trsl.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li M., Jiang W., Wang Z., Lu Y., Zhang J. New insights on IL-36 in intestinal inflammation and colorectal cancer (Review) Exp. Ther. Med. 2023;25:275. doi: 10.3892/etm.2023.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu P., Guan H., Xiao W., Sun L. The role of IL-36 subfamily in intestinal disease. Biochem. Soc. Trans. 2022;50:223–230. doi: 10.1042/BST20211264. [DOI] [PubMed] [Google Scholar]
- 200.Heys S.D., Deehan D.J., Eremin O. Interleukin 2 treatment in colorectal cancer: current results and future prospects. Eur. J. Surg. Oncol. 1994;20:622–629. [PubMed] [Google Scholar]
- 201.Song X., Traub B., Shi J., Kornmann M. Possible Roles of Interleukin-4 and -13 and Their Receptors in Gastric and Colon Cancer. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22020727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zarogoulidis P., Lampaki S., Yarmus L., Kioumis I., Pitsiou G., Katsikogiannis N., Hohenforst-Schmidt W., Li Q., Huang H., Sakkas A., et al. Interleukin-7 and interleukin-15 for cancer. J. Cancer. 2014;5:765–773. doi: 10.7150/jca.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Conciatori F., Bazzichetto C., Falcone I., Ferretti G., Cognetti F., Milella M., Ciuffreda L. Colorectal cancer stem cells properties and features: evidence of interleukin-8 involvement. Cancer Drug Resist. 2019;2:968–979. doi: 10.20517/cdr.2019.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Taniguchi K., Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 205.Engel M.A., Neurath M.F. Anticancer properties of the IL-12 family--focus on colorectal cancer. Curr. Med. Chem. 2010;17:3303–3308. doi: 10.2174/092986710793176366. [DOI] [PubMed] [Google Scholar]
- 206.Neurath M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019;45:1–8. doi: 10.1016/j.cytogfr.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 207.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014;2014 doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wei H.X., Wang B., Li B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020;11:1315. doi: 10.3389/fimmu.2020.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Niess J.H., Hruz P., Kaymak T. The Interleukin-20 Cytokines in Intestinal Diseases. Front. Immunol. 2018;9:1373. doi: 10.3389/fimmu.2018.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hurtado C.G., Wan F., Housseau F., Sears C.L. Roles for Interleukin 17 and Adaptive Immunity in Pathogenesis of Colorectal Cancer. Gastroenterology. 2018;155:1706–1715. doi: 10.1053/j.gastro.2018.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wu D., Wu P., Huang Q., Liu Y., Ye J., Huang J. Interleukin-17: a promoter in colorectal cancer progression. Clin. Dev. Immunol. 2013;2013 doi: 10.1155/2013/436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jou E., Rodriguez-Rodriguez N., McKenzie A.N.J. Emerging roles for IL-25 and IL-33 in colorectal cancer tumorigenesis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.981479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Aliper A.M., Frieden-Korovkina V.P., Buzdin A., Roumiantsev S.A., Zhavoronkov A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014;3:737–746. doi: 10.1002/cam4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maryam S., Krukiewicz K., Haq I.U., Khan A.A., Yahya G., Cavalu S. Interleukins (Cytokines) as Biomarkers in Colorectal Cancer: Progression, Detection, and Monitoring. J. Clin. Med. 2023;12 doi: 10.3390/jcm12093127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee H.M., Lee H.J., Chang J.E. Inflammatory Cytokine: An Attractive Target for Cancer Treatment. Biomedicines. 2022;10 doi: 10.3390/biomedicines10092116. [DOI] [PMC free article] [PubMed] [Google Scholar]