Abstract
Objective
to (1) systematically review the chronic effect of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) on maximal fat oxidation (MFO) in overweight and obese adults, and (2) explore MFO influencing factors and its dose-response relationships with HIIT and MICT.
Methods
Studies using a between-group design involving overweight and obese adults and assessing the effect of HIIT and MICT on MFO were included. A meta-analysis on MFO indices was conducted, and the observed heterogeneities were explored through subgroup, regression, and sensitivity analyses.
Results
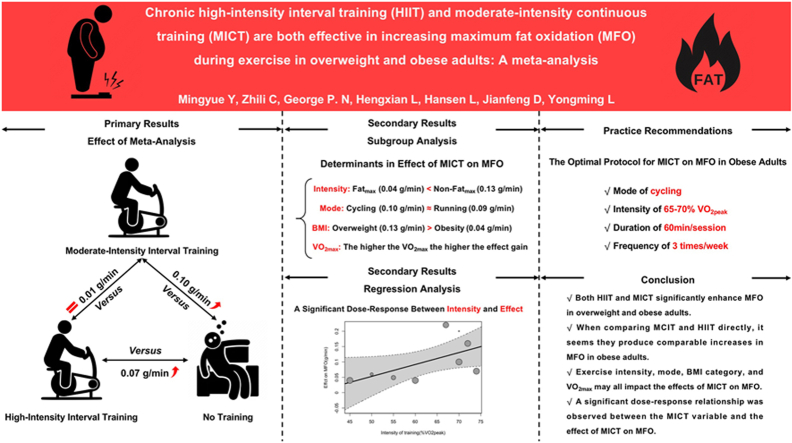
Thirteen studies of moderate to high quality with a total of 519 overweight and obese subjects were included in this meta-analysis (HIIT, n = 136; MICT, n = 235; Control, n = 148). HIIT displayed a statistically significant favorable effect on MFO compared to no-training (MD = 0.07; 95%CI [0.03 to 0.11]; I2 = 0%). Likewise, MICT displayed a statistically significant favorable effect on MFO compared to no-training (MD = 0.10; 95%CI [0.06 to 0.15]; I2 = 95%). Subgroup and regression analyses revealed that exercise intensity (Fatmax vs. non-Fatmax; %VO2peak), exercise mode, BMI, and VO2peak all significantly moderated MICT on MFO. When analyzing studies that have directly compared HIIT and MCIT in obese people, it seems there is no difference in the MFO change (MD = 0.01; 95%CI [-0.02 to 0.04]; I2 = 64%). No publication bias was found in any of the above meta-analyses (Egger's test p > 0.05 for all).
Conclusion
Both HIIT and MICT are effective in improving MFO in overweight and obese adults, and they have similar effects. MCIT with an intensity of 65–70% VO2peak, performed 3 times per week for 60 min per session, will optimize MFO increases in overweight and obese adults. Given the lack of studies examining the effect of HIIT on MFO in overweight and obese adults and the great diversity in the training protocols in the existing studies, we were unable to make sound recommendations for training.
Keywords: High-intensity interval training (HIIT), Moderate-intensity continuous training (MICT), Maximal fat oxidation (MFO), Meta-analysis, Obesity, Metabolism
Graphical abstract
1. Introduction
In recent years, overweight and obesity have been viewed as the largest chronic disease that has affected 1.9 billion adults worldwide, thereby posing challenges to public health.1,2 Physical inactivity, poor diet, and stress due to the COVID-19 pandemic have further exacerbated the problem, indicated by the observed increases in weight, BMI, and obesity rates in adults.3 Ectopic deposition of fat is a hallmark of overweight and obesity and is closely associated with metabolic diseases, including insulin resistance.4 This can also impair the function of tissues like skeletal muscles5 and ultimately increase the risk of cardiovascular diseases.1,2
Obesity is a multifactorial condition, but the primary etiology involves the chronic imbalance between energy intake and expenditure.6,7 Exercise represents a fundamental therapeutic approach to preventing and managing obesity,8 and exercise intensity is a major influence in modulating changes in fat oxidation during exercise and fat oxidation follows a parabolic curve with intensity.9 Earlier studies found that MFO was at 25% VO2max by constant-load continuous exercise at several different intensities (85/65/25% VO2max),9 but this test methodology makes it difficult to cover the full range of intensities and is less feasible and susceptible to extraneous variables over multiple days. To better elucidate and accurately measure MFO, Achten et al. "Fatmax"10 defined the intensity of MFO in the year 2001 and developed a graded incremental load test11 to determine Fatmax and MFO in the next year.
Over the past two decades, Fatmax and MFO have gained significant importance in weight management, metabolic health, and personalized exercise interventions. Firstly, a 4-year follow-up study found a strong positive association between higher MFO and clinical weight loss maintenance, suggesting that increasing MFO may facilitate weight loss maintenance. Secondly, an elevated MFO is associated with improving insulin sensitivity and lowering metabolic risk factors.12 Additionally, fat oxidation during exercise serves as an indicator of metabolic flexibility,13,14 and overweight and obese adults did have greater metabolic inflexibility compared to normal-weight individuals.15 Finally, Fatmax plays a crucial role in exercise intensity or threshold for improving or controlling body weight. Exercise with Fatmax maximizes the activation of lipid metabolic pathways throughout to increase lipid oxidation and achieve maximum fat utilization,10 and higher MFO helps to burn more fat during exercise.
Although Maunder's16 work on MFO (overweight/obese males: 0.28 ± 0.14 g/min; females: 0.16 ± 0.05 g/min) and Fatmax (overweight/obese males: 43 ± 18%VO2max; females: 61 ± 10%VO2max) yielded a standard reference value, significant variations were still found in the MFO values across existing studies. These inconsistencies could come from multiple factors of MFO, such as age,17,18 gender,19,20 training background,21, 22, 23, 24 BMI,25,26 exercise mode,27, 28, 29, 30, 31 nutritional status,32 assessment methods,33 etc. Notably, training experiences/status may also be a factor. Early cross-sectional studies also suggested that MFO increases with fitness level in well-trained males34 and is higher in well-trained people21,22 compared with sedentary counterparts.
The existing literature suggests that MFO is a modifiable parameter that can be enhanced through moderate-intensity continuous training (MICT) or high-intensity interval training (HIIT).16 MICT-induced increases in MFO result from adaptations in adipose tissue lipolysis, NEFA transport to skeletal muscle, skeletal muscle NEFA uptake, muscle triglyceride lipolysis, and/or mitochondrial uptake of fatty acids, given fat oxidation may be limited by fatty acid delivery to skeletal muscle or mitochondrial fatty acid uptake.9,35,36 HIIT has also been shown to enhance MFO in recent years,37 and the mechanism of action differs, as high-intensity or all-out sprinting training protocols increase mitochondrial enzyme activity and protein content,38, 39, 40 muscle membrane fatty acid transport protein content,41,42 and lipolytic enzyme protein content.42
However, relevant findings concerning HIIT and MFO are still inconsistent. While many scholars observed significant improvements in MFO in overweight and obese adults,43,44 there are also reports with null results.45 Similar inconsistencies are also present in the studies on MICT and MFO.45,46 These controversies underline the necessity to obtain more solid evidence for this topic.
Theoretically, systematic reviews and meta-analyses can offer stronger evidence than controlled trials. In 2018, Astorino37 and Maunder16 conducted a qualitative summary of the effects of exercise on MFO and concluded that HIIT and MICT were effective in improving MFO. However, this study only assessed the effectiveness of the two training, leaving many questions unanswered. For example, can these interventions fit overweight and obese adults? Which one is more beneficial? These can be essential for prescribing personalized exercise protocol, particularly among populations with metabolic inflexibility.47 Furthermore, the utilization of meta-analysis can contribute to addressing and bridging several critical knowledge gaps by 1) providing robust evidence for the effect of HIIT versus MICT on MFO in overweight and obese adults; 2) conducting subgroup analysis to gain clarity on the variables that may affect these results; 3) providing recommendations on the optimal exercise protocols to boost MFO.
Therefore, we conducted this systematic review and meta-analysis to examine and compare the effects of the two training on MFO in overweight and obese adults. We also aimed to explore the factors that may affect the results and the dose-response relationships between training settings and MFO. Our hypotheses were (a) Both HIIT and MICT are effective in improving MFO; (b) HIIT and MICT are comparative in improving MFO; (c) This effect is influenced by subject characteristics and has a dose-response relationship with training programs.
2. Methods
This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines48 and preregistered in the PROSPERO database (ID: CRD42023397504).
2.1. Search strategy
PubMed/MEDLINE, EBSCOhost. Cochrane Library and Web of Science (Core Collection) were searched from the database inception to March 01, 2023. Using the Web of Science as an example, we searched for the following terms by title, abstract, and keywords: TS=(("fat oxidation" OR "maximum fat oxidation" OR "MFO" OR "fat utilization" OR "fat utilization" OR "lipid oxidation" OR "lipid utilization" OR "lipid utilization" OR "CHO oxidation" OR "carbohydrate oxidation" OR "carbohydrate utilization" OR "carbohydrate utilization" OR "CHO utilization" OR "CHO utilization" OR "substrate oxidation" OR "substrate utilization" OR "substrate utilization") AND ("interval training" OR "high-intensity aerobic training" OR HIIT OR SIT OR "continuous training " OR "moderate-intensity continuous training" OR MICT)), additional databases are available in online supplemental file 1. Search terms for different databases are available in supplemental file 1. The reference lists of relevant meta-analyses and articles were also screened. Two reviewers (YMY and LHX) independently assessed the identified publications for eligibility, with any disagreements being resolved by a third reviewer (LYM).
2.2. Eligibility criteria
Studies were eligible for inclusion according to the following criteria: (a) the type of study was controlled between groups and consisted of a parallel randomized controlled trial or a pre-and post-randomized crossover trial; (b) participants were overweight (BMI = 25∼30 kg/m2) and obese (BMI>30 kg/m2) adults (18–60 years)who included apparently healthy; athletes or well-trained adults (participated in regular structured training protocol for at least 3 months prior to the intervention period) are excluded; (c) the training intervention was supervised, and consisted of HIIT/SIT (HIIT consists of several high-intensity training [approximately ≥75% of VO2peak, peak power out, HRmax] with intervals through active/passive recovery; SIT perform all-out sprints 2-6 30s with a resistance of 5–10% of body weight]) or MICT (continuous training at moderate intensity [45~75% VO2peak]), and did not include other types of exercise (e.g., combined exercise intervention), with the intervention lasting at least 2 weeks; (d) a comparator group involving a no-training control; (e) MFO was assessed during continuous submaximal or incremental graded exercise tests with indirect calorimetry before and after the intervention. MFO testing involves methodological issues such as different exercise modes, load increment protocols, and data collection and analysis methods, and these differences may have influenced the final MFO effect sizes pooled.33 Therefore, we fully disclose these details from the included studies in Supplementary Table 5 for reader review.
2.3. Data extraction
From the included studies, the following data were extracted by two independent authors (YMY and LHX) and checked by a third reviewer (LYM): the lead author's name, year of publication, participant characteristics, study design, training protocol, type of exercise testing used to assess MFO, training mode, means and standard deviations of MFO (g/min) at pre-and post-intervention. If information was missing, an attempt was made to contact the study investigators to obtain the necessary data. If the study authors were unresponsive or unreachable, the study was excluded.
2.4. Methodological quality of included studies and certainty of the evidence
The Physiotherapy Evidence Database (PEDro) scale was used to assess the risk of bias and methodological quality of included studies.49 The PEDro scale scores study on a scale of 0–10; studies scoring ≥6 are considered high quality, those scoring 4–5 are considered moderate quality, and those scoring ≤3 are considered low quality.50 Two authors (YMY and LHX) evaluated the studies, and a third author (LYM) double-checked the assigned scores. Evidence of effectiveness for each study was combined with quality scores for use in discussing the results. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology is used to rate the certainty of the evidence as “high”, “moderate”, “low” or “very low”.51 GRADE was completed by one reviewer (LHX) and reviewed by a second (YMY).
2.5. Data synthesis and statistical analysis
Statistical analyses were conducted using the “meta” and “metafor” in the statistical software R (V.4.2.0). The meta-analysis was performed using a generic inverse-variance pooling method and pooled effect sizes with a random-effects model using the DerSimonia-Laird approach52 to summarize the effects of HIIT and MICT on MFO. The effects were presented as a mean difference (MD) and standard mean difference (SMD) with estimated Hedges’ g. SMD were classified as trivial (0.2), small (0.2–0.5), medium (0.5–0.8), and large (>0.8). Statistical significance was set at p < 0.05.
We calculated a 95% confidence interval (CI). Numerous variables are currently used to assess heterogeneity (Cochrane's Q, I2 statistic, tau^2, Tau), but most of the available textbooks and recent literature support the use of I2 statistic (I2) as the primary source of information on the degree of heterogeneity.53 Therefore, the main analysis reports I2 with the following interpretations: 0%–40%, might not be important; 30%–60%, may represent moderate heterogeneity; 50%–90%, may represent substantial heterogeneity; and 75%–100%, considerable heterogeneity.54
With reference to previous studies, we selected the following variables for subgroup analyses: (a) training intensity, (b) training mode, (c) BMI category, (d) age, (e) sex, and (f) VO2max/VO2peak. Among these, the baseline values related to clinically relevant cutoff points were defined as follows: (1) BMI are <25 kg m−2, 25–30 kg m−2, and >30 kg m−2; (2) CRF are <30 mL kg−1·min−1, 30–35 mL kg−1·min−1, >35 mL kg−1·min−1; (3) age are 18–50 yr old and >50 yr old.
To explore the dose-response effects of HIIT and MICT on MFO, we conducted a set of meta-regression analyses based on random effects models, each including the modified variables associated with the protocol: (1) intensity; (2) duration per session; (3) number sessions per week; (4) total duration per week: (5) length of training; (6) total training time; (7) total training sessions.
Sensitivity analyses and exclusion methods were conducted to explore single case studies that caused heterogeneity in overall outcomes, and differences in corresponding study designs, intervention protocols, and populations to look for potential sources of heterogeneity. In addition, we used the contour-enhanced funnel plot and Egger's asymmetry test55 to check for publication bias and the p > 0.05 was considered without any publication bias.
3. Results
3.1. Search results
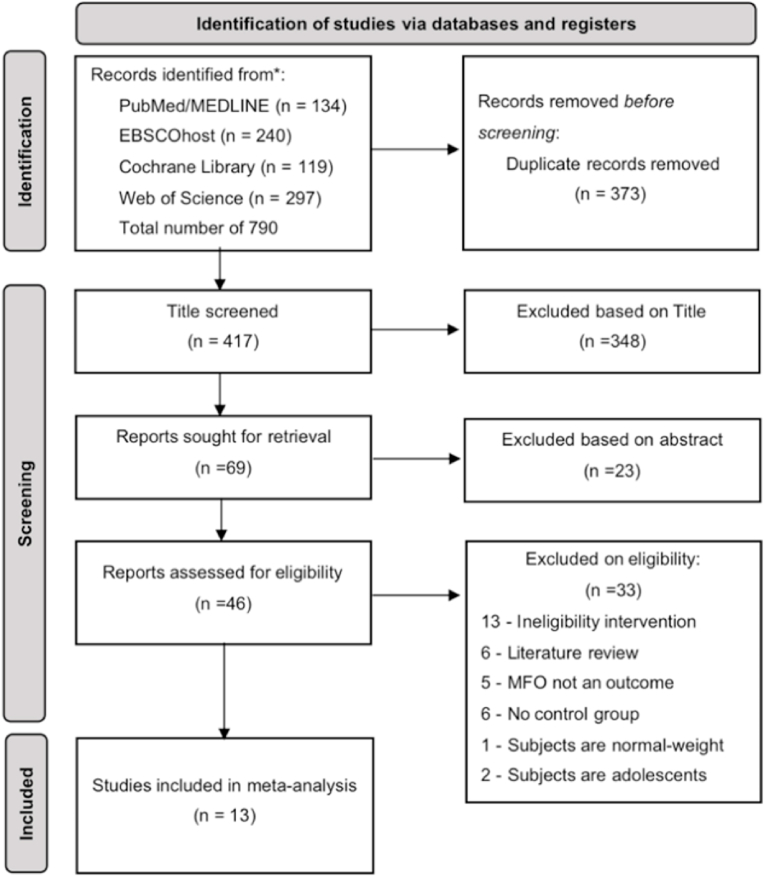
We systematically searched four databases and the initial search yielded 790 publications. Subsequently, we screened them, resulting in 13 papers43, 44, 45, 46,56, 57, 58, 59, 60, 61, 62, 63, 64 for systematic review and meta-analysis (Fig. 1).
Fig. 1.
PRISMA flow diagram for included and excluded studies.
3.2. Study characteristics
A total of 519 individuals (245 male and 274 female; age range: 19–57 years) participated in the included studies (HIIT, n = 136; MICT, n = 235; Control, n = 148). Three articles43,44,58 assessed the effects of HIIT against a non-training control group. Six46,57,60,62, 63, 64 articles included both MICT and non-training control groups. Four articles45,56,59,61 compared the effects of HIIT and MICT on MFO. Additional features of these studies, including detailed descriptions of the subjects, study design, intervention protocol arrangements, and methods for assessing MFO, can be found in the Supplementary Table 2.
3.3. Methodological quality of included studies
The obtained PEDro scores ranged from moderate to high quality (5–7) for the systematic review and meta-analysis. Supplementary Table 4 provides a detailed summary of the methodological quality assessment, including individual PEDro scores for each study.
3.4. Effects of HIIT on MFO compared with no-training
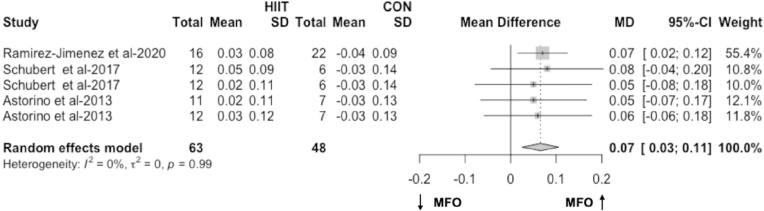
The meta-analysis including results from three articles43,44,58 (5 RCTs) found a significant pooled effect of HIIT on MFO compared to no-training in overweight and obese people (MD = 0.07 g/min, 95% CI 0.03 to 0.11, p < 0.01, Fig. 2; SMD = 0.59, 95% CI 0.20 to 0.99, p < 0.01). Heterogeneity between the studies might not be important (I2 = 0%, p = 0.99, Fig. 2). Sensitivity analysis confirms HIIT is improving MFO compared to no-training (MD = 0.07 g/min and p < 0.01; Supplemental Figs. 1–A). Further subgroup and regression analyses were not possible due to two reasons: 1) the limited number of studies on HIIT and 2) the heterogeneity of these HIIT interventions aimed at increasing MFO in overweight and obese adults might not be important.
Fig. 2.
The effect of HIIT on MFO compared with no-training (g/min). The effects are presented as mean difference (95% CI) in grams per minute.
3.5. Effects of MICT on MFO with no-training
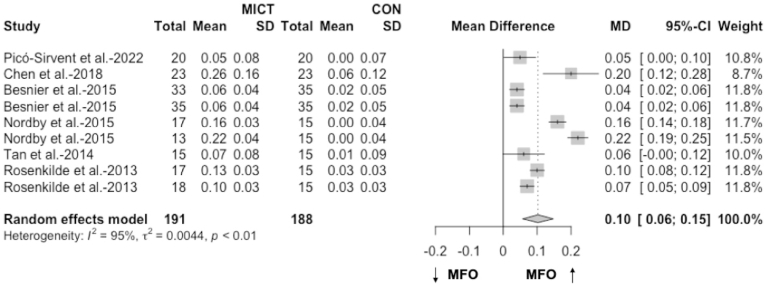
The meta-analysis including results from six articles46,57,60,62, 63, 64 (9 RCTs) found a significant pooled effect of MICT on MFO compared to no-training in overweight and obese people (MD = 0.10 g/min, 95% CI 0.06 to 0.15, p < 0.01, Fig. 3; SMD = 2.08, 95% CI 1.00 to 3.16, p < 0.01). Heterogeneity between the studies was substantial (I2 = 95%, p < 0.01, Fig. 3). Sensitivity analysis confirms MICT is improving MFO compared to no-training (MD = 0.10 g/min and p < 0.01; Supplemental Figs. 1–B).
Fig. 3.
The effect of MICT on MFO compared with no-training (g/min). The effects are presented as mean difference (95% CI) in grams per minute.
The following are the results (Table 1) of the subgroup analysis of the effect of MICT on MFO compared to no-training and are described in online Supplementary File 3. We found that training Intensity, training mode, BMI category, and VO2max were significant moderators of the effect of MICT on MFO.
Table 1.
The subgroup meta-analyses of MICT on MFO (g/min) compared with no-training.
| n | MD | 95% CI† | pd | I2 | ph | pt | |
|---|---|---|---|---|---|---|---|
| Intensity | |||||||
| Fatmax (34–55%VO2max) | 138 | 0.04 | 0.02 to 0.06 | <0.01 | 0% | 0.80 | <0.01 |
| non-Fatmax (55~70%VO2max) | 241 | 0.13 | 0.07 to 0.19 | <0.01 | 96% | <0.01 | |
| Mode | |||||||
| Cycling | 271 | 0.10 | 0.04 to 0.16 | <0.01 | 97% | <0.01 | 0.05 |
| Running | 62 | 0.09 | 0.06 to 0.12 | <0.01 | 33% | 0.22 | |
| gymnastics | 46 | n/a | n/a | n/a | n/a | n/a | |
| BMI category (kg/m2) | |||||||
| 25∼30 kg/m2 | 201 | 0.13 | 0.08 to 0.19 | <0.01 | 94% | <0.01 | <0.01 |
| >30 kg/m2 | 178 | 0.04 | 0.03 to 0.06 | <0.01 | 0% | 0.94 | |
| Age | |||||||
| 18∼50yr | 349 | 0.11 | 0.06 to 0.16 | <0.01 | 96% | <0.01 | n/a |
| >50yr | 30 | n/a | n/a | n/a | n/a | n/a | |
| Sex | |||||||
| Male | 125 | 0.14 | 0.07 to 0.20 | <0.01 | 96% | <0.01 | 0.09 |
| Female | 254 | 0.07 | 0.02 to 0.12 | <0.01 | 72% | <0.01 | |
| VO2max/VO2peak (ml.min−1.kg−1) | |||||||
| <30 | 138 | 0.04 | 0.02 to 0.06 | <0.01 | 0% | 1.0 | |
| 30-35 | 138 | 0.09 | 0.03 to 0.15 | <0.01 | 72% | 0.01 | <0.01 |
| >35 | 103 | 0.16 | 0.09 to 0.23 | <0.01 | 95% | <0.01 | |
MD: Pooled mean difference (g/min) between the observed effects of MICT and no-training. A positive value indicates a larger increase in MFO as a result of MICT compared with no-training.95% CI†: 95% confidence interval for pooled mean difference. pd: p-value for the pooled mean difference. I2(%): a measure of heterogeneity between studies expressed as a percentage. ph: p-value from the test of heterogeneity. pt: p-value for the test of moderation effect.
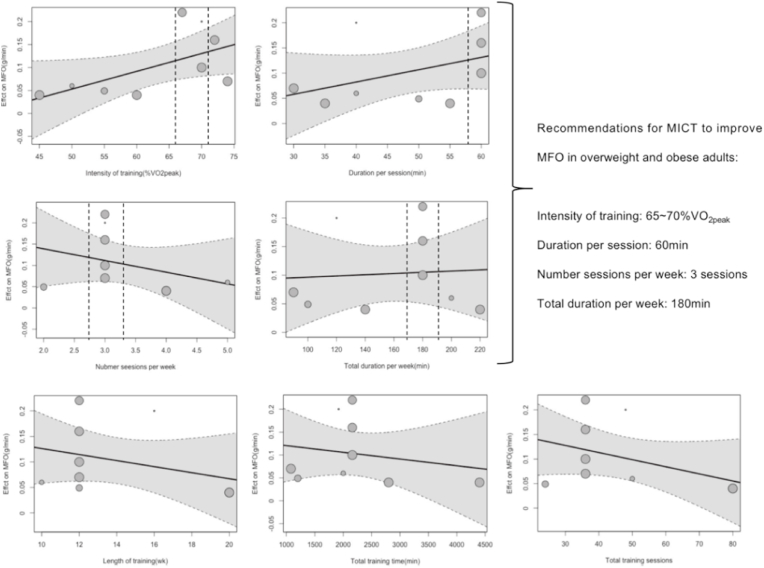
We found a significant dose-response relationship (β = 0.004, 95% CI 0.00 to 0.01, p = 0.05, Fig. 4) between the intensity of exercise (%VO2peak) and the effect of MICT on MFO, with MICT performed at an intensity of 65–70%VO2peak yielding greater improvements in MFO. We did not find a significant dose-response relationship between other exercise variables and the effect of MICT on MFO (p > 0.05 for all, Fig. 4). We give recommendations for the use of MICT to improve MFO in overweight and obese people based on the regression results.
Fig. 4.
Dose–response effects of MICT on MFO: results of meta-regression analysis for predictors related to the training. The effects are presented as the mean difference in grams per minute. A positive value indicates a larger increase in MFO because of MICT. The dashed line represents the 95% CI of the regression line.
3.6. Differences between effects of HIIT and MICT on MFO
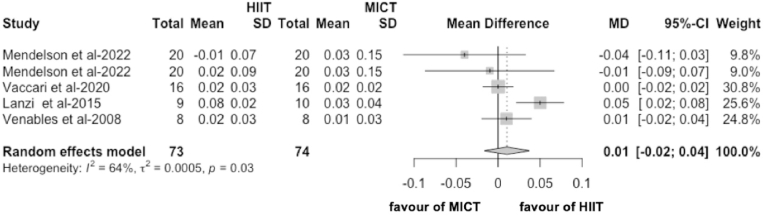
In the meta-analysis including results from four articles45,56,59,61 (5 RCTs), we found no difference between HIIT and MICT on MFO in obese people and the effect was not statistically significant (MD = 0.01 g/min, 95% CI -0.02 to 0.04, p = 0.44, Fig. 5; SMD = 0.17, 95% CI -0.36 to 0.70, p = 0.52). Heterogeneity between the studies was moderate (I2 = 64%, p = 0.03, Fig. 5). Sensitivity analysis indicates no difference in the efficacy of HIIT to MICT in improving MFO (MD = 0.01 g/min and p = 0.44; Supplemental Figs. 1–C).
Fig. 5.
The effect of HIIT on MFO compared with MICT (g/min). The effects are presented as mean difference (95% CI) in grams per minute.
The following are the results (Table 2) of the subgroup analysis of the effect of HIIT on MFO compared to MICT and are described in online Supplementary File 4. We did not find a significant moderating effect for the following influences (p > 0.05 for all).
Table 2.
The subgroup meta-analyses of HIIT on MFO (g/min) compared with MICT.
| n | MD | 95% CI† | pd | I2 | ph | pt | |
|---|---|---|---|---|---|---|---|
| Intensity | |||||||
| Fatmax (44–52%VO2max) | 35 | 0.03 | −0.01 to 0.07 | 0.13 | 73% | 0.05 | 0.13 |
| non-Fatmax (60%VO2max) | 112 | 0.00 | −0.02 to 0.01 | 0.69 | 0% | 0.57 | |
| Mode | |||||||
| Cycling | 115 | 0.01 | −0.02 to 0.05 | 0.49 | 62% | 0.05 | n/a |
| Running | 32 | n/a | n/a | n/a | n/a | n/a | |
| BMI category (kg/m2) | |||||||
| 30∼35 kg/m2 | 96 | 0.00 | −0.03 to 0.03 | 0.96 | 0% | 0.44 | 0.42 |
| >35 kg/m2 | 51 | 0.02 | −0.03 to 0.07 | 0.34 | 89% | <0.01 | |
| Age | |||||||
| 18∼50yr | 67 | 0.02 | −0.01 to 0.05 | 0.56 | 77% | <0.01 | 0.15 |
| >50yr | 80 | −0.03 | −0.08 to −0.03 | 0.34 | 0% | 0.58 | |
| Sex | |||||||
| Male | 35 | 0.03 | 0.00 to 0.06 | 0.13 | 73% | 0.05 | 0.13 |
| Male + Female | 112 | 0.00 | −0.02 to 0.01 | 0.76 | 0% | 0.57 | |
MD: Pooled mean difference (g/min) between the observed effects of HIIT and MICT.
3.7. Publication bias
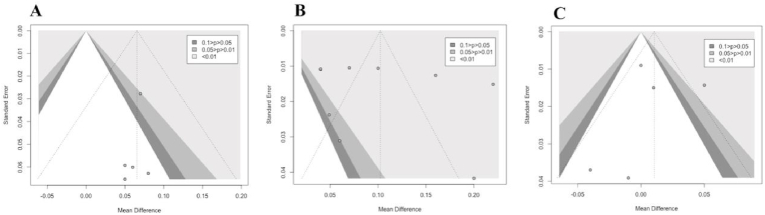
The publication bias was investigated using Egger's test for the included studies' impact comparison of HIIT to no-training (Fig. 6-A), MICT to no-training (Fig. 6-B), and HIIT to MICT (Fig. 6-C). The results of this analysis did not suggest the presence of publication bias for any of the comparisons (p = 0.37 for HIIT vs. no-training; p = 0.51 for MICT vs. no-training; and p = 0.82 for HIIT vs. MICT). This implies that the selected studies included in this meta-analysis were not affected by publication bias and the resultant conclusions have an enhanced level of reliability.
Fig. 6.
Funnel plot for the meta-analysis on the effect of training on MFO (g/min).
3.8. Certainty of evidence
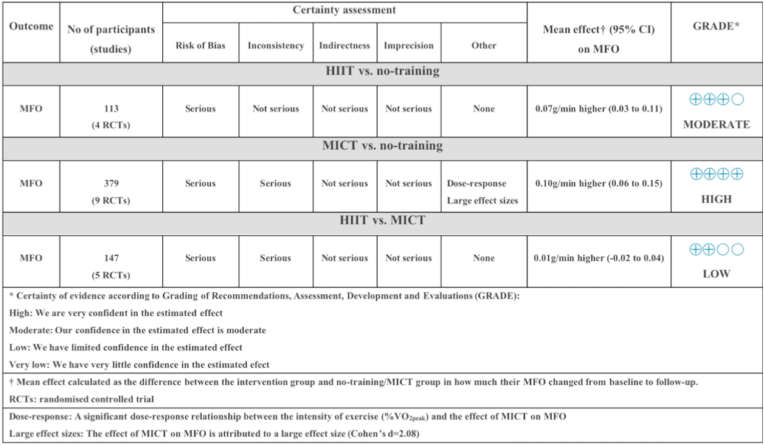
The summary of GRADE results (Fig. 7) provides an evaluation of the certainty of evidence assessment based on the various outcomes analyzed and subsequent performance. According to the GRADE assessment, certainty of the evidence for the effects of HIIT on MFO can be considered as ‘moderate’, and it was downgraded because of methodological bias in the inclusion of some studies. MICT on MFO can be considered "high”, which was downgraded because of methodological bias in some of the included studies and high heterogeneity in the pooled results. However, we improved the certainty of evidence due to the significant dose-response relationship between the intensity and MICT on MFO, and the large effect size of the pooled results. The certainty of the evidence for the difference between HIIT and MICT on MFO can be regarded as "low" due to methodological bias in some of the included studies and high heterogeneity in the pooled results.
Fig. 7.
Certainty of evidence on (1) the effectiveness of HIIT on MFO (2) the effectiveness of MICT on MFO and (3) the difference between the effects of HIIT and MICT.
4. Discussion
This study represents the first meta-analysis to systematically evaluate the effects of HIIT and MICT on MFO. Our results revealed that both HIIT and MICT were effective in improving MFO during exercise in overweight and obese adults. No significant differences between HIIT and MICT in obese adults, but no conclusion could be made for the overweight persons due to the low sample size.
4.1. Effects of HIIT on MFO
The pooled differences between the effect of HIIT on MFO in overweight and obese adults compared to no-training found in our meta-analysis can be categorized as moderate65 (SMD = 0.59). This is consistent with the previous finding by Atakan66 et al., where a moderate pooled effect of HIIT was found on fat oxidation (Cohen's d = 0.54). There is no widely accepted threshold for interpreting training-induced increases in MFO in a practical or clinical significance. Nevertheless, based on Atakan's66 findings, any increase greater than 0.07 g/min (Cohen's d = 0.20) could be deemed a significant increase in fat oxidation. Therefore, our results (0.07 g/min increase in MFO) can suggest a clinically significant increase in MFO under the HIIT intervention.
4.2. Effects of MICT on MFO
Our meta-analyses showed a very large, pooled effect of MICT on MFO compared to no-training controls (SMD = 2.08). It should be noted that the populations included in this meta-analysis were overweight and obese, so the effect sizes may not be applicable to normal-weight adults. Our findings are along the lines of previous meta-analyses showing that MICT can improve BMI, body fat, and waist circumference in overweight and obese people.67, 68, 69, 70 Overall, our findings can be summarized as a very significantly improved MFO level in overweight and obese people who underwent MICT interventions.
Our subgroup analyses investigated whether the MICT-MFO relationships were altered by potential moderators (compared to the no-training group). We found that training intensity, training mode, BMI category, and VO2max were significant moderators of the MICT effect on MFO improvement in overweight and obese people.
The present results indicated that non-Fatmax MICT was more effective in improving MFO in overweight and obese people than Fatmax (0.13 ± 0.03 g/min for non-Fatmax MICT and 0.04 ± 0.01 g/min for Fatmax MICT) compared to the no-training group. Fatmax has been used as a common MICT training intensity in exercise intervention protocol,10 the previous meta-analyses confirmed that Fatmax could improve body weight, body fat, waist circumference, and VO2max.71,72 In fact, in the overweight and obese populations, the intensity of Fatmax ranged between 43% and about 61% VO2max. In contrast, our data show that the optimal intensity to induce an increase in MFO during exercise is 65–70% of VO2max (Fig. 4).
Our results showed that there was no significant difference between running MICT and cycling MICT regarding exercise training-induced increases in MFO (0.09 ± 0.02 g/min with running and 0.10 ± 0.03 g/min with cycling). Previous cross-sectional studies consistently found that running was able to elicit a greater MFO than cycling.27,29,73 This may be related to the amount and type of muscle primarily recruited by the different exercise modes. However, this potential mechanism is beyond the scope of our review, so we refer the interested reader to previous research on the effects of different exercise modes on fat oxidation.27, 28, 29, 30, 31 In conclusion, our subgroup analysis rejects the hypothesis that running is more effective in improving MFO than cycling. However, due to the weight-bearing nature of running and its higher risk of injury,74 we still recommend the non-weight-bearing nature of cycling for overweight and obese adults.
Our results show that MICT improves MFO to a greater extent in the overweight (0.13 ± 0.03 g/min) compared to the obese population (0.04 ± 0.01 g/min). Previous cross-sectional studies found a significant positive correlation (r = 0.49, p < 0.05) between MFO and BMI,25 with higher MFO observed in obese adults compared to normal weight and overweight adults,75 suggesting that the overweight population has greater potential to enhance MFO compared to obese adults following exercise training.
Only one study included people above the 50th years of age, so we were unable to conduct a relevant subgroup analysis. Frandsen18 et al. found that although MFO was significantly higher in trained young males than in untrained young males, the difference was almost completely eliminated in the middle-aged male group (>50yr). This could be attributed to a decrease in mitochondrial oxidative capacity at age.76 In addition, based on the regression analysis of Rothschild et al.,19 the relationship between age and fat oxidation capacity (RER) would be in a "U"-shape. Therefore, the effect of age on improving MFO deserves further attention.75
Our results show that overweight and obese individuals with higher VO2max show a higher improvement in MFO with MICT compared to those with lower VO2max. As previously suggested,21, 22, 23, 24 individuals with high VO2max have enhanced mitochondrial biogenesis capacity, the tricarboxylic acid cycle, electron transport chain activity, and fatty acid transport during exercise.77 These may help explain this finding.
4.3. Effects of HIIT and MICT on MFO
The observed differences in the effects of HIIT and MICT on MFO can be categorized as very small65 (SMD = 0.17). This confirms the previous findings that a very small difference is present between HIIT and MICT in improving fat oxidation,66 body composition,78,79 and blood pressure.80 However, HIIT was found to be more effective than MICT in increasing VO2max and cardiorespiratory biomarkers.81, 82, 83 Our meta-analysis regarding “HIIT versus MICT” did not reveal any significant difference between the two training protocols in improving MFO. Furthermore, the selection of HIIT or MICT should not only be based on the physiological effects but also on participants’ preferences. This will probably ensure better long-term adherence to the exercise training program participant.84
Firstly, time constraint has been reported as a barrier to exercise participation85, 86, 87 and this has led to the growing popularity of HIIT as a more efficient alternative given its time-efficient nature.66 The present meta-analysis supports the idea that HIIT can be a time-efficient tool to increase MFO. However, taking into account that most of the included studies on HIIT interventions have been conducted in healthy volunteers, more evidence is needed on the feasibility and effectiveness of its use in overweight and obese populations.47 Second, the challenges in the implementation of HIIT interventions in obese adults must be considered.88 For example, the nature of HIIT is more perceptually and physiologically taxing with less pleasure89 than MICT,90 and a higher risk of lower limb muscle and joint injury in overweight and obese people.91,92 Moreover, it is important to note that certain studies include the use of SIT as a protocol,44 and SIT is not a suitable choice for obese adults due to its all-out sprinting attributes that can lead to adverse effects like dizziness and nausea in subjects.40 Finally, in the comparison of long-term adherence to HIIT and MICT, the evidence predominantly favors MICT. Ekkekakis and Biddle93 found that in 8 studies comparing HIIT vs. MICT over a 12-month follow-up period, individuals initially assigned HIIT tended to exercise at lower than prescribed intensities, and the HIIT demonstrated no advantage in long-term adherence.
Our subgroup analysis investigated the effects of different moderators on the impact of HIIT and MICT on MFO. We did not find any factor that significantly moderated the effect of HIIT versus MICT on MFO.
We used sensitivity analysis and leave-one-out to identify the sources of heterogeneity. The studies by Mendelson56 et al. and Lanzi61 et al. were identified as the main sources of heterogeneity. The methodological quality of Mendelson's56 study designs was high. However, unlike others, Mendelson's study56 allowed the participants to independently choose the length of the recovery bout based on self-perception, making it a possible source of heterogeneity. Furthermore, Lanzi's study61 intervention protocol only contains eight intervention sessions, which could be a potential source of heterogeneity.
4.4. Practical implications
The results of this study highlight the efficacy of HIIT and MICT in improving MFO during exercise in overweight and obese populations. Assuming that an overweight person's MFO during exercise is raised from 0.3 g/min to 0.4 g/min with MICT after 12 weeks of training, this means that it is possible to burn approximately 33% additional fat per MICT session (assuming the entire session is performed at Fatmax intensity).
When choosing HIIT or MICT, it is important to bear in mind the individual's preferences and the pros and cons of each training. Since both MCIT and HIIT produce a similar effect in MFO, the other features or advantages should be considered, such as the higher time efficiency of HIIT. On the other side, HIIT may not be as pleasant as MCIT for the overweight and obese, and this will affect their long-term adherence to exercise. It also seems that both cycling and running are equally effective in increasing MFO, but the non-weight-bearing nature of cycling may circumvent some risks of joint injury. Intervention designers should also focus on increasing VO2max, as it may enhance MFO gains.
There are various non-exercise strategies that can significantly acutely increase MFO during exercise, combining these strategies can also assist participants in achieving maximal fat oxidation during a single exercise session. Acute intake of fat,32 p-synephrine,94 and caffeine95 has been demonstrated to increase MFO. Additionally, MFO is influenced by diurnal variations, with higher MFO levels observed in the afternoon compared to the morning.95 Moreover, temperature plays a crucial role in influencing MFO, as colder (4.3 °C) temperatures tend to promote higher MFO levels than normothermic (18.3 °C)96 or hotter (34.1 °C) conditions.97
Furthermore, it seems that an exercise program of MCIT with an intensity of 65–70% VO2peak, performed 3 times per week with 60 min per session, will aid in optimizing MFO increases in overweight and obese adults. Lastly, due to limited research and small sample sizes in HIIT studies, it is challenging to develop an optimal intervention protocol. However, the following protocol has demonstrated effectiveness: each session lasts for 15–43 min, with 3–5 repetitions lasting 20 s to 4 min, the ranges from 85%HRmax to “all out”, with recovery periods lasting 60–180 s, and the intervention is applied 3 times per week.
4.5. Strengths and limitations of the review
This is the first systematic review and meta-analysis of studies examining the effect of HIIT and MICT on MFO in overweight and obese adults. Nevertheless, several limitations should be noted. Although we performed subgroup, regression, and sensitivity analysis, interpretation of the results remains challenging due to the different interventions and studies' designs. Additionally, the quality of study plan execution and the presence of measurement errors or unmeasured/unreported factors may contribute to increased heterogeneity. The limited number of available studies has prevented us from carrying out some further meta-analyses and subgroup analyses, particularly on the effect of HIIT on MFO in overweight and obese adults, and limited our ability to draw further recommendations on the optimal protocol of HIIT to improve MFO. We evaluated the effect sizes in our meta-analysis based on Cohen's effect size categories, which may not be applicable to the interpretation of MFO and therefore may influence the certainty of the evidence. Finally, studies included in the meta-analysis had methodological issues in measuring MFO (i.e., testing protocols33), which may have increased heterogeneity between studies.
5. Conclusion
The present systematic review and meta-analysis present evidence that both HIIT and MICT significantly enhance MFO in overweight and obese adults. Exercise intensity (Fatmax versus non-Fatmax), exercise mode (running versus cycling), BMI category, and VO2max may all impact the effects of MICT on MFO. Additionally, a significant dose-response relationship was observed between the MICT variable and the effect of MICT on MFO. When comparing MCIT and HIIT directly, it seems they produce comparable increases in MFO in obese adults. Therefore, it is recommended that utilizing MICT at 65–70% VO2peak, performed 3 times per week for 60 min per session, will optimize MFO in overweight and obese adults. Given the lack of studies examining the effect of HIIT on MFO in overweight and obese adults and the great diversity in the training protocols, we were unable to make an optimal protocol.
Author contributions
YMY designed the study and search strategy. YMY and LHX performed abstract and full-text screening, methodological quality, and GRADE assessment, and contributed to the completion of screening and data extraction for all data within this manuscript. YMY and CZL designed and calculated meta-analyses, subgroup analyses, regression analyses, sensitivity analyses, and publication bias, and created images and tables. YMY wrote the original draft preparation, performed review and editing, and prepared the final draft. GPN contributed to the critical evaluation of the findings and the drafting of the manuscript. CZL, GPN, LHS, DJF, and LYM contributed to editing and revising the manuscript in its final version. All authors read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Funding
No sources of funding were used to assist in this article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consented to the publication of the manuscript.
Declaration of competing interest
No conflicts and interests relevant to the content of this review.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jesf.2023.08.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ng M., Fleming T., Robinson M., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray G.A., Frühbeck G., Ryan D.H., Wilding J.P.H. Management of obesity. Lancet. 2016;387(10031):1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 3.Anderson L.N., Yoshida-Montezuma Y., Dewart N., et al. Obesity and weight change during the COVID-19 pandemic in children and adults: a systematic review and meta-analysis. Obes Rev. Published online January. 2023;31 doi: 10.1111/obr.13550. [DOI] [PubMed] [Google Scholar]
- 4.Savage D.B., Petersen K.F., Shulman G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe R.R. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 6.Brun Jean-Frédéric, Myzia Justine, Varlet-Marie Emmanuelle, Raynaud de Mauverger Eric, Mercier Jacques. Beyond the calorie paradigm: taking into account in practice the balance of fat and carbohydrate oxidation during exercise? Nutrients. 2022;14(8):1605. doi: 10.3390/nu14081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galgani J., Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes. 2008;32:S109–S119. doi: 10.1038/ijo.2008.246. Suppl 7(Suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swift D.L., Johannsen N.M., Lavie C.J., Earnest C.P., Church T.S. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–447. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romijn J.A., Coyle E.F., Sidossis L.S., et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265(3 Pt 1):E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 10.Jeukendrup A., Fatmax Achten J. A new concept to optimize fat oxidation during exercise? Eur J Sport Sci - EUR J SPORT SCI. 2001;1 doi: 10.1080/17461390100071507. [DOI] [Google Scholar]
- 11.Achten J., Gleeson M., Jeukendrup A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc. 2002;34(1):92–97. doi: 10.1097/00005768-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Purdom T., Kravitz L., Dokladny K., Mermier C. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr. 2018;15:3. doi: 10.1186/s12970-018-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster B.H., Sparks L.M. Metabolic flexibility in health and disease. Cell Metabol. 2017;25(5):1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W.H., Park J.H., Park S.Y., Park Y. Energetic contributions including gender differences and metabolic flexibility in the general population and athletes. Metabolites. 2022;12(10):965. doi: 10.3390/metabo12100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.San-Millán I., Brooks G.A. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sports Med. 2018;48(2):467–479. doi: 10.1007/s40279-017-0751-x. [DOI] [PubMed] [Google Scholar]
- 16.Maunder E., Plews D.J., Kilding A.E. Contextualising maximal fat oxidation during exercise: determinants and normative values. Front Physiol. 2018;9:599. doi: 10.3389/fphys.2018.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malone J.J., Bassami M., Waldron S.C., et al. Carbohydrate oxidation and glucose utilisation under hyperglycaemia in aged and young males during exercise at the same relative exercise intensity. Eur J Appl Physiol. 2019;119(1):235–245. doi: 10.1007/s00421-018-4019-4. [DOI] [PubMed] [Google Scholar]
- 18.Frandsen J., Amaro-Gahete F.J., Landgrebe A., et al. The influence of age, sex and cardiorespiratory fitness on maximal fat oxidation rate. Appl Physiol Nutr Metabol. 2021;46(10):1241–1247. doi: 10.1139/apnm-2021-0080. [DOI] [PubMed] [Google Scholar]
- 19.Rothschild J.A., Kilding A.E., Stewart T., Plews D.J. Factors influencing substrate oxidation during submaximal cycling: a modelling analysis. Sports Med. 2022;52(11):2775–2795. doi: 10.1007/s40279-022-01727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venables M.C., Achten J., Jeukendrup A.E. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol. 2005;98(1):160–167. doi: 10.1152/japplphysiol.00662.2003. [DOI] [PubMed] [Google Scholar]
- 21.Nordby P., Saltin B., Helge J.W. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity? Scand J Med Sci Sports. 2006;16(3):209–214. doi: 10.1111/j.1600-0838.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 22.Stisen A.B., Stougaard O., Langfort J., Helge J.W., Sahlin K., Madsen K. Maximal fat oxidation rates in endurance trained and untrained women. Eur J Appl Physiol. 2006;98(5):497–506. doi: 10.1007/s00421-006-0290-x. [DOI] [PubMed] [Google Scholar]
- 23.Bassami M., Ahmadizad S., Doran D., MacLaren D.P.M. Effects of exercise intensity and duration on fat metabolism in trained and untrained older males. Eur J Appl Physiol. 2007;101(4):525–532. doi: 10.1007/s00421-007-0523-7. [DOI] [PubMed] [Google Scholar]
- 24.Lima-Silva A.E., Bertuzzi R.C.M., Pires F.O., et al. Relationship between training status and maximal fat oxidation rate. J Sports Sci Med. 2010;9(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- 25.Chávez-Guevara I.A., Hernández-Torres R.P., Trejo-Trejo M., et al. Exercise fat oxidation is positively associated with body fatness in men with obesity: defying the metabolic flexibility paradigm. Int J Environ Res Publ Health. 2021;18(13):6945. doi: 10.3390/ijerph18136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arad A.D., Basile A.J., Albu J., DiMenna F.J. No influence of overweight/obesity on exercise lipid oxidation: a systematic review. Int J Mol Sci. 2020;21(5):1614. doi: 10.3390/ijms21051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knechtle B., Müller G., Willmann F., Kotteck K., Eser P., Knecht H. Fat oxidation in men and women endurance athletes in running and cycling. Int J Sports Med. 2004;25(1):38–44. doi: 10.1055/s-2003-45232. [DOI] [PubMed] [Google Scholar]
- 28.Brown G.A., Cook C.M., Krueger R.D., Heelan K.A. Comparison of energy expenditure on a treadmill vs. an elliptical device at a self-selected exercise intensity. J Strength Condit Res. 2010;24(6):1643–1649. doi: 10.1519/JSC.0b013e3181cb2854. [DOI] [PubMed] [Google Scholar]
- 29.Chenevière X., Malatesta D., Gojanovic B., Borrani F. Differences in whole-body fat oxidation kinetics between cycling and running. Eur J Appl Physiol. 2010;109(6):1037–1045. doi: 10.1007/s00421-010-1443-5. [DOI] [PubMed] [Google Scholar]
- 30.Egan B., Ashley D.T., Kennedy E., O'Connor P.L., O'Gorman D.J. Higher rate of fat oxidation during rowing compared with cycling ergometer exercise across a range of exercise intensities: fat oxidation during rowing vs cycling. Scand J Med Sci Sports. 2016;26(6):630–637. doi: 10.1111/sms.12498. [DOI] [PubMed] [Google Scholar]
- 31.Filipovic M., Munten S., Herzig K.H., Gagnon D.D. Maximal fat oxidation: comparison between treadmill, elliptical and rowing exercises. J Sports Sci Med. 2021;20(1):170–178. doi: 10.52082/jssm.2021.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achten J., Jeukendrup A.E. The effect of pre-exercise carbohydrate feedings on the intensity that elicits maximal fat oxidation. J Sports Sci. 2003;21(12):1017–1024. doi: 10.1080/02640410310001641403. [DOI] [PubMed] [Google Scholar]
- 33.Amaro-Gahete F.J., Sanchez-Delgado G., Jurado-Fasoli L., et al. Assessment of maximal fat oxidation during exercise: a systematic review. Scand J Med Sci Sports. 2019;29(7):910–921. doi: 10.1111/sms.13424. [DOI] [PubMed] [Google Scholar]
- 34.Achten J., Jeukendrup A.E. Maximal fat oxidation during exercise in trained men. Int J Sports Med. 2003;24(8):603–608. doi: 10.1055/s-2003-43265. [DOI] [PubMed] [Google Scholar]
- 35.van Loon L.J., Greenhaff P.L., Constantin-Teodosiu D., Saris W.H., Wagenmakers A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536(Pt 1):295–304. doi: 10.1111/j.1469-7793.2001.00295.x. 10.1111/j.1469-7793.2001. 00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romijn J.A., Coyle E.F., Sidossis L.S., Zhang X.J., Wolfe R.R. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. J Appl Physiol. 1995;79(6):1939–1945. doi: 10.1152/jappl.1995.79.6.1939. [DOI] [PubMed] [Google Scholar]
- 37.Astorino T.A., Schubert M.M. Changes in fat oxidation in response to various regimes of high intensity interval training (HIIT) Eur J Appl Physiol. 2018;118(1):51–63. doi: 10.1007/s00421-017-3756-0. [DOI] [PubMed] [Google Scholar]
- 38.Gibala M.J., McGee S.L. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev. 2008;36(2):58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- 39.Little J.P., Safdar A., Bishop D., Tarnopolsky M.A., Gibala M.J. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1303–R1310. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 40.Little J.P., Safdar A., Wilkin G.P., Tarnopolsky M.A., Gibala M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry C.G.R., Heigenhauser G.J.F., Bonen A., Spriet L.L. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metabol. 2008;33(6):1112–1123. doi: 10.1139/H08-097. [DOI] [PubMed] [Google Scholar]
- 42.Talanian J.L., Holloway G.P., Snook L.A., Heigenhauser G.J.F., Bonen A., Spriet L.L. Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299(2):E180–E188. doi: 10.1152/ajpendo.00073.2010. [DOI] [PubMed] [Google Scholar]
- 43.Astorino T.A., Schubert M.M., Palumbo E., Stirling D., Mcmillan D.W. Effect of two doses of interval training on maximal fat oxidation in sedentary women. Med Sci Sports Exerc. 2013;45(10):1878–1886. doi: 10.1249/MSS.0b013e3182936261. [DOI] [PubMed] [Google Scholar]
- 44.Schubert M.M., Clarke H.E., Seay R.F., Spain K.K. Impact of 4 weeks of interval training on resting metabolic rate, fitness, and health-related outcomes. Appl Physiol Nutr Metabol. 2017;42(10):1073–1081. doi: 10.1139/apnm-2017-0268. [DOI] [PubMed] [Google Scholar]
- 45.Venables M.C., Jeukendrup A.E. Endurance training and obesity: effect on substrate metabolism and insulin sensitivity. Med Sci Sports Exerc. 2008;40(3):495–502. doi: 10.1249/MSS.0b013e31815f256f. [DOI] [PubMed] [Google Scholar]
- 46.Tan S., Wang J., Cao L., Guo Z., Wang Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin Physiol Funct Imag. 2014;36(3):225–230. doi: 10.1111/cpf.12217. [DOI] [PubMed] [Google Scholar]
- 47.Nassis G.P. High-intensity interval training: how much pain to get a gain? Br J Sports Med. 2017;51(6):492–493. doi: 10.1136/bjsports-2016-097144. [DOI] [PubMed] [Google Scholar]
- 48.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 50.de Morton N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 51.Schünemann H.J., Higgins J.P., Vist G.E., et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; 2019. Completing ‘Summary of findings’ tables and grading the certainty of the evidence; pp. 375–402. [DOI] [Google Scholar]
- 52.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 53.Nakagawa S., Noble D.W.A., Senior A.M., Lagisz M. Meta-evaluation of meta-analysis: ten appraisal questions for biologists. BMC Biol. 2017;15(1):18. doi: 10.1186/s12915-017-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochrane Handbook for Systematic Reviews of Interventions. Accessed April 1, 2023. https://training.cochrane.org/handbook.
- 55.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendelson M., Chacaroun S., Baillieul S., et al. Effects of high intensity interval training on sustained reduction in cardiometabolic risk associated with overweight/obesity. A randomized trial. J Exerc Sci Fit. 2022;20(2):172–181. doi: 10.1016/j.jesf.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Picó-Sirvent I., Manresa-Rocamora A., Aracil-Marco A., Moya-Ramón M. A combination of aerobic exercise at Fatmax and low resistance training increases fat oxidation and maintains muscle mass, in women waiting for bariatric surgery. Obes Surg. 2022;32(4):1130–1140. doi: 10.1007/s11695-022-05897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramirez-Jimenez M., Morales-Palomo F., Ortega J.F., et al. Effects of exercise training during christmas on body weight and cardiometabolic health in overweight individuals. Int J Environ Res Publ Health. 2020;17(13):4732. doi: 10.3390/ijerph17134732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaccari F., Passaro A., D'Amuri A., et al. Effects of 3-month high-intensity interval training vs. moderate endurance training and 4-month follow-up on fat metabolism, cardiorespiratory function and mitochondrial respiration in obese adults. Eur J Appl Physiol. 2020;120(8):1787–1803. doi: 10.1007/s00421-020-04409-2. [DOI] [PubMed] [Google Scholar]
- 60.Effects Chen. Of a 16-week aerobic exercise intervention on fat oxidation kinetics in overweight female university students. Journal of Shenyang Institute of Sports. 2018;37(5):87–91. [Google Scholar]
- 61.Lanzi S., Codecasa F., Cornacchia M., et al. Short-term HIIT and Fat max training increase aerobic and metabolic fitness in men with class II and III obesity. Obesity. 2015;23(10):1987–1994. doi: 10.1002/oby.21206. [DOI] [PubMed] [Google Scholar]
- 62.Besnier F., Lenclume V., Gérardin P., et al. Individualized exercise training at maximal fat oxidation combined with fruit and vegetable-rich diet in overweight or obese women: the LIPOXmax-réunion randomized controlled trial. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0139246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nordby P., Rosenkilde M., Ploug T., et al. Independent effects of endurance training and weight loss on peak fat oxidation in moderately overweight men: a randomized controlled trial. J Appl Physiol. 2015;118(7):803–810. doi: 10.1152/japplphysiol.00715.2014. [DOI] [PubMed] [Google Scholar]
- 64.Rosenkilde M., Reichkendler M.H., Auerbach P., et al. Changes in peak fat oxidation in response to different doses of endurance training. Scand J Med Sci Sports. 2013;25(1):41–52. doi: 10.1111/sms.12151. [DOI] [PubMed] [Google Scholar]
- 65.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 66.Atakan M.M., Guzel Y., Shrestha N., et al. Effects of high-intensity interval training (HIIT) and sprint interval training (SIT) on fat oxidation during exercise: a systematic review and meta-analysis. Br J Sports Med. 2022;56(17):988–996. doi: 10.1136/bjsports-2021-105181. [DOI] [PubMed] [Google Scholar]
- 67.Armstrong A., Jungbluth Rodriguez K., Sabag A., et al. Effect of aerobic exercise on waist circumference in adults with overweight or obesity: a systematic review and meta-analysis. Obes Rev. 2022;23(8) doi: 10.1111/obr.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Donoghue G., Blake C., Cunningham C., Lennon O., Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev. 2021;22(2) doi: 10.1111/obr.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morze J., Rücker G., Danielewicz A., et al. Impact of different training modalities on anthropometric outcomes in patients with obesity: a systematic review and network meta-analysis. Obes Rev. 2021;22(7) doi: 10.1111/obr.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thorogood A., Mottillo S., Shimony A., et al. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am J Med. 2011;124(8):747–755. doi: 10.1016/j.amjmed.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 71.Romain A.J., Carayol M., Desplan M., et al. Physical activity targeted at maximal lipid oxidation: a meta-analysis. J Nutr Metab. 2012;2012 doi: 10.1155/2012/285395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chávez-Guevara I.A., Urquidez-Romero R., Pérez-León J.A., González-Rodríguez E., Moreno-Brito V., Ramos-Jiménez A. Chronic effect of Fatmax training on body weight, fat mass, and cardiorespiratory fitness in obese subjects: a meta-analysis of randomized clinical trials. Int J Environ Res Publ Health. 2020;17(21):7888. doi: 10.3390/ijerph17217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Achten J., Venables M.C., Jeukendrup A.E. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism. 2003;52(6):747–752. doi: 10.1016/s0026-0495(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 74.Colberg S.R., Albright A.L., Blissmer B.J., et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42(12):2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 75.Amaro-Gahete F.J., Sanchez-Delgado G., Ruiz J.R. Commentary: contextualising maximal fat oxidation during exercise: determinants and normative values. Front Physiol. 2018;9:1460. doi: 10.3389/fphys.2018.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conley K.E., Jubrias S.A., Esselman P.C. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabol. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Sultana R.N., Sabag A., Keating S.E., Johnson N.A. The effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: a systematic review and meta-analysis. Sports Med. 2019;49(11):1687–1721. doi: 10.1007/s40279-019-01167-w. [DOI] [PubMed] [Google Scholar]
- 79.Keating S.E., Johnson N.A., Mielke G.I., Coombes J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–964. doi: 10.1111/obr.12536. [DOI] [PubMed] [Google Scholar]
- 80.Batacan R.B., Duncan M.J., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017;51(6):494–503. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 81.Ramos J.S., Dalleck L.C., Tjonna A.E., Beetham K.S., Coombes J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45(5):679–692. doi: 10.1007/s40279-015-0321-z. [DOI] [PubMed] [Google Scholar]
- 82.Milanović Z., Sporiš G., Weston M. Effectiveness of high-intensity interval training (hit) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469–1481. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 83.Costa E.C., Hay J.L., Kehler D.S., et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. 2018;48(9):2127–2142. doi: 10.1007/s40279-018-0944-y. [DOI] [PubMed] [Google Scholar]
- 84.Ekkekakis P., Vallance J., Wilson P.M., Garber C.E. Extraordinary claims in the literature on high-intensity interval training (HIIT): III. Critical analysis of four foundational arguments from an interdisciplinary lens. Psychol Sport Exerc. 2023;66 doi: 10.1016/j.psychsport.2023.102399. [DOI] [PubMed] [Google Scholar]
- 85.Trost S.G., Owen N., Bauman A.E., Sallis J.F., Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34(12):1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 86.Kimm S.Y.S., Glynn N.W., McMahon R.P., Voorhees C.C., Striegel-Moore R.H., Daniels S.R. Self-perceived barriers to activity participation among sedentary adolescent girls. Med Sci Sports Exerc. 2006;38(3):534–540. doi: 10.1249/01.mss.0000189316.71784. dc. [DOI] [PubMed] [Google Scholar]
- 87.Downs D., Hausenblas H. Elicitation studies and the theory of planned behavior: a systematic review of exercise beliefs. Psychol Sport Exerc. 2005;6:1–31. doi: 10.1016/j.psychsport.2003.08.001. [DOI] [Google Scholar]
- 88.Biddle Sj, Batterham Am. High-intensity interval exercise training for public health: a big HIT or shall we HIT it on the head? Int J Behav Nutr Phys Activ. 2015;12 doi: 10.1186/s12966-015-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zenko Z., Ekkekakis P., Ariely D. Can you have your vigorous exercise and enjoy it too? Ramping intensity down increases postexercise, remembered, and forecasted pleasure. J Sport Exerc Psychol. 2016;38(2):149–159. doi: 10.1123/jsep.2015-0286. [DOI] [PubMed] [Google Scholar]
- 90.Sun S., Zhang H., Kong Z., Shi Q., Tong T.K., Nie J. Twelve weeks of low volume sprint interval training improves cardio-metabolic health outcomes in overweight females. J Sports Sci. 2019;37(11):1257–1264. doi: 10.1080/02640414.2018.1554615. [DOI] [PubMed] [Google Scholar]
- 91.Rynecki N.D., Siracuse B.L., Ippolito J.A., Beebe K.S. Injuries sustained during high intensity interval training: are modern fitness trends contributing to increased injury rates? J Sports Med Phys Fit. 2019;59(7):1206–1212. doi: 10.23736/S0022-4707.19.09407-6. [DOI] [PubMed] [Google Scholar]
- 92.Hamer O., Larkin D., Relph N., Dey P. Fear-related barriers to physical activity among adults with overweight and obesity: a narrative synthesis scoping review. Obes Rev. 2021;22(11) doi: 10.1111/obr.13307. [DOI] [PubMed] [Google Scholar]
- 93.Ekkekakis P. Is HIIT Associated with Higher Long-Term Exercise Adherence? Psychology of Sport & Exercise. Published online; 2023. Extraordinary claims in the literature on high-intensity interval training (HIIT): IV. [DOI] [PubMed] [Google Scholar]
- 94.Ruiz-Moreno C., Del Coso J., Giráldez-Costas V., González-García J., Gutiérrez-Hellín J. Effects of p-synephrine during exercise: a brief narrative review. Nutrients. 2021;13(1):233. doi: 10.3390/nu13010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramírez-Maldonado M., Jurado-Fasoli L., Del Coso J., R Ruiz J., Amaro-Gahete F.J. Caffeine increases maximal fat oxidation during a graded exercise test: is there a diurnal variation? J Int Soc Sports Nutr. 2021;18(1):5. doi: 10.1186/s12970-020-00400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gagnon D.D., Perrier L., Dorman S.C., Oddson B., Larivière C., Serresse O. Ambient temperature influences metabolic substrate oxidation curves during running and cycling in healthy men. Eur J Sport Sci. 2020;20(1):90–99. doi: 10.1080/17461391.2019.1612949. [DOI] [PubMed] [Google Scholar]
- 97.Ruíz-Moreno C., Gutiérrez-Hellín J., González-García J., GiráLdez-Costas V., Brito de Souza D., Del Coso J. Effect of ambient temperature on fat oxidation during an incremental cycling exercise test. Eur J Sport Sci. 2021;21(8):1140–1147. doi: 10.1080/17461391.2020.1809715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.