Abstract
Apart from advances in pharmaceutical antidiabetic agents, efforts are being made toward hypoglycemic agents derived from natural sources. Cinnamon has been reported to have significant benefits for human health, particularly as an anti-inflammatory, antidiabetic, and anti-hypertriglyceridemic agent. The phytochemicals in cinnamon can be extracted from different parts of plant by distillation and solvent extraction. These chemicals help in decreasing insulin resistance and can act against hyperglycemia and dyslipidemia, inflammation and oxidative stress, obesity, overweight, and abnormal glycation of proteins. Cinnamon has shown to improve all of these conditions in in vitro, animal, and/or human studies. However, the mechanism of action of active ingredients found in cinnamon remains unclear. The current review presents the outstanding ability of cinnamon derivatives to control diabetes by various pathways modulating insulin release and insulin receptor signaling. It was also found that the type and dosage of cinnamon as well as subject characteristics including drug interactions are likely to affect the response to cinnamon. Future research directions based on this review include the synergistic usage of various cinnamon derivatives in managing and/or preventing diabetes and possible other relevant chronic diseases.
Keywords: cinnamon, diabetes mellitus, fasting blood glucose, hyperglycemia
Background
Diabetes mellitus (DM) is one of the most important and key public health issues which is affecting more than 400 million people globally. 1 Diabetes mellitus type 2 (T2DM) is more common with more than 90% diabetic patients suffering from it. DM is a metabolic disorder which gradually leads to other life-threatening and chronic complications including neuropathic, macrovascular, and microvascular disorders. It is caused by a number of factors including secretion of insulin, insulin resistance related to non-use of insulin, or damage of pancreatic β-cell. Other important risk factors of DM among people globally are unhealthy food habits, sedentary lifestyle, and obesity. The prevalence of diabetes for all age groups worldwide was estimated to be 2.8% in 2000 and 4.4% in 2030. The number of diabetic patients is increasing on a daily basis, and cases in elderly population (>65 years) are expected to rise up to 366 million in 2030.2,3 In addition, the expected rise in the urban population in developing countries furthers the potential prevalence of this disease. 3
T2DM is a chronic condition which is characterized by insulin resistance, reduced insulin production, failure of pancreatic β-cells, and failure of metabolic control.4,5 The uncontrolled T2DM results in various life-threatening complications including cardiovascular disorders, microvascular complications, neuropathy, nephropathy, and renal complications with high mortality rates. 6
Burden of T2DM
Diabetes is one of the largest public health concerns worldwide which imposes a great burden on health care resources and economy. The occurrence of diabetes in developing and developed countries has been on a rise.7–9 Almost 537 million adults aged 20 to 79 years have diabetes. This accounts for 1 in every 10 adults of this age group suffering from this condition.10,11 The number is expected to increase up to 643 million in 2030 and 783 million in 2045.10,11 In every 5 seconds, around 6.7 million deaths have occurred due to diabetes in 2021.10,11 Diabetes resulted in 316% increase in health expenditure of causing USD 966 billion in the last 15 years. Around 541 million adults are at a high risk of T2DM due to impaired and reduced glucose tolerance (IGT).10,11
A community-based survey was carried out in Pakistan which showed that the occurrence of T2DM was 16.98% and prediabetes was 10.91%. 12 The analysis of subgroup showed that the prevalence of diabetes in males was more than in females (13.1 vs 12.4%). It was lower in rural than in urban patients (15.1 vs 1.6%) and in HbA1c than in OGTT tests (23.9 vs 14.4%). On the other hand, the WHO and ADA criteria for diabetes were almost similar (13.8 vs 13.5%).13,14
Pathogenesis of Type 2 Diabetes and Insulin Resistance
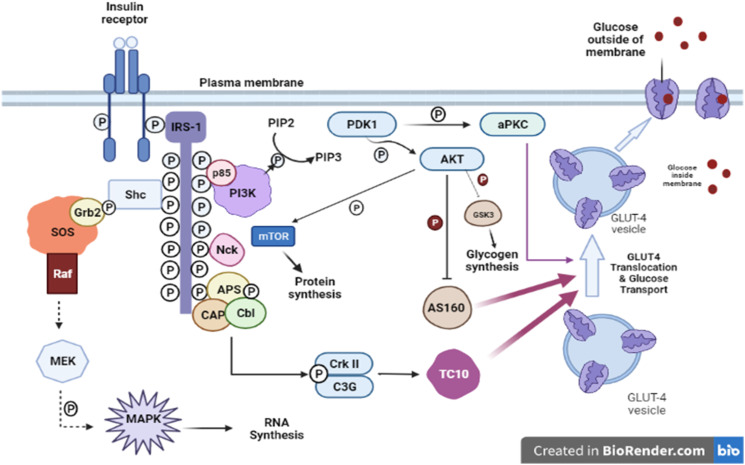
T2DM is a complicated disorder which is characterized by reduced insulin secretion, insulin sensitivity, and action on the adipose tissue and skeletal muscles. Signaling of insulin results in a cascade of events begin by binding of insulin to the receptor of its cell surface (Figure 1). The receptor comprises 2 β- and 2 α-subunits that is linked through a disulfide bridge into a hetero-tetrameric complex. The β-subunit domain of intracellular tyrosine kinase is activated by binding of insulin to the extracellular α-subunits. After that, the activation of receptor tyrosine kinases along with receptor auto-phosphorylation happens which leads to phosphorylation of tyrosine of insulin receptor substrates (IRSs). These substrates include IRS1, IRS2, IRS3, IRS4, Shc, and Gab1. 15 The β-subunit causes serine/threonine phosphorylation that results in decreasing its capacity to auto-phosphorylate to start the phosphorylation of insulin receptor substrates of insulin-resistant humans and animal models. The glucose transporter 4 (GLUT4) is translocated to the cell membrane, and this process is induced by activation of AMP-activated protein kinase (AMPK). 16 Previous research has shown that the signaling pathways of AMPK and AMPK are possible molecular targets in drug development for the treatment of obesity and T2DM. 17
Figure 1.
Insulin signaling pathway. Protein-tyrosine phosphatase 1B; PTP1B, insulin receptor substrate; IRS, ROCK, PIP, Rho-kinase; PTEN, phosphatase, phosphatidylinositol phosphate; and tension homologue deleted on chromosome 10; Pleckstrin homology domain; PDK, PH domain, GβL, phosphoinositide-dependent protein kinase; G-protein beta subunit like; mTOR, substrate; PKCλ/ζ, protein kinase C λ and mammalian target of rapamycin; AS160, 160 kDa Akt ζ; GLUT4, glucose transporter 4. 15
Insulin-mediated GLUT-4 translocation from intracellular vesicles toward the plasma membrane occurs due to IRSs which facilitates the entry of glucose. In this process, receptor of insulin is inactivated by dephosphorylation which is done by tyrosine phosphatase protein. Therefore, the biological action of insulin is dependent on phosphorylation and dephosphorylation.
Phosphatidylinositol 3-kinase (PI3K) is an important factor of the insulin-signaling cascade, responsible for the metabolic effects of insulin on GLUT4 translocation and glucose transport.15,18
Diabetes and insulin resistance occur due to the downstream signaling and reduced activation of the phosphatidylinositol 3-kinase which is due to derangement of insulin signaling pathways.
Pharmacological Management of T2DM
Observational research studies demonstrate that higher risks of mortality and complication are associated with inpatient hyperglycemia with and without diabetes. Sufficient evidence demonstrates that mortality in critically ill patients postsurgery and general medicine as well as the hospital complications is reduced by alteration of hyperglycemia through insulin administration. 19
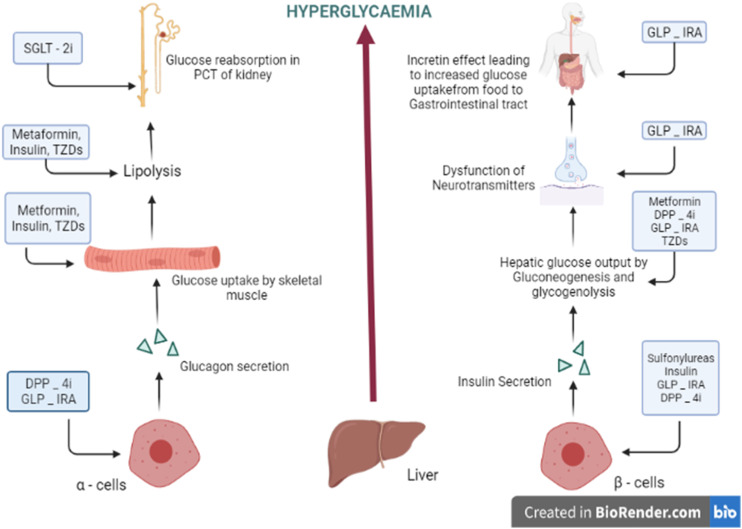
T2DM can be treated by emergence of a number of non-insulin-based oral therapies. These are characterized as Biguanides, insulin secretagogues, Alpha Glucosidase Inhibitors, Insulin Sensitizers, Amylin antagonists, Incretin mimetics, and SGLT2 inhibitors 20 (Figure 2).
Figure 2.
Target treatment for T2DM (DPP – 4i, dipeptidyl peptide – 4 inhibitor; TZDs, thiazolidinediones; SGLT-2i, sodium–glucose co-transporter 2 inhibitor; GLP-1RA, glucagon-like peptide – 1 receptor agonist). 20
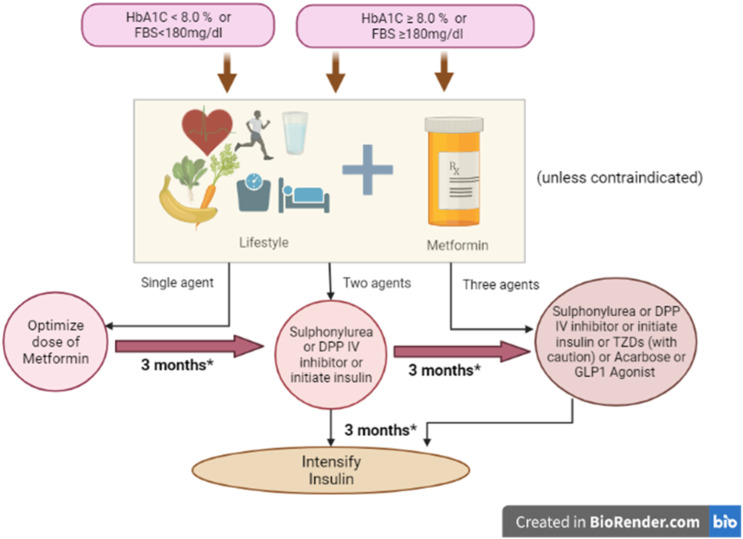
Pakistan has always met a great deal of health challenges due to diabetes because of its increased risk of complications and high prevalence. The Diabetic Association of Pakistan (DAP) recently established the National Clinical Practice Guidelines named “Pakistan’s Recommendations for Optimal Management of diabetes from Primary to Tertiary care level” (PROMPT). The foremost agenda of this document is to develop National Guidelines in order to manage T2DM in Pakistan in resource-controlled settings.
According to the PROMPT guidelines, if there are no contraindications, all patients should be prescribed metformin along with the modifications in lifestyles, regardless of their weight status in terms of BMI. 21 These guidelines are illustrated in Figure 3. The most common side effects of this therapy are nausea, anorexia, metallic taste, and diarrhea. Metformin should be taken with meals in order to reduce these side effects. Sulphonylureas, insulin, or dipeptidyl peptidase IV (DPP4) inhibitors may be used as an alternative when metformin is contraindicated. Repaglinide, glucagon-like peptide 1 (GLP-1), alpha glucosidase inhibitor, and thiazolidinedione (TZDs) can also be used as alternative drugs (Figure 1). 22
Figure 3.
PROMPT guidelines for managing T2DM. 2
Side Effects of Conventional Antidiabetic Drugs
Several antidiabetic medicines can be used in order to manage a chronic disease like diabetes which is a metabolic disorder. Long-lasting medications are a requirement for diabetes. However, the therapeutic success is difficult to achieve due to long-term safety profile of patients. The medications which are available in the market have side effects such as weight gain, hypoglycemia, and gastrointestinal adverse effects19,23 (Table 1).
Table 1.
Conventional antidiabetic drugs and their major adverse effects. 19
| S. No. | Drug class | Example of drugs | Adverse effects |
|---|---|---|---|
| 1 | Insulin and analogues | Regular insulin | Hypoglycemia, weight gain, insulin allergy, and lipodystrophy at injection sites |
| 2 | Sulphonylureas | Glibenclamide | Hypoglycemia, weight gain, cardiovascular risk, rash, cholestatic jaundice, bone marrow damage, and photosensitivity |
| 3 | Meglitinides | Repaglinide | Hypoglycemia and sensitivity reactions |
| 4 | Biguanides | Metformin | Gastrointestinal effects and lactic acidosis |
| 5 | GLP-1 agonists | Exenatide | Gastrointestinal effects, pancreatitis risks for cancer, and cardiovascular events |
| 6 | DPP-4 inhibitors | Saxagliptin | Pancreatitis, risk for cancer, acute hepatitis, and kidney impairment |
| 7 | Thiazolidinediones | Pioglitazone | Hepatitis, cardiovascular risk, bladder cancer, water retention, and weight gain |
| 8 | Dual PPAR agonists | Saroglitazar | Gastritis, asthenia, and pyrexia |
| 9 | Alpha-glucosidase inhibitors | Acarbose | Gastrointestinal effects and hepatitis |
| 10 | Amylin analogues | Pramlintide | Hypoglycemia and allergy |
| 11 | SGLT 2 inhibitors | Canagliflozin | Glycosuria and cardiovascular concern |
There are some adverse effects which are uncommon but can be bothersome to some patients. The adverse effects may prevent patients to stick to medications which lead to the failure of treatment. Few findings suggest that sulfonylureas are related with cholestatic jaundice and metformin is related with lactic acidosis. Pioglitazone may cause worse effect of pulmonary edema that may or may not result in congestive heart failure. Acarbose may result in ileus or subileus in many patients. Pancreatitis can be caused by glucagon-like peptide-1 receptor-like liraglutide.19,24
Nutraceuticals as an Alternative or Complementary Medicine
Nutraceutical is any ingredient or substance which is a part of food or a food particle and provides health or medical benefits. In the past, people have looked for nutritional and additional health benefits of food ingredients 25 such as minerals, vitamins, phytosterols, probiotics, and antioxidants. 26 Polyphenols, flavanone, and other natural chemical groups were examined as an adjuvant in the possible management of chronic diseases such as T2DM. 27
There are Several Sources of Such Active Ingredients. This Review Focuses on the Impact of Cinnamon Consumption on Diabetes Control
Search Strategy Used for Current Review
A thorough search for the literature on “Cinnamon and diabetes” was done using Google Scholar, PubMed, and Clinical Trials.gov. Key word alternates used for searching the exposure variable included cinnamon, cinnamon extract, cinnamon oil, cinnamon phytochemicals, and cinnamon phenolic compounds while those for the outcome variable included diabetic control, insulin resistance, insulin signaling, and cellular glucose uptake. Published literature demonstrating the link of cinnamon with diabetes mellitus was included in this review.
Cinnamon
Cinnamon (genus Cinnamomum and family Lauraceae) is a spice which is utilized as a nutraceutical. It has rich contents of phytochemically active compounds which have diverse structures and antioxidant properties. 28 Cinnamon is defined as a sweet wood which comes from a Greek word. It is a part of inner bark of evergreen tropical cinnamon trees. 29
Cinnamomum (cinnamon) belongs to Lauraceae family, and a lot of members of this family are utilized as spices. 30 Almost 250 cinnamon species have been known to date and 4 species are used to extract consumable cinnamon.
Cinnamomum verum (also famous as Ceylon cinnamon, Cinnamomum zeylanicum, or true cinnamon) is a small tree found in Chinese cassia and Sri Lanka. Other species such as Cinnamon (Cinnamomum cassia) are the most commonly accessible species in the entire world. 31 Although cinnamon is not produced in Pakistan, but people living in Pakistan commonly use cinnamon as a spice in their kitchens, around 90% of Pakistan’s cinnamon. The other imports come from Sri Lanka (3%) and India (6.5%). Cinnamon is a most commonly used flavoring agent in the beverage and food industry.
Cinnamon is prepared when tree’s outer bark is stripped and inner bark is dried. After that, the inner bark is curled into its cinnamon customary quills. It is very well known for its good medicinal properties.32,33 In a research study, it was stated that after hydro-distillation, volatile fractions of common spices along with remains of spent material of plants may be viewed as rich sources of bioactive molecules along with multi-enzymatic and antioxidant inhibitory effects. 34
Phytochemical Phenolic Composition of Cinnamon
Isolation of various bioactive compounds found in plants is a very time-consuming and laborious process. In a study, Jayaprakasha et al (2011) tried to isolate volatile oils present in the bark and leaves of cinnamon using hydro-distillation methods. Acetate, camphor, cinnamaldehyde, cinnamyl copane, and eugenol were some major constituents found in the extracted oils in addition to other compounds. 31 Essential oils can be easily extracted from barks of cinnamon using solvent extraction and distillation.35–41 A number of factors can affect the chemical composition of volatile oils found in cinnamon. In a study, it was suggested that antioxidant activity and flavonoid content differ considerably in various cinnamon species. 42 However, no visible effect was noted in either antioxidant capacity or flavonoid content due to difference in growing method (conventional or organic). 42 Chemical compositions of volatile oils are considerably influenced due to age of leaves and bark of cinnamon plant.43,44 In a study, it was confirmed that chemical constituents of essential oil extracted from leaves of cinnamon plants harvested on different dates were influenced. 45 The extraction method and solvent type have a significant effect on antioxidant activity and chemical composition of cinnamon-extracted oil.
Water-soluble polyphenol polymers that have the ability to enhance insulin-dependent in vitro glucose metabolism by approximately 20-fold and exhibit antioxidant activity were isolated from cinnamon and further characterized using mass spectroscopy and nuclear magnetic resonance. These polymers were made up of monomeric units having 288 molecular mass. 46 These compounds are known as tetramers and trimmers of catechin, epicatechin, and flavonoids. Contrary to other cinnamon components, cinnamon polyphenols with double-bonded procyanidin type-A polymers seem to exhibit insulin-like activity. 47 Other components like cinnamic acid, eugenol, cinnamide, 2-methoxy-cinnamaldehyde, and cinnamyl alcohol displayed little to no insulin-like activity. Difference in specie does not affect the insulin-like activity of cinnamon extract. The phytochemical and phenolic compounds extracted from cinnamon bark are described in Table 2 while the molecular structure is illustrated in Table 3.
Table 2.
Phytochemical and phenolic compounds extracted from cinnamon bark.
| Species | Type of extract | Compounds | Ref |
|---|---|---|---|
| Cinnamomum cassia | 60% Ethanol extract | Cinnamic acid, sinapic acid, p-coumaric acid, vanillin, caffeic acid, protocatechuic acid, and 3-4-dihydroxybenzaldehyde | 40 |
| C cassia | Boiling water extract | Quercetin-3-rhamnoside, kaempferol, coumaric acid, syringic acid, and tannic acid | 48 |
| Cinnamomum zeylanicum and C cassia | 60% Ethanol extract | Gallic acid, p-hydroxybenzoic acid, p-hydroxybenzaldehyde, protocatechuic acid, | 49 |
| salicylic acid, syringic acid, vanillic acid vanillin, caffeic acid, chlorogenic acid, ferulic acid, p-coumaric acid, cinnamic acid, and sinapic acid | |||
| C cassia | Essential oil | Trans-cinnamaldehyde (73.56%) benzene, 1,3-dimethyl, styrene, benzaldehyde, camphene, linalool, cis-cinnamaldehyde | 50 |
| acetophenone, camphor, borneol, benzenepropanal, decanal | |||
| decane, 3-ethyl-3-methyl-, 2-propen-1-ol, 3-phenylacetate, trimethyl-eugenol | |||
| copaene, geranyl acetate, caryophyllene, benzene, and cinnamyl acetate | |||
| Cinnamomum verum | Essential oil | Trans-cinnamaldehyde (74.49%) benzene, 1,3-dimethyl, styrene, benzaldehyde, camphene, linalool, and cis-cinnamaldehyde | 50 |
| acetophenone, camphor, borneol, benzene propanal, decanal | |||
| decane, 3-ethyl-3-methyl-, 2-propen-1-ol, 3-phenylacetate, trimethyl-eugenol | |||
| copaene, geranyl acetate, caryophyllene, benzene, and cinnamyl acetate | |||
| Cinnamomum loureiroi | Essential oil | Trans-cinnamaldehyde (81.97%) benzene, 1,3-dimethyl, styrene, benzaldehyde, camphene, linalool, cis-cinnamaldehyde | 50 |
| acetophenone, camphor, borneol, benzenepropanal, decanal | |||
| decane, 3-ethyl-3-methyl-, 2-propen-1-ol, 3-phenylacetate, trimethyl-eugenol | |||
| copaene, geranyl acetate, caryophyllene, benzene, and cinnamyl acetate |
Table 3.
Molecular structure of compounds commonly found in cinnamon (the structures were retrieved from PubChem database. 51
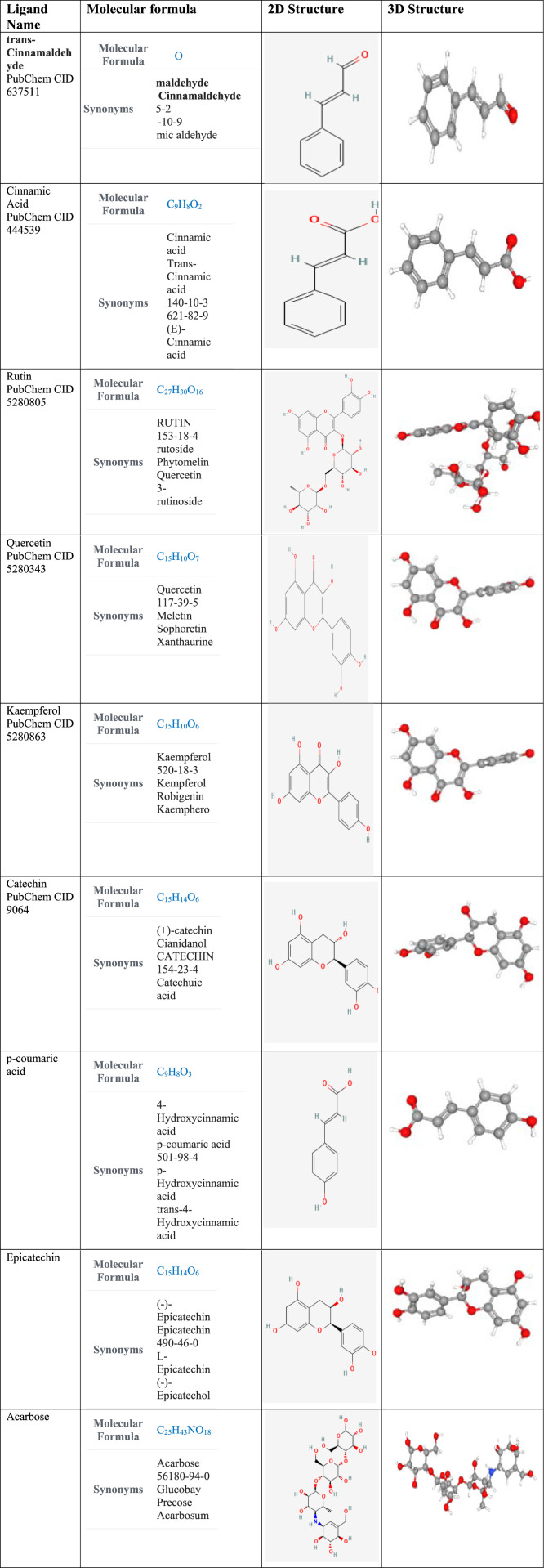
|
Mechanism of Action of Cinnamon Polyphenols on Insulin Signaling Pathway
Insulin receptors (IRs) are activated by cinnamon-extracted polyphenols due to enhanced tyrosine phosphorylation activity and decreased phosphatase activity (responsible for receptor inactivation) facilitated by these polyphenols. Furthermore, cinnamon polyphenols also enhance the synthesis and accumulation of glycogen by increasing the amount of GLUT-4 proteins and insulin receptor-β. It also reduces glycogen synthetase (GS) kinase-3 β (GSK3β) activity and enhances the levels of tristetraprolin protein. Cinnamon polyphenols might inhibit GSK3β activity resulting in decreased phosphorylation of tristetraprolin protein subsequently leading to increase in its activity. 52
Effect of Cinnamon for the Activation of Insulin Receptors
Major cause of metabolic syndrome, diabetes mellitus (type 2), and obesity is insulin resistance. Insulin receptor is responsible for mediating cellular response to insulin. It is a protein having two α-subunits (extracellular) which are responsible for binding insulin and two β-subunits which exhibit the activity of tyrosine kinase inside the cell. 53 Binding of insulin to α-subunit results in activation of tyrosine kinase in β-subunit leading to autophosphorylation of tyrosine residues in β-subunit. 54 Insulin sensitivity is enhanced when autophosphorylation increases and dephosphorylation decreases in the insulin receptor. 55 A proanthocyanidin (Cinnamtannin B1) extracted from the stem of Ceylon cinnamon facilitates the activation of β-subunit phosphorylation in various insulin receptors including adipocytes. 56 In a study, it was reported that cinnamon extract (CE) has the ability to enhance the insulin receptor (IR)-β (which is stimulated by insulin), IRS1/phosphoinositide 3-kinase (PI3K), and IR substrate-1 (IRS1) tyrosine phosphorylation levels in skeletal muscles of rats fed with chow diet. Furthermore, it was also revealed that utilization of glucose in rats fed high fructose diet (HFD) improved due to CE. 57
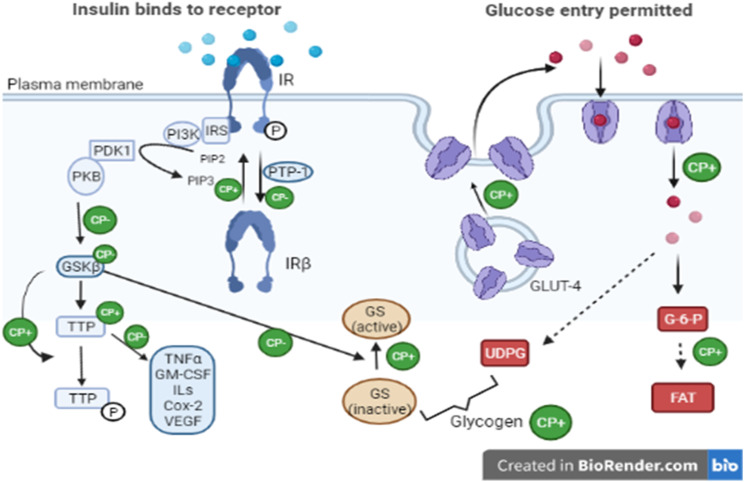
In HFD-fed rats, CE also improves the IRS1 associated with PI3K, IRS1 tyrosine phosphorylation levels, and reduced insulin-stimulated IRβ significantly. These findings suggest that insulin resistance development is prevented by CE to a certain extent by increasing insulin signaling and likely through NO pathway in skeletal muscle. In another research, it was reported that insulin sensitivity is improved due to an aqueous cinnamon extract in humans. 58 The mechanism is described in Figure 4.
Figure 4.
A model of actions of cinnamon polyphenols (CPs) in the insulin signal transduction pathway leading to beneficial effects in subjects with glucose intolerance or type 2 diabetes. 53 IRS, insulin receptor substrate; PI3K, 1-phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PTP-1, protein-tyrosine phosphatase-1; PDK1, phosphatidylinositol-dependent protein kinase 1; FAT, fat; G-6-P, glucose 6-phosphate; PKB, protein kinase B; UDPG, uridine diphosphoglucose; GM-CSF, granulocyte–macrophage colony-stimulating factor; Cox2, cyclooxygenase-2; VEGF, vascular endothelial growth factor; –, negative effect; +, positive effect. 52
Cinnamon Extract Upregulates GLUT4 Expression and Glucose Uptake
The transport of glucose in the adipose tissue and skeletal muscle is facilitated by GLUT4 (a transporter controlled by insulin). The transport of GLUT4 of cell membrane from intracellular compartments is promoted by insulin. 59 In diabetes mellitus, a decrease in GLUT4 is caused due to unavailability or deficiency of insulin sensitivity. In a study, it was reported that in C2C12 skeletal muscle cells treated with cinnamaldehyde, the GLUT4 receptor and its mRNA expression were upregulated in Real-Time PCR. 60 In various studies, the expression of GLUT4 and uptake of glucose in 3T3-L1 adipose cells were enhanced due to CE. It is also reported that a cinnamon water extract (Cinnulin PF®) facilitated the reduction of blood glucose, a novel insulin sensitivity marker known as soluble cluster of differentiation 36 (CD36) and plasma insulin.61,62 Expression of retinol binding protein, which is an adipokine involved in insulin resistance in adipose and plasma tissues, is also inhibited by cinnamon extract. 63 Increased levels of RBP4 are observed in serum of rodents and human who are insulin resistant, subsequently affecting the production of glucose in liver and mediating resistance of insulin in muscle.55,56,64 An inverse correlation is observed between levels of RBP4 in plasma and GLUT4 expression in the adipose tissue.55,56,64 Regulation of genes involved in glucose uptake (expression of GLUT1, glycogen synthesis 1 GLUT4, and glycogen synthase kinase 3β mRNA in the adipose tissue) takes place due to the consumption of CE. 61
In a recent research, it was testified that type 2 diabetes is improved by cinnamon extract due to its capability of translocating GLUT4 through the AMPK signaling pathway. It has also been reported that drugs for obesity and diabetes (type 2) can be potentially developed by targeting AMPK and the signaling pathway related to it because AMPK activation helps in translocating GLUT4 to the plasma membrane. 17 Amounts of Insulin Receptor (IR), Insulin Receptor substrates, and GLUT4 receptors are increased due to cinnamon which enables the entry of glucose into the cells. 56 A study revealed that Cinnamomum zeylanicum extracts enhance the translocation and production of GLUT4 to the plasma membrane in the adipose tissue. 65 This has been summarized in Table 4.
Table 4.
Summary of in vitro studies showing anti-glycemic effects of cinnamon.
| Year | Extract | Type of cinnamon | Part used | Result |
|---|---|---|---|---|
| 2007 52 | Ethanol extract | Cinnamomum burmannii | Increase in the amount of GLUT4 | |
| IRβ and TTP | ||||
| 2010 66 | Aqueous extract | Cinnamomum zeylanicum | Bark | Anti-α-glucosidase activity and inhibition of sucrase and maltase |
| 2010 66 | Aqueous extract | C zeylanicum | Bark | Anti-α-amylase activity |
| 2011 67 | Aqueous extract | Cinnamomum aromaticum | Bark | Anti-α-amylase activity |
| 2011 67 | Aqueous extract | C zeylanicum | Bark | Anti-α-amylase activity |
| 2011 67 | Aqueous extract | C zeylanicum | Bark | Anti-α-glucosidase activity and inhibition of sucrase and maltase |
| 2011 67 | Aqueous extract | Cinnamomum loureiroi | Bark | Anti-α-glucosidase activity and inhibition of sucrase and maltase |
| 2011 67 | Aqueous extract | C loureiroi | Bark | Anti-α-amylase activity |
| 2011 87 | Aqueous extract | C aromaticum | Bark | Anti-α-glucosidase activity and inhibition of sucrase and maltase |
| 2012 68 | Ethanol extract | C zeylanicum | Bark | Inhibitory activity of AChE and BChE |
| 2012 69 | Methanol extract | Cinnamomum verum | Bark | Anti-glycation activity |
| 2014 70 | Methanol extract | C zeylanicum | Leaf | Inhibitory activity of AChE and BChE |
| 2014 71 | Hydro-alcoholic extract | C zeylanicum | Bark | Anti-α-amylase activity |
| 2020 72 | Ethanol extract | C zeylanicum | Bark | Anti-α-glucosidase activity and anti-α-amylase activity |
Cinnamon Extracts Upregulate the Expression of PPARs
Diabetes and dyslipidemia can be treated by targeting peroxisome proliferator-activated receptors (PPARs). 73 PPARs are nuclear hormones (ligand-activated) including 3 isoforms, that is, PPARα, PPARγ, and PPARδ/β. PPARα is mostly expressed in the liver and brown adipose tissue, PPARγ in the adipose tissue, whereas PPARδ/β in various tissues. 74 HDL cholesterol levels in plasma are elevated and triglycerides lowered when PPARα is activated. 75 Insulin sensitivity is enhanced when PPARγ is activated resulting in antidiabetic effects. 76 Expression of PPARα and PPARγ is enhanced by cinnamon which elevates the insulin sensitivity. 77 In the adipose tissue of mouse (in vivo and in vitro), cinnamon extract is capable of inducing PPARα and PPARγ expressions. Moreover, 3 T3-L1 pre-adipocytes were differentiated into adipocytes in mouse when treated with CE. 77
Effect of Cinnamon Extracts on Enzyme Activity Inhibition
Extensive research has been conducted on antidiabetic usage of cinnamon. In vitro research has revealed that CE improves diabetes by enhancing glycogen synthesis, increasing uptake of glucose, modulating sensitivity and response of insulin, preventing the activity of gastro-intestinal enzymes, and gluconeogenesis. 78 In a study, four types of cinnamon species were tested for inhibitory effect on intestinal sucrase and maltase, pancreatic α-amylase separately, and in presence of acarbose. Intestinal maltase was potently inhibited by Thai cinnamon extract, whereas intestinal sucrase and pancreatic amylase were effectively inhibited by Ceylon cinnamon. However, acarbose was a lot more effective in inhibiting these two enzymes than Ceylon cinnamon. Yet, additional inhibitory effect was provided by these cinnamon extracts when used in combination with acarbose for all three enzymes. 67 A study revealed that pancreatic α-amylase and α-glucosidase are inhibited very effectively in a dose-dependent manner by Ceylon cinnamon. 66 Postprandial glucose can be potentially controlled in diabetic patients by using extracts obtained from the bark of cinnamon via inhibition of pancreatic α-amylase and intestinal α-glucosidase. A number of studies have witnessed the correlations between inhibitory effects of enzyme and polyphenol content found in natural products, 79 which is supported by another research where the inhibition of α-glucosidase by r = −.90; P < .001 and α amylase by r = −.59; P = .045 was correlated to polyphenol content obtained from natural extracts. 80
Table 5 summarizes the effects of cinnamon on insulin resistance.
Table 5.
Summary of clinical trials showing the effects of cinnamon on insulin resistance and T2DM.
| Year | Extract/oil | Type of cinnamon | Part | Dosage | Model | Follow-up duration | Result |
|---|---|---|---|---|---|---|---|
| 2010 81 | Powder | Cinnamomum cassia | Bark | 2 g/d | Human | 12 weeks | Reduction in HbA1C and FPG |
| 2007 82 | Capsule | C cassia | 1 g/d | Human | 3 months | No significant effect on insulin levels, A1C, or fasting glucose | |
| 2009 83 | Capsule | C cassia | 1 g/d | Human | 90 days | Reduction in HbA1C | |
| 2018 84 | Powder filled in a capsule | Cinnamomum verum | Bark | 1 g/d | Human | 3 months | Improvement in FPG, HbA1C, insulin resistance, fasting insulin, and 2 hpp |
| 2018 85 | Extract | C verum | Cinnamon stick | 3 g/d | Human | 90 days | - |
| 2014 86 | Sticks | C verum | Inner bark | Human | 8 weeks | Reduction in fasting blood sugar (FBS) | |
| 2020 87 | Extract encapsulated in a capsule | Cinnamomum zeylanicum | Bark | 225 mg/d | Human | 12 weeks | Reduction in FBS, HbA1C, and insulin resistance |
| 2016 88 | Capsule | C verum | Bark | 1 g/d | Human | 12 weeks | Reduction in FBS and HbA1C |
| 2015 89 | Alcohol extract | C zeylanicum | 50 mg/kg to 200 mg/kg body weight | Rats | 4 weeks | Reduction in glucose level | |
| 2015 90 | Aqueous extract | C cassia | Bark | 60 mg/kg | Rats | 15 days | Reduction in glucose level |
| 2010 91 | Aqueous extract | Cinnamomum tamala | Leaf | 125 mg/kg and 250 mg/kg body weight (twice a day) | Rats | 20 days | Reduction in fasting blood glucose level and an increase in glycogen level |
Conclusion
Cinnamon has been used as a natural traditional medicine in numerous cultures throughout the world. Cinnamon can be a promising natural medicine for the regulation of blood glucose levels. From the findings of various studies, it can be concluded that the oral administration of cinnamon extracts has a valuable nutraceutical effect on blood glucose levels through a range of metabolic pathways The incorporation of cinnamon powder or its phytochemical extract in nutraceutical preparations as well as in common food recipes would be of interest to future researchers. This opens up further research on the development of functional foods, incorporation of its powder as well as extract in local food recipes and the potential nutraceutical preparations for prevention and management of DM. Such adjunctive approach may inspire to reduce the burden of DM and possibly other related chronic illnesses.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Afifa Tanweer https://orcid.org/0000-0002-0174-2941
References
- 1.Khursheed R, Singh S, Wadhwa S, et al. Treatment strategies against diabetes: success so far and challenges ahead. Eur J Pharmacol. 2019;862:172625. DOI: 10.1016/j.ejphar.2019.172625. [DOI] [PubMed] [Google Scholar]
- 2.William J, John P, Mumtaz MW, et al. 2019 antioxidant activity, _-glucosidase inhibition and phytochemical profiling of Hyophorbe lagenicaulis leaf extracts. PeerJ 2019;4:1-16 DOI 10.7717/peerj.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. DOI: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020: Estimates of Diabetes and its Burden in the United States. US: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (2020). [Google Scholar]
- 5.DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 6.Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the diabetes & aging study). Diabetes Care. 2019;42(3):416-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia 2019;62:408-417. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer-Lindgren L, Mackenbach JP, Van Lenthe FJ, Flaxman AD, Mokdad AH. Diagnosed and undiagnosed diabetes prevalence by County in the U.S., 1999–2012. Diabetes Care. 2016;39:1556-1562. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Saeedi P, Karuranga S, et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence Esti-Mates for 2021 and projections for 2045, Diabetes Res Clin Pract. 2022;183:109119. DOI: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. DOI: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aamir AH, Ul-Haq Z, Mahar SA, et al. Diabetes prevalence survey of Pakistan (DPS-PAK): prevalence of type 2 diabetes mellitus and prediabetes using HbA1c: a population-based survey from Pakistan. BMJ Open. 2019;9:e025300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adnan M, Aasim M. Prevalence of type 2 diabetes mellitus in adult population of Pakistan: a meta-analysis of prospective cross-sectional surveys. Annals of Global Health. 2020;86(1):7. DOI: 10.5334/aogh.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meo SA, Zia I, Bukhari IA, Arain SA. Type 2 diabetes mellitus in Pakistan: current prevalence and future forecast. J Pak Med Assoc. 201;66(12):1637-1642 [PubMed] [Google Scholar]
- 15.Choi K, Kim YB. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med. 2010;25(2):119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Phys. 1999;277:H643-H647. [DOI] [PubMed] [Google Scholar]
- 17.Shen Yito Y, Muraki E, Honoso T, Seki T. Cinnamon extract enhances glucose uptake in 3 T3–L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS One. 2014;9(2):e87894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance Physiol Rev. 2018;98:2133–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlos EM, Guillermo EU. Pharmacotherapy for hyperglycemia in noncritically Ill hospitalized patients Diabetes Spectrum. 2014;27(3):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santwana P, Amit Kumar N, Anindita B. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomedicine & Pharmacotherapy. 2020;131:110708. [DOI] [PubMed] [Google Scholar]
- 21.Al-Shareef MA, Abdoulie FN, Abdullah SA. Clinical effect of metformin in children and adolescents with type 2 diabetes mellitus: a systematic review and meta-analysis. J Family Community Med. 2012;192:68-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shera AS, Basit A, Team P. Pakistan's recommendations for optimal management of diabetes from primary to tertiary care level (PROMPT). Pak J Med Sci. 2017;33(5):1279-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osadebe PO, Odoh EU, Uzor PF. Natural products as potential sources of antidiabetic drugs. British journal of pharmaceutical research. 2014;4:2075-2095. [Google Scholar]
- 24.Mohiuddin GS, Palaian S, Shankar PR, Sam KG, Kumar M. Uncommon side effects of commonly used anti-diabetics: time. To Monitor Them IJPSR. 2019;10(9):4145-4148. [Google Scholar]
- 25.Menrad K. Market and marketing of functional food in Europe. J Food Eng. 2003;56(2):181-188. [Google Scholar]
- 26.Bröring S. Innovation strategies for functional foods and supplements. challenges of the positioning between foods and drugs. Food Science and Technology (IFST), Sheffield. 2010;7(8):111-123. [Google Scholar]
- 27.Yeung AWK, Tzvetkov NT, Durazzo A, et al. Natural products in diabetes research: quantitative literature analysis. Nat Prod Res. 2020;1:1-15. [DOI] [PubMed] [Google Scholar]
- 28.Udayaprakash NK, Ranjithkumar M, Deepa S, Sripriya N, Al-Arfaj AA, Bhuvaneswari S. Antioxidant, free radical scavenging and GC–MS composition of cinnamomum iners reinw. Ex Blume. Industrial Crops and Products. 2015;69:175-179. [Google Scholar]
- 29.Vinitha M, Ballal M. In vitro anticandidal activity of Cinnamomum verum. J Med Sci. 2008;8:425-428. [Google Scholar]
- 30.Shan B, Cai YZ, Brooks DJ. Antibacterial properties and major bioactive component of cinnamon stick (Cinnamomum burmannii): activity against food has borne pathogenic bacteria. J Agric Food Chem. 2007;55:5484-5490. [DOI] [PubMed] [Google Scholar]
- 31.Jayaprakasha GK, Rao LJ. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr. 2011;51(6):547-562. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Su B, Jiang H, et al. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): a review. Fitoterapia. 2020;146:104675. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Kumari R, Mishra S. Pharmacological properties and their medicinal uses of Cinnamomum: a review. J Pharm Pharmacol. 2019;71:1735-1761. [DOI] [PubMed] [Google Scholar]
- 34.Trifan A, ZenginBrebu GM, Skalicka-Wo´zniak K, Luca SV. Phytochemical characterization and evaluation of the antioxidant and anti-enzymatic activity of five common spices: focus on their essential oils and spent material Extractives. Plants. 2021;10:2692. DOI: 10.3390/plants10122692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung Y-T, Chua M-T, Wang S-Y, Chang S-T. Anti-inflammation activities of essential oil and its constituents from Indigenous cinnamon (Cinnamomum Osmophloeum) twigs. Bioresour Technol. 2008;99(9):3908-3913. [DOI] [PubMed] [Google Scholar]
- 36.Lin C-T, Chen C-J, Lin T-Y, Tung JC, Wang S-Y. Anti-inflammation activity of fruit essential oil from Cinnamomum Insularimontanum Hayata. Bioresour Technol. 2008;99(18):8783-8787. [DOI] [PubMed] [Google Scholar]
- 37.Anis Z, Sulaiman O, Hashim R, Mehdi SH, Ghalib RM. Radical scavenging activity, total phenol content and antifungal activity of Cinnamomum iners wood. Iran J Energy Environ. 2012;3:74-78. [Google Scholar]
- 38.Jayaprakasha GK, Negi PS, Jena BS, Rao LJM. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J Food Compos Anal. 2007;20(3):330-336. [Google Scholar]
- 39.Dvorackova E, Snoblova M, Chromcova L, Hrdlicka P. Effects of extraction methods on the phenolic compounds contents and antioxidant capacities of cinnamon extracts. Food Sci Biotechnol. 2015;24(4):1201-1207. [Google Scholar]
- 40.Jiao L, Zhang X, Huang L, et al. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem Toxicol. 2013;56:398-405. [DOI] [PubMed] [Google Scholar]
- 41.Li Y-Q, Kong D-X, Wu H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind Crop Prod. 2013;41:269-278. [Google Scholar]
- 42.Prasad KN, Yang B, Dong X, et al. Flavonoid contents and antioxidant activities from Cinnamomum species. Innovative Food Sci Emerging Technol. 2009;10(4):627-632. [Google Scholar]
- 43.Lv J, Huang H, Yu L, et al. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and Peppermint. Food Chem. 2012;132(3):1442-1450. [DOI] [PubMed] [Google Scholar]
- 44.Li Y-Q, Kong D-X, Huang R-S, Liang H-L, Xu C-G, Wu H. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind Crop Prod. 2013;47:92-101. [Google Scholar]
- 45.Pragadheesh VS, Saroj A, Yadav A, Chanotiya CS, Alam M, Samad A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind Crop Prod. 2013;49:628-633. [Google Scholar]
- 46.Geng S, Cui Z, Huang X, Chen Y, Xu D, Xiong P. Variations in essential oil yield and composition during Cinnamomum Cassia bark growth. Ind Crop Prod. 2011;33(1):248-252. [Google Scholar]
- 47.Anderson RA, Broadhurst CL, Polnasky MM, et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52(1):65-70. [DOI] [PubMed] [Google Scholar]
- 48.Helal A, Tagliazucchi D, Verzelloni E, Conte A. Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J Funct Foods. 2014;7:506-516. [Google Scholar]
- 49.Klejdus B, Kováčik J. Quantification of phenols in cinnamon: a special focus on “total phenols” and phenolic acids including DESI-Orbitrap MS detection. Ind Crop Prod. 2016;83:774-780. [Google Scholar]
- 50.Kumar S, Sharma S, Vasudeva N. Chemical compositions of Cinnamomum tamala oil from two different regions of India. Asian Pacific Journal of Tropical Disease. 2012;2:S761-S764. [Google Scholar]
- 51.https://pubchem.ncbi.nlm.nih.gov/ National Center for Biotechnology Information U.S. National Library of Medicine. Retreived from:
- 52.Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys. 2007;459:214-222. [DOI] [PubMed] [Google Scholar]
- 53.White FM, Kahn CR. The insulin signaling system. J Biochem. 1994;269(1):1-4. [PubMed] [Google Scholar]
- 54.Imparl-Radosevich J, Deas S, Polansky MM, et al. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signalling. Horm Res. 1998;50(3):177-182. [DOI] [PubMed] [Google Scholar]
- 55.Taher M, Fadzilah Adibah AM, Mohomad RS. A proanthocyanidin from Cinnamomum zeylanicum stimulates phosphorylation of insulin receptor in 3 T3-L1 adipocytes. J Teknologi. 2006;44:53–68. 29. [Google Scholar]
- 56.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62(3):139-148. [DOI] [PubMed] [Google Scholar]
- 57.Wang JG, Anderson RA, Graham GM, 3rd, et al. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: a pilot study. Fertil Steril. 2007;88(1):240-243. [DOI] [PubMed] [Google Scholar]
- 58.Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341(4):248-257. [DOI] [PubMed] [Google Scholar]
- 59.Nikzamir A, Palangi A, Kheirollaha A, et al. Expression of glucose transporter 4 (GLUT4) is increased by cinnamaldehyde in C2C12 mouse muscle cells. Iranian Red Cresecent Med J. 2014;16(2):e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin B, Polansky MM, Anderson RA. Cinnamon extract regulates plasma levels of adipose-derived factors and expression of multiple genes related to carbohydrate metabolism and lipogenesis in adipose tissue of fructose-fed rats. Horm Metab Res. 2010;42(3):187-193. [DOI] [PubMed] [Google Scholar]
- 61.Handberg A, Levin K, Hojlund K, Beck-Nielsen H. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation. 2006;114(11):1169-1176. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Zhou L, Gu Y, et al. Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. Br J Nutr. 2007;98(4):720-726. [DOI] [PubMed] [Google Scholar]
- 63.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354(24):2552-2563. [DOI] [PubMed] [Google Scholar]
- 64.Polonsky KS. Retinol-binding protein 4, insulin resistance, and type 2 diabetes. N Engl J Med. 2006;354(24):2596-2598. [DOI] [PubMed] [Google Scholar]
- 65.Shen Y, Fukushima M, Ito Y, et al. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem. 2010;74:2418-2425. [DOI] [PubMed] [Google Scholar]
- 66.Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol. 2010;101:4676-4689. [DOI] [PubMed] [Google Scholar]
- 67.Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum Nutr. 2011;66:143-148. [DOI] [PubMed] [Google Scholar]
- 68.Kumar S, Brijeshlata, Dixit S. Screening of traditional indian spices for inhibitory activity of acetylcholinesterase and butyrylcholinesterase enzymes. Int J Pharma Bio Sci. 2012;3(1):P59–P65. [Google Scholar]
- 69.Ho S, Chang P. Inhibitory effects of several spices on inflammation caused by advanced glycation endproducts. Am J Plant Sci. 2012;3(7A):995–1002. [Google Scholar]
- 70.Dalai MK, Bhadra S, Chaudhary SK, Chanda J, Bandyopadhyay A, Mukherjee PK. Anticholinesterase activity of Cinnamomum zeylanicum L. leaf extract. Tang [Humanitas Medicine]. 2014;4(2):11.1–11.6. [Google Scholar]
- 71.Beejmohun V, Peytavy-Izard M, Mignon C, et al. Acute effect of Ceylon cinnamon extract on postprandial glycemia: alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Compl Alternative Med. 2014;14(1):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wariyapperuma WN, Kannangara S, Wijayasinghe YS, Subramanium S, Jayawardena B. In vitro anti-diabetic effects and phytochemical profiling of novel varieties of Cinnamomum zeylanicum (L.) extracts. PeerJ. 2020;8:e10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anand P, Murali K, Tandon V, Murthy PS, Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chemico-Biolog Interact 2010;186:72–81. 2 [DOI] [PubMed] [Google Scholar]
- 74.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. [DOI] [PubMed] [Google Scholar]
- 75.Steiner G, Hamsten A, Hosking J, et al. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the diabetes atherosclerosis intervention study, a randomised study. Lancet. 2001;357(9260):905-910. [PubMed] [Google Scholar]
- 76.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J Clin Investig. 2000;106(4):467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008;2008:581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kazeem MI, Davies TC. Anti-diabetic functional foods as sources of insulin secreting, insulin sensitizing and insulin mimetic agents. J Funct Foods. 2016;20:122-138. [Google Scholar]
- 79.Mai TT, Thu NN, Tien PG, Van Chuyen N. Alphaglucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J Nutr Sci Vitaminol. 2007;53:267-276. [DOI] [PubMed] [Google Scholar]
- 80.Hayward NJ, McDougall GJ, Farag S, et al. Cinnamon shows antidiabetic properties that are species-specific: effects on enzyme activity inhibition and starch digestion. Plant Foods Hum Nutr. 2019;74:544-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure‐lowering effect of cinnamon in multi‐ethnic Type 2 diabetic patients in the UK: a randomized, placebo‐controlled, double‐blind clinical trial. Diabet Med. 2010;27(10):1159-1167. [DOI] [PubMed] [Google Scholar]
- 82.Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabet. Care. 2007;30:2236-2237. [DOI] [PubMed] [Google Scholar]
- 83.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. 2009;22:507-512. [DOI] [PubMed] [Google Scholar]
- 84.Zare R, Nadjarzadeh A, Zarshenas MM, Shams M, Heydari M. Efficacy of cinnamon in patients with type II diabetes mellitus: a randomized controlled clinical trial. Clin Nutr. 2018;11:30114-30116. [DOI] [PubMed] [Google Scholar]
- 85.Neto JCGL, Damasceno MMC, Ciol MA, et al. Analysis of the effectiveness of cinnamon (Cinnamomum verum) in the reduction of glycemic and lipidic levels of adults with type 2 diabetes: a study protocol. Medicine. 2020;99(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azimi P, Ghiasvand R, Feizi A, Hariri M, Abbasi B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud: Reg Dev Stud. 2014;11(3):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parham M, Bagherzadeh M, Asghari M, et al. Evaluating the effect of a herb on the control of blood glucose and insulin-resistance in patients with advanced type 2 diabetes (a double-blind clinical trial). Caspian journal of internal medicine. 2020;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: a randomized, placebo-controlled clinical trial. Journal of intercultural ethnopharmacology. 2016;5(2):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajesh P, Sharon SS, Vijayabhaskara RY, Shiva Kumar M. Antidiabetic profile of CINNAMON powder extract in experimental diabetic animals. Int J Pharmacol. 2015;2(4):21-25. [Google Scholar]
- 90.Kamble S, Rambhimaiah S. Antidiabetic activity of aqueous extract of Cinnamomum cassia in alloxan-induced diabetic rats. Biomedical and Pharmacology Journal. 2015;6(1):83-88. [Google Scholar]
- 91.Chakraborty U, Das H. Antidiabetic and antioxidant activities of Cinnamomum tamala leaf extracts in STZ-treated diabetic rats. Global Journal of Biotechnology & Biochemistry. 2010;5(1):12-28. [Google Scholar]