Abstract
Objective:
To determine risk factors and time course for repeat procedures after ureteroscopy (URS) or shockwave lithotripsy (SWL) procedure using a large employer-based claims database.
Methods:
We identified all patients who underwent treatment for ureteral or renal stone with URS or SWL from 1/1/2007 to 12/31/2014 using the IBM MarketScan Commercial Database. Repeat stone procedure was evaluated after a 90-day grace period from the index procedure. Patients were followed until 12/31/2017. We performed multivariate analyses using Cox proportional hazards to determine independent risk factors for repeat procedure after the initial stone removal.
Results:
A total of 189,739 patients underwent a SWL or URS and were included in the study. The incidence of repeat procedure per 100 person years was 6.8, and 4.4 after SWL and URS, respectively. The median time to reoperation was 12.5 months for SWL and 14.6 months for URS. On multivariable analysis, SWL was associated with an increased risk of repeat procedure compared to URS. (HR = 1.63). Paralysis, neurogenic bladder and inflammatory bowel disease were also associated with an increased risk of repeat procedure (HR=1.66, 1.40, and 1.36 respectively)
Conclusions:
In a large national cohort, patients with paralysis and neurogenic bladder had a significantly higher risk of repeat stone procedure. SWL was associated with higher risk of repeat procedure than URS. Urologists can use these data to identify and counsel patients at high risk for need for recurrent procedure.
Keywords: Nephrolithiasis, Surgery, Ureteroscopy, Shockwave Lithotripsy
INTRODUCTION:
Stone recurrence is an unfortunate but common event for patients with urinary stone disease (USD). After the initial procedure, it is estimated that over 40% of stone formers will have a recurrence.1-4 Stone recurrence that leads to repeat surgery been found to result in decreased quality of life1. From a patient perspective, it is critically important to determine which treatment modality will result in fewer procedures and which patients are optimal candidates for each. Shockwave lithotripsy (SWL) and ureteroscopy with laser lithotripsy (URS) are the most commonly performed procedures to treat USD. AUA guidelines recommend both URS and SWL for stones less than 2cm not located in the lower pole. 5 Prior study of SWL and URS have revealed there is a lower stone-free and repeat treatment-free rate after SWL in the short term, however there is a paucity of data regarding patient-specific risk factors and long-term need for repeat procedure after these two treatment modalities.6-10
We perform an exploratory time-to-event analysis study using a large administrative claims database to assess risk factors for repeat procedure following URS versus SWL in a real-world clinical practice cohort. The variables utilized are known to be predictive of stone occurrence and recurrence, however, to date have not been examined to determine whether they will result in repeat operative procedures. Given the growing prevalence of USD, impact on patient quality of life, and the already exorbitant direct annual expense of treatment, these findings may be beneficial both for patient counseling and for surgical planning.
METHODS:
This study using deidentified administrative claims data was declared exempt by the Washington University Institutional Review Board. The IBM MarketScan Commercial Database includes national commercial claims data, from persons insured by employer-sponsored and individual health plans. This database was queried for all patients who underwent a renal or ureteral stone removal procedure from 1/1/2007 to 12/31/2014. Shockwave lithotripsy (SWL) and ureteroscopy (URS) were identified as stone procedures using Current Procedural Terminology, Fourth Edition (CPT) codes. (Supplementary 1)
Information used to confirm surgical procedures included procedure claims from both a provider and facility (either CPT-4 or International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes for inpatient surgeries), or a procedure claim for surgery along with either a provider anesthesia claim or facility revenue codes for operating room or anesthesia services.
The first stone surgery in our database was considered the index procedure. Patients were excluded if they did not have continuous insurance enrollment for a minimum of 1 year before and after the index event. Dichotomous variables were created for index SWL or URS. A 90-day grace period after the index procedure was used to exclude procedures intended to treat the same stones as a staged procedure. Recurrent procedures were classified as another procedure coded as PCNL, SWL or URS taking place after the grace period, up to a maximum of 10 years after the index procedure (through 12/31/2017).
ICD-9-CM diagnosis codes were used to identify comorbidities, as defined by Elixhauser.9 Comorbidities were captured within the year preceding and during the index surgery admission and required either two outpatient claims coded at least 30 days apart or one inpatient claim, as described previously.11 Morbid obesity (BMI>40), gout, paralysis, neurogenic bladder, Crohn’s disease, ulcerative colitis, and multiple sclerosis were identified with ICD-9-CM diagnosis codes listed in supplementary data.
Descriptive statistics were performed using Chi-square, Student’s t-test, and Mann-Whitney U tests, as appropriate. We performed multivariate analyses using Cox proportional hazards models. Patients were censored at the earliest of loss of health insurance enrollment, patient death, or end of study period. A multivariable Cox-proportional hazard model was used for time to repeat procedure by type of index procedure adjusting for sex, age, inflammatory bowel disease (IBD), gout, morbid obesity, neurogenic bladder, paralysis, multiple sclerosis, hypertension, and geographical region. Using scaled Schoenfeld’s residuals, procedure type, sex, diabetes, morbid obesity, hypertension, and region were all determined to violate the proportional hazards assumption. As a result, the final model was an extended Cox model with Heaviside functions in 6-month intervals. Interactions terms by time were included for each variable that violated the assumption of proportional hazards. Subset analysis was repeated separately for each index procedure type to determine risk factors specific to patients who underwent that procedure.
RESULTS:
A total of 189,739 patients underwent a stone removal procedure in the eight-year study period and were included in the study. Slightly more patients underwent URS (95,216) compared to SWL (94,523). Patients undergoing URS were, on average younger than those undergoing SWL with mean age 47.4 years for URS versus 48.3 for SWL. There were more male patients who underwent both SWL (57.0%) and URS (56.4%) (Table 1).
Table 1:
Baseline Demographics and 2nd Procedure Characteristics
| Variables | N | URS (95216) | ESWL (94523) | p-value |
|---|---|---|---|---|
| Mean age, years (SD) | 47.4 (11.1) | 48.3 (10.6) | <0.001 | |
| Region | <0.001 | |||
| Northeast | 23445 | 12347 (13.0%) | 11098 (11.7%) | |
| North Central | 48831 | 25387 (26.7%) | 23444 (24.8%) | |
| South | 88356 | 42831 (45.0%) | 45525 (48.2%) | |
| West | 25213 | 12540 (13.2%) | 12673 (13.4%) | |
| Unknown | 3894 | 2111 (2.2%) | 1783 (1.9%) | |
| Sex | 0.004 | |||
| Male | 107580 | 53702 (56.4%) | 53878 (57.0%) | |
| Female | 82159 | 41514 (43.6%) | 40645 (43.0%) | |
| Coronary Artery Disease | 6642 | 3523 (3.7%) | 3119 (3.3%) | <0.001 |
| Chronic Kidney Disease | 1519 | 857 (0.9%) | 662 (0.7%) | <0.001 |
| Diabetes | 23147 | 11521 (12.1%) | 11626 (12.3%) | 0.139 |
| Gout | 1884 | 921 (1.0%) | 963 (1.0%) | 0.258 |
| Hypertension | 43417 | 21417 (22.5%) | 22000 (23.3%) | <0.001 |
| Morbid Obesity | 7958 | 4423 (4.6%) | 3535 (3.7%) | <0.001 |
| Multiple Sclerosis | 689 | 362 (0.38%) | 327 (0.35%) | 0.215 |
| Neurogenic Bladder | 678 | 366 (0.4%) | 312 (0.3%) | 0.047 |
| Paralysis | 606 | 354 (0.4%) | 252 (0.3%) | <0.001 |
| Crohn’s Disease | 1138 | 571 (0.6%) | 567 (0.6%) | 0.104 |
| Ulcerative Colitis | 638 | 334 (0.35%) | 304 (0.32%) | 0.273 |
| Inflammatory Bowel Disease | 1708 | 857 (0.9%) | 851 (0.9%) | 0.056 |
| Subset of Observations who proceeded to 2nd Procedure | ||||
| N | URS (12038) | ESWL (17692) | p-value | |
| Median time til 2nd procedure, months (IQR) | 14.6 (7.1-27.4) | 12.5 (5.7-26.0) | ||
| Repeat procedures per | 4.4 | 6.8 | ||
| 100-person years | ||||
| 2nd Procedure URS | 11738 | 6236 (51.8%) | 5502 (31.1%) | <0.001 |
| 2nd Procedure ESWL | 17654 | 5694 (47.3%) | 11960 (67.6%) | <0.001 |
| 2nd Procedure PCNL | 856 | 325 (2.7%) | 531 (3.0%) | 0.218 |
| 2nd Procedure Non-Endo | 101 | 48 (0.4%) | 53 (0.3%) | 0.083 |
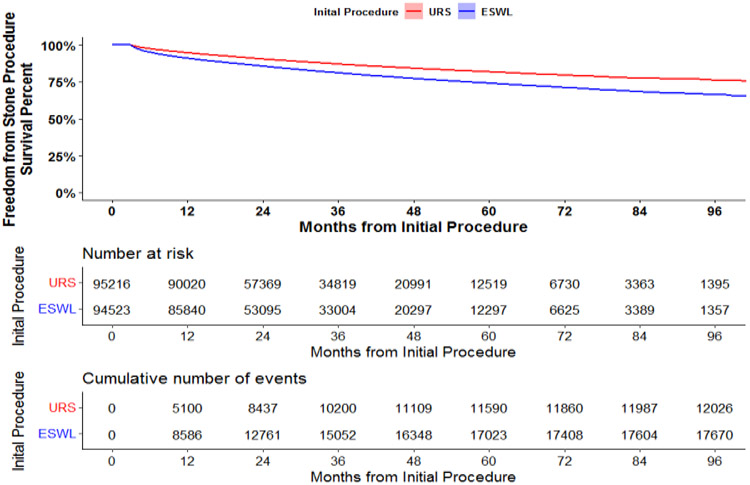
Patients undergoing URS were less likely to have undergone a repeat stone procedure. (Figure 1) The incidence of repeat procedure per 100 person years was 6.8 and 4.4 for SWL and URS, respectively. Patients who underwent URS as their initial procedure were more likely to have a repeat URS while patients undergoing SWL first were more likely to undergo a repeat SWL. In a multivariable Cox proportional hazard model, initial SWL was associated with 1.63-fold increased risk of repeat procedure (95% CI 1.58 – 1.68) compared to those who underwent URS as their initial procedure. The median time to reoperation was 12.5 (IQR 5.7-26.0) months for SWL and 14.6 (IQR 7.1-27.4) months for URS. The median length of follow up for each patient until end of data or censor date was 31.8 months (IQR 20.5 – 50.6). (Table 1)
Figure 1:
Survival from 2nd Procedure Analysis
Paralysis and neurogenic bladder were found to be risk factors for a repeat procedure after either SWL or URS with HRs of 1.66 (CI, 1.41-1.95) and 1.40 (CI,1.19 – 1.65), respectively. There was a high degree of correlation between neurogenic bladder and paralysis (Tetrachoric correlation coefficient = 0.84). IBD, Crohn’s disease or ulcerative colitis, had a HR of 1.36 (CI, 1.23 - 1.51). Diabetes mellitus, morbid obesity, and gout each increased the risk of repeat procedure with HRs of 1.25 (CI, 1.20 – 1.31), 1.28 (CI 1.20 – 1.37), and 1.19 (CI, 1.07 – 1.32). Female sex was also associated with a slightly increased risk of repeat procedure compared to males with a HR of 1.17 (CI, 1.13 – 1.20). We did not find multiple sclerosis or coronary artery disease to be associated with recurrent procedure. Regions outside of the northeast were associated with decreased risk of repeat procedure compared to the Northeast US. (Table 2)
Table 2:
Cox Proportional Hazard's Model - Modeling the Hazard of 2nd Stone
| Variable | Hazard's Ratio | 95% Lower |
95% Upper | p-value |
|---|---|---|---|---|
| Initial Procedure: SWL vs. URS | 1.63 | 1.58 | 1.68 | <0.001 |
| Female vs. Male | 1.17 | 1.13 | 1.20 | <0.001 |
| Age: 10-year increase | 1.05 | 1.04 | 1.06 | <0.001 |
| Inflammatory Bowel Disease | 1.36 | 1.23 | 1.51 | <0.001 |
| Diabetes | 1.25 | 1.20 | 1.31 | <0.001 |
| Gout | 1.19 | 1.07 | 1.32 | 0.001 |
| Morbid Obesity | 1.28 | 1.20 | 1.37 | <0.001 |
| Neurogenic Bladder | 1.40 | 1.19 | 1.65 | 0.002 |
| Paralysis | 1.66 | 1.41 | 1.95 | <0.001 |
| Multiple Sclerosis | 1.14 | 0.96 | 1.35 | 0.363 |
| Hypertension | 1.11 | 1.07 | 1.15 | <0.001 |
| Coronary Artery Disease | 1.04 | 0.98 | 1.11 | 0.179 |
| Region: North Central vs. Northeast | 0.82 | 0.78 | 0.86 | <0.001 |
| Region: South vs. Northeast | 0.82 | 0.79 | 0.86 | <0.001 |
| Region: West vs. Northeast | 0.72 | 0.67 | 0.76 | <0.001 |
| Region: Unknown vs. Northeast | 0.75 | 0.67 | 0.85 | <0.001 |
| SWL Subset | URS Subset | |||
| Variable | HR (95% CI) | p- value |
HR (95% CI) | p-value |
| Female vs. Male | 1.11 (1.06, 1.15) | <0.001 | 1.30 (1.23, 1.36) | <0.001 |
| Age: 10-year increase | 1.06 (1.04, 1.07) | <0.001 | 1.05 (1.03, 1.07) | <0.001 |
| Inflammatory Bowel Disease | 1.23 (1.06, 1.43) | 0.006 | 1.53 (1.31, 1.78) | <0.001 |
| Diabetes | 1.28 (1.21, 1.35) | <0.001 | 1.24 (1.15, 1.34) | <0.001 |
| Gout | 1.18 (1.03, 1.35) | 0.020 | 1.22 (1.03, 1.45) | 0.022 |
| Morbid Obesity | 1.31 (1.19, 1.44) | <0.001 | 1.31 (1.17, 1.46) | <0.001 |
| Neurogenic Bladder | 1.14 (0.90, 1.46) | 0.281 | 1.56 (1.21, 2.00) | <0.001 |
| Paralysis | 1.95 (1.54, 2.45) | <0.001 | 1.66 (1.29, 2.14) | <0.001 |
| Multiple Sclerosis | 1.05 (0.82, 1.33) | 0.714 | 1.13 (0.87, 1.47) | 0.364 |
| Hypertension | 1.10 (1.05, 1.15) | <0.001 | 1.14 (1.07, 1.21) | <0.001 |
| Coronary Artery Disease | 1.06 (0.98, 1.15) | 0.131 | 1.02 (0.92, 1.12) | 0.746 |
| Region: North Central vs. Northeast | 0.79 (0.74, 0.85) | <0.001 | 0.86 (0.79, 0.93) | <0.001 |
| Region: South vs. Northeast | 0.79 (0.74, 0.83) | <0.001 | 0.86 (0.80, 0.93) | <0.001 |
| Region: West vs. Northeast | 0.67 (0.62, 0.73) | <0.001 | 0.77 (0.69, 0.85) | <0.001 |
| Region: Unknown vs. Northeast | 0.70 (0.60, 0.82) | <0.001 | 0.86 (0.71, 1.04) | 0.111 |
In subset analysis, neurogenic bladder was not associated with significantly increased risk of repeat procedure for SWL, although it was associated with increased risk of repeat procedure for URS. (Table 2)
DISCUSSION:
Using a large dataset of commercial claims data, including persons insured by employer-sponsored and individual health plans, we examined predictors of repeat stone procedure after SWL and URS. To our knowledge, this is the largest cohort of patients evaluated for procedure-free survival after a stone procedure. The MarketScan database offers the advantage of capturing stone recurrence nationwide if that patient continues to remain covered by employer health insurance or an individual commercial health plan. Therefore, it provides an accurate account of recurrent stone procedures in a real-world clinical practice cohort, regardless of whether health care providers or hospital location have changed. Regarding claims data itself, the precision of upper tract calculi diagnosis based on ICD-9 codes was over 95% in one study.12 It would stand to reason that procedure coding would be equally if not more accurate.
Although we cannot comment on initial stone characteristics or stone-free rate after procedure, as these clinical data are not reported in the administrative database, we found that patients who underwent SWL were 63% more likely to require another stone procedure after a 90-day grace period compared to patients who underwent URS. A recent study using the New York State Department of Health Statewide Planning and Research Cooperative System also found that SWL had a higher chance of treatment failure compared with URS 90 and 180 days after treatment.9 Using the MarketScan database in 2014, Scales et al. evaluated the need for a second procedure 120 days after treatment also finding that SWL required more repeat procedures.6 However, they did not evaluate specific comorbid conditions as risk factors for recurrence, nor did they assess procedure-free survival after 120 days. Our data demonstrates SWL is associated with an increased risk of repeat procedure and further, the majority of those occur 10 months after the index procedure.
We found regional differences in repeat procedures as well. Patients from southern and western regions were less likely to undergo repeat procedure when compared with patients in the northeast. The reason for this is not entirely clear and perhaps counterintuitive. It has previously been shown that southern and central regions had a higher prevalence of stone disease while western regions had comparatively lower.13 Therefore, we believe this discrepancy is more likely due to regional differences in urologist practice patterns. For example, there may be more aggressive treatment of asymptomatic stones in the northeast.
Regarding comorbid risk factors, morbid obesity, diabetes, gout and hypertension are metabolic disorders that have been found to be associated with nephrolithiasis. Data from the Nurses’ Health Study revealed that obesity, diabetes, gout, and hypertension were associated with an increased risk of incident kidney stone disease.14-17 We found these each contributed an additive risk for repeat procedure after index URS and SWL. Our findings broadly confirm that these metabolic disorders continue to be risk factors for stone recurrence after initial treatment. Although these are potentially modifiable risk factors, it is unknown the degree to which treatment of these comorbidities through medication or lifestyle modifications affects urinary stone risk after initial stone event. Urologists should continue to counsel patients about these known risk factors, in order to minimize recurrence.
Despite males having a higher prevalence of kidney stone disease, we find that females had slightly higher risk of repeat procedure. In a single institution study by Iremashvili and colleagues, females were also found to have a higher risk of repeat stone procedure.8 Our study reinforces this in a larger national clinical practice cohort. Additionally, prior examination of stone compositions has revealed that females are more likely to have hydroxyapatite or struvite stone which can exhibit rapid growth and recurrence.18 This may explain why females who do have stones are at increased risk for a repeat procedure.
The pathophysiology of Inflammatory Bowel Disease (IBD) and its relationship to stone disease is explained by malabsorption from the inflamed bowel or surgical resection leading to hyperoxaluria, diarrhea and dehydration. Indeed, the prevalence of kidney stone disease is higher in this population.19 We find in this large dataset that IBD increased the risk for recurrent stone removal procedure after the index procedure by 36%, and was the strongest predictor of recurrence after neurogenic bladder and paralysis. This supports the fact that IBD not only increases stone incidence but also predicts for recurrence following URS and SWL.
We find that neurogenic bladder and paralysis conferred an increased risk of repeat surgery for USD. There was a high degree of correlation between neurogenic bladder and paralysis. Spinal cord injury and congenital cord abnormalities are among the most common causes of paralysis and neurogenic bladder.20 Patients with SCI are at increased risk of stone formation due to frequent urinary instrumentation, urinary stasis, urinary tract infections, and immobilization hypercalcemia. SCI has been shown to increase the risk of kidney stone, particularly early after injury and with the use of indwelling catheters.21 Our data show that after initial stone treatment, these patients continue to be at increased risk for another stone procedure. Interestingly, subgroup analysis revealed that paralysis but not neurogenic bladder conferred a high risk of repeat procedure after SWL. This may be due to immobility and inability to clear stone fragments which requires shifts in gravity dependent position.1
Patients with Multiple Sclerosis (MS) are at increased risk for larger stone operations such as PCNL when compared with matched patients without MS.22. However, we found that MS was not a risk factor for repeat surgery in this cohort, possibly due to the fact that the MS diagnosis covers a large spectrum of symptoms, many of which may not increase risk of nephrolithiasis. Although many patients with MS experience lower urinary tract symptoms, this mainly consists of incontinence,23 and it is unclear how many have urinary retention requiring catheterization, known to influence stone formation.
Although we did not find significant correlation, coronary artery disease and nephrolithiasis share similar risk factors and have been associated in several studies. One cohort study found that history of kidney stones was associated with a 50% increased incidence of cardiovascular event over a 10 year follow up period.24 This was also found to be true in the Nurses’ Health Study I and II.25 In this large national cohort, an increased risk of recurrent stone procedure in individuals with coronary artery disease was not demonstrated. This could be due to reluctance of urologists to repeat surgery in patients with a history of heart disease; approximately 4% of this cohort, who perhaps have additional comorbidities, or are on anticoagulation. Alternatively, this could also represent adequate medical treatment of individuals with a diagnosis code of coronary artery disease. For example, two studies found that usage of statins in hyperlipidemic patients was associated with reduced risk of developing urolithiasis.26,27 It is possible that widespread usage of statins in this population could have attenuated the risk of recurrence and repeat stone procedure.
As mentioned, a major limitation of administrative data use is lack of access to stone characteristics (size, location, number, composition), details of the stone procedure and perioperative outcomes such as stone free rate or results of metabolic evaluation. We recognize that lack of laterality represents a significant limitation of this study as a repeat procedure on the contralateral side may not necessarily reflect initial treatment failure. Further, we are unable to determine whether patients who presented initially had asymptomatic stones and whether concurrent asymptomatic stones were targeted as part of initial treatment. This is relevant in light of recent data showing that treatment of asymptomatic stones during initial stone surgery resulted in decreased risk of relapse event and increased time until relapse event.28 Last, we were unable to determine whether patients were recurrent stone formers or whether they had stone removal procedures prior to enrolling in insurance, or prior to the start of data collection. While we did not evaluate the cost of these procedures and subsequent healthcare utilization, that would be a suitable future use of this database.
Despite these limitations, this is the largest national cohort study to evaluate the time course and risk factors for stone recurrence with several years of follow up. These findings expand our understanding of comorbid risk factors as they relate to repeat surgery after index URS or SWL. Together this knowledge can serve as a useful tool for urologists to counsel patients who are at higher risk for a repeat procedure.
CONCLUSION:
In this large study, we identified risk factors for a repeat stone removal procedure after initial URS and SWL. SWL had a higher rate of repeat procedure than URS over time with a median time to recurrence of 12 months. Patients with neurogenic bladder or paralysis were at highest risk for repeat procedure. Alternatives to SWL should be considered for immobile or paralyzed patients. Urologists can use this data to identify and counsel patients at high risk for recurrence.
Supplementary Material
Supplementary 1: Index of codes used for data acquisition
Acknowledgements:
This study was supported by the Center for Administrative Data Research (CADR) which is supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamihira O, Ono Y, Katoh N, et al. Long-Term Stone Recurrence Rate After Extracorporeal Shock Wave Lithotripsy. J Urology. 1996;156(4):1267–1271. doi: 10.1016/s0022-5347(01)65566-6 [DOI] [PubMed] [Google Scholar]
- 2.Rule AD, Lieske JC, Li X, et al. The ROKS Nomogram for Predicting a Second Symptomatic Stone Episode. J Am Soc Nephrol. 2014;25(12):ASN.2013091011. doi: 10.1681/asn.2013091011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iremashvili V, Li S, Penniston KL, et al. Role of Residual Fragments on the Risk of Repeat Surgery After Flexible Ureteroscopy and Laser Lithotripsy: Single Center Study. J Urology. 2018;201(2):358–363. doi: 10.1016/j.juro.2018.09.053 [DOI] [PubMed] [Google Scholar]
- 4.Kim SD, Yang WJ, Chung JY. Recurrence Rate and Risk Factors for Stone Recurrence after Successful Extracorporeal Shock Wave Lithotripsy: 5-year-follow-up Study. Korean J Urology. 2006;48(1):49–53. doi: 10.4111/kju.2007.48.1.49 [DOI] [Google Scholar]
- 5.Streeper NM, Galida M, Boltz S, et al. Is Stone-free Status After Surgical Intervention for Kidney Stones Associated With Better Health-related Quality of Life? - A Multicenter Study From the North American Stone Quality of Life Consortium. Urology. 2021;148:77–82. doi: 10.1016/j.urology.2020.09.058 [DOI] [PubMed] [Google Scholar]
- 6.Scales CD, Lai JC, Dick AW, et al. Comparative Effectiveness of Shock Wave Lithotripsy and Ureteroscopy for Treating Patients With Kidney Stones. Jama Surg. 2014;149(7):648–653. doi: 10.1001/jamasurg.2014.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson JF, Lardas M, Scrimgeour D, et al. Systematic Review and Meta-analysis of the Clinical Effectiveness of Shock Wave Lithotripsy, Retrograde Intrarenal Surgery, and Percutaneous Nephrolithotomy for Lower-pole Renal Stones. Eur Urol. 2015;67(4):612–616. doi: 10.1016/j.eururo.2014.09.054 [DOI] [PubMed] [Google Scholar]
- 8.Fankhauser CD, Hermanns T, Lieger L, et al. Extracorporeal shock wave lithotripsy versus flexible ureterorenoscopy in the treatment of untreated renal calculi. Clin Kidney J. 2018;11(3):sfx151-. doi: 10.1093/ckj/sfx151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedlander DF, Brant A, McClure TD, et al. Real-world comparative effectiveness of shockwave lithotripsy versus ureterorenoscopy for the treatment of urinary stones. World J Urol. 2021;39(6):2177–2182. doi: 10.1007/s00345-020-03430-6 [DOI] [PubMed] [Google Scholar]
- 10.Geraghty RM, Jones P, Herrmann TRW, et al. Ureteroscopy is more cost effective than shock wave lithotripsy for stone treatment: systematic review and meta-analysis. World J Urol. 2018;36(11): 1783–1793. doi: 10.1007/s00345-018-2320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- 12.Semins MJ, Trock BJ, Matlaga BR. Validity of Administrative Coding in Identifying Patients With Upper Urinary Tract Calculi. J Urology. 2010; 184(1): 190–192. doi: 10.1016/j.juro.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 13.Soucie JM, Thun MJ, Coates RJ, et al. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46(3):893–899. doi: 10.1038/ki.1994.347 [DOI] [PubMed] [Google Scholar]
- 14.Kramer HJ, Choi HK, Atkinson K, et al. The association between gout and nephrolithiasis in men: The Health Professionals’ Follow-Up Study. Kidney Int. 2003;64(3): 1022–1026. doi: 10.1046/j.1523-1755.2003.t01-2-00171.x [DOI] [PubMed] [Google Scholar]
- 15.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68(3):1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x [DOI] [PubMed] [Google Scholar]
- 16.Taylor EN, Stampfer MJ, Curhan GC. Obesity, Weight Gain, and the Risk of Kidney Stones. Jama. 2005;293(4):455–462. doi: 10.1001/jama.293.4.455 [DOI] [PubMed] [Google Scholar]
- 17.Madore F, Stampfer MJ, Rimm EB, et al. Nephrolithiasis and Risk of Hypertension. Am J Hypertens. 1998;11(1):46–53. doi: 10.1016/s0895-7061(97)00371-3 [DOI] [PubMed] [Google Scholar]
- 18.Kravdal G, Helgø D, Moe MK. Kidney stone compositions and frequencies in a Norwegian population. Scand J Urol. 2019;53(2-3): 139–144. doi: 10.1080/21681805.2019.1606031 [DOI] [PubMed] [Google Scholar]
- 19.Parks JH, Worcester EM, O’Connor RC, et al. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int. 2003;63(1):255–265. doi: 10.1046/j.1523-1755.2003.00725.x [DOI] [PubMed] [Google Scholar]
- 20.Dorsher PT, McIntosh PM. Neurogenic Bladder. Adv Urology. 2012;2012:816274. doi: 10.1155/2012/816274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, DeVivo M, Roseman J. Current trend and risk factors for kidney stones in persons with spinal cord injury: a longitudinal study. Spinal Cord. 2000;38(6):346–353. doi: 10.1038/sj.sc.3101008 [DOI] [PubMed] [Google Scholar]
- 22.Ganesan V, Chen WM, Jain R, et al. Multiple sclerosis and nephrolithiasis: a matched-case comparative study. Bju Int. 2017;119(6):919–925. doi: 10.1111/bju.13820 [DOI] [PubMed] [Google Scholar]
- 23.Akkoç Y, Ersöz M, Yüceyar N, et al. Overactive bladder symptoms in patients with multiple sclerosis: Frequency, severity, diagnosis and treatment. J Spinal Cord Medicine. 2016;39(2):229–233. doi: 10.1179/2045772315y.0000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CY, Chen YT, Huang PH, et al. The association between urinary calculi and increased risk of future cardiovascular events: A nationwide population-based study. J Cardiol. 2016;67(5):463–470. doi: 10.1016/j.jjcc.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 25.Ferraro PM, Taylor EN, Eisner BH, et al. History of Kidney Stones and the Risk of Coronary Heart Disease. Jama. 2013;310(4):408–415. doi: 10.1001/jama.2013.8780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sur RL, Masterson JH, Palazzi KL, et al. Impact of statins on nephrolithiasis in hyperlipidemic patients: a 10-year review of an equal access health care system. Clin Nephrol. 2013;79(05):351–355. doi: 10.5414/cn107775 [DOI] [PubMed] [Google Scholar]
- 27.Cohen AJ, Adamsky MA, Nottingham CU, et al. Impact of Statin Intake on Kidney Stone Formation. Urology. 2019;124:57–61. doi: 10.1016/j.urology.2018.01.029 [DOI] [PubMed] [Google Scholar]
- 28.Sorensen MD, Harper JD, Borofsky MS, et al. Removal of Small, Asymptomatic Kidney Stones and Incidence of Relapse. New Engl J Med. 2022;387(6):506–513. doi: 10.1056/nejmoa2204253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1: Index of codes used for data acquisition