Abstract
Background
The GNAO1 gene encodes the α-subunit (Gαo) of the heterotrimeric guanine nucleotide-binding protein (G protein). The aim of this study was to explore the clinical characteristics of patients with GNAO1 pathogenic variations.
Methods
Ten patients with pathogenic variations in GNAO1 were enrolled from the Shenzhen Children's Hospital. Clinical data from several cases previously reported from China were also included and analyzed.
Results
Twenty-seven patients with variations in GNAO1 were analyzed (10 patients from Shenzhen Children's Hospital, 17 patients from previously published studies) including 12 boys and 15 girls. The median age of onset was 3 months with moderate to severe global developmental delay. Nineteen different GNAO1 heterozygous variants were identified. Epilepsy was observed in 18 patients (67%, 18/27), movement disorder (MD) was observed in 22 patients (81%, 22/27), and both were seen in 13 patients (48%, 13/27). Seizures typically presented as focal seizures in all patients with epilepsy. MD typically presented as dystonia and chorea. Loss-of-function (LOF) or partial loss-of-function (PLOF) mutations were more frequent in patients with developmental and epileptic encephalopathy (p = 0.029). Interictal electroencephalograms showed multifocal or diffuse epileptiform discharges. The most common magnetic resonance imaging finding was widened extracerebral space. In contrast to MD, in which improvements were not common, seizures were easily controlled by anti-seizure medications. Severe dystonia in three patients was effectively treated by deep brain stimulation. Seven (26%, 7/27) patients died of respiratory complications, status dystonicus, choreoathetosis, or sudden unexpected death in epilepsy.
Conclusion
We analyzed clinical data of 27 cases of GNAO1-related encephalopathy in China. MD seemed to be the central feature and was most difficult to control. LOF or PLOF variants were significantly associated with developmental and epileptic encephalopathy. The active intervention of severe dystonia may prevent death due to status dystonicus. However, future studies with larger samples are needed to confirm these results.
Keywords: GNAO1, gene, epilepsy, movement disorder, encephalopathy, status dystonicus
1. Introduction
The GNAO1(MIM 139311) gene maps on chromosome 16q13 and encodes the Gαo subunit of heterotrimeric guanine nucleotide-binding protein (G protein), which is present in many important G protein-coupled receptors, such as γ-aminobutyric acid receptor type B, dopamine receptor D2, and A1 adenosine and α2-adrenergic receptors (1). The typical function of Gαo is the inhibition of cyclic adenosine monophosphate (cAMP). GNAO1 is widely expressed in the brain, especially in the hippocampus, striatum, and cerebellum, and plays an important role in neural development and synaptic transmission (1, 2). Animal experiments have shown that GNAO1 knockout mice showed neurological symptoms such as tremors, seizures, abnormal behaviors, and movement disorders (MDs) and often died early (3).
In 2013, GNAO1-related encephalopathy was first reported in patients with Ohtahara syndrome and early infantile epileptic encephalopathy 17 (EIEE17; Online Mendelian Inheritance in Man 615473) (2). More recently, about 150 cases of GNAO1 variants associated with MD with or without seizures have been reported worldwide (4–8). Interestingly, Feng et al. reported a biochemical analysis of 15 different GNAO1 mutant alleles that revealed that loss-of-function (LOF) GNAO1 alleles were associated with epilepsy based on an inability to suppress cAMP production, whereas gain-of-function (GOF) or normal-function (NF) GNAO1 alleles were associated with MDs (9). In addition, developmental and epileptic encephalopathy (DEE) was correlated with LOF variants in a genotype–phenotype association analysis of 58 patients (4).
In the present study, we analyzed the genotypes and clinical phenotypes of ten patients with GNAO1 gene variants and data from 17 previously reported GNAO1-related encephalopathy cases from China (10–15). We analyzed possible genotype–phenotype correlations in patients with GNAO1 variants based on recent studies on the functional behavior of several GNAO1 variants (4, 9).
2. Materials and methods
2.1. Case collection
Ten patients with GNAO1 encephalopathy were enrolled from September 2020 to November 2022 at Shenzhen Children's Hospital (Shenzhen, China). The study protocol and consent documents were approved by the Institutional Review Board of Shenzhen Children's Hospital (No:202202001). Written informed consent was obtained from the parents or legal guardians of the patients, and assent was obtained when appropriate. Patients were followed-up at the pediatric neurology clinic or via telephone.
2.2. Genetic assessment
Blood samples were collected from participants and both of their parents for whole-exome sequencing, whole-genome sequencing, or targeted capture next-generation sequencing. Sanger sequencing was performed for validation. Variant prediction and interpretation followed the American College of Medical Genetics and Genomics guidelines (16). We classified all variants into LOF, partial-loss-of-function (PLOF), GOF, NF, and unknown-function based on the inhibition of cAMP production in Gαo mutants (4, 9). Furthermore, GNAO1 variants associated with disease were assessed for pathogenicity using several predictive bioinformatic tools: Mutation Taster (http://www.mutationtaster.org), Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/), and SIFT (http://sift.jcvi.org).
2.3. Clinical assessment
Clinical information was collected retrospectively from face-to-face and/or telephone interviews with patients, their families, or treating physicians. A questionnaire was developed to gather detailed information regarding sex, age at onset, family history, pre- and perinatal events, neurological symptoms, psychomotor development, cognitive function, neurological examination, treatments, and outcomes. Magnetic resonance imaging (MRI) and electroencephalography (EEG) data were also collected and reviewed by experienced pediatric neurologists. The follow-up period was terminated on January 30th, 2023 or at death.
2.4. Literature review
Previously reported cases in the Chinese population were identified via the China Knowledge Network (https://cnki.net/), Wanfang Data (https://wanfangdata.com.cn/), and PubMed search using the keywords “GNAO1” and “China.” Clinical and molecular genetic data were obtained from the respective references after removing duplicate individuals.
2.5. Statistical analysis
The clinical data for all patients were collected and analyzed using descriptive statistics. Continuous data are presented as median (range), and categorical data are presented as frequency (percentage). To test whether the associations of symptoms differed between GOF and LOF/PLOF, Fisher's exact test (2 × 2 contingency table) was used to analyze nonrandom associations between two categorical variables. Fisher's exact test was reliable for the small sample size in the present study. Statistical analysis was performed using STATA SE (v16.0; StataCorp, College Station, TX, USA). All statistical tests were two-sided, and p < 0.05 was considered statistically significant.
3. Results
3.1. Demographic information
Ten patients were enrolled including three boys and seven girls. All patients were unrelated with negative family history for developmental delay (DD), epilepsy, or MD. Pregnancy and birth history were unremarkable. In addition, clinical data from 17 patients with GNAO1 variants from previously published articles were included. A summary of the individuals with GNAO1 variants is provided in Supplementary Table S1.
3.2. Genetic results
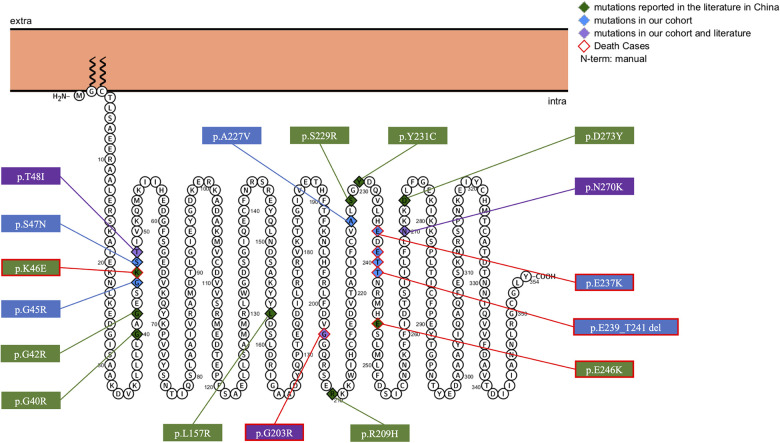
Eight different variant types were observed in the ten included patients, including six reported variants, c.607G>A(p.G203R), c.709G>A(p.E237K), c.680C>T(p.A227V), c.143C>T(p.T48l), c.808A>G(p.N270D), c.133G>C(p.G45R), and two novel variants, c.140G>A(p.S47N), and c.717_723+1del. We identified 19 different GNAO1 heterozygous variants, including 17 missense variants, one deletion, and one splicing variant. The variants were found to occur de novo after testing the parental DNA in all patients. The most common variants identified were c.607G>A(p.Gly203Arg) (n = 4), c.709G>A(p.Glu237Lys) (n = 3), c.724-8G>A (n = 3), and c.143C>T(p.Thr48Ile) (n = 2). Membrane topology and distribution of their GNAO1 variants were predicted using the PROTTER program (http://wlab.ethz.ch/protter/start/; Figure 1). All variants were tested using the in silico variant prediction tools PolyPhen-2, Mutation Taster, and SIFT, and predicted to be pathogenic or likely pathogenic variants. More detailed information on the GNAO1 gene is shown in Table 1.
Figure 1.
Membrane topology and distribution for GNAO1 variants predicted by PROTTER online tool (P09471, GNAO_HUMAN).
Table 1.
The information of genotype and clinical phenotype in patients associated with GNAO1 gene mutations in China.
| ID | Gender | Onset age | Age at last follow-up | GNAO1 mutations | Mutation type | Pathogenicity | Function of mutation | Seizure types | Epileptic syndrome | MD (symptom) | DD | Interictal EEG | MRI | Treatment | Seizures or MD at last follow-up | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| epilepsy | MD | Dystonia | Chorea | Other | ||||||||||||||
| 1 | F | 3m | 2y | 4y | c.607G>A (p.G203R) | Missense | Pathogenic | GOF | FS, GTCS, SE | - | + (hypotonia) | + | - | delay | MFD, FWR | Widened extracerebral space | VPA, LEV, LD, GBP | Died |
| 2 | F | - | 8m | 2y5m | c.709G>A (p.E237K) | Missense | Pathogenic | UF | - | - | + (appendicular hypertonia) | + | - | delay | N | Widened extracerebral space | None | Died |
| 3 | F | 31d | 5m | 4y1m | c.680C>T (p.A227V) | Missense | Pathogenic | PLOF | FS, MS, TS | EIDEE | + (hypotonia, appendicular hypertonia) | - | - | delay | SSW | Widened subdural space | VPA, LEV, PB | Seizure-relief, but dystonia persisted |
| 4 | F | 4m | 1y2m | 2y3m | c.140G>A (p.S47N) | Missense | Likely pathogenic | UF | FS, GTCS, SE | IESS | + (axial and appendicular hypertonia) | - | - | delay | MFD, SSW | Subdural effusion, widened extracerebral space | VPA, LEV, TPM, OXC, PB, LTG, PER, VGB, TBZ, KD | FS occurred 2–10 times a day, severe dystonia |
| 5 | M | 12y | 12y | 12y9m | c.717_723+1del | Deletion | Pathogenic | LOF | FS, TS | - | - | + | orofacial dyskinesia | delay | MFD | Abnormal corpus callosum | VPA, ACTH, CZP, RPD, LD | Died |
| 6 | F | 30d | 6m | 2y3m | c.143C>T (p.T48I) | Missense | Pathogenic | UF | FS, GTCS | EIDEE | + (axial and appendicular hypertonia, opisthotonos position) | - | - | delay | MFD, SSW | Arachnoid cyst, Smaller temporal lobe gyrus | VPA, LEV, TPM, PB, CZP, VGB, PER, KD | Seizure-free for 10 months, but MD persisted |
| 7 | F | - | 5m | 4y7m | c.709G>A (p.E237K) | Missense | Pathogenic | UF | - | - | + (thumb adduction) | + | - | delay | N | N | LD | Severe chorea and dystonia |
| 8 | M | - | 9m | 3y7m | c.709G>A (p.E237K) | Missense | Pathogenic | UF | - | - | + (axial and appendicular hypertonia, opisthotonos position) | + | - | delay | N | N | THX, TIZ | Severe chorea and dystonia |
| 9 | M | 2m | 5m | 7m | c.808A>G (p.N270D) | Missense | Likely pathogenic | LOF | ES, TS, GTCS | IESS | + (axial and appendicular hypertonia) | - | - | delay | BS, hypsarrhythmia | N | VPA, TPM, LD, VGB, PER, CZP, ATCH | Seizure occurred 2–3 times a day, and dystonia persisted |
| 10 | F | 5d | 2m | 1y5m | c.133G>C (p.G45R) | Missense | Likely pathogenic | UF | FS, ES, TS | IESS | + (appendicular hypertonia) | - | - | delay | MFD, SSW, BS | N | VPA, LEV, OXC, VGB, TPM, LTG | Poor seizure control |
| 11 (15) | F | 12d | - | 1y | c.607G>A (p.G203R) | Missense | Pathogenic | GOF | FS, ES | IESS | - | - | - | delay | hypsarrhythmia | N | LEV, VPA, TPM, VGB | Died |
| 12 (14) | M | 5m | NA | NA | c.607G>A (p.G203R) | Missense | Pathogenic | GOF | FS | - | + | + | Orofacial dyskinesia | delay | MFD, SB | Widened extracerebral space | TPM, VPA, OXC, CZP | Seizure-relief, but MD persisted |
| 13 (12) | F | 1m23d | - | 5m17d | c.143C>T (p.T48I) | Missense | Pathogenic | UF | FS | - | - | - | - | delay | SB, FWR | Increased water content in the white matter | TPM, LEV | Poor seizure control |
| 14 (13) | F | 6h | - | 1y11m | c.136A>G (p.K46E) | Missense | Pathogenic | UF | FS, ES, TS | IESS | - | - | - | delay | FMD, DSW, SB, hypsarrhythmia | N | VPA, TPM, PB, LEV, ACTH | Died |
| 15 (13) | M | 1.5m | NA | 1y9m | c.687C>G (p.S229R) | Missense | Pathogenic | UF | FS, ES, AAS | IESS | + | - | - | delay | hypsarrhythmia, DSW, MFD | White matter delayed myelination | VPA, TPM, ACTH, VGB | Seizure occurred 10 times a day, and dystonia persisted |
| 16 (13) | F | 1d | NA | 1y5 m | c.470T>C (p.L157R) | Missense | Pathogenic | UF | FS, ES | IESS | + | - | - | delay | hypsarrhythmia, BS | N | VGB, TPM, ACTH | Seizure-free, but dystonia persisted |
| 17 (13) | M | 4m | - | 1y1m | c.118G>C (p.G40R) | Missense | Pathogenic | LOF | FS, ES | IESS | - | - | - | delay | hypsarrhythmia, SB, SSW | Widened extracerebral space | TPM, VPA, LEV | Seizure-free |
| 18 (13) | M | 9d | NA | 1y7m | c.810C>A (p.N270K) | Missense | Pathogenic | LOF | FS, ES, TS | IESS | + | - | - | delay | BS, hypsarrhythmia | N | TPM, VPA, VGB, PER, LEV, LCM, PB, ACTH, LD | Seizure occurred 10 times a day, and dystonia persisted |
| 19 (13) | F | 2d | NA | 3y5m | c.817G>T (p.D273Y) | Missense | Pathogenic | UF | FS, GTCS | EIDEE | + | - | - | delay | DSW, SB | N | VPA, LEV, CZP, KD | Seizure-free, but dystonia persisted |
| 10 (13) | F | 3d | NA | 8m | c.692A>G (p.Y231C) | Missense | Pathogenic | PLOF | FS, ES | EIDEE | + | - | - | delay | DSW, FWR, MFD | Widened extracerebral space | VPA, TPM, VGB | Seizure-free, but dystonia persisted |
| 21 (13) | M | 12d | NA | 10m | c.607G>A (p.G203R) | Missense | Pathogenic | GOF | FS | - | + | - | - | delay | MFD | N | TPM, LD | Died |
| 22 (13) | M | - | - | 2y3m | c.736G>A (p.E246K) | Missense | Pathogenic | GOF | - | - | - | - | - | delay | N | Widened extracerebral space, white matter developmental delay | None | Died |
| 23 (13) | M | - | 4m | 7y3m | c.724-8G>A | Splice site | Pathogenic | UF | - | - | + | - | - | delay | SB | N | LD | MD persisted |
| 24 (10) | F | - | 3m | 8m | c.626G>A (p.R209H) | Missense | Pathogenic | NF | - | - | - | + | - | delay | N | N | None | NA |
| 25 (11) | F | - | 6m | 6y | c.124G>A (p.G42R) | Missense | Pathogenic | GOF | - | - | + | - | trunk torsion | delay | N | N | GPI-DBS | BFMDRS score from 77 dropped to 63 after 24 months, useful grasping |
| 26 (11) | M | - | 6m | 6y | c.724-8G>A | Splice site | Pathogenic | UF | - | - | + | - | trunk torsion | delay | N | N | STN-DBS | BFMDRS score from 62 dropped to 35 after 15 months, walk independently |
| 27 (11) | M | - | 2y | 18y | c.724-8G>A | Splice site | Pathogenic | UF | - | - | + | - | trunk torsion | NA | N | N | LD, CZP, THX, GPI-DBS | BFMDRS score from 86 dropped to 41 after 14 months |
m, months; y, years; d, days; h, hours; GOF, gain-of-function; UF, unknown-function; PLOF, partial-loss-of-function; LOF, loss-of-function; NF, normal-function; FS, focal seizure; CTCS, generalized tonic clonic seizure; SE, status epilepticus; MS, myoclonic seizure; TS, tonic seizure; ES, epileptic spasm; AAS, atypical absence seizures; EIDEE, early infantile developmental and epileptic encephalopathy; IESS, infantile epileptic spasm syndrome; MD, movement disorders; DD, developmental delay; MFD, multifocal discharge; FWR, fast wave rhythm; SSW, sharp slow wave; BS, burst-suppression pattern; SB, slow background; DSW, diffuse slow wave; MRI, magnetic resonance imaging; ASMs, anti-seizure medications; VPA, valproate; LEV, levetiracetam; LD, levodopa; GBP, gabapentin; PB, phenobarbital; TPM, topiramate; OXC, oxcarbazepine; LTG, lamotrigine; PER, perampanel; VGB, vigabatrin; TBZ, tetrabenazine; KD, ketogenic diet; ACTH, adrenocorticotropic hormone; CZP, clonazepam; LCM, lacosamide; RPD, risperidone.; THX, trihexyphenidyl; TIZ, tizanidine; GPI-DBS, globus pallidus interna-deep brain stimulation; STN-DBS, subthalamic nucleus-deep brain stimulation; -, absent; NA, not available; N, normal value.
3.3. Clinical data
The phenotypes of 27 patients (12 male, 15 female) with GNAO1 gene variants included 22 (81%, 22/27) patients with MD, 18 (67%, 18/27) patients with epilepsy, and 13 (48%, 13/27) patients with both MD and epilepsy. The median age of onset of symptoms was 3 months (range, 6 h to 4 years). The most common initial symptom was epileptic seizure (63%, 17/27) with a median onset age of 30 days. The median age of symptoms onset for the MD was 6 months. The clinical characteristics of the patients are summarized in Table 1.
MDs presented as mixed hyperkinetic syndrome including dystonia, chorea, and orofacial dyskinesia. The median age of onset of MD was 8 months. Provoking factors for episodes included fever (patients 1 and 5) and emotional stimulus (patients 4, 8 and 9). Dystonia (91%, 20/22) was the most common form of MD, followed by chorea (32%, 7/22). Patients with variant sites in c.709G>A or c.724-8G>A only had MD but not epilepsy. In our cohort, two patients (patients 1 and 3) presented with generalized hypotonia, and patient 3 presented with appendicular hypertonia. Axial and appendicular hypertonia was observed in four patients (patients 4, 6, 8, and 9), with opisthotonos position in patients 6 and 8. Other children with dystonia showed single appendicular hypertonia (patient 2, 10) and thumb adduction (patient 7). Moreover, three patients presented with severe chorea (patients 1, 5, and 8).
Seizures typically presented as focal seizures in all patients with epilepsy, then progressed to multiple seizure types including epileptic spasm (50%, 9/18) and tonic seizures (33%, 6/18). The median age of oneset of epilepsy was 3 months. Two patients had status epilepticus events. Thirteen children developed DEE including nine patients with Infantile Epileptic Spasm Syndrome (IESS), and four with EIDEE. In our cohort, two patients presented with epilepsy in infancy, followed by chorea (patient 1) and dystonia in early childhood (patient 6). For patient 5, delay in motor and cognitive functions during childhood was observed by the parents. However, the patient developed episodic MD after fever at the age of 12, manifested by orofacial dyskinesia with sustained torsional movements of the extremities, and epileptic seizures began 2 months later.
Almost all patients had mild to severe DD, manifesting with motor, speech, and cognitive deficits in the first year of life. In our cohort, seven patients (patient 1, 4, 6, 7, 8, 9 and 10) displayed severe global DD, with absence of head control, absence of speech, and intellectual disability throughout the entire disease course. In the other children, motor and language development, including head control, sitting, standing, walking, and speech skills, were also delayed but were better than in the other seven patients. Moreover, patient 5 was able to stand with help and speak (“ba ba, ma ma”) until the age of 12 years, but was unable to walk independently. However, after the onset of MD, the patient was observed to have development regression manifesting with progressive inability to raise his head stably and loss of language function due to severe dystonia and chorea.
3.4. Genotype–phenotype correlations
Genotype–phenotype associations were evaluated. Most of the mutation sites in patients with MD were located within or near the mutational hotspots (207–246 amino acid region). Of the 27 included cases, six carried GOF variants, six PLOF or LOF, one NF, and the remaining 14 carried variants whose functional effects were uncertain. Four clinical features, including epilepsy, DEE, MD, and death as final outcome were used to evaluate associations with different variant functions. LOF or PLOF variants were associated with a higher frequency of DEE compared with GOF or NF variants (p = 0.029). However, there were no statistically significant differences between GOF/NF and PLOF/LOF variants regarding epilepsy, MD, and death (Table 2).
Table 2.
Association between genotype and phenotype in patients carrying different functional variants.
| GOF/NF (n = 7) | LOF/POLF (n = 6) | p | |
|---|---|---|---|
| Epilepsy | 4 | 6 | 0.192 |
| DEE | 1 | 5 | 0 . 029 |
| MD | 5 | 5 | 1.000 |
| Death | 4 | 1 | 0.266 |
DEE, development and epileptic encephalopathy; GOF, gain-of-function; LOF, loss-of-function; PLOF, partial-loss-of-function.
The bold font indicates the difference was significant statistically.
3.5. Electroencephalography and neuroimaging findings
Interictal EEG showed abnormalities in 19 patients. Focal or multifocal discharges involving the frontal, temporal, or occipital lobe or the central area were seen in all patients with epilepsy. Slow background activity was present in six patients, hypsarrhythmia in seven, and burst-suppression in four. MRI in most individuals showed normal results or no specific findings. Widened extracerebral space was seen in eight patients; other MRI findings included abnormal corpus callosum, arachnoid cysts, smaller temporal lobe gyrus, and white matter abnormalities.
3.6. Treatment
The patients were followed-up for at least 3 months, and their ages ranged from 7 months to 18 years at the last follow-up. Among all available medications to control MDs, the most commonly used was levodopa (Table 2); others included gabapentin, tetrabenazine, trihexyphenidyl, and tizanidine. However, these medications did not effectively improve MD symptoms. Three patients (patients 25–27) with MD underwent deep brain stimulation (DBS) (11). Globus pallidus interna (GPI) DBS was performed in patients 25 and 27, and subthalamic nucleus (STN) DBS was performed in patient 26. Following surgery, both GPI and STN DBS were effective in improving dystonia symptoms. Seventeen children were treated with more than two anti-seizure medications (ASMs). The most frequently prescribed ASMs were valproate, topiramate, and levetiracetam. In addition, three patients were prescribed ketogenic diets but discontinued due to poor efficacy. At last follow-up, seizure-free status was obtained by treatment with multiple ASMs in five patients, seizure remission (reduction of seizure frequency >50%) occurred in two patients, and poor seizure control was seen in six patients.
3.7. Cause of death
Seven patients (26%) with GNAO1 variants experienced death (13, 15), 3 patients from Shenzhen Children's Hospital and 4 patients from previously published studies, including 3 boys and 4 girls. Of these deceased patients, the median age at death was 2 years 3 months (ranged 10 months to 12 years 9 months). Three patients had the same variant (p.G203R) located in the hotspot of variants (1). The common causes of death were respiratory complications (3/7) and status dystonicus (SD) (3/7) (Table 3). Another cause of death was sudden unexpected death in epilepsy. Pyrexia/infection is the commonest trigger for SD. In our cohort, Patient 1 and 2 presented with exacerbations of dystonia that were triggered by infection, lasting for hours to days ending up in dystonic state with rising creatine kinase and renal failure causing multiple organ dysfunction and death. Due to the young age, we were not able to perform deep brain stimulation (DBS). Patient 5 experienced hyperkinesia with dystonia and continuous choreoathetosis of the limbs and trunk at 12 years old. At age 12 years 9 months, he developed dyskinetic state triggered by an infection, requiring intensive care, with deep sedation. SD persisted, with rise in creatine kinase, leading to multisystemic deterioration and death.
Table 3.
Causes of death in patients with GNAO1 gene variants.
| ID | Gender | Onset age | Age at death | Provoking factor | Immediate cause of death | Underlying cause of death |
|---|---|---|---|---|---|---|
| 1 | F | 3m | 4y | Pyrexia | Renal failure due to rhabdomyolysis | Status dystonicus |
| 2 | F | 8m | 2y5m | Pyrexia | Renal failure due to rhabdomyolysis | Status dystonicus |
| 5 | M | 3m | 12y9m | Pyrexia | Renal failure due to rhabdomyolysis | Status dyskinetic |
| 11 (15) | F | 12d | 1y | Pyrexia and cough | NA | Severe pneumonia |
| 14 (13) | F | 6h | 1y11m | NA | NA | SUDEP |
| 21 (13) | M | 12d | 10m | NA | NA | Milk choking and asphyxia |
| 22 (13) | M | 4m | 2y3m | NA | NA | Severe pneumonia and respiratory failure |
F, female; M, male; m, months; d, days; h, hours; y, years; NA, not available; SUDEP, sudden unexpected death in epilepsy.
4. Discussion
Gαo, encoded by the GNAO1 gene, could be involved in signal transduction processes of G proteins. Gα binds with Gβγ and GDP in its initial state. Upon activation, Gα exchanges GDP for GTP. Both the active Gα–GTP and Gβγ subunits carry out separate downstream signaling (17). Many downstream targets of Gαo could lead to symptoms of epilepsy or MDs, including inhibiting the expression of adenylyl cyclase, which decreases cyclic cAMP production; N-type (Cav2.2) and P/Q type calcium channel (Cav2.1) activation; and stimulating the opening of inward rectifying potassium channels (1). cAMP signaling and neurotransmitter release affect many ongoing neural functions and neurological development (18). This could explain why most patients with GNAO1 variants exhibit DD.
Data were available on 27 patients with GNAO1 variants from China. The age of onset in most individuals was infancy. Our study showed that the DD phenotype was seen in almost all patients, consistent with previous reports (4, 8). MD seemed to be the central feature of GNAO1 encephalopathy with high incidence (81%), and it was more difficult to control (4–6). The symptoms of attacks included dystonia, chorea, and orofacial dyskinesia. Triggers of attacks included fever and emotional agitation. In a recent article including 157 cases with GNAO1 variants, GNAO1-related MD usually starts during infancy or childhood, with a median age at onset of 26 months (8). We observed that the age of onset of MD was 8 months, which is earlier than previously published cases. At last follow up we found no parkinsonian features and ocular movement abnormalities which Novelli and colleagues reported (8). More than half of patients (67%) experienced seizures with multiple seizure types including focal seizures, tonic seizures, epileptic spasm, and myoclonic seizures. Among them, focal seizures were the most common seizure type. Epilepsy in the present cases was more easily controlled compared to MD, consistent with previous reports (19). GNAO1 encephalopathy was associated with IESS and EIDEE. EEGs were characterized by focal and multifocal epileptiform discharges, generalized sharp slow waves, hypsarrhythmia, and slow activity. However, the pathogenic variant frequency of GNAO1 in the Chinese population patients is still unknown due to a lack of large multicenter prospective cohort studies.
In our genotype–phenotype correlation study, we found that LOF or PLOF variants were associated with DEE, which is in agreement with previous observations (4). However, we found no statistical correlation between GOF variants and MD symptoms. Considering the small sample size collected herein, further study with a larger sample of patients with GNAO1 encephalopathy will be required to confirm the observed genotype–phenotype correlations. In a previous study, LOF variants, mostly involving GTPase, led to enhanced cAMP-mediated signaling and calcium channel activity, increasing excitatory neurotransmission and neuron hyperexcitability, which may explain the pathogenesis of GNAO1-related epilepsy. On the contrary, GOF variants, near the ribose and phosphate moieties of the bound GDP, may reduce the release of neurotransmitters, leading to MD (1, 9). However, gene function is still unknown in about half of the patients. Obviously, regarding the biological activity of the GNAO1 gene, the ability to suppress cAMP production may not be present in all cases. Regarding striatal pathways, another mechanism to explain MDs relies on modifying inhibitory and stimulatory G protein-coupled receptor signaling to cAMP in Gαo. In addition, a model has been proposed through a combination of two principal mechanisms in GNAO1 disease, loss of signaling ability and dominant-negative interference (20).
Brain MRI showed no structural alterations and displayed widened extracerebral space in many patients with GNAO1 encephalopathy. According to most findings reported in the literature, brain MRI in many patients demonstrated delayed myelination, corpus callosum dysplasia, and brain atrophy (6, 8, 21). Symptoms worsen with age in some patients, accompanied by progressive brain atrophy (6, 21, 22). An autopsy was performed on a deceased child with the findings of periventricular gliosis, suggesting that a chronic neurodegenerative process could happen in patients with worsening MD (21).
In the data analyzed in the present study, nineteen patients with epilepsy received treatment with a combination of multiple ASMs. The most frequent ASMs were valproate, levetiracetam, and topiramate. For most of the patients, seizure remission was obtained with varying degrees of efficacy. Because norepinephrine-induced calcium-current inhibition is mediated by Gαo, Nakamura et al. suggested that epileptic seizures associated with GNAO1 variants might be improved by calcium-channel modulators such as pregabalin and gabapentin, which act as selective calcium-channel blockers, and topiramate, which modulates high-voltage-activated calcium channels in dentate granule cells (2). On the contrary, almost all patients with MD did not respond well to treatment. It has been reported that tetrabenazine and DBS were effective therapies for the treatment of MD associated with GNAO1 (13, 21–28). Tetrabenazine can deplete multiple amine neurotransmitters (dopamine, norepinephrine, and serotonin). Moreover, variable effects on MDs have been reported with gabapentin, topiramate, and levodopa (19, 21, 22, 29). Based on the fact that Gαo has been implicated in the regulation of many signals involved in epilepsy and MDs, it has been proposed that different approaches to therapy for different variants (agonists for LOF and antagonists for GOF mutants) may be a new therapeutic target for patients with GNAO1 variants (9). Also, while several drugs have shown efficacy in controlling symptoms of epilepsy or MDs, none of these drugs appeared to be able to alleviate developmental delays.
Among the cases of GNAO1 encephalopathy reported in China, three patients were treated with DBS (11). Patient 26 was first reported to receive STN DBS, and the postoperative Burk–Fahn–Marsden Dystonia Rating Scale score improved by 32% compared with the preoperative score, suggesting that STN may be used as the target of DBS (11). It has been previously reported that it is difficult to control status dystonicus even after one or more doses of drugs or sedatives (19, 22, 25). The fundamental goal of DBS is to alter pathological neural activity within the basal ganglia–thalamocortical circuit. About 20 patients reported a reduced frequency and severity of MD exacerbations after GPI DBS (19, 22–28). Patients undergoing GPI DBS were followed-up for 8–10 years by Koy et al. (22) and Benato et al. (24). The results showed that MD symptoms were controlled well and there were no more episodes of status dystonicus, which demonstrated that DBS had a potential role to control and prevent status dystonicus.
Patients with GNAO1 pathogenic variants showed poor prognosis with a high mortality rate (26%). Seven patients died of SD and respiratory complications in individuals with GNAO1 variants in China. To date, 14 patients with variants died because of MD exacerbations, respiratory failure, and neurological deterioration (8). Several cases with similar causes of death have also been reported in the literature (2, 19, 21). Hence, we believe that SD and respiratory complications may be the most common causes of death. Pyrexia/infection is the most provoking factor. Therefore, it is important to prevent infections in children with drug-refractory MD. The active intervention of severe dystonia may prevent death due to SD. DBS has been shown to control and prevent refractory hyperkinetic crises, which can improve prognosis by reducing the length of hospitalization and preventing complications associated with disease and treatment (22, 24, 26). However, generally accepted inclusion criteria for DBS included unequivocal diagnosis of dystonia, medical treatment failure, and sufficient disability to the patient with more than 7 years old (30). For young children (less than 7 years old), it is worth thinking about considering early DBS for patients with drug-refractory MD. Finally, particular attention should also be paid to motor and speech development, mental health, and nutritional status.
5. Conclusions
In the present study, our data support the findings that patients with GNAO1 variants usually present with MD, epilepsy, and DD at varying degrees. The age of onset in most individuals was infancy. MD seemed to be the central feature of GNAO1 encephalopathy, with an earlier age of onset than previously reported. LOF or PLOF variants were significantly associated with DEE. SD and respiratory complications were the main cause of GNAO1-related death. Further studies with a larger number of patients are needed to evaluate the possible genotype–phenotype correlations.
Acknowledgments
We would like to thank all patients and their parents for participating in the study.
Funding Statement
This study was financially supported by Basic research of Shenzhen science and technology plan project (JCYJ20210324135211030), Guangdong High-level Hospital Construction Fund and Shenzhen Key Medical Discipline Construction Fund (Grant Number: SZXK033).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Shenzhen Children's Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL and DC designed the study. DC obtained funding. HC, XD, XC, and LC assisted in data collection and patient recruitment. LL provided help in the genetic analysis. YL and HC drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1086970/full#supplementary-material
References
- 1.Feng H, Khalil S, Neubig RR, Sidiropoulos C. A mechanistic review on GNAO1-associated movement disorder. Neurobiol Dis. (2018) 116:131–41. 10.1016/j.nbd.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 2.Nakamura K, Kodera H, Akita T, Shiina M, Kato M, Hoshino H, et al. De novo mutations in GNAO1, encoding a Gαo subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet. (2013) 93:496–505. 10.1016/j.ajhg.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang M, Gold MS, Boulay G, Spicher K, Peyton M, Brabet P, et al. Multiple neurological abnormalities in mice deficient in the G protein go. Proc Natl Acad Sci U S A. (1998) 95:3269–74. 10.1073/pnas.95.6.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schirinzi T, Garone G, Travaglini L, Vasco G, Galosi S, Rios L, et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: results from an analytical review. Parkinsonism Relat Disord. (2019) 61:19–25. 10.1016/j.parkreldis.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Shim Y, Ko YJ, Park S, Jang SS, Lim BC, et al. Spectrum of movement disorders in GNAO1 encephalopathy: in-depth phenotyping and case-by-case analysis. Orphanet J Rare Dis. (2020) 15:343. 10.1186/s13023-020-01594-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly M, Park M, Mihalek I, Rochtus A, Gramm M, Pérez-Palma E, et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate-binding region. Epilepsia. (2019) 60:406–18. 10.1111/epi.14653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galosi S, Pollini L, Novelli M, Bernardi K, Di Rocco M, Martinelli S, et al. Motor, epileptic, and developmental phenotypes in genetic disorders affecting G protein coupled receptors-cAMP signaling. Front Neurol. (2022) 13:886751. 10.3389/fneur.2022.886751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novelli M, Galosi S, Zorzi G, Martinelli S, Capuano A, Nardecchia F, et al. GNAO1-related movement disorder: an update on phenomenology, clinical course, and response to treatments. Parkinsonism Relat Disord. (2023) 111:105405. 10.1016/j.parkreldis.2023.105405 [DOI] [PubMed] [Google Scholar]
- 9.Feng H, Sjögren B, Karaj B, Shaw V, Gezer A, Neubig RR. Movement disorder in GNAO1 encephalopathy associated with gain-of-function mutations. Neurology. (2017) 89:762–70. 10.1212/wnl.0000000000004262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang HB, Wen YX, Zhang QP, Bao XH. Genetic and phenotypic analysis of five patients with GNAO 1 gene related disorders. J Clin Pediatr. (2022) 40:361–5. 10.12372/jcp.2022.21e0985 [DOI] [Google Scholar]
- 11.Liu Y, Zhang Q, Wang J, Liu J, Yang W, Yan X, et al. Both subthalamic and pallidal deep brain stimulation are effective for GNAO1-associated dystonia: three case reports and a literature review. Ther Adv Neurol Disord. (2022) 15:17562864221093507. 10.1177/17562864221093507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JLHWG, Lan MP, Li SX, Liu P. GNAO1 encephalopathy: a case report and literature review. Chin J Child Health Care. (2022) 30:806–8. 10.11852/zgetbjzz2021-0975 [DOI] [Google Scholar]
- 13.Yang X, Niu X, Yang Y, Cheng M, Zhang J, Chen J, et al. Phenotypes of GNAO1 variants in a Chinese cohort. Front Neurol. (2021) 12:662162. 10.3389/fneur.2021.662162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang J, Li Y, Wang D, Zhang G, Guo H. GNAO1 encephalopathy with epilepsy and dyskinesia: a case report and literature review. J Clin Pediatr. (2020) 38:691–4. 10.3969/j.issn.1000-3606.2020.09.012 [DOI] [Google Scholar]
- 15.Xiong J, Peng J, Duan HL, Chen C, Wang XL, Chen SM, et al. Recurrent convulsion and pulmonary infection complicated by psychomotor retardation in an infant. Zhongguo Dang Dai Er Ke Za Zhi. (2018) 20:154–7. 10.7499/j.issn.1008-8830.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17:405–24. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. (2005) 85:1159–204. 10.1152/physrev.00003.2005 [DOI] [PubMed] [Google Scholar]
- 18.Leung CCY, Wong YH. Role of G protein-coupled receptors in the regulation of structural plasticity and cognitive function. Molecules. (2017) 22:1239. 10.3390/molecules22071239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, et al. GNAO1 encephalopathy: broadening the phenotype and evaluating treatment and outcome. Neurol Genet. (2017) 3:e143. 10.1212/nxg.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muntean BS, Masuho I, Dao M, Sutton LP, Zucca S, Iwamoto H, et al. Gαo is a major determinant of cAMP signaling in the pathophysiology of movement disorders. Cell Rep. (2021) 34:108718. 10.1016/j.celrep.2021.108718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananth AL, Robichaux-Viehoever A, Kim YM, Hanson-Kahn A, Cox R, Enns GM, et al. Clinical course of six children with GNAO1 mutations causing a severe and distinctive movement disorder. Pediatr Neurol. (2016) 59:81–4. 10.1016/j.pediatrneurol.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 22.Koy A, Cirak S, Gonzalez V, Becker K, Roujeau T, Milesi C, et al. Deep brain stimulation is effective in pediatric patients with GNAO1 associated severe hyperkinesia. J Neurol Sci. (2018) 391:31–9. 10.1016/j.jns.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 23.Yamashita Y, Ogawa T, Ogaki K, Kamo H, Sukigara T, Kitahara E, et al. Neuroimaging evaluation and successful treatment by using directional deep brain stimulation and levodopa in a patient with GNAO1-associated movement disorder: a case report. J Neurol Sci. (2020) 411:116710. 10.1016/j.jns.2020.116710 [DOI] [PubMed] [Google Scholar]
- 24.Benato A, Carecchio M, Burlina A, Paoloni F, Sartori S, Nosadini M, et al. Long-term effect of subthalamic and pallidal deep brain stimulation for status dystonicus in children with methylmalonic acidemia and GNAO1 mutation. J Neural Transm. (2019) 126:739–57. 10.1007/s00702-019-02010-2 [DOI] [PubMed] [Google Scholar]
- 25.Waak M, Mohammad SS, Coman D, Sinclair K, Copeland L, Silburn P, et al. GNAO1-related movement disorder with life-threatening exacerbations: movement phenomenology and response to DBS. J Neurol Neurosurg Psychiatry. (2018) 89:221–2. 10.1136/jnnp-2017-315653 [DOI] [PubMed] [Google Scholar]
- 26.Honey CM, Malhotra AK, Tarailo-Graovac M, van Karnebeek CDM, Horvath G, Sulistyanto A. GNAO1 mutation-induced pediatric dystonic storm rescue with pallidal deep brain stimulation. J Child Neurol. (2018) 33:413–6. 10.1177/0883073818756134 [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz S, Turhan T, Ceylaner S, Gökben S, Tekgul H, Serdaroglu G. Excellent response to deep brain stimulation in a young girl with GNAO1-related progressive choreoathetosis. Childs Nerv Syst. (2016) 32:1567–8. 10.1007/s00381-016-3139-6 [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni N, Tang S, Bhardwaj R, Bernes S, Grebe TA. Progressive movement disorder in brothers carrying a GNAO1 mutation responsive to deep brain stimulation. J Child Neurol. (2016) 31:211–4. 10.1177/0883073815587945 [DOI] [PubMed] [Google Scholar]
- 29.Arya R, Spaeth C, Gilbert DL, Leach JL, Holland KD. GNAO1-associated epileptic encephalopathy and movement disorders: c.607G>A variant represents a probable mutation hotspot with a distinct phenotype. Epileptic Disord. (2017) 19:67–75. 10.1684/epd.2017.0888 [DOI] [PubMed] [Google Scholar]
- 30.Larsh T, Wu SW, Vadivelu S, Grant GA, O’Malley JA. Deep brain stimulation for pediatric dystonia. Semin Pediatr Neurol. (2021) 38:100896. 10.1016/j.spen.2021.100896 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.