Abstract
Anxiety and depression are highly prevalent psychiatric disorders, affecting approximately 18% of the United States population. Evidence indicates that central oxytocin mediates social cognition, social bonding, and social anxiety. Although it is well-established that oxytocin ameliorates social deficits, less is known about the therapeutic effects of oxytocin in non-social contexts. We hypothesized that positive effects of oxytocin in social contexts are attributable to intrinsic effects of oxytocin on neural systems that are related to emotion regulation. The present study investigated the effect of intracerebroventricular (ICV) oxytocin administration (i.e., central action) on anxiety- and depression-like behavior in C57Bl/6J mice using non-social tests. Male and female mice received an ICV infusion of vehicle or oxytocin (100, 200, or 500 ng), then were tested in the elevated zero maze (for anxiety-like behavior) and the tail suspension test (for depression-like behavior). Oxytocin dose-dependently increased open zone occupancy and entries in the elevated zero maze and reduced immobility duration in the tail suspension test in both sexes. Oxytocin decreased anxiety and depression-like behavior in male and female mice. The observed effect of oxytocin on anxiolytic-like behavior appeared to be driven by the males. Given the smaller anxiolytic-like effect of oxytocin in the female mice and the established interaction between oxytocin and reproductive hormones (estrogen and progesterone), we also explored whether oxytocin sensitivity in females varies across estrous cycle phases and in ovariectomized females that were or were not supplemented with estrogen or progesterone. Oxytocin reduced anxiety-like behavior in female mice in proestrus/estrus, ovariectomized females (supplemented or not with estrogen or progesterone), but not females in metestrus/diestrus. Additionally, oxytocin reduced depression-like behavior in all groups tested with slight differences across the various hormonal statuses. These results suggest that the effect of oxytocin in depression- and anxiety-like behavior in mice can be influenced by sex and hormonal status.
Keywords: Oxytocin, Sex differences, Anxiety, Depression, Estrous cycle, Reproductive hormones
Graphical abstract
Highlights
-
•
Oxytocin reduces anxiety-like behavior in male and female mice, with a suggestion of a larger effect in males.
-
•
Oxytocin reduces anxiety-like behavior in females in proestrus/estrus, but not in females in metestrus/diestrus.
-
•
Oxytocin reduces anxiety-like behavior in ovariectomized female mice, regardless of hormone supplementation.
-
•
Oxytocin reduces depression-like behavior in males and in females, regardless of hormonal status.
1. Introduction
Depression and anxiety disorders are leading causes of disability worldwide (World Health Organization, 2017). In the United States, approximately 64 million adults are diagnosed with a depression or anxiety disorder annually (Anxiety and Depression Association of America, 2018). These disorders are highly comorbid with other mental and physical ailments, which gives rise to increased prevalence and severity (Kaufman and Charney, 2000). Women are disproportionately affected by these disorders, being twice as likely to be diagnosed with depression and/or anxiety than men (Do and Schnittker, 2022). A recent review demonstrates that several antidepressant medications from different pharmacological classes have been approved to treat mood disorders (Jakobsen et al., 2020). Although these pharmacotherapies show some efficacy, effect sizes are modest (Ioannidis, 2008, Pigott et al., 2010). Furthermore, there are disparities in the prescription and therapeutic efficacy of these drugs, which may be attributable to sex-based physiological and behavioral differences (Do and Schnittker, 2022, Sramek et al., 2022). Thus, it is important to identify additional biological targets to treat depression and anxiety.
The oxytocin system has been studied and assessed as a potential target for medication development (Carter et al., 2020). Oxytocin is a nonapeptide (i.e., a peptide chain with nine amino acid residues) that binds to class A G-protein-coupled oxytocin receptors and is synthesized in the paraventricular nucleus, supraoptic nucleus, and accessory nuclei of the hypothalamus (Jurek and Neumann, 2018). It is then transported to the posterior pituitary, where it can be released as a peripheral hormone, and to the rest of the brain, where it can function as a neuromodulator (Knobloch et al., 2012). Central oxytocin is important for the development and regulation of social bonding, social recognition, and social memory (Froemke and Young, 2021). Within the last two decades, protective and regulatory roles of oxytocin in response to stress have been reported (Carter et al., 2020). One hypothesis is that clinical studies suggest that anti-stress effects of oxytocin mediate its prosocial effects (Uvnäs-Moberg, 1998), and another is that oxytocin is important for emotional allostasis, such that it maintains stability through changing environments regardless of context. Altogether, these theories demonstrate an interdependent relationship between oxytocin, anxiety, and attachment (Tops et al., 2007).
While several rodent studies have investigated the effects of oxytocin on anxiety- and depression-like behaviors, sex differences are rarely studied (Yoon and Kim, 2020). In general, these studies demonstrated that exogenous oxytocin functions as an anti-stress neuromodulator in males, but not females, and that disruption of the endogenous oxytocin system produces stress-like effects in females, but not males. For example, Ring et al. demonstrated that peripherally and centrally administered oxytocin (Ring et al., 2006) and oxytocin receptor agonists (Ring et al., 2010) were shown to be anxiolytic-like in four-plate and elevated zero maze tests and reduced stress-induced hyperthermia in male mice and rats. These tests have yet to be replicated in female mice and rats under the same conditions. A few independent studies demonstrate that exogenous oxytocin may not effectively reduce anti-stress behaviors in female mice (Lukas et al., 2011; Lukas and Neumann, 2014; Steinman et al., 2016). For example, centrally administered oxytocin reversed stress-induced social withdrawal in male, but not female, mice and rats that were exposed to social defeat stress (Lukas et al., 2011; Lukas and Neumann, 2014; Steinman et al., 2016). In contrast, when the endogenous oxytocin system is disrupted, females showed increased anxiety-like behavior, whereas males did not (Mantella et al., 2003). This was demonstrated in oxytocin-deficient male and female mice and wildtype female mice treated with an oxytocin receptor antagonist (Mantella et al., 2003). Of note, others have shown that when exogenous oxytocin is administered to stressed female California mice it promotes anxiogenic-like behaviors such as social avoidance and vigilance, whereas oxytocin receptor antagonists increase social approach (Trainor et al., 2011; Steinman et al., 2016; Duque-Wilckens et al., 2018; Duque-Wilckens et al., 2020; Williams et al., 2020). In male and unstressed female California mice, oxytocin receptor antagonists reduce social approach. These data further highlight important sex differences in oxytocin system function. One possibility for the discrepancy between sexes is that the endogenous oxytocin system in females plays a more constitutive role in depression- and anxiety-like behavior and thus, is more sensitive to oxytocin antagonists and less sensitive to the effects of exogenous oxytocin (Mantella et al., 2003). The first purpose of the present study was to characterize the response of male and female mice in the elevated zero maze and tail suspension tests.
Another possibility is that the effects of exogenous oxytocin in females are influenced by reproductive hormones that fluctuate throughout the estrous cycle and have complex interactions with the oxytocin system as demonstrated in a recent review of the literature (Jirikowski et al., 2017). Previous studies demonstrated that intracerebroventricular (ICV) oxytocin administration produced an anxiolytic-like response in males (Uvnäs-Moberg et al., 1994; Ring et al., 2006; Yoshida et al., 2009) and in estrogen-treated, but not vehicle-treated, ovariectomized females (McCarthy et al., 1996). However, unknown is the effect of ICV oxytocin administration in naturally cycling females, across the estrous cycle and in ovariectomized females with progesterone supplementation.
Thus, the second purpose of the present study was to test the novel hypothesis that the effect of centrally administered oxytocin on anxiety- and depression-like behavior is different across the estrous cycle in naturally cycling female mice. To test this hypothesis, we compared the effects of ICV oxytocin and vehicle (control) administration prior to testing in the elevated zero maze and tail suspension tests in seven experimental groups. Tests that compared oxytocin and vehicle were conducted across sex, i.e., in males and females, and across hormonal status in females, i.e., in naturally cycling females (in proestrus/estrus, or in metestrus/diestrus), and ovariectomized females (supplemented or not with estrogen or progesterone). We hypothesized that oxytocin would be effective in males and in females with high levels of estrogen (i.e., females in proestrus/estrus and estrogen-treated ovariectomized females).
2. Methods
2.1. Subjects
Forty-four male and two hundred-fifty female C57Bl/6J mice were tested in this study. Females, 8–12 weeks old weighing 18–24 g, and males, 8–12 weeks old weighing 22–30 g, were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The mice were habituated to the laboratory for at least 1 week before the experiments began. The mice were housed in same-sex groups (2–4 per cage) in plastic cages (28 cm width × 17 cm length × 12 cm height) with free access to food and water. Temperature (24 °C ± 2 °C) and humidity (50–60%) were controlled, with a 12 h/12 h light/dark cycle (lights on at 6 a.m.). Animal care and use were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois Chicago (UIC) and National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) Animal Care and Use Committee.
2.2. Surgical procedures: cannulation, ovariectomy, and hormone replacement
All surgeries followed aseptic procedures. Each mouse was anesthetized by the inhalation of 1.5–3% isoflurane at NIDA IRP (Biomedical Research Center, Baltimore, MD, USA) or by intraperitoneal administration of ketamine (100 mg/kg) and xylazine (10 mg/kg) at UIC (Department of Psychology, Chicago, IL, USA). They were placed on a heating pad to prevent hypothermia and treated with eye ointment to prevent drying of the eyes. When relevant, ovariectomies, hormone supplement implantation, and ICV cannula implantations were performed during the same surgery. Most surgeries were conducted at NIDA. The results of experiments that were performed at UIC and NIDA were comparable. For the open zone occupancy measure, MeanUIC = 17.6%, SEMUIC = 3.46%; MeanNIDA = 21.3%, SEMNIDA = 1.31%. For the open zone entries measure, MeanUIC = 11.9, SEMUIC = 1.83; MeanNIDA = 15.8, SEMNIDA = 3.30. The results showed no significant difference in vehicle measures in mice from the two sites, i.e., using two-tailed t-tests (UIC v. NIDA): occupancy; t12 = 0.6529, p = 0.5261; entries; t12 = 1.230, p = 0.2421.
For ovariectomy, a 3 cm × 3 cm area on the back of each mouse was shaved and disinfected with betadine and 70% isopropyl alcohol. A 2 cm midline incision was made, and the skin was dissected from the underlying fascia. Approximately 1 cm lateral from the midline, another incision was made through the fascia and dissected laterally. Once the ovary was identified, the uterine horns and vessels were ligated, the ovary was removed, and the fascia incision was closed using absorbable sutures. A similar incision was then made on the contralateral side, and the procedure was repeated. The midline incision was then closed using removable sutures or wound clips.
Mice that received hormone supplements were implanted with a 17β-estradiol or progesterone capsule in a subcutaneous pocket prior to closing the midline incision. Sterilized silastic tubing (2 cm length) was filled with a 35 μg/ml 17β-estradiol (Millipore Sigma, Burlington, MA, USA) or 1 mg/ml progesterone (Millipore Sigma, Burlington, MA, USA) solution in sesame oil (Millipore Sigma, Burlington, MA, USA). 17β-estradiol and progesterone doses were selected based on previously established protocols (McCarthy et al., 1996; Ström et al., 2012; Sovijit et al., 2021) that demonstrate physiological ranges of estrogen and progesterone were achieved. The silastic tubing was stoppered with 3 mm wooden applicator sticks and allowed to incubate in the hormone solution overnight before implantation. Each mouse that underwent ovariectomy with or without hormone replacement was allowed to recover before behavioral testing. Each mouse received hormone supplement via the silastic capsule for at least 14 days prior to testing and throughout the study period. All mice were treated with meloxicam (5 mg/kg) postoperatively on the day of and 3 days following the procedure.
For ICV cannulation, a 1 cm × 1 cm area on the scalp midline of an anesthetized mousewas shaved before it was secured to the stereotaxic instrument. The skin on top of the head was disinfected with betadine and 70% isopropyl alcohol. After making an incision to access the skull and implanting three small skull screws, a 26-gauge, 5 mm stainless steel guide cannula (Plastics One, Roanoke, VA, USA) was unilaterally implanted into the lateral ventricle using the following coordinates relative to bregma: −0.1 mm (anterior/posterior), −0.9 mm (medial/lateral), −1.8 mm (dorsal/ventral). The coordinates were based on Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates (Paxinos and Franklin, 2019). The cannula was fixed to the skull using the implanted screws and dental acrylic, then covered using a stylet and an aluminum cap. To minimize pain and discomfort, each mouse was treated with meloxicam (5 mg/kg) postoperatively to reduce inflammation and placed in a warm cage for at least 2 h before being returned to the housing room. Each mouse was allowed to recover for at least 5 days before the experiment.
2.3. Microinfusion procedure
On the day of the experiment, the dust caps and dummy cannulae were replaced with 33-gauge infusion cannulae that extended 1 mm beyond the guide cannulae. The infusion cannulae were attached to polyethylene tubes (PE-50; Plastics One, Roanoke, VA, USA) that were connected to 10 μl Hamilton syringes. The syringes were driven by a microinfusion pump, with solutions infused in a volume of 2 μl at a rate of 0.5 μl/min. Oxytocin (Tocris Bioscience, Minneapolis, MN, USA; 100, 200, or 500 ng) or saline (vehicle; VEH) controls were assigned to separate groups of randomly selected animals. The mice were allowed to roam freely during the infusion, and the infusion cannulae were left in place for at least 5 min to allow drug diffusion. Infusion cannulae were then replaced by stylets and caps, and the mice were allowed to roam freely until they were behaviorally tested 12 min later. The doses of oxytocin used in this study are similar to those used in previously published studies (Kent et al., 2013).
2.4. Elevated zero maze
The elevated zero maze (EZM) used was a circular maze (60 cm diameter) that was elevated 50 cm above the floor. The zero maze was divided into four quadrants (two open quadrants with 1 cm high translucent edges that were separated by two closed quadrants with 15 cm high opaque walls; lanes were 5 cm wide). Testing began immediately after the experimenter placed the mouse on the maze in either of the open zones. Each mouse was allowed to explore the elevated zero maze for 5 min, and behavior was recorded using a wall-mounted Stoelting USB camera that was connected to AnyMaze tracking software (Wood Dale, IL, USA). Behavior was analyzed in real time by AnyMaze tracking software. The percent time that the animal spent in the open quadrants (open zone occupancy) and number of entries into the open quadrants (open zone entries) were used as measures of anxiety-like behavior. Increases in open zone occupancy and entries were interpreted as a reduction of anxiety-like behavior. Testing occurred in a dimly lit room (55 ± 15 lux) between 8 a.m. and 5 p.m. The elevated zero maze has predictive validity such that FDA-approved anxiolytic drugs increase open zone occupancy and entries in mice in this task (Shepherd et al., 1994).
2.5. Tail suspension test
Using a box (40 cm length × 25 cm width × 50 cm height) with a stainless-steel bar that was 45 cm above the base, each mouse was suspended from the tail using 10 cm long adhesive labeling tape. One end of the labeling tape was sealed around the tail of each mouse, and the other end was sealed around the stainless-steel bar. A short plastic straw was placed on the mouse's tail between the base and the tip to prevent tail climbing. The duration of the test was 6 min. Testing began immediately after the experimenter's hand was slowly and gently removed from under the mouse to suspend it. Each test was recorded using a Stoelting USB camera that was mounted to a tripod and connected to a computer. The time each animal spent immobile (immobility duration) was used as a measure of depression-like behavior. This measure was scored by other experimenters (LAG, MT) who were blind to group assignment. Testing occurred in a dimly lit room (55 ± 15 lux) between 8 a.m. and 5 p.m. The tail suspension test has predictive validity such that FDA-approved antidepressant drugs reduce immobility duration in this task (Steru et al., 1985).
2.6. Estrous cycle verification
The estrous cycle was monitored using methods adapted from a previous report (McLean et al., 2012). Approximately 100 μl of sterile saline in a sterile Pasteur pipette was used to collect vaginal samples. Each mouse was scruffed and any urine or feces were wiped away. The tip of the saline-filled pipette was placed at the opening of the vaginal canal, and the pipette bulb was gently depressed and released 2–3 times. The sample was placed on a glass slide, allowed to dry at room temperature, and stained using crystal violet. Microscopic images were taken, and estrous cycle phases were determined by two experimenters who were blind to group assignment.
2.7. Histological verification of injection site
After the final day of experimentation, each mouse was administered with 2 μl of Chicago Blue Dye solution (0.1% w/v) to the cannulated region using an infusion cannula. At UIC, mice were euthanized with FatalPlus (390 mg/ml sodium pentobarbital; 39 mg/kg), perfused with 0.9% saline and 20% formalin. Brains were then extracted and stored in formalin at 4 °C until further analysis. At NIDA IRP, mice were euthanized by isoflurane saturation followed by cervical dislocation. Brains were then extracted, fresh frozen in isopentane on dry ice and stored at -80 °C until further analysis. At both institutes, each brain was sliced into 50 μm thick sections until the cannula track and/or dye indication appeared. Using a microscope and Paxinos and Watson's the Mouse Brain in Stereotaxic Coordinates (Paxinos and Franklin, 2019) for guidance, the location of the cannula was identified. Brains that were accurately cannulated displayed blue stain throughout the lateral, third, and fourth ventricles. Data from mice that were not successfully cannulated were excluded.
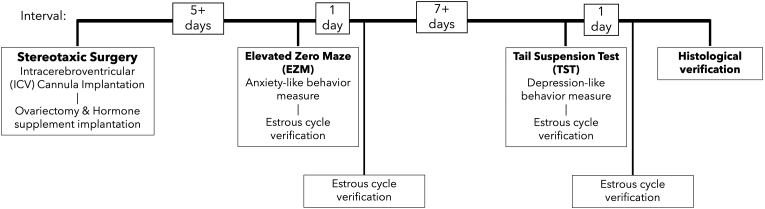
Fig. 1 shows the procedure and experiment timeline.
Fig. 1.
Procedure & experiment timeline.
2.8. Statistical analysis
The statistical analyses were conducted using Prism software (GraphPad, San Diego, CA, USA). Elevated zero maze (open zone occupancy and entries) and tail suspension (immobility duration) test data were analyzed using two-way analysis of variance (ANOVA) with experimental category (sex, estrous cycle phase or hormone supplementation) and treatment as between-subjects factors. ANOVAs that yielded a significant main effect of treatment were further analyzed using the Holm-Sidak's multiple-comparison test between means collapsed by sex. ANOVAs that yielded significant interaction effects or interaction effects that approached significance (p ≦ 0.1) were also followed by Holm Sidak's multiple comparison tests between means for each sex, and by interaction contrasts analyses across each dose using a two-tailed t-test. One-way ANOVAs were used to investigate differences in baseline measures for all experimental groups for the elevated zero maze and tail suspension test. Values of p < 0.05 were considered statistically significant. Data are expressed as the mean ± SEM.
3. Results
3.1. Male and female mice demonstrate sensitivity to oxytocin in the elevated zero maze
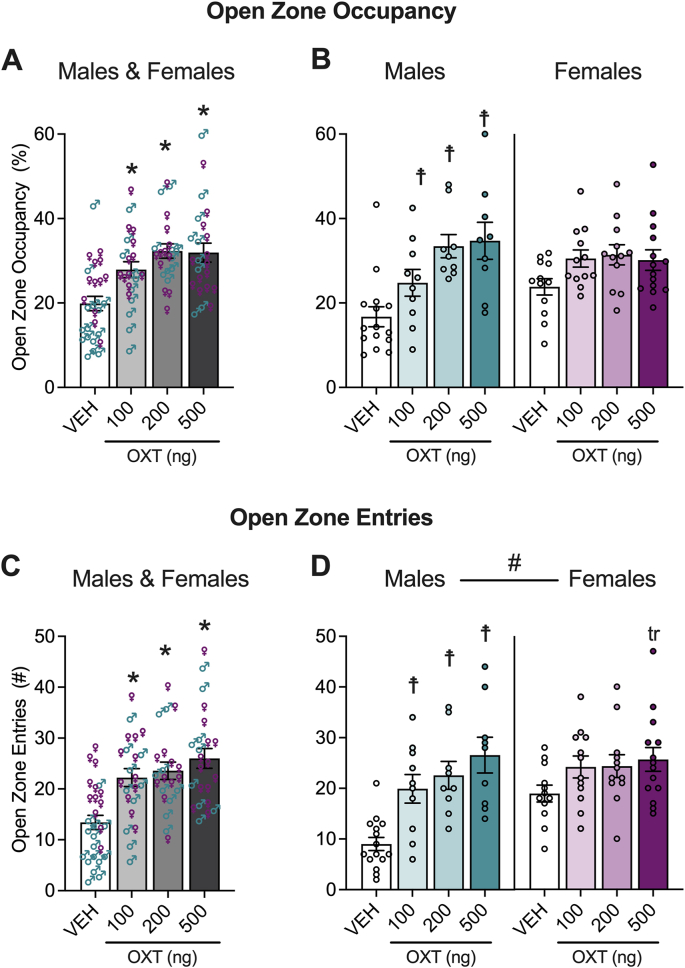
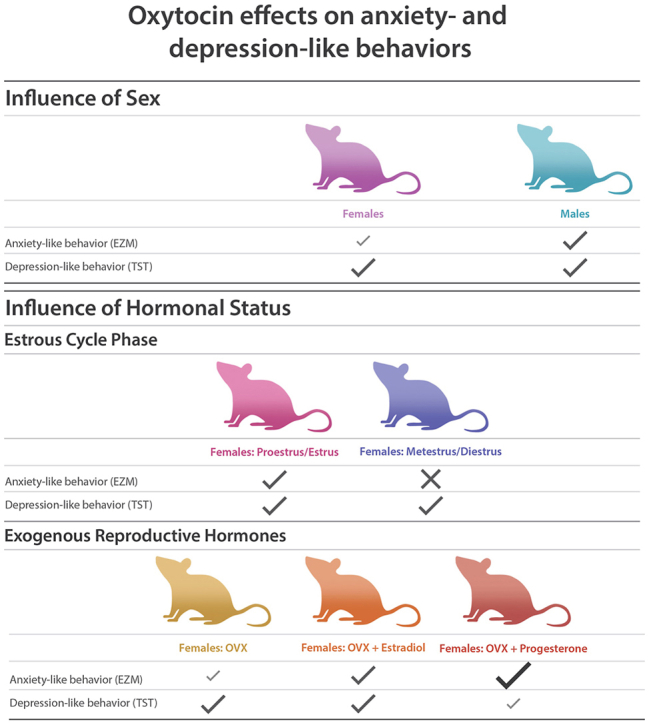
We examined the effect of vehicle (saline) or oxytocin (100, 200 or 500 ng) administered to the lateral ventricles of males (n, vehicle = 15, 100 ng oxytocin = 10, 200 ng oxytocin = 10, 500 ng oxytocin = 9) and females (n, vehicle = 12, 100 ng oxytocin = 12, 200 ng oxytocin = 13, 500 ng oxytocin = 14) on open zone occupancy and entries using the elevated zero maze test (Fig. 2). The ANOVA of open zone occupancy showed no main effect of sex (F1,86 = 0.6833, p = 0.4107), but it showed a main effect of treatment (F3,86 = 10.17, p < 0.0001) and the ANOVA of open zone entries showed main effects of treatment (F3,85 = 11.20, p < 0.0001) and sex (M < F; F1,85 = 5.256, p = 0.0243). Follow-up analyses using Holm-Sidak's multiple comparison tests on the male-female pooled data demonstrated that the 100 ng (occupancy: p = 0.0054; entries: p = 0.0005), 200 ng (occupancy: p < 0.0001; entries: p = 0.0002) and 500 ng (occupancy: p < 0.0001; entries: p < 0.0001) doses of oxytocin significantly increased open zone occupancy and entries. For open zone occupancy and entries, the treatment × sex interaction effect approached, but did not reach, statistical significance (F3,86 = 2.385, p = 0.0747 and F3,85 = 2.149, p = 0.1000, respectively). To investigate simple effects in males and females, we performed Holm-Sidak's multiple comparison tests on individual means within each sex. These analyses demonstrated that all doses of oxytocin increased open zone occupancy and entries in males (occupancy: 100 ng, p = 0.0306; 200 ng, p < 0.0001; 500 ng, p < 0.0001; entries: 100 ng, p = 0.0009; 200 ng, p = 0.0002; 500 ng, p < 0.0001), but not females (occupancy: 100 ng, p = 0.1348; 200 ng, p = 0.1178; 500 ng, p = 0.1348; entries: 100 ng, p = 0.1757; 200 ng, p = 0.1757; 500 ng, p = 0.0898). To further investigate whether the magnitude of the treatment effect was larger in males for open zone occupancy and entries, we performed an interaction contrast analysis across each dose using a two-tailed t-test. This analysis demonstrated that the difference from vehicle was larger in males than females for the 200 ng (occupancy: t21 = 2.668, p = 0.0144; entries: t19 = 2.312, p = 0.0321) and 500 ng (occupancy: t21 = 2.526, p = 0.0196; entries: t21 = 2.667, p = 0.0144) doses, but not the 100 ng dose (occupancy: t20 = 0.3617, p = 0.7214; entries: t20 = 1.618, p = 0.1214) of oxytocin, see Supplemental Fig. S1. Of note, the effect on open zone entries in females that were administered 500 ng oxytocin approached significance.
Fig. 2.
Oxytocin (OXT) decreased anxiety-like behavior in the elevated zero maze, with a suggestion of a larger effect in males. Oxytocin dose-dependently increased open zone occupancy (A, B) and the number of open zone entries (C, D) in male and female mice. The plots show pooled male and female data for open zone occupancy (A) and entries (C) with females represented as ♀ and males as ♂. Separate plots for male and female open zone occupancy (B) and entries (D) are also shown. Two-way ANOVAs were calculated to analyze the male vs. female data. #p < 0.05, main effect of sex. The significant main effect of treatment for open zone occupancy and entries was followed by the Holm-Sidak's multiple comparison test. *p < 0.05, difference from vehicle (VEH) in pooled plots. Because the sex × treatment interaction approached significance for open zone occupancy and entries, the Holm-Sidak's multiple comparison test was used to further assess the male and female data separately; trp = 0.09, ☨p < 0.05, difference from VEH in separate plots. See Section 3.1 (Results) for more details.
3.2. Oxytocin sensitivity in female mice is influenced by hormonal status in the elevated zero maze
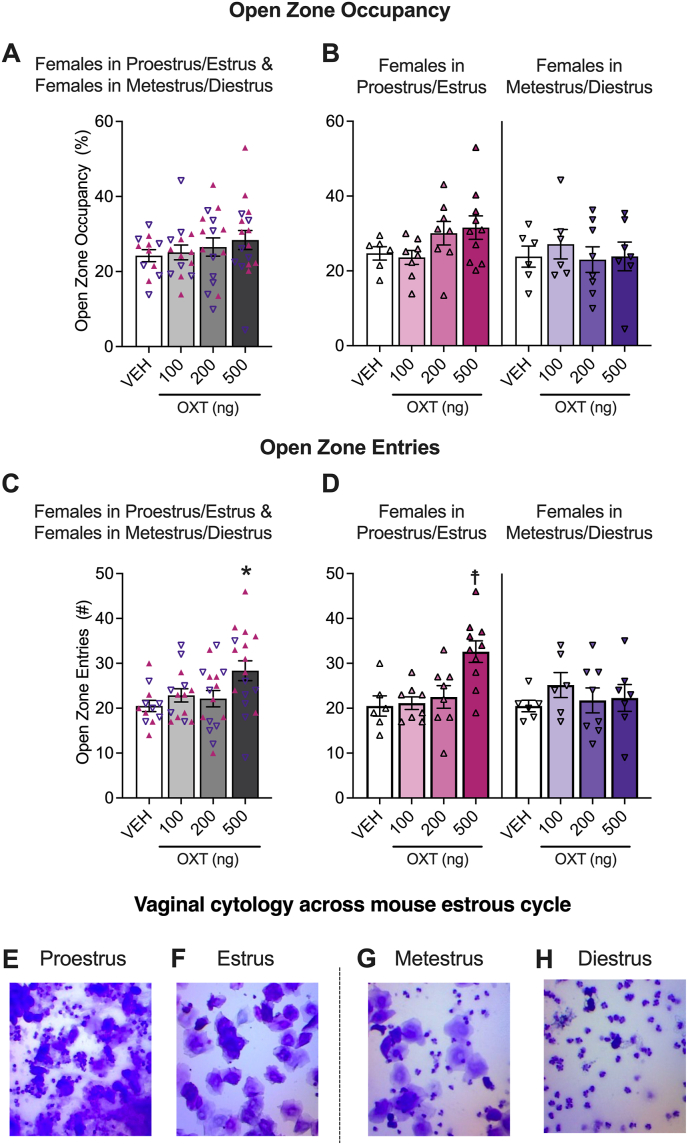
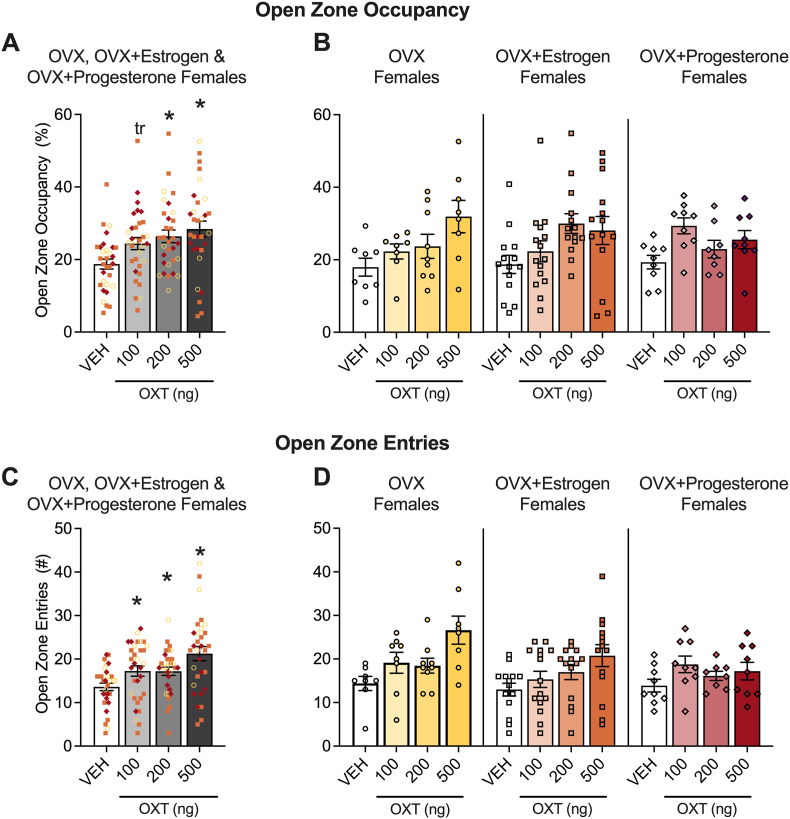
Using the same test of anxiety-like behavior, we then examined open zone occupancy and entries in females in proestrus/estrus and metestrus/diestrus and in ovariectomized females that were treated or not with 17β-estradiol or progesterone. The means for these measures across estrous cycle phases are shown in Fig. 3, and across ovariectomized females supplemented or not with exogenous hormones are shown in Fig. 4. Females tested in these experiments are different mice from females tested in the experiment reported in Fig. 2. Group sample sizes were as follows: proestrus/estrus (n, vehicle = 6, 100 ng oxytocin = 8, 200 ng oxytocin = 8, 500 ng oxytocin = 10), metestrus/diestrus (n, vehicle = 6, 100 ng oxytocin = 6, 200 ng oxytocin = 8, 500 ng oxytocin = 6), ovariectomized females, without hormone supplementation (n, vehicle = 14, 100 ng oxytocin = 15, 200 ng oxytocin = 14, 500 ng oxytocin = 14), with estrogen supplementation (n, vehicle = 8, 100 ng oxytocin = 8, 200 ng oxytocin = 9, 500 ng oxytocin = 8), and with progesterone supplementation (n, vehicle = 9, 100 ng oxytocin = 9, 200 ng oxytocin = 8, 500 ng oxytocin = 9).
Fig. 3.
Oxytocin decreased anxiety-like behavior in females in proestrus/estrus, but not in females in metestrus/diestrus. Oxytocin increased open zone entries (C, D), but not open zone occupancy (A, B) in pooled data. Further analysis demonstrates that oxytocin increased open zone entries in females in proestrus/estrus, but not in females in metestrus/diestrus. The plots show pooled data for females in proestrus/estrus and females in metestrus/diestrus for open zone occupancy (A) and entries (C) with female in proestrus/estrus represented as ▲ and females in metestrus/diestrus represented as ▽. Separate plots for females in proestrus/estrus and females in metestrus/diestrus in open zone occupancy (B) and entries (D) are also shown. Two-way ANOVAs were calculated to analyze females in proestrus/estrus vs. females in metestrus/diestrus data. The Holm-Sidak's multiple comparison test followed the main effect of treatment for open zone entries. *p < 0.05, difference from vehicle (VEH) in pooled plots. Because the estrous cycle phase × treatment interaction approached significance for open zone entries, Holm Sidak's multiple comparison tests were used to further assess the females in proestrus/estrus and females in metestrus/diestrus data separately; ☨p < 0.05, difference from VEH in separate plots. See Section 3.2 (Results) for details. Representative pictures of vaginal cytology across estrous cycle phases are shown in E (proestrus), F (estrus), G (metestrus), and H (diestrus).
Fig. 4.
Oxytocin decreased anxiety-like behavior in ovariectomized (OVX) females in the elevated zero maze. The pooled data demonstrate that oxytocin increased open zone occupancy and open zone entries in the elevated zero maze. The plots show pooled data for non-supplemented, estrogen-supplemented and progesterone-supplemented OVX females for open zone occupancy (A) and entries (C) with non-supplemented ovariectomized females represented as ○, estrogen-supplemented ovariectomized females represented as □, and progesterone-supplemented ovariectomized females represented as ◇. Separate plots for non-supplemented, estrogen-supplemented, and progesterone-supplemented ovariectomized females in open zone occupancy (B) and entries (D) are also shown. Two-way ANOVAs were calculated to analyze the non-supplemented vs. estrogen-supplemented vs. progesterone-supplemented ovariectomized female data. Holm-Sidak's multiple comparison tests were used to follow-up the significant main effect of treatment for open zone occupancy and entries. *p < 0.05, trp = 0.07, difference from vehicle (VEH) in pooled plots. See Section 3.2 (Results) for details.
For proestrus/estrus vs. metestrus/diestrus females, the ANOVA of open zone occupancy showed no main effect of treatment (F3,51 = 0.4347, p = 0.7291), no main effect of estrous cycle phase (F1,51 = 1.837, p = 0.1812) and no estrous cycle phase × treatment interaction effect (F3,51 = 1.437, p = 0.2430). However, the ANOVA of open zone entries demonstrated a main effect of treatment (F3,51 = 3.021, p = 0.0380), but no main effect of estrous cycle phase (F1,51 = 1.010, p = 0.3197). The Holm-Sidak's multiple comparison test performed on the main treatment effect demonstrated that 500 ng (p = 0.0234), but not 100 ng (p = 0.5321) nor 200 ng (p = 0.5321) oxytocin significantly increased open zone entries. The ANOVA also showed an estrous cycle phase × treatment interaction effect (F3,51 = 3.263, p = 0.0288). The Holm-Sidak's multiple comparisons test demonstrated that the 100 ng (proestrus/estrus, p = 0.8617; metestrus/diestrus, p = 0.5381) and 200 ng (proestrus/estrus, p = 0.8217; metestrus/diestrus, p = 0.8626) doses of oxytocin did not increase open zone entries in either cycle phase, but that the 500 ng dose increased open zone entries in females in proestrus/estrus (p = 0.0026), but not females in metestrus/diestrus (p = 0.8626).
For ovariectomized females, the ANOVAs showed a main effect of treatment (occupancy: F3,113 = 5.224, p = 0.0021; entries: F3,113 = 6.945, p = 0.0002) and a trend toward a main effect of hormone supplementation (occupancy: F2,113 = 0.06769, p = 0.9346; entries: F2,113 = 2.755, p = 0.0679) in both elevated zero maze measures. The Holm-Sidak's multiple comparison test performed on the pooled data demonstrated that 200 ng (p = 0.0224) and 500 ng (p = 0.0006) oxytocin significantly increased open zone occupancy and 100 ng doses of oxytocin approached statistical significance (p = 0.0511). All 3 doses of oxytocin (100 ng, p = 0.0416; 200 ng, p = 0.0472; 500 ng, p < 0.0001) increased open zone entries. We followed up the trend toward a main effect of hormone supplementation in the open zone entries measure via a comparison of row means using ovariectomized females as the control in the Holm-Sidak's multiple comparison test. This analysis demonstrated that the difference between non-supplemented and estrogen-supplemented ovariectomized females was trending (p = 0.0630), as was the difference between non-supplemented and progesterone-supplemented ovariectomized females (p = 0.0630). This suggests that overall the hormone supplementation blunted the anxiolytic-like effect.
The ANOVAs demonstrated no hormone supplementation × treatment interaction effect (occupancy: F6,113 = 1.442, p = 0.2048; entries: F6,113 = 1.108, p = 0.3622).
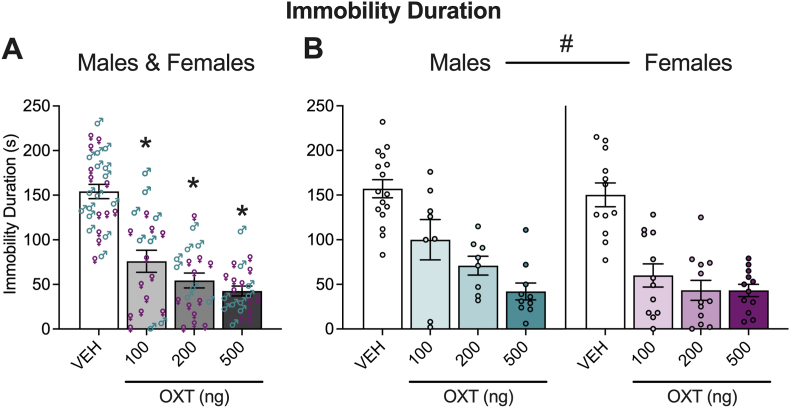
3.3. Male and female mice demonstrate sensitivity to oxytocin in the tail suspension test
Using the tail suspension test, we examined sex differences in immobility duration. Similar to the elevated zero maze, vehicle (saline) or oxytocin (100, 200 or 500 ng) was administered to the lateral ventricles of males (n, vehicle = 16, 100 ng oxytocin = 8, 200 ng oxytocin = 8, 500 ng oxytocin = 10) and females (n, vehicle = 12, 100 ng oxytocin = 12, 200 ng oxytocin = 12, 500 ng oxytocin = 12). The means of immobility durations of males and females in the tail suspension test are shown in Fig. 5. The two-way ANOVA showed main effects of treatment (F3,82 = 36.75, p < 0.0001) and sex (M > F; F1,82 = 4.393, p = 0.0392). The Holm-Sidak's multiple comparison test on the main effect of treatment on the male-female pooled data indicated that administration of 100 ng (p < 0.0001), 200 ng (p < 0.0001) or 500 ng (p < 0.0001) of oxytocin reduced the immobility duration compared to vehicle. The ANOVA did not show a sex × treatment interaction effect (F3,82 = 1.123; p = 0.3446).
Fig. 5.
Oxytocin (OXT) decreased depression-like behavior in the tail suspension test in male and female mice. Both males and females exhibited a significant decrease in immobility duration following treatment with oxytocin (A, B). The plots show male and female pooled data (A) using ♂ to represent males and ♀ to represent females. Separate data for males and females are also shown (B). Two-way ANOVAs were calculated to analyze the male vs. female data; #p < 0.05, main effect of sex. The Holm-Sidak's multiple comparison test followed the significant main effect of treatment for immobility duration. *p < 0.05, difference from vehicle (VEH) in pooled plots. See Section 3.3 (Results) for details.
3.4. Oxytocin sensitivity is influenced by hormonal status in female mice in the tail suspension test
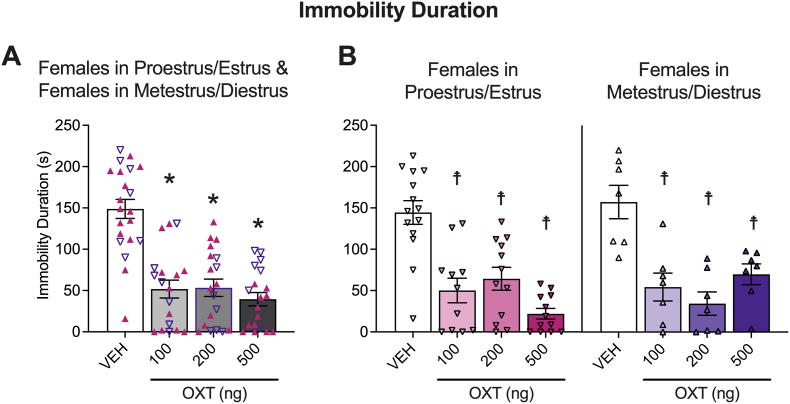
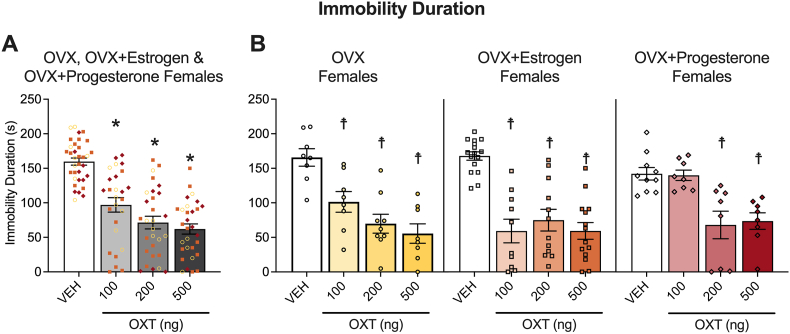
We also examined whether estrous cycle phase in naturally cycling mice or hormone supplementation in ovariectomized mice would affect oxytocin induced antidepression-like behavior. The means of immobility duration for females in proestrus/estrus and metestrus/diestrus are shown in Fig. 6, and for non-supplemented, estrogen-supplemented, and progesterone-supplemented ovariectomized females are shown in Fig. 7. Group sample sizes are as follows: proestrus/estrus (n, vehicle = 14, 100 ng oxytocin = 11, 200 ng oxytocin = 12, 500 ng oxytocin = 12), metestrus/diestrus (n, vehicle = 7, 100 ng oxytocin = 7, 200 ng oxytocin = 7, 500 ng oxytocin = 7), ovariectomized females, with estrogen supplementation (n, vehicle = 16, 100 ng oxytocin = 10, 200 ng oxytocin = 12, 500 ng oxytocin = 14), without hormone supplementation (n, vehicle = 8, 100 ng oxytocin = 8, 200 ng oxytocin = 9, 500 ng oxytocin = 8 estrogen supplementation), and with progesterone supplementation (n, vehicle = 10, 100 ng oxytocin = 8, 200 ng oxytocin = 8, 500 ng oxytocin = 8).
Fig. 6.
Oxytocin (OXT) decreased depression-like behavior in the tail suspension test in female mice in proestrus/estrus and metestrus/diestrus phases of the estrous cycle. Oxytocin reduced immobility duration of females regardless of estrous cycle. The plots show pooled data (A) for females in proestrus/estrus, represented as ▲, and females in metestrus/diestrus, represented as ▽. Separate plots for females in proestrus/estrus and females in metestrus/diestrus are also shown (B). Two-way ANOVAs were calculated to analyze the females in proestrus/estrus vs. females in metestrus/diestrus data. The Holm-Sidak's multiple comparison test followed the main effect of treatment for immobility duration.*p < 0.05, difference from vehicle (VEH) in pooled plots. Because the estrous cycle phase × treatment interaction approached significance for immobility duration, Holm-Sidak's multiple comparison tests were used to further assess the females in proestrus/estrus and females in metestrus/diestrus data separately; ☨p < 0.05, difference from VEH in separate plots. See Section 3.4 (Results) for details.
Fig. 7.
Oxytocin decreased depression-like behavior in the tail suspension test in ovariectomized female mice. Pooled data demonstrate that oxytocin reduce immobility duration for all ovariectomized female groups tested. The plots show pooled data for ovariectomized female mice, supplemented or not (○) with estrogen (□) or progesterone (◇) for immobility duration (A). Separate plots for non-supplemented, estrogen-supplemented, and progesterone-supplemented ovariectomized female mice are also shown (B). Two-way ANOVAs were calculated to analyze the non-supplemented vs. estrogen-supplemented vs. progesterone-supplemented ovariectomized female data. The Holm-Sidak's multiple comparison test followed the significant main effect of treatment for immobility duration. *p < 0.05, difference from vehicle (VEH) in pooled plots. Because the hormone supplementation × treatment interaction approached significance for open zone entries, Holm-Sidak's multiple comparison tests were used to further assess the non-supplemented, estrogen-supplemented, and progesterone-supplemented ovariectomized female data separately; ☨p < 0.05, difference from VEH in separate plots. See Section 3.4 (Results) for details.
The ANOVA demonstrated a main effect of treatment (F3,69 = 24.09, p < 0.0001) but no main effect of estrous cycle phase (F1,69 = 0.6941, p = 0.4076). This was followed by the Holm-Sidak's multiple comparison test on the proestrus/estrus-metestrus/diestrus pooled data. This analysis demonstrated that 100 ng (p < 0.0001), 200 ng (p < 0.0001) and 500 ng (p < 0.0001) doses of oxytocin decreased immobility duration across estrous cycle phase. The ANOVA also demonstrated that the estrous cycle phase × treatment interaction approached significance (F3,69 = 2.285, p = 0.0865). To investigate simple effect across estrous cycle phases, we performed Holm-Sidak's multiple comparison tests on females in proestrus/estrus and females in metestrus/diestrus separately. The post hoc comparisons demonstrated that all 3 doses of oxytocin decreased immobility duration in females in proestrus/estrus (100 ng, p < 0.0001; 200 ng, p < 0.0001; 500 ng, p < 0.0001) and metestrus/diestrus (100 ng, p = 0.0001; 200 ng, p < 0.0001; 500 ng, p = 0.0005).
For ovariectomized females supplemented or not with exogenous hormones, the ANOVA showed a main effect of oxytocin treatment (F3,107 = 33.48, p < 0.0001) and no main effect of hormone supplementation (F2,107 = 1.484, p = 0.2313) for immobility duration. The Holm-Sidak's multiple comparison test followed the main effect of treatment on the ovariectomized-supplementation pooled data demonstrated that all 3 doses of oxytocin (100 ng, p < 0.0001; 200 ng, p < 0.0001; 500 ng, p < 0.0001) decreased immobility duration across all ovariectomized female groups. The ANOVA also demonstrated an oxytocin hormone supplementation × treatment interaction effect (F6, 107 = 3.215, p = 0.0061). The Holm-Sidak's test demonstrated that all three doses of oxytocin decreased immobility duration in non-supplemented (100 ng, p = 0.0023; 200 ng, p < 0.0001; 500 ng, p < 0.0001) and estrogen-supplemented (100 ng, p < 0.0001; 200 ng, p < 0.0001; 500 ng, p < 0.0001) ovariectomized females. In the progesterone supplemented ovariectomized females, 200 ng (p = 0.0007) and 500 ng (p = 0.0013), but not 100 ng (p = 0.9094) of oxytocin decreased immobility duration.
3.5. Sex and hormonal status influence baseline anxiety-like, but not baseline depression-like, behavior in mice
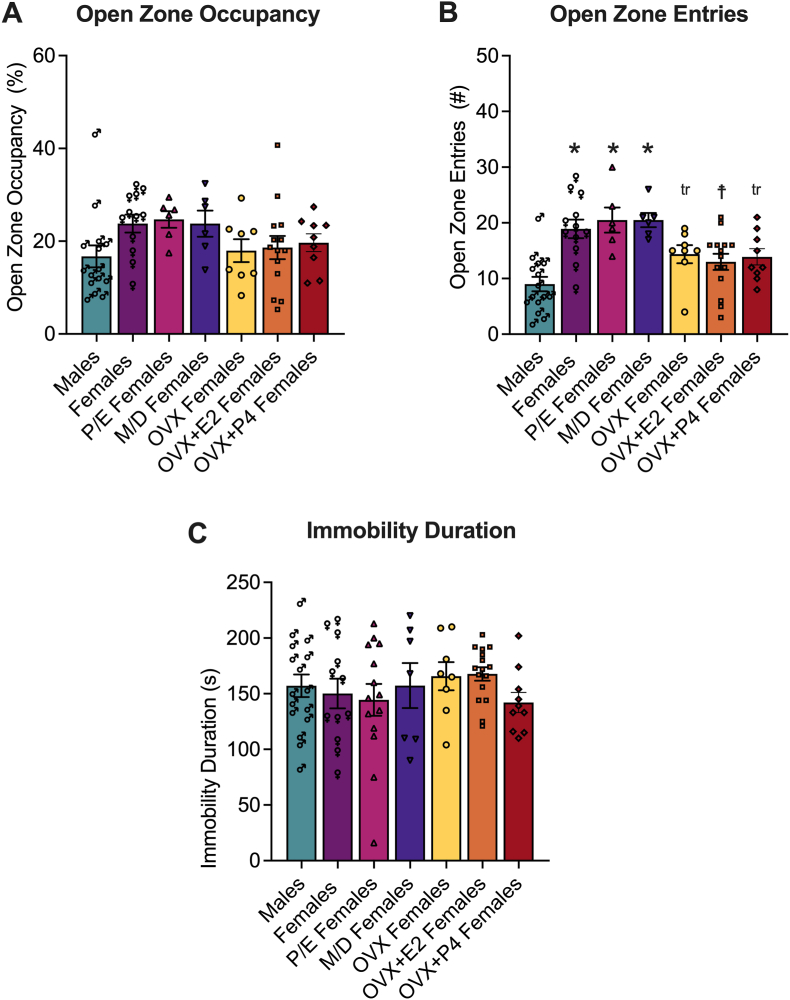
The means of open zone occupancy and entries for all experimental groups that were treated with vehicle are shown in Fig. 8A and B, respectively. The one-way ANOVA did not indicate a significant effect of sex on baseline open zone occupancy (F6,63 = 1.705, p = 0.1345) but indicated a significant effect of sex on open zone entries (F6,63 = 7.564, p < 0.0001). The post hoc comparisons indicated that females that were not tested for estrous cycle phase (p < 0.0001) and females in proestrus/estrus (p < 0.0001) and metestrus/diestrus (p < 0.0001) spent more time in the open zone than males at baseline (i.e., vehicle-treated). The post hoc comparisons also indicated that compared to naturally cycling females that were not tested for estrous cycle phase, ovariectomized females that were supplemented with estrogen (p = 0.0120), ovariectomized females that were not supplemented with hormones (p = 0.0534), or ovariectomized females that were supplemented with progesterone (p = 0.0534) showed decreased and trends for decreased open zone entries.
Fig. 8.
Sex and hormonal status influence baseline anxiety-like behavior in the elevated zero maze, but not baseline depression-like behavior in the tail suspension test. There was a significant effect of sex, estrous cycle, and hormone supplementation on open zone entries in vehicle-treated mice. Naturally cycling female mice demonstrated increased open zone entries compared to male mice. Whereas ovariectomized female mice (supplemented or not with estrogen or progesterone) demonstrated decreased open zone entries compared to naturally cycling female mice and not significant difference compared to male mice. The open zone occupancy measure in the elevated zero maze and the immobility duration measure in the tail suspension test demonstrated no significant effect of sex, estrous cycle or hormone supplementation in vehicle-treated mice. The plots represent baseline open zone occupancy, open zone entries and immobility duration of males (♂), females (♀), females in proestrus/estrus (P/E females; ▲), females in metestrus/diestrus (M/D females; ▽), non-supplemented ovariectomized females (OVX; ○), estrogen supplemented ovariectomized females (OVX + E2; □) and progesterone supplemented ovariectomized females (OVX + P4; ◇). One-way ANOVAs were used to analyze the effect of experimental group on baseline open zone occupancy, entries, and immobility duration. The Holm’Sidak's multiple comparison test followed the significant main effect of experimental group for open zone entries. *p < 0.05, compared with males; ☨p < 0.05, trp = 0.05, compared with females. See Section 3.5 (Results) for details.
The means of immobility duration for all experimental groups that were treated with vehicle are shown in Fig. 8C. The one-way ANOVA did not indicate a significant effect of sex on immobility duration (F6,76 = 0.7057, p = 0.6459).
4. Discussion
Central oxytocin is known for its role in social bonding and social cognition. However, relatively little research has explored the effect of centrally administered oxytocin in non-social measures of anxiety- and depression-like behavior in both sexes, see below. The present study found that exogenous oxytocin significantly decreased anxiety-like behavior in male and female mice, with a suggestion of a larger effect in males, based on follow-up tests, see Supplemental Fig. S1 and the interaction contrast analysis. Further experiments with female mice revealed that the effects of oxytocin were influenced by the estrous cycle phase. Indeed, the effect of oxytocin on anxiolytic-like behavior was significant in females that were in the proestrus/estrus phase but not in females that were in the metestrus/diestrus phase. Ovariectomized females, with and without hormone supplementation, exhibited a decrease in anxiety-like behavior in response to oxytocin.
We also found that exogenous oxytocin reduced depression-like behavior in male and female mice. This effect was significant across all groups. Further analyses demonstrated that this antidepressant-like effect was influenced by the estrous cycle in naturally cycling females and by hormone supplementation in ovariectomized females. Altogether, these data suggest that oxytocin reduces anxiety- and depression-like behavior in a manner that is influenced by sex and hormonal status.
The observation that the magnitude of differences in the elevated zero maze measures following ICV oxytocin administration was greater in males is consistent with previous studies that reported anti-stress effects of oxytocin (Uvnäs-Moberg et al., 1994; Windle et al., 1997; Ring et al., 2006; Yoshida et al., 2009; Ring et al., 2010). For example, Windle et al. found that oxytocin (1–100 ng/h, ICV) inhibited stress-induced increases in corticosterone levels in male rats (Windle et al., 1997) and Ring et al. found that oxytocin (1 μg/mouse, ICV) (Ring et al., 2006) and peripheral administration of the oxytocin receptor agonist, WAY-267464 (Ring et al., 2010), reduced measures of anxiety-like behavior in male mice. Our findings of a blunted response in females are also consistent with previous findings that intranasal (0.8 IU/kg) (Steinman et al., 2016) or ICV (100 ng/rat) (Lukas et al., 2011; Lukas and Neumann, 2014) oxytocin administration reversed stress-induced social withdrawal in male but not female California mice and rats. Additionally, that the effect in open zone entries in females approached significance suggests that higher doses of oxytocin may produce an effect in females that is comparable to males (Dumais et al., 2013). This magnitude difference in sensitivity may be explained by sex differences in number or activity of oxytocin neurons, oxytocin receptor expression and/or hormonal status (Dumais et al., 2013). Previous studies indicate that females exhibit higher levels of oxytocin, increased number of oxytocin neurons and decreased oxytocin receptor expression compared with males in numerous brain regions (Dumais et al., 2013). As such, these differences may account for the greater influence of constitutively acting oxytocin in females, which may explain the decreased baseline anxiety-like behavior of females compared to males demonstrated in Fig. 8A and B. This difference in baseline anxiety-like behavior is well-established and has been recently reviewed (Lovick and Zangrossi, 2021).
The magnitude difference in oxytocin sensitivity may also be explained by interactions between oxytocin and reproductive hormones. In the present study, we observed an estrous cycle phase-dependent response to oxytocin in the anxiety-like measures. This suggests that the effects of exogenous oxytocin are influenced by reproductive hormones. Our literature survey did not detect other studies that examined the effects of oxytocin throughout the estrous cycle, but other studies demonstrated that females in proestrus/estrus were more sensitive to effects of a selective serotonin reuptake inhibitor, citalopram (Sayin et al., 2014). One hypothesis is that these differences depend on regional differences in the effects of oxytocin and/or cyclic changes in endogenous reproductive hormones. For example, one study showed greater activity in the amygdala, a brain region that is important for anxiety-like behavior, in citalopram-treated female rats in metestrus/diestrus compared with female rats in proestrus (Sayin et al., 2014). Higher levels of oxytocin receptor binding were detected in the ventromedial nucleus of the hypothalamus, nucleus accumbens, bed nucleus of the stria terminalis, and medial preoptic area in the females in proestrus/estrus phase compared with females in the metestrus/diestrus phase (Bale et al., 2001; Dumais et al., 2013). Additionally, virgin, pregnant, lactating, and ovariectomized female rats which are known to have different levels of estrogen, progesterone and endogenous oxytocin, and demonstrate distinct responses to oxytocin receptor antagonists and changes in oxytocin neuron quantity and activity (Jirikowski et al., 1989; Neumann et al., 1999).
Another hypothesis is that magnitude differences between females in proestrus/estrus and females in metestrus/diestrus may be explained by cyclic changes in estrogen, progesterone, and their metabolites, such as allopregnanolone (Bailey, 1987; Corpechot et al., 1997), which have been shown to interact with oxytocin and modulate emotion (Richard and Zingg, 1990; Young et al., 1998; Nomura et al., 2002; Bishop, 2013; Nisbett and Pinna, 2018). For example, the metabolism of progesterone to allopregnanolone increases during the proestrus and estrus phases (Bailey, 1987). Although plasma and central progesterone levels were not shown to be different across the estrous cycle, levels of allopregnanolone were (Corpechot et al., 1997). Allopregnanolone may facilitate oxytocin function (Juif et al., 2013), have anti-stress effects (Nisbett and Pinna, 2018), and was recently approved for the treatment of postpartum depression (Meltzer-Brody and Kanes, 2020). Additionally, estrogen may enhance oxytocin receptor expression, facilitate oxytocin receptor binding, potentiate behavioral effects of oxytocin, and play a role in oxytocin synthesis (Nissenson et al., 1978; Richard and Zingg, 1990; Young et al., 1998; Nomura et al., 2002). In contrast, progesterone may inhibit oxytocin-mediated functions and oxytocin receptor signaling (Nissenson et al., 1978; Spitz et al., 2000; Davis et al., 2010; Bishop, 2013). Consistent with these observations, we found that oxytocin reduced anxiety-like behavior in ovariectomized females regardless of hormone supplementation. However, examination of the means demonstrated that ovariectomized females that received estrogen or progesterone supplementation exhibited a response to oxytocin at lower doses than ovariectomized females that did not receive supplementation. Examination of the individual means reveals that open zone occupancy was maximally increased in progesterone-supplemented ovariectomized females at 100 ng (29.9%) and in estrogen-supplemented ovariectomized females at 200 ng (29.9%), compared to non-supplemented ovariectomized females that showed a maximal response at 500 ng (31.9%). The present estrogen supplementation findings are supported by previous studies by demonstrating 1) that estradiol (10 μg/mouse, administered 49 and 24 h prior to testing) enhanced the anxiolytic action of peripherally (3 mg/kg) and centrally (8 μg/mouse) administered oxytocin in ovariectomized female mice in the elevated plus maze (McCarthy et al., 1996); and 2) that ovariectomized females supplemented with estrogen (17β-estradiol:cholesterol, 1:1; silastic capsule implant) exhibited a slight but not statistically significant anxiolytic-like response to intracerebroventricular oxytocin administration (100–1000 ng) in the elevated zero maze (Kent et al., 2013). Further, oxytocin levels and oxytocin receptor binding significantly increased after estrogen treatment in numerous brain regions of naturally cycling and/or ovariectomized female rats and mice (Jirikowski et al., 1988; Patchev et al., 1993; McCarthy et al., 1996; Young et al., 1998; Bale et al., 2001; Champagne et al., 2001; Murakami, 2016). Additionally, estrogen may influence the transcription and translation of oxytocin (Fuchs et al., 1983a, Fuchs et al., 1983b; Fuchs et al., 1983; Caldwell et al., 1989; Jirikowski et al., 2017), cellular dynamics in oxytocinergic neurons via non-transcriptional mechanisms, (Owman et al., 1996; Hazell et al., 2009; Jirikowski et al., 2017) or oxytocin receptor internalization (Conti et al., 2009).
Notably, in the present study, baseline open zone entries in the ovariectomized female groups were lower than in cycling females and were comparable to males. We acknowledge that the greater sensitivity to oxytocin may result from this increase in baseline anxiety-like behavior. This further supports the possibility that the naturally fluctuating gonadal hormones mediates baseline anxiety levels in female mice through their constitutive actions (Österlund et al., 2005). As such, the removal of endogenous reproductive hormones and/or the disruption of their natural cycle via ovariectomy may reduce the constitutive action of their respective receptors resulting in lower baseline anxiety-like behavior, in contrast to cycling females. Thus, a deficit of this constitutive activity in males may explain their increased sensitivity to oxytocin despite their lower estrogen levels.
The finding that oxytocin produced an antidepressant-like effect in the tail suspension test across all experimental groups with modest differences in sex is consistent with the literature (Arletti and Bertolini, 1987; Nowakowska et al., 2002; Ring et al., 2010). Unlike outcomes in the elevated zero maze, females in metestrus/diestrus exhibited an antidepressant-like effect of oxytocin. Also, in the present study, no difference in immobility duration was found between the vehicle-treated groups (Fig. 8C), contrary to previous studies using the forced swim test (Frye and Wawrzycki, 2003, Kokras et al., 2012). Additionally, in the present study, males and ovariectomized females exhibited similar response patterns to oxytocin, such that the efficacy of oxytocin increased similarly with each dose in both groups. Although we could not find studies investigating the effect of oxytocin on depression-like behaviors in females, these results are consistent with previous studies in male rodents. Centrally- (300 ng/mouse) and peripherally- (30 mg/kg) administered oxytocin previously reduced immobility duration in the tail suspension test in male mice (Ring et al., 2006), and acute (1 μg/kg, 0.25–1 mg/kg) and chronic (0.5 mg/kg; 10-days) peripheral oxytocin administration reduced immobility in the forced swim test and reduced escape latency in the learned helplessness test in male rats (Arletti and Bertolini, 1987; Nowakowska et al., 2002). Notably, all three doses of oxytocin showed opposing effects in the elevated zero maze and the tail suspension test in progesterone-supplemented ovariectomized females. This finding is striking and further highlights potential differences in the mechanisms underlying anxiety and depression-like behavior as well as the importance and complexity of hormone interaction with oxytocin. That the antidepressant-like effect of oxytocin was largely unaffected by estrous cycle phase and hormone supplementation compared to results from the elevated zero maze, also supports the hypothesis that behavioral measures captured by the elevated zero maze and the tail suspension test are mediated by different mechanisms and different neural networks.
We also acknowledge several limitations of the study. First, considering that progesterone and estrogen are released in varying ratios and levels across the reproductive cycle in a cyclical manner (Marcondes et al., 2001), we acknowledge that the hormone-supplemented experiments do not exactly mimic hormonal fluctuations. Second, the anorexigenic effects of oxytocin limits the examination of anti-anhedonia effects of oxytocin using the sucrose preference or splash tests of depression-like behavior. Third, the elevated zero maze measures are inextricably linked with locomotor activity, as such, the observed anxiolytic-like effects of oxytocin may be influenced by changes in locomotion (Miller et al., 2021). Note that the antidepressant-like effect of oxytocin could not be completely blocked by the centrally administered oxytocin receptor antagonist, WAY-162720, in a previous study (Ring et al., 2010), further suggesting that the role of oxytocin in depression involves other interacting systems, like vasopressin (Morales-Medina et al., 2016). As antagonists of the respective receptors were not utilized in this study, we cannot rule out the role of the vasopressin systems in the sex and hormonal status differences we observed. Additionally, given that relatively high doses of oxytocin were used in the current study, the potential for the vasopressin system (via vasopressin 1a and 1b receptors) to be engaged in the anxiolytic- and antidepressant-like effect of oxytocin cannot be ruled out. However, vasopressin receptor agonists have demonstrated anxiogenic-like effects (Mak et al., 2012), and vasopressin 1b receptor antagonists have demonstrated anxiolytic-like effects (Griebel et al., 2002; Gal et al., 2005; Griebel et al., 2005; Griebel and Holmes, 2013).
Numerous studies have investigated how oxytocin could be utilized as a therapeutic (CJ Liu et al., 2012; De Cagna et al., 2019). Administered intranasally, oxytocin improved anxiety symptoms (Kou et al., 2021; Voncken et al., 2021), self-perception (Cardoso et al., 2012), and attentional bias (Domes et al., 2016) in men and women, and postnatal depression in mothers (Baron-Cohen et al., 2022). Oxytocin has been successfully used as an adjunct to pharmacotherapeutics in treating major depressive disorder and treatment-resistant major depressive disorder (Scantamburlo et al., 2011; Scantamburlo et al., 2015) and as an aid to psychotherapy in the treatment of anxiety disorders (Guastella et al., 2009). However, some studies have reported negative (MacDonald et al., 2013; Mah et al., 2013) or no (Clarici et al., 2015; Baron-Cohen et al., 2022) effect of oxytocin. Given the inverse relationship between sociality and depression and anxiety, the systems that mediate social interaction and bonding may be dysregulated in individuals who are diagnosed with these disorders (Janeček and Dabrowska, 2019; Matsushita et al., 2019; Yoon and Kim, 2020). Our study highlights the importance of examining the effects of FDA-approved drugs, and new investigational drugs, across the various hormonal levels such as natural hormonal cycle, hormone replacement and hormone blockers in preclinical and clinical studies.
In conclusion, our study supports the larger literature, which suggests that responses to oxytocin are influenced by sex and hormonal status (Jirikowski et al., 2017). We found highly significant effects of oxytocin in producing anxiolytic-like effects in the elevated zero maze and antidepressant-like effects in the tail suspension test in mice. We also found that sex, estrous cycle, and hormone levels influenced the effects of oxytocin in C57Bl/6J mice in animal models of anxiety and depression. We demonstrated a key differential effect of oxytocin in cycling females and across the estrous cycle in an anxiety-like test. The present study highlights the importance of assessing the effects of potential psychopharmacotherapies across both sexes and as a function of reproductive cycles and hormonal status to better understand whether and how FDA-approved drugs should be prescribed for optimal clinical efficacy.
CRediT Author Statement
Khalin E. Nisbett: Conceptualization, Methodology, Formal Analysis, Investigation, Writing (Original draft, review, editing), Visualization, Funding Acquisition; Marina Teruel: Investigation, Writing (review); Luis A. Gonzalez: Investigation, Writing (review). C. Sue Carter: Methodology, Writing (review, editing); Leandro F. Vendruscolo: Conceptualization, Methodology, Writing (review, editing), Visualization; Michael E. Ragozzino: Conceptualization, Methodology, Validation, Resources, Writing (review, editing), Funding Acquisition; George F. Koob: Conceptualization, Methodology, Resources, Writing (review, editing), Funding Acquisition.
Funding
This work was supported by the University of Illinois Chicago Graduate College Pathway to an Inclusive Faculty Fellowship (KEN), the University of Illinois Chicago Provost Graduate Research Award (KEN), the National Institutes of Health grant HD084289 (MER), the National Institute on Drug Abuse Intramural Research Program (GFK, LFV, KEN), and the National Institutes on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research (LFV).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Amie Severino of the National Institute on Drug Abuse Histology and Imaging Core and Eleanor B. Towers of the University of Virginia Psychiatry and Neurobehavioral Sciences Department for technical assistance, Marc Raley of National Institute on Drug Abuse Visual Media for graphic design assistance, and Michael Arends for proofreading the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2023.100567.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Anxiety and Depression Association of America Facts & Statistics. 2018. https://adaa.org/about-adaa/press-room/facts-statistics Retrieved December 6, 2022, from.
- Arletti R., Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41(14):1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- Bailey K. Diurnal progesterone rhythms in the female mouse. J. Endocrinol. 1987;112(1):15–21. doi: 10.1677/joe.0.1120015. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Davis A.M., Auger A.P., Dorsa D.M., McCarthy M.M. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J. Neurosci. 2001;21(7):2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen K.L., Feldman R., Fearon P., Fonagy P. Intranasal oxytocin administration improves mood in new mothers with moderate low mood but not in mothers with elevated symptoms of postnatal depression: a randomised controlled trial. J. Affect. Disord. 2022;300:358–365. doi: 10.1016/j.jad.2021.11.062. [DOI] [PubMed] [Google Scholar]
- Bishop C.V. Progesterone inhibition of oxytocin signaling in endometrium. Front. Neurosci. 2013;7:138. doi: 10.3389/fnins.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J., Brooks P., Jirikowski G., Barakat A., Lund P., Pedersen C. Estrogen alters oxytocin mRNA levels in the preoptic area. J. Neuroendocrinol. 1989;1(4):273–278. doi: 10.1111/j.1365-2826.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Cardoso C., Ellenbogen M.A., Linnen A.-M. Acute intranasal oxytocin improves positive self-perceptions of personality. Psychopharmacology. 2012;220(4):741–749. doi: 10.1007/s00213-011-2527-6. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Kenkel W.M., MacLean E.L., Wilson S.R., Perkeybile A.M., Yee J.R., Ferris C.F., Nazarloo H.P., Porges S.W., Davis J.M. Is oxytocin “nature's medicine”. Pharmacol. Rev. 2020;72(4):829–861. doi: 10.1124/pr.120.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F., Diorio J., Sharma S., Meaney M.J. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. USA. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CJ Liu J., McErlean R.A., R Dadds M. Are we there yet? The clinical potential of intranasal oxytocin in psychiatry. Curr. Psychiatr. Rev. 2012;8(1):37–48. [Google Scholar]
- Clarici A., Pellizzoni S., Guaschino S., Alberico S., Bembich S., Giuliani R., Short A., Guarino G., Panksepp J. Intranasal adminsitration of oxytocin in postnatal depression: implications for psychodynamic psychotherapy from a randomized double-blind pilot study. Front. Psychol. 2015;6:426. doi: 10.3389/fpsyg.2015.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Sertic S., Reversi A., Chini B. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 “short cycle”. Am. J. Physiol. Endocrinol. Metabol. 2009;296(3):E532–E542. doi: 10.1152/ajpendo.90590.2008. [DOI] [PubMed] [Google Scholar]
- Corpechot C., Collins B., Carey M., Tsouros A., Robel P., Fry J. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766(1–2):276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Davis T.L., Bott R.C., Slough T.L., Bruemmer J.E., Niswender G.D. Progesterone inhibits oxytocin-and prostaglandin F2alpha-stimulated increases in intracellular calcium concentrations in small and large ovine luteal cells. Biol. Reprod. 2010;82(2):282–288. doi: 10.1095/biolreprod.109.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cagna F., Fusar-Poli L., Damiani S., Rocchetti M., Giovanna G., Mori A., Politi P., Brondino N. The role of intranasal oxytocin in anxiety and depressive disorders: a systematic review of randomized controlled trials. Clin. Psychopharmacol. Neurosci. 2019;17(1):1. doi: 10.9758/cpn.2019.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do D., Schnittker J. Pharmaceutical side effects and the sex differences in depression and distress. Am. J. Prev. Med. 2022;63(2):213–224. doi: 10.1016/j.amepre.2022.01.036. [DOI] [PubMed] [Google Scholar]
- Domes G., Normann C., Heinrichs M. The effect of oxytocin on attention to angry and happy faces in chronic depression. BMC Psychiatr. 2016;16(1):1–8. doi: 10.1186/s12888-016-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais K.M., Bredewold R., Mayer T.E., Veenema A.H. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region-and sex-specific ways. Horm. Behav. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Duque-Wilckens N., Steinman M.Q., Busnelli M., Chini B., Yokoyama S., Pham M., Laredo S.A., Hao R., Perkeybile A.M., Minie V.A. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol. Psychiatr. 2018;83(3):203–213. doi: 10.1016/j.biopsych.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N., Torres L.Y., Yokoyama S., Minie V.A., Tran A.M., Petkova S.P., Hao R., Ramos-Maciel S., Rios R.A., Jackson K. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc. Natl. Acad. Sci. USA. 2020;117(42):26406–26413. doi: 10.1073/pnas.2011890117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke R.C., Young L.J. Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci. 2021;44:359–381. doi: 10.1146/annurev-neuro-102320-102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C.A., Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm. Behav. 2003;44(4):319–326. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Fuchs A.-R., Periyasamy S., Alexandrova M., Soloff M.S. Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: effects of ovarian steroids. Endocrinology. 1983;113(2):742–749. doi: 10.1210/endo-113-2-742. [DOI] [PubMed] [Google Scholar]
- Fuchs A.-R., Periyasamy S., Soloff M. Systemic and local regulation of oxytocin receptors in the rat uterus, and their functional significance. Can. J. Biochem. Cell Biol. 1983;61(7):615–624. doi: 10.1139/o83-077. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Howard A.L., Dadds M.R., Mitchell P., Carson D.S. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34(6):917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Hazell G.G., Yao S.T., Roper J.A., Prossnitz E.R., O'Carroll A.-M., Lolait S.J. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol. 2009;202(2):223. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philos. Ethics Humanit. Med. 2008;3(1):1–9. doi: 10.1186/1747-5341-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen J.C., Gluud C., Kirsch I. Should antidepressants be used for major depressive disorder? BMJ Evid.-Based Med. 2020;25(4) doi: 10.1136/bmjebm-2019-111238. 130-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeček M., Dabrowska J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies—potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST) Cell Tissue Res. 2019;375(1):143–172. doi: 10.1007/s00441-018-2889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirikowski G., Caldwell J., Pedersen C., Stumpf W. Estradiol influences oxytocin-immunoreactive brain systems. Neuroscience. 1988;25(1):237–248. doi: 10.1016/0306-4522(88)90022-x. [DOI] [PubMed] [Google Scholar]
- Jirikowski G., Caldwell J., Pilgrim C., Stumpf W., Pedersen C. Changes in immunostaining for oxytocin in the forebrain of the female rat during late pregnancy, parturition and early lactation. Cell Tissue Res. 1989;256(2):411–417. doi: 10.1007/BF00218899. [DOI] [PubMed] [Google Scholar]
- Jirikowski G.F., Ochs S.D., Caldwell J.D. Oxytocin and steroid actions. Behav. Pharmacol. Neuropept.: Oxytocin. 2017:77–95. doi: 10.1007/7854_2017_9. [DOI] [PubMed] [Google Scholar]
- Juif P.-E., Breton J.-D., Rajalu M., Charlet A., Goumon Y., Poisbeau P. Long-lasting spinal oxytocin analgesia is ensured by the stimulation of allopregnanolone synthesis which potentiates GABAA receptor-mediated synaptic inhibition. J. Neurosci. 2013;33(42):16617–16626. doi: 10.1523/JNEUROSCI.3084-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B., Neumann I.D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 2018;98(3):1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Charney D. Comorbidity of mood and anxiety disorders. Depress. Anxiety. 2000;12(S1):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kent K., Arientyl V., Khachatryan M.M., Wood R.I. Oxytocin induces a conditioned social preference in female mice. J. Neuroendocrinol. 2013;25(9):803–810. doi: 10.1111/jne.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch H.S., Charlet A., Hoffmann L.C., Eliava M., Khrulev S., Cetin A.H., Osten P., Schwarz M.K., Seeburg P.H., Stoop R. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kokras N., Dalla C., Sideris A.C., Dendi A., Mikail H.G., Antoniou K., Papadopoulou-Daifoti Z. Behavioral sexual dimorphism in models of anxiety and depression due to changes in HPA axis activity. Neuropharmacology. 2012;62(1):436–445. doi: 10.1016/j.neuropharm.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Kou J., Zhang Y., Zhou F., Gao Z., Yao S., Zhao W., Li H., Lei Y., Gao S., Kendrick K.M. Anxiolytic effects of chronic intranasal oxytocin on neural responses to threat are dose-frequency dependent. Psychother. Psychosom. 2021:1–12. doi: 10.1159/000521348. [DOI] [PubMed] [Google Scholar]
- Lovick T.A., Zangrossi H., Jr. Effect of estrous cycle on behavior of females in rodent tests of anxiety. Front. Psychiatr. 2021;1492 doi: 10.3389/fpsyt.2021.711065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M., Neumann I.D. Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvement of brain oxytocin and vasopressin. J. Neurosci. Methods. 2014;234:101–107. doi: 10.1016/j.jneumeth.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Lukas M., Toth I., Reber S.O., Slattery D.A., Veenema A.H., Neumann I.D. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K., MacDonald T.M., Brüne M., Lamb K., Wilson M.P., Golshan S., Feifel D. Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology. 2013;38(12):2831–2843. doi: 10.1016/j.psyneuen.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Mah B.L., Van IJzendoorn M.H., Smith R., Bakermans-Kranenburg M.J. Oxytocin in postnatally depressed mothers: its influence on mood and expressed emotion. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2013;40:267–272. doi: 10.1016/j.pnpbp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Mak P., Broussard C., Vacy K., Broadbear J.H. Modulation of anxiety behavior in the elevated plus maze using peptidic oxytocin and vasopressin receptor ligands in the rat. J. Psychopharmacol. 2012;26(4):532–542. doi: 10.1177/0269881111416687. [DOI] [PubMed] [Google Scholar]
- Mantella R.C., Vollmer R.R., Li X., Amico J.A. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144(6):2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- Marcondes F.K., Miguel K.J., Melo L.L., Spadari-Bratfisch R.C. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol. Behav. 2001;74(4–5):435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Matsushita H., Latt H.M., Koga Y., Nishiki T., Matsui H. Oxytocin and stress: neural mechanisms, stress-related disorders, and therapeutic approaches. Neuroscience. 2019;417:1–10. doi: 10.1016/j.neuroscience.2019.07.046. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M., McDonald C.H., Brooks P.J., Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 1996;60(5):1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McLean A.C., Valenzuela N., Fai S., Bennett S.A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. 2012;67 doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S., Kanes S.J. Allopregnanolone in postpartum depression: role in pathophysiology and treatment. Neurobiol. Stress. 2020;12 doi: 10.1016/j.ynstr.2020.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.K., Halbing A.A., Patisaul H.B., Meitzen J. Interactions of the estrous cycle, novelty, and light on female and male rat open field locomotor and anxiety-related behaviors. Physiol. Behav. 2021;228 doi: 10.1016/j.physbeh.2020.113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Medina J.C., Witchey S.K., Caldwell H.K. Melatonin, Neuroprotective Agents and Antidepressant Therapy. Springer; 2016. The role of vasopressin in anxiety and depression; pp. 667–685. [Google Scholar]
- Murakami G. Distinct effects of estrogen on mouse maternal behavior: the contribution of estrogen synthesis in the brain. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I.D., Torner L., Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 1999;95(2):567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Nisbett K.E., Pinna G. Emerging therapeutic role of PPAR–α in cognition and emotions. Front. Pharmacol. 2018;9:998. doi: 10.3389/fphar.2018.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissenson R., Flouret G., Hechter O. Opposing effects of estradiol and progesterone on oxytocin receptors in rabbit uterus. Proc. Natl. Acad. Sci. USA. 1978;75(4):2044–2048. doi: 10.1073/pnas.75.4.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., McKenna E., Korach K.S., Pfaff D.W., Ogawa S. Estrogen receptor-β regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Mol. Brain Res. 2002;109(1–2):84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- Nowakowska E., Kus K., Bobkiewicz-Kozlowska T., Hertmanowska H. Role of neuropeptides in antidepressant and memory improving effects of venlafaxine. Pol. J. Pharmacol. 2002;54(6):605–614. [PubMed] [Google Scholar]
- Österlund M.K., Witt M.-R., Gustafsson J.-Å. Estrogen action in mood and neurodegenerative disorders. Endocrine. 2005;28(3):235–241. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- Owman C., Blay P., Nilsson C., Lolait S.J. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt's lymphoma and widely distributed in brain and peripheral tissues. Biochem. Biophys. Res. Commun. 1996;228(2):285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- Patchev V., Schlosser S., Hassan A., Almeida O. Oxytocin binding sites in rat limbic and hypothalamic structures: site-specific modulation by adrenal and gonadal steroids. Neuroscience. 1993;57(3):537–543. doi: 10.1016/0306-4522(93)90003-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B. Academic press; 2019. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Pigott H.E., Leventhal A.M., Alter G.S., Boren J.J. Efficacy and effectiveness of antidepressants: current status of research. Psychother. Psychosom. 2010;79(5):267–279. doi: 10.1159/000318293. [DOI] [PubMed] [Google Scholar]
- Richard S.p., Zingg H. The human oxytocin gene promoter is regulated by estrogens. J. Biol. Chem. 1990;265(11):6098–6103. [PubMed] [Google Scholar]
- Ring R.H., Malberg J.E., Potestio L., Ping J., Boikess S., Luo B., Schechter L.E., Rizzo S., Rahman Z., Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185(2):218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Ring R.H., Schechter L.E., Leonard S.K., Dwyer J.M., Platt B.J., Graf R., Grauer S., Pulicicchio C., Resnick L., Rahman Z. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58(1):69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Sayin A., Derinöz O., Yüksel N., Şahin S., Bolay H. The effects of the estrus cycle and citalopram on anxiety-like behaviors and c-fos expression in rats. Pharmacol. Biochem. Behav. 2014;124:180–187. doi: 10.1016/j.pbb.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G., Ansseau M., Geenen V., Legros J.-J. Intranasal oxytocin as an adjunct to escitalopram in major depression. J. Neuropsychiatry Clin. Neurosci. 2011;23(2) doi: 10.1176/jnp.23.2.jnpe5. E5–E5. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G., Hansenne M., Geenen V., Legros J.-J., Ansseau M. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: an open trial. Eur. Psychiatr. 2015;30(1):65–68. doi: 10.1016/j.eurpsy.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Shepherd J.K., Grewal S.S., Fletcher A., Bill D.J., Dourish C.T. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116(1):56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Sovijit W.N., Sovijit W.E., Pu S., Usuda K., Inoue R., Watanabe G., Yamaguchi H., Nagaoka K. Ovarian progesterone suppresses depression and anxiety-like behaviors by increasing the Lactobacillus population of gut microbiota in ovariectomized mice. Neurosci. Res. 2021;168:76–82. doi: 10.1016/j.neures.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Spitz I.M., Van Look P.F., Bennink H.J.C. The use of progesterone antagonists and progesterone receptor modulators in contraception. Steroids. 2000;65(10–11):817–823. doi: 10.1016/s0039-128x(00)00199-9. [DOI] [PubMed] [Google Scholar]
- Sramek J.J., Murphy M.F., Cutler N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2022 doi: 10.31887/DCNS.2016.18.4/ncutler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman M.Q., Duque-Wilckens N., Greenberg G.D., Hao R., Campi K.L., Laredo S.A., Laman-Maharg A., Manning C.E., Doig I.E., Lopez E.M. Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol. Psychiatr. 2016;80(5):406–414. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Ström J.O., Theodorsson A., Ingberg E., Isaksson I.-M., Theodorsson E. Ovariectomy and 17β-estradiol replacement in rats and mice: a visual demonstration. JoVE. 2012;(64) doi: 10.3791/4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M., Van Peer J.M., Korf J., Wijers A.A., Tucker D.M. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44(3):444–449. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Trainor B.C., Pride M.C., Landeros R.V., Knoblauch N.W., Takahashi E.Y., Silva A.L., Crean K.K. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K., Ahlenius S., Hillegaart V., Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol. Biochem. Behav. 1994;49(1):101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Voncken M.J., Dijk C., Stöhr F., Niesten I.J., Schruers K., Kuypers K.P. The effect of intranasally administered oxytocin on observed social behavior in social anxiety disorder. Eur. Neuropsychopharmacol. 2021;53:25–33. doi: 10.1016/j.euroneuro.2021.07.005. [DOI] [PubMed] [Google Scholar]
- Williams A.V., Duque-Wilckens N., Ramos-Maciel S., Campi K.L., Bhela S.K., Xu C.K., Jackson K., Chini B., Pesavento P.A., Trainor B.C. Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology. 2020;45(9):1423–1430. doi: 10.1038/s41386-020-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle R.J., Shanks N., Lightman S.L., Ingram C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2017. Depression and Other Common Mental Disorders: Global Health Estimates. [Google Scholar]
- Yoon S., Kim Y.-K. The role of the oxytocin system in anxiety disorders. Adv. Exp. Med. Biol. 2020;1191:103–120. doi: 10.1007/978-981-32-9705-0_7. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takayanagi Y., Inoue K., Kimura T., Young L.J., Onaka T., Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.J., Wang Z., Donaldson R., Rissman E.F. Estrogen receptor α is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998;9(5):933–936. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.