This cohort study examines the mortality, morbidity, and other outcomes associated with reproductive characteristics and lifestyle factors among female nurses who participated in the Nurses’ Health Study II.
Key Points
Question
Is gestational diabetes associated with a greater long-term risk of mortality?
Findings
In this cohort study of 91 426 participants in the Nurses’ Health Study II, a history of gestational diabetes was associated with an increased risk of mortality, particularly cardiovascular disease mortality, even in the absence of subsequent type 2 diabetes. A higher risk of mortality was observed among participants who adopted less healthy lifestyles, experienced gestational diabetes in 2 or more pregnancies, had gestational diabetes in initial and subsequent pregnancies, and concurrently reported chronic diseases and other adverse pregnancy or birth outcomes.
Meaning
Findings of this study suggest that participants with gestational diabetes may have an elevated risk of total and cardiovascular disease mortality.
Abstract
Importance
Gestational diabetes has been associated with numerous chronic diseases. However, few studies have examined the association of gestational diabetes with long-term mortality risk.
Objective
To investigate the associations between gestational diabetes and long-term risks of total and cause-specific mortality.
Design, Setting, and Participants
This cohort study analyzed participants of the Nurses’ Health Study II who were followed for 30 years (1989-2019). Participants included US female nurses aged 25 to 42 years who reported at least 1 pregnancy (≥6 months) at 18 years or older across their reproductive life span. Data were analyzed from May 1, 2022, to May 25, 2023.
Exposure
Gestational diabetes across the reproductive life span.
Main Outcomes and Measures
Hazard ratios (HRs with 95% CIs) for total and cause-specific mortality were estimated by Cox proportional hazards regression models.
Results
A total of 91 426 parous participants were included, with a mean (SD) age of 34.9 (4.7) years and a body mass index of 24.1 (4.7) at baseline. During a follow-up period of 2 609 753 person-years, 3937 deaths were documented, including 255 deaths from cardiovascular disease and 1397 from cancer. Participants with a history of gestational diabetes had a higher crude mortality rate than those without a history of gestational diabetes (1.74 vs 1.49 per 1000 person-years; absolute difference = 0.25 per 1000 person-years). The corresponding HR for total mortality was 1.28 (95% CI, 1.13-1.44), which did not materially change after additional adjustment for potential confounders and lifestyle factors during the reproductive life span (HR, 1.25; 95% CI, 1.11-1.41). The association persisted regardless of the subsequent development of type 2 diabetes and was more robust among participants who adopted less healthy lifestyles; experienced gestational diabetes in 2 or more pregnancies (HR, 1.48; 95% CI, 0.99-2.19); had gestational diabetes both in the initial and subsequent pregnancies (HR, 1.71; 95% CI, 1.11-2.63); and concurrently reported hypertensive disorders in pregnancy (HR, 1.80; 95% CI, 1.21-2.67), preterm birth (HR, 2.46; 95% CI, 1.66-3.64), or low birth weight (HR, 2.11; 95% CI, 1.21-3.68). Cause-specific mortality analyses revealed that gestational diabetes was directly associated with the risk of mortality due to cardiovascular disease (HR, 1.59; 95% CI, 1.03-2.47). Additionally, gestational diabetes was inversely associated with cancer mortality (HR, 0.76; 95% CI, 0.59-0.98); however, it was only evident among participants who later developed type 2 diabetes.
Conclusions and Relevance
Results of this cohort study suggest that participants who reported a history of gestational diabetes exhibited a small but elevated risk of subsequent mortality over 30 years. The findings emphasize the importance of considering gestational diabetes as a critical factor in later-life mortality risk.
Introduction
Gestational diabetes is increasingly prevalent and estimated to affect 14.2% of pregnant individuals on a global standardized scale.1 In the US, the age-standardized rate of gestational diabetes has increased from 47.6 in 2011 to 63.5 in 2019 per 1000 live births across all racial and ethnic groups.2 Growing evidence has associated gestational diabetes with type 2 diabetes (T2D),3 cancer,4 chronic hypertension,5 and cardiovascular disease (CVD).6 Nevertheless, the association of gestational diabetes with long-term risks of total and cause-specific mortality, a key focus in public health, has been rarely evaluated in prospective studies.7,8 Although individuals with a history of gestational diabetes have an almost 10-fold increased risk of developing T2D,3 it remains uncertain whether the association of gestational diabetes with mortality is primarily influenced by the subsequent development of T2D and its associated comorbidities and complications. It is also uncertain whether different patterns of gestational diabetes, such as recurrence across multiple pregnancies and co-occurrence with other adverse pregnancy or perinatal outcomes, have a similar association with mortality risk. Therefore, we investigated the associations of gestational diabetes with the long-term risks of total and cause-specific mortality among participants in the Nurses’ Health Study (NHS) II. The NHS II has followed female nurses of reproductive age for more than 3 decades, collecting periodic updates of reproductive characteristics and lifestyle factors.
Methods
Study Population
The NHS II is an ongoing prospective cohort initiated in 1989 by enrolling 116 429 US female nurses aged 25 to 42 years,9 with a follow-up rate of more than 90% for each biennial cycle. The Mass General Brigham and Harvard T.H. Chan School of Public Health Institutional Review Boards approved the study procedures. Participants provided written informed consent via their returned completed questionnaires. The present cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The present analysis included NHS II participants who either reported at enrollment a pregnancy of at least 6 months at 18 years or older or became pregnant during follow-up through 2009 when most participants had completed their reproductive years (Figure 1). We excluded participants who reported a history of CVD, diabetes, or cancer before their first pregnancy and those who reported a diagnosis of T2D before a diagnosis of gestational diabetes (Figure 1).
Figure 1. Cohort Design, Data Collection, and Exclusion Criteria for the Nurses’ Health Study II.
CVD indicates cardiovascular disease; RN, registered nurse; and T2D, type 2 diabetes.
Gestational Diabetes and Mortality Ascertainment
Participants’ history of physician-diagnosed gestational diabetes was initially ascertained in 1989 and was updated biennially until 2001.10 In 2009, participants retrospectively reported all of their previous pregnancies and associated adverse maternal and fetal pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy (HDP), preterm birth, and low birth weight. Among a random subgroup of 114 participants, a previous validation study found that 94% of self-reported gestational diabetes events were confirmed by medical records.11 A high degree of gestational diabetes surveillance has also been assessed in a different subgroup of 100 parous participants without gestational diabetes.12
Deaths were systematically ascertained from a search of state vital statistics records and the National Death Index or report from family members or the US postal system by matching first and last names, Social Security Number, birth date, and state of residence.13,14 This process was able to ascertain more than 98% of the deaths.15 Physicians who were unaware of participants’ history of gestational diabetes reviewed death certificates and, if required, medical records (obtained through contacting hospitals with permission from participant’s next of kin) or autopsy reports9 to classify the causes of death using codes from the eighth and ninth revisions of the International Classification of Diseases. We classified participants into 19 major death categories (eTable 1 in Supplement 1).16,17
Covariates
Participants’ demographic characteristics were self-reported at recruitment, including race and ethnicity (categorized as American Indian or Alaska Native, Asian, Black or African American, Hispanic, Native Hawaiian or Other Pacific Islander, White, and other). Race and ethnicity data were collected and included in analysis because of the differences in pregnancy complications and the burden of chronic diseases across race and ethnicity subgroups.
Lifestyles; health-related factors; diabetes drugs (eg, insulin, metformin, empagliflozin [Jardiance; Boehringer Ingelheim], canagliflozin [Invokana; Janssen], and sitagliptin); and physician-diagnosed depression, chronic obstructive pulmonary disease, chronic hypertension, and hypercholesterolemia were ascertained biennially during follow-up. Type 2 diabetes was self-reported biennially and ascertained through validated supplemental questionnaires.18 Cardiovascular disease was self-reported biennially and subsequently ascertained by reviewing medical records.19 Diet, including alcohol intake, was assessed by validated food frequency questionnaires every 4 years since 1991.20 We identified the overall dietary quality by calculating the 2010 Alternative Healthy Eating Index (AHEI) score (range: 0-110, with a higher score indicating a healthier diet).21 Data on physical activity were collected every 2 to 4 years since 1989. The reliability of self-reported lifestyle factors and health conditions has been verified among participants from NHS II and the original NHS.22,23,24,25,26,27
Statistical Analysis
Hazard ratios (HRs) for total and cause-specific mortality according to the time-varying history of gestational diabetes were estimated using Cox proportional hazards regression models.28 Participants’ person-time of follow-up began from the date of return of the baseline or follow-up questionnaires when a pregnancy (≥6 months) was reported until the end of follow-up (June 30, 2019) or the date of death, whichever occurred first. Exposure status was updated every 2 years from 1989 to 2009 (gestational diabetes occurring between 2004 and 2009 was ascertained in 2009), and participants were considered exposed to gestational diabetes from the age at which they initially reported a pregnancy complicated by gestational diabetes.
We also conducted a secondary analysis to evaluate total mortality risk according to the number of pregnancies with gestational diabetes over the reproductive life span; change in gestational diabetes status between pregnancies; and co-occurrence of gestational diabetes with HDP, preterm birth, or low birth weight among 52 729 participants who responded to the 2009 questionnaire. The 2009 questionnaire captured participants’ lifetime reproductive characteristics and thus permitted us to avoid double-counting pregnancies that were collected throughout follow-up and to more accurately match pregnancies to specific outcomes.
In the primary multivariable model (model 1), we included only potential confounders, such as race and ethnicity, age at first birth, age at menarche, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) at age 18 years, history of infertility, and parental history of diabetes. In the final multivariable model (model 2), we also adjusted for time-varying parity, oral contraceptive use, BMI change before and after pregnancy, smoking status, exercise at moderate to high intensity, and AHEI score during the reproductive life span. Covariates with missing values at a given biennial cycle were carried forward using the most recent data; otherwise, a missing indicator was created.29
We evaluated whether the association of gestational diabetes with mortality is mainly affected by subsequent T2D by jointly classifying participants according to time-varying gestational diabetes and subsequent T2D. The proportion of associations mediated by T2D was further estimated. Stratified analyses were conducted according to time-varying modifiable lifestyle factors during the reproductive life span, including BMI, smoking status, diet quality (AHEI score), physical activity, and alcohol intake, using cutoffs identified in previous studies.18,30,31 We also stratified the analyses according to time-varying chronic disease conditions during adulthood (ie, CVD, chronic obstructive pulmonary disease, hypercholesterolemia, chronic hypertension, and depression).
The multiplicative interaction between covariates was conducted by adding cross-product terms between exposure and any tested factors in multivariable Cox proportional hazards regression models.18,32 The additive interaction was estimated by calculating the relative excess risk due to interaction.33 To assess the robustness of the findings, we reanalyzed the association of gestational diabetes with total mortality by excluding multiple gestations; using time-varying lifestyle factors during adulthood instead of across the reproductive life span; excluding participants who never returned follow-up questionnaires; restricting analysis for premature deaths before age 70 years34; using the Markov chain Monte Carlo method of multiple imputations procedure to handle covariates with missing data; excluding deaths attributed to complications of pregnancy, childbirth, and the puerperium; and adjusting for the use of diabetes drugs. Additionally, we reanalyzed the association of gestational diabetes with cause-specific mortality using competing-risk Cox proportional hazards regression models, which permit the estimation of separate associations of gestational diabetes with different causes of death and to conduct a heterogeneity test for all estimations.35,36 We conducted a stratified analysis of the association between gestational diabetes and total mortality among non-Hispanic White participants compared with American Indian or Alaska Native, Asian, Black or African American, Hispanic, Native Hawaiian or Other Pacific Islander, and other groups to test the heterogeneity of estimations using Cochran Q and I2 statistics. All Cox proportional hazards regression models demonstrated adherence to the proportional hazards assumption.
All analyses were conducted from May 1, 2022, to May 25, 2023, using SAS, version 9.4 (SAS Institute Inc). Statistical significance was set at a 2-tailed P < .05.
Results
In the primary analysis, a total of 91 426 parous participants were included, with a mean (SD) age of 34.9 (4.7) years and BMI of 24.1 (4.7) at baseline. A total of 6.6% participants (6021 of 91 426) reported gestational diabetes either at baseline or during follow-up across their reproductive life span (Table 1). Most baseline characteristics were similar among participants with vs without gestational diabetes except for the prevalence of obesity, HDP, and parental history of diabetes.
Table 1. Age-Standardized Characteristics by Gestational Diabetes Status at Baseline or Follow-Up of Nurses’ Health Study II Parous Participantsa,b.
| Characteristic | Participants, No. (%) (N = 91 426) | |
|---|---|---|
| Without gestational diabetes | With gestational diabetes | |
| Sample | 85 405 (93.4) | 6021 (6.6) |
| Age, mean (SD), yc | 34.9 (4.7) | 33.9 (4.5) |
| Age at first birth, mean (SD), y | 26.6 (4.6) | 27.5 (4.8) |
| Age at menarche, mean (SD), y | 12.4 (1.4) | 12.3 (1.5) |
| Parity (pregnancies ≥6 mo), mean (SD)d | 2.07 (0.88) | 2.13 (0.99) |
| History of HDPd | 10 661 (14) | 1413 (28) |
| BMI at age 18 y | ||
| <18.5 | 12 551 (15) | 919 (15) |
| 18.5-24.9 | 65 743 (77) | 4396 (73) |
| 25-29.9 | 5699 (7) | 517 (9) |
| ≥30 | 1412 (2) | 189 (3) |
| Current BMI | ||
| <18.5 | 2687 (3) | 141 (2) |
| 18.5-24.9 | 55 017 (64) | 3076 (50) |
| 25-29.9 | 19 627 (23) | 1794 (29) |
| ≥30 | 8074 (9) | 1010 (18) |
| Non-Hispanic White race and ethnicity | 78 934 (92) | 5363 (89) |
| Other race and ethnicitye | 6471 (8) | 658 (11) |
| Total physical activity, mean (SD), h/wk | 3.269 (4.88) | 2.97 (4.65) |
| 2010 AHEI dietary score, mean (SD) | 47.36 (10.56) | 46.96 (10.35) |
| Alcohol intake, mean (SD), g/d | 2.875 (5.66) | 2.329 (5.05) |
| Current or ever oral contraceptive use | 72 763 (85) | 5065 (84) |
| Parental history of diabetes | 24 643 (29) | 2667 (45) |
| Smoking status | ||
| Never | 53 094 (66) | 3713 (66) |
| Past | 17 475 (22) | 1166 (22) |
| Current | 10 318 (13) | 722 (13) |
Abbreviations: AHEI, Alternative Healthy Eating Index (score range: 0-110, with a higher score indicating a healthier diet); BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDP, hypertensive disorders in pregnancy.
Means (SD) for continuous variables and No. (%) for categorical variables were standardized to the age distribution of the study population except for age.
A total of 4938 participants (5%) had missing data on baseline smoking status and 20 155 participants (22%) had missing data on diet (including alcohol intake).
Value was not age adjusted.
Analyses were conducted among 80 588 parous participants.
Other included American Indian or Alaska Native, Asian, Black or African American, Hispanic, Native Hawaiian or Other Pacific Islander, and other.
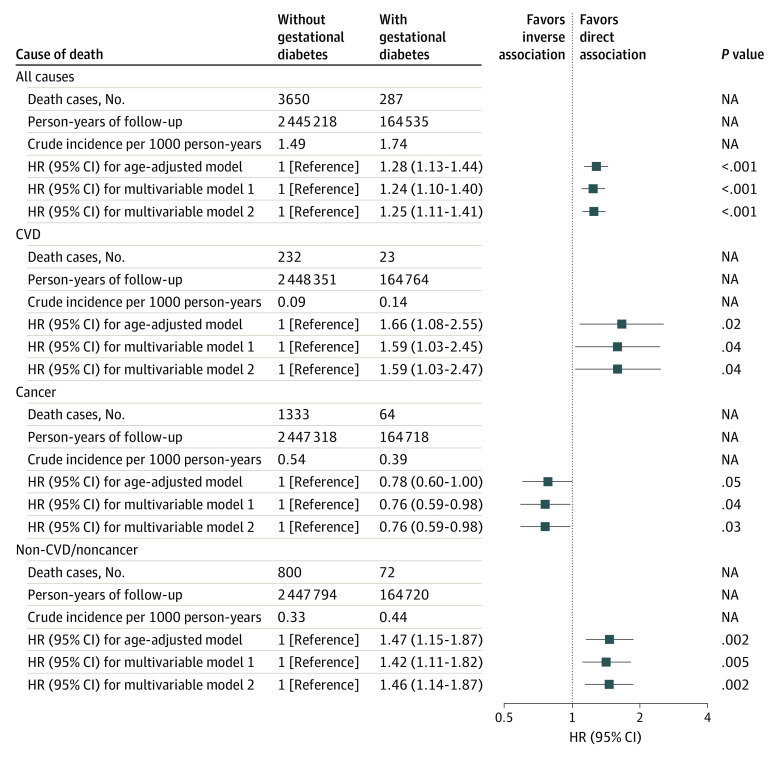
During the 2 609 753 person-years of follow-up, 3937 deaths were documented, including 255 deaths from CVD and 1397 from cancer (eTable 1 in Supplement 1). Participants with a history of gestational diabetes had a higher crude mortality rate than those without a history of gestational diabetes (1.74 vs 1.49 per 1000 person-years; absolute difference = 0.25 per 1000 person-years). The corresponding HR for total mortality was 1.28 (95% CI, 1.13-1.44), which did not materially change after additional adjustment for potential confounders and lifestyle factors during the reproductive life span (HR, 1.25; 95% CI, 1.11-1.41) (Figure 2). In fully adjusted cause-specific mortality analyses, gestational diabetes was inversely associated with cancer mortality risk (HR, 0.76; 95% CI, 0.59-0.98) but directly associated with the risk of mortality due to CVD (HR, 1.59; 95% CI, 1.03-2.47) and all other ascertained causes (HR, 1.46; 95% CI, 1.14-1.87). When cancer and non-CVD or noncancer deaths were further disaggregated and analyzed for diagnostic categories with at least 50 deaths (eTable 2 in Supplement 1), gestational diabetes was directly associated with mortality specifically attributed to infectious and parasitic diseases (HR, 3.31; 95% CI, 1.56-7.02) as well as senility and ill-defined diseases (HR, 1.84; 95% CI, 1.06-3.19).
Figure 2. Risk of Total and Cause-Specific Mortality by Gestational Diabetes Status Among the Nurses’ Health Study II Participants .
Age-adjusted model controlled for age, calendar time, and possible interactions between 2 timescales. Multivariable model 1 further adjusted for race and ethnicity, age at first birth, age at menarche, body mass index (BMI) at age 18 years, history of infertility, and parental history of diabetes. Multivariable model 2 further adjusted for parity, oral contraceptive use, BMI change before and after pregnancy, smoking status, exercise at moderate to high intensity, and Alternative Healthy Eating Index score. Error bars represent 95% CIs. The vertical line at 1 represents the reference line. CVD indicates cardiovascular disease; HR, hazard ratio; and NA, not applicable.
The direct association between gestational diabetes and total mortality persisted regardless of the subsequent development of T2D (eTable 3 in Supplement 1). Cause-specific mortality analyses revealed that the reduced risk of cancer mortality was observed only among participants who experienced both gestational diabetes and T2D; the risk of CVD and non-CVD or noncancer mortality, however, was elevated among participants who reported gestational diabetes only and those who reported both gestational diabetes and subsequent T2D. Mediation analyses showed that 16.2% (95% CI, 4.9%-42.0%) of the inverse association between gestational diabetes and cancer mortality was explained by subsequent T2D. When we restricted the analyses to 52 729 participants who responded to the 2009 questionnaire, the direct association of gestational diabetes with total mortality persisted through 10 years of follow-up (HR, 1.31; 95% CI, 1.04-1.66) (Table 2). Additionally, we found an association of gestational diabetes with total mortality among participants who experienced gestational diabetes in 2 or more pregnancies (HR, 1.48; 95% CI, 0.99-2.19); had gestational diabetes both in the first and subsequent pregnancies (HR, 1.71; 95% CI, 1.11-2.63); and simultaneously experienced HDP (HR, 1.80; 95% CI, 1.21-2.67), preterm birth (HR, 2.46; 95% CI, 1.66-3.64), or low birth weight (HR, 2.11; 95% CI, 1.21-3.68).
Table 2. Mortality by Gestational Diabetes Status Across Multiple Pregnancies Among Nurses’ Health Study II Parous Participants Who Responded to a 2009 Questionnaire .
| Gestational diabetes status | Death cases, No. | Crude incidence per 1000 person-years | HR (95% CI) | ||
|---|---|---|---|---|---|
| Age-adjusted modela | Multivariable model 1b | Multivariable model 2c | |||
| With gestational diabetes | |||||
| No | 1024 | 2.15 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 81 | 2.74 | 1.49 (1.19-1.87) | 1.44 (1.14-1.81) | 1.31 (1.04-1.66) |
| Total No. of pregnancies with gestational diabetes | |||||
| Without history of gestational diabetes | 1024 | 2.15 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 1 | 55 | 2.59 | 1.40 (1.07-1.84) | 1.36 (1.03-1.79) | 1.25 (0.95-1.65) |
| ≥2 | 26 | 3.11 | 1.72 (1.16-2.54) | 1.64 (1.11-2.43) | 1.48 (0.99-2.19) |
| Coexposure to gestational diabetes and preterm birth across the reproductive life span | |||||
| Without gestational diabetes or preterm birth | 853 | 2.08 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Preterm birth only | 171 | 2.52 | 1.20 (1.01-1.41) | 1.19 (1.01-1.40) | 1.14 (0.96-1.34) |
| Gestational diabetes only | 54 | 2.25 | 1.24 (0.94-1.64) | 1.21 (0.92-1.60) | 1.10 (0.83-1.45) |
| Both gestational diabetes and preterm birth | 27 | 4.89 | 2.86 (1.94-4.21) | 2.68 (1.82-3.96) | 2.46 (1.66-3.64) |
| Coexposure to gestational diabetes and low birth weight across the reproductive life span | |||||
| Without gestational diabetes or low birth weight | 909 | 2.09 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Low birth weight only | 115 | 2.76 | 1.27 (1.05-1.55) | 1.27 (1.04-1.54) | 1.18 (0.97-1.44) |
| Gestational diabetes only | 68 | 2.55 | 1.42 (1.11-1.82) | 1.37 (1.07-1.76) | 1.25 (0.97-1.61) |
| Both gestational diabetes and low birth weight | 13 | 4.43 | 2.51 (1.45-4.35) | 2.37 (1.36-4.11) | 2.11 (1.21-3.68) |
| Coexposure to gestational diabetes and HDP across the reproductive life span | |||||
| Without gestational diabetes or HDP | 882 | 2.09 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| HDP only | 142 | 2.56 | 1.29 (1.08-1.54) | 1.24 (1.03-1.48) | 1.15 (0.96-1.37) |
| Gestational diabetes only | 55 | 2.43 | 1.35 (1.03-1.78) | 1.31 (1-1.73) | 1.20 (0.91-1.58) |
| Both gestational diabetes and HDP | 26 | 3.78 | 2.18 (1.47-3.23) | 2.04 (1.38-3.03) | 1.80 (1.21-2.67) |
| Gestational diabetes status in the first and subsequent pregnancies | |||||
| Without gestational diabetes in any pregnancy | 1024 | 2.15 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Without gestational diabetes in the first birth but with gestational diabetes in subsequent pregnancy | 45 | 2.72 | 1.46 (1.08-1.97) | 1.41 (1.04-1.90) | 1.28 (0.94-1.73) |
| With gestational diabetes only in the first pregnancy | 14 | 2.06 | 1.13 (0.67-1.92) | 1.11 (0.66-1.88) | 1.04 (0.61-1.77) |
| With gestational diabetes both in the first and subsequent pregnancies | 22 | 3.53 | 1.96 (1.28-3.00) | 1.88 (1.22-2.87) | 1.71 (1.11-2.63) |
Abbreviation: HDP, hypertensive disorders in pregnancy; HR, hazard ratio.
In the age-adjusted model, age in months (continuous) at the start of follow-up and calendar year of the current questionnaire cycle were included as stratified variables to control for age, calendar time, and any possible interactions between these 2 timescales.
Based on age-adjusted models, multivariable model 1 was further adjusted for race and ethnicity (non-Hispanic White or other), age at first birth (≤25, 26-35, or >35 years), age at menarche (continuous), body mass index (calculated as weight in kilograms divided by height in meters squared) at age 18 years (<18.5, 18.5-24.9, 25-29.9, or ≥30), history of infertility (no or yes), and parental history of diabetes (no or yes).
Based on multivariable model 1, multivariable model 2 was further adjusted for parity (≤1, 2, or ≥3), oral contraceptive use (never or ever), body mass index change before and after pregnancy (loss >1.0, within 1.0, or gain >1.0), smoking status (never, former, current 1-34 cigarettes/d, or current ≥35 cigarettes/d), exercise at moderate to high intensity (0, 0.1-1.0, 1.1-3.4, 3.5-5.9, or ≥6 hours/wk), and Alternative Healthy Eating Index score (5 categories) during the reproductive life span.
The association of gestational diabetes with total mortality was found among participants with a low-quality diet (bottom 60% AHEI score: HR, 1.32; 95% CI, 1.15-1.52), heavy smoking habits (≥20 cigarettes/d: HR, 1.40; 95% CI, 1.01-1.94), overweight or obesity (BMI ≥25: HR, 1.37; 95% CI, 1.19-1.58), low levels of physical activity (<30 minute/d: HR, 1.31; 95% CI, 1.15-1.50), nonmoderate alcohol intake (<5 or >15 g/d: HR, 1.31; 95% CI, 1.12-1.51), hypercholesterolemia (HR, 1.35; 95% CI, 1.16-1.58), chronic hypertension (HR, 1.24; 95% CI, 1.05-1.46), and CVD (HR, 1.52; 95% CI, 1-2.32) (Table 3). The association of gestational diabetes with total or cause-specific mortality was materially unchanged in numerous sensitivity analyses (eTables 4 and 5 in Supplement 1). Homogeneous results were observed when we assessed the association of gestational diabetes with total mortality separately in non-Hispanic White participants and in American Indian or Alaska Native, Asian, Black or African American, Hispanic, Native Hawaiian or Other Pacific Islander, and other participants (eTable 6 in Supplement 1).
Table 3. Risk of Total Mortality Among Nurses’ Health Study II Participants, Stratified by Lifestyle Factors and Chronic Diseasesa,b.
| Lifestyle factor and chronic disease | HR (95% CI) | RERI (95% CI) | P value for additive interaction | P value for multiplicative interaction | |
|---|---|---|---|---|---|
| Without gestational diabetes | With gestational diabetes | ||||
| Diet quality | |||||
| Top 40% AHEI score (n = 1078 deaths) | 1 [Reference] | 1.07 (0.83 to 1.38) | 0.32 (−0.04 to 0.67) | .08 | .21 |
| Bottom 60% AHEI score (n = 2859 deaths) | 1 [Reference] | 1.32 (1.15 to 1.52) | |||
| Smoking status | |||||
| Never (n = 1976 deaths) | 1 [Reference] | 1.22 (1.03 to 1.45) | 0.13 (−0.01 to 0.28) | .07 | .91c |
| Current or former: 1-19 cigarettes/d (n = 1445 deaths) | 1 [Reference] | 1.21 (0.98 to 1.50) | |||
| Current or former: ≥20 cigarettes/d (n = 516 deaths) | 1 [Reference] | 1.40 (1.01 to 1.94) | |||
| BMI | |||||
| <25 (n = 1509 Deaths) | 1 [Reference] | 1.01 (0.78 to 1.29) | 0.36 (0.05 to 0.68) | .02 | .04 |
| ≥25 (n = 2428 Deaths) | 1 [Reference] | 1.37 (1.19 to 1.58) | |||
| Physical activity | |||||
| ≥30 min/d (n = 762 Deaths) | 1 [Reference] | 1.07 (0.78 to 1.47) | 0.30 (−0.09 to 0.69) | .13 | .25 |
| <30 min/d (n = 3175 Deaths) | 1 [Reference] | 1.31 (1.15 to 1.50) | |||
| Alcohol intake | |||||
| 5-15 g/d (n = 1498 Deaths) | 1 [Reference] | 1.11 (0.89 to 1.38) | 0.31 (−0.04 to 0.65) | .08 | .22 |
| <5 or >15 g/d (n = 2439 Deaths) | 1 [Reference] | 1.31 (1.12 to 1.51) | |||
| Depression | |||||
| No (n = 2875 deaths) | 1 [Reference] | 1.23 (1.06 to 1.42) | 0.16 (−0.23 to 0.55) | .42 | .66 |
| Yes (n = 1062 deaths) | 1 [Reference] | 1.28 (1.03 to 1.59) | |||
| Chronic hypertension | |||||
| No (n = 2135 deaths) | 1 [Reference] | 1.12 (0.93 to 1.35) | 0.33 (−0.04 to 0.70) | .08 | .23 |
| Yes (n = 1802 deaths) | 1 [Reference] | 1.24 (1.05 to 1.46) | |||
| Hypercholesterolemia | |||||
| No (n = 1928 deaths) | 1 [Reference] | 1.07 (0.87 to 1.31) | 0.37 (0.06 to 0.67) | .02 | .03 |
| Yes (n = 2009 deaths) | 1 [Reference] | 1.35 (1.16 to 1.58) | |||
| COPD | |||||
| No (n = 3813 deaths) | 1 [Reference] | 1.25 (1.11 to 1.42) | 0.24 (−1.87 to 2.34) | .83 | .94 |
| Yes (n = 124 deaths) | 1 [Reference] | 1.14 (0.30 to 4.34) | |||
| CVD | |||||
| No (n = 3610 deaths) | 1 [Reference] | 1.20 (1.05 to 1.36) | 1.12 (−0.59 to 2.82) | .20 | .50 |
| Yes (n = 327 deaths) | 1 [Reference] | 1.52 (1 to 2.32) | |||
Abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HR, hazard ratio; RERI, relative excess risk due to interaction.
In multivariable models, age in months (continuous) at the start of follow-up and calendar year of the current questionnaire cycle were included as stratified variables to control for age, calendar time, and any possible interactions between these 2 timescales, with adjustment for race and ethnicity (non-Hispanic White or other), age at first birth (≤25, 26-35, or >35 years), age at menarche (continuous), BMI at age 18 years (<18.5, 18.5-24.9, 25-29.9, or ≥30), history of infertility (no or yes), and parental history of diabetes (no or yes) as well as time-varying parity (≤1, 2, or ≥3), oral contraceptive use (never or ever), BMI change before and after pregnancy (loss >1.0, within 1.0, or gain >1.0), smoking status (never, former, current 1-34 cigarettes/d, or current ≥35 cigarettes/d), exercise at moderate to high intensity (0, 0.1-1.0, 1.1-3.4, 3.5-5.9, or ≥6 h/wk), and AHEI score (5 categories) during the reproductive life span except for the stratified variables.
Lifestyle factors were updated during the reproductive life span.
A likelihood ratio test was conducted to compare a model including multiplicative interaction terms between gestational diabetes and smoking status with a model without such terms.
Discussion
Among the parous participants of NHS II with more than 30 years of follow-up data, we found that gestational diabetes was associated with a greater subsequent risk of mortality during adulthood. Mortality due to CVD was a primary factor in this association, and it persisted regardless of the subsequent development of T2D. We also observed an inverse association between the history of gestational diabetes and cancer mortality, which persisted only among participants who reported both gestational diabetes and T2D after a pregnancy with gestational diabetes.
While gestational diabetes has been directly associated with the morbidity of various chronic diseases during adulthood, particularly CVD morbidity,8,37 a limited number of studies have investigated the association of gestational diabetes with long-term risk of mortality. Supporting these findings, Cirillo and Cohn8 reported a direct association between glycosuria during pregnancy and CVD mortality among 14 062 participants in the Child Health and Development Studies with more than 50 years of follow-up. In a more recent prospective cohort study of 48 197 pregnant individuals from 12 US clinical centers, Hinkle and colleagues7 reported a greater risk of mortality due to total and infectious diseases among participants with gestational diabetes or impaired glucose tolerance. However, Hinkle and colleagues7 found that gestational diabetes or impaired glucose tolerance was not associated with CVD mortality. The observed discrepancy may be attributed, at least partially, to variations in the timeframes of pregnancies examined (1989-2009 in the present study vs 1959-1966 in the previous study), population characteristics, exposure definitions, and clinical screening practices. The existing evidence of the association between gestational diabetes and cancer morbidity and mortality is limited and has controversial findings.4 For instance, several studies found an inverse association between gestational diabetes and the risk of breast cancer morbidity,38,39,40 but direct associations with ovarian, uterine, and breast cancer have also been reported.41,42 In the present study, while we observed an inverse association between gestational diabetes and cancer mortality, gestational diabetes was not associated with any type-specific cancer mortality. Similarly, Hinkle and colleagues7 did not find any association between gestational diabetes and cancer mortality. Instead, they reported direct associations with mortality due to diabetes and kidney disease, which were not investigated in the present study due to the limited number of death cases.7
Individuals with a history of gestational diabetes face an almost 10-fold increased subsequent risk of T2D.3 Therefore, we explored whether the increased mortality risk was associated with the development of T2D. Reduced cancer risk was observed only among participants who simultaneously experienced gestational diabetes and subsequent T2D. Mediation analyses showed that T2D mediated the association between gestational diabetes and cancer mortality. These findings suggest that gestational diabetes itself may not be associated with cancer mortality. In contrast, the direct associations between gestational diabetes and total and CVD mortality were not entirely attributable to subsequent T2D, which persisted independent of the subsequent occurrence of T2D. Existing evidence has yielded conflicting results on whether CVD morbidity was dependent on the intercurrent development of T2D.37,43,44,45,46,47 However, individuals who have experienced both gestational diabetes and T2D consistently exhibit the highest risk of CVD morbidity.37,47 We observed a similar risk of CVD mortality among participants who reported gestational diabetes only and those who reported both gestational diabetes and T2D. We suspected that the risk of CVD mortality among participants with a history of gestational diabetes and intercurrent T2D might have been underestimated because most NHS II participants were relatively young by the end of follow-up and may not have yet developed T2D. Additionally, we found an association between gestational diabetes and total mortality among participants with a low-quality diet, heavy smoking habits, overweight or obesity, low levels of physical activity, nonmoderate alcohol intake, hypercholesterolemia, chronic hypertension, and CVD. A greater risk of mortality was observed among participants with a history of gestational diabetes in 2 or more pregnancies, who had gestational diabetes both in the first and subsequent pregnancies, and who experienced other adverse pregnancy or birth outcomes. These findings were not unexpected given that individuals with less healthy lifestyle patterns, recurrent gestational diabetes, and multiple adverse maternal and fetal pregnancy outcomes may have more severe metabolic disorders (eg, hypercholesterolemia and chronic hypertension) that may interact synergistically with gestational diabetes, eventually playing a role in increased risk of mortality later in life.
Gestational diabetes may be etiologically linked to a greater risk of mortality, particularly CVD mortality, through shared mechanistic pathways that predate pregnancy, such as systemic inflammation, delayed insulin secretion, heightened insulin resistance, vascular changes, endothelial dysfunction, and dyslipidemia.6,48,49 Meanwhile, the increased risk of CVD mortality may also be accelerated by reduced sex hormone–binding globulin levels, insulin resistance, and increased release of inflammatory cytokines implicated in the cause of gestational diabetes during pregnancy.49,50,51 Together, these data support the emerging concept that individuals who develop adverse pregnancy outcomes may have greater underlying susceptivity to chronic metabolic disorders before or during pregnancy that can be unveiled by increased physiological stress during pregnancy.52 The reasons for the inverse associations between gestational diabetes and cancer are less clear. Results from the cause-specific proportional hazards models suggest that this inverse association is unlikely to be associated with the competing risk of mortality from other causes. Instead, it could be partly explained by early detection, management of medical conditions, and lifestyle interventions (eg, BMI reduction or maintenance, healthy diet, and physical activity) that are often recommended to patients with T2D. For instance, in a systematic review consisting of 41 observational studies, Franciosi and colleagues53 reported that metformin, the first-line oral antidiabetic medication, was associated with lower risk of total and cause-specific cancer. However, uncontrolled residual confounding cannot be fully ruled out.
Strengths and Limitations
The strengths of this study include its prospective research design, extensive follow-up period, high response rates, large sample size, and the collection of various time-varying lifestyle and health-related factors. The study also has several limitations. First, participants’ history of physician-diagnosed gestational diabetes was self-reported, which may have led to exposure misclassification. However, self-reported gestational diabetes has been confirmed to be highly accurate (94%) among NHS II participants.11 Meanwhile, the rate of gestational diabetes in the NHS II (6.6%) was comparable with the reported rates in the US population based on the National Diabetes Data Group criteria (3%-6%).54,55 Therefore, we suspected that the exposure misclassification of the present study would be minimal. Second, most of the NHS II participants self-identified as non-Hispanic White, which may have limited the representativeness of these findings. One study found that White individuals at first live birth exhibited a faster increasing rate of gestational diabetes in 2011 to 2019 than those from racial and ethnic minority populations in the US.2 Differences in pregnancy complications and the burden of chronic diseases across race and ethnicity groups have also been reported among US individuals with a history of gestational diabetes.56,57 However, the present stratified analysis showed homogeneous associations between gestational diabetes and total mortality between White and racial and ethnic minority participants. Third, there was a limited number of deaths from non-CVD or noncancer causes, which may have generated imprecise estimations.58 Fourth, we did not collect data on the presence of prediabetes, gestational age of gestational diabetes onset, and disease severity, which may be useful to identify individuals at the highest risk. Fifth, the study’s observational design did not allow us to establish any causal associations.
Conclusions
In this cohort study, we observed a greater risk of mortality over a 30-year follow-up among participants who reported a history of gestational diabetes vs those without a history of gestational diabetes, with an absolute difference in the mortality rate of 0.25 per 1000 person-years. The findings emphasize the importance for health care professionals to consider gestational diabetes as a critical factor when evaluating the later-life mortality risk of their patients.
eTable 1. Categories for Causes of Death
eTable 2. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk Cause-Specific Mortality (At Least 50 Deaths Attributed), According to the Occurrence of GDM Among 91,426 Women (NHS II, 1989-2019)
eTable 3. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk of Total and Cause-Specific Mortality According to the Join Categories of the Occurrence of GDM and T2DM Among 91,426 Women (NHS II, 1989-2019)
eTable 4. Sensitivity Analyses for the Associations Between GDM and Risk of Total Mortality (NHS II, 1989-2019)
eTable 5. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk of Cause-Specific Mortality Based on Competing-Risk Regression Models (NHS II, 1989-2019)
eTable 6. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk of Total Mortality Among White Versus non-White Nurses (NHS II, 1989-2019)
Data Sharing Statement
References
- 1.Wang H, Li N, Chivese T, et al. ; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group . IDF Diabetes Atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s criteria. Diabetes Res Clin Pract. 2022;183:109050. doi: 10.1016/j.diabres.2021.109050 [DOI] [PubMed] [Google Scholar]
- 2.Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326(7):660-669. doi: 10.1001/jama.2021.7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Yan P, Fu T, et al. The association between gestational diabetes mellitus and cancer in women: a systematic review and meta-analysis of observational studies. Diabetes Metab. 2020;46(6):461-471. doi: 10.1016/j.diabet.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011;34(7):1582-1584. doi: 10.2337/dc11-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905-914. doi: 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- 7.Hinkle SN, Schisterman EF, Liu D, et al. Pregnancy complications and long-term mortality in a diverse cohort. Circulation. 2023;147(13):1014-1025. doi: 10.1161/CIRCULATIONAHA.122.062177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation. 2015;132(13):1234-1242. doi: 10.1161/CIRCULATIONAHA.113.003901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the Three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573-1581. doi: 10.2105/AJPH.2016.303338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YX, Wang S, Mitsunami M, et al. Pre-pregnancy menstrual cycle regularity and length and the risk of gestational diabetes mellitus: prospective cohort study. Diabetologia. 2021;64(11):2415-2424. doi: 10.1007/s00125-021-05531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon CG, Willett WC, Rich-Edwards J, et al. Variability in diagnostic evaluation and criteria for gestational diabetes. Diabetes Care. 1996;19(1):12-16. doi: 10.2337/diacare.19.1.12 [DOI] [PubMed] [Google Scholar]
- 12.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078-1083. doi: 10.1001/jama.1997.03550130052036 [DOI] [PubMed] [Google Scholar]
- 13.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837-839. doi: 10.1093/oxfordjournals.aje.a113804 [DOI] [PubMed] [Google Scholar]
- 14.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax nationwide death search. Am J Epidemiol. 1994;140(11):1016-1019. doi: 10.1093/oxfordjournals.aje.a117191 [DOI] [PubMed] [Google Scholar]
- 15.Wang YX, Mínguez-Alarcón L, Gaskins AJ, et al. Association of spontaneous abortion with all cause and cause specific premature mortality: prospective cohort study. BMJ. 2021;372(530):n530. doi: 10.1136/bmj.n530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriyama IM. The eighth revision of the International Classification of Diseases. Am J Public Health Nations Health. 1966;56(8):1277-1280. doi: 10.2105/AJPH.56.8.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.StatLine. Deaths; underlying cause of death (shortlist), sex, age. Accessed May 25, 2023. https://opendata.cbs.nl/statline/#/CBS/en/dataset/7052eng/table?dl=4BCB4
- 18.Wang YX, Shan Z, Arvizu M, et al. Associations of menstrual cycle characteristics across the reproductive life span and lifestyle factors with risk of type 2 diabetes. JAMA Netw Open. 2020;3(12):e2027928. doi: 10.1001/jamanetworkopen.2020.27928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YX, Mínguez-Alarcón L, Gaskins AJ, et al. Pregnancy loss and risk of cardiovascular disease: the Nurses’ Health Study II. Eur Heart J. 2022;43(3):190-199. doi: 10.1093/eurheartj/ehab737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for Women. Am J Epidemiol. 2018;187(5):1051-1063. doi: 10.1093/aje/kwx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf AMH, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991-999. doi: 10.1093/ije/23.5.991 [DOI] [PubMed] [Google Scholar]
- 23.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52(5):828-832. doi: 10.1161/HYPERTENSIONAHA.108.117630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894-900. doi: 10.1093/oxfordjournals.aje.a114319 [DOI] [PubMed] [Google Scholar]
- 25.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570-572. [PubMed] [Google Scholar]
- 26.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570-584. doi: 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue Y, Yuan C, Wang DD, et al. Reproducibility and validity of diet quality scores derived from food-frequency questionnaires. Am J Clin Nutr. 2022;115(3):843-853. doi: 10.1093/ajcn/nqab368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YX, Arvizu M, Rich-Edwards JW, et al. Hypertensive disorders of pregnancy and subsequent risk of premature mortality. J Am Coll Cardiol. 2021;77(10):1302-1312. doi: 10.1016/j.jacc.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song M, Zhou X, Pazaris M, Spiegelman D. The missing covariate indicator method is nearly valid almost always. arXiv. Preprint posted online October 30, 2021. doi: 10.48550/arXiv.2111138 [DOI]
- 30.Li Y, Schoufour J, Wang DD, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368:l6669. doi: 10.1136/bmj.l6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan MS, Freiberg MS, Greevy RA Jr, Kundu S, Vasan RS, Tindle HA. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA. 2019;322(7):642-650. doi: 10.1001/jama.2019.10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YX, Li Y, Rich-Edwards JW, et al. Associations of birth weight and later life lifestyle factors with risk of cardiovascular disease in the USA: a prospective cohort study. EClinicalMedicine. 2022;51:101570. doi: 10.1016/j.eclinm.2022.101570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele TJ, Tchetgen Tchetgen EJ. Attributing effects to interactions. Epidemiology. 2014;25(5):711-722. doi: 10.1097/EDE.0000000000000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YX, Arvizu M, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ. 2020;371:m3464. doi: 10.1136/bmj.m3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35(5):782-800. doi: 10.1002/sim.6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524-532. doi: 10.2307/2532940 [DOI] [PubMed] [Google Scholar]
- 37.Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12):1735-1742. doi: 10.1001/jamainternmed.2017.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powe CE, Tobias DK, Michels KB, et al. History of gestational diabetes mellitus and risk of incident invasive breast cancer among parous women in the Nurses’ Health Study II prospective cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(3):321-327. doi: 10.1158/1055-9965.EPI-16-0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bejaimal SA, Wu CF, Lowe J, Feig DS, Shah BR, Lipscombe LL. Short-term risk of cancer among women with previous gestational diabetes: a population-based study. Diabet Med. 2016;33(1):39-46. doi: 10.1111/dme.12796 [DOI] [PubMed] [Google Scholar]
- 40.Rollison DE, Giuliano AR, Sellers TA, et al. Population-based case-control study of diabetes and breast cancer risk in Hispanic and non-Hispanic White women living in US southwestern states. Am J Epidemiol. 2008;167(4):447-456. doi: 10.1093/aje/kwm322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs O, Sheiner E, Meirovitz M, Davidson E, Sergienko R, Kessous R. The association between a history of gestational diabetes mellitus and future risk for female malignancies. Arch Gynecol Obstet. 2017;295(3):731-736. doi: 10.1007/s00404-016-4275-7 [DOI] [PubMed] [Google Scholar]
- 42.Perrin MC, Terry MB, Kleinhaus K, et al. Gestational diabetes and the risk of breast cancer among women in the Jerusalem Perinatal Study. Breast Cancer Res Treat. 2008;108(1):129-135. doi: 10.1007/s10549-007-9585-9 [DOI] [PubMed] [Google Scholar]
- 43.Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078-2083. doi: 10.2337/dc05-2482 [DOI] [PubMed] [Google Scholar]
- 44.Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care. 2017;40(1):101-108. doi: 10.2337/dc16-1400 [DOI] [PubMed] [Google Scholar]
- 45.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669. doi: 10.2337/dc08-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun J, Kim GR, Lee SJ, Kim HC. Gestational diabetes mellitus and the role of intercurrent type 2 diabetes on long-term risk of cardiovascular events. Sci Rep. 2021;11(1):21140. doi: 10.1038/s41598-021-99993-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, Soohoo M, Sørensen HT, Li J, Arah OA. Gestational diabetes mellitus and the risks of overall and type-specific cardiovascular diseases: a population- and sibling-matched cohort study. Diabetes Care. 2022;45(1):151-159. doi: 10.2337/dc21-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunderson EP, Sun B, Catov JM, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA Study. Circulation. 2021;143(10):974-987. doi: 10.1161/CIRCULATIONAHA.120.047320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green JB. Cardiovascular consequences of gestational diabetes. Circulation. 2021;143(10):988-990. doi: 10.1161/CIRCULATIONAHA.120.052995 [DOI] [PubMed] [Google Scholar]
- 50.Wolf M, Sauk J, Shah A, et al. Inflammation and glucose intolerance: a prospective study of gestational diabetes mellitus. Diabetes Care. 2004;27(1):21-27. doi: 10.2337/diacare.27.1.21 [DOI] [PubMed] [Google Scholar]
- 51.Thadhani R, Wolf M, Hsu-Blatman K, Sandler L, Nathan D, Ecker JL. First-trimester sex hormone binding globulin and subsequent gestational diabetes mellitus. Am J Obstet Gynecol. 2003;189(1):171-176. doi: 10.1067/mob.2003.343 [DOI] [PubMed] [Google Scholar]
- 52.Parikh NI, Gonzalez JM, Anderson CAM, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council . Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143(18):e902-e916. doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 53.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8(8):e71583. doi: 10.1371/journal.pone.0071583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magee MS, Walden CE, Benedetti TJ, Knopp RH. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA. 1993;269(5):609-615. doi: 10.1001/jama.1993.03500050087031 [DOI] [PubMed] [Google Scholar]
- 55.Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the Carpenter and Coustan plasma glucose thresholds. Diabetes Care. 2002;25(9):1625-1630. doi: 10.2337/diacare.25.9.1625 [DOI] [PubMed] [Google Scholar]
- 56.Venkatesh KK, Lynch CD, Powe CE, et al. Risk of adverse pregnancy outcomes among pregnant individuals with gestational diabetes by race and ethnicity in the United States, 2014-2020. JAMA. 2022;327(14):1356-1367. doi: 10.1001/jama.2022.3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bower JK, Butler BN, Bose-Brill S, Kue J, Wassel CL. Racial/ethnic differences in diabetes screening and hyperglycemia among US women after gestational diabetes. Prev Chronic Dis. 2019;16:E145. doi: 10.5888/pcd16.190144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang YX, Sun Y, Missmer SA, et al. Association of early life physical and sexual abuse with premature mortality among female nurses: prospective cohort study. BMJ. 2023;381:e073613. doi: 10.1136/bmj-2022-073613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Categories for Causes of Death
eTable 2. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk Cause-Specific Mortality (At Least 50 Deaths Attributed), According to the Occurrence of GDM Among 91,426 Women (NHS II, 1989-2019)
eTable 3. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk of Total and Cause-Specific Mortality According to the Join Categories of the Occurrence of GDM and T2DM Among 91,426 Women (NHS II, 1989-2019)
eTable 4. Sensitivity Analyses for the Associations Between GDM and Risk of Total Mortality (NHS II, 1989-2019)
eTable 5. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk of Cause-Specific Mortality Based on Competing-Risk Regression Models (NHS II, 1989-2019)
eTable 6. Hazard Ratio (HR) (95% Confidence Interval (CI) for the Risk of Total Mortality Among White Versus non-White Nurses (NHS II, 1989-2019)
Data Sharing Statement