Abstract
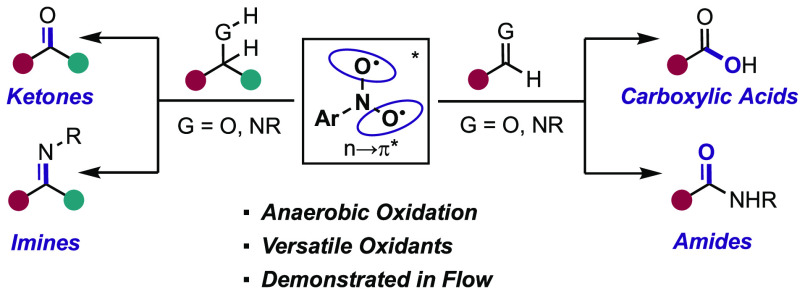
Herein, we report a protocol for the anaerobic oxidation of alcohols, amines, aldehydes, and imines promoted by photoexcited nitroarenes. Mechanistic studies support the idea that photoexcited nitroarenes undergo double hydrogen atom transfer (HAT) steps with alcohols and amines to provide the respective ketone and imine products. In the presence of aldehydes and imines, successive HAT and oxygen atom transfer (OAT) events occur to yield carboxylic acids and amides, respectively. This transformation is amenable to a continuous-photoflow setup, which led to reduced reaction times.
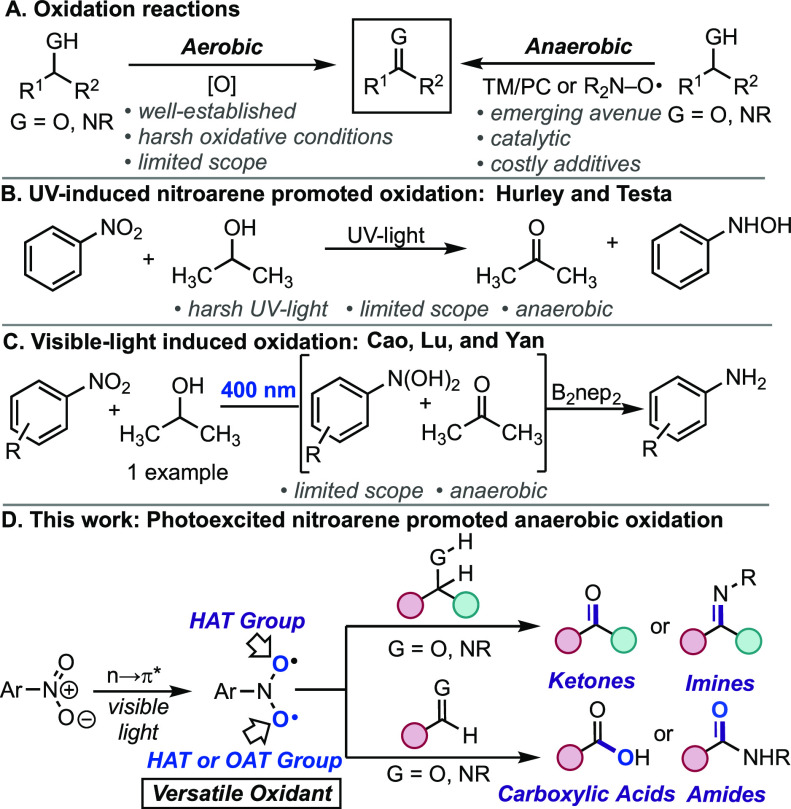
Oxidations of C(sp3)– and C(sp2)–heteroatom systems are essential transformations in organic chemistry (Scheme 1).1 Classical oxidation methods such as Jones,2 Swern,3 and Baeyer–Villiger4 are powerful; however, they are mostly conducted under super stoichiometric amounts of reagents. Furthermore, these reactions are often highly exothermic and can lead to undesired side products, like overoxidation, which limit the substrate scope (Scheme 1A). Hypervalent iodine-based reagents like IBX5 and DMP6 offer milder reaction conditions but are limited in large-scale applications due to the issues of solubility, cost, and explosive nature. Recently, oxidative approaches employing nitroxyl radicals can be achieved catalytically under milder aerobic or anaerobic conditions.7−9 The latter approach can lead to an expansion of the substrate scope that complements classical oxidation strategies. However, the employment of N-hydroxyl-based catalytic systems can suffer from the limitations of high catalyst loading and poor functional group tolerance.7 Hence, a complementary anaerobic oxidation protocol that is economical, practical, and sustainable is highly warranted.
Scheme 1. Prior Methods and Hypothesized Work.
In 1966, Hurley and Testa (Scheme 1B)10 and others11−13 studied the intermolecular oxidation of alcohol solvents in the presence of nitroarenes under UV irradiation. The authors uncovered that two sequential hydrogen atom transfer (HAT) events occur during the redox event with an alcohol solvent. Very recently, the groups of Cao, Lu, and Yan redirected the aforementioned reactivity toward the visible-light region for the photoreduction of nitroarenes with concomitant oxidation (Scheme 1C).14,15 Though limited in scope, both approaches illustrate that photoinduced nitroarenes are capable of anaerobic alcohol oxidation.13,16,17 Based on our previous work on hydrocarbon oxidation using nitroarene photochemistry,18−20 we hypothesized the possibility of harnessing multiple HAT events with nitroarenes to promote the anaerobic oxidation of heteroatom systems under visible-light irradiation. Herein, we illustrate that the photoexcited state of the nitroarene can trigger a double HAT event with C(sp3)–heteroatom systems to generate valuable ketone and imines and a successive HAT and oxygen atom transfer (OAT) event at C(sp2)–heteroatom systems to furnish synthetically useful carboxylic acids and amides in a general, mild, and cost-effective manner compared to established oxidation protocols.
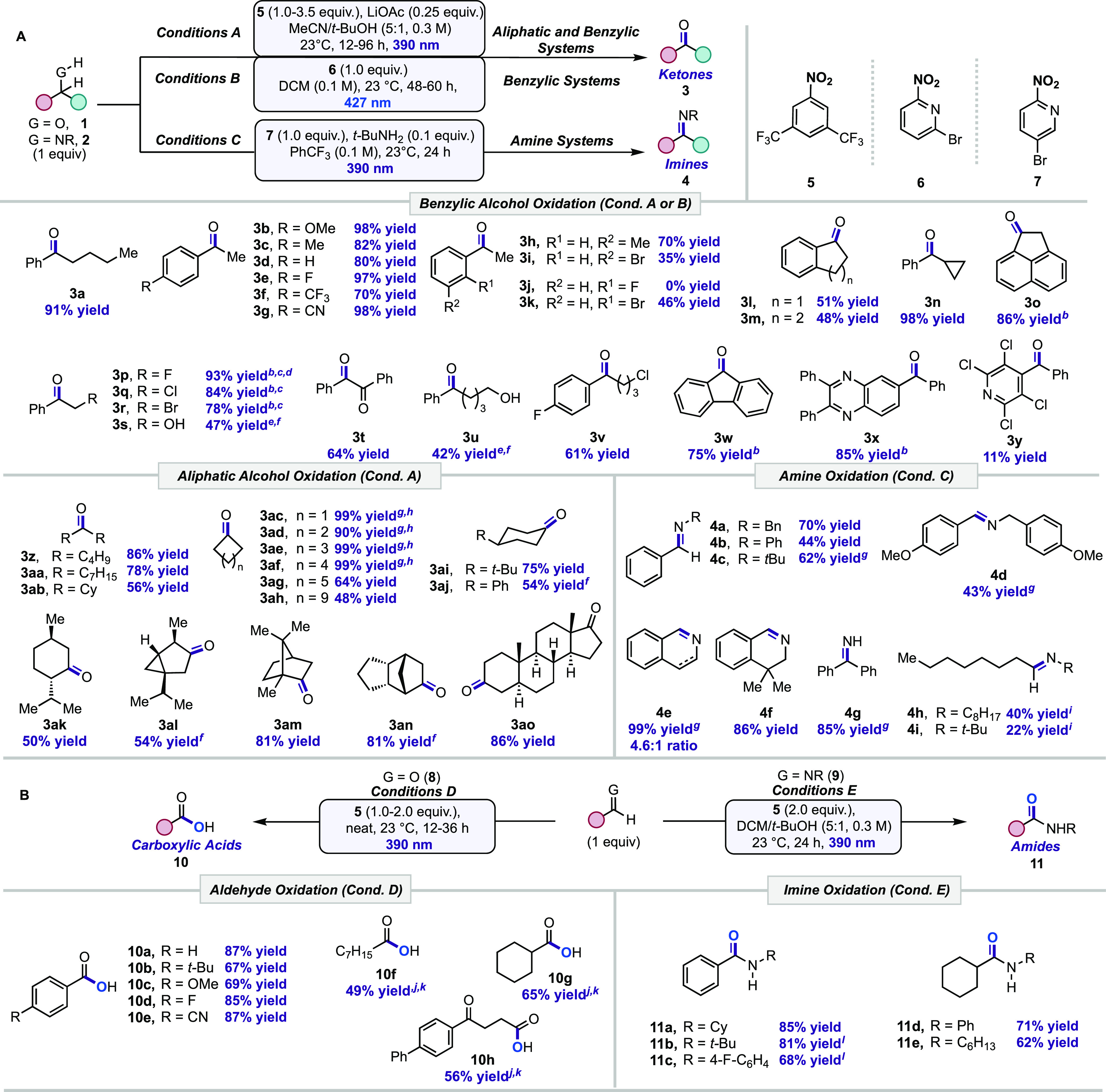
We began our investigation by testing the conversion of 1-phenylpentan-1-ol 1a to ketone 3a under our previously reported conditions featuring 2-chloro-4-nitropyridine under 390 nm.19 The oxidation was successful, resulting in a 61% yield of 3a. After an extensive optimization campaign (Tables S1–4), the yield was increased to 91% with 3,5-bis(trifluoromethyl)nitrobenzene (5) under 390 nm irradiation at 0.1 mmol scale. After the optimized reaction conditions were discovered, the electronic effect of the oxidation reaction was investigated with 4-substituted-phenyl-1-ethanol derivatives (Table 1A, 1b–g). It was found that the transformation was not sensitive to the electronic pattern, as substrates possessing both electron-rich and -deficient groups resulted in good to excellent yields of the oxidation products 3b–g. This could be attributed to small differences in the bond dissociation energy for α-C(sp3)–H of electronically different alcohols. Meta- and ortho-substituted benzylic alcohols were also tested. 1h,i,k gave 3h,i,k in low to good yields; however, to our surprise, 1j did not convert. We believe that hydrogen bonding between the O–H and ortho F-substituent in 1j may strengthen the α-C(sp3)–H bond and disfavor HAT with the photoexcited nitroarene.21 Cyclic benzylic alcohol systems, such as indanol and tetrahydronaphthalenol, resulted in a moderate yield of oxidation products 3l–m. Acyclic α-substituted benzylic alcohols containing sensitive and important functional handles, such as cyclopropyl 1n, halogen 1p–r, and carbonyl groups 1t, all resulted in corresponding oxidation products in good yields under conditions B (3o–r) and A (3t). Notably, secondary benzylic alcohol 1s and 1u possessing a free aliphatic alcohol unit underwent site-selectivity oxidation at the benzylic position (3u), which typically cannot be accessed from the Stevens-Stahl protocol.7−9 Haloperidol (3v), a common antipsychotic,22 was synthesized from 1v under this protocol in 61% yield. Finally, diaryl substituted ketones of medicinal relevance (3w,x)23,24 as well as halogenated heterocycle 3y were afforded in low to good yields under the reaction conditions, highlighting the synthetic utility for late-stage oxidation.
Table 1. Scope of the Photoinduced Nitroarene Promoted Oxidation of (A) Alcohols and Amines as Well as (B) Aldehydes and Iminesa.
Conditions B.
Using 2-bromo-4-nitropyridine.
Under 390 nm.
In MeCN/H2O (1:1, 0.3 M).
No LiOAc.
Denotes 1H NMR yield using CH2Br2 as an internal standard.
Isolated as a hydrazone derivative (see SI).
Denotes 1H NMR yield using CH2Cl2 as an internal standard.
MeCN (1 M).
H2O (1.0 equiv).
Neat.
Next, unactivated aliphatic alcohols were studied under conditions A (Table S5). Secondary acyclic alcohols containing linear hydrocarbon chains yielded the oxidation products with good efficiency under the reaction conditions (3z, 3aa). However, sterically encumbered dicyclohexylmethanol 1ab resulted in a moderate yield of 3ab. Cyclic alcohols featuring small to large ring sizes gave good to excellent yields under the reaction conditions (3ac–3ag); however, a decreased yield of 48% for 3ah was observed due to the overoxidation of secondary C(sp3)–H sites. Next, 4-substituted cyclohexanol substrates were exposed to the reaction conditions, resulting in fair to moderate yields of desired ketone products 3ai–3aj. The oxidations of naturally occurring terpenes 1ak–1an and steroid 1ao were tested. Oxidation of L-(−)-menthol and thujone precursor proceeded moderately well 3ak–3al, while the oxidation of borneol was highly efficient under the reaction conditions 3am. Corodane (3an) was obtained in 81% yield via the oxidation of 1an under our conditions, which is comparable to the Jones oxidation25 (84%) and Stahl’s protocol26 (78%). Lastly, the reaction of trans-androsterone 1ao generated 3ao in 86% yield.
Then, we investigated if amines (2)27−29 could be oxidized in the presence of photoexcited nitroarenes (Table 1A, 2 → 4). Exposure of conditions A and B to dibenzyl amine 2a resulted in a low yield of the desired oxidation product 4a. Further optimization revealed the use of nitroarene 7 in PhCF3 as a solvent under 390 nm irradiation led to higher yields (Conditions C, Table S6–7). Other benzylic amines 2b–c were tested, giving the corresponding imines 4b–c from moderate to good yields. Electron-rich amine 2d was tolerated under the reaction conditions, but 4d was prone to hydrolysis. Cyclic amines 2e and 2f gave the corresponding imines in good yields (4e–f). Amine 2e led to the dihydroisoquinoline 4e and overoxidized aromatic isoquinoline (4e′) in a 4.6:1 ratio with a 99% total NMR yield. Free amine 2g gave the corresponding imine 4g in 85% NMR yield, whereas reported oxidation of free primary amines can result in undesirable homocoupling.30 Furthermore, aliphatic amines reacted quickly and generated the desired oxidation product 4h–i in low yield with concomitant overoxidation to the amide (vide infra).
Classical transformations for the oxidation of C(sp2)–heteroatom systems, such as aldehydes and imines, would often suffer from low reactivity as well as poor substrate scope and require the use hazardous oxidants and expensive additives or transition metals.31−38 Hence, we questioned whether the oxidation of aldehydes (8) and imines (9) could be achieved under our mild protocol (Table 1B). It was discovered that the employment of nitroarene 5 under 390 nm irradiation promoted the effective oxidation of aldehydes to acids (8 → 10, Conditions D, Tables S8–9). Oxidation of benzaldehyde 8a resulted in an 87% yield of benzoic acid 10a under the optimized conditions. Varying the electronic pattern of aromatic aldehydes did not affect the reaction yields (10b–e). Oxidation of octanal 8f and cyclohexanecarboxaldehyde 8g afforded the corresponding products 10f and 10g in 49 and 65% yields, respectively. To illustrate the synthetic utility of the transformation, the synthesis of therapeutic fenbufen39,40 (10h) was achieved in 56% yield via oxidation of 8h. Finally, the oxidation of imines to amides was examined (9 → 11). Under conditions E (Table S10), N-cyclohexyl-1-phenylmethanimine (9a) afforded N-cyclohexylbenzamide (11a) in 85% yield. Benzyl imines such as N-alkyl (9b) and aryl imine (9c) generated the expected amide products (11b–c) in a good yield. Aliphatic imines containing N-phenyl and -hexyl substituents were subjected to the reaction conditions and resulted in 71 and 62% yields of amides 11d and 11e, respectively.
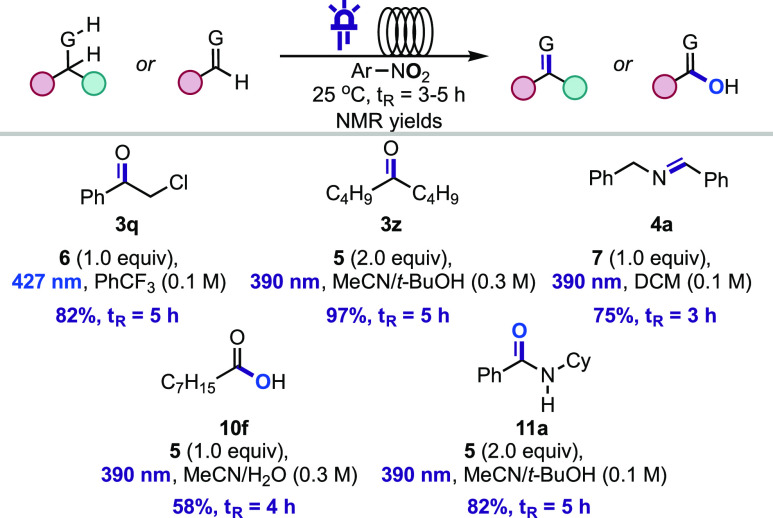
While the reported approach provides a complementary method to existing oxidations, the extended reaction times provide an opportunity for improvement. We postulated that reduced reaction times could be achieved under continuous-photoflow conditions (Scheme 2).41 A flow reactor consisted of a syringe pump to control residence time (tR), and a coil of fluorinated ethylene propylene (FEP) Teflon tubing irradiated by two LED lamps (general procedure F, see SI) was used to test the oxidation of one representative molecule from each substrate class assessed in batch (Scheme 2). Markedly, it was found that in all cases a 4- to 25-fold productivity improvement in mmol/h of the desired products was obtained leading to reduced reaction times.
Scheme 2. Continuous-Photoflow Oxidations.
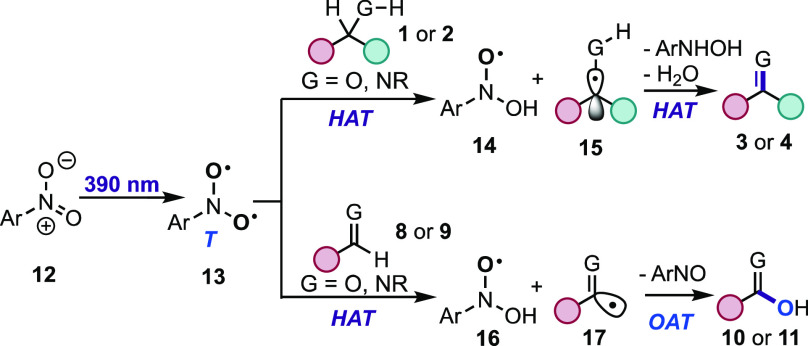
Based on the mechanistic studies from our lab,18−20 and others,42,43 the following mechanism is proposed (Scheme 3). Visible-light irradiation of the nitroarene 12 results in triplet diradical intermediate19,4413 that engages in HAT of the α-C(sp3)–H bond of 1 or 2 to generate α-hydroxyl radical 15 and N-hydroxy-N-phenylhydroxylamine radical 14. Kinetic isotope effect (KIE) studies9 and a radical clock probe test45 support that HAT participates in the rate-limiting step of the transformation and the formation of the α-hydroxyl radical intermediate, respectively (see SI). Subsequent HAT of intermediates 14 and 15 results in the desired oxidation products 3 or 4 and N-phenylhydroxylamine byproduct (see SI). An alternative pathway involving recombination of 14 and 15 and successive fragmentation leading to the oxidation products (3 or 4) is not supported based on 18O-labeling studies (see SI). For oxidation of C(sp2)–heteroatom systems, we propose that the HAT of 8 or 9 yields acyl radical 17 and N-hydroxy-N-phenylhydroxylamine radical 16. Radical recombination of the latter intermediates generates the OAT products 10 or 11 and condensation byproducts stemming from the nitrosoarene.46
Scheme 3. Proposed Mechanism.
In conclusion, we have illustrated that nitroarenes are potent photo-oxidants capable of oxidizing C(sp3)– and C(sp2)– heteroatom systems to generate synthetically useful ketones, imines, carboxylic acids, and amides with good reaction efficiency. Notably, our transformation can target vicinal and extended diols, contrary to aerobic N-hydroxyl-based protocols. Furthermore, we are able to oxidize free amines to imines without homocoupling and produce amides from imines under milder conditions. Also, this approach precludes the use of precious transition metals and expensive additives, thereby providing an opportunity for late-stage oxidation of medicinally relevant compounds in a cost-effective manner. The synthetic utility of the transformation is highlighted by its amenability to continuous-photoflow setup. Due to the anaerobic nature of the transformation and the practicality of using nitroarene oxidants, this protocol provides a sustainable alternative complementary to established oxidation methods.
Acknowledgments
Funding was provided through the generous start-up funds from the Department of Chemistry at New York University (NYU), the American Chemical Society Petroleum Research Fund (65501-DNI1), and the National Institute of General Medical Sciences of the National Institutes of Health (1R35GM150777-01). Ms. Sharon Lim is acknowledged for her assistance in the optimization studies during the early stages of the project.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
(The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c02292.
Experimental details, optimization studies, characterization data, and NMR spectra (PDF)
Author Contributions
J.K.M and W.A.H contributed equally to this work.
Author Contributions
A.H.B and R.M.O contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Hudlicky M.Oxidations in Organic Chemistry; American Chemical Society: Washington, DC, 1990. [Google Scholar]
- Bowden K.; Heilbron I. M.; Jones E. R. H.; Weedon B. C. L. Researches on Acetylenic Compounds. Part I. The Preparation of Acetylenic Ketones by Oxidation of Acetylenic Carbinols and Glycols. J. Chem. Soc. 1946, 39–45. 10.1039/jr9460000039. [DOI] [Google Scholar]
- Sharma A. K.; Swern D. Trifluoroacetic Anhydride: A New Activating Agent for Dimethyl Sulfoxide in the Synthesis of Iminosulfuranes. Tetrahedron Lett. 1974, 15, 1503–1506. 10.1016/S0040-4039(01)93121-1. [DOI] [Google Scholar]
- Baeyer A.; Villiger V. Einwirkung Des Caro’schen Reagens Auf Ketone. Ber. Dtsch. Chem. Ges. 1899, 32, 3625–3633. 10.1002/cber.189903203151. [DOI] [Google Scholar]
- Corey E. J.; Palani A. A Method for the Selective Oxidation of 1,4-Diols to Lactols. Tetrahedron Lett. 1995, 36, 3485–3488. 10.1016/0040-4039(95)00571-S. [DOI] [Google Scholar]
- Dess D. B.; Martin J. C. Readily Accessible 12-I-5 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156. 10.1021/jo00170a070. [DOI] [Google Scholar]
- Hoover J. M.; Stahl S. S. Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. 10.1021/ja206230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J. M.; Ryland B. L.; Stahl S. S. Mechanism of Copper(I)/TEMPO-Catalyzed Aerobic Alcohol Oxidation. J. Am. Chem. Soc. 2013, 135, 2357–2367. 10.1021/ja3117203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steves J. E.; Stahl S. S. Copper(I)/ABNO-Catalyzed Aerobic Alcohol Oxidation: Alleviating Steric and Electronic Constraints of Cu/TEMPO Catalyst Systems. J. Am. Chem. Soc. 2013, 135, 15742–15745. 10.1021/ja409241h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley R.; Testa A. C. Photochemical n → π* Excitation of Nitrobenzene. J. Am. Chem. Soc. 1966, 88, 4330–4332. 10.1021/ja00971a005. [DOI] [Google Scholar]
- Hashimoto S.; Sunamoto J.; Fujii H.; Kano K. Photochemical reduction of nitrobenzene and its reduction intermediates. III. The photochemical reduction of nitrobenzene. Bull. Chem. Soc. Jpn. 1968, 41, 1249–1251. 10.1246/bcsj.41.1249. [DOI] [Google Scholar]
- Hashimoto S.; Kano K. The photochemical reduction of nitrobenzene and its reduction intermediates. X. The photochemical reduction of the monosubstituted nitrobenzenes in 2-propanol. Bull. Chem. Soc. Jpn. 1972, 45, 549–553. 10.1246/bcsj.45.549. [DOI] [Google Scholar]
- Hashimoto S.; Kano K.; Ueda K. Photochemical Reduction of Nitrobenzene and Its Reduction Intermediates. IX. The Photochemical Reduction of 4-Nitropyridine in a Hydrochloric Acid-Isopropyl Alcohol Solution. Bull. Chem. Soc. Jpn.. 1971, 44, 1102–1106. 10.1246/bcsj.44.1102. [DOI] [Google Scholar]
- Wang B.; Ma J.; Ren H.; Lu S.; Xu J.; Liang Y.; Lu C.; Yan H. Chemo-, Site-Selective Reduction of Nitroarenes under Blue-Light, Catalyst-Free Conditions. Chin. Chem. Lett. 2022, 33, 2420–2424. 10.1016/j.cclet.2021.11.023. [DOI] [Google Scholar]
- Wang B.; Ren H.; Cao H.-J.; Lu C.; Yan H. A Switchable Redox Annulation of 2-Nitroarylethanols Affording N-Heterocycles: Photoexcited Nitro as a Multifunctional Handle. Chem. Sci. 2022, 13, 11074–11082. 10.1039/D2SC03590A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klán P.; Šolomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J. Electronic Effects of Nitro, Nitroso, Amino and Related Groups. Chemistry of Amino, Nitroso, Nitro and Related Groups 1996, 479. 10.1002/047085720X.ch11. [DOI] [Google Scholar]
- Wise D. E.; Gogarnoiu E. S.; Duke A. D.; Paolillo J. M.; Vacala T. L.; Hussain W. A.; Parasram M. Photoinduced Oxygen Transfer Using Nitroarenes for the Anaerobic Cleavage of Alkenes. J. Am. Chem. Soc. 2022, 144, 15437–15442. 10.1021/jacs.2c05648. [DOI] [PubMed] [Google Scholar]
- Paolillo J. M.; Duke A. D.; Gogarnoiu E. S.; Wise D. E.; Parasram M. Anaerobic Hydroxylation of C(Sp3)-H Bonds Enabled by the Synergistic Nature of Photoexcited Nitroarenes. J. Am. Chem. Soc. 2023, 145, 2794–2799. 10.1021/jacs.2c13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise D. E.; Parasram M. Photoexcited Nitroarenes as Anaerobic Oxygen Atom Transfer Reagents. Synlett 2023, 34, 1655. 10.1055/s-0042-1751443. [DOI] [Google Scholar]
- Galeotti M.; Salamone M.; Bietti M. Electronic Control over Site-Selectivity in Hydrogen Atom Transfer (HAT) Based C(Sp3)-H Functionalization Promoted by Electrophilic Reagents. Chem. Soc. Rev. 2022, 51, 2171–2223. 10.1039/D1CS00556A. [DOI] [PubMed] [Google Scholar]
- Tyler M. W.; Zaldivar-Diez J.; Haggarty S. J. Classics in Chemical Neuroscience: Haloperidol. ACS Chem. Neurosci. 2017, 8, 444–453. 10.1021/acschemneuro.7b00018. [DOI] [PubMed] [Google Scholar]
- Lampe J. W.; Jagdmann G. E.; Johnson M. G.; Lai Y. S.; Lowden C. T.; Lynch M. P.; Mendoza J. S.; Murphy M. M.; Wilson J. W.; Ballas L. M.; Carter K.; Biggers C. K.; Darges J. W.; Davis J. E.; Hubbard F. R.; Stamper M. L.; Defauw J. M.; Fog lesong R. J.; Hall S. E.; Heerding J. M.; Hollinshead S. P.; Hu H.; Hughes P. F. Synthesis and Protein Kinase Inhibitory Activity of Balanol Analogues with Modified Benzophenone Subunits. J. Med. Chem. 2002, 45, 2624–2643. 10.1021/jm020018f. [DOI] [PubMed] [Google Scholar]
- Wu S. B.; Long C.; Kennelly E. J. Structural Diversity and Bioactivities of Natural Benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174. 10.1039/C4NP00027G. [DOI] [PubMed] [Google Scholar]
- Masjedizadeh M. R.; Dannecker-Doerig I.; Little R. D. Linearly Fused vs Bridged Regioselection in the Intramolecular 1,3-diyl Trapping Reaction. J. Org. Chem. 1990, 55, 2742–2752. 10.1021/jo00296a035. [DOI] [Google Scholar]
- Kato M.; Hammam M. A. S.; Taniguchi T.; Suga Y.; Monde K. What is the True Structure of D609, a Widely Used Lipid Related Enzyme Inhibitor?. Org. Lett. 2016, 18, 768–771. 10.1021/acs.orglett.6b00025. [DOI] [PubMed] [Google Scholar]
- Largeron M. Protocols for the Catalytic Oxidation of Primary Amines to Imines. Eur. J. Org. Chem. 2013, 2013, 5225–5235. 10.1002/ejoc.201300315. [DOI] [Google Scholar]
- Yamamoto Y.; Kodama S.; Nomoto A.; Ogawa A. Innovative Green Oxidation of Amines to Imines under Atmospheric Oxygen. Org. Biomol. Chem. 2022, 20, 9503–9521. 10.1039/D2OB01421A. [DOI] [PubMed] [Google Scholar]
- Purohit M.; Kalla S.; Jangir R. A Comprehensive Review on Cu-Catalysed Aerobic Oxidation of Amines to Imines. ChemistrySelect 2023, 8, e202300386 10.1002/slct.202300386. [DOI] [Google Scholar]
- Largeron M. Protocols for the Catalytic Oxidation of Primary Amines to Imines. Eur. J. Org. Chem. 2013, 2013, 5225–5235. and references therein 10.1002/ejoc.201300315. [DOI] [Google Scholar]
- Corey E. J.; Gilman N. W.; Ganem B. E. New Methods for the Oxidation of Aldehydes to Carboxylic Acids and Esters. J. Am. Chem. Soc. 1968, 90, 5616–5617. 10.1021/ja01022a059. [DOI] [Google Scholar]
- Liao Y.; Aspin A.; Yang Z. Anaerobic Oxidation of Aldehydes to Carboxylic Acids under Hydrothermal Conditions. RSC Adv. 2022, 12, 1738–1741. 10.1039/D1RA08444E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H. A.; Cho Y. H.; Lee Y. S.; Cheon C. H. Formation of Amides from Imines via Cyanide-Mediated Metal-Free Aerobic Oxidation. J. Org. Chem. 2015, 80, 11993–11998. 10.1021/acs.joc.5b01922. [DOI] [PubMed] [Google Scholar]
- Nagaraaj P.; Vijayakumar V. Oxidation of Amine α-Carbon to Amide: A Review on Direct Methods to Access the Amide Functionality. Org. Chem. Front. 2019, 6, 2570–2599. 10.1039/C9QO00387H. [DOI] [Google Scholar]
- Han L.; Xing P.; Jiang B. Selective Aerobic Oxidation of Alcohols to Aldehydes, Carboxylic Acids, and Imines Catalyzed by a Ag-NHC Complex. Org. Lett. 2014, 16, 3428–3431. 10.1021/ol501353q. [DOI] [PubMed] [Google Scholar]
- Jeong D.; Kim H.; Cho J. Oxidation of Aldehydes into Carboxylic Acids by a Mononuclear Manganese(III) Iodosylbenzene Complex through Electrophilic C-H Bond Activation. J. Am. Chem. Soc. 2023, 145, 888–897. 10.1021/jacs.2c09274. [DOI] [PubMed] [Google Scholar]
- Ramarao J.; Yadav S.; Satyam K.; Suresh S. N-Heterocyclic Carbene (NHC)-Catalyzed Oxidation of Unactivated Aldimines to Amides via Imine Umpolung Under Aerobic Conditions. RSC Adv. 2022, 12, 7621–7625. 10.1039/D2RA00897A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S.; Ma Y.; Chen W.; Luo J. Pd-Catalyzed Oxidation of Aldimines to Amides. Synlett 2018, 29, 2191–2194. 10.1055/s-0037-1610653. [DOI] [Google Scholar]
- Brtogden R. N.; Heel R. C.; Speight T. M.; Avery G. S. Fenbufen: A Review of Its Pharmalogical Properties and Therapeutic Use in Rheumatic Dieases and Acute Pain. Drugs 1981, 21, 1–22. 10.2165/00003495-198121010-00001. [DOI] [PubMed] [Google Scholar]
- Kerwar S. S. Pharmacologic Properties of Fenbufen. Am. J. Med. 1983, 75, 62–69. 10.1016/0002-9343(83)90330-3. [DOI] [PubMed] [Google Scholar]
- Cambié D.; Bottecchia C.; Straathof N. J. W.; Hessel V.; Noël T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]
- Libman J.; Berman E. Photoexcited Nitrobenzene for Benzylic Hydroxylation: The Synthesis of 17β-Acetoxy-9α-Hydroxy-3-Methoxy-Estra-1,3,5(10)-Triene. Tetrahedron Lett. 1977, 18, 2191–2192. 10.1016/S0040-4039(01)83717-5. [DOI] [Google Scholar]
- Negele S.; Wieser K.; Severin T. Photochemical Oxidation of Hydrocarbons by Nitropyridinium Salts. J. Org. Chem. 1998, 63, 1138–1143. 10.1021/jo971617a. [DOI] [Google Scholar]
- Ruffoni A.; Hampton C.; Simonetti M.; Leonori D. Photoexcited Nitroarenes for the Oxidative Cleavage of Alkenes. Nature 2022, 610, 81–86. 10.1038/s41586-022-05211-0. [DOI] [PubMed] [Google Scholar]
- Newcomb M.Radical Kinetics and Clocks. In Encyclopedia of Radicals in Chemistry, Biology and Materials; 2012. 10.1002/9781119953678.rad007 [DOI] [Google Scholar]
- An alternative mechanism proposed by Hurley and Testa involves a secondary oxidation step via photoinduced homolysis of the N-hydroxy-N-phenylhydroxylamine byproduct and subsequent HAT of the formed N- and O-centered radicals with the C(sp3)–H heteroatom systems cannot be ruled out at this stage. See ref (10) andFrolov A. N.; Kuznetsova N. A.; El’tsov A. V. Intermolecular Photochemical Reduction of Aromatic Nitro-Compounds. Russ. Chem. Rev. 1976, 45, 1024–1034. 10.1070/RC1976v045n11ABEH002755. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.