Abstract
Background
Our aim was to explore the prognostic value of anthropometric parameters in a large population of patients treated with immunotherapy.
Methods
We retrospectively included 623 patients with advanced non-small cell lung cancer (NSCLC) (n=318) or melanoma (n=305) treated by an immune-checkpoint-inhibitor having a pretreatment (thorax-)abdomen-pelvis CT scan. An external validation cohort of 55 patients with NSCLC was used. Anthropometric parameters were measured three-dimensionally (3D) by a deep learning software (Anthropometer3DNet) allowing an automatic multislice measurement of lean body mass, fat body mass (FBM), muscle body mass (MBM), visceral fat mass (VFM) and sub-cutaneous fat mass (SFM). Body mass index (BMI) and weight loss (WL) were also retrieved. Receiver operator characteristic (ROC) curve analysis was performed and overall survival was calculated using Kaplan-Meier (KM) curve and Cox regression analysis.
Results
In the overall cohort, 1-year mortality rate was 0.496 (95% CI: 0.457 to 0.537) for 309 events and 5-year mortality rate was 0.196 (95% CI: 0.165 to 0.233) for 477 events. In the univariate Kaplan-Meier analysis, prognosis was worse (p<0.001) for patients with low SFM (<3.95 kg/m2), low FBM (<3.26 kg/m2), low VFM (<0.91 kg/m2), low MBM (<5.85 kg/m2) and low BMI (<24.97 kg/m2). The same parameters were significant in the Cox univariate analysis (p<0.001) and, in the multivariate stepwise Cox analysis, the significant parameters were MBM (p<0.0001), SFM (0.013) and WL (0.0003). In subanalyses according to the type of cancer, all body composition parameters were statistically significant for NSCLC in ROC, KM and Cox univariate analysis while, for melanoma, none of them, except MBM, was statistically significant. In multivariate Cox analysis, the significant parameters for NSCLC were MBM (HR=0.81, p=0.0002), SFM (HR=0.94, p=0.02) and WL (HR=1.06, p=0.004). For NSCLC, a KM analysis combining SFM and MBM was able to separate the population in three categories with the worse prognostic for the patients with both low SFM (<5.22 kg/m2) and MBM (<6.86 kg/m2) (p<0001). On the external validation cohort, combination of low SFM and low MBM was pejorative with 63% of mortality at 1 year versus 25% (p=0.0029).
Conclusions
3D measured low SFM and MBM are significant prognosis factors of NSCLC treated by immune checkpoint inhibitors and can be combined to improve the prognostic value.
Keywords: Immunotherapy, Non-Small Cell Lung Cancer, Melanoma
WHAT IS ALREADY KNOWN ON THIS TOPIC
Survival outcomes are significantly longer in overweight/obese patients (as determined by higher body mass index) for patients with cancer treated with immune checkpoint inhibitors.
WHAT THIS STUDY ADDS
It is shown in this study that an automatic three-dimensional (3D) determination of body composition, more accurate than the body mass index, is possible in clinical routine from diagnostic scans and that 3D-measured low muscle mass is a significant prognosis factor of melanoma treated by immune checkpoint inhibitors while 3D-measured low subcutaneous fat mass and muscle mass are significant prognosis factors of non-small cell lung cancer treated by immune checkpoint inhibitors and both can be combined to improve the prognostic value.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Determination of body composition may help to better define the prognosis of cancers treated with immunotherapy. In the long-term, therapeutic adaptation according to body composition could be possible.
Introduction
In 2020, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred worldwide.1 Among them, lung cancers represented 11.4% of new cases and 18.0% of deaths and melanoma 1.7% of new cases and 0.6% of deaths.1
Cancers have the ability to disrupt immune response by interfering with adaptive immunity.2 Immunotherapy using immune checkpoint inhibitors (ICI), notably anti-PD-1 (programmed cell death protein-1) and anti-PDL-1 (PD-1 ligand) antibody, has been shown to improve outcome of stage IIIb/IV non-small cell lung cancer (NSCLC) and of high-risk resected stage III/IV melanoma, which made it a new standard of care.3 Compared with cytotoxic chemotherapy and targeted therapy, ICIs have been shown to induce a durable response (plateau) in a fraction of patients with advanced or metastatic cancer, even after their interruption.4
Despite the clinical success of ICI in many patients with cancer, with some durable response, better understanding of determinants affecting response is required, thus ICI response rates in general are around 20% in unselected NSCLC.5 Some biomarkers are focused on the immune tumor microenvironment6 or on the gut microbiome while others focus on the disease and/or the host as a whole.
Thus, total tumor burden, as evaluated by positron emission tomography (PET)/CT at the start of treatment, has been shown to be a predictor of patient survival. People with a higher total tumor burden tend to have a worse prognosis and a lower likelihood of surviving compared with those with a lower total tumor burden.7–9
Patient-centered parameters also appear to be prognostic for survival. Therefore, body mass index (BMI) was higher in patients with melanoma treated by anti-PD-1 checkpoint inhibitors who had a better survival10 or had early acute limiting toxicity.11 Moreover, in a large study involving 976 patients with NSCLC (65.1%) and other cancer types, the response rate was significantly higher and survival outcomes were significantly longer in overweight/obese patients, for both sex.12
Although BMI is an interesting parameter to describe the overall mass of patients, it does not describe body composition. Some studies exploring the impact of body composition determined on CT have shown the pejorative prognostic value of low subcutaneous fat mass13 or sarcopenia14 for NSCLC treated with ICI. For melanoma, comparable results obtained by body composition analysis were identified with several features with improved clinical outcomes.15 16
Most of the time, these body composition estimates were based on a two-dimensional (2D) basis,17 with a segmentation at the L3 abdominal level. However, 2D estimates can be less accurate than three-dimensional (3D) multislice measurements.18 For example, it has been shown that during weight loss, changes in visceral and subcutaneous adipose tissue are poorly evaluated on 2D imaging,19 while 3D imaging gives good results for intra-abdominal fat.20 Therefore, multislice segmentation is preferable,21 but needs automatic processing to avoid a time-consuming manual segmentation.19
To accurately assess anthropometric parameters on CT, including CT of PET/CT, automatic, multislice measurement techniques have been developed.18 22 One of these algorithms has been tested on a limited number of patients with stage IV NSCLC who received immunotherapy. The results showed that subcutaneous fat mass can be a prognostic factor for this patient population.13
The main objective of this study was to evaluate the prognostic value of anthropometric parameters evaluated on CT scan using an automatic 3D deep learning software, on a large database of patients with NSCLC and melanoma treated with ICI.
Materials and methods
Population
The study included adult patients with advanced melanoma or NSCLC tumors who received ICI at Gustave Roussy cancer center in France between June 2014 and December 2018. Population characteristics are presented in table 1. Included patients were extracted from a previous database23 with the condition of having a wide-field CT scan (of PET/CT or thoraco-abdominopelvic or abdominopelvic diagnostic CT scan) within 90 days before immunotherapy. For the external validation, patients with stage IV NSCLC treated by nivolumab after at least one chemotherapy session in pulmonology, thoracic oncology, and respiratory intensive care department of Rouen University Hospital between February 2015 and October 2017 were included.
Table 1.
Characteristics of the population
| Characteristic | All patients (n=623, 100%) |
Non-small cell lung cancer (n=318, 51.0%) |
Melanoma (n=305, 49.0%) |
| Sex, n (%) Male Female |
353 (56.7) 270 (43.3) |
201 (63.2) 117 (36.8) |
152 (49.8) 153 (50.2) |
| Age (years) Median range |
63 (22–92) | 63 (34–92) | 62 (22–91) |
| Immunotherapy, n (%) Pembrolizumab Nivolumab Nivolumab+ipilimumab Durvalumab |
318 (51.0) 286 (45.9) 10 (1.6) 9 (1.4) |
76 (23.9) 233 (73.3) – 9 (2.8) |
242 (79.3) 53 (17.4) 10 (3.3) – |
| Stage, n (%) IV III II |
536 (86.0) 85 (13.6) 2 (0.3) |
276 (86.58) 41 (12.9) 1 (0.3) |
260 (85.2) 44 (14.4) 1 (0.3) |
| Metastasis Bone Liver Brain |
205 (32.9) 165 (26.5) 183 (29.4) |
144 (45.3) 87 (27.4) 87 (27.4) |
61 (20.0) 78 (25.6) 96 (31.5) |
| Prior treatment Systemic Radiotherapy Prior surgery |
431 (69.2) 275 (44.1) 335 (53.7) |
296 (93.1) 165 (51.9) 98 (30.8) |
135 (44.3) 110 (36.1) 237 (77.7) |
| Outcome | |||
| 1-year mortality rate 95% CI Events |
0.496 (0.457 to 0.537) 309 |
0.411 (0.359 to 0.47) 182 |
0.582 (0.529 to 0.64) 127 |
| 5-year mortality rate 95% CI Events |
0.196 (0.165 to 0.233) 477 |
0.11 (0.0762 to 0.158) 264 |
0.279 (0.23 to 0.337) 213 |
| Overall survival (months) Median IQR |
11.8 2.9–37.3 |
8.7 2.1–21.1 |
19.7 5.6–73.3 |
| Follow-up (months) Median IQR |
10.7 2.7–32.3 |
7.0 2.0–19.0 |
18.8 5.3–46.8 |
| Morphometric data | |||
| BMI (kg/m²) Mean (±SD) (min–max) |
24.1 (±4.7) (13.7–41.4) |
23.2 (±4.7) (13.7–39.9) |
25.0 (±4.5) (16.0–41.4) |
| FBM (kg/m²) Mean (±SD) (min–max) |
5.7 (±3.5) (0.1–21.9) |
4.9 (±3.2) (0.1–18.3) |
6.6 (±3.6) (0.8–21.9) |
| SFM (kg/m²) Mean (±SD) (min–max) |
4.8 (±3.0) (0.1–20.7) |
4.1 (±2.7) (0.1–15.7) |
5.5 (±3.1) (0.8–20.7) |
| VFM (kg/m²) Mean (±SD) (min–max) |
0.9 (±0.7) (0.01–3.4) |
0.9 (±0.7) (0.01–3.4) |
1.0 (±0.7) (0.02–3.3) |
| MBM (kg/m²) Mean (±SD) (min–max) |
5.8 (±1.5) (1.2–14.1) |
5.4 (±1.4) (1.2–9.9) |
6.2 (±1.6) (2.9–14.1) |
| LBM (kg/m²) Mean (±SD) (min–max) |
18.3 (±2.8) (4.9–36.1) |
18.2 (±2.6) (11.7–26.6) |
18.4 (±3.0) (4.9–36.1) |
BMI, body mass index; FBM, fat body mass; LBM, lean body mass; MBM, muscle body mass; SFM, subcutaneous fat mass; VFM, visceral fat mass.
Body composition
Height and weight measurements taken just before treatment and within 6 months prior to starting ICI treatment were collected from medical records and used to calculate BMI and weight loss (WL). Anthropometer3DNet, an updated version of Anthropometer3D,13 24 25 was used to extract body composition parameters from CT images. This software, which is available for research purposes on www.oncometer3d.com, can automatically measure 3D fat body mass (FBM), subcutaneous fat mass (SFM), visceral fat mass (VFM), muscle body mass (MBM) and lean body mass (LBM) (in kilograms) on multislice CT scans in less than 5 min (see figure 1). This software performs a deep learning-based segmentation26 of fat (visceral and subcutaneous) and muscle voxels. For parts outside the acquisition area, it uses adaptive extrapolation factors24 for the tissues of interest to determine the whole-body mass. The software is described in a previous publication.27
Figure 1.
Graphical representation of the automatic report generated by Anthropometer3DNet with subcutaneous fat (red), muscle (blue) and visceral fat (green) voxels on axial and frontal views.
Endpoints and assessments
The following baseline clinical data were collected: age, sex, type of cancer, and type of ICI. The primary endpoint was overall survival (OS), defined as the time from the beginning of immune therapy to death or last follow-up.
Statistical analysis
Descriptive statistics were calculated for the study population, with continuous variables reported as mean and SD, and categorical variables as frequencies and percentages. Spearman’s correlation coefficient was used to evaluate correlations between the different body composition parameters. The predictive accuracy of the parameters for 5-year survival was assessed using receiver operator characteristic (ROC) analysis and the area under the curve (AUC). The optimal cut-off value was determined by maximizing specificity and sensitivity using Youden’s Index. Two-sided tests were considered significant at the 5% level. For parameters with an AUC greater than 0.5, the Kaplan-Meier method was used to estimate survival functions, and the log-rank test was used to evaluate significance. Cox proportional hazards models were used to assess the relationship between study variables and survival rates. Analyses were performed in the overall population, as well as in the subgroups of patients with NSCLC or melanoma.
Results
Patient characteristics
A total of 623 patients (318 NSCLC and 305 melanoma) were included in this retrospective study; their characteristics, notably according to the tumor type and ICI received, are described in table 1. The average number of days between imaging and the start of immunotherapy was 31 days (median: 26 days), with a minimum of 0 days and a maximum of 90 days. In the overall cohort, 1-year mortality rate was 0.496 (95% CI: 0.457 to 0.537) for 309 events and 5-year mortality rate was 0.196 (95% CI: 0.165 to 0.233) for 477 events. For the external validation, 55 patients with NSCLC treated by nivolumab were included, with details of the population previously published.13
Whole population analysis
Correlations
Concerning the body composition parameters and their correlations, as visible in the correlogram (figure 2), FBM and SFM were very highly correlated (ρ=0.99), MBM and SFM slightly correlated (ρ=0.33) and BMI moderately-to-highly correlated with the other parameters (from ρ=0.49 with MBM to ρ=0.80 with FBM).
Figure 2.
Spearman’s correlations between anthropometric parameters (LBM, FBM, MBM, VFM and SFM) and BMI. BMI, body mass index; FBM, fat body mass; LBM, lean body mass; MBM, muscle body mass; SFM, subcutaneous fat mass; VFM, visceral fat mass.
ROC curve analysis
The ROC curve analysis of the anthropometric parameters for OS are summarized in table 2 with figures in online supplemental data 1. All body composition parameters appear significant, including MBM (AUC=0.69, p=<0.001) and SFM (AUC=0.60, p=0.01). Figure 3 shows the Kaplan-Meier analysis with log-rank tests by using cut-offs determined on the ROC curve analysis for BMI, FBM, SFM, VFM, MBM and LBM. All parameters, except LBM, were found as significant risk factors for OS (p<0.001), considering cut-off values of 5.26 kg/m2, 3.95 kg/m2, 0.91 kg/m2, 5.85 kg/m2 and 24.97 kg/m2 for FBM, SFM, VFM, MBM and BMI, respectively.
Table 2.
Diagnostic performance, clinical and PET metrics, and anthropometric parameters measured on 18FDG PET/CT for 5-year overall survival using an ROC analysis
| Whole population | NSCLC | Melanoma | |||||||
| Cut-offs | AUC | P value | Cut-offs | AUC | P value | Cut-offs | AUC | P value | |
| Age at diagnosis | NA | NA | 0.74 | NA | NA | 0.92 | NA | NA | 0.51 |
| BMI | 25.0 | 0.61 | 0.004 | 26.83 | 0.78 | 0.002 | NA | NA | 0.57 |
| FBM | 5.26 | 0.62 | 0.002 | 6.30 | 0.70 | 0.02 | NA | NA | 0.41 |
| SFM | 3.95 | 0.61 | 0.006 | 5.22 | 0.68 | 0.03 | NA | NA | 0.56 |
| VFM | 0.91 | 0.64 | <0.001 | 0.91 | 0.73 | 0.009 | NA | NA | 0.06 |
| MBM | 5.85 | 0.70 | <0.001 | 6.86 | 0.82 | <0.001 | 5.85 | 0.60 | 0.01 |
| LBM | NA | NA | 0.09 | 20.53 | 0.79 | 0.002 | NA | NA | 0.59 |
AUC, area under the curve; BMI, body mass index; FBM, fat body mass; 18FDG, 18-Fluorodeoxyglucose; 18FDG, 18F-Fluorodesoxyglucose; LBM, lean body mass; MBM, muscle body mass; NA, not available; NSCLC, non-squamous cell lung cancer; PET, positron emission tomography; ROC, receiver operator characteristics; SFM, subcutaneous fat mass; VFM, visceral fat mass.
Figure 3.
Kaplan-Meier estimates of overall survival according to the threshold determined on ROC analysis of FBM, SFM, VFM, MBM, LBM and BMI for (A) the whole population and (B) NSCLC. BMI, body mass index; FBM, fat body mass; LBM, lean body mass; MBM, muscle body mass; NSCLC, non-small cell lung cancer; ROC, receiver operator characteristic; SFM, subcutaneous fat mass; VFM, visceral fat mass.
jitc-2023-007315supp001.pdf (120.6KB, pdf)
Cox analysis
Table 3 shows the results of the Cox analysis. In a univariate study, all body composition parameters, except LBM, were significantly associated with survival. Some clinical parameters (cerebral-meningeal metastasis status and WL) were significant as well. In the multivariate stepwise Cox analysis using only the univariate statistically significant parameters from the univariate study, the significant parameters were cerebral-meningeal metastasis status, WL, BMI, SFM and MBM.
Table 3.
Univariate and multivariate Cox analysis using continuous values for clinical and significant PET metrics and anthropometric parameters measured on 18FDG PET/CT
| Whole population | NSCLC | Melanoma | ||||
| Univariate Cox analysis | ||||||
| HR | P value | HR | P value | HR | P value | |
| Age | 1.00 | 0.80 | 1.00 | 0.74 | 1.00 | 0.82 |
| Sex | 1.12 | 0.22 | 0.99 | 0.95 | 1.09 | 0.55 |
| Cerebral-meningeal metastasis | 1.25 | 0.02 | 1.01 | 0.96 | 1.56 | 0.002 |
| BMI | 0.96 | 0.0003 | 0.94 | 8×10–05 | 1.00 | 0.75 |
| Weight loss | 1.07 | 9×10–05 | 1.07 | 0.002 | 1.07 | 0.049 |
| FBM | 0.95 | 8×10–05 | 0.92 | 0.0001 | 0.99 | 0.74 |
| SFM | 0.94 | 0.0002 | 0.91 | 0.0003 | 0.99 | 0.81 |
| VFM | 0.79 | 0.0006 | 0.71 | 0.0005 | 0.94 | 0.53 |
| MBM | 0.82 | 3×10–10 | 0.80 | 1×10–6 | 0.89 | 0.01 |
| LBM | 0.99 | 0.44 | 0.94 | 0.02 | 1.02 | 0.38 |
| Multivariate stepwise Cox analysis | ||||||
| Cerebral-meningeal metastasis | 1.21 | 0.053 | NA | NA | 1.50 | 0.004 |
| BMI | 1.03 | 0.10 | NA | NA | 1.03 | 0.06 |
| Weight loss | 1.07 | 0.0003 | 1.06 | 0.004 | 1.06 | 0.10 |
| FBM | NA | NA | NA | NA | NA | NA |
| SFM | 0.93 | 0.013 | 0.94 | 0.02 | NA | NA |
| VFM | NA | NA | NA | NA | NA | NA |
| MBM | 0.81 | 3×10–08 | 0.83 | 0.0002 | 0.90 | 0.02 |
BMI, body mass index; FBM, fat body mass; 18FDG, 18F-fluorodesoxyglucose; LBM, lean body mass; MBM, muscle body mass; MTV, metabolic tumor volume; NA, not available; NSCLC, non-small cell lung cancer; PET, positron emission tomography; SFM, subcutaneous fat mass; VFM, visceral fat mass.
Subgroup analysis : NSCLC
For the subgroup of patients with NSCLC, on the ROC curve analysis (table 2) and in the Kaplan-Meier analysis with log-rank tests (figure 3), all body composition parameters, except LBM, were statistically significant (p<0.05). In the univariate Cox analysis, all of them were statistically significant. In the multivariate analysis, the weight loss, SFM and MBM were statistically significant (p=0.004, p=0.02 and p=0.0002, respectively) (table 3).
Subgroup analysis: melanoma
Contrary to the NSCLC subanalyses, only MBM was statistically significant (p<0.05) for melanoma in ROC curve analyses (table 2), Kaplan-Meier analysis with log-rank tests (not shown) and univariate and multivariate Cox analysis (table 3).
Combination of MBM and SFM
Because of their relatively low reciprocal correlations and their prognostic values observed in the different univariate and multivariate analyses performed, a combination of two parameters (MBM and SFM) was performed.
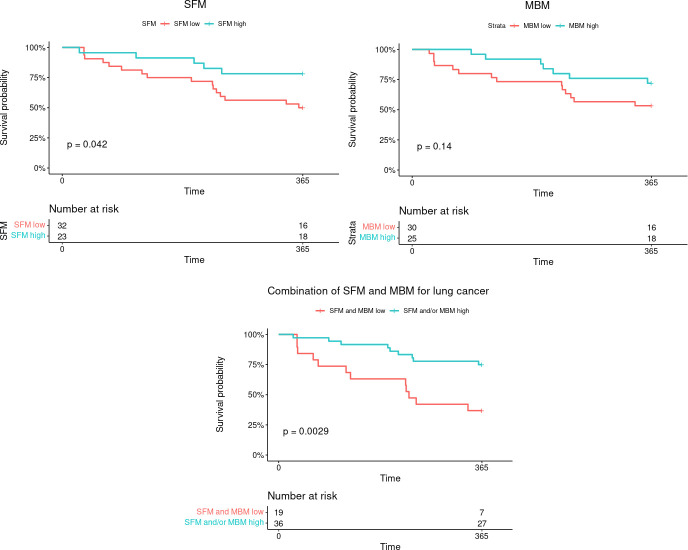
Figure 4 shows a Kaplan-Meier survival analysis with a prognostic stratification model combining two adverse parameters: MBM and SFM with three identified risk-groups. For the whole population, a high-risk group comprizing patients with low MBM and low SFM had significantly worse prognosis (p<0.001) than low-risk patients with both high MBM/SFM or intermediate-risk patients with only one high parameter. Similar results were observed for NSCLC (p<0.001).
Figure 4.
Kaplan-Meier estimates of overall survival according to a prognostic stratification model combining two adverse parameters: MBM and SFM with three identified risk-groups. MBM, muscle body mass; SFM, subcutaneous fat mass.
External validation
Figure 5 shows a Kaplan-Meier survival analysis on the external database of 55 patients with NSCLC using parameters and their cut-offs (SFM<5.22 kg/m² and MBM<6.86 kg/m²) determined on the NSCLC population. A significant prognostic value was found for SFM alone (p=0042) and for the combination of SFM and MBM with a worse prognosis when both SFM and MBM were low with 63% of mortality at 1 year versus 25% (p=0.0029).
Figure 5.
Kaplan-Meier estimates on a external validation cohort of overall survival at 1 year according to SFM, MBM and a prognostic stratification model combining two adverse parameters: MBM and SFM. MBM, muscle body mass; SFM, subcutaneous fat mass.
Discussion
The aim of the present study was to investigate the prognostic value of anthropometric parameters in a large population of patients treated with immunotherapy for NSCLC and melanoma. In a population of 623 patients (n=318 with NSCLC and n=305 for melanoma), anthropometric parameters were measured on pretherapeutic 3D CT scan by an automatic 3D software (Anthropometer3DNet). In our study, only MBM was a significant predictor of survival in melanoma, while all of the body composition parameters were significant in NSCLC. In multivariate Cox analysis, the significant predictors of survival in NSCLC were MBM (HR=0.81, p<0.0001), SFM (HR=0.93, p=0.01), and WL (HR=1.06, p=0.004). Kaplan-Meier analysis combining SFM and MBM was able to stratify patients into three prognostic categories, with the worst prognosis observed in those with both low SFM (<3.95 kg/m²) and MBM (<5.85 kg/m²) showing a complementary prognostic value of the combination of the two parameters. We confirmed the prognostic value of the combination of SFM and MBM in an external validation cohort of 55 patients with NSCLC treated by nivolumab.
Sarcopenia is a condition characterized by age-related loss of muscle mass and function.28 In a meta-analysis of 2501 patients from 26 studies many cancers treated by ICIs, a negative association between sarcopenia and efficacy of ICI was found with poor survival and poor response in patients with sarcopenia,29 justifying its assessment in clinical practice to select patients who may respond to ICIs pre-therapeutically.29 Similar results were observed in an other systematic review and meta-analysis where patients with sarcopenia tended to have a lower response rate than those without the disease (30.5% vs 15.9%; p=0.095) and a significantly shorter 1-year progression-free survival (PFS) rate (32% vs 10.8%; risk ratio (RR), 1.31; p<0.001) and 1-year OS rate (66 vs 43%; RR, 1.71; p<0.001).30 Our results concerning MBM are therefore consistent with those observed in the literature.31 From a physiopathological standpoint, that observation could be explained by a vicious circle of chronic inflammation and malnutrition leading up to cachexia and sarcopenia in patients with cancer and affecting immune system and response to therapy.32
Many methods exist to measure muscle indices and have proven the impact of sarcopenia for cancers treated by ICI, like two-photon absorptiometry14 of psoas muscle index measured on 2D CT scan.33 The use of 3D rather than 2D segmentation to determine body composition on CT is recent22 24 27 and allows a more accurate measurement of body composition, especially for fat, than the 2D segmentation computed at the L3 abdominal level.24 27 34 The quality of the 3D measurements compared with the 2D measurements may explain the prognostic value found of the association of muscle mass and subcutaneous fat mass for lung cancer in multivariate analysis despite the addition of weight loss as a covariable that does not cancel out the effect of subcutaneous fat mass in the current study.23
Among the studied prognostic factors, ‘sarcopenic obesity’ is a particular entity joining obesity and sarcopenia, with high fat mass and low muscle mass,35 which has notably been associated with increased toxicity with ICI.11 This association could be related to the pharmacokinetics of immunotherapy, with a significant variability depending on body composition. Concerning survival, the ‘obesity paradox’ has however been described in renal cell carcinoma,36 colorectal cancer37 and NSCLC.38 It refers to the improved survival among overweight/obese patients compared with normal weight patients, which appears to be related to ICI alone and not to the combination of chemotherapy and ICI.39 Some authors think that this effect could be explained by visceral obesity40 as visceral adipose tissue appears to be a prognostic parameter for several cancers, including lung cancer.41 However, the prognostic value of visceral adipose tissue seems to be observed for patients with lung cancers treated with chemotherapy42 and not for those treated with immunotherapy.43 44 In the case of immunotherapy, a team observed recently in a population of 52 patients with NSCLC treated with ICIs that low ‘lumbar skeletal muscle index’ and low ‘subcutaneous fat index’ were predictive of inferior OS.44 These results are consistent with ours, established on a larger population of 318 patients, plus 55 for the external validation cohort, with a 3D automatic method. While this ‘obesity paradox’ effect is observed in our study for NSCLC, this was not the case for melanoma, possibly due to the fact that melanoma have better OS than NSCLC (in our study, median OS 19.7 months vs 8.7 months, respectively), which could limit the impact of one fat measurement performed before treatment. However, these results are inconsistent with other studies, such the one of McQuade et al where, for patients with metastatic melanoma, obesity was associated with improved PFS and OS compared with those outcomes in patients with normal BMI.10
One particularly interesting finding of our study was the ability of a combination of SFM and MBM to stratify patients into different prognostic categories. In the whole study population and in NSCLC specifically, Kaplan-Meier analysis combining SFM and MBM was able to stratify patients into three prognostic categories, with the worst prognosis observed in those with both low SFM and MBM. This suggests that a combination of these two body composition variables may provide more prognostic information than either alone.
One of the advantages of the 3D analysis performed by Anthropometer3DNet is that it uses extrapolation factors to expand on the data beyond the scope of acquisition, resulting in a total mass measurement instead of just an area or index. This total mass measurement has potential therapeutic implications and is obtainable through automatic segmentation and a wide range of Hounsfield units. Furthermore, the software, compatible with both injected and non-injected scanners, is currently accessible for research purposes on the Oncometer3D.com platform through an online service. As the measurements are automatic and take only a few seconds, routine use could be envisaged in the future. For example, these measurements could be taken automatically when a patient undergoes a diagnostic or follow-up CT scan, and recorded in the patient’s report. They would then be available to any clinician who wished to use it.
While our study identified several body composition parameters that were significantly associated with survival in patients with NSCLC treated with ICIs, it is important to consider the potential limitations of these findings. First, we studied morphological parameters prior to treatment and did not perform follow-up measurements of these parameters to see their prognostic value. A recent study showed that, during treatment, visceral adipose tissue increased while subcutaneous adipose tissue and musculature decreased, although this variation did not correlate significantly with survival.32 However, these results could be studied on a larger population with a 3D measurement. Moreover, with a median delay of 26 days between imaging and the start of immunotherapy, variation in morphological parameters is possible within the interval. Although, in our study, this does not call into question the superiority of anthropometric parameters over BMI measured at the time of treatment, a maximum duration between imaging and the start of immunotherapy will probably have to be considered if these anthropometric parameters are to be taken into account when tailoring treatments. Second, our study population consisted primarily of patients with NSCLC, with no clear effect of body composition parameters, except muscle body mass, for patients with melanoma, and it is not clear if the same body composition parameters would have similar prognostic value in other cancer types.
Despite these limitations, our study shows that 3D-measured SFM and MBM may be useful prognostic factors in patients with NSCLC treated with ICIs. One potential approach to improving the prediction of outcomes in patients with cancer treated with ICIs could be to combine anthropometric parameters with other known prognostic factors. For example, multivariate statistical models or machine learning algorithms could be used to build predictive models based on a combination of multiple factors, including body composition parameters, tumor volume, tumor mutational burden (TMB),45 microsatellite instability (MSI), neutrophil-to-lymphocyte ratio,9 and other relevant variables. By considering multiple factors simultaneously, it may be possible to more accurately predict outcomes in patients with cancer treated with ICIs, and to inform personalized treatment decisions in these patients.
In conclusion, the present study suggests that 3D-measured low SFM (<5.22 kg/m2) and low MBM (<6.86 kg/m2) may be useful prognostic factors in patients with NSCLC treated with ICIs. Further research is needed to validate these findings and to determine the optimal management strategies for patients with low SFM and MBM. In addition to body composition parameters, other factors such as TMB and MSI may also be important prognostic factors in patients with cancer treated with ICIs, and should be considered in the management of these patients. Combining anthropometric parameters with other known prognostic factors may help to improve the prediction of outcomes in patients with cancer treated with ICIs, and could inform personalized treatment decisions in these patients.
Footnotes
Contributors: Conceptualization: PD, SA and NL. Methodology: PD, LL, YB and NL. Software: PD and LM. Validation: PD and AdP. Formal analysis: PD, AdP, HT and P-HC. Investigation: SA, YB, LL, AM, DP, TI, FG, CR and FB. Resources: AM, DP, SF, TI, FG, CR, FB, PV and NL. Data curation: PD, LL, YB, AdP and NL. Writing—original draft preparation: PD. Writing—review and editing: All authors. Visualization: PD. Supervision: NL and PV. Project administration: NL and PV. Funding acquisition: PD, PV and NL. Guarantor : PD. All authors have read and agreed to the published version of the manuscript.
Funding: This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors. The platform Oncometer3D with the software Anthropometer3DNet was advised by the Health Data Hub (HDH) and has received European Regional Development Fund (ERDF) with the region Normandy.
Competing interests: No, there are no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by institutional research ethics board and the scientific committee of Gustave Roussy Cancer Campus, registered in the French National institute for health data (INDS Institut Nationale des Données de Santé, Protocol 5722030719). Participants gave informed consent to participate in the study before taking part.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Peters S, Kerr KM, Stahel R. PD-1 blockade in advanced NSCLC: A focus on Pembrolizumab. Cancer Treat Rev 2018;62:39–49. 10.1016/j.ctrv.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 3. Carlino MS, Larkin J, Long GV. Immune Checkpoint inhibitors in melanoma. Lancet 2021;398:1002–14. 10.1016/S0140-6736(21)01206-X [DOI] [PubMed] [Google Scholar]
- 4. Hirsch L, Zitvogel L, Eggermont A, et al. PD-LOMA: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br J Cancer 2019;120:3–5. 10.1038/s41416-018-0294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321–8. 10.1093/annonc/mdz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542–51. 10.1016/S1470-2045(16)30406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eude F, Guisier F, Salaün M, et al. Prognostic value of total tumour volume, adding necrosis to metabolic tumour volume, in advanced or metastatic non-small cell lung cancer treated with first-line pembrolizumab. Ann Nucl Med 2022;36:224–34. 10.1007/s12149-021-01694-5 [DOI] [PubMed] [Google Scholar]
- 8. Dall’Olio FG, Marabelle A, Caramella C, et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol 2022;19:75–90. 10.1038/s41571-021-00564-3 [DOI] [PubMed] [Google Scholar]
- 9. Belkouchi Y, Nebot-Bral L, Lawrance L, et al. Predicting Immunotherapy outcomes in patients with MSI tumors using NLR and CT global tumor volume. Front Oncol 2022;12:982790. 10.3389/fonc.2022.982790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018;19:310–22. 10.1016/S1470-2045(18)30078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heidelberger V, Goldwasser F, Kramkimel N, et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-Pd1 checkpoint inhibitors in Melanoma patients. Invest New Drugs 2017;35:436–41.:537. 10.1007/s10637-017-0475-7 [DOI] [PubMed] [Google Scholar]
- 12. Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 2019;7:57. 10.1186/s40425-019-0527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popinat G, Cousse S, Goldfarb L, et al. Sub-cutaneous fat mass measured on Multislice computed tomography of pretreatment PET/CT is a prognostic factor of stage IV non-small cell lung cancer treated by nivolumab. Oncoimmunology 2019;8:e1580128. 10.1080/2162402X.2019.1580128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tenuta M, Gelibter A, Pandozzi C, et al. Impact of Sarcopenia and inflammation on patients with advanced non-small cell lung cancer (NCSCL) treated with immune checkpoint inhibitors (Icis): a prospective study. Cancers (Basel) 2021;13:6355. 10.3390/cancers13246355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martini DJ, Kline MR, Liu Y, et al. Adiposity may predict survival in patients with advanced stage cancer treated with Immunotherapy in phase 1 clinical trials. Cancer 2020;126:575–82. 10.1002/cncr.32576 [DOI] [PubMed] [Google Scholar]
- 16. Young AC, Quach HT, Song H, et al. Impact of body composition on outcomes from anti-Pd1 +/- anti-CTLA-4 treatment in melanoma. J Immunother Cancer 2020;8:e000821. 10.1136/jitc-2020-000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blanc-Durand P, Schiratti J-B, Schutte K, et al. Abdominal musculature segmentation and surface prediction from CT using deep learning for sarcopenia assessment. Diagn Interv Imaging 2020;101:789–94. 10.1016/j.diii.2020.04.011 [DOI] [PubMed] [Google Scholar]
- 18. Decazes P, Rouquette A, Chetrit A, et al. Automatic measurement of the total visceral Adipose tissue from computed tomography images by using a multi-Atlas Segmentation method. J Comput Assist Tomogr 2018;42:139–45. 10.1097/RCT.0000000000000652 [DOI] [PubMed] [Google Scholar]
- 19. Shen W, Chen J, Gantz M, et al. A single MRI slice does not accurately predict visceral and subcutaneous adipose tissue changes during weight loss. Obesity 2012;20:2458–63. 10.1038/oby.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas EL, Bell JD. Influence of undersampling on magnetic resonance imaging measurements of intra-abdominal Adipose tissue. Int J Obes Relat Metab Disord 2003;27:211–8. 10.1038/sj.ijo.802229 [DOI] [PubMed] [Google Scholar]
- 21. Schaudinn A, Linder N, Garnov N, et al. Predictive accuracy of Single- and multi-slice MRI for the estimation of total visceral adipose tissue in overweight to severely obese patients. NMR Biomed 2015;28:583–90. 10.1002/nbm.3286 [DOI] [PubMed] [Google Scholar]
- 22. Lee YS, Hong N, Witanto JN, et al. Deep neural network for automatic volumetric Segmentation of whole-body CT images for body composition assessment. Clin Nutr 2021;40:5038–46. 10.1016/j.clnu.2021.06.025 [DOI] [PubMed] [Google Scholar]
- 23. Antoun S, Lanoy E, Ammari S, et al. Protective effect of obesity on survival in cancers treated with Immunotherapy vanishes when controlling for type of cancer, weight loss and reduced skeletal muscle. Eur J Cancer 2023;178:49–59. 10.1016/j.ejca.2022.10.013 [DOI] [PubMed] [Google Scholar]
- 24. Decazes P, Tonnelet D, Vera P, et al. Anthropometer3D: automatic multi-slice Segmentation software for the measurement of anthropometric parameters from CT of PET/CT. J Digit Imaging 2019;32:241–50. 10.1007/s10278-019-00178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mallet R, Decazes P, Modzelewski R, et al. Prognostic value of low Skeletal muscle mass in patient treated by exclusive curative radiochemotherapy for a NSCLC. Sci Rep 2021;11:10628. 10.1038/s41598-021-90187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional networks for BIOMEDICAL image segmentation. Available: http://arxiv.org/abs/1505.04597 [Accessed 1 Feb 2020].
- 27. Decazes P, Ammari S, De Prévia A, et al. Body composition to define prognosis of cancers treated by anti-angiogenic drugs. Diagnostics (Basel) 2023;13:205. 10.3390/diagnostics13020205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010;39:412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takenaka Y, Oya R, Takemoto N, et al. Predictive impact of Sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle 2021;12:1122–35. 10.1002/jcsm.12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deng H-Y, Chen Z-J, Qiu X-M, et al. Sarcopenia and prognosis of advanced cancer patients receiving immune Checkpoint inhibitors: a comprehensive systematic review and meta-analysis. Nutrition 2021;90:111345. 10.1016/j.nut.2021.111345 [DOI] [PubMed] [Google Scholar]
- 31. Roch B, Coffy A, Jean-Baptiste S, et al. Cachexia - Sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 2020;143:19–26. 10.1016/j.lungcan.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 32. Baldessari C, Guaitoli G, Valoriani F, et al. Impact of body composition, nutritional and inflammatory status on outcome of non-small cell lung cancer patients treated with Immunotherapy. Clin Nutr ESPEN 2021;43:64–75. 10.1016/j.clnesp.2021.02.017 [DOI] [PubMed] [Google Scholar]
- 33. Bolte FJ, McTavish S, Wakefield N, et al. Association of Sarcopenia with survival in advanced NSCLC patients receiving concurrent Immunotherapy and chemotherapy. Front Oncol 2022;12:986236. 10.3389/fonc.2022.986236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pu L, Ashraf SF, Gezer NS, et al. Estimating 3-D whole-body composition from a chest CT scan. Med Phys 2022;49:7108–17. 10.1002/mp.15821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of Sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. The Lancet Oncology 2008;9:629–35. 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 36. Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and Genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 2013;105:1862–70. 10.1093/jnci/djt310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS study). Cancer Epidemiol Biomarkers Prev 2017;26:1008–15. 10.1158/1055-9965.EPI-17-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2017;104:52–7. 10.1016/j.lungcan.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 39. Cortellini A, Ricciuti B, Vaz VR, et al. Prognostic effect of body mass index in patients with advanced NSCLC treated with chemoimmunotherapy combinations. J Immunother Cancer 2022;10:e004374. 10.1136/jitc-2021-004374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barbi J, Patnaik SK, Pabla S, et al. Visceral obesity promotes lung cancer progression-toward resolution of the obesity paradox in lung cancer. J Thorac Oncol 2021;16:1333–48. 10.1016/j.jtho.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li L, Li W, Xu D, et al. Association between visceral fat area and cancer prognosis: a population-based multicenter prospective study. Am J Clin Nutr 2023. 10.1016/j.ajcnut.2023.07.001 [DOI] [PubMed] [Google Scholar]
- 42. Nattenmüller J, Wochner R, Muley T, et al. Prognostic impact of CT-quantified muscle and fat distribution before and after first-line-chemotherapy in lung cancer patients. PLoS ONE 2017;12:e0169136. 10.1371/journal.pone.0169136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minami S, Ihara S, Komuta K. Sarcopenia and visceral Adiposity are not independent Prognostic markers for extensive disease of small-cell lung cancer: a single-centered retrospective cohort study. World J Oncol 2020;11:139–49. 10.14740/wjon1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Makrakis D, Rounis K, Tsigkas A-P, et al. Effect of body tissue composition on the outcome of patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. PLoS One 2023;18:e0277708. 10.1371/journal.pone.0277708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–104. 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007315supp001.pdf (120.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request.