Abstract
Introduction
Perioperative myocardial injury evidenced by elevated cardiac biomarkers (both natriuretic peptides and troponin) is common after major non-cardiac surgery. However, it is unclear if the rise in cardiac biomarkers represents global or more localised cardiac injury. We have previously shown isolated right ventricular (RV) dysfunction in patients following lung resection surgery, with no change in left ventricular (LV) function. Given that perioperative RV dysfunction (RVD) can manifest insidiously, we hypothesise there may be a substantial burden of covert yet clinically important perioperative RVD in other major non-cardiac surgical groups. The Incidence, impact and Mechanisms of Perioperative Right VEntricular dysfunction (IMPRoVE) study has been designed to address this knowledge gap.
Methods and analysis
A multicentre prospective observational cohort study across four centres in the West of Scotland and London. One hundred and seventy-five patients will be recruited from five surgical specialties: thoracic, upper gastrointestinal, vascular, colorectal and orthopaedic surgery (35 patients from each group). All patients will undergo preoperative and postoperative (day 2–4) echocardiography, with contemporaneous cardiac biomarker testing. Ten patients from each surgical specialty (50 patients in total) will undergo T1-cardiovascular magnetic resonance (CMR) imaging preoperatively and postoperatively. The coprimary outcomes are the incidence of perioperative RVD (diagnosed by RV speckle tracking echocardiography) and the effect that RVD has on days alive and at home at 30 days postoperatively. Secondary outcomes include LV dysfunction and clinical outcomes informed by Standardised Endpoints in Perioperative Medicine consensus definitions. T1 CMR will be used to investigate for imaging correlates of myocardial inflammation as a possible mechanism driving perioperative RVD.
Ethics and dissemination
Approval was gained from Oxford C Research Ethics Committee (REC reference 22/SC/0442). Findings will be disseminated by various methods including social media, international presentations and publication in peer-reviewed journals.
Trial registration number
Keywords: Echocardiography, Adult anaesthesia, SURGERY, Magnetic resonance imaging
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first study to investigate the incidence of perioperative right ventricular dysfunction (RVD) after major non-cardiac surgery, and the association between RVD and patient outcomes in this group.
T1-cardiovascular MR substudy to investigate whether inflammation is a mechanism underlying perioperative RVD.
A large prospective multicentre study with appropriate statistical power analysis.
It is difficult to predict the incidence of perioperative RVD in surgical groups other than lung resection since there are such limited data.
Introduction
Perioperative myocardial injury (PMI) is common after major non-cardiac surgery, with a recent large international observational study demonstrating an elevated postoperative high-sensitivity troponin level in 19.7% of patients undergoing major non-cardiac surgery.1 Perioperative PMI has also been shown to be associated with poor cardiovascular outcomes in patients undergoing non-cardiac surgery.2 Similarly, natriuretic peptides increase following surgery, and this is associated with an increased risk of cardiovascular complications and mortality.3 Our group has demonstrated that peak postoperative brain natriuretic peptide is associated with postoperative complications and length of hospital stay after thoracic surgery.4 Although an increase in cardiac biomarkers after major non-cardiac is well described, there has been little research to investigate the location of the myocardial injury (although it is frequently attributed to injury of the left ventricle with little evidence to substantiate this assertion). A study in a mixed surgical population requiring ‘rescue’ echocardiography demonstrated that postoperative right ventricular (RV) dysfunction (RVD) was as prevalent as left ventricular (LV) dysfunction (LVD), occurring in 24.1% of patients.5 Postoperative RVD is difficult to diagnose, manifesting with subtle clinical signs; it is therefore unsurprising that its importance may have been overlooked.6 In addition to postoperative RVD, it has been shown that there may be a considerable burden of preoperative RVD in patients undergoing non-cardiac surgery. A study in patients undergoing major vascular surgery found a prevalence of preoperative RVD of 10%, and this was associated with postoperative major cardiac complications.7 The incidence and significance of perioperative RVD in other non-cardiac surgical populations has been poorly described. We have previously shown that patients undergoing lung resection experience significant impairment of RV function postoperatively with no change in LV function.8 Further research is needed to investigate the incidence and impact of perioperative RVD on patient outcomes in other non-cardiac surgery groups. Additionally, the mechanisms underlying perioperative RVD require elucidation to allow effective preventative and treatment strategies to be devised. The Incidence, impact and Mechanisms of Perioperative Right VEntricular dysfunction (IMPRoVE) study has been conceived to address this gap in our understanding of perioperative RVD.
Potential mechanisms of perioperative RVD
The mechanisms of postoperative RVD likely reflect a complex interplay between pre-existing RVD, patient susceptibility, surgical risk and a multitude of potential perioperative insults.
As described above,7 RVD may predate surgery. In the general population, RVD is more prevalent in the elderly, and in people with hypertension, diabetes mellitus, ischaemic heart disease and lung disease9; risk factors which are over-represented in the surgical population. As anticipated, in our previous thoracic surgery cohort we found a high prevalence of pre-existing RVD of 50%.8
The perioperative period exposes patients to many insults that may contribute to RVD. Excess preload may occur in the form of injudicious intravenous fluid administration, resulting in RV distension and tricuspid regurgitation.6 10 Impaired contractility may occur due to myocardial ischaemia. RV afterload may increase by many mechanisms, including:
Pulmonary thromboembolism: Occurring subclinically in up to 28% of patients undergoing elective intermediate to high-risk non-cardiac surgery.11
Lung injury and inflammation: Due to pre-existing lung disease and the combined deleterious effects of ventilator induced lung injury, systemic inflammation and fluid overload.6
Positive-pressure mechanical ventilation: Especially one-lung ventilation (OLV).6
Lung resection: Recently, we have demonstrated the pulsatile component of RV afterload significantly increases after lung resection.12
Inflammation and PMI
Although it is widely hypothesised that PMI results predominantly from ischaemia secondary to myocardial oxygen supply/demand imbalance, this hypothesis remains unproven and is challenged by important observations; in excess of 90% of patients with PMI have no ischaemic symptoms to support a diagnosis of myocardial infarction,1 and the extent and severity of coronary artery disease does not correlate closely with the occurrence of PMI.13
The inflammatory response is an important contributor to the myocardial injury seen after myocardial infarction and cardiac surgery, but the extent to which systemic inflammation is involved in the pathogenesis of PMI after non-cardiac surgery is not known. Ackland et al recently demonstrated that PMI was associated with an elevated neutrophil-to-lymphocyte ratio, suggesting systemic inflammation may predispose patients to PMI.14 Using T1-weighted cardiovascular magnetic resonance (T1-CMR) imaging, our group has described the presence of imaging correlates of perioperative RV (but not LV) myocardial inflammation in patients following lung resection.15
In summary, with greater understanding of the incidence, impact and underlying mechanisms of perioperative RVD provided through this investigation, preventative interventions targeted at patients at greatest risk may offer a unique therapeutic opportunity to provide a personalised approach to perioperative management and improve patient outcomes across a wide range of surgical populations.
Hypotheses
RVD after major non-cardiac surgery is a common covert contributor to perioperative morbidity.
Inflammatory injury to the RV is a significant contributing factor to PMI.
Methods and analysis
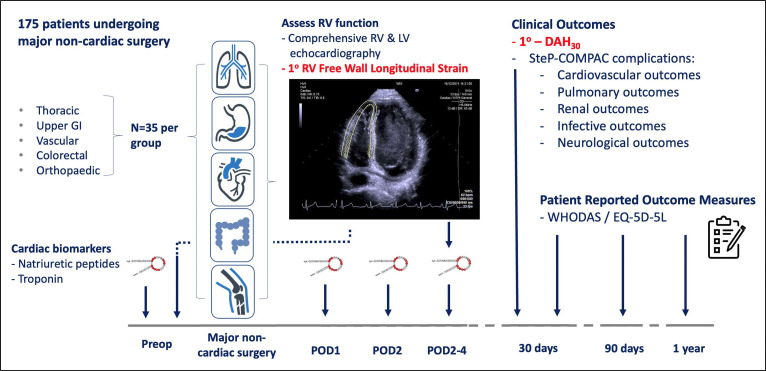
Summary: A multicentre prospective observational cohort study in patients undergoing major non-cardiac surgery in five surgical specialties. Main study: 175 patients to undergo transthoracic echocardiography (TTE) preoperatively and postoperatively (figure 1). Substudy: 50 patients to undergo T1-CMR preoperatively and postoperatively.
Figure 1.
Overview of IMPRoVE Main Study. One hundred and seventy-five patients (35 from each surgical group) will undergo echocardiography and cardiac biomarker testing preoperatively and postoperatively. Coprimary outcomes are the incidence of RV dysfunction, diagnosed by RV free wall longitudinal strain, and DAH30 (shown in red). DAH30, days alive and at home at 30 days postoperatively; EQ-5D-5L, EuroQol-5 Dimension-5 Level; GI, gastrointestinal; IMPRoVE, Incidence, impact and Mechanisms of Perioperative Right VEntricular dysfunction; LV, left ventricular; POD, postoperative day; RV, right ventricular; StEP-COMPAC, Standardised Endpoints and Core Outcome Measures for Perioperative and Anaesthetic Care; WHODAS, WHO Disability Assessment Schedule 2.0.
Centres: Three hospitals in the West of Scotland (Golden Jubilee National Hospital (GJNH), Queen Elizabeth University Hospital and Glasgow Royal Infirmary) and one London hospital (Royal London Hospital).
Study status: Grant funding was secured on 12th February 2021, with ethical approval on 12th January 2023 (REC reference 22/SC/0442). Recruitment commenced in May 2023 with an anticipated study duration of 36 months.
Selection of study subjects
Inclusion criteria
Patient aged >18 years.
Patient undergoing planned elective primary hip or knee joint replacement under spinal anaesthesia, major colorectal, major vascular surgery or major surgery requiring OLV with or without lung resection.
Provision of informed consent.
Main study exclusion criteria
Pregnancy.
Ongoing participation in any investigational research which could undermine the scientific basis of the study.
Major surgery within previous 3 months.
Previous participation in the IMPRoVE study.
Inadequate comprehension of English resulting in inability to comply with instructions while undergoing interventions required for main study and substudy.
Risk factors for RVD are likely to be over-represented in patients presenting for surgery and participants with pre-existing RVD could represent an important population that may face greater consequences of acute perioperative insults to the RV. For this reason, although not a specific inclusion or exclusion criteria, patients with pre-existing RVD, including when identified on preoperative echocardiography will be included in the study.
T1-CMR substudy exclusion criteria
Contraindication to T1-CMR (see online supplemental material).
Atrial fibrillation at baseline.
Acute or chronic kidney disease.
Allergy to intravenous contrast.
bmjopen-2023-074687supp001.pdf (44.8KB, pdf)
Study conduct
Recruitment
Patients will be identified from hospital waiting lists. Patients will be informed of the study, offered a patient information sheet and invited to participate at the earliest possible opportunity after they have been informed of their decision for surgery. Following appropriate time to consider participation, informed consent will be obtained by a member of the research team.
Consent
Written informed consent will be obtained, following a face-to-face discussion about the study by a member of the study team. Signing of consent form and preoperative blood sampling and imaging may take place at any time in the 30 days prior to surgery or on the day of surgery.
Medical management
Medical management will be according to the standard of care at each treating site and is not influenced by this study protocol.
Study interventions
Table 1 shows the general schedule of assessments/study interventions that patients will undergo.
Table 1.
Schedule of assessments for all patients enrolled into IMPRoVE study
| Visit window | Preoperative | Day of surgery (day 0) | POD1 | POD2 | Day of echocardiography (POD 2–4) | Discharge | 30 days | 3 months | 12 months |
| Informed consent | x | ||||||||
| Inclusion/exclusion criteria | x | ||||||||
| Baseline demographics and risk scoring | x | ||||||||
| BNP/HsTn | x | x | x | x | |||||
| NT-proBNP | x | ||||||||
| Immediate perioperative data | x | ||||||||
| Laboratory data | x | x | x | x | |||||
| Echocardiography | x | x | |||||||
| T1-CMR* | x | x | |||||||
| QoR-15 | x | x | |||||||
| Organ-specific complications (Clavien-Dindo ≥2) |
x | x | x | x | x | ||||
| Unplanned ICU admission | x | x | x | x | |||||
| Length of hospital stay | x | ||||||||
| Length of ICU/HDU stay | x | x | |||||||
| Mortality | x | x | x | x | x | ||||
| DAH30 | x | ||||||||
| Hospital readmission | x | ||||||||
| EQ-5D-5L | x | x | x | x | |||||
| WHODAS 2.0 | x | x | x | x |
*Ten patients from each of the five surgical groups (50 in total).
BNP, brain natriuretic peptide; DAH30, days alive and at home at 30 days; EQ-5D-5L, EuroQol-5 Dimension-5 Level; HDU, high-dependency unit; HsTn, high sensitivity troponin; ICU, intensive care unit; IMPRoVE, Incidence, impact and Mechanisms of Perioperative Right VEntricular dysfunction; NT-proBNP, N-terminal prohormone of BNP; POD, Post operative day; QoR-15, quality of recovery-15 score; T1-CMR, T1 cardiovascular magnetic resonance; WHODAS 2.0, WHO Disability Assessment Schedule 2.0.
Echocardiography conduct and analysis
TTE will be performed on all 175 patients by a British Society of Echocardiography (BSE) accredited echocardiographers preoperatively and between postoperative days 2 and 4. Echocardiography will acquire the minimum BSE image dataset.16 In addition to this minimum image dataset, we will acquire an RV focused apical four chamber view for RV free-wall peak longitudinal strain (FWLS) analysis (optimising feasibility as per consensus guidelines).17 18 All echocardiography study images will be sent centrally for offline analysis: anonymised images will be transferred via routine clinical imaging systems to the GJNH.
A full echocardiography data set will be used to assess for RVD (the primary outcome) and LVD. Offline RV and LV two-dimensional (2D) speckle tracking strain analysis will be performed using Tomtec 2D Cardiac Performance Analysis software. Twenty echocardiography scans will be randomly selected and re-reported by the same reporter a minimum of 2 weeks after initial reporting, and reported by a second reporter, to allow assessment of intraobserver and interobserver agreement. Reproducibility will be assessed by intraclass correlation coefficient using two-way mixed effects with absolute agreement and Bland-Altmann plots.
T1-CMR conduct
A subcohort of 50 patients (10 from each surgical group) will undergo a preoperative T1-CMR scan and a single postoperative scan (between postoperative days 2–4). Replicating our previous protocol,8 CMR will be undertaken on a 1.5 or 3.0 Tesla scanner, by band 7 Health and Care Professions Council accredited radiographers. T1-weighted scans will be performed preintravenous and postintravenous gadolinium administration. Postprocessing will be protocolised and dual reported by blinded observers.
Laboratory sampling
Where possible samples will be drawn contemporaneously with routine clinical blood tests. Cardiac biomarkers will be batch analysed at University of Glasgow Laboratories.
DAH30 conduct
Days alive and at home at 30 days postoperatively (DAH30) will be assessed by telephone on postoperative day 30 (up to +5 days). A script will be used to ensure that DAH30 is reliably and consistently recorded.
Data collection will be performed by the local study team on case report forms (CRFs), which will be filed and securely stored at participating sites. The data will be anonymised at site and a unique numeric study number allocated. Completed CRFs will be entered onto a secure online database in a linked anonymised form. Electronic data will be stored in an encrypted and anonymised format for 15 years following the completion of the trial. At the end of this period, the dataset will be destroyed according to DoD 5220.22-M standards. All data will be held in accordance with the General Data Protection Regulation (2018).
Laboratory data
Laboratory data (full blood count, urea and electrolytes, liver function tests and C reactive protein) will be obtained from the local biochemistry and haematology laboratory reporting systems perioperatively, on the day of echocardiography and if clinically indicated, at follow-up.
Clinical data
Baseline demographic information will be collected including chronic comorbidities. We will specifically gather information on sleep apnoea status and previous COVID-19 infection since these may affect baseline RV function. Preoperative data will include previous pulmonary function tests, cardiopulmonary exercise testing, CT thorax imaging (for coronary artery calcium scoring), American College of Surgeons National Surgical Quality Improvement Programme risk scoring, and baseline questionnaires (Duke Activity Status Index, quality of recovery-15, EuroQol-5 Dimension-5 Level (EQ-5D-5L) and WHO Disability Assessment Schedule 2.0 (WHODAS 2.0)). Immediate perioperative data will include the operation performed, duration of surgery and anaesthesia, duration of OLV (if applicable), and use of vasopressor/inotropic support.
Study outcomes
Coprimary outcomes
Incidence of postoperative RVD
RVD defined as:
2D-speckle tracking derived RV free wall longitudinal strain (RVFWLS) less negative than −20%.17 19
(When RVFWLS is not available) two of tricuspid annular plane systolic excursion <16 mm, S’ wave velocity at the tricuspid annulus <10 cm/s or tissue doppler RV index of myocardial performance >0.55.20
Clinical impact of postoperative RVD
Days alive and at home at 30 days postoperatively (DAH30). DAH30 is a continuous number between 0 and 30 which reflects, out of the 30 days following surgery, the total number of those days that a patient spends alive and at home. If a patient dies within those 30 days, their value is set to 0.
Justification for coprimary outcomes
Incidence of postoperative RVD
There is currently no consensus on how best to measure RV function in the context of clinical trials.21 However, recent work (by our group and others) has demonstrated the superiority and increased reproducibility of RVFWLS in identifying RVD compared with ‘conventional indices’.22–24 A recent American Thoracic Society Research Statement has advocated the use of RVFWLS due to its ability to assess RVD at an early stage and to detect differences when other traditional measurements fail to do so.21
Clinical impact of perioperative RV
Days alive and at home at 30 days postoperatively (DAH30) is a novel, well-validated clinical endpoint describing all facets of the perioperative experience and has been recommended as a patient-centred outcome by the Standardising Endpoints in Perioperative (StEP) Medicine initiative. DAH30 is sensitive to prolonged stay due to complications, discharge to a rehabilitation or nursing care facility, readmission to hospital after discharge and mortality thus integrating efficacy, quality and safety.25 26
Exploratory outcomes
Exploratory outcomes that we will investigate are shown in table 2.
Table 2.
Exploratory outcomes
| Left ventricular dysfunction | Defined by two-dimensional echocardiography derived biplane ejection fraction |
| Cardiac biomarkers | NT-proBNP, BNP, hsTn |
| Clinical outcomes informed by StEP trials consensus definitions: | |
| Cardiovascular outcomes27 | Myocardial infarction Myocardial injury Cardiac death Non-fatal cardiac arrest Coronary revascularisation Major adverse cardiac event |
| Pulmonary outcomes28 | Pneumonia Atelectasis Acute respiratory distress syndrome Pulmonary aspiration |
| Renal outcomes29 | Acute kidney injury Need for renal replacement therapy |
| Infection outcomes30 | Fever Clinical suspicion of infection |
| Neurological outcomes31 | Delirium Stroke |
| Major complications | Sequential Organ Failure Assessment Score |
| Clinical indicators | Need for unplanned HDU or ICU admission Requirement for new invasive or non-invasive ventilation Length of postoperative critical care and hospital stay Mortality at 30 days |
| Patient quality of recovery | QoR-15 |
| Patient-centred outcomes | EQ-5D-5L WHODAS 2.0 (assessed at 30 days, 3 months and 12 months postoperatively) |
| T1-CMR | Preoperative and postoperative T1-CMR. T1 weighted CMR preintravenous and postintravenous gadolinium to calculate T1 signal and extracellular volume (imaging correlates of myocardial inflammation) |
BNP, brain natriuretic peptide; EQ-5D-5L, EuroQol-5 Dimension-5 Level; HDU, high-dependency unit; HsTn, high sensitivity troponin; ICU, intensive care unit; NT-proBNP, N-terminal prohormone of BNP; QoR-15, quality of recovery-15; StEP, Standardised Endpoints in Perioperative; WHODAS 2.0, WHO Disability Assessment Schedule 2.0.
Statistical considerations
All statistical analyses will be performed in conjunction with the Robertson Centre for Biostatistics at the University of Glasgow.
Analysis of coprimary outcomes
Incidence of perioperative RVD
The identified incidence of postoperative RVD will be compared with the null hypothesis that the incidence equals zero using a one-sample binomial test; 95% CIs for the incidence will be defined using the Clopper-Pearson method. In addition, we will perform sensitivity analyses to identify the incidence of patients that develop new postoperative RVD, and identify the incidence of those that have pre-existing RVD maintained through to the postoperative period. Subgroup analyses will estimate the incidence rate of postoperative LVD and RVD within surgical subgroups and compare the incidence in patients with chronic obstructive pulmonary disease (COPD) versus no COPD, in operations involving mechanical ventilation and no mechanical ventilation (orthopaedic surgery under spinal anaesthesia), OLV versus no OLV, in videoscopic versus open surgeries, and in patients with ischaemic heart disease (IHD) versus no IHD. Secondary analyses will explore the association between preoperative and postoperative cardiac biomarker levels and perioperative LVD and RVD.
With a one sample binomial test at a one-sided significance level of 5% with 80% power, 31 patients would be required to confidently identify an incidence of postoperative RVD of 5% as different from zero in any individual surgical subgroup. As such, recruiting 35 patients per group provides a 10% margin for lost to follow-up and withdrawals. This results in a total sample size of 175.
Clinical impact of perioperative RVD
Assuming the incidence of RVD is proven to be different from zero in any group (highly likely given our previous findings8), then additional analyses will be performed in pooled data across all surgical groups to assess the clinical impact of postoperative LVD or RVD. Sensitivity analyses will be performed to assess the clinical impact of RVD on patients that develop new postoperative RVD, compared with the clinical impact of pre-existing RVD, which is maintained through to the postoperative period.
DAH30 postoperatively will be compared between the groups with and without postoperative RVD using negative binomial regression analysis adjusting for age and other known predictors of DAH30.25 26 It will also be explored how adjustment for further variables (including cardiac biomarker profile) affects results. We will conduct the same DAH30 analysis on the sensitivity analysis groups described above.
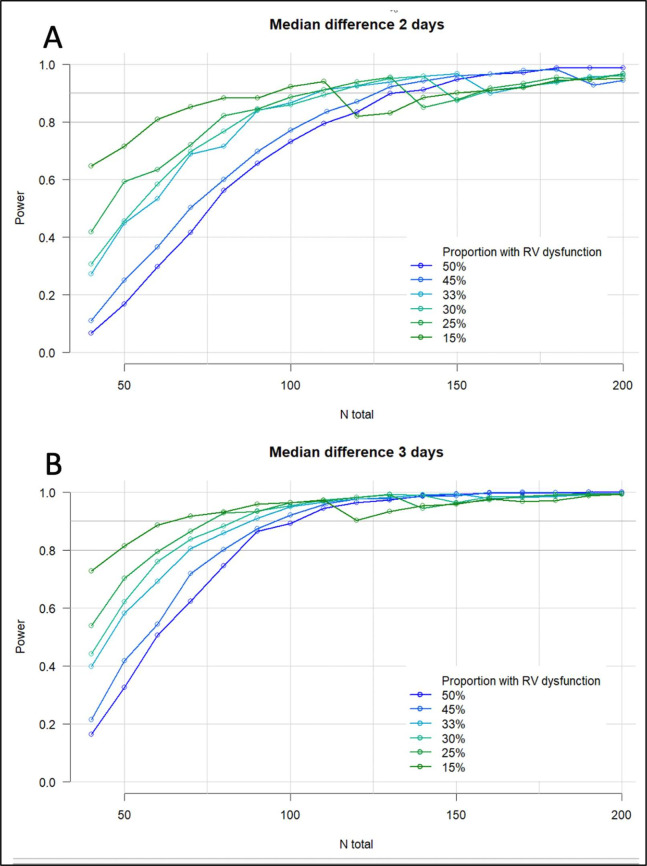
Performing power analysis for this comparison is challenging given the large number of unknowns in terms of the incidence of RVD, and the potential effect size. As such, an indicative power analysis was performed exploring sample sizes from 50 to 200 patients, an incidence of RVD 15%–50% and for a difference in DAH30 of 2 or 3 days. The anticipated power is in excess of 0.8 in all simulations containing over 125 patients suggesting that in the 175-patient sample should have sufficient power in most conceivable scenarios (figure 2).
Figure 2.
Simulated power analysis for impact of RVD on days alive and at home at 30 days. Assuming for 1% of the patients DAH30=0, for the remainder DAH30 follows a negative binomial distribution with parameters chosen such that the median DAH30 is 24/25 in one group and 27 in the other group and the shape of the distribution is similar to that seen in the validation cohorts. The simulated DAH30 was then compared between groups using negative binomial regression (repeated) in 1000 samples. The figures show the resulting estimated power for incidences of postoperative RVD from 15% to 50%#, and for a clinical effect size of 2 (A) or 3 (B) days difference in DAH30*. #In our previous work the incidence of postoperative RVD was 50% in thoracic surgical patients but may be significantly less in, for example, an orthopaedic population. *In Chou et al’s study preoperative RV dysfunction prolonged hospital length of stay by over 50%, but this cohort was a very high-risk vascular surgical population.7 DAH30, days alive and at home at 30 days postoperatively; RVD, right ventricular dysfunction.
Exploratory outcomes
LVD will be analysed analogously to RVD as a secondary analysis.
Secondary outcomes from the postoperative period will be used to compare their incidence in patients with and without the primary outcome (RVD). We will assess for association between RVD and PMI (via cardiac biomarkers), cardiovascular complications, major complications, patient recovery, length of intensive care unit and hospital stay. Analysis of intraoperative data will be used with the aim to identify mechanisms by which RVD may have arisen. Where appropriate, multivariate analysis will be used.
We will use 30-day mortality as our primary survival end point and will assess for association with RVD via appropriate survival analyses.
We will also assess the intermediate-term and long-term impact of RVD on patients by assessing association between RVD and health-related quality of life (via EQ-5D-5L) and functional status (via WHODAS 2.0) at 30-day, 3-month and 1-year postoperatively.
Preoperative and postoperative T1-CMR will explore for association between imaging correlates of myocardial inflammation (T1 and extracellular volume) and both RVD and PMI. This substudy will also aim to confirm our previous findings of elevated postoperative T1/extracellular volume in patients after thoracic surgery,15 and replicate this in other surgical groups.
Patient and public involvement
Our programme of work was presented to the Society of Cardiothoracic Surgeons ‘RESOLVES’ Patient and Public Involvement (PPI) group with very positive feedback. This PPI group was unanimously in favour of our research and its obvious benefits to patients.
Ethics and dissemination
The study will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the Good Clinical Practice Guidelines. UK wide ethical approval was obtained from the South Central- Oxford C Research Ethics Committee (REC reference 22/SC/0442) and will comply with all applicable UK legislation. Local research and development approval was obtained from each participating site. All local site Standardised Operating Procedures (SOPs) will be followed.
All publications and presentations relating to this study will be authorised by the trial chief investigator (BS). Authorship will be determined according to the international committee of medical journal editors’ recommendations. The results of the study will be first reported to study collaborators. Subsequently, we will communicate our results by reporting them to the funder and presentation at national meetings, with publication in appropriate peer-reviewed journals. Further details about the trial results and final report will be available on request to the scientific community in a timely manner.
Supplementary Material
Footnotes
Twitter: @philipmccall
Contributors: All authors contributed significantly to the submitted work. TK and JM wrote the initial draft of the protocol. BS and PM conceived the study and BS is the grant holder. CB contributed to study design and initial funding application. RK and SM are co-principal investigators at Glasgow Royal Infirmary and contributed to study design. MW is principal investigator and IR co-investigator at the Queen Elizabeth University Hospital, both contributed to study design. GA is coinvestigator at the Royal London Hospital and contributed to study design. KER is a coinvestigator and lead interventional cardiologist for the study and was involved in study design. NG advised on statistical analyses for the study. All authors read and approved the final manuscript.
Funding: This study is supported by the National Institute of Academic Anaesthesia/Royal College of Anaesthetists British Oxygen Company Chair of Anaesthesia Research Grant. CB is supported by the BHF Centre of Research Excellence grant (reference number RE/18/6/34217). GA is supported by the NIHR Advanced Fellowship (NIHR300097).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642. 10.1001/jama.2017.4360 [DOI] [PubMed] [Google Scholar]
- 2.Puelacher C, Gualandro DM, Glarner N, et al. Long-term outcomes of perioperative myocardial infarction/injury after non-cardiac surgery. Eur Heart J 2023;44:1690–701. 10.1093/eurheartj/ehac798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodseth RN, Biccard BM, Chu R, et al. Postoperative B-type natriuretic peptide for prediction of major cardiac events in patients undergoing noncardiac surgery: systematic review and individual patient meta-analysis. Anesthesiology 2013;119:270–83. 10.1097/ALN.0b013e31829083f1 [DOI] [PubMed] [Google Scholar]
- 4.Lafferty B, McCall P, Shelley B. BNP for prediction of outcome following lung resection surgery (PROFILES); an interim analysis. J Cardiothorac Vasc Anesth 2020;34:S6–7. 10.1053/j.jvca.2020.09.008 [DOI] [Google Scholar]
- 5.Markin NW, Gmelch BS, Griffee MJ, et al. A review of 364 perioperative rescue echocardiograms: findings of an anesthesiologist-staffed perioperative echocardiography service. J Cardiothorac Vasc Anesth 2015;29:82–8. 10.1053/j.jvca.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 6.Murphy E, Shelley B. Clinical presentation and management of right ventricular dysfunction. BJA Educ 2019;19:183–90. 10.1016/j.bjae.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou J, Ma M, Gylys M, et al. Preexisting right ventricular dysfunction is associated with higher postoperative cardiac complications and longer hospital stay in high-risk patients undergoing nonemergent major vascular surgery. J Cardiothorac Vasc Anesth 2019;33:1279–86. 10.1053/j.jvca.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCall PJ, Arthur A, Glass A, et al. The right ventricular response to lung resection. J Thorac Cardiovasc Surg 2019;158:556–65. 10.1016/j.jtcvs.2019.01.067 [DOI] [PubMed] [Google Scholar]
- 9.Modin D, Møgelvang R, Andersen DM, et al. Right ventricular function evaluated by tricuspid annular plane systolic excursion predicts cardiovascular death in the general population. J Am Heart Assoc 2019;8:e012197. 10.1161/JAHA.119.012197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy E, Shelley B. The right ventricle-structural and functional importance for anaesthesia and intensive care. BJA Educ 2018;18:239–45. 10.1016/j.bjae.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobben RB, van Waes JAR, Leiner T, et al. Unexpected cardiac computed tomography findings in patients with postoperative myocardial injury. Anesth Analg 2018;126:1462–8. 10.1213/ANE.0000000000002580 [DOI] [PubMed] [Google Scholar]
- 12.Glass A, McCall P, Arthur A, et al. Pulmonary artery wave reflection and right ventricular function after lung resection. Br J Anaesth 2023;130:e128–36. 10.1016/j.bja.2022.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth T, Chan M, Butler C, et al. Prognostic capabilities of coronary computed tomographic angiography before non-cardiac surgery: prospective cohort study. BMJ 2015;350:h1907. 10.1136/bmj.h1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackland GL, Abbott TEF, Cain D, et al. Preoperative systemic inflammation and perioperative myocardial injury: prospective observational multicentre cohort study of patients undergoing non-cardiac surgery. Br J Anaesth 2019;122:180–7. 10.1016/j.bja.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy E, Glass A, McCall P, et al. Myocardial inflammation after major non-cardiac thoracic surgery. Br J Anaesth 2021;126:e80–1. 10.1016/j.bja.2020.11.012 [DOI] [Google Scholar]
- 16.Robinson S, Rana B, Oxborough D, et al. A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: the British society of echocardiography minimum dataset. Echo Res Pract 2020;7:G59–93. 10.1530/ERP-20-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Zaidi A, Oxborough D, Augustine DX, et al. Echocardiographic assessment of the tricuspid and pulmonary valves: a practical guideline from the British society of echocardiography. Echo Res Pract 2020;7:G95–122. 10.1530/ERP-20-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Addetia K, Miyoshi T, Citro R, et al. Two-dimensional echocardiographic right ventricular size and systolic function measurements stratified by sex, age, and ethnicity: results of the world alliance of societies of echocardiography study. J Am Soc Echocardiogr 2021;34:1148–57. 10.1016/j.echo.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 20.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr 2010;23:685–713; 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 21.Lahm T, Douglas IS, Archer SL, et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. an official American thoracic society research statement. Am J Respir Crit Care Med 2018;198:e15–43. 10.1164/rccm.201806-1160ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Focardi M, Cameli M, Carbone SF, et al. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 2015;16:47–52. 10.1093/ehjci/jeu156 [DOI] [PubMed] [Google Scholar]
- 23.McCall P, Soosay A, Kinsella J, et al. The utility of transthoracic echocardiographic measures of right ventricular systolic function in a lung resection cohort. Echo Res Pract 2019;6:7–15. 10.1530/ERP-18-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 25.Myles PS, Shulman MA, Heritier S, et al. Validation of days at home as an outcome measure after surgery: a prospective cohort study in Australia. BMJ Open 2017;7:e015828. 10.1136/bmjopen-2017-015828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerath A, Austin PC, Wijeysundera DN. Days alive and out of hospital: validation of a patient-centered outcome for perioperative medicine. Anesthesiology 2019;131:84–93. 10.1097/ALN.0000000000002701 [DOI] [PubMed] [Google Scholar]
- 27.Beattie WS, Lalu M, Bocock M, et al. Systematic review and consensus definitions for the standardized endpoints in perioperative medicine (step) initiative: cardiovascular outcomes. Br J Anaesth 2021;126:56–66. 10.1016/j.bja.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 28.Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018;120:1066–79. 10.1016/j.bja.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 29.McIlroy DR, Bellomo R, Billings FT, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine (step) initiative: renal endpoints. Br J Anaesth 2018;121:1013–24. 10.1016/j.bja.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 30.Barnes J, Hunter J, Harris S, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine (step) initiative: infection and sepsis. Br J Anaesth 2019;122:500–8. 10.1016/j.bja.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haller G, Bampoe S, Cook T, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine initiative: clinical indicators. Br J Anaesth 2019;123:228–37. 10.1016/j.bja.2019.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074687supp001.pdf (44.8KB, pdf)