Abstract
Background:
Hypertension is a modifiable risk factor for cardiovascular disease and is responsible for major deaths due to stroke and coronary heart disease. Several pharmacological and non-pharmacological interventions for reducing blood pressure have been tried earlier. Modulating brain regions such as prefrontal cortex (PFC) to channelize activities is an effective tool to target blood pressure.
Purpose:
Prefrontal cortex (PFC) exerts inhibitory control over sympathoexcitatory circuits, which was explored using a novel reaction time paradigm.
Methods:
Thirty participants of both genders in the age group 40–70 years with established hypertension were included. A structured reaction time paradigm was designed to include psychomotor and visuomotor elements with integrated sensory attention and motor performance tasks. Blood pressure, Lead II ECG, and EEG from F3 and F4 were recorded. A paired t-test was used to examine the variations in these parameters across tasks.
Results:
A significant reduction in mean arterial pressure by 4.04 mmHg (p = .0232) during the visuomotor task and a reduction of 3.38 mmHg during the auditory cue task (p = .0446) were observed. Analysis of the difference in heart rate has shown a profound decrease after passive listening tasks by 3.7 beats (p < .0001*). Spectral analysis from F3 and F4 shows high power in low-frequency zone of EEG indicating a relaxed state during auditory cues and passive listening.
Conclusion:
The reaction time paradigm, when applied to hypertensives, helped decrease blood pressure and heart rate and improved the high frequency (HF) component of heart rate variability, indicating parasympathetic dominance. Such reward-oriented paradigms may act as biofeedback modules that cause hyperactivity of the PFC to suppress the sympathoexcitatory circuit with increased parasympathetic activity beneficial to hypertensive individuals.
Keywords: Blood pressure, Hedonic pleasure, Hypertension, Prefrontal cortex, Reaction time, Sensorimotor tasks
Introduction
Hypertension is a modifiable risk factor for cardiovascular disease and is the leading cause of death globally. 1 It is responsible for major deaths due to stroke and coronary heart disease in India.2–4 While several guidelines are released from time to time and implemented, the burden of hypertension continues globally and is more prevalent in developing countries.5–7 Imaging studies in humans suggest that essential hypertension is a disease of both the brain and the vasculature and is a risk factor for cerebrovascular disease leading to cognitive impairment.8–10
Several pharmacological and non-pharmacological interventions for reducing blood pressure in adults have been applied. Non-pharmacological interventions have been assessed in numerous randomized controlled trials and meta-analyses. Still, it is not clear which ones are most efficacious. The dietary approaches to stop hypertension (DASH) diet has been directly associated with blood pressure reduction, but it has also been associated with outcome-related bias.11, 12 It is worth exploring novel non-pharmacological interventions to alleviate blood pressure in hypertensives. One such model is the neurovisceral integration model that establishes a relationship between central nervous system and cardiac activity. It is largely accepted that the heart influences the brain and behavior, and this relationship is reciprocal. 13 Forebrain structures are important in this regulation as they modulate brain regions involved in the regulation of ANS activity. 14 Both parasympathetic and sympathetic branches of the nervous system are mediated by cortical-subcortical pathways that involve the prefrontal cortex (PFC), the anterior cingulate cortex, the insula, the hypothalamus, and the brainstem. 15 The PFC exerts its inhibitory control over sympathoexcitatory subcortical circuits and helps individuals respond to different demanding situations.15, 16
The PFC has a crucial role in cognitive control, executive function, and sensory processing. 17 It has a central integrative function for motor control and behavior through its diverse neuronal connections to several different motor regions such as the pre-motor cortices, supplementary motor area, cerebellum, and basal ganglia. 18
The contextual interference effect is a well-established motor learning phenomenon that refers to interference, which is experienced when practicing multiple skills, or variations of a skill, within a single practice session. The contextual interference can be high or low with high interference emerging when multiple skills are practiced successively. 19 However, contextual interference is high during set-shifting, leading to low performance, but this is expected to change to low contextual interference with repetitive practice.20–22 Furthermore, animal studies have indicated that PFC plays a crucial role in motor selection decisions for adapting to multiple behavioral rules. 23 Studies have also suggested that the lateral PFC is involved in multitasking processes. 24
In the present study, the authors attempted to explore the role of the PFC using the phenomenon of contextual interference through the application of a structured reaction time protocol to regulate blood pressure in humans. The protocol involves holding one stimulus constant, namely, responding to visual cues, while altering other input factors to vary the impact of pleasure. Tracking the effects of changes in pleasure after a stimulus presentation using autonomic parameters like changes in blood pressure makes the study more objective.
The study was designed to answer the following questions:
Can integrated attention tasks involving low and high mental activities modify blood pressure and heart rate (HR)?
What is the visible change in blood pressure and HR?
Is the change favorable or not?
What is the role of higher brain areas in producing this favorable change, if any?
Methods
A cross-sectional interventional study with 30 participants of both genders in the age group of 40–70 years were included in the study. Participants with established primary hypertension on a similar combination of drugs attending the General Medicine outpatient department were randomly selected and included in the study. Those with neurological disorders with sensorimotor incoordination, secondary hypertension, any other comorbidities, partial or complete loss of vision and hearing, and those routinely practicing any relaxation techniques were excluded from the study. ESIC Medical College, Hyderabad Institutional Ethics Committee’s permission (ESICMC/SNR/IEC-F0120/04/2019) was obtained, and written informed consent was obtained from all participants. A detailed history was taken, and an initial clinical examination was performed. After obtaining the demographic data and recording vital signs, the participants were administered the pre-designed structured reaction time paradigm.
Reaction Time Paradigm
Integration of attention tasks involving low and high mental activities to modify physiological parameters like blood pressure and HR is a relatively new concept. No such pre-structured protocols are available. Hence, a structured protocol was designed to include psychomotor and visuomotor elements with integrated sensory attention and motor performance. The protocol was pre-validated before being administered to the research participants. The tasks were designed with human – device and human – device – human interactions. Blood pressure was recorded at defined time intervals. Lead II ECG was recorded by placing pre-gel electrodes over the chest. Simultaneously, EEG was also recorded from F3 and F4 (10–20 international system) to visualize PFC activity (Figure 1). The ADI Power Lab 8/35 Lab Chart Pro v8.1.9 was used for recording. The data is reflected in the inbuilt data pad of software for analysis.
Figure 1. Reaction Time Paradigm.
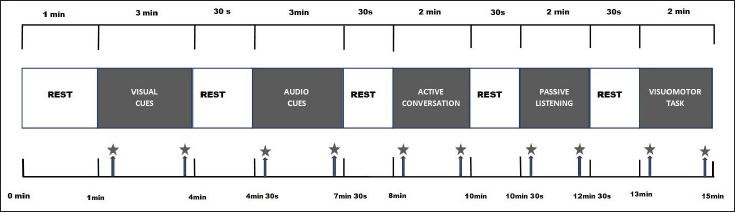
Note: Represents blood pressure measurement, 20 s after the task commencement and 20 s before the task.
Description of Tasks and Recording
A total of five tasks were incorporated into the paradigm to be administered successively spread over a duration of 15 min with an intertrial interval of 30 s. The procedure was explained to all the participants, and before the actual recording, the participants were given 1-min to accustom themselves to the device and lab environment, during which period the basal parameters were recorded. While the ECG recording continued for the entire duration, the blood pressure was recorded at defined time intervals: 20 seconds after the start and 20 seconds before the end of each task as shown in Figure 1.
The mean arterial pressure (MAP) was calculated, and the ECG was analyzed by the software to obtain the heart rate variability (HRV) parameters. The variance in these parameters across tasks in all subjects was further analyzed.
Tasks Included
Responding to visual cues alone: 3 min
Responding to auditory cues and integrating with visual cues: 3 min
Responding to visual cues while involved in active conversation/interaction: 2 min
Responding to visual cues while passively listening to music: 2 min
Responding to visual cues while performing a visuomotor activity: 2 min.
The participants were required to trace a maze to reach the target.
The mean and SD were determined for systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate before and after each task. The difference in values of the parameters before and after each task was calculated. The paired t-test was used to determine the level of significance, and a p value < .05 was considered significant.
Results
The mean age of the participants was 57.4 (±7.88) years. The mean basal SBP of participants was 146.26 ± 7.8 mmHg. There was a fall in the SBP after each intervention except after an active conversation, where there was a rise in the SBP. The maximum reduction of 4.86 mmHg was found after the auditory cue task, which was significant (p = .0302), followed by visuomotor activity with a fall in blood pressure of 4.27 mmHg that was close to a significant level (p = .0610). The active conversation alone caused a significant increase in SBP (8.07 mmHg) (Table 1).
Table 1. SBP Before and After Every Task.
| Intervention | SBP Before (mmHg) ± SD | SBP After (mmHg) | Difference in Mean SBP (mm Hg) | p value |
| Basal | 146.26 ± 7.8 | – | ||
| VC | 146.26 ± 7.8 | 143.1 ± 8.69 | Less by 3.16 | .14337 |
| AC | 148.43 ± 8.36 | 143.57 ± 8.58 | Less by 4.86 | .0302* |
| ACV | 145.63 ± 10.21 | 153.7 ± 8.96 | More by 8.07 | .0019* |
| PL | 147.43 ± 8.58 | 145.9 ± 7.45 | Less by 1.53 | .4638 |
| VM | 147.5 ± 8.49 | 143.23 ± 8.82 | Less by 4.27 | .0610 |
| Mean SBP (without basal) | 147.05 ± 8.69 | 146.6 ± 8.5 | Less by 0.45 | .8400 |
Notes: *Represents p value < .05.
SBP: Systolic blood pressure; VC: Visual cue; AC: Auditory cue; ACV: Active conversation; PL: Passive listening; VM: Visuomotor activity.
No significant change in DBP was observed in four of the five tasks except for visuomotor activity that caused a significant fall in DBP (Table 2).
Table 2. DBP Before and After Every Task.
| Intervention | DBP Before (mmHg) ± SD | DBP After (mmHg) ± SD | Difference in Mean DBP (mmHg) | p value |
| Basal | 94.13 ± 5.14 | – | ||
| VC | 94.13 ± 5.14 | 91.67 ± 5.29 | Less by 2.46 | .0729 |
| AC | 93.17 ± 5.24 | 90.53 ± 5.4 | Less by 2.64 | .0596 |
| ACV | 93.57 ± 5.6 | 93.9 ± 5.25 | More by 0.33 | .5919 |
| PL | 93.77 ± 5.41 | 92.67 ± 5.41 | Less by 1.1 | .4342 |
| VM | 93.4 ± 5.83 | 89.47 ± 5.83 | Less by 3.93 | .0115* |
| Mean DBP (without basal) | 93.61 ± 5.44 | 91.65 ± 5.43 | Less by 1.96 | .1678 |
Notes: *Represents p value < .05.
DBP: Diastolic blood pressure; VC: Visual cue; AC: Auditory cue; ACV: Active conversation; PL: Passive listening; VM: Visuomotor activity.
The heart rate was significantly reduced after visual cue (p = .0175) and after passive listening (p < .0001). There was a significant increase in heart rate after active conversation (p = .0003). Analysis of difference in HR has shown a profound decrease after passive listening by 3.7 beats per minute (Table 3).
Table 3. The Heart Rate Before and After Every Task.
| Activity/Task | Before (bpm) | After (bpm) | Difference in HR | p value |
| Basal | 79.1 ± 2.75 | – | ||
| VC | 81.09 ± 2.68 | 79.4 ± 2.67 | Less by 1.69 | .0175* |
| AC | 78.77 ± 2.61 | 77.67 ± 2.77 | Less by 1.1 | .1188 |
| ACV | 81.03 ± 2.85 | 83.8 ± 2.71 | More by –2.77 | .0003* |
| PL | 81.17 ± 2.88 | 77.47 ± 3.31 | Less by 3.7 | <.0001* |
| VM | 83.06 ± 2.95 | 82.5 ± 2.76 | Less by 0.56 | .4508 |
| Mean HR (without basal) | 81.19 ± 2.79 | 80.17 ± 2.84 | Less by 1.02 | .1659 |
Notes: *Represents p value < .05.
HR: Heart rate; VC: Visual cue; AC: Auditory cue; ACV: Active conversation; PL: Passive listening; VM: Visuomotor activity.
The MAP in relation to the tasks was decreased after each task except after active conversation where it increased. The decrease was significant after auditory cue and visuomotor activity (Figure 2).
Figure 2. MAP Before and After Each Task.
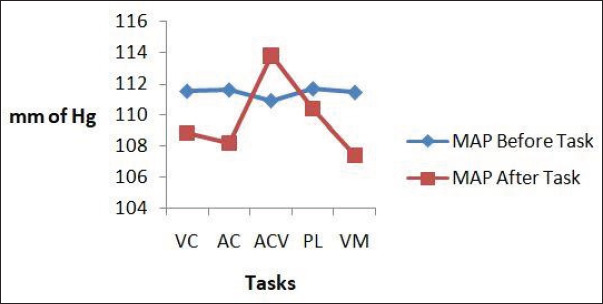
Note: MAP: Mean arterial pressure; VC: Visual cue; AC: Auditory cue; ACV: Active conversation; PL: Passive listening; VM: Visuomotor activity.
When the mean blood pressure and heart rate were determined across the tasks, they were decreased or showing a tendency to decrease though not significant (Table 4).
Table 4. Mean Values of Blood Pressure in All Participants Before and After All the Interventions.
| Parameter | Mean Value of All Participants Before the Activities | Mean Value of All Participants After the Activities | Difference | p value |
| SBP | 147.05 ± 8.69 | 146.6 ± 8.5 | Less by 0.45 | .8400 |
| DBP | 93.61 ± 5.44 | 91.65 ± 5.43 | Less by 1.96 | .1678 |
| PP | 52.13 ± 2.67 | 51.43 ± 3.4 | Less by 0.7 | .3788 |
| MAP | 111.42 ± 6.44 | 109.73 ± 6.35 | Less by 1.69 | .3103 |
Note: SBP: Systolic blood pressure; DBP: Diastolic blood pressure; PP: Pulse pressure; MAP: Mean arterial pressure.
HRV parameters including HF (%), HF (nu), and SD1 indicated high parasympathetic tone across the activities (Table 5).
Table 5. The Mean ± SD of HF and HF (nu) and SD1 Values of HRV.
| Parameter | Mean ± SD | Physiological Significance |
| HF (%) | 59.16 ± 2.4 | Parasympathetic tone, in self-regulatory activities |
| HF (nu) | 65.08 ± 3 | |
| SD1 | 109.08 ± 1.4 | Sensitive to rapid high-frequency variations in HR |
Note: HF: High frequency; HR: Heart rate; HRV: Heart rate variability.
Spectral analysis from F3 and F4 has shown high power in the low-frequency zone of the EEG, indicating a relaxed state of the participant during tasks involving response to auditory cues and passive listening. Also, high power in the high-frequency zone was observed during visuomotor activity, indicating stimulation of the PFC (Figure 3).
Figure 3. EEG Recording From F3 and F4 Montages.

Note: A: During Audio Cues; B: During Passive Listening; C: During Active Conversation; D: During Visuomotor Task.
Discussion
Autonomic nervous system (ANS) is responsible for the maintenance of homeostasis by responding to both changes in the internal milieu of the body and the environment. The ANS is known for its role in controlling many visceral functions such as digestion, respiration, papillary response, sexual arousal, urination, and cardiovascular functions. 25 It is long known fact that the ANS is involved in the short-term regulation of blood pressure. The sympathetic and parasympathetic limbs of the ANS operate in a harmonious manner to maintain a balance in blood pressure. An imbalance of these two limbs of the ANS contributes to the development of hypertension.26, 27
Structured tasks such as reaction time protocols involve observing and anticipating an action, forming mental imagery from evoked information followed by motor performance. Each mental construct leading to the formation of motor representation and performance in response to stimuli is associated with specific autonomic patterns. 28 Success and failure with regard to time taken to respond to various stimuli show linkage between autonomic parameters like heart rate and blood pressure with mental processes. 29 An anticipated cardiovascular adaptation of physiological processes is elicited in the preparation phase itself of an action. It is hypothesized that extending the basic concept of linking such motor representation to sensorimotor engagement can favorably influence changes in autonomic responses. This concept has been explored in the present study to see if hypertensive individuals, when provided with engaging non-invasive sensorimotor tasks, show a decrease in blood pressure. Generally, assessment of reaction time is performed using discrete tests involving responses to visual or auditory cues. The present study is more structured in that the discrete tests are combined successively to be administered at specific time intervals while simultaneously recording the physiological parameters. Such a structured reaction time paradigm allows the participant to engage in the tasks continuously despite set-shifting.
Visual Cues
Participants on average have shown a decrease in SBP of 3.16 mmHg and a decrease in DBP of 2.46 mmHg. Studies relating visual reaction time and blood pressure are limited to a single report of an increase in SBP in a group of 12 individuals when subjected to visual reaction time, but these were performed on normotensive subjects. 30 The fall in blood pressure in the present study is attributed to the stimulation of the PFC, as evidenced by the high-frequency output spectral power from F3 and F4 montages. This is comparable to the induction of more neural resources from the PFC by inducing an electrophysiological excitatory effect using HF repetitive transcranial magnetic stimulation (rTMS) over the left PFC. 31
Auditory Cues
When auditory cues were given to the individuals, a similar reduction in blood pressure was seen with slightly more fall in systolic than DBP, and SBP and DBP were reduced by 4.86 and 2.64 mmHg, respectively. Response to auditory cues is faster than visual reaction time explained by the fact that auditory signals reach brain three or four times faster compared to visual signals.32, 33 Anticipation and preparedness to respond to auditory cues are better along with improved engagement with the task provided. The auditory signals from the early part of the cortical auditory system, the auditory belt cortex, project densely to prefrontal regions. 34 Such stimulation of PFC might be responsible for fall in blood pressure.
Active Conversation
When the individuals were engaged in active conversation, it resulted in increased blood pressure, particularly systolic, by a significant 8.07 mmHg and only a marginal rise of 0.33 mmHg in DBP. Active conversation as such increases the blood pressure in general, and individuals with higher baseline blood pressure tend to show a larger increase. Studies have shown that increases in blood pressure of more than 25–40% occurred in certain hypertensive individuals within 30 s of the start of human speech. 35 Another report shows that MAPs rose significantly by 5.4 mmHg and 5.2 mmHg compared to their resting MAPs. 36
Passive Listening
The participants, when allowed to listen to soft instrumental music, showed a substantial fall in both systolic and DBPs. This is supported by many previous studies where relaxing music tends to reduce both SBP and DBP.37–39 But there has been no cause-effect relationship established so far. However, focus and engagement with an activity become easier with no active interference by other stimuli. If pleasing to the individual, engagement with the task become stronger with a beneficial outcome on blood pressure. Being an economical and non-pharmacological therapy for hypertension, it is widely encouraged.
Visuomotor Tasks
These tasks were designed to be a multitasking activity wherein the individual is engaged with both hands performing different tasks. Responding to visual cues continues as in the previous tasks with the right hand, while with the other hand, the participant is required to trace the maze to reach the target. It poses a challenge to the individual to perform multiple tasks by moving back and forth between two different tasks described as set-shifting. This more complex task was the last part of the paradigm. The participants were administered tasks with increasing complexity to provide an opportunity to get accustomed to and probably anticipate some newness in the upcoming tasks. Probably, the desire to accomplish all tasks and obtain the feeling of contentment and reward keeps the individual engaged in the task. Engagement in this activity has shown a fall in blood pressure, with a substantial fall in MAP of 4.04 mmHg, a significant fall in DBP of 3.93 mmHg, and a near-significant fall in SBP of 4.27 mmHg. This clearly shows that effective engagement with multitasking activities that provide hedonic pleasure leads to stimulation of parasympathetic division, which contributes significantly to the improvement of the HF parameter of heart rate variability.
Heart Rate and Variability
The heart rate was found to be reduced after visual cue, auditory cue, passive listening, and visuomotor activity and found to be increased after active conversation. The heart rate or rather HRV is an autonomic correlate of reaction time performance. 40 However, in the present study, the primary focus was to observe changes in physiological parameters rather than judging the performance of the participants. The heart rate seems to be increased before the onset of each activity that might be due to the anticipation and anxiety of the task to be performed. Nevertheless, once the individual starts performing the activity, the ease of the activity and engagement with the task, along with the desire to be rewarded, act as incentives to stabilize the heart rate at a lower level. A significant decrease in heart rate was observed after the visual cue and passive listening. There are similar reports of a reduction in heart rate after passive listening to music.41, 42 Overall, on average, there was a decrease in heart rate, except during active conversation. Analysis of the difference in HR with each task has shown a profound decrease of 3.7 beats per minute in passive listening. Integrating attention tasks involving sensory and motor components for engaging outcomes is seen to stimulate the parasympathetic system, producing the dominant high-frequency component in HRV. 43 In the present study also, parasympathetic dominance was evident from the values of the high-frequency component.
The phenomenon of set-shifting is clearly demonstrated in the administered paradigm. In order to accomplish the task, the individual is self motivated to give the best performance to reach targets. Pleasure mediated by mesocorticolimbic circuitry serves adaptive functions. 44 This gives a sense of hedonic pleasure to the individual and keeps them engaged with the administered protocol. 45 The present study reinforces the results of earlier neuroimaging studies that suggested many diverse rewards can activate a shared brain system with similar patterns of brain activity.46–53 These shared reward networks include anatomical regions of the PFC, including portions of the orbitofrontal and insula. Hyper-activation of the PFC inhibits the sympathoexcitatory circuit of the amygdala, which is known to have outputs relevant to autonomic regulation. 54 This in turn reduces sympathetic activity and removes parasympathetic suppression, culminating in a reduction in blood pressure when it is high. It is also clear from neuroimaging studies that the brain is spontaneously active and constantly switching between different states of rest and activity. 55 Also, the areas in the brain responsible for causing intense pleasure are more widely distributed. Facilitating this transition from one state to another while channelizing the brain activity through structured protocols to arouse these areas that have a beneficial effect on the parasympathetic nervous system is the novelty of the present study.
The limitation of the present study was its small sample size, and hence administering these types of protocols to a wider sample will further enable the study to establish its full potential. These protocols can be designed and improvised from time to time, and they can be used therapeutically in hypertensives.
Conclusion
The novelty of this study is that the individuals with hypertension, when gradually subjected to a series of tasks with increasing complexity in a paradigm, adapt themselves to the challenging environment. Administering this novel paradigm to hypertensives has helped decrease blood pressure and heart rate and improve the high-frequency component of heart rate variability, indicating parasympathetic dominance. Comprehensive and integrated activities like the present one also act as biofeedback reward-oriented paradigms that possibly cause hyperactivity of the PFC to suppress the amygdala’s sympathoexcitatory circuit, resulting in increased parasympathetic activity beneficial to hypertensive individuals.
Abbreviations
PFC: Prefrontal Cortex
ANS: Autonomic Nervous System
BP: Blood Pressure
HR: Heart Rate
HRV: Heart Rate Variability
SBP: Systolic Blood Pressure
DBP: Diastolic Blood Pressure
MAP: Mean Arterial Pressure
VC: Visual Cues
AC: Auditory Cues
ACV: Active Conversation
PL: Passive Listening
VM: Visuomotor
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Madhuri Taranikanti  https://orcid.org/0000-0002-4458-3514
https://orcid.org/0000-0002-4458-3514
Archana Gaur  https://orcid.org/0000-0002-1380-4679
https://orcid.org/0000-0002-1380-4679
Kalpana Medala  https://orcid.org/0000-0002-1357-7584
https://orcid.org/0000-0002-1357-7584
Varatharajan Sakthivadivel  https://orcid.org/0000-0002-5691-670X
https://orcid.org/0000-0002-5691-670X
Authors’ Contribution
Study Conception and Design: MT, AM, & SS. Data Collection: AK, AD, RG, & RK. Analysis and Interpretation of Results: MT, AG, MU, VG, SV, & KM. Writing & Revision of Manuscript: MT, AG, SV, & MU.
Statement of Ethics
ESIC Medical College, Hyderabad Institutional Ethics Committee permission (ESICMC/SNR/IEC-F0120/04/2019) was obtained. The present research complies with the guidelines for human studies and includes evidence that the research was conducted in accordance with the World Medical Association Declaration of Helsinki. Informed consent was obtained from the participating subjects.
ICMJE Statement
ICMJE disclosure form attached.
Informed Consent
ESIC Medical College, Hyderabad Institutional Ethics Committee’s permission (ESICMC/SNR/IEC-F0120/04/2019) was obtained, and written informed consent was obtained from all participants.
References
- 1.Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018. Nov 10; 392(10159): 1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta R. Trends in hypertension epidemiology in India. J Hum Hypertens 2004. Feb; 18(2): 73–78. [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 2016. Aug 9; 134(6): 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou B, Bentham J, Cesare MD, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. The Lancet 2017. Jan 7; 389(10064): 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kario K and Wang JG. Could 130/80 mm Hg be adopted as the diagnostic threshold and management goal of hypertension in consideration of the characteristics of Asian populations? Hypertens Dallas Tex 1979 2018. Jun; 71(6): 979–984. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa N and Hasebe N. Impact of the 2017 American College of Cardiology/American Heart Association blood pressure guidelines on the next blood pressure guidelines in Asia. Curr Hypertens Rep 2019. Jan 10; 21(1): 2. [DOI] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertens Dallas Tex 1979 2018. Jun; 71(6): 1269–1324. [DOI] [PubMed] [Google Scholar]
- 8.Huijts M, Duits A, Staals J, et al. Basal ganglia enlarged perivascular spaces are linked to cognitive function in patients with cerebral small vessel disease. Curr Neurovasc Res 2014. May; 11(2): 136–141. [DOI] [PubMed] [Google Scholar]
- 9.Østergaard L, Engedal TS, Moreton F, et al. Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2016. Feb; 36(2): 302–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010. Jul; 9(7): 689–701. [DOI] [PubMed] [Google Scholar]
- 11.Filippou CD, Tsioufis CP, Thomopoulos CG, et al. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 2020; 11(5): 1150–1160. https://pubmed.ncbi.nlm.nih.gov/32330233/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savica V, Bellinghieri G, Kopple JD.. The effect of nutrition on blood pressure. Annu Rev Nutr 2010. Aug 21; 30: 365–401. [DOI] [PubMed] [Google Scholar]
- 13.Sgoifo A, Carnevali L, Grippo AJ.. The socially stressed heart. Insights from studies in rodents. Neurosci Biobehav Rev 2014. Feb; 39: 51–60. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer F, McCraty R, Zerr CL.. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol 2014. Sep 30; 5: 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thayer JF, Hansen AL, Saus-Rose E, et al. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med Publ Soc Behav Med 2009. Apr; 37(2): 141–153. [DOI] [PubMed] [Google Scholar]
- 16.Heatherton TF and Wagner DD.. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci 2011. Mar; 15(3): 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui SV, Chatterjee U, Kumar D, et al. Neuropsychology of prefrontal cortex. Indian J Psychiatry 2008; 50(3): 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller EK and Cohen JD.. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202. [DOI] [PubMed] [Google Scholar]
- 19.Shea JB and Morgan RL.. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol [Hum Learn] 1979; 5(2): 179–187. [Google Scholar]
- 20.Barreiros J, Figueiredo T, Godinho M.. The contextual interference effect in applied settings. Eur Phys Educ Rev 2007. Jun 1; 13(2): 195–208. [Google Scholar]
- 21.Brady F. A Theoretical and empirical review of the contextual interference effect and the learning of motor skills. Quest 1998. Aug 1; 50(3): 266–293. [Google Scholar]
- 22.Magill RA and Hall KG.. A review of the contextual interference effect in motor skill acquisition. Hum Mov Sci 1990. Sep 1; 9(3): 241–289. [Google Scholar]
- 23.Hoshi E, Shima K, Tanji J.. Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J Neurophysiol 2000. Apr; 83(4): 2355–2373. [DOI] [PubMed] [Google Scholar]
- 24.Tachibana A, Noah JA, Bronner S, et al. Activation of dorsolateral prefrontal cortex in a dual neuropsychological screening test: an fMRI approach. Behav Brain Funct 2012. May 28; 8(1): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waxenbaum JA, Reddy V, & Varacallo M.. Anatomy, autonomic nervous system. In: StatPearls [Internet], Treasure Island (FL), StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK539845/ (2022, accessed 7 Mar 2022). [PubMed] [Google Scholar]
- 26.Brook RD, & Julius S.. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens 2000. Jun 1; 13(S4): 112S–122S. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, & Grassi G.. The autonomic nervous system and hypertension. Circ Res 2014. May 23; 114(11): 1804–1814. [DOI] [PubMed] [Google Scholar]
- 28.Collet C, Di Rienzo F, El Hoyek N, et al. Autonomic nervous system correlates in movement observation and motor imagery. Front Hum Neurosci . 2013. Jul 30; 7: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delliaux S, Delaforge A, Deharo JC, et al. Mental workload alters heart rate variability, lowering non-linear dynamics. Front Physiol 2019; 10: 565. https://www.frontiersin.org/article/10.3389/fphys.2019.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paller K and Shapiro D. Systolic blood pressure and a simple reaction time task. Psychophysiology 1983. Sep; 20(5): 585–588. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Wang L, Jia M, et al. The effects of high-frequency rTMS over the left DLPFC on cognitive control in young healthy participants. PLoS One . 2017. Jun 14; 12(6): e0179430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghuntla TP, Mehta HB, Gokhale PA, et al. A comparison and importance of auditory and visual reaction time in basketball players. Saudi J Sports Med 2014. Jan 1; 14(1): 35. [Google Scholar]
- 33.Jain A, Bansal R, Kumar A, et al. A comparative study of visual and auditory reaction times on the basis of gender and physical activity levels of medical first year students. Int J Appl Basic Med Res 2015; 5(2): 124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plakke B and Romanski LM. Auditory connections and functions of prefrontal cortex. Front Neurosci 2014; 8: 199. https://www.frontiersin.org/article/10.3389/fnins.2014.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch JJ, Long JM, Thomas SA, et al. The effects of talking on the blood pressure of hypertensive and normotensive individuals. Psychosom Med 1981. Feb; 43(1): 25–33. [DOI] [PubMed] [Google Scholar]
- 36.Zheng D, Giovannini R, & Murray A.. Effect of talking on mean arterial blood pressure: Agreement between manual auscultatory and automatic oscillometric techniques. In: 2011 Computing in cardiology , IEEE Conference Publication, IEEE Xplore [Internet], https://ieeexplore.ieee.org/document/6164697 (2011, accessed 11 Mar 2022). [Google Scholar]
- 37.Kühlmann AYR, Etnel JRG, Roos-Hesselink JW, et al. Systematic review and meta-analysis of music interventions in hypertension treatment: a quest for answers. BMC Cardiovasc Disord 2016. Apr 19; 16(1): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mir IA, Chowdhury M, Islam RM, et al. Relaxing music reduces blood pressure and heart rate among pre-hypertensive young adults: A randomized control trial. J Clin Hypertens 2020. Dec 21; 23(2): 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siritunga S, Wijewardena K, Ekanayaka R, et al. Effect of music on blood pressure, pulse rate and respiratory rate of asymptomatic individuals: A randomized controlled trial. Health 2013. Apr 18; 5(4A): 59–64. http://www.scirp.org/journal/PaperInformation.aspx?PaperID=29924 [Google Scholar]
- 40.Porges SW. Heart rate variability: an autonomic correlate of reaction time performance. Bull Psychon Soc 1973; 1(4): 270–272. [Google Scholar]
- 41.Bora B, Krishna M, & Phukan KD.. The effects of tempo of music on heart rate, blood pressure and respiratory rate: A study in Gauhati Medical College. Indian J Physiol Pharmacol 2017; 61(4): 445–448. [Google Scholar]
- 42.Ubrangala KK, Vijayadas Kunnavil R, et al. Acute effects of passive listening to Indian musical scale on blood pressure and heart rate variability among healthy young individuals: A randomized controlled trial. bioRxiv 2020. https://www.biorxiv.org/content/10.1101/2020.05.03.073916v1
- 43.Bellato A, Arora I, Hollis C, et al. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci Biobehav Rev . 2020. Jan 1; 108: 182–206. [DOI] [PubMed] [Google Scholar]
- 44.Berridge KC and Kringelbach ML.. Pleasure systems in the brain. Neuron 2015. May 6; 86(3): 646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naqvi NH and Bechara A.. The hidden island of addiction: the insula. Trends Neurosci 2009. Jan; 32(1): 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cacioppo S, Bianchi-Demicheli F, Frum C, et al. The common neural bases between sexual desire and love: a multilevel kernel density fMRI analysis. J Sex Med 2012. Apr; 9(4): 1048–1054. [DOI] [PubMed] [Google Scholar]
- 47.Georgiadis JR and Kringelbach ML.. The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Prog Neurobiol 2012. Jul; 98(1): 49–81. [DOI] [PubMed] [Google Scholar]
- 48.Kringelbach ML, Stein A, & van Hartevelt TJ.. The functional human neuroanatomy of food pleasure cycles. Physiol Behav 2012. Jun 6; 106(3): 307–316. [DOI] [PubMed] [Google Scholar]
- 49.Parsons CE, Young KS, Murray L, et al. The functional neuroanatomy of the evolving parent-infant relationship. Prog Neurobiol 2010. Jul; 91(3): 220–241. [DOI] [PubMed] [Google Scholar]
- 50.Salimpoor VN, Benovoy M, Larcher K, et al. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci 2011. Feb; 14(2): 257–262. [DOI] [PubMed] [Google Scholar]
- 51.Vartanian O and Skov M. Neural correlates of viewing paintings: evidence from a quantitative meta-analysis of functional magnetic resonance imaging data. Brain Cogn 2014. Jun; 87: 52–56. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Aron A, Brown L, et al. Reward and motivation systems: a brain mapping study of early-stage intense romantic love in Chinese participants. Hum Brain Mapp 2011. Feb; 32(2): 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeki S and Romaya JP. The brain reaction to viewing faces of opposite- and same-sex romantic partners. PloS One 2010. Dec 31; 5(12): e15802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thayer JF and Sternberg E.. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 2006. Nov; 1088: 361–372. [DOI] [PubMed] [Google Scholar]
- 55.Cabral J, Kringelbach ML, & Deco G.. Exploring the network dynamics underlying brain activity during rest. Prog Neurobiol 2014. Mar; 114: 102–131. [DOI] [PubMed] [Google Scholar]


