Abstract
The authors performed this study to investigate the efficacy and safety of a rosuvastatin (RSV)/amlodipine (AML) polypill compared with those of atorvastatin (ATV)/AML polypill. We included 259 patients from 21 institutions in Korea. Patients were randomly assigned to 1 of 3 treatment groups: RSV 10 mg/AML 5 mg, RSV 20 mg/AML 5 mg, or ATV 20 mg /AML 5 mg. The primary endpoint was the efficacy of the RSV 10.20 mg/AML 5 mg via percentage changes in LDL‐C after 8 weeks of treatment, compared with the ATV 20 mg /AML 5 mg. There was a significant difference in the mean percentage change of LDL‐C at 8 weeks between the RSV 10 mg/AML 5 mg and the ATV 20 mg/AML 5 mg (full analysis set [FAS]: −7.08%, 95% CI: −11.79 to −2.38, p = .0034, per‐protocol analysis set [PPS]: −6.97%, 95% CI: −11.76 to −2.19, p = .0046). Also, there was a significant difference in the mean percentage change of LDL‐C at 8 weeks between the RSV 20 mg/AML 5 mg and the ATV 20 mg/AML 5 mg (FAS: −10.13%, 95% CI: −15.41 to −4.84, p = .0002, PPS: −10.96%, 95% CI: −15.98 to −5.93, p < .0001). There was no significant difference in the adverse events rates between RSV 10 mg/AML 5 mg, RSV 20 mg/AML 5 mg, and ATV 20 mg/AML 5 mg. In conclusion, while maintaining safety, RSV 10 mg/AML 5 mg and the RSV 20 mg/AML 5 mg more effectively reduced LDL‐C compared with the ATV 20 mg /AML 5 mg (Clinical trial: NCT03951207).
Keywords: amlodipine, atorvastatin, dyslipidemia, hypertension, rosuvastatin
1. INTRODUCTION
Hypertension and dyslipidemia are major risk factors for cardiovascular disease (CVD). 1 They can synergistically increase the rate of CVD. 2 Therefore, the control of both of these factors is important. Adherence is a crucial factor decreasing the rate of CVD and improve clinical outcomes. Previous studies have demonstrated that polypill can improve drug adherence compared to single pills in patients with hypertension and dyslipidemia. 3 , 4 Lin and colleagues demonstrated polypill of atorvastatin (ATV)/amlodipine (AML) improve drug adherence and clinical outcomes compared to FEC of AML and ATV. 5 Rosuvastatin (RSV) is another potent statin for patients with dyslipidemia. Kim and colleagues presented the polypill of RSV/AML effectively reduced blood pressure and low‐density lipoprotein cholesterol (LDL‐C) levels while maintaining safety in hypertensive patients with dyslipidemia. 6 However, RSV/AML polypill and the ATV/AML polypill had never been clinically compared. Therefore, we performed this study to investigate the efficacy and safety of RSV/AML polypill compared with those of the ATV/AML polypill in hypertensive patients with dyslipidemia.
2. METHODS
2.1. Study population
This study was conducted in patients with hypertension and dyslipidemia over the age of 19 years who met the following criteria: (a) If the patient is not taking antihypertensive medications, patients with mean sitting systolic blood pressure (msSBP) 140–179 mm Hg and mean sitting diastolic blood pressure (msDBP) < 110 mm Hg; (b) If the patient is taking antihypertensive medications, patients with msSBP < 140 mm Hg; (c) patients who are eligible for dyslipidemia medication by meeting the LDL‐C criteria according to the risk group classification (Table S1), and (d) patients with voluntary written consent. Patients with the following history or laboratory abnormalities were excluded: (a) patients with triglycerides (TG) ≥400 mg/dL at the time of screening; (b) patients who need to administer antihypertensive drugs other than AML, β‐blocker, and renin‐angiotensin system (RAS) inhibitors; (c) patients with a SBP difference of 20 mm Hg or more or a DBP difference of 10 mm Hg or more between the arms at the time of screening; (d) patients with a history of muscle disease or rhabdomyolysis due to statin use; (e) patients who have had a hypersensitivity reaction to statin or AML; (f) patients with renal insufficiency (estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2); (g) patients with aspartate aminotransferase or alanine aminotransferase levels > 3 times the upper limit of normal or active liver disease; (h) patients with creatinine phosphokinase > 5 times the upper limit of normal; (i) patients who are participating in clinical trials of other medications; or (j) other than the above, patients who judged the investigator to be inappropriate to participate in this clinical trial.
2.2. Study design
This multicenter, randomized, open‐label, parallel‐group phase IV clinical trial was conducted at 21 institutions in Korea from May 2019 to September 2021. All 21 participating hospitals are tertiary referral hospitals. The principal and sub‐investigators of each hospital recruited outpatients who met the inclusion and exclusion criteria. Subjects who met the inclusion/exclusion criteria were instructed to make therapeutic lifestyle changes from the time of screening. Subjects were administered (run‐in) AML 5 mg for 4 weeks (± 4 days) prior to randomization. Subjects receiving dyslipidemia treatment including statin had a wash‐out period of 4 weeks (± 4 days) before randomization. Subjects receiving β‐blockers or RAS inhibitors as antihypertensives maintained their dose unchanged from 4 weeks (± 4 days) prior to randomization until the end of the study. After a wash‐out/run‐in period, patients eligible for randomization were finally enrolled and randomly assigned to 1 of 3 treatment groups: RSV 10 mg/AML 5 mg, RSV 20 mg/AML 5 mg, or ATV 20 mg/AML 5 mg. Randomization was performed in a 1:1:1 ratio by using SAS version 9.4 (SAS Institute Inc). During the 8‐week treatment period, the assigned medications were administered once a day at the same time (morning) if possible. All patients were asked to visit the institution at 4 and 8 weeks after randomization to assess the efficacy and safety (Figure 1). In this study, medication compliance of 80% or more during the run‐in period (4 weeks) and after randomization should be obtained. Subjects whose medication compliance less than 80% was excluded.
FIGURE 1.
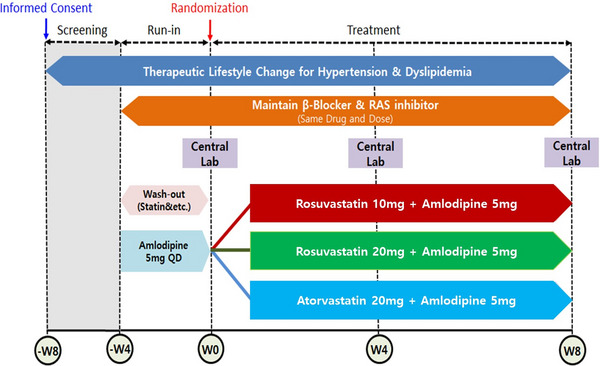
progress of clinical trial. RAS, renin angiotensin system.
2.3. Efficacy and safety assessment
The primary endpoints were the non‐inferiority of RSV 10 mg/AML 5 mg compared to ATV 20 mg/AML 5 mg in the LDL‐C % change rate, and the superiority of RSV 20 mg/AML 5 mg compared to ATV 20 mg/AML 5 mg in the LDL‐C % change rate after 8 weeks of administration. We determined LDL‐C concentrations by homogeneous enzymatic colorimetric assay using Roche Cobas 8000 c702 (Roche Diagnostics GmbH, Mannheim, Germany). The secondary end points were the percentage change in LDL‐C from baseline after 4 weeks of treatment; the percentage of patients who reached the LDL‐C treatment goal after 8 weeks of treatment (Group I: < 160 mg/dL, Group II: < 130 mg/dL, Group III: < 100 mg/dL) (Table S1); the percentage change in total cholesterol (TC), TG, high‐density lipoprotein cholesterol (HDL‐C), apolipoprotein B (Apo B), apolipoprotein A1 (Apo A‐1), Apo B/Apo A‐1, lipoprotein(a) (Lp (a)), high‐sensitivity C‐reactive protein (hsCRP), fasting blood glucose, hemoglobin a1c (HbA1c), homeostatic model assessment for insulin resistance (HOMA‐IR) from baseline after 4 and 8 weeks of treatment; the change in msSBP and msDBP of both arms from baseline after 4 and 8 weeks of treatment; and the change in msSBP difference and msDBP difference between both arms from baseline after 4 and 8 weeks of treatment. We assessed the safety by collecting the records of adverse events (AEs) and checking vital signs, laboratory tests, electrocardiograms, and physical examination results at each visit. The treatment‐emergent adverse events (TEAEs), adverse drug reactions (ADRs), and serious adverse events (SAEs) were compared among the treatment groups. In addition, the incidence of myopathies and the proportion of patients who had serum alanine aminotransferase increased or blood creatine phosphokinase increased were compared. An AE was defined as any harmful and unintended sign, symptom, or disease that occurred in the patient, regardless of whether it was related to the study drug. TEAEs were defined as: (a) AEs that occurred after the first administration of the study drug; and (b) symptoms that occurred prior to the first administration of the study drug, with severity worse after the first administration of the study drug. ADRs were defined as all harmful and unintended reactions that occur with any dose of the study drug, which can be suspected to be causally related to the drug. SAEs were defined as any of the following AEs occurring at any dose of the study drug: (a) AE that resulted in death or life‐threatening condition; (b) AE that required the patient to be admitted or need to extend the length of hospitalization; (c) AE that led to permanent or significant disability; and (d) other cases of medically important situations such as drug dependence, abuse, or blood diseases.
2.4. Statistical analysis
Data were expressed as number (%) and mean ± standard deviation. Categorical data were compared using the Chi‐square test or Fisher's exact test. Continuous variables were compared using the Student's t‐test and Kruskal–Wallis H test when they were normally and non‐normally distributed, respectively. A p‐value < .05 was considered statistically significant. Statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA).
2.4.1. Calculation of sample size
We estimated the power of the test based on the assumption of a difference between the test and control groups in percent change in LDL‐C at 8 weeks. In terms of LDL‐C percent changes, we planned the enrolment of 324 patients (108 per treatment group) by following criterions: (1) Level of significance, α = 0.05 (superiority), α = 0.025 (non‐inferiority), (2) power of test 80%, Type II error (β) = 0.2, (3) standard deviation 14%, and (4) loss to follow‐up 20%. We set the non‐inferiority criterion for the LDL‐C percent changes at 6% in the primary efficacy endpoint, as in the previous statin trials.
2.4.2. Data set analyzed
We screened 381 subjects and excluded 122 subjects with following reason: inappropriate inclusion/exclusion criteria: n = 93, withdrawal of subject consent: n = 24, Etc: n = 5 (Figure 2). Finally, a total of 259 subjects were randomly assigned to each group: 86 subjects in RSV 10 mg/AML 5 mg (test group 1), 87 subjects in RSV 20 mg/AML 5 mg (test group 2), and 86 subjects in ATV 20 mg /AML 5 mg (control group). Among 259 subjects, one subjects in each of test group 1 and test group 2 was excluded from the safety set as “no administration of clinical trial drugs,” and a total of 257 patients were used as safety evaluation analysis data. Among the safety set, one person in test group 2 was excluded as “missing efficacy evaluation after baseline,” and a total of 256 persons were used as full analysis set (FAS). One of the subjects assigned to the control group received RSV 20/AML 5 mg, and the subject was classified as test group 2 group in the safety analysis and the control group in the FAS analysis. Among FAS, 9 cases of “dropout” and 10 cases of “significant protocol violation” were excluded, and a total of 237 subjects were used as per‐protocol set (PPS) (Figure 2). Background factor analysis including demographic characteristics was conducted on a randomized set, and safety analysis including medication adherence was performed on a safety set. When testing the superiority of primary efficacy evaluation, FAS was used. When testing the non‐inferiority of primary efficacy evaluation, PPS was used. Both FAS and PPS were applied to all efficacy data.
FIGURE 2.
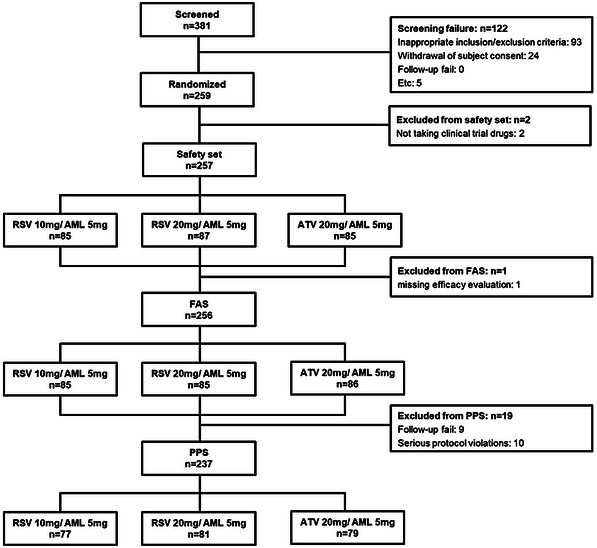
Enrollment flow chart for analysis. AML, amlodipine; ATV, atorvastatin; FAS, full analysis set; PPS, per‐protocol set; RSV, rosuvastatin.
2.4.3. Efficacy analysis
Primary efficacy analysis: Rate of LDL‐C change from baseline to week 8
We applied the same statistical analysis model to the non‐inferiority test and the significance test to determine whether test group 1 was non‐inferior to the control group and whether test group 2 was superior to the control group. Efficacy was analyzed using an analysis of covariance (ANCOVA) model with the primary efficacy as the dependent variable, the treatment group as the fixed factor, and the baseline LDL‐C as the covariate. Using the ANCOVA model, we presented the adjusted mean of the LDL‐C change rate of each group and the difference between the adjusted mean LDL‐C change rate in the test group and the control group. The ANCOVA model results showed a least‐square mean (LSM), standard deviation, LSM difference between the treatment group (test group vs. control group), corresponding 95% confidence interval (CI), and p‐value. Test group 1 is determined to be non‐inferior to the control group if the upper limit of the 95% two‐sided CI for (adjusted mean LDL‐C percent change of test group 1—adjusted mean LDL‐C percent change of the control group) is less than the preset non‐inferiority criterion (6%). Test group is determined to be superior to the control group if the upper limit of the 95% two‐sided CI for (adjusted mean LDL‐C percent change of test group—adjusted mean LDL‐C percent change of the control group) is less than 0 and p‐value is less than .05.
2.4.4. Secondary efficacy analysis
Non‐inferiority tests were not performed for all secondary efficacy analyses. Only significance tests were performed on the differences between test group 1 and the control group and between test group 2 and the control group. For the percentage change in LDL‐C from baseline after 4 weeks of treatment; the percentage of patients who reached the LDL‐C treatment goal after 8 weeks of treatment (Group I: < 160 mg/dL, Group II: < 130 mg/dL, Group III: < 100 mg/dL); the percentage change in TC, TG, HDL‐C, Apo B, Apo A‐1, Apo B/Apo A‐1, Lp (a), hsCRP, fasting blood glucose, HbA1c, HOMA‐IR from baseline after 4 and 8 weeks of treatment; the change in msSBP and msDBP of both arms from baseline after 4 and 8 weeks of treatment; the change in msSBP difference and msDBP difference in both arms from baseline after 4 and 8 weeks of treatment, the same statistical analysis method as that applied to the primary efficacy evaluation was applied.
2.4.5. Safety analysis
TEAEs occurred after administration of the clinical trial medication were analyzed, and the analysis was divided into the clinical trial medication taken during the run‐in period and the clinical trial medication taken during the treatment period. All AEs, SAEs, AEs for which a causal relationship with the clinical trial medication cannot be ruled out, and AEs that resulted in discontinuation after administration of the clinical trial medication were summarized by system organ class (SOC) and preferred terms (PT) in the medical dictionary for regulatory activities (MedDRA) coding dictionary. Also, the significance of the difference in the incidence of AEs between (test group 1‐control group) and (test group 2‐control group) was tested using Fisher's exact test. Clinical laboratory test values were evaluated with descriptive statistics for each treatment group and visit time. For vital signs, descriptive statistics were evaluated for each treatment group and visit time.
3. RESULTS
To 259 randomized subjects, demographic information was analyzed, the distribution between treatment groups was similar (Table 1). Adherence rate was as follow; (Run‐in period: 96.67% in test group 1, 96.43% in test group 2, 96.43% in the control group. Treatment period: 98.21% in test group 1, 96.61% in test group 2, 98.36% in the control group).
TABLE 1.
Patient demographics and baseline characteristics (randomized set).
| RSV10 mg/AML5 mg | RSV20 mg/AML5 mg | ATV20 mg/AML5 mg | P‐value 1 | P‐value 2 | |
|---|---|---|---|---|---|
| Total patients, n | 86 | 87 | 86 | ||
| Male | 58 (67.4) | 56 (64.4) | 54 (62.8) | .5222a | .8293a |
| Age, years | 62.79 ± 10.72 | 62.56 ± 10.45 | 62.06 ± 10.85 | .7745d | .8494d |
| Hypertension medication | 76 (88.4) | 78 (89.7) | 75 (87.2) | .8158a | .6149a |
| Duration of hypertension, years | 9.12 ± 7.80 | 8.48 ± 6.71 | 9.40 ± 7.12 | .6052d | .4158d |
| Dyslipidemia medication | 51 (59.3) | 55 (63.2) | 47 (54.7) | .5379a | .2521a |
| Duration of dyslipidemia, years | 6.12 ± 5.25 | 5.86 ± 5.67 | 6.13 ± 5.31 | .9804d | .5390d |
| Diabetes mellitus | 34 (39.5) | 25 (28.7) | 31 (36.1) | .6371a | .3041a |
| Smoking a month ago | 22 (25.6) | 19 (21.8) | 19 (22.1) | .5914a | .9678a |
| Drinking a month ago | 38 (44.2) | 37 (42.5) | 46 (53.5) | .2223a | .1491a |
| BMI, kg/m2 | 26.43 ± 3.87 | 26.48 ± 3.55 | 26.13 ± 3.19 | .9013d | .4941c |
| Myocardial infarction | 8 (9.3) | 11 (12.6) | 13 (15.1) | .2442a | .6381a |
| Angina | 18 (20.9) | 26 (29.9) | 24 (27.9) | .2869a | .7741a |
| Coronary revascularization | 10 (11.6) | 13 (14.9) | 15 (17.4) | .2794a | .6554a |
| PAD, AAA, symptomatic carotid disease | 2 (2.3) | 8 (9.2) | 6 (7.0) | .2774b | .5926a |
| Framingham Risk Score | .5555a | .6064a | |||
| <10% | 35 (40.7) | 35 (40.2) | 40 (46.5) | ||
| 10%–20% | 43 (50.0) | 43 (49.4) | 36 (41.9) | ||
| >20% | 8 (9.3) | 9 (10.3) | 10 (11.6) | ||
| Family history of premature CAD | 4 (4.7) | 4 (4.6) | 10 (11.6) | .0943a | .0900a |
| Categories of risk | .6832a | .6911a | |||
| Group I | 15 (17.4) | 13 (14.9) | 12 (14.0) | ||
| Group II | 9 (10.5) | 16 (18.4) | 12 (14.0) | ||
| Group III | 62 (72.1) | 58 (66.7) | 62 (72.1) |
Note: Values are given as mean ± standard deviation or n (%).
Abbreviations: Apo A1, apolipoprotein A1; Apo B, apolipoprotein; BMI, body mass index; CAC, coronary artery calcification; CAD, coronary artery disease; DBP, diastolic blood pressure; EF, ejection fraction; FBG, fasting blood glucose; FRS, Framingham Risk Score; Hb, hemoglobin; HDL, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LP(a), lipoprotein (a); PCE, Pooled Cohort Equations; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
RSV10 mg/AML5 mg vs. ATV20 mg/AML5 mg (aChi‐square test, bFisher's exact test, cTwo‐sample t‐test, dWilcoxon rank sum test).
RSV20 mg/AML5 mg vs. ATV20 mg/AML5 mg (aChi‐square test, bFisher's exact test, cTwo‐sample t‐test, dWilcoxon rank sum test).
3.1. Efficacy
3.1.1. Primary efficacy
The mean percentage change in LDL‐C from baseline after 8 weeks was (FAS: −48.11 ± 12.13%, PPS: −48.53 ± 11.97%) in the test group 1, (FAS: −51.93 ± 17.07%, PPS: −52.99 ± 14.91%) in the test group 2, and (FAS: −41.82 ± 18.04%, PPS: −42.20 ± 17.57%) in the control group. As a result of analyzing the primary efficacy in PPS for the non‐inferiority test, there was a significant difference in the adjusted mean percentage change of LDL‐C at 8 weeks between test group 1 and the control group (PPS: −6.97%, 95% CI: −11.76 to −2.19, p = .0046). The upper limit (−2.19%) of the 95% two‐sided CI for the difference in the adjusted mean percentage change of LDL‐C from the ANCOVA model was smaller than the preset non‐inferiority criterion (6%), indicating that test group 1 was non‐inferior to the control group. As a result of analyzing the primary efficacy endpoints in the FAS for the superiority, there was a significant difference in the adjusted mean percentage change of LDL‐C at 8 weeks between test group 1 and the control group (FAS: −7.08%, 95% CI: −11.79 to −2.38, p = .0034), indicating that test group 1 was superior to the control group in terms of LDL‐C percent changes. As a result of the comparison between test group 2 and the control group, there was a significant difference in the adjusted mean percentage change of LDL‐C at 8 weeks between test group 2 and the control group (FAS: −10.13%, 95% CI: −15.41 to −4.84, p = .0002, PPS: −10.96%, 95% CI: −15.98 to −5.93, p < .0001), indicating that test group 2 was superior to the control group in terms of LDL‐C percent changes (Table 2). The percent changes in LDL‐C according to treatment group at baseline, week 4, and week 8 are presented in Figure 3.
TABLE 2.
Changes in LDL‐C levels from baseline to after 8 weeks of treatment (FAS and PPS population).
| FAS | RSV 10 mg /AML 5 mg test group 1, N = 85 | RSV 20 mg /AML 5 mg test group 2, N = 85 | ATV 20 mg /AML 5 mg control group, N = 86 | |
|---|---|---|---|---|
| Baseline | n | 85 | 85 | 86 |
| Mean ± SD | 147.66 ± 28.55 | 156.35 ± 27.22 | 155.71 ± 32.99 | |
| Median | 146.00 | 154.00 | 153.50 | |
| Min, Max | 88.00, 212.00 | 104.00, 219.00 | 83.00, 222.00 | |
| Week 8 | n | 81 | 84 | 84 |
| Mean ± SD | 76.69 ± 21.23 | 74.89 ± 29.14 | 89.51 ± 28.37 | |
| Median | 77.00 | 70.50 | 88.00 | |
| Min, Max | 20.00, 130.00 | 34.00, 199.00 | 32.00, 199.00 | |
| % change (w8‐baseline) | n | 81 | 84 | 84 |
| Mean ± SD | −48.11 ± 12.13 | −51.93 ± 17.07 | −41.82 ± 18.04 | |
| Median | −50.00 | −56.17 | −46.79 | |
| Min, Max | −80.58, −11.56 | −72.13, 18.45 | −72.65, 32.20 | |
| ANCOVA test group 1 – control group | Adjusted Mean ± SE | −48.52 ± 1.69 | −41.43 ± 1.66 | |
| Difference between mean | −6.29 | |||
| Difference between adjusted mean | −7.08 | |||
| 95% CI | (−11.79, −2.38) | |||
| p‐value | .0034 | |||
| ANCOVA test group 2‐control group | Adjusted Mean ± SE | −51.94 ± 1.89 | −41.81 ± 1.89 | |
| Difference between mean | −10.11 | |||
| Difference between adjusted mean† | −10.13 | |||
| 95% CI | (−15.41, −4.84) | |||
| p‐value | .0002 | |||
| PPS | RSV 10 mg /AML 5 mg test group 1, N = 77 | RSV 20 mg /AML 5 mg test group 2, N = 81 | ATV 20 mg /AML 5 mg control group, N = 79 | |
|---|---|---|---|---|
| Baseline | n | 77 | 81 | 79 |
| Mean ± SD | 148.87 ± 28.73 | 156.11 ± 27.80 | 157.87 ± 32.25 | |
| Median | 147.00 | 153.00 | 154.00 | |
| Min, Max | 88.00, 212.00 | 104.00, 219.00 | 83.00, 222.00 | |
| Week 8 | n | 77 | 81 | 79 |
| Mean ± SD | 76.55 ± 21.76 | 72.94 ± 24.99 | 90.04 ± 28.65 | |
| Median | 77.00 | 70.00 | 88.00 | |
| Min, Max | 20.00, 130.00 | 34.00, 189.00 | 32.00, 199.00 | |
| % Change (w8‐baseline) | n | 77 | 81 | 79 |
| Mean ± SD | −48.53 ± 11.97 | −52.99 ± 14.91 | −42.20 ± 17.57 | |
| Median | −50.58 | −56.17 | −46.79 | |
| Min, Max | −80.58, −11.56 | −72.13, 9.01 | −72.65, 32.20 | |
| ANCOVA test group 1‐control group | Adjusted Mean ± SE | −48.85 ± 1.71 | −41.88 ± 1.69 | |
| Difference between mean | −6.33 | |||
| Difference between adjusted mean | −6.97 | |||
| 95% CI | (−11.76, −2.19) | |||
| p‐value | .0046 | |||
| ANCOVA test group 2‐control group | Adjusted Mean ± SE | −53.07 ± 1.79 | −42.12 ± 1.81 | |
| Difference between mean | −10.79 | |||
| Difference between adjusted mean | −10.96 | |||
| 95% CI | (−15.98, −5.93) | |||
| p‐value | <.0001 | |||
Note: Values are given as mean ± standard deviation or mean ± standard error or n (%).
Abbreviations: AML, amlodipine; ANCOVA, analysis of covariance; ATV, atorvastatin; CI, confidence interval; FAS, full analysis set; PPS, per protocol set; RSV, rosuvastatin; SD, standard deviation; SE, standard error.
FIGURE 3.
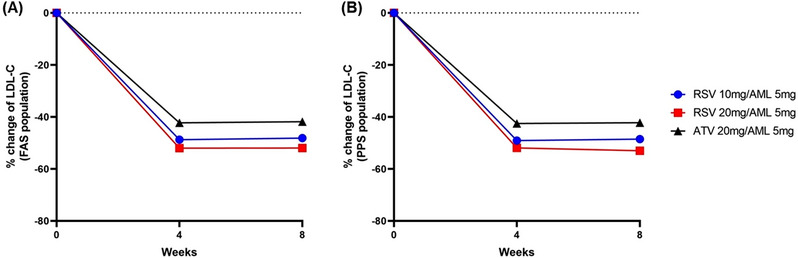
The percent changes in LDL‐C at baseline, week 4, and week 8 in FAS population (A) and PPS population (B). AML, amlodipine; ATV, atorvastatin; FAS, full analysis set; LD L‐C, low‐density lipoprotein cholesterol; PPS, per‐protocol set; RSV, rosuvastatin.
3.1.2. Secondary efficacy
As a result of the comparison between test group 1 and the control group, test group 1 showed a significant improvement in the following values compared to the control group in FAS and PPS analysis; the percentage change in LDL‐C from baseline after 4 weeks of treatment, the percentage change in Apo A‐1 from baseline after 8 weeks of treatment, and the percentage change in Apo B/Apo A‐1 from baseline after 4 and 8 weeks of treatment. On the other hand, test group 1 did not show any improvement in the percentage change of Lp (a) at 4 weeks and 8 weeks from baseline in FAS and PPS analysis. Compared to the control group, test group 1 showed a significant increase from baseline in the percentage change of Lp (a) at 4 weeks in FAS analysis and at 8 weeks in FAS and PPS analysis (Tables 3 and 4). As a result of the comparison between test group 2 and the control group, test group 2 showed a significant improvement in the following values compared to the control group in FAS and PPS analysis; the percentage change in LDL‐C from baseline after 4 weeks of treatment, the percentage change in TC from baseline after 4 and 8 weeks of treatment, the percentage change in Apo A‐1 from baseline after 8 weeks of treatment, the percentage change in Apo B from baseline after 4 and 8 weeks of treatment, and the percentage change in Apo B/Apo A‐1 from baseline after 4 and 8 weeks of treatment. On the other hand, test group 2 did not show any improvement in the percentage change of Lp (a) at 4 weeks and 8 weeks from baseline in FAS and PPS analysis. There was no significant difference of the percentage change of Lp (a) at 4 and 8 weeks from baseline in FAS and PPS analysis (Tables 3 and 4). The proportion of patients who satisfied the LDL‐C target goal according to risk classification was significantly higher in both test group 1 and test group 2 compared to the control group in PPS analysis (Figure 4). After replacing AML used during the run‐in period with polypill of RSV/AML and ATV/AML, the change in blood pressure was insignificant, less than 1%. In the FAS and PPS analysis, there was no significant difference in blood pressure change at 4 and 8 weeks from baseline between the test groups and the control group. (Tables 5 and 6). In the evaluation variables other than the secondary efficacy endpoints listed above, there was no statistical significance for the difference between the test groups and the control group.
TABLE 3.
Changes in lipoprotein, lipid and glucose levels from baseline to after 4 weeks of treatment (FAS and PPS population).
| RSV10 mg/AML5 mg test group 1, N = 85 | RSV20 mg/AML5 mg test group 2, N = 85 | ATV20 mg/AML5 mg control group, N = 86 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Lipids/lipoprotein/glucose (FAS) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w4‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w4‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w4‐baseline) | ANCOVA test group 1 – Control group | ANCOVA test group 2– Control group |
| LDL‐C | 147.7 ± 28.6 | −48.7 ± 12.7 | 156.4 ± 27.2 | −52.0 ± 15.8 | 155.7 ± 33.0 | −42.3 ± 15.7 | .0013 | <.0001 |
| TC | 212.8 ± 32.3 | −33.2 ± 8.7 | 221.1 ± 30.2 | −35.4 ± 11.6 | 220.8 ± 34.3 | −30.8 ± 11.7 | .0605 | .0075 |
| HDL‐C | 46.1 ± 11.7 | 10.0 ± 17.7 | 48.0 ± 11.1 | 9.1 ± 14.4 | 47.2 ± 11.9 | 9.16 ± 14.8 | .9127 | .8837 |
| TG | 183.1 ± 93.7 | −14.6 ± 39.4 | 166.2 ± 70.9 | − 17.6 ± 31.0 | 193.7 ± 225.3 | −22.0 ± 29.7 | .1923 | .5145 |
| Apo B | 129.8 ± 23.2 | −40.2 ± 10.5 | 135.9 ± 21.9 | −43.5 ± 12.8 | 134.6 ± 23.1 | −37.1 ± 12.5 | .0575 | .0012 |
| Apo A‐1 | 143.0 ± 25.6 | 6.9 ± 12.0 | 144.1 ± 22.2 | 7.0 ± 12.0 | 143.5 ± 22.6 | 4.5 ± 9.1 | .1661 | .1165 |
| Apo B/Apo A‐1 | 0.94 ± 0.25 | −43.2 ± 11.8 | 0.97 ± 0.23 | −47.2 ± 15.0 | 0.96 ± 0.24 | −39.0 ± 12.8 | .0223 | .0002 |
| Lp (a) | 40.8 ± 50.7 | 17.8 ± 37.5 | 48.0 ± 68.1 | 13.7 ± 43.3 | 34.0 ± 44.3 | 2.2 ± 31.6 | .0045 | .0620 |
| hs‐CRP | 1.3 ± 2.0 | 23.1 ± 128.4 | 1.4 ± 1.6 | −10.8 ± 87.0 | 1.2 ± 1.4 | 42.7 ± 254.2 | .5406 | .0723 |
| FBG | 118.0 ± 23.4 | −0.18 ± 11.6 | 114.2 ± 26.8 | 0.53 ± 9.6 | 117.6 ± 23.1 | 2.3 ± 13.5 | .2128 | .3286 |
| HbA1c | 6.1 ± 0.74 | 0.24 ± 4.4 | 6.1 ± 0.79 | 0.25 ± 3.5 | 6.3 ± 0.91 | −0.27 ± 3.2 | .4005 | .2259 |
| HOMA‐IR | 3.2 ± 2.2 | 4.1 ± 42.3 | 6.9 ± 36.2 | 26.2 ± 123.7 | 2.9 ± 2.2 | 26.6 ± 111.7 | .1168 | .9933 |
| RSV10 mg/AML5 mg test group 1, N = 77 | RSV20 mg/AML5 mg test group 2, N = 81 | ATV20 mg/AML5 mg control group, N = 79 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Lipids/lipoprotein/glucose (PPS) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w4‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w4‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w4‐baseline) | ANCOVA test group 1 – Control group | ANCOVA test group 2– Control group |
| LDL‐C | 148.9 ± 28.7 | −49.1 ± 11.7 | 156.1 ± 27.8 | −51.9 ± 16.0 | 157.9 ± 32.3 | −42.5 ± 15.5 | .0013 | .0001 |
| TC | 213.7 ± 32.1 | −33.7 ± 8.2 | 220.8 ± 30.8 | −35.4 ± 11.8 | 223.8 ± 32.6 | −31.2 ± 11.3 | .0614 | .0122 |
| HDL‐C | 45.7 ± 11.6 | 9.9 ± 18.1 | 47.8 ± 11.3 | 9.3 ± 14.4 | 47.6 ± 12.0 | 8.5 ± 13.6 | .7793 | .7037 |
| TG | 183.4 ± 96.8 | −15.0 ± 36.9 | 167.0 ± 72.0 | −17.6 ± 31.2 | 196.2 ± 234.1 | −22.7 ± 27.7 | .1611 | .4219 |
| Apo B | 130.9 ± 23.3 | −40.4 ± 9.8 | 135.9 ± 22.3 | −43.5 ± 13.0 | 136.1 ± 22.3 | −37.2 ± 12.1 | .0524 | .0015 |
| Apo A‐1 | 141.8 ± 25.2 | 6.5 ± 11.8 | 143.7 ± 22.5 | 7.0 ± 12.1 | 144.8 ± 21.9 | 3.8 ± 8.1 | .1180 | .0546 |
| Apo B/Apo A‐1 | 0.96 ± 0.26 | −43.8 ± 11.2 | 0.98 ± 0.23 | −47.2 ± 15.2 | 0.96 ± 0.23 | −38.7 ± 12.0 | .0069 | .0002 |
| Lp (a) | 40.6 ± 48.1 | 18.3 ± 37.3 | 48.2 ± 68.9 | 11.2 ± 39.1 | 35.5 ± 45.8 | 3.4 ± 31.9 | .0095 | .2002 |
| hs‐CRP | 1.40 ± 2.1 | 1.12 ± 1.3 | 1.40 ± 1.6 | 0.96 ± 1.3 | 1.2 ± 1.4 | 2.1 ± 7.8 | .4579 | .0773 |
| FBG | 117.7 ± 23.1 | 0.33 ± 11.6 | 113.9 ± 27.2 | 0.83 ± 9.5 | 117.0 ± 20.2 | 2.54 ± 14.0 | .2971 | .3567 |
| HbA1c | 6.2 ± 0.7 | 0.15 ± 4.3 | 6.0 ± 0.8 | 0.32 ± 3.4 | 6.2 ± 0.6 | −0.15 ± 3.1 | .6158 | .1782 |
| HOMA‐IR | 3.2 ± 2.2 | 5.8 ± 40.8 | 7.1 ± 37.1 | 27.6 ± 124.9 | 2.9 ± 2.2 | 28.2 ± 115.8 | .1402 | .9958 |
Note: Values are given as mean ± standard deviation.
Abbreviations: AML, amlodipine; ANCOVA, analysis of covariance; Apo A‐1, apolipoprotein A1; Apo B, apolipoprotein B; ATV, atorvastatin; CI, confidence interval; Hba1c, hemoglobin a1c; FAS, full analysis set; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HOMAR‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; Lp (a), lipoprotein (a); TC, total cholesterol; TG, triglyceride; PPS, per protocol set; RSV, rosuvastatin; SD, standard deviation.
TABLE 4.
Changes in lipoprotein, lipid and glucose levels from baseline to after 8 weeks of treatment (FAS and PPS population).
| RSV10 mg/AML5 mg test group 1, N = 85 | RSV20 mg/AML5 mg test group 2, N = 85 | ATV20 mg/AML5 mg control group, N = 86 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Lipids/lipoprotein/glucose (FAS) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w8‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w8‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w8‐baseline) | ANCOVA test group 1 – Control group | ANCOVA test group 2– Control group |
| TC | 212.8 ± 32.3 | −32.7 ± 9.2 | 221.1 ± 30.2 | −34.9 ± 12.7 | 220.8 ± 34.3 | −30.5 ± 13.1 | .1115 | .0246 |
| HDL‐C | 46.1 ± 11.7 | 11.3 ± 15.2 | 48.0 ± 11.1 | 10.1 ± 14.9 | 47.2 ± 11.9 | 7.6 ± 16.0 | .2056 | .2272 |
| TG | 183.1 ± 93.7 | −15.3 ± 40.3 | 166.2 ± 70.9 | −16.4 ± 30.7 | 193.7 ± 225.3 | −16.6 ± 35.6 | .9521 | .7266 |
| Apo B | 129.8 ± 23.2 | −39.7 ± 9.6 | 135.9 ± 21.9 | −42.9 ± 14.6 | 134.6 ± 23.1 | −36.1 ± 15.1 | .0548 | .0038 |
| Apo A‐1 | 143.0 ± 25.6 | 7.8 ± 13.5 | 144.1 ± 22.2 | 7.3 ± 12.5 | 143.5 ± 22.6 | 3.3 ± 9.4 | .0175 | .0174 |
| Apo B/Apo A‐1 | 0.94 ± 0.25 | −44.1 ± 11.0 | 0.97 ± 0.23 | −46.8 ± 15.3 | 0.96 ± 0.24 | −36.9 ± 15.5 | .0006 | <.0001 |
| Lp (a) | 40.8 ± 50.7 | 15.9 ± 32.4 | 48.0 ± 68.1 | 8.7 ± 40.3 | 34.0 ± 44.3 | 0.78 ± 33.9 | .0048 | .2094 |
| hs‐CRP | 1.3 ± 2.0 | 13.2 ± 127.9 | 1.4 ± 1.6 | −4.2 ± 175.4 | 1.2 ± 1.4 | 90.9 ± 522.4 | .2128 | .1353 |
| FBG | 118.0 ± 23.4 | 1.1 ± 11.8 | 114.2 ± 26.8 | 1.8 ± 13.8 | 117.6 ± 23.1 | 1.7 ± 12.3 | .7548 | .8854 |
| HbA1c | 6.1 ± 0.74 | 0.59 ± 6.2 | 6.1 ± 0.79 | 0.99 ± 5.5 | 6.3 ± 0.91 | 0.25 ± 4.7 | .7144 | .2370 |
| HOMA‐IR | 3.2 ± 2.2 | 12.7 ± 76.3 | 6.9 ± 36.2 | 33.1 ± 82.5 | 2.9 ± 2.2 | 42.4 ± 105.2 | .0539 | .5861 |
| RSV10 mg/AML5 mg test group 1, N = 77 | RSV20 mg/AML5 mg test group 2, N = 81 | ATV20 mg/AML5 mg control group, N = 79 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Lipids/lipoprotein/glucose (PPS) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w8‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w8‐baseline) | Baseline level mean ± SD, mg/dL | % change, mean ± SD (w8‐baseline) | ANCOVA test group 1 – Control group | ANCOVA test group 2– Control group |
| TC | 213.7 ± 32.1 | −33.1 ± 9.0 | 220.8 ± 30.8 | −35.7 ± 11.3 | 223.8 ± 32.6 | −30.8 ± 12.8 | .1305 | .0074 |
| HDL‐C | 45.7 ± 11.6 | 11.7 ± 15.4 | 47.8 ± 11.3 | 10.3 ± 15.1 | 47.6 ± 12.0 | 8.5 ± 15.8 | .3246 | .4221 |
| TG | 183.4 ± 96.8 | −15.6 ± 40.7 | 167.0 ± 72.0 | −17.8 ± 30.2 | 196.2 ± 234.1 | −17.4 ± 35.7 | .9072 | .6214 |
| Apo B | 130.9 ± 23.3 | −40.1 ± 9.3 | 135.9 ± 22.3 | −43.9 ± 12.8 | 136.1 ± 22.3 | −36.5 ± 14.6 | .0695 | .0008 |
| Apo A‐1 | 141.8 ± 25.2 | 8.2 ± 13.7 | 143.7 ± 22.5 | 7.2 ± 12.6 | 144.8 ± 21.9 | 3.5 ± 9.3 | .0171 | .0340 |
| Apo B/Apo A‐1 | 0.96 ± 0.26 | −44.8 ± 10.6 | 0.98 ± 0.23 | −47.8 ± 13.6 | 0.96 ± 0.23 | −37.5 ± 14.7 | .0004 | <.0001 |
| Lp (a) | 40.6 ± 48.1 | 16.0 ± 32.0 | 48.2 ± 68.9 | 8.9 ± 40.5 | 35.5 ± 45.8 | −0.34 ± 33.3 | .0026 | .1410 |
| hs‐CRP | 1.40 ± 2.1 | 13.4 ± 130.3 | 1.40 ± 1.6 | −5.6 ± 178.3 | 1.2 ± 1.4 | 94.7 ± 538.1 | .2221 | .1351 |
| FBG | 117.7 ± 23.1 | 1.7 ± 11.5 | 113.9 ± 27.2 | 2.1 ± 14.0 | 117.0 ± 20.2 | 1.7 ± 12.1 | .9900 | .9488 |
| HbA1c | 6.2 ± 0.7 | 0.75 ± 6.2 | 6.0 ± 0.8 | 1.1 ± 5.5 | 6.2 ± 0.6 | 0.6 ± 4.5 | .8572 | .3196 |
| HOMA‐IR | 3.2 ± 2.2 | 13.7 ± 77.5 | 7.1 ± 37.1 | 34.1 ± 83.6 | 2.9 ± 2.2 | 44.0 ± 106.0 | .0572 | .5766 |
Note: Values are given as mean ± standard deviation.
Abbreviations: AML, amlodipine; ANCOVA, analysis of covariance; Apo A‐1, apolipoprotein A1; Apo B, apolipoprotein B; ATV, atorvastatin; CI, confidence interval; Hba1c, hemoglobin a1c; FAS, full analysis set; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HOMAR‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; Lp (a), lipoprotein (a); TC, total cholesterol; TG, triglyceride; PPS, per protocol set; RSV, rosuvastatin; SD, standard deviation.
FIGURE 4.
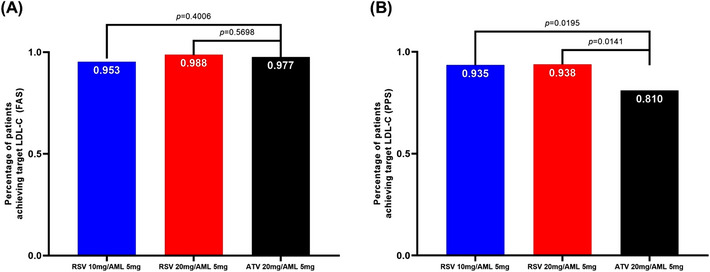
The proportion of patients who satisfied the LDL‐C target goal according to risk classification. AML, amlodipine; ATV, atorvastatin; FAS, full analysis set; LDL‐C, low‐density lipoprotein cholesterol; PPS, per‐protocol set; RSV, rosuvastatin.
TABLE 5.
Change in blood pressure from baseline to after 4 weeks of treatment (FAS and PPS population).
| RSV10 mg/AML5 mg Test group 1, N = 85 | RSV20 mg/AML5 mg Test group 2, N = 85 | ATV20 mg/AML5 mg Control group, N = 86 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Blood pressure (FAS) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w4‐baseline) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w4‐baseline) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w4‐baseline) | ANCOVA test group 1 – Control group | ANCOVA test group 2– Control group |
| Lt arm msSBP | 133.8 ± 12.9 | −0.66 ± 9.9 | 132.6 ± 11.7 | −2.4 ± 10.0 | 132.5 ± 12.2 | 0.17 ± 9.3 | .7053 | .0724 |
| Rt arm msSBP | 132.8 ± 13.3 | −0.42 ± 9.6 | 131.4 ± 11.9 | −1.3 ± 10.6 | 131.3 ± 13.1 | 0.7 ± 9.6 | .5797 | .1666 |
| Lt arm msDBP | 83.3 ± 9.5 | −0.54 ± 6.7 | 81.4 ± 8.5 | −0.92 ± 6.8 | 80.8 ± 8.3 | 0.6 ± 6.0 | .6537 | .1587 |
| Rt arm msDBP | 82.7 ± 9.2 | −0.3 ± 6.0 | 80.5 ± 8.2 | −0.7 ± 6.4 | 80.3 ± 9.4 | 0.4 ± 6.0 | .8200 | .2308 |
| msSBP difference in both arms | −0.98 ± 5.6 | 0.23 ± 6.0 | −1.2 ± 5.5 | 1.1 ± 6.9 | −1.12 ± 5.2 | 0.5 ± 6.5 | .8047 | .6345 |
| msDBP difference in both arms | −0.69 ± 3.6 | 0.23 ± 4.5 | −0.88 ± 3.8 | 0.20 ± 5.5 | −0.42 ± 3.9 | −0.14 ± 4.5 | .7826 | .9506 |
| RSV10 mg/AML5 mg test group 1, N = 77 | RSV20 mg/AML5 mg test group 2, N = 81 | ATV20 mg/AML5 mg control group, N = 79 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Blood pressure (PPS) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w4‐baseline) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w4‐baseline) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w4‐baseline) | ANCOVA test group 1 – Control group | ANCOVA test group 2– Control group |
| Lt arm msSBP | 134.2 ± 13.2 | −0.79 ± 9.7 | 132.6 ± 11.8 | −2.7 ± 10.0 | 132.4 ± 11.8 | 0.18 ± 9.2 | .7066 | .484 |
| Rt arm msSBP | 133.2 ± 13.5 | −0.74 ± 8.8 | 131.4 ± 12.0 | −1.6 ± 10.5 | 131.2 ± 13.0 | 0.7 ± 9.4 | .4966 | .1274 |
| Lt arm msDBP | 83.4 ± 9.6 | −0.80 ± 6.2 | 81.6 ± 8.5 | −1.2 ± 6.7 | 81.0 ± 8.1 | 0.67 ± 6.0 | .3687 | .0753 |
| Rt arm msDBP | 82.7 ± 9.3 | −0.55 ± 5.5 | 81.0 ± 8.1 | −1.0 ± 6.1 | 80.6 ± 9.2 | 0.45 ± 5.8 | .5445 | .1122 |
| msSBP difference in both arms | −1.0 ± 5.8 | 0.05 ± 6.0 | −1.2 ± 5.4 | 1.1 ± 7.0 | −1.2 ± 5.3 | 0.5 ± 6.7 | .6851 | .5134 |
| msDBP difference in both arms | −0.75 ± 3.7 | 0.25 ± 4.6 | −0.7 ± 3.8 | 0.13 ± 5.5 | −0.4 ± 3.9 | −0.22 ± 4.6 | .7542 | .8913 |
Note: Values are given as mean ± standard deviation.
Abbreviations: AML, amlodipine; ANCOVA, analysis of covariance; msSBP, mean sitting systolic blood pressure; msDBP, mean sitting diastolic blood pressure; SD, standard deviation.
TABLE 6.
Change in blood pressure from baseline to after 8 weeks of treatment (FAS and PPS population).
| RSV10 mg/AML5 mg test group 1, N = 85 | RSV20 mg/AML5 mg test group 2, N = 85 | ATV20 mg/AML5 mg control group, N = 86 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Blood pressure (FAS) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w8‐baseline) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w8‐baseline) | Baseline BP mean ± SD, mmHg | % change, mean ± SD (w8‐baseline) | ANCOVA test group 1 – control group | ANCOVA test group 2– control group |
| Lt arm msSBP | 133.8 ± 12.9 | 0.27 ± 9.9 | 132.6 ± 11.7 | −0.74 ± 10.9 | 132.5 ± 12.2 | −0.8 ± 9.4 | .2963 | .9157 |
| Rt arm msSBP | 132.8 ± 13.3 | 1.1 ± 10.6 | 131.4 ± 11.9 | −0.87 ± 11.8 | 131.3 ± 13.1 | −0.12 ± 10.2 | .2899 | .6777 |
| Lt arm msDBP | 83.3 ± 9.5 | −0.45 ± 5.6 | 81.4 ± 8.5 | −0.03 ± 6.4 | 80.8 ± 8.3 | −0.64 ± 6.2 | .3154 | .4250 |
| Rt arm msDBP | 82.7 ± 9.2 | −0.01 ± 5.4 | 80.5 ± 8.2 | −0.44 ± 7.1 | 80.3 ± 9.4 | −0.49 ± 6.5 | .2600 | .9593 |
| msSBP difference in both arms | −0.98 ± 5.6 | 0.81 ± 6.9 | −1.2 ± 5.5 | −0.13 ± 7.8 | −1.12 ± 5.2 | 0.68 ± 7.8 | .8386 | .3213 |
| msDBP difference in both arms | −0.69 ± 3.6 | 0.44 ± 4.4 | −0.88 ± 3.8 | −0.40 ± 5.6 | −0.42 ± 3.9 | 0.15 ± 4.6 | .9898 | .0707 |
| RSV10 mg/AML5 mg test group 1, N = 77 | RSV20 mg/AML5 mg test group 2, N = 81 | ATV20 mg/AML5 mg control group, N = 79 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Blood pressure (PPS population) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w8‐baseline) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w8‐baseline) | Baseline BP mean ± SD, mm Hg | % change, mean ± SD (w8‐baseline) | ANCOVA test group 1 – control group | ANCOVA test group 2– control group |
| Lt arm msSBP | 134.2 ± 13.2 | 0.22 ± 10.0 | 132.6 ± 11.8 | −1.3 ± 10.7 | 132.4 ± 11.8 | −0.92 ± 9.4 | .2779 | .8218 |
| Rt arm msSBP | 133.2 ± 13.5 | 1.2 ± 10.7 | 131.4 ± 12.0 | −1.4 ± 11.5 | 131.2 ± 13.0 | −0.3 ± 10.1 | .2007 | .5478 |
| Lt arm msDBP | 83.4 ± 9.6 | −0.46 ± 5.7 | 81.6 ± 8.5 | −0.13 ± 6.5 | 81.0 ± 8.1 | −0.73 ± 6.3 | .3159 | .4325 |
| Rt arm msDBP | 82.7 ± 9.3 | 0.13 ± 5.5 | 81.0 ± 8.1 | −0.85 ± 6.6 | 80.6 ± 9.2 | −0.63 ± 6.5 | .1805 | .8682 |
| msSBP difference in both arms | −1.0 ± 5.8 | 0.98 ± 7.0 | −1.2 ± 5.4 | −0.04 ± 7.9 | −1.2 ± 5.3 | 0.6 ± 7.9 | .5864 | .4972 |
| msDBP difference in both arms | −0.75 ± 3.7 | 0.58 ± 4.6 | −0.7 ± 3.8 | −0.72 ± 5.3 | −0.4 ± 3.9 | 0.1 ± 4.8 | .7537 | .0523 |
Note: Values are given as mean ± standard deviation.
Abbreviations: AML, amlodipine; ANCOVA, analysis of covariance; msSBP, mean sitting systolic blood pressure; msDBP, mean sitting diastolic blood pressure; SD, standard deviation.
3.2. Safety
As a result of analyzing the incidence of TEAE, test group 1 showed 22 cases in 18.82% (16/85 patients), test group 2 showed 14 cases in 11.49% (10/87 patients), and the control group showed 13 cases in 9.41% (8/85 patients). There was no significant difference in the incidence of TEAEs between test group 1 and the control group and test group 2 and the control group (p = .0781 and p = .6555, respectively). As a result of analyzing the incidence of all ADRs with the clinical trial medication in each treatment group, test group 1 showed 6 cases in 5.88% (5/85 patients), test group 2 showed 5 cases in 2.30% (2/87 patients), and the control group showed 1 case in 1.18% (1/85 patients). There was no significant difference in the incidence of ADRs between test group 1 and the control group and test group 2 and the control group (p = .2104 and p = 1.0000, respectively). An SAE occurred in 1 patient (cholelithiasis) in test group 2, but it was judged not to be related to the clinical trial drug. As for the AEs that caused clinical trial discontinuation, each 1 case of “Myalgia,” “Alanine aminotransferase increased” and “Blood creatine phosphokinase increased” occurred in test group 1, and 1 case of “Myalgia” occurred in test group 2. In the control group, there were no AEs that resulted in the discontinuation of the investigational medications. The relationship between the 4 reported AEs that resulted in the discontinuation of clinical trials and investigational medications is as follows. Three cases of “possible” (2 cases of Myalgia, 1 case of increased Alanine aminotransferase) were classified as ADRs, and 1 case of “low possibility” (increased blood creatine phosphokinase) was not classified as ADRs. There was no significant difference for the incidence of ADRs, SAEs, and AEs that resulted in clinical trial discontinuation between test group 1 and the control group and test group 2 and the control group (Table 7).
TABLE 7.
Adverse events (Safety set).
| RSV10 mg/AML5 mg Test group 1, N = 85 | RSV20 mg/AML5 mg Test group 2, N = 87 | ATV20 mg/AML5 mg Control group, N = 85 | P‐value | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Patients, n | Events, n | Patients, n | Events, n | Patients, n | Events, n | ANCOVA test group 1 vs. control group | ANCOVA test group 2 vs. control group | |
| TEAE | 16 (18.82) | 22 | 10 (11.49) | 14 | 8 (9.41) | 13 | .0781 | .6555 |
| ADR | 5 (5.88) | 6 | 2 (2.3) | 5 | 1 (1.18) | 1 | .2104 | 1.000 |
| SAE | 0 | 0 | 1 (1.15) | 1 | 0 | 0 | 1.000 | |
| AEs leading to discontinuation of treatment | 3 (3.53) | 3 | 1 (1.15) | 1 | 0 | 0 | .2456 | 1.000 |
| Pretreatment AEs | 6 (7.06) | 7 | 3 (3.45) | 4 | 3 (3.53) | 3 | .4958 | 1.000 |
Note: Values are given as n (%).
Abbreviations: ADR, adverse drug reaction; AE, adverse event; ANCOVA, analysis of covariance; AML, amlodipine; ATV, atorvastatin; RSV, rosuvastatin; SAE, serious adverse event; TEAE, treatment‐emergent adverse events.
4. DISCUSSION
The primary findings of present study were as follows: 1) After replacing the AML in the run‐in period with the polypill of RSV/AML, there was little change in blood pressure. There was no significant difference in blood pressure changes between RSV 10 mg, 20 mg/AML 5 mg and ATV 20 mg/AML 5 mg; 2) Not only RSV 20 mg/AML 5 mg but also RSV 10 mg/AML 5 mg lowered LDL‐C more and achieved more LDL‐C goals than ATV 20 mg/AML 5 mg; 3) In addition to LDL‐C, RSV 10 mg/AML 5 mg improved other atherosclerotic lipid factors including Apo A‐1 and Apo B/Apo A‐1 than ATV 20 mg/AML 5 mg. In addition to LDL‐C, RSV 20 mg/AML 5 mg improved other atherosclerotic lipid factors including TC, Apo A‐1, Apo B, and Apo B/Apo A‐1 than ATV 20 mg/AML 5 mg.4) In safety evaluation, there were no significant differences in TEAE, ADR, and SAE rate between polypill of RSV/AML and ATV 20 mg/AML 5 mg; 5) The adherence rate of polypill of RSV/AML was good at over 95%.
Hypertension and dyslipidemia are important diseases that increase CVD risk. 1 , 2 Therefore, simultaneous treatment is very important, and in this respect, the use of polypill can be a reliable option. It has been demonstrated that polypill can reduce CVD risk by increasing adherence to medication. 7 , 8 AML is recommended as a first‐line agent for hypertensive patients because of its excellent efficacy and safety, which is supported by strong evidence from large‐scale randomized clinical trials. 9 ATV is also a representative medication for dyslipidemia patients and is recommended as a first‐line agent for both primary and secondary prevention. Its efficacy and safety have been proven over a long period of time. 10 , 11 The polypill of AML and ATV has been used for a long time, and its advantages and safety have been proven 12 , 13 RSV is also another representative statin. There are many existing studies that have compared RSV and ATV alone, 14 , 15 , 16 however, few studies have compared the efficacy and side effects of these two medications in combination with AML.
In the present study, after replacing the AML in the run‐in period with the polypill of RSV/AML, there was little change in blood pressure. There was no significant difference in blood pressure changes between the polypill of RSV/AML 5 mg and the polypill of ATV 20 mg/AML 5 mg. Therefore, it is judged that the blood pressure‐lowering ability of AML is maintained even when the single formulation of AML is changed to the polypill of RSV/AML 5 mg. Previous studies have suggested that statin may have blood pressure‐lowering effects due to its effects such as improvement of endothelial function, influence of inflammatory response, stabilization of plaque, and reduction of risk of blood clots. 17 , 18 Liu and colleagues demonstrated in their meta‐analysis that statins have beneficial effects in reducing both SBP and DBP. However, they included only prospective randomized, controlled trials that had a minimum follow‐up of at least 2 months. Considering that the blood pressure follow‐up period in our study was 8 weeks after statin use, it seems that there was not enough time to confirm the blood pressure‐lowering effect of statin. 19
In the present study, not only RSV 20 mg/AML 5 mg but also RSV 10 mg/AML 5 mg achieved more percent reduction of LDL‐C and more LDL‐C goals than ATV 20 mg/AML 5 mg. This result was similar to the previous comparison of RSV and ATV alone. Wlodarczyk and colleagues demonstrated that RSV was more efficacious than the same dose of ATV (1:1 dose ratio) or a 2 times higher dose (1:2 dose ratio) of ATV. 15 Lowering LDL‐C is a major goal in treating dyslipidemia. For this purpose, the use of statins is recommended as the first medication. 20 Two LDL‐C level reduction treatment strategies are being used to lower LDL‐C: "treat‐to‐target" and "percent reduction". 21 Our study showed that RSV 10 mg/AML 5 mg and RSV 20 mg/AML 5 mg were more effective than ATV 20 mg/AML 5 mg for both strategies. In the present study, RSV 10 mg/AML 5 mg showed more improvement in Apo A‐1 and Apo B/Apo A‐1 than ATV 20 mg/AML 5 mg. RSV 20 mg/AML 5 mg showed more improvement in TC, Apo A‐1, Apo B, and Apo B/Apo A‐1 than ATV 20 mg/AML 5 mg. Apo B is a key structural protein component of all major atherosclerotic lipoproteins (LDL‐C, very‐low‐density lipoprotein cholesterol (VLDL‐C), intermediate‐density lipoproteins cholesterol (IDL‐C), and Lp (a)). The Apo A‐1 is an anti‐atherosclerotic lipoprotein and the main apolipoprotein incorporated into HDL‐C. Therefore, the Apo B/A1 ratio represents the cholesterol balance between atherogenic and anti‐atherogenic lipoproteins. 22 Several previous studies have reported that Apo B predicts CVD better than LDL‐C. 23 , 24 The Apo B/A1 ratio is also reported as a good predictor of CVD. Several prospective studies, including the INTERHEART and AMORIS studies, have demonstrated a strong relationship between incidence of CVD and the Apo B/A1 ratio. 23 , 24 Taken together, reduction of overall atherosclerotic lipid levels by using polypill of RSV/AML is expected to further CVD reduction. On the other hand, there was no improvement in Lp(a) level in polypill of RSV/AML but rather increased. Lp(a) is comprised of a low‐density lipoprotein particle with Apo B covalently bound to Apo A. 25 Lp(a) level is genetically determined in more than 90%, and high Lp(a) level is known to increase the risk of CVD. In the sub‐analysis of JUPITER study, it was announced that the residual CVD risk after sufficiently lowering LDL‐C can be explained by the Lp(a) level. 26 , 27 Interestingly, an association with an increased risk of CVD was demonstrated in people with high Lp(a) levels, however, medications known to effectively lower LDL‐C, such as statins, failed to lower Lp(a) levels. Recently PCSK9 inhibitor treatment has significantly lowered the Lp(a) level and significantly reduced the risk of CVD, showing potential as a treatment for patients with high Lp(a). 28 , 29 However, the mechanism by which polypill of RSV/AML increases Lp(a) is not well known, so further research is needed.
In the present study, RSV 10 mg/AML 5 mg significantly improved Apo A‐1 and Apo B/Apo A‐1 ratios as well as LDL‐C compared to ATV 20 mg/AML 5 mg. In addition, RSV 10 mg/AML 5 mg showed no significant difference compared to ATV 20 mg/AML 5 mg in the incidence of side effects and showed excellent adherence of over 95%. In actual clinical practice, the moderate‐intensity statin should be considered in patients for whom high‐intensity statin is not tolerable. Therefore, RSV 10 mg/AML 5 mg may have advantages over ATV 20 mg/AML 5 mg in hypertensive patients with dyslipidemia who require moderate‐intensity statin. Many dyslipidemias guideline recommend the use of high‐intensity statin for patients at high risk of CVD. 20 , 30 RSV 20 mg is a high‐intensity statin that can be expected to reduce LDL‐C by more than 50%, and its effectiveness has been demonstrated in a single formulation. 31 , 32 However, the effect of RSV 20 mg as the polypill of RSV/AML is not well known. In this study, the mean LDL‐C percent reduction at week 8 of RSV 20 mg/AML 5 mg was −51.93 ± 17.07% in FAS and −52.99 ± 14.91% in PPS, proving its effectiveness. In terms of safety, it was confirmed that RSV 20 mg/AML 5 mg did not significantly increase the incidence of ADR, SAE, and AEs leading to discontinuation of treatment than RSV 10 mg/AML 5 mg. In conclusion, it is judged that increasing the statin dose of polypill of RSV/AML increases the LDL‐C lowering efficacy while maintaining safety.
This study had several limitations. First, a relatively small number of patients were evaluated over a short period of time. Second, the study population was only comprised of Koreans; studies with other races are necessary to confirm and generalize our findings. Third, this study was open‐label study. Therefore, there could be several limitations such as possible higher patient dropout, and concerns regarding the internal validity of the study including possible patient underreporting of adverse events. 33 However, this study has several strengths. First, this study is the first study comparing the polypill of RSV/AML and the polypill of ATV/AML. Second, this study evaluated changes in various atherosclerotic lipid profiles and glucose metabolism profiles as well as LDL‐C following the use of polypill of RSV/AML. Third, we selected only patients with both hypertension and dyslipidemia and who had a compliance rate of 80% or more. In particular, we limited patients with dyslipidemia to those who met the LDL‐C criteria according to the risk group classification (Table S1). These strict patient selection criteria were helpful in evaluating the effects of the polypill of RSV/AML on patients who needed treatment for hypertension and dyslipidemia in real clinical practice.
5. CONCLUSIONS
While maintaining safety and blood pressure lowering effect, the polypill of RSV 10 mg/AML 5 mg and RSV 20 mg/AML 5 mg is more efficacious than the polypill of ATV 20 mg/AML 5 mg in terms of LDL‐C lowering, LDL‐C goal achievement, and atherogenic lipid profile improvement. Simultaneous improvement of blood pressure and atherosclerotic lipid profile using the polypill of RSV/AML may further reduce CVD risk.
AUTHOR CONTRIBUTIONS
H.W.J. wrote sections of the initial draft and performed analyses and interpreted results. S.P.H., H.J.B., J.Y.C., J.K.R., and J.B.L. provided substantive suggestions for revision, or critically reviewed the manuscript. K.H.L., K.R.H., D.H.Y., C.G.P., G.W.Y., M.Y.R., S.J.P., M.S.H., J.H.S., B.K.H., H.Y.J., S.Y.L., S.H.S., S.R.L., S.Y.K., K.J.L., E.J.C., C.W.N., T.H.P., and U.K. performed data gathering and provided substantive suggestions for revision. K.S.K. conceived, designed or planned the study, interpreted the results, and provided substantive suggestions for revision or critically reviewed the manuscript. All authors reviewed and approved the final version of the paper.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CONSENT
Written informed consent was obtained from all individual participants included in the study. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This study was funded by the Yuhan Corporation. The sponsor, Yuhan Corporation supported the supply of the study drug, laboratory tests, data collection, and data analysis. The sponsor had no role in data interpretation, the writing of the original draft of manuscript, or the decision to submit the article for publication.
Jung HW, Kim C‐Y, Hong S‐P, et al. Randomized, multicenter, parallel, open, phase 4 study to compare the efficacy and safety of rosuvastatin/amlodipine polypill versus atorvastatin/amlodipine polypill in hypertension patient with dyslipidemia. J Clin Hypertens. 2023;25:828–844. 10.1111/jch.14715
DATA AVAILABILITY STATEMENT
Data will be available based on the request from the corresponding author.
REFERENCES
- 1. Zhang Y, Vittinghoff E, Pletcher Mark J, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74(3):330‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang C, Du Z, Ye N, et al. Hyperlipidemia and hypertension have synergistic interaction on ischemic stroke: insights from a general population survey in China. BMC Cardiovasc Disord. 2022;22(1):47‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parati G, Kjeldsen S, Coca A, Cushman WC, Wang J. Adherence to single‐pill versus free‐equivalent combination therapy in hypertension. Hypertension. 2021;77(2):692‐705. [DOI] [PubMed] [Google Scholar]
- 4. Weisser B, Predel H‐G, Gillessen A, et al. Single pill regimen leads to better adherence and clinical outcome in daily practice in patients suffering from hypertension and/or dyslipidemia: results of a meta‐analysis. High Blood Press Cardiovasc Prev. 2020;27(2):157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin CP, Tung YC, Hsiao FC, et al. Fixed‐dose combination of amlodipine and atorvastatin improves clinical outcomes in patients with concomitant hypertension and dyslipidemia. J Clin Hypertens (Greenwich). 2020;22(10):1846‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim W, Chang K, Cho EJ, et al. A randomized, double‐blind clinical trial to evaluate the efficacy and safety of a fixed‐dose combination of amlodipine/rosuvastatin in patients with dyslipidemia and hypertension. J Clin Hypertens (Greenwich). 2020;22(2):261‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed‐dose combinations improve medication compliance: a meta‐analysis. Am J Med. 2007;120(8):713‐719. [DOI] [PubMed] [Google Scholar]
- 8. Webster R, Patel A, Selak V, et al. Effectiveness of fixed dose combination medication ('polypills') compared with usual care in patients with cardiovascular disease or at high risk: a prospective, individual patient data meta‐analysis of 3140 patients in six countries. Int J Cardiol. 2016;205:147‐156. [DOI] [PubMed] [Google Scholar]
- 9. Fares H, DiNicolantonio JJ, O'Keefe JH, Lavie CJ. Amlodipine in hypertension: a first‐line agent with efficacy for improving blood pressure and patient outcomes. Open Heart. 2016;3(2):e000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones PH, McKenney JM, Karalis DG, Downey J. Comparison of the efficacy and safety of atorvastatin initiated at different starting doses in patients with dyslipidemia. Am Heart J. 2005;149(1):e1. [DOI] [PubMed] [Google Scholar]
- 11. Adams SP, Tsang M, Wright JM. Lipid‐lowering efficacy of atorvastatin. Cochrane Database Syst Rev. 2015;2015(3):Cd008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Preston RA, Harvey P, Herfert O, et al. A randomized, placebo‐controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of coadministered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the respond trial. J Clin Pharmacol. 2007;47(12):1555‐1569. [DOI] [PubMed] [Google Scholar]
- 13. Erdine S, Ro YM, Tse HF, et al. Single‐pill amlodipine/atorvastatin helps patients of diverse ethnicity attain recommended goals for blood pressure and lipids (the Gemini‐AALA study). J Hum Hypertens. 2009;23(3):196‐210. [DOI] [PubMed] [Google Scholar]
- 14. Clearfield MB, Amerena J, Bassand JP, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high‐risk patients with hypercholesterolemia—Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials. 2006;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wlodarczyk J, Sullivan D, Smith M. Comparison of benefits and risks of rosuvastatin versus atorvastatin from a meta‐analysis of head‐to‐head randomized controlled trials. Am J Cardiol. 2008;102(12):1654‐1662. [DOI] [PubMed] [Google Scholar]
- 16. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med. 2008;168(7):721‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu HT, Deng NH, Wu ZF, et al. Statin's role on blood pressure levels: meta‐analysis based on randomized controlled trials. J Clin Hypertens. 2023;25(3):238‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082‐e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bangalore S, Fayyad R, Kastelein JJ, et al. 2013 cholesterol guidelines revisited: percent LDL cholesterol reduction or attained LDL cholesterol level or both for prognosis? Am J Med. 2016;129(4):384‐391. [DOI] [PubMed] [Google Scholar]
- 22. Behbodikhah J, Ahmed S, Elyasi A, et al. Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Metabolites. 2021;11(10):690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A‐I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358(9298):2026‐2033. [DOI] [PubMed] [Google Scholar]
- 24. McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case‐control study. Lancet. 2008;372(9634):224‐233. [DOI] [PubMed] [Google Scholar]
- 25. Patel AP, Wang M, Pirruccello JP, et al. Lp(a) (Lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease. ATVB. 2021;41(1):465‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamstrup PR, Tybjærg‐Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331‐2339. [DOI] [PubMed] [Google Scholar]
- 27. Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129(6):635‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsimikas S, Witztum JL, Miller ER, et al. High‐dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B‐100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110(11):1406‐1412. [DOI] [PubMed] [Google Scholar]
- 29. O'Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483‐1492. [DOI] [PubMed] [Google Scholar]
- 30. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111‐188. [DOI] [PubMed] [Google Scholar]
- 31. Karlson BW, Wiklund O, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Variability of low‐density lipoprotein cholesterol response with different doses of atorvastatin, rosuvastatin, and simvastatin: results from VOYAGER. Eur Heart J Cardiovasc Pharmacother. 2016;2(4):212‐217. [DOI] [PubMed] [Google Scholar]
- 32. Luvai A, Mbagaya W, Hall AS, Barth JH. Rosuvastatin: a review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin Med Insights Cardiol. 2012;6:17‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kushnir I, Clemons M, Fergusson D, Bossé D, Reaume MN. Attitudes towards open‐label versus placebo‐control designs in oncology randomized trials: a survey of medical oncologists. J Eval Clin Pract. 2022;28(3):495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Data will be available based on the request from the corresponding author.


