Abstract
Vitamin A has long been associated with bladder cancer, and many exogenous vitamin A supplements, vitamin A derivatives, and synthetic drugs have been investigated over the years. However, the effectiveness of these strategies in clinical practice has not met expectations, and they have not been widely adopted. Recent medical research on intestinal flora has revealed that bladder cancer patients exhibit reduced serum vitamin A levels and an imbalance of gut microbiota. In light of the close relationship between gut microbiota and vitamin A, one can speculate that a complex regulatory mechanism exists between the two in the development and occurrence of bladder cancer. As such, further exploration of their interaction in bladder cancer may help guide the use of vitamin A for preventive purposes. During the course of this review, attention is paid to the influence of intestinal microbiota on the vitamin A metabolism and the RA signaling pathway, as well as the mutual promotion relationships between them in the prevention of bladder cancer, In addition, it emphasizes the importance of intestinal microbiota for bladder cancer prevention and treatment.
Keywords: vitamin A, retinoic acid, gut microbiota, lipopolysaccharides, bladder cancer
1. Introduction
There has been an increase in the incidence of bladder cancer in recent years, particularly among women. It affects the urinary system and can be fatal. In 2020, it was reported to account for 573,278 new cases and 212,536 deaths worldwide, ranking ninth and 13th in terms of incidence and mortality among malignant tumors, respectively (1). Bladder cancer includes a variety of pathological types, including urothelial carcinoma, squamous cell carcinoma and adenocarcinoma, among which urothelial carcinoma is the most common pathological type (2). In reality, approximately 75% of patients with bladder cancer are afflicted with non-muscle invasive bladder cancers (NMIBC), which can have varying levels of risk for recurrence and progression. Generally, the 5-year survival rate for NMIBC exceeds 90%, contributing to the high incidence and low mortality rates of bladder cancer. Nonetheless, most NMIBC patients require long-term surveillance and preventive interventions, such as cystoscopy, which significantly impact their quality of life and impose a financial burden (3, 4). Therefore, chemoprophylaxis and other strategies to reduce postoperative bladder cancer recurrence have been widely employed in clinical practice, with retinoic acid (RA) being the most commonly used chemoprophylaxis drug. RA possesses remarkable anti-tumor properties. As early as 1990, it was discovered that RA could arrest hematopoietic cell cycle and induce cell differentiation into hematopoietic terminal cells, leading to its application in treating acute promyelocytic leukemia (5). Other cancer types, such as thyroid and prostate cancer, have also been shown to respond to RA’s anti-tumor effects, including inhibition of cell proliferation and induction of cell differentiation (6, 7). Vitamin A has been shown to prevent and treat bladder cancer in numerous studies conducted over the past 50 years. A review of these studies on vitamin A and bladder cancer is presented in Table 1 . Researchers have confirmed that patients with bladder cancer have lower levels of serum vitamin A than healthy people, as shown in a substantial body of research. In addition, low vitamin A levels are increasingly regarded as risk factors for bladder cancer. Several in vivo experiments on vitamin A are summarized in Table 2 . Almost all studies have shown positive results, mainly manifested as vitamin A can inhibit apoptosis, reduce tumor size, and inhibit the progression of bladder cancer. However, although in vivo experiments showed consistent promising results, the results of several clinical trials of vitamin A supplementation were not as expected (19–21). As a result, enhancing the efficiency of vitamin A in bladder cancer prevention and treatment would be an important research endeavor.
Table 1.
Clinical trials investigation on vitamin A and bladder cancer.
| Country | Study period | Age(years) | Case/subjects | Pathology | Main Findings | Reference |
|---|---|---|---|---|---|---|
| Sweden | 1985 to 1987 | 40 to 74 | 418/929 | urothelial carcinoma | Vitamin A supplement plays a certain preventive effect on urothelial carcinoma. | (8) |
| Eypt | 1957 to 1965 | Not mentioned | 70/144 | Not mentioned | Vitamin A levels were significantly lower in bladder cancer patients with squamous cell carcinoma than in normal individuals | (9) |
| Japan | 1990 to 2007 | >40 | 42/1666 | urothelial carcinoma | High serum carotene levels reduce the risk of bladder cancer | (10) |
| USA | 1957 to 65 | 40 to 89 | -/8606 | Not mentioned | Dietary vitamin A is associated with a reduced risk of squamous epithelial carcinoma | (11) |
| Turkey | Not mentioned | 40 to 79 | 23/91 | urothelial carcinoma | Compared to the control group, patients had significantly lower serum vitamin A levels | (12) |
| USA | 2001 to 2004 | 30 to 79 | 1418/2589 | urothelial carcinoma | Elevated plasma carotene levels significantly reduce the risk of bladder cancer | (13) |
| USA | 1993 to 2007 | 45 to 75 | 581/185885 | Not mentioned | Women with high vitamin A and carotene intake have a lower risk of bladder cancer. | (14) |
| USA | 1971 to 1995 | 52 to 71 | 111/222 | urothelial carcinoma | No significant correlation between serum carotene levels and bladder cancer risk after adjustment with smoking. | (15) |
| Japan | 1971 to 1975 | Not mentioned | 27/6800 | Not mentioned | Serum vitamin A levels were not associated with bladder cancer risk | (16) |
| Belgium | 1999 to 2004 | Not mentioned | 178/540 | urothelial carcinoma | Retinol intake was not significantly associated with bladder cancer. | (17) |
| Netherlands | 1981 to 1989 | 55 to 69 | 569/3692 | Not mentioned | There was no association between bladder cancer and dietary or supplemental intake of vitamin A and most carotenoids | (18) |
| USA | 1981 to 1989 | Not mentioned | 335/11580 | Not mentioned | Supplementing with β-carotene and vitamin A did not reduce bladder cancer risk significantly | (19) |
| USA | 1980 to 2000 | 30 to 55 | 237/88796 | Not mentioned | Vitamin A and carotene intake were not associated with bladder cancer risk. | (20) |
| USA | 2000 to 2007 | 50 to 76 | 330/77050 | urothelial carcinoma | Supplementation of carotene and retinol cannot effectively prevent the occurrence of urothelial carcinoma. | (21) |
Table 2.
In vivo studies investigating the effects of vitamin A in animal models of bladder cancer.
| In Vivo Model–Carcinogen | Species | Outcome | Reference |
|---|---|---|---|
| MNNG | Rat | Bladder cancer incidence induced by MNNG was higher in rats with low vitamin A diet | (22) |
| FANFT | Rat | Vitamin A deficiency accelerated the carcinogenic efficiency of FANFT, but high vitamin A did not significantly inhibit FANFT-induced bladder cancer | (23) |
| BBN | Rat | Hyperretinemia inhibited the incidence of BBN-induced transitional cell carcinoma and neoplasms of the bladder | (24) |
| BBN | Rat | Vitamin A diet could reduce the progression of early bladder cancer by reducing BBN-induced urothelial atypia. | (25) |
| BBN | Rat | Vitamin A supplementation reduce the incidence of tumor and tumor size. | (26) |
| BBN | Mouse | Vitamin A treatment reduce the urothelial atypia and apoptosis in early bladder cancer. | (25) |
MNNG, N-methyl-N’-nitro-N-nitrosoguanidine; FANFT, N-[4-(5-nitro-2-furyl)-2-thiazolyl] formanmide; BBN, N-butyl-N-(4-hydroxybutyl) nitrosamine.
Intestinal microbiomes are collections of microorganisms found in the gastrointestinal tract. Advancements in technologies such as 16S rRNA sequencing have revealed the significant role of gut microbiota in non-infectious diseases, particularly in tumor diseases. It is becoming increasingly clear that gut microbiota influences immunity and inflammation in intricate ways, implying its complex involvement in tumor occurrence and development (27). In addition, studies have reported enhanced anti-cancer effects associated with gut microbiota (28). Consequently, there is increasing attention on the role of gut flora in cancer. In a case-control study, bladder cancer patients’ gut microbiota was compared to that of healthy individuals. According to the findings, patients with bladder cancer showed a significant reduction in the abundance of specific bacteria in their gut. Additionally, this work used real-time qPCR to analyze the differences among 12 major Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria bacteria. The findings demonstrated that the numbers of domain Bacteria, Clostridium cluster XI and Prevotella in patients were significantly lower than those in healthy group (29). Some studies (30–32) have achieved positive results through the application of intestinal probiotics such as Bifidobacterium pseudolongum, Lactobacillus johnsonii and Lactobacillus rhamnosus preparation in the prevention and treatment of bladder cancer. We have observed that the abundance of some of these probiotics such as Lactobacillus in the intestine is closely related to RA, either promoting or inhibiting (33, 34). So, the relationship between RA, gut flora, and bladder cancer is very subtle, and it is therefore necessary to further explore the interaction between these three factors in order to gain a deeper understanding.
2. Vitamin A metabolism and its role in bladder cancer
2.1. Absorption, transport and metabolism of vitamin A
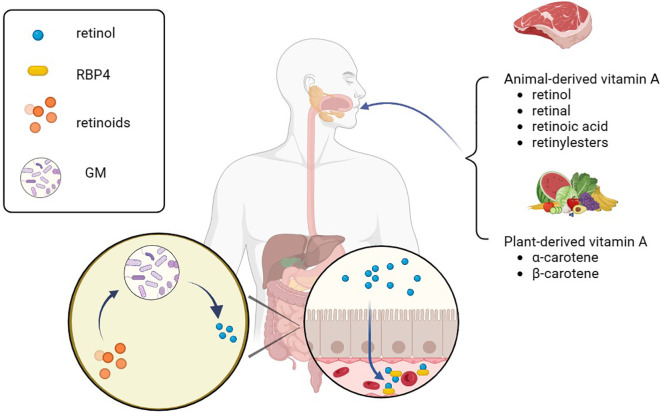
The human body lacks the ability to directly synthesize RA, so we primarily obtain it from our diet. It is possible to absorb preformed vitamin A directly from animal foods, such as liver or fish, in the form of retinol, retinal, RA, and retinyl esters, which can be directly absorbed into the bloodstream by the gut and stored in the liver (35). However, for our bodies, food sources rich in β-carotene are the primary source of vitamin A. In the gastrointestinal tract, β-carotene is broken down and released, subsequently converted into retinal and retinol with the help of β-carotene oxygenase (BCO) (36). It then binds to retinol-binding protein 4(RBP4) and is transported through the bloodstream to the liver for further metabolism. In addition, retinoids and β-carotenes can be directly absorbed from food, packaged as chylomicrons, and enter the bloodstream through the lymphatic system (37) ( Figure 1 ).
Figure 1.
Vitamin A absorption. Various animal-derived vitamin A is first converted into retinol in the gastrointestinal tract, then absorbed into the blood through the intestine, combined with RBP4 for transport in the blood, and finally transported to the liver for storage. A part of phytogenic vitamin A can be converted into retinol by β-carotene oxygenase and absorbed, and the other part can be integrated into chylomicron with retinol and enter the blood circulation through lymphatic reflux. RBP4, retinol-binding protein 4; GM, gut microbiota.
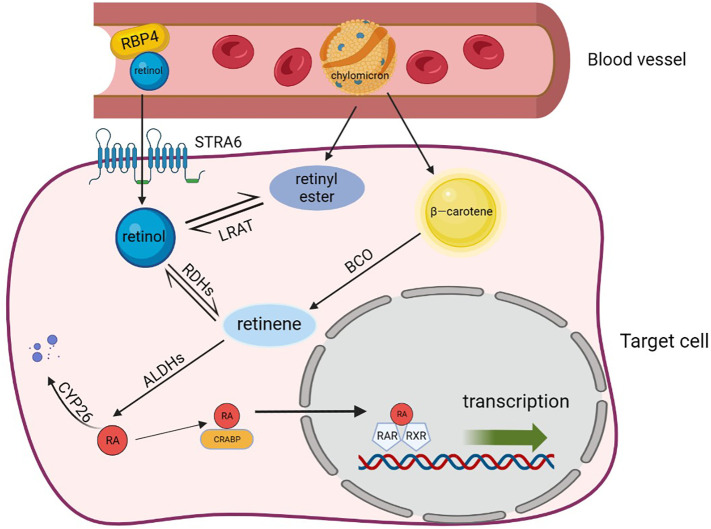
Target cells take up retinol that binds RBP4 from the blood through retinoic acid 6 (STRA6). Alternatively, retinol and β-carotenes from chylomicrons are taken up by target cells through lipoprotein-specific receptors (38). After entering the target cells, β-carotenes are converted to retinol by BCO. However, retinol has poor water solubility, so to enhance its transportation within cells, it binds with cellular retinol-binding proteins (CRABPs), which helps it exert its metabolic activity more effectively (39). Then, retinol is converted into RA through the action of retinol dehydrogenases (RDHs) and aldehyde dehydrogenases (ALDHs) (37, 40). The enzyme lecithin retinol acyltransferase (LRAT) finally converts RA and retinol into esterified products (36). RA, being the most active molecule among retinoids and the primary component of metabolically active vitamin A, activates the RA signaling pathway, which controls cell proliferation, differentiation, and apoptosis (41). It can be oxidized into non-biologically active compounds by the enzyme cytochrome P450 (CYP26) or transported to the nucleus by binding with CRABP or fatty acid binding protein 5 (FABP5) to activate the RA signaling pathway and exert its biological activity (42, 43).
RARs are classified as members of the steroid/thyroid hormone nuclear receptor superfamily, and RXRs are their indispensable eheterodimerization partners, all of which exist in the form of three para-homologs (RARα, RARβ, and RARγ as well as RXRα, RXRβ, and RXRγ) (44). There are more than 500 genes currently dependent on RA signaling, and activation of different isomers can lead to different biological effects (45–47). After entering the nucleus through CRABP, RA binds to RAR-RXR heterodimers and affects gene expression, which can be described as a molecular switch (48). RARs attach to the co-repressors NcoR1 and NcoR2 when RA is absent, and the co-repressors serve as bridges to connect a polymer complex with histone deacetylase activity (49). The complex has the ability to remove the acetyl group from the end of the histone to retain the chromatin’s condensation state and prevent the target gene from being transcribed. Conversely, co-repressors are dissociated from the RAR-RXR heterodimers and replaced by the co-activators such as nuclear receptor co-activator (NcoA1, NcoA2, and NcoA3) when RA binds to RAR. These co-activators may acetylate lysine residues in histone H3 and H4 or act as a platform to let other proteins or complexes on DNA change dynamically and rebuild nucleosomes (50). Finally, RA triggered modification of the chromatin, activation of the transcription machinery, and transcription of the target gene ( Figure 2 ). Furthermore, it has been reported that after being transported to the nucleus through FABP5, RA can also bind to the peroxisome proliferator activating receptor (PPARβ/δ) to regulates the expression of genes that control cell proliferation, metabolism, and other vital functions (51, 52). But this conclusion is still controversial.
Figure 2.
Retinoic acid metabolism. Retinol, β-carotene and chylomicron enter target cells through specific receptors and are transformed into the most active RA after a multi-step enzymatic reaction. RA binds different protein transporters and then interacts with different receptors. It also binds to CRABP and is transported to the RAR/RXR dimer to activate transcription, which can regulate the expression of genes such as cell proliferation and metabolism. In addition, RAs that are not transported to the nucleus are eventually degraded by CYP26 and lose functional activity. RBP4, retinol-binding protein 4; STRA6, stimulated by retinoic acid gene 6 protein; LRAT, lecithin retinol acyltransferase; RDHs, retinol dehydrogenases; ALDHs, aldehyde dehydrogenases; RA, retinoic acid; FAB5, fatty acid binding protein; CRABP, cellular retinol-binding proteins; PPAR, peroxisome proliferator activating receptor; RAR, retinoic acid receptor; CYP26, cytochrome P450 family 26.
2.2. The effect of RA signaling pathway in bladder cancer
It has been demonstrated that RA induces the differentiation of mouse embryonic stem cells (ESC) into urinary tract epithelial cells in a vitro environment (53). Gandhi et al. (54) emphasized the crucial role of RA in maintaining adult urothelial homeostasis and confirmed the involvement of the RA signaling pathway in urothelial specification, homeostasis, and regeneration. Their study suggested that RA synthesized in stromal compartments acts as a critical regulator of urothelial maintenance through Wnt, Bmp, and Shh signaling. Moreover, RA also plays a significant role in tumor invasion and migration. A study (55) revealed that RA effectively inhibits the expression of matrix metalloproteinase-13 (MMP-13) mRNA, which is known to promote tumor invasion and metastasis by enhancing extracellular matrix degradation during tumor growth. Based on Wang et al. (56) experiments, he demonstrated that synthetic RA 4-HPR increases E-cadherin expression, as well as increased cell adhesion, promoting its translocation to the nucleus and inducing epithelioid cell transformation while reducing cell invasion capability. Additionally, RA can reverse epithelial-mesenchymal transition (EMT) and inhibit the invasion and migration of bladder cancer cells.
Table 3 summarizes the in vitro study results of retinoids in bladder cancer cell lines. The RA plays a significant role in the proliferation, differentiation, migration, and invasion of bladder cancer cells, which makes it a key player in the disease’s development and progression. As a result, low serum levels of carotene and retinol are often seen among bladder cancer patients (9, 10, 12). In light of the important role of the retinoid signaling pathway in bladder cancer, restoring retinoid function could be a potential therapeutic option to prevent and treat bladder cancer. The different signal transduction pathways in retinoids have been shown to interfere with cell cycle progression in a variety of human cancer cells, particularly by regulating cyclins, CDKs, and cell cycle inhibitors (63, 64). Wang et al. (65) co-cultured RA with bladder cancer cells EJ and found that RA could significantly inhibit the growth of bladder cancer cells and reduce the expression of mutant P53 in cells. Zou et al. (62) evaluated the effects of three types of retinoids, namely all-trans-retinoic acid (ATRA), N-4-hydroxyphenyl-retinamide (4-HPR), and 6-[3-(1-adamantyl)-4 hydroxyphenyl]-2-naphthalene carboxylic acid (CD437), on the growth, apoptosis, cell cycle, and receptor expression of bladder cancer cells. They found that cells exposed to all three retinoids exhibited varying levels of apoptosis, G1 cell cycle arrest, and growth inhibition. Numerous animal experiments have also evaluated the chemoprophylaxis and treatment effects of RA on animal models of bladder cancer. Among the carcinogens, N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) is commonly used, as it is closely related to certain carcinogens found in tobacco smoke and exhibits remarkable bladder specificity (66, 67). Several studies (26, 68) have demonstrated that RA can significantly reduce urothelial atypia and apoptosis, decrease the incidence of urothelial carcinoma, and effectively inhibit BBN-induced urothelial carcinoma.
Table 3.
The effects of retinoids in bladder cancer cell lines.
| Retinoids | Application | Molecular and phenotypic effects | Reference |
|---|---|---|---|
| ATRA | RT112 | Inhibition of epidermal growth factor-induced cell proliferation. | (57) |
| HT-1376 | Inhibits cell proliferation by inhibiting the activity of related transcription factors | (58) | |
| T24 | Inhibition of cellular retinol-binding protein-II expression Direct inhibition peroxisome proliferator-activated receptor PPARβ/δ |
(59) | |
| 4-HPR | T24 | Promote the expression of E-cadherin and promote the transfer of β-catenin from the nucleus to the cytoplasm | (56) |
| RT4 UM-UC-9/10/14 |
Inhibition of cell growth and the induction of apoptosis | (60) | |
| 13-cis-RA | NHU | inhibition of squamous metaplasia and reverting to basal phenotype | (61) |
| ATRA CD437 4-HPR |
RT4 T24 UM-UC-2/3/6/10/13/14 |
Induction of apoptosis and G1 cell cycle arrest, and the inhibition of cell growth | (62) |
| ATRA 9-cis-RA 13-cis-RA |
RT4 T24 |
Inhibit the expression of matrix metalloproteinases | (55) |
ATRA, all-trans retinoic acid; 4-HPR, N-(4-Hydroxyphenyl)-retinamide or fenretinide; 9-cis-RA, 9-cis-retinoic acid; 13-cis-RA, 13-cis-retinoic acid.
Taken together, the RA signaling pathway is implicated in developing and progressing bladder cancer, and the use of exogenous RA supplementation may prove to be an effective method of preventing bladder cancer occurrence as well as postoperative recurrence. However, the current clinical use of RA supplementation is limited due to variations in the expression and distribution of PPAR and RXR subtypes in the human urothelium and the potential toxic effects of vitamin A (69, 70). In addition, pharmacological applications of RA also have limitations, such as short half-life, poor water solubility, sensitivity to light, heat and oxidants, and rapid degradation during digestion, leading to low bioavailability and bioaccessibility (71, 72). Therefore, addressing these challenges is crucial for improving the effectiveness of RA supplementation.
3. Promoting effect of gut microbiota on RA pathway
Therefore, it is evident that vitamin A undergoes a complex series of pathways encompassing absorption, metabolism, RA production, and subsequent activation of the RA signaling pathway. Any disruption in these processes can potentially impact the efficacy of vitamin A. Consequently, this review provides a comprehensive overview of the influence exerted by intestinal flora on these intricate pathways.
3.1. Promotes RA absorption through bile acids
As mentioned above, various forms of vitamin A precursors are present in different foods, and in the small intestine, they are absorbed mostly at the proximal part. β-carotene from plant-based foods is absorbed by small intestinal epithelial cells through passive diffusion after forming micelles with bile acids and dietary fats (73). However, animal-derived retinyl esters must be converted to retinol by retinyl ester hydrolases (REHs) before being absorbed by intestinal cells, and they are not directly absorbed by the intestine as retinol esters in the intestinal lumen (74). The primary enzymes involved in retinol ester hydrolysis in the intestinal lumen include pancreatic triglyceride lipase (PTL), carboxyl ester lipase (CEL) and the intestinal brush border membrane enzyme phospholipase B (PLB) (75), among which PTL is the most important REH in the intestinal cavity (76). It has been shown that bile acid sequestrants can lower serum levels of total carotenoids in humans, and bile acids can enhance the activities of PTL and CEL enzymes, promoting the absorption of retinol and its derivatives from animal-derived foods. In addition to absorption by intestinal epithelial cells, retinoids and β-carotenes can also be incorporated into chylomicrons along with triglycerides, cholesterol esters, phospholipids, cholesterol, and proteins in the Golgi apparatus of intestinal epithelial cells. These chylomicrons are then transported into the lymphatic circulation and subsequently re-enter the bloodstream through the lymph system. This process is also influenced by bile acids, as impaired chylomicron excretion has been observed in the absence of bile acids (59). In a mouse model of chylomicron retention disease, severely impaired fats and vitamins A and E absorption were observed, along with significantly reduced growth rates (77). Therefore, it is essential for the digestion, absorption, and dissolution of fat-soluble vitamin A from food that the concentration of bile acids in the small intestine is high.
Cholesterol is the raw material used to synthesize bile acids, which are a class of cholenoic acids. By passing through the tubule membrane of the gallbladder, they are synthesized in the liver and secreted into bile. The duodenum releases cholecystokinin after eating, stimulating the contraction of the gallbladder, which releases bile acids into the intestinal cavity for digestion (78). Approximately 95% of bile acids are then reabsorbed into the ileum and return to the liver through the portal vein, where they are once again secreted into bile (79). However, a portion of bile acids (approximately 200 to 800 mg per day in humans) escapes reabsorption in the gut and reaches the colon, where they are further metabolized by the gut flora, resulting in the production of secondary bile acids with increased hydrophilicity (80, 81).
Bile acids are metabolized by the gut microbiota, leading to secondary bile acids after three modifications are completed: uncoupling, 7α-dehydroxylation, and differential isomerism. An enzyme known as bile salt hydrolase (BSH) works on bile acids conjugated with glycine or taurine, which is required to perform 7α-dehydroxylation. Song et al. (82) investigated individuals from 11 groups across six continents and reported that the classification and identification of intestinal bacteria BSHs differed in taxonomy and abundance of BSHs in the human intestinal microbiome. The decoupled free bile acids were further transformed into secondary bile acids by 7α-dehydroxylation by microorganisms. Michael et al. (83) analyzed the signaling pathway of cholic acid dehydroxylation and found that certain strains, such as Bacteroides, Clostridium, Escherichia, Eubacterium and Lactobacillus, with the core bai gene cluster could induce dehydroxylation. In addition, the molecular modification of bile acids by intestinal bacteria also includes differential isomerization, which is the main process to enrich the diversity of intestinal bile acids.
It can thus be concluded that gut flora can influence bile acid pool size and bile acid composition in secondary forms, as confirmed in a study by Swann et al. (84), who observed that bile acid diversity was significantly reduced in sterile or antibiotic-treated rats while taurine-binding bile acid abundance was significantly increased. Further comprehensive studies (85) on gut microbiota, bile acid and vitamin A metabolism revealed that remodeling or alteration of gut microbiota resulted in lower bile acid levels, consequently reducing the absorption of vitamin A. Moreover, these studies support the hypothesis that the entire gut flora has a role in vitamin A metabolism.
3.2. Gut microbiota affects RA content by influencing the content of Ra-related enzymes
The content of RA is not only influenced by intestinal absorption but is also closely related to the levels and activities of RA synthesizing and degrading enzymes, such as ALDHs and CYP26. ALDHs are primarily found in the liver and intestines and comprise three main subtypes, namely ALDH1A1, ALDH1A2 and ALDH1A3, among which ALDH1A1 is the most abundant (37). The main function of ALDH1A1 is to participate in the second step of retinol oxidation, which oxidizes the retinol transported into cells into RA. Comparatively, CYP26 also comprises three subtypes, namely CYP26A1, CYP26B1 and CYP26C1, among which CYP26A1 has the strongest catalytic activity and can degrade RA into inactive hydroxylated and oxidized derivatives (86). An in vivo study (42) on trans-retinoic acid and colon cancer found decreased ALDH1A1 and ALDH1A3 protein expression, while ALDH1A2 protein expression remained unchanged in colon cancer progression with alterations in the gut microbiome. In addition, CYP26A1 colon transcription levels increase 3-8 times during the progression of the disease. In addition, the decrease of ALDH1A1 and increase of CYP26A1 were also corrected to a certain extent after the recovery of gut microbiota with antibiotics. Another study (87) found that feeding mice with Bifidobacterium infantis 35624 increased ALDH content in dendritic cells of the intestinal tract, resulting in a further rise in RA content.
The above studies found that gut microbiota was strongly correlated with retinoic acid (RA) metabolic enzymes. Further investigations have shown that this effect is mediated through lipopolysaccharides (LPS), a microbial product and Gram-negative bacteria’s outer membrane component (88). LPS interacts with toll-like receptor 4 (TLR4), its natural immune receptor, leading to the activation of signaling pathways. TLR4 signaling cascades activate the PI3K/Akt and NF-κB signaling pathways, resulting in subsequent biological effects (89). In an in vivo study, CYP26A1 and CYP26B1 mRNA expression was significantly suppressed in the liver of rats treated with LPS of P. aeruginosa in the presence of RA. Furthermore, Song et al. demonstrated the induction of dysbiosis in a mouse intestinal model through LPS injection, followed by subsequent administration of LPS into chicken embryos which resulted in an upregulation of retinal dehydrogenase 2 (RALDH2) mRNA expression (90). Additionally, quantitative PCR analysis revealed decreased expression levels of cytochrome P450 enzymes, namely Cyp26a1 and Cyp26c1, which are involved in RA metabolism. Furthermore, the study examined antioxidant enzymes and found that LPS treatment up-regulated mRNA expression of antioxidant enzymes such as glutathione peroxidase (GPX1), catalase (CAT) and NAD(P)H quinone dehydrogenase 1 (NQO1). Based on these, the researchers proposed that LPS induces oxidative stress by activating TLR, thereby influencing the levels of RA metabolic enzymes. Another in vitro experiment (91) showed similar results and attributed the results to LPS activation of the NF-κB pathway.
In summary, the gut microbiota can increase RA content by promoting RA synthesis enzymes and inhibiting RA degrading enzymes. This action is likely the result of the interaction between bacterial LPS and TLR signaling. It is worth noting that LPS can be further converted into fat micelles in the gastrointestinal tract, promoting the absorption of β-carotene and retinol.
3.3. Gut microbiota promotes the conversion of β-carotene to retinol in the intestine
After consuming fruits or vegetables rich in β-carotene, the compounds undergo various physical and chemical metabolic processes in the digestive tract, such as chewing and fermentation. Some of them can be processed into chylomicrons, which enter the bloodstream and are eventually transported to target cells. Within the target cells, β-carotene is enzymatically broken down into retinol. Other β-carotenes must be converted into retinol within the intestine before being combined with RBP4 for absorption. In short, plant-derived β-carotene needs to be converted into retinol to exert its biological functions, and this conversion process is primarily mediated by BCO, which is a highly potent enzyme found in various tissues of mammals, including jejunal epithelial cells, intestinal mucosa, liver, kidney, lung and brain. There are three paralogs of BCO: 15,15’-β-carotene oxygenase (BCO1), 9’,10’-β-carotene oxygenase (BCO2), and RPE65 (92). In the context of liver and intestinal tissues, BCO catalyzes the cleavage of β-carotene, splitting it in the middle to produce two retinal molecules. These retinal molecules are further oxidized to form RA (93).
As early as 1998, Grolier et al. (94) studied the biotransformation of carotenoids into retinoids in rat intestines and investigated the relationship between their bioavailability and the abundance of intestinal flora. Their results suggested that gut microbiota might influence absorption of carotenoids and retinoids, as well as their bioactivities. However, there have been no studies confirming the direct regulation of BCO by the gut microbiome. So far, only a metagenomics study (95) identified a gene in the human gut genomic library that shares homology with BCO. In a subsequent metagenomic study (96) of the human gut, certain Gram-positive and Gram-negative bacteria were found to possess brp/blh genes encoding bacteriorhodopsin-related protein-like homolog protein (Blh) and bacterioopsin-related protein (Brp), which is not homologous to BCO but has similar activity to BCO. Further experiments (97) were conducted to construct strains with brp/blh gene deletions, and the retinoid levels in the medium were measured. The results demonstrated that β-carotene levels were 3.8 times higher and retinol levels were 3.7 times lower than the wild type, thus validating the genomic prediction results. In recent years, brp/blh genes have been reported in proteobacteria, including Sphingopyxis alaskensis, Novosphingobium aromaticivorans and mycobacteria such as Mycobacterium tuberculosis (98). These genes encode enzymes that convert β-carotene into retinal, which could explain the role of the gut microbiota in facilitating vitamin A metabolism. Therefore, the gut microbiota may enhance the efficiency of vitamin A absorption from food by converting β-carotene to retinal in the gut or liver and encoding enzymes that exhibit similar effects to BCO, which may thus enhance the effect of RA downstream.
3.4. Regulating the expression and activity of RAR/RXR
Once transported into the nucleus by CRABP, RA exerts its signaling function by binding to various nuclear receptors and regulating downstream gene transcription. These nuclear receptors include RARs α, β, and γ, RXRs α, β and γ, and PPAR β/δ. In an investigational study (99), the mRNA expression of RAR was compared between normal and malignant bladder tissue specimens from human patients. The findings revealed a significant reduction in the level of RAR mRNA, particularly RARβ2 mRNA, among individuals with bladder cancer. RARs serve as substrates for various serine/threonine kinases, including PKA, PKC, and CDK7, which can phosphorylate them. The level of phosphorylation significantly impacts the activity of RARs. A study (100) revealed that Akt interacts with RARα and phosphorylates its DNA binding domain at Ser (90) residue, leading to a significant inhibition of its activity. Thus, the number and activity of RA receptors are also crucial for proper RA signaling.
Notably, the gut microbiota has been found to be closely associated with the content and activity of these nuclear receptors. Yuan et al. established an animal model of gut microbiota dysbiosis through administration of antibiotic mixtures to mice and observed a significant increase in serum IGF-1 levels due to dysbiosis-induced elevation of SCFAs. Consequently, this led to the activation of the IGF-1/Akt pathway and subsequent regulation of RAR phosphorylation. suggesting that SCFAs inhibit the RA response by enhancing RAR phosphorylation through the IGF-1/Akt pathway in cases of disrupted intestinal microecology (101). Moreover, additional studies (90, 91), by exposing chicken embryos to dysbacteriosis-derived LPS, revealed that intestinal microbiota can influence RA receptor activity through SCFAs and affect RA receptor expression through LPS induction. qPCR data showed that the mRNA levels of RAR (α, β, γ) and RXR (α, β, γ) in the cells were significantly changed after exposure to LPS. Another in vitro study (102) showed that the mRNA expressions of RARα and RARγ in hepatic stellate cells (HSCs) were significantly decreased following LPS treatment with an autophagy regulator. The expression of RARα and RARγ was restored after pretreatment with autophagy inhibitors, confirming that LPS may reduce RA receptor levels by activating autophagy.
4. Potential effect of gut microbiota on bladder cancer, also related to vitamin A
In summary, the intestinal microbiota plays a significant role in the absorption, synthesis, degradation, and regulation of retinoic acid (RA) and its receptors, highlighting the critical role for gut microbiota in RA signaling. However, the impact of the gut microbiota extends beyond RA metabolism. Emerging research has revealed that the gut microbiota can influence bladder cancer through various mechanisms. It is important to note that retinoic acid can also intervene in the anti-tumor effects mediated by the intestinal microbiota. This further reveals the close relationship among gut microbiota, retinoic acid, and bladder cancer, highlighting the critical role of the gut microbiota in this context.
4.1. Effects of gut microbiota on tumor
Intestinal microbiota refers to the trillions of microorganisms, including phages, viruses, bacteria, protists, worms and fungi, that colonize the intestinal tract. According to statistics, about 3.8 × 1013 bacteria colonize the intestinal tract, mainly comprising bacteroidetes and actinomyces (103, 104). Advances in 16SRNA gene sequencing and bioinformatics analysis have deepened our understanding of the intestinal microbiota, revealing its crucial role in human physiology and health (105). In addition to facilitating the digestive process and helping the body absorb nutrients from food (106), the gut microbiome also influences host metabolism (107), produces antibacterial substances that mediate the integrity of the intestinal barrier to protect the host from pathogens (108), and regulates host immunity to aid in the removal of harmful substances from the gut (109). In a number of studies (110, 111), it has been shown that gut microbiota diversity plays a critical role in human health. Under physiological or pathological conditions, an imbalance or disruption in the balance of the intestinal microbiome, known as gut microbiota disorder, can occur. Besides impairing the intestinal microbiome’s functions, this disorder may also contribute to cancer’s development and progression. For instance, an imbalance in the gut flora can cause inflammation that leads to colon cancer (112), and recent research on esophageal cancer similarly reported an imbalance in the flora (113).
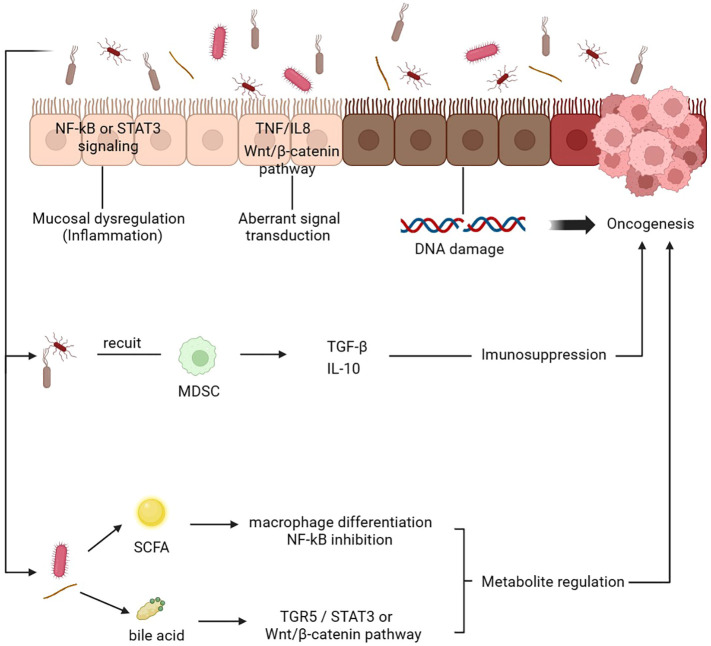
It has been shown that a dysbiotic intestinal microbiota contributes to the onset and progression of cancer in several studies. The impact of intestinal microbiota on tumors can be categorized into several aspects. Firstly, it is thought that the gut microbiome contributes to tumor progression by inducing chronic inflammation and immunological responses. Through antigen presentation and activation of pattern recognition receptors, such as toll-like receptors, NOD-like receptors, and G-protein-coupled receptors, the intestinal microbiota can activate immunoinflammatory signaling pathways and influence inflammatory immune response (114). This imbalance of intestinal flora can regulate changes in inflammatory factors, thereby promoting tumor progression. For instance, certain intestinal bacteria may activate the NF-κB or STAT3 pathway to induce the production of cytokines such as IL-10 and IL-17, which are believed to promote tumor cell proliferation and metastasis (115). Secondly, gut microbiota produces specific metabolites, including short-chain fatty acids, tryptophan metabolites and secondary bile acids, which can either promote or inhibit tumor occurrence and development (116, 117). In colorectal cancer, intestinal secondary bile acids have been found to activate carcinogenic pathways such as TGR5/STAT3, WNT/beta-catenin, and NF-kB signaling, thus promoting tumorigenesis (116, 118, 119). Conversely, some short-chain fatty acids, particularly acetate, propionate, and butyrate, have been shown to inhibit the development of colorectal cancer. A recent meta-genomic and metabonomic analysis revealed decreased levels of butyrate-producing bacteria in colorectal cancer patients, suggesting the potential role of butyrate levels in colorectal cancer development (120). Furthermore, the gut microbiome can promote tumor development by causing DNA damage, promoting cell growth and apoptosis, and modulating the immune response. Notably, E. coli is a prominent example, as it can directly cause genomic instability and DNA damage (121) ( Figure 3 ).
Figure 3.
Mechanism of gut microbiota mediating tumor development. 1. The gut microbiota induces chronic inflammation by activating the NF-kB or STAT3 pathways and various tumorigenic-related pathways. 2. Abnormal signaling pathways TNF/IL-8 and Wnt/β-catenin promote the metastasis and invasion of tumor cells. 3. The gut microbiota induces DNA damage and cell proliferation. 4. Gut microbiota recruit MDSCs release active mediators, thus mediating immunosuppression and promoting tumorigenesis. 5. The gut microbiota can change the content of various metabolites. For example, SCFA can induce the differentiation of macrophages and inhibit the NF-kB pathway, while bile acids can activate multiple pathways to affect the tumor microenvironment and the occurrence and development of tumors. DC, dendritic cell; SCFA, short-chain fatty acid; MDSC, myeloid-derived suppressor cells.
Furthermore, the impact of gut microbiota on tumors is also manifested in its influence on the tumor immune microenvironment. The tumor microenvironment (TME) serves as an internal milieu for the survival and proliferation of cancer cells, comprising various immune cells such as T lymphocytes, B lymphocytes, natural killer cells, and tumor-associated macrophages. The human immune system functions to conduct immune surveillance by identifying and eliminating abnormal cells; henceforth, tumors must evade or suppress this immunosurveillance to sustain their progression. TME is highly conducive to microbial invasion, colonization, and proliferation. A study (122) has demonstrated that intestinal microorganisms can migrate to the TME and induce immunosuppression. Similarly, Zhang et al. have confirmed that gut flora can prompt hepatocytes to recruit myeloid-derived suppressor cells (MDSCs) and produce tumor-promoting and anti-inflammatory chemicals such as TGF-β and IL-10, thereby establishing an immunosuppressive microenvironment that ultimately contributes to the development of cholangiocarcinoma (123). Furthermore, metabolites derived from gut microbiota also impede anti-cancer immunity. For instance, tryptophan metabolite produced by Lactobacillus, can activate aromatic hydrocarbon receptors in tumor-associated macrophages, thus inhibiting the infiltration of cytokines and immune cells in pancreatic cancer (124).
4.2. Effects of gut microbiota on bladder cancer, needs further exploration
It is worth noting that one study (125) found that immune cells, intestinal microbiota, metabolites, and cytokines can leave the intestinal tract through the blood circulation and induce corresponding pathological changes, indicating that the role of intestinal microbiota in promoting tumors may extend beyond the gastrointestinal tract to other areas, including bladder cancer. However, current studies have mainly focused on the relationship between bladder cancer and the urinary microbiome, leading to a relatively limited number of studies on the intestinal microbiome. Although it has been proposed that there might be a correlation between the intestinal microbiome and urinary microbiome, no direct comparisons have been made between the changes in the urinary and intestinal microbiomes in the same patient to confirm this conjecture (126). The most direct evidence linking the gut microbiota and bladder cancer comes from a study comparing the gut microbiota composition of bladder cancer patients with that of a normal population, which revealed alterations in the gut microbiota composition and significant differences in metabolite concentrations, such as butyric acid (29). In addition, the influence of dietary intervention on the intestinal microbial composition of mice on bladder cancer has been assessed in several in vivo experiments (28, 127, 128). The results demonstrate that normalizing the intestinal microbial composition through dietary intervention repairs the intestinal physiological barrier, reduces inflammation and immune response, inhibits bladder cancer progression, and enhances sensitivity to radiotherapy and chemotherapy. Lactic acid bacteria have been shown to be beneficial in avoiding the return of superficial bladder cancer in two clinical trials that compared their effectiveness to that of other biologics in preventing tumor recurrence following transurethral excision of bladder tumors (30, 129). These studies have offered preliminary evidence of the tight association between bladder cancer and gut microbiota, but further study is required to clarify the precise processes behind this association.
4.3. Auxiliary effects of vitamin A on tumor toxicity of gut microbiota
Interestingly, it has been observed that not only does the stable intestinal microbiota have a positive effect on the RA signaling pathway, but the level of vitamin A also has an important impact on the homeostasis of the intestinal microbiota, suggesting a mutually reinforcing positive feedback relationship. Micronutrient food sources, such as vitamins A, appear unlikely to have a significant impact on the gut microbiome, but studies have suggested that certain micronutrient signals, such as vitamin A, may first be amplified by inducing secretory mediators in intestinal epithelial cells and other stromal cells, leading to a stronger signal and influence on luminal microbes (130). This notion is supported by studies showing that vitamin A-deficient mice have impaired intestinal structural integrity and reduced Paneth cell numbers but increased secretion of goblet cells and mucins and that these secreted mucins, antimicrobial peptides and proteins have specific effects on the microbiome of these animals (131). Since mucin formation by goblet cells and low levels of antimicrobial peptides are two examples of how vitamin A deficiency affects the phenotypic and function of intestinal epithelial cells, it follows that these changes can impact the number and makeup of symbiotic bacteria in the gut. Studies have shown that vitamin A alleviates inflammation, enhances intestinal epithelial barrier function, and influences gut bacterial diversity in vivo (132, 133). Vitamin A deficiency leads to a specific reduction in the gut microbiome, ecological imbalance, impaired immune system function, and increased susceptibility to gastrointestinal infections or injuries (134). Mice treated with vitamin A or RA have shown higher gut microbiota diversity and altered bacterial composition (33, 135). Similar results have also been reported in clinical studies, which revealed significant differences in the gut microbiota composition among vitamin A intake groups (136). A study (34) on the stage-dependent effect of all-trans retinoic acid on lupus found that after two weeks of all-trans retinoic acid treatment, the abundance of intestinal lactobacillus decreased while clostridium increased, indicating that treatment with all-trans retinoic acid significantly altered the abundance of bacteria in the gut.
5. Conclusion
There is clear evidence indicating that the gut microbiota can enhance the absorption of RA by facilitating the transformation of vitamin A and influencing bile acid metabolism. It can also modulate the levels of RA by affecting key enzymes involved in RA synthesis and degradation. In addition, as summarized in the third part of the article, studies have also highlighted the potential impact of intestinal flora on bladder cancer through the production of specific metabolites or modulation of urethral microbiota, and RA has demonstrated a certain efficacy in modulating tumor-associated gut microbiota. Overall, the intestinal microbiota can contribute to the anti-tumor effects of the RA signaling pathway at multiple levels. However, direct evidence linking intestinal microbiota to enhanced inhibitory effects of RA in bladder cancer is currently lacking. Furthermore, the toxic effects of intestinal microbiota on bladder cancer have been demonstrated, and RA has been shown to play a significant role in the anti-tumor effects of the gut microbiota. The interaction between RA and the gut microbiome enhances the anti-tumor effects of each other. Consequently, any alterations in retinoic acid or the gut microbiota can disrupt this positive cycle, leading to an adverse feedback loop. Therefore, solely supplementing exogenous vitamin A may not provide optimal preventive effects for bladder cancer patients, as their gut microbiome may undergo alterations during the development and progression of the disease. Currently, synthetic retinoic acid drugs are being utilized in clinical practice to overcome the limitations of short half-life and poor patient tolerance. Encouragingly, these drugs have exhibited satisfactory therapeutic effects while maintaining good patient tolerability (137, 138). Additionally, promising feedback has been obtained from studies investigating intestinal probiotics (30–32). Although no reports exist regarding their combined application for bladder cancer treatment, it is reasonable to anticipate that further elucidation of the interplay between these three factors will pave the way for novel strategies in bladder cancer prevention and treatment.
Author contributions
BQ conceived the manuscript, and PL performed the literature search and drafted the manuscript. JZ and TC edited tables and figures. WL and QC collected the data. JRZ and LZ reviewed and polished the manuscript. All authors have approved the final version submitted and agree to its submission to this journal.
Acknowledgments
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service. All figures are created with BioRender.com.
Funding Statement
This work was supported by the Health Commission Research Project of China (No. HDSL202001057), the Jiangxi Provincial Health Commission Research Project (No. SKJP20203656), and the Jiangxi Province 2021 Postgraduate Innovation Special Fund Project (No. YC2022-S959).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Lobo N, Afferi L, Moschini M, Mostafid H, Porten S, Psutka SP, et al. Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol (2022) 5(6):628–39. doi: 10.1016/j.euo.2022.10.003 [DOI] [PubMed] [Google Scholar]
- 2. Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, et al. Epidemiology of bladder cancer: A systematic review and contemporary update of risk factors in 2018. Eur Urol (2018) 74(6):784–95. doi: 10.1016/j.eururo.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Zhang H, Guo Y, Cai H, Li X, He J, et al. A qualitative transcriptional signature for predicting recurrence risk of stage I-III bladder cancer patients after surgical resection. Front Oncol (2019) 9:629. doi: 10.3389/fonc.2019.00629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Catto JWF, Downing A, Mason S, Wright P, Absolom K, Bottomley S, et al. Quality of life after bladder cancer: A cross-sectional survey of patient-reported outcomes. Eur Urol (2021) 79(5):621–32. doi: 10.1016/j.eururo.2021.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature (1990) 347(6293):558–61. doi: 10.1038/347558a0 [DOI] [PubMed] [Google Scholar]
- 6. Cristiano MC, Cosco D, Celia C, Tudose A, Mare R, Paolino D, et al. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf B Biointerfaces (2017) 150:408–16. doi: 10.1016/j.colsurfb.2016.10.052 [DOI] [PubMed] [Google Scholar]
- 7. Kocher HM, Basu B, Froeling FEM, Sarker D, Slater S, Carlin D, et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun (2020) 11(1):4841. doi: 10.1038/s41467-020-18636-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steineck G, Hagman U, Gerhardsson M, Norell SE. Vitamin A supplements, fried foods, fat and urothelial cancer. A case-referent study in Stockholm in 1985-87. Int J Cancer (1990) 45(6):1006–11. doi: 10.1002/ijc.2910450604 [DOI] [PubMed] [Google Scholar]
- 9. El-Aaser AA, El-Merzabani MM, Abdel-Reheem KA, Hamza BM. A study on the etiological factors of bilharzial bladder cancer in Egypt: 4. beta-carotene and vitamin A level in serum. Tumori (1982) 68(1):19–22. doi: 10.1177/030089168206800104 [DOI] [PubMed] [Google Scholar]
- 10. Ozasa K, Ito Y, Suzuki K, Watanabe Y, Hayashi K, Mikami K, et al. Serum carotenoids and other antioxidative substances associated with urothelial cancer risk in a nested case-control study in Japanese men. J Urol (2005) 173(5):1502–6. doi: 10.1097/01.ju.0000154614.58321.e6 [DOI] [PubMed] [Google Scholar]
- 11. Middleton B, Byers T, Marshall J, Graham S, Dietary vitamin A. and cancer–a multisite case-control study. Nutr Cancer (1986) 8(2):107–16. doi: 10.1080/01635588609513883 [DOI] [PubMed] [Google Scholar]
- 12. Yalçin O, Karataş F, Erulaş FA, Ozdemir E. The levels of glutathione peroxidase, vitamin A, E, C and lipid peroxidation in patients with transitional cell carcinoma of the bladder. BJU Int (2004) 93(6):863–6. doi: 10.1111/j.1464-410X.2003.04729.x [DOI] [PubMed] [Google Scholar]
- 13. Wu JW, Cross AJ, Baris D, Ward MH, Karagas MR, Johnson A, et al. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br J Cancer (2012) 106(11):1891–8. doi: 10.1038/bjc.2012.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park S-Y, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, Kolonel LN. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr (2013) 143(8):1283–92. doi: 10.3945/jn.113.174920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nomura AMY, Lee J, Stemmermann GN, Franke AA. Serum vitamins and the subsequent risk of bladder cancer. J Urol (2003) 170(4 Pt 1):1146–50. doi: 10.1097/01.ju.0000086040.24795.ad [DOI] [PubMed] [Google Scholar]
- 16. Nomura AM, Stemmermann GN, Heilbrun LK, Salkeld RM, Vuilleumier JP. Serum vitamin levels and the risk of cancer of specific sites in men of Japanese ancestry in Hawaii. Cancer Res (1985) 45(5):2369–72. [PubMed] [Google Scholar]
- 17. Kellen E, Zeegers M, Buntinx F. Selenium is inversely associated with bladder cancer risk: a report from the Belgian case-control study on bladder cancer. Int J Urol (2006) 13(9):1180–4. doi: 10.1111/j.1442-2042.2006.01526.x [DOI] [PubMed] [Google Scholar]
- 18. Zeegers MP, Goldbohm RA, van den Brandt PA. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study. Br J Cancer (2001) 85(7):977–83. doi: 10.1054/bjoc.2001.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer (1992) 66(4):673–9. doi: 10.1038/bjc.1992.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holick CN, De Vivo I, Feskanich D, Giovannucci E, Stampfer M, Michaud DS. Intake of fruits and vegetables, carotenoids, folate, and vitamins A, C, E and risk of bladder cancer among women (United States). Cancer Causes Control CCC (2005) 16(10):1135–45. doi: 10.1007/s10552-005-0337-z [DOI] [PubMed] [Google Scholar]
- 21. Hotaling JM, Wright JL, Pocobelli G, Bhatti P, Porter MP, White E. Long-term use of supplemental vitamins and minerals does not reduce the risk of urothelial cell carcinoma of the bladder in the VITamins And Lifestyle study. J Urol (2011) 185(4):1210–5. doi: 10.1016/j.juro.2010.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narisawa T, Reddy BS, Wong CQ, Weisburger JH. Effect of vitamin A deficiency on rat colon carcinogenesis by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res (1976) 36(4):1379–83. [PubMed] [Google Scholar]
- 23. Cohen SM, Wittenberg JF, Bryan GT. Effect of avitaminosis A and hypervitaminosis A on urinary bladder carcinogenicity of N-(4-(5-Nitro-2-furyl)-2-thiazolyl)formamide. Cancer Res (1976) 36(7 pt 1):2334–9. [PubMed] [Google Scholar]
- 24. Miyata Y, Tsuda H, Matayoshi-Miyasato K, Fukushima S, Murasaki G, Ogiso T, et al. Effect of vitamin A acetate on urinary bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in rats. Gan (1978) 69(6):845–8. [PubMed] [Google Scholar]
- 25. Zupančič D, Korać-Prlić J, Kreft ME, Franković L, Vilović K, Jeruc J, et al. Vitamin A rich diet diminishes early urothelial carcinogenesis by altering retinoic acid signaling. Cancers (Basel) (2020) 12(7):1712. doi: 10.3390/cancers12071712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lubet RA, Clapper ML, McCormick DL, Pereira MA, Chang WCL, Steele VE, et al. Chemopreventive efficacy of Targretin in rodent models of urinary bladder, colon/intestine, head and neck and mammary cancers. Oncol Rep (2012) 27(5):1400–6. doi: 10.3892/or.2012.1673 [DOI] [PubMed] [Google Scholar]
- 27. Shim JA, Ryu JH, Jo Y, Hong C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int J Biol Sci (2023) 19(4):1178–91. doi: 10.7150/ijbs.79430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science (2020) 369(6510):1481–9. doi: 10.1126/science.abc3421 [DOI] [PubMed] [Google Scholar]
- 29. He C, Li B, Huang L, Teng C, Bao Y, Ren M, et al. Gut microbial composition changes in bladder cancer patients: A case-control study in Harbin, China. Asia Pac J Clin Nutr (2020) 29(2):395–403. doi: 10.6133/apjcn.202007_29(2).0022 [DOI] [PubMed] [Google Scholar]
- 30. Aso Y, Akazan H. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urol Int (1992) 49(3):125–9. doi: 10.6133/apjcn.202007_29(2).0022 [DOI] [PubMed] [Google Scholar]
- 31. Seow SW, Rahmat JN, Mohamed AA, Mahendran R, Lee YK, Bay BH. Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium Bovis (bacillus Calmette-Guerin). J Urol (2002) 168(5):2236–9. doi: 10.1016/S0022-5347(05)64362-5 [DOI] [PubMed] [Google Scholar]
- 32. Tomita K, Akaza H, Nomoto K, Yokokura T, Matsushima H, Homma Y, et al. [Influence of Lactobacillus casei on rat bladder carcinogenesis]. Nihon Hinyokika Gakkai Zasshi (1994) 85(4):655–63. doi: 10.5980/jpnjurol1989.85.655 [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Chen J, Wang X, Guo C, Peng X, Liu Y, et al. Novel investigations in retinoic-acid-induced cleft palate about the gut microbiome of pregnant mice. Front Cell Infect Microbiol (2022) 12:1042779. doi: 10.3389/fcimb.2022.1042779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdelhamid L, Cabana-Puig X, Swartwout B, Lee J, Li S, Sun S, et al. Retinoic acid exerts disease stage-dependent effects on pristane-induced lupus. Front Immunol (2020) 11:408. doi: 10.3389/fimmu.2020.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al Binali HA. Night blindness and ancient remedy. Heart Views (2014) 15(4):136–9. doi: 10.4103/1995-705X.151098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys (2015) 572:112–25. doi: 10.1016/j.abb.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 37. Chlapek P, Slavikova V, Mazanek P, Sterba J, Veselska R. Why differentiation therapy sometimes fails: molecular mechanisms of resistance to retinoids. Int J Mol Sci (2018) 19(1):132. doi: 10.3390/ijms19010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Henning P, Conaway HH, Lerner UH. Retinoid receptors in bone and their role in bone remodeling. Front Endocrinol (Lausanne) (2015) 6:31. doi: 10.3389/fendo.2015.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Napoli JL. Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol Ther (2017) 173:19–33. doi: 10.1016/j.pharmthera.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hurst RJ, Else KJ. Retinoic acid signalling in gastrointestinal parasite infections: lessons from mouse models. Parasite Immunol (2012) 34(7):351–9. doi: 10.1111/j.1365-3024.2012.01364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zieger E, Garbarino G, Robert NSM, Yu JK, Croce JC, Candiani S, et al. Retinoic acid signaling and neurogenic niche regulation in the developing peripheral nervous system of the cephalochordate amphioxus. Cell Mol Life Sci (2018) 75(13):2407–29. doi: 10.1007/s00018-017-2734-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhattacharya N, Yuan R, Prestwood TR, Penny HL, DiMaio MA, Reticker-Flynn NE, et al. NorMalizing microbiota-induced retinoic acid deficiency stimulates protective CD8(+) T cell-mediated immunity in colorectal cancer. Immunity (2016) 45(3):641–55. doi: 10.1016/j.immuni.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Isoherranen N, Zhong G. Biochemical and physiological importance of the CYP26 retinoic acid hydroxylases. Pharmacol Ther (2019) 204:107400. doi: 10.1016/j.pharmthera.2019.107400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghyselinck NB, Duester G. Retinoic acid signaling pathways. Development (2019) 146(13):dev167502. doi: 10.1242/dev.167502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gan W-J, Wang J-R, Zhu X-L, He X-S, Guo P-D, Zhang S, et al. RARγ-induced E-cadherin downregulation promotes hepatocellular carcinoma invasion and metastasis. J Exp Clin Cancer Res CR (2016) 35(1):164. doi: 10.1186/s13046-016-0441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gattu S, Bang Y-J, Pendse M, Dende C, Chara AL, Harris TA, et al. Epithelial retinoic acid receptor β regulates serum amyloid A expression and vitamin A-dependent intestinal immunity. Proc Natl Acad Sci United States America (2019) 116(22):10911–6. doi: 10.1073/pnas.1812069116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwartz DM, Farley TK, Richoz N, Yao C, Shih H-Y, Petermann F, et al. Retinoic acid receptor alpha represses a th9 transcriptional and epigenomic program to reduce allergic pathology. Immunity (2019) 50(1):106–20.e10. doi: 10.1016/j.immuni.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Osz J, McEwen AG, Bourguet M, Przybilla F, Peluso-Iltis C, Poussin-Courmontagne P, et al. Structural basis for DNA recognition and allosteric control of the retinoic acid receptors RAR-RXR. Nucleic Acids Res (2020) 48(17):9969–85. doi: 10.1093/nar/gkaa697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jepsen K, Hermanson O, Onami TM, Gleiberman AS, Lunyak V, McEvilly RJ, et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell (2000) 102(6):753–63. doi: 10.1016/S0092-8674(00)00064-7 [DOI] [PubMed] [Google Scholar]
- 50. Bourguet W, Germain P, Gronemeyer H. Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol Sci (2000) 21(10):381–8. doi: 10.1016/S0165-6147(00)01548-0 [DOI] [PubMed] [Google Scholar]
- 51. Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol (2009) 29(12):3286–96. doi: 10.1128/MCB.01742-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rieck M, Meissner W, Ries S, Müller-Brüsselbach S, Müller R. Ligand-mediated regulation of peroxisome proliferator-activated receptor (PPAR) beta/delta: a comparative analysis of PPAR-selective agonists and all-trans retinoic acid. Mol Pharmacol (2008) 74(5):1269–77. doi: 10.1124/mol.108.050625 [DOI] [PubMed] [Google Scholar]
- 53. Mauney JR, Ramachandran A, Yu RN, Daley GQ, Adam RM, Estrada CR. All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PloS One (2010) 5(7):e11513. doi: 10.1371/journal.pone.0011513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell (2013) 26(5):469–82. doi: 10.1016/j.devcel.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boström PJ, Ravanti L, Reunanen N, Aaltonen V, Söderström KO, Kähäri VM, et al. Expression of collagenase-3 (matrix metalloproteinase-13) in transitional-cell carcinoma of the urinary bladder. Int J Cancer (2000) 88(3):417–23. doi: [DOI] [PubMed] [Google Scholar]
- 56. Wang E, Li J, Yang G, Zhong S, Liu T. Impact of 4HPR on the expression of E-Cad in human bladder transitional epithelial cancer cells T24. J Huazhong Univ Sci Technol Med Sci (2012) 32(2):237–41. doi: 10.1007/s11596-012-0042-6 [DOI] [PubMed] [Google Scholar]
- 57. Nutting C, Chowaniec J. Evaluation of the actions and interactions of retinoic acid and epidermal growth factor on transformed urothelial cells in culture: implications for the use of retinoid therapy in the treatment of bladder cancer patients. Clin Oncol (R Coll Radiol) (1992) 4(1):51–5. doi: 10.1016/S0936-6555(05)80778-2 [DOI] [PubMed] [Google Scholar]
- 58. Lin F, Kolluri SK. Chen, G.-q.; Zhang, X.-k., Regulation of retinoic acid-induced inhibition of AP-1 activity by orphan receptor chicken ovalbumin upstream promoter-transcription factor. J Biol Chem (2002) 277(24):21414–22. doi: 10.1074/jbc.M201885200 [DOI] [PubMed] [Google Scholar]
- 59. Costantini L, Molinari R, Farinon B, Lelli V, Timperio AM, Merendino N. Docosahexaenoic acid reverted the all-trans retinoic acid-induced cellular proliferation of T24 bladder cancer cell line. J Clin Med (2020) 9(8):2494. doi: 10.3390/jcm9082494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clifford JL, Sabichi AL, Zou C, Yang X, Steele VE, Kelloff GJ, et al. Effects of novel phenylretinamides on cell growth and apoptosis in bladder cancer. Cancer Epidemiol Biomarkers Prev (2001) 10(4):391–5. [PubMed] [Google Scholar]
- 61. Southgate J, Hutton KA, Thomas DF, Trejdosiewicz LK. Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab Invest (1994) 71(4):583–94. [PubMed] [Google Scholar]
- 62. Zou C, Zhou J, Qian L, Feugang JM, Liu J, Wang X, et al. Comparing the effect of ATRA, 4-HPR, and CD437 in bladder cancer cells. Front Biosci J Virtual Library (2006) 11:2007–16. doi: 10.2741/1942 [DOI] [PubMed] [Google Scholar]
- 63. Nasr RR, Hmadi RA, El-Eit RM, Iskandarani AN, Jabbour MN, Zaatari GS, et al. ST1926, an orally active synthetic retinoid, induces apoptosis in chronic myeloid leukemia cells and prolongs survival in a murine model. Int J Cancer (2015) 137(3):698–709. doi: 10.1002/ijc.29407 [DOI] [PubMed] [Google Scholar]
- 64. Lou S, Gao H, Hong H, Zhu Z, Zhao H. Inhibition of retinoic acid receptor α phosphorylation represses the progression of triple-negative breast cancer via transactivating miR-3074-5p to target DHRS3. J Exp Clin Cancer Res (2021) 40(1):141. doi: 10.1186/s13046-021-01941-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Z, Zhang Z, Liu Y, Chen Y, Li Q, Duanguolan, et al. Effect of retinoic acid and its complexes with transition metals on human bladder cancer cell line EJ in vitro . Urol Res (2000) 28(3):191–5. doi: 10.1007/s002409900090 [DOI] [PubMed] [Google Scholar]
- 66. Oliveira PA, Vasconcelos-Nóbrega C, Gil da Costa RM, Arantes-Rodrigues R. The N-butyl-N-4-hydroxybutyl nitrosamine mouse urinary bladder cancer model. Methods Mol Biol (2018) 1655:155–67. doi: 10.1007/978-1-4939-7234-0_13 [DOI] [PubMed] [Google Scholar]
- 67. He Z, Kosinska W, Zhao ZL, Wu XR, Guttenplan JB. Tissue-specific mutagenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine as the basis for urothelial carcinogenesis. Mutat Res (2012) 742(1-2):92–5. doi: 10.1016/j.mrgentox.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 68. Qian L, Ding H, Zhou J, Wang X, Shao P, Wu S, et al. Intravesical N-(4-hydroxyphenyl) retinamide and adriamycin induces apoptosis in bladder cancer. Front Biosci (2006) 11:2045–51. doi: 10.2741/1946 [DOI] [PubMed] [Google Scholar]
- 69. Chopra B, Hinley J, Oleksiewicz MB, Southgate J. Trans-species comparison of PPAR and RXR expression by rat and human urothelial tissues. Toxicol Pathol (2008) 36(3):485–95. doi: 10.1177/0192623308315672 [DOI] [PubMed] [Google Scholar]
- 70. Ertugrul S, Yucel C, Sertoglu E, Ozkan Y, Ozgurtas T. Development and optimization of simultaneous determination of fat soluble vitamins by liquid chromatography tandem mass spectrometry. Chem Phys Lipids (2020) 230:104932. doi: 10.1016/j.chemphyslip.2020.104932 [DOI] [PubMed] [Google Scholar]
- 71. Assi S, Hajj HE, Hayar B, Pisano C, Saad W, Darwiche N. Development and challenges of synthetic retinoid formulations in cancer. Curr Drug Delivery (2023) 20(9):1314–26. doi: 10.2174/1567201819666220810094708 [DOI] [PubMed] [Google Scholar]
- 72. Ferreira R, Napoli J, Enver T, Bernardino L, Ferreira L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat Commun (2020) 11(1):4265. doi: 10.1038/s41467-020-18042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shilpa S, Shwetha HJ, Perumal MK, Ambedkar R, Hanumanthappa M, Baskaran V, et al. Turmeric, red pepper, and black pepper affect carotenoids solubilized micelles properties and bioaccessibility: Capsaicin/piperine improves and curcumin inhibits carotenoids uptake and transport in Caco-2 cells. J Food Sci (2021) 86(11):4877–91. doi: 10.1111/1750-3841.15926 [DOI] [PubMed] [Google Scholar]
- 74. Grumet L, Taschler U, Lass A. Hepatic retinyl ester hydrolases and the mobilization of retinyl ester stores. Nutrients (2016) 9(1):13. doi: 10.3390/nu9010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schreiber R, Taschler U, Preiss-Landl K, Wongsiriroj N, Zimmermann R, Lass A. Retinyl ester hydrolases and their roles in vitamin A homeostasis. Biochim Biophys Acta (2012) 1821(1):113–23. doi: 10.1016/j.bbalip.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van Bennekum AM, Fisher EA, Blaner WS, Harrison EH. Hydrolysis of retinyl esters by pancreatic triglyceride lipase. Biochemistry (2000) 39(16):4900–6. doi: 10.1021/bi9927235 [DOI] [PubMed] [Google Scholar]
- 77. Davidson NO, Kollmer ME, Glickman RM. Apolipoprotein B synthesis in rat small intestine: regulation by dietary triglyceride and biliary lipid. J Lipid Res (1986) 27(1):30–9. doi: 10.1016/S0022-2275(20)38864-7 [DOI] [PubMed] [Google Scholar]
- 78. Fuchs CD, Trauner M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol (2022) 19(7):432–50. doi: 10.1038/s41575-021-00566-7 [DOI] [PubMed] [Google Scholar]
- 79. Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol (2023) 21(4):236–47. doi: 10.1038/s41579-022-00805-x [DOI] [PubMed] [Google Scholar]
- 80. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab (2016) 24(1):41–50. doi: 10.1016/j.cmet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 81. Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens (2013) 3(1):14–24. doi: 10.3390/pathogens3010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y, et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome (2019) 7(1):9. doi: 10.1186/s40168-019-0628-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Funabashi M, Grove TL, Wang M, Varma Y, McFadden ME, Brown LC, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature (2020) 582(7813):566–70. doi: 10.1038/s41586-020-2396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U.S.A. (2011) 108 Suppl 1(Suppl 1):4523–30. doi: 10.1073/pnas.1006734107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang T, Sun P, Geng Q, Fan H, Gong Y, Hu Y, et al. Disrupted spermatogenesis in a metabolic syndrome model: the role of vitamin A metabolism in the gut-testis axis. Gut (2022) 71(1):78–87. doi: 10.1136/gutjnl-2020-323347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ono K, Keller J, López Ramírez O, González Garrido A, Zobeiri OA, Chang HHV, et al. Retinoic acid degradation shapes zonal development of vestibular organs and sensitivity to transient linear accelerations. Nat Commun (2020) 11(1):63. doi: 10.1038/s41467-019-13710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Konieczna P, Ferstl R, Ziegler M, Frei R, Nehrbass D, Lauener RP, et al. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PloS One (2013) 8(5):e62617. doi: 10.1371/journal.pone.0062617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem (2002) 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao K, Song X, Huang Y, Yao J, Zhou M, Li Z, et al. Wogonin inhibits LPS-induced tumor angiogenesis via suppressing PI3K/Akt/NF-κB signaling. Eur J Pharmacol (2014) 737:57–69. doi: 10.1016/j.ejphar.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 90. Song J, Wang C, Long D, Li Z, You L, Brand-Saberi B, et al. Dysbacteriosis-induced LPS elevation disturbs the development of muscle progenitor cells by interfering with retinoic acid signaling. FASEB J (2020) 34(5):6837–53. doi: 10.1096/fj.201902965R [DOI] [PubMed] [Google Scholar]
- 91. You L, Zhu L, Li PZ, Wang G, Cai H, Song J, et al. Dysbacteriosis-derived lipopolysaccharide causes embryonic osteopenia through retinoic-acid-regulated DLX5 expression. Int J Mol Sci (2020) 21(7):2518. doi: 10.3390/ijms21072518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Srinivasan K, Buys EM. Insights into the role of bacteria in vitamin A biosynthesis: Future research opportunities. Crit Rev Food Sci Nutr (2019) 59(19):3211–26. doi: 10.1080/10408398.2018.1546670 [DOI] [PubMed] [Google Scholar]
- 93. Poliakov E, Uppal S, Rogozin IB, Gentleman S, Redmond TM. Evolutionary aspects and enzymology of metazoan carotenoid cleavage oxygenases. Biochim Biophys Acta Mol Cell Biol Lipids (2020) 1865(11):158665. doi: 10.1016/j.bbalip.2020.158665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Grolier P, Borel P, Duszka C, Lory S, Alexandre-Gouabau MC, Azais-Braesco V, et al. The bioavailability of alpha- and beta-carotene is affected by gut microflora in the rat. Br J Nutr (1998) 80(2):199–204. doi: 10.1017/S0007114598001111 [DOI] [PubMed] [Google Scholar]
- 95. Culligan EP, Sleator RD, Marchesi JR, Hill C. Functional metagenomics reveals novel salt tolerance loci from the human gut microbiome. Isme J (2012) 6(10):1916–25. doi: 10.1038/ismej.2012.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature (2010) 464(7285):59–65. doi: 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Peck RF, Echavarri-Erasun C, Johnson EA, Ng WV, Kennedy SP, Hood L, et al. brp and blh are required for synthesis of the retinal cofactor of bacteriorhodopsin in Halobacterium salinarum. J Biol Chem (2001) 276(8):5739–44. doi: 10.1074/jbc.M009492200 [DOI] [PubMed] [Google Scholar]
- 98. Liang MH, Zhu J, Jiang JG. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit Rev Food Sci Nutr (2018) 58(14):2314–33. doi: 10.1080/10408398.2017.1322552 [DOI] [PubMed] [Google Scholar]
- 99. Boorjian S, Scherr DS, Mongan NP, Zhuang Y, Nanus DM, Gudas LJ. Retinoid receptor mRNA expression profiles in human bladder cancer specimens. Int J Oncol (2005) 26(4):1041–8. doi: 10.3892/ijo.26.4.1041 [DOI] [PubMed] [Google Scholar]
- 100. Srinivas H, Xia D, Moore NL, Uray IP, Kim H, Ma L, et al. Akt phosphorylates and suppresses the transactivation of retinoic acid receptor alpha. Biochem J (2006) 395(3):653–62. doi: 10.1042/BJ20051794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yuan X, Tang H, Wu R, Li X, Jiang H, Liu Z, et al. Short-chain fatty acids calibrate RARα Activity regulating food sensitization. Front Immunol (2021) 12:737658. doi: 10.3389/fimmu.2021.737658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen M, Liu J, Yang W, Ling W. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy (2017) 13(11):1813–27. doi: 10.1080/15548627.2017.1356550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ji BW, Sheth RU, Dixit PD, Tchourine K, Vitkup D. Macroecological dynamics of gut microbiota. Nat Microbiol (2020) 5(5):768–75. doi: 10.1038/s41564-020-0685-1 [DOI] [PubMed] [Google Scholar]
- 104. Llinás-Caballero K, Caraballo L. Helminths and bacterial microbiota: the interactions of two of humans' "Old friends". Int J Mol Sci (2022) 23(21):13358. doi: 10.3390/ijms232113358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lagier JC, Dubourg G, Million M, Cadoret F, Bilen M, Fenollar F, et al. Culturing the human microbiota and culturomics. Nat Rev Microbiol (2018) 16:540–50. doi: 10.1038/s41579-018-0041-0 [DOI] [PubMed] [Google Scholar]
- 106. Gabanyi I, Lepousez G, Wheeler R, Vieites-Prado A, Nissant A, Chevalier G, et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science (2022) 376(6590):eabj3986. doi: 10.1126/science.abj3986 [DOI] [PubMed] [Google Scholar]
- 107. Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med (2021) 27(2):321–32. doi: 10.1038/s41591-020-01183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cai J, Zhou L, Song X, Yin M, Liang G, Xu H, et al. Alteration of intestinal microbiota in 3-deoxyglucosone-induced prediabetic rats. BioMed Res Int (2020) 2020:8406846. doi: 10.1155/2020/8406846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell (2019) 178(4):795–806.e12. doi: 10.1016/j.cell.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Galley JD, Parry NM, Ahmer BMM, Fox JG, Bailey MT. The commensal microbiota exacerbate infectious colitis in stressor-exposed mice. Brain Behav Immun (2017) 60:44–50. doi: 10.1016/j.bbi.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun (2020) 11(1):5206. doi: 10.1038/s41467-020-18871-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Saus E, Iraola-Guzmán S, Willis JR, Brunet-Vega A, Gabaldón T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol Aspects Med (2019) 69:93–106. doi: 10.1016/j.mam.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ishaq HM, Mohammad IS, Sher Muhammad K, Li H, Abbas RZ, Din Sindhu ZU, et al. Gut microbial dysbiosis and its association with esophageal cancer. J Appl BioMed (2021) 19(1):1–13. doi: 10.32725/jab.2021.005 [DOI] [PubMed] [Google Scholar]
- 114. Brown EM, Kenny DJ, Xavier RJ. Gut microbiota regulation of T cells during inflammation and autoimmunity. Annu Rev Immunol (2019) 37:599–624. doi: 10.1146/annurev-immunol-042718-041841 [DOI] [PubMed] [Google Scholar]
- 115. Zhao S, Wu D, Wu P, Wang Z, Huang J. Serum IL-10 predicts worse outcome in cancer patients: A meta-analysis. PloS One (2015) 10(10):e0139598. doi: 10.1371/journal.pone.0139598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mao J, Chen X, Wang C, Li W, Li J. Effects and mechanism of the bile acid (farnesoid X) receptor on the Wnt/β-catenin signaling pathway in colon cancer. Oncol Lett (2020) 20(1):337–45. doi: 10.3892/ol.2020.11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hanus M, Parada-Venegas D, Landskron G, Wielandt AM, Hurtado C, Alvarez K, et al. Immune system, microbiota, and microbial metabolites: the unresolved triad in colorectal cancer microenvironment. Front Immunol (2021) 12:612826. doi: 10.3389/fimmu.2021.612826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jin D, Huang K, Xu M, Hua H, Ye F, Yan J, et al. Deoxycholic acid induces gastric intestinal metaplasia by activating STAT3 signaling and disturbing gastric bile acids metabolism and microbiota. Gut Microbes (2022) 14(1):2120744. doi: 10.1080/19490976.2022.2120744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yao Y, Li X, Xu B, Luo L, Guo Q, Wang X, et al. Cholecystectomy promotes colon carcinogenesis by activating the Wnt signaling pathway by increasing the deoxycholic acid level. Cell Commun Signal (2022) 20(1):71. doi: 10.1186/s12964-022-00890-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kong C, Liang L, Liu G, Du L, Yang Y, Liu J, et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut (2023) 72(6):1129–42. doi: 10.1136/gutjnl-2022-327156 [DOI] [PubMed] [Google Scholar]
- 121. Tomkovich S, Yang Y, Winglee K, Gauthier J, Mühlbauer M, Sun X, et al. Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Res (2017) 77(10):2620–32. doi: 10.1158/0008-5472.CAN-16-3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discovery (2018) 8(4):403–16. doi: 10.1158/2159-8290.CD-17-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discovery (2021) 11(5):1248–67. doi: 10.1158/2159-8290.CD-20-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hezaveh K, Shinde RS, Klötgen A, Halaby MJ, Lamorte S, Ciudad MT, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity (2022) 55(2):324–40.e8. doi: 10.1016/j.immuni.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, et al. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front Microbiol (2020) 11:814. doi: 10.3389/fmicb.2020.00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Martin A, Woolbright BL, Umar S, Ingersoll MA, Taylor JA. 3rd, Bladder cancer, inflammageing and microbiomes. Nat Rev Urol (2022) 19(8):495–509. doi: 10.1038/s41585-022-00611-3 [DOI] [PubMed] [Google Scholar]
- 127. He C, Huang L, Lei P, Liu X, Li B, Shan Y. Sulforaphane norMalizes intestinal flora and enhances gut barrier in mice with BBN-induced bladder cancer. Mol Nutr Food Res (2018) 62(24):e1800427. doi: 10.1002/mnfr.201800427 [DOI] [PubMed] [Google Scholar]
- 128. Then CK, Paillas S, Wang X, Hampson A, Kiltie AE. Association of Bacteroides acidifaciens relative abundance with high-fibre diet-associated radiosensitisation. BMC Biol (2020) 18(1):102. doi: 10.1186/s12915-020-00836-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol (1995) 27(2):104–9. doi: 10.1159/000475138 [DOI] [PubMed] [Google Scholar]
- 130. Sirisinha S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac J Allergy Immunol (2015) 33(2):71–89. [PubMed] [Google Scholar]
- 131. Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, et al. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol (2010) 184(12):6799–806. doi: 10.4049/jimmunol.0902944 [DOI] [PubMed] [Google Scholar]
- 132. Lv Z, Wang Y, Yang T, Zhan X, Li Z, Hu H, et al. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr (2016) 59(2):113–21. doi: 10.3164/jcbn.15-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Xiao S, Li Q, Hu K, He Y, Ai Q, Hu L, et al. and retinoic acid exhibit protective effects on necrotizing enterocolitis by regulating intestinal flora and enhancing the intestinal epithelial barrier. Arch Med Res (2018) 49(1):1–9. doi: 10.1016/j.arcmed.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 134. Tian Y, Nichols RG, Cai J, Patterson AD, Cantorna MT. Vitamin A deficiency in mice alters host and gut microbial metabolism leading to altered energy homeostasis. J Nutr Biochem (2018) 54:28–34. doi: 10.1016/j.jnutbio.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pang B, Jin H, Liao N, Li J, Jiang C, Shi J. Vitamin A supplementation ameliorates ulcerative colitis in gut microbiota-dependent manner. Food Res Int (2021) 148:110568. doi: 10.1016/j.foodres.2021.110568 [DOI] [PubMed] [Google Scholar]
- 136. Pan P, Atkinson SN, Taylor B, Zhu H, Zhou D, Flejsierowicz P, et al. Retinoic acid signaling modulates recipient gut barrier integrity and microbiota after allogeneic hematopoietic stem cell transplantation in mice. Front Immunol (2021) 12:749002. doi: 10.3389/fimmu.2021.749002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sabichi AL, Lerner SP, Atkinson EN, Grossman HB, Caraway NP, Dinney CP, et al. Phase III prevention trial of fenretinide in patients with resected non-muscle-invasive bladder cancer. Clin Cancer Res (2008) 14(1):224–9. doi: 10.1158/1078-0432.CCR-07-0733 [DOI] [PubMed] [Google Scholar]
- 138. Pili R, Salumbides B, Zhao M, Altiok S, Qian D, Zwiebel J, et al. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br J Cancer (2012) 106(1):77–84. doi: 10.1038/bjc.2011.527 [DOI] [PMC free article] [PubMed] [Google Scholar]