Take Home Message
Beneficial effects of hyperbaric oxygen or formalin on pain, quality of life, and functional symptoms were seen in patients with certain chronic pelvic pain subtypes. However, the current evidence level is too weak to allow recommendations about the use of any pharmacological treatment for postradiation pelvic pain.
Keywords: Chronic pelvic pain, Radiotherapy, Postradiation, Chronic post cancer treatment pain, Chronic post radiotherapy pain, Chronic painful radiation-induced neuropathy, Pelvic radiation disease
Abstract
Context
Radiotherapy of the pelvis is a widely used method for the treatment of malignancies, and local complications including pain following pelvic radiation therapy are acknowledged complications.
Objective
The primary objective is to assess the clinical effectiveness and safety of pharmacological therapies on postradiation pelvic pain.
Evidence acquisition
A systematic review of the use of different pharmacological treatments in the management of post-radiation pelvic pain was conducted (PROSPERO-ID: CRD42021249026). Comprehensive searches of EMBASE, Medline, and Cochrane library were performed for publications between January 1980 and April 2021. The primary outcomes were improvement in pain and adverse events following treatment. The secondary outcomes included quality of life, bowel function, and urinary function.
Evidence synthesis
After screening 1514 abstracts, four randomised controlled trials were identified, enrolling 355 patients with bladder and anorectal subtypes of postradiotherapy chronic pelvic pain (CPP). A narrative synthesis was performed as heterogeneity of included studies precluded a meta-analysis. A single study reported a significant reduction in pain after 6 mo in patients with bladder pain syndrome treated with hyaluronic acid or hyperbaric oxygen. Anorectal pain was reported to be reduced by the application of 4% formalin, but the use of hyperbaric oxygen in postradiotherapy anorectal pain remains controversial. Adverse event reporting was generally poor. Studies looking at medications used routinely in guidelines for neuropathic pain, such as gabapentin, pregabalin, amitriptyline, and duloxetine, were absent or of poor quality when it came to postradiation pelvic pain.
Conclusions
Beneficial effects of hyperbaric oxygen or formalin on pain, quality of life, and functional symptoms were seen in patients with certain CPP subtypes, but the current evidence level is too weak to allow recommendations about the use of any pharmacological treatment for postradiation pelvic pain.
Patient summary
Different pharmacological treatments are used to treat pain after radiotherapy, but current studies are of insufficient quality to determine whether these should be recommended and many chronic pelvic pain subtypes are not covered. Further research is needed.
1. Introduction
Radiotherapy is widely used for the treatment of malignancies located in the pelvis. Up to 35% of newly diagnosed cancers in men and up to 18% in women involve organs of the pelvis (bladder, prostate, uterus, cervix, colon, and rectum) [1]. Although techniques of administering radiation are continuously improving, external beam radiotherapy and brachytherapy can lead to adverse effects depending on the field of radiation and dose administered to the organs. Local complications of the bladder or rectum following pelvic radiotherapy are reported to occur in up to 15% of cases [2], [3]. Molecular effects of radiotherapy can be divided into immediate cell death (due to lipid peroxidation by reactive radicals) [4] and late-onset injuries (after months to years) with the development of an obliterative endarteritis [5]. Clinical symptoms caused by the immediate effects of radiotherapy are often self-limiting, but the delayed molecular impact of radiotherapy can lead to organ-specific adverse effects such as haemorrhage, reduced capacity, ulcerations, or ischaemia, including chronic painful radiation-induced neuropathy, and all these processes can result in pain. The syndrome encompassing these organ-specific adverse effects following pelvic radiotherapy is known as pelvic radiation disease (PRD) and includes radiation proctitis, cystitis, urethritis, osteitis, lumbar plexopathy, lymphoedema, and sexual dysfunction. In PRD, chronic pelvic pain (CPP), which is defined as continuous or recurrent pain related to organs or structures of the pelvis, may stem from any of the major pelvic organs [6]. Several pharmacological strategies are available for treating the late effects of pelvic radiotherapy, and these include oral and parenteral agents, intravesical or instillation agents, local injections, or hyperbaric oxygenation (HBO). This review aims to assess the evidence for the clinical effectiveness of the most commonly available strategies for treating postradiotherapy CPP, and to assess the associated adverse events and the impact on quality of life (QoL) and other functional outcomes. To the best of our knowledge, there are no prior systematic reviews (SRs) on this topic in the public domain.
2. Evidence acquisition
This SR was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7], and followed the key steps and methodology set out by the European Association of Urology (EAU) Guidelines Group (PROSPERO-ID: CRD42021249026) [8].
2.1. Data sources and searches
A comprehensive search of EMBASE, Medline, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews (via Ovid) was performed for papers published between January 1980 and April 2021. The search strategy and keywords used are available in the Supplementary material. Titles and abstracts were retained for screening after search results were combined and deduplicated.
2.2. Study selection
The primary study design included randomised controlled trials (RCTs) and prospective or retrospective comparative nonrandomised studies with a minimum of 3 mo of follow-up, as a shorter duration of clinical benefit would render repeat interventions impractical and hard to justify. Comparative studies with ten or fewer participants per arm or without at least one baseline measurement of interest were excluded. Single-arm case series were included only if no comparative studies were found. Case reports, editorial commentaries, and narrative reviews were excluded. The reference lists of relevant SRs were searched for additional relevant studies. There were no language restrictions.
The experimental interventions were any pharmacological treatments including oral or parenteral agents, intravesical treatments or instillations, injection therapies, HBO, or combinations of agents for treating postradiation pelvic pain. Control groups comprised no treatment, sham intervention, or placebo. Additional inclusion criteria were adult participants (aged ≥18 yr) with a history of radiation therapy (external beam or brachytherapy) for malignancies of the pelvis and who developed subsequent pelvic pain. All CPP subtypes such as bladder pain, prostate pain, anal pain, rectal pain, gynaecological pain, scrotal pain, and urethral pain were included.
Two reviewers (V.Z. and B.P.) independently screened titles and abstracts to identify potentially eligible studies, and then obtained and screened full-text papers to determine the finally included studies. A third reviewer (S.D.) was consulted for arbitration when needed. For studies with multiple publications, the main trial report was used.
2.3. Data extraction and risk of bias
Data were extracted using a standardised data extraction form. Collected data included the year of publication, study design, number of participants, subtype of pelvic pain, type of pharmacological intervention, and outcome measures recorded. The primary benefit was improvement in pain as defined by the trialist, while treatment-related adverse effects were considered primary harms. The secondary outcome measures were QoL, and urinary and bowel function. The Cochrane Risk of Bias Assessment Tool 2.0 was used to evaluate the risk of bias of included studies [9].
2.4. Data synthesis
When continuous measurements were used to assess the intervention effect, the mean difference or standardised mean difference was calculated for each included study. The effect size and corresponding 95% confidence intervals were calculated for the primary and secondary outcome measures. A risk of bias summary was generated using Cochrane Review Manager software version 5.3 (Cochrane, London, UK).
3. Evidence synthesis
3.1. Search results
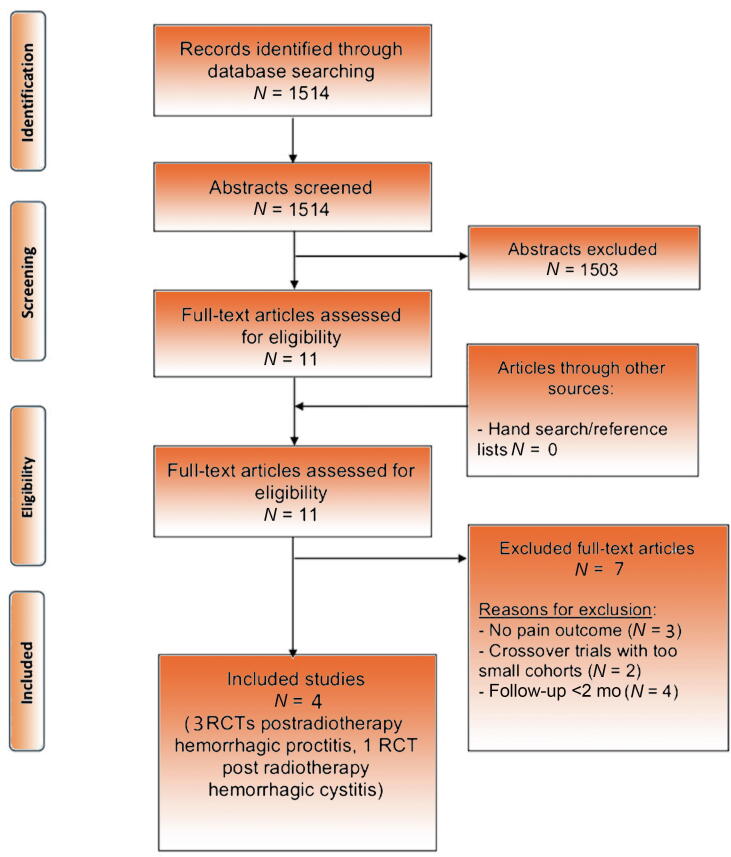
The PRISMA diagram illustrates the literature search and results (Fig. 1). The search identified 1514 abstracts, and following screening, 11 full-text papers were retrieved and assessed for eligibility. Seven full-text articles had to be excluded due to short follow-up, missing pain outcomes, or small cohort numbers. The final four included studies were all RCTs.
Fig. 1.
PRISMA flow diagram for systematic review of the benefits and harms of pharmacological treatment for postradiation pelvic pain. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCT = randomised controlled trial.
3.2. Study and patient characteristics
The characteristics of the included studies and their patient demographics are summarised in Table 1. One study assessed the use of hyaluronic acid instillations versus HBO in postradiation bladder pain [10], whereas the other three assessed treatments for postradiation anorectal pain. Two of these papers investigated HBO versus placebo/sham [11], [12], and one study compared an application of 10% formalin versus that of 4% formalin using a small soaked piece of gauze [13]. No studies assessing pharmacological treatments for other subtypes of CPP met the inclusion criteria of the review. Insufficient data were provided about funding sources and conflicts of interest.
Table 1.
Baseline characteristics of studies on treatment of postradiation pelvic pain
| Interventions (control vs experimental) | N | FU (mo), mean (SD), median (IQR) | Age (yr), mean (SD), median (IQR) | Gender M/F (%) | Type of medical therapy prior to study participation | Duration of symptoms prior to study participation (mo), mean (SD), median (IQR) | Pain assessment (as defined/used by trialist in study) | Quality of life assessment (as defined/used by trialist in study) | Voiding function/bladder capacity (as defined/used by trialist in study) | Bowel symptoms (as defined/used by trialist in study) | Symptom scores (as defined/used by trialist in study) | Outcomes measured | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shao et al. (2012) [10]; RCT; China; Nov 2004 to Dec 2008 | Intravesical hyaluronic acid | 16 | 18 | 59 (46–74) |
62/38 | NR | NR | VAS | NA | Urinary frequency | NA | NA | Haematuria/dysuria VAS Urinary frequency Adverse effects |
| Hyperbaric oxygen | 20 | 18 | 60 (39–77) |
60/40 | NR | NR | VAS | NA | Urinary frequency | NA | NA | ||
| Glover et al. (2016) [12]; RCT; UK; Aug 2009 to Oct 2012 | Hyperbaric oxygen | 55 | 12 | 64 (35–81) |
42/58 | NR | NR | LENT SOMA | EORTC QLQ-C30 EORTC QLQ-CR38 |
NA | IBDQ bowel function IBDQ rectal bleeding CTCAE GI scale |
IBDQ LENT SOMA |
IBDQ bowel function IBDQ rectal bleeding score LENT SOMA CTCAE GI scale EORTC QLQ-C30 EORTC QLQ-CR38 Adverse effects |
| Placebo/sham | 29 | 12 | 64 (54–70) |
48/52 | NR | NR | LENT SOMA | NA | |||||
| Guo et al. (2014) [13]; RCT; China; Jan 2009 to Dec 2012 | 10% formalin | 58 | 12 | 49 (26–70) |
0/100 | NR | 13.48 (6–25) |
RPSAS | NA | NA | RPSAS | RPSAS | RPSAS Vienna Rectoscopy Score Adverse effects |
| 4% formalin | 57 | 12 | 50 (25–69) |
0/100 | NR | 11.70 (6–23) |
RPSAS | NA | NA | RPSAS | RPSAS | ||
| Clarke et al. (2008) [11]; RCT; multiple countries | Hyperbaric oxygen | 33 | 60 | NR | 12/88 | NR | NR | LENT SOMA | EPCIC QoL | NA | SOMA LENT | LENT SOMA | LENT SOMA EPCIC QoL Adverse effects |
| Placebo | 39 | 60 | NR | NR | NR | LENT SOMA | EPCIC QoL | NA | LENT SOMA | LENT SOMA |
CTCAE GI = Common Terminology Criteria for Adverse Events gastrointestinal scale; EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer C30 core quality of life questionnaire; EORTC QLQ-CR38 = European Organisation for Research and Treatment of Cancer C30 core colorectal cancer–specific quality of life questionnaire; EPCIC QoL = Expanded Prostate Cancer Index Composite quality of life; F = female; FU = follow-up; IBDQ = Inflammatory Bowel Disease Questionnaire; IQR = interquartile range; LENT SOMA = Late Effects in Normal Tissues Subjective Objective Management and Analytic scales; M = male; NA = not assessed; NR = not reported; RCT = randomised controlled trial; RPSAS = Radiation Proctopathy System Assessments Scale; SD = standard deviation; VAS = visual analogue scale.
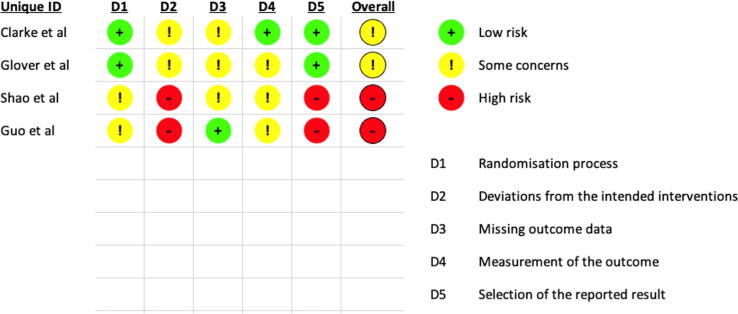
3.3. Risk of bias and confounding
There was a moderate to high risk of bias in all RCTs (Fig. 2). Power calculations were undertaken only in one study [12], and consequently, small population numbers were a frequent source of bias. For included RCTs, a risk of bias was found for all domains.
Fig. 2.
Risk of bias and confounding assessment of randomised controlled trial studies included in the systematic review.
3.4. Benefits and harms of pharmacological treatments
There was significant heterogeneity in design and outcome measures amongst the included studies; hence, a narrative review of the evidence was undertaken. Table 2, Table 3 summarise the reduction in pain scores and the reported adverse events for all the studies included in this review. The Grading of Recommendations Assessment, Development and Evaluation was not used to assess the quality of the evidence because of the high risk of bias and confounding amongst included RCTs [14].
Table 2.
Primary benefit outcome for included studies—reduction in pain score
| Study ID | CPPS type, study design | N | Group | Type and scale of pain score assessment | Pain scores |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time after treatment (mo) | Baseline pain | SD/IQR/range | Pain after | SD/IQR/range | p value | Mean diff Baseline vs after |
95% CI or SD/IQR of mean diff |
p value of mean diff | |||||
| Shao et al. (2012) [10] | BPS, RCT | 16 | Control HA |
VAS (mean, SD) |
6 | 2.75 | 2.24 | 1.88 | 1.41 | <0.05 | –0.88 | 1.41 | NR |
| 20 | Interv HBO |
6 | 2.5 | 2.24 | 1.60 | 1.79 | <0.01 | –0.90 | 0.79 | NR | |||
| 16 | Control HA |
VAS (mean, SD) |
12 | 2.75 | 2.24 | 1.44 | 1.36 | <0.01 | –1.31 | 1.3 | NR | ||
| 20 | Interv HBO |
12 | 2.5 | 2.24 | 1.60 | 1.88 | <0.01 | –0.9 | 1.02 | NR | |||
| 16 | Control HA |
VAS (mean, SD) |
18 | 2.75 | 2.24 | 1.25 | 1.53 | <0.01 | –1.50 | 1.21 | NR | ||
| 20 | Interv HBO |
18 | 2.5 | 2.24 | 1.35 | 1.69 | <0.01 | –1.15 | 1.22 | NR | |||
| Glover et al. (2016) [12] | Anorectal, RCT | 26 | Control Sham |
LENT SOMA (median, IQR) |
12 | 6 | 5–8 | 4.5 | 2–8 | 0.11 | –1.5 | –4 to 0 | 0.12 |
| 46 | Interv HBO |
12 | 6 | 4–8 | 5.0 | 3–8 | 0.11 | –1.0 | –2 to 1 | 0.12 | |||
| Guo et al. (2014) [13] | Anorectal, RCT | 57 | Control 4% FO |
RPSAS (median, range) |
3 | 20 | 7–30 | 10 | 6–26 | <0.001 | NR | NR | NR |
| 58 | Interv 10% FO |
3 | 21 | 7–30 | 9 | 6–25 | <0.001 | NR | NR | NR | |||
| Clarke et al. (2008) [11] | Anorectal, RCT | 56 | Control Sham |
LENT SOMA (mean) |
1.5 | 12.84 | NR | 10.23 | NR | 0.0019 | NR | NR | NR |
| 64 | Interv HBO |
1.5 | 12.55 | NR | 7.48 | NR | 0.0019 | NR | NR | NR | |||
BPS = bladder pain syndrome; CI = confidence interval; CPPS = chronic pelvic pain syndrome; diff = difference; FO = formalin; HA = hyaluronic acid; HBO = hyperbaric oxygen; Interv = intervention; IQR = interquartile range; LENT SOMA = Late Effects in Normal Tissues Subjective Objective Management and Analytic scale; NR = not reported; RCT = randomised controlled trial; SD = standard deviation; RPSAS = Radiation Proctopathy System Assessments Scale; VAS = visual analogue scale.
Table 3.
Primary harm outcome for included studies—adverse events
| Study ID | CPPS type, study design | Adverse events |
Other adverse effect | p value (Interv vs control) | Time point of assessment | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Group | Side effect 1 | Side effect 2 | Side effect 3 | Side effect 3 | |||||
| Shao et al. (2012) [10] | BPS, RCT | 16 | Control HA |
UTI (42.8%) | 6 mo | |||||
| 20 | Interv HBO |
UTI (10%) | 6 mo | |||||||
| Glover et al. (2016) [12] | Anorectal, RCT | 29 | Control Sham |
Eye refractive change (11%) | Fatigue (11%) | Ear/barotrauma (21%) | NR | |||
| 55 | Interv HBO |
Eye refractive change (30%) | Fatigue (4%) | Ear/barotrauma (28%) | NR | |||||
| Guo et al. (2014) [13] | Anorectal, RCT | 57 | Control 4% FO |
Pain after instillation (10.5%) | NR | |||||
| 58 | Interv 10% FO |
Pain after instillation (29.5%) | NR | |||||||
| Clarke et al. (2008) [11] | Anorectal, RCT | 56 | Control Sham |
NR | NR | |||||
| 64 | Interv HBO |
Transient myopia (3.3%) | Ear pain (15.8%) | NR | ||||||
BPS = bladder pain syndrome; CPPS = chronic pelvic pain syndrome; FO = formalin; HA = hyaluronic acid; HBO = hyperbaric oxygen; Interv = intervention; NR = not reported; RCT = randomised controlled trial; UTI = urinary tract infection.
3.4.1. Bladder pain
The only included study focusing on bladder pain compared HBO with intravesical instillation of hyaluronic acid for the treatment of radiation-induced cystitis [10] with a total of 36 participants (20 in the HBO group vs 16 in the hyaluronic acid group). The median age of participants was 60 (39–77) yr for the HBO treatment arm and 59 (46–74) yr for the hyaluronic acid group. In the HBO group, patients received 100% oxygen at a pressure of 2.5 atmosphere (atm) in a hyperbaric chamber. Treatment was given for 60 min and was performed once daily for at least a month. In the other group, 40 mg of hyaluronic acid was instilled slowly with a Foley catheter before clamping the catheter for 20 min. Instillations were performed weekly in the 1st month and then monthly for a further 2 mo. Therapeutic efficacy was evaluated every 6 mo, up to 18 mo after the start of treatment.
3.4.1.1. Pain
This study assessed pain at baseline and every 6 mo after treatment up to and including 18 mo using a visual analogue scale (VAS; ranging from 0 to 10). Baseline VAS was 2.5 ± 2.24 for the HBO group and 2.75 ± 2.24 for the hyaluronic acid group. It improved significantly in both groups (–0.9 ± 0.79 in the HBO group and –0.88 ± 1.41 in the hyaluronic acid group) after 6 mo. The effects seemed durable as the reduction in pain remained significant at the last follow-up reported at 18 mo.
3.4.1.2. Adverse events
The only adverse events reported in this study were urinary tract infections occurring significantly more often in the instillation group (42.8%) than in the HBO group (10%).
3.4.1.3. Quality of life
The impact of the interventions on participant QoL was not assessed in this study.
3.4.1.4. Functional outcomes
The impact of the interventions on the participants’ voiding frequency was assessed. Baseline voiding frequency was 9.8 ± 1.74 in the HBO group and 10.4 ± 1.8 in the hyaluronic acid group. It decreased significantly (–1 to 2 ± 1.06 in HBO group and –2.9 ± 1.7 in the hyaluronic acid group) in both groups after 6 mo, but the reduction in voiding frequency was no longer significant in either group at the 18-mo follow-up.
3.4.2. Anorectal pain
Three studies reported the impact of pharmacological treatments on postradiotherapy anorectal pain. Glover et al. [12] and Clarke et al. [11] assessed HBO treatment versus placebo/sham. In the Glover et al.’s [12] study, 55 patients received HBO during 40 pressure exposures at 2.4 standard atm breathing 100% oxygen for 90 min, whereas the control group of 29 patients received 40 exposures at 1.3 atm breathing 21% oxygen. The median age in both groups was 64 yr. The trial by Clarke et al. [11] compared 64 patients treated with HBO (100% oxygen at 2 atm once daily, five times weekly) with 56 patients treated with 21% oxygen (normal air, 1.1 atm once daily, five times weekly).
The third study by Guo et al. [13] compared 58 patients receiving 10% formalin with 57 patients receiving 4% formalin applied at sigmoidoscopy using a small soaked piece of gauze. The median age was 49 (26–70) yr in the 10% and 50 (25–69) yr in the 4% formalin group.
3.4.2.1. Pain
Glover et al. [12] and Clarke et al. [11] both reported changes in pain as a part of the Late Effects in Normal Tissues Subjective Objective Management and Analytic (LENT SOMA) scale. Glover et al. [12] found no significant change after 12 mo for LENT SOMA scores, whereas Clarke et al. [11] indicated a significant reduction in pain domains at the first evaluation for the HBO group. However, as all sham patients were subsequently crossed over to the intervention group (cross-over design), the comparative outcome measures were available only at 6 wk after intervention, which limited the strength of these results.
Guo et al. [13] reported improvement in pain as part of the Radiation Proctopathy System Assessments Scale (RPSAS) with baseline pain of 20 (7–30) and 21 (7–30) for the 4% formalin and 10% formalin groups, respectively. After 3 mo, a significant reduction in both groups was reported.
3.4.2.2. Adverse events
According to the reports from Clarke et al. [11] and Glover et al. [12], eye refractive changes (myopia) occurred, respectively, in 3.3% and up to 30% of patients treated with HBO. Ear pain and barotrauma were other common adverse effects of HBO (15.8–28%). Guo et al. [13] reported pain after formalin instillation in 29.5% of the 10% formalin group and 10.5% of the 4% formalin group.
3.4.2.3. Quality of life
Glover et al. [12] assessed changes in QoL using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-CR38 questionnaires. However, due to the nonsignificant change in LENT SOMA, the authors did not provide any descriptive analyses of the QoL scores. Clarke et al. [11] noted an improvement in bowel-specific QoL for the HBO group and for the placebo group after cross-over to HBO. Lastly, Guo et al. [13] did not assess or report QoL for the patients included in their study.
3.4.2.4. Functional outcomes
Glover et al. [12] assessed bowel symptoms with Inflammatory Bowel Disease Questionnaire and LENT SOMA, and found no significant improvement of overall bowel function or rectal bleeding. Clarke et al. [11] reported LENT SOMA as described above. Guo et al. [13] assessed functional outcomes with the RPSAS, showing a significantly improved score for both groups in their analysis.
3.5. Discussion
This SR addressed the efficacy and safety of pharmacological treatments used to treat patients with various CPP subtypes caused by pelvic radiotherapy. Included studies were generally of poor quality with significant risks of bias and confounding, warranting results to be interpreted with caution. Multicentre trials are needed, as currently most studies in this field are underpowered with small patient numbers. This limits the clinical generalisability of the study findings.
Although pain was the primary outcome of this review, most studies assessed pain as part of a composite outcome measure. Most of the screened studies focused on other clinically apparent and measurable side effects of radiotherapy such as bleeding or voiding/functional problems. It is out of contention that although postradiation pelvic pain is a recognised problem, the current literature does not focus on this aspect of postradiotherapy morbidity.
To facilitate comparison and interpretation of the data, included studies were separated according to the major subtypes of CPP. Even within these groups, there was considerable clinical and methodological heterogeneity, so only a narrative synthesis was possible.
The included papers investigated HBO, intravesical hyaluronic acid, and intrarectal formalin application. HBO seems to be beneficial for a reduction of pain at least in the short term. A summary estimate for an overall change in pain following HBO sessions was not possible, but some individual studies reported a statistically significant reduction in pain for patients with bladder [10] and anorectal [11] pain. However, one RCT with longer follow-up found no statistically significant benefit of HBO for anorectal pain [12], and consequently, it is not possible to make recommendations about the use of HBO in the management of postradiotherapy pain.
Intravesical hyaluronic acid was investigated in one trial [10] and led to a significant decrease of bladder pain, measured by VAS. Based on this single study, containing 16 participants who received hyaluronic acid, no recommendation can be made about its use for this indication.
The last included study [13] investigated two different strengths of formalin (4% vs 10%) applied topically to the rectum. Both strengths reduced pain significantly, but it is not possible to provide a definitive recommendation about the use of formalin for postradiotherapy anorectal pain based on a single small study. In terms of the reported adverse effects, 4% formalin seems to be better tolerated, with equivalent results to the higher strength.
Patient-centred secondary outcomes were reported poorly in the included studies, but functional outcomes such as voiding frequency or bowel symptoms generally also improved when an improvement in pain was recorded. Accurate reporting of adverse effects is important for establishing the safety profile of potentially novel uses of pharmacological treatments. The included studies show that each treatment has a specific adverse side-effect profile, such as urinary tract infections in bladder instillations or eye refractive changes and barotrauma in HBO. Future studies need to standardise the reporting of adverse events and the time of their occurrence, but it is important to remember that small trials may fail to reveal rare and potentially serious adverse effects.
In the panels’ experience, appropriately powered and well-designed RCTs with prolonged follow-up are often difficult to achieve in noncancer clinical trials. By the very nature of the condition, the patient population affected by CPP is often too heterogeneous to lend itself to RCT evaluation. Although there are many studies that focus on overall or cancer-specific survival following radiotherapy treatment, it is surprising that almost none of the screened studies provided any emphasis on postradiotherapy pain and its treatment. One explanation for the scarcity of data is the fact that radiotherapy can lead to a multitude of different symptoms such as bleeding, strictures, and changes in urinary and bowel frequency or continence that are clinically more evident and more easily assessed using objective measures than pain. The adverse events associated with radiation therapy may become increasingly evident long after the initial treatment and may consequently be overlooked in the limited follow-up period of most clinical trials. Anecdotally, members of this panel note that the pain may start many years after the radiation therapy. Postradiation morbidity can lead to a significant impact on QoL, with pain being a common problem in cancer survivors. To add new evidence on postradiotherapy pelvic pain and to reduce the deficiencies and biases of the currently published literature, the outcome variables measured, instruments used, and assessment time points need to be standardised. The inclusion of chronic post–cancer treatment pain (MG30.11), specifically chronic postradiotherapy pain and chronic painful radiation-induced neuropathy in ICD 11 [15], may highlight the issue and enable better data collection and, as a consequence, research.
Core outcomes of radiotherapy-induced pelvic pain trials should be identified by consensus of a panel of experts in collaboration with patient advocates using a similar methodology to that set out by the Core Outcome Measures in Effectiveness Trials (COMET) initiative [16], [17], [18]. A core outcome set would be an agreed minimum set of outcomes that should be measured and reported in all clinical trials of a specific disease or trial population [16]. Their adoption would bring methodological robustness to future trials and reduce the inconsistencies that hamper improved level of evidence.
The Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMMPACT) project published recommendations over 15 yr ago about the core outcome domains that should be considered in chronic pain trials [19]. Despite this, a CPP-specific core outcome set has yet to be developed. Standardisation of pain assessment, adverse event reporting, and QoL evaluation are key domains that should be measured. Secondary outcomes would depend on the CPP subtype but could include measures of sexual, urinary, and bowel function.
3.5.1. Recommendations
In the absence of evidenced-based guidelines specifically for pelvic pain after radiotherapy, the authors of this paper emphasise the importance of adhering to general principles as highlighted in the EAU CPP guidelines [20] and others. In particular, this panel advises the following key steps be adhered to when managing any patient with CPP after radiotherapy:
-
1.Screen all patients for chronic pain. The UK’s Faculty of Pain Medicine, the Royal College of Anaesthetists suggests an early prescreening and management tool to reduce the burden of pain [21]:
-
(a)Over the past 2 wk has pain been bad enough to interfere with your day to day activities?
-
(b)Over the past 2 wk have you felt worried or low in mood because of this pain?
-
(a)
-
2.
If the pain is having a significant impact on QoL, consider the underlying mechanisms. Treatment is more likely to be successful if the underlying mechanisms for the pain are understood. For instance, is the pain nociceptive, neuropathic, or nociplastic? Consider early referral to a specialist. Nociceptive pain, that is, pain secondary to an underlying cause (ICD11 secondary pain), requires referral to the appropriate specialist, such as a urologist, gynaecologist, or anal rectal specialist, for appropriate investigation and treatment. Pain thought to be neuropathic or nociplastic may require the involvement of a neurologist or pain medicine specialist.
-
3.
Treat according to established guidelines. It is not the aim of this paper for the authors to review all published guidelines on chronic pain. Recommendations for CPP and CPP syndromes can be found in the EAU CPP guidance [20]. The UK’s National Institute for Health and Care Excellence (NICE) and others have guidelines for neuropathic pain treatments that include amitriptyline, gabapentin, pregabalin, and duloxetine [22]. NICE also has guidelines on other treatments [23], and the UK’s Faculty of Pain Medicine has a resource on opioids [24]. The International Association for the Study of Pain also has resources such as their multidisciplinary pain centre development manual [25].
-
4.
Consider early referral to appropriate specialists. It is the clinical responsibility of all clinicians to be aware of chronic pain after radiotherapy for pelvic cancer. Once identified, it is every clinician's responsibility to ensure that the person living with pain sees the right specialist (who is aware of published guidelines) at the right time.
4. Conclusions
This review highlights the very scarce evidence and significant clinical and methodological heterogeneity of studies assessing pharmacological treatment of postradiotherapy pelvic pain. Clinical experience shows that pain syndromes can have a profound impact on the QoL of cancer survivors treated by pelvic radiotherapy. However, the published literature on adverse events of radiotherapy rarely focuses on the treatment of postradiotherapy pain and instead focuses on the treatment of bleeding or functional deficits. Larger-scale, multicentre, well-designed, and adequately powered studies with longer follow-up periods focusing on pain therapy are needed. Nonrandomised comparative or prospective case-control studies would generate useful information if well-conducted RCTs are not possible. In the meantime, adherence to other published guidelines is encouraged.
Author contributions: Valentin Zumstein had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zumstein, Parsons, Dabestani, Baranowski, Sacks, Yuan, Engeler.
Acquisition of data: Zumstein, Parsons.
Analysis and interpretation of data: Zumstein, Parsons, Dabestani, Engeler, Baranowski, Sacks.
Drafting of the manuscript: Zumstein, Parsons.
Critical revision of the manuscript for important intellectual content: Zumstein, Parsons, Dabestani, Baranowski, Tidman, Berghmans, Borovicka, Cottrell, Dinis-Oliveira, Elneil, Hughes, Messelink, Abreu-Mendes, Sacks, Yuan, Engeler.
Statistical analysis: Zumstein, Parsons, Yuan.
Obtaining funding: None.
Administrative, technical, or material support: Yuan.
Supervision: Engeler.
Other: None.
Financial disclosures: Valentin Zumstein certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgements: This SR was conducted through altruistic donation of time and knowledge from all involved parties. We want to thank Ms, Julie Darraugh at the European Association of Urology Guidelines Office for her help in the logistical management of this SR.
Associate Editor: Véronique Phé
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.08.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.American Cancer Society. Cancer facts & figures. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html.
- 2.Bennett M.H., Feldmeier J., Hampson N., Smee R., Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2012;5:CD005005. doi: 10.1002/14651858.CD005005.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Eifel P.J., Levenback C., Wharton J.T., Oswald M.J. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1995;32:1289–1300. doi: 10.1016/0360-3016(95)00118-I. [DOI] [PubMed] [Google Scholar]
- 4.Browne C., Davis N.F., Mac Craith E., et al. A narrative review on the pathophysiology and management for radiation cystitis. Adv Urol. 2015;2015 doi: 10.1155/2015/346812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathews R., Rajan N., Josefson L., Campoesi E., Makhuli Z. Hyperbaric oxygen therapy for radiation induced hemorrhagic cystitis. J Urol. 1999;161:435–437. [PubMed] [Google Scholar]
- 6.Zhou C., Adler D.C., Becker L., et al. Effective treatment of chronic radiation proctitis using radiofrequency ablation. Ther Adv Gastroenterol. 2009;2:149–156. doi: 10.1177/1756283X08103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 8.Knoll T., Omar M.I., Maclennan S., et al. Key steps in conducting systematic reviews for underpinning clinical practice guidelines: methodology of the European Association of Urology. Eur Urol. 2018;73:290–300. doi: 10.1016/j.eururo.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Sterne J.A.C., Savovic J., Page M.J, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 10.Shao Y., Lu G.-L., Shen Z.-J. Comparison of intravesical hyaluronic acid instillation and hyperbaric oxygen in the treatment of radiation-induced hemorrhagic cystitis. BJU Int. 2012;109:691–694. doi: 10.1111/j.1464-410X.2011.10550.x. [DOI] [PubMed] [Google Scholar]
- 11.Clarke R.E., Catalina Tenorio L.M., Hussey J.R., et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;72:134. doi: 10.1016/j.ijrobp.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Glover M., Smerdon G.R., Jervoise Andreyev H., et al. Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double-blind, sham-controlled phase 3 trial. Lancet Oncol. 2016;17:224–233. doi: 10.1016/S1470-2045(15)00461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo G.-H., Yu F.-Y., Wang X-J, et al. A randomized controlled clinical trial of formalin for treatment of chronic hemorrhagic radiation proctopathy in cervical carcinoma patients. Support Care Cancer. 2014;23:441–446. doi: 10.1007/s00520-014-2401-2. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. International statistical classification of diseases and related health problems. ed. 11. 2022. https://icd.who.int/en.
- 16.Prinsen C.A., Vohra S., Rose M.R., et al. Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a ‘core outcome set. Trials. 2014;15:247. doi: 10.1186/1745-6215-15-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson P.R., Altman D.G., Bagley H., et al. The COMET handbook: version 1.0. Trials. 2017;18(Suppl 3):280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson P., Clarke M. The COMET (Core Outcome Measures in Effectiveness Trials) initiative: its role in improving Cochrane reviews. Cochrane Database Syst Rev. 2012;5 doi: 10.1002/14651858.ED000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turk D.C., Dworkin R.H., Allen R.R., et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Engeler D., Baranowski A.P., Borovicka J., et al. EAU guidelines. Presented at the EAU Annual Congress Milan 2021; 2022.
- 21.Faculty of Pain Medicine of the Royal College of Anaesthetists. Ask 2 questions. https://www.fpm.ac.uk/standards-guidelines-innovations/ask2questions.
- 22.National Institute for Health and Care Excellence. Neuropathic pain in adults: pharmacological management in non-specialist settings (clinical guideline [CG173]). 2020. https://www.nice.org.uk/guidance/cg173. [PubMed]
- 23.National Institute for Health and Care Excellence. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain (NICE guideline [NG193]). 2021. https://www.nice.org.uk/guidance/ng193. [PubMed]
- 24.Faculty of Pain Medicine of the Royal College of Anaesthetists. Opioids aware. 2020. https://www.fpm.ac.uk/opioids-aware.
- 25.International Association for the Study of Pain. Pain management center. https://www.iasp-pain.org/resources/toolkits/pain-management-center.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.