Abstract
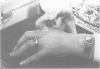
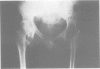
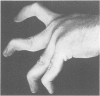
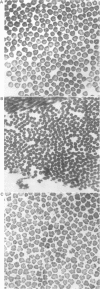
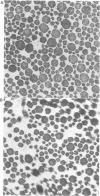
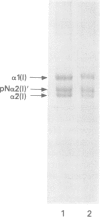
The features of a 32 year old woman with Ehlers-Danlos syndrome type VIIB and affected members of her family, resulting from a mutation in one COL1A2 allele, were studied. Her dermal type I collagen contained alpha 2(I) chains and mutant pN-alpha 2(I) chains in which the amino-terminal propeptide remained attached to the alpha 2(I) chain. She was heterozygous for an AG-->AC mutation at the splice acceptor site of intron 5 of the COL1A2 gene. The mutation activated a cryptic AG splice acceptor site corresponding to positions +14 and +15 of exon 6 of the COL1A2 gene. In contrast to previous reports only five, rather than all 18, amino acids encoded by exon 6 were deleted in the proband. The deleted peptide removed the amino-proteinase cleavage site, but not the nearby lysine cross linking site in the amino-telopeptide of the alpha 2(I) chain. She was born with bilateral hip dislocations, knee subluxations, and generalised joint hypermobility. Bilateral inguinal herniae and an umbilical hernia were present at birth. Facial features included a depressed nasal bridge with prominent paranasal folds. The skin was soft, moderately hyperelastic, and sagged over the face. Skin fragility and easy bruising were apparent from childhood. Skin wounds healed slowly and with broad, paper thin scars. Throughout her life, she had multiple fractures of the small bones of her hands and feet following moderate trauma. Electron microscopy of the proband's dermis as well as deep fascia and hip joint capsule from her affected brother showed that collagen fibrils in transverse section were nearly circular but with irregular margins. Light microscopy of bone from her affected brother and son showed normal Haversian systems and lamellar bone. All of these tissues contained approximately equal amounts of the normal and mutant alpha2(I) chains. The findings of this study confirm that loss of the amino-proteinase cleavage site of the pro alpha2(I) collagen chains, owing to anomalous splicing of exon 6 sequences in the conversion of pre-mRNA to mRNA, produces the clinical features of Ehlers-Danlos syndrome type VIIB. The history of frequent fractures found in this family is atypical and indicates an overlap with osteogenesis imperfecta.
Full text
PDF

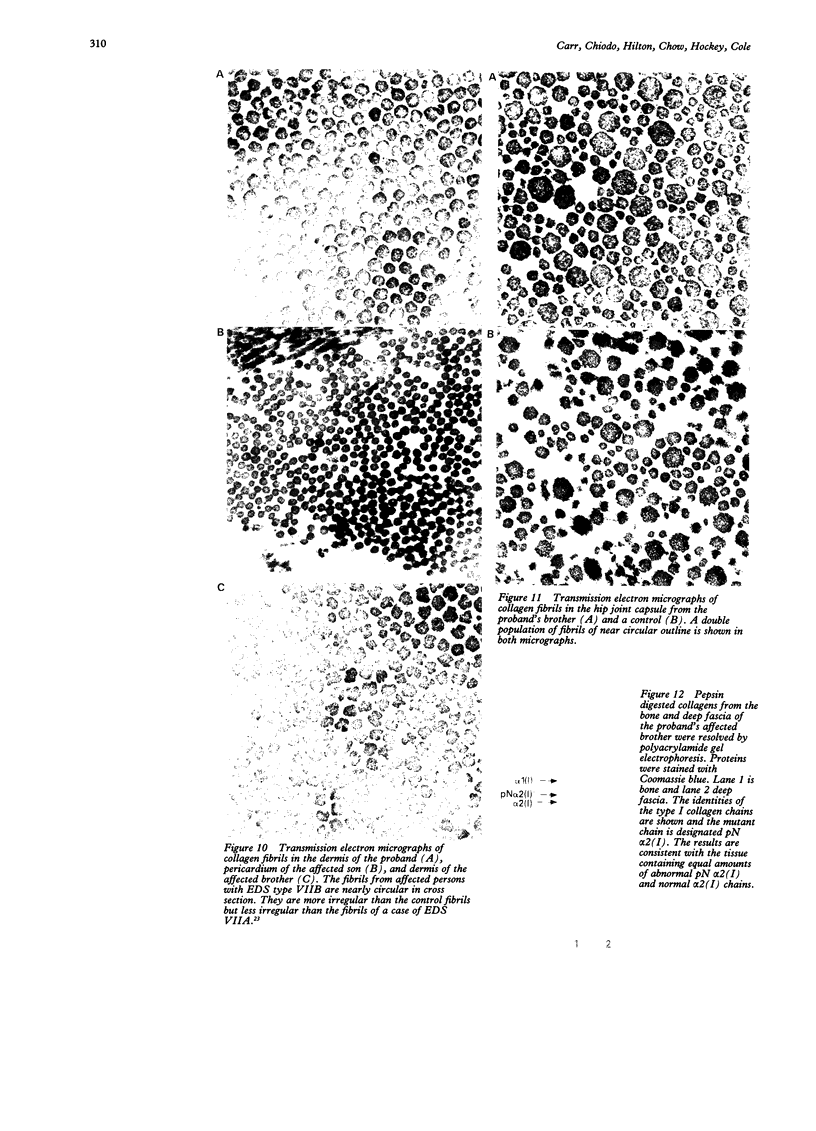

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball M. A., McCullough J. L., Weinstein G. D. Percutaneous absorption of methotrexate: effect on epidermal DNA synthesis in hairless mice. J Invest Dermatol. 1982 Jul;79(1):7–10. doi: 10.1111/1523-1747.ep12510415. [DOI] [PubMed] [Google Scholar]
- Beighton P., de Paepe A., Danks D., Finidori G., Gedde-Dahl T., Goodman R., Hall J. G., Hollister D. W., Horton W., McKusick V. A. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988 Mar;29(3):581–594. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Shapiro J. R., Rowe D. W., David K. E., Holbrook K. A. Abnormal alpha 2-chain in type I collagen from a patient with a form of osteogenesis imperfecta. J Clin Invest. 1983 Mar;71(3):689–697. doi: 10.1172/JCI110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo A. A., Hockey A., Cole W. G. A base substitution at the splice acceptor site of intron 5 of the COL1A2 gene activates a cryptic splice site within exon 6 and generates abnormal type I procollagen in a patient with Ehlers-Danlos syndrome type VII. J Biol Chem. 1992 Mar 25;267(9):6361–6369. [PubMed] [Google Scholar]
- Cole W. G., Chan D., Chambers G. W., Walker I. D., Bateman J. F. Deletion of 24 amino acids from the pro-alpha 1(I) chain of type I procollagen in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1986 Apr 25;261(12):5496–5503. [PubMed] [Google Scholar]
- Cole W. G., Evans R., Sillence D. O. The clinical features of Ehlers-Danlos syndrome type VII due to a deletion of 24 amino acids from the pro alpha 1(I) chain of type I procollagen. J Med Genet. 1987 Nov;24(11):698–701. doi: 10.1136/jmg.24.11.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts D. F., Byers P. H., Holbrook K. A., Hegreberg G. A. Dermatosparaxis in a Himalayan cat: I. Biochemical studies of dermal collagen. J Invest Dermatol. 1980 Feb;74(2):96–99. doi: 10.1111/1523-1747.ep12519991. [DOI] [PubMed] [Google Scholar]
- D'Alessio M., Bernard M., Pretorius P. J., de Wet W., Ramirez F., Pretorious P. J. Complete nucleotide sequence of the region encompassing the first twenty-five exons of the human pro alpha 1(I) collagen gene (COL1A1) Gene. 1988 Jul 15;67(1):105–115. doi: 10.1016/0378-1119(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Shapiro F. D., Aldridge J. F. A heterozygous collagen defect in a variant of the Ehlers-Danlos syndrome type VII. Evidence for a deleted amino-telopeptide domain in the pro-alpha 2(I) chain. J Biol Chem. 1985 Sep 15;260(20):11322–11329. [PubMed] [Google Scholar]
- Fjolstad M., Helle O. A hereditary dysplasia of collagen tissues in sheep. J Pathol. 1974 Mar;112(3):183–188. doi: 10.1002/path.1711120309. [DOI] [PubMed] [Google Scholar]
- Holbrook K. A., Byers P. H., Counts D. F., Hegreberg G. A. Dermatosparaxis in a Himalayan cat: II. Ultrastructural studies of dermal collagen. J Invest Dermatol. 1980 Feb;74(2):100–104. doi: 10.1111/1523-1747.ep12520000. [DOI] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Martin G. R., Kohn L. D., Byers P. H., McKusick V. A. Defect in conversion of procollagen to collagen in a form of Ehlers-Danlos syndrome. Science. 1973 Oct 19;182(4109):298–300. doi: 10.1126/science.182.4109.298. [DOI] [PubMed] [Google Scholar]
- Minor R. R., Sippola-Thiele M., McKeon J., Berger J., Prockop D. J. Defects in the processing of procollagen to collagen are demonstrable in cultured fibroblasts from patients with the Ehlers-Danlos and osteogenesis imperfecta syndromes. J Biol Chem. 1986 Jul 25;261(21):10006–10014. [PubMed] [Google Scholar]
- Nicholls A. C., Oliver J., Renouf D. V., McPheat J., Palan A., Pope F. M. Ehlers-Danlos syndrome type VII: a single base change that causes exon skipping in the type I collagen alpha 2(I) chain. Hum Genet. 1991 Jun;87(2):193–198. doi: 10.1007/BF00204180. [DOI] [PubMed] [Google Scholar]
- Nusgens B. V., Verellen-Dumoulin C., Hermanns-Lê T., De Paepe A., Nuytinck L., Piérard G. E., Lapière C. M. Evidence for a relationship between Ehlers-Danlos type VII C in humans and bovine dermatosparaxis. Nat Genet. 1992 Jun;1(3):214–217. doi: 10.1038/ng0692-214. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Wertelecki W., Milstone L. M., Petty E. M., Seashore M. R., Braverman I. M., Jenkins T. G., Byers P. H. Human dermatosparaxis: a form of Ehlers-Danlos syndrome that results from failure to remove the amino-terminal propeptide of type I procollagen. Am J Hum Genet. 1992 Aug;51(2):235–244. [PMC free article] [PubMed] [Google Scholar]
- Steinmann B., Tuderman L., Peltonen L., Martin G. R., McKusick V. A., Prockop D. J. Evidence for a structural mutation of procollagen type I in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1980 Sep 25;255(18):8887–8893. [PubMed] [Google Scholar]
- Vasan N. S., Kuivaniemi H., Vogel B. E., Minor R. R., Wootton J. A., Tromp G., Weksberg R., Prockop D. J. A mutation in the pro alpha 2(I) gene (COL1A2) for type I procollagen in Ehlers-Danlos syndrome type VII: evidence suggesting that skipping of exon 6 in RNA splicing may be a common cause of the phenotype. Am J Hum Genet. 1991 Feb;48(2):305–317. [PMC free article] [PubMed] [Google Scholar]
- Watson R. B., Wallis G. A., Holmes D. F., Viljoen D., Byers P. H., Kadler K. E. Ehlers Danlos syndrome type VIIB. Incomplete cleavage of abnormal type I procollagen by N-proteinase in vitro results in the formation of copolymers of collagen and partially cleaved pNcollagen that are near circular in cross-section. J Biol Chem. 1992 May 5;267(13):9093–9100. [PubMed] [Google Scholar]
- Weil D., Bernard M., Combates N., Wirtz M. K., Hollister D. W., Steinmann B., Ramirez F. Identification of a mutation that causes exon skipping during collagen pre-mRNA splicing in an Ehlers-Danlos syndrome variant. J Biol Chem. 1988 Jun 25;263(18):8561–8564. [PubMed] [Google Scholar]
- Weil D., D'Alessio M., Ramirez F., Eyre D. R. Structural and functional characterization of a splicing mutation in the pro-alpha 2(I) collagen gene of an Ehlers-Danlos type VII patient. J Biol Chem. 1990 Sep 15;265(26):16007–16011. [PubMed] [Google Scholar]
- Weil D., D'Alessio M., Ramirez F., Steinmann B., Wirtz M. K., Glanville R. W., Hollister D. W. Temperature-dependent expression of a collagen splicing defect in the fibroblasts of a patient with Ehlers-Danlos syndrome type VII. J Biol Chem. 1989 Oct 5;264(28):16804–16809. [PubMed] [Google Scholar]
- Weil D., D'Alessio M., Ramirez F., de Wet W., Cole W. G., Chan D., Bateman J. F. A base substitution in the exon of a collagen gene causes alternative splicing and generates a structurally abnormal polypeptide in a patient with Ehlers-Danlos syndrome type VII. EMBO J. 1989 Jun;8(6):1705–1710. doi: 10.1002/j.1460-2075.1989.tb03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz M. K., Glanville R. W., Steinmann B., Rao V. H., Hollister D. W. Ehlers-Danlos syndrome type VIIB. Deletion of 18 amino acids comprising the N-telopeptide region of a pro-alpha 2(I) chain. J Biol Chem. 1987 Dec 5;262(34):16376–16385. [PubMed] [Google Scholar]