Key Points
Question
Is there an association between sedentary behavior and risk of all-cause dementia in older adults?
Findings
In this retrospective study of prospectively collected data of 49 841 adults participating in the UK Biobank, more time spent in sedentary behaviors (determined through a machine learning–based analysis of wrist-worn accelerometer data) was significantly associated with higher risk of incident dementia.
Meaning
Among older adults, more time spent in sedentary behaviors was associated with higher risk of incident all-cause dementia.
Abstract
Importance
Sedentary behavior is associated with cardiometabolic disease and mortality, but its association with dementia is unclear.
Objective
To investigate whether accelerometer-assessed sedentary behavior is associated with incident dementia.
Design, Setting, and Participants
A retrospective study of prospectively collected data from the UK Biobank including 49 841 adults aged 60 years or older without a diagnosis of dementia at the time of wearing the wrist accelerometer and living in England, Scotland, or Wales. Follow-up began at the time of wearing the accelerometer (February 2013 to December 2015) and continued until September 2021 in England, July 2021 in Scotland, and February 2018 in Wales.
Exposures
Mean daily sedentary behavior time (included in the primary analysis) and mean daily sedentary bout length, maximum daily sedentary bout length, and mean number of daily sedentary bouts (included in the secondary analyses) were derived from a machine learning–based analysis of 1 week of wrist-worn accelerometer data.
Main Outcome and Measures
Incident all-cause dementia diagnosis from inpatient hospital records and death registry data. Cox proportional hazard models with linear and cubic spline terms were used to assess associations.
Results
A total of 49 841 older adults (mean age, 67.19 [SD, 4.29] years; 54.7% were female) were followed up for a mean of 6.72 years (SD, 0.95 years). During this time, 414 individuals were diagnosed with incident all-cause dementia. In the fully adjusted models, there was a significant nonlinear association between time spent in sedentary behavior and incident dementia. Relative to a median of 9.27 hours/d for sedentary behavior, the hazard ratios (HRs) for dementia were 1.08 (95% CI, 1.04-1.12, P < .001) for 10 hours/d, 1.63 (95% CI, 1.35-1.97, P < .001) for 12 hours/d, and 3.21 (95% CI, 2.05-5.04, P < .001) for 15 hours/d. The adjusted incidence rate of dementia per 1000 person-years was 7.49 (95% CI, 7.48-7.49) for 9.27 hours/d of sedentary behavior, 8.06 (95% CI, 7.76-8.36) for 10 hours/d, 12.00 (95% CI, 10.00-14.36) for 12 hours/d, and 22.74 (95% CI, 14.92-34.11) for 15 hours/d. Mean daily sedentary bout length (HR, 1.53 [95% CI, 1.03-2.27], P = .04 and 0.65 [95% CI, 0.04-1.57] more dementia cases per 1000 person-years for a 1-hour increase from the mean of 0.48 hours) and maximum daily sedentary bout length (HR, 1.15 [95% CI, 1.02-1.31], P = .02 and 0.19 [95% CI, 0.02-0.38] more dementia cases per 1000 person-years for a 1-hour increase from the mean of 1.95 hours) were significantly associated with higher risk of incident dementia. The number of sedentary bouts per day was not associated with higher risk of incident dementia (HR, 1.00 [95% CI, 0.99-1.01], P = .89). In the sensitivity analyses, after adjustment for time spent in sedentary behavior, the mean daily sedentary bout length and the maximum daily sedentary bout length were no longer significantly associated with incident dementia.
Conclusions and Relevance
Among older adults, more time spent in sedentary behaviors was significantly associated with higher incidence of all-cause dementia. Future research is needed to determine whether the association between sedentary behavior and risk of dementia is causal.
This study of prospectively collected data from the UK Biobank investigates whether accelerometer-assessed sedentary behavior is associated with incident dementia in adults aged 60 years or older without a diagnosis of dementia at the time of wearing the wrist accelerometer.
Introduction
Half of US adults spend more than 9.5 hours of their day sitting, including more than 80% of their leisure time.1 Previous work has detailed the potential links between sedentary behaviors and a range of health risks,2,3 including associations with both cognitive and structural brain aging.4,5 A sedentary behavior in this context is defined as “any waking behavior characterized by an energy expenditure ≤1.5 METs [metabolic equivalent units] while in a sitting or reclining posture.”6 In general, these include behaviors like sitting while using a computer, watching television, and driving.
Previous work found that self-reported leisure-time sedentary behaviors were associated with risk of developing all-cause dementia, and the direction of the risk depended on the activity done while sedentary (ie, cognitively passive TV watching vs cognitively active computer use).7 However, a recent study using accelerometer-derived sitting time did not find an association between sedentary behaviors and incident dementia in a sample of older women (n = 1277).8
In the present study, a machine learning algorithm was applied to wearable accelerometry data from a large cohort of older adults in the UK Biobank to derive an objective measure of time spent sedentary and to determine any associations between sedentary behaviors and incident dementia.
Methods
Study Design and Participants
Data from the UK Biobank (community-dwelling adults living in England, Scotland, or Wales) were used; the baseline data were collected between 2006 and 2010.9 All participants provided written informed consent and study approval was obtained from the National Health Service and the National Research Ethics Service. In a substudy10 conducted from 2013 to 2015, there were 103 684 adults who agreed to wear a 3-axis logging accelerometer (AX3; Axivity) for 24 hours per day for 7 days on their dominant wrist.
The current analysis was restricted to individuals who participated in the accelerometer substudy and were free of all-cause dementia prior to participating, who had at least 3 valid days (>16 hours/d) of wear time, and who were aged 60 years or older at the time of wearing the accelerometer. Participants were followed up from the accelerometer wear date until their first dementia diagnosis (incident dementia), death, lost to follow-up date, or to the last date of hospital admission from the respective database (September 30, 2021, in England; July 31, 2021, in Scotland; and February 28, 2018, in Wales).
Exposures
Sedentary behaviors were identified from raw accelerometer data using a previously published machine learning algorithm developed and validated for use with the UK Biobank.11 The algorithm was developed from a cohort of 152 adults (aged 18-91 years) who wore the AX3 accelerometer and a wearable camera, and kept a time-use diary during daily life. The researchers annotated accelerometer data with activities from the Compendium of Physical Activities12 and trained machine-learning models to classify behaviors in 30-second time windows of accelerometer data.11
In the present study, individuals with extreme values of sedentary behavior (>18 hours/d) were excluded. Sedentary behavior bouts were defined as more than 2 consecutive 30-second epochs classified as waking sedentary behaviors (sleep was not included in sedentary behavior time). This minimum was chosen to strike a balance between making arbitrary bout length decisions and ensuring that the classification of sedentary behavior was not confined to a single accelerometer epoch.
The mean daily sedentary behavior time (hours/day) was included in the primary analysis. The sedentary behavior pattern variables of mean number of daily sedentary bouts, mean daily sedentary bout length (determined for each day separately), and maximum daily sedentary bout length (the mean of the maximum sedentary bout length determined for each day) were included in the secondary analyses.
Outcomes
Inpatient hospital records and death registry data were used to determine incident all-cause dementia diagnoses.13 The International Classification of Diseases, Ninth Revision codes and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes were used to classify participants with dementia (eTable 1 in Supplement 1).
Statistical Analysis
We conducted complete case analyses and used Cox proportional hazard regression models to examine the associations of different sedentary behavior measures with incident all-cause dementia. The models were adjusted for a range of covariates. The minimally adjusted models, which included age and sex as covariates, were evaluated first.
Next, we evaluated the fully adjusted models, which included the additional covariates (measured at the baseline examination prior to the accelerometer study) of education level, Townsend Deprivation Index, presence of the APOE ε4 allele, ethnicity, chronic conditions, self-reported health, smoking status, alcohol use, body mass index, self-reported depression, and adherence to a healthy diet (eMethods in Supplement 1 for further description of covariates). In addition to these covariates, we included device-measured moderate to vigorous intensity physical activity (defined as behaviors with energy expenditures of ≥3 METs11) derived from the machine-learning algorithm in the fully adjusted models.
The study considered ethnicity as a potential confounder when assessing the association between sedentary behavior and the incidence of dementia. Ethnicity data were obtained from participant self-report using a set of sequential branching questions with fixed categories. The proportionality of hazards assumption was tested using Schoenfeld residuals14 (P > .05 for all models).
Potential nonlinear associations of sedentary behavior variables with incident all-cause dementia were tested using likelihood ratio tests. The fully adjusted Cox proportional hazard models with only linear terms were compared with models with cubic spline terms with 3 knots (eMethods in Supplement 1). The Akaike information criterion was used to determine whether linear or nonlinear terms provided the best model fit.
Shapes of nonlinear associations were estimated using restricted cubic splines with the reference value set at the median. The reference value was set at the median to ensure that the results were less sensitive to potential outliers or extreme values in the exposure distribution. Because total sedentary behavior is highly correlated with patterns of sedentary behavior accumulation, the sedentary behavior variables were analyzed in separate models for the secondary analyses.15 In addition, an analysis was included that used quartiles of sedentary behavior as an exposure.
In the sensitivity analyses, a landmark analysis was performed by moving the start of follow-up to 4 years after the accelerometer wear date and by excluding the individuals with diagnosed dementia or those who were censored prior to this new start date to account for potential reverse causality. In the sensitivity analyses of sedentary behavior pattern variables (the number of sedentary bouts per day, the mean bout length, and the maximum sedentary bout length), we adjusted for mean daily sedentary behavior to determine whether these variables added to dementia risk beyond mean daily sedentary behavior time. In an additional sensitivity analysis, we included self-reported and device-measured sleep as covariates in separate models.
To determine whether associations were driven by extreme values, we performed a sensitivity analysis using the median absolute deviation method and excluded values when the absolute values of the difference between the value and median, divided by median absolute deviation/0.6745, was greater than 2.4.16 In another analysis, missing covariate data were imputed to determine whether the complete case analyses were biased. Multiple imputations by chained equations with 40 imputations (based on the proportion of observations with missing values) were used to impute missing values using the MICE package in R (R Foundation for Statistical Computing).17
Given the lack of control for type I error in the secondary analyses, these analyses should be considered exploratory. All analyses were performed using R version 3.6.3. Statistical significance was determined by 2-tailed tests that yielded P values <.05.
Results
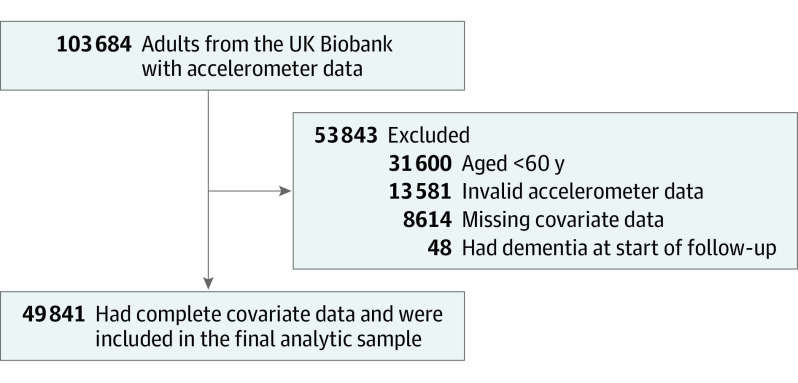
There were a total of 49 841 older adults (mean age, 67.19 [SD, 4.29] years; 54.7% were female) in the final analytic sample (Figure 1). The characteristics of the participants appear in Table 1 (the characteristics by sedentary behavior quartiles appear in eTable 2 in Supplement 1). There were 414 cases of incident dementia and more than 334 937 person-years of follow-up (mean follow-up time, 6.72 [SD, 0.95] years).
Figure 1. Flow of Study Participants.
Table 1. Cohort Characteristics From a Sample of UK Biobank Participants Wearing Accelerometersa.
| Incident dementia (n = 414) | No incident dementia (n = 49 427) | |
|---|---|---|
| Age, median (IQR), y | 71 (68 to 74) | 67 (64 to 70) |
| Sex, No. (%) | ||
| Female | 187 (45.2) | 27 053 (54.7) |
| Male | 227 (54.8) | 22 374 (45.3) |
| Education (college or higher), No. (%) | 167 (40.3) | 20 578 (41.6) |
| Townsend Deprivation Index, median (IQR)b | −2.6 (−4.0 to 0.1) | −2.7 (−3.9 to −0.6) |
| Ethnicity, No. (%)c | ||
| Asian | 3 (0.7) | 365 (0.7) |
| Black | 2 (0.5) | 139 (0.3) |
| Multiethnicd | 1 (0.2) | 146 (0.3) |
| Othere | 4 (1.0) | 197 (0.4) |
| White | 404 (97.6) | 48 580 (98.3) |
| APOE ε4 allele status, No. (%) | ||
| 1 allele | 171 (41.3) | 11 651 (23.6) |
| 2 alleles | 39 (9.4) | 1068 (2.2) |
| Body mass index, median (IQR)f | 26.2 (23.7 to 29.3) | 26.2 (23.8 to 29.0) |
| Smoking status, No. (%) | ||
| Current | 23 (5.6) | 2680 (5.4) |
| Former | 193 (46.6) | 19 855 (40.2) |
| Never | 198 (47.8) | 26 892 (54.4) |
| Alcohol consumption, No. (%)g | ||
| Excessive | 113 (27.3) | 14 923 (30.2) |
| Moderate | 187 (45.2) | 23 082 (46.7) |
| Never | 114 (27.5) | 11 422 (23.1) |
| Chronic condition present, No. (%)h | 194 (46.9) | 18 380 (37.2) |
| Self-reported health, No. (%) | ||
| Excellent | 61 (14.7) | 10 741 (21.7) |
| Good | 233 (56.3) | 30 494 (61.7) |
| Fair | 91 (22.0) | 7183 (14.5) |
| Poor | 29 (7.0) | 1009 (2.0) |
| Had depression, No. (%)i | 91 (22.0) | 8655 (17.5) |
| Adherence to a healthy diet, No. (%)j | 230 (55.6) | 28 550 (57.8) |
| Sedentary behavior, median (IQR), h/d | 9.7 (8.4 to 11.1) | 9.3 (8.1 to 10.4) |
| Sedentary bouts, median (IQR) | ||
| Frequency, bouts/d | 17.8 (15.3 to 40.9) | 17.5 (15.3 to 42.7) |
| Mean length, h/d | 0.5 (0.2 to 0.7) | 0.5 (0.2 to 0.6) |
| Maximum length, h/dk | 2.0 (1.5 to 2.5) | 1.9 (1.4 to 2.4) |
| Moderate to vigorous physical activity, median (IQR), h/d | 0.4 (0.1 to 0.8) | 0.5 (0.3 to 1.0) |
The accelerometer data were collected during a follow-up substudy (additional details appear in the Methods section). The demographic data were collected at the baseline visit (the data appear by sedentary behavior quartile in eTable 2 in Supplement 1).
A measure of socioeconomic status calculated using unemployment rate, no car ownership, no home ownership, and household overcrowding, which were obtained from census data. These variables were combined to create a single composite score, which was then standardized to have a mean of 0 and an SD of 1. Higher scores indicate higher levels of socioeconomic deprivation, whereas lower scores represent lower levels.
Obtained from self-report using a set of sequential branching questions with fixed categories.
The UK Biobank used the term mixed for participants who self-identified as multiethnic in their assessment.
Provided as a possible answer choice.
Calculated as weight in kilograms divided by height in meters squared.
Categories were determined using the methods of Lourida et al.13 Moderate defined as greater than 0 g/d to 14 g/d or less for women and greater than 0 g/d to 28 g/d or less for men.
Based on physician diagnosis of vascular or heart disease (eg, prior myocardial infarction, angina, or stroke or current high blood pressure), diabetes, or cancer.
Self-reported at baseline.
Determined using the methods of Lourida et al13 and was considered a diet that included at least 4 of the following 7 categories: (1) 3 or more servings/d of fruit; (2) 3 or more servings/d of vegetables; (3) 2 or more servings/wk of fish; (4) 1.5 servings/wk or less of unprocessed red meats; (5) 1 serving/wk or less of processed meats; (6) 3 or more servings/d of whole grains; and (7) 1.5 servings/d or less of refined grains.
Calculated as the mean of the daily maximum bout lengths.
The nonlinear model best described the relationship between mean daily sedentary behavior time and incident dementia (linear Akaike information criterion of 8217.20 and nonlinear Akaike information criterion of 8206.92 [χ21 = 12.29, P < .001]; Figure 2 and eFigures 1-2 in Supplement 1). These relationships were similar in the minimally and fully adjusted models that included time spent in device-measured moderate to vigorous intensity physical activity, and this pattern was also evident when sedentary behavior was grouped into quartiles (Table 2).
Figure 2. Associations Between Sedentary Behavior and Incident Dementia.
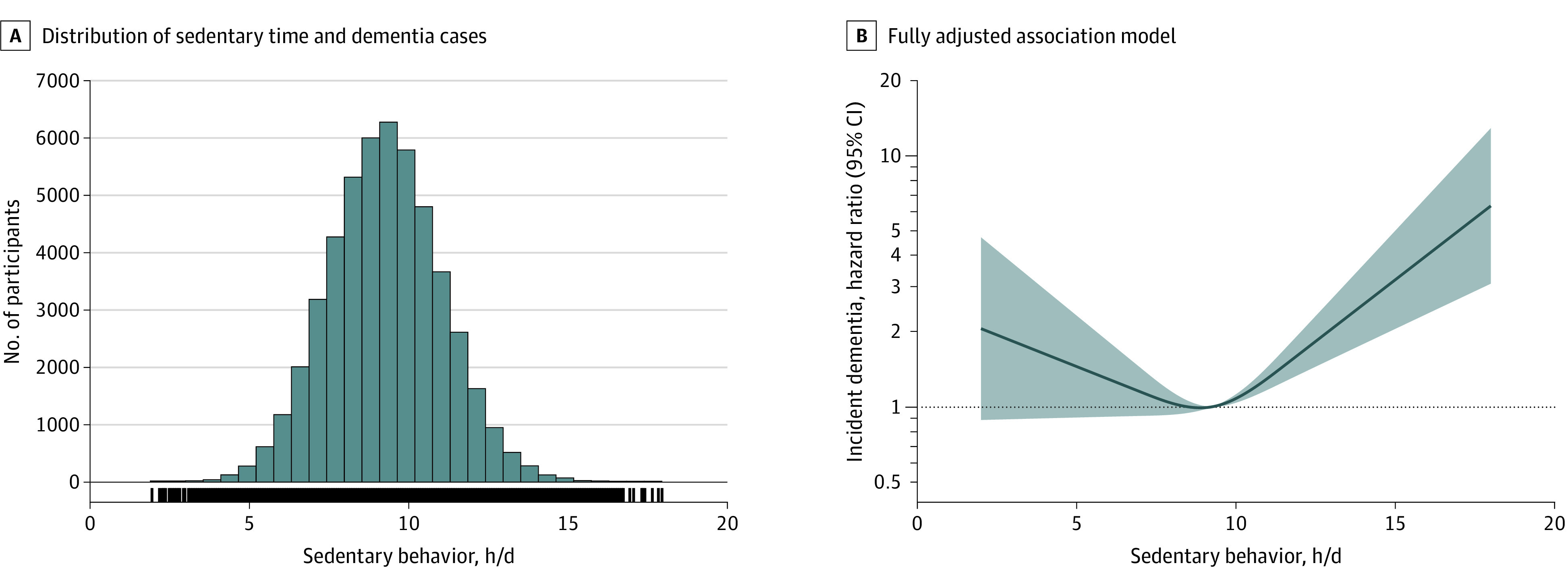
Sedentary behavior is defined as waking behaviors involving energy expenditure of 1.5 metabolic equivalent units or less while in a sitting or reclining posture and was determined using a machine learning–based analysis of 1 week of wrist-worn accelerometer data. A, Histogram shows participant counts across the range of mean daily sedentary behavior and vertical lines represent dementia cases. B, Model is fully adjusted (see Methods). The reference value (hazard ratio [HR] = 1; dotted horizontal line) was set by the median exposure variable (9.27 hours/d for sedentary behavior time) and the HRs are plotted on a log scale. The shaded areas reflect the 95% CIs for the HRs. The model depicted in part B was adjusted for age, sex, education, Townsend Deprivation Index, presence of APOE ε4 allele, ethnicity, chronic conditions (heart or vascular disease, diabetes, or cancer), self-reported health, smoking status, alcohol consumption, diet, body mass index, self-reported depression, and time spent engaged in moderate to vigorous physical activity.
Table 2. Risk of Incident Dementia According to Sedentary Behavior Quartiles.
| Sedentary behavior by quartile of mean daily time | ||||
|---|---|---|---|---|
| Quartile 1 (1.96-<8.08 h/d) | Quartile 2 (8.08-<9.27 h/d) | Quartile 3 (9.27-<10.44 h/d) | Quartile 4 (≥10.44 h/d) | |
| Incident dementia | ||||
| No. of cases | 82 | 82 | 96 | 154 |
| Person-years | 83 670 | 83 775 | 83 874 | 83 617 |
| Cases per 1000 person-years | 0.98 | 0.98 | 1.14 | 1.84 |
| Minimally adjusted modela | ||||
| HR (95% CI) | 1.11 (0.82-1.51) | 1 [Reference] | 1.14 (0.85-1.53) | 1.73 (1.32-2.27) |
| P value | .50 | .38 | <.001 | |
| Fully adjusted modelb | ||||
| HR (95% CI) | 1.14 (0.84-1.55) | 1 [Reference] | 1.09 (0.81-1.47) | 1.50 (1.14-1.99) |
| P value | .41 | .57 | .004 | |
Abbreviation: HR, hazard ratio.
Adjusted for age and sex.
Adjusted for age, sex, education, Townsend Deprivation Index, presence of APOE ε4 allele, ethnicity, chronic conditions (heart or vascular disease, diabetes, or cancer), self-reported health, smoking status, alcohol consumption, diet, body mass index, self-reported depression, and time spent engaged in moderate to vigorous physical activity.
In the nonlinear fully adjusted model, relative to a median of 9.27 hours/d for sedentary behavior, the hazard ratios (HRs) for dementia were 1.08 (95% CI, 1.04-1.12, P < .001) for 10 hours/d, 1.63 (95% CI, 1.35-1.97, P < .001) for 12 hours/d, and 3.21 (95% CI, 2.05-5.04, P < .001) for 15 hours/d. The adjusted incidence rate of dementia per 1000 person-years was 7.49 (95% CI, 7.48-7.49) for 9.27 hours/d of sedentary behavior, 8.06 (95% CI, 7.76-8.36) for 10 hours/d, 12.00 (95% CI, 10.00-14.36) for 12 hours/d, and 22.74 (95% CI, 14.92-34.11) for 15 hours/d. The HRs at the high end of sedentary behavior time included wide 95% CIs due to fewer individuals and dementia cases and should be interpreted in the context of this uncertainty.
In the linear fully adjusted model that included sedentary behavior quartiles, the reference group was the second quartile (8.08-<9.27 hours/d of sedentary behavior). The statistically significant increased HR was present only in the fourth quartile with 10.4 hours/d or greater of sedentary behavior (HR, 1.50 [95% CI, 1.14-1.99] relative to second quartile, P = .004; Table 2). The incidence rate of dementia (cases per 1000 person-years) was 0.98 for the first quartile, 0.98 for the second quartile, 1.14 for the third quartile, and 1.84 for the fourth quartile.
In the secondary analyses, the specific patterns of sedentary behavior showed no significant difference between linear and nonlinear associations with incident dementia (P > .05 for the likelihood ratio tests). For the linear relationships, the mean daily sedentary bout length (HR, 1.53 [95% CI, 1.03-2.27], P = .04 and 0.65 [95% CI, 0.04-1.57] more dementia cases per 1000 person-years for a 1-hour increase from the mean of 0.48 hours) and the maximum daily sedentary bout length (HR, 1.15 [95% CI, 1.02-1.31], P = .02 and 0.19 [95% CI, 0.02-0.38] more dementia cases per 1000 person-years for a 1-hour increase from the mean of 1.95 hours) were significantly associated with incident dementia in the fully adjusted model (Table 3). The number of bouts per day was not significantly associated with incident dementia in fully adjusted models (HR, 1.00 [95% CI, 0.99-1.01], P = .89).
Table 3. Linear Associations Between Sedentary Behavior Patterns and Incident Dementiaa.
| Mean, h | Minimally adjusted modelb | Fully adjusted modelc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence of dementia at mean (95% CI)d | Change in incidence (95% CI)e | HR (95% CI) | P value | Incidence of dementia at mean (95% CI)d | Change in incidence (95% CI)e | HR (95% CI) | P value | ||
| Sedentary bouts per day | 25.78 | 1.20 (1.03 to 1.41) | −0.001 (−0.009 to 0.006) | 1.00 (0.99 to 1.01) | .74 | 1.22 (1.03 to 1.45) | −0.001 (−0.009 to 0.008) | 1.00 (0.99 to 1.01) | .89 |
| Sedentary bout length | |||||||||
| Mean daily | 0.48 | 1.64 (1.36 to 1.97) | 0.99 (0.27 to 2.04) | 1.80 (1.22 to 2.65) | .003 | 1.51 (1.25 to 1.83) | 0.65 (0.04 to 1.57) | 1.53 (1.03 to 2.27) | .04 |
| Maximum daily | 1.95 | 1.83 (1.45 to 2.33) | 0.28 (0.10 to 0.47) | 1.22 (1.08 to 1.38) | .001 | 1.64 (1.29 to 2.09) | 0.19 (0.02 to 0.38) | 1.15 (1.02 to 1.31) | .02 |
Abbreviation: HR, hazard ratio.
There were 414 cases of dementia over 334 937 person-years.
Adjusted for age and sex.
Adjusted for age, sex, education, Townsend Deprivation Index, presence of APOE ε4 allele, ethnicity, chronic conditions (heart or vascular disease, diabetes, cancer), self-reported health, smoking status, alcohol consumption, diet, body mass index, depression, and time spent engaged in moderate to vigorous physical activity.
Calculated as dementia cases per 1000 person-years at the mean value for each variable.
Calculated as dementia cases per 1000 person-years for each additional hour of mean and maximum sedentary bout length, and for each additional sedentary bout per day.
The results were similar when including participants with more than 18 hours/d of sedentary behavior (eTable 3 and eFigure 1 in Supplement 1). The results were similar in the sensitivity analyses conducted to account for potential reverse causality (landmark analysis with 130 cases and 1483 controls excluded), in the models that adjusted for self-reported sleep, and in the models using imputed data for missing covariates (eFigures 3-4 and eTables 4-6 in Supplement 1). However, when including device-measured sleep (eTable 5 in Supplement 1) or mean daily sedentary behavior time in the models with sedentary behavior patterns as the exposure (eTable 7 in Supplement 1), the relationships between both mean daily sedentary bout length and maximum daily sedentary bout length and incident dementia were no longer significant.
Mean daily sedentary behavior time remained significantly associated with incident dementia when adjusted for sedentary behavior patterns (eFigure 5 in Supplement 1). The results for mean daily sedentary behavior time were similar in the models that excluded extreme values (25 cases and 931 controls excluded); however, the results for mean daily sedentary bout length and maximum daily sedentary bout length were not significant in these analyses (eFigure 6 and eTable 8 in Supplement 1).
Discussion
In this study, there was a nonlinear relationship between mean daily sedentary behavior time and incident dementia, with risks increasing after approximately 10 hours per day. In addition, patterns of sedentary behavior accumulation (mean and maximum daily sedentary bout lengths) were associated with increased incidence of dementia; however, these relationships were no longer significant when taking into account mean daily sedentary behavior time. In contrast, mean daily sedentary behavior time remained significantly associated with incident dementia when adjusting for patterns of sedentary behavior (mean and maximum daily sedentary bout lengths).
The mean time spent in sedentary behavior in the US in 2019 was approximately 9.5 hours,1 which falls close to the level when the risk of dementia began to increase in this study. Time spent physically active has been linked with lower risk of dementia in previous work.18,19 Similar to the results for mortality,3 the links between high levels of sedentary behavior and incident dementia remain strong when adjusting for time spent engaged in moderate to vigorous intensity physical activity. These study results complement previous work suggesting increased time in sedentary behavior is associated with reduced cognitive performance,20 and that some types of leisure-time sedentary behavior (eg, cognitively passive TV watching) are associated with increased dementia risk.7
Limitations
This study has several limitations. First, the observational study design may allow for residual or unmeasured confounding despite the large sample size and the wide range of covariates included in these analyses.
Second, although a landmark analysis was performed moving the start of follow-up to 4 years after the accelerometer wear date, reverse causality cannot be fully ruled out. Third, the use of hospital records and death registry data for dementia diagnoses without data on formal cognitive testing may provide less accurate diagnoses or may underestimate cases in this cohort.
Fourth, the accelerometer substudy was performed several years after the baseline measures were taken, and the accelerometer data were collected only once. Fifth, the participants in the accelerometer substudy were self-selected from a randomly invited subset of the larger UK Biobank cohort, which may introduce selection bias in studies of these participants. Despite the potential biases, it should be noted that the time spent in sedentary behavior in the sampled population was similar to previous estimations in US populations.1
Sixth, it is difficult to assess posture using wrist-based accelerometers and future studies should prioritize the use of thigh-mounted accelerometers to replicate these results. Seventh, the machine learning algorithm developed by Walmsley et al11 was internally validated in a sample of 152 adults that included a small subset of participants older than 60 years of age (n = 27), and future work should focus on further validating this algorithm on an external sample of older adults.
Eighth, there is a sparsity of both dementia cases and participants with very large amounts of sedentary behavior, which contributed to the wide 95% CIs. Ninth, the UK Biobank is a racially and ethnically homogeneous cohort, limiting the generalizability of these findings for other populations.
Conclusions
Among older adults, more time spent in sedentary behaviors was significantly associated with higher incidence of all-cause dementia. Future research is needed to determine whether the association between sedentary behavior and risk of dementia is causal.
Educational Objective: To identify the key insights or developments described in this article.
-
This study evaluated the association between sedentary behavior and dementia in cohort of adults aged 60 years or older. How was sedentary behavior determined?
Accelerometer detected energy expenditure ≤1.5 metabolic equivalent units while sitting or reclining.
Mobile application that randomly surveyed current activity averaging 1 question every 10 minutes during waking hours.
Participants maintained a daily journal in which they estimated time spent sitting or reclining vs standing upright or walking.
-
What did the authors find regarding the relationship between incidence of dementia and sedentary behavior?
In a fully adjusted model, more sedentary behavior was associated with greater incidence of dementia, but at the high end the 95% CIs were wide.
Sedentary behavior was found to be associated with incident dementia, but when adjusted for accelerometer-determined moderate to vigorous intensity physical activity, the hazard ratios were no longer statistically significant.
There was no statistically significant association between the 2 regardless of amount of sedentary behavior.
-
According to the authors, how does this work contribute to the understanding of sedentary behavior and dementia?
The findings confirm that patterns of sedentary behavior, including mean and maximum length of sedentary bouts, are more strongly associated with dementia than total daily sedentary time.
The mean time spent in sedentary behavior in the US, at approximately 9.5 hours, is well below the threshold for increased dementia risk.
The results complement prior work suggesting an association between sedentary behavior and reduced cognitive performance.
eMethods
eFigure 1. Associations of sedentary behavior time and incident dementia in the full sample including individuals with average daily sedentary behavior times greater than 18 hours/day
eFigure 2. Cumulative incidence of dementia across average daily sedentary behavior times (hrs)
eFigure 3. Association between sedentary behavior time and incident dementia for landmark analysis moving the start of follow-up to four years after accelerometer wear time (A) and when using imputed data (B)
eFigure 4. Association between average daily sedentary behavior time and incident dementia when including additional adjustment for A) self-reported sleep, and B) device measured sleep calculated from the machine learning algorithm
eFigure 5. Associations of sedentary behavior time and incident dementia when including an additional adjustment for A) mean sedentary bout length (hrs/day) and B) maximum sedentary bout length (hrs/day)
eFigure 6. Association between average daily sedentary behavior time and incident dementia excluding extreme values of sedentary behavior using the median absolute deviation method (MAD)
eTable 1. ICD 9 and ICD 10 codes used by the UK Biobank for determining all-cause dementia diagnoses
eTable 2. Cohort characteristics by sedentary behavior quartile
eTable 3. Associations between sedentary behavior patterns and incident dementia in the full sample including participants with average daily sedentary behavior times greater than 18 hours
eTable 4. Associations between sedentary behavior patterns and incident dementia in landmark analysis
eTable 5. Associations between sedentary behavior patterns and incident dementia with further adjustment for sleep
eTable 6. Dose-response for patterns of average daily sedentary behavior using imputed dataset
eTable 7. Associations between sedentary behavior patterns and incident dementia adjusted for average daily sedentary behavior time
eTable 8. Associations between SB patterns and incident dementia excluding extreme values of sedentary behavior using MAD method (see methods for further description)
eReference
Data sharing statement
References
- 1.Matthews CE, Carlson SA, Saint-Maurice PF, et al. Sedentary behavior in US adults: fall 2019. Med Sci Sports Exerc. 2021;53(12):2512-2519. doi: 10.1249/MSS.0000000000002751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellettiere J, LaMonte MJ, Evenson KR, et al. Sedentary behavior and cardiovascular disease in older women: the Objective Physical Activity and Cardiovascular Health (OPACH) study. Circulation. 2019;139(8):1036-1046. doi: 10.1161/CIRCULATIONAHA.118.035312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570. doi: 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakrania K, Edwardson CL, Khunti K, Bandelow S, Davies MJ, Yates T. Associations between sedentary behaviors and cognitive function: cross-sectional and prospective findings from the UK Biobank. Am J Epidemiol. 2018;187(3):441-454. doi: 10.1093/aje/kwx273 [DOI] [PubMed] [Google Scholar]
- 5.Siddarth P, Burggren AC, Eyre HA, Small GW, Merrill DA. Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PLoS One. 2018;13(4):e0195549. doi: 10.1371/journal.pone.0195549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tremblay MS, Aubert S, Barnes JD, et al. ; SBRN Terminology Consensus Project Participants . Sedentary Behavior Research Network (SBRN)–terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. doi: 10.1186/s12966-017-0525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raichlen DA, Klimentidis YC, Sayre MK, et al. Leisure-time sedentary behaviors are differentially associated with all-cause dementia regardless of engagement in physical activity. Proc Natl Acad Sci U S A. 2022;119(35):e2206931119. doi: 10.1073/pnas.2206931119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen S, LaCroix AZ, Hayden KM, et al. Accelerometer-measured physical activity and sitting with incident mild cognitive impairment or probable dementia among older women. Alzheimers Dement. 2023;19(7):3041-3054. doi: 10.1002/alz.12908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS One. 2017;12(2):e0169649. doi: 10.1371/journal.pone.0169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walmsley R, Chan S, Smith-Byrne K, et al. Reallocation of time between device-measured movement behaviours and risk of incident cardiovascular disease. Br J Sports Med. 2021;56(18):1008-1017. doi: 10.1136/bjsports-2021-104050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575-1581. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 13.Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430-437. doi: 10.1001/jama.2019.9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239-241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 15.Yerramalla MS, van Hees VT, Chen M, Fayosse A, Chastin SF, Sabia S. Objectively measured total sedentary time and pattern of sedentary accumulation in older adults: associations with incident cardiovascular disease and all-cause mortality. J Gerontol A Biol Sci Med Sci. 2022;77(4):842-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox RR. Introduction to Robust Estimation and Hypothesis Testing. 5th ed. Academic Press; 2022. [Google Scholar]
- 17.Van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 18.Del Pozo Cruz B, Ahmadi M, Naismith SL, Stamatakis E. Association of daily step count and intensity with incident dementia in 78 430 adults living in the UK. JAMA Neurol. 2022;79(10):1059-1063. doi: 10.1001/jamaneurol.2022.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabin JS, Klein H, Kirn DR, et al. Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 2019;76(10):1203-1210. doi: 10.1001/jamaneurol.2019.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falck RS, Davis JC, Liu-Ambrose T. What is the association between sedentary behaviour and cognitive function? a systematic review. Br J Sports Med. 2017;51(10):800-811. doi: 10.1136/bjsports-2015-095551 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Associations of sedentary behavior time and incident dementia in the full sample including individuals with average daily sedentary behavior times greater than 18 hours/day
eFigure 2. Cumulative incidence of dementia across average daily sedentary behavior times (hrs)
eFigure 3. Association between sedentary behavior time and incident dementia for landmark analysis moving the start of follow-up to four years after accelerometer wear time (A) and when using imputed data (B)
eFigure 4. Association between average daily sedentary behavior time and incident dementia when including additional adjustment for A) self-reported sleep, and B) device measured sleep calculated from the machine learning algorithm
eFigure 5. Associations of sedentary behavior time and incident dementia when including an additional adjustment for A) mean sedentary bout length (hrs/day) and B) maximum sedentary bout length (hrs/day)
eFigure 6. Association between average daily sedentary behavior time and incident dementia excluding extreme values of sedentary behavior using the median absolute deviation method (MAD)
eTable 1. ICD 9 and ICD 10 codes used by the UK Biobank for determining all-cause dementia diagnoses
eTable 2. Cohort characteristics by sedentary behavior quartile
eTable 3. Associations between sedentary behavior patterns and incident dementia in the full sample including participants with average daily sedentary behavior times greater than 18 hours
eTable 4. Associations between sedentary behavior patterns and incident dementia in landmark analysis
eTable 5. Associations between sedentary behavior patterns and incident dementia with further adjustment for sleep
eTable 6. Dose-response for patterns of average daily sedentary behavior using imputed dataset
eTable 7. Associations between sedentary behavior patterns and incident dementia adjusted for average daily sedentary behavior time
eTable 8. Associations between SB patterns and incident dementia excluding extreme values of sedentary behavior using MAD method (see methods for further description)
eReference
Data sharing statement