Key Points
Question
Does atrial fibrillation catheter ablation have an impact on mental health?
Findings
In this randomized clinical trial of 100 patients with symptomatic atrial fibrillation, catheter ablation with reduction in arrhythmia burden and antiarrhythmic drug use was associated with improvement in markers of psychological distress compared with medical therapy alone.
Meaning
Improvement of severe psychological distress may be an additional benefit of catheter ablation treatment for atrial fibrillation.
Abstract
Importance
The impact of atrial fibrillation (AF) catheter ablation on mental health outcomes is not well understood.
Objective
To determine whether AF catheter ablation is associated with greater improvements in markers of psychological distress compared with medical therapy alone.
Design, Setting, and Participants
The Randomized Evaluation of the Impact of Catheter Ablation on Psychological Distress in Atrial Fibrillation (REMEDIAL) study was a randomized trial of symptomatic participants conducted in 2 AF centers in Australia between June 2018 and March 2021.
Interventions
Participants were randomized to receive AF catheter ablation (n = 52) or medical therapy (n = 48).
Main Outcomes and Measures
The primary outcome was Hospital Anxiety and Depression Scale (HADS) score at 12 months. Secondary outcomes included follow-up assessments of prevalence of severe psychological distress (HADS score >15), anxiety HADS score, depression HADS score, and Beck Depression Inventory-II (BDI-II) score. Arrhythmia recurrence and AF burden data were also analyzed.
Results
A total of 100 participants were randomized (mean age, 59 [12] years; 31 [32%] women; 54% with paroxysmal AF). Successful pulmonary vein isolation was achieved in all participants in the ablation group. The combined HADS score was lower in the ablation group vs the medical group at 6 months (8.2 [5.4] vs 11.9 [7.2]; P = .006) and at 12 months (7.6 [5.3] vs 11.8 [8.6]; between-group difference, −4.17 [95% CI, −7.04 to −1.31]; P = .005). Similarly, the prevalence of severe psychological distress was lower in the ablation group vs the medical therapy group at 6 months (14.2% vs 34%; P = .02) and at 12 months (10.2% vs 31.9%; P = .01), as was the anxiety HADS score at 6 months (4.7 [3.2] vs 6.4 [3.9]; P = .02) and 12 months (4.5 [3.3] vs 6.6 [4.8]; P = .02); the depression HADS score at 3 months (3.7 [2.6] vs 5.2 [4.0]; P = .047), 6 months (3.4 [2.7] vs 5.5 [3.9]; P = .004), and 12 months (3.1 [2.6] vs 5.2 [3.9]; P = .004); and the BDI-II score at 6 months (7.2 [6.1] vs 11.5 [9.0]; P = .01) and 12 months (6.6 [7.2] vs 10.9 [8.2]; P = .01). The median (IQR) AF burden in the ablation group was lower than in the medical therapy group (0% [0%-3.22%] vs 15.5% [1.0%-45.9%]; P < .001).
Conclusion and Relevance
In this trial of participants with symptomatic AF, improvement in psychological symptoms of anxiety and depression was observed with catheter ablation, but not medical therapy.
Trial Registration
ANZCTR Identifier: ACTRN12618000062224
This randomized clinical trial evaluates the prevalence of psychological distress in participants referred for atrial fibrillation treatment.
Introduction
Observational studies have shed light on the relationship between atrial fibrillation (AF) and mental health.1,2,3 A 2018 report suggested that nearly one-third of patients with AF referred for AF management experienced severe depression and anxiety.3 Catheter ablation has been demonstrated to be superior to medical therapy alone in relieving the physical symptoms of AF and improving health-related quality of life among patients with AF.4,5,6 A number of nonrandomized and observational studies have suggested a positive impact of catheter ablation on measures of psychological distress (anxiety and depression).3,7,8 However, no prior randomized studies have assessed the impact of catheter ablation on markers of psychological distress including depression and anxiety as the primary outcome measure. The current study evaluated the prevalence of psychological distress in participants referred for AF management and compared the impact of catheter ablation vs medical management on markers of psychological distress (depression and anxiety). It was hypothesized that AF catheter ablation would result in greater improvement in markers of psychological distress compared to medical management.
Methods
Study Design
The REMEDIAL (Randomized Evaluation of the Impact of Catheter Ablation on Psychological Distress in Atrial Fibrillation) study was an investigator-initiated, multicenter, randomized clinical trial with a blinded evaluation of outcomes. Participants were recruited from 2 specialist AF centers in Australia, and ethics committee approval was obtained at each participating institution. In regard to ethical consideration of treating symptomatic patients with AF with medical therapy, there are no randomized data showing reduction in major cardiovascular events in patients treated with ablation compared with medical therapy.9 Furthermore, medical therapy retains a class 1A recommendation in international guidelines for maintenance of sinus rhythm in patients with AF.10 Nevertheless, at the completion of 12 months of follow-up, all patients in the medical therapy group were offered an ablation. In addition, patients in the medical therapy group could request to receive ablation.
The trial was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618000062224) on January 17, 2018, prior to enrollment of the first patient.
Study Population
Participants referred for AF treatment between June 2018 and March 2020 were screened for eligibility. Eligible participants were required to have paroxysmal or persistent AF (defined per 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society Guideline for the Management of Participants with Atrial Fibrillation),11 to be eligible for receipt of at least 2 antiarrhythmic drugs, to have the capacity to provide informed consent, and to be aged 18 to 80 years. Participants receiving treatment for severe depression were excluded (Table 1). All participants provided written informed consent to participate in the study.
Table 1. Baseline Clinical Participant Characteristics .
| Characteristic | No. (%) | |
|---|---|---|
| Ablation group (n = 49) | Medical group (n = 47) | |
| Demographics | ||
| Age, mean (SD), y | 58 (13) | 60 (11) |
| Men | 32 (65) | 33 (70) |
| Women | 17 (35) | 14 (30) |
| Persistent AF | 26 (53) | 18 (38) |
| CHA2DS2VASC score, median (IQR)a | 1.5 (1-3) | 1 (0-2) |
| Comorbidities | ||
| Hypertension | 26 (53) | 26 (55) |
| Vascular disease | 13 (27) | 7 (15) |
| Heart failure | 6 (12) | 2 (4) |
| Obstructive sleep apnea | 7 (14) | 12 (26) |
| Stroke | 4 (8) | 1 (2) |
| Diabetes | 3 (6) | 2 (4) |
| Risk factors | ||
| BMI, mean (SD) | 30 (5.5) | 31 (7) |
| Regular alcohol intake | 6 (12) | 9 (19) |
| Smoking | 6 (12) | 6 (13) |
| Medication use at enrollment | ||
| Anticoagulation | 44 (90) | 40 (85) |
| β-blockers | 18 (37) | 12 (26) |
| Calcium channel blockers | 5 (10) | 5 (11) |
| Digoxin | 7 (14) | 3 (6) |
| Amiodarone | 10 (20) | 6 (13) |
| Flecainide | 15 (31) | 12 (26) |
| Sotalol | 19 (39) | 24 (51) |
CHA2DS2VASC (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke/transient ischemic attack/thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], age 65-75 years, sex category [female]) score is measured on a sliding scale ranging from 0 to 10 to estimate the thromboembolic risk in nonvalvular atrial fibrillation.
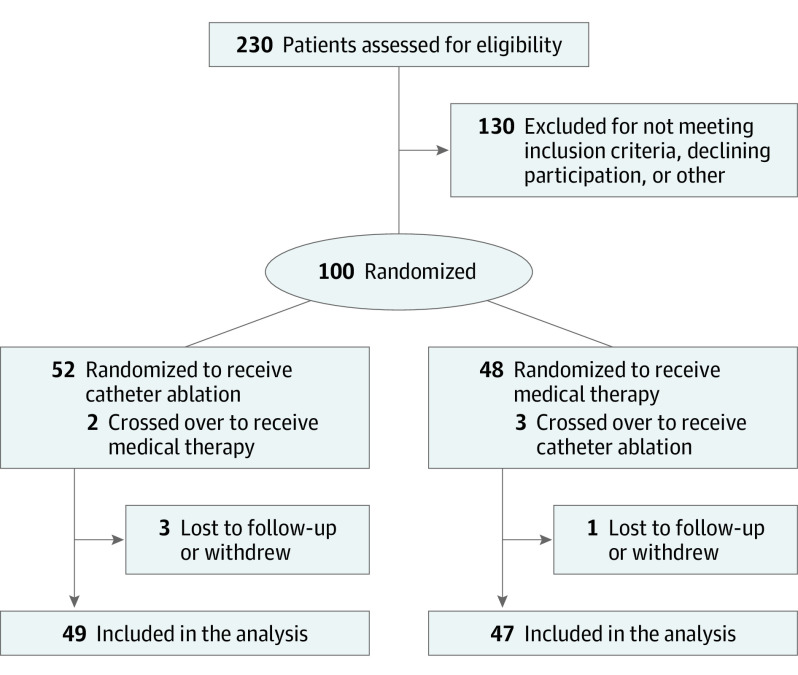
Following enrollment, participants underwent sequential randomization in a 1:1 ratio to receive medical therapy vs catheter ablation (Figure 1). Blinded randomization was achieved by using sequentially numbered, opaque sealed envelopes. Investigators following up participants and engaging in assessing psychological distress, AF symptoms, and AF burden were blinded to treatment group assignment. Crossover between the study groups was discouraged but was ultimately determined at the discretion of the treating physician.
Figure 1. Flow of Participants in a Trial of Atrial Fibrillation Catheter Ablation vs Medical Therapy and Psychological Distress.
Study Interventions
Medical Therapy
Participants randomized to receive medical therapy underwent optimization of antiarrhythmic drug (AAD) therapy in an effort to maintain sinus rhythm. If a participant was receiving a certain antiarrhythmic medication that failed to maintain sinus rhythm or was not tolerated, a second antiarrhythmic drug would be commenced and titrated to the maximal tolerated dose. Changes in medication regimens were recorded during each follow-up visit.
Catheter Ablation Procedure
Catheter ablation was performed within 1 month of randomization. All procedures were performed with patients receiving general anesthesia. Radiofrequency ablation of AF was performed with patients receiving general anesthesia with intraprocedural transesophageal echocardiogram to exclude left atrial appendage thrombus and guide transseptal access. Irrigated contact force–sensing ablation catheters (Thermocool SmartTouch ST [Biosense Webster] or TactiCath SE [Abbott Medical]) were used in all procedures.12 Ablation targets were guided by force-time integral, ablation index, or lesion size index. The goal of AF ablation was persistent isolation of all pulmonary veins. Additional ablation at the index procedure was performed at the physician’s discretion.
Oral anticoagulation was mandated for a minimum of 3 months after ablation. Antiarrhythmic drug use was continued during the 3-month blanking period after ablation then discontinued when there was no AF recurrence or low-burden asymptomatic AF.
Rhythm and AF Burden Assessment
Rhythm burden was assessed using twice-daily monitoring with the KardiaMobile (AliveCor) echocardiogram monitoring device as the primary follow-up method. In patients with an existing intracardiac monitoring device (implantable loop recorder [Reveal LINQ, Medtronic] or dual-chamber device), this device was used to evaluate burden. In patients unable to use the Alivecor device, 24-hour Holter monitoring was performed at 3, 6, and 12 months during follow-up and if symptoms occurred.
AF recurrence was defined as documented AF or atrial tachycardia more than 30 seconds’ duration. All arrhythmia recurrences after the blanking period in the trial (90 days after catheter ablation) were adjudicated by a dedicated committee consisting of clinicians specializing in cardiac arrhythmias/AF who were blinded to participant treatment group.
AF burden quantification was performed using 3 methods. For the KardiaMobile device, the time of the recorded AF event was taken as the onset of the episode, with the next recorded sinus rhythm taken as the offset. The duration of all episodes combined was then expressed as a percentage of a predefined monitoring period (ie, 0-30 days, 1-3 months, 3-6 months, 6-9 months, and 9-12 months) to derive the burden for that period.13
For the implantable cardiac device/loop recorder, the AF burden data were derived from transmitted data on remote monitoring networks or data from device interrogation during follow-up visits. Burden was recorded based on predefined monitoring periods (ie, 0-30 days, 1-3 months, 3-6 months, 6-9 months, and 9-12 months).
For the Holter monitoring method, AF burden was expressed as the percentage of time the patient was experiencing AF during a 24-hour Holter monitoring period.
Markers of Psychological Distress, Personality Traits, and Health-Related Quality of Life
Assessments of markers of psychological distress were performed at baseline (at the time of enrollment) and at 3, 6, and 12 months for all patients using the following self-administered questionnaires: the Hospital Anxiety and Depression Scale (HADS), Beck Depression Inventory-II (BDI-II) score, and Type D Scale-14.
HADS is a validated tool used for the initial diagnosis of psychological distress and to track progression (or improvement) in response to treatment in patients with physical comorbidities14,15 (eFigure in Supplement 2). The scale consists of 14 questions that assess how participants felt during the previous week. Responses are based on a 4-point Likert scale ranging from 0 to 3. Seven questions assess anxiety (range, 0-21) and 7 questions assess depression (range, 0-21). An overall combined score (depression HADS score plus anxiety HADS score) has been used to indicate overall psychological distress (range, 0-42). A combined HADS score greater than or equal to 7 indicates the presence of psychological distress and a score greater than or equal to 15 indicates severe psychological distress. An anxiety HADS score greater than or equal to 7 suggests the presence of anxiety and a depression HADS score greater than or equal to 7 suggests the presence of depression. The minimal clinically important difference for the HADS score in patients with cardiovascular disease is 1.7.16
The BDI-II is a 21-item self-reported rating inventory that measures attitudes and symptoms of depression and screens for self harm.17 The BDI-II score ranges from 0 to 63. A score of 0 to 13 suggests minimal depression; 14 to 19, mild depression; 20 to 28, moderate depression; and 29 to 63, severe depression.
Personality trait assessment was performed at baseline using the self-administered Type D Scale-14 questionnaire, a widely used diagnostic instrument for the assessment of type D personality, which has been established as a predictor of adverse clinical events and poorer cardiac prognosis in participants with heart disease.18 Patients are classified as having type D personality trait if they demonstrate both negative affectivity and social inhibition on the questionnaire.
Health-related quality of life assessments were performed for all patients using the following self-administered questionnaires: University of Toronto AF symptom severity score (AFSSS) and 36-Item Short Form Health Survey (SF-36).
AFSSS was assessed at baseline and at 3, 6, and 12 months. The score range is 0 to 35, with higher scores indicating the presence of physical symptoms of AF.
The SF-36 assessment was performed at baseline and 12 months. For each of the subscales, scores were coded and transformed to a scale ranging from 0 to 100, with higher scores indicating greater well-being. According to the SF-36 manual, a 5-point difference is defined as a “clinically and socially relevant” difference, a 10-point difference is defined as a “moderate” difference, and a 20-point difference is defined as a “very large” difference.4,19
Outcome Measures
The primary outcome was the combined anxiety and depression HADS score at 12 months.
The prespecified secondary outcomes (changes to the markers of psychological distress during follow-up) included the prevalence of severe psychological distress (defined as combined HADS score ≥15), the individual anxiety HADS score, the individual depression HADS score, and the BDI-II score.
Statistical Analysis
In a previous study of psychological distress in participants with AF,3 the mean combined HADS score (range, 0-42) was approximately 11 (indicating clinically significant symptoms). A meaningful clinical effect of catheter ablation would be to reduce the mean combined HADS score to be within the normal range, the upper limit of which is 7. This corresponds to a mean difference of 4 points. Using this mean difference and assuming an SD of 5.5, a minimum of 40 participants was required in each group for the study to reach a power of 80% with type I error of 5%. Using these data and allowing for a 20% dropout rate, 100 participants were recruited.
The analyses were based on assignment to treatments according to the principle of intention to treat. The estimand was derived from a comparison between the treatment groups in the mean scores at 12 months. Outcomes at each time (3, 6, and 12 months) were analyzed separately. The primary end point was 12 months. For most of the outcomes measured on a continuous scale, such as HADS scores, the summary statistics reported are mean (SD). For AF burden, the median (IQR) is reported, given the lack of normality. For categorical outcomes, such as severe psychological distress, the summary statistics are reported as frequencies and percentages. The Spearman correlation coefficient was used to determine the correlation between the BDI-II score and other clinical measures. Statistical inference to assess the impact of treatment was based the independent samples t test for continuous variables, except AF burden, for which the Mann-Whitney U test was used. For most continuous outcomes, such as the HADS score, a comparison of the mean score in each group (at each time) was used to describe the effect of the treatments (ablation vs medical therapy). The analysis of the treatment effect on categorical outcomes used the χ2 test.
We also analyzed the relationship between the change in HADS score at 12 months (12-month HADS score minus baseline HADS score) and AF-related outcomes including AF burden, AF recurrence, and AAD use.
P values <.05 indicated statistical significance. All statistical analyses were performed using SPSS, version 26 (IBM).
Results
A total of 100 participants were enrolled in the study. Fifty-two participants were randomized to receive catheter ablation and 48 were randomized to receive medical therapy. A total of 4 participants were lost to follow-up or withdrew from the study (1 in the medical therapy group and 3 and in the ablation group). Therefore, a total of 96 participants completed the study protocol and follow-up (49 in the ablation group and 47 in the medical group). This included 5 participants who crossed over study groups (2 from catheter ablation to medical therapy and 3 from medical therapy to catheter ablation) (Figure 1).
Baseline Patient Characteristics
The mean (SD) age for the study cohort was 59 (12) years and 31 (32%) were women. Persistent AF was present in 49% of the study cohort. There were no significant differences between the randomized parameters (Table 2), but there was a slightly higher prevalence of patients with persistent AF in the ablation group.
Table 2. Outcomes in the Atrial Fibrillation Treatment Groups.
| Measure | Mean (SD) | P value | |
|---|---|---|---|
| Ablation group (n = 49) | Medical group (n = 47) | ||
| Baseline | |||
| Combined HADS scorea | 12.3 (5.6) | 12.4 (7.1) | |
| Combined HADS score >15, No. (%) | 14 (29) | 17 (36) | |
| Anxiety HADS scoreb | 6.9 (3.8) | 6.9 (3.8) | |
| Depression HADS scorec | 5.4 (2.8) | 5.9 (3.9) | |
| BDI-II scored | 11.0 (7.7) | 12.7 (9.2) | |
| AFSSSe | 15.2 (8.0) | 16.5 (7.6) | |
| Suicidal thoughts, No. (%) | 7 (15) | 1 (2) | |
| Personality D trait, No. (%)f | 14 (29) | 16 (34) | |
| 3 Months | |||
| Primary outcomeg | |||
| Combined HADS score | 8.8 (5.5) | 11.2 (7.2) | .07 |
| Secondary outcomes | |||
| Combined HADS score >15, No. (%) | 7 (14.2) | 13 (27.6) | .09 |
| Anxiety HADS score | 5.0 (3.4) | 6.1 (4.0) | .19 |
| Depression HADS score | 3.7 (2.6) | 5.2 (4.0) | .04 |
| BDI-II score | 8.5 (7.4) | 10.7 (8.5) | .20 |
| AFSSS | 8.2 (6.1) | 13.7 (7.9) | .001 |
| 6 Months | |||
| Primary outcomeg | |||
| Combined HADS score | 8.2 (5.4) | 11.9 (7.2) | .006 |
| Secondary outcomes | |||
| Combined HADS score >15, No. (%) | 7 (14.2) | 16 (34) | .01 |
| Anxiety HADS score | 4.7 (3.3) | 6.4 (3.9) | .02 |
| Depression HADS score | 3.4 (2.7) | 5.5 (3.9) | .004 |
| BDI-II score | 7.2 (6.1) | 11.5 (9.0) | .01 |
| AFSSS | 8.1 (6.1) | 12.7 (8.1) | .005 |
| 12 Months | |||
| Primary outcomeg | |||
| Combined HADS score | 7.6 (5.3) | 11.8 (8.6) | .005 |
| Secondary outcomes | |||
| Combined HADS score >15, No. (%) | 5 (10.2) | 15 (31.9) | .01 |
| Anxiety HADS score | 4.5 (3.3) | 6.6 (4.8) | .01 |
| Depression HADS score | 3.1 (2.6) | 5.2(3.9) | .004 |
| BDI-II score | 6.6 (7.2) | 10.9 (8.2) | .01 |
| AFSSS | 7.7 (6.2) | 12.2 (7.7) | .004 |
Combined Hospital Anxiety and Depression Scale (HADS) score ranges from 0 to 42. A score ≥7 indicates presence of psychological distress. A score of ≥15 indicates severe psychological distress. The minimal clinically important difference (MCID) for the HADS score in patients with cardiovascular disease is 1.7.
Anxiety HADS score ranges from 0 to 21. A score of ≥7 suggests presence of anxiety.
Depression HADS score ranges from 0 to 21. A score of ≥7 suggests presence of depression.
Beck Depression Inventory-II (BDI-II) score ranges from 0 to 63. A score of 0-13 suggests minimal depression; 14-19, mild depression; 20-28, moderate depression; and 29-63, severe depression. An MCID of 17.5% reduction in scores from baseline has been reported.
Atrial fibrillation symptom severity score (AFSSS) ranges from 0 to 35. A higher score indicates the presence of more severe physical symptoms of atrial fibrillation.
Patients are classified as having type D personality if they demonstrate both negative affectivity and social inhibition.
Outcomes at each time point were analyzed separately with no adjustment for baseline values. P value comparisons at each time point were performed using independent samples t test.
Markers of Psychological Distress
The mean (SD) combined HADS score in the entire study cohort at baseline was 12.72 (6.82). Severe psychological distress (combined HADS score >15) was present in 31 of 96 participants (32%). The mean (SD) anxiety HADS score was 7.01 (3.97) and the mean (SD) depression HADS score was 5.71 (3.62). There was no relationship detected between severity of psychological distress and the AF phenotype (persistent vs paroxysmal) (eTable 1 in Supplement 2).
Personality D Trait
At baseline, 30 of 96 participants (31%) in the study cohort were identified as manifesting type D personality. The prevalence of type D personality was similar in the ablation and medical therapy groups (29% vs 34%; P = .82).
Variables associated with severe psychological distress (HADS score >15) at baseline included the presence of type D personality and AFSSS (eTable 1 in Supplement 2). In a multivariate model that included age, sex, elevated body mass index, type D personality, and AFSSS, only type D personality and AFSSS were independent predictors of psychological distress.
The mean (SD) BDI-II score for the study cohort was 11.86 (8.45). The BDI-II score correlated with the HADS score (r = 0.74; P = <.001), severity of AF symptoms (r = 0.6; P < .001), and presence of personality D trait (r = 0.5; P < .001). Suicidal thoughts were present in 8 of 96 participants (8%).
Procedural Characteristics
The baseline rhythm was AF in 17 participants (35%) and sinus rhythm in the remainder of participants. All 49 participants in the ablation group underwent successful pulmonary vein isolation. The mean ablation time was 36 minutes,12 and there were no major complications. During follow-up, the percentage of AliveCor, loop recorder or permanent pacemaker, and Holter monitoring in each of the 2 groups showed no significant differences (eTable 2 in Supplement 2).
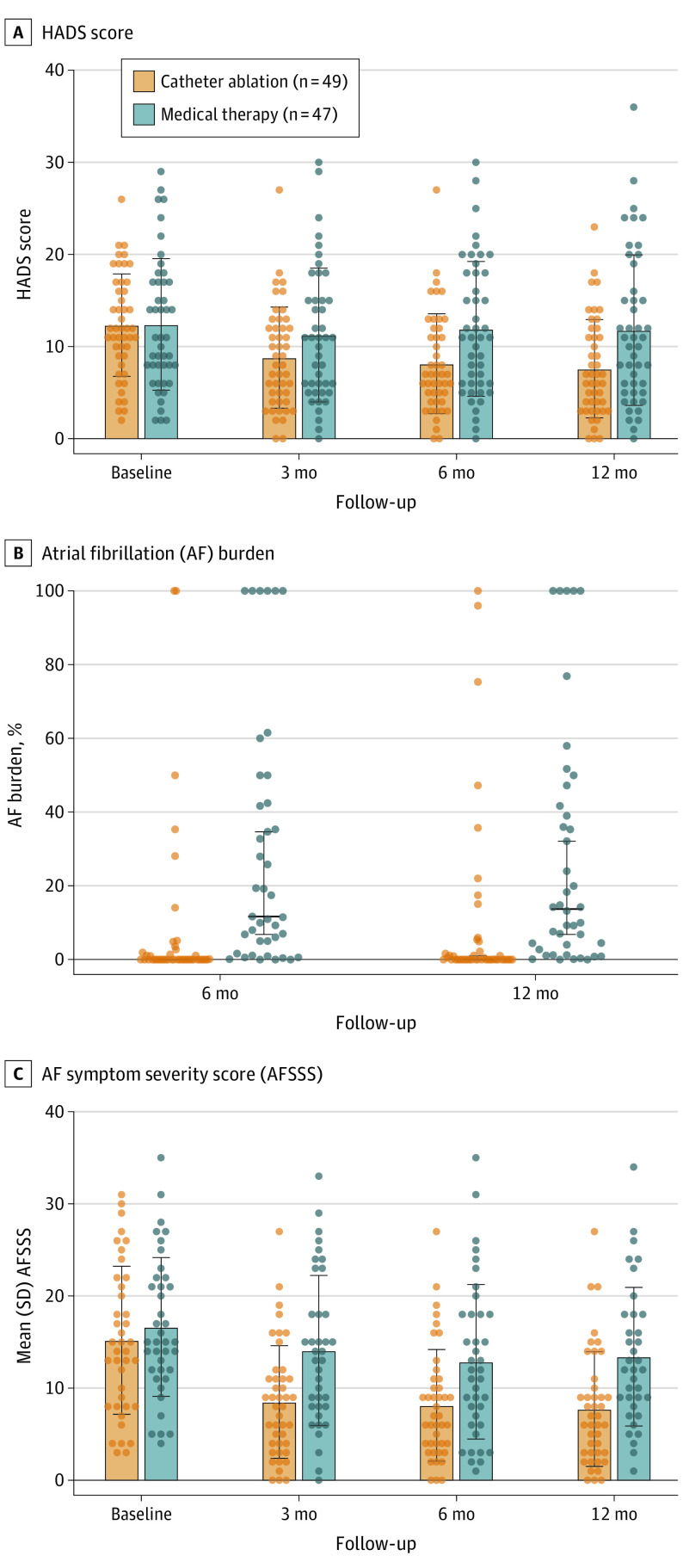
During 12 months of follow-up, 23 of 49 participants (47%) in the ablation group had documented AF during monitoring compared with 45 of 47 (96%) in the medical therapy group (P < .001). The median (IQR) AF burden in the ablation group was significantly lower compared with the medical group (0% [0%-3.22%] vs 15.5% [1%-45.9%]; P < .001) (Figure 2). The use of AADs in the ablation group reduced significantly during follow-up (90% at baseline, 53% at 3 months, and 30% at 12 months after ablation; McNemar test [between baseline and 12 months] P = .003). In 5 of 23 participants with AF recurrence, AAD use was ceased due to low-burden asymptomatic AF. In the medical group, no change in the requirement for AADs was recorded between baseline and 12 months (89% vs 85%; McNemar test P > .99). During follow-up, the use of β-blocker therapy reduced significantly from baseline to 12 months in the ablation group, but not the medical therapy group (ablation: 37% vs 4%; P < .001; medical: 26% vs 30%; P = .85).
Figure 2. Hospital Anxiety and Depression Scale Score, Atrial Fibrillation Burden, and Atrial Fibrillation Symptom Severity Score .
Outcomes
Primary Outcome
The primary outcome of the combined HADS score is shown in Table 2 and Figure 2. Compared with medical therapy, ablation was associated with a significantly lower mean (SD) combined HADS score at 6 months (11.9 [7.2] vs 8.2 [5.4]; P = .006) and 12 months (11.8 [8.6] vs 7.6 [5.3]; between-group difference, −4.17 [95% CI, −7.04 to −1.31]; P = .005).
In the ablation group, 20 participants experienced improvement in the combined HADS score between baseline and 12 months of more than 4 points, 14 participants had improvement of 2 to 4 points, and 15 had improvement of less than 2 points. In addition, both male and female participants in the ablation group demonstrated significant improvement in the combined HADS score at 12 months compared with baseline (men: −3.60 [95% CI, −6.42 to −0.77]; P = .01; women: −6.84 [95% CI, −10.60 to −3.08]; P < .001).
Secondary Outcomes
Compared with medical therapy, the ablation group demonstrated significant improvement in all prespecified secondary outcomes (Table 2).
Compared with medical therapy, the ablation group demonstrated improvement in the percentage of patients with combined HADS score of greater than or equal to 15 at 6 months (34% vs 14.2%; P = .02) and 12 months (31.9% vs 10.2%; P = .01).
In the ablation group, 10 of 14 participants with severe psychological distress at baseline demonstrated significant improvement in their combined HADS score at 12 months, while 1 participant had new severe psychological distress. In the medical therapy group, 6 of 17 participants with severe psychological distress at baseline demonstrated significant improvement in their combined HADS score at 12 months, while 4 had new severe psychological distress.
Compared with medical therapy, the ablation group had significantly lower mean (SD) anxiety HADS scores at 6 months (6.4 [3.9] vs 4.7 [3.3]; P = .02) and 12 months (6.6 [4.8] vs 4.5 [3.3]; P = .02).
Compared with medical therapy, the ablation group had significantly lower mean (SD) depression HADS scores at 3 months (5.2 [4.0] vs 3.7 [2.6]; P = .047), 6 months (5.5 [3.9] vs 3.4 [2.7]; P = .004), and 12 months (5.2 [3.9] vs 3.1 [2.6]; P = .004). Compared with medical therapy, the ablation group had significantly lower mean (SD) BDI-II score at 6 months (11.5 [9.0] vs 7.2 [6.1]; P = .01) and 12 months (10.9 [8.2] vs 6.6 [7.2]; P = .01).
Changes in Health-Related Quality of Life Assessments
Ablation was associated with significantly lower mean AFSSS compared with medical therapy at 3 months (8.2 [6.1] vs 13.7 [7.9]; P = .001), 6 months (8.1 [6.1] vs 12.7 [8.1]; P = .005), and 12 months (7.7 [6.2] vs 12.3 [7.7]; P = .004) (Table 2 and Figure 2). Scores of 4 of the 8 subscales of the SF-36, physical, emotional, vitality, and social functioning, improved in the ablation group compared with the medical group at 12 months (eTable 3 in Supplement 2).
Analyses of the Relationship Between Change in HADS Score and AF-Related Outcomes
The relationship between change in HADS score (HADS score at 12 months minus baseline) and AF burden was evaluated at 12 months using a cut-point of 0.1%, which has been previously shown to be an important marker of symptoms.20 Participants with a 12-month median AF burden less than 0.1% had significant improvement in HADS score (12 months minus baseline) vs participants with a median AF burden of greater than 0.1% (mean difference, −4.11 [95% CI, −6.30 to −1.92]; P < .001). Participants with no documented AF recurrence over 12 months of monitoring demonstrated significant improvement in the HADS score at 12 months (mean difference, −2.75 [95% CI, −5.04 to −0.46]; P = .02). Participants who were not receiving AAD therapy at 12 months experienced significant improvement in the HADS score at 12 months (12 months minus baseline) compared with participants who were receiving AAD therapy at 12 months (mean difference, −2.69 [95% CI, −4.83 to −0.56]; P = .01).
Discussion
At baseline, the prevalence of severe psychological distress (as determined by the combined HADS score) was 32%, and this correlated with both AF symptom severity and markers of personality-based vulnerability.
Effective catheter ablation with a significant and sustained reduction in AF burden and AAD usage at 12 months was associated with a highly significant improvement in markers of psychological distress (as measured by the HADS and BDI-II scores) compared with medical therapy.
Over the past decade, there has been a focus on the relationship between mental health, AF symptoms, and response to treatment. A high prevalence of depression and anxiety (up to 35%) has been observed among patients with AF in prior studies.3,21,22 In the current study, a similarly high rate (32%) of severe psychological symptoms (anxiety and depression) was observed. Importantly, a positive association was observed between psychological distress symptoms and reported AF-related symptoms. This was previously reported in a longitudinal analysis of 563 patients with AF in which depression was associated with a substantial increase in AF symptom burden (particularly uneasiness, nausea, and shortness of breath).23 This increase in AF symptom burden perception may translate to more frequent visits to seek medical care for AF.24 The relationship between AF and psychological distress could be bidirectional, whereby AF symptoms cause anxiety and depression and such psychological symptoms may amplify the perception of AF symptoms. Prior studies have shown the association between personality type D and psychological distress independent of AF symptoms.3 Thus, in patients with personality type D and AF, the dominant clinical manifestation may be psychological distress. In the current study, both type D personality and AF symptom severity were independent predictors of severe psychological distress.
The impact of catheter ablation on health-related quality of life has been a topic of extensive research in the last decade. Several randomized studies have consistently demonstrated the beneficial effects of catheter ablation over medical therapy on quality of life and symptoms of AF.4,5,6 However, there are no randomized data to date showing reduction in major cardiovascular events in patients treated with ablation compared with medical therapy.9 Furthermore, medical therapy retains a class 1A recommendation in international guidelines for maintenance of sinus rhythm in patients with atrial fibrillation.10 For these reasons, it was considered to be ethical to randomize consenting patients to undergo a 12-month trial of optimized medical therapy.
A number of nonrandomized and observational studies have suggested a positive impact of catheter ablation on measures of psychological distress (anxiety and depression).3,7,8 Interestingly, a recent nationwide registry-based study demonstrated the presence of a clear disparity between patients with AF with and without psychological distress with regards to access to rhythm control strategies including AADs, cardioversion, and catheter ablation.25
To the authors’ knowledge, this is the first randomized study assessing the impact of catheter ablation on the psychological symptoms of anxiety and depression as a primary end point. This study highlights the negative impact of AF on patients’ mental health. Compared with medical therapy, catheter ablation resulted in significant and sustained improvements in markers of psychological distress, further focusing attention on the importance of mental health assessment in patients with symptomatic AF. Importantly, this improvement was observed despite the presence of fixed personality-based vulnerability. A divergence in markers of psychological distress was observed starting at 6 months that continued to 12 months. The data indicate that low AF burden, absence of AF recurrence, and cessation of AAD/β-blocker therapy were all significantly associated with improvements in psychological distress. AADs and β-blocker therapy have been described to be associated with various neuropsychiatric effects including depression, insomnia, and fatigue.23,24 In addition, resumption of normal physical activities (as assessed by improvements in the SF-36 and AFSSS questionnaires) may have contributed to improvement in markers of psychological distress. The results of the current study support the role of catheter ablation in relieving symptoms of anxiety and depression in patients with AF.
Limitations
This study has limitations. First, participants were not blinded to treatment assignment. Therefore, the possibility of a differential procedure-related placebo effect on study outcomes could not be ruled out. A larger multicenter study with a sham group would more completely answer this question and further clarify the mechanisms associated with the improvement in mental health. Second, adherence to medical therapy among participants in the medical group was self-reported and may have not been accurately captured. Third, although intensive rhythm monitoring was utilized in the majority of the studied cohort, occult asymptomatic AF may have gone undetected.
Conclusion
In this contemporary study of patients with symptomatic AF, there was a high prevalence of psychological distress and 32% of participants experienced severe psychological symptoms of anxiety and depression. Catheter ablation was associated with a highly significant improvement in these markers of psychological distress compared with medical therapy. In patients with AF, the presence of significant psychological distress is an important factor in determining the optimal rhythm control strategy.
Trial protocol
eFigures and eTables
Data sharing statement
References
- 1.Schnabel RB, Michal M, Wilde S, et al. Depression in atrial fibrillation in the general population. PLoS One. 2013;8(12):e79109. doi: 10.1371/journal.pone.0079109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Severino P, Mariani MV, Maraone A, et al. Triggers for atrial fibrillation: the role of anxiety. Cardiol Res Pract. 2019;2019:1208505. doi: 10.1155/2019/1208505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters TE, Wick K, Tan G, et al. Psychological distress and suicidal ideation in patients with atrial fibrillation: prevalence and response to management strategy. J Am Heart Assoc. 2018;7(18):e005502. doi: 10.1161/JAHA.117.005502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Eisenhart Rothe AF, Goette A, Kirchhof P, et al. Depression in paroxysmal and persistent atrial fibrillation patients: a cross-sectional comparison of patients enroled in two large clinical trials. Europace. 2014;16(6):812-819. doi: 10.1093/europace/eut361 [DOI] [PubMed] [Google Scholar]
- 5.Blomström-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA. 2019;321(11):1059-1068. doi: 10.1001/jama.2019.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark DB, Anstrom KJ, Sheng S, et al. ; CABANA Investigators . Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1275-1285. doi: 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walfridsson H, Walfridsson U, Nielsen JC, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation: results on health-related quality of life and symptom burden: the MANTRA-PAF trial. Europace. 2015;17(2):215-221. doi: 10.1093/europace/euu342 [DOI] [PubMed] [Google Scholar]
- 8.Efremidis M, Letsas KP, Lioni L, et al. Association of quality of life, anxiety, and depression with left atrial ablation outcomes. Pacing Clin Electrophysiol. 2014;37(6):703-711. doi: 10.1111/pace.12420 [DOI] [PubMed] [Google Scholar]
- 9.Sang CH, Chen K, Pang XF, et al. Depression, anxiety, and quality of life after catheter ablation in patients with paroxysmal atrial fibrillation. Clin Cardiol. 2013;36(1):40-45. doi: 10.1002/clc.22039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlicek V, Wedegärtner SM, Millenaar D, et al. Heart-focused anxiety, general anxiety, depression and health-related quality of life in patients with atrial fibrillation undergoing pulmonary vein isolation. J Clin Med. 2022;11(7):1751. doi: 10.3390/jcm11071751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer DL, Mark DB, Robb RA, et al. ; CABANA Investigators . Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. doi: 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hindricks G, Potpara T, Dagres N, et al. ; ESC Scientific Document Group . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373-498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 13.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132. doi: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 14.Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. doi: 10.1056/NEJMoa1817591 [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence . Common Mental Health Disorders: Identification and Pathways to Care. British Psychological Society and The Royal College of Psychiatrists; 2011. [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 17.Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev. 2019;39(6):E6-E11. doi: 10.1097/HCR.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 19.Denollet J. DS14: standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosom Med. 2005;67(1):89-97. doi: 10.1097/01.psy.0000149256.81953.49 [DOI] [PubMed] [Google Scholar]
- 20.Ware JESK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Nimrod Press; 1993. [Google Scholar]
- 21.Andrade JG. Health-care utilisation and quality of life relative to af episode duration and burden: a CIRCA-DOSE sub-study. Eur Heart J. 2022. [Google Scholar]
- 22.Thrall G, Lip GY, Carroll D, Lane D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest. 2007;132(4):1259-1264. doi: 10.1378/chest.07-0036 [DOI] [PubMed] [Google Scholar]
- 23.von Eisenhart Rothe A, Hutt F, Baumert J, et al. Depressed mood amplifies heart-related symptoms in persistent and paroxysmal atrial fibrillation patients: a longitudinal analysis–data from the German Competence Network on Atrial Fibrillation. Europace. 2015;17(9):1354-1362. doi: 10.1093/europace/euv018 [DOI] [PubMed] [Google Scholar]
- 24.Gehi AK, Sears S, Goli N, et al. Psychopathology and symptoms of atrial fibrillation: implications for therapy. J Cardiovasc Electrophysiol. 2012;23(5):473-478. doi: 10.1111/j.1540-8167.2011.02264.x [DOI] [PubMed] [Google Scholar]
- 25.Koleck TA, Mitha SA, Biviano A, et al. Exploring depressive symptoms and anxiety among patients with atrial fibrillation and/or flutter at the time of cardioversion or ablation. J Cardiovasc Nurs. 2021;36(5):470-481. doi: 10.1097/JCN.0000000000000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigures and eTables
Data sharing statement