Abstract
The dysbiosis of the oral microbiome and vascular translocation of the periodontopathic microorganism to peripheral blood can cause local and systemic extra-oral inflammation. Microorganisms associated with the subgingival biofilm are readily translocated to the peripheral circulation, generating bacteremia and endotoxemia, increasing the inflammation in the vascular endothelium and resulting in endothelial dysfunction. This review aimed to demonstrate how the dysbiosis of the oral microbiome and the translocation of oral pathogen-induced inflammation to peripheral blood may be linked to cardiovascular diseases (CVDs). The dysbiosis of the oral microbiome can regulate blood pressure and activate endothelial dysfunction. Similarly, the passage of periodontal microorganisms into the peripheral circulation and their virulence factors have been associated with a vascular compartment with a great capacity to activate endothelial cells, monocytes, macrophages, and plaquettes and increase interleukin and chemokine secretion, as well as oxidative stress. This inflammatory process is related to atherosclerosis, hypertension, thrombosis, and stroke. Therefore, oral diseases could be involved in CVDs via inflammation. The preclinic and clinical evidence suggests that periodontal disease increases the proinflammatory markers associated with endothelial dysfunction. Likewise, the evidence from clinical studies of periodontal treatment in the long term evidenced the reduction of these markers and improved overall health in patients with CVDs.
Keywords: microbiota, dysbiosis, bacteremia, Porphyromonas gingivalis, nitric oxide, cardiovascular diseases
1. Introduction
The oral microbiota fulfills essential homeostasis functions in the body. An increase in systemic nitrite can improve nitric oxide (NO) concentrations via oral microbiome by its capacity to reduce dietary nitrates; the NO is a vasodilatory solid and anti-inflammatory molecule that maintains vascular homeostasis (1). The dysbiosis of the oral microbiome with increased anaerobic Gram-negative microorganisms, such as Porphyromonas gingivalis, displaces commensal microorganisms with a high nitrate-reduction capacity (NRC) (2). However, P. gingivalis cannot reduce nitrate, which is significantly affected by the high concentration of nitrates (3). In addition, the dysbiosis of the subgingival microbiome induces the innate immune response, which impacts the vascular endothelium (4). Periodontitis is a multifactorial disease characterized by an inflammatory response that may lead to endothelial dysfunction (5, 6). The microbiome dysbiosis and the bacteremia and endotoxemia caused by periodontopathic microorganisms have been reported after tooth brushing and periodontal treatment in individuals with periodontitis (7, 8). These microorganisms can increase proinflammatory activation at a distance and are found in the arteries of patients suffering from periodontitis with atherosclerosis and a high risk of CVD (9–11). However, some concerns have arisen, such as what are these doing here? Do they cause or potentiate vascular lesions? How are they accomplishing this? This review discusses these and other topics to demonstrate the role of oral microorganisms and their potential to cause or worsen CVDs.
2. Periodontal disease: a chronic inflammatory disease
Periodontitis is a multifactorial chronic inflammatory mediated by a biofilm dysbiosis that generates diverse grades of tissue destruction (12). Severe periodontitis affects more than 700 million people worldwide, with a global prevalence of 10.8% (13). However, less severe forms of the disease affect 12%–55% of the population, with the prevalence varying by population (14). Periodontitis is among the most prevalent diseases worldwide with local and systemic consequences (15). Periodontitis is characterized clinically by migrating the junctional epithelium, allowing proliferation and cell migration on an altered connective tissue substrate, and inflammatory tissular status generating the depth of the gingival sulcus forming periodontal pockets. In periodontitis, the subgingival biofilm in the gingival sulcus causes tissue destruction, which expands to the periodontal ligament and alveolar bone, causing protein degradation and proteoglycan reduction (16). The 2018 world workshop diagnostic criteria are based on a multidimensional system including stages in ranges from 1 to 4 and depend on the severity of the disease and the complexity of its treatment and rehabilitation, considering clinical attachment loss (the connective tissue of the root cementum), percentage of bone loss, pocket depth and teeth lost due to periodontal disease as critical factors. It also has a grading system that provides information on the progression based on risk factors such as smoking, and diabetes used as modifiers for progression risk (17, 18).
2.1. Dysbiosis of oral biofilm in periodontitis and their effect on the cardiovascular system
The human oral cavity and gastrointestinal tract harbor the most abundant microbiota (19). The oral microbiome contains up to 750 species of microorganisms, including bacteria and other important microorganisms such as archaea, protozoa, fungi, and viruses. These can grow on the tongue, buccal mucosa, tonsils, and palate or hard surfaces such as teeth or dental prostheses. In a healthy environment, there is a balance between these species, with stable diversity and composition. However, this balance is disrupted in dysbiosis, resulting in a composition with an increase of commensals and a reduction in beneficial microorganisms (20).
It has been reported that the nitrites generated in the metabolism in the eubiotic microbiome contribute to systemic health, stimulating the circulatory system related to cardiometabolic health (21). This link occurs because hemoglobin sequentially oxidizes NO in the blood to NO2− and NO3−. This NO3− concentrated in the salivary glands is mixed with nitrate ingested from the diet and reduced to nitrite NO2− by nitrate reductase enzymes oral bacteria. Salivary nitrite can be enzymatically reduced to NO, nitrous oxide (N2O), or dinitrogen (N2) in the mouth by denitrifying bacteria. Commensals such as Actinobacteria, including Actinomyces and Rothia; Firmicutes, such as Veillonella and Streptococcus; Bacteroidetes, such as Prevotella and some Proteobacteria that include the genus Neisseria and Haemophilus are the most critical microorganisms in eubiotic oral microbiome who improved the vasodilator function of the endothelium. The dysbiosis associated with periodontopathic microorganisms can move them and play an essential role in cardiovascular risk (21, 22).
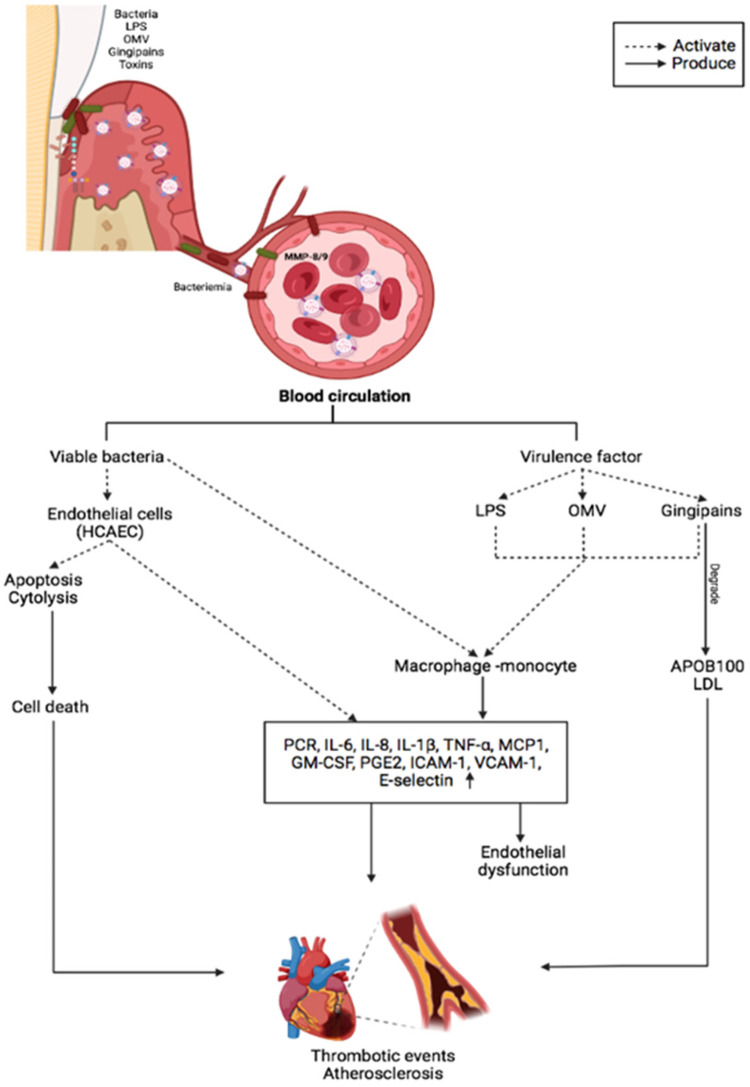
Pathobionts such as P. gingivalis, which cause dysbiosis and alter the bacterial community's relative abundances, can deregulate the inflammatory response (23–25). Subgingival microorganisms induce immune cell infiltration and inflammatory mediators in the periodontium (26, 27). Inflammatory markers can pass passively from the periodontal tissue to the peripheral circulation. Higher systemic circulating inflammatory burden of CVD risks such as interleukin (IL)-1β, IL-8, IL-6 and tumor necrosis factor-alpha (TNF-α) are directly associated with the periodontitis incipient lesions in adolescents and established periodontitis in adults (28, 29). Higher levels of circulating inflammatory markers have also been linked to patients with atherosclerosis with periodontitis (30). However, periodontal treatment reduces these biomarkers, particularly in those with CVD (31). These findings imply that periodontitis can elicit a systemic immune response that is not restricted to the localized lesion (32) (Figure 1).
Figure 1.
The main inflammatory effects of periodontopathogens and virulence factors on vascular endothelium and CVDs.
Porphyromonas species are proteolytic and degrade proteins and peptides into amino acids, which are degraded to produce short-chain fatty acids (SCFA), ammonia, sulfur compounds, and indole (22). Although SCFAs are considered metabolites that preserve endothelial function, this appears to be differential. Butyrate decreased Nlrp3 inflammasome activation in mice's carotid arterial wall after consuming a Western diet, while acetate-like propionate markedly enhanced Nlrp3 inflammasome generated an activation and carotid neointimal formation in mice in the carotid arteries (33). Due to the high metabolism of SACS in the oral microbiome, this mechanism has been proposed as another mechanism associated with increased cardiovascular risk in patients with periodontitis.
2.2. Atopobiosis of periodontopathic microorganisms and their effect on the cardiovascular system
Dysbiosis transforms the microbiome's composition into an inflammatory state, whereas atopobiosis can induce inflammation by translocating microorganisms to locations other than their usual location (34). Gut and oral dysbiosis have been linked to the pathogenesis of several immune-mediated inflammatory diseases (35, 36). The microorganisms will likely enter the bloodstream from oral niches via mechanisms that allow translocation and atopobiosis (20). Chewing, brushing, periodontal probing, scaling and root planning, and dental extractions can transfer the bacteria to capillaries and other small blood vessels, allowing bacteria to enter the systemic circulation (20, 37). P. gingivalis is the most frequent microorganism during oral bacteremia, especially in patients with periodontitis (8, 38). It is the most common microorganism in the amniotic fluid, placental tissues (39–41), brains, cerebrospinal fluid (42, 43), and vascular tissues (44, 45).
Other different routes of entry of oral organisms have been proposed, such as pathway phagocytes or dendritic cells. Many oral organisms in many tissues are related to these pathways. Periodontal pathogens such as P. gingivalis and F. nucleatum can achieve intracellular survival and disseminate to distant sites (46). P. gingivalis can invade several cell lines, including human umbilical vein endothelial cells, KB cells, and the human oral epidermoid cell line (47). P. gingivalis invasion of endothelial cells and phagocytic cells within the atheroma is also critical in atherosclerosis progression in mice (46, 48). During the invasion in endothelial cells, microvillus-like extensions are observed around bacteria, followed by the engulfment within vacuoles, using fimbriae and P. gingivalis proteases, which are essential for invasion; P. gingivalis invasion is considered an evasion mechanism of the host immune response (49).
P. gingivalis, can also colonize different oral mucosa cells, including the tongue, floor of the mouth, and buccal mucosa (50). P. gingivalis strain ATCC 33277 activates pro-survival phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT) pathway in the primary oral epithelial cells for its effective colonization; the microorganism regulates the inflammatory response promoting host cell survival through the PI3K/Akt pathway (51). P. gingivalis is associated with developing oral cancers by upregulating IL-8 and MMPs 1 and 2 levels and increasing metalloproteinase-9 stimulated by gingipains and by the induction of the PI3K/AKT signaling along with the epithelial-mesenchymal transition (EMT) (51). F. nucleatum also invades colorectal cancer cells (CRC) using galactose sugars, l-arginine, neutralizing membrane protein antibodies, or fap2 deletion and mobilizes immune cells, increasing IL-8 and CXCL1 production in tumors to generate their progression (52). Gal-GalNAc is overexpressed in CRC and recognized by Fap2 of F. nucleatum, identifies the tumors in a Fap2-dependent manner, and uses the hematogenous to reach the colorectal tumors (53).
During bacteremia, the innate response serves as the first line of defense (54). In healthy individuals, transient bacteremia of dental origin is common and is cleared rapidly and efficiently within a few minutes to an hour (55). Because of the blood speed and bacterial size, it is practically impossible for leukocytes to recognize, trap, and destroy bacteria in the bloodstream (56). Periodontal bacteria can sequester leukocytes and erythrocytes, which bind through complement 3b (C3b) via complement receptor type 1 and spread from the oral mucosa to the vascular tissues. The erythrocytes destroy the bacteria by oxidizing their membranes, and the Kupffer cells in the liver and the splenic lymphoid tissue macrophages engulf and digest them, resulting in their clearance (57). However, erythrocytes play a fundamental role in bacterial clearance due to their abundance. Therefore, they are the only cells capable of clearing the trillions of bacteria that can enter the blood during bacteremia in minutes (56). The complement system is another mechanism that aids in bacterial clearance and is an essential component of the innate immune response and is the first line of defense against bacterial infections. Despite its lack of specificity, complement selectively recognizes foreign pathogens and damaged cells damaged by using recognition molecules from the classical and alternate pathways and lectin receptors. Massive bacteremia has been observed in knockout mice lacking C5 and C3 due to their inability to induce phagocytosis or oxidative burst (58).
Recently, the atopobiosis of oral microorganisms was assessed by sequencing bacterial DNA and comparing it to subgingival biofilm samples and coronary balloons. Microbial diversity differed significantly between the two environments. However, the bacteria translocate between periodontal pockets and coronary arteries, and 17 phylotypes were identical between atheroma and subgingival samples (59). In vascular tissues, oral bacteria invade and activate endothelial cells and promote the transmigration of leukocytes that may harbor intracellular bacteria that induce the atheroma lesion formation (20). Another systemic mechanism of periodontitis is the presence of endotoxemia (60). The release of proinflammatory cytokines via lipopolysaccharide (LPS), LPS-binding protein (LBP), CD14, and toll-like receptor (TLR) activation (61). LPS-LBP is a complex that plays a crucial role in the innate immune response to bacterial challenges in the systemic and local environment by activating proinflammatory cytokines (62).
3. Periodontopathogens and CVD risk
Studies indicate that the adverse cardiovascular effects are due to a few putative or high-risk bacteria: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola or Fusobacterium nucleatum (63). P. gingivalis, and its virulence factors such as fimbriae (64, 65), LPS (66), gingipains (67), and outer membrane vesicles (OMVs) (68), A. actinomycetemcomitans with factors such as Leukotoxin (LtxA), cytolethal distending toxin (CDT), LPS and OMVs (69), and other microorganisms of dental biofilm as F. nucleatum, and Eikenella corrodens, and their virulence factors, migrate into the bloodstream and affect tissues and organs (7, 70, 71). Once in the systemic circulation, periodontal bacteria can be transported to distant sites, freely in the circulation or within circulating cells (36). Bacterial invasion can indirectly induce endothelial activation or generate endothelial dysfunction via systemic inflammation with increased acute-phase plasma proteins and proinflammatory cytokines. Furthermore, releasing bacterial products, such as OMVs, gingipains, or free soluble components, into circulation can induce proatherogenic responses in endothelial cells. Immune activation by the pathogen-derived GroEL heat shock protein (HSP) can also lead to an autoimmune response due to structural similarity between host HSP60 and GroEL (72, 73). F. nucleatum has been implicated in CVDs and is frequently detected in atherosclerotic plaques, as well as in a ruptured brain aneurysm (74). Genetic DNA from P. gingivalis and F. nucleatum in human aortic atherosclerotic lesions suggests a link between periodontal pathogens and CVDs (75).
OMVs also play a role in P. gingivalis-related systemic diseases. OMVs have been shown to activate Rho kinase in endothelial cells of umbilical veins via ERK1/2-and p38 mitogen-activated protein kinase, which may promote endothelial dysfunction and CVD (76). Preclinic experiments confirmed that P. gingivalis OMVs significantly increase vascular permeability. P. gingivalis can generate edema by altering the endothelial cell junctions such as platelet endothelial cell adhesion molecule-1 (77). The protease activity of P. gingivalis OMVs can cleave host proteins avoiding the immune response by degrading proinflammatory cytokines and disruption of the extracellular matrix, altering the tissue integrity, suggesting that these vesicles may play a vital role in the vascular damage (78). OMVs also promote vascular smooth muscle cell calcification via ERK1/2-RUNX2, inducing atherosclerosis (78, 79). In vitro, studies have demonstrated that OMVs from P. gingivalis can accelerate the foam cell formation in the murine macrophage and can induce the rupture of atherosclerotic plaque. P. gingivalis 381 also degrades fibrous caps and induce the matrix metalloproteinase (MMP)-9 activity in macrophages, which is involved in plaque disruption (80). In vitro, OMVs from P. gingivalis also induce platelet aggregation, which is essential in atherosclerotic plaque formation. In patients with periodontal disease, an increase in systemic inflammation, with elevated IL-1α and MMP-9 in plasma, causes a slight but significant decrease in cardiac function, dependent on MMP-9 (81). The proteolytic and oxidative activity of P. gingivalis also increased protein oxidation forming two apoB-100 N-terminal fragments that modified the LDL, which induce cell proliferation and could influence atherosclerosis (82). Likewise, the degradation of apoB-100 by Rgps gingipain plays a crucial role in promoting atherosclerosis by P. gingivalis infection (83) (Figure 1).
4. Endothelial dysfunction mediated by periodontopathic microorganisms
The vascular endothelium comprises monolayer endothelial cells that rest the intima formed by a matrix of proteoglycan. Endothelial cells perform various functions, including vascular tone, permeability, catabolic metabolism, SMC proliferation, angiogenesis, inflammation, white cell trafficking, fibrinolysis, thrombosis, and platelet activation (84). Therefore, the endothelial injury could alter their functionality, causing atherosclerosis (85). Furthermore, endothelial dysfunction can be caused by several factors, such as hyperlipidemia, oxidative stress, and inflammation due to bacteremia and endotoxemia (86).
Both periodontitis and CVDs are chronic inflammatory diseases. Biochemical and physiological analyses, including in vitro experiments, animal models, and clinical studies, establish a significant impact of periodontal pathogens, their virulence factors, and bacterial endotoxins on CVD mechanisms such as systemic inflammation, oxidative stress, endothelial dysfunction, foam cell formation, lipid accumulation, atherothrombosis, and vascular remodeling (6, 87). However, there is insufficient evidence to conclude that periodontitis causes CVDs. In this way, some studies have shown that periodontitis contributes to CVD through systemic bacterial exposure. For instance, the meta-analysis by Mustapha et al. demonstrated that elevated markers of systemic bacterial exposure, such as IgG to P. gingivalis, are associated with coronary heart diseases in periodontal patients (88).
In a population-based cohort study, Holtfreter et al. revealed a significant association between pocket probing depth and flow-mediated dilation of the brachial artery (6). Moreover, periodontal therapy has been shown to reduce inflammatory markers in CVDs. A previous meta-analysis demonstrated a significant weighted mean difference in CRP, proinflammatory cytokines, fibrinogen, total cholesterol, high-density lipoprotein-cholesterol, and improved endothelial function after periodontal therapy (32). In this context, we can speculate that periodontal pathogens promote endothelial dysfunction mechanisms to increase CVD risk.
Lipopolysaccharides (LPS) are biomolecules found in the outer membranes of anaerobic Gram-negative microorganisms composed of a lipidic (Lipid A) and a polysaccharide (O-antigen) region. Several studies have shown that Lipid A has heterogeneous structures; for example, some bacteria, such as Escherichia coli, have Hexa-acylated lipid A, whereas oral bacteria, such as P. gingivalis, have Penta- and Tetra-acylated lipid A (89, 90). The various structures of Lipid A from LPS are associated with multiple cell recognition and innate immune responses.
LPSs bind to TLRs in host cells, and several studies have shown that the classic structure of Hexa-acylated lipid A matches TLR-4, whereas Penta- and Tetra-acylated lipid A matches TLR-2. There are controversial studies on LPSs from P. gingivalis (LPS-Pg) and their TLRs; some studies show that LPS-Pg activates cells via TLR2, whereas others show that it activates cells via TLR4. Nonetheless, LPS-Pg heterogeneity enables recognition via Penta-acylated lipid A with TLR4 and Tetra-acylated lipid A with TLR2. LPSs from atherosclerosis-associated bacteria inhibit TLR4 and active endothelial cells via TLR2, resulting in a proinflammatory atherosclerotic response mediated by chemokines and cytokines (89, 90). Endothelial secretion of IL-8, monocyte chemoattractant protein-1 (MCP-1), IL-6, and TNF-α aids in leukocyte tracking and adhesion.
In bidimensional and three-dimensional models, human coronary artery endothelial cells (HCAECs) have low inflammatory responses to LPS-Pg (91). Nonetheless, when HCAECs were exposed to multiple doses of P. gingivalis, they produced significant amounts of MCP-1, IL-6and granulocyte-macrophage colony-stimulating factor (92). Other microorganisms, such as A. actinomycetemcomitans and E. corrodens, have a greater proinflammatory capacity than P. gingivalis. Treating HCAECs with A. actinomycetemcomitans and A.a-LPS IL-8 and IL-6 secretion increased via nuclear factor kappa beta-p65 (NF-κB p65) activation. However, this inflammatory response was inhibited by rosuvastatin due to the atheroprotective factor Kruppel-like factor 2 (93). A recent study demonstrated that A. actinomycetemcomitans infection increased transforming growth factor beta 1, IL-8, MCP-1, and IL-6 secretion in HCAECs in a dose-dependent manner in a 3D model. This proinflammatory response stimulated the THP-1 monocyte activation, adhesion, and migration (94). Similarly, E. corrodens-LPS activates HCAECs pathway TLR4, ERK, and NF-kB p65, inducing a pro-atherosclerotic endothelial response and monocyte adhesion (95). Previous evidence suggests that inflammation induced by oral pathogens and microorganisms may promote endothelial dysfunction and CVD progression (Table 1). A plausible mechanism for periodontopathogens' ability to induce endothelial dysfunction associated with CVDs and stroke is depicted in Figure 1.
Table 1.
Periodontopathogens factors related to endothelial dysfunction.
| Factors | Target | Mechanisms | Outcome | References |
|---|---|---|---|---|
| Dysbiosis of oral biofilm | Endothelial cells | Alteration in the reduction of nitrites of the diet. | Hypertension | (21) |
| Dysbiosis of oral biofilm | Endothelial cells | Proinflammatory cytokines. | Endothelial dysfunction and atherosclerosis | (23–32) |
| Dysbiosis of oral biofilm | Endothelial cells | Nrlp3 inflammasome activation by SCFAs. | Endothelial dysfunction | (121) |
| Bacteremia by P. gingivalis | Leukocytes and erythrocytes receptors | Sequestration and internalization of bacteria by complement receptor. | Invasion of the vascular tissues | (57) |
| Bacteremia by oral pathogens | Endothelial cells | Expression of cell adhesion molecules and chemokines, promoting the transmigration of leukocytes | Invasion of the vascular tissue | (20) |
| Bacteremia by oral pathogens | Platelets and erythrocytes | Activation and aggregation of cells. | Clot formation and pro coagulation | (103, 104) |
|
P. gingivalis, F. nucleatum A. actinomycetemcomitans Streptococcus sanguinis |
Platelets activation | Direct binding to platelet receptors, indirect binding to fibrinogen, fibronectin, and von Willebrand factors of LPS, gingipains, and OMVs. | Procoagulation | (106, 107) |
| Bacteremia P. gingivalis, A. actinomycetemcomitans | Endothelium, foam cell | Stress oxidative, ox-LDL, ROS. | Formation of atherosclerotic plaque | (119–121) |
| Endotoxemia by LPS | Endothelial cells and monocytes. | Activation via CD14. | Secretion of pro-inflammatory cytokines, adhesion, and migration monocyte | (62, 91–95, 122) |
| OMVs and gingipains | Endothelial cell | Cleave of endothelial adhesion molecules as a PECAM-1 (CD31) and VE-cadherin (CD144). Suppressed eNOS expression by Activation of ERK1/2 and p38 MAPK. | Increased endothelium permeability in vitro | (77, 78) |
5. Macrophage–monocyte activation by periodontopathogens and cardiovascular risk
Macrophages play an active role as effectors and regulators in different phases of inflammation. Together with other cells, they are phagocytic cells that effectively modulate innate and adaptive immune responses, promoting inflammatory resolution and tissue healing (96). In periodontitis, macrophages secrete chemotactic and different cytokines such as RANTES (Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted), MCP-1, macrophage inflammatory protein (MIP)-1α, MIP-3α, MIP-1β, and IL-8, which activate and migrate leukocytes, and proinflammatory cytokines such as IL-1β, TNF-α, and IL-6, which activate osteoclastogenesis processes and the degradation of periodontal tissue (97).
OMVs are essential virulence factors secreted into the external environment and serve as a generalized secretion and transport system for other virulence factors such as fimbriae, endotoxin as LPS, and enzymes, including gingipains. The role of this bacterium and its vesicles in the host immune response and tissue destruction by P. gingivalis infection has been well investigated. They have been shown in an in vitro model using the human macrophage cell line U937 the induction of proinflammatory cytokines associated with osteoclastogenesis, alveolar bone resorption, and tissue destruction (98, 99). In atherosclerosis-related endothelial dysfunction, monocytes migrate through the endothelium, differentiate into macrophages in the subendothelial tissue, and then convert to foam cells. LPSs from periodontal pathogenic bacteria induce acceleration in foam cell formation (100). LPSs from A. actinomycetemcomitans have been shown to synergistically interact.
LPSs play a role in developing atherosclerotic and CVD, including coronary artery disease, cerebrovascular disease, peripheral artery disease, and aortic atherosclerosis, increase TLR expression and respond to TLR agonists inducing significant inflammatory lesions (101). These roles and their plasticity make macrophages attractive targets for preventing and stabilizing existing atherosclerosis (102).
6. Prothrombotic state induced by periodontopathogens
Platelets play a role in various phases of the atherosclerotic process and vascular thrombosis leading to myocardial infarction. In periodontitis, low-grade systemic inflammation contributes to platelet activation, aggregation, and a procoagulant state (103, 104). Platelet activation is associated with the decrease in the release or inactivation of nitric oxide, as well as the release of platelet agonists. Platelet-derived substances, such as nitric oxide, cytokines, growth factors, chemokines, metalloproteinases, histamine, and selectins, actively participate in immune and inflammatory reactions (105, 106).
Epidemiological studies in patients with chronic periodontitis have revealed an increase in the mean platelet volume and platelet count, which has been linked to the production of cytokines such as IL-3 or IL-6, which in turn regulates megakaryocyte ploidy, producing more giant and reactive platelets (104, 105). Using in vitro studies, animal models, and clinical studies, P. gingivalis, F. nucleatum, and A. actinomycetemcomitans have been identified as the most commonly associated periodontopathogens with platelet activation and aggregation. The following mechanisms of pathogen–platelet interaction have been described: (1) direct bacterial binding to one of the platelet receptors; (2) indirect binding via other mediators such as fibrinogen, fibronectin, and von Willebrand factor (vWF); and (3) binding of bacterial products and toxins such as LPS, gingipains, and OMVs (80, 106–108).
Pathogens have been shown to express surface proteins, allowing them to bind directly to platelets. In this regard, P. gingivalis produces kazal-type (KSPI) serine proteasa and Arg-gingipains, RgpA, and RgpB, which, when bound to hemagglutinin/adhesion, stimulate platelets via Par-1 and Par-2, producing inositol trisphosphate and intracellular calcium downstream (107). Moreover, Naito et al. demonstrated that this microorganism could express the adhesin protein Hgp44, which can activate platelets by binding to glycoprotein (GP) Ib–IX–V, a vital platelet receptor essential in primary hemostasis due to its high affinity for vWF or the FcγRIIa receptor, a potent activator of hemostasis, followed by activated platelet elimination (108). Furthermore, S. sanguinis expresses highly glycosylated serine-rich protein A, which can bind to GPIbα (109), or indirectly induce platelet aggregation via the complement pathway. This interaction can aid in bacterial destruction, but it can also activate platelets inducing procoagulant factors such as the prothrombinase complex on the cell surface generating platelet–bacteria binding by complement molecules inducing an immunological mechanism rather than a hemostatic mechanism, highlighting platelets' dual role (110).
On the other hand, platelets can modulate the responses of other cells such as leukocytes and endothelial cells, via TLR2 and the GP ligand P-selectin/P-selectin (111). Platelets express TLR1, TLR2, TLR4, TLR6, TLR8, and TLR9, and the interaction of TLR2 and TLR4 with LPS from A. actinomycetemcomitans and P. gingivalis causes platelets to release cytokines such, as IL-1, TNF-α, and soluble CD40 ligand (sCD40l) (111, 112). Plasma sCD40l can predict recurrent cardiovascular events such as myocardial infarction and stroke, and arginine gingipains (RgpA and RgpB) of P. gingivalis can cause platelet activation and aggregation. Arg-ingipains enter the circulation and increase intracellular calcium levels in platelets by activating the protease-activated receptor-1 (PAR-1) and PAR-4 receptors, activate prothrombin, factor X and protein C, promoting thrombosis via thrombin release (113, 114). The vascular responses, upregulates endothelial cell adhesion molecule expression, and increases IL-1β, TNF-α, and thromboxane secretion, resulting in macrophage recruitment, aggregation, and platelet adhesion (114). Sharma et al. demonstrated that OMVs activate platelet aggregation. In contrast, fimbria is essential in bacterial adhesion to platelets via fibrinogen bridges that bind to the integrin receptor on the platelet surface. Most likely, this is what makes OMVs interact with platelet membrane receptors, which cause platelets to be stimulated and release dense and alpha granules and aggregate (80).
Platelets also link thrombosis and inflammation by producing platelet microparticles (PMPs). PMPs can release immunomodulatory factors such as RANTES, IL-1β, and CD40l. They can also modulate the activation of inflammatory cells such as neutrophils. In addition, bacterial infection with P. gingivalis induces PMP formation (112), so more studies are required to fully understand their role and association with periodontal pathogens. Studies have demonstrated that bacteria-induced platelet aggregation differs from that induced by conventional agonists. The above is recommended as a “binary” aggregation, which means that no aggregation is observed below a specific bacterial density, and aggregation is already maximal above that density (105). Therefore, it remains controversial. Several studies demonstrate that bacterial stimulation may not result in aggregation but rather in a more specific inflammatory response by activating the platelet TLR pathway (105).
We evaluated the effect of live-P. gingivalis W83 and live-A. Actinomycetemcomitans ATCC 29522 (106 CFU/kg) in an intraperitoneal infection model for six weeks in Wistar rats (ethical act No. 657), on platelet aggregation in platelet-rich plasma (PRP) using the spectrophotometric technique of aggregometry (115), against agonists adenosine diphosphate (ADP) (10 µM), collagen (10 µg/ml), arachidonic acid (AA) (150 µg/ml) and U46619 (2.10 µM thromboxane analog). The results demonstrate that after six weeks of treatment at the cumulative induction dose of live-P. gingivalis W83 and live-A. Actinomycetemcomitans on platelet aggregation in Wistar rats significantly increases platelet aggregation against agonists AA and U46619. In turn, it was shown that both periodontopathogens inhibit platelet aggregation in the presence of collagen (Table 2), which may be related to the “binary” effect of aggregation already described (105).
Table 2.
Effect on platelet aggregation.
| Treatment | ADP (%) | Collagen (%) | AA (%) | U46619 (%) |
|---|---|---|---|---|
| Control | 97.5 | 92.5 | 98.5 | 88.5 |
| 1 | 82.5 | 44* | 129.5* | 126.3* |
| 2 | 78.3 | 58.5* | 131.5* | 136* |
Effect on platelet aggregation induced by ADP (10 µM), collagen (10 µg/ml), AA (150 µg/ml), and U46619 (10 µM) in PRP from Wistar rats treated at cumulative doses for 6 weeks with 1. live-P. gingivalis, 2. live-A. Actinomycetemcomitans. Each point represents the average ± SE of n = 5; *p < 0.05 with respect to the control (uninfected rats).
7. Endothelial oxidative stress
Oxidative stress and chronic inflammation are significant contributors to atherosclerosis. The oxidation of polyunsaturated fatty acids (PUFAs) by free radical lipid peroxidation (LPO) in lipoproteins to phospholipids and cholesterol esters forms a fatty streak in atherogenesis. LDL-containing PUFAs are prime targets for reactive oxygen species (ROS), resulting in non-enzymatic oxidation processes known as LPO (116–118). LPO can be induced by multiple endogenous factors such as lipoxygenases, cyclooxygenases, myeloperoxidases, NADPH oxidases, and ROS generated by the mitochondrial electron transport chain (118).
Vascular oxidative stress and inflammation can be induced by oral microorganisms such as P. gingivalis and A. actinomycetemcomitans (119). A. actinomycetemcomitans was used to assess LPO production in the mouse aorta (73). The increase in oxidized LDL (ox-LDL) levels, with an increase in NADPH expression or oxidative stress in the aorta of Aa-infected mice, suggests that A. actinomycetemcomitans plays a significant role in oxidative stress and LDL oxidation. In spontaneously hyperlipidemic mice with apolipoprotein E deficiency exposed to LPS and A. actinomycetemcomitans, ROS production depends on NOX (nicotinamide adenine dinucleotide phosphate oxidase) and myeloperoxidase activities, accelerating the formation of atherogenic plaques (118).
In P. gingivalis, ROS generation is dependent on the interaction of bacterial gingipain R with platelets and platelets with neutrophils, which promotes foam cell formation by increasing ox-LDL absorption (73). In addition, it contributes to the progression of atherosclerotic plaques by stimulating MCP-1 release in vascular endothelial cells via NOX-mediated superoxide anion production, followed by activation of multiple signaling pathways such as p38, c-Jun N-terminal kinase, and NF-κB (118). Recent studies have shown that P. gingivalis OMVs are systemic proinflammatory and prooxidant effectors of endothelial responses and that they upregulate proinflammatory cytokines such as TNF-α and IL-6. Their secretion has also been linked to uncontrolled oxidative stress via eNOS downregulation and iNOS upregulation, implying an uncoupling of NO synthase (120).
8. Association of periodontitis with CVDs
CVDs are the leading cause of death globally (121). CVDs are pathological disorders that damage the heart and blood vessels, including coronary heart disease, cerebrovascular disease, and peripheral artery disease associated with atherosclerosis (122). In 1989, Mattila et al. were the first to identify oral infection, especially periodontal disease, as an independent predictor of myocardial infarction risk. Since then, several observational studies on the association between periodontal disease and CVDs have been published (123). Thus far, several systematic reviews have demonstrated that periodontal disease has a minor association with CVDs, with odds ratios (ORs) ranging from 1.14 to 1.34 (124, 125). However, subgroup analyses revealed that exposure to infection for more than 15 years was associated with an increased OR of 1.67 (95% confidence interval (CI): 1.27–2.2) (124). Moreover, systemic bacterial exposure based on IgG antibodies against periodontal pathogens raises the risk to 1.75 (95% CI: 1.32–2.34) (88). When periodontitis or edentulism was used as a mortality indicator, there was an increased risk of all-cause mortality and CVD mortality. However, a higher risk of heart disease (RR: 2.58, 95% CI: 2.20–.03) and cerebrovascular diseases (RR: 3.11, 95% CI: 2.42–3.98) were detected (126). Likewise, patients with periodontitis and erectile dysfunction presented a higher number of cardiovascular adverse events adjusted by age and previous cardiovascular disease (127).
Due to the difficulty of conducting clinical trials to assess the impact of periodontal treatment, only a multicenter clinical trial known as the Periodontitis and Vascular Events (PAVE) was conducted as a pilot study (128). Although no differences in the number of cardiovascular events were observed between periodontally treated patients and control over 24 months, periodontal care exhibited a significant reduction in the percentage of individuals with elevated high-sensitivity CRP (hs-CRP) over 3 mg/L at 6 months (129). However, a larger sample size and a longer time frame are required to assess the effect of periodontal treatment on new cardiovascular events.
The relationship between inflammation and endothelial dysfunction has been widely demonstrated (130, 131). Several clinical studies later confirmed this hypothesis, revealing a critical epidemiological association between inflammatory markers such as CRP and acute ischemic events (132–134). CRP has been used as a universal marker to predict the risk of coronary heart disease in intermediate-risk individuals (135). The effects of hs-CRP on vascular risk are linear, with higher values above 3 mg/L predicting worse cardiovascular outcomes (135). Moreover, reducing this marker with anti-inflammatory drugs such as statins reduced the risk of coronary events in patients with high CRP (>2 mg/L) but normal cholesterol levels (136). CRP is produced in the liver in response to inflammatory molecules, especially IL-6, activated mainly by innate immune responses to infectious agents or secondary to systemic inflammatory diseases (135–137). However, hs-CRP is produced in the SMCs of the coronary artery due to endothelial dysfunction (138). Because CRP is not produced in the periodontal tissue, hs-CRP detected in the crevicular fluid is most likely of systemic origin (139). However, in patients with periodontitis, local proinflammatory cytokines and transient bacteremia overexpression may be associated with increased oxidative stress and CRP levels (140). Recently, Machado et al. showed that chronic and aggressive periodontitis is consistently associated with higher hs-CRP levels. Patients with aggressive periodontitis had hs-CRP levels more than 50% higher than patients with chronic periodontitis. Intensive nonsurgical periodontal treatment increased hs-CRP levels immediately, followed by a decrease after treatment. Non-intensive treatment reduced hs-CRP for up to 180 days. These findings indicate that periodontitis is associated with systemic inflammation using the serum hs-CRP levels as a marker (141).
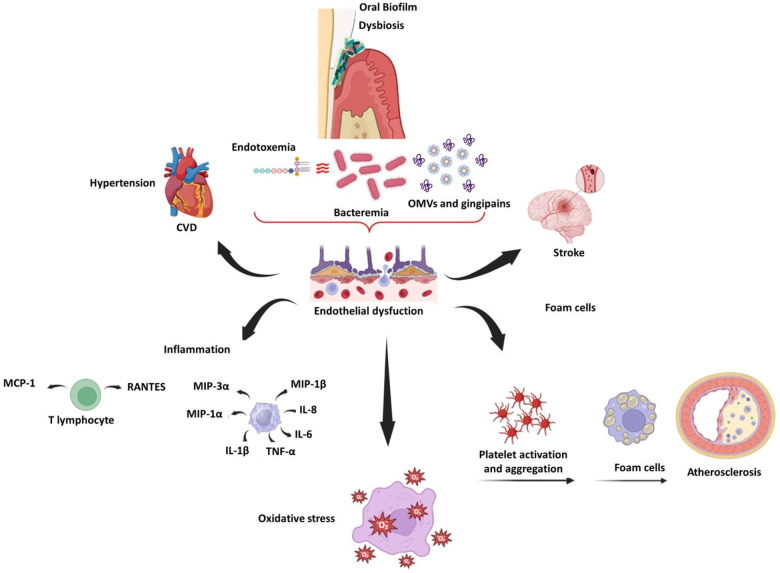
Periodontitis also induces endothelial dysfunction in rats, as evidenced by reduced eNOS expression and increased iNOS and COX-2 expression (142, 143). In humans, Tonetti et al. compared patients with severe periodontitis who received intensive periodontal treatment (IPT) to a control group who received community-based treatment. Endothelial function was assessed using flow-mediated dilatation (FMD), and inflammatory, coagulation, and endothelial activation markers were assessed before and after treatment. FMD was higher in the IPT group than in the control group by the second month. There was a significant correlation between the change in FMD and improved periodontal status. However, few changes were observed in inflammatory markers 6 months after therapy (144). After 6 months of intervention, the effects of periodontal treatment on endothelial function in patients with a recent ST-segment elevation myocardial infarction were observed in individuals with severe periodontitis (145). Similarly, periodontal therapy improved endothelial function in patients with a recent myocardial infarction without causing adverse clinical events (146). Figure 2 illustrates the main inflammatory effects of periodontopathogens and virulence factors on vascular endothelium and CVDs.
Figure 2.
Schematic representation of endothelial dysfunction related to oral dysbiosis, CVDs, and stroke.
9. Association between periodontal disease and hypertension
Another effect of periodontal disease in CVDs is arterial hypertension. Periodontitis causes inflammation and oxidative stress, which causes vascular changes such as arterial stiffness, vascular dysfunction, and hypertension (147, 148). Epidemiological observational studies have supported the association between periodontal disease and hypertension (149, 150). Moreover, IPT significantly reduced the systolic blood pressure (SBP) in patients with the periodontal disease after treatment compared to those who did not receive treatment, which correlated with periodontal status improvement. Periodontal treatment also improved diastolic pressure and endothelial function (FMD), with lower levels of circulating interferon-gamma and IL-6, as well as activated and immunosenescent CD8+ T-cells, both of which have been linked to hypertension (151, 152). In 2021, an integrative study of evidence based on eight randomized controlled trials found that IPT improved SBP and diastolic blood pressure in individuals with hypertension and prehypertension, with significant reductions in CRP and improvement in endothelial function after periodontal treatment. This evidence supports the role of periodontal treatment in improving endothelial dysfunction in patients with CVDs (149).
In a multiethnic cohort study on the direct assessment of subgingival periodontal bacterial load and its relationship to BP, which restricted clinical periodontal assessment bias, a strong positive association was demonstrated between increased subgingival colonization by, P. gingivalis, T. forsythia, T. denticola, and A. actinomycetemcomitans and arterial hypertension (148). To our knowledge, this was the first study to link bacterial load to increased blood pressure. Periodontitis is associated with systemic inflammation, the mediators can affect endothelial function (92). Clinical evidence suggests that periodontitis affects systemic endothelial function, which may affect hypertension, with some reports suggesting possible direct effects of bacteremia-related P. gingivalis in mediating vascular dysfunction and immune response, resulting in elevated BP, vascular inflammation, and endothelial dysfunction (147).
As discussed above, an imbalance in NO bioavailability associated with oral microbiome dysbiosis is associated with some cardiovascular and metabolic diseases (21, 22). A representative population study using data from the third National Health and Nutrition Examination Survey (NHANES III) demonstrate a significant association of elevated BP with antibodies against Campylobacter rectus, Veillonella parvula, and Prevotella melaninogenica. C. rectus resulted in the strongest association with BP (153). In another study, in periodontitis patients, high BP was associated with higher P. intermedia, P. gingivalis, and F. nucleatum counts (154). This dysbiosis can displace the denitrifying bacteria, reduce the NO, and alter arterial vasodilatation (21). Different publications have been realized to establish the impact of mouthwashes on the elevation of BP; The use of chlorhexidine, even at lower concentrations, inhibited the nitrate activity and the Veillonella dispar counts, however, the activity of salivary nitrite production was not affected (155). Other mouthwashes, such as essential oil, povidone-iodine, and cetylpyridinium chloride, have little effect on nitrate-reducing activity (156). The use of chlorhexidine mouthwash for one-week generated changes in the microbiome of the tongue and changes in systolic pressure in normotensive individuals. Otherwise, the individuals who cleaned the tongue regularly resulted in an enrichment of nitrate-reducing bacteria on the tongue (157). However, the tongue clean may disrupt the papillary surface and favor chlorhexidine penetration, resulting in a significant alteration of bacterial community and greater SBP (157).
Regarding the local renin–angiotensin system in gingival tissue, there may be another pathogenic link between the two conditions under investigation (147), in addition to a possible variable risk of periodontitis among different renin–angiotensin system genetic polymorphisms. Moreover, Viafara et al. demonstrated that repeated exposure to live P. gingivalis or LPSs induces the release of proinflammatory cytokines and angiotensin II in HCAECs and mediators of systemic inflammation such as CRP, IL-6, and TNF-α, contributing both to endothelial dysfunction (92). This could be linked to the Th1 response induced by bacterial antigens such as P. gingivalis by increasing sensitivity to the suppressive pro-hypertensive insult evoked by low doses of angiotensin II (147). Therefore, it supports the “two-hit” hypothesis, which states that immune activation at sites of chronic inflammation exacerbates responses to low-dose angiotensin II, establishing a link between chronic immune activation and hypertension.
10. Conclusions
Periodontitis and CVDs are both inflammatory diseases caused by the systemic circulation of periodontopathogens and their virulent factors, which can cause endothelial dysfunction via ILs, cytokines, oxidative stress, monocyte and macrophage activation, plaque aggregation, and cellular proliferation. These events could be related to CVDs such as atherosclerosis and hypertension. Periodontal treatment reduces inflammatory markers related to CVDs and may reduce the risk of CVD events. Dysbiosis of the oral microbiome in saliva and tongue in periodontal patients plays a vital role in the loss of balance that regulates blood pressure mediated by NO, which induces endothelial dysfunction. A controlled preventive therapy on other cardiovascular markers could significantly contribute to managing cardiovascular patients with periodontitis. This effect should be demonstrated in the future.
Funding Statement
The Vicerrectoria de Investigaciones-Universidad El Bosque funded this article. PCI-2017-9568 and PCI- 2019-10705.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit Care Clin. (2020) 36:307–21. 10.1016/j.ccc.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosier BT, Moya-Gonzalvez EM, Corell-Escuin P, Mira A. Isolation and characterization of nitrate-reducing bacteria as potential probiotics for oral and systemic health. Front Microbiol. (2020) 11:555465. 10.3389/fmicb.2020.555465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosier BT, Buetas E, Moya-Gonzalvez EM, Artacho A, Mira A. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep. (2020) 10(1):12895. 10.1038/s41598-020-69931-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suárez LJ, Garzón H, Arboleda S, Rodríguez A. Oral dysbiosis and autoimmunity: from local periodontal responses to an imbalanced systemic immunity. A review. Front Immunol. (2020) 11:591255. 10.3389/fimmu.2020.591255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- 6.Holtfreter B, Empen K, Gläser S, Lorbeer R, Völzke H, Ewert R, et al. Periodontitis is associated with endothelial dysfunction in a general population: a cross-sectional study. PLoS One. (2013) 8:e84603. 10.1371/journal.pone.0084603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lafaurie GI, Mayorga-Fayad I, Torres MF, Castillo DM, Aya MR, Barón A, et al. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J Clin Periodontol. (2007) 34:873–9. 10.1111/j.1600-051X.2007.01125.x [DOI] [PubMed] [Google Scholar]
- 8.Castillo DM, Sánchez-Beltrán MC, Castellanos JE, Sanz I, Mayorga-Fayad I, Sanz M, et al. Detection of specific periodontal microorganisms from bacteraemia samples after periodontal therapy using molecular-based diagnostics. J Clin Periodontol. (2011) 38:418–27. 10.1111/j.1600-051X.2011.01717.x [DOI] [PubMed] [Google Scholar]
- 9.Armingohar Z, Jørgensen JJ, Kristoffersen AK, Abesha-Belay E, Olsen I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol. (2014) 15:6. 10.3402/jom.v6.23408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. (2005) 25:e17–8. 10.1161/01.ATV.0000155018.67835.1a [DOI] [PubMed] [Google Scholar]
- 11.Reyes L, Herrera D, Kozarov E, Roldá S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Periodontol. (2013) 84:S30–50. 10.1902/jop.2013.1340012 [DOI] [PubMed] [Google Scholar]
- 12.Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. (2018) 89(Suppl 1):S74–84. 10.1002/JPER.17-0719 [DOI] [PubMed] [Google Scholar]
- 13.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. (2014) 93:1045–53. 10.1177/0022034514552491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dye BA. Global periodontal disease epidemiology. Periodontol 2000. (2012) 58:10–25. 10.1111/j.1600-0757.2011.00413.x [DOI] [PubMed] [Google Scholar]
- 15.Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. (2017) 44:456–62. 10.1111/jcpe.12732 [DOI] [PubMed] [Google Scholar]
- 16.Haapasalmi K, Mäkelä M, Oksala O, Heino J, Yamada KM, Uitto VJ, et al. Expression of epithelial adhesion proteins and integrins in chronic inflammation. Am J Pathol. (1995) 147:193–206. [PMC free article] [PubMed] [Google Scholar]
- 17.Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification. J Clin Periodontol. (2018) 45:S1–8. 10.1111/jcpe.12935 [DOI] [PubMed] [Google Scholar]
- 18.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. (2018) 89:S173–82. 10.1002/JPER.17-0721 [DOI] [PubMed] [Google Scholar]
- 19.Verma D, Garg PK, Dubey AK. Insights into the human oral microbiome. Arch Microbiol. (2018) 200:525–40. 10.1007/s00203-018-1505-3 [DOI] [PubMed] [Google Scholar]
- 20.Metsäniitty M, Hasnat S, Salo T, Salem A. Oral microbiota-A new frontier in the pathogenesis and management of head and neck cancers. Cancers (Basel). (2021) 14:46. 10.3390/cancers14010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morou-Bermúdez E, Torres-Colón JE, Bermúdez NS, Patel RP, Joshipura KJ. Pathways linking oral bacteria, nitric oxide metabolism, and health. J Dent Res. (2022) 101:623–31. 10.1177/00220345211064571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi N. Oral microbiome metabolism: from “who are they?” to “what are they doing?”. J Dent Res. (2015) 94:1628–37. 10.1177/0022034515606045 [DOI] [PubMed] [Google Scholar]
- 23.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. (2015) 15:30–44. 10.1038/nri3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajishengallis G, Krauss JL, Liang S, McIntosh ML, Lambris JD. Pathogenic microbes and community service through manipulation of innate immunity. Adv Exp Med Biol. (2012) 946:69–85. 10.1007/978-1-4614-0106-3_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajishengallis G, Diaz PI. Porphyromonas gingivalis: immune subversion activities and role in periodontal dysbiosis. Curr Oral Health Rep. (2020) 7:12–21. 10.1007/s40496-020-00249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slocum C, Kramer C, Genco CA. Immune dysregulation mediated by the oral microbiome: potential link to chronic inflammation and atherosclerosis. J Intern Med. (2016) 280:114–28. 10.1111/joim.12476 [DOI] [PubMed] [Google Scholar]
- 27.Shaik-Dasthagirisaheb YB, Huang N, Gibson FC, III. Inflammatory response to porphyromonas gingivalis partially requires interferon regulatory factor (IRF) 3. Innate Immun. (2014) 20:312–9. 10.1177/1753425913492180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palm E, Khalaf H, Bengtsson T. Porphyromonas gingivalis downregulates the immune response of fibroblasts. BMC Microbiol. (2013) 13:155. 10.1186/1471-2180-13-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro CCC, Carmo CDS, Benatti BB, Casarin RVC, Alves CMC, et al. Systemic circulating inflammatory burden and periodontitis in adolescents. Clin Oral Investig. (2021) 25:5855–65. 10.1007/s00784-021-03891-y [DOI] [PubMed] [Google Scholar]
- 30.Gomes MS, Blattner TC, Sant'Ana Filho M, Grecca FS, Hugo FN, Fouad AF, et al. Can apical periodontitis modify systemic levels of inflammatory markers? A systematic review and meta-analysis. J Endod. (2013) 39:1205–17. 10.1016/j.joen.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 31.Almeida APCPSC, Fagundes NCF, Maia LC, Lima RR. Is there an association between periodontitis and atherosclerosis in adults? A systematic review. Curr Vasc Pharmacol. (2018) 16:569–82. 10.2174/1570161115666170830141852 [DOI] [PubMed] [Google Scholar]
- 32.Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D'Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. (2014) 4:70–9. 10.1111/jcpe.12171 [DOI] [PubMed] [Google Scholar]
- 33.Yuan X, Wang L, Bhat OM, Lohner H, Li PL. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: antioxidant action of butyrate. Redox Biol. (2018) 16:21–31. 10.1016/j.redox.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potgieter M, Bester J, Kell DB, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev. (2015) 39:567–91. 10.1093/femsre/fuv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forbes JD, Chen CY, Knox NC, Marrie RA, El-Gabalawy H, de Kievit T, et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome. (2018) 6:221. 10.1186/s40168-018-0603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. (2021) 21:426–40. 10.1038/s41577-020-00488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parahitiyawa NB, Jin LJ, Leung WK, Yam WC, Samaranayake LP. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin Microbiol Rev. (2009) 22:46–64. 10.1128/CMR.00028-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horliana AC, Chambrone L, Foz AM, Artese HP, Rabelo Mde S, Pannuti CM, et al. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. (2014) 9:e98271. 10.1371/journal.pone.0098271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasegawa-Nakamura K, Tateishi F, Nakamura T, et al. The possible mechanism of preterm birth associated with periodontopathic Porphyromonas gingivalis. J Periodontal Res. (2011) 46:497–504. 10.1111/j.1600-0765.2011.01366.x [DOI] [PubMed] [Google Scholar]
- 40.Vanterpool SF, Been JV, Houben ML, Nikkels PG, De Krijger RR, Zimmermann LJ, et al. Porphyromonas gingivalis within placental villous mesenchyme and umbilical cord stroma is associated with adverse pregnancy outcome. PLoS One. (2016) 11(1):e0146157. 10.1371/journal.pone.0146157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gómez LA, De Avila J, Castillo DM, Montenegro DA, Trujillo TG, Suárez LJ, et al. Porphyromonas gingivalis placental atopobiosis and inflammatory responses in women with adverse pregnancy outcomes. Front Microbiol. (2020) 11:591626. 10.3389/fmicb.2020.591626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. (2013) 36:665–77. 10.3233/JAD-121918 [DOI] [PubMed] [Google Scholar]
- 43.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. (2019) 5:eaau3333. 10.1126/sciadv.aau3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szulc M, Kustrzycki W, Janczak D, Michalowska D, Baczynska D, Radwan-Oczko M. Presence of periodontopathic bacteria DNA in atheromatous plaques from coronary and carotid arteries. Biomed Res Int. (2015) 2015:825397. 10.1155/2015/825397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcelino SL, Jr G-JE, Nakano V, Canônico LA, Nunes FD, Lotufo RF, et al. Presence of periodontopathic bacteria in coronary arteries from patients with chronic periodontitis. Anaerobe. (2010) 16:629–32. 10.1016/j.anaerobe.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 46.Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. (2020) 83:90–106. 10.1111/prd.12304 [DOI] [PubMed] [Google Scholar]
- 47.Dorn BR, Burks JN, Seifert KN, Progulske-Fox A. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett. (2000) 187:139–44. 10.1111/j.1574-6968.2000.tb09150.x [DOI] [PubMed] [Google Scholar]
- 48.Amar S, Wu SC, Madan M. Is Porphyromonas gingivalis cell invasion required for atherogenesis? Pharmacotherapeutic implications. J Immunol. (2009) 182:1584–92. 10.4049/jimmunol.182.3.1584 [DOI] [PubMed] [Google Scholar]
- 49.Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. (1998) 66:5337–43. 10.1128/IAI.66.11.5337-5343.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yilmaz Ö. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology (Reading. (2008) 154:2897–903. 10.1099/mic.0.2008/021220-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. (2004) 72:3743–51. 10.1128/IAI.72.7.3743-3751.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casasanta MA, Yoo CC, Udayasuryan B, Sanders BE, Umaña A, Zhang Y, et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci Signal. (2020) 13:eaba9157. 10.1126/scisignal.aba9157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abed J, Emgård JE, Zamir G, Faroja M, Almogy G, Grenov A, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed gal-GalNAc. Cell Host Microbe. (2016) 20:215–25. 10.1016/j.chom.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. (2013) 14:785–92. 10.1038/ni.2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. (2008) 117:3118–25. 10.1161/CIRCULATIONAHA.107.758524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minasyan H. Erythrocyte and blood antibacterial defense. Eur J Microbiol Immunol (Bp). (2014) 4:138–43. 10.1556/EuJMI.4.2014.2.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belstrøm D, Holmstrup P, Damgaard C, Borch TS, Skjødt MO, Bendtzen K, et al. The atherogenic bacterium Porphyromonas gingivalis evades circulating phagocytes by adhering to erythrocytes. Infect Immun. (2011) 79:1559–65. 10.1128/IAI.01036-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flierl MA, Rittirsch D, Nadeau BA, Day DE, Zetoune FS, Sarma JV, et al. Functions of the complement components C3 and C5 during sepsis. FASEB J. (2008) 22:3483–90. 10.1096/fj.08-110595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serra e Silva Filho W, Casarin RC, Nicolela EL, Jr, Passos HM, Sallum AW, Gonçalves RB. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS One. (2014) 9:e109761. 10.1371/journal.pone.0109761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geerts SO, Nys M, De MP, Charpentier J, Albert A, Legrand V, et al. Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J Periodontol. (2002) 73:73–8. 10.1902/jop.2002.73.1.73 [DOI] [PubMed] [Google Scholar]
- 61.Marcano R, Rojo MÁ, Cordoba-Diaz D, Garrosa M. Pathological and therapeutic approach to endotoxin-secreting bacteria involved in periodontal disease. Toxins (Basel). (2021) 13:533. 10.3390/toxins13080533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding PH, Jin LJ. The role of lipopolysaccharide-binding protein in innate immunity: a revisit and its relevance to oral/periodontal health. J Periodontal Res. (2014) 49:1–9. 10.1111/jre.12081 [DOI] [PubMed] [Google Scholar]
- 63.Bale BF, Doneen AL, Vigerust DJ. High-risk periodontal pathogens contribute to the pathogenesis of atherosclerosis. Postgrad Med J. (2017) 93:215–20. 10.1136/postgradmedj-2016-134279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasegawa Y, Nagano K. Porphyromonas gingivalis FimA and Mfa1 fimbriae: current insights on localization, function, biogenesis, and genotype. Jpn Dent Sci Rev. (2021) 57:190–200. 10.1016/j.jdsr.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pérez-Chaparro PJ, Lafaurie GI, Gracieux P, et al. Distribution of Porphyromonas gingivalis fimA genotypes in isolates from subgingival plaque and blood sample during bacteremia. Biomedica. (2009) 29:298–306. 10.7705/biomedica.v29i2.31 [DOI] [PubMed] [Google Scholar]
- 66.Xu W, Zhou W, Wang H, Liang S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol. (2020) 120:45–84. 10.1016/bs.apcsb.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imamura T. The role of gingipains in the pathogenesis of periodontal disease. J Periodontol. (2003) 74:111–8. 10.1902/jop.2003.74.1.111 [DOI] [PubMed] [Google Scholar]
- 68.Amano A, Takeuchi H, Furuta N. Outer membrane vesicles function as offensive weapons in host-parasite interactions. Microbes Infect. (2010) 12:791–8. 10.1016/j.micinf.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 69.Belibasakis GN, Maula T, Bao K, Lindholm M, Bostanci N, Oscarsson J, et al. Virulence and pathogenicity properties of Aggregatibacter actinomycetemcomitans. Pathogens. (2019) 8:222. 10.3390/pathogens8040222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stokowa-Sołtys K, Wojtkowiak K, Jagiełło K. Fusobacterium nucleatum—friend or foe? J Inorg Biochem. (2021) 224:111586. 10.1016/j.jinorgbio.2021.111586 [DOI] [PubMed] [Google Scholar]
- 71.Umaña A, Sanders BE, Yoo CC, Casasanta MA, Udayasuryan B, Verbridge SS, et al. Utilizing whole Fusobacterium genomes to identify, correct, and characterize potential virulence protein families. J Bacteriol. (2019) 201:e00273–19. 10.1128/JB.00273-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, Liu D, Liu S, Zhang S, Pan Y. The role of Porphyromonas gingivalis outer membrane vesicles in periodontal disease and related systemic diseases. Front Cell Infect Microbiol. (2021) 10:585917. 10.3389/fcimb.2020.585917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurita-Ochiai T, Yamamoto M. Periodontal pathogens and atherosclerosis: implications of inflammation and oxidative modification of LDL. Biomed Res Int. (2014):595981. 10.1155/2014/595981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. (2015) 23:141–7. 10.1016/j.mib.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chukkapalli SS, Easwaran M, Rivera-Kweh MF, Velsko IM, Ambadapadi S, Dai J, et al. Sequential colonization of periodontal pathogens in induction of periodontal disease and atherosclerosis in LDLRnull mice. Pathog Dis. (2017) 75:ftx003. 10.1093/femspd/ftx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jia Y, Guo B, Yang W, Zhao Q, Jia W, Wu Y. Rho kinase mediates Porphyromonas gingivalis outer membrane vesicle-induced suppression of endothelial nitric oxide synthase through ERK1/2 and p38 MAPK. Arch Oral Biol. (2015) 60:488–95. 10.1016/j.archoralbio.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 77.Farrugia C, Stafford GP, Murdoch C. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J Dent Res. (2020) 99:1494–501. 10.1177/0022034520943187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang WW, Guo B, Jia WY, Jia Y. Porphyromonas gingivalis-derived outer membrane vesicles promote calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Open Bio. (2016) 6:1310–9. 10.1002/2211-5463.12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuramitsu HK, Qi M, Kang IC, Chen W. Role for periodontal bacteria in cardiovascular diseases. Ann Periodontol. (2001) 6:41–7. 10.1902/annals.2001.6.1.41 [DOI] [PubMed] [Google Scholar]
- 80.Sharma A, Novak EK, Sojar HT, Swank RT, Kuramitsu HK, Genco RJ. Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Microbiol Immunol. (2000) 15:393–6. 10.1034/j.1399-302x.2000.150610.x [DOI] [PubMed] [Google Scholar]
- 81.DeLeon-Pennell KY, de Castro Brás LE, Iyer RP, Bratton DR, Jin YF, Ripplinger CM, et al. P. gingivalis lipopolysaccharide intensifies inflammation post-myocardial infarction through matrix metalloproteinase-9. J Mol Cell Cardiol. (2014) 76:218–26. 10.1016/j.yjmcc.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bengtsson T, Karlsson H, Gunnarsson P, Skoglund C, Elison C, Leanderson P, et al. The periodontal pathogen Porphyromonas gingivalis cleaves apoB-100 and increases the expression of apoM in LDL in whole blood leading to cell proliferation. J Intern Med. (2008) 263:558–71. 10.1111/j.1365-2796.2007.01917.x [DOI] [PubMed] [Google Scholar]
- 83.Hashimoto M, Kadowaki T, Tsukuba T, Yamamoto K. Selective proteolysis of apolipoprotein B-100 by arg-gingipain mediates atherosclerosis progression accelerated by bacterial exposure. J Biochem. (2006) 140:713–23. 10.1093/jb/mvj202 [DOI] [PubMed] [Google Scholar]
- 84.Félétou M. The endothelium: Part 1: Multiple functions of the endothelial cells—focus on endothelium-derived vasoactive mediators. San Rafael (CA): Morgan & Claypool Life Sciences; (2011). [PubMed] [Google Scholar]
- 85.Matsubara T, Ishibashi T, Hori T, Ozaki K, Mezaki T, Tsuchida K, et al. Association between coronary endothelial dysfunction and local inflammation of atherosclerotic coronary arteries. Mol Cell Biochem. (2003) 249:67–73. 10.1023/A:1024778421491 [DOI] [PubMed] [Google Scholar]
- 86.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. (2016) 118:620–36. 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chistiakov DA, Orekhov AN, Bobryshev YV. Links between atherosclerotic and periodontal disease. Exp Mol Pathol. (2016) 100:220–35. 10.1016/j.yexmp.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 88.Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol. (2007) 78:2289–302. 10.1902/jop.2007.070140 [DOI] [PubMed] [Google Scholar]
- 89.Triantafilou M, Gamper FG, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, et al. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol. (2007) 9:2030–9. 10.1111/j.1462-5822.2007.00935.x [DOI] [PubMed] [Google Scholar]
- 90.Erridge C, Spickett CM, Webb DJ. Non-enterobacterial endotoxins stimulate human coronary artery but not venous endothelial cell activation via toll-like receptor 2. Cardiovasc Res. (2007) 73:181–9. 10.1016/j.cardiores.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 91.Gualtero DF, Lafaurie GI, Fontanilla MR. Differential responses of endothelial cells on three-dimensional scaffolds to lipopolysaccharides from periodontopathogens. Mol Oral Microbiol. (2019) 34:183–93. 10.1111/omi.12263 [DOI] [PubMed] [Google Scholar]
- 92.Viafara-García SM, Morantes SJ, Chacon-Quintero Y, Castillo DM, Lafaurie GI, Buitrago DM. Repeated Porphyromonas gingivalis W83 exposure leads to release pro-inflammatory cytokynes and angiotensin II in coronary artery endothelial cells. Sci Rep. (2019) 9:19379. 10.1038/s41598-019-54259-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gualtero DF, Viafara-Garcia SM, Morantes SJ, Buitrago DM, Gonzalez OA, Lafaurie GI. Rosuvastatin inhibits interleukin (IL)-8 and IL-6 production in human coronary artery endothelial cells stimulated with Aggregatibacter actinomycetemcomitans serotype b. J Periodontol. (2017) 88:225–35. 10.1902/jop.2016.160288 [DOI] [PubMed] [Google Scholar]
- 94.Torres MA, Gualtero DF, Lafaurie GI, Fontanilla MR. Aggregatibacter actinomycetemcomitans induces a pro-atherosclerotic response in a three-dimensional collagen scaffold model in human endothelial cells. Mol Oral Microbiol. (2021) 36:58–66. 10.1111/omi.12326 [DOI] [Google Scholar]
- 95.Viafara-Garcia SM, Gualtero DF, Avila-Ceballos D, Lafaurie GI. Eikenella corrodens lipopolysaccharide stimulates the pro-atherosclerotic response in human coronary artery endothelial cells and monocyte adhesion. Eur J Oral Sci. (2018) 126:476–84. 10.1111/eos.12580 [DOI] [PubMed] [Google Scholar]
- 96.Hasturk H, Kantarci A, Van Dyke TE. Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front Immunol. (2012) 3:118. 10.3389/fimmu.2012.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. (2015) 15:375–87. 10.1038/nri3837 [DOI] [PubMed] [Google Scholar]
- 98.Castillo Y, Castellanos JE, Lafaurie GI, Castillo DM. Porphyromonas gingivalis outer membrane vesicles modulate cytokine and chemokine production by gingipain-dependent mechanisms in human macrophages. Arch Oral Biol. (2022) 140:105453. 10.1016/j.archoralbio.2022.105453 [DOI] [PubMed] [Google Scholar]
- 99.Nakao R, Takashiba S, Kosono S, Yoshida M, Watanabe H, Ohnishi M, et al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. (2014) 16:6–16. 10.1016/j.micinf.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 100.Gualtero DF, Lafaurie GI, Fontanilla MR. Two-dimensional and three-dimensional models for studying atherosclerosis pathogenesis induced by periodontopathogenic microorganisms. Mol Oral Microbiol. (2018) 33:29–37. 10.1111/omi.12201 [DOI] [PubMed] [Google Scholar]
- 101.Barrett TJ. Macrophages in atherosclerosis regression. Arterioscler Thromb Vasc Biol. (2020) 40:20–33. 10.1161/ATVBAHA.119.312802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol. (2002) 7:72–8. 10.1902/annals.2002.7.1.72 [DOI] [PubMed] [Google Scholar]
- 103.Loos BG, Teeuw WJ, Nicu EA. Plausible mechanisms explaining the association of periodontitis with cardiovascular diseases. In: Lynge Pedersen A, editors. Oral infections and general health (2016). p. 19–33. 10.1007/978-3-319-25091-5_3 [DOI] [Google Scholar]
- 104.López R, Loos BG, Baelum V. Hematological features in adolescents with periodontitis. Clin Oral Investig. (2012) 16:1209–16. 10.1007/s00784-011-0628-6 [DOI] [PubMed] [Google Scholar]
- 105.Hamzeh-Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O. Platelets and infections—complex interactions with bacteria. Front Immunol. (2015) 6:82. 10.3389/fimmu.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pham K, Feik D, Hammond BF, Rams TE, Whitaker EJ. Aggregation of human platelets by gingipain-R from Porphyromonas gingivalis cells and membrane vesicles. Platelets. (2002) 13:21–30. 10.1080/09537100120104863 [DOI] [PubMed] [Google Scholar]
- 107.Lourbakos A, Yuan YP, Jenkins AL, Travis J, Andrade-Gordon P, Santulli R, et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood. (2001) 97:3790–7. 10.1182/blood.v97.12.3790 [DOI] [PubMed] [Google Scholar]
- 108.Naito M, Sakai E, Shi Y, Ideguchi H, Shoji M, Ohara N, et al. Porphyromonas gingivalis-induced platelet aggregation in plasma depends on Hgp44 adhesin but not rgp proteinase. Mol Microbiol. (2006) 59:152–67. 10.1111/j.1365-2958.2005.04942.x [DOI] [PubMed] [Google Scholar]
- 109.Plummer C, Wu H, Kerrigan SW, Meade G, Cox D, Ian Douglas CW. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br J Haematol. (2005) 129:101–9. 10.1111/j.1365-2141.2005.05421.x [DOI] [PubMed] [Google Scholar]
- 110.Hally K, Fauteux-Daniel S, Hamzeh-Cognasse H, Larsen P, Cognasse F. Revisiting platelets and toll-like receptors (TLRs): at the interface of vascular immunity and thrombosis. Int J Mol Sci. (2020) 21:6150. 10.3390/ijms21176150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cognasse F, Lafarge S, Chavarin P, Acquart S, Garraud O. Lipopolysaccharide induces sCD40l release through human platelets TLR4, but not TLR2 and TLR9. Intensive Care Med. (2007) 33:382–4. 10.1007/s00134-006-0488-8 [DOI] [PubMed] [Google Scholar]
- 112.Assinger A, Laky M, Badrnya S, Esfandeyari A, Volf I. Periodontopathogens induce expression of CD40l on human platelets via TLR2 and TLR4. Thromb Res. (2012) 130:e73–8. 10.1016/j.thromres.2012.04.017 [DOI] [PubMed] [Google Scholar]
- 113.Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. (2003) 4:397–407. 10.2174/1389203033487036 [DOI] [PubMed] [Google Scholar]
- 114.McNicol A. Bacteria-induced intracellular signalling in platelets. Platelets. (2015) 26:309–16. 10.3109/09537104.2015.1014470 [DOI] [PubMed] [Google Scholar]
- 115.Buitrago DM, Ramos G, Rincón GM. Actividad antiagregante del extracto etanólico de Solanum tuberosum en plaquetas humanas. Vitae. (2007) 14:49–54. [Google Scholar]
- 116.Zhong S, Li L, Shen X, Li Q, Xu W, Wang X, et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. (2019) 144:266–78. 10.1016/j.freeradbiomed.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 117.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. (2011) 111:5944–72. 10.1021/cr200084z [DOI] [PubMed] [Google Scholar]
- 118.Di Pietro M, Filardo S, Falasca F, Turriziani O, Sessa R. Infectious agents in atherosclerotic cardiovascular diseases through oxidative stress. Int J Mol Sci. (2017) 18:2459. 10.3390/ijms18112459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jia R, Kurita-Ochiai T, Oguchi S, Yamamoto M. Periodontal pathogen accelerates lipid peroxidation and atherosclerosis. J Dent Res. (2013) 92:247–52. 10.1177/0022034513475625 [DOI] [PubMed] [Google Scholar]
- 120.Bugueno IM, Zobairi El-Ghazouani F, Batool F, El Itawi H, Anglès-Cano E, Benkirane-Jessel N, et al. Porphyromonas gingivalis triggers the shedding of inflammatory endothelial microvesicles that act as autocrine effectors of endothelial dysfunction. Sci Rep. (2020) 10:1778. 10.1038/s41598-020-58374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.OMS 2. Available at: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1
- 122.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2020) 21:141. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mattila KJ, Nieminen MS, Valtonen VV, Rasi VP, Kesäniemi YA, Syrjälä SL, et al. Association between dental health and acute myocardial infarction. Br Med J. (1989) 298:779–81. 10.1136/bmj.298.6676.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. (2008) 23:2079–86. 10.1007/s11606-008-0787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res. (2021) 7:109–22. 10.1002/cre2.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Romandini M, Baima G, Antonoglou G, Bueno J, Figuero E, Sanz M. Periodontitis, edentulism, and risk of mortality: a systematic review with meta-analyses. J Dent Res. (2021) 100:37–49. 10.1177/0022034520952401 [DOI] [PubMed] [Google Scholar]
- 127.Mesa F, Arrabal-Polo MA, Magan-Fernandez A, Arrabal M, Martin A, Muñoz R, et al. Patients with periodontitis and erectile dysfunction suffer a greater incidence of major adverse cardiovascular events: a prospective study in a spanish population. J Periodontol. (2022) 93:1233–42. 10.1002/JPER.21-0477 [DOI] [PubMed] [Google Scholar]
- 128.Beck JD, Couper DJ, Falkner KL, Graham SP, Grossi SG, Gunsolley JC, et al. The periodontitis and vascular events (PAVE) pilot study: adverse events. J Periodontol. (2008) 79:90–6. 10.1902/jop.2008.070223 [DOI] [PubMed] [Google Scholar]
- 129.Offenbacher S, Beck JD, Moss K, Mendoza L, Paquette DW, Barrow DA, et al. Results from the periodontitis and vascular events (PAVE) study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. (2009) 80:190–201. 10.1902/jop.2009.080007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Becker L, Prado K, Foppa M, Martinelli N, Aguiar C, Furian T, et al. Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J Crit Care. (2012) 27:316.e9–e3. 10.1016/j.jcrc.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 131.Hänsel S, Lässig G, Pistrosch F, Passauer J. Endothelial dysfunction in young patients with long-term rheumatoid arthritis and low disease activity. Atherosclerosis. (2003) 170:177–80. 10.1016/s0021-9150(03)00281-8 [DOI] [PubMed] [Google Scholar]
- 132.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. (1997) 336:973–9. 10.1056/NEJM199704033361401 [DOI] [PubMed] [Google Scholar]
- 133.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. (2000) 342:836–43. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 134.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. (2010) 375:132–40. 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. (2016) 118:145–56. 10.1161/CIRCRESAHA.115.306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. (2009) 373:1175–82. 10.1016/S0140-6736(09)60447-5 [DOI] [PubMed] [Google Scholar]
- 137.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. (2018) 70:61–75. 10.1016/j.bbi.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 138.Calabró P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. (2003) 108:1930–2. 10.1161/01.CIR.0000096055.62724.C5 [DOI] [PubMed] [Google Scholar]
- 139.Megson E, Fitzsimmons T, Dharmapatni K, Bartold PM. C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation. J Clin Periodontol. (2010) 37:797–804. 10.1111/j.1600-051X.2010.01603.x [DOI] [PubMed] [Google Scholar]
- 140.Singer RE, Moss K, Kim SJ, Beck JD, Offenbacher S. Oxidative stress and IgG antibody modify periodontitis-CRP association. J Dent Res. (2015) 94:1698–705. 10.1177/0022034515602693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Machado V, Botelho J, Escalda C, Hussain SB, Luthra S, Mascarenhas P, et al. Serum C-reactive protein and periodontitis: a systematic review and meta-analysis. Front Immunol. (2021) 12:706432. 10.3389/fimmu.2021.706432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brito LC, DalBó S, Striechen TM, Farias JM, Olchanheski LR, Jr, Mendes RT, et al. Experimental periodontitis promotes transient vascular inflammation and endothelial dysfunction. Arch Oral Biol. (2013) 58:1187–98. 10.1016/j.archoralbio.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 143.Campi P, Herrera BS, de Jesus FN, Napolitano M, Teixeira SA, Maia-Dantas A, et al. Endothelial dysfunction in rats with ligature-induced periodontitis: participation of nitric oxide and cycloxygenase-2-derived products. Arch Oral Biol. (2016) 63:66–74. 10.1016/j.archoralbio.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 144.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. (2007) 356:911–20. 10.1056/NEJMoa063186 [DOI] [PubMed] [Google Scholar]
- 145.Gugnani N, Gugnani S. Can treatment of severe periodontitis in patients with ST-segment elevation myocardial infarction improve endothelial function? Evid Based Dent. (2021) 22:5–7. 10.1038/s41432-021-0157-3 [DOI] [PubMed] [Google Scholar]
- 146.Lobo MG, Schmidt MM, Lopes RD, Dipp T, Feijó IP, Schmidt KES, et al. Treating periodontal disease in patients with myocardial infarction: a randomized clinical trial. Eur J Intern Med. (2020) 71:76–80. 10.1016/j.ejim.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 147.Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. (2019) 40:3459–70. 10.1093/eurheartj/ehz646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. (2020) 116:28–39. 10.1093/cvr/cvz201 [DOI] [PubMed] [Google Scholar]
- 149.Sharma S, Sridhar S, McIntosh A, Messow CM, Aguilera EM, Del Pinto R, et al. Periodontal therapy and treatment of hypertension-alternative to the pharmacological approach. A systematic review and meta-analysis. Pharmacol Res. (2021) 166:105511. 10.1016/j.phrs.2021.105511 [DOI] [PubMed] [Google Scholar]
- 150.Tsakos G, Sabbah W, Hingorani AD, Netuveli G, Donos N, Watt RG, et al. Is periodontal inflammation associated with raised blood pressure? Evidence from a national US survey. J Hypertens. (2010) 28:2386–93. 10.1097/HJH.0b013e32833e0fe1 [DOI] [PubMed] [Google Scholar]
- 151.Kawabata Y, Ekuni D, Miyai H, Kataoka K, Yamane M, Mizutani S, et al. Relationship between prehypertension/hypertension and periodontal disease: a prospective cohort study. Am J Hypertens. (2016) 29:388–96. 10.1093/ajh/hpv117 [DOI] [PubMed] [Google Scholar]
- 152.Desvarieux M, Demmer RT, Jacobs JD, Jr, Rundek T, Boden-Albala B, Sacco RL, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. (2010) 28:1413–21. 10.1097/HJH.0b013e328338cd36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pietropaoli D, Del Pinto R, Ferri C, Ortu E, Monaco A. Definition of hypertension-associated oral pathogens in NHANES. J Periodontol. (2019) 90:866–76. 10.1002/JPER.19-0046 [DOI] [PubMed] [Google Scholar]
- 154.Silveira TMD, Silva CFE, Vaucher RA, Angst PDM, Casarin M, Pola NM. Higher frequency of specific periodontopathogens in hypertensive patients. A pilot study. Braz Dent J. (2022) 33(5):64–73. 10.1590/0103-6440202204914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Woessner M, Smoliga JM, Tarzia B, Stabler T, Van Bruggen M, Allen JD. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide. (2016) 1:1–7. 10.1016/j.niox.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 156.Mitsui T, Harasawa R. The effects of essential oil, povidone-iodine, and chlorhexidine mouthwash on salivary nitrate/nitrite and nitrate-reducing bacteria. J Oral Sci. (2017) 59:597–601. 10.2334/josnusd.16-0593 [DOI] [PubMed] [Google Scholar]
- 157.Tribble GD, Angelov N, Weltman R, Wang BY, Eswaran SV, Gay IC, et al. Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front Cell Infect Microbiol. (2019) 9:39. 10.3389/fcimb.2019.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]