Abstract
The approval of ketamine for treatment-resistant depression has created a model for a novel class of rapid-acting glutamatergic antidepressants. Recent research into other novel rapid-acting antidepressants – most notably serotonergic psychedelics (SPs) – has also proven promising. Presently, the mechanisms of action of these substances are under investigation to improve these novel treatments, which also exhibit considerable side effects such as dissociation. This chapter lays out the historical development of ketamine as an antidepressant, outlines its efficacy and safety profile, reviews the evidence for ketamine’s molecular mechanism of action, and compares it to the proposed mechanism of SPs. The evidence suggests that although ketamine and SPs act on distinct primary targets, both may lead to rapid restoration of synaptic deficits and downstream network reconfiguration. In both classes of drugs, a glutamate surge activates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) throughput and increases in brain-derived neurotrophic factor (BDNF) levels. Taken together, these novel antidepressant mechanisms may serve as a framework to explain the rapid and sustained antidepressant effects of ketamine and may be crucial for developing new rapid-acting antidepressants with an improved side effect profile.
Keywords: Antidepressants, Brain-derived neurotrophic factor, Glutamate, Synaptic plasticity, Treatment resistant depression
1. Introduction
1.1. Clinical Studies Leading to the Approval of Esketamine as an Antidepressant: A Historical Overview
Since it was first approved by the US Food and Drug Administration (FDA) in 1970, ketamine1 has become a crucial and versatile drug and is listed as an essential medicine by the World Health Organization (World Health Organization 2019). Originally developed as a derivative of phencyclidine (PCP) by the pharmaceutical company Parke Davis, ketamine has been used for decades as an anesthetic and analgesic agent; more recently, esketamine (racemic ketamine’s (S)-stereoisomer) received FDA approval for treatment-resistant depression (TRD) for adults with major depressive disorder (MDD) as well as with acute suicidal ideation or behavior. TRD is commonly defined by nonresponse to at least two adequate antidepressant trials.
PCP was first trialed in humans as an anesthetic, but researchers soon realized that its unpleasant side effect profile – which included loss of feeling in limbs and prolonged sensory deprivation post-treatment – precluded its clinical use. Short-acting PCP derivatives were subsequently synthesized. After pharmacological testing, ketamine – which has similar anesthetic potential to PCP but a more favorable side-effect profile – was selected for human trials. Though ketamine and PCP are both noncompetitive N-methyl-D-aspartate receptor (NMDAR) inhibitors, notable pharmacological differences exist. For example, PCP has pro-convulsive effects while ketamine does not, and ketamine has a faster induction rate of anesthesia than PCP but a shorter duration of action (McCarthy et al. 1965). At the time of its development as an anesthetic, PCP’s sensory deprivation effects led researchers to investigate it in a model of schizophrenia (Cohen et al. 1959; Rosenbaum 1959); indeed, a study of nine schizophrenic and nine non-schizophrenic patients showed that PCP had psychotomimetic effects in both groups (Rosenbaum 1959). As a result, such effects were assessed during ketamine’s development. Though both drugs exerted psychotomimetic effects, ketamine’s were less intense, and of shorter duration, than PCP’s (Domino et al. 1965). Nonetheless, Parke Davis worried that ketamine’s classification as a psychotomimetic drug would hinder its development and thus employed an internal psychiatrist to observe patients after application of ketamine anesthesia.
In recent decades, our understanding of mood disorders and depression has evolved, and researchers realized that the pathophysiology of mood disorders extends beyond monoaminergic neurotransmission. By the 1990s, emerging preclinical work supported the hypothesis that glutamate and its ionotropic NMDAR might be involved in the pathophysiology of affective disorders and, commensurately, that its study might lead to the development of new classes of antidepressants (Skolnick et al. 1996). Within the context of this preclinical evidence, as well as a broad understanding of ketamine’s mechanism of action, Berman and colleagues were the first to administer ketamine to individuals with depression in a placebo-controlled, double-blind trial (Berman et al. 2000). The trial included seven participants with major depression who were treated intravenously with a subanesthetic dose of 0.5 mg/kg ketamine hydrochloride or a saline solution on 2 days 1 week apart. The results showed that ketamine infusion had rapid-acting antidepressant effects, as assessed by its ability to significantly improve depression rating scale scores within hours. Moreover, these antidepressant effects continued for at least 3 days after ketamine’s acute dissociative effects had faded.
In retrospect, these results marked a milestone. However, they did not attract lasting interest after publication (Gallagher et al. 2021). Explanations included skepticism that the robust and rapid antidepressant effects observed were due to the drug rather than its intravenous application, as previous work had similarly demonstrated rapid response to an intravenously administered tricyclic antidepressant (Berman et al. 2000; Malhotra and Santosh 1996; Sallee et al. 1997). Another factor was the low number of participants enrolled in the trial. Finally, Berman and colleagues had mentioned that a potential limitation associated with clinical applications for ketamine would be its abuse potential and its well-established psychotomimetic profile, suggesting that these properties would need to be eliminated before further testing could be conducted. Nevertheless, due to persistent interest in ketamine’s potential antidepressant effects, in 2006 Zarate and colleagues replicated the findings of the original trial by Berman and colleagues in a randomized, double-blind, placebo-controlled study of 18 patients with TRD (Zarate et al. 2006).
Since then, research into ketamine’s mechanism of action has sought to understand the results of these initial clinical trials and the possible connection between the glutamatergic system and the underlying pathophysiology of depression and other stress-related disorders. Notably, the surge of interest in ketamine’s antidepressant effects did not occur until independent, placebo-controlled studies substantiated the results of these initial trials underscoring the importance of funding replication studies. Indeed, a recent study that compared publications that were initially highly cited with their replication rate found that nearly half of these publications were not replicated in the following 10 years (Aarts et al. 2015).
Despite ketamine’s potential as a rapid-acting antidepressant, the impracticalities of intravenous application in outpatient clinics and private settings led investigators to research alternative routes of administration. Lapidus and colleagues conducted the first trial of intranasally-delivered ketamine and found that this application led to sufficiently high plasma concentrations of the agent to induce antidepressant effects (Lapidus et al. 2014). In combination with positive clinical trials, these findings led to a patent for the intranasal administration of esketamine and culminated in the FDA approval of esketamine in 2019 for adults with TRD and in 2020 for adults with acute suicidal ideation and behavior. Esketamine showed roughly four-fold higher NMDAR binding affinity than the R(+)enantiomer but retained ketamine’s anesthetic and dissociative properties (Fukumoto et al. 2017; Zhang et al. 2014).
Results of the initial clinical trials with ketamine also led to a surge in research to uncover or develop agents with similar rapid-acting antidepressant properties but fewer psychotomimetic side effects. This effort has, to date, been unsuccessful, suggesting that ketamine might have unique pharmacological characteristics. Nonetheless, the search for new and similar substances continues, as do efforts to improve ketamine’s own side effect profile (Kadriu et al. 2020).
1.2. The Need for Rapid-Acting Antidepressants
First-line antidepressant medications such as selective serotonin reuptake inhibitors (SSRIs) target the monoaminergic system. Despite their positive safety profile, these agents have some key limitations. First, SSRIs exhibit response rates in first-line trials of about 30–60%, underscoring that a significant number of patients with major depression do not respond to these agents. The large NIMH-funded Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that, in a group of approximately 4,000 patients with MDD who received as many as four different psychotropic treatment combinations, about 33% did not respond to any of the standard medications (Gaynes et al. 2009). Such treatment-resistant patients are vulnerable to disease-associated debilitating impairments in psychosocial functioning, personal relationships, working capacity, and general well-being, and are at particularly high risk for suicidal behavior (Whiteford et al. 2013). Second, even when they will ultimately prove successful, standard antidepressant treatments have a lag time of several weeks before symptom alleviation; this long delay to reach full therapeutic potential makes them relatively ineffective for immediate treatment of emergencies, including suicidal behavior and acute relief of depressive symptoms. Third, side effects, genetic polymorphisms, and other related factors may all critically affect drug efficacy and general drug adherence; interactions with other drugs also remain a key consideration (Goethe et al. 2007). As an example, side effects such as nausea might occur before the onset of antidepressant response given the significant lag time associated with standard antidepressants.
In this context, the discovery of a rapid-acting antidepressant like ketamine was a major paradigm shift in the development of novel antidepressants and the treatment of patients with depression. The potential to treat previous non-responders, including individuals with TRD who experience unremitting depressive episodes is a major advantage, especially considering the high prevalence of depression in community settings. However, it should be noted that, compared with standard antidepressant agents, ketamine’s long-term side effects require further research because most of this research has been conducted only in short-term ketamine trials.
1.3. Safety of Ketamine as an Antidepressant
Given that ketamine is one of the most commonly used anesthetics, safety data on single use applications of anesthetic doses were more readily available than for newly developed drugs. Nevertheless, given that anesthetic and antidepressant ketamine doses are quite different, ketamine’s antidepressant side effect profile needed to be considered separately.
A systematic assessment of ketamine’s side effects across five separate studies (n = 188) found that single-dose IV ketamine (0.5 mg/kg) was associated with several, mostly transient, side effects compared to placebo (Acevedo-Diaz et al. 2020). Dissociative side effects were most commonly reported (e.g., feeling strange (80%), a feeling of floating (>50%), and visual distortion (>50%)). Less common side effects included difficulty speaking (>50%), numbness (>40%), confusion (>40%), euphoria (>20%), and blurred vision (>20%) (Acevedo-Diaz et al. 2020). Side effects that occurred in fewer than 20% of patients included hypertension, dry mouth, tingling, difficulty concentrating, changes in body temperature, hallucinations, headaches, and gastrointestinal problems. As noted above, most of these side effects were transient, resolving after 2 h and peaking within an hour of ketamine administration. No significant cognitive or memory deficits or long-lasting side effects were observed in association with a single-dose infusion (Acevedo-Diaz et al. 2020).
As mentioned previously, most randomized clinical trials have monitored ketamine’s short-term side effects, with relatively fewer data available regarding the safety of frequent or long-term use (Short et al. 2018). However, clinical trials surged (Bahr et al. 2019) with the development and FDA approval of the esketamine nasal spray, raising potential safety concerns about repeat- dose applications. Few studies have systematically assessed the side effects of long-term, repeat-dose ketamine and, to date, no clinical trials have monitored patients receiving ketamine for longer than one year. The extant data, however, suggest that ketamine’s dissociative side effects remain transient and seem to decrease with repeated dosing; in addition, side effects such as dizziness and nausea vary depending on dose (Bahr et al. 2019). Interestingly, some data have come from substance abuse disorder studies of recreational ketamine users; while such data are not directly comparable to intravenously administered, antidepressant-dose ketamine administered in controlled settings, they do provide information regarding the risks and side effects associated with long-term ketamine use. Most notably, these data suggest a risk for ulcerative cystitis in chronic ketamine users (Jhang et al. 2015), though a recent study found that neither single nor repeat-dose esketamine led to urothelial toxicity (Findeis et al. 2020). Long-term recreational ketamine use has also been associated with impaired cognitive functioning, including episodic and semantic memory (Curran et al. 2001) as well as cognitive processing speed and verbal learning (Chan et al. 2013); these deficits persisted after matching with polydrug controls. Researchers have suggested that these effects may be at least partially explained by the observed upregulation of D1 receptors in the dorsolateral prefrontal cortex of long-term ketamine users (Narendran et al. 2005). Studies in rodents have also suggested that repeated subanesthetic administration of ketamine may have neurotoxic effects (Li et al. 2017; McIntyre et al. 2021).
With regard to esketamine in particular, one Phase 3 long-term study of over 800 enrolled patients assessed the long-term safety and efficacy of esketamine nasal spray plus a novel oral antidepressant for a year. The most common side effects were dizziness (32,9%), dissociation (27,6%), nausea (25,1%), and headache (24,9%). Cognitive function increased or remained stable at baseline throughout the study. Most side effects were mild or moderate, occurred on dosing day, and resolved on the same day; dissociative symptoms typically resolved 1.5 h after dosing (Wajs et al. 2020). Although some patients reported urinary tract side effects (17%), symptoms were mild to moderate and resolved after two weeks despite continued esketamine treatment. After discontinuation, the most common (>20%) new or worsening “withdrawal” symptoms were fatigue-lethargy/lack of energy and insomnia (Wajs et al. 2020). Intranasal esketamine also holds a risk for abuse and misuse as well as an increased risk of suicidal ideation and behavior in adolescents and children (Bahr et al. 2019); however, it is important to note that no abuse has been reported to date, and no patients requested an increase in dosing frequency in long-term studies of adults (Wajs et al. 2020). Recent findings also suggest that esketamine nasal spray appears to have neither short-term nor long-term adverse effects on nasal tolerability or olfactory function (Doty et al. 2021). However, cardiovascular and cerebrovascular conditions sensitive to increases in blood pressure should be treated as a contraindication or caution, as esketamine can increase blood pressure and heart rate.
2. Pharmacodynamics of Ketamine as Antidepressant
2.1. Central Mechanisms of Action
Ketamine’s main pharmacological target is use-dependent antagonism of the ionotropic NMDAR and eventual decrease of calcium ion influx (Fig. 1). Crucially, the block is closely linked to prior α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-linked depolarization of the post-synapse and the removal of Mg2+ ions that block the channel pore (Hansen et al. 2018). At subanesthetic, antidepressant doses, ketamine facilitates glutamate release in the medial prefrontal cortex (mPFC), while higher doses decrease glutamate release (Moghaddam et al. 1997). This process occurs via the selective inhibition of NMDARs on gamma-aminobutyric acid (GABA)-ergic interneurons, which in turn disinhibits glutamatergic neurotransmission of pyramidal neurons in the mPFC (Duman et al. 2019; Miller et al. 2016). This hypothesis is supported by the finding that knockdown of the GluN2B subunit of NMDARs on GABAergic interneurons produced antidepressant effects in rodent models (Gerhard et al. 2020). The same effect was also found for somatostatin- and parvalbumin-expressing subtypes, but not glutamatergic pyramidal (CAMK2a) neurons (Gerhard et al. 2020).
Fig. 1.
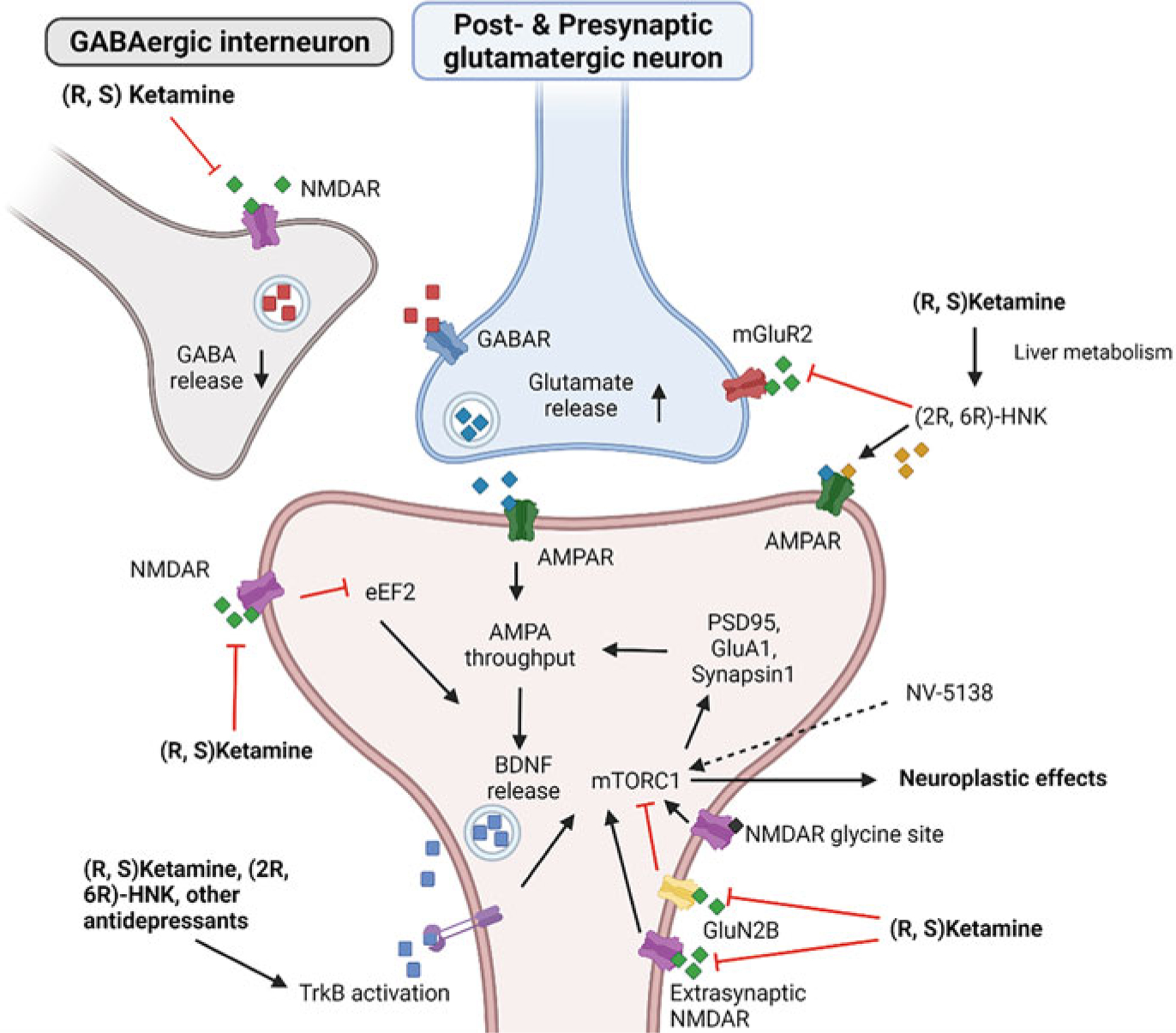
Ketamine’s general mechanism of action. Ketamine blocks the N-methyl-D-aspartate glutamate receptor (NMDAR) on gamma-aminobutyric acid (GABA)-ergic interneurons, thereby disinhibiting glutamate release by pyramidal neurons. Glutamate in turn binds to postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) which mediates brain-derived neurotrophic factor (BDNF) release. BDNF binds primarily to tropomyosin-related kinase B (TrkB), leading to downstream activation of mammalian target of rapamycin complex 1 (mTORC1) and, consequently, neuroplastic effects. mTORC1 activation may, in turn, be associated with an increase in synaptic proteins such as postsynaptic density protein 95 (PSD95), synapsin 1, and the AMPAR subunit GluA, further increasing AMPAR throughput. Alternatively, ketamine may directly bind to postsynaptic NMDARs, reducing the amount of phosphorylated elongation factor 2 (eEF2) that, in turn, increases BDNF translation. Evidence also suggests that ketamine activates mTORC1 via direct TrkB activation and antagonism of extrasynaptic NMDARs. Phosphorylation of mTORC1 may also be increased by agonists binding to the glycine site of NMDARs or directly via NV-5138. In addition, the ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) may increase downstream neuroplasticity by blocking mainly presynaptic metabotropic glutamate receptor 2 (mGluR2), thereby increasing glutamate release. Created with biorender.com
An alternative hypothesis states that ketamine’s rapid antidepressant effects are due to direct blocking of postsynaptic NMDAR activity in glutamatergic pyramidal neurons of the hippocampus and mPFC (Autry et al. 2011; Miller et al. 2016; Monteggia et al. 2013). This block, in turn, suppresses eukaryotic elongation factor 2 kinase (eEF2K), reducing the amount of phosphorylated elongation factor 2 (eEF2) and thus increasing brain-derived neurotrophic factor (BDNF) (Autry et al. 2011). This hypothesis is supported by evidence demonstrating that selective eEF2K inhibition in mice increased BDNF levels and led to subsequent antidepressant-like effects. Inhibition of protein synthesis – and, thus, BDNF synthesis – via anisomycin prevented ketamine-mediated rapid behavioral response (Autry et al. 2011).
In addition to its ability to modulate glutamate, ketamine affects a range of other neurotransmitter systems, including the GABAergic, dopaminergic, and serotonergic systems. With regard to GABAergic neurotransmission, ketamine was found to reverse reductions in GABA-synthesizing enzymes, proteins, and co-expressed neuropeptides due to chronic stress (Ghosal et al. 2017, 2020; Ren et al. 2016). In rats, a single intraperitoneal dose of ketamine led to a dose-dependent increase in GABA, glutamate, and glutamine cycling (Chowdhury et al. 2017). In addition, in mice, the prophylactic administration of ketamine prior to stress exposure was associated with an increase in precursors to inhibitory neurotransmitters and a decrease in precursors to excitatory neurotransmitters in response to a stressor (McGowan et al. 2018). This change in precursors did not occur when no stressor was presented, suggesting that ketamine might selectively enhance resilience to stressful events.
Ketamine’s positive effects on motivation may be at least partially explained by its effect on the dopaminergic reward circuit (Abdallah et al. 2017; Mkrtchian et al. 2020). In rodents, ketamine increased activity in dopaminergic neurons in the ventral tegmental area (VTA) as well as extracellular dopamine in the nucleus accumbens and prefrontal cortex (PFC) (Witkin et al. 2016). The mediating role of AMPARs is underscored by the fact that the AMPAR antagonist NBQX (1,2,3,4-tetrahydro-6-nitro-2,3-dioxo- (9CI)-benzo[f]quinoxaline-7-sulfonamide) abolished both increased dopaminergic activity and antidepressant-like behavioral effects. Because activation of the D1 receptor increases expression and excitability of NMDARs and AMPARs (Gao and Wolf 2008; Lavin and Grace 2001; Sun et al. 2005) as well as excitatory input to the PFC (Björkholm et al. 2017; Gonzalez-Islas and Hablitz 2003; Gurden et al. 2000), this effect might explain ketamine’s impact on synaptogenesis and synaptic potentiation via increased BDNF and, thus, stimulation of the mechanistic target of rapamycin complex 1 (mTORC1). With regard to the behavioral effects of D1 receptor activity, repeated stress was found to reduce D1 receptor expression in the rodent mPFC, which in turn led to depression-related behaviors (Shinohara et al. 2018). Along these lines, D1 receptor-expressing pyramidal cells in the rodent mPFC produced a rapid antidepressant and anxiolytic response, while disruption of D1 receptor activity via the D1 receptor antagonist SCH39166 blocked ketamine’s antidepressant-like effects (Hare et al. 2019).
Recent studies further suggest that serotonergic neurotransmission may be involved in ketamine’s antidepressant effects. In rodents, subcutaneous ketamine injection increased prefrontal serotonin levels (Nishitani et al. 2014). This effect seemed to be mediated by AMPARs, given that injection of an AMPAR antagonist into the dorsal raphe nucleus (DRN) attenuated this effect while administration of an AMPAR agonist increased prefrontal serotonin. In addition, the prior infusion of a tryptophan hydroxylase inhibitor depleted serotonin levels, and direct application of the 5-HT1A antagonist WAY100635 into the mPFC abolished ketamine’s antidepressant effects (Fukumoto et al. 2016, 2018; Gigliucci et al. 2013). Conversely, administration of the 5-HT1A agonist 8-OH-DPAT into the rodent mPFC mimicked the rapid antidepressant effects of ketamine and increased BDNF and subsequent mTORC1 signaling, ultimately increasing synaptic protein levels (Fukumoto et al. 2020). However, binding of the serotonin transporter (SERT) – as seen with SSRIs – is unlikely, given that positron emission tomography (PET) studies using the radioligand [11C]N,N-dimethyl-2-(2-amino-4- cyanophenylthio)-benzylamine ([11C]DASB) to measure SERT binding showed no measurable occupancy of the SERT after administering subanesthetic-dose ketamine (Spies et al. 2018).
Ketamine may also decrease the activity of extrasynaptic GluN2B containing NMDARs, which directly increases mTOR phosphorylation. Miller and colleagues demonstrated that deletion of GluN2B from principal cortical neurons abolished ketamine’s antidepressant effects in mice (Miller et al. 2014). In addition, preclinical evidence suggests that ketamine’s enantiomers are metabolically active. For instance, (2R,6R)-hydroxynorketamine (HNK) appears to have antidepressant-like and neuroplastic effects in animal models but lack ketamine’s dissociative effects. Fewer dissociative side effects would be clinically beneficial and facilitate more widespread ketamine use. Interestingly, (2R,6R)-HNK’s effects also seem to rely on AMPAR transmission, given that (2R,6R)-HNK administration led to both increased frequency and amplitude of AMPAR-mediated excitatory potentials and that its antidepressant effects were abolished by administering an AMPAR antagonist (Zanos et al. 2016). Increased glutamate release via presynaptic metabotropic glutamate receptor 2 (mGluR2) blockade by (2R,6R)-HNK further increased AMPAR transmission (Zanos et al. 2019). Additional evidence also suggests that (2R,6R)-HNK and ketamine – as well as traditional antidepressants – bind directly to tropomyosin receptor kinase B (TrkB), facilitating activation via BDNF and, thus, synaptic plasticity (Casarotto et al. 2021).
Beyond HNK, several trials have investigated novel glutamatergic compounds that might exhibit similar antidepressant effects to ketamine while avoiding its dissociative effects. Ketamine also possesses a glycine binding site in addition to its main NMDAR binding site, which presents a possible antidepressant target. In rats, GLYX-13, a partial glycine site agonist, was found to have antidepressant effects 24 h and seven days after administration (Burgdorf et al. 2013, 2015). However, recent trials with AV-101, a prodrug of 7-chlorokynurenic acid (a glycine site antagonist) failed to demonstrate antidepressant effects for patients with TRD (Park et al. 2020) or as adjunctive treatment in MDD (VistaGen Therapeutics 2019).
2.2. Ketamine’s Central and Peripheral Actions Beyond Glutamate
Increasing evidence suggests that ketamine’s biological effects transcend the glutamatergic synapse and affect other relevant neuropathological pathways, including neuroinflammatory pathways. One example is the kynurenine (KYN) pathway (Kadriu et al. 2019) (Fig. 2). In particular, overactivation of the neurotoxic branch of this pathway has been associated with mood disorders (Birner et al. 2017). Proinflammatory cytokines (e.g., interleukin-6 (IL-6) and quinolinic acid (QA), a key metabolite of the neurotoxic branch, were also found to be elevated in the cerebrospinal fluid of suicidal patients (Bay-Richter et al. 2015). Conversely, kynurenic acid (KYNA), a metabolite of the neuroprotective branch, was negatively associated with depressive symptoms (Bay-Richter et al. 2015).
Fig. 2.
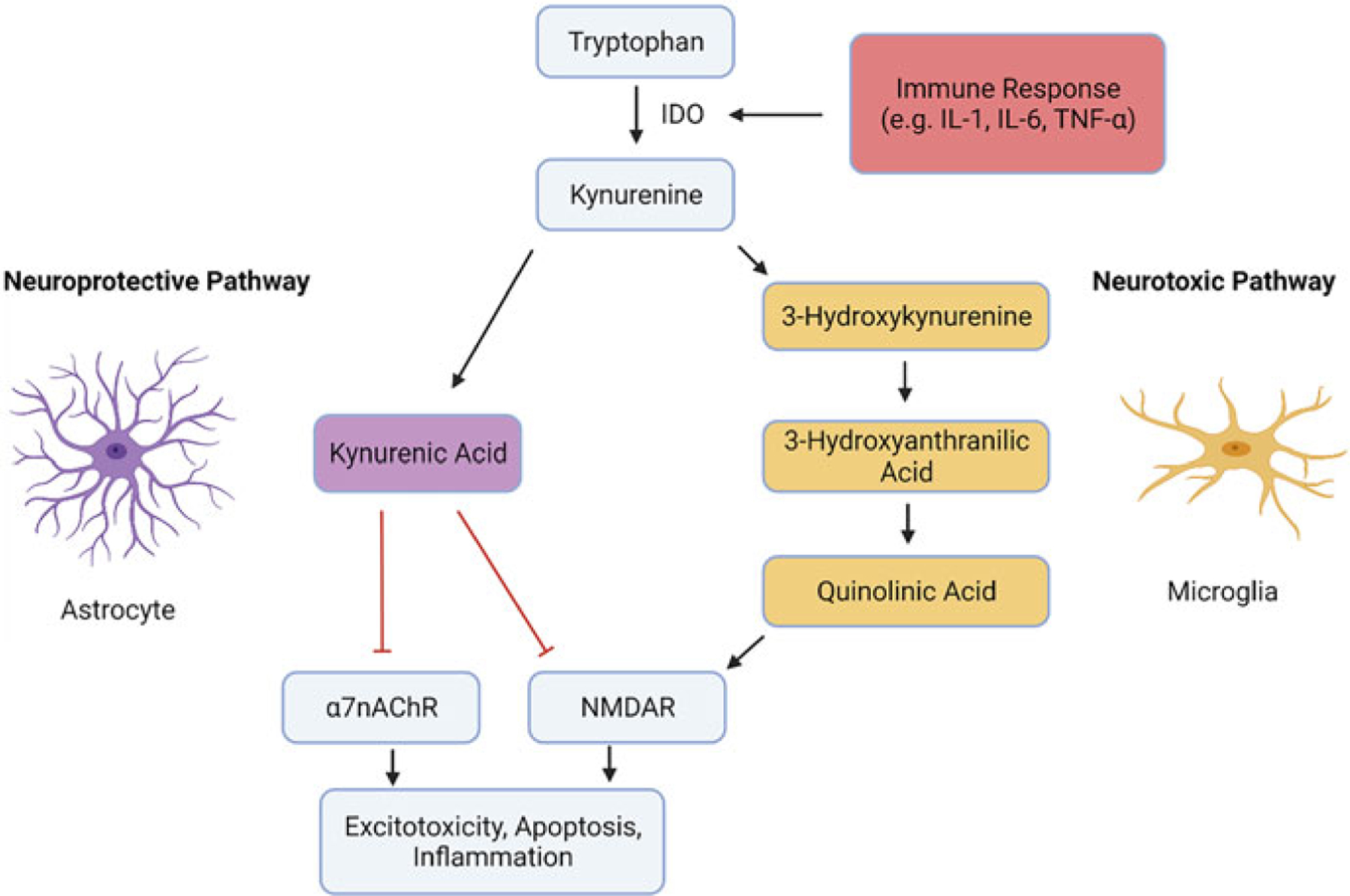
The kynurenine pathway. The figure depicts the two branches of the kynurenine pathway. In the neuroprotective branch, mainly associated with astrocytes, kynurenine is transformed into kynurenic acid. Kynurenic acid acts as an N-methyl-D-aspartate glutamate receptor (NMDAR) and alpha 7 nicotinic acetylcholine receptor (α7nAChR) antagonist, thereby decreasing inflammation and excitotoxicity. In the neurotoxic pathway, mainly associated with microglia, kynurenine is metabolized into quinolinic acid, which acts as an NMDAR agonist, increasing excitotoxicity and neuronal apoptosis. Crucially, activation of rate-limiting enzymes such as indoleamine 2,3-dioxygenase (IDO), which transforms tryptophan into kynurenine via inflammation or psychological stress shifts the pathway toward its neurotoxic branch
More specifically, depression and psychological stress are hypothesized to lead to an increased immune response (e.g., IL-6, tumor necrosis factor alpha (TNF-α)), activating the enzyme indoleamine 2,3-dioxygenase (IDO) and thereby increasing the conversion of tryptophan (TRP) into KYN (Heisler and O’Connor 2015). In the neuroprotective branch of the KYN pathway, KYN is processed by astrocytes into KYNA, which acts as an NMDAR and α7nAChR antagonist and thus has positive effects on synaptic plasticity and neuronal protection, possibly via anti-inflammatory processes (Moroni et al. 2012) and the prevention of glutamate spill-over (Haroon et al. 2016; Vécsei et al. 2013). In the neurotoxic branch of the KYN pathway, KYN is processed by activated microglia into metabolites such as QA, which acts as a potent NMDAR agonist promoting excitotoxicity and neuronal apoptosis (Birner et al. 2017; Heyes et al. 1992).
Regarding ketamine, some evidence suggests that a single ketamine infusion may reduce circulating levels of proinflammatory cytokines in the blood of patients with TRD within hours of administration (Kiraly et al. 2017). Furthermore, in mice, a low dose of intraperitoneally-administered ketamine abolished depressive behaviors usually induced by lipopolysaccharide (LPS) via QA production (Walker et al. 2013). Similarly, in humans, a double-blind, placebo-controlled study of TRD patients found that KYN levels and the KYN/TRP ratio were decreased in ketamine responders 4 h post-ketamine infusion (Moaddel et al. 2018). Further corroborating this finding, Kadriu and colleagues found that KYNA levels were increased and IDO levels were decreased after a single ketamine infusion in patients with treatment-resistant bipolar disorder (Kadriu et al. 2019).
In addition, Kadriu et al. (2018) showed that ketamine also reduces markers of bone inflammation usually found in patients with long-term MDD. The study demonstrated that ketamine normalized an abnormal osteoprotegerin/RANKL ratio and plasma osteoprotegerin levels, possibly counteracting the loss of bone mineral density found in patients with MDD.
2.3. Common Downstream Mechanisms Engaged by Rapid-Acting Antidepressants
Spurred by the paradigm-shifting nature of ketamine research, investigators have examined other candidate drugs with potentially rapid antidepressant effects, including serotonergic psychedelics (SPs). These substances – most prominently psilocybin, lysergic acid diethylamide (LSD), and N,N-dimethyltryptamine (DMT) – have been shown to be potentially effective in treating conditions ranging from substance dependence (Johnson et al. 2014) to terminal cancer anxiety (Grob et al. 2011) as well as depression (Palhano-Fontes et al. 2019).
The exact underlying mechanisms of SPs are under investigation, but agonism of the 5-HT2a receptor has been proposed as a central mechanism underlying the efficacy of these compounds. For instance, administering the 5-HT2a receptor blocker ketanserin diminished the subjective psychedelic effects of LSD (Preller et al. 2017) and psilocybin (Vollenweider et al. 1998) in humans, as well as drug discrimination in animal models (Appel and Callahan 1989; Cunningham and Appel 1987). A blockade of the effects of DMT administered as ayahuasca proved only partially effective (Valle et al. 2016). This may be due to the additional monoamine oxidase (MAO) inhibition as well as beta-carboline alkaloids, which have been shown to interact with a variety of molecular targets (Deecher et al. 1992; Husbands et al. 2001). 5-HT2a receptors are expressed in a variety of brain regions, most notably the neocortex, amygdala, striatum, mammillary nucleus, and claustrum (Pasqualetti et al. 1996; Pazos et al. 1985; Weber and Andrade 2010). 5-HT2a agonism has been proposed to lead to the desynchronization of brain areas and, more specifically, the default mode network, leading to the psychedelic effects of these compounds (Carhart-Harris et al. 2014). However, recent evidence has also questioned the contribution of 5-HT2a receptors to the antidepressant effect of SPs, suggesting instead that 5-HT2a receptor-independent mechanisms such as induction of neuronal plasticity may play a role by directly binding to TrkB (Casarotto et al. 2021; Hesselgrave et al. 2021).
Another notable advance in clinical research regarding psychoactive substances is a recent Phase 3 study demonstrating the efficacy of 3,4-Methylenedioxymethamphetamine (MDMA)-assisted therapy in post-traumatic stress disorder (Mitchell et al. 2021). However, the mechanism of action and consequent effects of MDMA are distinct; this agent is thought to act primarily by increasing serotonin levels via binding of presynaptic serotonin transporters (Rudnick and Wall 1992). Given the monoamine hypothesis, this mechanism suggests a putative usefulness of MDMA for treating MDD, although little evidence for this indication presently exists (see Patel and Titheradge 2015 for a review).
SPs typically produce similarly rapid antidepressant effects despite possessing mechanistically and pharmacodynamically distinct properties (Muttoni et al. 2019). In particular, research on the molecular mechanisms that mediate rapid antidepressant effects expand our understanding of antidepressant mechanisms, with the ultimate goal of identifying future drug targets with similar or better rapid antidepressant effects than ketamine and a better side-effect profile. In this context, investigating the downstream mechanisms of other rapid-acting drugs could provide valuable insights. While common downstream mechanisms of action between ketamine and drugs such as SPs remain largely speculative, several mechanisms, discussed below, have been proposed that may account for the rapid antidepressant effects of both substance classes.
2.3.1. Rapid Restoration of Synaptic Deficits Due to Stress
Considerable evidence suggests that chronic stress and depression are associated with a decrease in dendritic spines, spine synapse connections, and activity in regions implicated in depression, such as the hippocampus and the PFC (Duman et al. 2016; Kang et al. 2012; McEwen et al. 2015; McEwen and Morrison 2013). One mechanism proposed to account for rapid antidepressant effects is the rapid and selective restoration of stress-induced neuronal deficits. In a mouse model, ketamine infusions reversed stress-related loss of dendritic spines (Moda-Sava et al. 2019). This effect was associated with a change in neuronal systems in the PFC two days post-treatment as well as more delayed antidepressant effects. Another study detected reversal of apical dendritic spine deficits in the hippocampal CA1 region of Flinders sensitive rats 60 min after ketamine treatment (Treccani et al. 2019). Höflich and colleagues similarly found changes in hippocampal subfield volumes after ketamine infusion in healthy controls, with peaking effects in CA1 (Höflich et al. 2021). These effects may be mediated by the aforementioned BDNF-dependent activation of mTORC1 and the consequent neuroplastic effects.
2.3.2. Rapid Glutamate Release
Both ketamine and SPs lead to rapid glutamate release post-administration (Razoux et al. 2007; Vollenweider and Kometer 2010). With regard to SPs, rodent studies found that both LSD and 2,5-Dimethoxy-4-iodoamphetamine (DOI) increased glutamate levels in the prefrontal and somatosensory cortices, an effect that was abolished by blocking the 5-HT2A receptor (Muschamp et al. 2004; Scruggs et al. 2003). Martin and Nichols (2016) further showed that DOI administration increased early-activation genes and the indicator for neuronal activity cFos, especially in cerebral regions with a high density of glutamate-releasing pyramidal neurons.
It should be noted that ketamine’s ability to increase glutamate levels has been directly confirmed using carbon-13 magnetic resonance spectroscopy (Abdallah et al. 2018), but less evidence exists for SPs. However, one study found that, compared to placebo, psilocybin increased glutamate levels in the PFC (Mason et al. 2020).
2.3.3. Rapid Stimulation of AMPA Throughput
In the case of ketamine, the glutamate surge is known to stimulate AMPAR throughput, which mediates BDNF release and the downstream activation of mTORC1 (Duman 2018; Olson 2018). Administration of an AMPAR antagonist again abolished ketamine’s rapid antidepressant effects and mTORC1 activation (Maeng et al. 2008; Moghaddam et al. 1997). While this link is less clear with SPs, in rodents DOI induced behavioral effects like head shakes that were subsequently blocked by administration of AMPAR antagonists (C. Zhang and Marek 2008).
2.3.4. Release of Neurotrophins
In preclinical studies, ketamine, LSD, and DOI all increased BDNF levels in the hippocampus and other cortical areas (Autry et al. 2011; Garcia et al. 2008; Silva Pereira et al. 2017; Ly et al. 2018). BDNF, in turn, is associated with neuronal growth and plasticity. Shifts in BDNF levels are known to increase both spinogenesis and neuritogenesis (Cohen-Cory et al. 2010). Conversely, in Val66Met knock-in mice, where BDNF messenger RNA transport is impaired, ketamine had neither synaptogenic nor antidepressant effects (Liu et al. 2012). Ly and colleagues further demonstrated that the TrkB antagonist ANA-12 abolished the neuroplastic effects of SPs (Ly et al. 2018). BDNF has a high affinity for binding TrkB, which in turn mediates neuronal plasticity via mTORC1 (Jaworski et al. 2005; Kumar et al. 2005). Thus, rapamycin administration – which inhibits mTORC1 – also abolished the neuroplastic effects of SPs (Ly et al. 2018). Finally, the selective 5-HT2A antagonist ketanserin also inhibited spinogenesis and neuritogenesis after SP administration, highlighting the importance of this receptor in initiating downstream effects. When directly compared to ketamine, some SPs proved to be more efficacious (e.g., MDMA) or potent (e.g., LSD) in promoting neuritogenesis, which may account for the prolonged antidepressant effect of these substances (Ly et al. 2018).
It should be noted, however, that the role of BDNF in rapidly decreasing depressive symptoms in humans is still under debate. Especially with regard to ketamine, the evidence has been mixed (Duncan et al. 2013; Haile et al. 2014; Machado-Vieira et al. 2009; Laje et al. 2012). Similarly mixed findings have also been found for SPs (de Almeida et al. 2019; Holze et al. 2020).
2.3.5. Stimulation of Intracellular Neuroplasticity Cascades
Both ketamine and SPs have been shown to increase neuroplasticity via dendritic growth and new synapse formation as well as strengthen preexisting synaptic connections (Kadriu et al. 2021). A single dose of ketamine increased levels of pre-and postsynaptic proteins such as PSD95, synapsin 1, and the AMPAR GluA subunit in the rodent PFC, which in turn was associated with the increased number and function of synapses (Li et al. 2010). The increase in synaptic proteins was detected as soon as 2 h after ketamine administration and lasted for at least 72 h, which may help explain ketamine’s rapid antidepressant effects. Neuroplasticity genes, including for Bdnf, Homer1a, and activity related cytoskeletal protein (Arc) have also been shown to be activated via glutamatergic signaling (Bagot et al. 2017; De Bartolomeis et al. 2013).
In rodent models, both DOI and LSD administration similarly increased a wide array of neuroplasticity genes, including Bdnf, Arc, egr-1, Nor1, Ania3, sgk, C/EBP-β, and Iκβ-α (González-Maeso et al. 2007; Martin et al. 2014; Martin and Nichols 2016; Nichols et al. 2003; Nichols and Sanders-Bush 2002). However, in the case of SPs, gene expression is mediated via 5-HT2A receptor activation, which in turn affects neuroplasticity via G-protein-coupled receptor pathways.
Considerable similarities between SPs and ketamine also exist with regard to neuritogenesis and spinogenesis. Like ketamine, LSD, DOI, psilocybin, DMT, and noribogaine (a metabolite of ibogaine) all increased dendritic arbor complexity in cultured cortical neurons in vitro, both in terms of increasing the total length of arbors and the number of dendritic branches (Ly et al. 2018). LSD, DOI, and DMT also increased the number of dendritic spines (Ly et al. 2018). In vivo, treating Drosophila larvae with LSD and DOI increased dendritic branching (Ly et al. 2018). In rats, the intraperitoneal administration of DMT rapidly increased dendritic spines on cortical pyramidal neurons in the PFC 24 h after administration, comparable to an equivalent dose of ketamine (Ly et al. 2018). Although these effects remain poorly understood, they seem to be mediated by mTORC1, TrkB, and 5-HT2A signaling pathways. Because the effects were demonstrated in vivo in both vertebrate and invertebrate models, they suggest an evolutionarily conserved mechanism. Interestingly, in rodents, alterations in synaptic plasticity and ketamine’s antidepressant effects have both been demonstrated to disappear when either mTORC1 or AMPARs are blocked (Li et al. 2010). Conversely, mTORC1 activation via NV-5138 produces rapid antidepressant effects in rodent models.
3. Conclusion
The evidence reviewed above describes the novel biochemical mechanisms of action that underlie ketamine’s antidepressant effects as well as those of SPs. It should be noted, however, that recent attempts to identify or develop glutamatergic drugs that mimic ketamine’s antidepressant qualities but lack its dissociative psychotomimetic effects have largely proven futile (Kadriu et al. 2020). Furthermore, while both the safety profile of ketamine and the lack of abuse potential associated with a single dose have been relatively well established (Acevedo-Diaz et al. 2020), only limited safety data exist regarding the long-term effects of repeated ketamine administration. Similarly, few data exist regarding the development of tolerance to chronic antidepressant ketamine use. Given that the recommended dosing regimen of esketamine for depression is one to three times a week for the first two months and once every week or every two weeks thereafter, such long-term data are crucially needed (Canuso et al. 2018).
A key possible mechanism of action for the antidepressant effects of both ketamine and SPs is the ability of these compounds to increase neuroplasticity. Underscoring the plausibility of this hypothesis is that postmortem studies have demonstrated that individuals with depression have lower BDNF mRNA and protein levels (Castrén 2005; Thoenen 1995), serum BDNF protein levels (Sen et al. 2008; Shimizu et al. 2003) as well as decreased hippocampal volume (Sheline et al. 1996; Videbech and Ravnkilde 2004). However, neural plasticity may not be an exclusively beneficial state, regardless of the circumstances in which it occurs (Branchi 2011). For example, Belsky and colleagues demonstrated that polymorphisms of monoamine oxidase-A, dopamine receptor D4, or a 5-hydroxytryptamine-linked polymorphic region were linked to either beneficial or adverse outcomes depending on the environment (Belsky et al. 2009). Indeed, at least in the case of SPs, the importance of context or even psychotherapeutic care appears to be key for the beneficial effect of the neuroplastic state produced by these compounds to manifest (Carhart-Harris et al. 2018). Similarly, Chiarotti and colleagues identified a dose-dependent interaction between the SSRI escitalopram and the environmental context of patients (Chiarotti et al. 2017).
It is interesting to note that the US Food and Drug Administration (FDA) recently approved the rapid-acting antidepressant brexanolone (SAGE-547), a positive allosteric GABAA receptor modulator, for use in postpartum depression (U.S. Food and Drug Administration 2019). Research in mice further suggests that impairment of GABAARs can lead to depressive-like behaviors as well as downregulation of both AMPA and NMDA receptors and consequent glutamatergic transmission, which can be normalized by ketamine infusion (Ren et al. 2016). In rats, the administration of brexanolone prevented a decrease in BDNF levels and consequent impairment of hippocampal neurogenesis due to stress (Evans et al. 2012).
The rapid-acting antidepressant effects of ketamine, SPs, and possibly also brexanolone/zuranolone suggest that downstream commonalities on glutamatergic systems may underlie the mechanism of action of all three agents, even if their initial targets differ. The emergence of rapid-acting antidepressants as treatments for depression offers investigators new and potentially fruitful avenues for exploration, both in terms of identifying novel biochemical mechanisms underlying the mechanism of action of successful rapid-acting antidepressants and developing novel therapeutics. Together, such evidence can be used to investigate and develop alternate and possibly more rapid-acting antidepressant therapies with the goal of helping numerous patients who suffer from treatment-resistant forms of mood disorders.
Acknowledgements
Ioline Henter (NIMH) provided invaluable editorial assistance.
Disclosures
Funding for this work was provided in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857). Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.-S. government but will share a percentage of any royalties that may be received by the government. C. Kraus has received honoraria from Janssen, LivaNova, AOP Orphan, and Roche Austria. Dr. Kadriu is full-time employee at Janssen Research & Development, LLC and owns stock and stock options in Johnson & Johnson.
All other authors have no conflict of interest to disclose, financial or otherwise.
Footnotes
Unless otherwise specified, the term ketamine refers to racemic (R,S)-ketamine.
Contributor Information
Marina Kojic, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria.
Johan Saelens, Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria.
Bashkim Kadriu, Section on the Neurobiology and Treatment of Mood Disorders, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA; Department of Neuroscience, Janssen Research & Development, LLC, San Diego, CA, USA.
Carlos A. Zarate, Jr, Section on the Neurobiology and Treatment of Mood Disorders, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA.
Christoph Kraus, Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria; Section on the Neurobiology and Treatment of Mood Disorders, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA.
References
- Aarts AA, Anderson JE, Anderson CJ, Attridge PR, Attwood A, Axt J, Babel M, Bahník Š, Baranski E, Barnett-Cowan M, Bartmess E, Beer J, Bell R, Bentley H, Beyan L, Binion G, Borsboom D, Bosch A, Bosco FA et al. (2015) Estimating the reproducibility of psychological science. Science 349(6251). 10.1126/science.aac4716 [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Jackowski A, Salas R, Gupta S, Sato JR, Mao X, Coplan JD, Shungu DC, Mathew SJ (2017) The nucleus accumbens and ketamine treatment in major depressive disorder. Neuropsychopharmacology 42(8):1739–1746. 10.1038/npp.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GMI, Purohit P, de Graaf RA, Esterlis I, Juchem C, Pittman BP, Krystal JH, Rothman DL, Sanacora G, Mason GF (2018) The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 43(10):2154–2160. 10.1038/s41386-018-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CA, Park LT (2020) Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord 263:568–575. 10.1016/j.jad.2019.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel JB, Callahan PM (1989) Involvement of 5-HT receptor subtypes in the discriminative stimulus properties of mescaline. Eur J Pharmacol 159(1):41–46. 10.1016/0014-2999(89)90041-1 [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475(7354):91–96. 10.1038/nature10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, LaBonté B, Peña CJ, Shen L, Wittenberg GM, Nestler EJ (2017) Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry 81(4): 285–295. 10.1016/j.biopsych.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr R, Lopez A, Rey JA (2019) Intranasal esketamine (SpravatoTM) for use in treatment-resistant depression in conjunction with an oral antidepressant. Pharm Ther 44(6):340–375 [PMC free article] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Träskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L (2015) A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain Behav Immun 43:110–117. 10.1016/j.bbi.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R (2009) Vulnerability genes or plasticity genes? Mol Psychiatry 14(8):746–754. 10.1038/mp.2009.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354. 10.1016/S0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- Birner A, Platzer M, Bengesser SA, Dalkner N, Fellendorf FT, Queissner R, Pilz R, Rauch P, Maget A, Hamm C, Herzog-Eberhard S, Mangge H, Fuchs D, Moll N, Zelzer S, Schütze G, Schwarz M, Reininghaus B, Kapfhammer HP, Reininghaus EZ (2017) Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS One 12(2):1–14. 10.1371/journal.pone.0172699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkholm C, Marcus MM, Konradsson-Geuken Å, Jardemark K, Svensson TH (2017) The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol 27(4):411–417. 10.1016/j.euroneuro.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Branchi I (2011) The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover (for ketamine analogues). Psychoneuroendocrinology 36(3):339–351. 10.1016/j.psyneuen.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, David Leander J, Stanton PK, Gross AL, Kroes RA, Moskal JR (2013) GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38(5):729–742. 10.1038/npp.2012.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Weiss C, Gross A, Boikess SR, Kroes RA, Khan MA, Burch RM, Rex CS, Disterhoft JF, Stanton PK, Moskal JR (2015) The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience 308:202–211. 10.1016/j.neuroscience.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatr 175(7): 620–630. 10.1176/appi.ajp.2018.17060720 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, Nutt D (2014) The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8(1 FEB):1–22. 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Haijen E, Erritzoe D, Watts R, Branchi I, Kaelen M (2018) Psychedelics and the essential importance of context. J Psychopharmacol 32(7):725–731. 10.1177/0269881118754710 [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, Biojone C, Cannarozzo C, Sahu MP, Kaurinkoski K, Brunello CA, Steinzeig A, Winkel F, Patil S, Vestring S, Serchov T, Diniz CRAF, Laukkanen L, Cardon I et al. (2021) Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 184(5):1299–1313.e19. 10.1016/j.cell.2021.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E (2005) Is mood chemistry? Nat Rev Neurosci 6(3):241–246 [DOI] [PubMed] [Google Scholar]
- Chan KWS, Lee TMC, Siu AMH, Wong DPL, Kam C, Tsang SKM, Chan CCH (2013) Addictive Behaviors effects of chronic ketamine use on frontal and medial temporal cognition. Addict Behav 38(5):2128–2132. 10.1016/j.addbeh.2013.01.014 [DOI] [PubMed] [Google Scholar]
- Chiarotti F, Viglione A, Giuliani A, Branchi I (2017) Citalopram amplifies the influence of living conditions on mood in depressed patients enrolled in the STAR * D study. Transl Psychiatry 7(3):e1066. 10.1038/tp.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GMI, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL, Sanacora G (2017) Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22(1):120–126. 10.1038/mp.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BD, Rosenbaum G, Dobie SI, Gottlieb JS (1959) Sensory isolation: hallucinogenic effects of a brief procedure. J Nerv Ment Dis 129(5):486–491. 10.1097/00005053-195911000-00009 [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70(5):271–288. 10.1002/dneu.20774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Appel JB (1987) Neuropharmacological reassessment of the discriminative stimulus properties of d-lysergic acid diethylamide (LSD). Psychopharmacology 91(1):67–73. 10.1007/BF00690929 [DOI] [PubMed] [Google Scholar]
- Curran HV, Monaghan L, Curran HV, Monaghan L (2001) In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction 96(5):749–760. 10.1080/09652140020039116 [DOI] [PubMed] [Google Scholar]
- de Almeida RN, Galvão AC, Da Silva FS, Silva EA, Palhano-Fontes F, Maia-de-Oliveira JP, de Araújo DB, Lobão-Soares B, Galvão-Coelho NL (2019) Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front Psychol 10(JUN):1–13. 10.3389/fpsyg.2019.01234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bartolomeis A, Sarappa C, Buonaguro EF, Marmo F, Eramo A, Tomasetti C, Iasevoli F (2013) Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog Neuro-Psychopharmacol Biol Psychiatry 46:1–12. 10.1016/j.pnpbp.2013.06.010 [DOI] [PubMed] [Google Scholar]
- Deecher DC, Teitler M, Soderlund DM, Bornmann WG, Kuehne ME, Glick SD (1992) Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies. Brain Res 571(2):242–247. 10.1016/0006-8993(92)90661-R [DOI] [PubMed] [Google Scholar]
- Domino EF, Chodoff P, Corssen G (1965) Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther 6(3):279–291. 10.1002/cpt196563279 [DOI] [PubMed] [Google Scholar]
- Doty RL, Popova V, Wylie C, Fedgchin M, Daly E, Janik A, Ochs R, Rosanne R, Pilar L, Kim L, Rama C, Carol M, Jaskaran J (2021) Effect of esketamine nasal spray on olfactory function and nasal tolerability in patients with treatment - resistant depression: results from four multicenter, randomized, double - blind, placebo - controlled, phase III studies. CNS Drugs 35(7):781–794. 10.1007/s40263-021-00826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS (2018) Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide [version 1; referees: 3 approved]. F1000Research 7(May):1–10. 10.12688/f1000research.14344.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22(3):238–249. 10.1038/nm.4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Shinohara R, Fogaça MV, Hare B (2019) Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry 24(12):1816–1832. 10.1038/s41380-019-0400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA (2013) Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol 16(2): 301–311. 10.1017/S1461145712000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Sun Y, McGregor A, Connor B (2012) Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology 63(8). 10.1016/j.neuropharm.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Findeis H, Sauer C, Cleare A, Bauer M, Ritter P (2020) Urothelial toxicity of esketamine in the treatment of depression. Psychopharmacology 237(11):3295–3302. 10.1007/S00213-020-05611-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S (2016) The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 41(4):1046–1056. 10.1038/npp.2015.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S (2017) Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 361(1):9–16. 10.1124/jpet.116.239228 [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Funakoshi T, Chaki S (2018) Role of 5-HT 1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol 21(4):371–381. 10.1093/ijnp/pyx116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaça MV, Liu RJ, Duman CH, Li XY, Chaki S, Duman RS (2020) Medial PFC AMPA receptor and BDNF signaling are required for the rapid and sustained antidepressant-like effects of 5-HT1A receptor stimulation. Neuropsychopharmacology 45(10):1725–1734. 10.1038/s41386-020-0705-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher B, Neiman A, Slattery M-C, McLoughlin DM (2021) Online news media reporting of ketamine as a treatment for depression from 2000 to 2017. Ir J Psychol Med. 10.1017/IPM.2021.47 [DOI] [PubMed] [Google Scholar]
- Gao C, Wolf ME (2008) Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J Neurochem 106(6):2489–2501. 10.1111/j.1471-4159.2008.05597.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuro-Psychopharmacol Biol Psychiatry 32(1):140–144. 10.1016/j.pnpbp.2007.07.027 [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ (2009) What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 60(11):1439–1445. 10.1176/ps.2009.60.11.1439 [DOI] [PubMed] [Google Scholar]
- Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, Taylor SR, Duman CH, Delpire E, Picciotto M, Wohleb ES, Duman RS (2020) GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Investig 130(3):1336–1349. 10.1172/JCI130808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Hare BD, Duman RS (2017) Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr Opin Behav Sci 14:1–8. 10.1016/j.cobeha.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Duman CH, Liu RJ, Wu M, Terwilliger R, Girgenti MJ, Wohleb E, Fogaca MV, Teichman EM, Hare B, Duman RS (2020) Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol Dis 134: 104669. 10.1016/j.nbd.2019.104669 [DOI] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A (2013) Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology 228(1):157–166. 10.1007/s00213-013-3024-x [DOI] [PubMed] [Google Scholar]
- Goethe JW, Woolley SB, Cardoni AA, Woznicki BA, Piez DA (2007) Selective serotonin reuptake inhibitor discontinuation: side effects and other factors that influence medication adherence. J Clin Psychopharmacol 27(5):451–458. 10.1097/jcp.0b013e31815152a5 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ (2003) Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci 23(3):867–875. 10.1523/jneurosci.23-03-00867.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron 53(3):439–452. 10.1016/j.neuron.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstad AL, Greer GR (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68(1):71–78. 10.1001/archgenpsychiatry.2010.116 [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM (2000) Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci 20(22):1–5. 10.1523/jneurosci.20-22-j0003.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney JJ, De La Garza R, Charney DS, Newton TF, Mathew SJ (2014) Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 17(2):331–336. 10.1017/S1461145713001119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ, Traynelis SF (2018) Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol 150(8):1081–1105. 10.1085/jgp.201812032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS (2019) Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun 10(1):1–12. 10.1038/s41467-018-08168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, Hu XP, Miller AH (2016) Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry 21(10):1351–1357. 10.1038/mp.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler JM, O’Connor JC (2015) Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun 50:115–124. 10.1016/j.bbi.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM (2021) Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A 118(17):1–7. 10.1073/pnas.2022489118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJP, Lackner A, Larsen SA, Lee K, Leonard HL, Markey SP, Martin A, Milstein S, Mouradian MM, Pranzatelli MR, Quearry BJ et al. (1992) Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 115(5):1249–1273. 10.1093/brain/115.5.1249 [DOI] [PubMed] [Google Scholar]
- Höflich A, Kraus C, Pfeiffer RM, Seiger R, Rujescu D, Zarate CA, Kasper S, Winkler D, Lanzenberger R (2021) Translating the immediate effects of S-ketamine using hippocampal subfield analysis in healthy subjects-results of a randomized controlled trial. Transl Psychiatry 11(1). 10.1038/s41398-021-01318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holze F, Vizeli P, Müller F, Ley L, Duerig R, Varghese N, Eckert A, Borgwardt S, Liechti ME (2020) Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology 45(3):462–471. 10.1038/s41386-019-0569-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands SM, Glennon RA, Gorgerat S, Gough R, Tyacke R, Crosby J, Nutt DJ, Lewis JW, Hudson AL (2001) B-carboline binding to imidazoline receptors. Drug Alcohol Depend 64(2): 203–208. 10.1016/S0376-8716(01)00123-5 [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M (2005) Control of dendritic arborization by the phosphoinositide-3′-kinase- Akt-mammalian target of rapamycin pathway. J Neurosci 25(49):11300–11312. 10.1523/JNEUROSCI.2270-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhang J, Hsu Y, Kuo H (2015) Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. Int J Urol 22(9):816–825. 10.1111/iju.12841 [DOI] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeau A, Cosimano MP, Griffiths RR (2014) Pilot study of the 5-HT2A R agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 28(11):983–992. 10.1177/0269881114548296.Pilot [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Gold PW, Luckenbaugh DA, Lener MS, Ballard ED, Niciu MJ, Henter ID, Park LT, De Sousa RT, Yuan P, Machado-Vieira R, Zarate CA (2018) Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol Psychiatry 23(7):1626–1631. 10.1038/mp.2017.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Farmer CA, Yuan P, Park LT, De Deng Z, Moaddel R, Henter ID, Shovestul B, Ballard ED, Kraus C, Gold PW, Machado-Vieira R, Zarate CA (2019) The kynurenine pathway and bipolar disorder: intersection of the monoaminergic and glutamatergic systems and immune response. Mol Psychiatry. 10.1038/s41380-019-0589-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, De Deng Z, Kraus C, Henter ID, Lisanby SH, Zarate CA (2020) Not so fast: recent successes and failures in treating depression. J Clin Psychiatry 81(4). 10.4088/JCP.19ac13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Greenwald M, Henter ID, Gilbert JR, Kraus C, Park LT, Zarate CA (2021) Ketamine and serotonergic psychedelics: common mechanisms underlying the effects of rapid-acting antidepressants. Int J Neuropsychopharmacol 24(1):8–21. 10.1093/ijnp/pyaa087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS (2012) Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18(9):1413–1417. 10.1038/nm.2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, Patel M, Hodes GE, Russo SJ, Merad M, Iosifescu DV, Charney DS, Murrough JW (2017) Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry 7(3). 10.1038/tp.2017.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY (2005) Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 25(49): 11288–11299. 10.1523/JNEUROSCI.2284-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C (2012) Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 72(11):e27–e28. 10.1016/j.biopsych.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 76(12):970–976. 10.1016/j.biopsych.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Grace AA (2001) Stimulation of D1-type dopamine receptors enhances excitability in prefrontal cortical pyramidal neurons in a state-dependent manner. Neuroscience 104(2): 335–346. 10.1016/S0306-4522(01)00096-3 [DOI] [PubMed] [Google Scholar]
- Li N, Boyoung L, Rong-Jian L, Mounira B, Jason MD, Masaaki I, Xiao-Yuan L, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(August):959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shen R, Wen G, Ding R, Du A, Zhou J (2017) Effects of ketamine on levels of inflammatory cytokines IL-6, IL-1 β, and TNF- α in the hippocampus of mice following acute or chronic administration. Front Pharmacol 8:139. 10.3389/fphar.2017.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK (2012) Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71(11):996–1005. 10.1016/j.biopsych.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep 23(11):3170–3182. 10.1016/j.celrep.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA (2009) Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry 70(12):1662–1666. 10.4088/JCP.08m04659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63(4):349–352. 10.1016/j.biopsych.2007.05.028 [DOI] [PubMed] [Google Scholar]
- Malhotra S, Santosh PJ (1996) Loading dose imipramine - new approach to pharmacotherapy of melancholic depression. J Psychiatr Res 30(1):51–58. 10.1016/0022-3956(95)00042-9 [DOI] [PubMed] [Google Scholar]
- Martin DA, Nichols CD (2016) Psychedelics recruit multiple cellular types and produce complex transcriptional responses within the brain. EBioMedicine 11:262–277. 10.1016/j.ebiom.2016.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Marona-Lewicka D, Nichols DE, Nichols CD (2014) Chronic LSD alters gene expression profiles in the mPFC relevant to schizophrenia. Neuropharmacology 83:1–8. 10.1016/j.neuropharm.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason NL, Kuypers KPC, Müller F, Reckweg J, Tse DHY, Toennes SW, Hutten NRPW, Jansen JFA, Stiers P, Feilding A, Ramaekers JG (2020) Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 45(12):2003–2011. 10.1038/s41386-020-0718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DA, Chen G, Kaump DH, Ensor C (1965) General anesthetic and other pharmacological properties of 2-(O-chlorophenyl)-2-methylamino cyclohexanone HCl (CI-581). J New Drugs 5(1):21–33. 10.1002/j.1552-4604.1965.tb00219.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH (2013) The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79(1):16–29. 10.1016/j.neuron.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C (2015) Mechanisms of stress in the brain. Nat Neurosci 18(10):1353–1363. 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JC, Hill C, Mastrodonato A, Lagamma CT, Kitayev A, Brachman RA, Narain NR, Kiebish MA, Denny CA (2018) Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology 43(9):1813–1821. 10.1038/s41386-018-0043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, Brietzke E, Dodd S, Gorwood P, Ho R, Iosifescu DV, Jaramillo CL, Kasper S, Kratiuk K, Lee JG, Lee Y, Lui LMW, Mansur RB, Papakostas GI et al. (2021) Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatr 178(5):383–399. 10.1176/APPI.AJP.2020.20081251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ (2014) GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. elife 2014(3):1–3. 10.7554/eLife.03581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ (2016) Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 100:17–26. 10.1016/j.neuropharm.2015.07.028 [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Bogenschutz M, Lilienstein A, Harrison C, Kleiman S, Parker-Guilbert K, Ot’alora GM, Garas W, Paleos C, Gorman I, Nicholas C, Mithoefer M, Carlin S, Poulter B, Mithoefer A, Quevedo S, Wells G, Klaire SS, van der Kolk B et al. (2021) MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med 27(6): 1025–1033. 10.1038/s41591-021-01336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtchian A, Evans JW, Kraus C, Yuan P, Kadriu B, Nugent AC, Roiser JP, Zarate CA (2020) Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol Psychiatry. 10.1038/s41380-020-00878-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S, Morris PJ, Yuan P, Thomas CJ, Gould TD, Ferrucci L, Zarate CA (2018) Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology 235(10):3017–3030. 10.1007/s00213-018-4992-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C (2019) Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364(6436). 10.1126/science.aat8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17(8):2921–2927. 10.1523/jneurosci.17-08-02921.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia L, Gideons E, Kavalali E (2013) The role of eEF2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 73(12):1199–1203. 10.1016/j.biopsych.2012.09.006.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Sili M, Mannaioni G (2012) Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm 119(2):133–139. 10.1007/s00702-011-0763-x [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA (2004) Lysergic acid diethylamide and [−]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res 1023(1):134–140. 10.1016/j.brainres.2004.07.044 [DOI] [PubMed] [Google Scholar]
- Muttoni S, Ardissino M, John C (2019) Classical psychedelics for the treatment of depression and anxiety: a systematic review. J Affect Disord 258(July):11–24. 10.1016/j.jad.2019.07.076 [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Keefe R, Ph D, Gil R, Martinez D, Slifstein M, Ph D, Kegeles LS, Ph D, Talbot PS, Huang Y, Ph D, Hwang D, Ph D, Khenissi L, Cooper TB, Laruelle M, Abi-dargham A (2005) Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatr 162(12):2352–2359 [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E (2002) A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26(5):634–642. 10.1016/S0893-133X(01)00405-5 [DOI] [PubMed] [Google Scholar]
- Nichols CD, Garcia EE, Sanders-Bush E (2003) Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Mol Brain Res 111(1–2): 182–188. 10.1016/S0169-328X(03)00029-9 [DOI] [PubMed] [Google Scholar]
- Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S (2014) Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 17(8):1321–1326. 10.1017/S1461145714000649 [DOI] [PubMed] [Google Scholar]
- Olson DE (2018) Psychoplastogens: a promising class of plasticity-promoting neurotherapeutics. J Exp Neurosci 12. 10.1177/1179069518800508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, Mota-Rolim SA, Osório FL, Sanches R, Dos Santos RG, Tófoli LF, De Oliveira Silveira G, Yonamine M, Riba J, Santos FR, Silva-Junior AA, Alchieri JC, Galvão-Coelho NL, Lobão-Soares B et al. (2019) Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med 49(4):655–663. 10.1017/S0033291718001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LT, Kadriu B, Gould TD, Zanos P, Greenstein D, Evans JW, Yuan P, Farmer CA, Oppenheimer M, George JM, Adeojo LW, Snodgrass HR, Smith MA, Henter ID, MacHado-Vieira R, Mannes AJ, Zarate CA (2020) A randomized trial of the N-methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int J Neuropsychopharmacol 23(7):417–425. 10.1093/IJNP/PYAA025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetti M, Nardi I, Ladinsky H, Marazziti D, Cassano GB (1996) Comparative anatomical distribution of serotonin 1A, 1Dα and 2A receptor mRNAs in human brain postmortem. Mol Brain Res 39(1–2):223–233. 10.1016/0169-328X(96)00026-5 [DOI] [PubMed] [Google Scholar]
- Patel R, Titheradge D (2015) MDMA for the treatment of mood disorder: all talk no substance? Ther Adv Psychopharmacol 5(3):179–188. 10.1177/2045125315583786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Cortés R, Palacios JM (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res 346(2):231–249. 10.1016/0006-8993(85)90857-1 [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stämpfli P, Liechti ME, Seifritz E, Vollenweider FX (2017) The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol 27(3):451–457. 10.1016/j.cub.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Razoux F, Garcia R, Léna I (2007) Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology 32(3):719–727. 10.1038/sj.npp.1301057 [DOI] [PubMed] [Google Scholar]
- Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, Luscher B (2016) Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry 80(6):457–468. 10.1016/j.biopsych.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum G (1959) Comparison of sernyl with other drugs. A.M.A. Arch Gen Psychiatry 1(6). 10.1001/archpsyc.1959.03590060113013 [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC (1992) The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A 89(5):1817–1821. 10.1073/pnas.89.5.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Vrindavanam NS, Deas-Nesmith D, Carson SW, Sethuraman G (1997) Pulse intravenous clomipramine for depressed adolescents: double- blind, controlled trial. Am J Psychiatr 154(5):668–673. 10.1176/ajp.154.5.668 [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY (2003) The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett 346(3):137–140. 10.1016/S0304-3940(03)00547-0 [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G (2008) Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64(6):527–532. 10.1016/j.biopsych.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 93(9):3908–3913. 10.1073/pnas.93.9.3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada SI, Iyo M (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54(1):70–75. 10.1016/S0006-3223(03)00181-1 [DOI] [PubMed] [Google Scholar]
- Shinohara R, Taniguchi M, Ehrlich AT, Yokogawa K, Deguchi Y, Cherasse Y, Lazarus M, Urade Y, Ogawa A, Kitaoka S, Sawa A, Narumiya S, Furuyashiki T (2018) Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol Psychiatry 23(8):1717–1730. 10.1038/mp.2017.177 [DOI] [PubMed] [Google Scholar]
- Short B, Fong J, Galvez V, Shelker W, Loo CK (2018) Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5(1):65–78. 10.1016/S2215-0366(17)30272-9 [DOI] [PubMed] [Google Scholar]
- Silva Pereira V, Elfving B, Joca SRL, Wegener G (2017) Ketamine and aminoguanidine differentially affect Bdnf and Mtor gene expression in the prefrontal cortex of adult male rats. Eur J Pharmacol 815(May):304–311. 10.1016/j.ejphar.2017.09.029 [DOI] [PubMed] [Google Scholar]
- Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R (1996) Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry 29(1):23–26. 10.1055/s-2007-979537 [DOI] [PubMed] [Google Scholar]
- Spies M, James GM, Berroterán-Infante N, Ibeschitz H, Kranz GS, Unterholzner J, Godbersen M, Gryglewski G, Hienert M, Jungwirth J, Pichler V, Reiter B, Silberbauer L, Winkler D, Mitterhauser M, Stimpfl T, Hacker M, Kasper S, Lanzenberger R (2018) Assessment of ketamine binding of the serotonin transporter in humans with positron emission tomography. Int J Neuropsychopharmacol 21(2):145–153. 10.1093/ijnp/pyx085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME (2005) Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci 25(32):7342–7351. 10.1523/JNEUROSCI.4603-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H (1995) Neurotrophins and neuronal plasticity. Science 270(5236):593–598. 10.1126/science.270.5236.593 [DOI] [PubMed] [Google Scholar]
- Treccani G, Ardalan M, Chen F, Musazzi L, Popoli M, Wegener G, Nyengaard JR, Müller HK (2019) S-ketamine reverses hippocampal dendritic spine deficits in flinders sensitive line rats within 1 h of administration. Mol Neurobiol 56(11):7368–7379. 10.1007/s12035-019-1613-3 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (2019) FDA approves first treatment for post-partum depression
- Valle M, Maqueda AE, Rabella M, Rodríguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mañanas MT, Barker S, Friedlander P, Feilding A, Riba J (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26(7):1161–1175. 10.1016/j.euroneuro.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Vécsei L, Szalárdy L, Fülöp F, Toldi J (2013) Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 12(1):64–82. 10.1038/nrd3793 [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B (2004) Reviews and overviews hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatr 161:1957–1966 [DOI] [PubMed] [Google Scholar]
- VistaGen Therapeutics (2019) VistaGen reports topline phase 2 results for AV-101 as an adjunctive treatment of major depressive disorder. https://www.prnewswire.com/news-releases/vistagen-reports-topline-phase-2-results-for-av-101-as-an-adjunctive-treatment-of-major-depressive-disorder-300958317.html
- Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11(9):642–651. 10.1038/nrn2884 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9(17):3897–3902. 10.1097/00001756-199812010-00024 [DOI] [PubMed] [Google Scholar]
- Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, George JE, Morrison RL, Sanacora G, Young AH (2020) Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry 81(3). 10.4088/JCP.19M12891 [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R (2013) NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38(9):1609–1616. 10.1038/npp.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ET, Andrade R (2010) Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci 4(AUG):1–12. 10.3389/fnins.2010.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJL, Vos T (2013) Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382(9904):1575–1586. 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. In. J Pharmacol Exp Ther 358(1). 10.1124/jpet.116.233627 [DOI] [PubMed] [Google Scholar]
- World Health Organization, Geneva (2019) World Health Organization model list of essential medicines, 21st List, 2019. https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604):481–486. 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, Morris PJ, Thomas CJ, Moaddel R, Zarate CA, Gould TD (2019) (2R,6R)-hydroxynorketamine exerts mGlu2 receptordependent antidepressant actions. Proc Natl Acad Sci U S A 116(13):6441–6450. 10.1073/pnas.1819540116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8). 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- Zhang C, Marek GJ (2008) AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog Neuro-Psychopharmacol Biol Psychiatry 32(1):62–71. 10.1016/j.pnpbp.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K (2014) R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. 10.1016/j.pbb.2013.11.033 [DOI] [PubMed] [Google Scholar]


