Abstract
Platelets are small anucleated blood cells primarily known for their vital hemostatic role. Allogeneic platelet concentrates (PCs) collected from healthy donors are an essential cellular product transfused by hospitals to control or prevent bleeding in patients affected by thrombocytopenia or platelet dysfunctions. Platelets fulfill additional essential functions in innate and adaptive immunity and inflammation, as well as in wound-healing and tissue-repair mechanisms. Platelets contain mitochondria, lysosomes, dense granules, and alpha-granules, which collectively are a remarkable reservoir of multiple trophic factors, enzymes, and signaling molecules. In addition, platelets are prone to release in the blood circulation a unique set of extracellular vesicles (p-EVs), which carry a rich biomolecular cargo influential in cell–cell communications. The exceptional functional roles played by platelets and p-EVs explain the recent interest in exploring the use of allogeneic PCs as source material to develop new biotherapies that could address needs in cell therapy, regenerative medicine, and targeted drug delivery. Pooled human platelet lysates (HPLs) can be produced from allogeneic PCs that have reached their expiration date and are no longer suitable for transfusion but remain valuable source materials for other applications. These HPLs can substitute for fetal bovine serum as a clinical grade xeno-free supplement of growth media used in the in vitro expansion of human cells for transplantation purposes. The use of expired allogeneic platelet concentrates has opened the way for small-pool or large-pool allogeneic HPLs and HPL-derived p-EVs as biotherapy for ocular surface disorders, wound care and, potentially, neurodegenerative diseases, osteoarthritis, and others. Additionally, allogeneic platelets are now seen as a readily available source of cells and EVs that can be exploited for targeted drug delivery vehicles. This article aims to offer an in-depth update on emerging translational applications of allogeneic platelet biotherapies while also highlighting their advantages and limitations as a clinical modality in regenerative medicine and cell therapies.
Keywords: Platelet, Allogeneic platelet concentrate, Human platelet lysate, Extracellular vesicles, Regenerative medicine, Cell therapy
Introduction
For a long time, allogeneic platelet concentrates (PCs) have been used in transfusion medicine to prevent or treat bleeding episodes in patients with low platelet counts or platelet dysfunction [1, 2]. Over the years, autologous platelet-rich plasma (PRP) gained increasing–sometimes empiric–popularity, used alone or in combination with biomaterials, as adjunct treatment for various pathologies that required support for healing soft and hard tissues and cartilage [3–5]. Recently, however, interest has sharply grown in evaluating the potential clinical translation of biomaterials derived from allogeneic PCs, such as human platelet lysates (PLs; HPLs), platelet extracellular vesicles (EVs; p-EVs), and formulated platelets [6–8], for applications in cell therapy [6, 9–12], regenerative medicine [13–15], and targeted drug delivery (TDD) [16] as needed for aging societies.
Several reasons contribute to the growing interest in platelet biomaterials made from allogeneic donations rather than autologous sources. First, certain patients may face challenges in donating 50–100 mL of their own blood due to various health conditions, lack of venous access, the presence of comorbidities such as thrombocytopenia (a low platelet count), platelet dysfunctions, diabetes, peripheral arterial and neuropathic disease [17–19], or the use of antithrombotic medications, which can impact the blood-clotting process and platelet functions [20]. Secondly, the variability in autologous platelet derivatives, affecting growth factor content and immune cell and inflammatory cytokine profile, further complicates their use [21]. Lastly, significant variations in platelet count enrichment depending on the type of medical devices and procedures used for isolation contribute to the complexity of autologous platelet-derived biomaterials [21–23]. Considering these challenges, exploring the benefits and addressing the challenges associated with allogeneic sources to prepare platelet biomaterials as “off-the-shelf” products becomes an appealing approach for further investigation [24]. The successful use of allogeneic PRP has been previously documented in the treatment of chronic skin wounds, diabetic foot ulcers [17, 18, 25–27], and osteoarthritis [28]. Similarly, allogeneic serum has shown therapeutic value for dry eye syndrome and other ocular surface pathologies [26, 29–31] especially in situations where autologous materials was unsuitable or inconvenient. Notably, there is a growing interest in the use of allogeneic PCs obtained from healthy donors who meet stringent donation criteria and are processed by blood establishments following licensed monitoring procedures adhering to good manufacturing practices (GMPs) principles and meeting consistent quality criteria [9, 32]. Moreover, the use of allogeneic PCs can offer a more cost-effective alternative compared to autologous sources, as the latter often requires additional handling and testing that can increase overall cost. In contrast, allogeneic PCs undergo thorough screening and testing procedures in regulated blood establishments [33], ensuring a high level of safety as source material of biomaterials.
These developments have involved academic researchers, clinicians, as well as medical device, biotechnology, and cell and biotherapy industries, highlighting the strong translational potential and clinical expectations. Such translational developments have benefited from the vast scientific, medical, and regulatory experience gained over several decades by the well-established blood-transfusion and plasma-fractionation community in supporting scientifically based and safe production and clinical use of allogeneic blood-derived cell and protein products [1, 34], as highlighted throughout this manuscript.
The last few years have seen a growing recognition that allogeneic PCs prepared by blood establishments can be repositioned as a valuable source for various biotherapies. The primary aim of this article is thus to provide a comprehensive overview of the scientific, clinical, and regulatory rationale for considering allogeneic platelets as a valuable cellular source material in human medicine. Similar to mesenchymal stromal cells (MSCs) and other cell types, allogeneic platelets hold significant potential for novel applications. In this context, we conduct a critical analysis of the recent advancements in clinical applications involving allogeneic platelets, HPLs, and p-EVs) in the fields of cell therapy (as xeno-free supplement of growth media used to expand therapeutic cells), regenerative medicine, and as targeted drug delivery systems (TDDS).
Throughout this review, we not only emphasize the various advantages and constraints associated with these translational developments but also draw valuable lessons from the blood transfusion and plasma fractionation industry, which can contribute to the safe and effective translational development of allogeneic platelet-based therapies. By highlighting the safety measures and quality control protocols applied in the blood and plasma products industry, we aim to provide valuable insights into ensuring the safety and efficacy of allogeneic platelet-derived biotherapies. In addition to examining the current state of the field, we also explore the potential future therapeutic applications of allogeneic platelets, HPLs, and p-EVs. By encompassing a broad range of topics, this article aims to serve as a comprehensive resource for researchers, clinicians, and stakeholders looking at novel application of platelets.
Platelet structure, functions, and clinical use
Platelets: essential therapeutic cells for bleeding disorders and beyond
Understanding the structural and functional roles of platelets under physiological and pathological conditions allows us to realize the growing therapeutic value of platelet-derived biomaterials and biotherapies in the fields of cell therapy, regenerative medicine, and TDD. The scientific rationale for these applications derives from the potent functionality of platelet membranes and the richness of their contents.
Platelets are the first blood cells to respond to an injury and play critical roles in blood clotting, wound healing, and tissue repair. They are, after red blood cells (RBCs), the second most abundant cells in blood circulation with a number ranging (150–400) × 106 cells/mL in healthy human individuals [35], and account to close to 5% of total cells in the body [36]. Resting platelets have a lenticular shape with a diameter of 2–4 μm and a thickness of 0.5 μm. Platelets have a relatively short residence time of 7–10 days in the blood circulation under normal physiological conditions and are continually produced by megakaryocytes in the bone marrow for release into the blood as anucleated cells.
Platelets are known most specifically for their vital function in maintaining blood hemostasis. This primarily role of platelets explains why PCs collected from allogeneic blood donors are essential in transfusion medicine to treat bleeding disorders resulting from thrombocytopenia and platelet function defects [37]. As such, platelet concentrates are featured on the World Health Organization (WHO)'s model lists of essential medicines for adults and children [38]. Our understanding of platelet biology, storage methods, and transfusion techniques has significantly improved, leading to the establishment of standardized protocols for PC production from allogeneic donors and evidence-based transfusion therapy [32]. Various international guidelines and studies have been developed to ensure clinicians’ best practices in transfusing PCs to safely and efficiently address bleeding complications and maintain hemostasis in individuals with platelet-related disorders or those undergoing surgical procedures [2, 37, 39]. A similar approach should be followed to guide the production and clinical use of allogeneic platelet biomaterials, including HPLs and p-EVs.
Biochemical bases of platelets’ functions in hemostasis, blood coagulation, tissue repair, and immunity
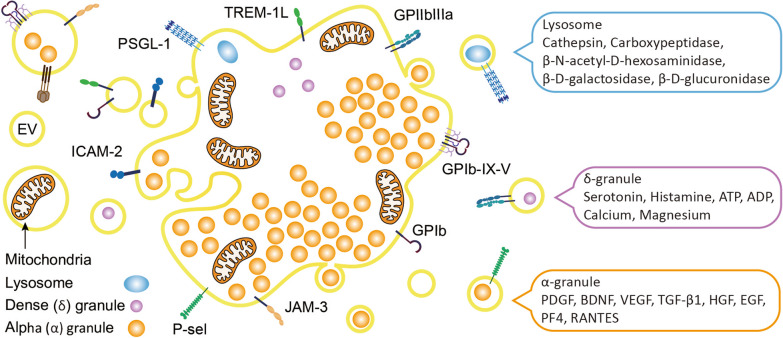
Platelets play a crucial role as the keeper of the integrity of the blood vasculature due to the intimate involvement of their membrane receptors at various stages of the blood-coagulation cascade [40], a series of biochemical reactions that occur in the body in response to injury or damage to blood vessels. It is a complex biochemical process that involves activation of several clotting factors and ultimately results in the conversion of fibrinogen into fibrin, and the formation of a thrombus that helps stop bleeding. The blood coagulation cascade is traditionally divided into two main pathways: an intrinsic pathway, which is activated by contact of blood with a negatively charged surface, such as the surface of a damaged blood vessel, and an extrinsic pathway which is activated by the release of tissue factor (TF) from damaged tissues [40]. Both converge to form thrombin, which converts fibrinogen to fibrin, and leads to a hemostatic platelet fibrin plug [35, 40–44]. A schematic representation of platelets and their biochemical content is provided in Fig. 1.
Fig. 1.
Schematic illustration of the structure, important membrane markers, and cargo of platelets, platelet granules, and platelet extracellular vesicles. ADP adenosine diphosphate, ATP adenosine triphosphate, BDNF brain derived neurotrophic factor, EV extracellular vesicles, GP glycoprotein, HGF hepatocyte growth factor, ICAM intercellular adhesion molecule, JAM-3 junctional adhesion molecule, PF4 platelet factor 4, P-sel P-selectin, PDGF platelet-derived growth factor, PSGL-1 P-selectin glycoprotein ligand-1, RANTES regulated upon activation, normal T-cell expressed and secreted, TGF-B1 transforming growth factor-ß1, TREM triggering receptors expressed on myeloid cells, VEGF vascular endothelium growth factor
Platelet membranes
Platelets have a highly functional and dynamic external membrane that expresses various glycoproteins, integrins, and antigens [45]. These membrane components are pivotal in orchestrating the complex crosstalk existing between platelets and subendothelial structures exposed by an injured blood vessel wall. Biomolecules expressed on platelet membranes also interact with plasma coagulation factors and activators, and protein components of fibrin clots. Membrane glycoproteins are instrumental in platelet adhesion and platelet activation, and recognize blood clotting factors. GPIIb/IIIa, GPIb-IX-V, GPVI, and P2Y12, which are vital in the hemostatic process preceding the wound-healing phase, are highly expressed by platelet membranes [44]. In particular, GPIIb-IIIa (also known as integrin alphaIIb-beta3 or cluster of differentiation 41 (CD41)/CD61, a specific platelet marker), GPIb-IX-V, and GPVI bind to fibrinogen, von Willebrand factor (vWF), and collagen, respectively. Also, P-Selectin, which is expressed by activated platelets, binds to P-selectin glycoprotein ligand (PSGL)-1 which is present, in particular, on leucocytes. The P2Y12 receptor binds to adenosine diphosphate (ADP) which leads to platelet activation and the release of platelet molecules. The lipid bilayer membrane contains negatively charged phospholipids such as phosphatidyl serine (PS), which triggers coagulation upon platelet activation. The rapid exposure of PS upon platelet activation leads to the binding of the activated coagulation factors Va, VIIa, and Xa, the formation of a prothrombinase complex, and the rapid generation of thrombin that converts fibrinogen into the hemostatic three-dimensional (3D) fibrin scaffold enriched with platelet trophic factors (see below) [40, 42, 43]. This natural fibrin-trophic factor biomaterial sustains tissue repair and remodeling by stimulating “in situ” chemotaxis. The activation process of platelets, which leads to their “degranulation”, is accompanied by a sudden change in structure, a fourfold increase in size, the formation of pseudopods, the translocation of receptors, and the secretion of synergistic trophic factors that are stored in inner granules [35].
It is also now well-accepted that various populations of functional EVs are released during this platelet-activation process and can contribute to coagulation and, beyond that, to inflammation but also tissue repair functions [46, 47]. This dynamic phenomenon occurs because the platelet submembrane cytoskeleton is made of actin filaments and myosin and is supported by microtubules present in the sol–gel zone. Microtubules help move the dense granules and alpha granules in the center of the platelets and facilitate the release of their contents upon activation.
Platelet cargo
Platelets contain a rather complex mix of organelles: mitochondria (about five per platelet), lysosomes (one or two/platelet), 150-nm dense granules (ca. five to eight/platelet), and 200–500-nm alpha-granules (ca. 50–80/platelet) and a tubular system that is similar to the endoplasmic reticulum (ER) [45, 48, 49]. The various granules allow the specialized storage of bioactive substances (Fig. 1), which at least for alpha-granules is differentially guided by megakaryocytes [50]. Recent proteomic analyses estimated the number of proteins in platelets to exceed 5000, and platelets were demonstrated to have the capacity to perform some limited translational processes in spite of lacking a nucleus [51]. Mitochondria are vital as a source of energy for platelet metabolism [44, 52]. Lysosomes contain various hydrolases (glucosidase and galactosidase) and proteolytic enzymes (cathepsin D and E, elastase, collagenase, lysosomal-associated membrane protein (LAMP)-2, and CD63 tetraspanin) [45]. Dense granules store adenosine triphosphate (ATP), ADP, Ca++, polyphosphate, magnesium, and several bioactive amines like the serotonin and dopamine neurotransmitters which originate from the extracellular environment and contribute to amplifying platelet activation [53, 54]. Alpha-granules are small, membrane-bound organelles with a complex composition of hundreds of proteins encompassing coagulation factors, adhesion molecules, inflammatory molecules, and a range of cytokines, chemokines, and growth factors [44, 55, 56]. These proteins are either synthesized by megakaryocytes or loaded within platelets by endocytosis. These contents are essential for hemostasis and play vital roles in the inflammatory response and initiation of wound healing and tissue repair. Multiple growth factors are present in platelets and include platelet-derived growth factor (PDGF)-AA, -AB, and -BB, vascular endothelium growth factor (VEGF), transforming growth factor (TGF)-β, brain-derived neurotrophic factor (BDNF), epidermal growth factor (EGF), basic fibroblast growth factor (b-FGF), connective tissue growth factor (CTGF), and hepatocyte growth factor (HGF). Other functionally important cytokines and chemokines in alpha-granules include C-X-C motif chemokine ligand 4 (CXCL4 or platelet factor 4 (PF4)), CCL5 (RANTES), interleukin (IL)-1, and tumor necrosis factor (TNF), which are involved in the inflammatory response. Other proteins in alpha-granules include adhesion molecules, such as fibrinogen and vWF, and enzymes, such as metalloproteinases which play roles in breaking down the extracellular matrix (ECM) during tissue repair. Overall, platelet alpha-granules are thus a rich source of biomolecules that play critical roles in platelet functions, hemostasis, and tissue repair. Recent experimental studies using mouse megakaryocyte cultures confirmed the suspicion that different subpopulations of alpha-granules, with dedicated compositions, exist because of differential sorting and packaging by megakaryocytes [57]. The release of the alpha-granule content can occur in a specific manner depending upon the triggering factor for platelet activation [58]. Interestingly, platelets possess an open canalicular system (OCS) that occupies roughly 1% of their volume [44, 59] and allows two-way communication between the inner platelet compartment, especially alpha-granules, and the blood. The OCS is a route for exchanging biomolecules with the platelet environment, and leads to the storage of functional biomolecules, such as fibrinogen, within platelet alpha-granules [60]. The OCS also contributes to storing calcium ions, and other free ions, in platelets [19], which is vital for platelet aggregation. Furthermore, the OCS regulates p-EV release in response to platelet aggregation. Platelets also contain micro (mi)RNAs which can regulate gene expressions in target cells [61, 62].
Overall, platelets play critical roles in modulating pro- and anti-inflammatory processes and tissue-repair mechanisms. They respond quickly to injury, and their actions help protect the body from blood loss and promote the healing process. In the last stage of the coagulation cascade when activated platelets are entrapped within the hemostatic fibrin network, which act as a powerful scaffold for cell migration and growth, and they release a myriad of trophic factors (growth factors, chemokines, cytokines, enzymes, signaling molecules, etc.) into the tissue microenvironment. These factors bind to specific receptors on membranes of target cells and modulate signaling pathways and gene expressions [13]. These events are essential to stimulate inflammation, local MSC proliferation and differentiation, and angiogenesis, and thereby orchestrate overall tissue-repair and tissue-remodeling mechanisms. As such, platelets play a pivotal role as the most potent “healing cells” of the body. These functions are supported by the fact that only one-tenth of the one trillion platelets in the blood circulation of healthy individuals is needed to control bleeding, and that many of the components of the platelet inner compartment are not directly involved in primary hemostasis [56, 63].
Numerous studies have demonstrated the leading contribution of platelets in inflammation, atherogenesis, immune defense, tumor growth and metastasis, or neuroinflammation [44, 64–71], and one of the best recognized functional roles of platelets is to sustain tissue repair and wound healing [63, 68]. It should be kept in mind that upon activation, platelets release their cargo of trophic factors and signaling molecules in a soluble and free form or loaded within EVs which express platelet membrane markers [72–74]. This feature supports the rationale that free platelet trophic factors, obtained as platelet lysates, and p-EVs have therapeutic value in cell therapy and regenerative medicine and, as described below, as drug carriers. Table 1 provides an overview of the multifaceted functional roles of platelets in tissue repair.
Table 1.
Multifaceted roles of platelets in supporting tissue repair
| Functional event | Key factors involved | References |
|---|---|---|
| Hemostasis/clot formation | Platelets, through their interaction with coagulation factors, are an essential component of a cascade of biochemical reactions leading to the formation of fibrin clots, which help prevent excessive bleeding from injured blood vessels. This platelet–fibrin clot with a tri-dimensional structure acts not only as a temporary hemostatic barrier, but also as a functional scaffold for tissue repair and tissue remodeling | [14, 357, 358] |
| Growth factor release | Platelets store in their alpha-granules a diverse range of growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), vascular endothelium growth factor (VEGF), and insulin-like growth factor (IGF), which can be selectively released upon platelet activation in a free form or packaged into extracellular vesicles. These growth factors promote cell proliferation, angiogenesis, and tissue regeneration | [8, 14, 55, 56, 63, 359] |
| Stimulation of angiogenesis | Platelet-released factors, including VEGF, PDGF, hepatic growth factor (HFG), and basic fibroblast growth factor (bFGF), support the formation of new blood vessels. Angiogenesis helps deliver oxygen, nutrients, and other biological entities, to sites of injury, facilitating tissue repair | [360, 361] |
| Remodeling of the extracellular matrix (ECM) | Platelets release enzymes and molecules involved in ECM degradation and remodeling, such as matrix metalloproteinase (MMP)-2 and MMP-9. MMP-2 and MMP-9 break down proteins like collagen and facilitate tissue remodeling during wound healing. Platelets also release fibronectin and vitronectin that are instrumental glycoproteins involved in cell adhesion, migration, and wound healing. Fibronectin binds to the ECM and promotes the attachment and migration of cells during tissue repair | [63, 362] |
| Modulation of immune responses | Platelets interact with immune cells and release immune-modulating molecules, including chemokines and cytokines, which contribute to regulating inflammation and recruiting immune cells to sites of injury | [63, 362] |
| Recruitment and differentiation of cells | Platelet-derived trophic factors can attract and stimulate the differentiation of various cells involved in tissue repair, such as mesenchymal stem cells and fibroblasts, promoting their migration to and proliferation at sites of injury | [63, 362] |
| Antibacterial defense | Platelets possess antimicrobial peptides and enzymes that aid in combating bacteria multiplication at sites of tissue damage, reducing the risk of secondary infections | [362–365] |
Interactions of intact platelets with other cells
While attention in regenerative therapy is currently focused on the use of HPLs or naïve p-EVs, as a source of various trophic factors, known interactions of intact platelets with other cells can justify exploration of new clinical indications. Indeed, the functions of both intact and activated platelets are intimately linked to their ability to interact with other cell types and biomolecules in the body, which support the use of loaded platelet- or p-EV-based therapies as DDSs [8, 16, 75]. For instance, as mentioned above, platelets interact with endothelial cells to form a physical barrier that helps maintain vascular integrity. Activation of the endothelium triggers platelet adhesion and aggregation, which is crucial for hemostasis and wound healing by promoting tissue repair and preventing excessive bleeding. Activated platelets can enhance leukocyte recruitment to sites of inflammation and infection, influencing the immune response, playing an instrumental role in the development of some diseases like atherosclerosis [76, 77], and modulating both innate and adaptive immune responses [78]. Platelets and neutrophils are increasingly recognized as playing synergistic roles, involving the stimulation of neutrophil extracellular traps (NETs), in thromboinflammation and pathogenesis of neuroinflammatory diseases [71]. Additionally, platelets interact with various extracellular matrix components, such as collagen and vWF, which facilitate platelet adhesion and activation during injury or vessel damage and promote tissue regeneration and repair [63]. Platelets play a central role in the coagulation cascade by interacting with coagulation factors, such as fibrinogen and thrombin, leading to clot formation and hemostasis. Thus, therapies using platelets or p-EVs as DDSs can be engineered in a tailor-made fashion to target these interactions and further enhance clot formation or, on the contrary, prevent thrombosis [79]. p-EVs, which carry a diverse cargo of bioactive molecules, can be released by platelets during interactions with other body cells, including cancer cells [80]. These p-EVs can interact with target cells and modulate cellular processes.
The understanding and control of platelet interactions with different cell types and biomolecules may thus help tailor therapies for specific clinical applications in regenerative medicine and cellular/subcellular therapies to promote tissue repair, regulate immune responses, enhance angiogenesis, modulate coagulation, and treat pathologies.
Allogeneic PC collection and properties
Collection procedures
Platelet derivatives, including PRP and “platelet gels” used for regenerative medicine, are still typically obtained from autologous patient-specific blood fractions [81, 82]. However, there is now an increasing focus on considering allogeneic (donor-derived whole blood or apheresis) PCs prepared by blood establishments and hospital blood banks as starting materials to make platelet-derived biomaterials [17–19, 24–27, 83–86]. Preparation procedures of PCs from allogeneic blood donors by blood establishments (a term equivalent to blood centers) using whole-blood donations or plateletpheresis procedures were described in previous publications [9, 10, 87–89] and are summarized below.
Whole blood collection
Allogeneic PCs can be obtained from whole blood donations, where a donor donates by venipuncture a unit of whole blood (typically 200–450 mL depending upon the jurisdiction) that contains RBCs, plasma, and platelets. Whole blood-derived PCs, known as “random-donor” platelets, are obtained as byproducts of RBC preparations. These platelets can be processed by the PRP or the buffy coat (BC) separation procedures [9]. To collect whole blood-derived platelets, 450 mL of whole blood, containing RBCs, white blood cells (WBCs), platelets, and plasma is collected in a polyvinyl chloride bag containing 63 mL of an anticoagulant solution typically composed of citrate, glucose, and adenine. It is important to complete the donation procedure in less than 12–15 min to minimize the risk of activating blood coagulation and platelets. The donated blood can be stored at 22 ± 2 °C for up to 24 h. Indeed, if PCs are being prepared, the blood should not be cooled down to 2–6 °C, as this can result in the loss of platelet viability and the formation of platelet and leukocyte microaggregates. The first centrifugation step of whole blood plays a crucial role in determining PC characteristics. It is important to apply precise conditions of g-forces, acceleration, time, and deceleration to ensure the separation of different blood components and obtain PCs with specific and consistent compositions [90].
In the PRP method, the whole-blood donation undergoes light spinning centrifugation (typically 1000 × g) for around 10 min at 21–22 °C, using validated acceleration and deceleration curves. Afterward, the PC is left at room temperature for about 1 h and then resuspended in 50–70 mL of plasma [90]. The PRP method is the predominant approach for producing platelets from whole blood in the United States.
The BC procedure involves subjecting anticoagulated whole blood to vigorous centrifugation at approximately 3000 × g for 5 min. Thirty milliliters of plasma is returned to the BC layer and gently mixed before undergoing another round of centrifugation. This time, light spinning centrifugation at approximately 1000 × g for 6 min at 21–22 °C is applied, employing validated acceleration and deceleration curves [90]. The resulting supernatant is subsequently placed in a designated storage bag and stored at 22 ± 2 °C. In more-recent techniques, a platelet additive solution (PAS), containing sodium/potassium chloride, citrate, phosphate, and mannitol, can be employed to replace a portion of the plasma. The BC method is particularly favored in Europe due to its ability to effectively deplete leukocytes through BC removal, thereby reducing the incidence of febrile transfusion reactions and facilitating efficient leukodepletion when combined with leukocyte filtration. To obtain an adult therapeutic dose equivalent to that of a single apheresis platelet procedure, it is necessary to pool the platelets collected from four to six whole blood donations.
Plateletpheresis collection
Apheresis PCs can be prepared using automated cell separators and a closed collection and processing system. These dedicated extracorporeal procedures employ intermittent or continuous centrifugation to separate and collect platelets. Multiple cell separators with varying collection principles are available for platelet collection [89]. At the point of withdrawal from the donor, whole blood is anticoagulated in a controlled manner with a blood/anticoagulant ratio of 9:1–11:1: Typically, RBCs are returned to the donor, and, potentially, the plasma can be recovered in the separate bag (“concurrent plasma”) and used for clinical applications or fractionation. The volume of such PCs is typically 200–300 mL per donation, with an enrichment in platelets of about 6–eightfold compared to blood. Apheresis PCs are obtained from a single donor and are not pooled for transfusion purposes. During processing, the PC may or may not be subjected to a leukoreduction step, usually by dedicated filtration, to reduce the content of WBCs, and may be formulated in 100% plasma or in a combination of plasma and PAS. Plateletpheresis procedures yield a higher concentration of platelets compared to whole-blood donations. Indeed, a larger number of platelets can be collected in a single plateletpheresis session. The plateletpheresis procedure takes longer donation times and may require specific vein accessibility for the apheresis machine. Plateletpheresis frequency varies based on individual health and regulatory guidelines but is typically up to 24 per year in most jurisdictions [91]. Regardless of the procedure used, production of PC should comply with GMPs to meet established quality, safety and consistency criteria for transfusion use [32].
Variables in PC collection
Several variables in PC production methods can influence the platelet, leukocyte, and protein contents. For instance, apheresis PCs usually have significantly lower WBC contamination than standard BC-derived or PRP-derived PCs [92]. Procedures for preparing PCs may include, as mentioned above, a leukoreduction step using different types of leukoreduction filters [93, 94]. When leukocytes are present in PCs, the resulting lysates may contain higher amounts of cytokines. In addition, until recently, PCs were suspended in 100% plasma. However, residual plasma may mediate transfusion-related adverse reactions in recipients when used intravenously. Therefore, PAS may be used to substitute for part, usually 2/3, of the plasma volume [95, 96], decreasing the protein content and the presence of plasma nutrients. Treatments of PC by gamma-irradiation, to inactivate leucocytes and prevent a risk of graft-versus-host disease upon transfusion, or by photochemical treatments, to inactivate pathogens, may affect the platelet proteome or stimulate the release of cytokines [88, 97, 98]. All these variables in the preparation of allogeneic PC may potentially impact their performance in cell therapy or regenerative medicine applications and should be thoroughly recorded and monitored to draw scientifically-based conclusions in the use of platelet-derived biomaterials.
Pathogen reduction treatments of PCs
The implementation of pathogen reduction treatment is a determining factor in the preparation of PCs as it may significantly impact the virus safety of pooled platelet-derived materials [99, 100]. Indeed, in some jurisdictions, individual PC donations intended for transfusion can be subjected to a licensed pathogen-reduction step aimed at inactivating bacteria, viruses, and/or parasites. Pathogen reduction is achieved by adding to the PC donation a small molecule, like psoralen [101] or riboflavin [102], that can penetrate cells and nuclei, bind to nucleic acids and, upon UV light activation, induce alterations making the viruses noninfectious [103–106]. Such photochemical treatments are possible because platelets are anucleated. Extensive validation studies conducted by the developers of pathogen-reduction treatments have generally demonstrated their effectiveness in inactivating a wide range of pathogens [107, 108] and reducing the risk of transmission of viruses (e.g., HIV, hepatitis B and C viruses), bacteria, and parasites. However, some limitations in the extent of inactivation of enveloped and non-enveloped blood-borne viruses resistant to UV-based treatments were also reported [109, 110], suggesting that certain viruses may escape complete inactivation when platelet donations contain infectious titers above the inactivation threshold provided by the technique. Such potential residual viral infectivity, which is even more relevant when the PC are not subjected to a pathogen-reduction treatment, raises specific concerns for the viral safety of allogeneic platelet-derived HPLs or p-EVs when they are made from PC pools as more recipients are at risk of a blood-borne infection. Furthermore, these pathogen-reduction steps might not eliminate the risk of transmission of emerging or unknown viruses, as some viruses may exhibit varying levels of susceptibility to the employed technologies. Therefore, countermeasures to enhance the virus safety margins include limitation of the pool size [100], testing of mini-pool and manufacturing platelet pool for relevant virus markers, or additional dedicated virus-reduction treatments [6, 99], as described below.
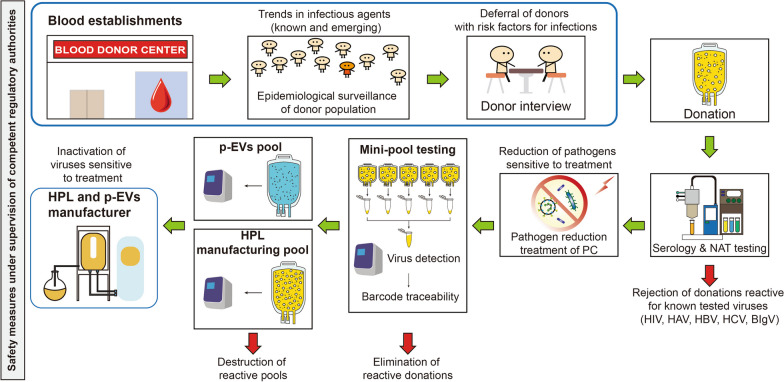
The expertise developed by the blood transfusion industry over the last 4 decades in establishing measures to protect the blood supply against known and emerging viruses, including the development, validation, clinical evaluation, licensing, and implementation of dedicated pathogen reduction treatment [103, 111–113], can be of great value to the development of safe and efficient allogeneic PCs as source materials for biomaterials. The various complementary safety measures that can be considered for the production of pooled platelet-derived materials, based on experience developed by the plasma fractionation industry [34], are summarized in Fig. 2.
Fig. 2.
Overview of the various virus safety measures in place in the blood transfusion and industrial plasma fractionation industry, and supervised by regulatory authorities, as potential models to be implemented for optimal virus safety margin of pooled human platelet lysates and platelet extracellular vesicles for clinical applications. p-EV platelet extracellular vesicles, HPL human platelet lysate, NAT nucleic acid testing, PC platelet concentrate
Additionally, the safety of PCs and the effectiveness of licensed pathogen-reduction treatments of PCs for transfusion are continuously being assessed in advanced countries through hemovigilance programs overseen by regulatory authorities to ensure timely implementation of appropriate countermeasures, such as donor exclusion criteria and screening, as well as donation testing, to minimize the transmission of infectious agents in allogeneic PCs [113–115].
Storage of PC
Platelets undergo physiological and functional changes upon storage which may affect therapeutic benefits to the recipient, or further applications in cell therapy or clinical use. PCs are stored at 20–24 °C for up to 5–7 days, depending upon the jurisdiction and specificities of the collection and test procedures [9, 10, 87–89]. They should be stored under continuous gentle agitation in a bag made of cell-compatible plastic with enhanced oxygen permeability and diffusion of carbon dioxide. The pH should remain above pH 6.4. Temperatures below 20 °C may induce platelet activation and release platelet storage components into the plasma. Proper storage conditions limit the risk of activation and maintain an unaltered hemostatic state for intravenous infusion. Agitation enables good mixing and gas exchange. Folding of the bags and foaming should be avoided. Platelets used for transfusion can typically be stored for up to 5–7 days, depending upon national regulations, after which time they can no longer be used for transfusions and are currently discarded. However, such expired (or outdated) PCs can be used as source material to prepare lysates for propagation of therapeutic human cells [6, 116]. The PC are then frozen at − 20 °C or colder until being processed into HPLs.
Table 2 summarizes the most significant characteristics of allogeneic PCs available from blood establishments using current production procedures. Briefly, all therapeutic PC unit should have a total platelet count exceeding 2 × 1011. Limits in the acceptable residual leukocyte content (of < 109 to < 106) depend upon whether the PC is stored in 100% plasma or a combination of plasma (30–40%) and additive solution (60–70%), and whether a leukoreduction step is used.
Table 2.
Characteristics of the various types of allogeneic platelet concentrates prepared by blood establishments for transfusion purposes
| Processing method | Suspension and storage solution | Leuco-reduction step | Cell count/PC unit | ||
|---|---|---|---|---|---|
| Plasma (%) | Platelet additive solution (%) | Platelets | Leukocytes | ||
| Whole blood (buffy coat or PRP methods) | 100 | > 2 × 1011 | < 109 | ||
| 100 | Yes | < 106 | |||
| 30–40 | 60–70 | 0.3 × 109 | |||
| 30–40 | 60–70 | Yes | < 106 | ||
| Plateletpheresis | 100 | < 0.3 × 109 | |||
| 100 | Yes | < 106 | |||
| 30–40 | 60–70 | < 0.3 × 109 | |||
| 30–40 | 60–70 | Yes | < 106 | ||
PC platelet concentrate, PRP platelet-rich plasma
Regulatory oversight of PC collection
In countries with a mature regulatory system, blood establishments responsible for producing blood components, including platelet fractions, should have a strict quality system in place based on GMP principles and should comply with local regulations [117, 118] to ensure the quality and consistency in meeting specifications. Anticoagulants, any additive solutions, and collection and storage bags should be licensed. Preparation equipment should be validated and premises maintained under clean and hygienic conditions and monitored. Preparation of PCs and pooling should be done using aseptic procedures, and either multiple bag configurations or sterile connecting devices used following a validated procedure for closed-system processing. Although variability linked to individual donors cannot be eliminated, such requirements ensure a relative consistency in the characteristics of the various types of allogeneic PCs, particularly platelet and leukocyte counts, used to prepare PLs for MSC expansion, which should contribute to higher reproducibility.
Advantages and constraints of allogeneic PCs as source materials for platelet-derived biomaterials
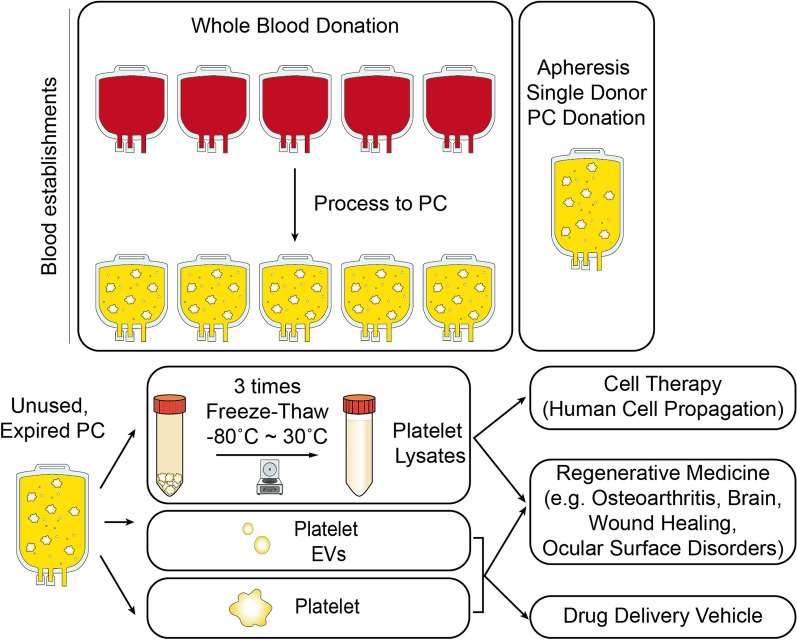
Figure 3 provides a typical scheme of the production steps of allogeneic PCs and their processing for preparing different classes of derivatives (HPL, EVs, isolated platelets) for application in cell therapy industry, regenerative medicine, and drug delivery, as discussed in the following section of this article.
Fig. 3.
Overview how allogeneic PCs may be used for preparing platelet lysates, extracellular vesicles, or purified platelets for novel applications in cell therapy for human cell propagation, regenerative medicine in various clinical indications, and as drug delivery vehicles. PC platelet concentrate, EVs extracellular vesicles
Objective reasons justify the interest in using allogeneic PCs to prepare platelet-derived biomaterials for translational cell therapy and regenerative medicine. First, the use of PCs from a donor represents a readily available clinical-grade therapeutic source that is highly valuable when the patients themselves are physically unable or unwilling to donate blood for their own treatment [17–19]. In addition, allogeneic PCs donations are available in substantially larger volumes (ca. 250 mL) than autologous PCs (20–100 mL), which allows for a wider range of clinical benefits, including larger dose sizes and multiple administrations to patients. One valid argument often mentioned supporting autologous platelet donations is the absence of risks of pathogen transmission. However, in a regulated blood-collection setting from volunteer-screened repeat blood donors, as is the case for licensed blood establishments in regulated countries, a single-donor allogeneic PC has a high margin of viral safety [119]. Individual blood donor candidates are screened to assess their suitability for blood donations, and they should have low risk factors for transfusion-transmitted viral infections. When a donor is eligible, dedicated mandatory blood testing of each individual donation is performed to verify non-reactivity (such as the absence of antibody markers or viral nucleic acids) against human immunodeficiency virus (HIV), hepatitis B virus (HBV), HCV, and, in some jurisdictions, other viruses such as West Nile virus or parvovirus B19 [120]. Any reactive donations are discarded, and the blood donor deferred, temporarily or permanently, from subsequent donations depending upon the specificity of the infection identified. Additionally, in wealthy countries with advanced blood-donation systems, PCs are subjected to dedicated pathogen reduction using a licensed photochemical treatment, as mentioned above [99, 100]. The product is then labeled, sealed, and shipped to hospitals for clinical use. Thus, using PCs from blood establishments presents the advantage of guaranteeing safety and consistency in total platelet counts, with 4–sixfold enrichment in platelet counts compared to the baseline level in the blood circulation, and with production methods that can yield minimal residual RBC and white blood cell (WBC) contamination [32]. Controlled operating procedures contribute to minimizing platelet damage, providing an optimal material as a TDD vehicle. Additionally, allogeneic PCs have a well-understood clinical safety and efficacy background for transfusion to trauma and surgical patients, facilitating translation of PCs to emerging clinical indications. Lastly, mounting experimental evidence has shown that 5–7-day outdated allogeneic PCs can be frozen and further processed into derivatives, such as HPLs as a growth medium supplement for human cell propagation [6, 10, 121]. The possibility of processing frozen outdated allogeneic PCs is valuable to facilitate the supply, avoids wastage of precious blood resources, and supports the sustainability of blood establishments as it avoids wastage of this blood component and cost of its destruction [6].
Although allogeneic PCs prepared by a given mode of production provide consistent specifications [32], some variations in preparation procedures, as highlighted above, including use of a PAS in place of plasma [122] or pathogen reduction [103–105], can impact the PC characteristics, and therefore those of biomaterials made from the PCs. For instance, PCs suspended in 100% plasma retain the integral composition and concentration of the native plasma. This implies that HPLs derived from such unprocessed PCs also contain, in addition to platelet components, valuable plasma proteins, such as albumin, immunoglobulins, the full range of coagulation factors, including fibrinogen, and physiologically important anticoagulants, protease inhibitors, and plasma-borne growth factors including most of the IGF-1 present in the blood circulation [123, 124], as well as plasma-borne lipids and EVs [125–128]. This type of HPL, with a total protein content close to 60 mg/ml was found by numerous studies to be optimal for expanding human cells [6]. In contrast, PCs suspended in a mixture of plasma and PAS are partially depleted of plasma protein components proportionally to the extent of the substitution by PAS, but concentrations of platelet-derived components remain unaltered in the corresponding HPLs as the platelet count is unchanged. Experimental data illustrated that the use of PAS can affect the capacity of the resulting HPLs to expand MSCs [129], although their performance in cell propagation still remained superior to that obtained with FBS supplementation [128, 130]. Similarly, the total content of EVs derived from the blood circulation at the time of collection is expected to be higher in PCs suspended in plasma than in a mixture of plasma and PAS, as the collected plasma contains EVs originating from erythrocytes, platelets, megakaryocytes, and leukocytes [131]. Platelets stored in plasma may maintain superior functionality, as plasma provides a physiological environment for preserving platelet viability and activity. On the other hand, platelets stored in PAS may exhibit some functional alterations due to the absence of certain plasma components. One study identified that the type of PAS used can have a significant impact on the level of platelet activation, exposure to CD62P, content of soluble glycoprotein V (sGPV) and the size and number of p-EVs as found by nanoparticle tracking analysis [131]. However, impacts on the storage of platelets in plasma compared to storage in plasma/PAS mixtures or various types of PAS deserve further analysis. From another angle, the use of PAS was recently found to be associated with lower accumulation of complement activation products compared to plasma, which could result in less risk of side effects, at least for clinical transfusions [132], and potentially some therapeutic indications of platelet biomaterials.
Another important factor potentially affecting characteristics of PCs is implementation of pathogen-reduction treatments. Although photochemical inactivation and UV light treatment are considered to have minimal impacts on platelets, it was shown that riboflavin/UVB induced protein upregulation on EVs at day 7 of storage, reflecting the presence of platelet storage lesions potentially predictive of adverse transfusion reactions [133]. In conclusion, the choice of preparation method and pathogen-reduction steps can influence the storage duration and conditions of PCs and their derivatives. Some methods may extend the shelf life of platelets by reducing the risk of bacterial transmission, allowing for longer storage periods, but also possibly impacting platelet viability and functionality and use in cell therapy and regenerative medicine. Documented monitoring of process variables is therefore needed to optimize consistency in functional outcomes of platelet derivatives.
Use of allogeneic HPL for cell propagation
Preparation of the platelet lysate or releasate
Allogeneic PCs can be used to prepare “lysates” by breaking down or “releasate” by activating platelets to release their contents of growth factors, cytokines, enzymes, signaling molecules, and other biomolecules valuable for propagating and differentiating therapeutic human cells for transplantation [6, 9, 10] (Fig. 3). This is achieved by combining physical, mechanical, and chemical treatments such as freeze–thaw cycles [134], ultrasound or sonication [135], or solvent/detergent (S/D) [136], respectively, followed by downstream centrifugation and filtration [6, 137]. Fibrinogen seroconversion into fibrin may additionally be performed to prevent growth medium gelation during cell culturing. This can be achieved by adding calcium chloride [6, 10], or preferably a combination of calcium chloride and glass beads as synergistic catalysts of the coagulation [126, 127, 138, 139], thereby avoiding the need for the addition of an anticoagulant such as heparin to avoid gelation of the cell culture medium [10, 121].
Virus inactivation or removal during HPL processing
Recent articles [99, 100], inspired by vast knowledge on complementary measures needed to guarantee the viral safety of fractionated pooled plasma-derived medicinal products [34, 140, 141], stressed the fact that HPLs, when made from pooled PCs, inherently have statistically higher risks of contamination by blood-borne viruses. It was demonstrated that lysates made from PCs pathogen-reduced by psoralen/ultraviolet (UV) A [142–145], riboflavin/UV treatment [146] or short-wave UV light treatment [147] can maintain their functionality to support the propagation of therapeutic MSC in vitro. This is vital considering that pooling of over 50 donations [6, 12, 148], which increases the risks of contamination by blood-borne viruses [99, 100, 149], has been considered a suitable pool size to provide consistency in HPL quality and improved productivity.
As is the case with the manufacture of pooled plasma protein products, the manufacture of large-pool HPLs requires the application of GMPs and a combination of appropriate procedures to minimize risks of viral transmission to recipients, including platelet donor screening, donation and mini-pool and manufacturing HPL pool virus testing and virus-reduction treatment(s) (Fig. 2). As the efficiency of pathogen-reduction treatments of PC donations has some limits in the range of viruses inactivated, as mentioned above [109, 150, 151], virus-reduction treatments of pooled HPLs must be considered when feasible. Measures implemented should be based on scientific evidence and integrate risk assessments that consider the HPL pool size [99, 100].
Here again, the experience gained with pooled human plasma products is of interest as authorities regulating industrial plasma products recommend manufacturers to implement two robust validated orthogonal (i.e. different and complementary) virus-reduction treatments [152, 153]. The plasma fractionation industry has thus developed several robust virus-reduction procedures applicable to complex or purified plasma protein products. Historically, heat treatment in the liquid state at 60 °C for 10 h, known as “pasteurization”, was applied to human serum albumin, a relatively heat-stable protein, stabilized by low doses of caprylate or acetyltryptophanate to inactivate HBV and other blood-borne enveloped and non-enveloped viruses [154]. Various formulations of stabilizers combining high doses of sugars, polyols, and/or amino acids were also developed to pasteurize coagulation factors, protease inhibitors, and immunoglobulins [154]. In most cases, pasteurization requires a subsequent step of tangential flow filtration to remove the stabilizers [34, 140, 155]. Dry-heat virus inactivation treatment refers to heating a lyophilized protein preparation at temperatures ranging from 60 to 100 °C for durations varying between 0.5 and 96 h depending upon the proteins and the viruses targeted [34, 140, 156]. Low-pH (at ca. pH 4–4.5) treatment in the presence of small quantities of pepsin was demonstrated by the plasma fractionation industry to be a potent virus-reduction treatment of lipid-enveloped viruses in intravenous immunoglobulin preparations [34, 140, 157]. Chemical S/D treatment using combinations of a solvent (tri-n-butyl phosphate, TnBP) and various non-ionic detergents (e.g. Tween-80, Triton X-100, and Triton X-45) at temperatures ranging–20–37 °C for 1–6 h is extremely efficient at inactivating lipid-enveloped viruses and is currently applied to whole plasma [158, 159] and to a large range of PDMPs [160]. The S/D agents can be removed by chromatography and/or oil extraction [140, 158]. Finally, the plasma fractionation industry has largely adopted a dedicated virus-removal step, typically known as “nanofiltration” that consists of filtering plasma protein solutions through specifically designed virus filters with pore sizes of 15, 20, 35 nm, or equivalent [161, 162], to remove enveloped and non-enveloped viruses based on size-exclusion [163]. Among those virus-reduction treatments of plasma products, only solvent/detergent (S/D) can be directly applied to complex protein solutions like HPLs [126, 130, 136, 164] as other methods would most likely denature or precipitate most of HPL components. Other virus-reduction treatments developed for pooled HPLs include gamma-irradiation [146, 164, 165] and electron (e)-beam irradiation [166]. Virus removal by sequential 35–20-nm nanofiltration of 10% HPL-supplemented growth media has been proven to be feasible [128, 167], providing a procedure that also avoids the risks of contaminating the cells by adventitious viruses during media preparation. Combining psoralen/UVA treatment of PC donations and S/D treatment of pooled HPLs was shown to be technically feasible [130], as is nanofiltration of growth media supplemented with HPLs made from psoralen/UVA-treated PCs [128]. These methods can effectively inactivate or remove a broad range of enveloped and non-enveloped viruses, including HIV, HBV, HCV, West Nile virus, hepatitis A virus (HAV), and parvovirus B19. Therefore, technical solutions exist to ensure the needed margin of safety of large-pool HPLs used for human cell propagation, thus complying with existing regulations of blood-derived products [99, 100]. Additionally, the starting HPL donations, assembled as an experimental mini-pool, and HPL manufacturing pool can be tested to confirm that virus contamination is not detectable, as done in the plasma-fractionation industry [34]. Mini-pool testing makes it possible to eliminate reactive donations prior to assembling the whole donations into a large-scale manufacturing pool, therefore serving as a precautionary measure to limit the risks of destroying a manufacturing pool ultimately found to be contaminated by a virus (Fig. 2).
Allogeneic HPL-based propagation of human cells for cell therapy
HPL can serve as a substitute for FBS as a supplement in growth media for propagating various therapeutic cells, with most of the experimental data obtained thus far focused on MSC expansion, but now expanding to differentiated cells and immune cells [121], as explained below.
HPLs made from clinical-grade allogeneic PCs, including those that are “outdated” (i.e., after 5–7 days of storage at 20–24 °C after collection), are a proven efficient growth medium supplement to successfully substitute for FBS for in vitro xeno-free MSC expansion [6, 10–12]. MSCs can be isolated from bone marrow and adipose tissues and expanded successfully in HPLs, exhibiting proliferation, immunophenotype, and differentiation properties that meet the criteria of the International Society of Cell and Gene Therapy. MSCs expanded in media supplemented with HPLs grow faster than in FBS (meaning a shorter doubling time), maintain clonogenicity and typical MSC immunophenotypes, and exhibit an unaltered differentiation capacity into the three referenced lineages (chondrocytes, osteocytes, and adipocytes) as well as immunosuppressive functionality [137, 149, 165, 168]. Better performance using HPL than FBS may possibly be due to the human origin of the HPL and/or the higher concentration of platelets in PC than in bovine blood, resulting in a higher concentration of growth factors. It was observed in some studies that supplementation with HPLs versus FBS favors MSC differentiation into chondrocytes [126, 128, 130, 169]. HPLs are also a suitable supplement to expand MSCs from other tissue sources such as Wharton jelly [126, 170], umbilical cords [171–174], amniotic fluid [175, 176], dental pulp [177–179], periodontal ligaments [180], and others [121]. HPLs are therefore emerging as a universal supplement for in vitro xeno-free expansion of MSCs [12, 121, 181] for clinical transplantation. Studies to identify which types of HPL provide optimal expansion of a given MSC isolate are however recommended [6].
The number of publications describing the use of HPLs for expanding differentiated cells is also growing [121], most studies evaluating whether such xeno-free growth culture conditions can sustain the quality of in vitro-expanded cells for optimal therapeutic outcomes. One focus has been on expanding primary articular chondrocytes intended for treatment of joint alterations, cartilage damage, and osteoarthritis. HPLs stimulate proteoglycan and glycosaminoglycan (GAG) production, as well as the synthesis of collagen type II by chondrocytes and upregulation of SRY-box transcription factor 9 (SOX9) gene expression [182]. Better expansion of human chondrocytes was also stimulated by HPLs, although chondrogenic differentiation was more pronounced with FBS [183]. Mixed results were identified in other studies with regards to the capacity of HPLs to support cartilage matrix formation in vitro or to enhance chondrogenesis [121, 184–186]. Recent studies found that HPLs can stimulate the propagation of pediatric auricular cartilage stem/progenitor cells, enhanced the preservation of a spindle-like morphology, with good preservation of surface marker expression, and supported expressions of collagen type II and aggrecan. However, defects in matrix deposition were observed [187]. More systematic studies are deemed necessary to understand the functional effects of HPLs and determine the type and dose of HPLs optimal for chondrocyte expansion. Several studies have consistently pointed out the value of HPLs for xeno-free expansion of human corneal endothelial cells [188–191]. HPL-expanded cells present a good adhesion capacity, hexagonal morphology, and viability, and express vital functional membrane markers such as Na–K ATPase a1, zona occludens-1, phospho-connexin 43, and N-cadherin [188–190, 192]. However, not unexpectedly, not all HPL types are fully equivalent as a supplement for corneal endothelium cells (CEC) expansion. For instance, 1-h treatment of platelet lysate materials at 56 °C was found to enhance the proliferation of CECs [188, 193]. Recent data suggest that HPLs may be of value for safe clinical-grade expansion of dermal fibroblasts for cell therapy and tissue-engineering intended for the treatment of facial scars and burns [194] as well as for the expansion of tenocytes [195].
HPLs also appear to be useful for xeno-free expansion of immune cells like macrophages [196], dendritic cells (DCs) [197], T-lymphocytes, and chimeric antigen receptor (CAR)-T cells [196, 198]. HPL-expanded DCs exhibit good viability, a normal morphology, a satisfactory endocytic capacity, normal phenotypes, and good functional plasticity [197]. They may, however, have a lower type-1 polarization capacity, possibly affecting the suitability to be used as DC vaccines [199], further highlighting that more studies are needed to optimize culture conditions. HPLs can also help in expanding T-cells and CAR-T cells [200–203]. Medium supplementation with one pathogen-reduced allogeneic HPL decreased the doubling-time of a certain type of CAR-T cells [204], increased the percentage of TCM cell subsets, and improved cell functionality, their in vivo antitumor effects, and otherwise overall performance [198, 202, 205, 206]. A pathogen-reduced HPL showed benefits for preparing T cell- and CAR-T cell-based therapies, with a higher proliferative capacity of T-cells and increased CD4 + and CD8 + cell numbers [207]. By decreasing the doubling time of CAR-T cells, HPLs allow cells to replicate more quickly and multiply in greater numbers, thus increasing the treatment efficacy. Additionally, by increasing the percentage of central memory T-cells (TCM) cells, which have greater antitumor effects, HPLs may further increase the therapeutic efficacy. Further research into the use of pathogen-reduced allogeneic HPLs will help define how researchers can increase the efficacy of CAR-T cell therapy in cancer treatment, while research to improve the productivity and performance of CAR-T technology may help expand this cell-based therapy to other clinical domains [200], including for treating solid tumors. These data encourage further evaluation of how pathogen-reduced allogeneic HPL culture medium supplementation can help comply with the clinical safety of T-cell and CAR-T cell therapies and also enhance their efficacy and domains of application.
While HPLs demonstrates robust benefits in facilitating clinical-grade expansion of human cells for transplantation, keeping track of the manufacturing attributes and biochemical characteristics of the HPLs is needed to further improve standardization of use and clinical outcomes [6, 11]. More data are needed to confirm that outdated and pathogen-reduced allogeneic PCs can successfully be used to prepare HPLs to efficiently expand differentiated cells and immune cells for transplantation.
Allogeneic HPLs for regenerative medicine
While many studies have reported the use of PRP as a growth factor-rich surgical adjunct for direct clinical applications in implantology, dermatology, orthopedic surgery, sports medicine, and other fields (see [208–211] for review), only limited preclinical or clinical evaluations of the use of HPLs made from allogeneic PCs have so far been conducted.
Ocular treatments
The first example of a move from an autologous to an allogeneic product is seen with the preparation of serum eye drops (SEDs) and other eye drops of human origin (EDHOs), including HPLs, for treatment of dry-eye syndrome and other ocular surface disorders [30, 31, 84, 212, 213]. This situation can be explained by the fact that blood establishments producing SEDs realized the limit and logistical constraints of autologous serum production, and the incapacity of frail patients, or those unable to travel to a blood donation or apheresis center, to donate blood [85]. Indeed, relying on autologous serum presents several challenges. First, the collection and processing of blood from individual patients can be difficult due to limitations of donor eligibility, such as poor venous access or underlying health conditions, making blood donation a challenging process. Second, the production of autologous serum requires repeated blood draws from the same patient at regular intervals, which might not be feasible or practical for long-term use. This process can be burdensome and inconvenient for both patients and healthcare providers. Moreover, there may be inherent variability in the composition of autologous serum, including growth factors, cytokines, and other bioactive molecules, depending on the patient’s health condition. This variability in composition may potentially impact the effectiveness of the resulting treatment. Finally, the volume of blood collected from each individual patient is limited, which can impact the overall cost of the production process, including expenses associated with individual patient’s blood processing, monitoring, and customization [31]. These reasons make production from allogenic donations more cost-effective and better standardized and compliant with GMPs [83, 86, 213].
Therefore, a trend was seen towards developing small-pool allogeneic HPLs or serum, including with the inclusion of pathogen-reduction by riboflavin/UV treatment [214], for treatment of ocular surface disorders, but to our knowledge, using outdated PCs as source material has not been reported. Experimental studies demonstrated the possibility of performing S/D virus inactivation treatment of SEDs without substantially affecting their functional properties [215]. Further research can be conducted to establish whether any virally inactivated pooled HPLs from outdated PCs can be a therapeutic alternative for treating ocular surface disorders [31, 216]. Such research will need to consider various aspects to demonstrate feasibility, efficacy, and safety through well-designed preclinical and clinical studies aiming at assessing the effects on corneal epithelial healing, wound closure, inflammation reduction, and an overall improvement in ocular surface health [31, 217], with reference to existing guidelines [218]. Investigating the underlying mechanisms of action of such allogeneic HPLs by elucidating specific bioactive factors, growth factors, potent EVs, and molecular pathways involved [219, 220], and determining potential side-effects can provide insights into the regenerative and anti-inflammatory mechanisms of allogeneic HPL eye drops, and help determine key quality control tests and specifications [31]. Studies should also conduct stratification approaches based on disease severity, etiology, or other relevant factors to unveil patient subpopulations most benefiting from a particular type of allogeneic HPL preparation.
Neurological disorders
Interestingly, allogeneic, including outdated and pathogen-reduced, HPLs are being investigated in preclinical animal models for treating neurological disorders of the central nervous system (CNS) (see [7, 70] for recent reviews) including neurodegenerative diseases, such as Parkinson’s disease (PD) [221–223], Alzheimer’s disease (AD) [224, 225], amyotrophic lateral sclerosis (ALS) [226], as well as stroke [227]. and traumatic brain injury (TBI) [222, 228–230]. These HPLs are a pleiotropic source of trophic growth factors, such as PDGF, TGF-β, brain derived neurotrophic factor (BDNF), and antioxidant and anti-inflammatory molecules [7, 219, 229]. These factors exert neuroprotective, neuroregenerative, and neurotrophic properties, supporting the survival, growth, and function of neurons in the CNS [7]. Additionally, allogeneic HPLs contain bioactive molecules with anti-inflammatory, anti-oxidative and immunomodulatory effects, which are valuable in combating neuroinflammation and immune dysfunction associated with neurological disorders [7]. By modulating the inflammatory response and immune activity in the CNS, allogeneic HPLs may help reduce inflammation-induced neuronal damage and create a conducive environment for neuronal repair and regeneration [231].
Allogeneic HPLs are also a reservoir of EVs, including exosomes released by platelets during collection and storage processes, or generated during the preparation of the lysate [127]. These vesicles carry a cargo of bioactive molecules, including micro (mi)RNAs, proteins, and lipids, which can be transferred to recipient cells, including neurons [127]. EVs and exosomes play crucial roles in intercellular communication, neuronal survival, and synaptic plasticity. They can modulate disease processes, support neuronal function, cross various brain barriers, and diffuse into the brain, making them likely contributors for therapeutic interventions in neurological disorders. From a practical perspective, allogeneic HPLs can be produced from a pooled source of outdated, potentially pathogen-reduced PCs that are no longer suitable for transfusion [222, 229, 230]. This provides a readily available and standardized source of platelet-derived factors, ensuring consistency and quality in the manufacturing process. The use of outdated allogeneic HPLs simplifies production and reduces the potential constraints and inadequacies associated with using autologous sources from severely ill patients [7]. Moreover, allogeneic HPLs can undergo pathogen-reduction, such as Intercept treatment [230], mild heat treatment [221], and nanofiltration [222] and (b) purification steps [221] to adhere to established safety guidelines and regulatory standards, ensuring the safety and efficacy of the final product. Preclinical developments are on-going to establish the safety and efficacy of dedicated HPLs made from pathogen-reduced allogenic outdated PCs for treatment of ALS [226].
Other clinical indications
It can be anticipated that the next few years will see additional preclinical and clinical development of applications of HPLs made from allogeneic PCs collected by blood establishments in clinical domains where autologous platelet materials, like PRP or platelet gels, are currently used, such as osteoarthritis [232, 233] or the treatment of recalcitrant wounds [234]. Published clinical reports using allogeneic platelet materials or HPL in the treatment of hip and knee osteoarthritis [235], bone reconstruction [236], or wound healing [237], including diabetic ulcers [238], show the trends towards the development of standardized products for improved consistency of treatment.
Emerging interest in stand-alone p-EV-based biotherapies
Scientific and clinical rationale of p-EVs-based biotherapy
Developments seen in the use of allogeneic platelet lysates in regenerative medicine provide scientific and technical rationales for looking at the possibility of using p-EVs, which (a) are abundantly present in HPLs, (b) may contribute to their functionality, and (c) are relatively easy to isolate [74, 127, 239, 240] as stand-alone biotherapy [8]. The relative ease of access to HPLs may indeed provide a source of EVs that is more convenient than other cell types, including MSCs, for which various technology and regulatory challenges still need to be addressed before they can emerge as an abundant, economical therapy [8, 241]. Clinical-grade platelet lysates represent a potentially abundant and complementary source of EVs that could serve specific needs in human regenerative medicine and targeted treatment of diseases [8]. Table 3 summarizes various translational advantages exhibited by p-EVs prepared from allogeneic PC from blood establishments for applications in regenerative medicine and for use as TDDS, as well as various points to consider, as developed below.
Table 3.
Translational advantages and points to consider about platelet extracellular vesicles (p-EVs) made from allogeneic platelet concentrates (PCs) for regenerative medicine or as drug-delivery system (DDS)
| Features | Comments | |
|---|---|---|
| Supply |
• Allogenic single-donor or pooled PCs collected by blood establishments following GMPs • Autologous PCs obtained from a patient using plateletpheresis collection procedure (or alternatively whole blood collection) |
• Allogeneic PCs are a known cellular product on the WHO Model List of Essential Medicines • Platelets are concentrated 3–fivefold in PCs compared to their basal level in the blood circulation, providing a concentrated cellular source for EV production • Outdated PCs, no longer suitable for transfusion, can be a source of p-EVs, therefore not competing with transfusion needs. If needed, allogeneic PC collection dedicated to p-EV preparation is technically feasible • PCs can be used fresh, or alternatively stored frozen and used directly as source material to generate and isolate p-EVs • The lack of ex vivo expansion to generate p-EVs facilitates clinical translation |
| Pathogen safety |
• Donors of allogeneic PCs are screened and donations are tested by serological and/or NAT to limit the risks of TTI (e.g., HIV, HBV, and HCV) • PCs can be subjected to licensed photochemical treatments to inactivate most bacteria, viruses, and parasites |
• The collection process and storage of PCs are conducted under aseptic conditions using dedicated licensed single-use medical devices. There is a residual incidence of bacterial contamination of approximately 1 in 2000 in PCs occurring during venipuncture • Risks of viral contamination (due to window phase donations of known and tested viruses or emerging, untested, viruses) cannot be excluded. The risk is approximately 1 in 1–2 million when PCs are collected from healthy donors in countries with a regulated blood system • p-EVs made from pools of multiple donations should preferably be prepared from pathogen-reduced PCs • The lack of a nucleus in platelets makes it feasible to use photochemical treatments designed to alter nucleic acids and inactivate blood-borne pathogens |
| Immunological safety | • The PC material should be tested for the presence of platelet antigens to avoid alloimmunization by recipients |
• Platelets express antigens (e.g., ABO, HLA class 1, or HPA antigens) that can cause alloimmunization in incompatible recipients. These antigens may be present on p-EVs and may potentially be clinically significant • Leukoreduction of PCs decreases risks of contamination of p-EV preparations by leukocytes expressing HLA class I and HLA class II |
| Procoagulant activity | • PCs are collected in the presence of a citrate anticoagulant solution and stored for up to 5 to 7 days following licensed procedures intended to minimize platelet activation | • Generation methods of p-EVs may generate p-EVs with exacerbated procoagulant activity. Process validation and quality control tests can be used to check the prothrombogenic activity of p-EVs |
HLA human leukocyte antigen, HPA human platelet antigen, NRA national regulatory authority, TTI transfusion-transmitted infection, WHO World Health Organization, LMICs low- and middle-income countries, NAT nucleic acid testing, HIV human immunodeficiency virus, HBV hepatitis B virus, HCV hepatitis C virus, GMPs good manufacturing practices
Briefly, allogeneic PCs provide a readily available and abundant source for the production of p-EVs, facilitating large-scale production and the development of standardized manufacturing processes without considering additional specific production and regulatory issues (a GMP facility, compliance and suitability of growth medium) associated with obtaining EVs as byproducts of cell cultures [242, 243]. Using allogeneic PCs collected using already approved standardized protocols can provide better control over the composition and quality of p-EVs. The fact that blood donors are screened, and donations are tested and potentially undergo pathogen-reduction steps contribute to ensuring the safety of derived p-EVs. Finally, the existing infrastructure for the preparation of PCs, based on the nearly 120 million blood donations collected each year at the global level [244], provides an abundant possible supply for scalability, optimal cost-effectiveness, and potential widespread clinical translation using local platelet resources [8].
However, points to consider include the fact that little is still known about the impacts of donor variations and processing in terms of cargo (miRNAs, proteins, and lipids), potency, and therapeutic effects of p-EVs. There is indeed a possibility that p-EV compositions vary depending on donor characteristics, the platelet activation status, and manufacturing processes [8]. Identifying and characterizing specific cargoes and their functional implications are important for targeted applications. It is also essential, as for any type of EV, to develop, validate, and carefully monitor standardized protocols for the isolation, purification, characterization, and safety testing of allogeneic p-EVs [8, 74, 240]. Meeting compliance with regulatory guidelines and obtaining appropriate approvals for the use of allogeneic p-EVs in regenerative medicine or as TDDSs will also be necessary [8]. Preclinical and clinical studies, most preferably conducted following double-blind placebo-controlled protocols [245], will be required to evaluate the efficacy and safety of allogeneic p-EVs and obtain marketing authorization. Aspects to evaluate, among others, should include demonstrated therapeutic potential, dose and frequency of administration, administration routes, and potential adverse effects. Recently, initial results of a double-blind placebo-controlled clinical study of allogeneic small-pool p-EVs for wound healing were reported, demonstrating feasibility of this approach [246]. Such allogeneic p-EV, manufactured from PC collected from healthy donors, have the potential to emerge as an effective and standardized biotherapy [8].
Source material, generation, and purification of p-EVs
Blood, from which PCs and HPLs are prepared, contains up to 108–109 EVs/µl [46, 247] under normal physiological conditions, with platelets and megakaryocytes jointly contributing to close to 50% of the EV blood pool [72, 248, 249]. It should also be kept in mind, however, that blood is a very complex fluid, and determining the total number of EVs in PCs and HPLs is challenging due to possible co-isolation of lipoprotein particles [250]. PCs contain approximately (2–5) × 109 platelets/ml, and their supernatants can yield about 1011–1012 “EV-like” events/ml depending upon the methods used for EV generation [74, 251–253] and the assessment method [133, 254–257]. Studies have shown that the mean size of most EVs in plasma and serum is in the 100–300-nm range [47, 258]. It is expected that EVs present in PCs have diverse cellular origins, representing the EV population in the donor’s blood at the time of blood collection [72, 247, 248], being derived from erythrocytes, WBCs, platelets, megakaryocytes, or vascular endothelial cells. This naturally occurring population of EVs is likely enriched by p-EVs released by platelets during PC collection, processing, and storage. Several processing steps performed during the preparation of PCs, such as whole blood collection versus apheresis, leukoreduction, pathogen reduction, and the duration of storage at 20–24 °C under agitation, may also influence the total number and type of EVs present in PCs [133, 255, 259]. Systematic studies to delineate the impacts of PC processing on the number and functionality of PC-EVs are thus still needed.
HPLs obtained by various processing methods, such as freeze–thaw or calcium chloride activation, were found to contain approximately 1012 EV-like nanoparticles/mL [127]. Two main types of EVs are thought to occur in HPLs, namely microvesicles and exosomes [260], but this is likely an over-simplification, and more research is needed to understand the complexity of p-EV populations. Microvesicles, with sizes ranging 100–300 nm and larger, are shed from plasma membranes [242], while exosomes, or small EVs, with a diameter of 30–100 nm, originate from endosomal multivesicular bodies and alpha-granules [239]. The zeta potential of p-EVs is slightly negative (-5 mV or less) [127], similar to values reported for EVs from other cell types [261]. Several treatments can be used to trigger the release of EVs from platelets present in PCs. These methods can be applied to whole PCs themselves (platelets suspended in plasma or plasma/PAS), or on platelets isolated from PCs by a centrifugation step at ca. 3000 × g for 20 min at 20–24 °C [8, 80, 127, 240]. Physico-mechanical methods, such as freeze–thaw cycles, shaking, and sonication [262] can be applied to disrupt platelet membranes leading to EV release. Such methods have been reported to lead to the release of as many as 100 to 500 p-EV (or p-EV-like events) per platelet [262]. Alternatively, chemical and biochemical methods involving incubation with Ca2+ ionophores (to activate the coagulation cascade), lipopolysaccharides, thrombin, collagen, or ADP, to activate platelets through binding to their receptors, can also stimulate the release of p-EVs [74, 263]. The ways that PCs are collected and HPLs prepared impact the type and proportions of EVs expressing different markers relative to CD41a, specific to p-EVs, confirming a diversity in p-EV populations [264]. Much work is thus needed to better characterize the nature of EV populations released by platelets.
Several procedures have been proposed for purifying EVs from PCs or from isolated platelets, including differential centrifugation, ultracentrifugation, and size-exclusion chromatography, as for other EV types [8, 74, 127, 240, 265, 266]. The most common methods used so far combine low (ca. 300 × g) and intermediate (ca. 3000 × g) speed centrifugation, to remove cell debris, with 20,000–100,000 × g ultracentrifugation to pelletize p-EVs, exploiting differences in size and density between cellular components and debris, protein components, and EVs to achieve their partitioning. The obtained EV pellet can be resuspended in a suitable buffer for further and final processing, formulation, bacterial filtration, and dispensing into the final container. The selection of an optimal isolation method may vary based on the sample source, the downstream specifications of purity, and the intended applications. For instance, some studies used size-exclusion chromatography with resins, such as Sepharose CL-2B [127, 264, 267], with a porosity of 35–75 nm to remove proteins and isolate EVs based on their size and shape, rather than their density. Defining an optimal method to produce p-EVs for clinical applications should therefore consider various factors such as the impacts of blood-collection procedures and mode of preparation of HPLs, process efficiencies, p-EV sub-population characterization, impacts on the downstream process, and complexity of regulatory approval for clinical evaluation [8]. Producing p-EVs from pools of allogeneic PCs or platelet lysates can be a powerful means to enhance the consistency of p-EV properties [8]. This, however, needs to address also other challenges such as guaranteeing pathogen safety.
Pathogen safety of p-EVs
Ensuring the virus safety of p-EVs derived from allogeneic PCs overlaps partially that needed for HPL, and is essential for clinical translation, as summarized in Table 4. It is vital that only allogeneic PCs meeting quality specifications for transfusion are used to prepare clinical-grade p-EVs. However, several virus-reduction treatments that provide robust safety for plasma protein products and HPLs, such as dissolution of lipid envelopes by S/D, or removal by 20-nm nanofiltration [34, 160, 163, 222], are not seen to be applicable to EV preparation. This is due to common structural features shared between EVs and viruses, including the presence of a lipid membrane and a similarity in nanometer size. Pathogen safety of allogeneic p-EVs should therefore currently be best assured by donors’ screening, donation testing, and pathogen-reduction treatment of the starting PC materials using photochemical or photoinactivation procedures [104, 113]. It is important to note that these methods do not guarantee protection against all viral risks [109], a concern for large-pool EV products that would not be subjected to one additional virus-reduction step. In addition, some experimental data suggest that photoinactivation treatments of PCs may affect the miRNA content [133, 268] but how this can influence the regenerative and protective functions of p-EVs is still unknown. Therefore, further research should be conducted to develop novel pathogen-reduction procedures that minimally affect p-EV functionality. Until such technological developments are achieved, it is crucial to rely on screened donors and clinical-grade donations that undergo rigorous screening and testing following international guidelines [33] to respectively ensure donor eligibility and detect potential infectious diseases. This is vital to help minimize the risk of transmitting viral infections through p-EV preparations especially when derived from pooled donations.
Table 4.
Examples of quality and safety requirements of allogeneic platelet lysates and platelet extracellular vesicles (EVs; p-EVs) as medicinal preparations
| Platelet lysates | Platelet extracellular vesicles | |
|---|---|---|
| Blood donors | Meet health requirements for donations of whole blood or platelet concentrates (PCs) | Meet health requirements for donations of whole blood or PCs |
| Whole blood or platelet concentrate donations | Meet overall quality and safety requirements for clinical use: testing of viral markers, blood cell counts within specifications | Meet overall quality and safety requirements clinical use: testing of viral markers, blood cell counts within specifications |
| Pathogen-reduction treatment of individual donations | Licensed pathogen-reduction treatment of PCs (e.g., photochemical treatment) | Pathogen-reduction treatment of PCs (e.g., photochemical treatment) demonstrated to not affect EV functional activities |
| Treatment of PCs | Freezing, thawing, and pooling | Freezing, thawing, and pooling |
| Optional processing steps | Fibrinogen conversion (e.g., CaCl2 activation); dedicated virus-reduction treatment (e.g., gamma-irradiation; electron-beam; solvent-detergent), low-speed centrifugation; depth filtration; bacterial filtration; aseptic filling; freezing | EV generation from PCs (freeze–thaw, sonication, CaCl2 activation); low-speed centrifugation; ultracentrifugation; size exclusion chromatography; bacterial filtration; aseptic filling; freezing |
| Quality control | Meet specifications: protein content; microbial sterility, endotoxins; pH; osmolality; representative growth factor content (e.g., PDGF-AB); functional assay (e.g., cell proliferation test) | Meet specifications: protein content; EV number; representative p-EV markers; microbial sterility; endotoxins; pH; osmolality; representative growth factor content (e.g., PDGF-AB); functional assay (e.g., cell proliferation test; internalization assay) |
PDGF platelet-derived growth factor
p-EVs as regenerative medicine biotherapy
Recently, the concept of p-EVs as a key effector of the regenerative activity of allogeneic HPLs has emerged, leading to mounting interest in evaluating p-EVs as a stand-alone clinical adjunct in various fields of regenerative medicine [8, 246]. Thanks to paracrine action associated with the broad repertoire of trophic factors they carry and transfer to recipient cells, p-EVs are capable, like MSC-EVs [269], of modulating biological pathways of tissues by directly activating target cells, homing in on injured or inflamed tissues [270] where they help maintain stem cell populations and prolong their lifespan in vitro [239], and inducing the release of biomolecules by neighboring cells [8, 271–276]. Thus, p-EVs may be capable of modulating the mechanisms of ageing-associated diseases [8, 242, 249, 277]. Functional molecules present in p-EVs encompass various growth factors, chemokines, cytokines, pro- and anti-inflammatory factors, pro- and antiangiogenic factors, pro- and anticoagulants, antioxidants, matrix metalloproteinases originally stored on platelet granules, in addition to lipids and nucleic acids (messenger (m)RNA and miRNA) [47, 73, 74, 220, 278–280]. Therefore, p-EVs may play critical roles in tissue regeneration by providing a wide range of synergistic biomolecules to promote cell proliferation, migration, differentiation, angiogenesis, and remodeling of the extracellular matrix and regulating gene expressions [8, 281–285].
For instance, VEGF, PDGF, and TGF-β released by p-EVs may promote cell proliferation, migration, and differentiation of fibroblasts, endothelial cells, and MSCs at the site of injury, as well as promoting the formation of new blood vessels to bring oxygen and nutrients to sites of injury as shown in a rat model [286]. Lipid components of p-EVs also contributed to tube formation by human umbilical vein endothelial cells (HUVECs) [287], and a proangiogenic effect, attributed to VEGF, b-FGF, and PDGF, was found in a rat model, allowing for better revascularization following chronic ischemia [286]. p-EVs isolated from HPLs contain miRNAs, including miRNA126, which can modulate expressions of genes involved in cellular mechanisms important for angiogenesis in a HUVEC model [280]. In an animal model of trauma, p-EVs contributed to maintaining hemodynamic stability and mitigated the development of ischemia and metabolic acidosis [288].
Recently, p-EVs were found to help heal wounds in diabetic rat models [289], repair endothelial corneal defects [220], treat intervertebral disc degeneration [290] and damaged tendons [273, 280] thanks in part to their inherent anti-inflammatory and antioxidative properties. Interestingly, p-EVs, rich in neurotrophic factors (e.g., PDGF, BDNF, and b-FGF), neurotransmitters, anti-inflammatory factors, and antioxidants [7, 220], may contribute to the emerging platelet-derived neuroprotective biotherapy of neurodegenerative disorders by promoting neuronal survival and reducing neuroinflammation [7]. Administration of p-EVs by topical/intracranial routes allowed their diffusion into the brain and stimulated neurogenesis and improved behavior in a stroke model [291], stimulated the differentiation of neural stem cells into glia and neurons [292], and generally promoted neurogenesis [221, 293, 294]. One p-EV population isolated from serum-converted HPLs by size exclusion chromatography promoted network formation in primary cortical neuronal cultures [127]. In addition, some EV subsets contain functionally active mitochondria [72, 254], supporting possible translation to treatment of mitochondrial abnormalities and the recovery of cell functions.
Identifying key molecular players will help the clinical development of p-EVs, but much work is still needed [295, 296]. In addition, to date, most of the assumptions as to possible applications of p-EVs in regenerative medicine are still relatively scarce and rely on laboratory-scale experiments using “homemade” preparations. Translational work is needed using p-EV preparations made from pooled allogeneic HPLs processed under scalable clinical-grade conditions eventually capable of meeting regulatory requirements. Also, one needs to understand how the methodology of p-EV generation and isolation may impact surface markers and cargo, and thus their safety and functionality [8, 74, 240]. Additionally, a comparative assessment of the functionality of p-EVs in relation to various MSC-EVs, considering their physiological cargo, would be highly interesting to identify their respective therapeutic values.
p-EVs as a targeted drug-delivery system (TDDS)
The rationale for using p-EVs as a TDDS is a logical progression of previous studies which demonstrated that platelets themselves are potent vehicles for drug delivery, especially in the context of cancer treatment [80, 242, 297–305] and immune and inflammatory diseases [242, 301, 302]. p-EVs' value as a targeted drug-delivery vehicle to cells and tissues is also linked to the specific range of surface markers originating from platelet parent cells. Due to their nanometer size, p-EVs are expected to have a better capacity to reach and be retained by tumors [80, 306], and at sites of inflammation such as with pneumonia [307].
P-EVs membrane markers important for tissue targeting
p-EVs are characterized by expressions of various glycoproteins/clusters of differentiation (CDs) originating from platelets that contribute to cellular targeting and cross-talk with pathological sites [308]. Instrumental p-EV membrane markers include CD41, also known as integrin αIIb, and CD61 (integrin β3) which together form the platelet-specific integrin alpha-IIb/beta-3 (GPIIb/IIIa) that plays crucial roles in platelet aggregation, adhesion, and cell targeting and retention. CD42 (glycoprotein Ib or GPIb) is a receptor for vWF and contributes to p-EVs' adhesive properties and interactions with specific cell types or endothelial surfaces. CD62P (P-selectin), a marker of platelet activation, is a cell adhesion molecule that interacts with the P-selectin glycoprotein ligand (PSGL)-1 on leukocytes during inflammation (e.g., atherosclerosis), immune responses, and thromboses, contributing to the recruitment and retention of p-EVs to pathological sites. Platelet endothelial cell adhesion molecule-1 (PECAM-1; CD31) plays a role in cell–cell adhesion, contributing to the adhesion and transmigration of platelets across endothelial cells during inflammation. Thus PECAM-1 may trigger p-EVs to adhere to the endothelium and migrate into underlying tissues [309]. These membrane markers are instrumental in cellular targeting [281, 283, 288] and in cross-talk with pathological sites [80, 310], and can thus facilitate the adhesion of p-EVs to specific cell types or surfaces and enable the delivery of cargoes, but more research is needed to elucidate how p-EV generation procedures impact expression levels of these markers.
Loading of drugs within p-EVs
The loading of hydrophobic and hydrophilic drugs can be achieved by various methods [8, 75, 242, 311]. In passive loading, isolated p-EVs are incubated with drugs, allowing them to enter through diffusion or a concentration gradient, taking advantage of the natural permeability of the lipid bilayer of p-EVs [262]. In active loading, methods such as electroporation, sonication, and extrusion are employed to disrupt the lipid bilayer of p-EVs, and create transient pores or channels through which drugs can be actively loaded to achieve higher encapsulation efficiencies [242, 262, 265, 266]. Pre-loading drugs into platelets, before the isolation of p-EVs, is another option. Platelets can be loaded with drugs via a combination of incubation, electroporation, freeze-thawing, calcium chloride activation, and sonication, with loaded platelets being activated to release drug-loaded p-EVs [8]. Removal of the unloaded drug typically involves processes based on size exclusion to segregate loaded p-EVs from the free drug [262]. An important element to consider for industrial and economic feasibilities is the yield of production of p-EVs from platelets. Recent studies showed that sonication and extrusion respectively generate means of 496 and 493 p-EVs per platelet, more than the 145 p-EVs by freeze/thawing and 33 p-EVs by 37 °C incubation [262]. The method by which p-EVs are generated and loaded can have detectable impacts on the structure and safety of p-EVs. For instance, recent data suggested that based on the level of expression of PS, p-EVs obtained by freeze-thawing of platelets would be less prothrombogenic than those generated by extrusion or sonication [75]. However, more studies are needed to delineate the impacts of the generation and drug loading procedures on p-EV procoagulant activity for any desired therapeutic application. Once loaded, the drugs are protected from degradation and are expected to be released slowly over time at the site of injury, ensuring sustained drug delivery.
Clinical developments of p-EVs as DDS
Clinical developments in the use of p-EVs as TDDSs are still limited. One expected area of clinical translation of drug-loaded p-EVs is for the delivery of anticancer agents based on the known pathogenic loop existing between tumors, platelets, and p-EVs [80, 281, 312–315] and a capacity for p-EVs to be internalized by cancer cells [80]. This expectation was confirmed by a recent study showing that drug-loaded p-EVs could be internalized and exhibited higher cytotoxicity than free drug in vitro against cancer cell lines [262] as well as in clinical samples from leukemia patients [305]. Other pathological targets are atherosclerosis and other diseases associated with inflammatory processes [75]. For instance, in an experimental model, p-EVs loaded with an NLRP3-inflammasome inhibitor allowed significant reduction in atherosclerotic plaques, a decrease in local inflammation and macrophages and T-cell proliferation at plaque sites in ApoE-knockout (KO) mice [316]. Similarly, p-EVs loaded with the [5-(p-fluorophenyl)-2-ureido]thiophene-3-carboxamide (TPCA-1) anti-inflammatory molecule could target pneumonia in a mouse model of acute lung injury, inhibiting expressions of inflammatory factors and the infiltration of pulmonary inflammatory cells, and making it possible to modulate cytokine storms [307].
Future prospects in using p-EVs as TDDS
Although still limited, these experimental data support the potential for p-EVs for use as TDDSs in various clinical applications [8]. p-EVs appear to be particularly potent vehicles to deliver their therapeutic payloads specifically to cancer cells [262, 317], with the potential to improve retention within the tumor microenvironment (TME), minimize off-target effects, and reduce systemic toxicity [8, 75, 317]. It was speculated that in inflammatory and autoimmune diseases involving a functional role a platelets, p-EVs could be used to deliver immunosuppressive agents or anti-inflammatory drugs to sites of inflammation or autoimmune activity, potentially improving the treatment of conditions like rheumatoid arthritis, inflammatory bowel disease, and atherosclerosis [8, 318]. In cardiovascular diseases, p-EVs can interact with endothelial cells, promote angiogenesis, and modulate cardiovascular functions. They can be utilized for targeted delivery of therapeutics to promote cardiac repair, neovascularization, and tissue regeneration, or modulate oxidative stress after myocardial infarction or peripheral artery disease [75, 319]. In neurological disorders, p-EVs, similar to other nanoformulations, can be loaded with neuroprotective or neuroregenerative factors, as well as antioxidants and targeted to specific cell types in the CNS, offering a promising avenue for treating conditions like neurodegenerative diseases, stroke, and TBIs [7, 320]. While the use of p-EVs as TDDSs appears safe and promising, additional preclinical studies followed by rigorous clinical trials, as recommended for “naïve” p-EVs [245], will be necessary to establish their efficacy, optimize their delivery strategies, and ensure their safety in various clinical applications such as those listed here [8]. In summary, compared to other DDSs, allogeneic p-EVs offer several practical and functional advantages over other types of EVs or DDSs, including (a) the ease of access to a standardized platelet source material, (b) a human origin that confers good biocompatibility and low immunogenicity, and (c) their natural targeting capabilities due to the presence of platelet membrane receptors and surface proteins making it possible for p-EVs to interact with specific target cells or tissues.
Lessons learned from the use of platelet membranes to prepare “p-EV-like” nanomedicines
Recently, there is a research trend in using platelet membrane coating to enhance the biocompatibility, residence time, targeting, and functional capacity of p-EV-like nanomedicines for applications in the treatment of various inflammatory diseases, including atherosclerosis, thrombosis or cancer [321–324]. Platelet membrane coating was shown in experimental models to be of special value to enhance the adhesion to the sub-endothelium, provides immune evasion, and interactions with pathogens, and, in general terms, improved homing and retention at the pathological sites for optimal drug release [324, 325].
Among other examples, one promising area of clinical development is the use of such platelet cloaking approach to treat myocardial infarction (MI), especially in a context where the heart shows very low uptake of therapeutics after MI [326]. The rationale lies on the fact that platelets play important biological roles in MI injury and repair. Firstly, platelets accumulate at the MI injury site, particularly at injured endothelial cells via binding to vWF using surface glycoproteins including GPIb, GPIIb, GPVI, GPIV [327]. Thereupon, they release cargo which modulates inflammation and immune cell recruitment. In addition, platelets have been shown to bind circulating CD34 + progenitor cells and macrophages, which are, in-turn, recruited to the injured heart [328]. Thus, using platelet membrane is a rational approach for use as drug delivery systems in MI. It was indeed shown that liposomes functionalized with platelet membranes expressing GPIIb and CD42c (GPIb-beta) were able to bind to circulating monocytes and could be recruited to the MI site in greater concentrations, and for prolonged periods of time [329]. The liposomes were used to carry an anti-inflammatory small molecule Cobalt protoporphyrin IX (CoPP), which reduced MI severity and improved cardiac function in a mouse model. Platelet “nanovesicles” could be used to enhance the delivery and retention of cardiosphere-derived stem cells (CSCs) by altering CSC membrane signatures [330]. These CSCs showed improved engraftment and resulted in better therapeutic outcomes in both rodent and porcine models of MI, compared to unmodified CSCs.
Another study modified MSC-derived EVs with platelet membranes to achieve improved endothelial cell targeting and angiogenesis in a mouse model of MI [331]. Modification of MSC -exosomes with platelet membranes reduced their uptake by macrophages, enhanced targeting to the injured heart and promoted micropinocytosis, resulting in enhanced cellular uptake by endothelial cells and cardiomyocytes. This resulted in improved cardiac functions in a mouse model of myocardial infarction injury [332]. Lastly, platelet membranes were used to functionalize PLGA nanoparticles carrying miRNA inhibitors [333], reducing infarct size and improving cardiac function in a rat MI model.
Therefore, platelet membrane coating of nanomedicines may offer several synergistic advantages [8, 75]. In brief, the enhanced biocompatibility can reduce the risk of immune recognition, clearance, and adverse reactions. Prolonged residence times can allow enhanced accumulation at the target site and improve the potential therapeutic efficacy. The presence of platelet membrane markers can improve the targeting ability to pathological sites or cell types, thereby enhancing internalization by target cells, improving the therapeutic potential and reducing off-target effects [323, 324, 334]. Altogether this field of preclinical research supports the scientific rationale towards the development of p-EVs as stand-alone carriers for TDDSs.
Platelet membrane-coated nanomedicines hold potential in human medicine but some possible trends can be considered to further improve the therapeutic value and domains of application, as well as production capacity. For instance, incorporation of additional ligands or functional molecules onto the platelet membrane surface may further enhance targeting specificity, synergistic effects, and multifunctionality for personalized medicine approaches of drug-loaded nanocarriers. While cancer therapy has been a primary focus, it may be valuable to expand the scope and impact of platelet membrane-coated nanomedicines to other areas, such as cardiovascular diseases, neurodegenerative disorders, and inflammatory conditions. Last but not least, manufacturing techniques should be refined to make it feasible to prepare platelet membranes under conditions meeting GMPs and allowing scalability.
Possible thrombotic risks associated with p-EVs
p-EVs are promising therapies, but one safety concern in using them in the clinic is their potential capacity to promote blood clotting. Some populations of p-EVs have a membrane 50–100-fold more procoagulant than that of activated platelets due to expressions of binding sites for activated factor V and factor VIII, and thrombin [335] or exposure of negatively charged PS and P-selectin (CD62P) [284, 336, 337]. Therefore, some p-EVs may increase the risk of thrombotic events, which is of specific concern for patients with pathologies like cancer associated with a procoagulant state. The extensive experience and safety testing developed in the human blood product industry may provide valuable insights and methodologies that can be applied to assess the quality and safety of p-EVs, particularly in relation to the risks of pro-coagulation and thrombogenicity. Several assays can be considered, including the thrombin generation assay (TGA) that measures the in vitro generation of thrombin [338] and has proven useful when adopted to predict the procoagulant potential of intravenous immunoglobulin preparations [339]. The TGA provides information about the potential procoagulant activity associated with p-EVs and their ability to initiate clot formation by documenting various parameters, such as the lag time, peak thrombin concentration, and endogenous thrombin potential, to evaluate the thrombotic potential of p-EVs [340, 341]. This assay can be used in conjunction with other tests to determine the exposure of PS and TF, including various clotting time assays, activated partial thromboplastin time (aPTT), prothrombin time (PT), phospholipid-dependent clot-based assays, and determination of platelet activation markers, such as CD62P [125, 342–345]. Animal models, such as venous or arterial thrombosis models, can be also be considered as preclinical models to assess the thrombotic potential of p-EVs by evaluating possible induced thrombus formation as has been done for some plasma products [346].
Another concern is a possible excessive proinflammatory effect of some p-EVs due to their content of proinflammatory cytokines like interleukin (IL)-1, IL-6, and TNF [347]. However, studies have observed that some p-EV populations are capable of suppressing inflammation through inhibiting the release of TNF by macrophages [348], and the release of TNF and IL-8 by plasmacytoid DCs [349]. Differences were found in the capacity of two p-EV populations to aggregate monocytes and induce NETs [336, 350]. Thus, p-EVs may play differential roles in triggering inflammation. As for any complex biotherapy, little is still known about any dose-dependent effects, which implies that the effects of treatment may vary depending on the dose used and the frequency of administration. This can make it challenging to determine the optimal safe and efficacious treatment within the scope of a specialized individual medicine. The long-term effects of p-EV therapy, as with any treatment based on EVs, are largely unknown, thereby highlighting the need for more preclinical research to understand their long-term safety and efficacy. As for any biologicals and EVs from other cellular sources [242], process validation and monitoring, as well as in-process and final product quality control and standardization in the preparation and characterization of p-EVs are vital to support consistency in quality, safety, and efficacy [8].
Future trends in p-EV-based biotherapies
Further research is needed to better delineate the functionality and optimal clinical applications of each type of allogeneic p-EV, also recognizing the advantages and potential constraints of this material. This is important as, compared to MSC-EVs, the use of allogeneic p-EVs has so far received relatively little attention, and no comparative assessments of safety and efficacy have been conducted to our knowledge. As is the case for HPLs, the use of clinical-grade blood donations as a source material for p-EVs has major advantages for clinical translation and to establish proof-of-concept of clinical safety and efficacy of EV therapy. Moving p-EVs towards clinical use can be facilitated when targeting clinical indications where the benefits of platelet biomaterials, themselves rich in p-EVs, have been demonstrated and where the presence of p-EVs has been shown to contribute to functional outcomes. In addition, rigorous preclinical methodologies should be followed to define the biophysical characteristics, safety, functionality, and optimal clinical applications of each type of purified allogeneic p-EV as summarized in Fig. 4.
Fig. 4.
Overview of a range of in vitro, cellular, and animal preclinical tests that can be conducted to characterize and select the optimal populations of platelet extracellular for clinical translation. DLS dynamic light scattering, ELISA enzyme-linked immunoassay, CCK-8 cell counting kit-8, EM electron microscopy, p-EVs platelet extracellular vesicles, FCM flow cytometry, MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, NTA nanoparticle tracking analysis, PS phosphatidyl serine, PC platelet concentrate, qRT-PCR quantitative reverse phase polymerase chain reaction, RNA ribonucleic acid, TGA thrombin generation assay, TF tissue factor, WB western blotting
Various in vitro biophysical and biochemical assays can be performed, as for any type of EVs, to ascertain the size, number, zeta potential, membrane marker expression, cargo composition (e.g., proteomic and miRNA contents), and possible procoagulant activity as a means to identify populations providing the optimal quality and safety profiles to best meet the intended clinical application [8, 74, 127, 220, 240, 242, 262, 279]. In vitro cell culture studies are invaluable in assessing the toxicity and the functional effects of different types of allogeneic p-EVs on various cell types or disease models, and comparing the capacity for cellular internalization, cellular responses, functional outcomes, and molecular mechanisms to understand specific functions and therapeutic potential [127, 220]. In case good outcomes are seen in cellular models, animal models could help to investigate the therapeutic efficacy and safety profiles of selected allogeneic p-EVs in vivo. These studies can be instrumental in assessing and comparing the regenerative and protective potential, immune responses, biodistribution, and therapeutic outcomes associated with specific p-EV preparations and determine, based on scientifically based and objective criteria, the most suitable allogeneic p-EV types for specific disease conditions. These could be further validated by well-designed clinical trials as crucial instruments for evaluating the efficacy, safety, and optimal clinical applications of allogeneic p-EVs and for monitoring clinical outcomes, disease progression, biomarker analyses, and adverse events in an objective scientifically based manner [245].
One possible approach to target clinical domains where p-EVs could be clinically beneficial is to scrutinize clinical indications where the benefits of platelet biomaterials, such as HPLs containing p-EVs, have been demonstrated. This includes treating ocular surface disorders such as dry eye syndrome, corneal ulcers, and ocular surface injuries, where EDHO and HPL rich in p-EVs, showed promising experimental and clinical results [29–31, 83, 86, 213, 215, 217] in promoting corneal epithelialization, reducing inflammation, and improving overall ocular surface health. Another clinical field is treating chronic wounds where various platelet biomaterials containing p-EVs have demonstrated efficacy in promoting healing, including diabetic foot ulcers and venous ulcers. This approach was confirmed by the fact that the first clinical trial of p-EVs was conducted in wound healing [246]. Similarly, treating osteoarthritis with p-EVs is expected to be evaluated in the near future, as this is one main indication of PRP and now, increasingly, allogeneic HPLs [19, 24, 351]. Finally, interest is emerging in evaluating p-EVs for treating various neurological disorders [127], based on exciting preclinical data showing preclinical benefits of HPLs for brain administration in various neurodegenerative diseases and trauma [7, 70].
Ethical considerations in using clinical-grade allogeneic PCs as source material for p-EVs
The use of clinical-grade blood or platelet donations as a source material for p-EVs therapeutics should fall within international ethical principles regulating the preparation of current therapeutic blood products, both cell-based and protein-based. These ethical principles are defined in several resolutions adopted by the World Health Assembly (WHA) [352, 353]. In particular WHA58.13 supports “the full implementation of well-organized, nationally coordinated, and sustainable blood programs with appropriate regulatory systems”, and stresses the role of voluntary non-remunerated blood donors from low-risk populations [354]. WHA resolution 63.12 supports efforts to ensure the availability, safety, and quality of blood products in response to major unmet public health needs. These resolutions have been translated into guidelines and regulations published by the WHO, indicating that blood and platelet donors should donate in blood establishments licensed and regularly inspected by national regulatory authorities to ascertain operations following GMPs [33]. Donations should be voluntary and non-remunerated (which according to the WHA should be the target for all countries), to avoid recruiting donors among at-risk populations. Donors should provide informed consent (e.g., by signing a donation information leaflet) for the use of their donations in research or therapeutic applications, and this should apply when donations are used to isolate p-EVs. Donors should be fully informed about the purpose, potential benefits, and possible risks (including infectious risks) of using their donated blood for p-EV production, as any other blood products, so they can refrain from donating if they become aware of a risk factor. The confidentiality and privacy of donor information should be preserved, and measures should be in place to protect donor identities, medical records, and any other sensitive information related to the use of their blood donations. However, full traceability should be in place to make it possible “to trace each individual unit of blood or blood component derived thereof from the donor to its final destination, whether this is a recipient, one or more batches of medicinal product or disposal. The term is used to describe forward tracing (donation to disposition) and reverse tracing (disposition to donation)” in a way similar to that which prevails for plasma utilized for fractionation [355]. Such procedures can allow to perform a “lookback” if it was found retrospectively that a donation from a high-risk donor should have been excluded from processing [355]. At the preclinical and clinical trial stages, collaborating with institutional review boards (IRBs), ethics committees, and regulatory authorities can ensure adherence to established ethical guidelines. In conclusion, the ethical framework already in place for currently marketed cellular and protein blood products should be readily applicable to the production of p-EVs from allogeneic PCs. In addition, the possibility of using outdated PCs not suitable for transfusion as a source material to isolate functional p-EVs, as already shown experimentally to be potentially feasible for the repair of corneal endothelium cells [220], can contribute to avoiding the wastage of precious PC donations and improving the sustainability of blood establishments.
Perspectives: challenges and solutions in using platelet-derived biomaterials and biotherapies and current solutions
The fact that allogeneic PCs are a therapeutic cellular product for transfusion presents opportunities for their repositioning towards regenerative medicine and cell therapy. However, several limitations commonly associated with human-derived platelet source materials need to be addressed to propose potential solutions and valorize the potential. One limitation is the relative variability observed in platelet quality, composition, and functional characteristics, which can impact the consistency and efficacy of platelet-derived products. To address this, rigorous exclusion criteria and screening of donors accepted for donations, as employed by blood establishments for transfusion purposes, can ensure a more-homogeneous donor pool. Additionally, standardized processing and quality control protocols can minimize batch-to-batch variations in PCs eventually used to make platelet-derived biomaterials. Pooling an adequate number of platelet donations further contributes to normalizing the characteristics of the starting platelet pool for HPL or p-EV production.
It is well-established that platelets are sensitive to storage conditions, potentially impacting the release of trophic factors and EVs. Although this release might not be detrimental to the production of some HPLs or EVs, careful monitoring of storage conditions is crucial to ensure reproducibility and maintain product specifications. Cryopreservation has shown promise in enhancing the shelf life of PCs for lysate production, but its suitability for p-EV preparation requires validation.
The field of allogeneic platelet-derived biomaterials and biotherapies is still evolving, and standardization of production methods, characterization assays, and regulatory frameworks is needed. Lack of standardized protocols and regulations can pose challenges for product development, clinical translation, and regulatory approval. Collaboration among researchers, clinicians, blood establishments, industry stakeholders, and regulatory bodies is essential to establish standardized protocols, characterization methods, and guidelines. Harmonization of regulatory requirements across jurisdictions can streamline approval processes and leverage the expertise of the blood and plasma product industry.
Ensuring an adequate supply of PCs is crucial for scaling up the production of PLs and p-EVs to meet clinical demands supported by scientific and clinical evidence. Donor recruitment and retention strategies, as well as optimizing the utilization of whole blood units, can help maintain a steady supply. Dedicated platelet collection procedures and the wider introduction of pathogen-reduction technologies can further enhance the availability of PCs, including outdated units that would otherwise be discarded. Cost optimization strategies, such as streamlining manufacturing processes and leveraging economies of scale, are essential for improving accessibility and affordability of platelet-derived products.
Robust research studies, well-designed preclinical and clinical trials, and evidence-based guidelines are crucial for justifying the clinical applications of HPLs and p-EVs. High-quality clinical data supporting their efficacy and safety will guide their use and integration into standard therapeutic approaches. Tailored preparations may be required to address the specificities of different clinical targets.
Addressing the challenges and limitations associated with platelet-derived biomaterials and biotherapies will require collaboration among various stakeholders. By streamlining production processes, sharing resources and expertise, facilitating technology transfer, and ensuring coordinated efforts, the accessibility, affordability, and appropriate clinical use of these therapies can be improved. Collaboration among blood establishments, research institutions, industry stakeholders, and regulatory bodies will play vital roles in overcoming these challenges and maximizing patient access to platelet-derived biotherapies.
Conclusions
Allogeneic clinical-grade PCs collected from repeat healthy blood donors by licensed blood establishments represent a readily available source of therapeutic cells, which, when not used to cover the needs for hospital transfusion, can serve rapidly growing clinical needs for cell therapy, regenerative medicine, and drug delivery. The beneficial use of allogeneic platelets in these therapeutic domains is currently illustrated by the demonstration that HPL is a safe and potent xeno-free substitute for FBS as a growth medium supplement for the clinical-grade expansion of human cells for transplantation, primarily MSCs, and potentially others like chondrocytes, DCs, or CAR-T cells. Currently, the use of allogeneic PCs as source material for the preparation of HPLs for direct clinical use is still at a relatively early stage; however, recent developments are demonstrating that allogeneic HPLs are a viable and safe alternative to traditional serum eye drops for the treatment of ocular surface disorders, including dry eye syndrome. One can logically anticipate that future preclinical and clinical studies will examine the possibility of using allogeneic HPLs in other degenerative pathologies such as treating recalcitrant skin wounds and degenerative joints, and even as biotherapy to modulate neurodegenerative diseases of the central nervous system. When considering such applications in regenerative medicine, it will remain vital to develop dedicated and standardized HPL preparations, which through careful scientifically based in vitro characterization and well-designed preclinical and clinical evaluations are demonstrated to be safe and best fit the clinical specificities of the pathology addressed for optimal efficacy. In this manuscript, we also highlighted the exciting potential offered by allogeneic PCs and HPLs as source materials, complementary to MSCs, for the isolation of various types of pooled p-EV preparations as subcellular therapeutic modalities for regenerative medicine or as vehicles for drug delivery. Establishing p-EVs as a recognized therapy requires, as for most EV sources [356], the resolution of various manufacturing and regulatory challenges. Challenges include, among others, the development of reliable and dedicated p-EV generation and downstream purification processes, including pathogen-reduction treatments, the capacity to provide suitable biochemical characterization of the molecular cargo and mechanism of action and/or targeting, the development of meaningful quality control assays to guarantee batch-to-batch consistency, safety, and efficacy, and the implementation of meaningful preclinical tests relevant to targeted clinical indications. In conclusion, we believe that allogeneic platelet-derived preparations are likely to have an increasingly prominent position in the future among the arsenal of biotherapies available for cell therapy, regenerative medicine, and drug delivery.
Acknowledgements
Not applicable.
Abbreviations
- ADP
Adenosine diphosphate
- ATP
Adenosine triphosphate
- BDNF
Brain-derived neurotrophic factor
- b-FGF
Basic fibroblast growth factor
- CAR
Chimeric antigen receptor
- CCL5
C–C motif chemokine ligand 5
- CD
Cluster of differentiation
- CEC
Corneal endothelium cells
- TCM
Central memory T-cells
- CTGF
Connective tissue growth factor
- CXCL4
C-X-C chemokine ligand 4
- DC
Dendritic cell
- DDS
Drug-delivery system
- EDHO
Eye drops of human origin
- EGF
Epidermal growth factor
- EV
Extracellular vesicle
- FBS
Fetal bovine serum
- FGF
Fibroblast growth factor
- GMP
Good manufacturing practice
- HAV
Hepatitis A virus
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HGF
Hepatocyte growth factor
- HIV
Human immunodeficiency virus
- HPL
Human platelet lysate
- HUVEC
Human umbilical vein endothelial cell
- IGF-I and II
Insulin-like growth factor I and II
- IL
Interleukin
- LAMP
Lysosomal-associated membrane protein
- MI
Myocardial infarction
- MSC
Mesenchymal stromal cell
- NETs
Neutrophil extracellular traps
- OSC
Open canalicular system
- PAS
Platelet additive solution
- PC
Platelet concentrate
- PDGF
Platelet-derived growth factor
- PF4
Platelet factor 4
- PL
Platelet lysate
- PRP
Platelet-rich plasma
- PSGL
P-selectin glycoprotein ligand
- RBC
Red blood cell
- S/D
Solvent/detergent
- SED
Serum eye drop
- SOX9
SRY-box transcription factor 9
- TDDS
Targeted drug delivery system
- TNF
Tumor necrosis factor
- TGF
Transforming growth factor
- UV
Ultraviolet
- VEGF
Vascular endothelial growth factor
- vWF
Von Willebrand factor
- WBC
White blood cell
Author contributions
TB wrote the first draft and the revision of the manuscript. MLC conceived and made the figures. DJL contributed to the section on myocardial infarction and revised the whole manuscript, EYC wrote the first draft of the section on platelet membrane coated nanomedicines, and CLT on applications to treat ocular diseases. HG contributed to ideas and concepts on the use of platelets and p-EVs as drug delivery vehicles. All authors read and approved the manuscript.
Authors’ information
TB is a member of the organizing committee and treasurer of the Working Party for Cellular Therapies” of the International Society of Blood Transfusion which has organized international workshops and published recommendations on the use of HPL for cell therapies and on the production of EDHO for treating ocular surface disorders.
Funding
This work was supported in part by Grants 111-2314-B-038-025 and 112-2923-E-038-001from the National Science and Technology Council (NSTC) of Taiwan, and Grants NHRI-EX111-10920E, NHRI-EX110-10920EI, and NHRI-EX109-10920EI from National Health Research Institute of Taiwan to TB’s laboratory. The funding bodies did not play a role in the content or writing of the manuscript.
Availability of data and materials
All materials are available from the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
TB is the inventor of patents owned by Taipei Medical University and is a founder of Invenis Biotherapies, a start-up company that develops applications of platelet lysates for brain administration and treatment of neurological disorders.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simon TL, et al. Rossi’s principles of transfusion medicine. Wiley Online Library; 2016. [Google Scholar]
- 2.Kaufman RM, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–213. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Paliczak A, Delgado D. Evidence-based indications of platelet-rich plasma therapy. Expert Rev Hematol. 2021;14(1):97–108. doi: 10.1080/17474086.2021.1860002. [DOI] [PubMed] [Google Scholar]
- 4.Everts P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi: 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dos Santos RG, et al. The regenerative mechanisms of platelet-rich plasma: a review. Cytokine. 2021;144:155560. doi: 10.1016/j.cyto.2021.155560. [DOI] [PubMed] [Google Scholar]
- 6.Schallmoser K, et al. Production and quality requirements of human platelet lysate: a position statement from the working party on cellular therapies of the international society of blood transfusion. Trends Biotechnol. 2020;38(1):13–23. doi: 10.1016/j.tibtech.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Nebie O, et al. Can the administration of platelet lysates to the brain help treat neurological disorders? Cell Mol Life Sci. 2022;79(7):379. doi: 10.1007/s00018-022-04397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson J, et al. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2021;39(6):598–612. doi: 10.1016/j.tibtech.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Shih DT, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotechnol. 2015;32(1):199–211. doi: 10.1016/j.nbt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnouf T, et al. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 11.Bieback K, et al. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: a joint publication from the AABB and the International Society for Cell & Gene Therapy. Transfusion. 2019;59(11):3448–3460. doi: 10.1111/trf.15483. [DOI] [PubMed] [Google Scholar]
- 12.Henschler R, et al. Human platelet lysate current standards and future developments. Transfusion. 2019;59(4):1407–1413. doi: 10.1111/trf.15174. [DOI] [PubMed] [Google Scholar]
- 13.De Pascale MR, et al. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev. 2015;29(1):52–61. doi: 10.1016/j.tmrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Burnouf T, et al. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27(2):77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Gomes FG, et al. Synergy of human platelet-derived extracellular vesicles with secretome proteins promotes regenerative functions. Biomedicines. 2022;10(2):238. doi: 10.3390/biomedicines10020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, et al. Platelet for drug delivery. Curr Opin Biotechnol. 2019;58:81–91. doi: 10.1016/j.copbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 17.He M, et al. The role of allogeneic platelet-rich plasma in patients with diabetic foot ulcer: current perspectives and future challenges. Front Bioeng Biotechnol. 2022;10:993436. doi: 10.3389/fbioe.2022.993436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao X, et al. Allogeneic platelet-rich plasma therapy as an effective and safe adjuvant method for chronic wounds. J Surg Res. 2020;246:284–291. doi: 10.1016/j.jss.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, et al. Efficacy of intra-articular injection of allogeneic platelet-rich plasma for knee osteoarthritis combined with hematologic blood dyscrasias with platelet dysfunction: protocol of a randomized, double-blind, placebo-controlled trial. BMC Musculoskelet Disord. 2022;23(1):1095. doi: 10.1186/s12891-022-06073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velier M, et al. Production of platelet-rich plasma gel from elderly patients under antithrombotic drugs: perspectives in chronic wounds care. Platelets. 2018;29(5):496–503. doi: 10.1080/09537104.2017.1336212. [DOI] [PubMed] [Google Scholar]
- 21.Niemann M, et al. Individual immune cell and cytokine profiles determine platelet-rich plasma composition. Arthritis Res Ther. 2023;25(1):6. doi: 10.1186/s13075-022-02969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison P, et al. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1895–1900. doi: 10.1111/jth.14223. [DOI] [PubMed] [Google Scholar]
- 23.Alsousou J, et al. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24(3):173–182. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 24.Anitua E, Prado R, Orive G. Allogeneic platelet-rich plasma: at the dawn of an off-the-shelf therapy? Trends Biotechnol. 2017;35(2):91–93. doi: 10.1016/j.tibtech.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Mastrogiacomo M, et al. Innovative cell and platelet rich plasma therapies for diabetic foot ulcer treatment: the allogeneic approach. Front Bioeng Biotechnol. 2022;10:869408. doi: 10.3389/fbioe.2022.869408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romano V, et al. Allogeneic solid platelet-rich plasma for persistent epithelial neurotrophic defects: a protocol and pilot study. Cornea. 2023;42(4):498–506. doi: 10.1097/ICO.0000000000003204. [DOI] [PubMed] [Google Scholar]
- 27.Akbarzadeh S, et al. Allogeneic platelet-rich plasma: is it safe and effective for wound repair? Eur Surg Res. 2021;62(1):1–9. doi: 10.1159/000514223. [DOI] [PubMed] [Google Scholar]
- 28.El-Gohary R, et al. Using intra-articular allogenic lyophilized growth factors in primary knee osteoarthritis: a randomized pilot study. Regen Med. 2021;16(2):113–115. doi: 10.2217/rme-2020-0104. [DOI] [PubMed] [Google Scholar]
- 29.Marchand M, et al. Serum drops for ocular surface disease: national survey of Canadian cornea specialists. Can J Ophthalmol. 2018;53(3):266–271. doi: 10.1016/j.jcjo.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Drew VJ, et al. Reflections on dry eye syndrome treatment: therapeutic role of blood products. Front Med (Lausanne) 2018;5:33. doi: 10.3389/fmed.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabriel C, et al. Eye drops of human origin-Current status and future needs: report on the workshop organized by the ISBT Working Party for Cellular Therapies. Vox Sang. 2023;118(4):301–309. doi: 10.1111/vox.13413. [DOI] [PubMed] [Google Scholar]
- 32.Council of Europe—20th Edition of the Guide to the preparation, use and quality assurance of blood components. https://www.edqm.eu/en/blood-guide, C.o. Europe, Editor. 2022: Strasbourg, France.
- 33.World Health Organization Guidelines on good manufacturing practices for blood establishments, Annex 4, TRS No 961. https://www.who.int/publications/m/item/gmp-for-blood-establishments-annex-4-trs-no-961. 2011.
- 34.Burnouf T. Modern plasma fractionation. Transfus Med Rev. 2007;21(2):101–117. doi: 10.1016/j.tmrv.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michelson AD, et al. Platelets. Academic press; 2019. [Google Scholar]
- 36.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estcourt L, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2016;176(3):365. doi: 10.1111/bjh.14423. [DOI] [PubMed] [Google Scholar]
- 38.WHO Model Lists of Essential Medicines 2021 https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists. 2021.
- 39.Al-Riyami AZ, et al. Quality of evidence-based guidelines for platelet transfusion and use: a systematic review. Transfusion. 2021;61(3):948–958. doi: 10.1111/trf.16257. [DOI] [PubMed] [Google Scholar]
- 40.Schenone M, Furie BC, Furie B. The blood coagulation cascade. Curr Opin Hematol. 2004;11(4):272–277. doi: 10.1097/01.moh.0000130308.37353.d4. [DOI] [PubMed] [Google Scholar]
- 41.Rapaport SI, Rao LVM. The tissue factor pathway: how it has become a “prima ballerina”. Thromb Haemost. 1995;74(07):007–017. [PubMed] [Google Scholar]
- 42.Norris LA. Blood coagulation. Best Pract Res Clin Obstet Gynaecol. 2003;17(3):369–383. doi: 10.1016/s1521-6934(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 43.Davie EW. Biochemical and molecular aspects of the coagulation cascade. Thromb Haemost. 1995;74(07):001–006. [PubMed] [Google Scholar]
- 44.Gremmel T, Frelinger AL III, Michelson AD. Platelet physiology. in Seminars in thrombosis and hemostasis. 2016. Thieme Medical Publishers. [DOI] [PubMed]
- 45.Thomas SG. The structure of resting and activated platelets. Platelets, 2019: p. 47–77.
- 46.Puhm F, Boilard E, Machlus KR. Platelet extracellular vesicles: beyond the blood. Arterioscler Thromb Vasc Biol. 2021;41(1):87–96. doi: 10.1161/ATVBAHA.120.314644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasecka A, Nieuwland R, Siljander PR-M. Platelet-derived extracellular vesicles, in Platelets. 2019, Elsevier. p. 401-416.
- 48.van der Meijden PE, Heemskerk JW. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 49.Rendu F, Brohard-Bohn B. The platelet release reaction: granules' constituents, secretion and functions. Platelets. 2001;12(5):261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 50.Battinelli EM, et al. Megakaryocytes package contents into separate α-granules that are differentially distributed in platelets. Blood Adv. 2019;3(20):3092–3098. doi: 10.1182/bloodadvances.2018020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang J, et al. Assessment of a complete and classified platelet proteome from genome-wide transcripts of human platelets and megakaryocytes covering platelet functions. Sci Rep. 2021;11(1):12358. doi: 10.1038/s41598-021-91661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melchinger H, et al. Role of platelet mitochondria: life in a nucleus-free zone. Front Cardiovasc Med. 2019;6:153. doi: 10.3389/fcvm.2019.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupuis A, et al. Platelet delta-storage pool disease: an update. J Clin Med. 2020;9(8):2508. doi: 10.3390/jcm9082508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNicol A, Israels SJ. Platelet dense granules: structure, function and implications for haemostasis. Thromb Res. 1999;95(1):1–18. doi: 10.1016/s0049-3848(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 55.Harrison P, Cramer EM. Platelet α-granules. Blood Rev. 1993;7(1):52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- 56.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Battinelli EM, et al. Megakaryocytes package contents into separate alpha-granules that are differentially distributed in platelets. Blood Adv. 2019;3(20):3092–3098. doi: 10.1182/bloodadvances.2018020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Italiano JE, Jr, et al. Angiogenesis is regulated by a novel mechanism: pro-and antiangiogenic proteins are organized into separate platelet α granules and differentially released. Blood. 2008;111(3):1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White JG, Michelson A. Platelet structure. Platelets. 2007;2:45–71. [Google Scholar]
- 60.White JG, Escolar G. The blood platelet open canalicular system: a two-way street. Eur J Cell Biol. 1991;56(2):233–242. [PubMed] [Google Scholar]
- 61.Dahiya N, et al. Platelet microRNAs: an overview. Transfus Med Rev. 2015;29(4):215–219. doi: 10.1016/j.tmrv.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stakos DA, et al. Platelet microRNAs: from platelet biology to possible disease biomarkers and therapeutic targets. Platelets. 2013;24(8):579–589. doi: 10.3109/09537104.2012.724483. [DOI] [PubMed] [Google Scholar]
- 63.Nurden AT, et al. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- 64.Gaertner F, Massberg S. Patrolling the vascular borders: platelets in immunity to infection and cancer. Nat Rev Immunol. 2019;19(12):747–760. doi: 10.1038/s41577-019-0202-z. [DOI] [PubMed] [Google Scholar]
- 65.Kapur R, et al. Nouvelle cuisine: platelets served with inflammation. J Immunol. 2015;194(12):5579–5587. doi: 10.4049/jimmunol.1500259. [DOI] [PubMed] [Google Scholar]
- 66.Maouia A, et al. The immune nature of platelets revisited. Transfus Med Rev. 2020;34(4):209–220. doi: 10.1016/j.tmrv.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcoux G, et al. Role of platelets and megakaryocytes in adaptive immunity. Platelets. 2021;32(3):340–351. doi: 10.1080/09537104.2020.1786043. [DOI] [PubMed] [Google Scholar]
- 68.Nurden AT. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front Biosci (Landmark Ed) 2018;23:726–751. doi: 10.2741/4613. [DOI] [PubMed] [Google Scholar]
- 69.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burnouf T, Walker TL. The multifaceted role of platelets in mediating brain function. Blood (accepted), 2022. [DOI] [PMC free article] [PubMed]
- 71.Chou ML, et al. Blood-brain crosstalk: the roles of neutrophils, platelets, and neutrophil extracellular traps in neuropathologies. Trends Neurosciences. 2023 doi: 10.1016/j.tins.2023.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Boilard E, Duchez AC, Brisson A. The diversity of platelet microparticles. Curr Opin Hematol. 2015;22(5):437–444. doi: 10.1097/MOH.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 73.Melki I, et al. Platelet microvesicles in health and disease. Platelets. 2017;28(3):214–221. doi: 10.1080/09537104.2016.1265924. [DOI] [PubMed] [Google Scholar]
- 74.Aatonen MT et al. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014; 3. [DOI] [PMC free article] [PubMed]
- 75.Dai Z, et al. Platelets and platelet extracellular vesicles in drug delivery therapy: a review of the current status and future prospects. Front Pharmacol. 2022;13:1026386. doi: 10.3389/fphar.2022.1026386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. 2015;6:98. doi: 10.3389/fimmu.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ali RA, Wuescher LM, Worth RG. Platelets: essential components of the immune system. Curr Trends Immunol. 2015;16:65. [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y-T, et al. Biomimetic platelet nanomotors for site-specific thrombolysis and ischemic injury alleviation. ACS Appl Mater Interfaces. 2023;15:32967. doi: 10.1021/acsami.3c06378. [DOI] [PubMed] [Google Scholar]
- 80.Wu YW, et al. Clinical-grade cryopreserved doxorubicin-loaded platelets: role of cancer cells and platelet extracellular vesicles activation loop. J Biomed Sci. 2020;27(1):45. doi: 10.1186/s12929-020-00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peter I, et al. Platelet-rich plasma. Phys Med Rehabil Clin. 2016;27(4):825–853. doi: 10.1016/j.pmr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Foster TE, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 83.van der Meer PF, Seghatchian J, de Korte D. Autologous and allogeneic serum eye drops. The Dutch perspective. Transfus Apher Sci. 2015;53(1):99–100. doi: 10.1016/j.transci.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J, et al. Characteristics of platelet lysate compared to autologous and allogeneic serum eye drops. Transl Vis Sci Technol. 2020;9(4):24. doi: 10.1167/tvst.9.4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Badami KG, McKellar M. Allogeneic serum eye drops: time these became the norm? Br J Ophthalmol. 2012;96(8):1151–1152. doi: 10.1136/bjophthalmol-2012-301668. [DOI] [PubMed] [Google Scholar]
- 86.Espinosa A, et al. Implementation of a standardised method for the production of allogeneic serum eye drops from regular blood donors in a Norwegian University Hospital: some methodological aspects and clinical considerations. Transfus Apher Sci. 2015;53(1):88–91. doi: 10.1016/j.transci.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 87.Singh RP, et al. Quality assessment of platelet concentrates prepared by platelet rich plasma-platelet concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods. Asian J Transfus Sci. 2009;3(2):86. doi: 10.4103/0973-6247.53882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tynngård N. Preparation, storage and quality control of platelet concentrates. Transfus Apheres Sci. 2009;41(2):97–104. doi: 10.1016/j.transci.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Burgstaler EA. Blood component collection by apheresis. J Clin Apheresis. 2006;21(2):142–151. doi: 10.1002/jca.20043. [DOI] [PubMed] [Google Scholar]
- 90.Anonymous, Guide to the preparation, use and quality assurance of blood components, 10th edition., ed. E.D.f.t.Q.o.M.a.H. Care. 2011, Strasbourg: Council of Europe Publishing. 411.
- 91.Katz L, et al. Frequent plateletpheresis does not clinically significantly decrease platelet counts in donors. Transfusion. 2007;47(9):1601–1606. doi: 10.1111/j.1537-2995.2007.01330.x. [DOI] [PubMed] [Google Scholar]
- 92.Singh RP, et al. Quality assessment of platelet concentrates prepared by platelet rich plasma-platelet concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods. Asian J Transfus Sci. 2009;3:86–94. doi: 10.4103/0973-6247.53882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pietersz RNI, et al. Preparation of leukodepleted platelet concentrates from pooled buffy coats: prestorage filtration with Autostop (TM) BC. Vox Sang. 1999;76(4):231–236. doi: 10.1159/000031057. [DOI] [PubMed] [Google Scholar]
- 94.van der Meer P, Pietersz R, Reesink H. Leucoreduced platelet concentrates in additive solution: an evaluation of filters and storage containers. Vox Sang. 2001;81(2):102–107. doi: 10.1046/j.1423-0410.2001.00084.x. [DOI] [PubMed] [Google Scholar]
- 95.Eriksson L, Hogman CF. Platelet concentrates in an additive solution prepared from pooled buffy coats. 1. In vitro studies. Vox Sang. 1990;59(3):140–145. doi: 10.1111/j.1423-0410.1990.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 96.Azuma H, et al. Platelet additive solution—electrolytes. Transfus Apheres Sci. 2011;44(3):277–281. doi: 10.1016/j.transci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Thiele T, et al. Profiling alterations in platelets induced by Amotosalen/UVA pathogen reduction and gamma irradiation–a LC-ESI-MS/MS-based proteomics approach. Blood Transfus. 2012;10(Suppl 2):s63–70. doi: 10.2450/2012.010S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marrocco C, et al. Proteomic analysis of platelets treated with gamma irradiation versus a commercial photochemical pathogen reduction technology. Transfusion. 2013;53(8):1808–1820. doi: 10.1111/trf.12060. [DOI] [PubMed] [Google Scholar]
- 99.Burnouf T, et al. Viral safety of human platelet lysate for cell therapy and regenerative medicine: moving forward, yes, but without forgetting the past. Transfus Apher Sci. 2019;58(6):102674. doi: 10.1016/j.transci.2019.102674. [DOI] [PubMed] [Google Scholar]
- 100.Blumel J, et al. Strategies toward virus and prion safe human platelet lysates. Transfusion. 2020;60(1):219–220. doi: 10.1111/trf.15581. [DOI] [PubMed] [Google Scholar]
- 101.Lin L, et al. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion. 1997;37(4):423–435. doi: 10.1046/j.1537-2995.1997.37497265344.x. [DOI] [PubMed] [Google Scholar]
- 102.Goodrich R. The use of riboflavin for the inactivation of pathogens in blood products. Vox Sang. 2000;78:211–215. [PubMed] [Google Scholar]
- 103.Cid J, Lozano M. Pathogen inactivation of platelets for transfusion. Platelets. 2022;33(1):23–26. doi: 10.1080/09537104.2021.1935838. [DOI] [PubMed] [Google Scholar]
- 104.Rebulla P, Prati D. Pathogen reduction for platelets-a review of recent implementation strategies. Pathogens. 2022;11(2):142. doi: 10.3390/pathogens11020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Escolar G, Diaz-Ricart M, McCullough J. Impact of different pathogen reduction technologies on the biochemistry, function, and clinical effectiveness of platelet concentrates: an updated view during a pandemic. Transfusion. 2022;62(1):227–246. doi: 10.1111/trf.16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dodd RY. Viral inactivation in platelet concentrates. Transfus Clin Biol. 1994;1(3):181–186. doi: 10.1016/s1246-7820(05)80026-6. [DOI] [PubMed] [Google Scholar]
- 107.Goodrich RP, et al. A laboratory comparison of pathogen reduction technology treatment and culture of platelet products for addressing bacterial contamination concerns. Transfusion. 2009;49(6):1205–1216. doi: 10.1111/j.1537-2995.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- 108.Sobral PM, et al. Viral inactivation in hemotherapy: systematic review on inactivators with action on nucleic acids. Rev Bras Hematol Hemoter. 2012;34(3):231–235. doi: 10.5581/1516-8484.20120056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kwon SY, et al. Pathogen inactivation efficacy of Mirasol PRT System and Intercept Blood System for non-leucoreduced platelet-rich plasma-derived platelets suspended in plasma. Vox Sang. 2014;107(3):254–260. doi: 10.1111/vox.12158. [DOI] [PubMed] [Google Scholar]
- 110.Kaiser-Guignard J, et al. The clinical and biological impact of new pathogen inactivation technologies on platelet concentrates. Blood Rev. 2014;28(6):235–241. doi: 10.1016/j.blre.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Hechler B, Ravanat C, Gachet C. Amotosalen/UVA pathogen inactivation technology reduces platelet activability, induces apoptosis and accelerates clearance. Haematologica. 2017;102(12):e502–e503. doi: 10.3324/haematol.2017.180539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garban F, et al. Comparison of the hemostatic efficacy of pathogen-reduced platelets vs untreated platelets in patients with thrombocytopenia and malignant hematologic diseases: a randomized clinical trial. JAMA Oncol. 2018;4(4):468–475. doi: 10.1001/jamaoncol.2017.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Osselaer JC, et al. An active haemovigilance programme characterizing the safety profile of 7437 platelet transfusions prepared with amotosalen photochemical treatment. Vox Sang. 2008;94(4):315–323. doi: 10.1111/j.1423-0410.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 114.Willaert B, Vo Mai MP, Caldani C. French haemovigilance data on platelet transfusion. Transfus Med Hemother. 2008;35(2):118–121. doi: 10.1159/000118887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Knutson F, et al. A prospective, active haemovigilance study with combined cohort analysis of 19 175 transfusions of platelet components prepared with amotosalen-UVA photochemical treatment. Vox Sang. 2015;109(4):343–352. doi: 10.1111/vox.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jonsdottir-Buch SM, Lieder R, Sigurjonsson OE. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS ONE. 2013;8(7):e68984. doi: 10.1371/journal.pone.0068984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Commission, E., Commission Directive 2003/94/EC of 8 October 2003 laying down the principles and guidelines of good manufacturing practice in respect of medicinal products doe human use and investigational medicinal products for human use. 14.10.2003, O.j.o.t.E. Union, Editor. 2003, European Commission: Brussels. p. 22–26.
- 118.WHO. Guidelines on good manufacturing practices for blood establishments. Annex 4. WHO Technical Report Series, 2011; 961: 148–214.
- 119.Organization WH. Global status report on blood safety and availability 2021. 2022.
- 120.Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood J Am Soc Hematol. 2019;133(17):1854–1864. doi: 10.1182/blood-2018-11-833996. [DOI] [PubMed] [Google Scholar]
- 121.Barro L, et al. Human platelet lysates for human cell propagation. Platelets. 2021;32(2):152–162. doi: 10.1080/09537104.2020.1849602. [DOI] [PubMed] [Google Scholar]
- 122.van der Meer PF, de Korte D. Platelet additive solutions: a review of the latest developments and their clinical implications. Transfus Med Hemother. 2018;45(2):98–102. doi: 10.1159/000487513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Burnouf T, et al. Solvent/detergent treatment of platelet concentrates enhances the release of growth factors. Transfusion. 2008;48(6):1090–1098. doi: 10.1111/j.1537-2995.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 124.Su CY, et al. A virally inactivated functional growth factor preparation from human platelet concentrates. Vox Sang. 2009;97(2):119–128. doi: 10.1111/j.1423-0410.2009.01180.x. [DOI] [PubMed] [Google Scholar]
- 125.Delila L, et al. Extensive characterization of the composition and functional activities of five preparations of human platelet lysates for dedicated clinical uses. Platelets. 2021;32(2):259–272. doi: 10.1080/09537104.2020.1849603. [DOI] [PubMed] [Google Scholar]
- 126.Chen MS, et al. Four types of human platelet lysate, including one virally inactivated by solvent-detergent, can be used to propagate Wharton jelly mesenchymal stromal cells. N Biotechnol. 2019;49:151–160. doi: 10.1016/j.nbt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 127.Nyam-Erdene A, et al. Characterization and chromatographic isolation of platelet extracellular vesicles from human platelet lysates for applications in neuroregenerative medicine. ACS Biomater Sci Eng. 2021;7(12):5823–5835. doi: 10.1021/acsbiomaterials.1c01226. [DOI] [PubMed] [Google Scholar]
- 128.Barro L, et al. Nanofiltration of growth media supplemented with human platelet lysates for pathogen-safe xeno-free expansion of mesenchymal stromal cells. Cytotherapy. 2020;22(8):458–472. doi: 10.1016/j.jcyt.2020.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glovinski PV, et al. Overcoming the bottleneck of platelet lysate supply in large-scale clinical expansion of adipose-derived stem cells: a comparison of fresh versus three types of platelet lysates from outdated buffy coat–derived platelet concentrates. Cytotherapy. 2017;19(2):222–234. doi: 10.1016/j.jcyt.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 130.Barro L, et al. A double-virally-inactivated (Intercept-solvent/detergent) human platelet lysate for in vitro expansion of human mesenchymal stromal cells. Transfusion. 2019;59(6):2061–2073. doi: 10.1111/trf.15251. [DOI] [PubMed] [Google Scholar]
- 131.Valkonen S, et al. Assessment of time-dependent platelet activation using extracellular vesicles, CD62P exposure, and soluble glycoprotein V content of platelet concentrates with two different platelet additive solutions. Transfus Med Hemother. 2019;46(4):267–275. doi: 10.1159/000499958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Wit YE, et al. Platelet concentrates in platelet additive solutions generate less complement activation products during storage than platelets stored in plasma. Blood Transfus. 2023;21(2):157. doi: 10.2450/2022.0323-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hermida-Nogueira L, et al. Proteomic analysis of extracellular vesicles derived from platelet concentrates treated with Mirasol® identifies biomarkers of platelet storage lesion. J Proteomics. 2020;210:103529. doi: 10.1016/j.jprot.2019.103529. [DOI] [PubMed] [Google Scholar]
- 134.Strandberg G, et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion. 2017;57(4):1058–1065. doi: 10.1111/trf.13998. [DOI] [PubMed] [Google Scholar]
- 135.Bernardi M, et al. Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2013;15(8):920–929. doi: 10.1016/j.jcyt.2013.01.219. [DOI] [PubMed] [Google Scholar]
- 136.Shih DTB, et al. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion. 2011;51(4):770–778. doi: 10.1111/j.1537-2995.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- 137.Schallmoser K, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47(8):1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 138.Kao YC, et al. Removal process of prion and parvovirus from human platelet lysates used as clinical-grade supplement for ex vivo cell expansion. Cytotherapy. 2016;18(7):911–924. doi: 10.1016/j.jcyt.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 139.Delabie W, et al. Single step method for high yield human platelet lysate production. Transfusion. 2023;63(2):373–383. doi: 10.1111/trf.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Burnouf T, Radosevich M. Reducing the risk of infection from plasma products: specific preventative strategies. Blood Rev. 2000;14(2):94–110. doi: 10.1054/blre.2000.0129. [DOI] [PubMed] [Google Scholar]
- 141.Farrugia A. The evolution of the safety of plasma products from pathogen transmission-a continuing narrative. Pathogens. 2023;12(2):318. doi: 10.3390/pathogens12020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jonsdottir-Buch S, et al. Expired pathogen inactivated platelet concentrates support differentiation and immunomodulation of mesenchymal stromal cells in culture. J Tissue Eng Regenerative Med. 2014;8:374. doi: 10.3727/096368914X683043. [DOI] [PubMed] [Google Scholar]
- 143.Fazzina R, et al. Culture of human cell lines by a pathogen-inactivated human platelet lysate. Cytotechnology. 2016;68(4):1185–1195. doi: 10.1007/s10616-015-9878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jonsdottir-Buch SM, et al. Expired and pathogen-inactivated platelet concentrates support differentiation and immunomodulation of mesenchymal stromal cells in culture. Cell Transplant. 2015;24(8):1545–1554. doi: 10.3727/096368914X683043. [DOI] [PubMed] [Google Scholar]
- 145.Christensen C, Jonsdottir-Buch SM, Sigurjonsson OE. Effects of amotosalen treatment on human platelet lysate bioactivity: a proof-of-concept study. PLoS ONE. 2020;15(4):e0220163. doi: 10.1371/journal.pone.0220163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Razani E, et al. A comparative study of pathogen inactivation technologies in human platelet lysate and its optimal efficiency in human placenta-derived stem cells culture. J Virol Methods. 2022;302:114478. doi: 10.1016/j.jviromet.2022.114478. [DOI] [PubMed] [Google Scholar]
- 147.Viau S, et al. Pathogen reduction through additive-free short-wave UV light irradiation retains the optimal efficacy of human platelet lysate for the expansion of human bone marrow mesenchymal stem cells. PLoS ONE. 2017;12(8):e0181406. doi: 10.1371/journal.pone.0181406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bieback K, et al. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: a joint publication from the AABB and the International Society for Cell & Gene Therapy. Cytotherapy. 2019;21(9):911–924. doi: 10.1016/j.jcyt.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 149.Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J Vis Exp. 2009(32). [DOI] [PMC free article] [PubMed]
- 150.Hauser L, et al. Hepatitis E transmission by transfusion of Intercept blood system-treated plasma. Blood. 2014;123(5):796–797. doi: 10.1182/blood-2013-09-524348. [DOI] [PubMed] [Google Scholar]
- 151.Gowland P, et al. Parvovirus B19 passive transmission by transfusion of intercept(R) blood system-treated platelet concentrate. Transfus Med Hemother. 2016;43(3):198–202. doi: 10.1159/000445195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.European Medicine Agency Guideline on plasma-derived medicinal products https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-plasma-derived-medicinal-products_en.pdf. Accessed June 24, 2023, E.M. Agency, Editor. 2011, EMA: London.
- 153.European Medicines Agency (EMA) Committee for proprietary medicinal products (CPMP), Note for guidance on virus validation studies: the design, contribution and interpretation of studies validating the inactivation and removal of viruses. CPMP/BWP/268/95. https://www.ema.europa.eu/documents/scientific-guideline/note-guidance-virus-validation-studies-design-contribution-interpretation-studies-validating_en.pdf, (accessed June 24, 2023). 1996.
- 154.Gröner A, et al. Effective inactivation of a wide range of viruses by pasteurization. Transfusion. 2018;58(1):41–51. doi: 10.1111/trf.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burnouf T, et al. Biochemical and biological properties of an alpha 1-antitrypsin concentrate. Vox Sang. 1987;52(4):291–297. doi: 10.1111/j.1423-0410.1987.tb04895.x. [DOI] [PubMed] [Google Scholar]
- 156.Levy JA, et al. Inactivation by wet and dry heat of AIDS-associated retroviruses during factor VIII purification from plasma. Lancet. 1985;1(8443):1456–1457. doi: 10.1016/s0140-6736(85)91888-4. [DOI] [PubMed] [Google Scholar]
- 157.Reid K, et al. Potential contribution of mild pepsin treatment at pH4 to the viral safety of human immunoglobulin products. Vox Sang. 1988;55(2):75–80. doi: 10.1111/j.1423-0410.1988.tb05140.x. [DOI] [PubMed] [Google Scholar]
- 158.Horowitz B, et al. Solvent/detergent-treated plasma: a virus-inactivated substitute for fresh frozen plasma. Blood. 1992;79(3):826–831. [PubMed] [Google Scholar]
- 159.Burnouf T, et al. A process for solvent/detergent treatment of plasma for transfusion at blood centers that use a disposable-bag system. Transfusion. 2006;46(12):2100–2108. doi: 10.1111/j.1537-2995.2006.01035.x. [DOI] [PubMed] [Google Scholar]
- 160.Dichtelmuller HO, et al. Robustness of solvent/detergent treatment of plasma derivatives: a data collection from Plasma Protein Therapeutics Association member companies. Transfusion. 2009;49(9):1931–1943. doi: 10.1111/j.1537-2995.2009.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burnouf T, Radosevich M. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia. 2003;9(1):24–37. doi: 10.1046/j.1365-2516.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- 162.Burnouf-Radosevich M, et al. Nanofiltration, a new specific virus elimination method applied to high-purity factor IX and factor XI concentrates. Vox Sang. 1994;67(2):132–138. doi: 10.1111/j.1423-0410.1994.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 163.Roth NJ, et al. Nanofiltration as a robust method contributing to viral safety of plasma-derived therapeutics: 20 years’ experience of the plasma protein manufacturers. Transfusion. 2020;60:2661. doi: 10.1111/trf.16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ren J, et al. Comparison of human bone marrow stromal cells cultured in human platelet growth factors and fetal bovine serum. J Transl Med. 2018;16(1):65. doi: 10.1186/s12967-018-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Doucet C, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 166.Charlebois S, Canestrari E, Harris S. Characterization of a pathogen reduced human platelet lysate. Cytotherapy. 2018;20(5):S61–S61. [Google Scholar]
- 167.Barro L, et al. Removal of minute virus of mice-mock virus particles by nanofiltration of culture growth medium supplemented with 10% human platelet lysate. Cytotherapy. 2021;23(10):902–907. doi: 10.1016/j.jcyt.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 168.Bieback K, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27(9):2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 169.Cowper M, et al. Human platelet lysate as a functional substitute for fetal bovine serum in the culture of human adipose derived stromal/stem cells. Cells. 2019;8(7):724. doi: 10.3390/cells8070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vennila R, et al. Effect of human platelet lysate in differentiation of Wharton’s jelly derived mesenchymal stem cells. Endocr Metab Immune Disord Drug Targets. 2019;19(8):1177–1191. doi: 10.2174/1871530319666190226165910. [DOI] [PubMed] [Google Scholar]
- 171.Kandoi S, et al. Evaluation of platelet lysate as a substitute for FBS in explant and enzymatic isolation methods of human umbilical cord MSCs. Sci Rep. 2018;8(1):12439. doi: 10.1038/s41598-018-30772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hassan G, et al. Platelet lysate induces chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells. Cell Mol Biol Lett. 2018;23:11. doi: 10.1186/s11658-018-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.de Soure AM, et al. Integrated culture platform based on a human platelet lysate supplement for the isolation and scalable manufacturing of umbilical cord matrix-derived mesenchymal stem/stromal cells. J Tissue Eng Regen Med. 2017;11(5):1630–1640. doi: 10.1002/term.2200. [DOI] [PubMed] [Google Scholar]
- 174.Fazzina R, et al. Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues. Stem Cell Res Ther. 2016;7(1):122. doi: 10.1186/s13287-016-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tancharoen W, et al. Human platelet lysate as an alternative to fetal bovine serum for culture and endothelial differentiation of human amniotic fluid mesenchymal stem cells. Mol Med Rep. 2019;19(6):5123–5132. doi: 10.3892/mmr.2019.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pasztorek M, et al. Influence of platelet lysate on 2D and 3D amniotic mesenchymal stem cell cultures. Front Bioeng Biotechnol. 2019;7:338. doi: 10.3389/fbioe.2019.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marrazzo P, et al. Highly efficient in vitro reparative behaviour of dental pulp stem cells cultured with standardised platelet lysate supplementation. Stem Cells Int. 2016;2016:7230987. doi: 10.1155/2016/7230987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abuarqoub D, Awidi A, Abuharfeil N. Comparison of osteo/odontogenic differentiation of human adult dental pulp stem cells and stem cells from apical papilla in the presence of platelet lysate. Arch Oral Biol. 2015;60(10):1545–1553. doi: 10.1016/j.archoralbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 179.Govindasamy V, et al. Human platelet lysate permits scale-up of dental pulp stromal cells for clinical applications. Cytotherapy. 2011;13(10):1221–1233. doi: 10.3109/14653249.2011.602337. [DOI] [PubMed] [Google Scholar]
- 180.Abuarqoub DA, et al. The effect of platelet lysate in culture of PDLSCs: an in vitro comparative study. PeerJ. 2019;7:e7465. doi: 10.7717/peerj.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Palombella S, et al. Systematic review and meta-analysis on the use of human platelet lysate for mesenchymal stem cell cultures: comparison with fetal bovine serum and considerations on the production protocol. Stem Cell Res Ther. 2022;13(1):142. doi: 10.1186/s13287-022-02815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kabiri A, et al. Platelet-rich plasma application in chondrogenesis. Adv Biomed Res. 2014;3:138. doi: 10.4103/2277-9175.135156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sykes JG, et al. Impact of human platelet lysate on the expansion and chondrogenic capacity of cultured human chondrocytes for cartilage cell therapy. Eur Cell Mater. 2018;35:255–267. doi: 10.22203/eCM.v035a18. [DOI] [PubMed] [Google Scholar]
- 184.Kaps C, et al. Human platelet supernatant promotes proliferation but not differentiation of articular chondrocytes. Med Biol Eng Comput. 2002;40(4):485–490. doi: 10.1007/BF02345083. [DOI] [PubMed] [Google Scholar]
- 185.Pötter N, et al. Evaluation of the influence of platelet-rich plasma (PRP), platelet lysate (PL) and mechanical loading on chondrogenesis in vitro. Sci Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-99614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mantripragada VP, Muschler GF. Improved biological performance of human cartilage-derived progenitors in platelet lysate xenofree media in comparison to fetal bovine serum media. Curr Res Transl Med. 2022;70(4):103353. doi: 10.1016/j.retram.2022.103353. [DOI] [PubMed] [Google Scholar]
- 187.Gardner OFW, et al. Human platelet lysate enhances proliferation but not chondrogenic differentiation of pediatric mesenchymal progenitors. Cytotherapy. 2023;25:286. doi: 10.1016/j.jcyt.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 188.Chou ML, Burnouf T, Wang TJ. Ex vivo expansion of bovine corneal endothelial cells in xeno-free medium supplemented with platelet releasate. PLoS ONE. 2014;9(6):e99145. doi: 10.1371/journal.pone.0099145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mishan MA, et al. Potential Effect of human platelet lysate on in vitro expansion of human corneal endothelial cells compared with Y-27632 ROCK inhibitor. J Ophthalmic Vis Res. 2021;16(3):349–356. doi: 10.18502/jovr.v16i3.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mishan MA, et al. Comparative culture of human corneal endothelial cells following treatment with human platelet lysate/fibrin hydrogel versus Y-27632 ROCK inhibitor: in vitro and ex vivo study. Int Ophthalmol. 2022;42(5):1469–1479. doi: 10.1007/s10792-021-02136-x. [DOI] [PubMed] [Google Scholar]
- 191.Wang TJ, et al. Comparison of three human platelet lysates used as supplements for in vitro expansion of corneal endothelium cells. Transfus Apher Sci. 2017;56(5):769–773. doi: 10.1016/j.transci.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 192.Thieme D, et al. Optimized human platelet lysate as novel basis for a serum-, xeno-, and additive-free corneal endothelial cell and tissue culture. J Tissue Eng Regen Med. 2018;12(2):557–564. doi: 10.1002/term.2574. [DOI] [PubMed] [Google Scholar]
- 193.Anitua E, et al. Effects of heat-treatment on plasma rich in growth factors-derived autologous eye drop. Exp Eye Res. 2014;119:27–34. doi: 10.1016/j.exer.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 194.Fernandez Munoz B, et al. A proprietary GMP human platelet lysate for the expansion of dermal fibroblasts for clinical applications. Platelets. 2022;33(1):98–109. doi: 10.1080/09537104.2020.1856356. [DOI] [PubMed] [Google Scholar]
- 195.Berger DR, Centeno CJ, Steinmetz NJ. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Joint Res. 2019;8(1):32–40. doi: 10.1302/2046-3758.81.BJR-2018-0164.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tylek T, et al. Platelet lysate outperforms FCS and human serum for co-culture of primary human macrophages and hMSCs. Sci Rep. 2019;9(1):3533. doi: 10.1038/s41598-019-40190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Švajger U. Human platelet lysate is a successful alternative serum supplement for propagation of monocyte-derived dendritic cells. Cytotherapy. 2017;19(4):486–499. doi: 10.1016/j.jcyt.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 198.Alonso-Camino V, et al. Expansion of human T cells using xenogeneic free and gamma irradiated human platelet lysate. Cancer Res. 2023;83(7_Supplement):1775–1775. [Google Scholar]
- 199.Tesic N, et al. Dendritic cells generated in the presence of platelet lysate have a reduced type 1 polarization capacity. Immunol Invest. 2019;49:215. doi: 10.1080/08820139.2019.1624768. [DOI] [PubMed] [Google Scholar]
- 200.Lukjanov V, Koutna I, Simara P. CAR T-cell production using nonviral approaches. J Immunol Res. 2021;2021:6644685. doi: 10.1155/2021/6644685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ghassemi S, et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol Res. 2018;6(9):1100–1109. doi: 10.1158/2326-6066.CIR-17-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Canestrari E, et al. Human platelet lysate media supplement supports lentiviral transduction and expansion of human t lymphocytes while maintaining memory phenotype. J Immunol Res. 2019;2019:3616120. doi: 10.1155/2019/3616120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ghassemi S, et al. Enhancing Chimeric Antigen Receptor (CAR)-T cell anti-tumor function through advanced media design. Mol Ther-Methods Clin Dev. 2020;18:595. doi: 10.1016/j.omtm.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nowakowska P, et al. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. Cancer Immunol Immunother. 2018;67(1):25–38. doi: 10.1007/s00262-017-2055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Torres Chavez A, et al. Expanding CAR T cells in human platelet lysate renders T cells with in vivo longevity. J Immunother Cancer. 2019;7(1):330. doi: 10.1186/s40425-019-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lopez-Cantillo G, et al. CAR-T cell performance: how to improve their persistence? Front Immunol. 2022;13:878209. doi: 10.3389/fimmu.2022.878209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alonso-Camino V et al. Expansion of Different Types of Therapeutic Cells Using Xenogeneic Free and Gamma Irradiated Human Platelet Lysate. in CYTOTHERAPY. 2022. ELSEVIER SCI LTD THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB.
- 208.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 209.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 210.Ehrenfest DMD, et al. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(1):3. [PMC free article] [PubMed] [Google Scholar]
- 211.Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in medical dermatology: a systematic review. J Am Acad Dermatol. 2019;81(3):834–846. doi: 10.1016/j.jaad.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 212.Franchini M, et al. Serum eye drops for the treatment of ocular surface diseases: a systematic review and meta-analysis. Blood Transfus. 2019;17(3):200. doi: 10.2450/2019.0080-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Marks DC, van der Meer PF, C. Biomedical Excellence for Safer Transfusion Serum eye drops: a survey of international production methods. Vox Sang. 2017;112(4):310–317. doi: 10.1111/vox.12502. [DOI] [PubMed] [Google Scholar]
- 214.Janus J, et al. What’s new in the field of serum-based eye drops. J Transfusion Med. 2023;16(1):23–26. [Google Scholar]
- 215.Tseng CL, et al. Solvent/detergent virally inactivated serum eye drops restore healthy ocular epithelium in a rabbit model of dry-eye syndrome. PLoS ONE. 2016;11(4):e0153573. doi: 10.1371/journal.pone.0153573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.van der Meer PF, Seghatchian J, Marks DC. Quality standards, safety and efficacy of blood-derived serum eye drops: a review. Transfus Apheres Sci. 2016;54(1):164–167. doi: 10.1016/j.transci.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 217.Tseng CL, Seghatchian J, Burnouf T. Animal models to assess the therapeutic efficacy of human serum and serum-converted platelet lysates for dry eye syndrome: seeing is believing. Transfus Apher Sci. 2015;53(1):95–98. doi: 10.1016/j.transci.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 218.Rauz S et al. The Royal College of Ophthalmologists guidelines on serum eye drops for the treatment of severe ocular surface disease: full report. Eye. 2017: 1–16. [DOI] [PubMed]
- 219.Widyaningrum R, et al. A purified human platelet pellet lysate rich in neurotrophic factors and antioxidants repairs and protects corneal endothelial cells from oxidative stress. Biomed Pharmacother. 2021;142:112046. doi: 10.1016/j.biopha.2021.112046. [DOI] [PubMed] [Google Scholar]
- 220.Widyaningrum R, et al. In vitro evaluation of platelet extracellular vesicles (PEVs) for corneal endothelial regeneration. Platelets. 2022;33(8):1237–1250. doi: 10.1080/09537104.2022.2105829. [DOI] [PubMed] [Google Scholar]
- 221.Chou ML, et al. Tailor-made purified human platelet lysate concentrated in neurotrophins for treatment of Parkinson’s disease. Biomaterials. 2017;142:77–89. doi: 10.1016/j.biomaterials.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 222.Delila L, et al. Neuroprotective activity of a virus-safe nanofiltered human platelet lysate depleted of extracellular vesicles in Parkinson’s disease and traumatic brain injury models. Bioeng Transl Med. 2023;8(1):e10360. doi: 10.1002/btm2.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Anitua E, et al. Intranasal PRGF-Endoret enhances neuronal survival and attenuates NF-kappaB-dependent inflammation process in a mouse model of Parkinson’s disease. J Control Release. 2015;203:170–180. doi: 10.1016/j.jconrel.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 224.Anitua E, et al. Plasma rich in growth factors (PRGF-Endoret) reduces neuropathologic hallmarks and improves cognitive functions in an Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35(7):1582–1595. doi: 10.1016/j.neurobiolaging.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 225.Anitua E, et al. Intranasal delivery of plasma and platelet growth factors using PRGF-Endoret system enhances neurogenesis in a mouse model of Alzheimer’s disease. PLoS ONE. 2013;8(9):e73118. doi: 10.1371/journal.pone.0073118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gouel F, et al. Whole and fractionated human platelet lysate biomaterials-based biotherapy induces strong neuroprotection in experimental models of amyotrophic lateral sclerosis. Biomaterials. 2022;280:121311. doi: 10.1016/j.biomaterials.2021.121311. [DOI] [PubMed] [Google Scholar]
- 227.Hayon Y, et al. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb Haemost. 2013;110(08):323–330. doi: 10.1160/TH12-11-0875. [DOI] [PubMed] [Google Scholar]
- 228.Nebie O, et al. Heat-treated human platelet pellet lysate modulates microglia activation, favors wound healing and promotes neuronal differentiation in vitro. Platelets. 2021;32(2):226–237. doi: 10.1080/09537104.2020.1732324. [DOI] [PubMed] [Google Scholar]
- 229.Nebie O, et al. Human platelet lysate biotherapy for traumatic brain injury: preclinical assessment. Brain. 2021;144(10):3142–3158. doi: 10.1093/brain/awab205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nebie O, et al. The neuroprotective activity of heat-treated human platelet lysate biomaterials manufactured from outdated pathogen-reduced (amotosalen/UVA) platelet concentrates. J Biomed Sci. 2019;26(1):89. doi: 10.1186/s12929-019-0579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Margraf A, Zarbock A. Platelets in inflammation and resolution. J Immunol. 2019;203(9):2357–2367. doi: 10.4049/jimmunol.1900899. [DOI] [PubMed] [Google Scholar]
- 232.Costa LAV et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2022: 03635465211062243. [DOI] [PubMed]
- 233.Tramś E, et al. Role of platelets in osteoarthritis—updated systematic review and meta-analysis on the role of platelet-rich plasma in osteoarthritis. Cells. 2022;11(7):1080. doi: 10.3390/cells11071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Qu S, et al. Clinical studies on platelet-rich plasma therapy for chronic cutaneous ulcers: a systematic review and meta-analysis of randomized controlled trials. Adv Wound Care. 2022;11(2):56–69. doi: 10.1089/wound.2020.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gupta A, Potty AG, Maffulli N. Allogenic platelet-rich plasma for treatment of knee and hip osteoarthritis. Front Pain Res. 2023;4:1216190. doi: 10.3389/fpain.2023.1216190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen TM, et al. Single-donor allogeneic platelet fibrin glue and osteoconductive scaffold in orbital floor fracture reconstruction. Ann Plast Surg. 2013;70(3):370–374. doi: 10.1097/SAP.0b013e31823b6880. [DOI] [PubMed] [Google Scholar]
- 237.Wang S et al. Allogeneic platelet gel therapy for refractory abdominal wound healing: a preliminary study. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University, 2023. [DOI] [PubMed]
- 238.He M, et al. Comparison of allogeneic platelet-rich plasma with autologous platelet-rich plasma for the treatment of diabetic lower extremity ulcers. Cell Transplant. 2020;29:0963689720931428. doi: 10.1177/0963689720931428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Torreggiani E, et al. Exosomes: novel effectors of human platelet lysate activity. Eur Cell Mater. 2014;28:137–151. doi: 10.22203/ecm.v028a11. [DOI] [PubMed] [Google Scholar]
- 240.Aatonen M et al. Isolation of platelet-derived extracellular vesicles. Exosomes and Microvesicles: Methods and Protocols, 2017: 177–188. [DOI] [PubMed]
- 241.Maumus M, et al. Mesenchymal stem cell-derived extracellular vesicles: opportunities and challenges for clinical translation. Front Bioeng Biotechnol. 2020;8:997. doi: 10.3389/fbioe.2020.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Agrahari V, et al. Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol. 2019;37(7):707–729. doi: 10.1016/j.tibtech.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 243.Burnouf T, Agrahari V, Agrahari V. Extracellular vesicles as nanomedicine: hopes and hurdles in clinical translation. Int J Nanomed. 2019;14:8847–8859. doi: 10.2147/IJN.S225453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.World Health Organization. Blood safety and availability. https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability. 2 June 2023 (accessed July 12). 2023: Geneva.
- 245.Camussi G, Lotvall J. The importance of controlled clinical trials with extracellular vesicles. J Extracell Vesicles. 2023;12(7):e12347. doi: 10.1002/jev2.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Johnson J, et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J Extracell Vesicles. 2023;12(7):12332. doi: 10.1002/jev2.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Arraud N, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12(5):614–627. doi: 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- 248.Berckmans RJ, et al. Extracellular vesicles and coagulation in blood from healthy humans revisited. J Extracell Vesicles. 2019;8(1):1688936. doi: 10.1080/20013078.2019.1688936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kao CY, Papoutsakis ET. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr Opin Biotechnol. 2019;60:89–98. doi: 10.1016/j.copbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 250.Simonsen JB. What are we looking at? Extracellular vesicles, lipoproteins, or both? Circ Res. 2017;121(8):920–922. doi: 10.1161/CIRCRESAHA.117.311767. [DOI] [PubMed] [Google Scholar]
- 251.Sung PS, Huang TF, Hsieh SL. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat Commun. 2019;10(1):2402. doi: 10.1038/s41467-019-10360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xu Y, Nakane N, Maurer-Spurej E. Novel test for microparticles in platelet-rich plasma and platelet concentrates using dynamic light scattering. Transfusion. 2011;51(2):363–370. doi: 10.1111/j.1537-2995.2010.02819.x. [DOI] [PubMed] [Google Scholar]
- 253.Rank A, et al. Apheresis platelet concentrates contain platelet-derived and endothelial cell-derived microparticles. Vox Sang. 2011;100(2):179–186. doi: 10.1111/j.1423-0410.2010.01385.x. [DOI] [PubMed] [Google Scholar]
- 254.Marcoux G, et al. Platelet-derived extracellular vesicles convey mitochondrial DAMPs in platelet concentrates and their levels are associated with adverse reactions. Transfusion. 2019;59(7):2403–2414. doi: 10.1111/trf.15300. [DOI] [PubMed] [Google Scholar]
- 255.Black A, et al. Analysis of platelet-derived extracellular vesicles in plateletpheresis concentrates: a multicenter study. Transfusion. 2017;57(6):1459–1469. doi: 10.1111/trf.14109. [DOI] [PubMed] [Google Scholar]
- 256.Marcoux G, et al. Microparticle and mitochondrial release during extended storage of different types of platelet concentrates. Platelets. 2017;28(3):272–280. doi: 10.1080/09537104.2016.1218455. [DOI] [PubMed] [Google Scholar]
- 257.Black A, et al. Platelet-derived extracellular vesicles in plateletpheresis concentrates as a quality control approach. Transfusion. 2015;55(9):2184–2196. doi: 10.1111/trf.13128. [DOI] [PubMed] [Google Scholar]
- 258.Brennan K, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10(1):1039. doi: 10.1038/s41598-020-57497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Javan MR, et al. In-line leukoreduction filters: a new source of microparticle for human and animal study. Transfus Apher Sci. 2022;62:103602. doi: 10.1016/j.transci.2022.103602. [DOI] [PubMed] [Google Scholar]
- 260.Heijnen HF, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 261.Midekessa G, et al. Zeta potential of extracellular vesicles: toward understanding the attributes that determine colloidal stability. ACS Omega. 2020;5(27):16701–16710. doi: 10.1021/acsomega.0c01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wu YW et al. Platelet extracellular vesicles are efficient delivery vehicles of doxorubicin, an anti-cancer drug: Preparation and in vitro characterisation (In press). Platelets. 2023. [DOI] [PubMed]
- 263.Zara M, et al. The impact of platelet isolation protocol on the release of extracellular vesicles. Front Biosci (Landmark Ed) 2022;27(5):161. doi: 10.31083/j.fbl2705161. [DOI] [PubMed] [Google Scholar]
- 264.Karimi N, et al. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma—contributions of platelet extracellular vesicles in plasma samples. J Extracell Vesicles. 2022;11(5):e12213. doi: 10.1002/jev2.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Vader P, et al. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 266.Walker S, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9(26):8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Böing AN, et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3(1):23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Diallo I, et al. Platelet pathogen reduction technologies alter the microRNA profile of platelet-derived microparticles. Front Cardiovasc Med. 2020;7:31. doi: 10.3389/fcvm.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mabrouk M et al. The pathophysiological role of platelet-derived extracellular vesicles. In Seminars in thrombosis and hemostasis. 2022. Thieme Medical Publishers, Inc. [DOI] [PubMed]
- 271.Antich-Rosselló M, et al. Platelet-derived extracellular vesicles for regenerative medicine. Int J Mol Sci. 2021;22(16):8580. doi: 10.3390/ijms22168580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wu J, et al. Platelet-rich plasma-derived extracellular vesicles: a superior alternative in regenerative medicine? Cell Prolif. 2021;54(12):e13123. doi: 10.1111/cpr.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Graca AL, et al. Therapeutic effects of platelet-derived extracellular vesicles in a bioengineered tendon disease model. Int J Mol Sci. 2022;23(6):2948. doi: 10.3390/ijms23062948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tao SC, Guo SC, Zhang CQ. Platelet-derived extracellular vesicles: an emerging therapeutic approach. Int J Biol Sci. 2017;13(7):828–834. doi: 10.7150/ijbs.19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Spakova T, Janockova J, Rosocha J. Characterization and therapeutic use of extracellular vesicles derived from platelets. Int J Mol Sci. 2021;22(18):9701. doi: 10.3390/ijms22189701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Boilard E, Bellio M. Platelet extracellular vesicles and the secretory interactome join forces in health and disease. Immunol Rev. 2022;312(1):38–51. doi: 10.1111/imr.13119. [DOI] [PubMed] [Google Scholar]
- 277.Mathieu M, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 278.Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J Lipid Res. 2018;59(11):2037–2046. doi: 10.1194/jlr.R084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Burnouf T, et al. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014;28(4):155–166. doi: 10.1016/j.blre.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 280.Bordin A, et al. Human platelet lysate-derived extracellular vesicles enhance angiogenesis through miR-126. Cell Prolif. 2022;55(11):e13312. doi: 10.1111/cpr.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Dyer MR, et al. Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. J Thromb Haemost. 2019;17(10):1733–1745. doi: 10.1111/jth.14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chimen M, et al. Appropriation of GPIbalpha from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica. 2020;105(5):1248–1261. doi: 10.3324/haematol.2018.215145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kerris EW, et al. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J Investig Med. 2020;68(4):813–820. doi: 10.1136/jim-2019-001195. [DOI] [PubMed] [Google Scholar]
- 285.Graca AL, et al. Controlling the fate of regenerative cells with engineered platelet-derived extracellular vesicles. Nanoscale. 2022;14(17):6543–6556. doi: 10.1039/d1nr08108j. [DOI] [PubMed] [Google Scholar]
- 286.Brill A, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67(1):30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 287.Kim HK, et al. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124(3):376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 288.Lopez E, et al. Platelet-derived- extracellular vesicles promote hemostasis and prevent the development of hemorrhagic shock. Sci Rep. 2019;9(1):17676. doi: 10.1038/s41598-019-53724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Guo SC, et al. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1):81. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Dai Z, et al. Platelet-derived extracellular vesicles ameliorate intervertebral disc degeneration by alleviating mitochondrial dysfunction. Materials Today Bio. 2023;18:100512. doi: 10.1016/j.mtbio.2022.100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hayon Y, et al. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr Neurovasc Res. 2012;9(3):185–192. doi: 10.2174/156720212801619018. [DOI] [PubMed] [Google Scholar]
- 292.Hayon Y, et al. Platelet microparticles promote neural stem cell proliferation, survival and differentiation. J Mol Neurosci. 2012;47(3):659–665. doi: 10.1007/s12031-012-9711-y. [DOI] [PubMed] [Google Scholar]
- 293.Leiter O, Walker TL. Platelets: the missing link between the blood and brain? Prog Neurobiol. 2019;183:101695. doi: 10.1016/j.pneurobio.2019.101695. [DOI] [PubMed] [Google Scholar]
- 294.Leiter O, Walker TL. Platelets in neurodegenerative conditions-friend or foe? Front Immunol. 2020;11:747. doi: 10.3389/fimmu.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. 2019;20(9):509–510. doi: 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 296.Borger V, et al. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy. 2020;22(9):482–485. doi: 10.1016/j.jcyt.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sarkar S, et al. Drug delivery using platelet cancer cell interaction. Pharm Res. 2013;30:2785–2794. doi: 10.1007/s11095-013-1097-1. [DOI] [PubMed] [Google Scholar]
- 298.Uslu D et al. Effect of platelet exosomes loaded with doxorubicin as a targeted therapy on triple-negative breast cancer cells. Mol Divers. 2022. [DOI] [PubMed]
- 299.Xu P, et al. Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci Rep. 2017;7:42632. doi: 10.1038/srep42632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Xu P, et al. Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: a novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget. 2017;8(35):58322–58337. doi: 10.18632/oncotarget.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yanez-Mo M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wiklander OPB et al. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019; 11(492). [DOI] [PMC free article] [PubMed]
- 303.Burnouf T, et al. Circulatory-cell-mediated nanotherapeutic approaches in disease targeting. Drug Discov Today. 2018;23(5):934–943. doi: 10.1016/j.drudis.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 304.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kailashiya J, Gupta V, Dash D. Engineered human platelet-derived microparticles as natural vectors for targeted drug delivery. Oncotarget. 2019;10(56):5835–5846. doi: 10.18632/oncotarget.27223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Goubran H, et al. Platelet microparticles and cancer: an intimate cross-talk. Transfus Apher Sci. 2015;53(2):168–172. doi: 10.1016/j.transci.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 307.Ma Q, et al. Calming cytokine storm in pneumonia by targeted delivery of TPCA-1 using platelet-derived extracellular vesicles. Matter. 2020;3(1):287–301. doi: 10.1016/j.matt.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kieffer N, Phillips D. Platelet membrane glycoproteins: functions in cellular interactions. Annu Rev Cell Biol. 1990;6(1):329–357. doi: 10.1146/annurev.cb.06.110190.001553. [DOI] [PubMed] [Google Scholar]
- 309.Woodfin A, Voisin M-B, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27(12):2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 310.Gomes FG, et al. Breast-cancer extracellular vesicles induce platelet activation and aggregation by tissue factor-independent and -dependent mechanisms. Thromb Res. 2017;159:24–32. doi: 10.1016/j.thromres.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 311.Tian YH, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 312.Goubran HA, et al. The platelet-cancer loop. Eur J Intern Med. 2013;24(5):393–400. doi: 10.1016/j.ejim.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 313.Goubran HA, et al. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40(3):296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 314.Lazar S, Goldfinger LE. Platelets and extracellular vesicles and their cross talk with cancer. Blood. 2021;137(23):3192–3200. doi: 10.1182/blood.2019004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Franco AT, Ware J. Pathophysiology 2: the role of platelets in cancer biology. Cancer Treat Res. 2019;179:37–54. doi: 10.1007/978-3-030-20315-3_3. [DOI] [PubMed] [Google Scholar]
- 316.Ma Q, et al. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J Control Release. 2021;329:445–453. doi: 10.1016/j.jconrel.2020.11.064. [DOI] [PubMed] [Google Scholar]
- 317.Meliciano A, et al. Clinically expired platelet concentrates as a source of extracellular vesicles for targeted anti-cancer drug delivery. Pharmaceutics. 2023;15(3):953. doi: 10.3390/pharmaceutics15030953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Desai C, et al. Beyond the thrombus: platelet-inspired nanomedicine approaches in inflammation, immune response, and cancer. J Thromb Haemost. 2022;20(7):1523–1534. doi: 10.1111/jth.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zaldivia MTK, et al. Platelet-derived microvesicles in cardiovascular diseases. Front Cardiovasc Med. 2017;4:74. doi: 10.3389/fcvm.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Agrahari V, et al. Nanoformulation properties, characterization, and behavior in complex biological matrices: challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential. Adv Drug Deliv Rev. 2019;148:146–180. doi: 10.1016/j.addr.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 321.Burnouf T, et al. Near-infrared-driven photoablation of lung cancer tumors utilizing biomimetic platelet-polyethyleneimine-polypyrrole drug-free nanoparticles. Mater Des. 2022;215:110481. [Google Scholar]
- 322.Chen L et al. Platelet membrane-coated nanocarriers targeting plaques to deliver anti-cd47 antibody for atherosclerotic therapy. Research. 2022; 2022. [DOI] [PMC free article] [PubMed]
- 323.Hu C-MJ, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Han H, et al. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur J Pharm Biopharm. 2022;172:1–15. doi: 10.1016/j.ejpb.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 325.Narain A, et al. Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine. 2017;12(21):2677–2692. doi: 10.2217/nnm-2017-0225. [DOI] [PubMed] [Google Scholar]
- 326.Lundy DJ, et al. Distribution of systemically administered nanoparticles reveals a size-dependent effect immediately following cardiac ischaemia-reperfusion injury. Sci Rep. 2016;6(1):25613. doi: 10.1038/srep25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8(9):502–512. doi: 10.1038/nrcardio.2011.91. [DOI] [PubMed] [Google Scholar]
- 328.Stellos K, et al. Circulating platelet–progenitor cell coaggregate formation is increased in patients with acute coronary syndromes and augments recruitment of CD34+ cells in the ischaemic microcirculation. Eur Heart J. 2013;34(32):2548–2556. doi: 10.1093/eurheartj/eht131. [DOI] [PubMed] [Google Scholar]
- 329.Cheng B, et al. Biomimicking platelet–monocyte interactions as a novel targeting strategy for heart healing. Adv Healthcare Mater. 2016;5(20):2686–2697. doi: 10.1002/adhm.201600724. [DOI] [PubMed] [Google Scholar]
- 330.Tang J, et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng. 2018;2(1):17–26. doi: 10.1038/s41551-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Li Q, et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics. 2021;11(8):3916. doi: 10.7150/thno.52496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hu S, et al. Platelet membrane and stem cell exosome hybrids enhance cellular uptake and targeting to heart injury. Nano Today. 2021;39:101210. doi: 10.1016/j.nantod.2021.101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wang T, et al. Platelet membrane-camouflaged nanoparticles carry microRNA inhibitor against myocardial ischaemia-reperfusion injury. J Nanobiotechnol. 2022;20(1):1–22. doi: 10.1186/s12951-022-01639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wang S, et al. Drug targeting via platelet membrane–coated nanoparticles. Small structures. 2020;1(1):2000018. doi: 10.1002/sstr.202000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sinauridze EI, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425–434. [PubMed] [Google Scholar]
- 336.Obeid S, et al. NanoBioAnalytical characterization of extracellular vesicles in 75-nm nanofiltered human plasma for transfusion: a tool to improve transfusion safety. Nanomedicine. 2019;20:101977. doi: 10.1016/j.nano.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 337.Chou ML, et al. Nanofiltration to remove microparticles and decrease the thrombogenicity of plasma: in vitro feasibility assessment. Transfusion. 2015;55(10):2433–2444. doi: 10.1111/trf.13162. [DOI] [PubMed] [Google Scholar]
- 338.Kintigh J, Monagle P, Ignjatovic V. A review of commercially available thrombin generation assays. Res Pract Thromb Haemost. 2018;2(1):e12048. doi: 10.1002/rth2.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Grundmann C et al. Modified thrombin generation assay: application to the analysis of immunoglobulin concentrates. 2010.
- 340.Tripisciano C, et al. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017;7(1):6522. doi: 10.1038/s41598-017-03262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Tripisciano C, et al. Extracellular vesicles derived from platelets, red blood cells, and monocyte-like cells differ regarding their ability to induce factor XII-dependent thrombin generation. Front Cell Dev Biol. 2020;8:298. doi: 10.3389/fcell.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Devalet B, et al. Application of a clot-based assay to measure the procoagulant activity of stored allogeneic red blood cell concentrates. Blood Transfus. 2018;16(2):163–172. doi: 10.2450/2017.0230-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Nielsen T, et al. Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation. J Extracell Vesicles. 2018;7(1):1454777. doi: 10.1080/20013078.2018.1454777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Amiral J, Seghatchian J. The diagnostic usefulness of capture assays for measuring global/specific extracellular micro-particles in plasma. Transfus Apher Sci. 2015;53(2):127–136. doi: 10.1016/j.transci.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 345.Laroche M, et al. Update on functional and genetic laboratory assays for the detection of platelet microvesicles. Platelets. 2017;28(3):235–241. doi: 10.1080/09537104.2016.1265925. [DOI] [PubMed] [Google Scholar]
- 346.Germishuizen W, et al. Quantifying the thrombogenic potential of human plasma-derived immunoglobulin products. Biologicals. 2014;42(5):260–270. doi: 10.1016/j.biologicals.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 347.Słomka A, et al. Large extracellular vesicles: have we found the holy grail of inflammation? Front Immunol. 2018;9:2723. doi: 10.3389/fimmu.2018.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Sadallah S, et al. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186(11):6543–6552. doi: 10.4049/jimmunol.1002788. [DOI] [PubMed] [Google Scholar]
- 349.Ceroi A, et al. The anti-inflammatory effects of platelet-derived microparticles in human plasmacytoid dendritic cells involve liver X receptor activation. Haematologica. 2016;101(3):e72–e76. doi: 10.3324/haematol.2015.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lin HC, et al. Platelet-derived microparticles trigger THP-1 monocytic cell aggregation and release of pro-coagulant tissue factor-expressing microparticles in vitro. Transfus Apher Sci. 2015;53(2):246–252. doi: 10.1016/j.transci.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 351.Southworth TM, et al. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J Knee Surg. 2019;32(01):037–045. doi: 10.1055/s-0038-1675170. [DOI] [PubMed] [Google Scholar]
- 352.Sixtty-third World Health Assembly resolution Availability, safety and quality of blood products. WHA 63.12. https://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R12-en.pdf. 2010.
- 353.World Health Organization Action framework to advance universal access to safe, effective and quality assured blood products 2020–2023. https://www.who.int/news/item/19-02-2020-who-action-framework-to-advance-universal-access-to-safe-effective-and-quality-assured-blood-products-2020--2023. 2020.
- 354.World Health Assemble 58.13. Blood safety: proposal to establish World Blood Donor Day. https://apps.who.int/iris/bitstream/handle/10665/20363/WHA58_13-en.pdf;jsessionid=039857C6DE671B3843E8E18CF0900E16?sequence=1. 2005.
- 355.World Health Organization recommendations for the production, control and regulation of human plasma for fractionation, Annex 4, TRS No 941. https://www.who.int/publications/m/item/annex4-ecbs-human-plasma-fractionation. 2005.
- 356.Lener T, et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958–965. [PubMed] [Google Scholar]
- 358.Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 359.Italiano J, Battinelli E. Selective sorting of alpha-granule proteins. J Thromb Haemost. 2009;7:173–176. doi: 10.1111/j.1538-7836.2009.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Martínez CE, Smith PC, Palma Alvarado VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood J Am Soc Hematol. 2013;122(15):2550–2554. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- 362.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(S06):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 363.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):449–458. doi: 10.1160/TH14-12-1067. [DOI] [PubMed] [Google Scholar]
- 364.Hamzeh-Cognasse H, et al. Platelets and infections–complex interactions with bacteria. Front Immunol. 2015;6:82. doi: 10.3389/fimmu.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ezzeroug Ezzraimi A, et al. The antibacterial effect of platelets on Escherichia coli strains. Biomedicines. 2022;10(7):1533. doi: 10.3390/biomedicines10071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All materials are available from the corresponding author.