Abstract
Liver diseases are a major health issue, and prolonged liver injury always progresses. Advanced liver disorders impair liver regeneration. Millions of patients die yearly worldwide, even with the available treatments of liver transplantation and artificial liver support system. With its abundant cell resources and significant differentiative potential, stem cell therapy is a viable treatment for various disorders and offers hope to patients waiting for orthotopic liver transplantation. Considering such plight, stem cell therapeutic strategies deliver hope to the patients. Moreover, we conclude intrinsic and acquired perspectives based on stem cell sources. The properties and therapeutic uses of these stem cells' specific types or sources were then reviewed. Owing to the recent investigations of the above cells, a safe and effective therapy will emerge for advanced liver diseases soon.
Keywords: Stem cell, Intrinsic, Acquired, Advanced liver diseases, Therapeutic strategy
1. Background
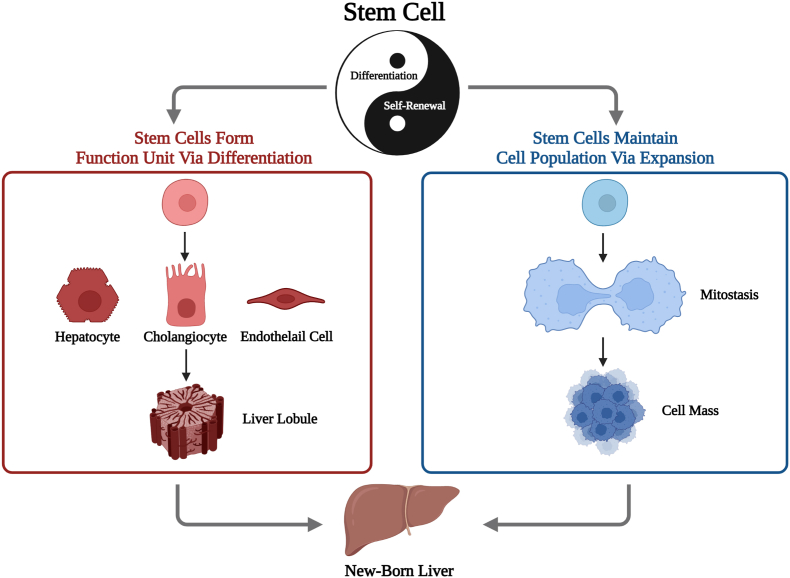
Liver illnesses caused by alcohol abuse, viral infection, and inborn metabolic abnormalities account for many global health issues and impose huge economic loads on sufferers (Asrani et al., 2019). Due to the limitations of current therapeutic methods, advanced liver diseases (ALD), defined as cirrhosis of any cause with one or more liver-related complications, is an inevitable end, and it is responsible for 560.4 age-standardized disability-adjusted life years per 100,000 population globally (Jepsen & Younossi, 2021; Naik et al., 2020). Due to the inconvenience and expensive cost of traditional artificial liver support systems, most patients view orthotopic liver transplantation (OLT) as the best approach to prolong their lives. As for the cell-based bio-artificial liver, primary porcine liver cells have been used to construct an extracorporeal hybrid liver support system clinically (Sauer et al., 2003). Another extracorporeal supporting system using human fibroblast-induced hepatocytes has been shown to benefit acute liver failure in pigs and humans (Wang et al., 2023; Shi et al., 2016). Yet, it has not been proven to rescue patients with chronic liver diseases once and for all. American liver transplantation has steadily increased to 8906 in 2020 (Jepsen & Younossi, 2021). However, owing to high-risk operations, possible utilization of lifelong immunosuppressants and specifically the scarcity of suitable deceased-donor, its clinical application is hindered from benefiting all. Split liver transplantation (SLT) and living donor liver transplantation (LDLT) have reduced mortality by narrowing the gap between patients on the waiting list and accessible livers. Still, they have not eliminated it (Fisher, 2017; Hackl et al., 2018). Hepatocyte transplantation (HT) and stem cell therapy, which are less invasive, have shown promise in reconstituting the functional parenchyma with fewer grafts, prolonging the bridging period to OLT, and boosting liver regeneration to reduce the need for organ transplantation (Dwyer et al., 2021). Stem cells' potential for self-renewal and differentiation can be described as a Taoist saying goes, “One produced two, two produced three, three produced all” (Fig. 1). Stem cells are also more likely to solve the difficulties than hepatocyte transplantation due to their wider range of origins and ability to survive cryogenic preservation (Zhang et al., 2021a). Initially, stem cell therapy was applied clinically to hematopathology. Then, to broaden its application range, it was investigated to have a profound prospect in neurology and ophthalmology as the immune privilege of brains and eyes simplified the trials. As for other solid organs with their more intricate niche, such as the liver, stem cell therapy still has a long way to go. The types of stem cells concerning the potential treatments of ALD can be roughly classified into 2 categories, intrinsic stem cells and acquired stem cells (Fig. 2). The “facultative stem cells” liver progenitor cells (LPCs) and biliary epithelial cells (BECs) can transdifferentiate into hepatocytes. However, the existence of preexisting hepatic tissue-specific stem cells is contested (Michalopoulos & Khan, 2015; Raven et al., 2017). The latter continues to be divided into three groups, including fetal stem cells (FSCs), mesenchymal stem cells (MSCs) and pluripotent stem cells (PSCs), which harbor the capability to replenish the liver parenchyma and simultaneously modulate the microenvironment of liver regeneration at some extent.
Fig. 1.
One produced two, two produced three, and three produced all things. Stem cells harbor self-renewal and differentiation abilities, which enable such cells to maintain the cell population and form functional units, thus leading to the future of providing all patients with appropriate newborn livers.
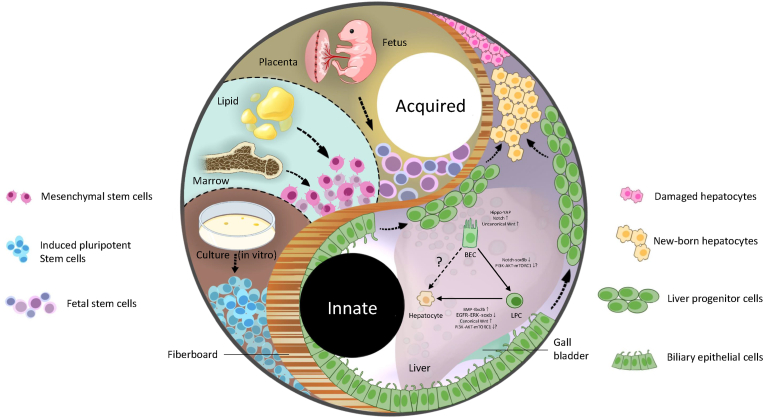
Fig. 2.
Potential intrinsic and acquired stem cell therapy. There are two stem cell therapies for advanced liver diseases, intrinsic and acquired, to treat the patients possibly. As for the former, the upregulation of Hippo-YAP, Notch and uncanonical Wnt pathways promote the expansion of BECs, then the downregulation of Notch-Sox9b pathways proceeds dedifferentiation of BEC, and at last, upregulation of BMP-tbx2b and canonical Wnt pathways and downregulation of EGFR-ERK-sox9 pathway assist the process of LPC-to-hepatocytes. In contrast, the role of the PI3K-AKT-mTORC1 pathway remains unclear. Cell sources for acquired stem cell therapy in advanced liver diseases include fetal-related tissue and adult tissue, from which mesenchymal and fetal stem cells can be isolated and induced pluripotent stem cells can be reprogrammed. For their application, there are indirect and direct approaches.
On the one hand, these cells are cultured into hepatocyte-like cells consequent to the differentiation in vitro. Contrarily, MSC and FSC can use in the patient directly since they could differentiate in vivo with relatively low risk. iPSC, induced pluripotent stem cell; MSC, mesenchymal stem cell; FLSC, fetal liver stem cell; BEC, biliary epithelial cell; LPC, liver progenitor cell.
This review presents numerous information regarding the main types of stem cells and their effects on ALD from innate and acquired perspectives. Simultaneously, we discuss the current progress of clinical research with its bottlenecks and prospects.
2. Intrinsic stem cell therapy
The liver’s parenchymal and nonparenchymal cells are all involved in hepatocyte regeneration, making it a highly regenerative organ (Fausto, 2004). To seek the cellular source of liver regeneration, it is reported that a group of telomerase high-expressing hepatocytes distribute throughout the liver lobule, repopulating the liver mass in homeostasis or injury (Lin et al., 2018). However, persistent liver injury, specifically in ALD with a loss of native hepatocytes and severe pathological alterations like cirrhosis, can decrease hepatocyte proliferating ability (Choi et al., 2014). Then targeting the intrinsic stem cells from non-hepatocytes residing in the liver seems to be a natural idea facing ALD. However, the existence of adult liver tissue-specific stem cells cannot be ascertained yet.
2.1. Liver progenitor cells and biliary epithelial cells
2.1.1. Controversial types of intrinsic stem cells
LPCs in the peribiliary glands and Canals of Hering, a population of quiescent liver cells, increase and differentiate when hepatocyte-driven regeneration is inhibited (Carpino et al., 2016; Kohn-Gaone et al., 2016). Moreover, with the single-cell RNA sequencing, a human liver cell atlas was provided to reveal a gradient expression of TROP2 that used to be identified as LPC markers among the liver cells, and a TROP2 intermediate expression subpopulation of bile duct cells strongly confirms the derivation of putative liver progenitor population after in situ location (Aizarani et al., 2019). Contrarily, LPCs activation was inhibited due to the implication of methylenedianiline that damages the bile duct; it is controversial whether LPCs exist in liver as an individual cell type (Ko et al., 2020). In various animals, BECs were also shown to supplement hepatocytes. BEC transdifferentiation replaces liver parenchyma in NTR/MTZ-induced zebrafish hepatocyte ablation models (He et al., 2014). A recent study observed that BECs converse into proliferative hepatocytes after short-term injury when blocking primary hepatocyte regeneration in hepatocyte-target p21 overexpressing or ltgb1 elimination mouse models (Raven et al., 2017). However, the mechanism how BECs sense the proliferation ability of hepatocytes is still not understood, and the triggering mechanism for such cells to play a stem-like role needs further exploration.
Moreover, in a mouse model induced by long-term thioacetamide (TAA) or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) treatment without genetic manipulations, a study implicated that up to 55.7% hepatocytes could be repopulated from the BECs by an HNF4α+CK19+ bi-phenotypic status without forming cancer nodules, which mimics the real progress in chronic liver injuries. Such phenotypic cells were also discovered in cirrhotic human livers (Deng et al., 2018). A study revealed that there was a transformation from K19+/GS+/EpCAM+/Heppar1− to K19−/GS−/EpCAM−/Heppar1+ of hepatocyte bud, a potential anatomic structure referring to the stem cell-based regeneration of the liver, in human cirrhosis specimens. In contrast, such buds are reduced in biliary diseases with bile duct loss; thus, it ensures the potential contribution of BECs in ALD (Stueck & Wanless, 2015). Cholangiocytes lose their transcriptional differences during organoid culture but retain their adaptability and in vivo signatures, which is appealing when transplanting the cholangiocytes organoid into the human liver to repair bile ducts (Sampaziotis et al., 2021), indicating that BECs harbor the potential stemness and their fate seems to be controlled by the specific types of liver injury.
2.1.2. Possible mechanisms of intrinsic stem cell-derived hepatocyte regeneration
Scientists are fascinated by the conceivable process which underpins potential therapeutic techniques, as the initiative events and inherent stem cell fate remain unknown. With the zebrafish model, Ko et al. revealed that all the preexisting BECs dedifferentiate into LPCs during the BEC-derived liver regeneration, indicating that it may contain two major steps, including BEC-to-LPC and LPC-to-hepatocyte in BECs-based liver regeneration. Unlike zebrafish, TAA-treated mouse models may regenerate biliary-to-hepatocytes directly based on new LPC markers (Deng et al., 2018). However, the process in mouse models is controversial since small Lgr5+ cells were still observed near bile ducts after the server liver damage (Huch et al., 2013). Thus, it seems that an indirect process is temporarily more conceivable, of which the potential molecular mechanisms are described below.
During biliary-to-hepatocyte differentiation, Notch signaling boosts the IGF1R to make BECs more sensitive to IGF1 and increase their quantity. However, persistent BEC proliferation prevents hepatocellular development, demonstrating that proliferation alone is insufficient (Minnis-Lyons et al., 2021). Remarkably, Ko et al. uncovered that Notch inhibition impels the conversion of LPCs to hepatocytes in vivo by sox9b (Russell et al., 2019). According to Ko et al., the Notch signaling pathway is transiently activated in the early stage of biliary-mediated differentiation and then intervenes before the following step (He et al., 2014). With the single-cell analysis, dynamic activation of YAP was uncovered among the BECs population, which was induced by the bile acid exposure level to maintain BECs' homeostatic (Pepe-Mooney et al., 2019). Considering that gut microbiota is involved in the progression of bile acid metabolism, it could also have a crucial role in the transdifferentiation of BECs via the liver-gut axis.
Moreover, the Hippo/YAP pathway was currently validated to promote the BECs expansion during the ductular reaction (DR) (Planas-Paz et al., 2019), which not only contains the BECs expansion and differentiation but also promotes intrahepatic angiogenesis (Coll et al., 2022). LPC-to-hepatocyte differentiation controls liver function restoration. BMP4 induces LPC mid- and late-phase hepatic markers (Fan et al., 2009; Wang, Yuan, & Shen, 2015), and BMP9 functions as a negative regulator of LPC expansion by decreasing cell expansion and increasing apoptosis (Addante et al., 2018). Then, Choi et al. certified that BMP signaling suppression did not hinder the dedifferentiation of BECs, but governs the LPCs differentiation into hepatocytes by tbx2b in a hepatocyte ablation model (Choi et al., 2017), which further validated the BMP role in LPC-to-hepatocytes differentiation. EGFR signaling not only regulates hepatocyte proliferation (Gonzalez et al., 2021), but is regarded as among cores of the regenerative process after the liver injury and involves cellular transformation associated with chronic damage (Gonzalez et al., 2021). In a zebrafish model, EGFR governs LPC-to-hepatocyte differentiation through the ERK-Sox9 axis, and EGFR inhibitors are more effective than Notch inhibitors (So et al., 2021), which further concludes a more promising target for regenerative medicine and reprogramming in vitro. Besides the above signaling, Hdac1 regulates LPC-to-hepatocyte differentiation by repressing sox9b expression (Ko et al., 2019). mTOR and Wnt signaling is thought to be involved in biliary-to-hepatocyte differentiation. Though experiments associated with the influence of mTOR on biliary-mediated regeneration are still limited, it is proven to take different effects in the thorough process during biliary-to-hepatocyte regeneration. In an NTR/MTZ-treated zebrafish model, mTORC1 inhibition suppresses BEC dedifferentiation partially through Urb2-mediated protein synthesis, which is vital for mTORC1 downstream effectors and LPC duplication. However, it only represses the proliferation of LPC-derived hepatocytes in the late stage (He et al., 2019).
Consequently, such evidence indicates that mTORC1 is truly involved in the BEC-driven regeneration, but the actual effects of mTORC1 urge further investigation. As for Wnt signaling, its uncanonical and canonical pathways are involved in BEC-driven regeneration, contributing to different processes. Contrarily, non-canonical Wnt signaling is considered significant merely for initiating DR (Okabe et al., 2016). However, Wnt3a-induced canonical Wnt/β-catenin signaling causes LPCs to become hepatocytes (Boulter et al., 2012). Canonical Wnt signaling may have contributed to other BEC-driven hepatocyte regeneration mechanisms. Recently, LGR4/5-mediated Wnt/β-catenin signaling is neither active nor necessary for DR (Planas-Paz et al., 2019). It is provided to assure further that the canonical Wnt/β-catenin signaling pathway mainly participates in the LPC redifferentiation into hepatocytes. DNA methylation at the p53 locus activates the dedifferentiation by inhibiting mTORC1 signaling and induces hepatocyte redifferentiation by repressing BMP signaling, which suggests that epigenetic modification also regulates the whole process (He et al., 2022). The degree of transdifferentiation determines whether intrinsic stem cells cause cancer, hence finding the essential regulator will lead to further research.
Besides, the stem cell niche has a tight relationship with cell plasticity and fate (Chacon-Martinez, Koester, & Wickstrom, 2018). Physically, the proliferation and liver organoid formation of BECs was reported to be negatively correlated with dynamical cell-cell contacts between them and PDGFRa+SCA1+ periportal mesenchymal cells (Cordero-Espinoza et al., 2021). Despite this, proliferating BECs recruit part of immune cells, myofibroblasts and endothelial cells by secreting the chemoattractant at the DR initiation. Macrophages help trigger the DR and promote biliary-mediated regeneration by the Wnt signaling pathway. (Boulter et al., 2012). Additionally, ECM, a complicated network composed of fibrous proteins and proteoglycans, can regulate the regeneration with matrix metalloproteinases (MMPs) which modulate collagenolytic activity (Roderfeld, 2018). Studies in humans and animals have shown that BECs can develop into hepatocytes after leaving a laminin-rich environment (Dubuquoy et al., 2015; Lorenzini et al., 2010). Due to complicated cellular or non-cellular components, the new understanding of the extracellular niche and BEC-driven regeneration cannot harness intrinsic cell plasticity and proliferative ability for ALD therapy.
In conclusion, LPC, or BEC-driven hepatocyte regeneration, is an exceedingly complex process with intricate networks of multiple signaling and factors inside the cells. Moreover, the regenerative niche around BECs also influences cell fate. The findings enable the invention of precise transdifferentiation-regulating regenerative therapies. Given their positive and negative effects, balancing the safety and effectiveness of intrinsic repair cautiously is still a challenge that urges us to address, and the more precise mechanism of biliary-to-hepatocyte transdifferentiation in humans must be further certified by a more appropriate and efficient model and other brand technologies to mine deep data.
2.1.3. Therapeutic applications
With the above data, researchers could not yet paint the picture of BEC-to-hepatocyte regeneration, much alone the proper dose and delivery techniques. Therefore, employing these cells in clinical stem cell therapy for ALD is not yet safe, which is still at the proof-of-concept stage.
For cell transplantation, research regarded CD45(−)/CD11b(−)/CD31(−)/MIC1-1C3(+)/CD133(+)/CD26(−) subpopulation as a prospective isolation strategy for adult hepatic stem cells (Dorrell et al., 2011). Later, Huch et al. designed a culture medium with the Wnt agonist RSPO1 to expand the mouse-derived single Lgr5+ liver stem cells. Then they certified that Wnt signals, cAMP activation, and Tgf-β inhibition were crucial for stable long-term expansion of human bile duct-derived stem cells (Huch et al., 2013, 2015). By precoating BECs with hyaluronic acid before transplantation, 11% of the liver parenchyma was restored, and ALB levels increased significantly, improving BEC engraftment (Nevi et al., 2017). Furthermore, with the progressing isolation, ex vivo expansion and long-term culture technologies, BECs have been successfully acquired from discarded human livers with excessive steatosis or fibrosis to transplant to rescue a mouse model of biliary diseases. It is also proved that transplantation of hBECs ameliorates the level of bilirubin, fibrosis and survival rate (Hallett et al., 2022), whereas it is unclear whether BECs repopulate the parenchyma. In addition, BECs regulate bile acid levels to enable remaining intact hepatocyte cell cycle progression because FXR and TGR5 preserve bile acids (Merlen et al., 2017). Nonetheless, scant evidence exists to support the direct interaction between BECs and the remaining healthy hepatocytes. This may be due to the fact that they are spatially distant from each other.
Moreover, for regenerative medicine, which is a non-invasive therapeutic strategy compared to cell transplantation, a recent study showed that farnesoid X receptor (FXR) activation inhibited the BEC-driven regeneration by suppressing PI3K-AKT-mTORC1 signaling and, therefore, downregulating LPC-to-hepatocyte differentiation rather than BEC-to-LPC dedifferentiation (Jung et al., 2021). In another work, FXR impaired BEC-driven regeneration by accumulating LPCs and preventing Notch-controlled hepatocyte production (Cai et al., 2021). Consequently, FXR blockers can function in vivo to impel the BEC-driven regeneration. Further, since FXR contributes to regulating the homeostasis of bile acid, whether metabolic homeostasis plays a pivotal role in such liver regeneration arouses huge interests and may lead to the development of medicines or therapies concerning metabolism. However, few regenerative medicine research targets BECs in situ to promote the biliary-to-hepatocyte transition without tumorigenicity.
3. Acquired stem cell therapy
Targeting intrinsic stem cells does not always improve liver function in all types of ALD because intrinsic stem cell-based healing happens step by step while some patients are on the brink of death. ALD patients with congenital aetiology, including inborn errors of metabolism (IEM), cannot undergo this type of correction since genetic mistakes persist after that. Thus, developing acquired cell-based treatment is considered a more promising method to deal with emergencies and correct these intrinsic conditions. Mature hepatocytes are obvious choices, and HT for IEM is currently regarded as a theoretical and practicable strategy. Though the HT clinical safety and short-term effectiveness were underpinned in a series of experiments with mouse models (Ambrosino et al., 2005; Celik et al., 2019; Overturf et al., 1997; Soltys et al., 2017; Stephenne et al., 2012), the clinical research elucidated that HT did not benefit as much as preclinical research (Iansante et al., 2018). Considering these drawbacks of HT, stem cell-based cell transplantation seems to be a more promising method.
3.1. Fetal stem cell
3.1.1. Sources and characteristics
Since the initial fetal tissue transplantation for diabetes mellitus, fetal stem cells have attracted significant research. Studies have shown that fetal stem cells are derived from the fetus and extra-embryonic tissues. The former includes cadaveric fetuses due to miscarriage, stillbirth, ectopic pregnancy and elective abortion (Ishii & Eto, 2014), and the latter includes amnion membrane, amniotic fluid, Wharton jelly and placenta (Marcus & Woodbury, 2008). Fetal stem cells derived from the two sources share the similar property of self-proliferation and reduced immunogenicity, but the differentiative potential of fetal stem cells is source dependent. Stem cells generated directly from the fetus are usually multipotent, meaning they can only differentiate into a few cell types. In contrast, those obtained from extra-embryonic tissues usually display pluripotency markers like Oct-4 and Sox2 (Bacakova et al., 2018). Therefore, extra-embryonic tissues derived stem cells seem to be more attractive.
Fetal liver stem cells (FLSCs), one type isolated from fetal livers, are regarded as the only choice in fetus-derived stem cells to effectively regenerate the almost normal liver (Oertel, 2011), representing an attractive stem cell therapy for liver diseases. These cells were found to contain a long-term compartment of colony-forming cells with multipotency, powerful self-renewal, and proliferative ability under extracorporeal culture conditions. They can also form hepatic-like tissue morphologically and phenotypically at the selective location according to liver injury (Yasen et al., 2019). Additionally, it is speculated that FLSCs possess less immunogenicity since they are immature cells without HLA class II antigen expression (Shi et al., 2005). Even if under long-term cryopreservation, they maintain the ability of proliferation and differentiation without much apoptosis (Ikeda et al., 2002). FLSCs require fewer cells for cell transplantation than mature hepatocytes to cure liver disorders effectively (Yoshida et al., 1996). Notwithstanding their limited availability, preprepared FLSCs are a viable alternative for acquired stem cell treatment.
The extra-embryonic tissue-derived stem cells comprise amniotic epithelial cells (AECs), amniotic fluid-derived stem cells (AFSCs), and the MSCs derived from amnion, amniotic fluid and placenta. These cells all harbor a strong ability for differentiation and proliferation (Marcus & Woodbury, 2008). These cells also have immunomodulatory effects, less ethical problems, low immunogenicity, and low tumorigenicity. Despite the wide application of extra-embryonic tissue-derived MSCs on liver diseases, other subpopulations of these fetal stem cells are also involved in some clinical research.
3.1.2. Therapeutic application
Compared to other disorders like hematopathy, ALD clinical trials using fetal stem cells are rare. The efficiency of FLSC transplantation for liver diseases has been demonstrated in animals and humans. In a DPPIV-deficient rat model, DPPIV+ HLCs occupied 60–80% of the primary liver after engraftment of DPPIV+ FLSCs (Sandhu et al., 2001). Recently, in rat models of chronic liver injury, another study observed that the portal vein injection of fetal liver stem cell-derived liver buds led to a level of 70% reconstitution of primary parenchyma and decreased the DR intensity, in which liver organoids were revealed settling down inside the liver rather than residing in other places (Tsuchida et al., 2019). The 6-month clinical observation of 25 FLSC-infused patients with liver cirrhosis of various etiologies demonstrates a significant rise in ALB and a decrease in SGOT, ALP, bilirubin, and MELD scores (Khan et al., 2010). Some studies just utilized AECs as a vector to carry therapeutic genes for immunodeficient liver diseases but did not detect whether these cells replenished the parenchyma of the liver (Saha & Jaenisch, 2009; Touboul et al., 2010). In liver fibrosis, AECs may block the activation of hepatic stellate cells and macrophages, acting as immunomodulators (Kuk et al., 2019) rather than replenishing the liver’s parenchyma (Cargnoni et al., 2018). These studies suggest fetal stem cells act in vivo in a complex way, and how to limit their use to predicted results is unknown.
3.2. Mesenchymal stem cells
3.2.1. Sources and characteristic
MSCs, a subtype of adult fibroblast-like cells, were initially described in 1968 by Friedenstein et al. (Friedenstein et al., 1968). The results of hitherto investigations verify that MSCs can move to injury sites, secrete regulatory factors, modulate immune response (Akker, de Jager, & Sluijter, 2013), differentiate into mesoderm (Pittenger et al., 1999), and self-renew. Various convenient sources are investigated to produce sufficient populations of MSCs like extra-embryonic, adipose and other adult tissues. Thus, MSCs are not scarce compared to the stem cell types mentioned above. Due to its widespread availability in adult tissues and low intrusive damage, adipose is the best MSC-based cell treatment candidate (Kolaparthy et al., 2015). These MSCs from various sources share similar properties on surface markers and morphological features (Mattar & Bieback, 2015). However, there are still discrepancies in differentiation potential, proliferative ability, clonality, tolerance for aging, and paracrine activity, among them underlying the sources (Jin et al., 2013). Several studies showed that umbilical cord-derived MSCs (UC-MSCs) expand more than MSCs derived from adult tissues (Baksh, Yao, & Tuan, 2007; Jin et al., 2013). As for the differentiation potential, UC-MSCs also seem to perform better than other types on multilineage potential, which have adipogenic, chondrogenic and osteogenic capacities and can differentiate into endothelial-like cells and neuron-like cells (Mushahary et al., 2018). Besides, even if the same source-derived MSCs are a constellation of heterogenous cells, each population has special advantages in the proliferative, differentiative and immunomodulatory activity of MSCs. Researchers sorted the CD34+ population of MSC in a TAA-induced liver fibrosis model. They found that they reduce collagen levels and mute hepatic stellate cells to prevent fibrosis and maintain liver function (Lee et al., 2016). Given the above advantages, distinct properties of some subtypes and the ability to well survive under cryopreservation, allogenic UC-MCSs are appropriate to be ‘off-the-shelf’ therapeutic products in case of diseases that sufficient cell quantities must be administrated promptly such as acute-on-chronic liver failure and even a more precise therapeutic application for ALD based on individual demands is possible.
3.2.2. How can MSCs exert therapeutic effects on advanced liver diseases?
MSCs have been demonstrated to ameliorate various liver diseases, whereas the actual mechanism remains unclear. Due to the pluripotency of MSCs, it seems apparent that they can replenish the functional hepatocytes. Schwartz and colleagues found that bone marrow-derived MSCs (BM-MSCs) treated with FGF-4 and HGF can develop into hepatocyte-like cells in vitro with limited hepatic capabilities, including as urea and albumin synthesis, phenobarbital-inducible CYP activity, and Dil-acil-LDL absorption (Schwartz et al., 2002). In vivo, however, some studies investigated that the bone marrow-derived stem cells hardly transdifferentiate into other types of cells (Castro et al., 2002; Wagers et al., 2002), indicating that there possibly be another mechanism in the above facts. Wang et al. pointed out that cell fusion is the crucial procedure contributing to increased bone marrow-derived hepatocytes. Afterwards, supplementary experiments were implemented and the result that hepatocytes expanses with donor surface markers further validates that viewpoint (Camargo, Finegold, & Goodell, 2004; Willenbring et al., 2004). MSCs also have pro- and anti-inflammatory actions to maintain the immunological microenvironment (Jiang & Xu, 2020), promoting the proliferation of remaining healthy hepatocytes. When the immune niche changes, MSCs sense the dynamic level of inflammatory factors such as IFN-γ, TNF-α, IL-1α and IL-1β and regulate the behavior of the adaptive and intrinsic immune cells and the activity of hepatic stellate cells (Tsuchiya et al., 2019). Besides the direct cell-to-cell contact in situ, MSCs can influence the remote immunological environment through the paracrine effect even if they are passively halted at the non-target site, which is now thought to be their main function rather than differentiation into HLCs (Alfaifi et al., 2018), suggesting that the secretions rather than the cells themselves will benefit more patients.
3.2.3. Therapeutic application
MSCs have been extensively studied, although their clinical use in liver disorders is still limited. For direct applications, a review summarized 321 MSC-based complete clinical trials. However, there were only 16 cases concerning liver diseases, which is much fewer than bone diseases (n = 62) or brain diseases (n = 53) (Yang et al., 2020). Currently, some clinical research focuses on subpopulations of ALDs, with some attempting to examine the positive and negative effects of MSC-based therapy. Though the effectiveness of MSCs from multiple sources is different due to their distinct characteristics on differentiation and proliferation, the collected researches show that MSCs derived from the umbilical cord, bone marrow and adipose tissue improve liver function and increase the survival rate of liver cirrhosis or liver failure caused by different etiologies (Amer et al., 2011; El-Ansary et al., 2012; Huang et al., 2019; Lanthier et al., 2017; Lin et al., 2017; Salama et al., 2014; Schacher et al., 2021; Shi et al., 2012, 2021). However, one study involved 58 patients with alcoholic liver cirrhosis, some of whom were administered BM-MSCs, and discovered that MSC-based therapy only has a limited effect on liver cirrhosis, activating the liver regenerative pathway but not expanding hepatocytes in liver biopsies (Zhang et al., 2012). Considering the powerful differentiation potential of MSCs, the chief problem of whether the application of MSCs is safe is crucial to be addressed. In vivo, tremendous research has revealed that MSC can be chemoattracted towards the tumor microenvironment and exert promotive and suppressive effects on tumor progression (Rosland et al., 2009; Yagi & Kitagawa, 2013).
Moreover, MSCs can multiply at the 15th passage in vitro and are stable in serum-free media without tumor transformation (Chen et al., 2014). Eventually, in collected clinical trials, the iatrogenic tumor formation was hardly observed after the infusion of MSCs, which did not simultaneously increase the risk of side effects. The MSCs' differentiation into HLC has also been reported in animals and humans (Banas et al., 2007). Thus, MSCs cultured in vitro can be an alternative source of hepatocyte transplantation in preclinical research, and the indirect application of MSCs attracts researchers. Both differentiated and undifferentiated BM-MSC transplantation helped cirrhosis patients, although statistical comparisons showed no meaningful difference (El-Ansary et al., 2012). Due to the complicated process of differentiating MSCs into HLC in vitro, infusing MSCs directly may be a better MSC-based therapy for ALD. More recently, evidence indicates that MSC-derived exosome emerges as a novel cell-free approach owing to the low risk of tumorigenicity and little toxicity of MSC-based cell therapy. The therapeutic effects of MSC-derived exosomes for liver fibrosis are confirmed in cirrhotic animal models, but the major mechanisms remain vague. UC-MSC exosomes suppressed the epithelial-mesenchymal transition and TGF-β/SMAD signaling pathway by lowering type I/III collagen in the CCl4-induced liver cirrhosis model (Li et al., 2013). Additionally, these exosomes were found to mitigate the acute injury and liver fibrosis by antioxidant effects (Jiang et al., 2018). In Schistosoma japonicum-infected mice, MSC-derived exosomes suppressed HSC activation and downregulated type I/III collagen, reducing liver fibrosis. However, the clinical applications of extracellular vesicles have not been fully implemented, which is waiting for an optimal purification protocol without contamination and damage.
3.3. Pluripotent stem cell
3.3.1. Sources and characteristics
Pluripotent stem cells include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Human embryonic stem cells (hESCs) from the embryo’s inner cell mass are used in regenerative medicine due to their pluripotency (Damdimopoulou et al., 2016). Recently, it was reported that hESC lines could be derived from single biopsied blastomeres of a human in vitro fertilization-embryo under utterly chemically defined and xeno-free conditions without destroying the embryo. Additionally, stable pig pregastrulation ESC lines have also been generated (Zhi et al., 2022), which provides a theoretical basis for regenerative medical research such as xenotransplantation. Takahashi and Yamanaka (Takahashi & Yamanaka, 2006) initially produced iPSCs from mouse fibroblasts in 2006 with infinite proliferation and differentiation capacity of three germ layers. Based on previous studies, they identified four reprogramming factors: Oct3/4, Sox2, Klf4, and c-Myc. Then, human iPSCs were generated from human dermal fibroblasts in the next year (Takahashi et al., 2007), which completely overcame the ethical plight of stem cell therapy and further underpinned the basis of iPSC clinical application in patients. Four other factors—OCT4, SOX2, NANOG, and LIN28—also transformed human somatic cells into iPSCs (Yu et al., 2007), therefore broadening the alternative sources of iPSCs. To properly apply the PSC in liver diseases, heterogeneity (Nishizawa et al., 2016), one of their characteristics, should be considered. A couple of studies suggest that genetic background and epigenetic changes explain the heterogeneity in differentiation capability across iPSC lines (Choi et al., 2015; Nishizawa et al., 2016), referring to the strong correlation between iPSCs and their original cell types. Despite maintaining the liver-specific transcriptional signature, iPSCs from human liver biopsies cannot develop into mature hepatocytes in vitro (Calabrese et al., 2019). These facts also suggest that the directed redifferentiation of intrinsic stem cells in vivo might undergo a more complex process.
Further, another study compared isogenic primary human hepatocyte and dermal fibroblast-derived iPSCs to assess their differentiation potential into hepatocytes, showing that there are no statistically significant differences in the liver-enriched HNF4α promotor region and the level of the HNF4α methylation was downregulated accompanied with the culture process (Heslop et al., 2017). Thus, personal genetics dominate iPSC differentiation (Kajiwara et al., 2012). The donor-dependent potential of iPSCs enlightens the possibility of aggregating a constellation of high-quality allogenic somatic cells as the reservation of effective iPSCs. Since iPSCs inherit the immunogenicity of the donors, patients may be at risk of immune rejection when utilizing these cells. And to overcome immune rejection issues, HLA-matched PSC lines were created by choosing common HLA types among diverse ethnicities (Yamanaka, 2020).
3.3.2. How can PSCs exert therapeutic effects on advanced liver diseases?
One of the PSCs' therapeutic strategies is to generate hepatocytes in vitro. To ensure liver disease treatment safety, it is important to avoid tumorigenicity, which may come from its tremendous differentiation potential and endless growth ability. Thus, unlike MSCs, iPSCs are rarely administrated directly without a differentiation process in vitro. For clinical application, ideal iPSC-induced hepatocytes are supposed to possess the surface markers and functions of mature human hepatocytes. Despite high hopes, hepatocytes produced from iPSCs express alpha-fetoprotein (AFP), a marker of immature hepatic cells, and exhibit immature cytochrome P450 enzyme function (Si-Tayeb et al., 2010). Thus, many researchers continue to improve differentiation strategies. Like ESCs, Actin A, Wnt 3a, and FGF2 signaling pathways also played a significant role in iPSC differentiation. Thus, ex vivo culture protocols emerge to influence these pathways with precise management of hormonal and chemokines, plating techniques, and transduction of key liver-specific transcription factors (Zhang et al., 2021b). The expression of albumin, CYP450 enzymes, and clotting factors V, VII, and IX shows that OSM and HGF play a key role in the ex vivo differentiation of iPSC-induced hepatocytes (Ang et al., 2018).
Moreover, recently, gene editing to overexpress microRNA and culturing with decellularized scaffolds were demonstrated to account for a higher-level expression of hepatic surface markers and more stable metabolic activities (Abazari et al., 2020; Jaafarpour et al., 2020; Mobarra et al., 2019, 2021). Though scientists have worked hard to enhance differentiation techniques, the impurity of iPSC-derived liver products limits clinical research and requires strict purification. With the development of good manufacturing practice-compatible cell sorting systems, a group of selective genes, such as CYP3A4 and CYP3A7, are committed to isolating the final mature hepatocytes from the mixed cells, mapping their specific traits during the differentiation process (Zabulica et al., 2019), thus lower the unexpected risk of tumorigenicity.
Liver organoids are another option. As indicated above, PSCs' ability to differentiate all three germ layers is their most desirable attribute. Wang et al. generated expandable hepatic organoids (hEHOs) from hESCs. hEHOs were demonstrated to differentiate into hepatic lineages restrictively in vivo and repopulated the damaged livers of mice (Wang et al., 2019). Sato and colleagues generated a crypt-villus organoid by a single Lgr5+ stem cell, developing under long-term culture conditions with Matrigel and growth factors (EGF and R-spondin 1) (Noggin et al.) (Sato et al., 2009). Thus, iPSC-derived liver organoids with all types of hepatic parenchymal and nonparenchymal cells and a three-dimensional shape like human livers appear promising.
Moreover, in 2013, a study generated an iPSC-derived liver bud (iPSC-LB) in vitro by recapitulating multicellular interactions (Takebe et al., 2013, 2014). Despite these promising results, iPSC-LBs have not shown human liver capabilities, specifically self-renewal, which is necessary for long-term use. A novel protocol was established with three specific mediums, including expansion, differentiation and hepatic medium, based on the expansion medium designed by Clevers' group and containing essential factors like Dexamethasone, OSM and insulin-transferrin-selenium (Huch et al., 2015). Consequently, the novel liver-like organoids exhibit self-renewal ability and maintain other physiological functions simultaneously (Mun et al., 2019). All evidence corroborates the belief that artificial organs can ultimately answer ALD.
3.3.3. Therapeutic application
Though PSC, a new stem cell therapy approach, lacks clinical data on direct application to liver illnesses, it nonetheless aids other therapeutic options. The hEHOs mentioned above were regarded as new models to mimic the pathophysiologic of alcoholic liver injury (Wang et al., 2019). It is essential to assess the efficiency of iPSC-derived HLCs in animal models before applying for clinical ALD. Indeed, a study showed that iPSC-derived HLC transplantation enhanced the secretion of total serum ALB and reduced LDH and bilirubin in the CCL4-induced cirrhosis mice models (Asgari et al., 2013). However, the number of research on ALD treated by indirect iPSC therapy is also limited. Unfortunately, due to the complex human microenvironment in vivo and insufficient methodologies to enable their application in humans, all of these findings are limited to cell or animal models, urging future research and achievements.
Following the generation of liver organoids whose structure is akin to the human organs, scientists have further transplanted the 3D spherical tissue mass on the mesentery of immunodeficient mice, which was validated to produce liver-specific proteins and exert metabolic functions like human beings' independently as consequences of a further maturation by the host blood perfusion. Furthermore, such a possible therapeutic method was utilized to ganciclovir-induced lethal liver failure models to improve survival and outperform fetal liver cell-derived liver buds and human hepatocytes (Takebe et al., 2013). The above-mentioned studies were rarely based on ALD but suggested that organoids from iPSCs could replace the OLT for ALD. Currently, most of the application of liver organoids is used to mimic the real response to diseases and drugs in vivo. These include steatohepatitis, virus hepatitis, Alagille syndrome, polycystic liver disease and Wilson’s disease et al. (Oliveira & Fiorotto, 2021), thereby assisting in understanding the latent mechanisms of liver diseases and evaluating new medicines' efficacy.
4. Conclusion
The liver self-regenerates and performs several metabolic tasks to maintain human homeostasis. Due to liver-damaged regenerative ability and restricted liver transplantation, ALD therapy is important. There has been promising progress in the intrinsic and acquired stem cell treatments for ALD since hepatocyte regeneration is curbed by harsh microenvironments (Table 1).
Table 1.
Application of intrinsic and acquired Stem cells in liver diseases.
| Cell source | Types of liver diseases | Patients or models | Follow-up | Principal improvements | References |
|---|---|---|---|---|---|
| BEC | Biliary diseases | Mouse model | 10 days | Bilirubin, survival rate, fibrosis | Hallett et al. (2022) |
| Severely combined immunodeficient condition | Mouse models | 4 weeks | Liver parenchyma, ALB | Nevi et al. (2017) | |
| FLSC | Alcoholic cirrhosis, HBV/HCV-induced cirrhosis | 25 treatments | 6 months | ALB, bilirubin, SGOT, ALP, MELD score | Khan et al. (2010) |
| Retrorsine/partial hepatectomy model | Rat models | 180 days | Survival rate, decrease of precancerous lesion | Tsuchida et al. (2019) | |
| UC-MSCs | HBV-induced decompensated liver failure | 111 treatments; 108 controls | 75 months | Survival rate, ALB, prothrombin activity, cholinesterase, total bilirubin | Shi et al. (2021) |
| HBV-induced decompensated liver cirrhosis | 30 treatments; 15 controls | 1 year | Ascites, ALB, TB. MELD | Zhang et al. (2012) | |
| HBV-induced acute-on-chronic liver failure | 24 treatments; 19 controls | 48 or 72 weeks | ALB, prothrombin time, total bilirubin, ALT, survival rates and MELD score | Shi et al. (2012) | |
| BM-MSCs | Alcoholic cirrhosis | 28 treatments; 30 controls | 4 weeks | Nothing but observation of macrophagic expansion | Lanthier et al. (2017) |
| Alcoholic cirrhosis | 34 treatments; 16 controls | 12 months | Collagen proportionate area, Child-Pugh score, ALT, AST, GGT, bilirubin | Suk et al. (2016) | |
| HCV-induced end-stage liver disease | 20 treatments; 20 controls | 6 months | ALB, bilirubin, INR, prothrombin concentration, ALT | Salama et al. (2014) | |
| HCV-induced liver cirrhosis | 15 treatments; 10 controls | 6 months | Encephalopathy, jaundice, lower limb edema, tremors, hematemesis, ascites. Laboratory data: Albumin, prothrombin, bilirubin. MELD score | El-Ansary et al. (2012) | |
| HCV-induced liver cirrhosis | 20 treatments; 20 controls | 6 months | Child-Pugh score, MELD score, fatigue scale, and performance status | Amer et al. (2011) | |
| iPSC | CCL4-induced cirrhosis | Mouse model | 5 weeks | ALB, LDH, bilirubin | Asgari et al. (2013) |
| Gancyclovir-induced liver failure | Mouse model | – | Survival rate | Takebe et al. (2013) |
BEC are the best intrinsic liver stem cells for repair, while LPC may be a transition form that transdifferentiates to repopulate wounded hepatocytes. Understanding the specific signaling pathways during each process of BEC-to-hepatocytes transdifferentiation, including Notch, Hippo/YAP, BMP, EGFR, Sox9b, mTORC1 and Wnt pathway, and interactions of cellular or non-cellular components in microenvironments benefits the designation for targets of regenerative medicine to treat the ALD.
Fetal stem cells, MSC, and PSCs can develop into hepatocytes for acquired stem cell treatment in vivo or in vitro. Liver organoids from iPSCs can eliminate the need for OLT. Moreover, with a benign precondition, MSCs can exert typical immunomodulatory function directly to regulate the inflammation level in the injured liver niche and facilitate the progression of cirrhosis. Moreover, with the iPSC-derived liver disease models, the precision of current and future medicines will probably be further improved. In cellular dynamics, hepatocytes are the most proliferative adult cell type. Given that cellular crosstalk exists in the self-replication of hepatocytes under normal conditions (Shu et al., 2022), intrinsic and acquired stem cells may also play a pivotal role in motivating and stimulating the proliferation of residual, undamaged healthy hepatocytes. This contribution increases the hepatocyte pool during liver regeneration through direct cell-cell connections or indirect signal transmission.
These results have partly been demonstrated to narrow the gap between normal people and patients with ALD in some clinical trials. However, standard application techniques for these stem cell-related therapies are unclear, and their long-term effects and safety are unknown. Therefore, future comparative and quantitative experiments are needed to establish a timely, low-risk and high-return therapeutic strategy.
Declaration of competing interest
The authors declare no competing interests.
Authors’ contributions
YS Song was the major contributor in reviewing the literature, writing the manuscript, and creating descriptive figures. ZY Lu was a major contributor to editing the manuscript and figures. All authors read and approved the final manuscript.
Acknowledgement
This work was supported by the National Key Research and Development Program of China (No.2021YFA1100502, No.2021YFA1100504); Key Program, National Natural Science Foundation of China (No. 81930016); Key Research & Development Program of Zhejiang Province (NO.2022C03108).
Contributor Information
Xuyong Wei, Email: 1315009@zju.edu.cn.
Xiao Xu, Email: zjxu@zju.edu.cn.
References
- Abazari M.F., Soleimanifar F., Enderami S.E., Nasiri N., Nejati F., Mousavi S.A., Soleimani M., Kiani J., Ghoraeian P., Kehtari M. Decellularized amniotic membrane Scaffolds improve differentiation of iPSCs to functional hepatocyte-like cells. Journal of Cellular Biochemistry. 2020;121(2):1169–1181. doi: 10.1002/jcb.29351. [DOI] [PubMed] [Google Scholar]
- Addante A., Roncero C., Almale L., Lazcanoiturburu N., Garcia-Alvaro M., Fernandez M., Sanz J., Hammad S., Nwosu Z.C., Lee S.J., Fabregat I., Dooley S., Ten Dijke P., Herrera B., Sanchez A. Bone morphogenetic protein 9 as a key regulator of liver progenitor cells in DDC-induced cholestatic liver injury. Liver International. 2018;38(9):1664–1675. doi: 10.1111/liv.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizarani N., Saviano A., Sagar, Mailly L., Durand S., Herman J.S., Pessaux P., Baumert T.F., Grun D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572(7768):199–204. doi: 10.1038/s41586-019-1373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaifi M., Eom Y.W., Newsome P.N., Baik S.K. Mesenchymal stromal cell therapy for liver diseases. Journal of Hepatology. 2018;68:1272–1285. doi: 10.1016/j.jhep.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Ambrosino G., Varotto S., Strom S.C., Guariso G., Franchin E., Miotto D., Caenazzo L., Basso S., Carraro P., Valente M.L., D'Amico D., Zancan L., D'Antiga L. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplantation. 2005;14:151–157. doi: 10.3727/000000005783983250. [DOI] [PubMed] [Google Scholar]
- Amer M.E., El-Sayed S.Z., El-Kheir W.A., Gabr H., Gomaa A.A., El-Noomani N., Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. European Journal of Gastroenterology and Hepatology. 2011;23:936–941. doi: 10.1097/MEG.0b013e3283488b00. [DOI] [PubMed] [Google Scholar]
- Ang L.T., Tan A.K.Y., Autio M.I., Goh S.H., Choo S.H., Lee K.L., Tan J., Pan B., Lee J.J.H., Lum J.J., Lim C.Y.Y., Yeo I.K.X., Wong C.J.Y., Liu M., Oh J.L.L., Chia C.P.L., Loh C.H., Chen A., Chen Q., et al. A roadmap for human liver differentiation from pluripotent stem cells. Cell Reports. 2018;22:2190–2205. doi: 10.1016/j.celrep.2018.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S., Moslem M., Bagheri-Lankarani K., Pournasr B., Miryounesi M., Baharvand H. Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rev Rep. 2013;9:493–504. doi: 10.1007/s12015-011-9330-y. [DOI] [PubMed] [Google Scholar]
- Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. Journal of Hepatology. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Bacakova L., Zarubova J., Travnickova M., Musilkova J., Pajorova J., Slepicka P., Kasalkova N.S., Svorcik V., Kolska Z., Motarjemi H., Molitor M. Stem cells, their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnology Advances. 2018;36:1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Baksh D., Yao R., Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- Banas A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Quinn G., Okochi H., Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- Boulter L., Govaere O., Bird T.G., Radulescu S., Ramachandran P., Pellicoro A., Ridgway R.A., Seo S.S., Spee B., Van Rooijen N., Sansom O.J., Iredale J.P., Lowell S., Roskams T., Forbes S.J. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nature Medicine. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai P., Mao X., Zhao J., Nie L., Jiang Y., Yang Q., Ni R., He J., Luo L. Farnesoid X receptor is required for the redifferentiation of bipotential progenitor cells during biliary-mediated zebrafish liver regeneration. Hepatology. 2021;74:3345–3361. doi: 10.1002/hep.32076. [DOI] [PubMed] [Google Scholar]
- Calabrese D., Roma G., Bergling S., Carbone W., Mele V., Nuciforo S., Fofana I., Campana B., Szkolnicka D., Hay D.C., Tchorz J., Bouwmeester T., Wieland S., Heim M.H. Liver biopsy derived induced pluripotent stem cells provide unlimited supply for the generation of hepatocyte-like cells. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo F.D., Finegold M., Goodell M.A. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. Journal of Clinical Investigation. 2004;113:1266–1270. doi: 10.1172/JCI21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnoni A., Farigu S., Cotti Piccinelli E., Bonassi Signoroni P., Romele P., Vanosi G., Toschi I., Cesari V., Barros Sant'Anna L., Magatti M., Silini A.R., Parolini O. Effect of human amniotic epithelial cells on pro-fibrogenic resident hepatic cells in a rat model of liver fibrosis. Journal of Cellular and Molecular Medicine. 2018;22:1202–1213. doi: 10.1111/jcmm.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino G., Renzi A., Franchitto A., Cardinale V., Onori P., Reid L., Alvaro D., Gaudio E. Stem/Progenitor cell Niches involved in hepatic and biliary regeneration. Stem Cells International. 2016;2016 doi: 10.1155/2016/3658013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R.F., Jackson K.A., Goodell M.A., Robertson C.S., Liu H., Shine H.D. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- Celik N., Squires J.E., Soltys K., Vockley J., Shellmer D.A., Chang W., Strauss K., McKiernan P., Ganoza A., Sindhi R., Bond G., Mazariegos G., Khanna A. Domino liver transplantation for select metabolic disorders, Expanding the living donor pool. JIMD Reports. 2019;48:83–89. doi: 10.1002/jmd2.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Martinez C.A., Koester J., Wickstrom S.A. Signaling in the stem cell niche, regulating cell fate, function and plasticity. Development. 2018:145. doi: 10.1242/dev.165399. [DOI] [PubMed] [Google Scholar]
- Chen G., Yue A., Ruan Z., Yin Y., Wang R., Ren Y., Zhu L. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T.Y., Khaliq M., Tsurusaki S., Ninov N., Stainier D.Y.R., Tanaka M., Shin D. Bone morphogenetic protein signaling governs biliary-driven liver regeneration in zebrafish through tbx2b and id2a. Hepatology. 2017;66:1616–1630. doi: 10.1002/hep.29309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Lee S., Mallard W., Clement K., Tagliazucchi G.M., Lim H., Choi I.Y., Ferrari F., Tsankov A.M., Pop R., Lee G., Rinn J.L., Meissner A., Park P.J., Hochedlinger K. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nature Biotechnology. 2015;33:1173–1181. doi: 10.1038/nbt.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T.Y., Ninov N., Stainier D.Y., Shin D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology. 2014;146:776–788. doi: 10.1053/j.gastro.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M., Arino S., Martinez-Sanchez C., Garcia-Pras E., Gallego J., Moles A., Aguilar-Bravo B., Blaya D., Vallverdu J., Rubio-Tomas T., Lozano J.J., Pose E., Graupera I., Fernandez-Vidal A., Pol A., Bataller R., Geng J.G., Gines P., Fernandez M., Sancho-Bru P. Ductular reaction promotes intrahepatic angiogenesis through Slit2-Roundabout 1 signaling. Hepatology. 2022;75:353–368. doi: 10.1002/hep.32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Espinoza L., Dowbaj A.M., Kohler T.N., Strauss B., Sarlidou O., Belenguer G., Pacini C., Martins N.P., Dobie R., Wilson-Kanamori J.R., Butler R., Prior N., Serup P., Jug F., Henderson N.C., Hollfelder F., Huch M. Dynamic cell contacts between periportal mesenchyme and ductal epithelium act as a rheostat for liver cell proliferation. Cell Stem Cell. 2021;28:1907–19021 e8. doi: 10.1016/j.stem.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damdimopoulou P., Rodin S., Stenfelt S., Antonsson L., Tryggvason K., Hovatta O. Human embryonic stem cells. Best Practice & Research Clinical Obstetrics & Gynaecology. 2016;31:2–12. doi: 10.1016/j.bpobgyn.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Deng X., Zhang X., Li W., Feng R.X., Li L., Yi G.R., Zhang X.N., Yin C., Yu H.Y., Zhang J.P., Lu B., Hui L., Xie W.F. Chronic liver injury induces conversion of biliary epithelial cells into hepatocytes. Cell Stem Cell. 2018;23:114–122 e3. doi: 10.1016/j.stem.2018.05.022. [DOI] [PubMed] [Google Scholar]
- Dorrell C., Erker L., Schug J., Kopp J.L., Canaday P.S., Fox A.J., Smirnova O., Duncan A.W., Finegold M.J., Sander M., Kaestner K.H., Grompe M. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes & Development. 2011;25:1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuquoy L., Louvet A., Lassailly G., Truant S., Boleslawski E., Artru F., Maggiotto F., Gantier E., Buob D., Leteurtre E., Cannesson A., Dharancy S., Moreno C., Pruvot F.R., Bataller R., Mathurin P. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64:1949–1960. doi: 10.1136/gutjnl-2014-308410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer B.J., Macmillan M.T., Brennan P.N., Forbes S.J. Cell therapy for advanced liver diseases: Repair or rebuild. Journal of Hepatology. 2021;74:185–199. doi: 10.1016/j.jhep.2020.09.014. [DOI] [PubMed] [Google Scholar]
- El-Ansary M., Abdel-Aziz I., Mogawer S., Abdel-Hamid S., Hammam O., Teaema S., Wahdan M. Phase II trial: Undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Reviews and Reports. 2012;8:972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]
- Fan J., Shen H., Dai Q., Minuk G.Y., Burzynski F.J., Gong Y. Bone morphogenetic protein-4 induced rat hepatic progenitor cell (WB-F344 cell) differentiation toward hepatocyte lineage. Journal of Cellular Physiology. 2009;220:72–81. doi: 10.1002/jcp.21731. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration and repair: Hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Fisher R.A. Living donor liver transplantation: Eliminating the wait for death in end-stage liver disease? Nature Reviews Gastroenterology & Hepatology. 2017;14:373–382. doi: 10.1038/nrgastro.2017.2. [DOI] [PubMed] [Google Scholar]
- Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- Gonzalez L., Diaz M.E., Miquet J.G., Sotelo A.I., Dominici F.P. Growth hormone modulation of hepatic epidermal growth factor receptor signaling. Trends in Endocrinology and Metabolism. 2021;32:403–414. doi: 10.1016/j.tem.2021.03.004. [DOI] [PubMed] [Google Scholar]
- Hackl C., Schmidt K.M., Susal C., Dohler B., Zidek M., Schlitt H.J. Split liver transplantation: Current developments. World Journal of Gastroenterology. 2018;24:5312–5321. doi: 10.3748/wjg.v24.i47.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett J.M., Ferreira-Gonzalez S., Man T.Y., Kilpatrick A.M., Esser H., Thirlwell K., Macmillan M.T., Rodrigo-Torres D., Dwyer B.J., Gadd V.L., Ashmore-Harris C., Lu W.Y., Thomson J.P., Jansen M.A., O'Duibhir E., Starkey Lewis P.J., Campana L., Aird R.E., Bate T.S.R., et al. Human biliary epithelial cells from discarded donor livers rescue bile duct structure and function in a mouse model of biliary disease. Cell Stem Cell. 2022;29:355–371. doi: 10.1016/j.stem.2022.02.006. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Chen J., Wei X., Leng H., Mu H., Cai P., Luo L. Mammalian target of rapamycin complex 1 signaling is required for the dedifferentiation from biliary cell to bipotential progenitor cell in zebrafish liver regeneration. Hepatology. 2019;70:2092–2106. doi: 10.1002/hep.30790. [DOI] [PubMed] [Google Scholar]
- He J., Lu H., Zou Q., Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789–800 e8. doi: 10.1053/j.gastro.2013.11.045. [DOI] [PubMed] [Google Scholar]
- He J., Zhou Y., Qian C., Wang D., Yang Z., Huang Z., Sun J., Ni R., Yang Q., Chen J., Luo L. DNA methylation maintenance at the p53 locus initiates biliary-mediated liver regeneration. NPJ Regenerative Medicine. 2022;7:21. doi: 10.1038/s41536-022-00217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop J.A., Kia R., Pridgeon C.S., Sison-Young R.L., Liloglou T., Elmasry M., Fenwick S.W., Mills J.S., Kitteringham N.R., Goldring C.E., Park B.K. Donor-Dependent and other nondefined factors have greater influence on the hepatic phenotype than the starting cell type in induced pluripotent stem cell derived hepatocyte-like cells. Stem Cells Translational Medicine. 2017;6:1321–1331. doi: 10.1002/sctm.16-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.C., Chuang M.H., Lin Z.S., Lin Y.C., Chen C.H., Chang C.L., Huang P.C., Syu W.S., Chiou T.W., Hong Z.H., Tsai Y.C., Harn H.J., Lin P.C., Lin S.Z. Transplantation with GXHPC1 for liver cirrhosis: Phase 1 trial. Cell Transplantation. 2019;28 doi: 10.1177/0963689719884885. 100S-11S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., Haft A., Vries R.G., Grompe M., Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M., Ellis E., van Wenum M., Fuchs S.A., de Ligt J., van de Wetering M., Sasaki N., Boers S.J., Kemperman H., de Jonge J., Ijzermans J.N., Nieuwenhuis E.E., Hoekstra R., Strom S., et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansante V., Mitry R.R., Filippi C., Fitzpatrick E., Dhawan A. Human hepatocyte transplantation for liver disease: Current status and future perspectives. Pediatric Research. 2018;83:232–240. doi: 10.1038/pr.2017.284. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Mitaka T., Harada K., Sugimoto S., Hirata K., Mochizuki Y. Proliferation of rat small hepatocytes after long-term cryopreservation. Journal of Hepatology. 2002;37:7–14. doi: 10.1016/s0168-8278(02)00069-7. [DOI] [PubMed] [Google Scholar]
- Ishii T., Eto K. Fetal stem cell transplantation: Past, present, and future. World Journal of Stem Cells. 2014;6:404–420. doi: 10.4252/wjsc.v6.i4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafarpour Z., Soleimani M., Hosseinkhani S., Geramizadeh B., Yaghmaei P., Mobarra N., Karimi M.H. Overexpression of microRNA-375 and microRNA-122 promotes the differentiation of human induced pluripotent stem cells into hepatocyte-like cells. Biologicals. 2020;63:24–32. doi: 10.1016/j.biologicals.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Jepsen P., Younossi Z.M. The global burden of cirrhosis: A review of disability-adjusted life-years lost and unmet needs. Journal of Hepatology. 2021;75(Suppl 1) doi: 10.1016/j.jhep.2020.11.042. S3-S13. [DOI] [PubMed] [Google Scholar]
- Jiang W., Tan Y., Cai M., Zhao T., Mao F., Zhang X., Xu W., Yan Z., Qian H., Yan Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int, 2018. 2018 doi: 10.1155/2018/6079642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Xu J. Immune modulation by mesenchymal stem cells. Cell Proliferation. 2020;53 doi: 10.1111/cpr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.J., Bae Y.K., Kim M., Kwon S.J., Jeon H.B., Choi S.J., Kim S.W., Yang Y.S., Oh W., Chang J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. International Journal of Molecular Sciences. 2013;14:17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Kim M., So J., Lee S.H., Ko S., Shin D. Farnesoid X receptor activation impairs liver progenitor cell-mediated liver regeneration via the PTEN-PI3K-AKT-mTOR Axis in zebrafish. Hepatology. 2021;74:397–410. doi: 10.1002/hep.31679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara M., Aoi T., Okita K., Takahashi R., Inoue H., Takayama N., Endo H., Eto K., Toguchida J., Uemoto S., Yamanaka S. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the U S A. 2012;109:12538–12543. doi: 10.1073/pnas.1209979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.A., Shaik M.V., Parveen N., Rajendraprasad A., Aleem M.A., Habeeb M.A., Srinivas G., Raj T.A., Tiwari S.K., Kumaresan K., Venkateswarlu J., Pande G., Habibullah C.M. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplantation. 2010;19:409–418. doi: 10.3727/096368910X498241. [DOI] [PubMed] [Google Scholar]
- Ko S., Russell J.O., Molina L.M., Monga S.P. Liver progenitors and adult cell plasticity in hepatic injury and repair: Knowns and unknowns. Annual Review of Pathology: Mechanisms of Disease. 2020;15:23–50. doi: 10.1146/annurev-pathmechdis-012419-032824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S., Russell J.O., Tian J., Gao C., Kobayashi M., Feng R., Yuan X., Shao C., Ding H., Poddar M., Singh S., Locker J., Weng H.L., Monga S.P., Shin D. Hdac1 regulates differentiation of bipotent liver progenitor cells during regeneration via Sox9b and Cdk8. Gastroenterology. 2019;156:187–202 e14. doi: 10.1053/j.gastro.2018.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn-Gaone J., Gogoi-Tiwari J., Ramm G.A., Olynyk J.K., Tirnitz-Parker J.E. The role of liver progenitor cells during liver regeneration, fibrogenesis, and carcinogenesis. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2016;310:G143–G154. doi: 10.1152/ajpgi.00215.2015. [DOI] [PubMed] [Google Scholar]
- Kolaparthy L.K., Sanivarapu S., Moogla S., Kutcham R.S. Adipose tissue - adequate, accessible regenerative material. International Journal of Stem Cells. 2015;8:121–127. doi: 10.15283/ijsc.2015.8.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk N., Hodge A., Sun Y., Correia J., Alhomrani M., Samuel C., Moore G., Lim R., Sievert W. Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. Journal of Gastroenterology and Hepatology. 2019;34:1441–1449. doi: 10.1111/jgh.14643. [DOI] [PubMed] [Google Scholar]
- Lanthier N., Lin-Marq N., Rubbia-Brandt L., Clement S., Goossens N., Spahr L. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: What is the impact on liver histology and gene expression patterns? Stem Cell Research & Therapy. 2017;8:88. doi: 10.1186/s13287-017-0541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.H., Tu C.T., Hsiao C.C., Tsai M.S., Ho C.M., Cheng N.C., Hung T.M., Shih D.T. Antifibrotic activity of human placental amnion membrane-derived CD34+ mesenchymal stem/progenitor cell transplantation in mice with thioacetamide-induced liver injury. Stem Cells Translational Medicine. 2016;5:1473–1484. doi: 10.5966/sctm.2015-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Yan Y., Wang B., Qian H., Zhang X., Shen L., Wang M., Zhou Y., Zhu W., Li W., Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells and Development. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B.L., Chen J.F., Qiu W.H., Wang K.W., Xie D.Y., Chen X.Y., Liu Q.L., Peng L., Li J.G., Mei Y.Y., Weng W.Z., Peng Y.W., Cao H.J., Xie J.Q., Xie S.B., Xiang A.P., Gao Z.L. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- Lin S., Nascimento E.M., Gajera C.R., Chen L., Neuhofer P., Garbuzov A., Wang S., Artandi S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556:244–248. doi: 10.1038/s41586-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini S., Bird T.G., Boulter L., Bellamy C., Samuel K., Aucott R., Clayton E., Andreone P., Bernardi M., Golding M., Alison M.R., Iredale J.P., Forbes S.J. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59:645–654. doi: 10.1136/gut.2009.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A.J., Woodbury D. Fetal stem cells from extra-embryonic tissues: Do not discard. Journal of Cellular and Molecular Medicine. 2008;12:730–742. doi: 10.1111/j.1582-4934.2008.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar P., Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Frontiers in Immunology. 2015;6:560. doi: 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlen G., Ursic-Bedoya J., Jourdainne V., Kahale N., Glenisson M., Doignon I., Rainteau D., Tordjmann T. Bile acids and their receptors during liver regeneration: "Dangerous protectors". Molecular Aspects of Medicine. 2017;56:25–33. doi: 10.1016/j.mam.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G.K., Khan Z. Liver stem cells: Experimental findings and implications for human liver disease. Gastroenterology. 2015;149:876–882. doi: 10.1053/j.gastro.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnis-Lyons S.E., Ferreira-Gonzalez S., Aleksieva N., Man T.Y., Gadd V.L., Williams M.J., Guest R.V., Lu W.Y., Dwyer B.J., Jamieson T., Nixon C., Van Hul N., Lemaigre F.P., McCafferty J., Leclercq I.A., Sansom O.J., Boulter L., Forbes S.J. Notch-IGF1 signaling during liver regeneration drives biliary epithelial cell expansion and inhibits hepatocyte differentiation. Science Signaling. 2021;14 doi: 10.1126/scisignal.aay9185. [DOI] [PubMed] [Google Scholar]
- Mobarra N., Raji S., Najafi S., Kafi F.K., Ferns G.A., Pakzad R. Hypoxia-Induced miR-210 overexpression promotes the differentiation of human-induced pluripotent stem cells to hepatocyte-like cells on random nanofiber poly-L-lactic acid/poly (epsilon-Caprolactone) scaffolds. Oxidative Medicine and Cellular Longevity. 2021;2021 doi: 10.1155/2021/4229721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarra N., Soleimani M., Ghayour-Mobarhan M., Safarpour S., Ferns G.A., Pakzad R., Pasalar P. Hybrid poly-l-lactic acid/poly(epsilon-caprolactone) nanofibrous scaffold can improve biochemical and molecular markers of human induced pluripotent stem cell-derived hepatocyte-like cells. Journal of Cellular Physiology. 2019;234:11247–11255. doi: 10.1002/jcp.27779. [DOI] [PubMed] [Google Scholar]
- Mun S.J., Ryu J.S., Lee M.O., Son Y.S., Oh S.J., Cho H.S., Son M.Y., Kim D.S., Kim S.J., Yoo H.J., Lee H.J., Kim J., Jung C.R., Chung K.S., Son M.J. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. Journal of Hepatology. 2019;71:970–985. doi: 10.1016/j.jhep.2019.06.030. [DOI] [PubMed] [Google Scholar]
- Mushahary D., Spittler A., Kasper C., Weber V., Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- Naik A.D., Arney J., Clark J.A., Martin L.A., Walling A.M., Stevenson A., Smith D., Asch S.M., Kanwal F. Integrated model for patient-centered advanced liver disease care. Clinical Gastroenterology and Hepatology. 2020;18:1015–1024. doi: 10.1016/j.cgh.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevi L., Carpino G., Costantini D., Cardinale V., Riccioni O., Di Matteo S., Melandro F., Berloco P.B., Reid L., Gaudio E., Alvaro D. Hyaluronan coating improves liver engraftment of transplanted human biliary tree stem/progenitor cells. Stem Cell Research & Therapy. 2017;8:68. doi: 10.1186/s13287-017-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Chonabayashi K., Nomura M., Tanaka A., Nakamura M., Inagaki A., Nishikawa M., Takei I., Oishi A., Tanabe K., Ohnuki M., Yokota H., Koyanagi-Aoi M., Okita K., Watanabe A., Takaori-Kondo A., Yamanaka S., Yoshida Y. Epigenetic variation between human induced pluripotent stem cell lines is an indicator of differentiation capacity. Cell Stem Cell. 2016;19:341–354. doi: 10.1016/j.stem.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Oertel M. Fetal liver cell transplantation as a potential alternative to whole liver transplantation? Journal of Gastroenterology. 2011;46:953–965. doi: 10.1007/s00535-011-0427-5. [DOI] [PubMed] [Google Scholar]
- Okabe H., Yang J., Sylakowski K., Yovchev M., Miyagawa Y., Nagarajan S., Chikina M., Thompson M., Oertel M., Baba H., Monga S.P., Nejak-Bowen K.N. Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice. Hepatology. 2016;64:1652–1666. doi: 10.1002/hep.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A.G., Fiorotto R. Novel approaches to liver disease diagnosis and modeling. Transl Gastroenterol Hepatol. 2021;6:19. doi: 10.21037/tgh-20-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf K., al-Dhalimy M., Ou C.N., Finegold M., Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. The American journal of pathology. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Pepe-Mooney B.J., Dill M.T., Alemany A., Ordovas-Montanes J., Matsushita Y., Rao A., Sen A., Miyazaki M., Anakk S., Dawson P.A., Ono N., Shalek A.K., van Oudenaarden A., Camargo F.D. Single-Cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration. Cell Stem Cell. 2019;25:23–38 e8. doi: 10.1016/j.stem.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Planas-Paz L., Sun T., Pikiolek M., Cochran N.R., Bergling S., Orsini V., Yang Z., Sigoillot F., Jetzer J., Syed M., Neri M., Schuierer S., Morelli L., Hoppe P.S., Schwarzer W., Cobos C.M., Alford J.L., Zhang L., Cuttat R., et al. YAP, but not RSPO-LGR4/5, signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell. 2019;25:39–53 e10. doi: 10.1016/j.stem.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Raven A., Lu W.Y., Man T.Y., Ferreira-Gonzalez S., O'Duibhir E., Dwyer B.J., Thomson J.P., Meehan R.R., Bogorad R., Koteliansky V., Kotelevtsev Y., Ffrench-Constant C., Boulter L., Forbes S.J. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderfeld M. Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biology. 2018;68–69:452–462. doi: 10.1016/j.matbio.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Rosland G.V., Svendsen A., Torsvik A., Sobala E., McCormack E., Immervoll H., Mysliwietz J., Tonn J.C., Goldbrunner R., Lonning P.E., Bjerkvig R., Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Research. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- Russell J.O., Ko S., Monga S.P., Shin D. Notch inhibition promotes differentiation of liver progenitor cells into hepatocytes via sox9b repression in zebrafish. Stem Cells International. 2019;2019 doi: 10.1155/2019/8451282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K., Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–595. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama H., Zekri A.R., Medhat E., Al Alim S.A., Ahmed O.S., Bahnassy A.A., Lotfy M.M., Ahmed R., Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Research & Therapy. 2014;5:70. doi: 10.1186/scrt459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaziotis F., Muraro D., Tysoe O.C., Sawiak S., Beach T.E., Godfrey E.M., Upponi S.S., Brevini T., Wesley B.T., Garcia-Bernardo J., Mahbubani K., Canu G., Gieseck R., 3rd, Berntsen N.L., Mulcahy V.L., Crick K., Fear C., Robinson S., Swift L.…Vallier L. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–846. doi: 10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu J.S., Petkov P.M., Dabeva M.D., Shafritz D.A. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. The American journal of pathology. 2001;159:1323–1334. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sauer I.M., Kardassis D., Zeillinger K., Pascher A., Gruenwald A., Pless G., Irgang M., Kraemer M., Puhl G., Frank J., Muller A.R., Steinmuller T., Denner J., Neuhaus P., Gerlach J.C. Clinical extracorporeal hybrid liver support--phase I study with primary porcine liver cells. Xenotransplantation. 2003;10:460–469. doi: 10.1034/j.1399-3089.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- Schacher F.C., Martins Pezzi da Silva A., Silla L., Alvares-da-Silva M.R. Bone marrow mesenchymal stem cells in acute-on-chronic liver failure grades 2 and 3: A phase I-II randomized clinical trial. Chinese Journal of Gastroenterology and Hepatology. 2021;2021 doi: 10.1155/2021/3662776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R.E., Reyes M., Koodie L., Jiang Y., Blackstad M., Lund T., Lenvik T., Johnson S., Hu W.S., Verfaillie C.M. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. Journal of Clinical Investigation. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.L., Gao Y., Yan Y., Ma H., Sun L., Huang P., Ni X., Zhang L., Zhao X., Ren H., Hu D., Zhou Y., Tian F., Ji Y., Cheng X., Pan G., Ding Y.T., Hui L. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Research. 2016;26:206–216. doi: 10.1038/cr.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Li Y.Y., Xu R.N., Meng F.P., Yu S.J., Fu J.L., Hu J.H., Li J.X., Wang L.F., Jin L., Wang F.S. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial. Hepatol International. 2021;15:1431–1441. doi: 10.1007/s12072-021-10199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Liang X.L., Lu B.X., Pan S.Y., Chen X., Tang Q.Q., Wang Y., Huang F. Diminution of toxic copper accumulation in toxic milk mice modeling Wilson disease by embryonic hepatocyte intrasplenic transplantation. World Journal of Gastroenterology. 2005;11:3691–3695. doi: 10.3748/wjg.v11.i24.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Zhang Z., Xu R., Lin H., Fu J., Zou Z., Zhang A., Shi J., Chen L., Lv S., He W., Geng H., Jin L., Liu Z., Wang F.S. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Translational Medicine. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W., Yang M., Yang J., Lin S., Wei X., Xu X. Cellular crosstalk during liver regeneration: Unity in diversity. Cell Communication and Signaling. 2022;20:117. doi: 10.1186/s12964-022-00918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F.K., Nagaoka M., Li J., Battle M.A., Duris C., North P.E., Dalton S., Duncan S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So J., Kim M., Lee S.H., Ko S., Lee D.A., Park H., Azuma M., Parsons M.J., Prober D., Shin D. Attenuating the epidermal growth factor receptor-extracellular signal-regulated kinase-sex-determining region Y-box 9 Axis promotes liver progenitor cell-mediated liver regeneration in zebrafish. Hepatology. 2021;73:1494–1508. doi: 10.1002/hep.31437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys K.A., Setoyama K., Tafaleng E.N., Soto Gutierrez A., Fong J., Fukumitsu K., Nishikawa T., Nagaya M., Sada R., Haberman K., Gramignoli R., Dorko K., Tahan V., Dreyzin A., Baskin K., Crowley J.J., Quader M.A., Deutsch M., Ashokkumar C.…Fox I.J. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. Journal of Hepatology. 2017;66:987–1000. doi: 10.1016/j.jhep.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenne X., Debray F.G., Smets F., Jazouli N., Sana G., Tondreau T., Menten R., Goffette P., Boemer F., Schoos R., Gersting S.W., Najimi M., Muntau A.C., Goyens P., Sokal E.M. Hepatocyte transplantation using the domino concept in a child with tetrabiopterin nonresponsive phenylketonuria. Cell Transplantation. 2012;21:2765–2770. doi: 10.3727/096368912X653255. [DOI] [PubMed] [Google Scholar]
- Stueck A.E., Wanless I.R. Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology. 2015;61:1696–1707. doi: 10.1002/hep.27706. [DOI] [PubMed] [Google Scholar]
- Suk K.T., Yoon J.H., Kim M.Y., Kim C.W., Kim J.K., Park H., Hwang S.G., Kim D.J., Lee B.S., Lee S.H., Kim H.S., Jang J.Y., Lee C.H., Kim B.S., Jang Y.O., Cho M.Y., Jung E.S., Kim Y.M., Bae S.H., Baik S.K. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.R., Ueno Y., Zheng Y.W., Koike N., Aoyama S., Adachi Y., Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- Takebe T., Zhang R.R., Koike H., Kimura M., Yoshizawa E., Enomura M., Koike N., Sekine K., Taniguchi H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature Protocols. 2014;9:396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- Touboul T., Hannan N.R., Corbineau S., Martinez A., Martinet C., Branchereau S., Mainot S., Strick-Marchand H., Pedersen R., Di Santo J., Weber A., Vallier L. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- Tsuchida T., Murata S., Matsuki K., Mori A., Matsuo M., Mikami S., Okamoto S., Ueno Y., Tadokoro T., Zheng Y.W., Taniguchi H. The regenerative effect of portal vein injection of liver organoids by retrorsine/partial hepatectomy in rats. International Journal of Molecular Sciences. 2019;21 doi: 10.3390/ijms21010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya A., Takeuchi S., Watanabe T., Yoshida T., Nojiri S., Ogawa M., Terai S. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as "conducting cells" for improvement of liver fibrosis and regeneration. Inflammation and Regeneration. 2019;39:18. doi: 10.1186/s41232-019-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker F., de Jager S.C., Sluijter J.P. Mesenchymal stem cell therapy for cardiac inflammation: Immunomodulatory properties and the influence of toll-like receptors. Mediators of Inflammation. 2013;2013 doi: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers A.J., Sherwood R.I., Christensen J.L., Weissman I.L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang X., Tan Z., Su Y., Liu J., Chang M., Yan F., Chen J., Chen T., Li C., Hu J., Wang Y. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Research. 2019;29:1009–1026. doi: 10.1038/s41422-019-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.M., Yuan X.H., Shen H. BMP-4 induced proliferation and oriented differentiation of rat hepatic oval cells into hepatocytes. Asian Pacific Journal of Tropical Medicine. 2015;8:412–416. doi: 10.1016/S1995-7645(14)60353-9. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zheng Q., Sun Z., Wang C., Cen J., Zhang X., Jin Y., Wu B., Yan T., Wang Z., Gu Q., Lv X., Nan J., Wu Z., Sun W., Pan G., Zhang L., Hui L., Cai X. Reversal of liver failure using a bioartificial liver device implanted with clinical-grade human-induced hepatocytes. Cell Stem Cell. 2023;30:617–631 e8. doi: 10.1016/j.stem.2023.03.013. [DOI] [PubMed] [Google Scholar]
- Willenbring H., Bailey A.S., Foster M., Akkari Y., Dorrell C., Olson S., Finegold M., Fleming W.H., Grompe M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nature Medicine. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- Yagi H., Kitagawa Y. The role of mesenchymal stem cells in cancer development. Frontiers in Genetics. 2013;4:261. doi: 10.3389/fgene.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- Yang X., Meng Y., Han Z., Ye F., Wei L., Zong C. Mesenchymal stem cell therapy for liver disease: Full of chances and challenges. Cell & Bioscience. 2020;10:123. doi: 10.1186/s13578-020-00480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasen A., Tuxun T., Apaer S., Li W., Maimaitinijiati Y., Wang H., Aisan M., Aji T., Shao Y., Hao W. Fetal liver stem cell transplantation for liver diseases. Regenerative Medicine. 2019;14:703–714. doi: 10.2217/rme-2018-0160. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Tokusashi Y., Lee G.H., Ogawa K. 'Intrahepatic transplantation of normal hepatocytes prevents Wilson's disease in Long-Evans cinnamon rats'. Gastroenterology. 1996;111:1654–1660. doi: 10.1016/s0016-5085(96)70029-x. [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin, Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zabulica M., Srinivasan R.C., Vosough M., Hammarstedt C., Wu T., Gramignoli R., Ellis E., Kannisto K., Collin de l'Hortet A., Takeishi K., Soto-Gutierrez A., Strom S.C. Guide to the assessment of mature liver gene expression in stem cell-derived hepatocytes. Stem Cells and Development. 2019;28:907–919. doi: 10.1089/scd.2019.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lin H., Shi M., Xu R., Fu J., Lv J., Chen L., Lv S., Li Y., Yu S., Geng H., Jin L., Lau G.K., Wang F.S. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. Journal of Gastroenterology and Hepatology. 2012;27(Suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ma X.J., Fei Y.Y., Han H.T., Xu J., Cheng L., Li X. Stem cell therapy in liver regeneration: Focus on mesenchymal stem cells and induced pluripotent stem cells. Pharmacology & Therapeutics. 2021 doi: 10.1016/j.pharmthera.2021.108004. [DOI] [PubMed] [Google Scholar]
- Zhang L., Pu K., Liu X., Bae S.D.W., Nguyen R., Bai S., Li Y., Qiao L. The application of induced pluripotent stem cells against liver diseases: An update and a review. Frontiers of Medicine. 2021;8 doi: 10.3389/fmed.2021.644594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi M., Zhang J., Tang Q., Yu D., Gao S., Gao D., Liu P., Guo J., Hai T., Gao J., Cao S., Zhao Z., Li C., Weng X., He M., Chen T., Wang Y., Long K., Jiao D., et al. Generation and characterization of stable pig pregastrulation epiblast stem cell lines. Cell Research. 2022;32:383–400. doi: 10.1038/s41422-021-00592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]