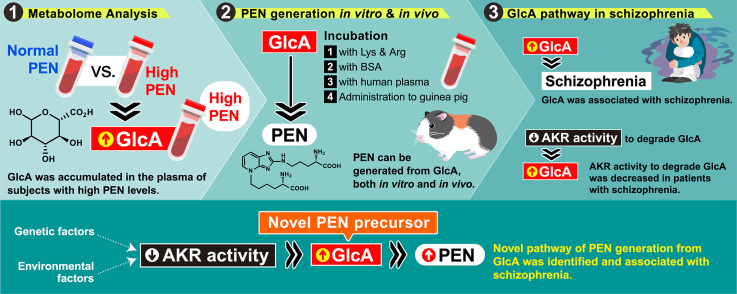
Abstract
Pentosidine (PEN) is an advanced glycation end-product (AGEs), where a fluorescent cross-link is formed between lysine and arginine residues in proteins. Accumulation of PEN is associated with aging and various diseases. We previously reported that a subpopulation of patients with schizophrenia showed PEN accumulation in the blood, having severe clinical features. PEN is thought to be produced from glucose, fructose, pentoses, or ascorbate. However, patients with schizophrenia with high PEN levels present no elevation of these precursors of PEN in their blood. Therefore, the molecular mechanisms underlying PEN accumulation and the molecular pathogenesis of schizophrenia associated with PEN accumulation remain unclear. Here, we identified glucuronic acid (GlcA) as a novel precursor of PEN from the plasma of subjects with high PEN levels. We demonstrated that PEN can be generated from GlcA, both in vitro and in vivo. Furthermore, we found that GlcA was associated with the diagnosis of schizophrenia. Among patients with high PEN, the proportion of those who also have high GlcA is 25.6%. We also showed that Aldo-keto reductase (AKR) activity to degrade GlcA was decreased in patients with schizophrenia, and its activity was negatively correlated with GlcA levels in the plasma. This is the first report to show that PEN is generated from GlcA. In the future, this finding will contribute to understanding the molecular pathogenesis of not only schizophrenia but also other diseases with PEN accumulation.
Keywords: Pentosidine, Glucuronic acid, Advanced glycation end-products, Schizophrenia, Aldo-keto reductase
Graphical abstract
Highlights
-
•
GlcA was accumulated in the plasma of subjects with high PEN levels.
-
•
PEN can be generated from GlcA, both in vitro and in vivo.
-
•
GlcA was associated with diagnosis of schizophrenia.
-
•
AKR activity to degrade GlcA was decreased in patients with schizophrenia.
1. Introduction
Abnormalities in glucose metabolism in the body cause non-enzymatic modifications called glycation (Maillard reactions) on proteins, nucleic acids, and lipids, forming advanced glycation end-products (AGEs) [1,2]. AGEs formation in biomolecules significantly affects their activity and physical properties. AGEs are also known to induce inflammatory responses via binding to a receptor for AGEs (RAGE) [3,4]. The adverse influence of AGEs accumulation is extensive throughout the body and can lead to various diseases [1,5,6]. The inhibition of AGE formation is expected to be an effective preventive and therapeutic strategy for these diseases.
Pentosidine (PEN), a type of AGEs with fluorescence, possesses a cross-linked structure between lysine and arginine residues in proteins by an imidazo (4,5b)-pyridinium ring (Fig. 1A). PEN accumulation is associated with aging [[7], [8], [9], [10]] and various diseases such as diabetes mellitus, chronic kidney dysfunction, cataracts, osteoporosis, and Alzheimer’s disease [8,[11], [12], [13], [14], [15], [16], [17]]. We previously reported that PEN levels in the peripheral blood of a subpopulation of patients with schizophrenia is significantly higher than that in healthy controls [18,19]. These results were replicated by other groups [[20], [21], [22]]. Severe clinical features observed in patients with PEN accumulation, such as a higher proportion of hospitalized patients, a longer duration of hospitalization, and larger prescribed doses of antipsychotic medication [23], are similar to those observed in treatment-resistant schizophrenia, as defined by Kane et al. [24]. Furthermore, we recently found that the plasma PEN levels are associated with impairment of cognitive performance [25]. These findings strongly suggest that PEN accumulation is one of the pathological mechanisms underlying schizophrenia.
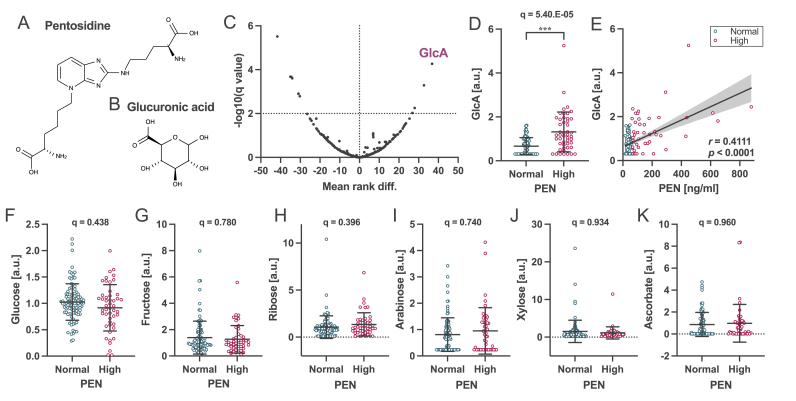
Fig. 1.
Differential plasma metabolites between subjects with high and normal pentosidine in cohort 1. Chemical structures of (A) PEN and (B) GlcA. (C) Volcano plot demonstrating the relationship between the mean rank difference in metabolites and the FDR (i.e., the q-value) of plasma metabolites. The high PEN group showed four upregulated and seven downregulated metabolites in the plasma compared to the normal PEN group. (D) Comparison of GlcA levels between high PEN and normal PEN groups. q = 5.40.E−05 using the Mann–Whitney test. (E) Correlation between GlcA and PEN in the plasma. Spearman’s correlation coefficient was r = 0.4111 (p < 0.0001). Comparison of known PEN-precursors levels between the high and normal PEN groups: (F) glucose, (G) fructose, (H) ribose, (I) arabinose, (J) xylose, and (K) ascorbate. Data are presented as the mean ± standard deviation (SD).
Early in vitro studies have shown that PEN is generated from glucose, fructose, pentoses, and ascorbate [7,26,27]; however, the details of its synthetic pathway remain unclear. None of these substances was elevated in the subpopulation of patients with schizophrenia, although they had high PEN levels. Patients with diabetes mellitus, renal dysfunction, Behcet’s disease, and chronic viral hepatitis type C were excluded, as these diseases are known to increase plasma PEN levels. In other words, it was unclear from what substance the increased PEN within patients was generated. Therefore, animal and cellular models that reproduce the pathophysiology of “PEN-accumulating schizophrenia” without increasing the already known PEN precursors have not been generated. Thus, the molecular mechanisms underlying the involvement of accumulated PEN in the pathophysiology of schizophrenia remain unclear.
In the present study, to identify novel precursors of PEN accumulated in patients with schizophrenia, we performed metabolome analysis using peripheral blood samples from two cohorts, including patients with high PEN levels. We found some metabolites that correlate with peripheral PEN levels and that are significantly elevated in the subjects with high PEN compared to those with normal PEN. We further validated whether the metabolites were novel PEN precursors by in vitro and in vivo studies.
2. Subjects and methods
2.1. Subjects
Cohorts 1 and 2 included 98 patients with schizophrenia and 50 healthy controls, and 120 patients with schizophrenia and 67 healthy controls, respectively (Suppl. Tables S1 and S4). Specimens were collected from both cohorts using the method described below. The experimental protocols were approved by the ethics committees of all participating institutions (Tokyo Metropolitan Matsuzawa Hospital and Tokyo Metropolitan Institute of Medical Science, approval no. 20-17). This study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All participants provided written informed consent. Patients were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Diagnoses were made by at least two experienced psychiatrists and randomly recruited from inpatients and outpatients. Patients with diabetes mellitus (hemoglobin A1c (HbA1c) ≥ 6.3), renal dysfunction (creatinine >1.04 mg/dl for men and >0.79 mg/dl for women or estimated glomerular filtration rate (eGFR) < 60.0 ml/min/1.73 m2), Behcet’s disease, and chronic viral hepatitis type C were excluded from this study as these diseases are known to increase plasma PEN levels. The cutoff point for high plasma PEN levels was set at 55.2 ng/ml, namely, the mean + 2 SDs of healthy controls, as determined in a previous report [18]. Values below this threshold were defined as normal values. Based on the definition of PEN, each cohort was divided into high and normal PEN groups. To identify PEN precursors independently of diagnosis, healthy subjects and patients with schizophrenia were included in the normal PEN group in the metabolome analysis. Cohort 1 included 48 and 100 subjects, and cohort 2 included 38 and 149 subjects with high and normal PEN, respectively.
Clinical studies were conducted using combined data from cohort 1 and 2. The glucuronic acid (GlcA) score in the metabolome data is expressed as a relative value within each cohort. Therefore, samples of two individuals (ID: SCZ138 and SCZ176) were measured in both cohorts. Using the mean values, the GlcA levels in each cohort were standardized and then combined.
2.2. Metabolome analysis using LC/MS and GC/MS
Metabolome analysis using plasma samples was conducted by Metabolon, Inc. (Durham, NC, USA). The sample preparation process was performed using an automated MicroLab STAR® system (Hamilton Company, Reno, NV). Sample preparation was conducted using a proprietary series of organic and aqueous extractions to remove the protein fraction while allowing the maximum recovery of small molecules. The resulting extract was divided into two fractions: one for liquid chromatography (LC) and one for analysis by gas chromatography (GC). The samples were briefly placed on a TurboVap® (Zymark: Hopkinton, MA) to remove the organic solvent. Each sample was frozen and dried under a vacuum. The samples were then prepared using an appropriate instrument, either LC-MS or GC-MS.
The LC-MS portion of the platform was based on an ACQUITY UPLC (Waters, Milford, MA) and an LTQ mass spectrometer (Thermo Fisher Scientific, Waltham, MA), which comprised an electrospray ionization (ESI) source and a linear ion-trap (LIT) mass analyzer. The sample extract was split into two aliquots, dried, and reconstituted in acidic or basic LC-compatible solvents, each of which contained 11 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic positive-ion optimized conditions and the other using basic negative-ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted under acidic conditions were gradient eluted using water and methanol containing 0.1% formic acid, while the basic extracts, which also used water/methanol, contained 6.5 mM ammonium bicarbonate. Mobile phase solvents under acidic condition consist of 0.1% formic acid in water (A) and in methanol (B), while under basic condition consist of 6.5 mM ammonium bicarbonate in water at pH 8 (A) and in 95:5 methanol/water (B). The gradient profile used for both conditions was from 0.5% B to 70% B in 4 min, from 70% B to 98% B in 0.5 min, and holding at 98% B for 0.9 min before returning to 0.5% B in 0.2 min. The flow rate was 350 μL/min. MS analysis was alternated between MS and data-dependent MS/MS scans using dynamic exclusion.
The samples destined for GC/MS analysis were re-dried under vacuum desiccation for a minimum of 24 h prior to derivatization under dried nitrogen using bistrimethyl-silyl-trifluoroacetamide (BSTFA). The GC column was 5% phenyl, and the temperature ramp was from 40 to 300 °C over 16 min. Samples were analyzed on a Trace DSQ fast-scanning single-quadrupole mass spectrometer (Thermo Scientific, Waltham, MA, USA) using electron impact ionization.
Metabolome data generated or analyzed during this study are included in this published article and its supplementary information files.
2.3. Quantification of PEN
An equal volume of 20% trichloroacetic acid (TCA) was added to plasma samples containing 1 mg protein. After centrifugation at 20,400×g at 4 °C for 10 min, the protein pellet was resolved using 1 N NaOH. The protein solution was hydrolyzed at 100 °C for 18 h with an equal concentration of hydrochloric acid (HCl) (6 N, final concentration). The pretreatment cartridge was prepared as previously described with minor modifications [28]. A cation-exchange extraction cartridge, Oasis MCX (3 ml; Waters), was used for the sample pretreatment. The cartridge was preconditioned with 1 ml of methanol and equilibrated with 1 ml of distilled water before loading the sample. The sample was applied to the cartridge, which was then washed with 3 ml of 2% formic acid. PEN was eluted from the cartridge with 3 ml of 7% ammonia solution (v/v). The eluate was evaporated to dryness under vacuum, and the residues were dissolved in the HPLC mobile phase to prepare 100 μl of analytical sample solutions. The solutions were filtered through a 0.20-μm Millex filter (Ultrafree-MC Hydrophilic PTFE membrane, Millipore). An aliquot (20 μl) of each sample was applied to the analytical HPLC system.
The HPLC method has been previously reported with slight modifications [29]. The HPLC system comprised a 1260 infinity liquid chromatography system (Agilent) with a Capcellpak C18 UG80 column (250 mm × 94.6 mm (i.d.); 5 lm particle size, Shiseido, Tokyo, Japan), and an RF-10A Shimadzu spectrofluorometric detector (Ex. 335 nm, Em. 385 nm). The mobile phase was 16% acetonitrile (v/v), containing 0.2% heptafluorobutyric acid (HFBA). The flow rate was maintained at 1.0 ml/min, and the column was kept at 40 °C.
2.4. In vitro PEN generation
In PEN generation in vitro, 10, 20, 100, or 200 mM GlcA was incubated with Nα-acetyl-l-lysine (Tokyo chemical industry Co., Tokyo, Japan) and Nα-acetyl-l-arginine (Sigma-Aldrich, MO) at the same concentration as GlcA in 300 mM phosphate buffer (pH 7.4) for one week at 37 °C. Moreover, Glucose, galactose, mannose, GlcA, galacturonic acid, mannoic acid, ascorbate, or ribose (20 mM each) were incubated with 20 mM Nα-acetyl-l-lysine and 20 mM Nα-acetyl-l-arginine in 300 mM phosphate buffer (pH 7.4) for one week at 37 °C. The yielded PEN were quantified by HPLC.
For PEN generation in BSA, BSA (20 μg/μl) was dissolved in 500 mM phosphate buffer at pH 7.4 and incubated at 37 °C with 200 mM GlcA for 0, 7, 14, 21, or 28 days. The yielded PEN in the incubated samples was quantified by HPLC, and PEN-BSA was detected by western blotting using a PEN antibody (1:500; Medical Chemistry Pharmaceutical Co., Sapporo, Japan).
For PEN generation in plasma, 0, 0.5, or 2 mM GlcA was incubated with human plasma for one week at 37 °C. PEN levels in the plasma were quantified using HPLC.
2.5. In vivo PEN generation
Ten adult male C57BL/6J mice and eight male Hartley guinea pigs (SLC Japan, Shizuoka, Japan) were subcutaneously treated with 300 mg/kg/day GlcA or saline (SAL) at eight and five weeks of age, respectively, for two weeks. No significant changes in body weight were observed following GlcA administration. Animals were housed in plastic cages and maintained in a regulated environment (25 °C ± 1 °C, 50% ± 5% humidity) under a 12 h light/dark cycle (lights switched on at 8:00 a.m. and off at 8:00 p.m.). Food and tap water were provided ad libitum.
2.6. Confirmation of PEN by LC-QTOF
PEN generated in vitro was fractionated by HPLC using a Mightysil RP-18GP column (250 mm × 10 mm (i.d.); 5 μm particle size, Kanto Kagaku, Tokyo, Japan) after the sample preparation described above. Samples (5 μl) were subjected to LC-QTOF mass spectrometry equipped with electrospray ionization (ESI) interface source (Bruker Daltonics, Germany), as described previously [30]. Briefly, LC was conducted by flow injection. The mobile phase comprised 80% acetonitrile and 0.1% formic acid. The flow rate was set at 0.2 ml/min. The ionization source temperature was 200 °C and the capillary voltage was 4500 V. The drying gas flow rate was 8.0 l/min and 1.6 the nebulizer pressure. MS/MS was conducted in the MRM mode at m/z 190.1080 (width 1.00), and its collision energy was set to 20.0 eV. The data were acquired with a stored mass range of m/z 50–1000 and analyzed using Compass DataAnalysis (Bruker Daltonics, Germany).
2.7. Measurement of AKR activity using whole blood cells
AKR activity was measured in the whole blood cells from 80 healthy participants and 209 schizophrenia patients in cohorts 1 and 2. AKR activity was measured as described previously [31]. A total of 150 μl of cOmplete Lysis-M EDTA-free (Merck, Darmstadt, Germany) was added to the pellet of human whole blood cells. The mixture was sonicated and centrifuged at 20,400×g for 15 min at 4 °C. The supernatant was collected from cell lysates. After protein quantification, 10 μg of protein was used for activity assay.
AKR activity was measured by monitoring NADPH consumption. The reaction mixture contained 100 mM HEPES (pH 7.4), 0.1 mM NADPH, and 10 mM GlcA as a substrate. Reactions were monitored by assessing the decrease in absorbance at 340 nm using an Epoch microplate spectrophotometer (BioTek Instruments, Winooski, VT, USA) at 37 °C. One unit of enzyme activity was defined as the amount of enzyme that catalyzed the oxidation of 1 μmol NADPH per min.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9 and R version 4.1.2. Metabolome data were compared using the Mann–Whitney test with individual variances for samples and a two-stage step-up (Benjamini, Krieger, and Yekutieli) false discovery rate (FDR) to account for multiple comparisons. Statistical significance was set at q < 0.01.
Statistical differences between the two groups were determined using Student’s t-test or the Mann–Whitney test. Statistical differences between more than three groups were determined using one-way or two-way analysis of variance (ANOVA) with repeated measures followed by Tukey’s multiple comparison test. A two-sided F-test confirmed normally distributed data. Correlations between GlcA and PEN levels in plasma, clinical scores, and AKR activity in whole blood cells were evaluated using Spearman’s rank test. Differences were considered statistically significant at P < 0.05.
Multiple regression analysis was conducted using the Statistical Package for Social Sciences version 24.0 (IBM Corp., New York, USA). The significance level (α) was set to 0.05 for two-tailed tests.
2.9. Study approval
In animal studies, the experimental procedures were approved by the Animal Experiment Committee of the Tokyo Metropolitan Institute of Medical Science (approval nos. 18006, 19003, 20005, 21011, and 22004).
3. Results
3.1. Differential plasma metabolites between subjects with high and normal pentosidine
To identify the metabolites that most affected the PEN level, metabolome analysis of the plasma samples from the subjects with high and normal PEN levels, regardless of their diagnosis, was performed. In cohort 1, the high PEN group included 48 patients with schizophrenia, showing a mean of 182.1 ± 173.3 ng/ml PEN in the plasma, whereas the normal PEN group included 50 patients with schizophrenia and 50 healthy subjects, with an average of 37.2 ± 9.2 ng/ml PEN (Suppl. Table S1). No significant differences in age, sex, HbA1c level, or eGFR were observed between the two groups. The metabolome results showed that four upregulated, and seven downregulated metabolites were identified in the high PEN group compared with the normal PEN group (q < 0.01; Suppl. Tables S2 and S3). GlcA was the most upregulated metabolite (Fig. 1B–D). Moreover, plasma PEN levels significantly correlated with plasma GlcA levels (Fig. 1E). In contrast, the levels of glucose, fructose, ribose, arabinose, xylose, and ascorbate, known precursor molecules of PEN, showed no significant differences (Fig. 1F–K and Suppl. Table S3). Furthermore, to confirm the differential plasma metabolites observed in cohort 1, we performed metabolome analysis using another independent cohort 2, including 38 and 149 subjects with high and normal PEN, respectively, and we confirmed the increased GlcA level in the high PEN group (Suppl. Fig. S1 and Suppl. Tables S4–S6). These findings suggest that GlcA may be a new PEN source in the peripheral blood.
3.2. PEN generation from GlcA
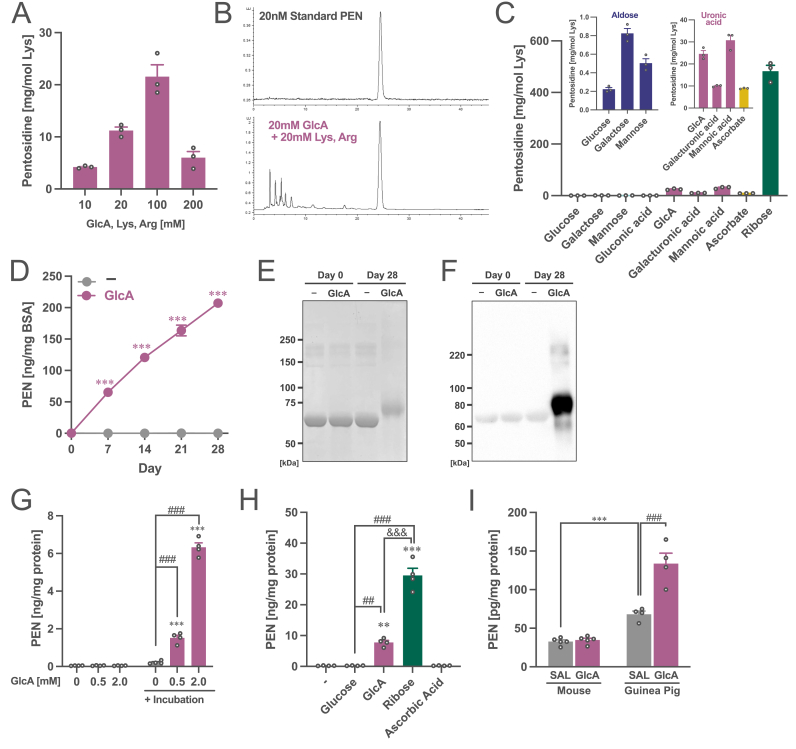
To investigate whether GlcA is a precursor of PEN, GlcA was incubated with lysine and arginine. After incubation for one week under physiological conditions (pH7.4, 37 °C), a product with fluorescence and retention times identical to those of PEN was obtained (Fig. 2A and B). To confirm whether this product was PEN, the product was fractionated and analyzed by liquid chromatography–quadrupole time-of-flight mass spectrometry (LC-QTOF) (Suppl. Figs. S2A–S2C). The m/z of PEN 190.1180 ± 0.005 (divalent ion), consistent with the composition formula of pentosidine C17H26N6O4, was detected in the GlcA-derived product. Furthermore, the fragment ion at m/z 190 detected in the GlcA-derived product was consistent with that in the PEN standard (Supp. Figs. S2D–S2G). Based on these findings, the GlcA-derived product was confirmed as PEN. Furthermore, the PEN generation ability of GlcA after incubation with lysine and arginine was higher than that of glucose and the same as that of ascorbate, whereas it was low compared to that of ribose (Fig. 2C). We also found that uronic acids other than GlcA, galacturonic acid, and mannuronic acid could also yield PEN to approximately the same extent as GlcA, depicting a higher ability of PEN generation than aldoses including glucose, galactose, and mannose. These findings suggest that PEN can be generated from uronic acid.
Fig. 2.
Generation of pentosidine from glucuronic acid. (A) Amount of the yielded PEN from GlcA by incubating with lysine and arginine. (B) Chromatograms of standard PEN and the incubate samples with GlcA, lysine, and arginine (20 mM each) for one week. (C) Amount of the yielded PEN from GlcA and aldoses, other uronic acids, and precursor molecules of PEN by incubating with lysine and arginine. Two enlarged figures of the data are included at the top. (D) Amount of the yielded PEN from GlcA by incubating with BSA. Two-way ANOVA: FInteraction(4,16) = 437, p < 0.001; FDay(4,16) = 436, p < 0.001; FGlcA(1,4) = 1296, p < 0.001. ***p < 0.001 (vs. control (−)) by Student’s t-test. (E) Staining with Coomassie brilliant blue and (F) western blotting with an anti-PEN antibody for BSA-PEN protein. (G) Amount of the yielded PEN from GlcA by incubating with human plasma. All the samples include plasma. Two-way ANOVA: FInteraction(2,6) = 858, p < 0.001; FGlcA(2,6) = 837, p < 0.001; FIncubation(1,3) = 446, p < 0.001. ***p < 0.001 (vs. without incubation), ###p < 0.001 (vs. 0 mM after incubation) by Tukey’s test. (H) Amount of the yielded PEN from GlcA and other precursor molecules of PEN by incubating with human plasma. One-way ANOVA: F(4,12) = 154, p < 0.001. ***p < 0.001, **p < 0.01 (vs. control (−)), ###p < 0.001, ##p < 0.01 (vs. Glucose) and &&&p < 0.001 (GlcA vs. Ribose) using Tukey’s multiple comparison test. (I) Quantification of PEN in plasma of saline (SAL)- or GlcA-treated mice and guinea pigs. Two-way ANOVA: FInteraction(1,14) = 24.6, p < 0.001; FSpecies(1,14) = 110, p < 0.001; FGlcA(1,14) = 27.8, p < 0.001. ***p < 0.001 (Mouse vs. Guinea pig with SAL), ###p < 0.001 (SAL vs. GlcA in Guinea pig) by Tukey’s test. The data are shown as mean ± SEM values. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, we demonstrated that PEN was generated when BSA was incubated with GlcA (Fig. 2D). The generation of PEN in BSA was also confirmed by western blotting using a PEN antibody (Fig. 2E and F). Moreover, PEN was produced in a dose-dependent manner by incubating GlcA with human plasma (Fig. 2G). Similar to the results in Fig. 2C, the PEN generation capacity in plasma was also higher; the order being ribose, GlcA, and glucose (Fig. 2H). Furthermore, PEN cannot be generated from 2-hydroxyisobutyrate (2-HIB), another metabolite that is significantly higher in the high PEN group than in the normal PEN group in both cohorts 1 and 2 (Suppl. Fig. S3A), and other metabolites upregulated in the high PEN group including xylitol, ribitol, pseudouridine, β-hydroxyisovaleric acid, and lactate (Suppl. Fig. S3B).
Finally, we investigated whether PEN was produced in vivo by GlcA treatment in rodents. After treating mice and guinea pigs with GlcA for two weeks, plasma PEN levels were measured. We found that the basal level of plasma PEN in guinea pigs before GlcA treatment was higher than that in the mice (Fig. 2I). Guinea pigs treated with GlcA showed PEN accumulation in the plasma, although GlcA-treated mice did not show this accumulation. These findings suggest that PEN can be generated from GlcA, both in vitro and in vivo in some animal models.
3.3. Plasma GlcA level in patients with schizophrenia
We previously reported a 1.7-fold higher mean plasma concentration of PEN in patients with schizophrenia than in control subjects [18]. Here, using the combined data from cohorts 1 and 2, we investigated whether GlcA, a precursor of PEN, is also increased in patients with schizophrenia. Table 1 presents a demographic summary of the control subjects and patients with schizophrenia. The mean (± standard deviation (SD)) age of 117 healthy subjects (male/female = 41/76) and 218 patients with schizophrenia (male/female = 116/102) were 39.8 ± 11.3 and 45.9 ± 11.5, respectively (Age, p < 0.0001: Sex, p = 0.0014). There were no significant differences in HbA1c and eGFR. Similar to previous findings, plasma PEN levels in patients with schizophrenia were higher than those in the controls (p < 0.0001). Patients also showed enhanced GlcA levels compared to healthy subjects (p < 0.0001). Furthermore, logistic regression analysis adjusted for age and sex demonstrated that the diagnosis was significantly associated with plasma GlcA levels (odds ratio = 1.94, p = 1.15 × 10−4) (Table 2). These findings suggest that GlcA may be a useful biomarker for identifying homogeneous populations with schizophrenia and providing appropriate treatment.
Table 1.
Demographic summary in control subjects and patients with schizophrenia.
| Control | Schizophrenia | p valuea | |
|---|---|---|---|
| N | 117 | 218 | |
| Age | 39.8 ± 11.3 | 45.9 ± 11.5 | <.0001*** |
| Sex (male/female) | 41/76 | 116/102 | 0.0014** |
| HbA1c (%) | 5.4 ± 0.3 | 5.4 ± 0.4 | 0.912 |
| eGFR (ml/min/1.73m2) | 88.6 ± 14.4 | 85.5 ± 14.3 | 0.063 |
| PEN (ng/ml) | 36.2 ± 8.7 | 77.6 ± 102.1 | <.0001*** |
| GlcA (Z-score)b | −0.325 ± 0.626 | 0.174 ± 1.12 | <.0001*** |
Abbreviations: PEN, pentosidine; GlcA, glucuronic acid; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate.
Statistical differences were determined using the Mann–Whitney test. Differences were considered statistically significant at P < 0.05.
GlcA values were normalized by z-score.
Table 2.
Association between plasma glucuronic acid and diagnosis for schizophrenia.
| Unadjusted model |
Adjusted modela |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P valuec | Odds Ratio (95% CI) | p valuec | |
| GlcA (Z-score)b | 2.04 (1.48–2.82) | 1.22 × 10−5 *** | 1.94 (1.39–2.72) | 1.15 × 10−4 *** |
| Age | 1.04 (1.02–1.06) | 1.95 × 10−4 *** | ||
| Sex | 1.83 (1.12–2.99) | 1.51 × 10−2 * | ||
Abbreviations: GlcA, Glucuronic acid; CI, Confidence interval.
Adjusted for age and sex.
GlcA values were normalized by z-score.
A value of p < 0.05 was considered statistically significant.
Moreover, we investigated the association between GlcA and biological features in patients with schizophrenia (Table 3 and Suppl. Table S8). GlcA levels were significantly associated with age, plasma PEN, and serum pyridoxal levels. There were no significant differences in sex, body mass index (BMI), HbA1c level, and eGFR. These findings suggest that GlcA accumulation in patients with schizophrenia is not related to physical diseases, such as diabetes mellitus and renal dysfunction.
Table 3.
Association between glucuronic acid and biological features in patient with schizophrenia.
| N | Scoresa | ρb | p valuec | |
|---|---|---|---|---|
| Age | 218 | 45.9 ± 11.5 | 0.170 | 0.012* |
| Sex (male/female) | 218 | 116/102 | 0.100 | 0.261 |
| BMI | 56 | 23.2 ± 5.35 | −0.050 | 0.713 |
| PEN (ng/ml) | 218 | 77.6 ± 102.1 | 0.344 | <0.0001*** |
| HbA1c (%) | 211 | 5.4 ± 0.4 | 0.132 | 0.056 |
| eGFR (ml/min/1.73m2) | 218 | 85.5 ± 14.3 | −0.109 | 0.108 |
| Pyridoxal (ng/ml) | 218 | 6.88 ± 3.94 | −0.211 | 0.002** |
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate.
Classification or mean ± standard deviation (SD) were shown.
Spearman’s rank correlation coefficients were shown.
Statistical differences were determined using Spearman’s rank correlation test. Differences were considered statistically significant at P < 0.05.
3.4. AKR activity in patients with schizophrenia
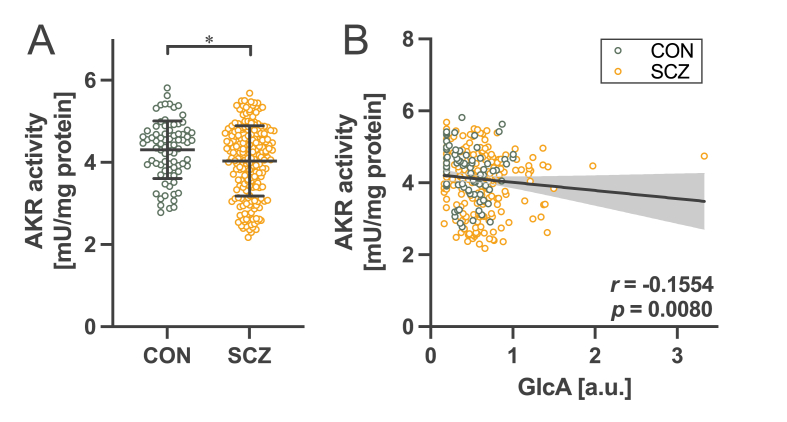
GlcA is metabolized to gulonic acid by AKR [32]. To assess whether increased GlcA levels in schizophrenia are associated with decreased AKR activity, we measured the enzymatic activity of AKR using whole blood cells obtained from the participants in cohorts 1 and 2. The results showed that AKR activity in patients with schizophrenia was significantly lower than that in healthy controls (Fig. 3A). Moreover, AKR activity was negatively correlated with plasma GlcA levels (Fig. 3B). These findings suggest that the decreased enzymatic activity of AKR in schizophrenia might increase GlcA levels, leading to PEN accumulation.
Fig. 3.
Decreased AKR activity in schizophrenia. (A) Comparison of AKR activity in whole blood cells between control subjects (CON) and patients with schizophrenia (SCZ). *p < 0.05 by Mann–Whitney test. (B) Correlation between GlcA in plasma and AKR activity. Spearman’s correlation coefficient r = −0.1554, p = 0.0080.
However, we found no significant difference in the AKR activity between the normal and high PEN groups (Suppl. Fig. S4A), and no correlation between AKR activity and plasma PEN levels (Suppl. Fig. S4B). Although these results may appear contradictory to the finding that GlcA is the precursor of PEN, this discrepancy might suggest that GlcA is not the only source of PEN in schizophrenia. A Venn diagram of patients with schizophrenia overlapped with high GlcA and high PEN is shown in Suppl. Fig. S4C, where the high GlcA group is defined by the cutoff point at 0.928 (z-score), namely, the mean + 2 SDs of the healthy controls, similar to the definition of the high PEN subjects. Among the patients in the high GlcA group, the proportion of those who also have high PEN is 71.0%, suggesting that high GlcA level often leads to high PEN levels. On the other hand, among those in the high PEN group, the proportion of individuals who also have high GlcA is 25.6%. This suggests that only 25% of patients with high PEN levels can be attributed to increased GlcA levels. The remaining 75% of patients with high PEN levels do not have high GlcA levels and the cause of PEN accumulation in these patients is still unknown. Furthermore, multiple regression analysis revealed that there was a significant association between plasma PEN and GlcA (Suppl. Table S7). The significant difference remained even after adjustments for possible confounding factors were made, including age and sex. However, the contribution of GlcA to the plasma PEN level was 15.4%. Based on these findings, it can be inferred that not all patients with PEN accumulation exhibit increased GlcA levels and that GlcA alone cannot account for all cases of PEN accumulation in schizophrenia. The presence of patients with high PEN not due to GlcA accumulation may be responsible for the lack of correlation between AKR activity and PEN. Further analysis with a different approach would be needed to elucidate the cause of the remaining 75% of patients with PEN accumulation.
4. Discussion
We identified GlcA as a novel PEN precursor by metabolomic analysis of plasma in the high PEN and normal PEN groups (Fig. 1C and D). Plasma PEN levels significantly correlated with plasma GlcA levels (Fig. 1E). The levels of glucose, ribose, pentose, and ascorbate, which are reported precursors of PEN [7,9], were not high across the board in the high-PEN group (Fig. 1F–K), suggesting that PEN was yielded from GlcA. Incubation of GlcA with human plasma increased PEN generation in a dose-dependent manner (Fig. 2G). The ability of GlcA to generate PEN in human plasma at 2 mM doses was approximately 0.26-fold lower than that of ribose but approximately 34-fold higher than that of glucose (Fig. 2H). According to the human metabolome database [33], the blood concentrations of GlcA and ribose in healthy subjects are approximately 165 μM and 2.3 μM, respectively. Considering their concentration ratio (GlcA/ribose = 71.7), it can be inferred that GlcA, rather than ribose, was the dominant PEN precursor in the blood. Furthermore, patients with diabetes have high levels of GlcA and glucose [[34], [35], [36], [37]]. GlcA accumulation has been reported in chronic kidney disease [38] and liver disease [39] in which PEN accumulation has been observed. Given its high synthetic capacity, GlcA, rather than glucose, may be the primary source of PEN in these diseases.
Moreover, not only PEN, but also GlcA accumulate in the body with age and are associated with longevity [10,40]. Our findings suggest that GlcA is responsible for PEN accumulation during aging. The levels of PEN and GlcA in the blood have been identified as a powerful predictor of mortality in healthy subjects. In other words, subjects with higher levels of PEN and GlcA tend to have shorter lifespans. AGEs, including PEN, can induce aberrant inflammation via binding to RAGE [3,4]. GlcA is also known to induce inflammation via activating Toll-like receptor 4 [41]. These suggest that both PEN and GlcA may be involved in accelerating aging via inflammation, since chronic inflammation is well-known to accelerate aging [42].
By incubation of GlcA with lysine and arginine, PEN could be generated under physiological conditions (Fig. 2A); although its generation increased in a dose-dependent manner up to 100 mM, it decreased at 200 mM GlcA, consistent with previous studies [43]. In addition to ribose, GlcA might inhibit the conversion of “early” Amadori rearrangement products to “late” AGEs. Moreover, it has been shown that PEN can be generated from GlcA and other uronic acids (Fig. 2C). However, the concentrations of galacturonic and mannuronic acids are markedly smaller than GlcA in the plasma, suggesting that these uronic acids might contribute little to the generation of PEN. Although “glucuronidine/LW-1” is a known AGE composed of GlcA [[44], [45], [46], [47]], it is completely different from PEN in terms of its molecular weight. Although there are no reports on the generation of PEN from GlcA, Horvat et al. reported the formation of Amadori compounds from GlcA (Suppl. Fig. 5) [48]. They predicted that the Amadori products from GlcA lead to β-keto acid intermediates via keto-enol tautomerization and carbonyl group migration along the саrbohydrate backbone. The unstable keto-acid intermediate eliminated CO2 at C-6, resulting in 4-ketopentose. Carbonyl group migration to C-5, followed by an intramolecular amino-carbonyl reaction and series of dehydrations, yielded the final 3-hydroxypyridinium compound. Because five carbons are required for synthesizing PEN, decarboxylation by β-keto acid is reasonable for synthesizing PEN from GlcA, which has six carbons. Moreover, the end product of this process, 3-hydroxypyridinium, is an AGE known as 2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine), which accumulates in the eyes of cataractous and aged subjects as well as in rodent models of diabetes [[49], [50], [51], [52], [53]]. If OP-lysine is also yielded from GlcA, its accumulation may be involved in a wider range of diseases. It is unclear whether OP-lysine is yielded by the reaction of GlcA and lysine and whether PEN can be generated by the reaction of OP-lysine and arginine. The predicted pathway for PEN generation from GlcA is shown in Suppl. Fig. 5. Further research is required to clarify the exact PEN-synthetic process of GlcA.
Furthermore, we successfully induced in vivo PEN generation by administering GlcA to guinea pigs for two weeks, whereas GlcA treatment in mice did not result in PEN accumulation (Fig. 2I). One reason why PEN can be generated by the administration of GlcA in guinea pigs, but not in mice, may involve an ascorbate generation system. Mice can generate ascorbate from GlcA in sufficient amounts, but guinea pigs cannot generate ascorbate because of a genetic mutation in gulono-γ-lactone oxidase (GLO), the last enzyme in the ascorbate biosynthesis pathway [54]. Thus, GlcA administered to mice was probably consumed for the generation of ascorbate, but in guinea pigs, it was not used and consequently accumulated in the body. We found that the basal level of PEN in guinea pigs was higher than that in mice before GlcA administration. Humans, like guinea pigs, cannot generate ascorbate from GlcA owing to deficiencies in the GLO gene [55]; humans may accumulate GlcA more readily than mice and other species, resulting in PEN accumulation.
We found that GlcA was significantly higher in patients with schizophrenia (Table 1). This significant difference remained even after adjusting for age and sex (Table 2). A single SD increase in GlcA was associated with an approximately 2-fold increase in the odds for schizophrenia. These findings suggest that GlcA might be a useful marker for stratifying patients with schizophrenia because it is easier to quantify than PEN. Little is known about how PEN accumulation relates to schizophrenic pathology because appropriate cellular or animal models that reproduce the pathology of PEN-accumulating schizophrenia have not been generated. Future analysis of the models constructed based on the novel pathway of PEN generation from GlcA is expected to advance our understanding of the pathophysiology of PEN accumulation in schizophrenia. Furthermore, GlcA accumulation itself may be involved in the pathogenesis of schizophrenia via neuroinflammation since GlcA is known to cause inflammation [41]. Therefore, it is necessary to elucidate the involvement of GlcA accumulation in the pathogenesis of schizophrenia [56,57].
Finally, we measured AKR activity using GlcA as a substrate in whole blood cells and showed that AKR activity was decreased in patients with schizophrenia and was negatively correlated with GlcA levels in the plasma (Fig. 3). These findings suggest the possibility that decreased AKR activity in patients with schizophrenia increases GlcA levels in the plasma, leading to PEN accumulation. These findings are consistent with a previous report that Akr1a1 knockout mice showed GlcA accumulation in the peripheral blood [58] and inhibition of AKR1A1 in mice increased the urinary output of GlcA [59]. We previously reported mutations in the AKR1A1 gene, a GlcA-degrading enzyme with reduced enzymatic activity [31]. Such genetic influences may also contribute to GlcA accumulation. Moreover, we found that the GlcA level was negatively associated with pyridoxal (Table 3). We have already reported that the pyridoxal levels in the peripheral blood of a subpopulation of patients with schizophrenia are significantly lower than that of healthy controls [18,23]. Furthermore, a recent review has shown the decreased pyridoxal in patients with schizophrenia as the most convincing evidence in peripheral biomarkers for major mental disorders [60]. Pyridoxal 5-phosphate (PLP), the phosphorylated form of pyridoxal, is a cofactor for over 150 enzymes (including representatives of every major enzyme class), which account for approximately 4% of known enzymes [61]. In fact, PLP has been reported to control AKR activity as a cofactor [62,63]. Thus, the decreased pyridoxal level in schizophrenia might downregulate AKR activity, leading to GlcA accumulation. These findings suggest that the AKR activity decreased as a result of genetic and environmental factors might be due to GlcA accumulation in schizophrenia.
Another possibility for GlcA accumulation is the enhancement of the glucuronidation system. GlcA is a key molecule involved in the detoxification of xenobiotic compounds. Many compounds, including pollutants, bilirubin, and drug metabolites, undergo hepatic glucuronidation in which they are conjugated to GlcA via the enzymatic action of UDP-glucuronosyltransferases (UGT). This chemical modification increases bile solubility, facilitates urinary excretion, and is a key step in the phase II metabolism of these compounds required for their effective clearance from the body. Owing to increased GlcA levels, activation of the glucuronidation system might cause the sequestration of GlcA in schizophrenia. In fact, our metabolomics data showed that the unconjugated bilirubin level in the high PEN group was lower than that in the normal PEN group in both cohorts, especially in cohort 2 (Suppl. Tables S2 and S5: q = 2.11 × 10−2 in cohort 1, q = 2.29 × 10−3 in cohort 2). The lower bilirubin levels suggest that the glucuronidation system might be chronically activated in patients with schizophrenia with high PEN. Conversely, decreased levels of PEN have been reported in Gilbert’s syndrome, caused by UGT dysfunction, leading to impairment of the glucuronidation system [64]. Gilbert’s syndrome is characterized by an increase in unconjugated bilirubin levels. These findings serve as evidence suggesting a potential role of the enhanced glucuronidation system in the accumulation of GlcA. Activation of the glucuronidation system is consistent with the clinical features of treatment resistance in patients with PEN accumulation [23]. Moreover, we identified “piperine” as one of the significantly decreased molecules in high PEN group in both the cohort 1 and 2 (Suppl. Tables S3 and S6). Piperine reportedly exerts a strong inhibitory effect on the glucuronidation system [65,66]. This finding suggests that the disinhibitory effect of glucuronidation by decreased piperine may be involved in the increase in GlcA, leading to the accumulation of PEN. Although the direct relationship between the enhanced glucuronidation system and the pathogenesis of schizophrenia is still unknown, the enhanced glucuronidation system might cause both PEN accumulation and treatment-resistant observed in patients with schizophrenia.
The generation and degradation of GlcA is predominantly in the liver. In Akr1a1 KO mice, a marked accumulation of GlcA has been observed within the liver [58]. Thus, some functional changes in the liver may result in local accumulation of GlcA and production of PEN in the liver without changes in the amount of GlcA in the blood. This possibility may explain why high plasma GlcA does not explain the high circulating levels of PEN in all patients with schizophrenia (only 25.6%). In the future, we believe that the quantification of GlcA and the activity of UGT in the liver of patients with schizophrenia need to be investigated.
Taken together, we found GlcA accumulation in subjects with high PEN. We also found that PEN was generated by incubation of GlcA with lysine and arginine, BSA, or human plasma. We also demonstrated that GlcA treatment increased PEN levels in the plasma of guinea pigs but not in that of mice. In view of the conversion of GlcA to PEN in vitro and the potential of GlcA to increase PEN in an animal model, it is likely that GlcA may act as a precursor of PEN in a subpopulation of patients with schizophrenia. Furthermore, GlcA has been associated with the diagnosis of schizophrenia. We showed that AKR activity to degrade GlcA was decreased in patients with schizophrenia, and its activity was negatively correlated with GlcA levels in the plasma, suggesting that the decreased AKR activity in patients with schizophrenia increases GlcA levels in the plasma, leading to PEN accumulation. Our findings are expected to make important contributions to our understanding of the aging process and pathogenesis of diseases related to PEN accumulation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
We thank the donors and their families for the samples used in this study. We are grateful for the expert technical assistance provided by Izumi Nohara and Yukiko Shimada. This work was supported by Japan Society for the Promotion of Science KAKENHI grant numbers, 18K06977, 19H04887, 20H03608, 22K07609, 23H02844; Japan Agency for Medical Research and Development grant JP20dm0107088; The Takeda Science Foundation, The Kanae Foundation for the Promotion of Medical Science; The Uehara Memorial Foundation; The Sumitomo Foundation; The SENSHIN Medical Research Foundation; The Mishima Kaiun Memorial Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102876.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Metabolome data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- 1.Chaudhuri J., Bains Y., Guha S., Kahn A., Hall D., Bose N., Gugliucci A., Kapahi P. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metabol. 2018;28(3):337–352. doi: 10.1016/j.cmet.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabbani N., Xue M., Thornalley P.J. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj. J. 2016;33(4):513–525. doi: 10.1007/s10719-016-9705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouhiainen A., Kuja-Panula J., Tumova S., Rauvala H. RAGE-mediated cell signaling. Methods Mol. Biol. 2013;963:239–263. doi: 10.1007/978-1-62703-230-8_15. [DOI] [PubMed] [Google Scholar]
- 5.Rabbani N., Thornalley P.J. Protein glycation - biomarkers of metabolic dysfunction and early-stage decline in health in the era of precision medicine. Redox Biol. 2021;42 doi: 10.1016/j.redox.2021.101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldin A., Beckman J.A., Schmidt A.M., Creager M.A. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 7.Sell D.R., Monnier V.M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J. Biol. Chem. 1989;264(36):21597–21602. [PubMed] [Google Scholar]
- 8.Sell D.R., Monnier V.M. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J. Clin. Invest. 1990;85(2):380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyer D.G., Blackledge J.A., Thorpe S.R., Baynes J.W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J. Biol. Chem. 1991;266(18):11654–11660. [PubMed] [Google Scholar]
- 10.Sell D.R., Lane M.A., Johnson W.A., Masoro E.J., Mock O.B., Reiser K.M., Fogarty J.F., Cutler R.G., Ingram D.K., Roth G.S., Monnier V.M. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc. Natl. Acad. Sci. U.S.A. 1996;93(1):485–490. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith M.A., Taneda S., Richey P.L., Miyata S., Yan S.D., Stern D., Sayre L.M., Monnier V.M., Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc. Natl. Acad. Sci. U.S.A. 1994;91(12):5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odetti P., Fogarty J., Sell D.R., Monnier V.M. Chromatographic quantitation of plasma and erythrocyte pentosidine in diabetic and uremic subjects. Diabetes. 1992;41(2):153–159. doi: 10.2337/diab.41.2.153. [DOI] [PubMed] [Google Scholar]
- 13.Lyons T.J., Bailie K.E., Dyer D.G., Dunn J.A., Baynes J.W. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J. Clin. Invest. 1991;87(6):1910–1915. doi: 10.1172/JCI115216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beisswenger P.J., Moore L.L., Brinck-Johnsen T., Curphey T.J. Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. J. Clin. Invest. 1993;92(1):212–217. doi: 10.1172/JCI116552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyer D.G., Dunn J.A., Thorpe S.R., Bailie K.E., Lyons T.J., McCance D.R., Baynes J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Invest. 1993;91(6):2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaraj R.H., Sell D.R., Prabhakaram M., Ortwerth B.J., Monnier V.M. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. U.S.A. 1991;88(22):10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hein G., Wiegand R., Lehmann G., Stein G., Franke S. Advanced glycation end-products pentosidine and N epsilon-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology (Oxford) 2003;42(10):1242–1246. doi: 10.1093/rheumatology/keg324. [DOI] [PubMed] [Google Scholar]
- 18.Arai M., Yuzawa H., Nohara I., Ohnishi T., Obata N., Iwayama Y., Haga S., Toyota T., Ujike H., Arai M., Ichikawa T., Nishida A., Tanaka Y., Furukawa A., Aikawa Y., Kuroda O., Niizato K., Izawa R., Nakamura K., Mori N., Matsuzawa D., Hashimoto K., Iyo M., Sora I., Matsushita M., Okazaki Y., Yoshikawa T., Miyata T., Itokawa M. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch. Gen. Psychiatr. 2010;67(6):589–597. doi: 10.1001/archgenpsychiatry.2010.62. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita M., Arai M., Yuzawa H., Niizato K., Oshima K., Kushima I., Hashimoto R., Fukumoto M., Koike S., Toyota T., Ujike H., Arinami T., Kasai K., Takeda M., Ozaki N., Okazaki Y., Yoshikawa T., Amano N., Miyata T., Itokawa M. Replication of enhanced carbonyl stress in a subpopulation of schizophrenia. Psychiatr. Clin. Neurosci. 2014;68(1):83–84. doi: 10.1111/pcn.12081. [DOI] [PubMed] [Google Scholar]
- 20.Katsuta N., Ohnuma T., Maeshima H., Takebayashi Y., Higa M., Takeda M., Nakamura T., Nishimon S., Sannohe T., Hotta Y., Hanzawa R., Higashiyama R., Shibata N., Arai H. Significance of measurements of peripheral carbonyl stress markers in a cross-sectional and longitudinal study in patients with acute-stage schizophrenia. Schizophr. Bull. 2014;40(6):1366–1373. doi: 10.1093/schbul/sbt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnuma T., Nishimon S., Takeda M., Sannohe T., Katsuta N., Arai H. Carbonyl stress and microinflammation-related molecules as potential biomarkers in schizophrenia. Front. Psychiatr. 2018;9:82. doi: 10.3389/fpsyt.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sannohe T., Ohnuma T., Takeuchi M., Tani E., Miki Y., Takeda M., Katsuta N., Takebayashi Y., Nakamura T., Nishimon S., Kimoto A., Higashiyama R., Shibata N., Gohda T., Suzuki Y., Yamagishi S.I., Tomino Y., Arai H. High doses of antipsychotic polypharmacy are related to an increase in serum levels of pentosidine in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;76:42–48. doi: 10.1016/j.pnpbp.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Miyashita M., Arai M., Kobori A., Ichikawa T., Toriumi K., Niizato K., Oshima K., Okazaki Y., Yoshikawa T., Amano N., Miyata T., Itokawa M. Clinical features of schizophrenia with enhanced carbonyl stress. Schizophr. Bull. 2014;40(5):1040–1046. doi: 10.1093/schbul/sbt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane J., Honigfeld G., Singer J., Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatr. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 25.Kobori A., Miyashita M., Miyano Y., Suzuki K., Toriumi K., Niizato K., Oshima K., Imai A., Nagase Y., Yoshikawa A., Horiuchi Y., Yamasaki S., Nishida A., Usami S., Takizawa S., Itokawa M., Arai H., Arai M. Advanced glycation end products and cognitive impairment in schizophrenia. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyer D.G., Blackledge J.A., Thorpe S.R., Baynes J.W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J. Biol. Chem. 1991;266(18):11654–11660. [PubMed] [Google Scholar]
- 27.Grandhee S.K., Monnier V.M. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J. Biol. Chem. 1991;266(18):11649–11653. [PubMed] [Google Scholar]
- 28.Tsukahara H., Sekine K., Uchiyama M., Kawakami H., Hata I., Todoroki Y., Hiraoka M., Kaji M., Yorifuji T., Momoi T., Yoshihara K., Beppu M., Mayumi M. Formation of advanced glycosylation end products and oxidative stress in young patients with type 1 diabetes. Pediatr. Res. 2003;54(3):419–424. doi: 10.1203/01.PDR.0000076662.72100.74. [DOI] [PubMed] [Google Scholar]
- 29.Yoshihara K., Nakamura K., Kanai M., Nagayama Y., Takahashi S., Saito N., Nagata M. Determination of urinary and serum pentosidine and its application to elder patients. Biol. Pharm. Bull. 1998;21(10):1005–1008. doi: 10.1248/bpb.21.1005. [DOI] [PubMed] [Google Scholar]
- 30.Ban I., Sugawa H., Nagai R. Protein modification with ribose generates N(delta)-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine. Int. J. Mol. Sci. 2022;23(3):1224. doi: 10.3390/ijms23031224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iino K., Toriumi K., Agarie R., Miyashita M., Suzuki K., Horiuchi Y., Niizato K., Oshima K., Imai A., Nagase Y., Kushima I., Koike S., Ikegame T., Jinde S., Nagata E., Washizuka S., Miyata T., Takizawa S., Hashimoto R., Kasai K., Ozaki N., Itokawa M., Arai M. AKR1A1 variant associated with schizophrenia causes Exon skipping, leading to loss of enzymatic activity. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.762999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penning T.M. The aldo-keto reductases (AKRs): overview. Chem. Biol. Interact. 2015;234:236–246. doi: 10.1016/j.cbi.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wishart D.S., Guo A., Oler E., Wang F., Anjum A., Peters H., Dizon R., Sayeeda Z., Tian S., Lee B.L., Berjanskii M., Mah R., Yamamoto M., Jovel J., Torres-Calzada C., Hiebert-Giesbrecht M., Lui V.W., Varshavi D., Varshavi D., Allen D., Arndt D., Khetarpal N., Sivakumaran A., Harford K., Sanford S., Yee K., Cao X., Budinski Z., Liigand J., Zhang L., Zheng J., Mandal R., Karu N., Dambrova M., Schioth H.B., Greiner R., Gautam V. HMDB 5.0: the human metabolome database for 2022. Nucleic Acids Res. 2022;50(D1):D622–D631. doi: 10.1093/nar/gkab1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiehn O., Garvey W.T., Newman J.W., Lok K.H., Hoppel C.L., Adams S.H. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suhre K., Meisinger C., Doring A., Altmaier E., Belcredi P., Gieger C., Chang D., Milburn M.V., Gall W.E., Weinberger K.M., Mewes H.W., Hrabe de Angelis M., Wichmann H.E., Kronenberg F., Adamski J., Illig T. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S., Sadanala K.C., Kim E.K. A metabolomic approach to understanding the metabolic link between obesity and diabetes. Mol. Cells. 2015;38(7):587–596. doi: 10.14348/molcells.2015.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saltzman A., Caraway W.T., Beck I.A. Serum glucuronic acid levels in diabetes mellitus. Metabolism. 1954;3(1):11–15. [PubMed] [Google Scholar]
- 38.Roshanravan B., Zelnick L.R., Djucovic D., Gu H., Alvarez J.A., Ziegler T.R., Gamboa J.L., Utzschneider K., Kestenbaum B., Himmelfarb J., Kahn S.E., Raftery D., de Boer I.H. Chronic kidney disease attenuates the plasma metabolome response to insulin. JCI Insight. 2018;3(16) doi: 10.1172/jci.insight.122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saltzman A., Caraway W.T. Cinnamic acid as a test substance in the evaluation of liver function. J. Clin. Invest. 1953;32(8):711–719. doi: 10.1172/JCI102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho A., Sinick J., Esko T., Fischer K., Menni C., Zierer J., Matey-Hernandez M., Fortney K., Morgen E.K. Circulating glucuronic acid predicts healthspan and longevity in humans and mice. Aging (Albany N. Y.) 2019;11(18):7694–7706. doi: 10.18632/aging.102281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis S.S., Hutchinson M.R., Zhang Y., Hund D.K., Maier S.F., Rice K.C., Watkins L.R. Glucuronic acid and the ethanol metabolite ethyl-glucuronide cause toll-like receptor 4 activation and enhanced pain. Brain Behav. Immun. 2013;30:24–32. doi: 10.1016/j.bbi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jurk D., Wilson C., Passos J.F., Oakley F., Correia-Melo C., Greaves L., Saretzki G., Fox C., Lawless C., Anderson R., Hewitt G., Pender S.L., Fullard N., Nelson G., Mann J., van de Sluis B., Mann D.A., von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalifah R.G., Todd P., Booth A.A., Yang S.X., Mott J.D., Hudson B.G. Kinetics of nonenzymatic glycation of ribonuclease A leading to advanced glycation end products. Paradoxical inhibition by ribose leads to facile isolation of protein intermediate for rapid post-Amadori studies. Biochemistry. 1996;35(15):4645–4654. doi: 10.1021/bi9525942. [DOI] [PubMed] [Google Scholar]
- 44.Holte K.B., Svanteson M., Hanssen K.F., Sveen K.A., Seljeflot I., Solheim S., Sell D.R., Monnier V.M., Berg T.J. Collagen methionine sulfoxide and glucuronidine/LW-1 are markers of coronary artery disease in long-term survivors with type 1 diabetes. The Dialong study. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sell D.R., Nemet I., Liang Z., Monnier V.M. Evidence of glucuronidation of the glycation product LW-1: tentative structure and implications for the long-term complications of diabetes. Glycoconj. J. 2018;35(2):177–190. doi: 10.1007/s10719-017-9810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sell D.R., Nemet I., Monnier V.M. Partial characterization of the molecular nature of collagen-linked fluorescence: role of diabetes and end-stage renal disease. Arch. Biochem. Biophys. 2010;493(2):192–206. doi: 10.1016/j.abb.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sell D.R., Sun W., Gao X., Strauch C., Lachin J.M., Cleary P.A., Genuth S., Group D.E.R., Monnier V.M. Skin collagen fluorophore LW-1 versus skin fluorescence as markers for the long-term progression of subclinical macrovascular disease in type 1 diabetes, Cardiovasc. Diabetologe. 2016;15(1):30. doi: 10.1186/s12933-016-0343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horvat S., Roscic M. Glycosylation of lysine-containing pentapeptides by glucuronic acid: new insights into the Maillard reaction. Carbohydr. Res. 2010;345(3):377–384. doi: 10.1016/j.carres.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 49.Argirov O.K., Lin B., Ortwerth B.J. 2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) is a newly identified advanced glycation end product in cataractous and aged human lenses. J. Biol. Chem. 2004;279(8):6487–6495. doi: 10.1074/jbc.M309090200. [DOI] [PubMed] [Google Scholar]
- 50.Argirov O.K., Lin B., Ortwerth B.J. Phototransformations of advanced glycation end products in the human eye lens due to ultraviolet A light irradiation. Ann. N. Y. Acad. Sci. 2005;1043:166–173. doi: 10.1196/annals.1333.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharyya J., Shipova E.V., Santhoshkumar P., Sharma K.K., Ortwerth B.J. Effect of a single AGE modification on the structure and chaperone activity of human alphaB-crystallin. Biochemistry. 2007;46(50):14682–14692. doi: 10.1021/bi701326b. [DOI] [PubMed] [Google Scholar]
- 52.Miwa I., Chen A.S., Taguchi T. Glyceraldehyde is present in rat lens and its level is increased in diabetes mellitus. Ophthalmic Res. 2009;41(2):98–101. doi: 10.1159/000187626. [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto S., Murakami Y., Miyake H., Hayase F., Watanabe H. Identification of a novel advanced glycation end product derived from lactaldehyde. Biosci. Biotechnol. Biochem. 2019;83(6):1136–1145. doi: 10.1080/09168451.2019.1585745. [DOI] [PubMed] [Google Scholar]
- 54.Nishikimi M., Kawai T., Yagi K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J. Biol. Chem. 1992;267(30):21967–21972. [PubMed] [Google Scholar]
- 55.Burns J.J. Missing step in man, monkey and Guinea pig required for the biosynthesis of L-ascorbic acid. Nature. 1957;180(4585):553. doi: 10.1038/180553a0. [DOI] [PubMed] [Google Scholar]
- 56.Comer A.L., Carrier M., Tremblay M.E., Cruz-Martin A. The inflamed brain in schizophrenia: the convergence of genetic and environmental risk factors that lead to uncontrolled neuroinflammation. Front. Cell. Neurosci. 2020;14:274. doi: 10.3389/fncel.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldsmith D.R., Rapaport M.H. Inflammation and negative symptoms of schizophrenia: implications for reward processing and motivational deficits. Front. Psychiatr. 2020;11:46. doi: 10.3389/fpsyt.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi M., Miyata S., Fujii J., Inai Y., Ueyama S., Araki M., Soga T., Fujinawa R., Nishitani C., Ariki S., Shimizu T., Abe T., Ihara Y., Nishikimi M., Kozutsumi Y., Taniguchi N., Kuroki Y. In vivo role of aldehyde reductase. Biochim. Biophys. Acta. 2012;1820(11):1787–1796. doi: 10.1016/j.bbagen.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Barski O.A., Papusha V.Z., Ivanova M.M., Rudman D.M., Finegold M.J. Developmental expression and function of aldehyde reductase in proximal tubules of the kidney. Am. J. Physiol. Ren. Physiol. 2005;289(1):F200–F207. doi: 10.1152/ajprenal.00411.2004. [DOI] [PubMed] [Google Scholar]
- 60.Carvalho A.F., Solmi M., Sanches M., Machado M.O., Stubbs B., Ajnakina O., Sherman C., Sun Y.R., Liu C.S., Brunoni A.R., Pigato G., Fernandes B.S., Bortolato B., Husain M.I., Dragioti E., Firth J., Cosco T.D., Maes M., Berk M., Lanctot K.L., Vieta E., Pizzagalli D.A., Smith L., Fusar-Poli P., Kurdyak P.A., Fornaro M., Rehm J., Herrmann N. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl. Psychiatry. 2020;10(1):152. doi: 10.1038/s41398-020-0835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Percudani R., Peracchi A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinf. 2009;10:273. doi: 10.1186/1471-2105-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morjana N.A., Lyons C., Flynn T.G. Aldose reductase from human psoas muscle. Affinity labeling of an active site lysine by pyridoxal 5'-phosphate and pyridoxal 5'-diphospho-5'-adenosine. J. Biol. Chem. 1989;264(5):2912–2919. [PubMed] [Google Scholar]
- 63.Bohren K.M., Page J.L., Shankar R., Henry S.P., Gabbay K.H. Expression of human aldose and aldehyde reductases. Site-directed mutagenesis of a critical lysine 262. J. Biol. Chem. 1991;266(35):24031–24037. [PubMed] [Google Scholar]
- 64.Kalousova M., Novotny L., Zima T., Braun M., Vitek L. Decreased levels of advanced glycation end-products in patients with Gilbert syndrome. Cell. Mol. Biol. (Noisy-le-grand) 2005;51(4):387–392. [PubMed] [Google Scholar]
- 65.Atal C.K., Dubey R.K., Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Therapeut. 1985;232(1):258–262. [PubMed] [Google Scholar]
- 66.Singh J., Dubey R.K., Atal C.K. Piperine-mediated inhibition of glucuronidation activity in isolated epithelial cells of the Guinea-pig small intestine: evidence that piperine lowers the endogeneous UDP-glucuronic acid content. J. Pharmacol. Exp. Therapeut. 1986;236(2):488–493. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metabolome data generated or analyzed during this study are included in this published article and its supplementary information files.