Abstract
Purpose:
To evaluate DS-6157a, an antibody–drug conjugate targeting G protein–coupled receptor 20 (GPR20), in gastrointestinal stromal tumors (GIST).
Patients and Methods:
In this phase I multicenter, open-label, multiple-dose study, patients with previously treated advanced GIST received intravenous DS-6157a on Day 1 of 21-day cycles, with a starting dose of 1.6 mg/kg. The primary objective evaluated the safety and tolerability of DS-6157a, while determining dose-limiting toxicity (DLT) and the MTD. Secondary objectives included plasma pharmacokinetics parameters, plasma antidrug antibodies (ADA), and efficacy.
Results:
A total of 34 patients enrolled. DS-6157a was well tolerated, with DLTs in 4 patients (11.8%) at doses of 6.4 mg/kg, 9.6 mg/kg, and 12.8 mg/kg; the MTD was determined to be 6.4 mg/kg. Treatment-emergent adverse events (TEAE) grade ≥3 occurred in 17 patients (50.0%), including decreased platelet count (23.5%), anemia (20.6%), decreased neutrophil count (14.7%), and decreased white blood cell count (11.8%). Four patients (11.8%) experienced serious adverse events related to DS-6157a. Six patients died with 5 due to disease progression and 1 due to DS-6157a-related TEAE. Tumor shrinkage was observed in 7 patients (20.6%), and 1 patient (2.9%) achieved a partial response. Plasma concentrations and exposure of intact DS-6157a, DXd, and total anti-GPR20 antibody all demonstrated a dose-dependent profile. No treatment-emergent ADAs were observed.
Conclusions:
Targeting GPR20 with DS-6157a was tolerated in patients with advanced GIST with tumor shrinkage demonstrated in KIT/PDGFRA wild-type GIST. However, the study did not proceed further due to lower efficacy outcomes than anticipated.
Translational Relevance.
Gastrointestinal stromal tumors (GIST) are largely characterized by mutations in KIT and/or PDGFRA. The orphan receptor G protein–coupled receptor 20 (GPR20) is highly expressed in GIST, regardless of genotype, and presents a novel therapeutic target in patients with limited treatment options. DS-6157a is the first antibody–drug conjugate (ADC) targeting GPR20 and comprises a humanized anti-GPR20 mAb that is covalently conjugated to an enzymatically cleavable linker and a novel exatecan derivative (DXd) payload. DS-6157a was well tolerated, with an adverse event profile comparable to other DXd-ADC therapies. Tumor shrinkage was observed in 7 of 34 patients enrolled, including 1 patient with a partial response to treatment, who had succinate dehydrogenase–deficient GIST with NF1 mutation (wild-type KIT/PDGFRA). The relationship between response to GPR20-targeted therapy and specific molecular subtypes of GIST warrants further investigation.
Introduction
Gastrointestinal stromal tumors (GIST) are the most common type of sarcoma, with a reported incidence of 0.7 per 100,000 people per year in the United States (1, 2). Notably, the incidence of GIST has been increasing since 2000 (3). GISTs are most often located in the stomach (∼60%) and small intestine (∼30%) but may metastasize, largely to other intra-abdominal locations (3–5). The majority of GISTs in adults are associated with mutations in KIT (∼80%) or PDGFRA (5%–7%; refs. 6–8), resulting in ligand-independent activation of their downstream signaling pathways (9). However, in a small percentage of adults (∼7%–10%), GIST is wild-type for both KIT and PDGFRA and such cancers are characterized by multiple different rarer pathologic mechanisms, including mutations in SDHx (resulting in SDH deficiency), NF1, BRAF, FGFR, and RAS genes; additionally, gene fusions such as ETV6-NTRK3 fusion have rarely been identified in GIST (6–11).
The orphan G protein–coupled receptor 20 (GPR20) is a 358 amino acid 7-pass transmembrane protein whose ligand has not been identified. When exogenously expressed in HEK293 cells, GPR20 constitutively activates its coupled G proteins without ligand stimulation (12). GPR20 has been detected in multiple brain regions, including the nuclei, thalamus, and putamen (12), and represents a novel therapeutic target in GIST, as it is selectively and abundantly expressed across different mutations (13–15). Studies in mice have demonstrated that GPR20 is expressed in subsets of the interstitial cells of Cajal, the pacemaker cells for peristaltic contractions of the gut and the GIST cell-of-origin (16, 17). The expression of GPR20 is regulated by FOXF1 and ETV1, which are transcription factors that play a central role in KIT-driven GIST initiation, proliferation, and survival (16, 18).
Antibody–drug conjugates (ADC) represent a promising treatment strategy that uses the specificity of a mAb to selectively deliver potent cytotoxic drugs to target antigen-expressing tumor cells (19, 20). The ADC binds to the cell-surface antigen, and then the complex undergoes internalization and translocation into the lysosomal compartment of the cell. The ADC is then cleaved by the lysosomal enzymes which releases the cytotoxic drug, causing apoptosis of the tumor cells (20).
DS-6157a is the first-in-class ADC to target GPR20 and comprises a humanized anti-GPR20 mAb that is covalently conjugated to an enzymatically cleavable linker, and a novel exatecan derivative (DXd) payload at a drug-to-antibody ratio (DAR) of ∼8. The ADC binding to GPR20 followed by intracellular release of the drug inhibits DNA topoisomerase I, resulting in apoptosis of target cells (15), and the cleaved payload can also diffuse out of the target cells expressing GPR20 and kill neighboring tumor cells with lower or absent GPR20 expression as a by-stander effect. Currently, the tyrosine kinase inhibitor (TKI) imatinib is the recommended first-line treatment of GIST with KIT mutations, either as (neo)adjuvant therapy before/after resection of the primary tumor or in advanced disease (21–24). Other TKIs [sunitinib (25), regorafenib (26), ripretinib (27), avapritinib (28)] are recommended as second- or later-line treatment options (21). DS-6157a was developed with the aim of providing a novel treatment option to patients whose GIST is resistant to TKIs. Here, we report results of the dose escalation phase of the first-in-human phase I study of DS-6157a in patients with advanced GIST.
Patients and Methods
Study design
This was a phase I multicenter, open-label, multiple-dose study (ClinicalTrials.gov identifier, NCT04276415) in patients with GIST conducted at 5 sites in the United States and Japan. The study plan included two phases—a dose escalation phase, and a dose expansion phase.
DS-6157a (Daiichi Sankyo, Tokyo, Japan; supplied as a lyophilized powder) was to be administered intravenously on Day 1 of each 21-day cycle. The dose escalation stage was to begin with a starting dose of 1.6 mg/kg DS-6157a infused over an approximately 90-minute period on the first day of the first treatment cycle, followed by a 21-day observation period, during which all relevant safety data were to be reviewed. Patients were to continue to receive DS-6157a every 3 weeks until unacceptable toxicity, disease progression, initiation of new anticancer treatment, death, or withdrawal of consent. If no infusion-related reaction (IRR) was observed following administration on Day 1 of Cycle 1, subsequent infusion duration could be reduced to approximately 30 minutes based on the clinical judgment of the investigator, but this reduced infusion rate was not mandatory.
Dose escalation was to be guided by a Bayesian logistic regression model (BLRM), following the escalation with overdose control (EWOC) principle (29–31). The next dose level was to be chosen by investigators based on the dose recommendation of the BLRM, a clinical assessment of the overall safety data, together with any pharmacokinetics (PK), pharmacodynamics, and efficacy data available. The dose level could not be increased by more than 100% even if the BLRM suggested an increase to a dose level greater than 100%. The final number of dose levels evaluated in the study was to be dependent on determination of the MTD of DS-6157a based on dose-limiting toxicities (DLT) reported during the DLT evaluation period (Days 1–21 of Cycle 1 in all dose escalation cohorts). Planned DS-6157a dose levels were 1.6 mg/kg, 3.2 mg/kg, 4.8 mg/kg, 6.4 mg/kg, and 9.6 mg/kg. The human starting dose of 1.6 mg/kg was chosen on the basis of preclinical toxicology and nonclinical pharmacology data per International Council for Harmonisation (ICH)-S9 guideline to ensure patient safety while affording a minimum level of efficacy (15).
The dose expansion phase was to enroll and treat patients at the recommended dose for expansion (RDE) in 2 cohorts: Cohort 1 with patients who received ≥1 post-imatinib treatment, and Cohort 2 with patients who did not receive a post-imatinib treatment. However, due to lower than anticipated efficacy in the dose escalation phase, the study did not proceed to dose expansion.
Patients
Patients were eligible to participate in the dose escalation phase if they were aged ≥20 years at the Japanese study sites, and aged ≥18 years at the U.S. sites; had an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1; left ventricular ejection fraction (LVEF) ≥50%; and adequate renal, hepatic, and hematologic functions. They were also required to have histopathologically documented unresectable and/or metastatic GIST following treatment with standard of care, including imatinib, and ≥ 1 measurable lesion per RECIST v1.1. An adequate washout period for previous treatments (major surgery, radiotherapy, systemic anticancer therapies) prior to study treatment was also necessary. Evidence of GPR20 expression was not required for enrollment into Part 1 of the study. However, patients were required to provide consent for collection of fresh tumor biopsy samples before and on DS-6157a treatment for biomarker testing.
Patients were excluded from the study if they had undergone allogeneic bone marrow or solid organ transplant within the 3 months prior to the study, were receiving concomitant treatment with medications known to increase the risk of Torsade de Pointes, or treatments supporting hematologic function. Patients were also ineligible to participate in the study if they had spinal cord compression, clinically active central nervous system metastases, a history of cardiac disease, corrected QT interval of >470 ms, interstitial lung disease (ILD)/pneumonitis, impaired respiratory function, clinically significant corneal disease, active human immunodeficiency virus, hepatitis B, hepatitis C, or severe or uncontrolled systemic diseases. Patients were excluded from the study if they had unresolved toxicities from previous anticancer therapies or prior or concurrent malignancy whose natural history or treatment had a potential to interfere with the efficacy or safety of study treatment.
The study was conducted in accordance with Good Clinical Practice and local regulatory requirements, and the protocol was reviewed and approved by the relevant institutional review boards. All patients provided written informed consent.
Study endpoints
The primary objective of the dose escalation phase was to evaluate the safety and tolerability of DS-6157a, with determination of the MTD and/or the RDE. Secondary objectives included plasma PK parameters of intact DS-6157a, total anti-GPR20 antibody and cytotoxic payload (DXd), incidence of plasma antidrug antibodies (ADA) against DS-6157a, and efficacy. In the dose expansion phase the primary objective was to assess the safety, tolerability, and efficacy of DS-6157a at the RDE.
Safety parameters included treatment-emergent adverse events (TEAE), serious adverse events (SAE), adverse events of special interest (AESI; ILD/pneumonitis and IRRs), ophthalmologic findings, electrocardiography, LVEF (measured by echocardiogram or multi-gated acquisition scan), physical examination, vital signs, and laboratory parameters. Potential cases of ILD or pneumonitis were to be evaluated by an independent adjudication committee. TEAEs were summarized according to the Medical Dictionary for Regulatory Activities v24.1 and severity graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0. All patients were followed for ≥30 days after the last dose of study treatment during the off-treatment period until all treatment-related toxicities resolved or before the start of another anticancer treatment. Those patients alive and without disease progression at the end of this period continued to be followed-up until disease progression, start of new anticancer treatment, death, lost to follow-up, withdrawal of consent, or investigator discretion.
DLTs were defined as grade 3 to 4 hematologic toxicities, grade 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) increase, and other AST/ALT abnormalities. Decline in LVEF/symptomatic congestive heart failure, grade ≥2 ILD/pneumonitis, grade 3 skin toxicity lasting >7 days or grade 4 for any duration, and grade ≥3 IRR. Full details of DLTs are presented in the Supplementary Appendix.
Blood samples for PK analysis were obtained predose on Day 1 of Cycle 1 after the end of infusion (EOI); 2, 4, and 7 hours after EOI; and 24 hours after the start of Day 1 infusion, with additional sampling on Days 4, 8, and 15. Similar sampling schedule was performed for Cycle 3. Analysis was conducted using validated bioanalytical assays. Plasma PK parameters of intact DS-6157a, total anti-GPR20 antibody, and DXd were estimated using standard noncompartmental analysis in Phoenix WinNonlin (v8.3.4; Certara USA, Inc., Princeton, NJ) and included maximum concentration (Cmax), time to maximum concentration (Tmax), trough concentration (Ctrough), terminal half-life (t1/2), and area under the concentration-time curve in the dosing interval (AUCtau) after the Cycle 1 and Cycle 3 doses. Blood samples for the determination of ADAs were obtained predose on Day 1 of Cycle 1 and again at Day 8 of Cycle 1, with further sampling at Day 1 of Cycles 2 to 4 and every 2 cycles thereafter through the end of study visit and a 30-day safety follow-up visit. ADAs was measured using a validated immunoassay. Immunogenicity was assessed as the number and percentage of patients with detectable treatment-emergent ADAs.
Tumor samples were to be obtained by core needle biopsy for retrospective measurement of GPR20 expression prior to the first dose of DS-6157a in the study, with further core biopsies obtained between Days 8 and 15 during Cycle 2 in clinically stable patients as judged by the investigator. GPR20 protein level in tumor biopsy samples was retrospectively examined by IHC at Roche Tissue Diagnostics (Tucson, AZ). GPR20 IHC assay was performed using the anti-GPR20 mAb clone 04–093 OcH1L1 provided by Daiichi Sankyo Co., Ltd. with RTD OptiView DAB detection kit on the RTD's VENTANA Benchmark ULTRA staining platform. The H-score was calculated according to the following formula: H-score = [(0 × %negative cells) + (1 × %weakly positive cells) + (2 × %moderately positive cells) + (3 × %strongly positive cells)]. DXd-ADC IHC (the detection of antibody binding to the DXd epitope in the intact ADC) was also evaluated.
Tumor responses were to be assessed according to RECIST 1.1 criteria by investigator and using computed tomography or magnetic resonance imaging of the chest and abdominopelvic cavity, conducted at 6-week intervals during the first 36 weeks and every 9 weeks thereafter. Outcome measures included overall response rate [ORR; proportion of patients with partial response (PR) or complete response (CR)], progression-free survival (PFS; time from start of study treatment to first documented radiologic progression or death from any cause), and best percent change in target lesion.
Statistical methodology
For the dose escalation phase, the MTD/RDE were to be determined following a recommendation from BLRM with EWOC criteria and a clinical assessment of the overall safety findings, together with any PK, pharmacodynamics, and efficacy results. The recommendation of BLRM was based on the accumulated DLT data from Cycle 1 in the DLT-evaluable set and the prior distribution. The DLT-evaluable set included patients who either experienced DLT in Cycle 1 or completed Cycle 1 without DLT.
Patients were enrolled in a cohort of 3 after each dose-titration decision. The safety analysis set comprised all patients who had any exposure to DS-6157a, while the DLT evaluation set included all patients enrolled in the dose escalation phase of the study who had a DLT or who received a full dose of DS-6157a in Cycle 1 and completed the DLT evaluation period. The PK analysis set included patients from the safety analysis set who had ≥1 blood sample with a measurable concentration of intact DS-6157a, total anti-GPR20 antibody, and DXd.
Data were summarized according to dose level. For continuous variables, descriptive statistics included mean, standard deviation, median, and range, while categorical data were summarized using frequency and percentages. The Kaplan–Meier method was used to obtain median PFS and PFS probabilities at various timepoints. No statistical hypothesis testing was planned.
Data availability
Anonymized individual participant data on completed studies and applicable supporting clinical study documents will be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/.
Results
Patient baseline demographics and clinical characteristics
A total of 34 patients were included in the dose escalation phase with a median age of 61 years (range, 29–81); 19 (56%) were male, and almost all were White or Asian (Table 1). Representativeness of study participants are presented in Supplementary Table S1. Nearly all patients [n = 32 (94.1%)] had previously received systemic therapy for metastatic GIST except for 2 patients who had no prior systemic therapy [median of 5 prior systemic anticancer regimens (range, 0–12)], with the primary tumor site mostly in the stomach or small intestine, and the median GPR20 H-score was 168 (range, 12–273). At the time of data cutoff (August 17, 2022), all patients had discontinued from the study. The primary reason for discontinuation was progressive disease [18 patients (52.9)], followed by physician decision [6 patients (17.6%); Supplementary Table S2].
Table 1.
Patient demographics and baseline disease characteristics.
| Patients (n = 34) | |
|---|---|
| Age, median (range), years | 60.5 (29–81) |
| Male, n (%) | 19 (55.9) |
| Country of enrollment, n (%) | |
| United States | 18 (52.9) |
| Japan | 16 (47.1) |
| Race, n (%) | |
| White | 16 (47.1) |
| Asian | 16 (47.1) |
| Black/African American | 1 (2.9) |
| Other | 1 (2.9) |
| ECOG performance status, n (%) | |
| 0 | 18 (52.9) |
| 1 | 16 (47.1) |
| Primary tumor site, n (%) | |
| Stomach | 13 (38.2) |
| Jejunum | 5 (14.7) |
| Ileum | 3 (8.8) |
| Duodenum | 2 (5.9) |
| Rectum | 2 (5.9) |
| Other | 9 (26.5) |
| Metastatic site at study entry, n (%) | |
| Liver | 23 (67.6) |
| Peritoneum | 23 (67.6) |
| Lung | 7 (20.6) |
| Bone | 3 (8.8) |
| Other | 14 (41.2) |
| Molecular subtype per most recent genetic test results, n (%) | |
| KIT | 20 (58.8) |
| Exon 9 | 4 (11.8) |
| Exon 11 | 16 (47.1) |
| Exon 13 | 4 (11.8) |
| Exon 17 | 6 (17.6) |
| PDGFRA, D842V | 1 (2.9) |
| SDH | 3 (8.8) |
| SDHA | 1 (2.9) |
| SDHB | 2 (5.9) |
| NF1 | 2 (5.9) |
| Unkown | 10 (29.4) |
| Number of prior systemic anticancer regimens, median | 5.0 |
| GPR20 H-score at baseline, median (range) | 168 (12–273) |
| GPR20 H-score values at baseline, n (%) | |
| 0–100 | 5 (14.7) |
| >100–200 | 14 (41.2) |
| >200–300 | 11 (32.4) |
| Not evaluable | 4 (11.8) |
Safety
All 34 patients received ≥1 dose of DS-6157a; 6 dose levels were administered, including 5 preplanned dose levels: 1.6 mg/kg (n = 4), 3.2 mg/kg (n = 4), 4.8 mg/kg (n = 5), 6.4 mg/kg (n = 13), 9.6 mg/kg (n = 6), as well as a dose escalation to 12.8 mg/kg (n = 2). The median number of infusions was 3.0 (range, 1–21), with a median cumulative dose of 19.3 mg/kg per patient (range, 2.93–133.38). The median relative dose intensity was 1.0 (range: 0.53–1.05).
TEAEs were experienced by all patients, with 32 patients (94.1%) experiencing TEAEs related to DS-6157a (Table 2). Grade ≥3 TEAEs, regardless of causality, were reported for 27 patients (79.4%), including 17 patients (50.0%) with DS-6157a related grade ≥3 TEAEs. The most commonly reported related grade ≥3 TEAEs were decreased platelet count (23.5%), anemia (20.6%), decreased neutrophil count (14.7%), and decreased white blood cell count (11.8). SAEs were experienced by 9 patients (26.5%), with the most common including abdominal pain, abnormal hepatic function, and decreased platelet count (2 patients each). Four patients (11.8%) experienced SAEs related to DS-6157a: grade 4 abnormal hepatic function, which progressed to grade 5 (1 patient); grade 4 intestinal perforation, grade 4 decreased neutrophil count, and grade 3 and grade 4 decreased platelet count (1 patient); grade 4 abnormal hepatic function, grade 4 decreased platelet count, and grade 3 renal disorder (1 patient); and grade 3 neutropenic fever and grade 3 pneumonia (1 patient). A total of 6 patients died during the study (2 were on treatment), 5 due to disease progression and 1 due to a DS-6157a-related TEAE (investigator assessed grade 4 hepatic dysfunction occurring on Day 8 in a patient at dose level 6.4 mg/kg that became fatal on Day 10). An external physician reviewed the report of the grade 4 abnormal hepatic function, which progressed to grade 5 and considered that the patient, who had significant liver disease at baseline, died due to septic shock secondary to biliary sepsis attributable to bactibilia resulting from the choledochojejunostomy performed in 2007 and more recent anastomotic obstruction.
Table 2.
Safety outcomes summary.
| n (%) | DS-6157a 1.6 mg/kg (n = 4) | DS-6157a 3.2 mg/kg (n = 4) | DS-6157a 4.8 mg/kg (n = 5) | DS-6157a 6.4 mg/kg (n = 13) | DS-6157a 9.6 mg/kg (n = 6) | DS-6157a 12.8 mg/kg (n = 2) | Total (n = 34) |
|---|---|---|---|---|---|---|---|
| Patients with any TEAE | 4 (100.0) | 4 (100.0) | 5 (100.0) | 13 (100.0) | 6 (100.0) | 2 (100.0) | 34 (100.0) |
| TEAEs associated with drug discontinuation | 0 | 0 | 0 | 3 (23.1) | 3 (50.0) | 0 | 6 (17.6) |
| TEAEs associated with dose interruption | 0 | 0 | 0 | 0 | 0 | 1 (50.0) | 1 (2.9) |
| TEAEs associated with dose reduction | 0 | 0 | 0 | 0 | 0 | 2 (100.0) | 2 (5.9) |
| TEAEs associated with death as outcome | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (2.9) |
| Treatment-related SAE | 0 | 0 | 0 | 1 (7.7) | 2 (33.3) | 1 (50.0) | 4 (11.8) |
| Adverse events of special interest | 0 | 0 | 0 | 4 (30.8) | 1 (16.7) | 0 | 5 (14.7) |
| ILD/pneumonitis | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (2.9) |
| IRRs | 0 | 0 | 0 | 3 (23.1) | 1 (16.7) | 0 | 4 (11.8) |
| DLTs (any grade by patient) | 0 | 0 | 0 | 1 (7.7) | 1 (16.7) | 2 (100.0) | 4 (11.8) |
| CTCAE maximum grade ≥3 treatment-related TEAEs | 0 | 1 (25.0) | 1 (20.0) | 8 (61.5) | 5 (83.3) | 2 (100.0) | 17 (50.0) |
| Anemia | 0 | 1 (25.0) | 1 (20.0) | 3 (23.1) | 1 (16.7) | 1 (50.0) | 7 (20.6) |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (50.0) | 2 (5.9) |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 1 (50.0) | 1 (2.9) |
| Intestinal perforation | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (2.9) |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 1 (50.0) | 1 (2.9) |
| Fatigue | 0 | 0 | 0 | 1 (7.7) | 0 | 1 (50.0) | 2 (5.9) |
| Peripheral edema | 0 | 0 | 0 | 0 | 0 | 1 (50.0) | 1 (2.9) |
| Hepatic function abnormal | 0 | 0 | 0 | 1 (7.7) | 1 (16.7) | 0 | 2 (5.9) |
| Pneumonia | 0 | 0 | 0 | 0 | 0 | 1 (50.0) | 1 (2.9) |
| IRR | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (2.9) |
| Decreased lymphocyte count | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (2.9) |
| Decreased neutrophil count | 0 | 0 | 0 | 2 (15.4) | 3 (50.0) | 0 | 5 (14.7) |
| Decreased platelet count | 0 | 0 | 0 | 3 (23.1) | 4 (66.7) | 1 (50.0) | 8 (23.5) |
| Decreased white blood cell count | 0 | 0 | 0 | 1 (7.7) | 3 (50.0) | 0 | 4 (11.8) |
| Muscular weakness | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (2.9) |
| Renal disorder | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (2.9) |
The most frequent TEAEs included nausea [28 patients (82.4%)], decreased appetite [21 patients (61.8%)], anemia [19 patients (55.9%)], fatigue [17 patients (50.0%)], and constipation [14 patients (41.2%); Supplementary Table S3]. All patients discontinued study treatment, and TEAEs were the primary reason for treatment discontinuation for 6 patients (17.6%), all of which were DS-6157a related. With respect to AESIs, 1 patient (60-year-old male in the 6.4 mg/kg dose level) was reported to have a grade 1 ILD that was later adjudicated as a non-ILD event by the ILD adjudication committee. Four patients (11.8%) experienced IRRs during the study, 3 of which occurred at the 6.4 mg/kg DS-6157a dose level (one grade 3 event and two grade 1 events), and 1 at the 9.6 mg/kg dose level (grade 1); all were related to study treatment.
Of 31 DLT-evaluable patients, DLTs occurred in 4 patients during the dose escalation phase at doses of 6.4 mg/kg, 9.6 mg/kg, and 12.8 mg/kg (Table 2). Of 11 DLT-evaluable patients at the 6.4 mg/kg dose level, 1 patient (9.1%) experienced non-serious DLTs of grade 3 anemia and grade 4 decreased platelet count and a serious DLT of abnormal hepatic function that was fatal. Of 6 DLT-evaluable patients at the 9.6 mg/kg dose level, 1 patient (16.7%) experienced non-serious DLTs of grade 3 anemia and grade 3 febrile neutropenia and serious DLTs of grade 4 hepatic function abnormality and grade 4 decreased platelet count. Two of 2 DLT-evaluable patients at the 12.8 mg/kg experienced DLTs: 1 experienced a serious DLT of grade 3 neutropenic fever and the other experienced non-serious DLTs of grade 2 nausea, grade 2 diarrhea, grade 2 vomiting, and grade 2 dehydration. The 12.8 mg/kg dose of DS-6157a was therefore not administered to any further patients. The DLT events at the 6.4 mg/kg and 9.4 mg/kg were observed when additional patients were enrolled at those dose levels to define RDE after identifying 12.8 mg/kg was not tolerable. Afterwards, the MTD was determined to be 6.4 mg/kg at cohort review meeting of the investigators and sponsor's clinical and safety team, as 9.4 mg/kg was also considered non-tolerable for long term use due to hematologic toxicities and nausea. No trends or clinically important changes in vital signs, ECOG performance status, or ECGs were observed.
Efficacy
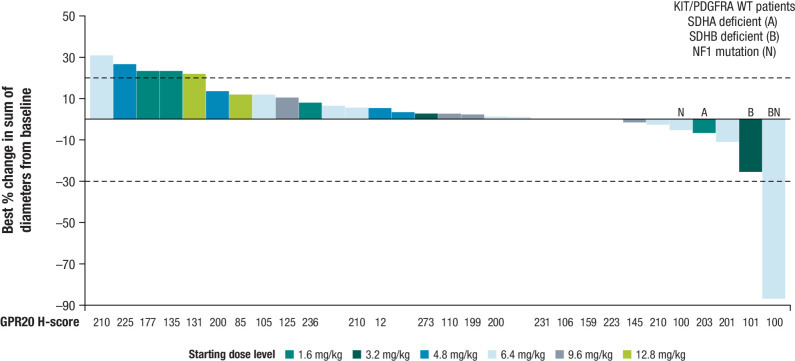
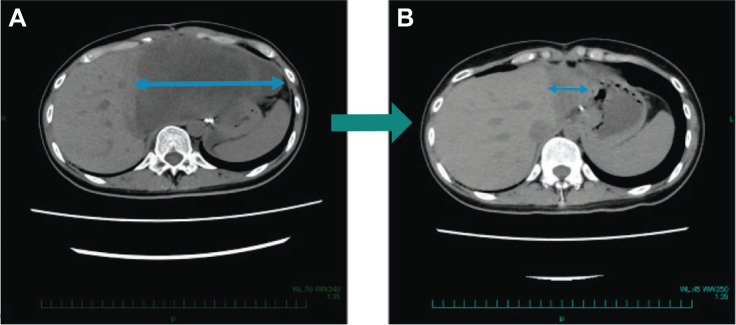
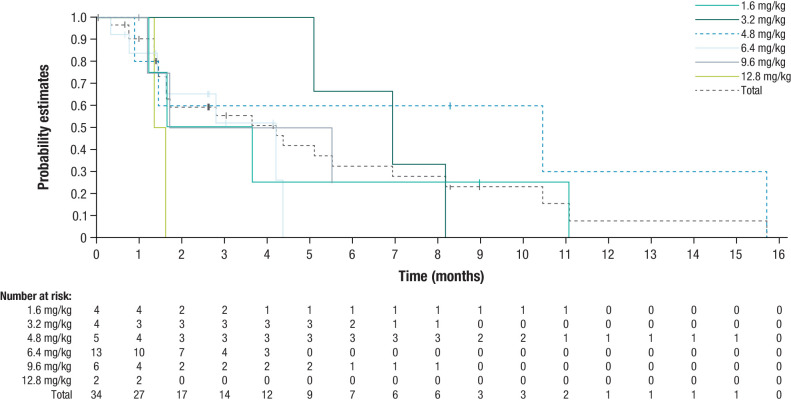
Tumor shrinkage was observed in 7 patients (20.6%), of which 4 patients (11.8%) had KIT/PDGFRA wild-type GIST (2 SDH deficient; 1 NF1 mutation; 1 SDH deficient and NF1 mutation; Fig. 1). One patient (2.9%) achieved a PR, 17 patients (50.0%) had stable disease, and 10 patients had a best overall response of progressive disease. No patients achieved a CR (Supplementary Table S4). The patient who achieved a PR after 2 cycles of treatment (a 29-year-old female in the 6.4 mg/kg dose level without any prior cancer systemic therapies) had SDH-deficient GIST diagnosed in 2020 with mutations to succinate dehydrogenase (SDHB) R90 (nonsense) and NF1 Y628FS*3 (Fig. 2). This patient then achieved a complete pathologic response per investigator assessment after surgical resection. Tumor response over time is presented in Supplementary Fig. S1. Median PFS was 4.2 months [95% confidence interval (CI), 1.6–6.9; Fig. 3]. On-treatment biopsies revealed that based on DXd IHC, DS-6157a was shown to reach the tumor target site (imaging presented in Supplementary Fig. S2).
Figure 1.
Waterfall plot of the best percent change in sum of diameters from baseline in target lesions. Tumor shrinkage was observed in 7 patients, of which 4 patients had KIT/PDGFRA wild-type GIST (2 SDH deficient; 1 NF1 mutation; 1 SDH deficient and NF1 mutation).
Figure 2.
SDH-deficient patient with pathologic PR. A 29-year-old female patient with SDH-deficient GIST diagnosed in 2020 without any prior cancer systemic therapies, demonstrated a maximum 87% decrease in tumor size, following treatment with DS-6157a, at the MTD confirmed dose of 6.4 mg/kg. The target lesion, an abdominal mass, was 150 mm at baseline (A) which decreased to 33 mm in Cycle 3 and decreased further to 20 mm 4 weeks later (B). The patient discontinued from study to undergo a surgical resection of the remaining small lesion to become tumor-free. The resected tumor, showed a pathologic CR per the investigator's assessment.
Figure 3.
Kaplan–Meier curve of PFS. Median PFS, or the time from start of study treatment to first documented radiologic progression or death from any cause, was 4.2 months (95% CI, 1.6–6.9).
PK
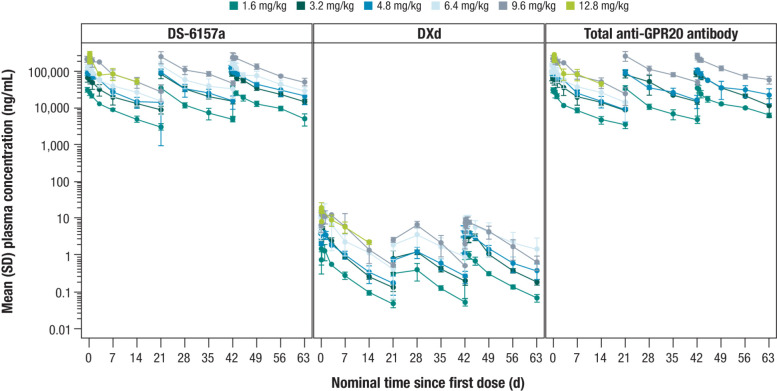
The PK analysis set included all 34 enrolled patients. Plasma concentrations and exposure (Cmax and AUCtau) of intact DS-6157a, DXd, and total anti-GPR20 antibody all demonstrated a dose-dependent profile (Fig. 4). Mean t1/2 of intact DS-6157a and total anti-GPR20 antibody was approximately 8 to 12 days for Cycle 1 (dose levels of 1.6 mg/kg–12.8 mg/kg) and 9 to 15 days in Cycle 3 (dose range of 1.6 mg/kg–9.6 mg/kg). With respect to DXd, mean t1/2 was approximately 4 to 7 days for Cycle 1 and 5 to 8 days for Cycle 3. Total anti-GPR20 antibody and intact DS-6157a exhibited a similar PK profile, indicating that DS-6157a is stable in circulation. Cmax, AUCtau, and Ctrough in Cycle 3 (across dose levels of 1.6 mg/kg–9.6 mg/kg) were much higher for intact DS-6157a than for DXd by 65- to 117-fold, 84- to 126-fold, and 191- to 297-fold, respectively.
Figure 4.
Mean plasma concentrations over time of intact DS-6157a, DXd, and total anti-GPR20 antibody. Plasma concentrations and exposure of intact DS-6157a (left), DXd (middle), and total anti-GPR20 antibody (right) all demonstrated a dose-dependent profile.
As Tmax is related to infusion and sampling time, it was shorter during Cycle 3 relative to Cycle 1, as investigators were permitted to decrease the infusion time from 90 minutes to 30 minutes in the absence of IRRs during Cycle 1. In general, Tmax was longer for DXd relative to intact DS-6157a and total anti-GPR20 antibody, which is likely due to the process of internalization of the ADC, cleavage of the linker, and release of payload process. Mean accumulation ratios (AUCtau, Cycle 3/AUCtau, Cycle 1) were between 1.1 and 1.7 across all dose levels for intact DS-6157a, total anti-GPR20 antibody, and DXd.
Immunogenicity
Across all 34 enrolled patients, only 1 patient had a positive ADA result at baseline. No treatment-emergent or treatment-induced ADAs were detected, including in the patient who had a positive result at baseline.
Discussion
In this open-label, phase I study evaluating DS-6157a in patients with advanced GIST, there were 2 planned phases, a dose escalation phase in patients in which the MTD and/or RDE of DS-6175a was to be determined, and a dose expansion phase to further evaluate safety, tolerability, and preliminary efficacy at RDE. Tumor shrinkage was observed in only 7 patients, of which 4 patients were with KIT/PDGFRA wild-type GIST treated at different doses. One patient with SDH-deficient GIST with both SDHB and NF1 mutations achieved a PR per CT scan, and then a complete pathologic response per investigator assessment after surgical resection. However, due to lack of sufficient number of responders during dose escalation, the dose expansion phase was not initiated.
DS-6157a was considered tolerable, with an MTD of 6.4 mg/kg. All 34 patients (100%) enrolled in the study experienced ≥1 TEAE and 4 patients experienced DLTs. The most common TEAEs included nausea, decreased appetite, anemia, fatigue, constipation, decreased platelet count, and vomiting, which have also been observed with other DXd-ADC therapies, including trastuzumab deruxtecan and patritumab deruxtecan (32–34). There were no reports of adjudicated drug-related ILD in this study. The frequency of IRRs was comparable with the other DXd ADC agents (32–35). Two patients experienced serious TEAEs of hepatic function abnormalities: 1 patient had grade 4 abnormal hepatic function (acute hepatocellular injury occurring 7 days after DS-6157a infusion); the other had grade 4 hepatic dysfunction occurring on Day 8, which progressed on grade 5 on Day 10 after DS-6157a infusion, both of which were considered to attribute to underlying liver disease and concurrent sepsis.
There were no patients that achieved a CR. One patient (2.9%) achieved a PR and 17 patients (50.0%) had stable disease. Tumor shrinkage was observed in 4 KIT/PDGFR wild-type patients; the patient that achieved a PR had SDH-deficient GIST, with pathologic CR observed after surgical resection. Although the mechanism associated with response in this patient is not fully understood, it may be hypothesized that this specific molecular subtype or the absence of prior treatment for GIST affects sensitivity to treatment with DS-6157a. The median PFS of 4.2 months (95% CI, 1.6–6.9) in this molecularly unselected population of patients with advanced GIST is similar to other agents evaluated in later-line therapies in this population (26, 27), although the small numbers of patients treated in the study limit the ability to make firm conclusions. PK results from 34 patients indicate that intact DS-6157a, total anti-GPR20 antibody, and DXd plasma concentrations generally increased in a dose-dependent manner. In general, the total anti-GPR20 antibody and intact DS-6157a have similar PK profiles, indicating DS-6157a is stable in circulation. PK parameters of DS-6157a were within the range of DAR 8 DXd-ADCs, such as trastuzumab deruxtecan (36). Although DS-6157a provided benefits to some patients, the overall efficacy across the study was lower than expected. As the overall efficacy targets were not met during the dose escalation phase, the study did not proceed to the planned dose expansion phase.
Previous studies have reported GPR20 expression in ∼90% of the GIST samples analyzed, with a significant positive correlation between GPR20 and KIT expression. Expression of GPR20 was not different between KIT-mutant and KIT/PDGFRA wild-type GIST but was lower in PDGFRA-mutant GIST (15, 37). This greater understanding of GPR20 expression across different molecular subtypes of GIST may present an opportunity for further exploration of GRP20 as a target for patients with either KIT/PDGFRA wild-type SDH-deficient GISTs (accounting for the majority of this subtype) or NF1-associated GISTs, for which there is no standard approved therapy (38, 39). This approach may also be of benefit to patients with GISTs harboring KIT mutations relapsing after multiple lines of TKI treatments who have few other treatment options (15). The current study evaluated a therapy with a novel mechanism of action, relying on GIST-specific expression of GPR20 for intracellular delivery of a DNA-damaging cytotoxic agent to control a disease that is generally resistant to chemotherapy (40). The PR in 1 patient observed in this study highlights the potential vulnerability of KIT wild-type/SDH-deficient GIST to DNA-damaging payload by a GPR20-specific ADC. However, this observation needs to be verified in additional GIST patients with this specific tumor biology. Of note, SDH-deficient tumors are felt to have deficient DNA repair ability, due to succinate inhibition of DNA repair enzymes. Targeted delivery of DNA damaging agents to SDH-deficient GIST by GPR20 ADC may represent a novel strategy for treatment of SDH-deficient GIST by lowering the systemic toxicities of therapy compared with intravenous infusion of the unmodified chemotherapy drug (41, 42). The reason for the lack of objective responses or significant tumor shrinkage in KIT-mutant GIST is currently unknown. Whether the resistance to the ADC downstream of KIT signaling might be due to inefficient ADC internalization and release of the payload or insensitivity to the DNA damaging payload itself need to be elucidated. The decision made to halt study continuation was based on study results (ORR, PFS, ORR, PK parameters, and biomarker expression) in a molecularly unselected population of GIST.
In conclusion, the results from this study, as well as those in literature, warrants further research of the potential for the ADC-mediated delivery of DNA-damaging cytotoxic payloads in treating specific subgroups of GIST patients, such as those with SDH-deficient GIST or in potential drug combinations that addresses the resistance mechanisms in KIT-mutant GIST.
Supplementary Material
Supplementary Appendix
Supplementary Figure S1. Swimmer plot of tumor response over time
Supplementary Figure S2. DXd immunohistochemistry in a patient treated with 6.4 mg/kg DS-6157a (A) pre-treatment and (B) on treatment (Cycle 2, Day 10)
Supplementary Tables 1-4
Acknowledgments
We thank all of the patients and family members for their participation in this trial. We thank all of the investigators and their support staff who participated in this work. Medical writing and editorial support were provided by Danyang Zhou, PharmD, of Lumanity Communications Inc., and funded by Daiichi Sankyo, Inc. The authors also thank Rakesh Mucha and colleagues (Sarah Cannon Development Innovations) for delivering biostatistics outputs, Sophia Xu (Daiichi Sankyo, Inc.) for her contribution to PK sample analysis, Maki Kobayashi, Tomoya Kakegawa, Tomoko Shibutani, Masanori Funabashi (Daiichi Sankyo RD Novare Co., Ltd.), Masatoshi Nishimura, Mitsuhiro Yazawa, Naoyuki Maeda, Motoko Nagata (Daiichi Sankyo Co., Ltd.) and Roche Tissue Diagnostics for conducting DXd-IHC or GPR20-IHC and their operational assistance to analyze tumor samples, Debra Hyde, Kiyoshi Arai, Steven Cohen, Yutaka Noguchi (Daiichi Sankyo, Inc.) for their contribution to alliance start-up and management, Terri McDonough (Sarah Cannon Development Innovations) for her assistance to ensure quality of all study documents from the medical writing perspective, Poul Nielsen (Daiichi Sankyo, Inc.) and the CRO project team from Sarah Cannon Development Innovations (Michele Vaughan-Endsley and colleagues) and Daiichi Sankyo RD Novare Co., Ltd. (Hitoshi Ushida and colleagues) for their best efforts to trial management and site monitoring. Funding: Daiichi Sankyo, Inc.; Daiichi Sankyo Co. Ltd.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
S. George reports nonfinancial support and other support from Daiichi Sankyo during the conduct of the study. S. George also reports personal fees from CStone, Blueprint Medicines, Deciphera Pharmaceuticals, and Kayothera; other support from Blueprint Medicines, Deciphera Pharmaceuticals, Daiiichi Sankyo, BioAtla, Merck, Eisai, Springworks, Theseus, IDRX, and Tracon; and personal fees from Immunicum outside the submitted work. In addition, S. George receives royalties as author for UpToDate, and reports President Alliance for Clinical Trials in Oncology Foundation as well as Interim Group Chair Alliance for Clinical Trials in Oncology. M.C. Heinrich reports other support from Daiichi during the conduct of the study. M.C. Heinrich also reports personal fees from Novartis, Deciphera Pharmaceuticals, Blueprint Medicines, Zai Labs, and CStone Pharmaceuticals; grants and personal fees from Cogent Pharmaceuticals; and personal fees from Theseus Pharmaceuticals outside the submitted work. In addition, M.C. Heinrich has a patent for Treatment of GI Stromal Tumors issued, licensed, and with royalties paid from Novartis. N. Somaiah reports personal fees from Aadi Biosciences, Epizyme, and Boehringer Ingelheim, as well as grants from AstraZeneca outside the submitted work. P. Oppelt reports grants from Merck and HiFiBio outside the submitted work. R. McLeod reports full time employee with Daiichi Sankyo. M.G. Kundu reports full-time employee and shareholder of Daiichi Sankyo Inc. X. Qian reports other support from Daiichi Sankyo Inc. during the conduct of the study, as well as other support from Daiichi Sankyo Inc. outside the submitted work. P. Kumar reports other support from Daiichi Sankyo Inc. outside the submitted work. A. Laadem reports other support from Daiichi Sankyo outside the submitted work. Y. Naito reports grants and personal fees from Daiichi Sankyo during the conduct of the study. Y. Naito also reports grants and personal fees from Chugai, Pfizer, AstraZeneca, Ono, Takeda, and Taiho; personal fees from Eli Lilly, Eisai, PDR Pharma, Novartis, Gardant, Bayer, Nihon Kayaku, Bristo Myers Squibb, and MSD; and grants from Roche, AbbVie, Boehringer Ingelheim, and Gilead outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
S. George: Conceptualization, resources, data curation, investigation, methodology, writing–review and editing. M.C. Heinrich: Resources, data curation, investigation, methodology, writing–review and editing. N. Somaiah: Resources, data curation, investigation, methodology, writing–review and editing. P. Oppelt: Resources, data curation, investigation, methodology, writing–review and editing. R. McLeod: Conceptualization, resources, methodology, writing–review and editing. S. Nishioka: Conceptualization, resources, data curation, methodology, writing–review and editing. M.G. Kundu: Conceptualization, resources, data curation, formal analysis, methodology. X. Qian: Conceptualization, resources, data curation, formal analysis, validation, visualization, methodology, writing–review and editing. P. Kumar: Conceptualization, resources, funding acquisition, methodology, writing–review and editing. A. Laadem: Resources, funding acquisition, writing–review and editing. Y. Lau: Conceptualization, resources, data curation, formal analysis, methodology, writing–review and editing. B.P. Tran: Resources, data curation, formal analysis, validation, visualization, writing–review and editing. M. Fallon: Resources, data curation, writing–review and editing. O. Dosunmu: Resources, methodology, writing–review and editing. J. Shi: Resources, data curation, formal analysis, validation, visualization, writing–review and editing. Y. Naito: Resources, data curation, investigation, methodology, writing–review and editing.
References
- 1. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001–2015: a United States cancer statistics analysis of 50 states. Cureus 2019;11:e4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ducimetiere F, Lurkin A, Ranchere-Vince D, Decouvelaere AV, Peoc'h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011;6:e20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan J, Ullah A, Waheed A, Karki NR, Adhikari N, Vemavarapu L, et al. Gastrointestinal stromal tumors (GIST): a population-based study using the SEER database, including management and recent advances in targeted therapy. Cancers 2022;14:3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer 2005;103:821–9. [DOI] [PubMed] [Google Scholar]
- 5. Garcia J, Valls E, Soler M, Fuertes S, Moragas M, Riera E, et al. Spread patterns of gastrointestinal stromal tumors (GISTs) with 18F-FDG-PET-CT. J Nucl Med 2011;52:1845.22080445 [Google Scholar]
- 6. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342–9. [DOI] [PubMed] [Google Scholar]
- 7. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol 2004;22:3813–25. [DOI] [PubMed] [Google Scholar]
- 8. Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23:5357–64. [DOI] [PubMed] [Google Scholar]
- 9. Mathias-Machado MC, de Jesus VHF, de Carvalho Oliveira LJ, Neumann M, Peixoto RD. Current molecular profile of gastrointestinal stromal tumors and systemic therapeutic implications. Cancers 2022;14:5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brenca M, Rossi S, Polano M, Gasparotto D, Zanatta L, Racanelli D, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol 2016;238:543–9. [DOI] [PubMed] [Google Scholar]
- 11. Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 2004;28:889–94. [DOI] [PubMed] [Google Scholar]
- 12. Hase M, Yokomizo T, Shimizu T, Nakamura M. Characterization of an orphan G protein–coupled receptor, GPR20, that constitutively activates Gi proteins*. J Biol Chem 2008;283:12747–55. [DOI] [PubMed] [Google Scholar]
- 13. Allander SV, Nupponen NN, Ringner M, Hostetter G, Maher GW, Goldberger N, et al. Gastrointestinal stromal tumors with KIT mutations exhibit a remarkably homogeneous gene expression profile. Cancer Res 2001;61:8624–8. [PubMed] [Google Scholar]
- 14. Antonescu CR, Viale A, Sarran L, Tschernyavsky SJ, Gonen M, Segal NH, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res 2004;10:3282–90. [DOI] [PubMed] [Google Scholar]
- 15. Iida K, Abdelhamid Ahmed AH, Nagatsuma AK, Shibutani T, Yasuda S, Kitamura M, et al. Identification and therapeutic targeting of GPR20, selectively expressed in gastrointestinal stromal tumors, with DS-6157a, a first-in-class antibody–drug conjugate. Cancer Discov 2021;11:1508–23. [DOI] [PubMed] [Google Scholar]
- 16. Ran L, Chen Y, Sher J, Wong EWP, Murphy D, Zhang JQ, et al. FOXF1 defines the core-regulatory circuitry in gastrointestinal stromal tumor. Cancer Discov 2018;8:234–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumors: origin and molecular oncology. Nat Rev Cancer 2011;11:865–78. [DOI] [PubMed] [Google Scholar]
- 18. Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, Shamu T, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumors. Nature 2010;467:849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parslow AC, Parakh S, Lee FT, Gan HK, Scott AM. Antibody–drug conjugates for cancer therapy. Biomedicines 2016;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pettinato MC. Introduction to antibody–drug conjugates. Antibodies 2021;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Comprehensive Cancer Network . 2022. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Gastrointestinal Stromal Tumors (GISTs). Version 2.2022. Available from:https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf. Accessed January 27, 2023. [Google Scholar]
- 22. Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localized, primary gastrointestinal stromal tumor: a randomized, double-blind, placebo-controlled trial. Lancet 2009;373:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demetri GD, von MM, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–80. [DOI] [PubMed] [Google Scholar]
- 24. Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–32. [DOI] [PubMed] [Google Scholar]
- 25. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumor after failure of imatinib: a randomized controlled trial. Lancet 2006;368:1329–38. [DOI] [PubMed] [Google Scholar]
- 26. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumors after failure of imatinib and sunitinib (GRID): an international, multicenter, randomized, placebo-controlled, phase III trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blay JY, Serrano C, Heinrich MC, Zalcberg J, Bauer S, Gelderblom H, et al. Ripretinib in patients with advanced gastrointestinal stromal tumors (INVICTUS): a double-blind, randomized, placebo-controlled, phase III trial. Lancet Oncol 2020;21:923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones RL, Serrano C, von Mehren M, George S, Heinrich MC, Kang YK, et al. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumors: Long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur J Cancer 2021;145:132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neuenschwander B, Branson M, Gsponer T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med 2008;27:2420–39. [DOI] [PubMed] [Google Scholar]
- 30. Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med 1998;17:1103–20. [DOI] [PubMed] [Google Scholar]
- 31. Rogatko A, Babb JS, Tighiouart M, Khuri FR, Hudes G. New paradigm in dose-finding trials: patient-specific dosing and beyond phase I. Clin Cancer Res 2005;11:5342–6. [DOI] [PubMed] [Google Scholar]
- 32. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomized, open-label, phase III trial. Lancet 2023;401:105–17. [DOI] [PubMed] [Google Scholar]
- 34. Janne PA, Baik C, Su WC, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov 2022;12:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powell CA, Modi S, Iwata H, Takahashi S, Smit EF, Siena S, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 2022;7:100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumor activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastroesophageal tumors: a phase I dose-escalation study. Lancet Oncol 2017;18:1512–22. [DOI] [PubMed] [Google Scholar]
- 37. Iida K, Abdelhamid AH, Nagatsuma AK, Shibutani T, Yasuda S, Kitamura M, et al. Abstract 5181: Therapeutic targeting of GPR20, selectively expressed in gastrointestinal stromal tumor (GIST), with DS-6157a, an antibody–drug conjugate (ADC). Cancer Res 2020;80:5181. [DOI] [PubMed] [Google Scholar]
- 38. Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2016;2:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zoller ME, Rembeck B, Oden A, Samuelsson M, Angervall L. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer 1997;79:2125–31. [PubMed] [Google Scholar]
- 40. Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumor. Lancet 2013;382:973–83. [DOI] [PubMed] [Google Scholar]
- 41. Sulkowski PL, Sundaram RK, Oeck S, Corso CD, Liu Y, Noorbakhsh S, et al. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat Genet 2018;50:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yebra M, Bhargava S, Kumar A, Burgoyne AM, Tang CM, Yoon H, et al. Establishment of patient-derived succinate dehydrogenase–deficient gastrointestinal stromal tumor models for predicting therapeutic response. Clin Cancer Res 2022;28:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix
Supplementary Figure S1. Swimmer plot of tumor response over time
Supplementary Figure S2. DXd immunohistochemistry in a patient treated with 6.4 mg/kg DS-6157a (A) pre-treatment and (B) on treatment (Cycle 2, Day 10)
Supplementary Tables 1-4
Data Availability Statement
Anonymized individual participant data on completed studies and applicable supporting clinical study documents will be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/.