Abstract
Background
Bladder cancer (BCa) is one of the most common urological malignancies worldwide. This study examines the global epidemiological profile of BCa incidence and mortality in 2020 and the projected burden to 2040.
Methods
The estimated number of BCa cases and deaths were extracted from the GLOBOCAN 2020 database. Age-standardised incidence rates (ASIRs) and age-standardised mortality rates (ASMRs) were calculated using the world standard. The predicted BCa incidence and mortality in 2040 was calculated based on demographic projections.
Results
Globally, approximately 573 000 new BCa cases and 213 000 deaths occurred in 2020, corresponding to ASIRs and ASMRs of 5.6 and 1.9 per 100 000, respectively. The incidence and mortality rates were approximately 4-fold higher in men (9.5 and 3.3 per 100 000, respectively) than women (2.4 and 0.9, respectively). Across world regions, incidence rates varied at least 12-fold among men and 8-fold among women, with the highest ASIRs for both men and women detected in Southern Europe (26.5 and 5.8 per 100 000, respectively) and Western Europe (21.5 and 5.8, respectively) and the lowest in Middle Africa (2.2) in men and South-Central Asia (0.7) in women. The highest ASMRs for both men and women were found in Northern Africa (9.2 and 1.8 per 100 000, respectively). By 2040, the annual number of new BCa cases and deaths will increase to 991 000 (72.8% increase from 2020) and 397 000 (86.6% increase), respectively.
Conclusions
Geographical distributions of BCa incidence and mortality uncovered higher risk of BCa incidence in Southern and Western European populations and higher risk of mortality in Northern African populations. Considering the predicted 73% and 87% increase in annual BCa cases and deaths by 2040 globally, respectively, there is an urgent need to develop and accelerate BCa control initiatives for high-risk populations to tackle global BCa burden and narrow its geographical disparities.
Bladder cancer (BCa) is the 10th most commonly diagnosed cancer and the thirteenth leading cause of cancer death worldwide [1,2]. A previous study showed that more than 60% of all BCa cases and about half of its deaths were observed in less developed countries in 2012 [3]. However, over the past decade, incidence of BCa in Europe has increased while mortality has decreased, whereas in Asia BCa incidence has decreased but mortality from BCa in men has increased [4]. Notably, distinct sex disparities were found for BCa, with three-quarters of cases occurring in men in 2012 [3]. Furthermore, compared to other urological cancers, 5-year survival from BCa has remained relatively low and there are considerable inequalities in survival e.g., 77% in Peru and 34% in Colombia [5,6].
BCa is a largely preventable disease due to its many modifiable risk factors. Tobacco smoking is the principal risk factor for BCa, accounting for about half of all BCa cases [7-9] and 37% of its deaths [10]. Current and former smokers are considered to have a 3.5- and 2.0-fold increased risk of BCa compared with nonsmokers, respectively [11]. The second greatest risk factor for BCa after smoking is occupational exposure to carcinogens [12]. Approximately 10% of all BCa cases can be attributed to occupational exposure to carcinogenic chemicals, such as aromatic amines, polycyclic aromatic hydrocarbons, and chlorinated hydrocarbons [12-14]. Another important risk factor for BCa is Schistosoma haematobium infection [15,16].
Given the recent changes in geographical disparities in the burden of BCa [3,4] and the strong association of BCa with its modifiable risk factors, understanding the current epidemiological profile of international variations in BCa incidence and mortality is important. This would allow policymakers to make evidence-based decisions for primary prevention and to optimise the allocation of resources to reduce the global burden of BCa. Moreover, BCa is more likely to affect older people [17], and considering the growing and ageing global population, predicting the future BCa burden is essential for cancer control planning. Although previous studies have reported the burden of BCa incidence and mortality in 2012 [3] and the changing epidemiological profiles of BCa burden over the past decades [4], recent and comprehensive epidemiological data on BCa incidence and mortality and quantitative projections of the future BCa burden are still lacking.
Herein, we aimed to better understand the current disease patterns across the world by describing the magnitude of BCa incidence and mortality and examining their geographic variations according to country, world region, and the four-tier Human Development Index (HDI), where HDI was used to assess the cancer burden at varying levels of development (low, medium, high and very high HDI), using estimates for 2020. These findings will help to better identify populations at higher risk of BCa and provide indications of the causal factors underlying reported geographic variations. Furthermore, we have predicted the future burden of new BCa cases and deaths up to 2040 based on demographic projections with the aim of supporting future planning requirements for optimising the allocation of resources for BCa screening, diagnosis, and therapy.
METHODS
Data sources
The number of new cases of, and deaths from, BCa (International Classification of Diseases tenth revision (ICD-10) C67) were extracted from the GLOBOCAN 2020 database for 185 countries or territories, by sex and 5-year age group (0-4, 5-9, …, 80-84, 85 and over) [1,2,18]. GLOBOCAN 2020 database are available from the Global Cancer Observatory [2] which includes facilities for the tabulation and graphical visualisation of the GLOBOCAN database, including explorations of the current burden in 2020 and the future burden by 2040 for 36 cancers (as well as all cancers combined) by sex and by 18 age groups (0-4, 5-9, …, 80-84, 85 and over) in 185 countries or territories. The GLOBOCAN 2020 database, collated by the International Agency for Research on Cancer, comprises national cancer incidence and mortality estimates derived from the best available recorded data available from national (or subnational) cancer registries and national vital registry systems worldwide [1,2,18]. Briefly, cancer incidence estimates were derived from national or subnational population-based cancer registry data including submissions to the Cancer Incidence in Five Continents (volume XI) from 2008 to 2012, and more recent data from the African Cancer Registry Network [1,2,18]. Cancer mortality estimates were obtained using the most recent national vital registration data from World Health Organization (WHO) [1,2,18]. The data sources and methods used in compiling the global cancer estimates for 2020 have been described in detail elsewhere [18]. The population data from the year 2020 to 2040 were retrieved from the United Nations (UN) website [19].
Statistical analysis
We present tables and figures of the estimated new cases and deaths, as well as two summary measures using direct standardisation, namely the age-standardised incidence rates (ASIRs) and age-standardised mortality rates (ASMRs) per 100 000 person-years based on the 1966 Segi-Doll World standard population [20,21] and the cumulative risk of being diagnosed with or dying from BCa before the age of 75 expressed as a percentage, assuming the absence of competing causes of death [22].
We predicted the future number of BCa cases and deaths worldwide and by the HDI groups up to the year 2040, based on demographic projections and scenarios of annually increasing (+1, +2, +3, +4%), stable (0%) or decreasing (-1, -2, -3, -4%) rates from the baseline year of 2020. We did not use scenarios of rates changing by ±5% or more because such changes would be unlikely to occur in real life. Predictions were calculated by applying the age-specific rates for the year 2020 (and each of the increasing or decreasing scenarios described) to the corresponding projected population data as estimated by the United Nations Development Programme.
The results are presented by country and aggregated across 20 UN-defined world regions [19] and six WHO regions and according to HDI in 2020 [23]. The estimated numbers of cases and deaths have been rounded to three significant figures to avoid spurious precision. In some cases, this creates small discrepancies with the displayed totals and percentages, which are based on the data before rounding. Data management and analyses were performed in R software (version 4.0.2) [24]. Figures were plotted using SigmaPlot software (version 12.5) [25]. Global maps of BCa incidence and mortality rates by country were retrieved from the Global Cancer Observatory, Cancer Today website [2]. Global maps of BCa incidence and mortality rates by country were depicted by ourselves using R software (version 4.0.2) [24] according to World Health Organization data [2].
RESULTS
Global burden of bladder cancer incidence and mortality
In 2020, an estimated 573 000 people were diagnosed with BCa worldwide, corresponding to an ASIR of 5.6 per 100 000 (Table 1). More men than women were diagnosed with BCa and the ASIRs were approximately 4-fold higher in men than women (Table S1 in the Online Supplementary Document). Globally, an estimated 213 000 people died from BCa, corresponding to an ASMR of 1.9 per 100 000 (Table 1). Mortality was also higher among men than women (Table S1 in the Online Supplementary Document). In addition, the cumulative risk of being diagnosed with and dying from BCa before the age of 75 was one in 53 and one in 112, respectively (Table 1).
Table 1.
Bladder cancer incidence and mortality in both sexes combined in 2020 by world region and human development index level
| Region | Population | Incidence |
Mortality |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
No. (%), in thousands
|
Number of cases
|
Percentage of world total
|
ASIR (95% CI)
|
Cumulative risk*
|
Number of deaths
|
Percentage of world total
|
ASMR (95% CI)
|
Cumulative risk*
|
|
Europe
|
|
|
|
|
|
|
|
|
|
| Northern Europe |
106 261 (1.4) |
23 333 |
4.1 |
8.7 (8.6-8.9) |
2.87 |
9563 |
4.5 |
2.7 (2.7-2.8) |
1.39 |
| Western Europe |
196 146 (2.5) |
68 143 |
11.9 |
13.0 (13.0-13.2) |
3.92 |
20 866 |
9.8 |
3.0 (3.0-3.0) |
1.40 |
| Southern Europe |
153 423 (2.0) |
61 480 |
10.7 |
15.3 (15.3-15.6) |
4.29 |
17 931 |
8.4 |
3.3 (3.2-3.4) |
1.48 |
| Central and Eastern Europe |
293 013 (3.8) |
51 027 |
8.9 |
8.6 (8.5-8.7) |
2.20 |
18 929 |
8.9 |
2.8 (2.7-2.8) |
1.00 |
|
America
|
|
|
|
|
|
|
|
|
|
| Northern America |
368 870 (4.7) |
89 997 |
15.7 |
10.9 (10.8-10.9) |
3.59 |
21 045 |
9.9 |
2.1 (2.1-2.2) |
1.04 |
| South America |
430 760 (5.5) |
27 159 |
4.7 |
4.8 (4.8-4.9) |
1.58 |
10 108 |
4.8 |
1.6 (1.6-1.7) |
0.73 |
| Central America |
179 670 (2.3) |
4119 |
0.7 |
2.2 (2.1-2.3) |
0.68 |
1690 |
0.8 |
0.8 (0.8-0.9) |
0.36 |
| Caribbean |
43 532 (0.6) |
2562 |
0.4 |
4.1 (3.9-4.2) |
1.36 |
1302 |
0.6 |
1.8 (1.8-2.0) |
0.82 |
|
Asia
|
|
|
|
|
|
|
|
|
|
| Eastern Asia |
1 678 090 (21.5) |
132 316 |
23.1 |
4.3 (4.3-4.4) |
1.46 |
54 206 |
25.5 |
1.6 (1.6-1.6) |
0.81 |
| South-Central Asia |
2 014 709 (25.8) |
35 949 |
6.3 |
1.9 (1.9-2.0) |
0.51 |
18 243 |
8.6 |
1.0 (1.0-1.0) |
0.34 |
| South-Eastern Asia |
668 620 (8.6) |
18 911 |
3.3 |
2.6 (2.5-2.60) |
0.85 |
10 327 |
4.9 |
1.4 (1.3-1.4) |
0.65 |
| Western Asia |
278 429 (3.6) |
20 915 |
3.6 |
8.9 (8.8-9.1) |
2.38 |
7834 |
3.7 |
3.2 (3.1-3.2) |
1.31 |
|
Oceania
|
|
|
|
|
|
|
|
|
|
| Australia and New Zealand |
30 322 (0.4) |
3923 |
0.7 |
5.5 (5.3-5.6) |
2.12 |
1615 |
0.8 |
1.8 (1.7-1.9) |
1.05 |
| Melanesia |
11 123 (0.1) |
201 |
<0.1 |
2.8 (2.4-3.3) |
0.79 |
104 |
<0.1 |
1.6 (1.3-1.9) |
0.56 |
| Micronesia/Polynesia |
1233 (<0.1) |
47 |
<0.1 |
3.8 (2.4-5.1) |
0.47 |
26 |
<0.1 |
2.1 (1.1-3.2) |
0.28 |
|
Africa
|
|
|
|
|
|
|
|
|
|
| Northern Africa |
246 233 (3.2) |
18 589 |
3.2 |
8.9 (8.8-9.0) |
2.52 |
10 787 |
5.1 |
5.2 (5.1-5.3) |
2.17 |
| Western Africa |
401 861 (5.2) |
4139 |
0.7 |
2.0 (1.9-2.0) |
0.49 |
2397 |
1.1 |
1.3 (1.2-1.3) |
0.37 |
| Southern Africa |
67 504 (0.9) |
2208 |
0.4 |
4.1 (3.9-4.3) |
1.28 |
853 |
0.4 |
1.6 (1.5-1.7) |
0.59 |
| Middle Africa |
179 595 (2.3) |
1366 |
0.2 |
1.6 (1.5-1.7) |
0.47 |
784 |
0.4 |
1.0 (0.9-1.0) |
0.35 |
| Eastern Africa |
445 406 (5.7) |
6894 |
1.2 |
3.2 (3.1-3.2) |
0.94 |
3926 |
1.8 |
1.9 (1.8-1.9) |
0.71 |
|
HDI
|
|
|
|
|
|
|
|
|
|
| Very high HDI |
1 564 286 (20.1) |
356 601 |
62.2 |
10.2 (10.2-10.2) |
3.13 |
108 713 |
51.2 |
2.5 (2.5-2.5) |
1.15 |
| High HDI |
2 909 468 (37.3) |
164 626 |
28.7 |
4.1 (4.1-4.1) |
1.26 |
75 297 |
35.4 |
1.8 (1.8-1.8) |
0.81 |
| Medium HDI |
2 327 556 (29.9) |
38 712 |
6.8 |
1.9 (1.9-1.9) |
0.49 |
20 719 |
9.8 |
1.0 (1.0-1.0) |
0.33 |
| Low HDI |
990 175(12.7) |
13 025 |
2.3 |
2.6 (2.5-2.6) |
0.76 |
7693 |
3.6 |
1.7 (1.7-1.8) |
0.61 |
|
WHO Region
|
|
|
|
|
|
|
|
|
|
| WHO Africa |
1 124 061 (14.4) |
17 526 |
3.1 |
3.1 (3.0-3.1) |
0.93 |
9655 |
4.5 |
1.8 (1.8-1.8) |
0.69 |
| WHO Americas |
1 024 551 (13.1) |
123 837 |
21.6 |
7.3 (7.3-7.4) |
2.52 |
34 145 |
16.1 |
1.8 (1.8-1.9) |
0.86 |
| WHO East Mediterranean |
764 753 (9.8) |
31 204 |
5.4 |
5.9 (5.9-6.0) |
1.65 |
16 468 |
7.8 |
3.2 (3.2-3.3) |
1.31 |
| WHO Europe |
929 738 (11.9) |
221 298 |
38.6 |
11.0 (11.0-11.1) |
3.22 |
73 363 |
34.5 |
3.0 (3.0-3.0) |
1.30 |
| WHO South-East Asia |
2 040 083 (26.1) |
39 118 |
6.8 |
2.0 (2.0-2.0) |
0.57 |
20 890 |
9.8 |
1.0 (1.0-1.0) |
0.40 |
| WHO Western Pacific |
1 928 975 (24.7) |
140 171 |
24.5 |
4.2 (4.2-4.3) |
1.41 |
57 965 |
27.3 |
1.6 (1.6-1.6) |
0.79 |
| World | 7 794 799 (100) | 573 278 | 100.0 | 5.6 (5.6-5.6) | 1.88 | 212 536 | 100.0 | 1.9 (1.9-1.9) | 0.89 |
ASIR – age-standardised incidence rate per 100 000 person-years, CI – confidence interval, ASMR – age-standardised mortality rate per 100 000 person-years, HDI – Human Development Index, WHO – World Health Organization
*Cumulative risk of being diagnosed with or dying from bladder cancer until 74 years of age in 2020.
Geographical variation in bladder cancer incidence and mortality by world region
The greatest number of cases and deaths of BCa in both sexes combined were observed in Eastern Asia, followed by Northern America and Western Europe (Figure 1, panel A and panel B). Among men and women separately, the top three world regions with the largest number of both BCa cases and deaths remained Eastern Asia, Northern America, and Western Europe (Figure S1 and Figure S2 in the Online Supplementary Document). A predominance in men in BCa cases and deaths was consistent across world regions (Table S1 in the Online Supplementary Document).
Figure 1.
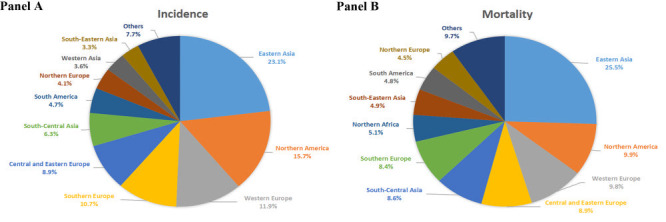
Panel A. Distribution of bladder cancer cases for both sexes combined by world region in 2020. Panel B. Distribution of bladder cancer deaths for both sexes combined by world region in 2020.
The incidence rates of BCa showed approximately 12-fold variation in men and 8-fold in women across world regions (Figure 2, panel A and panel B and Table S1 in the Online Supplementary Document). In men, the ASIRs were lowest in Middle Africa but the highest in Southern Europe, followed by Western Europe and Northern America. In women, the ASIRs were lowest in South-Central Asia but the highest in Western Europe, Southern Europe, and Northern America. Between world regions, the mortality rates of BCa varied approximately 7-fold in men and 5-fold in women (Figure 2, panel C and panel D and Table S1 in the Online Supplementary Document). In men, the highest ASMRs were detected in Northern Africa, followed by Central and Eastern Europe, Southern Europe, and Western Asia, and the lowest were in Middle Africa and Central America. In women, the highest ASMRs were found in Northern Africa, Northern Europe, Western Europe, and Eastern Africa, and the lowest were in South-Central Asia and Central America. Sex-related disparities were also noted, with BCa ASIRs and ASMRs higher in men than women across world regions. For example, the ASIR ratio of men to women ranged from 1.6 in Western Africa to 5.9 in Western Asia, and the ASMR ratio of men to women ranged from 1.5 in Western Africa to 6.7 in Central and Eastern Europe.
Figure 2.
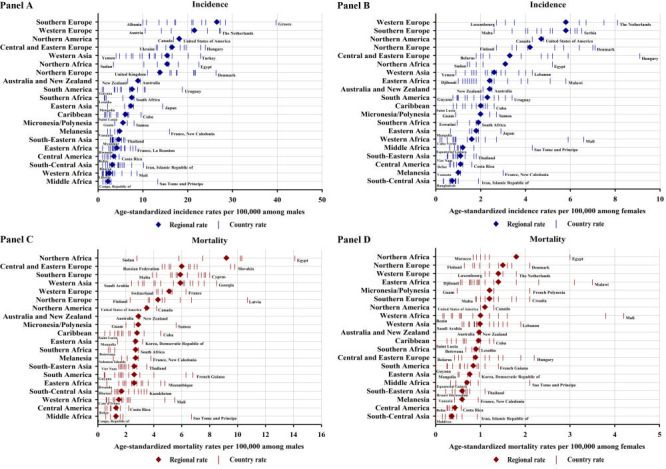
Panel A. Age-standardised incidence rates of bladder cancer in men by country within world region. Panel B. Age-standardised incidence rates of bladder cancer in women by country within world region. Panel C. Age-standardised mortality rates of bladder cancer in men by country within world region. Panel D. Age-standardised mortality rates of bladder cancer in women by country within world region. Ordered according to descending rates by world region.
By WHO region, the highest ASIR in both sexes combined was detected in Europe, while the highest ASMR was found in East Mediterranean (Table 1); the same was true in women (Table S1 in the Online Supplementary Document). However, the highest both ASIR and ASMR were observed in Europe (Table S1 in the Online Supplementary Document).
Geographical variations in bladder cancer incidence and mortality by country
At the national level, the highest ASIRs occurred in Greece, Italy, Spain, and the Netherlands in men and in Hungary, the Netherlands, and Germany in women (Figure 2, panel A and panel B and Figure 3, panel A and panel B). Notably, incidence rates varied within world regions, for example, ranging from 0.9 per 100 000 in Guyana to 18.8 in Uruguay in men within South America (high-to-low ASIR ratio = 20.9) and from 0.2 in Cabo Verde to 6.6 in Mali in women within Western Africa (high-to-low ASIR ratio = 33.0) (Figure 2, panel A and panel B). The incidence rates were also heterogeneous across countries in high-risk world regions, for example, ranging from 10.0 per 100 000 in Albania to 39.7 in Greece in men within Southern Europe (high-to-low ASIR ratio = 4.0) and from 2.7 in Luxembourg to 8.1 in the Netherlands in women within Western Europe (high-to-low ASIR ratio = 3.0). In terms of mortality, the highest ASMRs were observed in Egypt, Latvia, and Tunisia in men and in Mali, Burkina Faso, and Malawi in women (Figure 2, panel C and panel D and Figure 3, panel C and panel D). Considerable variations in BCa mortality were also evident within world regions. For example, the ASMRs in South America in men were as low as 0.5 per 100 000 in Guyana and Bolivia and as high as 6.8 in French Guiana (high-to-low ASMR ratio = 13.6) and the ASMRs in Western Africa in women were as low as 0.2 in Benin and Cabo Verde and as high as 4.2 in Mali (high-to-low ASMR ratio = 21.0) (Figure 2, panel C and panel D).
Figure 3.
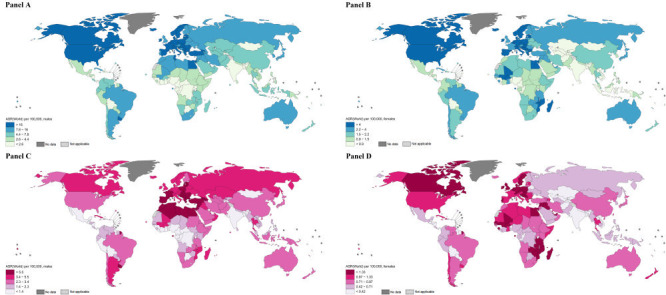
Panel A. Global map of age-standardised rates (ASR) of bladder cancer incidence per 100 000 person-years in men by country. Panel B. Global map of ASR of bladder cancer incidence per 100 000 person-years in women by country. Panel C. Global map of ASR of bladder cancer mortality per 100 000 person-years in men by country. Panel D. Global map of ASR of bladder cancer mortality per 100 000 person-years in women by country [2,24].
Bladder cancer incidence and mortality by level of human development
By HDI group, the vast majority of BCa cases and deaths occurred among the 57.4% of the world population living in high and very high HDI countries, representing 90.9% of new cases and 86.6% of deaths globally (Table 1). A gradient across HDI categories was observed for both BCa incidence and mortality. Bladder cancer incidence rates were substantially higher in countries with very high HDI and high HDI than those with medium and low HDI, while mortality rates were slightly higher in countries with very high HDI and high HDI than those with medium and low HDI (Table 1).
Predicted number and percentage increase of cases and deaths from bladder cancer
Worldwide, the number of new BCa cases were estimated to increase by approximately 72.8%, from 573 000 in 2020 to 991 000 in 2040, assuming global incidence rates in 2020 will remain constant (Figure 4, panel A). Moreover, a 1% annual increase in incidence rates from 2020 would more than double the total annual BCa cases by 2040. Notably, an annual decline of 3% in incidence rates would be required to achieve fewer BCa cases in the future compared to the estimated cases in 2020. In terms of mortality, the number of BCa deaths were estimated to increase by approximately 86.6%, from 213 000 in 2020 to 397 000 in 2040, assuming mortality rates in 2020 remained unchanged (Figure 4, panel B). A 4% annual decrease in mortality rates would be required to ensure that there would be fewer BCa deaths in 2040 than in 2020.
Figure 4.
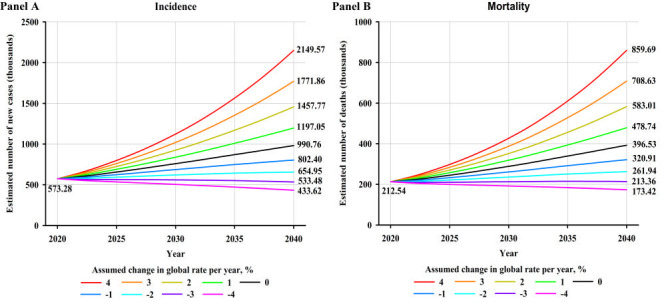
Panel A. Predicted number of new bladder cancer cases for both sexes combined assuming nine scenarios of annual change in global incidence rates between 2020 and 2040. Panel B. Predicted number of bladder cancer deaths for both sexes combined assuming nine scenarios of annual change in global mortality rates between 2020 and 2040.
By WHO region, the largest relative increase in new BCa cases and deaths will occur in Western Pacific, with 105.7% more cases and 115.4% more deaths per year by 2040 (Figure S3 and Figure S4 in the Online Supplementary Document), assuming the incidence and mortality rates in 2020 remain constant. It would require a 4%, 3%, 4%, 2%, 3%, and 3% annual decrease in incidence rates to achieve fewer BCa cases in 2040 compared to the estimated number of cases in 2020 for Africa, Americas, East Mediterranean, Europe, South-East Asia, and Western Pacific, respectively. Notably, decreases in mortality rates would need to be greater than 4%, 4%, 4%, 2%, 4%, and 4% to retain the predicted BCa deaths in 2040 at the level observed in 2020 for Africa, Americas, East Mediterranean, Europe, South-East Asia, and Western Pacific, respectively.
By HDI group, the largest absolute increase in new BCa cases and deaths will occur in very high HDI countries, with 43.7% more cases and 60.0% more deaths per year by 2040 (Figure 5, panel A and panel B), assuming the incidence and mortality rates in 2020 remain constant, reflecting the already high rates in the very high HDI countries and its large population which will continue to grow. However, the greatest relative increases in cases and deaths will occur in low HDI countries (103.5% and 105.3% increases, respectively) and high HDI countries (78.2% and 105.4% increases, respectively). A 1%, 1%, and 2% annual increase in incidence rates from 2020 would nearly double the total annual BCa cases by 2040 for medium, high, and very high HDI countries, respectively (Figure S5 in the Online Supplementary Document). Notably, decreases in incidence rates would need to be greater than 4%, 3%, 3%, and 2% to retain the predicted number of new BCa cases in 2040 at the level seen in 2020 for low, medium, high, and very high HDI countries, respectively. It would require a 4%, 4%, 4%, and 3% annual decrease in mortality rates to achieve fewer BCa deaths in 2040 compared to the estimated number of deaths in 2020 for low, medium, high, and very high HDI countries, respectively (Figure S6 in the Online Supplementary Document).
Figure 5.
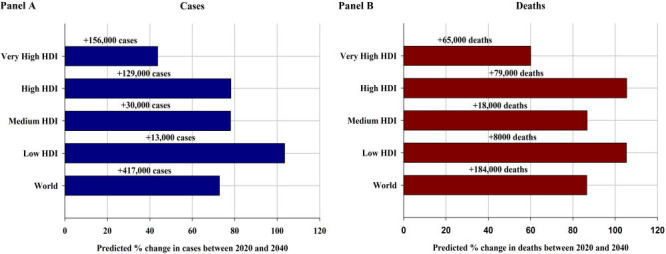
Panel A. Predicted percentage change (absolute numbers are shown above bars) of the number of new bladder cancer cases for both sexes combined from between 2020 and 2040, by HDI, assuming incidence rates in 2020 remained unchanged. Panel B. Predicted percentage change (absolute numbers are shown above bars) of the number of bladder cancer deaths for both sexes combined from between 2020 and 2040, by HDI, assuming mortality rates in 2020 remained unchanged. HDI – Human Development Index
DISCUSSION
Our study identifies high-risk populations for BCa and provides indications for cancer specialists and public health policymakers seeking to plan appropriate BCa control strategies and optimise the allocation of resources accordingly. Specifically, our study found that the higher risk of BCa incidence occurred in Southern and Western European and Northern American populations, while higher risks of mortality were detected in Northern African populations. Regarding sex disparities, men had higher risk than women across all world regions. Furthermore, our study found that BCa cases and deaths will increase by more than 70% over the next 20 years worldwide and highlights the need to develop and implement targeted interventions for high-risk populations to tackle global BCa burden.
We have shed light on the current global distribution of BCa cases and deaths yet the epidemiological profile of BCa burden across regions and countries has been changing. For example, the incidence rates of BCa for both men and women in most European countries have increased and those in most Asian, Oceanian, and South American countries have decreased during 1993-2012 [4]. Hence, the relatively high incidence rates of BCa observed in Europe and the relatively low incidence rates found in Asia, Oceania, and South America could be related to the corresponding ongoing transitions in the epidemiological profile of BCa over the past decade.
The disparities in BCa burden by geography and by sex might be mainly correlated with the different prevalence of its risk factors, such as tobacco smoking, occupational exposure to carcinogenic chemicals, and infection with Schistosoma haematobium. Considering a latency period of approximately 30 years from the initiation of smoking to the diagnosis of BCa [3,26], the relatively high incidence rates of BCa observed in European and Northern American countries could be largely attributed to the higher smoking prevalence in these regions in previous decades. For example, the age-standardised smoking prevalence (%) in men in 1980 and 1996 was as high as 54.7 and 53.8 in Greece, 44.3 and 32.2 in Italy, 44.4 and 41.0 in Spain, 34.5 and 29.9 in the Netherlands, and 33.2 and 23.1 in the United States, and in women was 27.4 and 24.1 in Hungary, 25.9 and 25.3 in the Netherlands, 27.8 and 23.2 in Germany, 39.3 and 34.9 in Denmark, 28.3 and 20.1 in the United States, respectively [27]. The higher BCa incidence in men than in women could be largely explained by the tobacco epidemic among women lagging behind that of men by several decades, and the public health effects of the epidemic have yet to mature in women [27].
The relatively elevated BCa incidence in high and very high HDI countries could also possibly be linked to higher rates of occupational exposure to carcinogens in these countries a few decades ago, such as the aromatic amines in the dye industry in the European Union [28]. The relatively high BCa burden in Northern Africa and Western Asia are more likely to be attributable to the higher population-level prevalence (more than 50%) of Schistosoma haematobium infection in these regions [29,30]. Furthermore, Northern African and Western Asian countries show between a 5- and 6-fold higher BCa risk in men than women; this finding could be interpreted as a result of the transmission of Schistosoma haematobium via agricultural activities typically undertaken by men [13]. Notably, compared with low and medium HDI countries, high and very high HDI countries have a higher proportion of the elderly in their populations [31], resulting in a higher burden of BCa which is more commonly diagnosed in older age [13,17]. Additionally, people in such countries have higher health awareness and can more easily obtain access to advanced diagnostic methods, such as urine cytology, cystoscopy, and computed tomography scan [32,33], which promotes the diagnosis of new BCa cases. However, the diagnostic opportunities and awareness could be the source of error since the HDI may not entirely capture the same point in the national health care development. Thus, the findings presented according to HDI should be interpreted with caution. In terms of BCa mortality, its geographical disparities could be largely explained by the differences in terms of health care systems, clinical practice, and access to diagnosis and treatment facilities between countries at different levels of development of resource levels [3,34].
We suggest that BCa incidence could be largely reduced if the prevalence of modifiable risk factors was reduced to a minimum in regions with larger population attributable fractions (PAFs). The PAF for BCa incidence due to tobacco smoking was estimated to be 39.5% in men and 22.6% in women in European countries, with the largest PAF being 45.3% in men in Central and Eastern European countries and 35.2% in women in Northern European countries [35]. The PAF for BCa incidence due to occupational exposure to carcinogens was estimated to be 10.6% in men and 11.4% in women in East Asian countries [36]. The PAF for the incidence of squamous cell carcinoma of the bladder due to Schistosoma haematobium infection was estimated to be 100% in most African and Western Asian countries [37]. With such a high contribution of modifiable risk factors to BCa incidence in certain regions, research to estimate the impact of changes in major risk factors on the future incidence of BCa would provide useful insight for BCa prevention strategies in varying contexts.
A previous study showed that the increases of new BCa cases from 2006-2016 worldwide were mainly due to population growth and ageing [38]. In our study, the projected number of cases and deaths from BCa are expected to increase to 981 000 and 392 000 by 2040 worldwide, respectively, as a result of population growth and aging alone. A 3% annual decrease in global incidence rates and a 4% annual decline in global mortality rates would be needed to halt the increasing BCa burden by 2040. Considering these changes, the reallocation of resources for BCa primary prevention programmes aimed at reducing population levels of tobacco smoking, occupational exposure to carcinogenic chemicals, and Schistosoma haematobium infection in certain regions and countries and the increased access to early detection modalities and health care services for high-risk populations identified by our study are crucial for reducing the global BCa burden.
The numbers and rates of BCa presented in this study are estimates based on the best available data (reviewed for their completeness, coverage and accuracy) from population-based cancer registries. Our study provides a comprehensive picture of the epidemiological profile of BCa incidence and mortality on a global scale in 2020, with an emphasis on region-, country- and sex-specific disparities as well as differences between HDI groups, which is highly relevant for cancer control and clinical practice. Furthermore, our study predicts the future BCa incidence and mortality worldwide and by HDI group up to the year 2040 based on scenarios of rates changing from -4 to 4% from the baseline year of 2020. These predictions might help optimise the allocation of resources for screening, diagnosis, and therapy and provide a benchmark for evaluating BCa prevention and control interventions. Nevertheless, several limitations should be addressed. First, although our findings are based on the best available and high-quality data, caution is warranted when interpreting the findings for countries with limited coverage from population-based cancer registries and where proxy data were used to obtain national estimates [18]. Second, the GLOBOCAN estimates did not account for the impact of the coronavirus 2019 (COVID-19) pandemic on cancer diagnoses because the GLOBOCAN estimates were based on extrapolations of previous years of cancer data [18]. Third, the projections of the future burden of BCa in 2040 neither considered recent changes in BCa incidence and mortality rates nor heterogeneity in BCa incidence and mortality trends between countries. Thus, the predictions in our study likely represent a conservative estimate (an underestimation) of the future BCa burden and should be interpreted with caution. Finally, our study was unable to distinguish the required changes in modifiable risk factors and their corresponding effects on predicted BCa incidence and mortality rates which suggests the need for further studies to clarify the impact of changes in exposure to modifiable risk factors on the burden of BCa.
CONCLUSIONS
Bladder cancer continues to be a considerable public health challenge worldwide. Distinct variation exists in BCa incidence and mortality across world regions and countries, possibly related to differences in tobacco smoking, occupational exposure to carcinogenic chemicals, and Schistosoma haematobium infection. The global share of both BCa cases and deaths was disproportionally high in high and very high HDI countries compared to low and medium HDI countries. Given the considerable geographic disparity of BCa burden across world regions and countries and the corresponding ongoing transition in its epidemiological profile, this study provides new insights into global BCa burden. Considering the predicted 73 and 87% increase in annual BCa cases and deaths by 2040 globally, respectively, this study also highlights the urgent need to promote the development of BCa control strategies and to prioritise allocation of BCa prevention and treatment resources for high-risk populations to tackle the global burden of BCa and narrow its geographical disparities.
Additional material
Acknowledgements
We gratefully acknowledge the contribution of all cancer registry staff in generating the cancer registry data used in our study. Where authors are identified as personnel of the International Agency for Research on Cancer and the World Health Organization (WHO), the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer and WHO.
Ethics approval: Ethics approval was not required for this study as the study used existing non-identifiable data that were aggregated and population-level.
Data availability: Data are available in a public, open access repository. Data are available on https://gco.iarc.fr/. Processed datasets used in the analysis are available from the corresponding author upon reasonable request.
Footnotes
Funding: YZ is supported by Funds for PhD Researcher of Guangdong Medical University (4SG23187G). ML is supported by National Natural Science Foundation of China (82204131). HY is supported by the Medical Research Foundation of Guangdong Province (A2021395). JN is supported by National Natural Science Foundation of China (82273709) and the Natural Science Foundation of Guangdong Province in China (2021A1515011038). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Authorship contributions: YZ: conceptualisation, supervision, project administration, data curation, methodology, formal analysis, software, visualisation, writing – original draft, review & editing. HR: validation, visualisation writing – review & editing. ML: review & editing. HY: review & editing. HP: review & editing. JN: review & editing.
Disclosure of interest: The authors completed the ICMJE Declaration of Interest Form (available upon request from the corresponding author) and disclose no relevant interest.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2020. Available: https://gco.iarc.fr/today. Accessed: 30 December 2022.
- 3.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F.Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96-108. 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 4.Teoh JY, Huang J, Ko WY, Lok V, Choi P, Ng CF, et al. Global Trends of Bladder Cancer Incidence and Mortality, and Their Associations with Tobacco Use and Gross Domestic Product Per Capita. Eur Urol. 2020;78:893-906. 10.1016/j.eururo.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Grayson M.Bladder cancer. Nature. 2017;551:S33. 10.1038/551S33a [DOI] [PubMed] [Google Scholar]
- 6.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859-66. 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 7.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC.Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737-45. 10.1001/jama.2011.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S, Jee SH, Shin HR, Park EH, Shin A, Jung KW, et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer. 2014;14:406. 10.1186/1471-2407-14-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agudo A, Bonet C, Travier N, González CA, Vineis P, Bueno-de-Mesquita HB, et al. Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study. J Clin Oncol. 2012;30:4550-7. 10.1200/JCO.2011.41.0183 [DOI] [PubMed] [Google Scholar]
- 10.Safiri S, Kolahi AA, Naghavi M.Global, regional and national burden of bladder cancer and its attributable risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease study 2019. BMJ Glob Health. 2021;6:e004128. 10.1136/bmjgh-2020-004128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumberbatch MG, Rota M, Catto JW, La Vecchia C.The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur Urol. 2016;70:458-66. 10.1016/j.eururo.2015.06.042 [DOI] [PubMed] [Google Scholar]
- 12.Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur Urol. 2018;74:784-95. 10.1016/j.eururo.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 13.Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A.Epidemiology of Bladder Cancer. Medical sciences (Basel, Switzerland). 2020;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rushton L, Bagga S, Bevan R, Brown TP, Cherrie JW, Holmes P, et al. Occupation and cancer in Britain. Br J Cancer. 2010;102:1428-37. 10.1038/sj.bjc.6605637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234-41. 10.1016/j.eururo.2012.07.033 [DOI] [PubMed] [Google Scholar]
- 16.Silverman DT, Koutros S, Figueroa JD, Prokunina-Olsson L, Rothman N. 977Bladder Cancer. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, editors. Cancer Epidemiology and Prevention: Oxford University Press; 2017. [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A.Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021. 10.1002/ijc.33588 [DOI] [PubMed] [Google Scholar]
- 19.United Nations Statistics Division Standard country or area codes for statistical use (M49). Available: https://population.un.org/wpp/. Accessed 30 December 2022.
- 20.Segi M, Kurihara M. Cancer mortality for selected sites in 24 countries (1950-1957). 2 edn. Sendai: Tohoku University of Medicine; 1960. [Google Scholar]
- 21.Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical report. New York: Springer; 1966. [Google Scholar]
- 22.Day NE.Cancer incidence in five continents. Cumulative rate and cumulative risk. IARC Sci Publ. 1992;(120):862-4. [PubMed] [Google Scholar]
- 23.United Nations Development Programme. Human development report 2020. New York: United Nations; 2020. [Google Scholar]
- 24.R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available: https://www.R-project.org/. Accessed 30 December 2022.
- 25.SigmaPlot version 12.5; from Systat Software, Inc., San Jose California USA. Available: https://systatsoftware.com. Accessed 30 December 2022.
- 26.Moolgavkar SH, Stevens RG.Smoking and cancers of bladder and pancreas: risks and temporal trends. J Natl Cancer Inst. 1981;67:15-23. [PubMed] [Google Scholar]
- 27.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183-92. 10.1001/jama.2013.284692 [DOI] [PubMed] [Google Scholar]
- 28.Kauppinen T, Toikkanen J, Pedersen D, Young R, Ahrens W, Boffetta P, et al. Occupational exposure to carcinogens in the European Union. Occup Environ Med. 2000;57:10-8. 10.1136/oem.57.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaghloul MS.Bladder cancer and schistosomiasis. J Egypt Natl Canc Inst. 2012;24:151-9. 10.1016/j.jnci.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 30.Parkin DM.The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-44. 10.1002/ijc.21731 [DOI] [PubMed] [Google Scholar]
- 31.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145-54. 10.1016/S0140-6736(15)00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF.The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8:1129. 10.1038/s41598-018-19199-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richters A, Aben KKH, Kiemeney L.The global burden of urinary bladder cancer: an update. World J Urol. 2020;38:1895-904. 10.1007/s00345-019-02984-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcos-Gragera R, Mallone S, Kiemeney LA, Vilardell L, Malats N, Allory Y, et al. Urinary tract cancer survival in Europe 1999-2007: Results of the population-based study EUROCARE-5. European journal of cancer (Oxford, England: 1990). 2015;51:2217-30. 10.1016/j.ejca.2015.07.028 [DOI] [PubMed] [Google Scholar]
- 35.Kulhánová I, Forman D, Vignat J, Espina C, Brenner H, Storm HH, et al. Tobacco-related cancers in Europe: The scale of the epidemic in 2018. European journal of cancer (Oxford, England: 1990). 2020;139:27-36. 10.1016/j.ejca.2020.07.024 [DOI] [PubMed] [Google Scholar]
- 36.Li P, Deng SS, Wang JB, Iwata A, Qiao YL, Dai XB, et al. Occupational and environmental cancer incidence and mortality in China. Occup Med (Lond). 2012;62:281-7. 10.1093/occmed/kqs016 [DOI] [PubMed] [Google Scholar]
- 37.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM.Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-90. 10.1016/S2214-109X(19)30488-7 [DOI] [PubMed] [Google Scholar]
- 38.Cai Q, Chen Y, Xin S, Zhang D, Pan J, Xie Z, et al. Temporal trends of bladder cancer incidence and mortality from 1990 to 2016 and projections to 2030. Transl Androl Urol. 2020;9:153-65. 10.21037/tau.2020.02.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


