Abstract
In 2010, the American Heart Association defined a novel construct of cardiovascular health to promote a paradigm shift from a focus solely on disease treatment to one inclusive of positive health promotion and preservation across the life course in populations and individuals. Extensive subsequent evidence has provided insights into strengths and limitations of the original approach to defining and quantifying cardiovascular health. In response, the American Heart Association convened a writing group to recommend enhancements and updates. The definition and quantification of each of the original metrics (Life’s Simple 7) were evaluated for responsiveness to interindividual variation and intraindividual change. New metrics were considered, and the age spectrum was expanded to include the entire life course. The foundational contexts of social determinants of health and psychological health were addressed as crucial factors in optimizing and preserving cardiovascular health. This presidential advisory introduces an enhanced approach to assessing cardiovascular health: Life’s Essential 8. The components of Life’s Essential 8 include diet (updated), physical activity, nicotine exposure (updated), sleep health (new), body mass index, blood lipids (updated), blood glucose (updated), and blood pressure. Each metric has a new scoring algorithm ranging from 0 to 100 points, allowing generation of a new composite cardiovascular health score (the unweighted average of all components) that also varies from 0 to 100 points. Methods for implementing cardiovascular health assessment and longitudinal monitoring are discussed, as are potential data sources and tools to promote widespread adoption in policy, public health, clinical, institutional, and community settings.
Keywords: AHA Scientific Statements, health promotion, healthy lifestyle, life change events, public health, social determinants of health
In 2010, after decades of declines in cardiovascular disease (CVD) death rates, the American Heart Association (AHA) expanded its focus from addressing existing CVD and risk factors to adding strategies that would also directly promote the health of the population and individuals. Central to this new approach was the creation of a novel and operational definition for the construct of cardiovascular health (CVH).1
THE CONCEPT OF CARDIOVASCULAR HEALTH
In defining CVH, the AHA’s 2010 writing group acknowledged that health is a broader, more positive construct than merely the absence of disease. It leveraged relevant existing data and emerging prevention concepts to formulate a definition that was intended to be accessible for all, with actionable components for individuals, practitioners, researchers, and policymakers to focus efforts for improvement in CVH for all. Readers are referred to that document for a more detailed discussion of the rationale and genesis of the CVH construct. The initial definition of CVH1 was based on 7 health behaviors and health factors that, when optimal, were associated with greater CVD-free survival and total longevity and higher quality of life. The 7 components of CVH, subsequently called Life’s Simple 7, included indicators of dietary quality, participation in physical activity (PA), exposure to cigarette smoking, and measures of body mass index, fasting blood glucose, total cholesterol, and blood pressure (BP) levels. Each metric was classified as poor, intermediate, or ideal on the basis of accepted clinical thresholds. Overall, ideal CVH was defined as having all 7 metrics at ideal levels. Ideal CVH also formed the basis of a new definition of optimal brain health published in 2017.2
The rich experience with and evidence in support of this powerful new health construct over the past 12 years have created an opportunity to update the measurement of CVH in the current context. Now is the time to enhance the approach to quantifying the original metrics and to assess the potential value of additional metrics and data sources to better represent the full range of CVH and to further motivate individual and population health improvement. This advisory presents an updated and enhanced approach to measuring, monitoring, and modifying CVH—now called Life’s Essential 8 (Figure 1) after the inclusion of sleep as a new CVH component—to catalyze ongoing efforts to improve CVH in all individuals and the population.
Figure 1. Life’s Essential 8.

Life’s Essential 8 includes the 8 components of cardiovascular health: healthy diet, participation in physical activity, avoidance of nicotine, healthy sleep, healthy weight, and healthy levels of blood lipids, blood glucose, and blood pressure.
REVIEW OF KNOWLEDGE GAINED SINCE 2010
To date, >2500 scientific articles have cited the original 2010 document describing the AHA’s construct of CVH and explored the prevalence, determinants, outcomes, and mechanisms of CVH in diverse populations across the life course. A number of findings are highlighted here.
Prevalence of CVH
In the United States, the prevalence of ideal CVH is exceedingly low (<1%) for all age groups studied, including among individuals as young as 12 years of age.3 Overall CVH declines with age: The prevalence of having ≥5 metrics at ideal levels is only 45% among US adolescents, 32% among adults 20 to 39 years of age, 11% among adults 40 to 59 years of age, and 4% among adults ≥60 years of age.3 Thus, although some individuals can preserve higher levels of CVH, most will require some attention to achieve and maintain it into later life. The prevalence of ideal diet (as defined in 2010) is consistently negligible (<1%) across all age groups, driving the overall low prevalence of ideal CVH. Population levels of CVH in the United States have been low and fairly stagnant over the past 15 to 20 years,4 but this overall observation conceals several important findings. First, although some segments of the population are experiencing modest improvements in CVH, other groups (generally those at a lower socioeconomic position) are experiencing worsening CVH, creating a bimodal distribution.5 Second, there are persistent differences in the prevalence of CVH levels by self-reported race and ethnicity, and these disparities are larger at younger ages.3 The prevalence of high CVH also varies geographically6 and is higher in those who live in urban7 areas compared with those who live in rural areas. Furthermore, recent data indicate that the prevalence of high CVH is <1 in 10 during pregnancy,8 and poor CVH in pregnancy is associated with poor CVH in offspring, suggesting that ideal CVH is not universal even at birth.9
Outcomes of CVH
Numerous studies have shown strong, stepwise, inverse associations between the number of ideal CVH metrics or overall CVH score and total CVD and CVD mortality, all-cause mortality, and a wide variety of non-CVD outcomes. In all studies, those with higher CVH have markedly lower risks for CVD events. In a meta-analysis of 9 prospective cohort studies, having the highest number of ideal CVH metrics (generally ≥5 versus 0 to 2) was associated with a relative risk of 0.20 for CVD (95% CI, 0.11–0.37), 0.31 for stroke (95% CI, 0.25–0.38), 0.25 for CVD mortality (95% CI, 0.10–0.63), and 0.55 for all-cause mortality (95% CI, 0.37–0.80).10 Similar associations are observed across all age groups, down to as young as 8 years of age,11–15 and regardless of race and ethnicity or socioeconomic position.
All 7 original CVH metrics contribute to risks for health outcomes,12 and the importance of CVH behaviors is underscored by the association of optimal CVH behaviors with nearly 50% lower risk for coronary events among individuals at high genetic risk.16,17 It is never too late to realize benefits from improvement in CVH.18–20 However, the earlier that CVH is optimized, the better the outcomes are. Having higher CVH is associated with favorable long-term health outcomes at every age, and improvement in CVH over time is associated with lower risk for CVD.18–20 A recent analysis estimated that if all US adults maintained high CVH (defined in that article as 12–14 of 14 points on the CVH score), 2.0 million CVD events would be prevented each year.21 Better CVH has also been associated with lower risks for cancer, dementia, end-stage renal disease, and chronic obstructive pulmonary disease; better cognitive function and quality of life; compression of morbidity (longer health span); and lower health care costs despite a longer life span, among many other positive outcomes.22,23
Determinants of CVH
The heritability of overall CVH is low, indicating that behavioral and environmental exposures are paramount in determining CVH.24 Indeed, pursuing and sustaining a healthier lifestyle from a young age is a successful strategy for maintaining higher CVH into middle age.25–31 However, one’s ability to choose healthy lifestyles across the life course is strongly influenced by psychological health factors32,33 and social and structural determinants,34 as addressed later in detail (in the Foundational Factors for CVH: Psychological Health/Well-Being and Social Determinants section).
Mechanisms of CVH
Investigations of mechanisms through which higher CVH is associated with lower CVD risk (or lower CVH with higher risk) have identified several potential pathways involving inflammation, endothelial function, atherosclerosis, cardiac stress and remodeling, hemostatic factors, and epigenetics,35,36 among others.13,37,38 Two studies examined multiple potential pathways from low CVH status to clinical CVD events and found only partial statistical attenuation of the relationship after adjustment for a wide array of subclinical disease measures and biomarkers presumed to be in the causal pathway.13,37 Thus, beyond known CVD risk pathways, the protection conferred by optimal CVH may be “more than the sum of its parts.”39
Taken together, the substantial body of knowledge gained about CVH indicates that it is uniquely positioned as a health outcome itself related to upstream genetic, social, behavioral, and environmental factors, and as a determinant of major downstream health outcomes. Across the life course, assessment of CVH status has been shown to be an effective means to monitor public and individual health and a strong indicator of the extraordinary potential of primordial prevention strategies to improve and extend countless lives.
LESSONS LEARNED ABOUT CVH AND RATIONALE FOR REDEFINITION
As is evident from the previous review, a number of lessons have been learned about the original construct of CVH through its application and study in diverse settings. The collective experience of the scientific and medical community in using the original CVH construct to measure and improve CVH suggested several important considerations for this update.
First, some features of CVH component definitions may not have allowed appreciation of the full scope of health behaviors and practices in the current environment. For example, the original diet metric assessed intake of only 5 foods or nutrients (fruit and vegetables, fish, whole grains, sugar-sweetened beverages, and sodium). These dietary components were selected to represent an overall healthy eating pattern such as the DASH (Dietary Approaches to Stop Hypertension) eating pattern from variables available at the time in NHANES (National Health and Nutrition Examination Surveys). However, those 5 components are not the only features of a contemporary healthful eating pattern. The new metrics attempt to allow credit for a broader scope of health in each CVH component.
Second, over time, application of the CVH construct has increasingly been used to assess individual- and population-level CVH. This application has revealed limitations in how the metrics are quantified. Specifically, the original definitions of ideal, intermediate, and poor CVH for each component are less sensitive to interindividual differences and intraindividual change than is desirable. For example, the PA metric quantified intermediate CVH as 1 to 149 minutes of moderate to vigorous activity per week. Thus, 2 distinct individuals with widely different amounts (eg, 1 min/wk versus 149 min/wk) would be categorized as having intermediate PA, and an individual who changed their own PA from 1 to 149 min/wk would receive no credit for the substantial improvement in this health behavior. The newer approach to quantification of CVH is designed to be more sensitive and responsive to these considerations. There were also large shifts in some individuals’ CVH scores as they transitioned from childhood to adult metrics (some of which is unavoidable) that we have attempted to address.
Third, although CVH was designed to measure and monitor health trajectories over time, a novel contribution of this construct, it has also been used effectively to predict future risk for CVD and other health outcomes across the life course. This has proved to be a useful feature, especially for younger individuals (for whom risk equations are typically unavailable).
Fourth, although the metrics used to measure and monitor CVH are useful for describing health status and trajectories, they should not necessarily be used to promote specific interventions. In the example of the original diet metric, clinicians could recommend and consumers should pursue numerous strategies to improve their eating pattern beyond focusing solely on the 5 original nutrients and food groups. Generally speaking, then, in the promotion of CVH, the specific metrics (eg, the diet components) should not be confused with the health messaging (ie, people should pursue an overall heart-healthy diet such as the DASH- or Mediterranean-style eating patterns).
Fifth, the data on CVH change and its benefits consistently suggest that maintaining the highest possible levels of CVH on all metrics will lead to the best outcomes. Clinicians and consumers should focus on strategies that reinforce success and maintain high levels of overall CVH over time. However, if >1 metric is suboptimal or trending worse, they do not all need to be addressed simultaneously. Picking 1 CVH component at a time to improve, particularly if it is aligned with patient motivation, can lead to successful outcomes (as discussed in Implementation of CVH in Clinical Practice section).
Last, the original writing group contemplated the inclusion of sleep, stress, and other factors as metrics and acknowledged their contributions to overall CVH. However, at the time, the means for reliably measuring these domains in individuals and populations were limited. In the ensuing years, improved assessment techniques and emerging evidence have bolstered the importance of sleep and psychological health/well-being for CVH. In addition, there is increased awareness of the critical importance for CVH of social determinants of health (SDOH) and the underlying societal and structural issues that create them. These factors were of significant importance in the deliberations of the writing group.
In the next sections, we introduce the AHA’s new Life’s Essential 8, including affirmation of the foundational roles that psychological health/well-being and SDOH play in achieving optimal and equitable CVH in the population (in the Foundational Factors for CVH: Psychological Health/Well-Being and Social Determinants section); a new CVH component metric related to sleep (in the Sleep Health as a New Component of CVH section); and novel, updated methods for defining and quantifying CVH (in the Updated Definitions and Novel Quantitative Assessment of CVH Metrics section).
FOUNDATIONAL FACTORS FOR CVH: PSYCHOLOGICAL HEALTH/WELL-BEING AND SOCIAL DETERMINANTS
Over the past decade, key findings have illuminated the essential, foundational context of psychological health and well-being and SDOH for maintaining or improving CVH (Figure 2). Positive psychological health characteristics such as optimism, purpose in life, environmental mastery, perceived reward from social roles, and resilient coping are associated with more favorable CVH40–42; conversely, greater psychosocial stress and depression are associated with poorer CVH.43–45 SDOH provides the daily context for CVH and often determine the lifelong potential for CVH preservation and success or failure of interventions to improve CVH. A variety of favorable individual-level socioeconomic and social indicators are associated with higher CVH such as higher income, educational attainment, occupational status, and subjective social status and less social isolation, fewer racial discrimination experiences, and less incarceration.46–50 Likewise, favorable neighborhood-level factors such as greater resources, social cohesion, and built environment are also associated with higher CVH, although fewer neighborhood and community health resources are associated with poorer CVH.48,51–54 The writing group therefore judged that the context of psychological health/well-being and SDOH must be considered in attempts to assess and improve CVH in any patient or population. We therefore highlight these 2 critical, foundational factors first.
Figure 2. The foundational context of CVH.
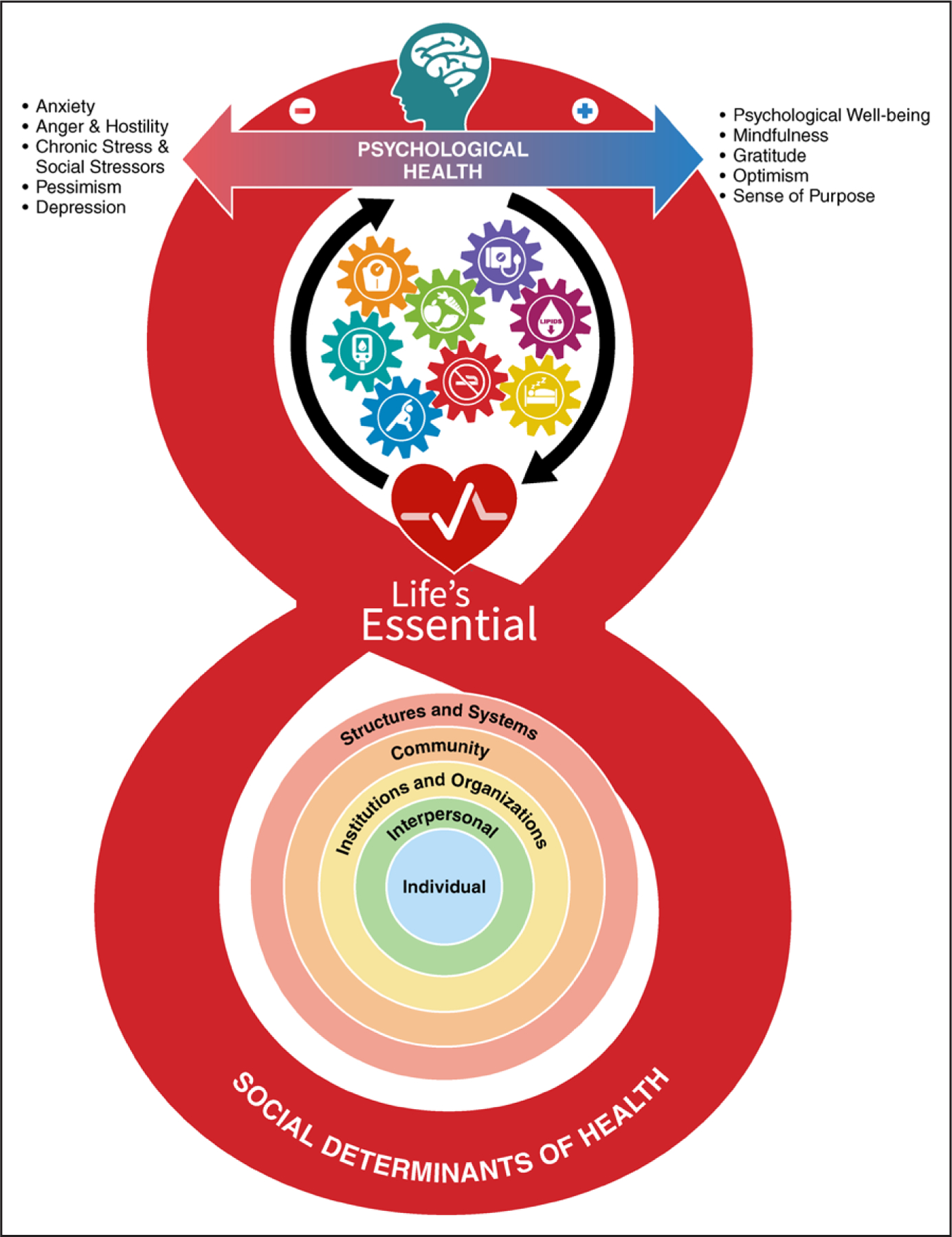
As depicted in the social-ecological model in the base of the figure 8, a variety of socioeconomic and structural determinants of health provide the foundational framework that affects an individual’s or a community’s ability to optimize cardiovascular health (CVH). Several interacting factors provide critical context for CVH, including structures and systems (general socioeconomic, cultural, and environmental conditions), community resources (ie, education, agriculture, food production, employment, water and sanitation, health care, and housing), institutions and organizations (in which people learn, grow, eat, sleep, play, and pray), interpersonal social and community networks, and individual genetic and behavioral factors. These foundational social-ecological factors work through and alongside an individual’s psychological health (represented at the top of the 8 in the figure) to provide the context for what is possible in improving or maintaining CVH. There is a continuous interplay of brain-mind-heart-body connections that can positively and negatively affect CVH, which is represented by the 8 component metrics (diet, physical activity, nicotine exposure, sleep, body mass index, blood lipids, blood glucose, and blood pressure) as interacting gears.
Psychological Health and Well-Being
A growing body of evidence supports the brain-mind-heart-body connection that can positively or negatively affect CVH, individual CVD risk factors, and cardiovascular outcomes. A recent AHA scientific statement33 reviewed a large number of studies that address a broad range of positive (eg, optimism, sense of purpose, happiness) and negative (eg, stress, depression, anxiety) psychological health factors and their significant associations with CVH and CVD risk. That statement guided much of the current writing group’s deliberations on the interactions of psychological health and well-being with CVH.
There are multiple direct and indirect pathways by which psychological health and well-being may influence CVH and CVD risk. These include physiological pathways (such as inflammatory response, glucose and lipid homeostasis, and coagulation) related to chronic stress, indirect effects on health behaviors that influence CVH, and changes in psychosocial resilience factors that promote or impair health or buffer detrimental effects of stressful experiences.33,55–59 The preponderance of data suggests that interventions to improve psychological health can have a beneficial impact on CVH.33 However, agreement on which psychological factors are the most robust predictors and correlates of CVH is lacking. Relatively simple questionnaires can be used by clinicians to assess psychological health status in the evaluation and management of patients with or at risk for CVD.33
On the basis of the reviewed evidence, the writing group judged that psychological health and well-being form a critical context and interact bidirectionally with the potential for preserving and improving CVH. Psychological health is multidimensional, and at this time, it is not clear how best to combine measures of psychological health or which indicator(s) may be most important for influencing CVH. When the writing group considered the nature of CVH metrics and improvement strategies, psychological health and well-being were judged to be more foundational, underlying all of the CVH metrics, rather than a distinct metric of CVH per se. For these reasons, the writing group elected to acknowledge the critical importance of psychological health and well-being and to strongly encourage more routine assessment and intervention in the clinical domain but not to include them as formal metrics of CVH at this time.
SDOH and Considerations for Equitably Improving CVH Across Contexts
SDOH is defined as the “structural determinants and conditions in which people are born, grow, live, work, and age” that affect health, functioning, and quality of life.60 There are 5 key domains of SDOH: economic stability, neighborhood and built environment, education, social and community context, and health and health care.61,62 Given this context, it is easy to understand how SDOH may directly and importantly affect an individual’s ability to optimize their CVH; the availability of healthy food and the ability to pay for it, safe places in which to pursue PA, health literacy, social support structures and networks, and access to and ability to pay for health care all directly influence CVH status.61
Disparities in CVH exist across a wide range of social strata, including race and ethnicity, socioeconomic position, geography, and rurality, among others,63,64 and inequities persist as a result of societal and structural barriers.34,64 The need to address CVH disparities has recently received considerable attention because persistence of these pervasive issues hinders achievement of health equity.34,65,66 Prior CVD disparities–focused interventions and initiatives have not proactively incorporated the full spectrum of complex psychosocial influences on CVH.67–71
Substantial research activity is currently focused on discovering the best means for representing and measuring SDOH in individuals and neighborhood environments. As with psychological health and well-being, the writing group judged that the best methods for measuring and quantifying SDOH are inadequately understood at this time, and the most important factors for preserving CVH in individuals and populations remain to be elucidated. Likewise, SDOH factors underlie much of the ability to optimize CVH rather than forming a single component of CVH. Accordingly, the writing group encourages further research on SDOH and CVH and urges consideration of SDOH in individual clinical attempts to improve CVH and in the design of community and population policies and interventions (see Implementation of CVH in Clinical Practice and Context and Opportunities for Improving CVH Going Forward).
SLEEP HEALTH AS A NEW COMPONENT OF CVH
Sleep is a foundational element of human biology and a requirement for life.72 Sleep is defined as “a naturally recurring, reversible state of perceptual disengagement, reduced consciousness, and relative immobility,”72 although its functions are wide ranging and affect nearly every physiological system.73 Numerous epidemiological studies have identified poor habitual sleep as a risk factor for all-cause mortality,74–83 and subsequent research has explored potential mechanisms,84–86 including implications for cardiometabolic health.
Much of the existing research has focused on sleep duration, although it should be noted that sleep health is a multidimensional construct with overlapping components, including duration, timing, regularity, efficiency, satisfaction, and impact on daytime alertness.87 Population-level studies have shown that inappropriate sleep duration (either shorter or longer than ideal) is associated with coronary heart disease.88 Sleep duration is associated with each of the original 7 components of CVH72,89–107 and with overall CVH score.108 Recent trends toward decreased sleep health in the population appear to account for some of the variance in changing cardiometabolic risk prevalence.109 Furthermore, recent evidence suggests that sleep metrics add independent predictive value for CVD events over and above the original 7 CVH metrics.110 It should be noted that poor sleep health is also known to be associated with poor psychological health111–114 and SDOH,84,115–120 important contextual drivers of CVH; therefore, for some individuals, sleep health assessment and intervention may require customized approaches that consider the surrounding context. As with many of the other CVH component metrics, sleep may also serve to integrate and mediate some of the effects of SDOH and psychological health on CVH. Several organizations have adopted sleep duration guidelines, recognizing the population health value of ≈7 to 8 hours of habitual sleep for adults and age-appropriate ranges of sleep duration for children.91,121–127
Although there is a paucity of evidence indicating that improving sleep duration or quality reduces CVD incidence, several other lines of evidence support its connection with CVH. For example, laboratory studies show that experimentally manipulated sleep affects BP, inflammation, glucose homeostasis, and other relevant factors. Larger observational studies show that small changes in sleep at the population level are associated with changes in CVD-related risk factors. Research indicates that real-world manipulation of sleep time is possible and that therefore sleep time is modifiable. Last, a limited number of studies demonstrate that real-world sleep manipulation is associated with changes in CVD-related risk factors.128,129 Nonetheless, overall, this is a research gap for which further investigation is warranted.
As a result of the above evidence, the ease and increasing reliability of measurement, and its comparable and independent contributions to overall and cardiometabolic health and health outcomes, the writing group elected to add sleep duration as an eighth metric to the formal definition of CVH. Its measurement and quantification are described in the next section with the other metrics.
UPDATED DEFINITIONS AND NOVEL QUANTITATIVE ASSESSMENT OF CVH METRICS
Here, we propose updated definitions and rescoring of the original 7 CVH metrics and the new sleep metric on a more continuous scale to better account for interindividual difference and intraindividual change (Table 1). The table should not be used as the sole guide for individuals to shape prevention or health promotion strategies. It is provided for the AHA, researchers, health systems, and policymakers to create standardized tools to measure and monitor CVH in individuals and populations. In the following paragraphs, descriptions of new or updated metric definitions are first presented, followed by a discussion of new measurement and quantification techniques.
Table 1.
New and Updated Metrics for Measurement and Quantitative Assessment of CVH (see Notes for implementation of each metric; See Supplemental Material for additional information on scoring of the Diet Metric, scoring in children at different ages, and examples of overall CVH scores in diverse scenarios)
| Domain | CVH metric | Method of measurement | Quantification of CVH metric: adults (≥20 y of age) | Quantification of CVH metric: children (up to 19 y of age) | ||
|---|---|---|---|---|---|---|
| Health behaviors | Diet | Measurement: Self-reported daily intake of a DASH-style eating pattern Example tools for measurement: DASH diet score130,131 (populations); MEPA132 (individuals) |
Quantiles of DASH-style diet adherence or HEI-2015 (population) | Quantiles of DASH-style diet adherence or HEI-2015 (population) or MEPA (individuals)*; ages 2–19 y (see Supplemental Material for younger ages) | ||
| Scoring (population): | Scoring (population): | |||||
| Points | Quantile | Points | Quantile | |||
| 100 | ≥95th percentile (top/ideal diet) | 100 | ≥95th percentile (top/ideal diet) | |||
| 80 | 75th–94th percentile | 80 | 75th–94th percentile | |||
| 50 | 50th–74th percentile | 50 | 50th–74th percentile | |||
| 25 | 25th–49th percentile | 25 | 25th–49th percentile | |||
| 0 | 1st–24th percentile (bottom/least ideal quartile) | 0 | 1st–24th percentile (bottom/least ideal quartile) | |||
| Scoring (individual): | Scoring (individual): | |||||
| Points | MEPA score (points) | Points | MEPA score (points) | |||
| 100 | 15–16 | 100 | 9–10 | |||
| 80 | 12–14 | 80 | 7–8 | |||
| 50 | 8–11 | 50 | 5–6 | |||
| 25 | 4–7 | 25 | 3–4 | |||
| 0 | 0–3 | 0 | 0–2 | |||
| PA | Measurement: Self-reported minutes of moderate or vigorous PA per week Example tools for measurement: NHANES PAQ-K questionnaire133 |
Metric: Minutes of moderate- (or greater) intensity activity per week: | Metric: Combustible tobacco use or inhaled NDS use at any age (per clinician discretion); or secondhand smoke exposure | |||
| Scoring: | Scoring: | |||||
| Points | Minutes | Points | Minutes | |||
| 100 | ≥150 | 100 | ≥420 | |||
| 90 | 120–149 | 90 | 360–419 | |||
| 80 | 90–119 | 80 | 300–359 | |||
| 60 | 60–89 | 60 | 240–299 | |||
| 40 | 30–59 | 40 | 120–239 | |||
| 20 | 1–29 | 20 | 1–119 | |||
| 0 | 0 | 0 | 0 | |||
| Nicotine exposure | Measurement: Self-reported use of cigarettes or inhaled NDS Example tools for measurement: NHANES SMQ134 |
Metric: Combustible tobacco use or inhaled NDS use; or secondhand smoke exposure | Metric: Combustible tobacco use or inhaled NDS use at any age (per clinician discretion); or secondhand smoke exposure | |||
| Scoring: | Scoring: | |||||
| Points | Status | Points | Status | |||
| 100 | Never smoker | 100 | Never tried | |||
| 75 | Former smoker, quit ≥5 y | 50 | Tried any nicotine product, but >30 d ago | |||
| 50 | Former smoker, quit 1–<5 y | |||||
| 25 | Former smoker, quit <1 y, or currently using inhaled NDS | 25 | Currently using inhaled NDS | |||
| 0 | Current smoker | 0 | Current combustible use (any within 30 d) | |||
| Subtract 20 points (unless score is 0) for living with active indoor smoker in home | Subtract 20 points (unless score is 0) for living with active indoor smoker in home | |||||
| Sleep health | Measurement: Self-reported average hours of sleep per night Example tools for measurement: “On average, how many hours of sleep do you get per night?” Consider objective sleep/actigraphy data from wearable technology if available | Metric: Average hours of sleep per night | Metric: Average hours of sleep per night (or per 24 h for age ≤5 y; see notes for age-appropriate ranges) | |||
| Scoring: | Scoring: | |||||
| Points | Level | Points | Level | |||
| 100 | 7–<9 | 100 | Age-appropriate optimal range | |||
| 90 | 9–<10 | 90 | <1 h above optimal range | |||
| 70 | 6–<7 | 70 | <1 h below optimal range | |||
| 40 | 5–<6 or ≥10 | 40 | 1–<2 h below or ≥1 h above optimal | |||
| 20 | 4–<5 | 20 | 2–<3 h below optimal range | |||
| 0 | <4 | 0 | ≥3 h below optimal range | |||
| Health factors | BMI | Measurement: Body weight (kilograms) divided by height squared (meters squared) Example tools for measurement: Objective measurement of height and weight |
Metric: BMI (kg/m2) | Metric: BMI percentiles for age and sex, starting in infancy; see Supplemental Material for suggestions for age <2 y | ||
| Scoring: | Scoring: | |||||
| Points | Level | Points | Level | |||
| 100 | <25 | 100 | 5th–<85th percentile | |||
| 70 | 25.0–29.9 | 70 | 85th–<95th percentile | |||
| 30 | 30.0–34.9 | 30 | 95th percentile–<120% of the 95th percentile | |||
| 15 | 35.0–39.9 | 15 | 120% of the 95th percentile–<140% of the 95th percentile | |||
| 0 | ≥40.0 | 0 | ≥140% of the 95th percentile | |||
| Blood lipids | Measurement: Plasma total and HDL cholesterol with calculation of non–HDL cholesterol Example tools for measurement: Fasting or nonfasting blood sample |
Metric: Non–HDL cholesterol (mg/dL) | Metric: Non–HDL cholesterol (mg/dL), starting no later than age 9–11 y and earlier per clinician discretion | |||
| Scoring: | Scoring: | |||||
| Points | Level | Points | Level | |||
| 100 | <130 | 100 | <100 | |||
| 60 | 130–159 | 60 | 100–119 | |||
| 40 | 160–189 | 40 | 120–144 | |||
| 20 | 190–219 | 20 | 145–189 | |||
| 0 | ≥220 | 0 | ≥190 | |||
| If drug-treated level, subtract 20 points | If drug-treated level, subtract 20 points | |||||
| Blood glucose | Measurement: FBG or casual HbA1c Example tools for measurement: Fasting (FBG, HbA1c) or non-fasting (HbA1c) blood sample |
Metric: FBG (mg/dL) or HbA1c (%) | Metric: FBG (mg/dL) or HbA1c (%), symptom-based screening at any age or risk-based screening starting at age ≥10 y of age or onset of puberty per clinician discretion | |||
| Scoring: | Scoring: | |||||
| Points | Level | Points | Level | |||
| 100 | No history of diabetes and FBG <100 (or HbA1c <5.7) | 100 | No history of diabetes and FBG <100 (or HbA1c < 5.7) | |||
| 60 | No diabetes and FBG 100–125 (or HbA1c 5.7–6.4) (prediabetes) | 60 | No diabetes and FBG 100–125 (or HbA1c 5.7–6.4) (prediabetes) | |||
| 40 | Diabetes with HbA1c <7.0 | 40 | Diabetes with HbA1c <7.0 | |||
| 30 | Diabetes with HbA1c 7.0–7.9 | 30 | Diabetes with HbA1c 7.0–7.9 | |||
| 20 | Diabetes with HbA1c 8.0–8.9 | 20 | Diabetes with HbA1c 8.0–8.9 | |||
| 10 | Diabetes with Hb A1c 9.0–9.9 | 10 | Diabetes with Hb A1c 9.0–9.9 | |||
| 0 | Diabetes with HbA1c ≥10.0 | 0 | Diabetes with HbA1c ≥10.0 | |||
| BP | Measurement: Appropriately measured systolic and diastolic BPs Example tools for measurement: Appropriately sized BP cuff |
Metric: Systolic and diastolic BPs (mm Hg) | Metric: Systolic and diastolic BP (mm Hg) percentiles for age through 12 y. For age ≥13 y, use adult scoring. Screening should start no later than age 3 y and earlier per clinician discretion | |||
| Scoring: | Scoring: | |||||
| Points | Level | Points | Level | |||
| 100 | <120/<80 (optimal) | 100 | Optimal (<90th percentile) | |||
| 75 | 120–129/<80 (elevated) | 75 | Elevated (≥90th–<95th percentile or ≥120/80 mm Hg to <95th percentile, whichever is lower) | |||
| 50 | 130–139 or 80–89 (stage 1 hypertension) | 50 | Stage 1 hypertension (≥95th–<95th percentile+12 mm Hg, or 130/80 to 139/89 mm Hg, whichever is lower) | |||
| 25 | 140–159 or 90–99 | 25 | Stage 2 hypertension (≥95th percentile+12 mm Hg, or ≥140/90 mm Hg, whichever is lower) | |||
| 0 | ≥160 or ≥100 | 0 | Systolic BP ≥160 or ≥95th percentile+30 mm Hg systolic BP, whichever is lower; and/or diastolic BP ≥100 or ≥95th percentile+20 mm Hg diastolic BP | |||
| Subtract 20 points if treated level | Subtract 20 points if treated level | |||||
| Points | Level (kg/m2) |
|---|---|
| 100 | 18.5–22.9 |
| 75 | 23.0–24.9 |
| 50 | 25.0–29.9 |
| 25 | 30.0–34.9 |
| 0 | ≥35.0 |
BMI indicates body mass index; BP, blood pressure; CVH, cardiovascular health; DASH, Dietary Approaches to Stop Hypertension; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HEI, Healthy Eating Index; MEPA, Mediterranean Eating Pattern for Americans; NDS, nicotine-delivery system; NHANES, National Health and Nutrition Examination Surveys; PA, physical activity; PAQ-K, Physical Activity Questionnaire K; and SMQ, smoking assessment.
Cannot meet these metrics until solid foods are being consumed.
Notes on implementation:
Diet: See Supplemental Material Appendix 1. For adults and children, a score of 100 points for the CVH diet metric should be assigned for the top (95th percentile) or a score of 15 to 16 on the MEPA (for individuals) or for those in the ≥95th percentile on the DASH score or HEI-2015 (for populations). The 75th to 94th percentile should be assigned 80 points, given that improvement likely can be made even among those in this top quartile. For individuals, the MEPA points are stratified for the 100-point scoring system approximately by quantiles. In children, a modified MEPA is suggested that is based on age-appropriate foods. The writing group recognizes that the quantiles may need to be adjusted or recalibrated at intervals with population shifts in eating patterns. In children, the scoring applies only once solid foods are being consumed. For now, the reference population for quantiles of HEI or DASH score should be the NHANES sample from 2015 to 2018. The writing group acknowledges that this may need to change or be updated over time. Clinicians should use judgment in assigning points for culturally contextual healthy diets. For additional notes on scoring in children, see Supplemental Material Appendix 2.
PA: Thresholds are based in part on US Physical Activity Guidelines. For adults, each minute of moderate activity should count as 1 minute and each minute of vigorous activity should count as 2 minutes toward the total for the week. For children, each minute of moderate or vigorous activity should count as 1 minute. The score for PA is not linear, given that there is a greater increase in health benefit for each minute of marginal exercise at the lower end of the range and the association tends to approach an asymptote at the higher end of the range.
If scoring is desired for children ≤5 years of age, see Supplemental Material. For additional notes on scoring in children, see Supplemental Material Appendix 2.
Nicotine exposure: The writing group recommends subtracting 20 points for children and adults exposed to indoor secondhand smoke at home, given its potential for long-term effects on cardiopulmonary health.135 For additional notes on scoring in children, see Supplemental Material Appendix 2.
Sleep health: Thresholds are based in part on sleep guidelines. Clinicians may consider subtracting 20 points from the sleep score for adults or children with untreated or undertreated sleep apnea if information is available. Note that overall scoring reflects the inverse-U–shaped association of sleep duration with health outcomes, such that excessive sleep duration is also considered to be suboptimal for CVH.
For children, age-appropriate optimal sleep durations are as follows121:
Age 4 to 12 months, 12 to 16 hours per 24 hours (includes naps);
Age 1 to 2 years, 11 to 14 hours per 24 hours;
Age 3 to 5 years, 10 to 13 hours per 24 hours;
Age 6 to 12 years, 9 to 12 hours; and
Age 13 to 18 years, 8 to 10 hours.
For additional notes on scoring in children, see Supplemental Material Appendix 2.
BMI: Thresholds are based in part on National Heart, Lung, and Blood Institute (NHLBI) guidelines. The writing group acknowledges that BMI is an imperfect metric for determining healthy body weight and body composition. Nonetheless, it is widely available and routinely calculated in clinical and research settings. BMI ranges may differ for individuals from diverse ancestries. For example, the World Health Organization has recommended different BMI ranges for individuals of Asian or Pacific ancestry. For individuals in these groups, point scores should be aligned as appropriate:
Clinicians may want to assign 100 points for overweight individuals (BMI, 25.0–29.9 kg/m2) who are lean with higher muscle mass. For underweight individuals (<18.5 kg/m2 in adults or below the fifth percentile in children), the writing group defers to clinician judgment in assigning points on the basis of individual assessment as to whether the underweight BMI is healthy or unhealthy. Conditions that should be considered unhealthy include chronic catabolic illnesses (eg, cancer), eating disorders, and growth failure (for children). For additional notes on scoring in children, see Supplemental Material Appendix 2.
Blood lipids: Thresholds are based in part on 2018 Cholesterol Clinical Practice Guideline.129a The levels of non–HDL cholesterol for adults were selected on the basis of current guideline recommendations and in concert with the observation that non–HDL cholesterol levels are generally ≈30 mg/dL higher than low-density lipoprotein cholesterol levels in normative ranges in the population. For children, thresholds for non–HDL cholesterol were chosen on the basis of NHLBI pediatric guidelines, pediatric low-density lipoprotein cholesterol thresholds for diagnosis of familial hypercholesterolemia phenotypes (+30 mg/dL), and current distributions of non–HDL cholesterol to smooth transitions to adult point scales. The writing group recommends subtracting 20 points from the blood lipid score if the level of non–HDL-cholesterol represents a treated value, given the residual risk present in those who require treatment. There may be a modest shift in point scores for this metric as individuals age from pediatric to adult metrics. For additional notes on scoring in children, see Supplemental Material Appendix 2.
Blood glucose: Thresholds are based in part on American Diabetes Association guidelines.129b If an individual patient with prediabetes (ie, not yet diagnosed formally with diabetes) is being treated with metformin to prevent the onset of diabetes and has normoglycemic levels, the writing group recommends clinician judgment for assigning point values (ie, consider subtracting 20 points). The maximal point value for patients with well-controlled diabetes was set at 40, given the residual risk present in those with diabetes. For additional notes on scoring in children, see Supplemental Material Appendix 2.
BP: Thresholds are based in part on the 2017 Hypertension Clinical Practice Guidelines and the guidelines for children.129c The writing group recommends subtracting 20 points from the BP score if the level of BP represents a treated value, given the residual risk present in those who require treatment. For additional notes on scoring in children, see Supplemental Material Appendix 2.
Several of the original 7 metrics have been redefined for consistency with newer clinical guidelines, to better represent their biological impact, or for compatibility with new measurement tools, as summarized here:
Diet: A new method is proposed for assessing dietary quality for both rapid individual assessment in clinical settings and population-level assessment in other settings, along with a suggested means for linking and aligning these assessments, when needed, through the Healthy Eating Index136 (see Supplemental Material for full details). The writing group supports the overall goal of pursuing DASH- and Mediterranean-style eating patterns as being most consistent with optimal CVH. That said, there is no one such eating pattern, and there are limited tools for assessing alignment with these eating patterns. The DASH-style eating pattern is more easily assessed at the population level for the United States, although it is more difficult at the individual level. Therefore, a rapid dietary assessment tool is suggested for individuals that is a modified Mediterranean Eating Pattern for Americans (MEPA). The writing group selected what we judge to be the best available tools and calls for directed research to advance the field and identify even better standardized and rapid assessment tools. This new approach provides a focus on individuals’ eating patterns and intake of whole foods, rather than nutrients, that should promote implementation in clinical and research settings.
Nicotine exposure: Use of inhaled nicotine-delivery systems (eg, e-cigarettes or vaping devices) has been added to the former metric, which included only combustible cigarette use, to reflect adult and childhood use of these products and their implications for long-term health.137,138 Secondhand tobacco smoke exposure has also been added to the definition to reflect its adverse impact on health.135,139
Sleep health: As noted, the writing group endorses the systematic assessment and inclusion of sleep duration as the current means for reflecting sleep health within the construct of CVH.
Blood lipids: The metric for blood lipids has been updated to consider non–high-density lipoprotein cholesterol as the metric of interest rather than total cholesterol because non–high-density lipoprotein cholesterol can be measured in the nonfasting state and reliably calculated in all patients (unlike low-density lipoprotein cholesterol) and because of the lifelong associations demonstrated for different atherogenic lipoprotein fractions, all of which are represented in the non–high-density lipoprotein cholesterol measurement.
Blood glucose: The metric for blood glucose has been expanded to include hemoglobin A1c measurement (in individuals with or without diabetes) and to better reflect glycemic control among diabetic patients.
PA, body mass index, and BP: The writing group discussed these metrics and elected to use the same metric definitions (but with updated scoring, as for all the metrics).
Childhood metrics were updated to reflect current pediatric guidelines, to extend to younger ages when appropriate, and to better align with transitions to adulthood. More detailed discussion of the approach to quantifying CVH in children, especially children <6 years of age, is provided in the Supplemental Material Appendix 2.
For ease of reference in clinical or research settings, the 8 metrics making up the new CVH definition have been grouped into the 2 domains of health behaviors (diet, PA, nicotine exposure, sleep) and health factors (body mass index, blood lipids, blood glucose, BP).
For the approach to quantification of the metrics and overall CVH, various methods were considered. Also considered was the desire for ease of programming metric scores to create applications (apps) and online CVH assessment tools, as well as for incorporation into electronic health records (EHRs) and other platforms. Because some metrics do not lend themselves to fully continuous quantification scales and because some associations of metrics with health are nonlinear, the writing group judged that an ordinal point scoring system for each metric (ranging from 0 to 100 points) was most appropriate. We used a modified Delphi approach among the expert panel members to arrive at point score levels, informed by health outcomes and risk associations. We also examined US population distributions (from recent NHANES) and resulting effects on the metric-specific and overall CVH scores to arrive at the final point assignments for each metric. The group also strove to ensure that, across metrics, similar point value differences were associated with approximately similar impacts on health. Final point scores for each metric are displayed in Table 1. The writing group acknowledges that these point scores are somewhat arbitrary, but we judge that this approach is a substantial improvement to be able to detect interindividual differences and population and individual changes in CVH over time. Further research is warranted to understand all of the implications of the algorithm. The writing group judged that use of categorical descriptors (ideal, intermediate, poor) for each metric was no longer desirable, electing instead for less pejorative and more supportive presentations of the entire spectrum of each metric, from lower to higher, to encourage intervention and change for improvement.
For overall CVH, the writing group continues to endorse a composite, aggregate score for measuring, monitoring, and assessing change in CVH. The new aggregate score is also scaled from 0 to 100 points, calculated as the unweighted average of all 8 component metric scores. Examples of CVH calculation for different scenarios are shown in the Supplemental Material. The writing group recommends that presentation of CVH score or status for individuals and populations should consider images or icons that reflect the entire spectrum of CVH, including some indication of more and less desirable states (such as red/yellow/green coloration; example shown in Figure 3) across a range of 0 to 100. For some purposes, it may continue to be useful to consider categorical consideration of overall CVH; the writing group recommends that overall CVH scores of 80 to 100 be considered high CVH; 50 to 79, moderate CVH; and 0 to 49 points, low CVH. New research is encouraged in samples from diverse populations, across the life course, and in diverse settings to assess the implications of the new scoring of the metrics and overall CVH. Further discussion of implementation considerations in various contexts is provided below (see Implementation of CVH in Clinical Practice and Context and Opportunities for Improving CVH Going Forward sections).
Figure 3. Example presentation of CVH score.
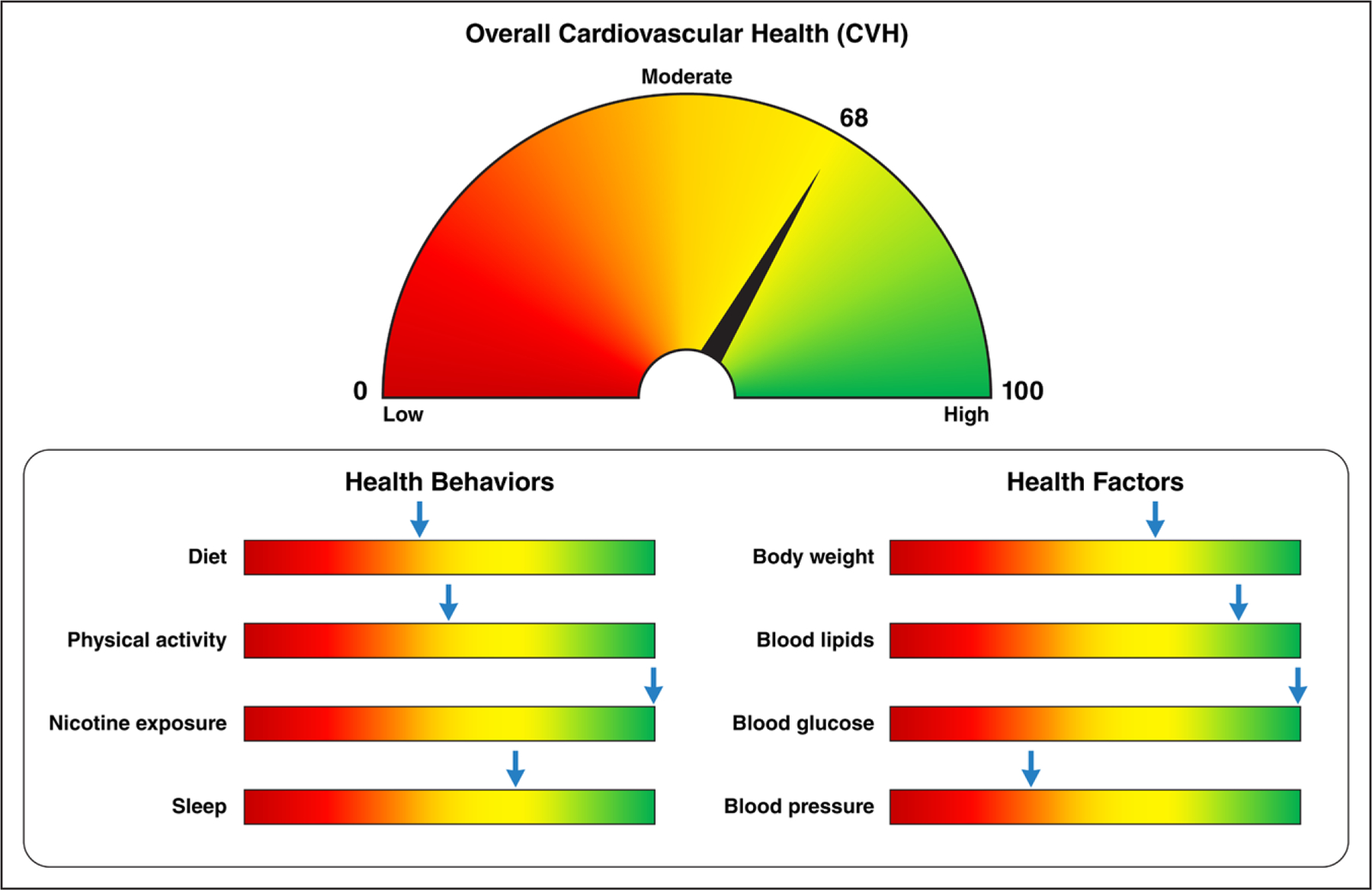
The figure provides an example of how to represent an individual’s cardiovascular health (CVH) assessment with the new Life’s Essential 8. The gauge at the top corresponds to the individual’s overall CVH score (which can range from 0–100 points), with higher scores shown toward the right (a “full tank” of CVH) in green. The individual’s status for each of the 8 component metrics is shown below, thus identifying health behaviors and risk factors on which the individual can focus to achieve and maintain a full tank of overall CVH. The overall CVH score is the unweighted average of the 8 component metric scores.
DATA SOURCES FOR MONITORING CVH
In the original 2010 monograph defining CVH, NHANES data were identified as the best available source with which to monitor population-level CVH. NHANES continues to have a number of advantages, including representative sampling across demographic groups, inclusion of all CVH metrics (including the newly added sleep metric) at most ages, in-person examinations, and sustainability as part of population health monitoring by the Centers for Disease Control and Prevention. NHANES data have limitations, including missing some CVH metrics at the youngest ages, small samples in any given year of some racial and ethnic subgroups, limited generalizability for individuals living in the most deprived conditions, and being limited to the US population. Additional data sources will be needed to fully understand the scope of CVH within these populations and beyond the United States. Alternative data sources such as cohort data, EHRs, national surveys, and registries may be useful in many instances but can have significant limitations that currently prevent their routine use for population monitoring of CVH. For now, it is recommended that NHANES remain the main data source for tracking the US population’s CVH over time, although focused efforts to optimize other data sources, as described below, are encouraged.
Major advances in data science and informatics should be harnessed to better understand CVH in individuals and populations going forward. Large health information exchanges such as the National Patient-Centered Clinical Research Network140 offer comprehensive EHR registries that can be used to track individuals over time. As these health information exchanges have evolved, many now include large swaths of the United States and comprehensively cover most major metropolitan areas, although information on CVH in rural populations may still be limited. Notably, EHRs include only individuals who seek care; they miss individuals who have difficulty accessing or paying for care, particularly well care. EHRs have been shown to provide reliable prevalence rates for some chronic conditions such as diabetes that require routine care, although their validity and reliability for other conditions such as obesity141 are lower. Although EHR systems have great potential, at present, they typically contain limited behavioral and lifestyle information; thus, they may need dedicated work to generate systematic and standardized inputs that include diet, PA, smoking, and sleep metrics of CVH and to structure these data in useful formats. The AHA can continue to lead in this arena by identifying brief and valid methods of assessing these lifestyle constructs that can then be documented within the medical records. Several are suggested in this document (Table 1). In addition, understanding and tracking the fundamental roles of SDOH and psychological health in CVH through EHRs remain challenging but important (see Implementation of CVH in Clinical Practice section).
Combining EHR data with lifestyle data collected via surveys or wearable technology offers the ability for individuals (or their health care professionals) to monitor their CVH over time and to help preserve high CVH when it exists or intervene early if declines in CVH are detected. A variety of strategies have been proposed to collect lifestyle data and house it within the EHR system,142 including the use of brief questionnaires on computers or tablets in the waiting room or through patient portals before a visit. Some investigators and companies have created standalone apps to collect self-reported lifestyle data,143,144 although these have the disadvantage of not being integrated within the EHR. As technology continues to evolve, wearable devices may offer the ability to replace self-reported data on PA, BP, and sleep with objectively measured data. Ultimately, pragmatic— and automated if feasible—data collection is central to simple and effective individual and population monitoring of CVH over time. In turn, the aforementioned health technology platforms can provide motivation to individuals to engage in behavior change to favorably affect CVH. The writing group encourages the AHA to be a leader in the development and dissemination of these technologies to improve CVH.
Taken together, these advances offer the possibility of monitoring CVH across an individual’s life span and for surveillance within the population in the near future. These more systematically collected and comprehensive data may ultimately assist the AHA and policymakers in providing insight into population CVH metrics. Other opportunities may arise through existing or new large-scale crowd-sourcing efforts to monitor health (eg, Project Baseline, Health eHeart).
IMPLEMENTATION OF CVH IN CLINICAL PRACTICE
In addition to the social-ecological context for CVH promotion (Figure 2), clinical implementation is crucial. With the proliferation of health technologies noted previously, health systems, individuals, and families can participate in the pragmatic collection of CVH data. In turn, the AHA or health systems need to provide platforms that assist individual patients and their health care teams in assessing their CVH and tracking their progress over time through online websites or apps. These same health technology platforms can serve to aggregate CVH data for population health monitoring and risk prediction or for intervening in CVH by motivating behavior change among diverse populations.145
Implementing the CVH Metric in Diverse Populations and Settings and Leveraging Health Technologies for Pragmatic Data Collection and CVH Intervention
Most CVH metrics (body mass index, BP, cholesterol, fasting glucose/hemoglobin A1c, and smoking status) are captured as structured fields in the EHR. Health systems are well positioned to leverage the EHR for population health monitoring, risk prediction, and intervening on CVH in patient populations across the life course.146 Examples of CVH data visualization tools that have been integrated with the EHR in learning health care systems include Stroke Prevention in Healthcare Delivery Environments and Priorities Wizard, among others.147 Clinicians have the opportunity to use these tools with patients at the point of care to raise awareness of CVH and use shared decision-making approaches to help patients preserve or achieve optimal CVH.148 For greater success, it is suggested that clinicians use motivational interviewing strategies to help patients identify those metrics that would benefit from improvement and for which the patient expresses some motivation for change and can envision the means to do so. Better skills training for clinicians in these areas is critical for success. Likewise, it may be helpful to have the patient focus on a single health behavior or health factor at a time for improvement rather than trying to change too many things at once, which may risk the patient feeling overwhelmed or experiencing a sense of failure.
Universal efforts and tailored, culturally appropriate methods will be needed to direct individuals to resources for improving or maintaining CVH, potentially ameliorating the negative impacts of SDOH and psychological health and promoting positive social and psychological assets.143,149 Ubiquitous health technology can put important CVH information in the hands of traditionally underserved populations. However, contextual factors (SDOH, access to health care and health technology, health literacy, and psychological health) mediate individuals’ access to health technology and information and can affect their ability to maintain or achieve optimal CVH.145 The AHA and other stakeholders will need to be mindful of these issues in the design of data collection and intervention tools.
Communication of the New CVH Score With Patients
Clinical and general populations alike are accustomed to hearing health status framed as risk (implying negative or bad) for a given disease or condition. When the new CVH, Life’s Essential 8, is implemented in diverse settings, it should be presented in a positive manner through a lay-friendly format to ensure accurate interpretation, to indicate its strong associations with favorable health outcomes, and to provide motivation for behavior change as needed. Indeed, high CVH is a positive outcome in and of itself and can bring about immediate health benefits for an individual. Each assessment and reassessment serve as reinforcement of CVH metrics that have remained favorable with an opportunity to arrest decline in others. Tools from the AHA and its partners for clear communication will be needed so that it can be delivered by health care professionals, EHRs, and health technologies and accompanied by suggested steps one can take to improve CVH and monitor progress over time. Health care system and government programs should also be designed to catalyze implementation of CVH improvement strategies in venues beyond the clinic such as through evidence-based individual, family, or group interventions. Newer strategies of patient self-management also show promise for engaging and empowering patients to improve aspects of their CVH.150
CONTEXT AND OPPORTUNITIES FOR IMPROVING CVH GOING FORWARD
In this section and Table 2, we highlight selected examples of successful and promising strategies across the spheres of influence of the social-ecological model (Figure 2), aimed at preserving and improving population-wide CVH across the life course. Taken together, these interventions can serve as road maps for policymakers, health systems, institutions, clinicians, researchers, and communities for the future development and translation of SDOH-informed, equitable solutions to ensure attainment of CVH equity for diverse populations.
Table 2.
Multilevel Efforts to Improve CVH Across Social-Ecological Contexts
| Ecological level | Selected examples | Examples of key gaps and needed directions |
|---|---|---|
| Policies | ||
| Federal | FDA regulation of tobacco products Robust school nutrition standards and healthy school meals for all Active transportation infrastructure investment Ensuring affordable, equitable, adequate access to health insurance for all151 Public health infrastructure investment, data modernization, and surveillance systems upgrade152,153 |
Premarket approval of newer tobacco products154 Regulating synthetic nicotine155 Removing all characterizing flavors from all tobacco products156 Continued support for implementation and increasing sodium reduction, promotion of whole grains; developing an added sugars standard Ensuring that federal appropriations flow effectively to the state and local levels for biking, walking, and rolling, reaching all people equitably, particularly those in the most underserved and underinvested communities157 Preserving and building on the Affordable Care Act Optimizing value-based insurance design158,159 Continued federal investment of the data modernization and surveillance systems upgrade to ensure seamless integration across all levels of government and health systems Protecting and expanding the public health workforce |
| State | Tobacco end game strategies (eg, comprehensive smoke-free air laws, tobacco excise taxes, comprehensive coverage and access to tobacco cessation services, tobacco retail strategies, and removing all characterizing flavors from all tobacco products)138 Medicaid expansion and Medicaid coverage of extended postpartum coverage, self-measured BP, telehealth160–162 |
Effective coordination and engagement across public health, social justice, and equity partners Need for robust public and private investment in the tobacco end game, overcoming industry product innovation, targeted marketing, and positioning Housing, income, and transportation issues for the Medicaid population Ensuring that states can use all means at their disposal to offset costs of expansion163 and to increase access to services |
| Local | Sugar-sweetened beverage taxes164,165 Increasing access to early care and education166 | Combatting industry opposition and preemption efforts Significant commitment to funding for advocacy campaigns and ground softening efforts State preemption of local efforts Inadequate workforce compensation167,168 Disruption caused by the COVID-19 pandemic169 |
| Advocacy groups | AHA Voices for Healthy Kids grantees policy work | |
| Public health programs | ||
| Federal | Healthy People 2020 and 2030 Million Hearts WISEWOMAN NHLBI ENRICH/home visiting program partnership Head Start |
Tailored sociocultural messaging for diverse populations in partnership with relevant stakeholders |
| State/national | AHA’s Go Red for Women | Tailored sociocultural messaging for diverse populations in partnership with relevant stakeholders |
| Local | Mass media campaigns to promote healthy behaviors and risk factor control170,171 | |
| Institutions | ||
| Early childcare/education | Chicago Child-Parent Center Education Program Longitudinal Study172 | Broader implementation |
| Schools and colleges | AHA/NFL Play60 AHA/Clinton Foundation Alliance for a Healthier Generation AHA Kids Heart Challenge School-based tobacco prevention,171,173 PA promotion, sugar-sweetened beverage reduction |
Delineate specific intervention components most effective in promoting CVH and best approaches to implementation174 |
| Workplaces | AHA Workforce Well-Being Playbook and Corporate Recognition Program175 NIOSH Total Worker Health Centers of Excellence |
Implementation, particularly including workplaces more likely to employ individuals who may be socioeconomically impacted |
| Health care systems (eg, insurance/payers, hospitals, practitioners) | AHA Get With The Guidelines SPHERE176 | Broader implementation |
| Neighborhoods and communities | ||
| Community-serving programs | Strong Hearts, Healthy Communities intervention177,178 AHA SFRN-funded Hearts & Parks/Bull City Fit intervention179 CDC Prevention Research Centers |
Outreach to broader rural communities with geographic barriers to access |
| Private community settings | Faith-based interventions (FAITH trial, FAITH!) Barbershop interventions | Evaluation of design elements sufficient for large-scale dissemination and implementation in community settings and broad population health impact180 Expansion of rigorously tested CVH promotion interventions to other community venues (eg, hair salons,181 community centers) in partnership with civic organizations182 (eg, sororities, fraternities) |
| Neighborhood environments | Green space, corner store interventions183,184 | Development of methods to increase consumer demand and to foster sustainability of corner store interventions in various neighborhood/environmental contexts (eg, urban vs rural)185 Specific assessment of impacts of green space interventions on health equity and potential adverse effects (eg, gentrification and reduced access)186 |
| Virtual communities | Interactive, group social media interventions187 | Culturally responsive interventions to promote CVH |
| Families and individuals | ||
| Parents/children | AHA Simple Cooking With Heart for Kids AHA/Aramark Health for Life nutrition education curriculum INSIGHT intervention STRIP intervention |
|
| Adults | Health-partner intervention Mobile technology for stroke prevention |
|
AHA indicates American Heart Association; BP, blood pressure; CDC, Centers for Disease Control and Prevention; CVH, cardiovascular health; ENRICH, Early Intervention to Promote Cardiovascular Health of Mothers and Children; FAITH, Faith-Based Approaches in the Treatment of Hypertension; FAITH!, Fostering African-American Improvement in Total Health; FDA, US Food and Drug Administration; INSIGHT, Intervention Nurses Start Infants Growing on Healthy Trajectories; NFL, National Football League; NHLBI, National Heart, Lung, and Blood Institute; NIOSH, National Institute for Occupational Safety and Health; PA, physical activity; SFRN, Strategically-Focused Research Network; SPHERE, Stroke Prevention in Healthcare Delivery Environments; STRIP, Special Turku Coronary Risk Factor Intervention Project; and WISEWOMAN, Well-Integrated Screening and Evaluation for Women Across the Nation.
Policies
Over the past decade, the AHA has partnered with other volunteer science organizations on presidential advisories, clinical practice guidelines, and health policy statements focused on primordial, primary, and secondary prevention and promotion of optimal CVH throughout the life course. These efforts have also increased awareness about the SDOH and their driving influence on CVH disparities and have provided recommendations for addressing them.2,33,71,147,161,188–192 Although interventions focused on the entire construct of CVH are exceedingly rare, health policy statements have focused on several important structural, contextual, and intergenerational factors that can promote overall optimal CVH (Table 2), including but not limited to access to quality health care, healthy foods, and recreational facilities for leisure-time PA.193 Ongoing advocacy efforts at the federal, state, and community levels must continue for improvement in population-wide CVH. Indeed, policy-level solutions are often the only ways to address issues such as health care reform (for CVH monitoring and intervention), transformation of PA and healthy meal programs in schools, regulation of tobacco/nicotine products, and accessibility to a healthier food supply.
The writing group particularly acknowledges the emerging evidence on the critical importance of preconception maternal health, gestational health, pregnancy outcomes, and follow-up peripartum care to improve the health of women and children to launch successively healthier generations.161,194,195 Further research and expanded programs are needed, including better metrics for monitoring progress in addressing disparities in maternal health outcomes. Likewise, a number of successful policies for promoting and sustaining better health from childhood through adolescence have been developed, demonstrated, and supported by the AHA and its partners.193 Future efforts are needed, given the COVID-19 pandemic and its associated worsening health inequities.
Public Health Programs
Several successful public health programs have been developed to address CVH disparities in recent decades. In alignment with the AHA, the US Department of Health and Human Services–led Healthy People 2020 and 2030 public health initiatives have identified identical indicators for overall CVH for nationwide health improvement goals, including CVD prevention.196 The systematic, evidence-based approach encourages cross-sector community collaborations for health promotion, including state-specific benchmarks, with an overarching mission to achieve health equity for all population groups. Nonetheless, disparities by race and ethnicity and geographic regions have persisted, indicating the complexity of SDOH and psychological health as key barriers and facilitators to optimal CVH.197,198 Additional broad-based initiatives focused on risk factor control in individuals (eg, the Million Hearts and Well-Integrated Screening and Evaluation for Women Across the Nation programs) complement these efforts.199–201 The AHA’s Go Red for Women and similar programs have been instrumental in raising heart disease awareness to promote CVH among women.125,127,202–204 Room for optimization of these initiatives exists through enhanced cross-fertilization efforts across key stakeholder groups for tailored sociocultural messaging among high-priority populations.205–208 The writing group urges the AHA to engage all of its partners in new broad-based communication strategies to raise awareness and to enhance engagement with the new CVH construct across all sectors to improve population CVH.
Institutions
Institutions such as early childhood care or education centers, schools, and workplaces have unique opportunities to preserve and promote optimal CVH through engagement of their large, captive populations. For example, preschool programs providing comprehensive educational and family support172 can improve multigenerational CVH and positively affect numerous life course outcomes other than CVH such as socioeconomic position, justice-system involvement, and addiction.209 School-based programs such as health education and screenings retain their influence through adolescence and young adulthood, including at colleges and universities, to promote positive CVH behaviors among youth,171,173 although widespread implementation remains a challenge.174 Starting in adolescence and extending through most of adulthood, workplace wellness programs gain importance. Workplace programs align employee and employer incentives; they can generate savings not only from reduced health care costs but also reduced absenteeism and improved employee engagement.210 The AHA Workplace Wellness Playbook offers recognition for workplaces with high-quality programs.210,211 Implementation of such programs across all types of workplaces, including those most relevant to individuals who may be socioeconomically impacted, needs focused attention.
Neighborhoods and Communities
Promising interventions to promote CVH have demonstrated the importance of going beyond traditional venues (outside of the clinical setting or academia) to places where individuals actually live, learn, work, play, and pray within neighborhoods and communities.212 This paradigm of “meeting people where they are” while considering the sociocultural context of individuals and their families is at the heart of several community-based interventions. These programs have successfully leveraged social capital and trust building with individuals belonging to traditionally underserved and marginalized groups, especially for underrepresented racial and ethnic populations.213–218 Successful examples of multidisciplinary hypertension interventions for Black men based in barbershops and other community venues are justifiably celebrated. In addition, culturally tailored interventions incorporating faith-based tenets and delivered in partnership with churches have resulted in significant improvements in CVH.219–226 Other community-serving programs with a clinic-to-community link by way of municipal parks and recreation centers179,227,228 have aimed to build a culture of health for youth by providing community-centered support for a lifelong commitment to healthy lifestyle.177,178 Furthermore, there is evidence to support multicomponent corner store interventions in addressing food insecurity in food deserts/swamps in both rural and urban areas.183,184,229–236
Of utmost importance are collaborative, equitable community health needs assessments to allow a better understanding of community priorities/needs, socioeconomic constraints/barriers, and strengths/assets to ensure that deployed interventions within underserved communities are relevant, meaningful, scalable, and sustainable.237–246 Through its Empowered to Serve initiative and significant investments through its Social Impact Funds, the AHA has galvanized a movement to reduce CVH disparities in underresourced communities by supporting community advocates, social justice leaders, social entrepreneurs, and locally owned businesses in implementing community-led models to improve SDOH.247
Life Course and Intergenerational Perspectives on CVH Promotion Across Ecological Levels
Figure 4 depicts key windows and transitions in the life course of CVH, along with examples of opportunities to preserve or improve CVH at every stage. Pregnancy and the periods around it (preconception, postpartum) set the stage for the offspring’s CVH potential and represent an important transition period and physiologic stressor for mothers. It is at once a period of great opportunity given universal coverage of (although not necessarily access to) health care during pregnancy but also a taxing and vulnerable time for mothers and families. Early childhood is key for establishing healthy CVH behaviors, with preschool age thought to be a particularly important window. Adolescence through very early adulthood is a period of rapid development physically, mentally, emotionally, and socially. The transition to full responsibility for self comes with competing priorities for limited resources of attention, often including parenthood, and appears to be a sensitive period for CVH loss.248 Contexts outside of the young adult’s own health care such as college, workplace, community, and family-based/child health care settings gain importance. Middle age may offer new perspectives and changing roles that can be leveraged by workplace, community, and health care contexts to control risk factors, improve CVH, and prevent CVD events. In older age, access to well-being supports through communities, neighborhoods, and health care systems can help prevent frailty, promote active living, extend healthy longevity, and improve quality of life through CVH promotion.
Figure 4. Life course of CVH.
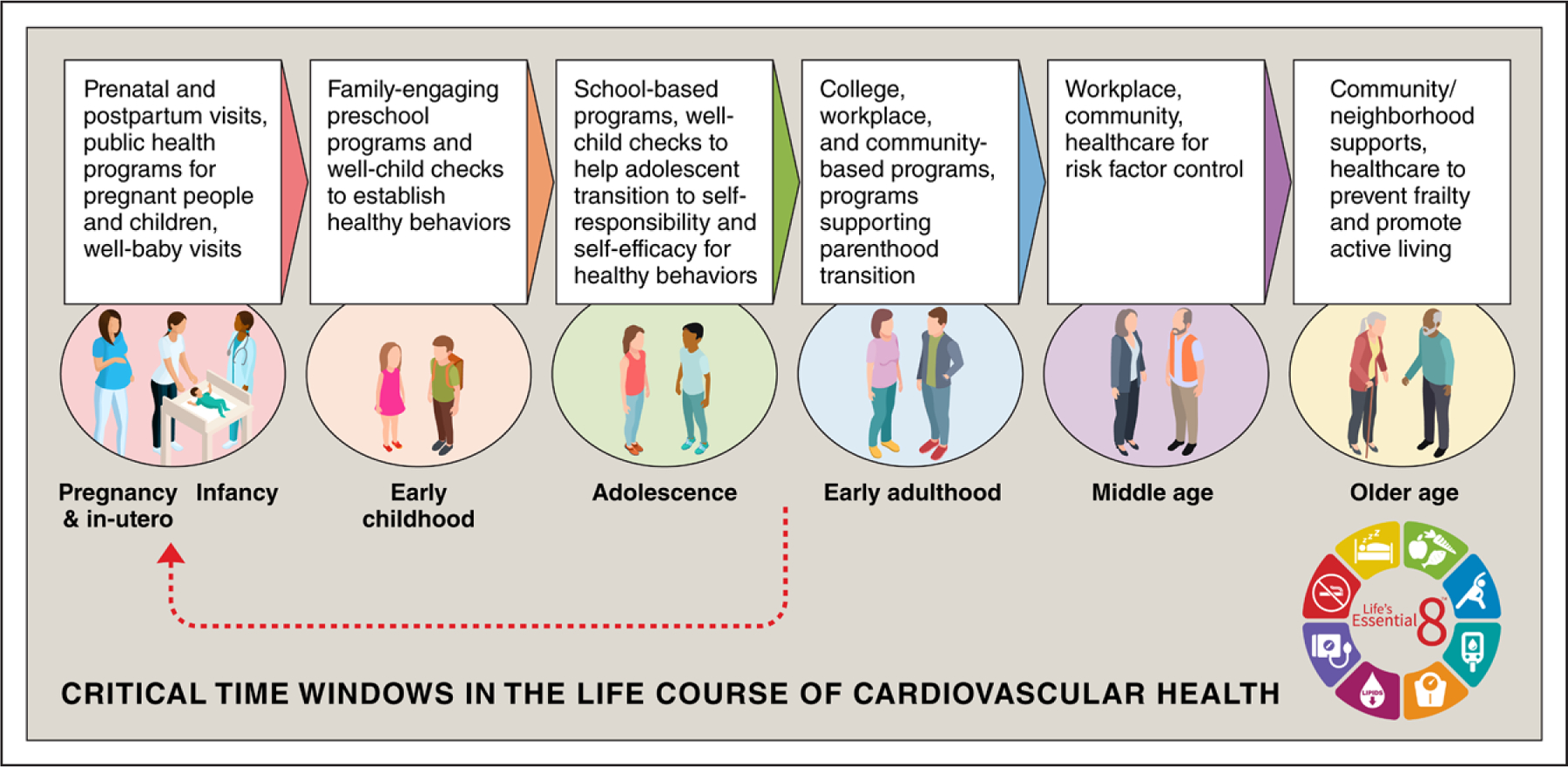
The figure describes sensitive periods and transitions for cardiovascular health (CVH) across the life span, along with example opportunities for intervention to preserve or promote CVH at each age or stage. Opportunities to improve CVH occur across public health and policy, institutional, neighborhood- and community-level, and clinical contexts. Red arrow indicates the feedback loop of primordial prevention strategies that can maintain CVH through early life, leading to healthier parents before conception and a subsequent generation of healthier children.
RESEARCH GAPS AND FUTURE DIRECTIONS
The extensive knowledge gained about the construct of CVH since 2010 provided the basis for the current update and enhancement. Nonetheless, numerous knowledge gaps and research opportunities remain to ensure that the utility, implementation, and impact of CVH can be optimized. In addition, there is now need for study of this new approach to measuring and monitoring CVH in diverse settings. Some research gaps and proposed future directions identified by the writing group are presented in Table 3.
Table 3.
Example Research Needs and Future Directions
| Enhanced definition and scoring and potentially different metrics for CVH in pregnancy, at birth, and during the earliest years of life (especially <2 y of age); enhanced data collection at individual and population levels during these life stages |
| Assessment of the new CVH scoring algorithm across the life course and in diverse populations to understand its utility for describing individual and population health, strengths and limitations, and trends in CVH over time |
| Focused research to tie classification of existing metrics at the youngest ages based on guidelines (eg, PA) to meaningful outcomes, eg, CVH or BMI at older ages in childhood or subclinical CVD in midlife |
| Investigation of novel measures or biomarkers to represent overall CVH at young age |
| Development and validation of short, clinically feasible surveys for each age group/developmental stage in children |
| Utility of new CVH score for predicting diverse health outcomes, including total mortality, healthy longevity (compression of morbidity), cardiovascular events, and other chronic disease outcomes, especially in younger people |
| Research driving to consensus on best tools for assessment of health behaviors in clinical settings |
| Greater routine and standardized assessment of diet, PA, nicotine exposure, and sleep health in clinical and population settings to facilitate CVH measurement and monitoring |
| Greater routine and standardized assessment of psychological health and well-being and SDOH, as well as their associations with CVH in clinical and population settings |
| Discovery, demonstration, and dissemination of successful strategies (eg, policies, clinical strategies, community interventions, individual behavioral changes) to preserve or improve overall CVH in individuals and in diverse settings and populations |
| Research on other metrics of sleep related to CVH and interventions to improve CVH through improved sleep health |
| Enhanced medical education and training to equip clinicians with the tools to assess CVH, to implement motivational interviewing, to assist patients with behavioral change or maintenance, to promote wellness strategies, and to avoid bias and stigma around adverse health behaviors and factors (eg, nicotine exposure or obesity) |
| Deployment of apps, online tools, and code for implementation of CVH scoring in consumer-facing, clinical, population, and research settings |
| Optimization of broad-based communication strategies to promote and preserve CVH and tailored communication strategies for diverse cultural settings and demographic groups |
Apps indicates applications; BMI, body mass index; CVD, cardiovascular disease; CVH, cardiovascular health; PA, physical activity; and SDOH, social determinants of health.
CONCLUSIONS AND VISION FOR LIFE’S ESSENTIAL 8
The formal definition of CVH in 2010 represented the culmination of decades of evolution in epidemiology, public health, clinical care, and prevention concepts. Supported by the AHA, the rapid uptake of the CVH concept by researchers, policymakers, funding agencies, communities, and, to a certain extent, patients and clinicians has provided a road map for reenergizing individual and population health promotion strategies and reducing the ongoing substantial burden of CVD and other chronic health conditions. The CVH construct provided the means for positive, actionable steps that could be taken to measure, monitor, and modify CVH through primordial, primary, and secondary prevention approaches. The knowledge gained over the past 12 years has reinforced the need for addressing limitations and enhancing utility to advance this construct even further. The goal is to unite efforts around improving a wide array of health outcomes and quality of life through a positively framed, responsive health construct of CVH used consistently across the life course and in diverse settings. Greater insight into the foundational aspects of SDOH and psychological health in which CVH occurs will likely lead to further refinements of CVH definitions in the future.
The present document presents a substantial update of and enhancement to the means for measuring and monitoring CVH to address the current context of public health and to assist the AHA and its partners in efforts to achieve greater health equity by promoting better CVH for all. The new means for defining and measuring CVH, Life’s Essential 8, in individuals and populations represents a major step forward in our ability to intervene by promoting and reinforcing healthy metrics while averting decline of those with potential for unfavorable trajectories. It should help re-energize efforts to develop, test, and disseminate interventions to maintain or improve CVH at every stage across the life course, in diverse settings, and for all people. Primordial prevention efforts focused on early life are likely to have particularly beneficial effects in initiating healthier trajectories of lifelong CVH and creating healthier parents of healthier babies in future generations.
In business management, it is often said that “if you cannot measure it, you cannot improve it.” With this enhanced measurement tool for CVH, the AHA and its numerous multisector partners in schools, communities, government, health care, business, and beyond have new opportunities to catalyze CVH improvement by raising awareness of its importance, promoting platforms for its measurement, funding research on interventions, and disseminating successful strategies. Capitalizing on these opportunities will be a critical step toward ensuring that every person has the opportunity for optimal CVH and a full, healthy life.
Supplementary Material
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Donald M. Lloyd-Jones | Northwestern University Feinberg School of Medicine | None | None | None | None | None | None | None |
| Norrina B. Allen | Northwestern University Feinberg School of Medicine | NIH†; AHA† | None | None | None | None | None | None |
| Cheryl A.M. Anderson | UCSD | None | None | None | None | None | WW†; McCormick Science Foundation† | None |
| Terrie Black | University of Massachusetts College of Nursing | None | None | None | None | None | None | None |
| LaPrincess C. Brewer | Mayo Clinic College of Medicine | AHA*; NIH*; CDC*; ACC* | None | None | None | None | None | ABC*; ACC*; Am J Prev Card*; J Am Med Assoc* |
| Randi E. Foraker | Washington University in St. Louis, School of Medicine | NIH/NCI (1R01CA226078)† | None | None | None | None | None | None |
| Michael A. Grandner | University of Arizona College of Medicine | None | None | None | None | None | None | None |
| Helen Lavretsky | UCLA | NIH (PI)†; PCORI (PI)†; NCCIH† | None | None | None | None | None | None |
| Amanda Marma Perak | Lurie Children’s Hospital and Northwestern University | NHLBI (K23 grant that examines molecular mechanisms of CVH in children)†; Northwestern University (multiple small pilot grants investigating CVH in pregnant people and youth)† | None | None | None | None | None | None |
| Wayne Rosamond | University of North Carolina Gillings School of Global Public Health | None | None | None | None | None | None | None |
| Garima Sharma | Johns Hopkins University School of Medicine | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Donna K. Arnett | University of Kentucky | None | None | None | None | None | None | None |
| Robert H. Eckel | University of Colorado Anschutz Medical Campus | None | None | None | None | None | None | None |
| Heather M. Johnson | Christine E. Lynn Women’s Health & Wellness Institute Charles E. Schmidt College of Medicine, Florida Atlantic University | NIH/NHLBI (coinvestigator)* | None | None | None | None | None | None |
| Amit Khera | UT Southwestern Medical Center | None | None | None | None | None | None | None |
| Latha P. Palaniappan | Stanford University | NIH (K24 Award)† | None | None | None | None | None | None |
| Linda V. Van Horn | Northwestern University, Feinberg School of Medicine | NIH† | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Footnotes
Supplemental material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIR.0000000000001078.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This advisory was approved by the American Heart Association Science Advisory and Coordinating Committee on May 9, 2022, and the American Heart Association Executive Committee on May 16, 2022. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215–356-2721 or email Meredith.Edelman@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, Rosamond W; on behalf of the American Heart Association. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/aboutus/statements-and-policies/copyright-request-form).
Disclosures
Writing Group Disclosures
Reviewer Disclosures
Contributor Information
Donald M. Lloyd-Jones, Northwestern University Feinberg School of Medicine.
Norrina B. Allen, Northwestern University Feinberg School of Medicine.
Cheryl A.M. Anderson, UCSD.
Terrie Black, University of Massachusetts College of Nursing.
LaPrincess C. Brewer, Mayo Clinic College of Medicine.
Randi E. Foraker, Washington University in St. Louis, School of Medicine.
Michael A. Grandner, University of Arizona College of Medicine.
Helen Lavretsky, UCLA.
Amanda Marma Perak, Lurie Children’s Hospital and Northwestern University.
Garima Sharma, Johns Hopkins University School of Medicine.
Wayne Rosamond, University of North Carolina Gillings School of Global Public Health.
REFERENCES
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. ; on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, et al. ; on behalf of the American Heart Association/American Stroke Association. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017;48:e284–e303. doi: 10.1161/STR.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 4.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 5.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation 2012;125:2595–2602. doi: 10.1161/CIRCULATIONAHA.111.070722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilkerton CS, Singh SS, Bias TK, Frisbee SJ. Changes in cardiovascular health in the United States, 2003–2011. J Am Heart Assoc 2015;4:e001650. doi: 10.1161/JAHA.114.001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence E, Hummer RA, Harris KM. The cardiovascular health of young adults: disparities along the urban-rural continuum. Ann Am Acad Pol Soc Sci 2017;672:257–281. doi: 10.1177/0002716217711426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd-Jones DM. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc 2020;9:e015123. doi: 10.1161/JAHA.119.015123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perak AM, Lancki N, Kuang A, Labarthe DR, Allen NB, Shah SH, Lowe LP, Grobman WA, Lawrence JM, Lloyd-Jones DM, et al. ; HAPO Follow-Up Study Cooperative Research Group. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA 2021;325:658–668. doi: 10.1001/jama.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang N, Jiang M, Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int J Cardiol 2016;214:279–283. doi: 10.1016/j.ijcard.2016.03.210 [DOI] [PubMed] [Google Scholar]
- 11.Perak AM, Ning H, Khan SS, Bundy JD, Allen NB, Lewis CE, Jacobs DR Jr, Van Horn LV, Lloyd-Jones DM. Associations of late adolescent or young adult cardiovascular health with premature cardiovascular disease and mortality. J Am Coll Cardiol 2020;76:2695–2707. doi: 10.1016/j.jacc.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polonsky TS, Ning H, Daviglus ML, Liu K, Burke GL, Cushman M, Eng J, Folsom AR, Lutsey PL, Nettleton JA, et al. Association of cardiovascular health with subclinical disease and incident events: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2017;6:e004894. doi: 10.1161/JAHA.116.004894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen NB, Krefman AE, Labarthe D, Greenland P, Juonala M, Kähönen M, Lehtimäki T, Day RS, Bazzano LA, Van Horn LV, et al. Cardiovascular health trajectories from childhood through middle age and their association with subclinical atherosclerosis. JAMA Cardiol 2020;5:557–566. doi: 10.1001/jamacardio.2020.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Kou F, Yang S, Wang S, He Y, Zhang W. Ideal cardiovascular health in the oldest-old and centenarians and its association with disability and health-related quality of life. Front Cardiovasc Med 2021;8:603877. doi: 10.3389/fcvm.2021.603877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasbani NR, Ligthart S, Brown MR, Heath AS, Bebo A, Ashley KE, Boerwinkle E, Morrison AC, Folsom AR, Aguilar D, et al. American Heart Association’s Life’s Simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation 2022;145:808–818. doi: 10.1161/CIRCULATIONAHA.121.053730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Yano Y, Cho SMJ, Lee HH, Kim DW, Lloyd-Jones DM, Kim HC. Associations of ideal cardiovascular health and its change during young adulthood with premature cardiovascular events: a nationwide cohort study. Circulation 2021;144:90–92. doi: 10.1161/CIRCULATIONAHA.121.054212 [DOI] [PubMed] [Google Scholar]
- 19.Gaye B, Tajeu GS, Vasan RS, Lassale C, Allen NB, Singh-Manoux A, Jouven X. Association of changes in cardiovascular health metrics and risk of subsequent cardiovascular disease and mortality. J Am Heart Assoc 2020;9:e017458. doi: 10.1161/JAHA.120.017458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Song L, Li D, Zhou Z, Chen S, Yang Y, Hu Y, Wang Y, Wu S, Tian Y. Ideal cardiovascular health metric and its change with lifetime risk of cardiovascular diseases: a prospective cohort study. J Am Heart Assoc 2021;10:e022502. doi: 10.1161/JAHA.121.022502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bundy JD, Zhu Z, Ning H, Zhong VW, Paluch AE, Wilkins JT, Lloyd-Jones DM, Whelton PK, He J, Allen NB. Estimated impact of achieving optimal cardiovascular health among US adults on cardiovascular disease events. J Am Heart Assoc 2021;10:e019681. doi: 10.1161/JAHA.120.019681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities study. Circulation 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen NB, Hwang S-J, Cupples LA, Levy D, Fox C, O’Donnell C, Lloyd-Jones D. The heritability of ideal cardiovascular health: the Framingham Heart Study. Circulation 2010;122:A17245–A17245. Abstract 17245. [Google Scholar]
- 25.Pahkala K, Laitinen TT, Niinikoski H, Kartiosuo N, Rovio SP, Lagström H, Loo BM, Salo P, Jokinen E, Magnussen CG, et al. Effects of 20-year infancy-onset dietary counselling on cardiometabolic risk factors in the Special Turku Coronary Risk Factor Intervention Project (STRIP): 6-year post-intervention follow-up. Lancet Child Adolesc Health 2020;4:359–369. doi: 10.1016/S2352-4642(20)30059-6 [DOI] [PubMed] [Google Scholar]
- 26.Matthews LA, Rovio SP, Jaakkola JM, Niinikoski H, Lagström H, Jula A, Viikari JSA, Rönnemaa T, Simell O, Raitakari OT, et al. Longitudinal effect of 20-year infancy-onset dietary intervention on food consumption and nutrient intake: the randomized controlled STRIP study. Eur J Clin Nutr 2019;73:937–949. doi: 10.1038/s41430-018-0350-4 [DOI] [PubMed] [Google Scholar]
- 27.Allen NB, Lloyd-Jones D, Hwang SJ, Rasmussen-Torvik L, Fornage M, Morrison AC, Baldridge AS, Boerwinkle E, Levy D, Cupples LA, et al. Genetic loci associated with ideal cardiovascular health: a meta-analysis of genome-wide association studies. Am Heart J 2016;175:112–120. doi: 10.1016/j.ahj.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, Moller AC, Lloyd-Jones DM. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation 2012;125:996–1004. doi: 10.1161/CIRCULATIONAHA.111.060681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 2014;130:10–17. doi: 10.1161/CIRCULATIONAHA.113.005445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gooding HC, Shay CM, Ning H, Gillman MW, Chiuve SE, Reis JP, Allen NB, Lloyd-Jones DM. Optimal lifestyle components in young adulthood are associated with maintaining the ideal cardiovascular health profile into middle age. J Am Heart Assoc 2015;4:e002048. doi: 10.1161/JAHA.115.002048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahkala K, Hietalampi H, Laitinen TT, Viikari JS, Rönnemaa T, Niinikoski H, Lagström H, Talvia S, Jula A, Heinonen OJ, et al. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] study). Circulation 2013;127:2088–2096. doi: 10.1161/CIRCULATIONAHA.112.000761 [DOI] [PubMed] [Google Scholar]
- 32.Whitaker KM, Jacobs DR Jr, Kershaw KN, Demmer RT, Booth JN 3rd, Carson AP, Lewis CE, Goff DC Jr, Lloyd-Jones DM, Gordon-Larsen P, et al. Racial disparities in cardiovascular health behaviors: the Coronary Artery Risk Development in Young Adults Study. Am J Prev Med 2018;55:63–71. doi: 10.1016/j.amepre.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine GN, Cohen BE, Commodore-Mensah Y, Fleury J, Huffman JC, Khalid U, Labarthe DR, Lavretsky H, Michos ED, Spatz ES, et al. ; on behalf of the American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Lifestyle and Cardiometabolic Health. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation 2021;143:e763–e783. doi: 10.1161/CIR.0000000000000947 [DOI] [PubMed] [Google Scholar]
- 34.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. ; on behalf of the American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 35.Joyce BT, Gao T, Zheng Y, Ma J, Hwang SJ, Liu L, Nannini D, Horvath S, Lu AT, Bai Allen N, et al. Epigenetic age acceleration reflects long-term cardiovascular health. Circ Res 2021;129:770–781. doi: 10.1161/CIRCRESAHA.121.318965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pottinger TD, Khan SS, Zheng Y, Zhang W, Tindle HA, Allison M, Wells G, Shadyab AH, Nassir R, Martin LW, et al. Association of cardiovascular health and epigenetic age acceleration. Clin Epigenetics 2021;13:42. doi: 10.1186/s13148-021-01028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014;130:1676–1683. doi: 10.1161/CIRCULATIONAHA.114.009273 [DOI] [PubMed] [Google Scholar]
- 38.Gaye B, Tafflet M, Arveiler D, Montaye M, Wagner A, Ruidavets JB, Kee F, Evans A, Amouyel P, Ferrieres J, et al. Ideal cardiovascular health and incident cardiovascular disease: heterogeneity across event subtypes and mediating effect of blood biomarkers: the PRIME study. J Am Heart Assoc 2017;6:e006389. doi: 10.1161/JAHA.117.006389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd-Jones DM. Cardiovascular health and protection against CVD: more than the sum of the parts? Circulation 2014;130:1671–1673. doi: 10.1161/CIRCULATIONAHA.114.012869 [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Islam SJ, Topel ML, Ko YA, Mujahid MS, Vaccarino V, Liu C, Sims M, Mubasher M, Searles CD, et al. Individual psychosocial resilience, neighborhood context, and cardiovascular health in Black adults: a multilevel investigation from the Morehouse-Emory Cardiovascular Center for Health Equity Study. Circ Cardiovasc Qual Outcomes 2020;13:e006638. doi: 10.1161/CIRCOUTCOMES.120.006638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart A, Barinas-Mitchell E, Matthews KA, Magnani JW, Khoudary SE, Jackson EA, Brooks MM . Social role stress, reward and the American Heart Association Life’s Simple 7 in midlife women: the Study of Women’s Health Across the Nation. Abstract. Circulation 2018;137:AMP56. Accessed February 15, 2022. 10.1161/circ.137.suppl_1.mp56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boehm JK, Soo J, Chen Y, Zevon ES, Hernandez R, Lloyd-Jones D, Kubzansky LD. Psychological well-being’s link with cardiovascular health in older adults. Am J Prev Med 2017;53:791–798. doi: 10.1016/j.amepre.2017.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brewer LC, Redmond N, Slusser JP, Scott CG, Chamberlain AM, Djousse L, Patten CA, Roger VL, Sims M. Stress and achievement of cardiovascular health metrics: the American Heart Association Life’s Simple 7 in Blacks of the Jackson Heart Study. J Am Heart Assoc 2018;7:e008855. doi: 10.1161/JAHA.118.008855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogunmoroti O, Osibogun O, Spatz ES, Okunrintemi V, Mathews L, Ndumele CE, Michos ED. A systematic review of the bidirectional relationship between depressive symptoms and cardiovascular health. Prev Med 2022;154:106891. doi: 10.1016/j.ypmed.2021.106891 [DOI] [PubMed] [Google Scholar]
- 45.Winning A, McCormick MC, Glymour MM, Gilsanz P, Kubzansky LD. Childhood psychological distress and healthy cardiovascular lifestyle 17–35 years later: the potential role of mental health in primordial prevention. Ann Behav Med 2018;52:621–632. doi: 10.1093/abm/kax001 [DOI] [PubMed] [Google Scholar]
- 46.Caleyachetty R, Echouffo-Tcheugui JB, Muennig P, Zhu W, Muntner P, Shimbo D. Association between cumulative social risk and ideal cardiovascular health in US adults: NHANES 1999–2006. Int J Cardiol 2015;191:296–300. doi: 10.1016/j.ijcard.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 47.Saiz AM Jr, Aul AM, Malecki KM, Bersch AJ, Bergmans RS, LeCaire TJ, Nieto FJ. Food insecurity and cardiovascular health: Findings from a statewide population health survey in Wisconsin. Prev Med 2016;93:1–6. doi: 10.1016/j.ypmed.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foraker RE, Bush C, Greiner MA, Sims M, Henderson K, Smith S, Bidulescu A, Shoben AB, Hardy NC, O’Brien E. Distribution of cardiovascular health by individual- and neighborhood-level socioeconomic status: findings from the Jackson Heart Study. Glob Heart 2019;14:241–250. doi: 10.1016/j.gheart.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 49.Shockey TM, Sussell AL, Odom EC. Cardiovascular health status by occupational group–21 states, 2013. MMWR Morb Mortal Wkly Rep 2016;65:793–798. doi: 10.15585/mmwr.mm6531a1 [DOI] [PubMed] [Google Scholar]
- 50.Piedra LM, Andrade FCD, Hernandez R, Perreira KM, Gallo LC, González HM, Gonzalez S, Cai J, Chen J, Castañeda SF, et al. Association of subjective social status with Life’s Simple 7s Cardiovascular Health Index among Hispanic/Latino people: results from the HCHS/SOL. J Am Heart Assoc 2021;10:e012704. doi: 10.1161/JAHA.119.012704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Islam SJ, Kim JH, Baltrus P, Topel ML, Liu C, Ko YA, Mujahid MS, Vaccarino V, Sims M, Mubasher M, et al. Neighborhood characteristics and ideal cardiovascular health among Black adults: results from the Morehouse-Emory Cardiovascular (MECA) Center for Health Equity. Ann Epidemiol 2022;65:120.e1–120.e10. doi: 10.1016/j.annepidem.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unger E, Diez-Roux AV, Lloyd-Jones DM, Mujahid MS, Nettleton JA, Bertoni A, Badon SE, Ning H, Allen NB. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic Study of Atherosclerosis. Circ Cardiovasc Qual Outcomes 2014;7:524–531. doi: 10.1161/CIRCOUTCOMES.113.000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laitinen TT, Pahkala K, Venn A, Woo JG, Oikonen M, Dwyer T, Mikkilä V, Hutri-Kähönen N, Smith KJ, Gall SL, et al. Childhood lifestyle and clinical determinants of adult ideal cardiovascular health: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Princeton Follow-Up Study. Int J Cardiol 2013;169:126–132. doi: 10.1016/j.ijcard.2013.08.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthews KA, Boylan JM, Jakubowski KP, Cundiff JM, Lee L, Pardini DA, Jennings JR. Socioeconomic status and parenting during adolescence in relation to ideal cardiovascular health in Black and White men. Health Psychol 2017;36:673–681. doi: 10.1037/hea0000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maia DB, Marmar CR, Mendlowicz MV, Metzler T, Nóbrega A, Peres MC, Coutinho ES, Volchan E, Figueira I. Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. J Affect Disord 2008;107:259–263. doi: 10.1016/j.jad.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schins A, Honig A, Crijns H, Baur L, Hamulyák K. Increased coronary events in depressed cardiovascular patients: 5-HT2A receptor as missing link? Psychosom Med 2003;65:729–737. doi: 10.1097/01.psy.0000088596.42029.10 [DOI] [PubMed] [Google Scholar]
- 57.von Känel R, Hepp U, Buddeberg C, Keel M, Mica L, Aschbacher K, Schnyder U. Altered blood coagulation in patients with posttraumatic stress disorder. Psychosom Med 2006;68:598–604. doi: 10.1097/01.psy.0000221229.43272.9d [DOI] [PubMed] [Google Scholar]
- 58.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 2014;140:774–815. doi: 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sumner JA, Nishimi KM, Koenen KC, Roberts AL, Kubzansky LD. Posttraumatic stress disorder and inflammation: untangling issues of bidirectionality. Biol Psychiatry 2020;87:885–897. doi: 10.1016/j.biopsych.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Thiene D, Marceca M. Closing the gap in a generation: health equity through action on the social determinants of health: a challenge for the international community [in Italian]. Ann Ig 2008;20:595–601. [PubMed] [Google Scholar]
- 61.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, et al. ; on behalf of the American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132:873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 62.US Department of Health and Human Services. Social determinants of health: Healthy People 2020 Accessed January 30, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
- 63.Diaz CL, Shah NS, Lloyd-Jones DM, Khan SS. State of the nation’s cardiovascular health and targeting health equity in the United States: a narrative review. JAMA Cardiol 2021;6:963–970. doi: 10.1001/jamacardio.2021.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao X, Kershaw KN, Barber S, Schreiner PJ, Do DP, Diez Roux AV, Mujahid MS. Associations between residential segregation and incident hypertension: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2022;11:e023084. doi: 10.1161/JAHA.121.023084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lloyd-Jones DM, Elkind M, Albert MA. American Heart Association’s 2024 Impact Goal: every person deserves the opportunity for a full, healthy life. Circulation 2021;144:e277–e279. doi: 10.1161/CIRCULATIONAHA.121.057617 [DOI] [PubMed] [Google Scholar]
- 66.Angell SY, McConnell MV, Anderson CAM, Bibbins-Domingo K, Boyle DS, Capewell S, Ezzati M, de Ferranti S, Gaskin DJ, Goetzel RZ, et al. The American Heart Association 2030 Impact Goal: a presidential advisory from the American Heart Association. Circulation 2020;141:e120–e138. doi: 10.1161/CIR.0000000000000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Health, United States, 2011: With Special Feature on Socioeconomic Status and Health National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 68.Purnell TS, Calhoun EA, Golden SH, Halladay JR, Krok-Schoen JL, Appelhans BM, Cooper LA. Achieving health equity: closing the gaps in health care disparities, interventions, and research. Health Aff (Millwood) 2016;35:1410–1415. doi: 10.1377/hlthaff.2016.0158 [DOI] [PubMed] [Google Scholar]
- 69.Crook ED, Bryan NB, Hanks R, Slagle ML, Morris CG, Ross MC, Torres HM, Williams RC, Voelkel C, Walker S, et al. A review of interventions to reduce health disparities in cardiovascular disease in African Americans. Ethn Dis 2009;19:204–208. [PMC free article] [PubMed] [Google Scholar]
- 70.Davis AM, Vinci LM, Okwuosa TM, Chase AR, Huang ES. Cardiovascular health disparities: a systematic review of health care interventions. Med Care Res Rev 2007;64(suppl):29S–100S. doi: 10.1177/1077558707305416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kris-Etherton PM, Petersen KS, Després JP, Braun L, de Ferranti SD, Furie KL, Lear SA, Lobelo F, Morris PB, Sacks FM; on behalf of the American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Stroke Council; Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Hypertension. Special considerations for healthy lifestyle promotion across the life span in clinical settings: a science advisory from the American Heart Association. Circulation 2021;144:e515–e532. doi: 10.1161/CIR.0000000000001014 [DOI] [PubMed] [Google Scholar]
- 72.Grandner MA, Fernandez FX. The translational neuroscience of sleep: a contextual framework. Science 2021;374:568–573. doi: 10.1126/science.abj8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kryger MH, Roth T, Goldstein CA. Principles and Practice of Sleep Medicine Elsevier Health Sciences; 2021. [Google Scholar]
- 74.Chastin S, McGregor D, Palarea-Albaladejo J, Diaz KM, Hagströmer M, Hallal PC, van Hees VT, Hooker S, Howard VJ, Lee IM, et al. Joint association between accelerometry-measured daily combination of time spent in physical activity, sedentary behaviour and sleep and all-cause mortality: a pooled analysis of six prospective cohorts using compositional analysis. Br J Sports Med 2021;55:1277–1285. doi: 10.1136/bjsports-2020-102345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pienaar PR, Kolbe-Alexander TL, van Mechelen W, Boot CRL, Roden LC, Lambert EV, Rae DE. Associations between self-reported sleep duration and mortality in employed individuals: systematic review and meta-analysis. Am J Health Promot 2021;35:853–865. doi: 10.1177/0890117121992288 [DOI] [PubMed] [Google Scholar]
- 76.He M, Deng X, Zhu Y, Huan L, Niu W. The relationship between sleep duration and all-cause mortality in the older people: an updated and dose-response meta-analysis. BMC Public Health 2020;20:1179. doi: 10.1186/s12889-020-09275-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.García-Perdomo HA, Zapata-Copete J, Rojas-Cerón CA. Sleep duration and risk of all-cause mortality: a systematic review and meta-analysis. Epidemiol Psychiatr Sci 2019;28:578–588. doi: 10.1017/S2045796018000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin J, Jin X, Shan Z, Li S, Huang H, Li P, Peng X, Peng Z, Yu K, Bao W, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc 2017;6:e005947. doi: 10.1161/JAHA.117.005947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu TZ, Xu C, Rota M, Cai H, Zhang C, Shi MJ, Yuan RX, Weng H, Meng XY, Kwong JS, et al. Sleep duration and risk of all-cause mortality: a flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev 2017;32:28–36. doi: 10.1016/j.smrv.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 80.Shen X, Wu Y, Zhang D. Nighttime sleep duration, 24-hour sleep duration and risk of all-cause mortality among adults: a meta-analysis of prospective cohort studies. Sci Rep 2016;6:21480. doi: 10.1038/srep21480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33:585–592. doi: 10.1093/sleep/33.5.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x [DOI] [PubMed] [Google Scholar]
- 83.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grandner MA. Sleep, health, and society. Sleep Med Clin 2020;15:319–340. doi: 10.1016/j.jsmc.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 85.Paudel ML, Taylor BC, Ancoli-Israel S, Blackwell T, Stone KL, Tranah G, Redline S, Cummings SR, Ensrud KE; Osteoporotic Fractures in Men (MrOS) Study. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol Int 2010;27:363–377. doi: 10.3109/07420520903419157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hossin MZ. From habitual sleep hours to morbidity and mortality: existing evidence, potential mechanisms, and future agenda. Sleep Health 2016;2:146–153. doi: 10.1016/j.sleh.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 87.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep 2014;37:9–17. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang X, Chen H, Li S, Pan L, Jia C. Association of sleep duration with the morbidity and mortality of coronary artery disease: a meta-analysis of prospective studies. Heart Lung Circ 2015;24:1180–1190. doi: 10.1016/j.hlc.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 89.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 90.Grandner MA. Sleep and obesity risk in adults: possible mechanisms; contextual factors; and implications for research, intervention, and policy. Sleep Health 2017;3:393–400. doi: 10.1016/j.sleh.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 91.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL; on behalf of the American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grandner MA, Seixas A, Shetty S, Shenoy S. Sleep duration and diabetes risk: population trends and potential mechanisms. Curr Diab Rep 2016;16:106. doi: 10.1007/s11892-016-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. The association between sleep and metabolic syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12:773646. doi: 10.3389/fendo.2021.773646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bock JM, Vungarala S, Covassin N, Somers VK. Sleep duration and hypertension: epidemiological evidence and underlying mechanisms. Am J Hypertens 2022;35:3–11. doi: 10.1093/ajh/hpab146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Makarem N, Alcántara C, Williams N, Bello NA, Abdalla M. Effect of sleep disturbances on blood pressure. Hypertension 2021;77:1036–1046. doi: 10.1161/HYPERTENSIONAHA.120.14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Makarem N, Shechter A, Carnethon MR, Mullington JM, Hall MH, Abdalla M. Sleep duration and blood pressure: recent advances and future directions. Curr Hypertens Rep 2019;21:33. doi: 10.1007/s11906-019-0938-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu H, Yang Q, Tian F, Lyu Y, He H, Xin X, Zheng X. A meta-analysis of a cohort study on the association between sleep duration and type 2 diabetes mellitus. J Diabetes Res 2021;2021:8861038. doi: 10.1155/2021/8861038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med Rev 2016;30:11–24. doi: 10.1016/j.smrv.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 99.Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol 2021;252:125–141. doi: 10.1530/JOE-21-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barragán R, Zuraikat FM, Tam V, Scaccia S, Cochran J, Li S, Cheng B, St-Onge MP. Actigraphy-derived sleep is associated with eating behavior characteristics. Nutrients 2021;13:852. doi: 10.3390/nu13030852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Makarem N, Sears DD, St-Onge MP, Zuraikat FM, Gallo LC, Talavera GA, Castaneda SF, Lai Y, Mi J, Aggarwal B. Habitual nightly fasting duration, eating timing, and eating frequency are associated with cardiometabolic risk in women. Nutrients 2020;12:E3043. doi: 10.3390/nu12103043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.St-Onge MP, Zuraikat FM. Reciprocal roles of sleep and diet in cardiovascular health: a review of recent evidence and a potential mechanism. Curr Atheroscler Rep 2019;21:11. doi: 10.1007/s11883-019-0772-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tracy EL, Reid KJ, Baron KG. The relationship between sleep and physical activity: the moderating role of daily alcohol consumption. Sleep 2021;44:zsab112. doi: 10.1093/sleep/zsab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baron KG, Reid KJ, Zee PC. Exercise to improve sleep in insomnia: exploration of the bidirectional effects. J Clin Sleep Med 2013;9:819–824. doi: 10.5664/jcsm.2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosenberger ME, Fulton JE, Buman MP, Troiano RP, Grandner MA, Buchner DM, Haskell WL. The 24-hour activity cycle: a new paradigm for physical activity. Med Sci Sports Exerc 2019;51:454–464. doi: 10.1249/MSS.0000000000001811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patterson F, Grandner MA, Malone SK, Rizzo A, Davey A, Edwards DG. Sleep as a target for optimized response to smoking cessation treatment. Nicotine Tob Res 2019;21:139–148. doi: 10.1093/ntr/ntx236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Catoire S, Nourredine M, Lefebvre S, Couraud S, Gronfier C, Rey R, Peter-Derex L, Geoffroy PA, Rolland B. Tobacco-induced sleep disturbances: a systematic review and meta-analysis. Sleep Med Rev 2021;60:101544. doi: 10.1016/j.smrv.2021.101544 [DOI] [PubMed] [Google Scholar]
- 108.Makarem N, St-Onge MP, Liao M, Lloyd-Jones DM, Aggarwal B. Association of sleep characteristics with cardiovascular health among women and differences by race/ethnicity and menopausal status: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. Sleep Health 2019;5:501–508. doi: 10.1016/j.sleh.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tubbs AS, Ghani SB, Valencia D, Jean-Louis G, Killgore WDS, Fernandez F-X, Grandner MA. Racial/ethnic minorities have greater declines in sleep duration with higher risk of cardiometabolic disease: an analysis of the U.S. National Health Interview Survey. Sleep Epidemiol 2022;2:100022. doi: 10.1016/j.sleepe.2022.100022 [DOI] [Google Scholar]
- 110.Makarem N, Castro-Diehl C, St-Onge M-P, Redline S, Shea S, Lloyd-Jones DM, Ning H, Aggarwal B. The role of sleep as a cardiovascular health metric: does it improve cardiovascular disease risk prediction? Results from the Multi-Ethnic Study of Atherosclerosis. Abstract. Circulation 2020;141:A36. Accessed February 15, 2022. 10.1161/circ.141.suppl_1.36 [DOI] [Google Scholar]
- 111.Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Jansson-Fröjmark M, Palagini L, Rücker G, Riemann D, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev 2019;43:96–105. doi: 10.1016/j.smrv.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 112.Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, Reynolds CF, Riemann D. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull 2016;142:969–990. doi: 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tubbs AS, Gallagher R, Perlis ML, Hale L, Branas C, Barrett M, Gehrels JA, Alfonso-Miller P, Grandner MA. Relationship between insomnia and depression in a community sample depends on habitual sleep duration. Sleep Biol Rhythms 2020;18:143–153. doi: 10.1007/s41105-020-00255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hall MH, Brindle RC, Buysse DJ. Sleep and cardiovascular disease: emerging opportunities for psychology. Am Psychol 2018;73:994–1006. doi: 10.1037/amp0000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Etindele Sosso FA, Holmes SD, Weinstein AA. Influence of socioeconomic status on objective sleep measurement: a systematic review and meta-analysis of actigraphy studies. Sleep Health 2021;7:417–428. doi: 10.1016/j.sleh.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 116.Tomfohr-Madsen L, Cameron EE, Dhillon A, MacKinnon A, Hernandez L, Madigan S, Tough S. Neighborhood socioeconomic status and child sleep duration: a systematic review and meta-analysis. Sleep Health 2020;6:550–562. doi: 10.1016/j.sleh.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 117.Chattu VK, Chattu SK, Spence DW, Manzar MD, Burman D, Pandi-Perumal SR. Do disparities in sleep duration among racial and ethnic minorities contribute to differences in disease prevalence? J Racial Ethn Health Disparities 2019;6:1053–1061. doi: 10.1007/s40615-019-00607-7 [DOI] [PubMed] [Google Scholar]
- 118.Rangaraj VR, Knutson KL. Association between sleep deficiency and cardiometabolic disease: implications for health disparities. Sleep Med 2016;18:19–35. doi: 10.1016/j.sleep.2015.02.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jackson CL, Walker JR, Brown MK, Das R, Jones NL. A workshop report on the causes and consequences of sleep health disparities. Sleep 2020;43:zsaa037. doi: 10.1093/sleep/zsaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.American Academy of Pediatrics. Healthy sleep habits: how many hours does your child need? 2016. Accessed January 28, 2022. https://www.healthychildren.org/English/healthy-living/sleep/Pages/healthy-sleep-habits-how-many-hours-does-your-child-need.aspx
- 122.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015;38:843–844. doi: 10.5665/sleep.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Adams Hillard PJ, Katz ES, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health 2015;1:233–243. doi: 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 124.Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, Malow BA, Maski K, Nichols C, Quan SF, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med 2016;12:785–786. doi: 10.5664/jcsm.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.National Heart, Lung, and Blood Institute. The heart truth Accessed January 10, 2022. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/heart-truth
- 126.Eunice Kennedy Shriver National Institute of Child Health and Human Development. How much sleep do I need? Accessed January 31, 2022. https://www.nichd.nih.gov/health/topics/sleep/conditioninfo/how-much
- 127.American Heart Association. Go Red for Women 2022. Accessed January 10, 2022. https://www.goredforwomen.org/
- 128.Hale L, Troxel W, Buysse DJ. Sleep health: an opportunity for public health to address health equity. Annu Rev Public Health 2020;41:81–99. doi: 10.1146/annurev-publhealth-040119-094412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: a review of the literature. Prog Cardiovasc Nurs 2004;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x [DOI] [PubMed] [Google Scholar]
- 129a.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, RS Blumenthal, LT Braun, S de Ferranti, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129b.American Diabetes Association. Practice Guidelines Resources Accessed February 15, 2022. https://professional.diabetes.org/content-page/practice-guidelines-resources
- 129c.Whelton PK Carey RM, Aronow WS, Casey DE Jr Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018;71:e140–e144]. Hypertension 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 130.Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med 2008;168:308–314. doi: 10.1001/archinternmed.2007.119 [DOI] [PubMed] [Google Scholar]
- 131.Gao S. Diet and Exercise: Behavioral Management of Hypertension and Diabetes [dissertation] Seattle: University of Washington, 2006. [Google Scholar]
- 132.Cerwinske LA, Rasmussen HE, Lipson S, Volgman AS, Tangney CC. Evaluation of a dietary screener: the Mediterranean Eating Pattern for Americans tool. J Hum Nutr Diet 2017;30:596–603. doi: 10.1111/jhn.12451 [DOI] [PubMed] [Google Scholar]
- 133.National Health and Nutrition Examination Survey. Physical activity and physical fitness: PAQ-K 2019. Accessed February 15, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2019-2020/questionnaires/PAQ_K.pdf
- 134.National Health and Nutrition Examination Survey. Smoking and tobacco use: SMQ 2015. Accessed February 15, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/questionnaires/SMQ_I.pdf
- 135.Raghuveer G, White DA, Hayman LL, Woo JG, Villafane J, Celermajer D, Ward KD, de Ferranti SD, Zachariah J; on behalf of the American Heart Association Committee on Atherosclerosis, Hypertension, and Obesity in the Young of the Council on Cardiovascular Disease in the Young; Behavior Change for Improving Health Factors Committee of the Council on Lifestyle and Cardiometabolic Health and Council on Epidemiology and Prevention; and Stroke Council. Cardiovascular consequences of childhood secondhand tobacco smoke exposure: prevailing evidence, burden, and racial and socioeconomic disparities: a scientific statement from the American Heart Association [published correction appears in Circulation. 2016;134:e366]. Circulation 2016;134:e336–e359. doi: 10.1161/CIR.0000000000000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.US Department of Agriculture. Healthy Eating Index (HEI) 2020. Accessed January 27, 2022. https://www.fns.usda.gov/healthy-eating-index-hei
- 137.Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N; on behalf of the American Heart Association Advocacy Coordinating Committee, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation 2014;130:1418–1436. doi: 10.1161/CIR.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bhatnagar A, Whitsel LP, Blaha MJ, Huffman MD, Krishan-Sarin S, Maa J, Rigotti N, Robertson RM, Warner JJ. New and emerging tobacco products and the nicotine endgame: the role of robust regulation and comprehensive tobacco control and prevention: a presidential advisory from the American Heart Association. Circulation 2019;139:e937–e958. doi: 10.1161/CIR.0000000000000669 [DOI] [PubMed] [Google Scholar]
- 139.Centers for Disease Control and Prevention, Office on Smoking and Health. Health effects of secondhand smoke 2020. Accessed April 26, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/secondhand_smoke/health_effects/index.htm
- 140.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc 2014;21:578–582. doi: 10.1136/amiajnl-2014-002747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klompas M, Cocoros NM, Menchaca JT, Erani D, Hafer E, Herrick B, Josephson M, Lee M, Payne Weiss MD, Zambarano B, et al. State and local chronic disease surveillance using electronic health record systems. Am J Public Health 2017;107:1406–1412. doi: 10.2105/AJPH.2017.303874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lobelo F, Rohm Young D, Sallis R, Garber MD, Billinger SA, Duperly J, Hutber A, Pate RR, Thomas RJ, Widlansky ME, et al. ; on behalf of the American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Cardiovascular Surgery and Anesthesia; and Stroke Council. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation 2018;137:e495–e522. doi: 10.1161/CIR.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 143.Montgomery RM, Boucher EM, Honomichl RD, Powell TA, Guyton SL, Bernecker SL, Stoeckl SE, Parks AC. the effects of a digital mental health intervention in adults with cardiovascular disease risk factors: analysis of real-world user data. JMIR Cardio 2021;5:e32351. doi: 10.2196/32351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, et al. ; on behalf of the American Heart Association Publications Committee of the Council on Epidemiology and Prevention, Behavior Change Committee of the Council on Cardiometabolic Health, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, Council on Quality of Care and Outcomes Research, and Stroke Council. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association [published correction appears in Circulation. 2015;132:e233]. Circulation 2015;132:1157–1213. doi: 10.1161/CIR.0000000000000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kozik M, Isakadze N, Martin SS. Mobile health in preventive cardiology: current status and future perspective. Curr Opin Cardiol 2021;36:580–588. doi: 10.1097/HCO.0000000000000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schorr EN, Gepner AD, Dolansky MA, Forman DE, Park LG, Petersen KS, Still CH, Wang TY, Wenger NK; on behalf of the American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Lifestyle and Cardiometabolic Health. Harnessing mobile health technology for secondary cardiovascular disease prevention in older adults: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes 2021;14:e000103. doi: 10.1161/HCQ.0000000000000103 [DOI] [PubMed] [Google Scholar]
- 147.Foraker RE, Benziger CP, DeBarmore BM, Cené CW, Loustalot F, Khan Y, Anderson CAM, Roger VL; on behalf of the American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Lifestyle and Cardiometabolic Health. Achieving optimal population cardiovascular health requires an interdisciplinary team and a learning healthcare system: a scientific statement from the American Heart Association. Circulation 2021;143:e9–e18. doi: 10.1161/CIR.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bombard Y, Baker GR, Orlando E, Fancott C, Bhatia P, Casalino S, Onate K, Denis JL, Pomey MP . Engaging patients to improve quality of care: a systematic review. Implement Sci 2018;13:98. doi: 10.1186/s13012-018-0784-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brewer LC, Fortuna KL, Jones C, Walker R, Hayes SN, Patten CA, Cooper LA. Back to the future: achieving health equity through health informatics and digital health. JMIR Mhealth Uhealth 2020;8:e14512. doi: 10.2196/14512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, Bradburn P, Farmer A, Grant S, Greenfield SM, et al. ; TASMINH4 Investigators. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet 2018;391:949–959. doi: 10.1016/S0140-6736(18)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Warner JJ, Benjamin IJ, Churchwell K, Firestone G, Gardner TJ, Johnson JC, Ng-Osorio J, Rodriguez CJ, Todman L, Yaffe K, et al. ; on behalf of the American Heart Association Advocacy Coordinating Committee. Advancing healthcare reform: the American Heart Association’s 2020 statement of principles for adequate, accessible, and affordable health care: a presidential advisory from the American Heart Association. Circulation 2020;141:e601–e614. doi: 10.1161/CIR.0000000000000759 [DOI] [PubMed] [Google Scholar]
- 152.Roger VL, Sidney S, Fairchild AL, Howard VJ, Labarthe DR, Shay CM, Tiner AC, Whitsel LP, Rosamond WD; on behalf of the American Heart Association Advocacy Coordinating Committee. Recommendations for cardiovascular health and disease surveillance for 2030 and beyond: a policy statement from the American Heart Association. Circulation 2020;141:e104–e119. doi: 10.1161/CIR.0000000000000756 [DOI] [PubMed] [Google Scholar]
- 153.Hagan CN, Holubowich EJ, Criss T; Council of State and Territorial Epidemiologists. Driving public health in the fast lane: the urgent need for a 21st century data superhighway 2019. Accessed June 1, 2022. https://debeaumont.org/wp-content/uploads/2019/09/DSI-White-Paper_v15-Spreads.pdf
- 154.US Food and Drug Administration. Premarket tobacco product applications 2022. https://www.fda.gov/tobacco-products/market-and-distribute-tobacco-product/premarket-tobacco-product-applications Accessed February 10, 2022.
- 155.Jordt SE. Synthetic nicotine has arrived [published online September 7, 2021]. Tob Control doi: 10.1136/tobaccocontrol-2021-056626. https://tobaccocontrol.bmj.com/content/early/2021/09/07/tobaccocontrol-2021-056626.long [DOI] [PMC free article] [PubMed]
- 156.American Heart Association. New and emerging tobacco products and the nicotine endgame: the role of robust regulation and comprehensive tobacco control and prevention 2019. Accessed June 1, 2022. https://www.heart.org/-/media/Files/About-Us/Policy-Research/Policy-Positions/Tobacco-and-Clean-Air/New-and-Emerging-Tobacco-Products-and-the-Nicotine-Endgame--Policy-In-Brief.pdf [DOI] [PubMed]
- 157.Young DR, Cradock AL, Eyler AA, Fenton M, Pedroso M, Sallis JF, Whitsel LP; on behalf of the American Heart Association Advocacy Coordinating Committee. Creating built environments that expand active transportation and active living across the United States: a policy statement from the American Heart Association. Circulation 2020;142:e167–e183. doi: 10.1161/CIR.0000000000000878 [DOI] [PubMed] [Google Scholar]
- 158.Stecker EC, Ayanian JZ, Fendrick AM. Value-based insurance design: aligning incentives to improve cardiovascular care. Circulation 2015;132:1580–1585. doi: 10.1161/CIRCULATIONAHA.114.012584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Joynt Maddox KE, Bleser WK, Das SR, Desai NR, Ng-Osorio J, O’Brien E, Psotka MA, Wadhera RK, Weintraub WS, Konig M. Value in Healthcare Initiative: summary and key recommendations. Circ Cardiovasc Qual Outcomes. 2020;13:e006612. doi: 10.1161/CIRCOUTCOMES.120. 006612 [DOI] [PubMed] [Google Scholar]
- 160.Buchmueller TC, Cliff BQ, Levy H. The benefits of Medicaid expansion. JAMA Health Forum 2020;1:e200879–e200879. doi: 10.1001/jamahealthforum.2020.0879 [DOI] [PubMed] [Google Scholar]
- 161.Mehta LS, Sharma G, Creanga AA, Hameed AB, Hollier LM, Johnson JC, Leffert L, McCullough LD, Mujahid MS, Watson K, et al. ; on behalf of the American Heart Association Advocacy Coordinating Committee. Call to action: maternal health and saving mothers: a policy statement from the American Heart Association. Circulation 2021;144:e251–e269. doi: 10.1161/CIR.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 162.Shimbo D, Artinian NT, Basile JN, Krakoff LR, Margolis KL, Rakotz MK, Wozniak G; on behalf of the American Heart Association and the American Medical Association. Self-measured blood pressure monitoring at home: a joint policy statement from the American Heart Association and American Medical Association [published correction appears in Circulation. 2020;142:e64]. Circulation 2020;142:e42–e63. doi: 10.1161/CIR.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 163.Ward B; The Commonwealth Fund. The impact of Medicaid expansion on states’ budgets 2020. Accessed June 1, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/may/impact-medicaid-expansion-states-budgets
- 164.Lee Y, Mozaffarian D, Sy S, Liu J, Wilde PE, Marklund M, Abrahams-Gessel S, Gaziano TA, Micha R. Health impact and cost-effectiveness of volume, tiered, and absolute sugar content sugar-sweetened beverage tax policies in the United States: a microsimulation study. Circulation 2020;142:523–534. doi: 10.1161/CIRCULATIONAHA.119.042956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Powell LM, Andreyeva T, Isgor Z. Distribution of sugar-sweetened beverage sales volume by sugar content in the United States: implications for tiered taxation and tax revenue. J Public Health Policy 2020;41:125–138. doi: 10.1057/s41271-019-00217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.RAND Education and Labor. Improving access to early childhood education Accessed February 10, 2022. https://www.rand.org/capabilities/solutions/improving-access-to-early-childhood-education.html
- 167.Maier M Investing in the early care and education workforce 2020. Accessed February 10, 2022. https://www.mdrc.org/publication/investing-early-care-and-education-workforce#:~:text=Inadequate%20compensation%3B%20demanding%2C%20often%20stressful%20 working%20conditions%3B%20and,and%20turnover%20that%20are%20exacerbated%20by%20the%20pandemic
- 168.US Department of Health and Human Services, Administration for Children and Families: Office of Child Care. Federal and state funding for child care and early learning 2014. Accessed June 1, 2022. https://childcareta.acf.hhs.gov/sites/default/files/public/federal_and_state_funding_for_child_care_and_early_learning_edited.pdf
- 169.Impact of the COVID-19 pandemic on early childhood care and education. Early Child Educ J 2020;48:533–536. doi: 10.1007/s10643-020-01082-0 [DOI] [Google Scholar]
- 170.Bala MM, Strzeszynski L, Topor-Madry R. Mass media interventions for smoking cessation in adults. Cochrane Database Syst Rev 2017;11:CD004704. doi: 10.1002/14651858.CD004704.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.von Philipsborn P, Stratil JM, Burns J, Busert LK, Pfadenhauer LM, Polus S, Holzapfel C, Hauner H, Rehfuess E. Environmental interventions to reduce the consumption of sugar-sweetened beverages and their effects on health. Cochrane Database Syst Rev 2019;6:CD012292. doi: 10.1002/14651858.CD012292.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reynolds AJ, Ou SR, Eales L, Mondi CF, Giovanelli A. Assessment of a comprehensive early childhood education program and cardiovascular disease risk in midlife. JAMA Netw Open 2021;4:e2120752. doi: 10.1001/jamanetworkopen.2021.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.MacArthur G, Caldwell DM, Redmore J, Watkins SH, Kipping R, White J, Chittleborough C, Langford R, Er V, Lingam R, et al. Individual-, family-, and school-level interventions targeting multiple risk behaviours in young people. Cochrane Database Syst Rev 2018;10:CD009927. doi: 10.1002/14651858.CD009927.pub2 [DOI] [PMC free article] [PubMed]
- 174.Wolfenden L, Nathan NK, Sutherland R, Yoong SL, Hodder RK, Wyse RJ, Delaney T, Grady A, Fielding A, Tzelepis F, et al. Strategies for enhancing the implementation of school-based policies or practices targeting risk factors for chronic disease. Cochrane Database Syst Rev 2017;11:CD011677. doi: 10.1002/14651858.CD011677.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.American Heart Association. Workplace health playbook Accessed January 30, 2022. https://playbook.heart.org/
- 176.Foraker RE, Shoben AB, Kelley MM, Lai AM, Lopetegui MA, Jackson RD, Langan MA, Payne PR. Electronic health record-based assessment of cardiovascular health: the Stroke Prevention in Healthcare Delivery Environments (SPHERE) study. Prev Med Rep 2016;4:303–308. doi: 10.1016/j.pmedr.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Seguin RA, Paul L, Folta SC, Nelson ME, Strogatz D, Graham ML, Diffenderfer A, Eldridge G, Parry SA. Strong Hearts, Healthy Communities: a community-based randomized trial for rural women. Obesity (Silver Spring) 2018;26:845–853. doi: 10.1002/oby.22158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Seguin-Fowler RA, Strogatz D, Graham ML, Eldridge GD, Marshall GA, Folta SC, Pullyblank K, Nelson ME, Paul L. The Strong Hearts, Healthy Communities Program 2.0: an RCT examining effects on Simple 7. Am J Prev Med 2020;59:32–40. doi: 10.1016/j.amepre.2020.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Armstrong SC, Windom M, Bihlmeyer NA, Li JS, Shah SH, Story M, Zucker N, Kraus WE, Pagidipati N, Peterson E, et al. Rationale and design of “Hearts & Parks”: study protocol for a pragmatic randomized clinical trial of an integrated clinic-community intervention to treat pediatric obesity. BMC Pediatr 2020;20:308. doi: 10.1186/s12887-020-02190-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dunn CG, Wilcox S, Saunders RP, Kaczynski AT, Blake CE, Turner-McGrievy GM. Healthy eating and physical activity interventions in faith-based settings: a systematic review using the reach, effectiveness/efficacy, adoption, implementation, maintenance framework. Am J Prev Med 2021;60:127–135. doi: 10.1016/j.amepre.2020.05.014 [DOI] [PubMed] [Google Scholar]
- 181.Linnan LA, D’Angelo H, Harrington CB. A literature synthesis of health promotion research in salons and barbershops. Am J Prev Med 2014;47:77–85. doi: 10.1016/j.amepre.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brown AG, Hudson LB, Chui K, Metayer N, Lebron-Torres N, Seguin RA, Folta SC. Improving heart health among Black/African American women using civic engagement: a pilot study. BMC Public Health 2017;17:112. doi: 10.1186/s12889-016-3964-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ayala GX, Baquero B, Laraia BA, Ji M, Linnan L. Efficacy of a store-based environmental change intervention compared with a delayed treatment control condition on store customers’ intake of fruits and vegetables. Public Health Nutr 2013;16:1953–1960. doi: 10.1017/S1368980013000955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Minkler M, Estrada J, Thayer R, Juachon L, Wakimoto P, Falbe J. Bringing healthy retail to urban “food swamps”: a case study of CBPR-informed Policy and neighborhood change in San Francisco. J Urban Health 2018;95:850–858. doi: 10.1007/s11524-018-0234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Robles B, Barragan N, Smith B, Caldwell J, Shah D, Kuo T. Lessons learned from implementing the Supplemental Nutrition Assistance Program Education Small Corner Store project in Los Angeles County. Prev Med Rep 2019;16:100997. doi: 10.1016/j.pmedr.2019.100997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.World Health Organization. Urban green space interventions and health: a review of impacts and effectiveness 2017. Accessed June 1, 2022. https://www.cbd.int/health/who-euro-green-spaces-urbanhealth.pdf
- 187.Petkovic J, Duench S, Trawin J, Dewidar O, Pardo Pardo J, Simeon R, DesMeules M, Gagnon D, Hatcher Roberts J, Hossain A, et al. Behavioural interventions delivered through interactive social media for health behaviour change, health outcomes, and health equity in the adult population. Cochrane Database Syst Rev 2021;5:CD012932. doi: 10.1002/14651858.CD012932.pub2 [DOI] [PMC free article] [PubMed]
- 188.Spring B, Ockene JK, Gidding SS, Mozaffarian D, Moore S, Rosal MC, Brown MD, Vafiadis DK, Cohen DL, Burke LE, et al. ; on behalf of the American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation 2013;128:2169–2176. doi: 10.1161/01.cir.0000435173.25936.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, Fonarow GC, Fortmann SP, Franklin BA, Galloway JM, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention. American Heart Association guide for improving cardiovascular health at the community level, 2013 update: a scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation 2013;127:1730–1753. doi: 10.1161/CIR.0b013e31828f8a94 [DOI] [PubMed] [Google Scholar]
- 190.Kris-Etherton PM, Petersen KS, Després JP, Anderson CAM, Deedwania P, Furie KL, Lear S, Lichtenstein AH, Lobelo F, Morris PB, et al. ; on behalf of the American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Stroke Council; Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Hypertension. Strategies for promotion of a healthy lifestyle in clinical settings: pillars of ideal cardiovascular health: a science advisory from the American Heart Association. Circulation 2021;144:e495–e514. doi: 10.1161/CIR.0000000000001018 [DOI] [PubMed] [Google Scholar]
- 191.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 192.Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK; on behalf of the American Heart Association and the American College of Obstetricians and Gynecologists. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation 2018;137:e843–e852. doi: 10.1161/CIR.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 193.American Heart Association. Our policy positions Accessed January 6, 2022. https://www.heart.org/en/get-involved/advocate/policy-research/our-policy-positions
- 194.Eliason EL. Adoption of Medicaid expansion is associated with lower maternal mortality. Womens Health Issues 2020;30:147–152. doi: 10.1016/j.whi.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 195.Sharma G, Grandhi GR, Acquah I, Mszar R, Mahajan S, Khan SU, Javed Z, Mehta LS, Gulati M, Cainzos-Achirica M, et al. Social determinants of suboptimal cardiovascular health among pregnant women in the United States. J Am Heart Assoc 2022;11:e022837. doi: 10.1161/JAHA.121.022837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pahigiannis K, Thompson-Paul AM, Barfield W, Ochiai E, Loustalot F, Shero S, Hong Y. Progress toward improved cardiovascular health in the United States. Circulation 2019;139:1957–1973. doi: 10.1161/CIRCULATIONAHA.118.035408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.US Department of Health and Human Services. Social determinants: Healthy People 2020 Accessed January 10, 2022. https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Social-Determinants
- 198.US Department of Health and Human Services. Social determinants of health: Healthy People 2030 Accessed January 10, 2022.https://health.gov/healthypeople/objectives-and-data/social-determinants-health
- 199.Frieden TR, Berwick DM. The “Million Hearts” initiative: preventing heart attacks and strokes. N Engl J Med 2011;365:e27. doi: 10.1056/NEJMp1110421 [DOI] [PubMed] [Google Scholar]
- 200.Wright JS, Wall HK, Ritchey MD. Million Hearts 2022: small steps are needed for cardiovascular disease prevention. JAMA 2018;320:1857–1858. doi: 10.1001/jama.2018.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Centers for Disease Control and Prevention. Wisewoman locations 2021. Accessed February 15, 2022. https://www.cdc.gov/wisewoman/locations/
- 202.Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA; on behalf of the American Heart Association Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on High Blood Pressure. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation 2013;127:1254–1263, e1–e29. doi: 10.1161/CIR.0b013e318287cf2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.St-Onge MP, Aggarwal B, Allison MA, Berger JS, Castañeda SF, Catov J, Hochman JS, Hubel CA, Jelic S, Kass DA, et al. Go Red for Women strategically focused research network: summary of findings and network outcomes. J Am Heart Assoc 2021;10:e019519. doi: 10.1161/JAHA.120.019519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Suero-Abreu GA, Barajas-Ochoa A, Perez-Peralta A, Rojas E, Berkowitz R. Assessment of the effect of the Go Red for Women campaign on search engine queries for cardiovascular disease in women. Cardiol Res 2020;11:348–352. doi: 10.14740/cr1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ma M, Ma A. Racial/ethnic differences in knowledge of personal and target levels of cardiovascular health indicators. J Community Health 2015;40:1024–1030. doi: 10.1007/s10900-015-0027-z [DOI] [PubMed] [Google Scholar]
- 206.Ma M, Dollar KM, Kibler JL, Sarpong D, Samuels D. The effects of priming on a public health campaign targeting cardiovascular risks. Prev Sci 2011;12:333–338. doi: 10.1007/s11121-011-0228-3 [DOI] [PubMed] [Google Scholar]
- 207.Revere D, Calhoun R, Baseman J, Oberle M. Exploring bi-directional and SMS messaging for communications between public health agencies and their stakeholders: a qualitative study. BMC Public Health 2015;15:621. doi: 10.1186/s12889-015-1980-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gooding HC, Brown CA, Liu J, Revette AC, Stamoulis C, de Ferranti SD. Will teens go red? Low cardiovascular disease awareness among young women. J Am Heart Assoc 2019;8:e011195. doi: 10.1161/JAHA.118.011195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reynolds AJ, Temple JA, Ou SR, Arteaga IA, White BA. School-based early childhood education and age-28 well-being: effects by timing, dosage, and subgroups. Science 2011;333:360–364. doi: 10.1126/science.1203618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fonarow GC, Calitz C, Arena R, Baase C, Isaac FW, Lloyd-Jones D, Peterson ED, Pronk N, Sanchez E, Terry PE, et al. ; on behalf of the American Heart Association. Workplace wellness recognition for optimizing workplace health: a presidential advisory from the American Heart Association. Circulation 2015;131:e480–e497. doi: 10.1161/CIR. 00000000 [DOI] [PubMed] [Google Scholar]
- 211.American Heart Association. Workplace health Accessed January 6, 2022. https://www.heart.org/en/professional/workplace-health
- 212.Elgazzar R, Nolan TS, Joseph JJ, Aboagye-Mensah EB, Azap RA, Gray DM 2nd. Community-engaged and community-based participatory research to promote American Heart Association Life’s Simple 7 among African American adults: a systematic review. PLoS One 2020;15:e0238374. doi: 10.1371/journal.pone.0238374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Palmer KNB, Rivers PS, Melton FL, McClelland DJ, Hatcher J, Marrero DG, Thomson CA, Garcia DO. Health promotion interventions for African Americans delivered in U.S. barbershops and hair salons: a systematic review. BMC Public Health 2021;21:1553. doi: 10.1186/s12889-021-11584-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Turner J, Smith J, Bryant K, Haynes T, Stewart MK, Kuo DZ, Harris K, McCoy S, Lovelady N, Sullivan G, et al. Community building community: the distinct benefits of community partners building other communities’ capacity to conduct health research. Prog Community Health Partnersh 2017;11:81–86. doi: 10.1353/cpr.2017.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Spencer MS, Rosland AM, Kieffer EC, Sinco BR, Valerio M, Palmisano G, Anderson M, Guzman JR, Heisler M. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health 2011;101:2253–2260. doi: 10.2105/AJPH.2010.300106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liao Y, Siegel PZ, Zhou H, Grimm K, Njai R, Kent C, Giles W. Reduced disparity in vegetable consumption in 16 disadvantaged Black communities: a successful 5-year community-based participatory intervention. J Racial Ethn Health Disparities 2015;2:211–218. doi: 10.1007/s40615-014-0065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx-Drew D, et al. A cluster-randomized trial of blood-pressure reduction in Black barbershops. N Engl J Med 2018;378:1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Joseph JJ, Nolan TS, Williams A, McKoy A, Zhao S, Aboagye-Mensah E, Kluwe B, Odei JB, Brock G, Lavender D, et al. Improving cardiovascular health in Black men through a 24-week community-based team lifestyle change intervention: the Black Impact pilot study. Am J Prev Cardiol 2022;9:100315. doi: 10.1016/j.ajpc.2022.100315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Campbell MK, Hudson MA, Resnicow K, Blakeney N, Paxton A, Baskin M. Church-based health promotion interventions: evidence and lessons learned. Annu Rev Public Health 2007;28:213–234. doi: 10.1146/annurev.publhealth.28.021406.144016 [DOI] [PubMed] [Google Scholar]
- 220.Schoenthaler AM, Lancaster KJ, Chaplin W, Butler M, Forsyth J, Ogedegbe G. Cluster randomized clinical trial of FAITH (Faith-Based Approaches in the Treatment of Hypertension) in Blacks. Circ Cardiovasc Qual Outcomes 2018;11:e004691. doi: 10.1161/CIRCOUTCOMES.118.004691 [DOI] [PubMed] [Google Scholar]
- 221.Skolarus LE, Cowdery J, Dome M, Bailey S, Baek J, Byrd JB, Hartley SE, Valley SC, Saberi S, Wheeler NC, et al. Reach Out Churches: a community-based participatory research pilot trial to assess the feasibility of a mobile health technology intervention to reduce blood pressure among African Americans. Health Promot Pract 2018;19:495–505. doi: 10.1177/1524839917710893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Flórez KR, Payán DD, Palar K, Williams MV, Katic B, Derose KP. Churchbased interventions to address obesity among African Americans and Latinos in the United States: a systematic review. Nutr Rev 2020;78:304–322. doi: 10.1093/nutrit/nuz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ralston PA, Wickrama KKAS, Coccia CC, Lemacks JL, Young-Clark IM, Ilich JZ . Health for Hearts United longitudinal trial: improving dietary behaviors in older African Americans. Am J Prev Med 2020;58:361–369. doi: 10.1016/j.amepre.2019.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brown DL, Conley KM, Sánchez BN, Resnicow K, Cowdery JE, Sais E, Murphy J, Skolarus LE, Lisabeth LD, Morgenstern LB . A multicomponent behavioral intervention to reduce stroke risk factor behaviors: the Stroke Health and Risk Education cluster-randomized controlled trial. Stroke 2015;46:2861–2867. doi: 10.1161/STROKEAHA.115.010678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brewer LC, Balls-Berry JE, Dean P, Lackore K, Jenkins S, Hayes SN. Fostering African-American Improvement in Total Health (FAITH!): an application of the American Heart Association’s Life’s Simple 7 among Midwestern African-Americans. J Racial Ethn Health Disparities 2017;4:269–281. doi: 10.1007/s40615-016-0226-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brewer LC, Hayes SN, Jenkins SM, Lackore KA, Breitkopf CR, Cooper LA, Patten CA. Improving cardiovascular health among African-Americans through mobile health: the FAITH! app pilot study. J Gen Intern Med 2019;34:1376–1378. doi: 10.1007/s11606-019-04936-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bull City Fit: about us Accessed January 9, 2022. https://www.bullcityfit.org/about-us
- 228.Hoffman J, Frerichs L, Story M, Jones J, Gaskin K, Apple A, Skinner A, Armstrong S. An integrated clinic-community partnership for child obesity treatment: a randomized pilot trial. Pediatrics 2018;141:e20171444. doi: 10.1542/peds.2017-1444 [DOI] [PubMed] [Google Scholar]
- 229.Mozaffarian D, Afshin A, Benowitz NL, Bittner V, Daniels SR, Franch HA, Jacobs DR Jr, Kraus WE, Kris-Etherton PM, Krummel DA, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on Nutrition, Physical Activity and Metabolism, Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on the Kidney in Cardiovascular Disease, Council on Peripheral Vascular Disease, and the Advocacy Coordinating Committee. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation 2012;126:1514–1563. doi: 10.1161/CIR.0b013e318260a20b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gittelsohn J, Rowan M, Gadhoke P. Interventions in small food stores to change the food environment, improve diet, and reduce risk of chronic disease. Prev Chronic Dis 2012;9:E59. [PMC free article] [PubMed] [Google Scholar]
- 231.Wensel CR, Trude ACB, Poirier L, Alghamdi R, Trujillo A, Anderson Steeves E, Paige D, Gittelsohn J. B’more Healthy Corner Stores for Moms and Kids: identifying optimal behavioral economic strategies to increase WIC redemptions in small urban corner stores. Int J Environ Res Public Health 2018;16:E64. doi: 10.3390/ijerph16010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Thorndike AN, Bright OM, Dimond MA, Fishman R, Levy DE. Choice architecture to promote fruit and vegetable purchases by families participating in the Special Supplemental Program for Women, Infants, and Children (WIC): randomized corner store pilot study. Public Health Nutr 2017;20:1297–1305. doi: 10.1017/S1368980016003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Paek HJ, Oh HJ, Jung Y, Thompson T, Alaimo K, Risley J, Mayfield K. Assessment of a healthy corner store program (FIT Store) in low-income, urban, and ethnically diverse neighborhoods in Michigan. Fam Community Health 2014;37:86–99. doi: 10.1097/FCH.0000000000000014 [DOI] [PubMed] [Google Scholar]
- 234.Lawman HG, Vander Veur S, Mallya G, McCoy TA, Wojtanowski A, Colby L, Sanders TA, Lent MR, Sandoval BA, Sherman S, et al. Changes in quantity, spending, and nutritional characteristics of adult, adolescent and child urban corner store purchases after an environmental intervention. Prev Med 2015;74:81–85. doi: 10.1016/j.ypmed.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 235.Leak TM, Setiono F, Gangrade N, Mudrak E. Youth willingness to purchase whole grain snack packs from New York City corner stores participating in a healthy retail program. Int J Environ Res Public Health 2019;16:E3233. doi: 10.3390/ijerph16183233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Handbury J, Rahkovsky I, Schnell M. Is the focus on food deserts fruitless? Retail access and food purchases across the socioeconomic spectrum. National Bureau of Economic Research 2015. Accessed June 1, 2022. https://www.nber.org/papers/w21126
- 237.Wynn TA, Wyatt SB, Hardy CM, Walker SS, Thomas TF, Williams AG, Partridge EE. Using community feedback to improve community interventions: results from the Deep South Network for Cancer Control Project. Fam Community Health 2016;39:234–241. doi: 10.1097/FCH.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Smith SA, Whitehead MS, Sheats JQ, Ansa BE, Coughlin SS, Blumenthal DS. Community-based participatory research principles for the African American community. J Ga Public Health Assoc 2015;5:52–56. [PMC free article] [PubMed] [Google Scholar]
- 239.Su D, Garg A, Wiens J, Meyer E, Cai G. Assessing health needs in African American churches: a mixed-methods study. J Relig Health 2021;60:1179–1197. doi: 10.1007/s10943-019-00924-5 [DOI] [PubMed] [Google Scholar]
- 240.Azap RA, Nolan TS, Gray DM 2nd, Lawson K, Gregory J, Capers Q 4th, Odei JB, Joseph JJ. Association of socioeconomic status with ideal cardiovascular health in Black men. J Am Heart Assoc 2021;10:e020184. doi: 10.1161/JAHA.120.020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang K, Lovasi GS, Odden MC, Michael YL, Newman AB, Arnold AM, Kim DH, Wu C. Association of retail environment and neighborhood socioeconomic status with mortality among community-dwelling older adults in the United States: Cardiovascular Health Study [published online October 20, 2021]. J Gerontol A Biol Sci Med Sci doi: 10.1093/gerona/glab319. [DOI] [PMC free article] [PubMed]
- 242.Ko YA, Shen J, Kim JH, Topel M, Mujahid M, Taylor H, Quyyumi A, Sims M, Vaccarino V, Baltrus P, et al. Identifying neighbourhood and individual resilience profiles for cardiovascular health: a cross-sectional study of Blacks living in the Atlanta metropolitan area. BMJ Open 2021;11:e041435. doi: 10.1136/bmjopen-2020-041435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Savin KL, Roesch SC, Oren E, Carlson JA, Allison MA, Sotres-Alvarez D, Sallis JF, Jankowska MM, Talavera GA, Rodriguez TM, et al. Social and built neighborhood environments and blood pressure 6 years later: Results from the Hispanic Community Health Study/Study of Latinos and the SOL CASAS ancillary study. Soc Sci Med 2022;292:114496. doi: 10.1016/j.socscimed.2021.114496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lapidos A, Lapedis J, Heisler M. Realizing the value of community health workers: new opportunities for sustainable financing. N Engl J Med 2019;380:1990–1992. doi: 10.1056/NEJMp1815382 [DOI] [PubMed] [Google Scholar]
- 245.South EC, Hohl BC, Kondo MC, MacDonald JM, Branas CC. Effect of greening vacant land on mental health of community-dwelling adults: a cluster randomized trial. JAMA Netw Open 2018;1:e180298. doi: 10.1001/jamanetworkopen.2018.0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Branas CC, South E, Kondo MC, Hohl BC, Bourgois P, Wiebe DJ, MacDonald JM. Citywide cluster randomized trial to restore blighted vacant land and its effects on violence, crime, and fear. Proc Natl Acad Sci U SA 2018;115:2946–2951. doi: 10.1073/pnas.1718503115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.American Heart Association. Current impact: EmPOWERED to Serve’s impact on social determinants of health Accessed January 9, 2022. https://www.empoweredtoserve.org/en/about-empowered-to-serve/current-impact [Google Scholar]
- 248.Krefman AE, Labarthe D, Greenland P, Pool L, Aguayo L, Juonala M, Kähönen M, Lehtimäki T, Day RS, Bazzano L, et al. Influential periods in longitudinal clinical cardiovascular health scores. Am J Epidemiol 2021;190:2384–2394. doi: 10.1093/aje/kwab149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


