Abstract
The offspring of parents with mental disorders are at increased risk for developing mental disorders themselves. The risk to offspring may extend transdiagnostically to disorders other than those present in the parents. The literature on this topic is vast but mixed. To inform targeted prevention and genetic counseling, we performed a comprehensive, PRISMA 2020‐compliant meta‐analysis. We systematically searched the literature published up to September 2022 to retrieve original family high‐risk and registry studies reporting on the risk of mental disorders in offspring of parents with any type of mental disorder. We performed random‐effects meta‐analyses of the relative risk (risk ratio, RR) and absolute risk (lifetime, up to the age at assessment) of mental disorders, defined according to the ICD or DSM. Cumulative incidence by offspring age was determined using meta‐analytic Kaplan‐Meier curves. We measured heterogeneity with the I2 statistic, and risk of bias with the Quality In Prognosis Studies (QUIPS) tool. Sensitivity analyses addressed the impact of study design (family high‐risk vs. registry) and specific vs. transdiagnostic risks. Transdiagnosticity was appraised with the TRANSD criteria. We identified 211 independent studies that reported data on 3,172,115 offspring of parents with psychotic, bipolar, depressive, disruptive, attention‐deficit/hyperactivity, anxiety, substance use, eating, obsessive‐compulsive, and borderline personality disorders, and 20,428,575 control offspring. The RR and lifetime risk of developing any mental disorder were 3.0 and 55% in offspring of parents with anxiety disorders; 2.6 and 17% in offspring of those with psychosis; 2.1 and 55% in offspring of those with bipolar disorder; 1.9 and 51% in offspring of those with depressive disorders; and 1.5 and 38% in offspring of those with substance use disorders. The offspring's RR and lifetime risk of developing the same mental disorder diagnosed in their parent were 8.4 and 32% for attention‐deficit/hyperactivity disorder; 5.8 and 8% for psychosis; 5.1 and 5% for bipolar disorder; 2.8 and 9% for substance use disorders; 2.3 and 14% for depressive disorders; 2.3 and 1% for eating disorders; and 2.2 and 31% for anxiety disorders. There were 37 significant transdiagnostic associations between parental mental disorders and the RR of developing a different mental disorder in the offspring. In offspring of parents with psychosis, bipolar and depressive disorder, the risk of the same disorder onset emerged at 16, 5 and 6 years, and cumulated to 3%, 19% and 24% by age 18; and to 8%, 36% and 46% by age 28. Heterogeneity ranged from 0 to 0.98, and 96% of studies were at high risk of bias. Sensitivity analyses restricted to prospective family high‐risk studies confirmed the pattern of findings with similar RR, but with greater absolute risks compared to analyses of all study types. This study demonstrates at a global, meta‐analytic level that offspring of affected parents have strongly elevated RR and lifetime risk of developing any mental disorder as well as the same mental disorder diagnosed in the parent. The transdiagnostic risks suggest that offspring of parents with a range of mental disorders should be considered as candidates for targeted primary prevention.
Keywords: Familial risk, mental disorders, psychosis, depression, bipolar disorder, substance use disorders, eating disorders, anxiety disorders, transdiagnostic risk, targeted primary prevention
Mental disorders run in families. Decades of epidemiological research have documented that having an affected biological parent is a potent risk factor for mental disorders in the offspring. For some mental disorders, the relationship to offspring's risk is so strong that a parent's diagnosis has been considered as an indication for primary targeted prevention 1 , 2 . For example, preventive approaches have been developed for young offspring of individuals affected with psychosis, bipolar disorder or depressive disorder 1 , 3 , 4 , 5 , 6 . Another area of clinical application is genetic counselling, which helps people make meaning out of genetic information, including familial risk, and use that information in alignment with their wishes, needs and values, to manage their health in the face of uncertainty 7 , 8 .
The preventive potential of these approaches relies on accurate knowledge of the likelihood of mental disorders and their age of onset among offspring of affected parents. Such knowledge remains incomplete in several respects. First, while numerous studies examined offspring of parents with major depressive, bipolar or psychotic disorders, the impact of other parental disorders on offspring risk is less mapped out. Second, most prior publications focused on one parental mental disorder at a time (e.g., only examining risk in offspring of parents with bipolar disorder), making a comparison of risks associated with different parental disorders difficult.
Moreover, the findings differ among study designs, populations and settings, leaving uncertainty about the accuracy of estimates. For example, traditional family high‐risk studies and reports from national registries draw different conclusions about the magnitude and extent of familial risk. A synthesis drawing on the complementary strengths of family high‐risk and registry studies is therefore needed to provide accurate estimates for clinical practice and prevention.
Finally, both degree and specificity of familial risk is undetermined. The causes of mental disorders’ clustering in families include genetic variants, shared environment, and the interplay between genetic and environmental factors 9 , 10 . Most genetic variants and environmental risk factors are not specific to a particular diagnosis 11 , 12 , 13 . Common causal factors and high rates of comorbidity between disorders have motivated the move to transdiagnostic approaches in psychiatry 14 . Yet again, there are discrepancies between study designs. For example, some family high‐risk studies reported that increased risk in offspring was specific to the disorder diagnosed in their parent 15 , 16 , while analyses of nationwide registries suggest a pattern of non‐specific risk that extends across all mental disorders 10 , 17 . An earlier meta‐analysis by our group drew on data from 33 studies of 3,863 offspring of parents with schizophrenia, bipolar and major depressive disorders to reveal a pattern of partial specificity and broad transdiagnostic risks 18 .
The last decade has seen more publications on offspring of parents with a range of mental disorders. Additional meta‐analyses have focused on offspring of parents with bipolar disorder 19 , 20 , offspring of parents with anxiety disorders 21 , 22 , offspring of parents with attention‐deficit/hyperactivity disorder (ADHD) 23 , or anxiety and disruptive disorders among offspring of parents with multiple diagnoses 24 , 25 . However, there has been no comprehensive transdiagnostic synthesis across offspring of parents with various types of mental disorders that could inform clinical practice. Transdiagnostic approaches may be especially relevant to prevention, as early developmental manifestations of psychopathology often change in ways that cross diagnostic boundaries 26 , 27 .
The present study aims to fill this gap in the literature, by providing a transdiagnostic synthesis of the available studies in offspring of parents affected with all types of mental disorders to inform targeted prevention and genetic counselling. For the first time, we combine, compare and synthesize family high‐risk studies and registry studies. We compare the relative risk between offspring of affected and unaffected parents, and examine both transdiagnostic and diagnosis‐specific risk to offspring. We quantify the probability (absolute risk) of developing a range of mental disorders for offspring of affected parents up to the assessment age (lifetime). We further estimate the cumulative incidence by offspring age, and test the impact of study design. We then leverage the evidence to formulate recommendations for targeted primary prevention and genetic counselling. We conclude by drafting a research agenda for the next generation of studies in this field.
METHODS
We performed a systematic review and meta‐analysis of the available literature on the relationship between any mental disorder in parents and the risk of mental disorders in the offspring. We followed a protocol that was registered at PROSPERO (CRD42022358509) on September 22, 2022. We report the review process and results according to the PRISMA 2020 statement 28 .
Literature search
We searched Web of Science with a combination of terms tagging family studies (offspring, parent*, matern*, patern*) and terms capturing mental disorders, to identify publications from database inception until September 16, 2022, with no language restrictions. We validated the search strategy against a set of 62 relevant publications obtained through expert suggestions and a prior systematic review 18 . The search identified all 62 publications in this validation set.
Inclusion and exclusion criteria
Inclusion criteria were: a) original family high‐risk (cross‐sectional or prospective) or registry study that reported quantitative data on the relationship between one or more mental disorders in a parent and one or more mental disorders in their biological offspring; b) offspring sampled from the general population or selected based on parent diagnosis; c) definitions of mental disorders in parents and offspring based on the ICD or the DSM (any version), established with a diagnostic interview or a standard clinical assessment; d) published in any language.
Exclusion criteria were: a) inadequate study design, including adoption studies (because they systematically differ from family high‐risk studies in separating genetic from environmental aspects of familial risk), case reports (to avoid highly selective sampling), and intervention studies (in which the risk of disorders in offspring could be reduced through an intervention); b) offspring selection (where offspring were selected based on their own health or an environmental exposure, as such selection could inflate the risk of disorders in the offspring); c) lack of ICD/DSM parent diagnosis (when no ICD/DSM diagnosis in parents was reported, or parent assessment was limited to self‐report questionnaires that do not clearly identify ICD/DSM diagnoses); d) lack of ICD/DSM offspring diagnosis (when no ICD/DSM diagnosis in offspring was reported, or offspring assessment was limited to self‐report questionnaires that do not clearly identify ICD/DSM diagnoses); and e) lack of relevant data (when there was no numeric information on the relationship between ICD/DSM diagnoses in parents and in offspring, or data on offspring were only reported as part of a larger group of first‐degree relatives).
Selection of relevant publications
The selection of eligible publications proceeded in two stages, implemented in Covidence 29 . First, two independent reviewers screened all titles and abstracts against a list of eligibility criteria, to remove studies that were ineligible and select publications for full‐text review. Second, two independent reviewers went through full texts of the pre‐selected publications, to confirm that eligibility criteria were met and select a final list of publications for data extraction. At both stages, a senior investigator resolved discrepancies between the reviewers.
Data extraction
We extracted the information on parent‐offspring disorder relationships as relative risk and absolute risk, using Covidence extraction 2 interface 29 . To assess relative risk, we extracted the risk ratios (RR), odds ratios (OR) or hazard ratios (HR) reflecting the increased (values greater than 1) or decreased (values smaller than 1) rates of disorder in offspring of parents with a given diagnosis, relative to control offspring of parents without a diagnosis. We recorded the type of the relative risk (RR, OR or HR), and its 95% confidence interval (CI) or standard error (SE). To assess absolute risk, for each group of offspring defined by a given parental diagnosis, we extracted the number of offspring with and without each mental disorder and the total number of offspring assessed for the disorder. We extracted the absolute risk of the same disorders for control offspring of parents without a diagnosis, if such control group was included. We use the term “lifetime risk” to describe these absolute risks measured up to age at assessment.
In addition, we extracted the country of origin of the study, the study design (prospective, cross‐sectional, registry), the population (general, high‐risk), the diagnostic instruments and classification system used to make diagnoses in parents and in offspring, and the mean offspring age at assessment. For prospective studies, we extracted the offspring age at first and last assessment and additional information on the cumulative incidence of developing mental disorders by offspring age (from available Kaplan‐Meier plots, see the data analysis section).
Where two or more publications reported data on the same disorder from the same sample or a partially overlapping sample, we selected the report with the largest sample size. For prospective studies, we extracted data from all time points, to inform analyses of cumulative incidence by offspring age.
Study design
We defined the two primary study types based on their design: i.e., family high‐risk studies and registry studies. We further subdivided family high‐risk studies into cross‐sectional and prospective ones. Cross‐sectional studies are those where offspring are assessed only once for presence or absence of mental disorders 30 , 31 , 32 . Prospective studies are those where researchers follow the offspring over time and repeatedly assess them for mental disorders at two or more time points 33 , 34 , 35 . Registry studies are those where offspring are not systematically assessed for the presence or absence of diagnosis, but information on the presence of a mental disorder is obtained from a health care record database or national registry 9 , 17 .
Family high‐risk studies systematically assess offspring with diagnostic interviews covering the full range of mental disorders and including comorbidity (high psychometric validity). However, samples are often selected from clinical populations and therefore are prone to selection bias (low ecological validity). This is of particular concern in cross‐sectional studies, which recruit participants when the target disorders are already present. Prospective studies mitigate disorder‐related sources of selection bias by recruiting participants before they develop mental disorders of interest, but they may still be prone to selection bias and confounding because of factors pre‐dating enrolment and attrition of participants over time leading to incomplete follow‐up. Typically, each one of these studies is too small to individually provide conclusive results (low statistical power) 36 .
Registry studies avoid most sources of sampling bias and provide adequate statistical power to detect even weak relationships with high ecological validity, as they take advantage of data on an entire population 37 . However, registries only contain diagnostic information on mental disorders which received treatment, and this information is based on unstructured clinical assessments (low psychometric validity) 37 . Individuals who meet diagnostic criteria for a mental disorder but do not seek treatment are misclassified as not having a disorder 37 . This misclassification may result in significant underestimates of risk of mental disorders that often remain untreated or are not seen as the primary reason of hospital admissions or clinic visits.
Risk of bias
To capture the various sources of bias in prospective, cross‐sectional, and registry studies, we rated the risk of bias using the Quality In Prognosis Studies (QUIPS) tool 38 . For each included report, we rated six bias domains: participation, attrition, parent diagnosis assessment, offspring diagnosis assessment, blinding of offspring assessors to parent diagnosis, and analysis reporting. Each domain is rated as low, moderate or high risk of bias. A “high” score in any domain indicates that a study is at high risk of bias.
Transdiagnosticity assessment
To meet the TRANSD criteria, we defined the gold standard by including specific ICD/DSM diagnoses, acknowledged the primary outcome of the study, defined the transdiagnostic construct as relative or absolute risk, appraised it across ten diagnostic groups, performed three types of multiple comparative analyses (RR, absolute risk, and risk of having the same mental disorder as the parent vs. having any other mental disorder), and validated the findings by focusing on those supported by at least three independent studies (see below) 14 , 39 .
Outcome measures
We grouped parent and offspring disorders into ten diagnostic categories: psychosis (schizophrenia, schizophreniform, schizoaffective and other psychotic disorders); bipolar disorder (bipolar I, bipolar II, and other/not otherwise specified); depressive disorders (major depressive disorder, persistent depressive disorder, and dysthymia); anxiety disorders (generalized anxiety disorder, panic disorder, social anxiety disorder, and phobias); substance use disorders (alcohol or substance use disorder, excluding nicotine use disorder); borderline personality disorder; ADHD (inattentive, hyperactive/impulsive, combined); disruptive disorders (oppositional‐defiant disorder and conduct disorder); obsessive‐compulsive disorder (OCD); eating disorders (anorexia nervosa, bulimia nervosa, other/not otherwise specified eating disorder).
We also included “any mental disorder” where this was reported (here, “any mental disorder” refers to one or more mental disorder diagnoses; because of comorbidity, this number is distinct from a sum of individuals affected with specific disorders). When a study reported more than one specific disorder (e.g., several specific anxiety disorders), we used the one representing more affected individuals as a proxy for the number of individuals with any specific disorders, considering the high comorbidity between them. For specific eating disorders at the same time point, we added the number of individuals with anorexia and bulimia, as these diagnoses are mutually exclusive 40 .
Statistical analyses
For each parent and offspring disorder combination, we performed two random‐effect meta‐analyses.
First, we conducted a meta‐analysis of the RR of the target disorder among offspring of affected parents compared to control offspring (i.e., those with no affected parents). Specifically, we calculated RR as the disorder risk in the offspring of affected parents divided by the disorder risk in control offspring. When the statistic available was only a RR/HR/OR and its CI, we first used the “improve_ci” function of the “metaumbrella” R package 41 to unround the estimates, and then derived the SE. We forced estimated SEs to be at least 0.001, to avoid a few samples receiving exaggerated weights in the subsequent meta‐analyses. When the risk estimate reported was an OR, we imputed the equivalent RR using a modified version of the “estimate_n_from_or_and_n_cases” functions of the “metaumbrella” package 41 , 42 . Then, we used the imputed number of affected offspring to derive the RR. As these imputations are not free from error, we forced the imputed RR to be equal to or smaller (in absolute logarithmic terms) than the corresponding OR, while we retained the variance, so that the imputed RR was similar or slightly lower and had a similar or slightly lower statistical significance than the reported OR.
To meta‐analyze the RR, we used the “metafor” R package 43 to create random‐effects models of the log‐transformed RR. This package uses the restricted maximum likelihood (REML) to fit the model and adds 0.5 to any zero counts of affected and non‐affected offspring. While computationally necessary, the addition of 0.5 can distort rate estimates in very small samples; therefore we restricted this procedure to groups of 50 or more individuals. We interpreted p values smaller than 0.05 as statistically significant. We estimated the heterogeneity between studies with the I 2 statistic.
Second, we completed a meta‐analysis of the absolute risk, i.e., the proportion of offspring affected with the target mental disorder, which is the preferred metric in genetic counseling 7 . We followed the same methodology as for the RR meta‐analysis, except for using the logit instead of the log‐transform. We noted that some disorders are typically underdiagnosed in the population registries but frequently diagnosed in family high‐risk studies, leading to the absolute risks of clinically meaningful disorders being systematically underestimated in registry studies and overestimated in non‐registry studies. Since family high‐risk and registry studies differ in more ways that can be accounted for, and neither is free from bias, we meta‐analyzed registry and non‐registry studies separately and then combined the two meta‐analytic results, setting the weights to be 50% (rather than altering the variances). Of note, such weighting was not necessary for RR, under the assumption that under‐ and over‐diagnoses applied to both offspring of affected parents and control offspring.
To further characterize the age‐dependent risk, we performed a meta‐analytic Kaplan‐Meier assessment of the absolute risk (cumulative incidence) of severe mental disorders by offspring age. We first generated pseudo‐individual participant data (pseudo‐IPD), whose survival curve would be identical to the published survival curves, using an established methodology 44 as in previous meta‐analyses 45 , 46 . For a study 47 which reported separate Kaplan‐Meier plots for bipolar disorder and bipolar disorder not otherwise specified in the same sample, we matched the events of each curve with censors occurring at the same age in the other curve, to generate a single dataset. Second, we combined the datasets from the different studies to estimate a curve for the risk of psychosis in the offspring of parents with psychosis, a curve for the risk of bipolar disorder in the offspring of parents with bipolar disorder, and a curve for the risk of depressive disorders in the offspring of parents with depressive disorders. There were too few studies for other disorder combinations (all n≤3).
We then conducted some sensitivity analyses. First, we conducted meta‐analyses of the relative and absolute risks restricted to prospective studies that had followed the offspring at least until the typical age of each disorder onset or diagnosis (childhood for ADHD, disruptive disorders and OCD; adolescence for depressive, anxiety and eating disorders; adulthood for psychosis, bipolar, substance use and borderline personality disorders). For this purpose, we labeled the samples as “children” when the mean age was <12 years old, “adolescents” when it was ≥12 but <18 years old, and “adults” when it was ≥18 years old. When data from multiple age groups were available, we used multilevel random‐effects models, including the age group as a moderator. These multilevel models are conceptually the same as subgroup analyses by follow‐up age ranges, with the only difference being that they include studies with shorter follow‐ups in the model to improve fit. For these multilevel models, we calculated I 2 as recommended by the creator of the “metafor” package at https://www.metafor‐project.org/doku.php/tips.
Second, to formally assess whether absolute risks were significantly smaller in registry than in non‐registry studies, we calculated the difference in (logit‐transformed) absolute risks between registry and non‐registry meta‐analytic results, along with its variance and the (log‐transformed) risk ratio. Then, we conducted a meta‐analysis of these differences for each offspring disorder (across parental disorders and children age ranges) and applied the resulting weights to the (log‐transformed) relative risks. Risk ratios <1 mean that the absolute risk of a disorder is smaller in registry than in non‐registry studies.
Third, to address the risk of having the same mental disorder as the parent vs. having any other mental disorder, we conducted multilevel meta‐analyses of the RR of having a mental disorder other than the disorder of the parent. The reason to use multilevel models, with the sample as a random factor, was that we include several RR estimates from each sample (i.e., an estimate for each mental disorder in offspring). We discarded offspring disorders with less than two studies and “having any mental disorder” because this grouping includes the parent's disorder. We then meta‐analyzed the results of these meta‐analyses to have an overall estimate of the RR of developing the same mental disorder as the parent and an estimate of the RR of developing a different mental disorder from the parent. We conducted this analysis separately for family high‐risk and registry studies.
The main meta‐analytic (i.e., based on at least three independent studies) results were presented stratified according to clinical‐informative topics that may inform practice and prevention. The estimates based on fewer than three independent studies are reported in tables, but are not interpreted, as they are considered to be less reliable.
RESULTS
Meta‐analytic database
Of 20,964 unique records identified by the literature search, we selected 911 reports for full‐text review, and extracted data from 457 eligible publications (see Figure 1). Common reasons for exclusion at the full‐text review stage were offspring sample selection based on their own health or environmental factors, missing or inadequate information on diagnosis in parents or offspring, and publications that contained no original data on the relationship between parent diagnosis and offspring disorders.
Figure 1.
PRISMA 2020 flow chart
The 457 eligible publications reported data from 211 unique studies, including 3,172,115 offspring of parents with mental disorders. A subset of 157 studies reported data on 20,428,575 comparison offspring. Most studies were family high‐risk studies, but the 18 registry studies included a disproportionately large number of participants (see Table 1). The sample size of the included studies ranged from 19 to 8,951,763. Offspring were assessed at a mean age of 4 to 42 years. One hundred and thirty‐five (64%) studies reported data on children, 142 (67%) on adolescents, and 95 (44%) on adult offspring. Of the 211 included studies, 54% (n=113) were from the US, 23% (n=48) from Europe, 7% (n=15) from Asia, 7% (n=15) from Canada, 4% (n=8) from Australia, and 1% (n=3) from low‐ or middle‐income countries. We computed 88 RRs (10 for the same disorder and 78 for different disorder combinations) and 96 absolute risks (10 for the same disorder and 86 for different disorders or controls).
Table 1.
Included studies and participants by study type
| Offspring of affected parents | Control offspring | All offspring | |||
|---|---|---|---|---|---|
| Study type | n | N | n | N | N |
| Prospective family high‐risk | 81 | 21,477 | 62 | 11,389 | 32,866 |
| Cross‐sectional family high‐risk | 112 | 69,918 | 77 | 9,008 | 78,926 |
| Registry | 18 | 3,080,720 | 18 | 20,408,178 | 23,488,898 |
| Total | 211 | 3,172,115 | 157 | 20,428,575 | 23,600,690 |
How likely are the offspring of affected parents to develop any mental disorder?
Of the 211 eligible studies, 86 provided data on offspring's risk of developing any mental disorder. Compared to control offspring, the offspring of affected parents had a 1.5‐ to 3‐fold elevated RR for developing any mental disorder (see Table 2): 3.0 in offspring of parents affected with anxiety disorders; 2.6 in those of parents affected with psychosis; 2.1 in those of parents affected with bipolar disorder; 1.9 in those of parents affected with depressive disorders; and 1.5 in those of parents affected with substance use disorders. No or few data were available on the RR of any mental disorders in offspring of parents with other mental disorders.
Table 2.
Meta‐analytic estimates of the risk ratios (RRs) of DSM/ICD mental disorders in offspring of affected parents vs. offspring of unaffected parents
| Disorder in parents | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Psychosis | Bipolar disorder | Depressive disorders | Anxiety disorders | Substance use disorders | Borderline personality disorder | ADHD | Disruptive disorders | OCD | Eating disorders | Any mental disorder | ||
| Disorder in offspring | Psychosis | 5.8 (4.2‐7.9) (n=21, N=7,545,374) | 1.8 (0.6‐5.0) (n=11, N=3,924,359) | 2.0 (1.3‐3.1) (n=6, N=1,746,667) | 1.7 (0.1‐26.3) (n=2, N=521) | 2.2 (2.0‐2.5) (n=5, N=2,153,172) | 1.8 (1.2‐2.6) (n=2, N=348,808) | 1.6 (1.3‐2.0) (n=1, N=347,208) | 4.0 (2.3‐6.9) (n=8, N=3,677,788) | |||
| Bipolar disorder | 1.3 (0.3‐5.0) (n=6, N=4,286,168) | 5.1 (3.3‐8.1) (n=33, N=11,561,026) | 2.1 (0.9‐5.0) (n=12, N=6,318,061) | 1.0 (0.4‐2.6) (n=3, N=1,300) | 11.4 (1.3‐96.8) (n=2, N=459) | 3.1 (0.1‐73.0) (n=1, N=45) | 2.3 (1.9‐2.8) (n=3, N=4,486,959) | 1.6 (1.4‐1.8) (n=2, N=347,286) | 2.0 (0.6‐6.9) (n=1, N=970) | |||
| Depressive disorders | 1.9 (1.7‐2.2) (n=14, N=2,989,314) | 2.1 (1.5‐2.9) (n=39, N=9,296,154) | 2.3 (1.9‐2.6) (n=53, N=11,895,688) | 1.7 (1.4‐2.0) (n=12, N=6,360,668) | 1.8 (1.3‐2.3) (n=13, N=8,180) | 9.4 (1.3‐68.3) (n=1, N=45) | 2.2 (1.4‐3.6) (n=2, N=4,138,210) | 1.3 (1.1‐1.6) (n=1, N=2,764) | 3.7 (0.8‐15.9) (n=1, N=78) | 1.9 (1.6‐2.3) (n=5, N=7,336,515) | ||
| Anxiety disorders | 1.7 (1.0‐3.1) (n=13, N=283,363) | 2.1 (1.7‐2.5) (n=33, N=6,373) | 2.0 (1.7‐2.3) (n=33, N=9,807) | 2.2 (2.0‐2.5) (n=22, N=1,981,092) | 1.4 (1.1‐1.9) (n=12, N=4,576) | 9.3 (2.2‐39.1) (n=2, N=135) | 2.0 (0.4‐9.3) (n=1, N=59) | 2.0 (1.1‐3.8) (n=1, N=78) | 1.7 (1.6‐1.8) (n=5, N=1,078,763) | |||
| Substance use disorders | 2.0 (1.2‐3.3) (n=10, N=423,316) | 1.9 (1.6‐2.2) (n=20, N=4,693) | 2.4 (1.6‐3.8) (n=15, N=5,875) | 8.2 (0.8‐82.1) (n=2, N=607) | 2.8 (2.1‐3.6) (n=23, N=685,252) | 6.3 (0.8‐48.0) (n=1, N=45) | 2.0 (0.4‐9.9) (n=1, N=78) | 4.8 (2.4‐9.7) (n=5, N=992,098) | ||||
| Borderline personality disorder | 2.2 (0.9‐5.8) (n=3, N=11,873) | 3.0 (0.1‐71.7) (n=1, N=86) | 3.8 (0.9‐16.4) (n=1, N=44) | |||||||||
| ADHD | 2.8 (1.7‐4.7) (n=8, N=3,865,558) | 1.9 (1.7‐2.3) (n=28, N=7,913,589) | 2.0 (1.8‐2.3) (n=21, N=8,779,593) | 1.4 (0.9‐2.3) (n=7, N=243,711) | 1.9 (1.4‐2.6) (n=15, N=1,016,734) | 5.1 (1.5‐17.2) (n=2, N=89) | 8.4 (3.3‐21.8) (n=5, N=6,724,918) | 0.8 (0.4‐1.7) (n=1, N=78) | 1.8 (1.3‐2.3) (n=4, N=1,522,341) | |||
| Disruptive disorders | 3.0 (1.0‐9.1) (n=6, N=282,343) | 2.1 (1.6‐2.9) (n=18, N=4,010) | 1.8 (1.5‐2.2) (n=16, N=6,566) | 1.2 (0.8‐1.8) (n=8, N=2,079) | 2.7 (1.8‐4.1) (n=13, N=5,604) | 1.6 (0.5‐4.7) (n=1, N=59) | 1.2 (0.4‐4.1) (n=1, N=75) | 2.4 (1.4‐4.0) (n=2, N=1,545) | ||||
| OCD | 1.9 (0.3‐14.6) (n=2, N=225) | 2.0 (1.3‐3.1) (n=13, N=3,347) | 3.2 (1.8‐5.6) (n=9, N=4,224) | 3.1 (1.0‐9.0) (n=4, N=991) | 2.4 (0.4‐15.0) (n=2, N=417) | 2.7 (0.7‐10.8) (n=2, N=457) | 1.1 (0.3‐4.5) (n=1, N=970) | |||||
| Eating disorders | 1.1 (0.7‐1.7) (n=5, N=285,787) | 2.3 (1.6‐3.5) (n=8, N=145,391) | 3.9 (0.2‐79.1) (n=1, N=73) | 1.3 (0.1‐12.8) (n=2, N=242,834) | 2.0 (1.7‐2.4) (n=3, N=148,704) | 1.0 (0.0‐50.4) (n=1, N=45) | 5.7 (0.3‐107.3) (n=1, N=78) | 2.3 (1.4‐3.6) (n=3, N=886,377) | 1.1 (1.0‐1.3) (n=3, N=654,911) | |||
| Any mental disorder | 2.6 (1.6‐4.2) (n=12, N=2,115,213) | 2.1 (1.7‐2.5) (n=20, N=1,480,732) | 1.9 (1.5‐2.3) (n=19, N=1,480,550) | 3.0 (1.8‐5.0) (n=3, N=169) | 1.5 (1.4‐1.6) (n=9, N=136,727) | 8.4 (2.2‐32.2) (n=1, N=45) | 8.4 (0.8‐3.9) (n=1, N=59) | 2.3 (1.6‐3.4) (n=6, N=195,477) | ||||
Each RR is followed by 95% CI. Low‐confidence RR estimates based on less than three studies are in italics. Empty cells indicate lack of data. Diagonal (grey‐shaded) cells show RR for the same disorder that is present in the parent. Off‐diagonal cells show RR for offspring disorders other than that diagnosed in the parent. ADHD – attention‐deficit/hyperactivity disorder, OCD – obsessive‐compulsive disorder.
The absolute risk of any mental disorder among offspring of affected parents was 55% in offspring of parents affected with bipolar disorder or anxiety disorders; 51% in offspring of parents affected with depressive disorders; 38% in those of parents affected with substance use disorders, and 17% in those of parents affected with psychosis (see Table 3). In contrast, one in seven (14%) control offspring developed any mental disorder. No or few data were available on the lifetime risk of any mental disorders in offspring of parents with other mental disorders.
Table 3.
Meta‐analytic estimates of the absolute lifetime risk of DSM/ICD mental disorders in offspring by parent diagnosis
| Disorder in parents | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Psychosis | Bipolar disorder | Depressive disorders | Anxiety disorders | Substance use disorders | Borderline personality disorder | ADHD | Disruptive disorders | OCD | Eating disorders | Any mental disorder | ||
| Disorder in offspring | Psychosis | 1% (1‐2) (n=33, N=2,598,579) | 8% (4‐17) (n=26, N=20,403) | 1% (1‐2) (n=14, N=1,913) | 2% (1‐5) (n=6, N=734) | 1% (0‐8) (n=2, N=124) | 3% (2‐4) (n=4, N=15,863) | 8% (0‐72) (n=5, N=14,637) | |||||
| Bipolar disorder | 1% (0‐3) (n=42, N=2,254,022) | 2% (1‐8) (n=8, N=1,521) | 5% (1‐23) (n=46, N=102,980) | 5% (3‐9) (n=12, N=2,771) | 1% (0‐6) (n=2, N=135) | 4% (2‐8) (n=2, N=168) | 5% (1‐26) (n=1, N=22) | 1% (0‐4) (n=2, N=32,251) | 3% (1‐10) (n=1, N=87) | ||||
| Depressive disorders | 5% (2‐11) (n=99, N=1,017,601) | 7% (2‐20) (n=17, N=2,352) | 18% (15‐21) (n=49, N=4,282) | 14% (5‐36) (n=52, N=360,472) | 2% (0‐35) (n=11, N=545) | 12% (8‐18) (n=13, N=1,948) | 43% (29‐58) (n=2, N=42) | 3% (0‐19) (n=1, N=33) | 21% (11‐36) (n=1, N=43) | 37% (26‐49) (n=2, N=183) | |||
| Anxiety disorders | 7% (2‐22) (n=89, N=345,063) | 8% (2‐30) (n=15, N=1,062) | 26% (21‐31) (n=46, N=4,069) | 24% (20‐28) (n=39, N=5,908) | 31% (17‐49) (n=14, N=23,394) | 19% (14‐25) (n=16, N=2,069) | 26% (16‐39) (n=2, N=58) | 15% (6‐32) (n=1, N=33) | 51% (37‐66) (n=1, N=43) | 25% (16‐37) (n=3, N=5,638) | |||
| Substance use disorders | 3% (1‐17) (n=55, N=502,737) | 13% (8‐19) (n=13, N=1,122) | 14% (10‐20) (n=22, N=2,767) | 23% (15‐34) (n=16, N=4,909) | 2% (0‐35) (n=2, N=166) | 9% (2‐39) (n=20, N=42,167) | 27% (13‐49) (n=1, N=22) | 12% (5‐25) (n=1, N=43) | 6% (0‐93) (n=2, N=6,010) | ||||
| Borderline personality disorder | 2% (0‐17) (n=6, N=11,024) | 5% (1‐19) (n=5, N=991) | 2% (0‐10) (n=2, N=69) | 2% (1‐4) (n=1, N=507) | 33% (17‐55) (n=1, N=21) | ||||||||
| ADHD | 3% (1‐14) (n=65, N=2,898,200) | 11% (2‐43) (n=10, N=20,279) | 10% (3‐30) (n=40, N=69,902) | 10% (6‐18) (n=25, N=224,003) | 2% (0‐34) (n=9, N=787) | 13% (10‐15) (n=17, N=2,542) | 49% (34‐64) (n=2, N=43) | 32% (8‐71) (n=5, N=44,287) | 23% (13‐38) (n=1, N=43) | 9% (1‐39) (n=2, N=5,542) | |||
| Disruptive disorders | 5% (4‐6) (n=50, N=147,749) | 4% (1‐22) (n=9, N=666) | 14% (11‐19) (n=26, N=2,386) | 12% (8‐17) (n=16, N=1,329) | 7% (3‐14) (n=8, N=483) | 12% (9‐17) (n=15, N=2,355) | 24% (13‐42) (n=1, N=33) | 15% (7‐29) (n=1, N=41) | 10% (6‐15) (n=2, N=183) | ||||
| OCD | 2% (1‐3) (n=30, N=4554) | 3% (1‐7) (n=4, N=185) | 4% (2‐6) (n=17, N=2,152) | 3% (2‐4) (n=9, N=1,930) | 5% (1‐15) (n=3, N=128) | 2% (1‐4) (n=3, N=344) | 41% (13‐77) (n=2, N=172) | 2% (1‐9) (n=1, N=87) | |||||
| Eating disorders | 1% (0‐4) (n=19, N=1,599,968) | 5% (2‐12) (n=6, N=1,776) | 2% (1‐4) (n=11, N=1,892) | 5% (1‐18) (n=1, N=41) | 0% (0‐2) (n=2, N=412) | 2% (1‐6) (n=4, N=4,963) | 2% (0‐27) (n=1, N=22) | 7% (2‐20) (n=1, N=43) | 1% (0‐9) (n=3, N=3,089) | 2% (1‐7) (n=3, N=38,098) | |||
| Any mental disorder | 14% (3‐42) (n=56, N=762,381) | 17% (1‐82) (n=13, N=7,830) | 55% (48‐61) (n=27, N=2,278) | 51% (42‐59) (n=20, N=2,134) | 55% (37‐72) (n=4, N=94) | 38% (18‐64) (n=12, N=24,913) | 73% (51‐87) (n=1, N=22) | 39% (24‐57) (n=1, N=33) | 55% (7‐95) (n=5, N=15,146) | ||||
Each percentage absolute risk estimate is followed by 95% CI. Low‐confidence estimates based on less than three studies are in italics. Empty cells indicate lack of data. Diagonal (grey‐shaded) cells show the lifetime risk for the same disorder that is present in the parent. Off‐diagonal cells show the lifetime risk for offspring disorders other than that diagnosed in the parent. The first column shows the lifetime risks of disorders in offspring of parents without a mental disorder. Where both family high‐risk and registry studies were available, data were weighted equally in the meta‐analytic estimate. ADHD – attention‐deficit/hyperactivity disorder, OCD – obsessive‐compulsive disorder.
Sensitivity analyses restricted to prospective studies that had followed the offspring at least until the typical age of each disorder’s onset reported similar RRs, but substantially higher absolute risks of mental disorders (see supplementary information).
How likely are the offspring to develop the same mental disorder as their parents?
Across all mental disorders examined, the offspring had increased risk of the same type of disorder that was diagnosed in their parents, with RRs ranging from 2.2 for anxiety disorders to 8.4 for ADHD (see Table 2). The other RRs were 5.8 for psychosis, 5.1 for bipolar disorder, 2.3 for depressive disorders, 2.8 for substance use disorders, and 2.3 for eating disorders. Small datasets of offspring of parents with borderline personality disorder and OCD precluded establishing statistical significance. There were no data for disruptive disorders.
The absolute risks of the same disorder diagnosed in parents were 32% for ADHD, 31% for anxiety disorders, 14% for depressive disorders, 9% for substance use disorders, 8% for psychosis, 5% for bipolar disorder, and 1% for eating disorders. There were no or too little data to reliably estimate the risk of other mental disorders. In terms of absolute risk, control offspring had a low risk of developing specific mental disorders, with estimates ranging from 1% (psychosis, bipolar disorder, eating disorders) to 7% (anxiety disorders).
Sensitivity analyses restricted to prospective studies that had followed the offspring at least until the typical age of each disorder's onset confirmed the overall direction and pattern of results, but showed a higher RR of bipolar disorder in offspring of parents with bipolar disorder (RR=9.0) and 2‐ to 3‐fold higher absolute risks of disorders for which adequate data were available: 35% for substance use disorders, 34% for depressive disorders, 21% for psychosis, and 13% for bipolar disorder (see supplementary information). There were no prospective studies for ADHD.
How likely are the offspring to develop mental disorders other than those diagnosed in their parents?
The eligible studies provided data on 62 transdiagnostic relationships between parental mental disorders and the risk of a different mental disorder in the offspring (see the off‐diagonal cells with white background in Table 2). Of the 62 transdiagnostic RR estimates, 60 (97%) were greater than 1.0, and 37 (60%) were statistically significant. However, most of these RRs were of small magnitude, and only psychosis in offspring of parents affected with substance use disorder had a lower bound of the 95% CI of at least 2 (see Table 2).
Table 3 shows the absolute lifetime risks of developing mental disorders other than those diagnosed in parents (in the off‐diagonal white‐background cells). For example, 10‐13% of offspring of parents with psychosis, bipolar disorder, depressive disorders, or substance use disorders developed ADHD, but only 3% of offspring of parents without mental disorders did so. Notably, several RRs or absolute risk cells were characterized by small sample sizes, and there were little or no data on risk in offspring of parents with borderline personality disorder, ADHD, disruptive disorders, OCD and eating disorders.
Sensitivity analyses restricted to prospective studies that had followed the offspring at least until the typical age of each disorder's onset showed similar RRs and larger absolute risks of most disorders (see supplementary information). One notable difference was in the absolute risk of psychosis among offspring of parents with bipolar disorder, which was estimated at 1% in the overall analysis but at 4% in the sensitivity analysis of prospective studies.
How does the risk of having a mental disorder change with age?
Twenty‐one prospective family high‐risk studies provided detailed data on cumulative incidence of mental disorders in the form of Kaplan‐Meier curves based on repeated diagnostic assessments. These detailed data were limited to psychotic, bipolar and depressive disorders in the offspring of parents with the same disorder (see Figures 2, 3, 4 and Table 4).
Figure 2.

Meta‐analytic Kaplan‐Meier curve summarizing the cumulative incidence of DSM/ICD psychotic disorders in offspring of parents affected with those disorders (n=4) and control offspring (n=3). The shade in the curve represents 95% CI.
Figure 3.

Meta‐analytic Kaplan‐Meier curve summarizing the cumulative incidence of DSM/ICD bipolar disorder in offspring of parents affected with that disorder (n=4) and control offspring (n=4). The shade in the curve represents 95% CI.
Figure 4.

Meta‐analytic Kaplan‐Meier curve summarizing the cumulative incidence of DSM/ICD depressive disorders in offspring of parents affected with those disorders (n=5) and control offspring (n=6). The shade in the curve represents 95% CI.
Table 4.
Cumulative incidence by age of psychotic, bipolar and depressive disorders in offspring of parents affected with the same disorder
| Age (years) | Risk of mental disorder in offspring of parents with that disorder | ||
|---|---|---|---|
| Psychosis | Bipolar disorder | Depressive disorders | |
| 4 | 0% (0‐0) | 0% (0‐1) | 0% (0‐1) |
| 6 | 0% (0‐0) | 1% (1‐2) | 1% (0‐1) |
| 8 | 0% (0‐0) | 4% (2‐5) | 2% (1‐3) |
| 10 | 0% (0‐0) | 6% (4‐8) | 3% (2‐4) |
| 12 | 0% (0‐0) | 9% (7‐11) | 7% (5‐8) |
| 14 | 0% (0‐0) | 11% (9‐14) | 11% (9‐13) |
| 16 | 2% (1‐3) | 15% (12‐18) | 17% (15‐19) |
| 18 | 3% (2‐5) | 19% (16‐22) | 24% (22‐26) |
| 20 | 5% (3‐7) | 23% (19‐26) | 29% (27‐31) |
| 22 | 6% (4‐7) | 26% (22‐29) | 35% (32‐37) |
| 24 | 7% (5‐9) | 28% (24‐32) | 39% (37‐42) |
| 26 | 7% (6‐9) | 28% (24‐31) | 43% (41‐46) |
| 28 | 8% (6‐10) | 36% (30‐41) | 46% (43‐48) |
| 30 | 9% (6‐11) | 36% (30‐41) | 54% (47‐59) |
Estimates (with 95% CIs) are based on meta‐analytic Kaplan‐Meier curves
Among offspring of parents with psychosis, the onset of psychotic disorders became notable at age 16, increased to 3% at age 18, and continued to increase in an approximately linear fashion until age 30, when it reached 9%, then remaining stable (Figure 2). Among offspring of parents with bipolar disorder, the onset of that disorder became notable as early as age 5, increased to 9% at age 12, 19% at age 18, and 36% by age 28 (Figure 3). Among offspring of parents with depressive disorders, the onset of depressive disorders became notable at age 6, increased at first slowly, then accelerated around age 12, leading to a steep rise in cumulative incidence that continued until mid twenties, when it reached 43%, with sparse data indicating possible further increase beyond 50% (Figure 4).
Heterogeneity and risk of bias
Heterogeneity (I 2 ) ranged from 0 to 0.98, and 202 (96%) of included studies were at high risk of bias in one or more domains. The risk of bias was unevenly distributed across study types. The nine studies that had low or moderate risk of bias in all six domains were all prospective family high‐risk studies 15 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 .
What factors affect our knowledge about the risk to offspring?
Sensitivity analyses showed that study type (family high‐risk vs. registry study) was a key contributor to heterogeneity in absolute risks. Specifically, the comparison of absolute risks between registry and family high‐risk studies showed that the risk of any mental disorder was 5 times smaller in the former than in the latter. Of the specific mental disorders, the risks of bipolar disorder, depressive disorders, anxiety disorders, substance use disorders, borderline personality disorder, and ADHD were between 3 and 10 times lower in registry than in family high‐risk studies (see Figure 5).
Figure 5.
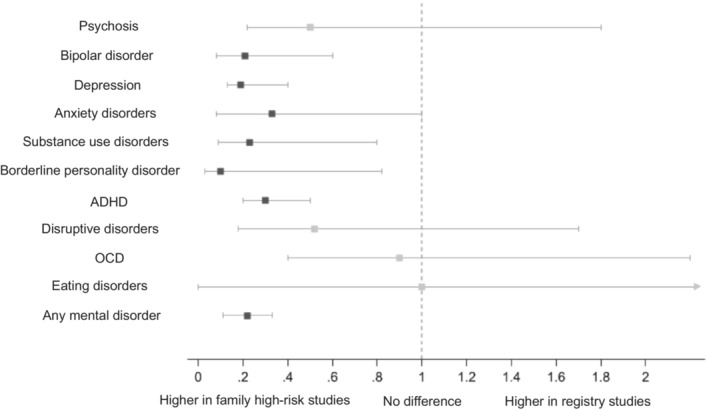
Comparison of the risk of mental disorders reported in registry studies vs. family high‐risk studies. For each mental disorder in offspring, the square shows the estimate and the horizontal line the 95% CI of the registry to family high‐risk ratio. Significant ratios are shown in dark grey squares; non‐significant ratios in pale grey squares. ADHD – attention‐deficit/hyperactivity disorder, OCD – obsessive‐compulsive disorder.
What is offspring's risk of developing the same mental disorder as the parent compared to the risk of developing any other mental disorder?
Across all examined mental disorders, the offspring of affected parents were 3‐fold more likely to develop the same disorder as the parent and 2‐fold more likely to develop a mental disorder other than that diagnosed in the parent, with little difference between family high‐risk and registry studies (see Table 5).
Table 5.
Relative risk (with 95% CI) of same or different mental disorder in offspring of parents with a mental disorder across family high‐risk and registry studies
| Same disorder in offspring | Different disorder in offspring | |||
|---|---|---|---|---|
| Disorder in parents | Family high‐risk studies | Registry studies | Family high‐risk studies | Registry studies |
| Psychosis |
4.4 (2.8‐6.8) (n=12, N=2,506) |
6.1 (4.1‐9.1) (n=9, N=7,542,868) |
1.9 (1.2‐3.1) (n=42, N=2,302) |
1.7 (0.9‐3.0) (n=23, N=11,553,211) |
| Bipolar disorder |
5.4 (3.5‐8.4) (n=28, N=5,234) |
4.4 (1.2‐16.9) (n=5, N=11,555,792) |
2.2 (1.9‐2.6) (n=70, N=6,698) |
1.2 (0.5‐2.8) (n=9, N=14,805,882) |
| Depressive disorders |
2.3 (2.0‐2.8) (n=47, N=22,121) |
2.1 (1.7‐2.6) (n=6, N=11,873,567) |
2.7 (2.1‐3.5) (n=26, N=6,682) |
1.3 (0.5‐3.2) (n=7, N=10,952,304) |
| Anxiety disorders |
2.1 (1.8‐2.6) (n=19, N=13,575) |
2.3 (2.0‐2.7) (n=3, N=1,967,517) |
1.3 (1.0‐1.7) (n=10, N=4,345) |
1.8 (1.7‐2.0) (n=2, N=6,356,323) |
| Substance use disorders |
3.0 (2.2‐4.2) (n=19, N=10,680) |
2.1 (1.3‐3.5) (n=4, N=674,572) |
9.4 (2.9‐30.1) (n=2, N=1,328) |
2.2 (2.0‐2.4) (n=3, N=2.151.844) |
| Overall |
3.1 (2.2‐4.4) (n=125, N=54,116) |
2.9 (1.8‐4.6) (n=27, N=33.614.316) |
2.2 (1.5‐3.3) (n=150, N=21,355) |
1.9 (1.6‐2.3) (n=44, N=45,819,564) |
Low‐confidence estimates based on fewer than three studies are shown in italics
DISCUSSION
The body of evidence on the risk of developing mental disorders in offspring of affected parents has increased dramatically over the past decade. The present meta‐analysis synthesizes data from 6 times more studies than the most inclusive prior analysis 18 . We present estimates of relative and absolute risks for 90 parent‐offspring disorder combinations, based on over 3 million offspring of affected parents and 20 million control offspring, originating from 211 family high‐risk (prospective, cross‐sectional) and registry studies. This data synthesis shows that the offspring of affected parents have strongly elevated relative and absolute risk of developing any mental disorder, as well as the same mental disorder that was diagnosed in the parent. In addition, the offspring of affected parents have moderately elevated transdiagnostic risk of most other disorders. We provide tables allowing clinicians to reference relative and absolute risks for parent‐offspring disorder combinations as well as meta‐analytic cumulative incidence by offspring age to inform clinical practice and prevention.
By systematically searching the global literature and summarizing evidence, this study has identified offspring who are at highest risk for mental disorders. We found that approximately one‐in‐two offspring of parents with anxiety, bipolar and depressive disorders will develop a mental disorder. Similarly, more than one third of offspring of parents with substance use disorder and one sixth of offspring of parents with psychosis will develop a mental disorder. Notably, offspring of parents with ADHD have 8‐fold increased risk of developing the same disorder; offspring of parents with psychotic and bipolar disorders have a 5‐fold increased risk; and offspring of parents with substance use, depressive and anxiety disorders about a 2‐fold increased risk.
Prospective studies reveal that the lifetime risk of offspring to develop the same disorder of parent is substantial, cumulating to 34% for offspring of parents affected with depressive disorder, 21% for offspring of parents with psychosis, and 13% for offspring of parents with bipolar disorder. These estimates are important for clinical practice, including genetic counselling and prevention in psychiatry. The results align with independent twin‐study literature showing that twin heritability is 77% for psychotic, 76% for bipolar, 40% for anxiety and 34% for depressive disorders 56 . There is also evidence for a dose‐response association in first‐degree relatives for psychotic (one proband: OR=7.69; two probands: OR=11.11), bipolar (one proband: RR=6.10, two probands: RR=29.1) and depressive (one proband: OR=2.14; two probands: OR=3.23) disorders 2 , 57 , 58 , 59 .
The magnitude of the meta‐analytic risks designates offspring of parents affected with psychotic, mood (bipolar and depressive), anxiety, substance use disorders and ADHD as a population that should be prioritized for systematic screening, monitoring and preventive interventions. To date, these efforts have been largely limited to young people at clinical high risk for psychotic, and more recently bipolar disorders 3 , 4 , 60 , 61 . While the clinical high‐risk paradigms include a subgroup of individuals with affected first‐degree relatives, screening of relatives is not routinely implemented 2 . Our meta‐analytic data urge professionals to systematically assess and address the mental health of offspring of patients affected with psychotic, mood, anxiety, substance use disorders and ADHD.
Based on the substantial risk, mental health screening of these offspring would be supported by sufficient evidence. A next step could involve the implementation of a periodic monitoring for additional risk indicators over time, coupled with targeted preventive approaches 62 . Emerging preventive approaches include needs‐based interventions; psychotherapy for offspring at risk of psychotic or anxiety disorders; physical activity for offspring at risk of depressive disorders; and genetic counselling for offspring at risk for bipolar or depressive disorders, and their parents 5 , 45 , 63 , 64 , 65 , 66 , 67 .
These interventions are not effective when administered to the whole population (universal prevention). For example, school‐based interventions designed to prevent anxiety and depressive disorders are ineffective 68 and may even cause harm to some adolescents 69 . However, interventions targeted to youth with a specific risk profile can have beneficial effects, including reduction in the risk of depressive disorder onset 70 , 71 . Preventive interventions may target symptomatic offspring of affected parents 72 , and include optimized treatment of parents 73 , both of which can reduce the risk of onset and burden of mental disorders in offspring.
The present report is also the most comprehensive summary of the transdiagnostic risk of developing mental disorders in offspring of affected parents. It has been debated whether the risk to offspring is specific to the disorder diagnosed in a parent or whether it extends transdiagnostically to most or all mental disorders. Typically, family high‐risk studies focus on disorder‐specific relationships 15 , 16 , but studies of national registries highlighted extensive transdiagnostic risks 10 , 17 . The present synthesis of family high‐risk and registry studies suggests broad transdiagnostic risks, although the magnitude of transdiagnostic RRs was smaller than for disorder‐specific estimates. Overall, the offspring of affected parents were 3 times more likely to develop the same disorder as their parent and, in addition, were 2 times more likely to develop a different disorder. These results were consistent across family high‐risk and registry studies, suggesting that discrepancies in prior literature might have been the result of limited statistical power.
The most robust transdiagnostic risk was observed for psychosis in offspring of parents with substance use disorder. There was substantial variation in transdiagnostic effect sizes and some indications of limited specificity. For example, the relative risk of anxiety disorders is elevated in offspring of parents with bipolar, depressive and substance use disorders, but not in offspring of parents with psychosis. On the other hand, the relative risk of ADHD is elevated in offspring of parents with bipolar disorder, depressive disorders, borderline personality disorder and psychosis, but not in offspring of parents with anxiety disorders. These variations to the broad transdiagnostic familial risks deserve attention, as they may hold clues to the structure of risks for mental disorders.
In the context of precision psychiatry, these findings can inform the development of new algorithms that can predict the transdiagnostic risk of onset across mental disorders 74 , 75 . In the context of genetic counseling, the provision of absolute risk estimates helps counter the common overestimation of familial risk and related fatalism among potential parents living with mental disorders 7 . In the context of public health, the common element in familial risk suggests that transdiagnostic approaches to targeted prevention can be more advantageous, as multiple outcomes can be potentially prevented with the same intervention.
The risk of mental disorders is age dependent. Information on the development of risk over age is essential to time‐targeted prevention efforts in clinical practice and to adjust risk information to the client's current age (for example, when providing genetic counselling) 76 . In this respect, longitudinal family high‐risk studies provide unique information on prospectively ascertained onsets over long developmental periods, that complements clinical high‐risk studies focused on individuals at an age close to the typical onset of major mental disorders. Our meta‐analyses of cumulative incidence show a rapid accumulation of onsets through adolescence and into mid‐to‐late twenties, aligning with a recent meta‐analysis which indicated that the peak age of onset of any mental disorder worldwide is of 14.5 years 77 . By age 28, just under one‐in‐ten offspring of parents with psychotic disorders, one‐in‐three offspring of parents with bipolar disorder, and one‐in‐two offspring of parents with depressive disorders will develop the same disorder themselves.
These cumulative incidence estimates exceed the absolute lifetime risk estimates derived from family high‐risk and registry studies. In line with the known differences in prevalence between prospective and retrospective ascertainment of mental disorders 78 , this discrepancy suggests that the actual risk of mental disorders in offspring of affected parents may be even higher than what is expected based on current family high‐risk literature. The relatively low incidence of psychosis onset in offspring aligns with the existing meta‐analytic evidence in samples at clinical high‐risk for psychosis, which indicates that the genetic risk and deterioration syndrome subgroup, which includes first‐degree relatives, has a lower short‐term risk of transitioning to psychosis than other clinical high‐risk groups 79 .
Our meta‐analytic cumulative incidence data are clinically informative. For example, a general practitioner might use them to predict the 5‐year likelihood of developing bipolar disorder in a 16 year‐old who has a parent affected with the same condition. However, the decision to communicate such information to individuals or families should take into account their preferences and priorities, as well as the availability of interventions and tools that can modify the risk. A clinician should explore existing perceptions of risk before providing new information, provide absolute rather than relative risks, contextualize the numbers provided, check understanding and emotional impact so as to promote positive outcomes (e.g., appropriate preventive intervention to mitigate risk for developing the condition) and avoid the potential for harms associated with this type of information (e.g., increasing stigma, or fatalism) 7 , 80 , 81 , 82 , 83 , 84 .
Although based on a vast body of literature, the present study has some limitations. First, there are considerable differences in the estimates reported by family high‐risk vs. registry studies. Since these two study designs are prone to different sources of selection and information bias, it may not be appropriate to declare one set of results as superior to the other. Accordingly, we gave family high‐risk and registry studies equal weight in our primary analyses and we qualified the estimates in sensitivity analyses. For several mental disorders, registry studies report absolute risks between 5 and 10 times lower than those seen in family high‐risk studies that systematically assess participants with diagnostic interviews. This difference is probably due to the fact that, in registry studies, diagnosis depends upon treatment seeking. Prospective studies suggest that the offspring of parents with psychotic, bipolar and depressive disorders have substantially elevated rates of mental disorders, that are discernable on repeated active inquiry even when some of them do not present for treatment. The clinical and societal significance of such undertreated disorders remains to be established.
Second, although we referred to a lifetime absolute risk of developing mental disorder, this estimate indexes the risk measured at the assessment point. The latter could widely vary from cross‐sectional to prospective studies. However, we have performed a meta‐analytic Kaplan‐Meier assessment that provides fine‐grained cumulative incidence of mental disorders by offspring age.
Third, the geographic distribution of available evidence is imbalanced: of the 211 eligible studies, only three originated from low‐ or middle‐income countries. Intensive work is needed to establish the global invariance or heterogeneity of familial risk. Fourth, the distribution of evidence over various mental disorders is uneven, and several comparisons were underpowered (i.e., less than three independent studies available). While extensive efforts have been dedicated to examining familial risk for psychotic and mood (bipolar, depressive) disorders, less evidence is available for anxiety and substance use disorders, and most of the other mental disorders remain unexplored. Fifth, we have not identified enough relevant data to examine the effects of having both parents affected with mental disorders 85 . With evidence of assortative mating 86 suggesting that cumulation of risk from two affected parents is common, targeted efforts are warranted to prospectively study the offspring of two parents with mental disorders.
Although the current study primarily informs clinical practice, especially in prevention and genetic counselling, it additionally paves the way for future research in this field. Research may next focus on filling the gaps in existing evidence, particularly relating to familial risk for borderline personality disorder, ADHD, disruptive disorders, eating disorders and OCD. The empty or low‐count cells in our tables highlight the specific parent‐offspring combinations that should be prioritized by future studies. Transdiagnostic risks to offspring growing up in low‐ or middle‐income countries also need to be determined. Although well‐designed prospective studies require substantial resources, recent interest in epidemiological research by several European funders, international research networks, and methodological innovation may facilitate this type of research 87 .
Examining mixed diagnostic groups of parents without diagnostic exclusions may prove particularly important. Additionally, potential sex‐specific patterns of transgenerational transmission of mental disorders – which have been reported for anxiety disorders, psychosis and ADHD 88 , 89 , 90 , 91 – should be examined transdiagnostically. A further research priority is better characterizing differences between family high‐risk and registry studies. This would benefit from validation of registry diagnoses that extends to “controls” without registry‐identified disorder and examines multiple comorbid mental disorders 37 . Future research may take advantage of family high‐risk studies nested within registries to understand the sources of information in national and health‐provider registries 50 . Only a few studies have been able to combine the advantages of the different study designs, through using a national registry as a basis for comprehensive recruitment into a prospective family high‐risk study 50 , 92 . These exceptionally well‐designed studies allow mapping the sources of selection and information bias to improve the interpretation of broader literature 93 .
In conclusion, this large meta‐analytic synthesis documents elevated risks for a range of mental disorders, including transdiagnostic risks, in offspring of parents affected with psychotic, mood (bipolar and depressive), anxiety and substance use disorders, as well as ADHD. While gaps in evidence motivate future research, the present knowledge robustly supports systematic screening in offspring of parents affected with these conditions. Urgent research is needed to identify effective targeted interventions to reduce risk for offspring of parents with these mental disorders, and to deliver them without exacerbating fatalism or stigma.
ACKNOWLEDGEMENTS
B. Pavlova, J. Radua, U. Provenzani and S. Najafi contributed equally to this work. Supplementary information on the study is available at https://www.offspringrisk.org/.
REFERENCES
- 1. Lannes A, Bui E, Arnaud C et al. Preventive interventions in offspring of parents with mental illness: a systematic review and meta‐analysis of randomized controlled trials. Psychol Med 2021;51:2321‐36. [DOI] [PubMed] [Google Scholar]
- 2. Fusar‐Poli P, Correll CU, Arango C et al. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry 2021;20:200‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catalan A, Salazar de Pablo G, Vaquerizo Serrano J et al. Annual Research Review: Prevention of psychosis in adolescents – systematic review and meta‐analysis of advances in detection, prognosis and intervention. J Child Psychol Psychiatry 2021;62:657‐73. [DOI] [PubMed] [Google Scholar]
- 4. Fusar‐Poli P, Salazar de Pablo G, Correll CU et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry 2020;77:755‐65. [DOI] [PubMed] [Google Scholar]
- 5. Loechner J, Starman K, Galuschka K et al. Preventing depression in the offspring of parents with depression: a systematic review and meta‐analysis of randomized controlled trials. Clin Psychol Rev 2018;60:1‐14. [DOI] [PubMed] [Google Scholar]
- 6. Havinga PJ, Maciejewski DF, Hartman CA et al. Prevention programmes for children of parents with a mood/anxiety disorder: systematic review of existing programmes and meta‐analysis of their efficacy. Br J Clin Psychol 2021;60:212‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Austin JC. Evidence‐based genetic counseling for psychiatric disorders: a road map. Cold Spring Harb Perspect Med 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Semaka A, Austin J. Patient perspectives on the process and outcomes of psychiatric genetic counseling: an “empowering encounter”. J Genet Couns 2019;28:856‐68. [DOI] [PubMed] [Google Scholar]
- 9. Kendler KS, Abrahamsson L, Ohlsson H et al. An extended Swedish adoption study of anxiety disorder and its cross‐generational familial relationship with major depression. Am J Psychiatry 2022;179:640‐9. [DOI] [PubMed] [Google Scholar]
- 10. Kendler KS, Ohlsson H, Sundquist J et al. An extended Swedish national adoption study of bipolar disorder illness and cross‐generational familial association with schizophrenia and major depression. JAMA Psychiatry 2020;77:814‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arango C, Dragioti E, Solmi M et al. Risk and protective factors for mental disorders beyond genetics: an evidence‐based atlas. World Psychiatry 2021;20:417‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uher R, Zwicker A. Etiology in psychiatry: embracing the reality of poly‐gene‐environmental causation of mental illness. World Psychiatry 2017;16:121‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munn‐Chernoff MA, Johnson EC, Chou YL et al. Shared genetic risk between eating disorder‐ and substance‐use‐related phenotypes: evidence from genome‐wide association studies. Addict Biol 2021;26:e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fusar‐Poli P, Solmi M, Brondino N et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry 2019;18:192‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parnas J, Cannon TD, Jacobsen B et al. Lifetime DSM‐III‐R diagnostic outcomes in the offspring of schizophrenic mothers. Results from the Copenhagen High‐Risk Study. Arch Gen Psychiatry 1993;50:707‐14. [DOI] [PubMed] [Google Scholar]
- 16. Preisig M, Strippoli MF, Castelao E et al. The specificity of the familial aggregation of early‐onset bipolar disorder: a controlled 10‐year follow‐up study of offspring of parents with mood disorders. J Affect Disord 2016;190:26‐33. [DOI] [PubMed] [Google Scholar]
- 17. Dean K, Stevens H, Mortensen PB et al. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Arch Gen Psychiatry 2010;67:822‐9. [DOI] [PubMed] [Google Scholar]
- 18. Rasic D, Hajek T, Alda M et al. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta‐analysis of family high‐risk studies. Schizophr Bull 2014;40:28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lau P, Hawes DJ, Hunt C et al. Prevalence of psychopathology in bipolar high‐risk offspring and siblings: a meta‐analysis. Eur Child Adolesc Psychiatry 2018;27:823‐37. [DOI] [PubMed] [Google Scholar]
- 20. Stapp EK, Mendelson T, Merikangas KR et al. Parental bipolar disorder, family environment, and offspring psychiatric disorders: a systematic review. J Affect Disord 2020;268:69‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lawrence PJ, Murayama K, Creswell C. Systematic review and meta‐analysis: anxiety and depressive disorders in offspring of parents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2019;58:46‐60. [DOI] [PubMed] [Google Scholar]
- 22. Micco JA, Henin A, Mick E et al. Anxiety and depressive disorders in offspring at high risk for anxiety: a meta‐analysis. J Anxiety Disord 2009;23:1158‐64. [DOI] [PubMed] [Google Scholar]
- 23. Uchida M, Driscoll H, DiSalvo M et al. Assessing the magnitude of risk for ADHD in offspring of parents with ADHD: a systematic literature review and meta‐analysis. J Atten Disord 2021;25:1943‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayano G, Betts K, Maravilla JC et al. The risk of anxiety disorders in children of parents with severe psychiatric disorders: a systematic review and meta‐analysis. J Affect Disord 2021;282:472‐87. [DOI] [PubMed] [Google Scholar]
- 25. Ayano G, Betts K, Maravilla JC et al. A systematic review and meta‐analysis of the risk of disruptive behavioral disorders in the offspring of parents with severe psychiatric disorders. Child Psychiatry Hum Dev 2021;52:77‐95. [DOI] [PubMed] [Google Scholar]
- 26. Caspi A, Houts RM, Ambler A et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. JAMA Netw Open 2020;3:e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGorry PD, Hartmann JA, Spooner R et al. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry 2018;17:133‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veritas Health Innovation . Covidence systematic review software. www.covidence.org.
- 30. McLaughlin KA, Gadermann AM, Hwang I et al. Parent psychopathology and offspring mental disorders: results from the WHO World Mental Health Surveys. Br J Psychiatry 2012;200:290‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clark DB, Cornelius J, Wood DS et al. Psychopathology risk transmission in children of parents with substance use disorders. Am J Psychiatry 2004;161:685‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Küng AL, Pham E, Cordera P et al. Psychiatric disorders among offspring of patients with bipolar and borderline personality disorder. J Clin Psychol 2019;75:1810‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cannon TD, Mednick SA. The schizophrenia high‐risk project in Copenhagen: three decades of progress. Acta Psychiatr Scand 1993;87(Suppl. 370):33‐47. [DOI] [PubMed] [Google Scholar]
- 34. Hillegers MH, Reichart CG, Wals M et al. Five‐year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord 2005;7:344‐50. [DOI] [PubMed] [Google Scholar]
- 35. Sandstrom A, MacKenzie L, Pizzo A et al. Observed psychopathology in offspring of parents with major depressive disorder, bipolar disorder and schizophrenia. Psychol Med 2020;50:1050‐6. [DOI] [PubMed] [Google Scholar]
- 36. Sandstrom A, Sahiti Q, Pavlova B et al. Offspring of parents with schizophrenia, bipolar disorder, and depression: a review of familial high‐risk and molecular genetics studies. Psychiatr Genet 2019;29:160‐9. [DOI] [PubMed] [Google Scholar]
- 37. Thygesen LC, Ersbøll AK. When the entire population is the sample: strengths and limitations in register‐based epidemiology. Eur J Epidemiol 2014;29:551‐8. [DOI] [PubMed] [Google Scholar]
- 38. Hayden JA, van der Windt DA, Cartwright JL et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280‐6. [DOI] [PubMed] [Google Scholar]
- 39. Fusar‐Poli P. TRANSD recommendations: improving transdiagnostic research in psychiatry. World Psychiatry 2019;18:361‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stein A, Woolley H, Cooper S et al. Eating habits and attitudes among 10‐year‐old children of mothers with eating disorders: longitudinal study. Br J Psychiatry 2006;189:324‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gosling CJ, Solanes A, Fusar‐Poli P et al. metaumbrella: the first comprehensive suite to perform data analysis in umbrella reviews with stratification of the evidence. BMJ Ment Health 2023;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dragioti E, Radua J, Solmi M et al. Impact of mental disorders on clinical outcomes of physical diseases: an umbrella review assessing population attributable fraction and generalized impact fraction. World Psychiatry 2023;22:86‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw 2010;36:1‐48. [Google Scholar]
- 44. Radua J, Grunze H, Amann BL. Meta‐analysis of the risk of subsequent mood episodes in bipolar disorder. Psychother Psychosom 2017;86:90‐8. [DOI] [PubMed] [Google Scholar]
- 45. Davies C, Cipriani A, Ioannidis JPA et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta‐analysis. World Psychiatry 2018;17:196‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salazar de Pablo G, Radua J, Pereira J et al. Probability of transition to psychosis in individuals at clinical high risk: an updated meta‐analysis. JAMA Psychiatry 2021;78:970‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eraso‐Osorio JJ, Palacio‐Ortiz JD, Quintero‐Cadavid CP et al. High risk for psychiatric disorders in bipolar offspring. A four years prospective study. Rev Colomb Psiquiatr 2021;50:273‐84. [DOI] [PubMed] [Google Scholar]
- 48. Axelson D, Goldstein B, Goldstein T et al. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: a longitudinal study. Am J Psychiatry 2015;172:638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duffy A, Goodday S, Keown‐Stoneman C et al. The emergent course of bipolar disorder: observations over two decades from the Canadian High‐Risk Offspring Cohort. Am J Psychiatry 2019;176:720‐9. [DOI] [PubMed] [Google Scholar]
- 50. Ellersgaard D, Plessen KJ, Jepsen JR et al. Psychopathology in 7‐year‐old children with familial high risk of developing schizophrenia spectrum psychosis or bipolar disorder – The Danish High Risk and Resilience Study ‐ VIA 7, a population‐based cohort study. World Psychiatry 2018;17:210‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lieb R, Isensee B, Höfler M et al. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective‐longitudinal community study. Arch Gen Psychiatry 2002;59:365‐74. [DOI] [PubMed] [Google Scholar]
- 52. Merikangas KR, Lieb R, Wittchen HU et al. Family and high‐risk studies of social anxiety disorder. Acta Psychiatr Scand 2003;108(Suppl. 417):28‐37. [DOI] [PubMed] [Google Scholar]
- 53. Palacio‐Ortiz JD, Peña‐Quintero CE, Gómez‐Valero MA et al. Lifetime psychiatric disorders: a comparison study between offspring of parents with bipolar disorder type‐I versus the offspring of community controls parents. Rev Colomb Psiquiatr 2017;46:129‐39. [DOI] [PubMed] [Google Scholar]
- 54. Rudaz D, Vandeleur CL, Gholam M et al. Psychopathological precursors of the onset of mood disorders in offspring of parents with and without mood disorders: results of a 13‐year prospective cohort high‐risk study. J Child Psychol Psychiatry 2021;62:404‐13. [DOI] [PubMed] [Google Scholar]
- 55. Weissman MM, Wickramaratne P, Gameroff MJ et al. Offspring of depressed parents: 30 years later. Am J Psychiatry 2016;173:1024‐32. [DOI] [PubMed] [Google Scholar]
- 56. Polderman TJ, Benyamin B, de Leeuw CA et al. Meta‐analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 2015;47:702‐9. [DOI] [PubMed] [Google Scholar]
- 57. Lo LE, Kaur R, Meiser B et al. Risk of schizophrenia in relatives of individuals affected by schizophrenia: a meta‐analysis. Psychiatry Res 2020;286:112852. [DOI] [PubMed] [Google Scholar]
- 58. Chen MH, Hsu JW, Huang KL et al. Risk and coaggregation of major psychiatric disorders among first‐degree relatives of patients with bipolar disorder: a nationwide population‐based study. Psychol Med 2019;49:2397‐404. [DOI] [PubMed] [Google Scholar]
- 59. Wilde A, Chan HN, Rahman B et al. A meta‐analysis of the risk of major affective disorder in relatives of individuals affected by major depressive disorder or bipolar disorder. J Affect Disord 2014;158:37‐47. [DOI] [PubMed] [Google Scholar]
- 60. Fusar‐Poli P, De Micheli A, Rocchetti M et al. Semistructured Interview for Bipolar At Risk States (SIBARS). Psychiatry Res 2018;264:302‐9. [DOI] [PubMed] [Google Scholar]
- 61. Salazar de Pablo G, Cabras A, Pereira J et al. Predicting bipolar disorder I/II in individuals at clinical high‐risk: results from a systematic review. J Affect Disord 2023;325:778‐86. [DOI] [PubMed] [Google Scholar]
- 62. Maciejewski D, Hillegers M, Penninx B. Offspring of parents with mood disorders: time for more transgenerational research, screening and preventive intervention for this high‐risk population. Curr Opin Psychiatry 2018;31:349‐57. [DOI] [PubMed] [Google Scholar]
- 63. Bosnjak Kuharic D, Kekin I, Hew J et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev 2019;11:CD012236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moreno‐Peral P, Conejo‐Cerón S, Rubio‐Valera M et al. Effectiveness of psychological and/or educational interventions in the prevention of anxiety: a systematic review, meta‐analysis, and meta‐regression. JAMA Psychiatry 2017;74:1021‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salazar de Pablo G, De Micheli A, Solmi M et al. Universal and selective interventions to prevent poor mental health outcomes in young people: systematic review and meta‐analysis. Harv Rev Psychiatry 2021;29:196‐215. [DOI] [PubMed] [Google Scholar]
- 66. Hu MX, Turner D, Generaal E et al. Exercise interventions for the prevention of depression: a systematic review of meta‐analyses. BMC Public Health 2020;20:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carrion P, Semaka A, Batallones R et al. Reflections of parents of children with 22q11.2 deletion syndrome on the experience of receiving psychiatric genetic counseling: ‘Awareness to Act’. J Genet Couns 2022;31:140‐52. [DOI] [PubMed] [Google Scholar]
- 68. Caldwell DM, Davies SR, Hetrick SE et al. School‐based interventions to prevent anxiety and depression in children and young people: a systematic review and network meta‐analysis. Lancet Psychiatry 2019;6:1011‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Foulkes L, Stringaris A. Do no harm: can school mental health interventions cause iatrogenic harm? BJPsych Bull 2023; doi: 10.1192/bjb.2023.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hetrick SE, Cox GR, Witt KG et al. Cognitive behavioural therapy (CBT), third‐wave CBT and interpersonal therapy (IPT) based interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev 2016;8:CD003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cuijpers P, Pineda BS, Quero S et al. Psychological interventions to prevent the onset of depressive disorders: a meta‐analysis of randomized controlled trials. Clin Psychol Rev 2021;83:101955. [DOI] [PubMed] [Google Scholar]
- 72. Garber J, Clarke GN, Weersing VR et al. Prevention of depression in at‐risk adolescents: a randomized controlled trial. JAMA 2009;301:2215‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weissman MM, Pilowsky DJ, Wickramaratne PJ et al. Remissions in maternal depression and child psychopathology: a STAR*D‐child report. JAMA 2006;295:1389‐98. [DOI] [PubMed] [Google Scholar]
- 74. Fusar‐Poli P, Werbeloff N, Rutigliano G et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent national health service trust. Schizophr Bull 2019;45:562‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oliver D, Wong CMJ, Bøg M et al. Transdiagnostic individualized clinically‐based risk calculator for the automatic detection of individuals at‐risk and the prediction of psychosis: external replication in 2,430,333 US patients. Transl Psychiatry 2020;10:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Austin JC, Palmer CG, Rosen‐Sheidley B et al. Psychiatric disorders in clinical genetics II: Individualizing recurrence risks. J Genet Couns 2008;17:18‐29. [DOI] [PubMed] [Google Scholar]
- 77. Solmi M, Radua J, Olivola M et al. Age at onset of mental disorders worldwide: large‐scale meta‐analysis of 192 epidemiological studies. Mol Psychiatry 2022;27:281‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moffitt TE, Caspi A, Taylor A et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med 2010;40:899‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fusar‐Poli P, Cappucciati M, Borgwardt S et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta‐analytical stratification. JAMA Psychiatry 2016;73:113‐20. [DOI] [PubMed] [Google Scholar]
- 80. Corcoran C, Malaspina D, Hercher L. Prodromal interventions for schizophrenia vulnerability: the risks of being “at risk”. Schizophr Res 2005;73:173‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fusar‐Poli P, Manchia M, Koutsouleris N et al. Ethical considerations for precision psychiatry: a roadmap for research and clinical practice. Eur Neuropsychopharmacol 2022;63:17‐34. [DOI] [PubMed] [Google Scholar]
- 82. Nery FG, Wilson AR, Schneider MR et al. Medication exposure and predictors of first mood episode in offspring of parents with bipolar disorder: a prospective study. Braz J Psychiatry 2020;42:481‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Post RM, Goldstein BI, Birmaher B et al. Toward prevention of bipolar disorder in at‐risk children: potential strategies ahead of the data. J Affect Disord 2020;272:508‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ryan J, Virani A, Austin JC. Ethical issues associated with genetic counseling in the context of adolescent psychiatry. Appl Transl Genom 2015;5:23‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gottesman II, Laursen TM, Bertelsen A et al. Severe mental disorders in offspring with 2 psychiatrically ill parents. Arch Gen Psychiatry 2010;67:252‐7. [DOI] [PubMed] [Google Scholar]
- 86. Greve AN, Uher R, Als TD et al. A nationwide cohort study of nonrandom mating in schizophrenia and bipolar disorder. Schizophr Bull 2021;47:1342‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fusar‐Poli P, Bauer M, Borgwardt S et al. European College of Neuropsychopharmacology Network on the Prevention of Mental Disorders and Mental Health Promotion (ECNP PMD‐MHP). Eur Neuropsychopharmacol 2019;29:1301‐11. [DOI] [PubMed] [Google Scholar]
- 88. Aylott A, Zwicker A, MacKenzie LE et al. Like father like daughter: sex‐specific parent‐of‐origin effects in the transmission of liability for psychotic symptoms to offspring. J Dev Orig Health Dis 2019;10:100‐7. [DOI] [PubMed] [Google Scholar]
- 89. Goldstein JM, Cherkerzian S, Seidman LJ et al. Sex‐specific rates of transmission of psychosis in the New England high‐risk family study. Schizophr Res 2011;128:150‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Goos LM, Ezzatian P, Schachar R. Parent‐of‐origin effects in attention‐deficit hyperactivity disorder. Psychiatry Res 2007;149:1‐9. [DOI] [PubMed] [Google Scholar]
- 91. Pavlova B, Bagnell A, Cumby J et al. Sex‐specific transmission of anxiety disorders from parents to offspring. JAMA Netw Open 2022;5:e2220919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gregersen M, Søndergaard A, Brandt JM et al. Mental disorders in preadolescent children at familial high‐risk of schizophrenia or bipolar disorder – a four‐year follow‐up study: the Danish High Risk and Resilience Study, VIA 11. J Child Psychol Psychiatry 2022;63:1046‐56. [DOI] [PubMed] [Google Scholar]
- 93. Krantz MF, Hjorthøj C, Ellersgaard D et al. Examining selection bias in a population‐based cohort study of 522 children with familial high risk of schizophrenia or bipolar disorder, and controls: the Danish High Risk and Resilience Study VIA 7. Soc Psychiatry Psychiatr Epidemiol 2023;58:113‐40. [DOI] [PubMed] [Google Scholar]


