Abstract
Critical data on the impacts of fluoride (F) in food systems along the Eastern Africa Rift Valley System (EARS) is needed for public health risk assessment and for the development of strategies for ameliorating its deleterious effects among the affected communities. Long-term F overexposure causes dental and skeletal fluorosis, and leads to neurotoxicity, which impacts several important body functions. Investigating F exposure pathways is of essence to inform and safeguard public health of the affected communities. The current study assessed the F levels in potatoes (Solanum tuberosum L.), beans (Phaseolus vulgaris L.) and garden peas (Possum sativa) from Nakuru County, Kenya, by potentiometric analysis using F ion-selective electrodes. It then evaluated the risk factors for excessive human exposure to F through contaminated foodstuffs. The mean F levels in the potatoes (8.50 ± 4.70 mg/kg), beans (8.02 ± 4.12 mg/kg) and peas (4.99 ± 1.25 mg/kg) exceeded recommended dietary allowances (RDA) level of 4 mg/kg endorsed by US Institute of Medicine for the different categories of people. The F distribution trends in beans and potatoes reflected the environmental patterns of F contamination of the study area but the spatial extent Fin the peas indicated existence of partial resistance of the pea plants to environmental F uptake. The results indicated that both the beans and the potatoes were more liable to accumulating greater amounts of F from the environment than garden peas and that all the three foodstuffs contained high F levels that posed greater risk of F overexposure and its deleterious impacts among the young children, male populations, and in people of greater body weight and high physical activity levels.
Subject terms: Environmental chemistry, Environmental sciences, Chemistry, Risk factors
Introduction
Prolonged fluoride (F) ingestion through foods and water causes adverse health effects in humans, animals and plants. The negative physiological effects of F in humans include dental and skeletal fluorosis, and a myriad of other harmful non-skeletal symptoms that range from mild disturbances in the gut caused by fluoride-induced hyperacidity to severe physiological disorders and toxicities. Dental and skeletal fluorosis develop when excessive fluoride stimulate osteoblastic activity leading to delayed mineralization of bone tissue during its development and resulting in malformed porous and brittle bone structure1.
The most common non-skeletal fluorosis appears to spring from the F interference in dietary calcium assimilation. A normal adult human requires approximately 450 mg of daily dietary calcium. Ca is a vital element to the body and it is required for maintaining total health, ensuring strong bones and teeth, maintaining the integrity of skeletal muscles, controlling nerve excitability and aiding in blood clotting2. It plays vital roles in spermatogenesis; and in sperm motility, capacitation, acrosome reaction and fertilization3. F can precipitae soluble Ca both in the gut and in the blood and lessen its availability to the body inducing its pseudo-deficiency. When blood Ca is too low, the body restores its balance by solubilizing bones’ Ca deposits and vice versa in a process controlled by secretions from the parathyroid glands.
So, F toxicity has been linked to osteoporosis of bones4; muscle wasting in animals5 and other complications related to carotid artery atherosclerosis6 and nephrolithiasis (urinary stones disease)7; neurotoxicity manifesting as intelligence suppression8–14 and emotional imbalance in children15; reproductive16,17 and embryo toxicity18; and thyroid dysfunction19 but even though the association between F toxicity and different kinds of cancers is subject of frequent discuss20,21, the causative role of F has not been established. In general, dental and skeletal fluorosis occur in communities depending on drinking water with at least 1.5 and about 3.0 mg/kg F content, respectively. One study reported F neurotoxic effects in 3–4 year-old infants at 0.34 mg daily F exposure13 but the most subtle neurological F effects have been reported for threshold drinking water F levels of 1.5 mg/L8–11,14,15 and some studies have indicated that structural soft-tissue toxic effects of F may appear at higher threshold drinking water F levels of at least 2.0 mg/L6,7
The Eastern African Rift Valley System (EARS) is one of the regions of the world where highest F concentrations of 2800 mg/L have been reported in natural waters22. The same has recently been reviewed and discussed extensively in the literature23. So, high F levels exceeding permissible standards of 1.5 mg/L for drinking water24,25 and reference dose (RfD) of 0.06 mg/kg/day for non-liquid foodstuffs26 have been reported27–29 among these areas. This has heightened food safety concerns prompting several investigations focused on evaluating the F content and safety for the key foodstuffs from the region30,31. Rizzu et al.31, for example, assessed the F levels in maize (Zea mays L.), tomato (Lycopersicon esculentum Mill.), and kale (Brassica sp. pl.) along the EARS around Arusha in Tanzania and reported high fluoride contents of 8.0–14.2 mg/kg for the foodstuffs. Despite the ongoing efforts, the F levels in other key staple foodstuffs including potatoes (Solanum tuberosum L.), beans (Phaseolus vulgaris L.) and garden peas (Possum sativa) and the associated risk factors related to consumption of F-contaminated foodstuffs among the most affected communities along the EARS remains unclear. This data is necessary for improving the understanding of the fluoride problem along the EARS high-fluoride belt, and for informing the strategies to deal with fluoride overexposure and ameliorate its deleterious effects among the affected communities.
The current study investigated the F content in Irish potatoes (Solanum tuberosum L.), beans (Phaseolus vulgaris L.) and peas (Possum sativa) from Nakuru County, Kenya, and also assessed the risk of F overexposure among the affected communities. It is anticipated that the results of the current work will contribute to improving knowledge of the local F problem and contribute to the ongoing global efforts to develop credible working solutions to the harmful effects of F overexposure among the communities living in high F regions of the world.
Material and methods
Study area
Figure 1 shows the map of the study area in relation to the map of Kenya and the map of Africa. The area, which comprises Nakuru County of Kenya is about 7500 km2 and it lies across latitudes 0º13′ S and 1º10′ S and longitudes 35º28′ E and 35º36′ E. It is divided into 9 administrative sub-counties including: Naivasha, Gilgil, Nakuru East, Nakuru West, Njoro, Nakuru North, Molo, and Rongai, which were the main focus of the current study.
Figure 1.
Map of the study area in Nakuru County relative to the map of Kenya and map of Africa.
The landscape is composed of a volcanic terrain32 and the climate is characterized by a bimodal annual rainfall pattern consisting of short rains in the months of October–December and long rains during the period of March–June33. The amount of annual rainfall decreases from over 1500 mm in parts of Njoro and Molo highlands at 2400 m altitude to less than 500 mm in Rongai, Naivasha, Gilgil, Nakuru East and Nakuru West sub-counties at the rift valley floor at about 1600 m elevation. The temperatures range from 12 to 30 °C with the coolest months being June–July and the hottest being December–March.
The varied climatic conditions make the Nakuru County to be one of the mainstay agricultural hubs in Kenya. With a population of close to 2.5 million people comprising 36% urban residents34, most of the foods produced are consumed locally with a surplus for external markets and processing.
Children under the age of 13 years, who are at highest risk of the adverse effects of F overdose35, make up to 38.2% of the total population of the area32.
Sample collection
Permissions or licenses were obtained from the relevant authorities as required. Field sampling was conducted during the months of August and September in 2019. A total of 165 samples consisting 75 Irish potatoes samples, 54 beans samples and 43 peas samples were collected directly from farms, where they are grown. The samples were handled and used in the present work in accordance with national and international guidelines36,37. The sample collection, which was done by random sampling, was carried out from sample points selected to cover the entire study area according to the criterion depicted in Table 138. The raw samples were collected directly into dry polythene zip lock bags and transported to the laboratory for processing and testing.
Table 1.
Distribution of sample collection points by the target sub-counties.
| Sub-counties | Foodstuffs | |||
|---|---|---|---|---|
| Potatoes (n) | Beans (n) | Peas (n) | Total (N) | |
| Molo | 10 | 5 | 8 | 23 |
| Njoro | 10 | 7 | 9 | 26 |
| Rongai | 9 | 6 | 6 | 21 |
| Nakuru N | 11 | 5 | 6 | 22 |
| Nakuru W | 10 | 4 | 4 | 18 |
| Nakuru E | 10 | 4 | 3 | 17 |
| Gilgil | 7 | 8 | 3 | 18 |
| Naivasha | 8 | 8 | 4 | 20 |
| Overall | 75 | 47 | 43 | 165 |
Sample preparation
At the laboratory, the samples were washed with excess doubly-deionized water (DDW) to remove soils and other impurities. The potatoes were sliced into cubes of about 1.5 cm dimensions and dried on a ULM-400 constant-temperature oven (Memmert, Germany) at 110 °C for 24 h. The peas and beans samples were dried in the same way without further treatment. The dry samples were pulverized to a fine powder using an SBG-301 Blender/Grinder (ASL Ltd, Kenya) and preserved in air-tight sample bottles for the subsequent tests.
Extraction of fluoride from the samples
A portion of 1.25 g of each of the powdered samples (beans, potatoes and peas) was digested with 10 mL of 6 M NaOH on a BKD-20F Kjeldahl Digestion Furnace (Biobase, China) until the samples dissolved in the base. The solution was allowed to cool to room temperature and neutralized by careful addition of 3 M H2SO4 to pH 7.1 ± 0.1 before it was transferred into a 50-mL volumetric flask and made up to the 50-mL volumetric mark with DDW.
Fluoride determination using ion-selective electrodes
Exactly 10.0 mL of each of the sample solutions was mixed with an equal volume of total ionic strength adjusting buffer (TISAB-III) solution39 and the F concentration measured using a Thermo Fisher Scientific 9609BNWP pH/Ion Meter (STAR) with a Thermo Fisher Fluoride ion selective electrode (Thermo Fischer Scientific, UK)38. All the experiments were conducted in triplicate.
Statistical analysis
The results were analyzed using IBM SPSS Statistics Software by determining the mean and standard deviations on replicate data and causal relationships determined by the Analysis of Variance (ANOVA) to determine whether there were any significant differences (p ≤ 0.05) between the concentrations of fluoride in peas, beans and potatoes from different regions of the study area. The paired t-test was then applied to determine significant difference at p ≤ 0.05 confidence level in fluoride level between peas, beans and potatoes and to compare means from different regions.
The results were used to calculate the daily F intake (DFI) in mg/kg/day for individual foodstuffs and to compute the estimated average daily F dosage (EADD) in mg/kg/day for the households.
Determination of the risk of fluoride overexposure
Daily food energy contribution (FEC) by the foodstuffs
The average daily household food intake (ADFCi) in kg for the particular ith foodstuff was determined from food conservation and dietary diversification data and from the food consumption recall data and production survey conducted previously in the study40,41. The energy contribution (FEC) of the ith foodstuff, which is the fractional/ percentage contribution of the particular type of foodstuff to the overall daily energy consumption of an overage individual/household was estimated according to Eq. (1) as:
| 1 |
where Ei is the calorific value (kcal/kg) of the ith foodstuff and DEI (kcal/day) is the total daily energy intake from all foodstuffs in the person’s or household daily menu. The daily energy intake (DEI) data for human energy requirements by age categories, body weights (BW) and physical activity levels (PAL) have been published and was obtained for the current calculations from a joint report of Food and Agriculture Organization (FAO), World Health Organization (WHO) and the United Nations University (UNU)42.
Daily fluoride intake, DFI, (mg/kg/day) from the foodstuffs
The daily fluoride intake, DFI, (mg/kg/day) through consumption of the particular ith foodstuff was then calculated from Eq. (2)30,31 based on the determined values of FECi and DEI as:
| 2 |
where, [F]i is the F content of ith foodstuff (mg/kg); AF is the F absorption factor estimated at 60% for most non-liquid foodstuffs43; whereas Ei is the dietary calorific value (kcal/kg) of the ith foodstuff. The F content of the foodstuff in mg/kg, [F]i, was determined experimentally for beans, peas and potatoes in the current work and the values for the other foodstuffs obtained from the relevant literature28,44.
The US Centers for Disease Control and Prevention (CDC) through the National Center for Health Statistics publishes Data Tables of Weight-for-age Charts that provide information on the distribution of body weights (BW) by human age categories45. The daily human energy requirements used for estimating FECi and DFIi according to Eqs. (1) and (2) above was obtained from the joint data report of FAO/WHO/UNU42 by adopting the 50th percentile weight in each age category as the representative weight for the particular age.
Estimated average daily fluoride dosage (EADDc)
The estimated daily fluoride dosage, EADDc30 for n number of food items in the average daily household menus was then computed by adding together the daily fluoride intake, DFI, (mg/kg/day) for individual foodstuffs according to Eq. (3) as:
| 3 |
The results were compared with the recommended dietary allowance (RDA) published in the literature for the different categories of people46.
Results
Spatial distribution of the fluoride levels in peas, beans and potatoes
The results of the analyses of F levels of the three foodstuffs by the sub-counties are presented in Table 2. The F content of the foodstuffs was found to follow the order:
| 4 |
where, [F]peas, [F]beans, and [F]potatoes are the F concentrations (mg/kg) of the respective foodstuffs. The highest mean F levels in peas were recorded in Nakuru East (5.64 ± 0.70 mg/kg) whereas the lowest Naivasha (3.51 ± 1.73 mg/kg) sub-counties. However, the highest mean F contents of the beans (14.50 ± 4.34) and of the potatoes (12.50 ± 5.53 mg/kg) were found among the samples collected from Nakuru North and Molo sub-counties, respectively. The general trends in the overall F levels of the three foodstuffs were as follows:
Table 2.
Spatial distribution of [F] mg/kg in the three foodstuffs.
| Sub County | [F] in Beans (mg/kg) | [F] in Potatoes (mg/kg) | [F] in Peas (mg/kg) | |||
|---|---|---|---|---|---|---|
| Range | Mean ± Stdev | Range | Mean ± Stdev | Range | Mean ± Stdev | |
| Gilgil | 2.97–8.92 | 5.81 ± 2.21 | 3.47–7.89 | 5.43 ± 1.52 | 3.86–4.18 | 3.97 ± 0.18 |
| Molo | 4.9–9.6 | 6.67 ± 1.60 | 3.99–18.88 | 12.50 ± 5.53 | 3.62–6.64 | 5.43 ± 1.06 |
| Naivasha | 2.15–5.66 | 4.27 ± 1.07 | 2.75–6.56 | 4.57 ± 1.63 | 1.94–5.28 | 3.51 ± 1.73 |
| Nakuru East | 6.14–10.59 | 8.24 ± 1.84 | 4.86–10.72 | 7.05 ± 1.95 | 4.88–6.35 | 5.64 ± 0.70 |
| Nakuru North | 10.51–21.44 | 14.50 ± 4.34 | 4.8–18.05 | 10.77 ± 4.81 | 3.77–6.89 | 5.37 ± 1.16 |
| Nakuru West | 8.88–18.2 | 12.90 ± 3.97 | 3.31–12.79 | 9.55 ± 2.58 | 3.42–7.12 | 5.41 ± 1.63 |
| Njoro | 3.74–5.99 | 4.98 ± 0.84 | 3.82–12.9 | 7.10 ± 3.10 | 3.57–5.94 | 4.89 ± 0.84 |
| Rongai | 10.59–17.47 | 14.21 ± 2.85 | 3.72–6.58 | 5.02 ± 0.89 | 3.57–7.89 | 4.85 ± 1.59 |
| Overall | 2.15–21.44 | 8.50 ± 4.70 | 2.75–18.88 | 8.02 ± 4.12 | 7.89 | 4.99 ± 1.25 |
High F levels in the three foodstuffs were detected in areas at the rift valley floor, which have been reported to be the fluoride hotspots47.
Statistical analyses
The results of the analysis of the variance (ANOVA) showed that there was no significant difference in the F content of peas samples between the 8 sub-counties (p = 0.436) of Nakuru County, Kenya. Nonetheless, the F levels of both the beans (p = 0.000) and potatoes (p = 0.000) were significantly different across the 8 sub-counties studied (p = 0.000).
The paired t-test was applied to compare the means of F levels between the peas and the beans, the peas and potatoes, and between the beans and the potatoes. It was found that the mean F levels were significantly different (p ˂ 0.05) between the peas and the beans (p = 0.033) and between peas and potatoes (p = 0.030) but that the means of the F levels between beans and potatoes (p = 0.067) were not significantly different from each other.
The paired t-test was then also applied to compare the mean F levels in the peas between Njoro and Naivasha and between Nakuru and Gilgil regions to test if significance differences existed within the same dataset across the regions. It was found that no significant differences existed in the mean F levels in the peas between Njoro and Naivasha regions (p = 0.051) but there was strong significance between the mean F levels in peas samples obtained from Nakuru and Gilgil regions (p = 0.00).
Then, by applying the paired t-test to compare the mean F levels in the beans and in the potatoes between Njoro and Naivasha and between Nakuru and Gilgil regions (p ˂ 0.05), it was found that the mean F levels in the beans were significantly different between Njoro and Naivasha (p = 0.000) and also between Nakuru and Gilgil (p = 0.020). In the same way, the results confirmed that the mean F levels in the potatoes were significantly different between Njoro and Naivasha (p = 0.001).
The foregoing results from application of the paired t-test agreed with the results obtained from the analysis of the variance (ANOVA) in this section.
Analyses of fluoride risk of overexposure
Daily food energy contribution (FEC)
In order to assess the potential of F overexposure through the foodstuffs, household dietary patterns of local communities were analyzed based on food diversification and the food production data for the area in order to identify the most used foodstuffs and estimate the food energy contribution (FEC) of the foodstuff to the daily energy intake as in Eq. (2) and the results depicted in Table 3.
Table 3.
The main foodstuffs contributing to daily household dietary energy requirement in Nakuru County, Kenya.
| Food items | Food energy content | Av. weekly food consumption | Av. daily food consumption | Daily food energy contribution | Food F content | Daily food energy contribution | Refernce |
|---|---|---|---|---|---|---|---|
| [F] | |||||||
| (kcal/kg) | (kg) | (kg) | (kcal) | (mg/kg) | (%) | ||
| Maize (Zea mays) | 1110 | 6.89 | 0.98 | 1092.56 | 13.6 | 27.9 | 44 |
| Irish potatoes (Solanum tuberosum L.) | 1050 | 6.5 | 0.93 | 975.00 | 8.2 | 24.9 | Current study |
| Garden peas (Possum sativa) | 1200 | 3.35 | 0.48 | 574.29 | 4.9 | 14.7 | Current study |
| Carrots (Daucus carota) | 320 | 2.8 | 0.40 | 128.00 | 3.3 | ||
| Cabbage (Brassica oleracea capitata) | 1000 | 2.1 | 0.30 | 300.00 | 7.7 | ||
| Onions (Allium cepa) | 420 | 2.1 | 0.30 | 126.00 | 3.2 | ||
| Sukuma (Brassica oleracea) | 540 | 2.1 | 0.30 | 162.00 | 13.3 | 4.1 | 28 |
| Tomatoes (Lycopersicum esculentum) | 280 | 2.1 | 0.30 | 84.00 | 12.9 | 2.1 | 28 |
| Beans (Phaseolus vulgaris L.) | 1090 | 1.5 | 0.21 | 233.57 | 8.7 | 6.0 | Current study |
| Others (e.g., tea beverage etc.) | 608 | 2.8 | 0.40 | 243.20 | 6.21 | ||
| Overall |
The average household size in Nakuru is 3.0–3.9 persons34. It was found that a typical household consumes about 3918.61 kcal of food daily in form of the popular local maize meal known as ugali (27.88%), Irish potatoes (24.88%), garden peas (14.66%), and leafy vegetables, especially cabbages and a popular local variety of kales known as Sukuma wiki. These foodstuffs, which are depicted in Table 4 contribute approximately 93.79% of the daily household energy needs of the members.
Table 4.
Calculated EADD values for the main foodstuffs consumed daily by residents in Nakuru County.
| Age category (years) | Recommended limits | Upper tolerable limits46 (mg/day) | Three foods* (mg/day) | Five foods ** (mg/day) | |||
|---|---|---|---|---|---|---|---|
| Male (mg/day) | Female (mg/day) | Male | Female | Male | Female | ||
| Mean ± Stdev | Mean ± Stdev | Mean ± Stdev | Mean ± Stdev | ||||
| 1–3 | 0.7 | 1.0 | 1.3 | 2.00 ± 0.28 | 1.85 ± 0.27 | 5.61 ± 0.77 | 5.17 ± 0.74 |
| 4–8 | 1.0 | 2.0 | 2.2 | 2.76 ± 0.39 | 2.53 ± 0.36 | 7.74 ± 1.08 | 7.09 ± 1.02 |
| 9–13 | 2.0 | 3.0 | 10.0 | 4.10 ± 0.64 | 3.72 ± 0.47 | 11.48 ± 1.79 | 10.42 ± 1.31 |
| 14–18 | 3.0 | 3.0 | 10.0 | 5.66 ± 0.47 | 4.45 ± 0.10 | 15.86 ± 1.31 | 12.47 ± 0.27 |
| ≥ 19 | 4.0 | 3.0 | 10.0 | 5.30 ± 1.05 | 4.47 ± 0.84 | 14.87 ± 2.95 | 12.54 ± 2.36 |
*Beans, potatoes and garden peas studied in the current work.
**Beans, potatoes and garden peas studied in the current work plus maize, tomatoes and sukuma wiki whose from content had been previous studied in the literature.
Daily fluoride intake (DFIi) and estimated average daily F dosage, EADD values
Based on the FEC ratios in Table 4, the daily fluoride intake, DFI, for the food items and the average daily dosage of fluoride (EADD) for the different age groups, gender, body weight (BW), and physical activities level (PAL) were estimated using Eq. (2) and (3) and the results presented in Fig. 2 and in Table 4.
Figure 2.
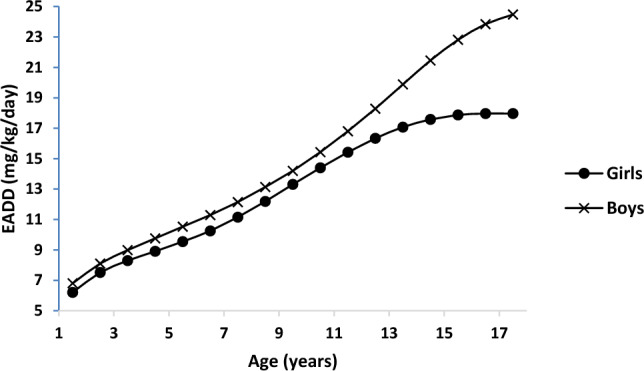
Amounts of dietary F exposure through food for boys and girls aged 1–18 years.
The mean EADD values were 14.87 ± 2.95 and 12.54 ± 2.36 mg/day for males and females above the age of 18 years, respectively, and 5.61 ± 0.77—11.48 ± 1.08 and 5.17 ± 0.74—10.42 ± 1.31 mg/day for male and female children in the age bracket 1–13 years, respectively. These results are in agreement with those of previous researchers who found that the daily F intake by a group of nursing mothers averaged 22.1 mg/day with a range of 9.5–37.2 with cooked food, water and tea contributing 11.7, 4.5 and 5.8 mg of F exposure daily48. For all the age categories and in both males and female, the calculated EADD values for the foodstuffs exceeded recommended dietary limits quoted in Table 446.
Effect of age and gender
From the results present in Fig. 2, it was observed that F exposure was higher in boys than in girls and that the estimated average daily F dosage (EADD) increased with increasing age of the children from one year of age and it leveled off at about age of 18 and 16 years for the boys and the girls, respectively.
It can be noted that in the age-bracket of 1–12 years, the EADD for boys was about 1.10 times higher than that for the girls at all ages. This ratio increased to about 1.36 times in the adolescent age bracket of 12–18 years, which was consistent with similar results reported elsewhere in the literature. In a previous survey involving 3771 adult males and 4495 adult females on the risks of F-instigated bone fractures among rural communities in China, for example, the authors indicated that the F-instigated bone fractures were 1.35 more prevalent among the male populations than among the corresponding female categories49. In an epidemiological survey on the pattern of abnormal urinary fluoride levels among populations with occupational fluoride exposure in Shanghai, it was reported that the prevalence rates of abnormally high urinary fluoride in the men were about 4.46 times greater than in the women. These values collaborate the results of the present work, which have revealed higher tendency of males to suffer greater impact of F than females under the same conditions of exposure.
Impact of body weight (BW) and physical activity levels (PAL)
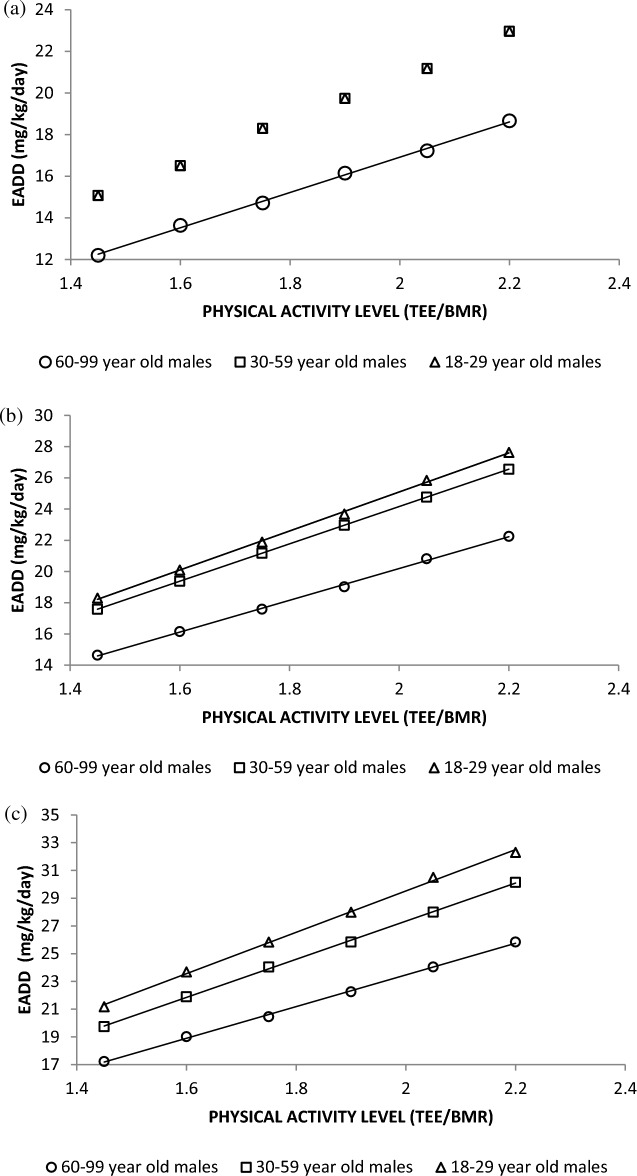
The levels of EADD were compared for the different categories of BW and PAL by gender and age-groups and the results presented in Fig. 3a–c.
Figure 3.
Representative plots of estimated daily F dosage (EADD) for different categories of BW and PAL by gender and by different age groups for: (a) 50 kg BW, (b) 70 kg BW, and (c) 90 kg BW males (NB: TEE is the total energy expenditure in 24 h and BMR is the basal metabolic rate).
The results showed that the risk of F overexposure through the foodstuffs increased with increasing BW and increasing PAL and that the potential of F overexposure was greater for the young adults (18–29 years) than among the older categories of people. It could be observed that at low BW of 50 kg, the F exposure levels for young men (18–29 years) was similar to those for men of 30–59 years of age but it differed from those for older men above 60 years of age. Figure 3b,c show that the value of EADD was different for the 18–29, 30–59, and for 60-and-above age categories of BW of 70 kg and above.
Discussion
As depicted in Table 4, the results in the current study showed that peas, beans and Irish potatoes from Nakuru County contained high levels of F above the recommended dietary allowance (RDA) for the different groups of people46. However, whereas high F was recorded in all the three foodstuffs obtained from all the regions of the current study area, the F levels in garden peas were much lower when compared to those in the beans and the potatoes. Furthermore, the results of the analysis of the variance (AVOVA), showed that F levels in the beans and in the potatoes were not statistically different even though both of them were significantly different between the regions of the current study area. The F content in the peas was, however, not statistically different between the regions despite the heterogeneity in spatial distribution of fluoride in the environment50.
High fluoride occurrence in beans and potatoes, depicted in Table 2, were consistent with the prevailing F distribution trends in environment47 and it was heightened within the fluoride pollution hotspots located at the hotter and drier areas of rift valley floor including Nakuru North, Nakuru West, Molo, Rongai and Nakuru East sub-counties47. This was attributed to uptake and accumulation of F in the bean51 and potato52 plants from F-enriched soils resulting from long-term sedimentation of high-fluoride debris by agents of erosion and deposition (accentuated by evaporative concentration due the hotter climate of the low altitude regions). This showed that people relying on these foods, derived from crops cultivated in these areas, were at greater potential of fluoride overexposure.
The differences in the mean F levels of the three foodstuffs (Table 2) produced within similar conditions can be explained, in part, on the variations in the local soil and soil–water F conditions and on dissimilarities in ecological conditions. Other than ecological conditions affecting the F accumulation of the respective crops, the prevailing land use forms and practices, and other human activities can influence the availability of soil–water F content to varying degrees53. Also, differences in the local geology and volcanic terrains resulting from presence of active geothermal activities54 can occur over limit geographical space55 causing minor variations in rock solubilization processes impacting soil geochemistry and the net spatial F concentration in the soil–water F56. This, in turn, controls the soil-F interactions with plant species57. The high F content in the foodstuffs obtained from higher altitude areas of Molo and Njoro regions could, therefore, be linked to the presence of high-F volcanic landscapes58 and to the resulting F soils enrichment due to the ascent of hydrothermal fluids of active geothermal activities occurring in parts of these areas59.
The diverse genotypic traits of plant species can also affect the extent to which the plant species absorb60 and tolerate F levels in the soils61. The low F concentrations in the pea’s samples across the regions of Nakuru County when compared to the F levels of the beans and the potatoes samples, indicated that F uptake by the pea’s plants was not influenced by the ecological, climatological and geological F conditions of the regions. It showed that the pea plant was somewhat resistant to F uptake from the environment than both the beans and the Irish potatoes, which can have implications on safe food production.
The results from the analysis of the risk of F exposure from the foodstuffs given in Fig. 2 and in Table 4 revealed that the male populations of the resident communities living in high F belts are at the greater risks of F overexposure compared to the corresponding female populations under matching conditions. It showed that under similar conditions, the male populations were prone to ingesting greater dietary amounts of F exposing themselves to greater prevalence and more severe F impacts than their female counterparts.
A summary of similar discrepancies in the effects of F overexposure between the male and female human populations reported in the literature from around the world are presented in Table 5.
Table 5.
Differential impact of fluoride on the male and female populations reported in the literature all over the world.
| Fluoride adverse health effect | Place of study | Sample size (n) | Female Index (If) | Male index (Im) | Units | Im/If | Literature source | Age bracket (years) | Age category |
|---|---|---|---|---|---|---|---|---|---|
| Dental fluorosis prevalence (< 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with < 1.5 mg/L F water | 244 | 4.58 | 6.67 | % | 1.46 | 62 | 0–4 | Children |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with > 1.5 mg/L F water | 315 | 7.04 | 7.55 | % | 1.07 | 62 | 0–4 | Children |
| Dental fluorosis prevalence | Ledhupur & Rustampur, Varanasi | 218 | 4.49 | 4.58 | % | 1.02 | 63 | 1–5 | Children |
| Dental fluorosis prevalence | Nawa tehsil, Nagaur district, Rajasthan (India) | 60 | 90 | 91.67 | % | 1.02 | 64 | 4–16 | Children |
| Blood serum/plasma F | Balavenkatapuram, Kalyandurg Mandal, Anantapur, Andhra Pradesh, India | 10 | 0.18 | 0.51 | ppm | 2.83 | 65 | 5–11 | Children |
| Urine F | Ayyagarlapalli, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 10 | 2.14 | 3.08 | ppm | 1.44 | 65 | 5–11 | Children |
| Urine F | Kanukuru, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 10 | 5.12 | 7.26 | ppm | 1.42 | 65 | 5–11 | Children |
| Urine F | Balavenkatapuram, Kalyandurg Mandal, Anantapur, Andhra Pradesh, India | 10 | 4.12 | 5.4 | ppm | 1.31 | 65 | 5–11 | Children |
| Blood serum/plasma F | Ayyagarlapalli, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 10 | 0.36 | 0.46 | ppm | 1.28 | 65 | 5–11 | Children |
| Dental fluorosis prevalence (< 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with < 1.5 mg/L F water | 663 | 36.76 | 38.01 | % | 1.03 | 62 | 5–11 | Children |
| Blood serum/plasma F | Kanukuru, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 10 | 0.64 | 0.64 | ppm | 1.00 | 65 | 5–11 | Children |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with > 1.5 mg/L F water | 533 | 63.94 | 56.67 | % | 0.89 | 62 | 5–11 | Children |
| Dental fluorosis prevalence (DMFT scores) | Thailand | 271 | 0.2 | 0.1 | DMFT score | 0.50 | 66 | 5–9 | Children |
| Dental fluorosis prevalence | Libyan Population | 6244 | 62.28 | 64.27 | % | 1.03 | 67 | 6–60 | All |
| Dental fluorosis prevalence | Rural Area, Tianjin, China | 709 | 46.26 | 53.74 | % | 1.16 | 68 | 6–13 | Children |
| Dental fluorosis prevalence | Ledhupur & Rustampur, Varanasi | 256 | 37.19 | 37.1 | % | 1.00 | 63 | 6–10 | Children |
| Dental fluorosis prevalence | Israel-administered Gaza Strip | 152 | 52.6 | 47.4 | % | 0.90 | 69 | 6–8 | Children |
| Dental fluorosis prevalence | Han, China | 182 | 9.33 | 10.28 | % | 1.10 | 70 | 8–15 | Adolescents |
| Dental fluorosis prevalence | Tibetan, China | 375 | 52.47 | 50.23 | % | 0.96 | 70 | 8–15 | Adolescents |
| Dental fluorosis prevalence | Alappuzha district, Kerala, India | 1124 | 39.2 | 31.3 | % | 0.80 | 71 | 10–17 | Adolescents |
| Dental fluorosis prevalence | Thailand | 299 | 0.8 | 0.4 | DMFT scores | 0.50 | 66 | 10–14 | Adolescents |
| Dental fluorosis prevalence | Ledhupur & Rustampur, Varanasi | 158 | 53.94 | 58.86 | % | 1.09 | 63 | 11–15 | Adolescents |
| Dental fluorosis prevalence | Fluorotic rural area, Turkey | 293 | 4.75 | 8.13 | %DMFT scores | 1.71 | 72 | 12–80 | All |
| Dental fluorosis prevalence (< 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with < 1.5 mg/L F water | 744 | 40.2 | 48.87 | % | 1.22 | 62 | 12–24 | Adolescents |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with > 1.5 mg/L F water | 599 | 71.83 | 83.74 | % | 1.17 | 62 | 12–24 | Adolescents |
| Blood serum/plasma F | Kanukuru, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 8 | 0.35 | 0.39 | ppm | 1.11 | 65 | 12–18 | Adolescents |
| Blood serum/plasma F | Ayyagarlapalli, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 10 | 0.17 | 0.18 | ppm | 1.06 | 65 | 12–18 | Adolescents |
| Urine F | Kanukuru, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 8 | 6.87 | 7.18 | ppm | 1.05 | 65 | 12–18 | Adolescents |
| Urine F | Ayyagarlapalli, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 10 | 3.58 | 2.67 | ppm | 0.75 | 65 | 12–18 | Adolescents |
| Dental fluorosis prevalence | Schools in Southern India | 1025 | 63.6 | 65.2 | % | 1.03 | 73 | 12–17 | Adolescents |
| Dental fluorosis prevalence | Nalgonda district, Andhra Pradesh, India | 775 | 77 | 78 | % | 1.01 | 74 | 12 | Adolescents |
| Dental fluorosis prevalence | Nalgonda district, Andhra Pradesh, India | 778 | 76.9 | 78.8 | % | 1.02 | 74 | 15 | Adolescents |
| Dental fluorosis prevalence | Ledhupur & Rustampur, Varanasi | 95 | 27.41 | 54.73 | % | 2.00 | 63 | 16–20 | Adults |
| Dental fluorosis prevalence | Nawa tehsil, Nagaur district, Rajasthan (India) | 28 | 81.82 | 95.93 | % | 1.17 | 64 | 17–28 | Adults |
| Urine F | Ayyagarlapalli, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 9 | 2.13 | 2.55 | ppm | 1.20 | 65 | 19–35 | Adults |
| Blood serum/plasma F | Ayyagarlapalli, Setturu Mandal, Anantapur District, Andhra Pradesh, India | 9 | 0.22 | 0.24 | ppm | 1.09 | 65 | 19–35 | Adults |
| Dental fluorosis prevalence (DMFT scores) | Thailand | 116 | 1.2 | 0.8 | DMFT scores | 0.67 | 66 | 20–24 | Adults |
| Dental fluorosis prevalence | Ledhupur & Rustampur, Varanasi | 91 | 22.41 | 42.83 | % | 1.91 | 63 | 21–25 | Adults |
| Dental fluorosis prevalence (DMFT scores) | Thailand | 261 | 0.9 | 0.6 | DMFT scores | 0.67 | 66 | 24–34 | Adults |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with > 1.5 mg/L F water | 302 | 60.27 | 67.3 | % | 1.12 | 62 | 25–44 | Adults |
| Dental fluorosis prevalence (< 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with < 1.5 mg/L F water | 442 | 40.62 | 46.77 | % | 1.15 | 62 | 25–44 | Adults |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with > 1.5 mg/L F water | 480 | 64.45 | 72.32 | % | 1.12 | 62 | 25–34 | Adults |
| Dental fluorosis prevalence (< 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with < 1.5 mg/L F water | 516 | 42.33 | 45.37 | % | 1.07 | 62 | 25–34 | Adults |
| Dental fluorosis prevalence | Ledhupur & Rustampur, Varanasi | 80 | 16.66 | 31.25 | % | 1.88 | 63 | 26–30 | Adults |
| Dental fluorosis prevalence | Nawa tehsil, Nagaur district, Rajasthan (India) | 49 | 95 | 96.43 | % | 1.02 | 64 | 29–40 | Adults |
| Dental fluorosis prevalence | Ledhupur & Rustampur, Varanasi | 299 | 5.12 | 16.72 | % | 3.27 | 63 | 31 | Adults |
| Dental fluorosis prevalence | Thailand | 202 | 1.5 | 1.5 | DMFT scores | 1.00 | 66 | 35–44 | Adults |
| Blood serum/plasma F | Xinhuai, Sihong County, Jiangsu Province, China | 36 | 0.073 | 0.077 | mg/L | 1.05 | 75 | 36–78 | Adults |
| Skeletal fluorosis prevalence | Wamiao, Sihong County, Jiangsu Province, China | 132 | 36.21 | 27.03 | % | 0.75 | 75 | 36–78 | Adults |
| Dental fluorosis prevalence | Nawa tehsil, Nagaur district, Rajasthan (India) | 20 | 94.44 | 100 | 1.06 | 64 | 40 < | Adults | |
| Dental fluorosis prevalence | Thailand | 297 | 8.1 | 9.2 | DMFT scores | 1.14 | 66 | 45 < | Adults |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with > 1.5 mg/L F water | 430 | 56.22 | 69.01 | % | 1.23 | 62 | 45–64 | Adults |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with < 1.5 mg/L F water | 156 | 27.17 | 37.5 | % | 1.38 | 62 | 65 < | Adults |
| Dental fluorosis prevalence (> 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with > 1.5 mg/L F water | 183 | 45.74 | 52.8 | % | 1.15 | 62 | 65 < | Adults |
| Dental fluorosis prevalence | Thailand | 125 | 1.1 | 0.8 | DMFT scores | 0.73 | 66 | , | Adolescents |
| Dental fluorosis prevalence (< 1.5 mg/L water F) | Villages in Vadodara district, Gujarat, India, with < 1.5 mg/L F water | 487 | 44.15 | 45.49 | % | 1.03 | 62 | Adults | |
| Health risk | Vattamalaikarai River basin, South India | 118 | 73 | 64 | % | 0.88 | 76 | Adults |
Past studies show that, under similar conditions of exposure, boys below puberty (up to 11 years of age) are likely to suffer stronger and more prevalent adverse effects of F overexposure than girls of the same age bracket. Likewise, the literature sources show greater potential of adolescent boys (12–18 year) to suffer more severe F impacts than the girls and this discrepancy appear to increase against the male populations in older cohorts than in the younger ones.
The literature data (Table 5) collaborated the results of the current study as well as those of similar studies on toxic trends of other environmental agents. Many studies assessing sex-related differences in human responses to toxic or pharmacologic agents have substantiated the existence of differential response between males and females77. In one study, the males exhibited a greater potential to suffer greater DNA-damage from exposure to DNA damaging agents78. Yet, in another study, the differential response to toxic heavy metals in men and women indicated that differences, such being reported, can emanate both from variation in the levels and structure of exposure to the deleterious agents and from differential physiological responses of the male and female bodies to the stimulus79.
The understanding of sex differences in susceptibility to toxic impacts of fluoride present information of considerable public health importance, which can have formidable socioecological and socioeconomic implications. Nonetheless, other than the age and sex of the subjects affecting the degree and impact of F exposure among the affected communities, the physical activity levels (PAL) and the body weight (BW) of the subjects appear to exert greater effect in influencing the levels and impacts of deleterious F exposure. The risk levels of F overexposure increased with increasing PAL and the effects were magnified by greater BW. This is because both the BW and PAL increase relative food consumption by individuals and heightens the F overexposure levels from the polluted foodstuffs. Thus, the high risk of F overexposure, and the prevalence of adverse F impacts, among male populations in the current study could linked to the greater average BW, and higher average PAL, among the males than among the corresponding female populations. Higher average BW, and PAL, cause the average males to consume greater quantities of food, and water, ingesting greater amounts of F from the F-contaminated diets and resulting in greater F exposure and toxicity.
Consequently, the combined influence of sex, age, PAL and BW on F exposure was greatest in young boys than in any other category of the populations.
Conclusions
According to the findings of the current work, the following conclusions could be drawn:
The study revealed high F levels exceeding the RDA level of 4 mg/kg in potatoes (Solanum tuberosum L.), beans (Phaseolus vulgaris L.) and peas sampled from Nakuru County.
High F was recorded in the three foodstuffs from all the sub regions of the current study area but the F levels in both the beans and the potatoes varied from region to region showing that they were controlled by conditions that control F availability in the environment but the F levels of peas (Possum sativa) samples were lower and uniform across the regions showing that they were not controlled by regional variations in F levels in the environment.
The risk of F overexposure through the contaminated foodstuffs was greater in younger children than in adult populations, in male populations than in the female populations, and in individuals with higher BW and PAL. As a results, the study found that young boys could be at the greatest risk of fluoride toxicity than any other groups, especially if they also presented symptoms of above average BW.
Elevated levels of fluorides in peas, beans and potatoes have adverse public health implications (of risk of fluoride overexpose) to the resident communities through food, which calls for public awareness campaigns, especially among farmers, food producers and consumers as well as for the development and disseminate appropriate prevention and F amelioration strategies.
Acknowledgements
Authors acknowledge the contribution and support of the late Prof. Giorgio Ghiglieri of the University of Cagliari, Sardinia, Italy, who was the coordinator of the EU’s H2020 FLOWERED Project.
Abbreviations
- [F]
Fluoride concentration (mg/kg or mg/L)
- AF
Absorption factor for foodstuffs
- ANOVA
Analysis of variance
- BMR
Basal metabolic rate
- DDW
Doubly-deionized water
- DEI
Daily energy intake through food (kcal/kg/day)
- E
Dietary calorific value (kcal/kg) of food
- EADD
Estimated average daily F dosage
- EARS
Eastern African Rift Valley System
- F
Fluoride
- FEC
Percentage daily energy contribution of food
- RDA
Recommended dietary allowance
- TEE
Total energy expenditure in 24 h
- TISAB
Total ionic strength adjustment buffer
Author contributions
D.N. contacted the field and laboratory data collection and analysis and wrote the main manuscript text; E.W.W. designed the initial field and laboratory experiments, and reviewed the field and laboratory results as well as the manuscript; and J.L.K. participated in the initial design of the experiments and in the field data collection.
Funding
This work was part of the FLOWERED Research project which was supported the EU’s Horizon 2020 research and innovation programme under grant agreement No 690378.
Data availability
Available by placing requests to Dr. Enos Wambu who is the Corresponding Author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mousny M, Omelon S, Wise L, Everett ET, Dumitriu M, Holmyard DP, Banse X, Devogelaer JP, Grynpas MD. Fluoride effects on bone formation and mineralization are in fl uenced by genetics. Bone. 2008;43:1067–1074. doi: 10.1016/j.bone.2008.07.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.P. Pravina, D. Sayaji, M.A.R. in P. and, undefined 2013, Calcium and its role in human body, Academia.Edu. 4 (2013) 659–668. http://www.academia.edu/download/32830082/8.pdf.
- 3.Beigi Harchegani A, Irandoost A, Mirnamniha M, Rahmani H, Tahmasbpour E, Shahriary A. Possible mechanisms for the effects of calcium deficiency on male infertility. Int. J. Fertil. Steril. 2019;12:267–272. doi: 10.22074/ijfs.2019.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.P.H.. J.B.M.. S.C.T. et al Gary, (1990) The New England Journal of Medicine Downloaded from nejm.org on April 1, 2015. For personal use only. No other uses without permission. Copyright © 1990 Massachusetts Medical Society. All rights reserved., New English J. Med. 323, 1120–1123.
- 5.Choubisa SL. Fluoride toxicosis in immature herbivorous domestic animals living in low fluoride water endemic areas of Rajasthan, India: An observational survey. Fluoride. 2013;46:19–24. [Google Scholar]
- 6.Liu H, Gao Y, Sun L, Li M, Li B, Sun D. International Journal of Hygiene and Assessment of relationship on excess fluoride intake from drinking water and carotid atherosclerosis development in adults in fluoride endemic areas, China. Int. J. Hyg. Environ. Health. 2014;217:413–420. doi: 10.1016/j.ijheh.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Singh P, Barjatiya M, Dhing S, Bhatnagar R, Kothari S, Dhar V. Evidence suggesting that high intake of fluoride provokes nephrolithiasis in tribal populations. Urol. Res. 2001;29:238–244. doi: 10.1007/s002400100192. [DOI] [PubMed] [Google Scholar]
- 8.Seraj B, Shahrabi M, Shadfar M, Ahmadi R, Fallahzadeh M, Eslamlu HF, Kharazifard MJ. Effect of high water fluoride concentration on the intellectual development of children in Makoo/Iran. J. Dent. 2012;9:221–229. [PMC free article] [PubMed] [Google Scholar]
- 9.Sebastian ST, Sunitha S. A cross-sectional study to assess the intelligence quotient (IQ) of school going children aged 10–12 years in villages of Mysore district, India with different fluoride levels. J. Indian Soc. Pedodontics Prev. Dent. 2015;33:307–311. doi: 10.4103/0970-4388.165682. [DOI] [PubMed] [Google Scholar]
- 10.Rocha-amador ST. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad Saude Publica. 2007;23:579–587. doi: 10.1590/S0102-311X2007001600018. [DOI] [PubMed] [Google Scholar]
- 11.Bashash M, Thomas D, Hu H, Martinez-mier EA, Sanchez BN, Basu N, Peterson KE, Ettinger AS, Wright R, Zhang Z, Liu Y, Schnaas L, Mercado-garcía A, Téllez-rojo MM, Hernández-avila M. Prenatal fluoride exposure and cognitive outcomes in children at 4 and 6–12 years of age in Mexico. Environ. Health Perspect. 2016;125:1–12. doi: 10.1289/EHP655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green R, Lanphear B, Hornung R, Flora D, Martinez-mier EA, Neufeld R, Ayotte P, Muckle G, Till C. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr. 2019;173:940–948. doi: 10.1001/jamapediatrics.2019.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Till C, Green R, Flora D, Hornung R, Martinez-mier EA, Blazer M, Farmus L, Ayotte P, Muckle G, Lanphear B. Fluoride exposure from infant formula and child IQ in a Canadian birth cohort. Environ. Int. 2020;134:105315. doi: 10.1016/j.envint.2019.105315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, An N, Huang H, Duan L, Ma J, Ding J, He T, Zhu J. Fluoride exposure and intelligence in school-age children: Evidence from different windows of exposure susceptibility. BMC Public Health. 2020;20(1):1–8. doi: 10.1186/s12889-020-09765-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Liu F, Ma J, Zhang H, Liu P, Liu Y, Xing B, Dang Y. Physiology & Behavior Fluoride exposure during development affects both cognition and emotion in mice. Physiol. Behav. 2014;124:1–7. doi: 10.1016/j.physbeh.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Zhang H, He J, Chen X, Ding Y, Wang Y, Liu X. Effects of sodium fluoride on reproductive function in female rats. Food Chem. Toxicol. 2013;56:297–303. doi: 10.1016/j.fct.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Wan S, Zhang J, Wang J. Effects of high fluoride on sperm quality and testicular histology in male rats. Fluoride. 2006;39:17–21. [Google Scholar]
- 18.Verma RJ, Sherlin DMG. Vitamin C ameliorates fluoride-induced embryotoxicity in pregnant rats. Hum. Exp. Toxicol. 2001;20:619–623. doi: 10.1191/096032701718890559. [DOI] [PubMed] [Google Scholar]
- 19.Malin AJ, Riddell J, Mccague H, Till C. Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environ. Int. 2018;121:667–674. doi: 10.1016/j.envint.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Weng C, Tsai C, Chu S, Sharma YC. Adsorption characteristics of copper (II) onto spent activated clay. Sep. Purif. Technol. 2007;54:187–197. doi: 10.1016/j.seppur.2006.09.009. [DOI] [Google Scholar]
- 21.Menya D, Maina SK, Kibosia C, Kigen N, Oduor M, Some F, Chumba D, Ayuo P, Middleton DRS, Osano O, Abedi-ardekani B, Schüz J, Mccormack VA. Dental fl uorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case—Control study and a pan-African perspective. Int. J. Cancer. 2019 doi: 10.1002/ijc.32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO, Fluorides and Oral Health -Report of a WHO Expert Committee on Oral Health Status and Fluoride Use, Geneva, 1994. [PubMed]
- 23.Gevera P, Mouri H. Natural occurrence of potentially harmful fluoride contamination in groundwater: An example from Nakuru County, the Kenyan Rift Valley. Environ. Earth Sci. 2018;77:265. doi: 10.1007/s12665-018-7466-7. [DOI] [Google Scholar]
- 24.WHO, Guidelines for drinking-water quality, third edition, incorporating first and second addenda, Geneva, 1984. [PubMed]
- 25.KEBS, Potable water -Specifications:, Nairobi, Kenya, 2011. 10.1007/springerreference_29699.
- 26.ATSDR, Toxicological Profile for Fluorides , Hydrogen Fluoride and Fluorine, Atlanta, Georgia, 2003. [PubMed]
- 27.Wambu EW, Muthakia GK. High fluoride water in the Gilgil area of Nakuru County, Kenya. Fluoride. 2011;44:37–41. [Google Scholar]
- 28.Maina M, Wambu E, Lusweti JK. Analysis of Fluoride in Kales (Brassicca oleracea) and Tomatoes (Lycopersicum esculentum) from Nakuru County, Kenya, Africa. Environ. Rev. 2020;4:35–42. [Google Scholar]
- 29.Asembo EO, Oliech GO, Wambu EW, Waddams KE, Ayieko PO. Dental fluorosis in ruminants and fluoride. Fluoride. 2021;54:141–155. [Google Scholar]
- 30.Gevera PK, Cave M, Dowling K, Gikuma-Njuru P, Mouri H. Potential fluoride exposure from selected food crops grown in high fluoride soils in the Makueni County, South-Eastern Kenya. Environ. Geochem. Health. 2022;44:4703–4717. doi: 10.1007/s10653-022-01240-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzu M, Tanda A, Canu L, Masawe K, Mtei K, Deroma MA, Roggeroa PP, Seddaiu G. Fluoride uptake and translocation in food crops grown in fluoride-rich soils. J. Sci. Food Agric. 2020;100:5498–5509. doi: 10.1002/jsfa.10601. [DOI] [PubMed] [Google Scholar]
- 32.County Government of Nakuru, County Integrated Development Plan (CIDP 2018–2022), Nakuru, Kenya, 2018.
- 33.Wakachala F, Shilenje ZW, Apondo W, Wakachala FM, Nguyo J, Shaka S. Statistical patterns of rainfall variability in the great rift valley of Kenya. J. Environ. Agric. Sci. 2015;5:17–26. [Google Scholar]
- 34.KNBS, 2019 Kenya Population and Housing Census Volume I : Population By County and Sub-County, 2019th ed., Kenya National Bureau of Statistics (KNBS), Nairobi, Kenya, 2019. http://www.knbs.or.ke.
- 35.Shorter JP, Massawe J, Parry N, Walker RW. Comparison of two village primary schools in northern Tanzania affected by fluorosis. Int. Health. 2010;2:269–274. doi: 10.1016/j.inhe.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Secretariat of the International Plant Protection Convention (IPPC), Methodologies for sampling of consignments, 31 (2008) 1–24.
- 37.Sampling soil, vegetables, fruit and grain for residue testing, n.d.
- 38.Wambu EW, Agong SG, Anyango B, Akuno W, Akenga T. High fluoride water in Bondo-Rarieda area of Siaya County, Kenya: A hydro-geological implication on public health in the Lake Victoria Basin. BMC Public Health. 2014 doi: 10.1186/1471-2458-14-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam M, Patel RKK. Evaluation of removal efficiency of fluoride from aqueous solution using quick lime. J. Hazard. Mater. 2007;143:303–310. doi: 10.1016/j.jhazmat.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 40.M. Rizzu, M.H. Akuno, P.P. Roggero, E.W.Wambu, K.M. Mtei, G. Seddaiu, Characterizing cropping systems affected by fluoride contamination in East African Countries, in: F. Ventura, G.Seddaiu (Eds.), Proc. XX ALAM Conf. XLVI SLA Conv. Milan, 12–14 Sept. 2017, Milan, Italy, 2017: pp. 219–221.
- 41.Action Against Hunger (ACF-USA) and Ministry of Health (KENYA), Nutritional Anthropometric Survey Final Report for Nakuru, Molo, Nakuru North aAnd Naivasha Districts, Rift Valley Province, Kenya, Action Against Hunger (ACF-USA) and Ministry of Health (KENYA), Nakuru, Kenya, 2008.
- 42.FAO/WHO/UNU Expert Consultation, Human energy requirements: report of a joint FAO/ WHO/UNU Expert Consultation, Rome 17-24th October, 2001, Rome, Italy, 2001.
- 43.Prystupa J. Fluorine—A current literature review. An NRC and ATSDR based review of safety standards for exposure to fluorine and fluorides. Toxicol. Mech. Methods. 2011;21:103–170. doi: 10.3109/15376516.2010.542931. [DOI] [PubMed] [Google Scholar]
- 44.Gudima VO, Wambu EW, Lagat G, Waddams KE. Fluoride in Chicken (Gallus domesticus) Feathers from Nakuru, Kenya. African Environ. Rev. J. 2021;4:73–81. [Google Scholar]
- 45.National Center for Health Statistics, Data Table of Weight-for-age Charts, Atlanta -Giorgia, 2000.
- 46.US Institute of Medicine . Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. National Academies Press; 2006. [Google Scholar]
- 47.Gevera P, Mouri H. Natural occurrence of potentially harmful fluoride contamination in groundwater: An example from Nakuru County, the Kenyan Rift Valley. Environ Earth Sci. 2018;77:1–19. doi: 10.1007/s12665-018-7466-7. [DOI] [Google Scholar]
- 48.Opinya GN, Bwibo N, Valderhaug J, Birkeland JM, Lökken PD. Intake of fluoride and excretion in mothers ’ milk in a high fluoride (9 ppm ) area in Kenya. Eur. J. Clin. Nutr. 1991;45:37–41. [PubMed] [Google Scholar]
- 49.Li Y, Liang C, Slemenda CW, Ji R, Sun S, Cao J, Emsley CL, Ma F, Wu Y, Ying PO, Zhang YAN, Gao S, Zhang WU, Katz BP, Niu S, Cao S, Johnston CC. Effect of long-term exposure to fluoride in drinking water on risks of bone fractures. J. Bone Miner. Res. 2001;16:932–939. doi: 10.1359/jbmr.2001.16.5.932. [DOI] [PubMed] [Google Scholar]
- 50.Montcoudiol N, Burnside NM, Mutia T, Boyce A, Györe D, Mariita N. Surface and groundwater hydrochemistry of the Menengai Caldera geothermal field and surrounding Nakuru County, Kenya. Energies. 2019;12:3131. doi: 10.3390/en12163131. [DOI] [Google Scholar]
- 51.Treshow M, Harner FM. Growth responses of Pinto bean and alfalfa to sublethal fluoride concentrations. Can. J. Bot. 1968;46:1207–1210. doi: 10.1139/b68-161. [DOI] [Google Scholar]
- 52.Das C, Dey U, Chakraborty D, Datta JK, Mondal NK. Fluoride toxicity effects in potato plant (solanum Solanum tuberosum L.) Grown in contaminated soils. Octa J. Environ. Res. 2015;3:136–143. [Google Scholar]
- 53.Li Y, Bi Y, Mi W, Xie S, Ji L. Land-use change caused by anthropogenic activities increase fluoride and arsenic pollution in groundwater and human health risk. J. Hazard. Mater. 2021 doi: 10.1016/j.jhazmat.2020.124337. [DOI] [PubMed] [Google Scholar]
- 54.P. Omenda, S.A. Oncaha, W.J. Ambusso, Ocuurrence and distribution of high temperature geothermal systems in Kenya, in: Proc. 15th NZ Geathermal Work., University of Aukland, Geothermal Institute, Auckland, Newzealand, 1993: pp. 241–246.
- 55.Leat PT. Volcanological development of the Nakuru area of the Kenya rift valley. J. African Earth Sci. (and Middle East) 1991;13:483–498. doi: 10.1016/0899-5362(91)90111-B. [DOI] [Google Scholar]
- 56.Arnesen AKM. Availability of fluoride to plants grown in contaminated soils. Plant Soil. 1997;191:13–25. doi: 10.1023/A:1004210713596. [DOI] [Google Scholar]
- 57.Takmaz-Nisancioglu S, Davison AW. Effects of aluminium on fluoride uptake by plants. New Phytol. 1988;109:149–155. doi: 10.1111/j.1469-8137.1988.tb03702.x. [DOI] [Google Scholar]
- 58.Ontumbi MG, Ucakuwun EK, Munyao MT. Variation of fluoride levels in surface geology: A study of river Njoro Catchment, Kenya. African J. Educ Sci. Technol. 2020;6:1–6. [Google Scholar]
- 59.G. Ghiglieri, S.D.A. Pelo, M. Pistis, M.T. Melis, F. Dessì, G. Oggiano, B. Abebe, T. Azazegn, Geological and hydrogeological features controlling mechanisms of fluoride enrichment in groundwater in the east african rift system, in: 3rd Natl. Meet. Hydrogeol. Cagliari, 14–16 June 2017, Flowpath and AH Italian Chapter, Cagliari, Sardinia, Italy, 2017: p. 1.
- 60.Ruan JY, Wong MH. Accumulation of fluoride and aluminium related to different varieties of tea. Environ. Geochem. Health. 2001;23:53–63. doi: 10.1023/A:1011082608631. [DOI] [Google Scholar]
- 61.Mutahir A, Bisht M, Baunthiyal M. Comparative assessment of fluoride tolerance in two genotypes of Zea mays. J. Med. Plants Stud. 2016;4:253–258. [Google Scholar]
- 62.Kotecha PV, Patel SV, Bhalani KD, Shah D, Shah VS, Mehta KG. Prevalence of dental fluorosis & dental caries in association with high levels of drinking water fluoride content in a district of Gujarat, India. Indian J. Med. Res. 2012;135:873–877. [PMC free article] [PubMed] [Google Scholar]
- 63.Ray SK, Ghosh S, Tiwari IC, Kaur P, Reddy DCS, Nagchaudhuri J. Dental fluorosis in Ledhupur and Rustampur villages near Varanasi. Indian J. Med. Res. 1983;77:112–118. [PubMed] [Google Scholar]
- 64.Gautam R, Bhardwaj N, Saini Y. Dental fluorosis-a case study from Nawa tehsil in Nagaur district, Rajasthan (India) Environmentalist. 2011;31:401–406. doi: 10.1007/s10669-011-9354-5. [DOI] [Google Scholar]
- 65.Bhagavan SVBK, Raghu V. Utility of check dams in dilution of fluoride concentration in ground water and the resultant analysis of blood serum and urine of villagers, Anantapur District, Andhra Pradesh, India. Environ. Geochem. Health. 2006;27:97–108. doi: 10.1007/s10653-004-0786-4. [DOI] [PubMed] [Google Scholar]
- 66.Leatherwood EC, George WA, Burnett DDS, Chandravejismarn R, Sirikaya P. Dental caries and dental fluorosis in Thailand. Am. J. Public Health. 1965;55:1792–1799. doi: 10.2105/AJPH.55.11.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sunil-Tejaswi KL, Suneeth S, Annapoorna BM, Pujari SC, Sarveshwar RP, Nandlal B. A pioneering study of dental fluorosis in the Libyan population. J. Int. Oral Health. 2013;5:67–72. [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S, Zhao Q, Li G, Wang M, Liu H, Yu X, Chen J, Li P, Dong L, Zhou G, Cui Y, Wang M, Liu L, Wang A. The cholinergic system, intelligence, and dental fluorosis in school-aged children with low-to-moderate fluoride exposure. Ecotoxicol. Environ. Saf. 2021;228:1–9. doi: 10.1016/j.ecoenv.2021.112959. [DOI] [PubMed] [Google Scholar]
- 69.Mann J, Mahmoud W, Ernest M, Sgan-Cohen H, Shoshan N, Gedaiia I. Fluorosis and dental caries in 6-8-year-old children in a 5 ppm fluoride area. Community Dent. Oral Epidemiol. 1990;18:77–79. doi: 10.1111/j.1600-0528.1990.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 70.Cao J, Bail X, Zhao Y, Liu J, Zhou D, Fang S, Jia M, Wu J. The relationship of fluorosis and brick tea drinking in Chinese Tibetans. Environ. Health Perspect. 1996;104:1340–1343. doi: 10.1289/ehp.961041340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gopalakrishnan P, Vasan RS, Sarma PS, Ravindran-Nair KS. Original articles. Natl. Med. J. India. 1999;12:99–103. [PubMed] [Google Scholar]
- 72.Keçeci AD, Kaya BÜ, Güldaş E, Sarıtekin E, E. Şener, B Isparta Evaluation of dental fluorosis in relation to DMFT rates in a fluoritic rural area of Turkey. Fluoride. 2014;47:119–132. [Google Scholar]
- 73.Verma A, Shetty BK, Guddattu V, Chourasia MK, Pundir P. High prevalence of dental fluorosis among adolescents is a growing concern: A school based cross-sectional study from Southern India, Environ. Health. Prev. Med. 2017;22:1–7. doi: 10.1186/s12199-017-0624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sukhabogi JR, Parthasarathi P, Anjum S, Chandra Shekar BR. Prevalence of dental caries and dental fluorosis among 12 and15 year-old school children in an endemic fluoride area of Nalgonda district, Andhra Pradesh, India. Ann. Trop. Med. Public Health. 2013;6:422–429. doi: 10.4103/1755-6783.127785. [DOI] [Google Scholar]
- 75.Xiang QY, Chen LS, Chen XD, Wang CS, Liang YX, Liao QL, Fan DF, Hong P, Zhang MF. Serum fluoride and skeletal fluorosis in two villages in Jiangsu Province, China. Fluoride. 2005;38:178–184. [Google Scholar]
- 76.Arya S, Subramani T, Vennila G, Karunanidhi D. Health risks associated with fluoride intake from rural drinking water supply and inverse mass balance modeling to decipher hydrogeochemical processes in Vattamalaikarai River basin, South India. Environ. Geochem. Health. 2021;43:705–716. doi: 10.1007/s10653-019-00489-y. [DOI] [PubMed] [Google Scholar]
- 77.Calabrese EJ. Sex differences in susceptibility to toxic industrial chemicals. Br. J. Ind. Med. 1986;43:577–579. doi: 10.1136/oem.43.9.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pero RW, Bryngelsson C, Mitelman F, Kornfalt R, Thulin T, Norden A. Interindividual variation in the responses of cultured human lymphocytes to exposure from dna damaging chemical agents. Mutat. Res. 1978;53:327–341. doi: 10.1016/0165-1161(78)90005-5. [DOI] [PubMed] [Google Scholar]
- 79.Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available by placing requests to Dr. Enos Wambu who is the Corresponding Author.