Abstract
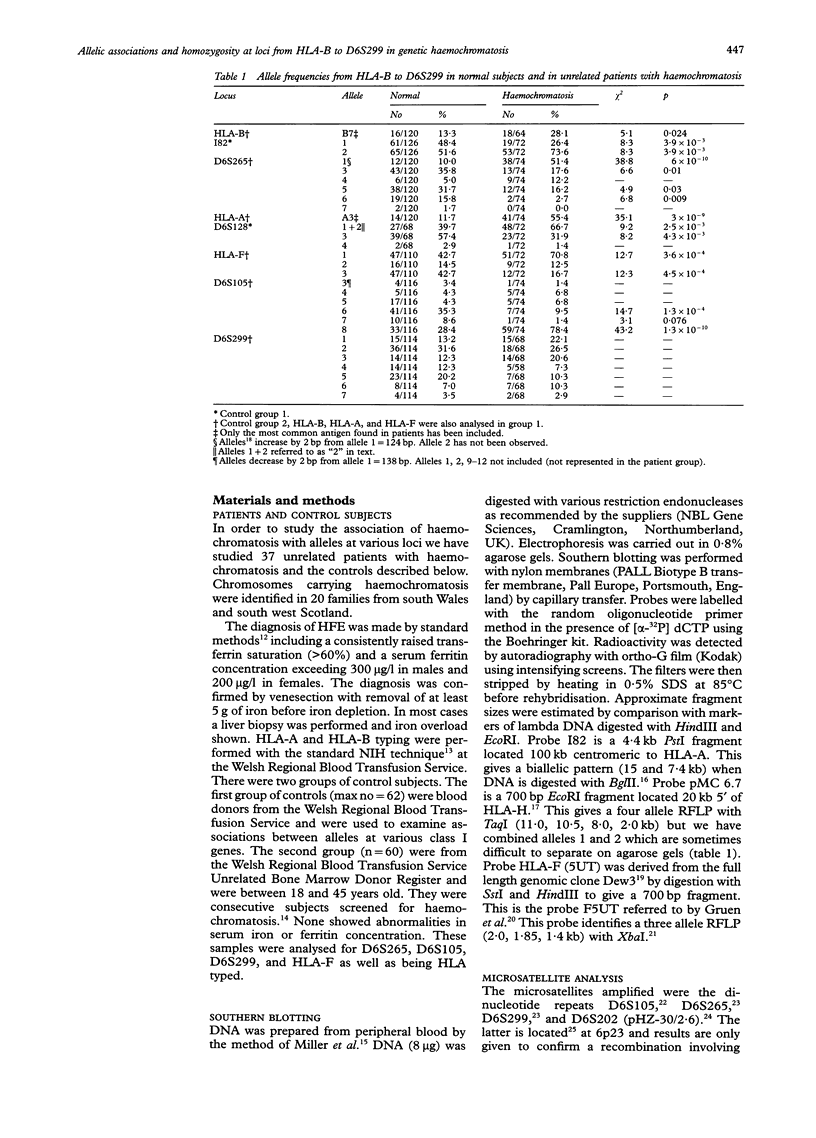
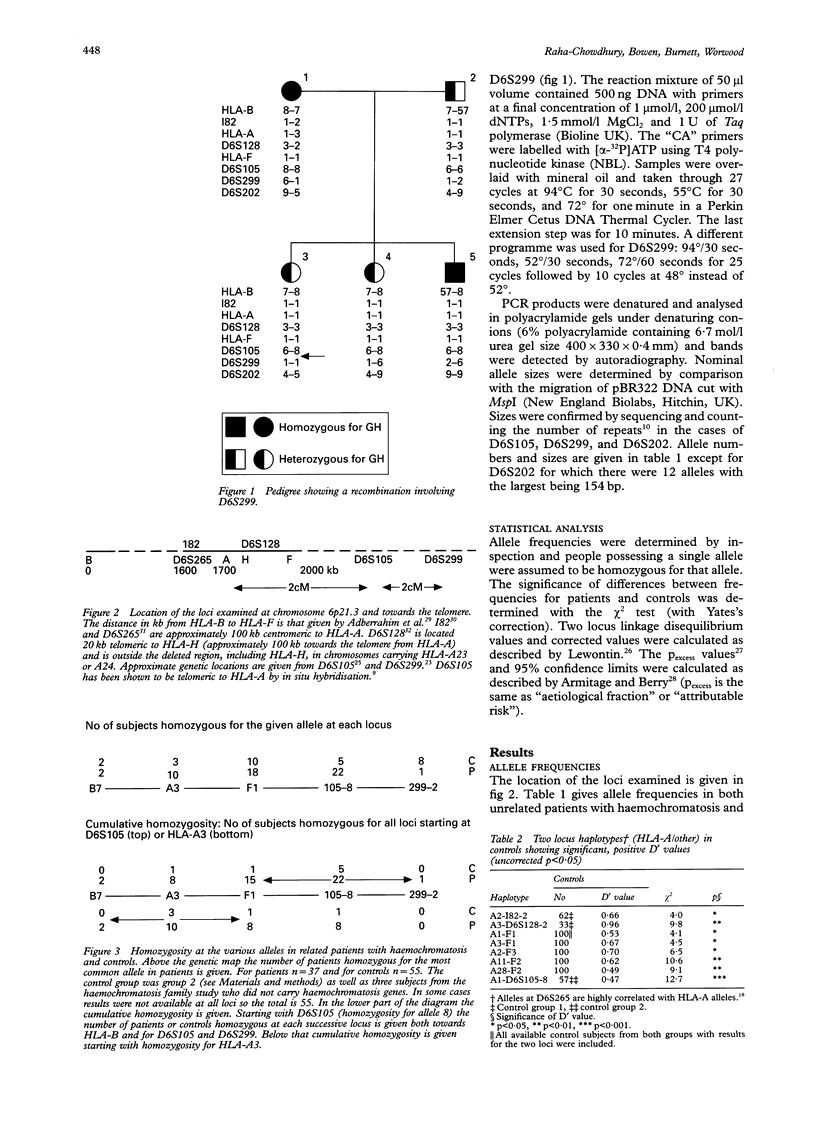
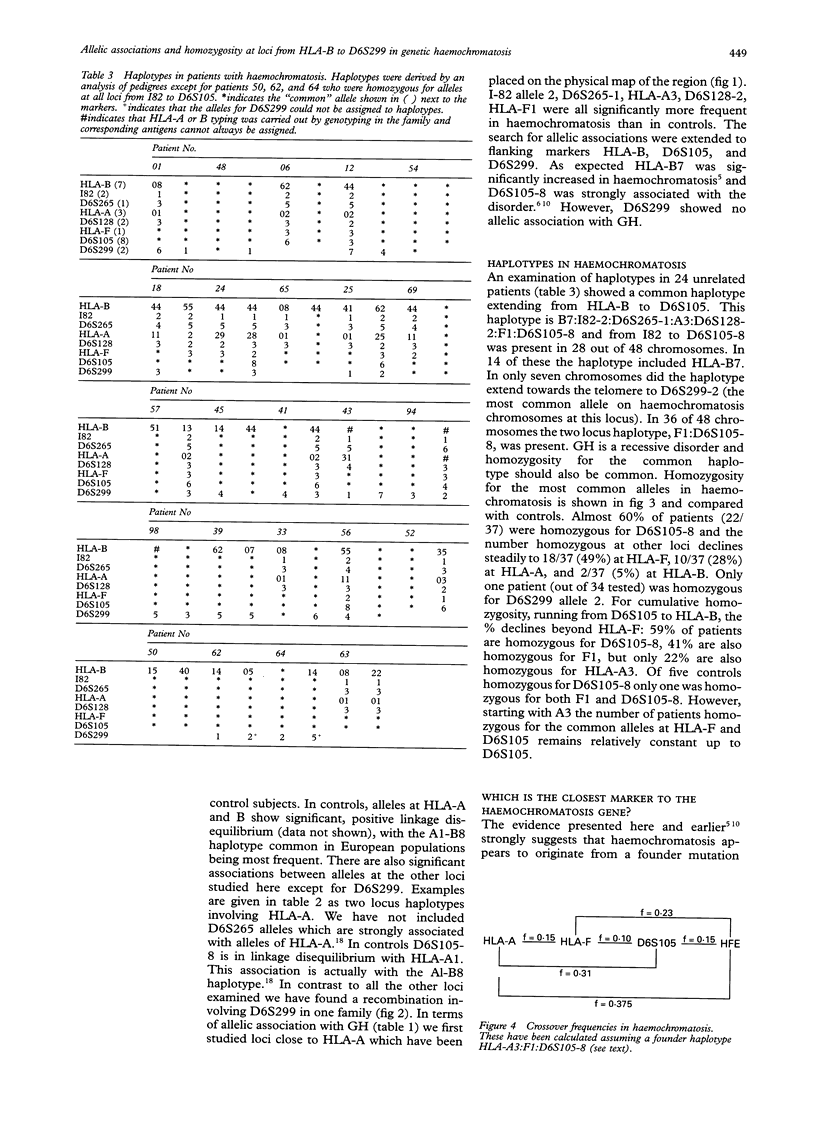
Haemochromatosis (GH) is an autosomal recessive disorder in which increased iron absorption causes iron overload. The gene (HFE) is closely linked to HLA-A on chromosome 6 (6p21.3) but has not yet been identified. We have examined eight polymorphic loci, HLA-B (most centromeric), I82, D6S265, HLA-A, D6S128, HLA-F, D6S105, and D6S299 (most telomeric) in 37 unrelated patients and 60 control subjects. There are also significant positive associations between GH and alleles at all loci except D6S299. Analysis of 48 GH chromosomes in which haplotypes could be established showed that the most common haplotype was I82-2:D6S265-1:HLA-A3:D6S128-2:HLA-F1:D6S105-8. This was present in 28 of 48 chromosomes. In 14 the haplotype included HLA-B7 but only in seven did this extend beyond the telomere to D6S299-2 (the most common allele on GH chromosomes at this locus). In 36 out of 48 chromosomes the two locus haplotype, F1:D6S105-8 was present. Since haemochromatosis appears to originate from a founder mutation we have examined linkage disequilibrium between these various loci and GH using calculations of pexcess. The maximum value (0.72, 95% CI 0.55-0.85) is given by D6S105-8 but is not significantly different from values for HLA-A3 and HLA-F1 (0.50, 95% CI 0.34-0.61 and 0.49, 0.25-0.66 respectively). However, both HLA-A and D6S105 give a value for pexcess which is significantly higher than that for the most centromeric marker, HLA-B (0.17, 95% CI 0.02-0.30). We have counted the number of patients who are homozygous for the common allele at each locus. At D6S105, 22 patients are homozygous for allele 8, with 18 homozygous for HLA-F1 and 10 homozygous for A3. The pattern of cumulative homozygosity suggests a gene location closer to D6S105 than HLA-A. We have also analysed our data for divergence from the apparent founder haplotype (A3:F1:105-8) and have calculated the theoretical frequencies of crossovers between loci. These data suggest a location telomeric to D6S105. A more precise localisation of the gene may be possible with the identification of new markers around D6S105.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abderrahim H., Sambucy J. L., Iris F., Ougen P., Billault A., Chumakov I. M., Dausset J., Cohen D., Le Paslier D. Cloning the human major histocompatibility complex in YACs. Genomics. 1994 Oct;23(3):520–527. doi: 10.1006/geno.1994.1538. [DOI] [PubMed] [Google Scholar]
- Boretto J., Jouanolle A. M., Yaouanq J., el Kahloun A., Mauvieux V., Blayau M., Perichon M., Le Treut A., Clayton J., Borot N. Anonymous markers located on chromosome 6 in the HLA-A class I region: allelic distribution in genetic haemochromatosis. Hum Genet. 1992 Apr;89(1):33–36. doi: 10.1007/BF00207038. [DOI] [PubMed] [Google Scholar]
- Chorney M. J., Sawada I., Gillespie G. A., Srivastava R., Pan J., Weissman S. M. Transcription analysis, physical mapping, and molecular characterization of a nonclassical human leukocyte antigen class I gene. Mol Cell Biol. 1990 Jan;10(1):243–253. doi: 10.1128/mcb.10.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. Q., Skolnick M. H., Kushner J. P. Hereditary hemochromatosis: contributions of genetic analyses. Prog Hematol. 1981;12:43–71. [PubMed] [Google Scholar]
- Geraghty D. E., Koller B. H., Hansen J. A., Orr H. T. The HLA class I gene family includes at least six genes and twelve pseudogenes and gene fragments. J Immunol. 1992 Sep 15;149(6):1934–1946. [PubMed] [Google Scholar]
- Geraghty D. E., Wei X. H., Orr H. T., Koller B. H. Human leukocyte antigen F (HLA-F). An expressed HLA gene composed of a class I coding sequence linked to a novel transcribed repetitive element. J Exp Med. 1990 Jan 1;171(1):1–18. doi: 10.1084/jem.171.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen J. R., Goei V. L., Summers K. M., Capossela A., Powell L., Halliday J., Zoghbi H., Shukla H., Weissman S. M. Physical and genetic mapping of the telomeric major histocompatibility complex region in man and relevance to the primary hemochromatosis gene (HFE). Genomics. 1992 Oct;14(2):232–240. doi: 10.1016/s0888-7543(05)80211-3. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hästbacka J., de la Chapelle A., Mahtani M. M., Clines G., Reeve-Daly M. P., Daly M., Hamilton B. A., Kusumi K., Trivedi B., Weaver A. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994 Sep 23;78(6):1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Jazwinska E. C., Lee S. C., Webb S. I., Halliday J. W., Powell L. W. Localization of the hemochromatosis gene close to D6S105. Am J Hum Genet. 1993 Aug;53(2):347–352. [PMC free article] [PubMed] [Google Scholar]
- Le Borgne-Demarquoy F., Kwiatowski T. J., Jr, Zoghbi H. Y. Two dinucleotide repeat polymorphisms at the D6S202 locus. Nucleic Acids Res. 1991 Nov 11;19(21):6060–6060. doi: 10.1093/nar/19.21.6060-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin R. C. On measures of gametic disequilibrium. Genetics. 1988 Nov;120(3):849–852. doi: 10.1093/genetics/120.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lury D., Epstein H., Holmes N. The human class I MHC gene HLA-F is expressed in lymphocytes. Int Immunol. 1990;2(6):531–537. doi: 10.1093/intimm/2.6.531. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C., Fischer R., Sonnenberg A., Stremmel W., Trampisch H. J., Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985 Nov 14;313(20):1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- Powell L. W., Summers K. M., Board P. G., Axelsen E., Webb S., Halliday J. W. Expression of hemochromatosis in homozygous subjects. Implications for early diagnosis and prevention. Gastroenterology. 1990 Jun;98(6):1625–1632. doi: 10.1016/0016-5085(90)91100-k. [DOI] [PubMed] [Google Scholar]
- Raha-Chowdhury R., Tigue N. J., Worwood M. Trinucleotide repeat microsatellite in the 5' untranslated region of HLA-F. Hum Mol Genet. 1994 Nov;3(11):2084–2084. [PubMed] [Google Scholar]
- Simon M., Le Mignon L., Fauchet R., Yaouanq J., David V., Edan G., Bourel M. A study of 609 HLA haplotypes marking for the hemochromatosis gene: (1) mapping of the gene near the HLA-A locus and characters required to define a heterozygous population and (2) hypothesis concerning the underlying cause of hemochromatosis-HLA association. Am J Hum Genet. 1987 Aug;41(2):89–105. [PMC free article] [PubMed] [Google Scholar]
- Stone C., Pointon J. J., Jazwinska E. C., Halliday J. W., Powell L. W., Robson K. J., Monaco A. P., Weatherall D. J. Isolation of CA dinucleotide repeats close to D6S105; linkage disequilibrium with haemochromatosis. Hum Mol Genet. 1994 Nov;3(11):2043–2046. [PubMed] [Google Scholar]
- Terasaki P. I., Bernoco D., Park M. S., Ozturk G., Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol. 1978 Feb;69(2):103–120. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- Venditti C. P., Chorney M. J. Class I gene contraction within the HLA-A subregion of the human MHC. Genomics. 1992 Dec;14(4):1003–1009. doi: 10.1016/s0888-7543(05)80123-5. [DOI] [PubMed] [Google Scholar]
- Volz A., Boyle J. M., Cann H. M., Cottingham R. W., Orr H. T., Ziegler A. Report of the Second International Workshop on Human Chromosome 6. Genomics. 1994 May 15;21(2):464–472. doi: 10.1006/geno.1994.1302. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E., Zoghbi H. Y. Dinucleotide repeat polymorphism at the D6S105 locus. Nucleic Acids Res. 1991 Feb 25;19(4):968–968. doi: 10.1093/nar/19.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Fan W. F., Xu H., Parimoo S., Shukla H., Chaplin D. D., Weissman S. M. Genes in one megabase of the HLA class I region. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11870–11874. doi: 10.1073/pnas.90.24.11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worwood M., Darke C. Serum ferritin, blood donation, iron stores and haemochromatosis. Transfus Med. 1993 Mar;3(1):21–28. doi: 10.1111/j.1365-3148.1993.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Worwood M., Dorak M. T., Raha-Chowdhury R. Haplotypes in linkage disequilibrium with the hemochromatosis gene. Am J Hum Genet. 1994 Sep;55(3):585–586. [PMC free article] [PubMed] [Google Scholar]
- Worwood M., Raha-Chowdhury R., Darke C. Distribution of alleles at D6S105 and D6S265 with possible HLA haplotype associations. Tissue Antigens. 1994 Nov;44(5):322–325. doi: 10.1111/j.1399-0039.1994.tb02403.x. [DOI] [PubMed] [Google Scholar]
- Worwood M., Raha-Chowdhury R., Dorak M. T., Darke C., Bowen D. J., Burnett A. K. Alleles at D6S265 and D6S105 define a haemochromatosis-specific genotype. Br J Haematol. 1994 Apr;86(4):863–866. doi: 10.1111/j.1365-2141.1994.tb04843.x. [DOI] [PubMed] [Google Scholar]
- Yaouanq J., Perichon M., Chorney M., Pontarotti P., Le Treut A., el Kahloun A., Mauvieux V., Blayau M., Jouanolle A. M., Chauvel B. Anonymous marker loci within 400 kb of HLA-A generate haplotypes in linkage disequilibrium with the hemochromatosis gene (HFE) Am J Hum Genet. 1994 Feb;54(2):252–263. [PMC free article] [PubMed] [Google Scholar]
- el Kahloun A., Chauvel B., Mauvieux V., Dorval I., Jouanolle A. M., Gicquel I., Le Gall J. Y., David V. Localization of seven new genes around the HLA-A locus. Hum Mol Genet. 1993 Jan;2(1):55–60. doi: 10.1093/hmg/2.1.55. [DOI] [PubMed] [Google Scholar]
- el Kahloun A., Jouanolle A. M., Chorney M., Mauvieux V., Gicquel I., Pontarotti P., David V. A new polymorphic probe close to HLA-A. Nucleic Acids Res. 1991 Sep 25;19(18):5100–5100. doi: 10.1093/nar/19.18.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]


